
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 04 January 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1337159
The purple sea urchin (Heliocidaris crassispina) is one of the main drivers of ecosystem dynamics, and its reproductive cycle plays an important role in population structure and size variability. In this study, we analyzed the population structure, gonadal developmental characteristics, reproductive cycle and other factors influencing wild purple sea urchins from December 2021 to November 2022 in Daya Bay, Guangdong, China. The results showed that purple sea urchins showed an allometric growth pattern, there were obvious seasonal variations in the population size, and fishing pressure reduced the sea urchin populations; two-factor analysis of variance (ANOVA) and correlation analyses showed negative correlations between month (M), test diameter (TD), weight (W) and gonadal index (GI) (P> 0.01), and a positive correlation between TD, weight (W), and GI (P<0.01); the gonadal development process can be divided into recovery, growth, prematuration, maturation, and late discharge, with almost synchronous development of male and female gametes; the spawning period is from April to November, which is longer than in the temperate zones, showing two spawning peaks in April and August. The time of spawning is influenced by temperature and food. Data on the breeding cycle of sea urchins in Daya Bay were obtained in this study. This information can offer theoretical assistance in the preservation and management of both artificial nurseries and wild resources.
Purple sea urchins (Heliocidaris crassispina) are marine invertebrates belonging to the order Echinodermata (G, J. S, 1936). They live mainly on the seabed of rocky reefs, gravel and sandy or rocky substrates in the shallow sea, feeding mainly on brown, red and green algae (Cárcamo, 2015). The gonads, the only edible part of the sea urchin (O’Hara and Thórarinsdóttir, 2021), contain high-quality proteins that are also rich in nutrients (Ciriminna et al., 2021) and have high commercial value. However, the number of wild sea urchins has been drastically reduced due to overfishing (Pais et al., 2011; Lawrence, 2013) and declining food resources (Yeruham et al., 2019). Currently, many studies on sea urchins have focused on reproduction (Hassan et al., 2022), food resources (Takagi et al., 2022) and the effects of environmental factors (Chi et al., 2021).
Sea urchin reproduction is influenced by various factors, including location (Urriago et al., 2016), season (O’Hara and Thórarinsdóttir, 2021), and food (Ciriminna et al., 2021). Additionally, gonadal qualities are affected by the reproductive cycle and spawning time (Xi et al., 2020). The fecundity of sea urchins is also influenced by various factors, such as their feeding patterns, food organisms, and the environments where they reside. Additionally, gonadal fecundity also varies according to shell diameter and weight, maturity of gonads, intestinal fullness, sex, and lipid content, among other factors (Zhao et al., 2016). Scholars worldwide have frequently employed the external morphology and internal organization of sea urchin gonads as the standard for the staging of gonadal development.
Fujisaki (1969) classified gonadal development in sea urchins into five distinct periods by analyzing the external morphology of the gonad and the characteristics of sectioned tissue. Similarly, Byrne (1990) subdivided gonadal development into six periods by focusing on morphological characteristics and the number of gametes. Urriago et al. (2016) identified four stages of sea urchin gonadal development by analyzing the changing activities of germ cells and trophoblasts. Environmental factors, particularly temperature and light, are crucial in influencing the reproductive cycle and spawning of sea urchins (Kong et al., 2017; Johnstone et al., 2019). Therefore, sea urchin spawning can be stimulated by manipulating these environmental factors appropriately. Temperature plays a significant role in the feeding rate, metabolic rate, and nutrient and energy utilization efficiency of marine organisms, which are processes essential for their growth (Han et al., 2023). However, the majority of current research on temperature and sea urchins is limited to laboratory settings (Wei et al., 2016; Gouda and Agatsuma, 2020), with less emphasis placed on studying the correlation between sea urchin gonadal development and temperature under natural conditions.
Sea urchins in China are predominantly studied in the northern region of the country, although the abundant sea urchin resources in the southern territory deserve research attention as well. The purple sea urchin is a vital commercially exploited species in the South China Sea (Liang et al., 2023). It resides in the low intertidal and shallow subtidal areas along the southern coast of China (Freeman, 2003) and adequately grows at a water temperature of 15~30°C and a salinity of 25~30 ppt. It has a narrow salinity adaptation range, and is a warm-temperature species of great commercial potential. Daya Bay, located in Guangdong Province, is one of the largest and most significant semienclosed bays on the coast of South China Sea (Zhu et al., 2020). Its favorable geomorphological and climatic conditions have transformed it into a crucial reserve of aquatic germplasm resources in the region (Li et al., 2016).
Currently, the majority of research on purple sea urchins in Daya Bay concentrates on artificial breeding (Chen et al., 2021), adult rearing (Chen et al., 2003), food (Xi et al., 2020), and feeding behavior (Mo et al., 2017). Insufficient attention has been given to the reproductive cycle, gonadal development and population structure. Hence, this investigation gathered specimens of purple sea urchins over various months in Daya Bay as the study area. By analyzing the changing population structure of purple sea urchins in Daya Bay, we have gained further insight into the characteristics of their gonadal development and reproductive cycle. We have also interpreted the effects of temperature and food on gonadal development. The study’s findings can provide fundamental data for the upcoming large-scale breeding of purple sea urchins.
Throughout this research, biological samples of purple sea urchins were collected in the Daya Bay area located in Guangdong Province, China, between December 2021 and November 2022 (Figure 1, 12 stations). Each time, a total of 30-35 adult purple sea urchins were obtained. To obtain the environmental data in the sampling area, we utilized a YSI water quality meter (YSI, USA) to measure seawater temperature (T), salinity (Sal), pH, and dissolved oxygen (DO). Additionally, seawater depth data were measured with a thermocline depth sensor (CTD, USA). The months of February and March 2022 were impacted by COVID-19, and no samples were obtained during this time.
A total of 301 purple sea urchins were collected between 2021 and 2022. The sea urchins were collected manually by divers close to the shore and immediately transported in foam boxes covered with wet cloths to the laboratory for processing. In the laboratory, any algae or shells attached to the sea urchins were removed. Measurements of diameter/length (TD mm) (horizontal test diameter excluding spines) and total body weight (TW g) were taken to the nearest 0.01 mm and 0.01 g, respectively.
The length-weight relationship of all specimens was estimated using the following equation:
where W is the body weight (g), L is the test diameter (TD cm), and a and b are constants. The a-value is a conditioning factor for growth, which is higher when environmental conditions such as food base and hydrology are better; the b-value is used to determine the type of growth, indicating uniform growth if there is no significant difference between the b-value and 3, and allometric growth if the opposite is true (Zhan, 1995).
Fifteen to twenty individuals of the more vigorous purple sea urchins were collected monthly for gonad dissection. Their gonads were removed using dissecting tools such as scissors and tweezers, and as the sex could not be determined with the naked eye, no sex examination or recording of the gonad samples was carried out. The gonads were weighed after dissection, and an intact piece of gonad was selected and fixed in 4% paraformaldehyde fixative for 24 hours and then sent to Wuhan Service Biologicals for tissue sectioning.
