- 1Department of Biological, Geological, and Environmental Sciences (BiGeA), Alma Mater Studiorum - University of Bologna, Bologna, Italy
- 2National Research Council - Institute for Marine Biological Resources and Biotechnologies (CNR-IRBIM), Ancona, Italy
- 3Department of Biology, University of Padova, Padova, Italy
- 4National Research Council - Institute of Polar Sciences (CNR-ISP), Bologna, Italy
Although fishing is considered the primary cause of the decline in fish populations, increasing evidence of the significant role of climate change has been provided recently in the Mediterranean Sea, which shows one of the highest warming trends in the world. In this area, the most important environmental driver is represented by the increase in seawater temperature. Though several studies have addressed the effects of sea warming on thermophilic species, little attention has been paid to cold-water species. Among these, blue whiting (Micromesistius poutassou) constitutes one of the most important traditional fisheries resources in the northern part of the basin, particularly in the central Adriatic Sea. This area has experienced intense fishing exploitation by the Italian and Croatian fishing fleets. Since 2015, the Pomo/Jabuka Pits area, the fleets’ main fishing ground, has been subject to a series of fishing regulations over time and space. In the present study, we investigated the age structure and growth performance (by means of otoliths) of blue whiting, comparing samples collected during 1985–86 and 2020–21 in the Pomo/Jabuka Pits. Our results show that the 2020–21 blue whiting specimens had a lower length-at-age compared to 1985–86. The asymptotic length estimate decreased from 29 cm TL in 1985–86 to 25 cm TL in 2020–21. The pattern observed might be related to a modification in the cold and dense water formation dynamics in the northern Adriatic Sea, as a consequence of climate change, resulting in higher temperatures and lower nutrient and oxygen exchange, which may have hampered the optimal growth of the species. Moreover, data on the historical trend of landings from the Adriatic Sea reveals a clear decline in catches starting from 2000 onwards. Although the introduction of a fishing ban in the Pomo/Jabuka Pits was an important milestone, the abundance of this species in the area remains at low levels, highlighting a potentially alarming situation for the stock of blue whiting in the central Adriatic Sea.
1 Introduction
Knowledge of life history traits and fish population dynamics represents a key issue in understanding how commercially exploited species cope with human-induced stressors, such as climate change and fishing (Hidalgo et al., 2022). This is particularly true for those showing narrow thermal ranges and distributions restricted to small areas, such as the cold-adapted species living in relatively deep waters of the Mediterranean Sea (Lloret et al., 2021). Among them, one of the most important fishing resources is blue whiting, Micromesistius poutassou (Risso, 1827), whose fishery was the third most important worldwide at the beginning of the twenty-first century (FAO, 2011). This mesopelagic gadoid is broadly distributed along the continental slope of the North Atlantic and the Mediterranean Sea, inhabiting the areas of continental slope and shelf from 150 to more than 1000 m (Bailey, 1982). It is known that this species undergoes seasonal migration in the North Atlantic, moving towards the northern edge of its distribution (Faroes, Iceland, and Norway) during the summer (Cohen et al., 1990). In the Mediterranean Sea, there are no indications of seasonal migrations, although it has been observed that the species performs diel vertical migrations and is captured more efficiently during daylight when it is closer to the bottom than at night (Martin et al., 2016). The spawning of this species occurs at temperatures between 11−13°C, which represent the minimum values in the Mediterranean Sea. While in the North Atlantic, the spawning season starts in January showing a wide temporal lag in relation to the latitudinal temperature gradient (Bailey, 1982), in the Mediterranean Sea it is restricted between January and March (Froglia and Gramitto, 1981; Serrat et al., 2019). Despite cold-water species living in deep habitats usually exhibit slow growth and late maturity (Lloret et al., 2021), the Mediterranean blue whiting shows fast growth and early maturity, being sexually mature at the end of the first year of life at around 20 cm TL (Froglia and Gramitto, 1981; Serrat et al., 2019; Mir-Arguimbau et al., 2020).
The blue whiting is the most important commercial species among gadids in the Mediterranean Sea and one of the most important traditional fishery resources in the northern areas of the basin (Gulf of Lions, Catalan, Ligurian, Adriatic, and Aegean Seas). The northern sectors of the Mediterranean Sea are key regions where intermediate and deep-water convection is regularly observed, leading to vertical recirculation, which is essential to sustain the Mediterranean thermohaline circulation (Roether et al., 1996). In particular, these cold areas contribute substantially to the deep-water formation and among them the Adriatic Sea plays a key role, being the area where the eastern Mediterranean Deep Water originates (Pollack, 1951). Here, during winter, low temperatures combined with the strong and dry north-easterly wind (Bora) trigger the formation of cold and dense waters in the northern part, which deepen and move southward carrying oxygen and nutrients to the deep layers, first reaching the middle Adriatic depression (Pomo/Jabuka Pits) and then the Bari Canyon (southern Adriatic) (Marini et al., 2016). Consequently, climate variations occurring in these regions can have huge impacts not only on the local circulation system but also at the Mediterranean scale, with serious consequences for the marine ecosystems, whose health status depends on the functioning of the cold-water generation system. Furthermore, the Adriatic Sea is one of the most exploited basins by trawl fisheries at a global scale (Pitcher et al., 2022) and the combined effect of fishing and climate change could represent a major threat to the long-term maintenance of the locally relevant cold-water species (e.g., whiting - Merlangius merlangus, blue whiting – M. poutassou, sprat – Sprattus sprattus, turbot – Scophthalmus maximus). Among these species, the blue whiting underwent the most dramatic decline in landings in the Adriatic Sea, showing an 80–90% decline from the 1980s to 2010s (FAO, 2020). Although the stock status of this species is not routinely assessed, and validated stock assessments are not available for this area, it could be hypothesized that the stock underwent high exploitation rates over the last decades. Indeed, the northern and central Adriatic Sea was identified as the most exploited in the Mediterranean area, showing values of fishing mortality higher than the fishing mortality at Maximum Sustainable Yield for almost all assessed commercial stocks(Colloca et al., 2017; FAO-GFCM, 2020). Recent studies recognized the effects of climate change on the productivity potential of several stocks (Free et al., 2019; Moullec et al., 2019), indicating stocks could be negatively affected by increased sea temperatures, although the response is species-specific. In fact, despite