Fixed purple sea urchin gonad tissues were cut into 1 cm thick sections, graded, dehydrated and embedded in paraffin according to conventional histological techniques (Delgado and Pérez-Camacho, 2005). Sections of 5 μm thickness were prepared and fixed on slides with neutral gum for H&E staining, followed by observation and recording of sex and stage of gonadal development using a light microscope (Olympus Corporation, CX22LEDRFS1, Tokyo, Japan). Gonadal development was divided into five stages according to the Fujisaki (Fujisaki, 1969) method: recovery, growth, prematuration, maturation and discharge.
The reproductive cycle of purple sea urchins is influenced by fluctuations in GI. GI considers gonadal wet weight and complete body wet weight. The formula for this calculation is as follows:
The data were initially entered using Excel 2019 and then subjected to normality and chi-square tests (at 95% confidence intervals) utilizing SPSS 27.0 statistical software. Two-way analysis of variance (ANOVA) was conducted for month and sex, and statistical significance was assumed when P<0.05. Pearson Correlation Analysis was then conducted for test diameter, weight, GI and month, correlation significance was assumed when P<0.01 (Two-tailed). A correlation coefficient greater than zero is positive, and a correlation coefficient less than zero is negative. A regression analysis was conducted on length-weight equations using Origin 2022 software to establish the length-weight relationship parameters. The b-value was used to determine the growth pattern and tested against b=3 (isometric growth) via the Bailey-t test. Origin 2022 was utilized for graphing.
Variation in the size of purple sea urchins in different months (Figure 2) indicated that the urchins grew gradually from December to April, reaching their largest size in April. From May to November, their growth rate was slower. In this study, the horizontal diameter (excluding spines) of the purple sea urchin tests ranged from 21.41-87.18 mm. The mean diameter was 49.94 ± 0.73 mm, with 74.41% of the samples falling in the 20-55 mm range (Figure 3).
The analysis of the length-weight (L-W) relationship involved the measurement of diameter (TD or L mm) and total body weight (TW or Wt g) of purple sea urchins (Figure 4). The L-W equation obtained is W = 0.0242L1.993 (R2 = 0.8421). A t test for the coefficient of allometric growth (b) yielded a statistically significant result at the 0.01 level of significance. The value of b<3 further confirms that the growth pattern of purple sea urchins are allometric at this stage.
Correlation analysis was used to examine the relationships among factors that affect population changes (Table 1, Figure 5). The findings indicate a negative correlation (P>0.01) between the variables of month (M), test diameter (TD), weight (W) and GI. Conversely, a positive correlation (P<0.01) was found between TD, weight (W), and GI.
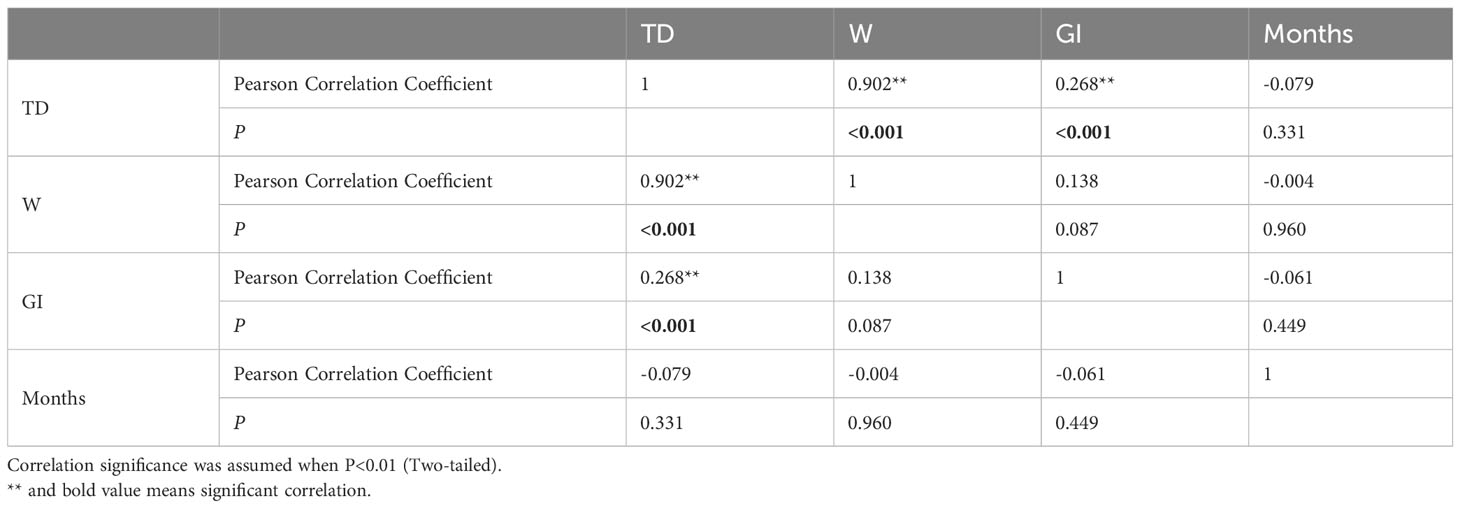
Table 1 Person correlation analysis results comparing H. crassispina test diameter, weight, GI and months.
The five stages of the gonadal development cycle of the sea urchin are characterized as follows (Figure 6):
Stage I: Recovery
Female and male gonads are not easily distinguishable from each other in appearance; the gonads are yellowish in color and emaciated.
Female: The ovarian follicular wall is dominated by oogonia with only a few primary oocytes. The oogonia are oval or slightly spindle-shaped with large nuclei.
Male: The testicular follicular cells are dominated by spermatogonia with only a few primary spermatocytes. The spermatogonia are subrounded, and the primary spermatocytes are easily stained.
Stage II: Growth
The gonads become larger in size, but the female and male gonads remain indistinguishable in appearance.
Female: oogonia are still present in small numbers but are significantly less numerous than in the recovery phase. Primary oocytes in the follicular wall increase in number and size.
Male: Spermatogonia and spermatogonia increase dramatically in number and size, but no spermatogenesis yet.
Stage III: Premature
Male and female gonads can be distinguished by their appearance and significant size increase.
Females: develop many oocytes that turn into pear-shaped secondary oocytes. Most primary oocytes are attached to the follicular wall in grape-like clusters, while a few secondary oocytes detach from the follicular wall and develop into oogonia in the follicular lumen.
Males: The quantity of spermatocytes and spermatids increases, leading to the formation of spermatid clusters in the follicular cavity by spermatozoa.
Stage IV: Mature
The gonads are extremely well developed and brightly colored.
Female: The follicular cavity is filled with mature ova.
Male: The follicular cavity is filled with mature sperm.
Stage V: Late Emission
The gonads become thin and dark due to the discharge of germ cells. As a consequence, the appearance of both female and male gonads becomes indistinguishable.
Female: there is an enlargement of the follicular lumen, and only a few oocytes remain. Phagocytes with pseudopods appear and gradually phagocytose the remaining oocytes. The wall of the follicle shrinks and thins, causing connective tissue to emerge in the lumen.
Male: the follicular space widens, resulting in the presence of only a few spermatozoa.
Purple sea urchins exhibited a cyclical process of reproduction and recovery, with a spawning peak in April and a rapid decline in May, followed by a gradual recovery, finally culminating in an August peak (Table 2) during the breeding cycle. Monthly mean GI peaks were observed in April (8.31 ± 3.45) and August (7.96 ± 3.78), with the lowest value recorded in October (2.15 ± 1.06). GI followed a comparable pattern across the year for both sexes, whereby males displayed a marginally higher mean GI than females in January, April, September, and December, while females exhibited a slightly higher mean GI than males in other months (Figure 7).
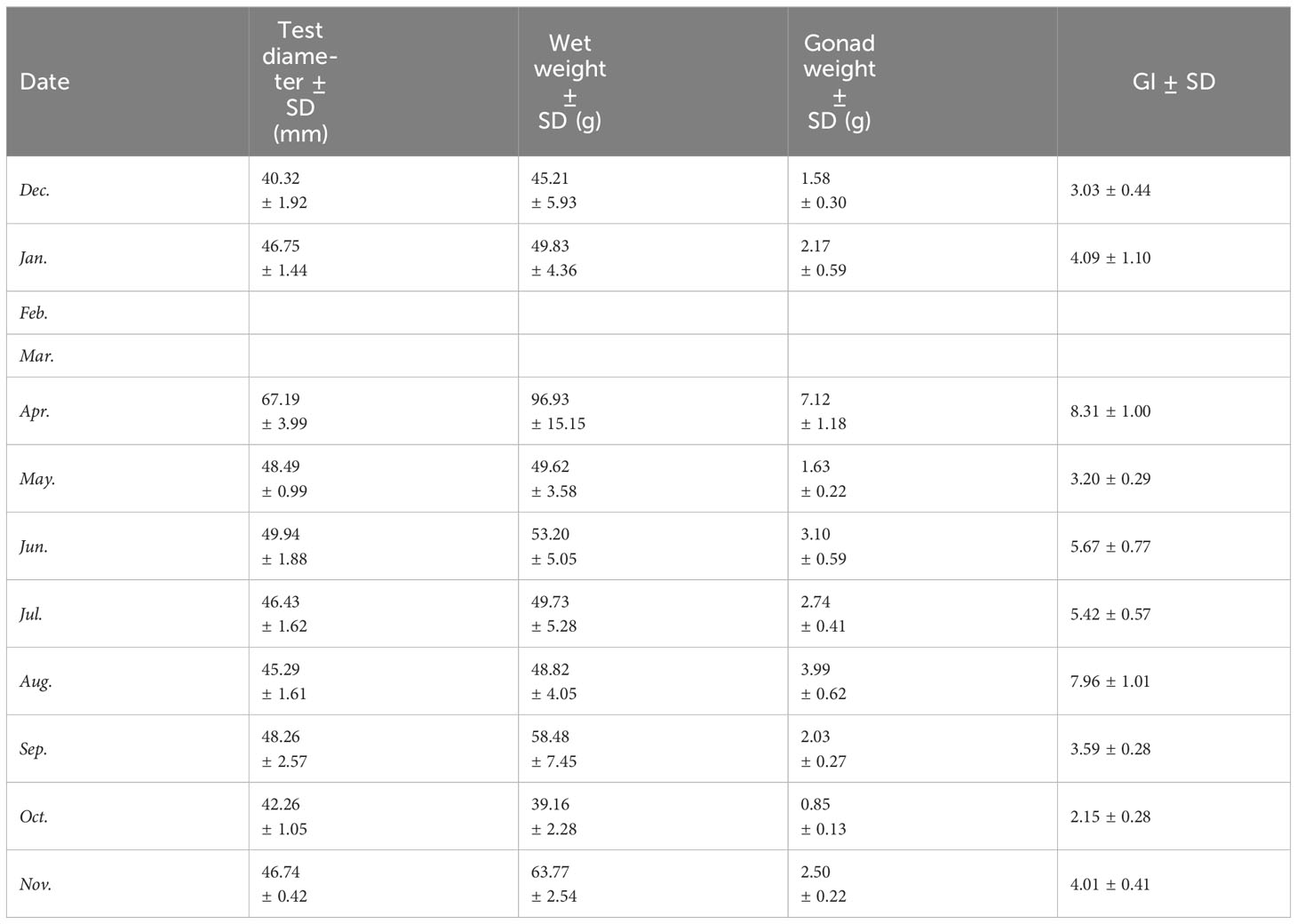
Table 2 Mean dimensions of randomly selected mature sea urchins, including wet weight, test diameter, gonad weight, and GI.
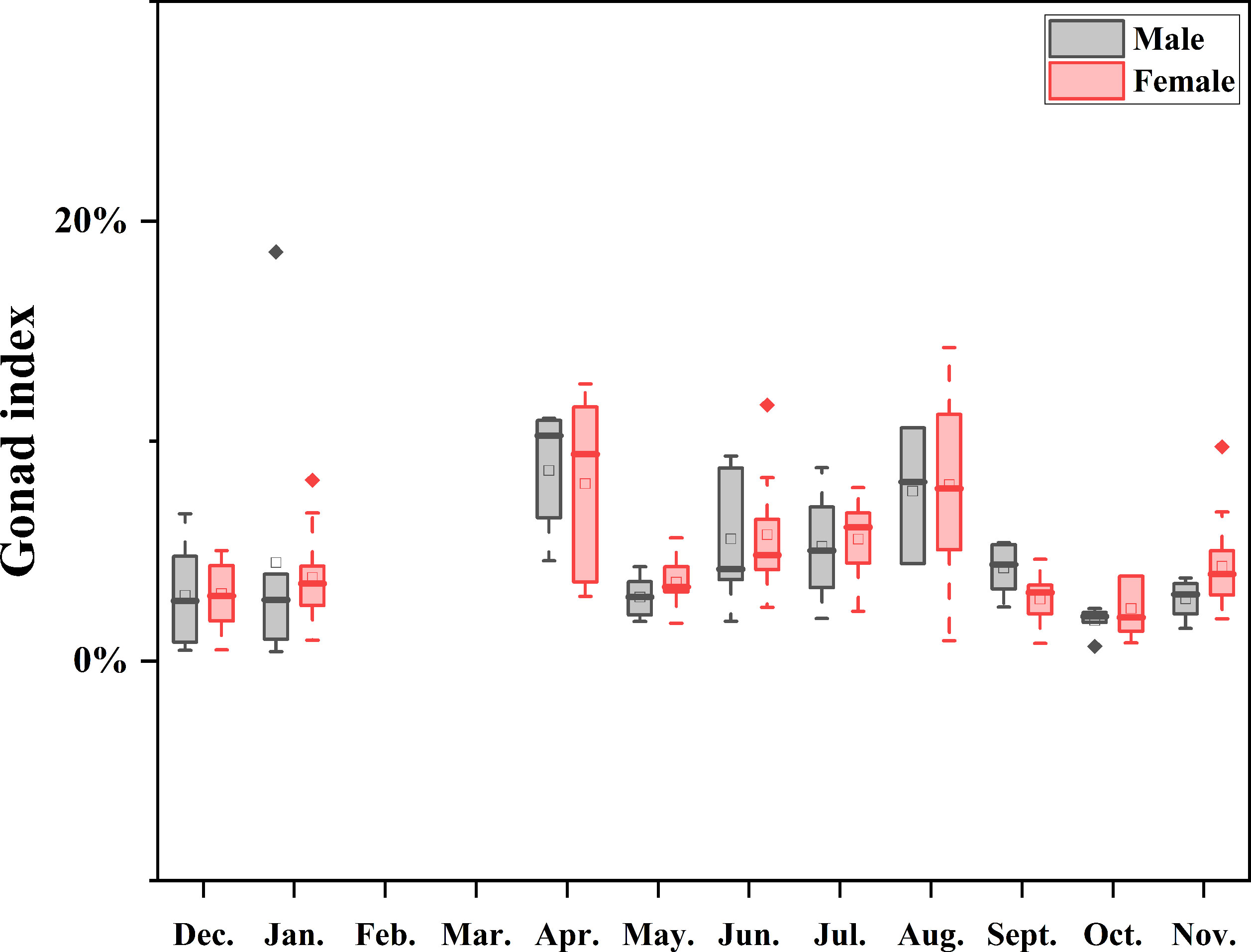
Figure 7 Boxplot of annual GI medians by sex, showing monthly variation, ranges, distribution skew, and outliers (white circles). Data were not available for February and March as indicated by the null line.
A significant disparity was found in the effect of month on GI (P<0.001) when assessing the differences between consecutive months by sex using a two-way ANOVA (Table 3). However, no statistically significant difference was observed in the effect of sex and the interaction of sex and month on GI (P>0.05).
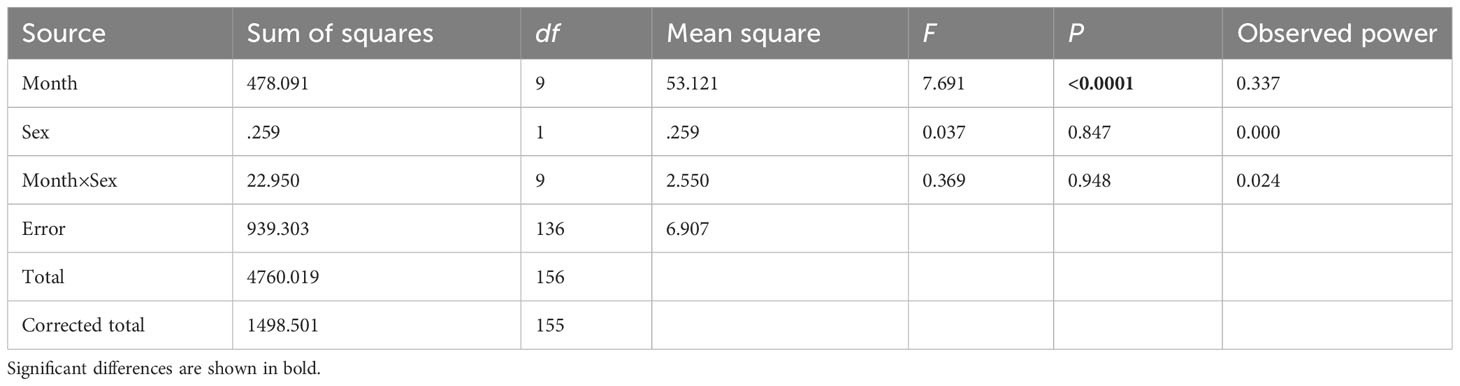
Table 3 ANOVA results comparing the H. crassispina monthly mean gonadal index between months (fixed factor, 10 levels) and sex (fixed factor, 2 levels), α = 0.01.
There was a strong correspondence between gametogenesis and GI, and similar patterns of gametogenesis and gonadal staging were observed between the sexes. In some months, there were overlapping stages (Figure 8). Gonadal development was predominantly in the recovery stage (Stage I) during the months of September to December, with corresponding lower and steadily increasing GI values. From May to November, the growth stage (Stage II) commenced, during which GI tended to decrease and then increase. Early maturing individuals (Stage III) demonstrated a significant increase in gonadal size from April to October, and mature individuals (Stage IV) appeared from April to November, indicating a longer spawning cycle. Mature Stage IV individuals exhibit dual peaks: one in April and the other in August. These are succeeded by late oviparous Stage V individuals from October to January.
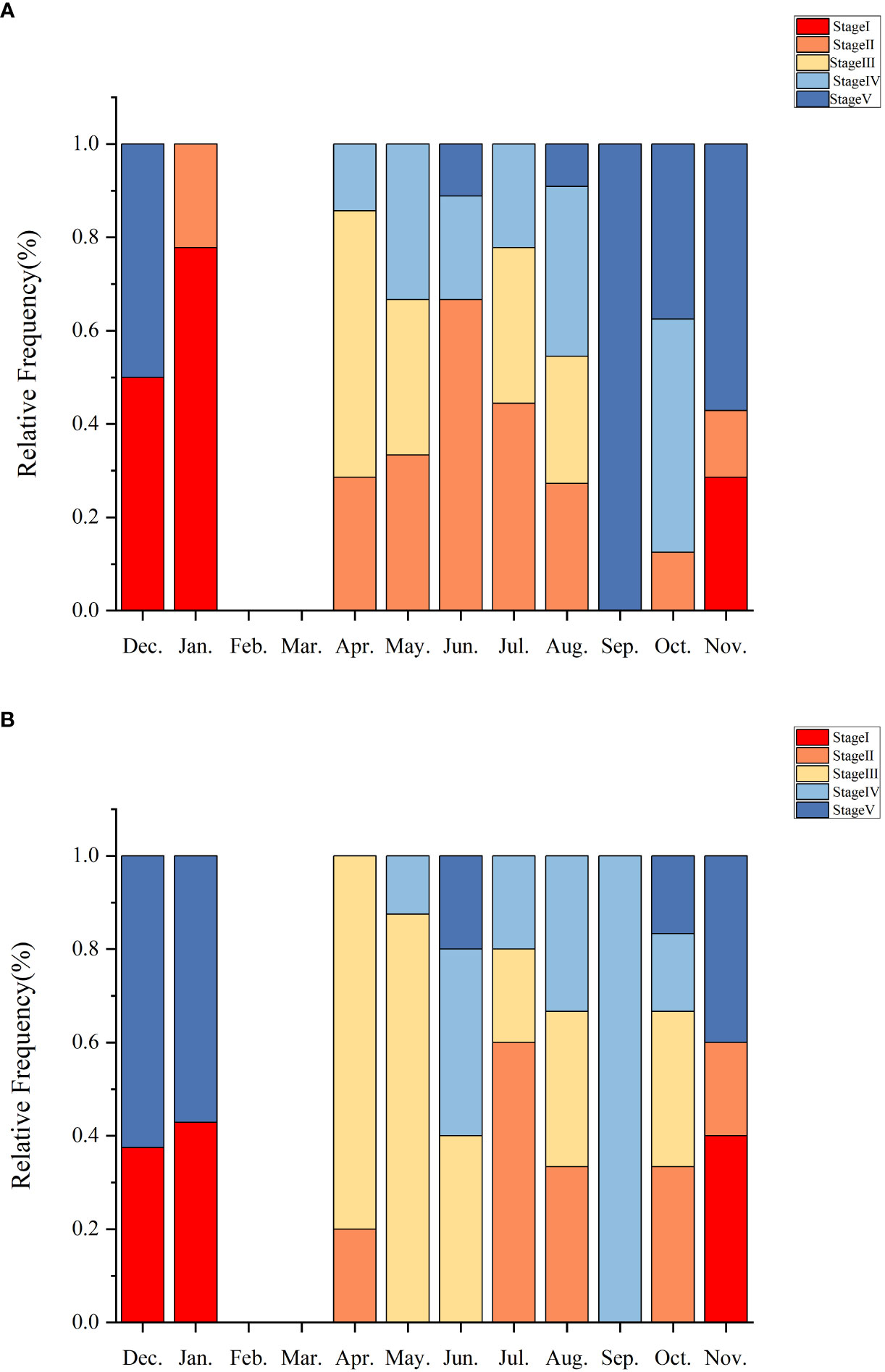
Figure 8 Histograms show the relative frequencies of the maturity stages of ovaries (A) and testes (B) observed in histological sections of sea urchin gonads from Daya Bay.
The mean annual temperature of the investigated sea area was 24.59°C, the mean salinity was 32.87 ppt, the mean pH was 8.35, and the mean DO concentration was 7.62 mg/L. It was found that reproductive seasonality is influenced by temperature and food abundance (O’Hara and Thórarinsdóttir, 2021). Nonetheless, there was no significant correlation observed between monthly seawater temperature and mean monthly GI values (P>0.05). The water temperature in Daya Bay (Figure 9; Table 4) varied throughout the year, with a peak of 31.17°C in August, coinciding with the second highest GI of purple sea urchins. Subsequently, the temperature gradually decreased along with a decrease in GI. In December, the water temperature reached 18.76°C, while most gonadal glands were in the late stage of oviposition and recovery. Subsequently, the water temperature gradually decreased, leading to the first peak GI in April when it was only 25.04°C.
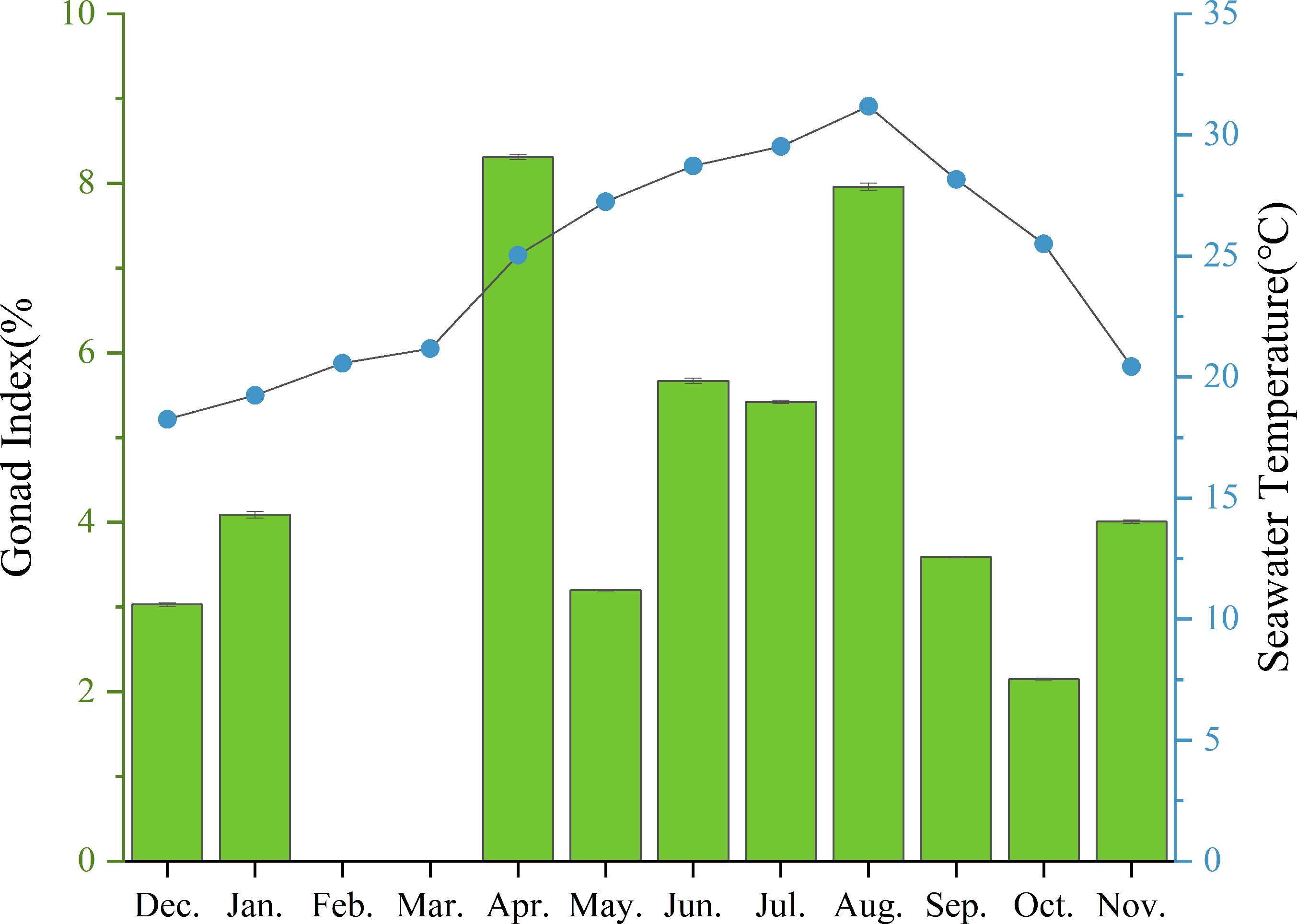
Figure 9 Monthly mean gonadal index values (mean ± SE) of H. crassispina and monthly mean seawater temperature (°C).
The distribution of the population structure and population size of marine organisms is generally affected by seasonal changes and human activities (Qin et al., 2020; Thompson et al., 2023). In this study, the diameters of purple sea urchins were significantly different between the different months, which was in line with the studies of Qian (2021) and Li et al. (2021), who found that the growth characteristics of cod and squid were significantly affected by seasonal factors in the sea environment. Correlation analyses showed significant correlations between month, size, and GI, with a gradual increase in the size of purple sea urchins from December to April and a slow increasing trend from May to November, which was due to the seasonal differences in the developmental stage of purple sea urchins. Gonad sections showed that purple sea urchins spawned in April of the following year, and in May, when the urchins were small and the gonads were in the growth phase, the urchins developed slowly, with the growth rate accelerating each month.
Addis et al. (2012) and Bertocci et al. (2014) reported low population sizes of large-sized sea urchins (> 50 mm) and Paracentrotus lividus in Sardinia and northern Portugal, respectively, and that they are only found in protected areas or marine reserves where fishing is prohibited. Guidetti (Guidetti et al., 2004) found that unregulated fishing of P. lividus on shallow rocky reefs in the Mediterranean Sea reduced the mean size and biomass of its individuals. In contrast, the area where this study was carried out is heavily influenced by human activities, especially as purple sea urchins are an important fished species in this area, and therefore, the low number of large sea urchins may be related to fishing in this area.
In this study, the investigation of the length-weight correlation in purple sea urchins revealed an allometric pattern of growth. This pattern indicated that the growth rate of the test diameter, similar to that of other sea urchin species, was higher than that of the total weight (Villalba Villalba et al., 2021; Elmasry et al., 2023). The allometric growth of purple sea urchins in the waters of Daya Bay could indicate suboptimal body conditions, which may be linked to a wide range of factors, such as pollution, low food supply, poor habitat conditions, increased fishing pressure and high predation rates. Notably, previous studies by Mon (2020) and Siddique and Ayub (2016) found that Echinometra mathaei and Liza tade in Mediterranean waters exhibited allometric growth patterns due to poor environments, supporting our current findings. Our results demonstrate fundamental consistency with previous research.
Differences in the gonadal development of organisms exist in various populations (Cui et al., 2013). In sea urchin gametogenesis, there is a close interaction between germ cells and nutrient phagocytes (NPs) (Villalba Villalba et al., 2021), and then NPs transfer nutrients to growing gametes (Rocha et al., 2019; Hernandez et al., 2020). In the context of gonadal development, the Daya Bay purple sea urchin experiences significant changes throughout the process, particularly in the primary spermatocytes, gamete counts, and the emergence of NPs. Documenting these changes is crucial to understanding the development of this species’ gonads. During the initial phases of gonadal development (stages I and II), a lower number of spermatocytes and oocytes were attached to the follicular wall. In the intermediate stages (stages III and IV), there was a gradual increase in both spermatocytes and oocytes, reaching maximum levels and completely filling up the follicular cavity, leading to a developmentally mature gonad. Phagocytosis is a notable characteristic of the final stage of ovarian development, initially emerging during the partially discharged phase (stage V) and ultimately breaking down any remaining oocytes during the emptying stage. The changes in these characteristics can precisely indicate the stage of gonadal growth of every individual purple sea urchin and the developmental status of the population as a whole during various seasons (Villalba Villalba et al., 2021).
In this study, we analyzed the differences in development periods between male and female purple sea urchins. Overall, there was not much variation in developmental rate between sexes. However, successful spawning of sea urchin colonies requires synchronous spawning for fertilization. Individual differences in development periods between males and females may be related to urchin size (Zhao et al., 2016), food (Lourenço et al., 2021), and temperature (Ding et al., 2020).
The spawning cycles of marine organisms exhibit diverse patterns. For instance, spawning in fish can be either one-time or multiple (Li, 1994). Conversely, the reproductive cycle of oyster gonads spans one year (Chen et al., 2011). In sea urchins, reproductive seasonality is uniform among populations, with gamete growth commencing in late autumn, and most individuals maturing in summer before spawning in late summer to early autumn (Urriago et al., 2016). Water temperature is the primary environmental factor that impacts the maturation of sea urchin gonads. Over a span of three (Yatsuya and Nakahara, 2004) or four months (Kobayashi, 1969), purple sea urchins spawn in temperate waters. The study area under consideration is situated in a subtropical zone characterized by elevated seawater temperatures. Moreover, the purple sea urchin that resides in the study area has a longer breeding cycle, spanning 8 months from April until November. The onset of its spawning takes place in April, thereby revealing a cyclical breeding pattern where it spawns and then resumes the breeding cycle again. This pattern is characterized by two distinct peaks of breeding, with the major peak occurring in April and the minor peak appearing in August. Purple sea urchins in Hong Kong also have a lengthy spawning period with two separate peaks in spawning, which occur in May and September (Urriago et al., 2016). Similarly, P. lividus, the sea urchins from northern Spain, exhibit a similar pattern with two spawning periods and a prolonged spawning time lasting seven months from March to September (González-Irusta et al., 2010).
Differences in food abundance impact the size of sea urchin gonads, with urchins from macroalgae-rich regions showing higher gonadal indices (Qi, 2018). There is a distinct seasonal pattern of macroalgae abundance across the purple sea urchin’s distribution range, with notable winter abundance in comparison to summer (Urriago et al., 2016). In contrast, there is a diversity of purple sea urchin food species in Daya Bay, with some studies showing an abundance of food species in the neighboring waters of Daya Bay (Mo et al., 2017), including Sargassum hemiphyllum, Ulva lactuca and Colpomenia sinuosa. Seasonal changes have had an impact on the feeding habits of purple sea urchins. During spring, their main source of food is macroalgae, but as summer progresses, the availability of macroalgae decreases due to decomposition, resulting in smaller particles that affect their feeding behavior (Zhang et al., 2008). In autumn, however, purple sea urchins mainly feed on suspended particulate organic matter (POM) (Mo et al., 2017). During the research, the spawning period of purple sea urchins ended from spring through autumn, and the source of nutrition changed from macroalgae to POM. It was observed that the various sources of food could provide sufficient energy for gonad development in sea urchins.
The population structure and size distribution of purple sea urchins in Daya Bay are influenced by seasonal fluctuations and fishing pressure, leading to an allometric growth pattern. Purple sea urchins breed between April and November, with peak breeding seasons in April and August. Therefore, it is pivotal to decrease fishing intensity during this time to safeguard mature individuals and enhance the reproductive rate. The study’s findings can provide theoretical support for the systematic artificial breeding of purple sea urchins, the selection of high-quality gonads, and the management and preservation of their natural resources.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Ethics Committee of South China Sea Fisheries Research Institute (CAFS) (nhdf2021-03). The study was conducted in accordance with the local legislation and institutional requirements.
CQ: Funding acquisition, Supervision, Writing – review & editing. XZ: Data curation, Writing – original draft, Writing – review & editing. XM: Investigation, Writing – review & editing. YG: Methodology, Resources, Writing – review & editing. JL: Validation, Writing – review & editing. ZM: Project administration, Writing – review & editing. GY: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 32160863), South Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (No. 2023XK01) and Sanya Yazhou Bay Science and Technology City 2021 Matching Grant Targeted Organisation Project (No. SKJC-2022-PTDX-014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Addis P., Secci M., Angioni A., Cau A. (2012). Spatial distribution patterns and population structure of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea), in the coastal fishery of western Sardinia: A geostatistical analysis. Scientia Marina 0 76 (4), 733–740. doi: 10.3989/scimar.03602.26B
Bertocci I., Dominguez R., MaChado I., Freitas C., Domínguez Godino J., Sousa-Pinto I., et al. (2014). Multiple effects of harvesting on populations of the purple sea urchin paracentrotus lividus in north Portugal. Fish. Res. 150, 60–65. doi: 10.1016/j.fishres.2013.10.010
Byrne M. (1990). Annual reproductive cycles of the commercial sea urchinParacentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 104, 275–289. doi: 10.1007/BF01313269
Cárcamo P. F. (2015). Effects of food type and feeding frequency on the performance of early juveniles of the sea urchin Loxechinus albus (Echinodermata: Echinoidea): Implications for aquaculture and restocking. Aquaculture 436, 172–178. doi: 10.1016/j.aquaculture.2014.10.045
Chen Y., Huang J., You X., Lin B., Pan Z. (2003). Experiments on the culture of purple sea urchin (Heliocidaris crassispina, Agassiz 1864) in land-based tanks. J. Aquacult. 05), 23–14.
Chen J., Xi S., Qin C., Guo Y., Pan W., Shao G. (2021). Effect of light intensity on the growth and digestive enzyme activities of purple sea urchin (Heliocidaris crassispina, Agassiz 1864) planktonic larvae. Prog. Fishery Sci. 42 (03), 125–131. doi: 10.19663/j.issn2095-9869.20201219001
Chen Z., Xiao S., Pan Y., Yu Z. (2011). Comparison of early development and growth of Hong Kong oyster(Crassostrea hongkongensis) families. South China Fish. Sci. 7 (06), 40–46.
Chi X., Shi D., Ma Z., Hu F., Sun J., Huang X., et al. (2021). Carryover effects of long-term high water temperatures on fitness-related traits of the offspring of the sea urchin Strongylocentrotus intermedius. Mar. Environ. Res. 169, 105371. doi: 10.1016/j.marenvres.2021.105371
Ciriminna L., Signa G., Vaccaro A. M., Visconti G., Mazzola A., Vizzini S. (2021). Turning waste into gold: Sustainable feed made of discards from the food industries promotes gonad development and colouration in the commercial sea urchin Paracentrotus lividus (Lamarck 1816). Aquacult. Rep. 21, 100881. doi: 10.1016/j.aqrep.2021.100881
Cui D., Liu Z., Liu N., Zhang Y., Zhang J. (2013). Histological study on the gonadal development of Scatophagus argus. J. Fish. China 37 (05), 696–704. doi: 10.3724/SP.J.1231.2013.38442
Delgado M., Pérez-Camacho A. (2005). Histological study of the gonadal development of Ruditapes decussatus (L.) (Mollusca: Bivalvia) and its relationship with available food. Scientia Marina 69, 87–97. doi: 10.3989/scimar.2005.69n187
Ding J., Zheng D., Sun J., Hu F., Yu Y., Zhao C., et al. (2020). Effects of water temperature on survival, behaviors and growth of the sea urchin Mesocentrotus nudus: new insights into the stock enhancement. Aquaculture 519, 734873. doi: 10.1016/j.aquaculture.2019.734873
Elmasry E., Abdelrazek F. A., El-Sayed A.-F. M. (2023). Growth pattern and population status of the sea urchin Paracentrotus lividus (Lamarck 1816) on the mediterranean coast of Egypt. Egyptian J. Aquat. Res. 49 (3), 409–416. doi: 10.1016/j.ejar.2023.07.001
Freeman S. M. (2003). Size-dependent distribution, abundance and diurnal rhythmicity patterns in the short-spined sea urchin Anthocidaris crassispina. Estuarine Coast. Shelf Sci. 58 (4), 703–713. doi: 10.1016/S0272-7714(03)00134-3
González-Irusta J. M., Goñi de Cerio F., Canteras J. C. (2010). Reproductive cycle of the sea urchin Paracentrotus lividus in the Cantabrian Sea (northern Spain): environmental effects. J. Mar. Biol. Assoc. United Kingdom 90 (4), 699–709. doi: 10.1017/S002531540999110X
Gouda H., Agatsuma Y. (2020). Effect of high temperature on gametogenesis of the sea urchin Strongylocentrotus intermedius in the Sea of Japan, northern Hokkaido, Japan. J. Exp. Mar. Biol. Ecol. 525, 151324. doi: 10.1016/j.jembe.2020.151324
Guidetti P., Terlizzi A., Boero F. (2004). Effects of the edible sea urchin, Paracentrotus lividus, fishery along the Apulian rocky coast (SE Italy, Mediterranean Sea). Fish. Res. 66 (2), 287–297. doi: 10.1016/S0165-7836(03)00206-6
Han L., Hao P., Wang W., Wu Y., Ruan S., Gao C., et al. (2023). Molecular mechanisms that regulate the heat stress response in sea urchins (Strongylocentrotus intermedius) by comparative heat tolerance performance and whole-transcriptome RNA sequencing. Sci. Total Environ. 901, 165846. doi: 10.1016/j.scitotenv.2023.165846
Hassan M. M., Pilnick A. R., Petrosino A. M., Harpring J., Schwab C. J., O’Neil K. L., et al. (2022). Growth and foraging behavior of hatchery propagated long-spined sea urchins, Diadema antillarum: Implications for aquaculture and restocking. Aquacult. Rep. 26, 101298. doi: 10.1016/j.aqrep.2022.101298
Hernandez E., Vázquez O. A., Torruco A., Rahman M. S. (2020). Reproductive cycle and gonadal development of the Atlantic sea urchin Arbacia punctulata in the Gulf of Mexico: changes in nutritive phagocytes in relation to gametogenesis. Mar. Biol. Res. 16 (3), 177–194. doi: 10.1080/17451000.2020.1731758
Johnstone J., Nash S., Hernandez E., Rahman M. S. (2019). Effects of elevated temperature on gonadal functions, cellular apoptosis, and oxidative stress in Atlantic sea urchin Arbacia punculata. Mar. Environ. Res. 149, 40–49. doi: 10.1016/j.marenvres.2019.05.017
Kobayashi J. (1969). Myoe's practice of buddhism. J. Indian Buddhist Stud. (Indogaku Bukkyogaku Kenkyu) 18 (1), 254–257. doi: 10.4259/ibk.18.254
Kong N., Liu X., Li J., Mu W., Lian J., Xue Y., et al. (2017). Effects of temperature and salinity on survival, growth and DNA methylation of juvenile Pacific abalone, Haliotis discus hannai Ino. Chin. J. Oceanol. Limnol. 35 (5), 1248–1258. doi: 10.1007/s00343-016-5185-z
Lawrence J. M. (2013). “Chapter 2 - sea urchin life history strategies,” in Developments in Aquaculture and Fisheries Science. Ed. Lawrence J. M. (Netherlands: Elsevier), 15–23.
Li S. (1994). Introduction to reproductive physiology of farmed fish in China. J. Fish. China 03), 198.
Li K., Yan Y., Yin J., Tan Y., Huang L. (2016). Seasonal occurrence of Calanus sinicus in the northern South China Sea: A case study in Daya Bay. J. Mar. Syst. 159, 132–141. doi: 10.1016/j.jmarsys.2016.03.014
Li N., Yu J., Fang Z., Chen X., Zhang Z. (2021). Day age, growth and population structure of Uroteuthis edulis(Hoyle 1885) in the East China Sea based on otolith day age information. J. Fish. China 45 (06), 887–898.
Liang Q., Zhang L., Huang S., Zhong Y., Xie Y., Wang G. (2023). Effects of salinity on fertilization, embryonic development, larval growthingestion and metamorphosis of Heliocidaris crassispina. J. Fish. China 47 (05), 84–96.
Lourenço S., Cunha B., Raposo A., Neves M., Santos P. M., Gomes A. S., et al. (2021). Somatic growth and gonadal development of Paracentrotus lividus (Lamarck 1816) fed with diets of different ingredient sources. Aquaculture 539, 736589. doi: 10.1016/j.aquaculture.2021.736589
Mo B., Qin C., Chen P., Li X., Feng X., Tong F., et al. (2017). Feeding habits of the purple sea urchin Heliocidaris crassispina based onstable carbon and nitrogen isotope analysis. J. Fishery Sci. China 24 (03), 566–575. doi: 10.3724/SP.J.1118.2017.16278
Mon E. (2020). Length-weight relationship, condition factor and sex ratio of tade mullet (Liza tade Forsskal 1775) from mawlamyine, mon state, Myanmar. J. Aquacult. Mar. Biol. 9, 107–112. doi: 10.15406/jamb.2020.09.00285
O’Hara T. E., Thórarinsdóttir G. G. (2021). A depth-dependent assessment of annual variability in gonad index, reproductive cycle (gametogenesis) and roe quality of the green sea urchin (Strongylocentrotus droebachiensis) in Breidafjördur, west Iceland. Regional Stud. Mar. Sci. 45, 101846. doi: 10.1016/j.rsma.2021.101846
Pais A., Serra S., Meloni G., Saba S., Ceccherelli G. (2011). Harvesting Effects on Paracentrotus lividus Population Structure: A Case Study from Northwestern Sardinia, Italy, before and after the Fishing Season. J. Coast. Res. 28 (3), 570–575. doi: 10.2112/jcoastres-d-10-00119.1
Qi S. (2018). Influence of microalgae and macroalgae baits on the growth of the Strongylocentrotus intermedius (China: Doctor, Nanjing Agricultural University).
Qian J. (2021). Climate warming alters Atlantic cod stock structure. NONG CUN SHI YONG JI SHU 01), 96–102.
Qin C., Chen P., Sarà G., Mo B., Zhang A., Li X. (2020). Ecological implications of purple sea urchin (Heliocidaris crassispina, Agassiz 1864) enhancement on the coastal benthic food web: evidence from stable isotope analysis. Mar. Environ. Res. 158, 104957. doi: 10.1016/j.marenvres.2020.104957
Rocha F., Baião L. F., Moutinho S., Reis B., Oliveira A., Arenas F., et al. (2019). The effect of sex, season and gametogenic cycle on gonad yield, biochemical composition and quality traits of Paracentrotus lividus along the North Atlantic coast of Portugal. Sci. Rep. 9 (2994), 1–13. doi: 10.1038/s41598-019-39912-w
Siddique S., Ayub Z. (2016). Length-Weight Relationships And Condition Factor Of The Sea Urchin Echinometra Mathaei (Echinoidea: Echinodermata) On Buleji Rocky Shore Of Karachi, Pakistan. (Pakistan: Pakistan Journal of Marine Sciences).
Takagi S., Murata Y., Sato Y., Kokubun A., Agatsuma Y. (2022). Gonad traits in the sea urchin Mesocentrotus nudus fed on the kelp Eisenia bicyclis: Gametogenesis promotion and lack of sea urchin-like taste. Algal Res. 66, 102824. doi: 10.1016/j.algal.2022.102824
Thompson B., Brooks P. R., Farrugia Drakard V., Kubin F., Earp H. S., Alvarez-Cienfuegos I., et al. (2023). Population structure and reproductive states of the dogwhelk Nucella lapillus differ between artificial structures and natural rocky shores. Mar. Environ. Res. 189, 106059. doi: 10.1016/j.marenvres.2023.106059
Urriago J. D., Wong J. C. Y., Dumont C. P., Qiu J.-W. (2016). Reproduction of the short-spined sea urchin Heliocidaris crassispina (Echinodermata: Echinoidea) in Hong Kong with a subtropical climate. Regional Stud. Mar. Sci. 8, 445–453. doi: 10.1016/j.rsma.2016.03.005
Villalba Villalba A. G., Chan Chan L. H., Lagarda Diaz I., Reyes Jiménez N. Y., Minjarez Osorio C., Castro Longoria R., et al. (2021). Reproductive cycle of sea urchin Echinometra vanbrunti (Echinodermata: Echinoidea) from the Gulf of California. Mar. Biol. Res. 17 (9-10), 838–852. doi: 10.1080/17451000.2022.2029901
Wei J., Zhang L., Zhao C., Feng W., Sun P., Chang Y. (2016). Correlation analyses of covering and righting behaviors to fitness related traits of the sea urchin Glyptocidaris crenularis in different environmental conditions. Chin. J. Oceanol. Limnol. 34 (6), 1183–1190. doi: 10.1007/s00343-016-5133-y
Xi S., Qin C., Ma Z., Yu G., Sun J., Pan W., et al. (2020). Effects of dietary microalgae on growth and survival of larval development of sea urchin (Anthocidaris crassispina). South China Fish. Sci. 16 (02), 115–120.
Yatsuya K., Nakahara H. (2004). Density, growth and reproduction of the sea urchin Anthocidaris crassispina (A. Agassiz) in two different adjacent habitats, the Sargassum area and Corallina area. Fish. Sci. 70 (2), 233–240. doi: 10.1111/j.1444-2906.2003.00796.x
Yeruham E., Abelson A., Rilov G., Ben Ezra D., Shpigel M. (2019). Energy budget of cultured Paracentrotus lividus under different temperatures. Aquaculture 501, 7–13. doi: 10.1016/j.aquaculture.2018.11.006
Zhang S. Y., Wang L., Wang W. D. (2008). Algal communities at Gouqi Island in the Zhoushan archipelago, China. J. Appl. Phycol. 20 (5), 853–861. doi: 10.1007/s10811-008-9338-0
Zhao C., Feng W., Wei J., Zhang L., Sun P., Chang Y. (2016). Effects of temperature and feeding regime on food consumption, growth, gonad production and quality of the sea urchin Strongylocentrotus intermedius. J. Mar. Biol. Assoc. United Kingdom 96 (1), 185–195. doi: 10.1017/S0025315415001617
Keywords: Heliocidaris crassispina, gonadal development, reproductive cycle, population status, Daya Bay
Citation: Zhao X, Mu X, Guo Y, Li J, Ma Z, Yu G and Qin C (2024) Assessment of annual variability in the population status and reproductive cycle of purple sea urchins (Heliocidaris crassispina, Agassiz, 1864) in Daya Bay, China. Front. Mar. Sci. 10:1337159. doi: 10.3389/fmars.2023.1337159
Received: 12 November 2023; Accepted: 15 December 2023;
Published: 04 January 2024.
Edited by:
Bin Xia, Qingdao Agricultural University, ChinaReviewed by:
Ji Liu, University of East Anglia, United KingdomCopyright © 2024 Zhao, Mu, Guo, Li, Ma, Yu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanxin Qin, cWluY3hAc2NzZnJpLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.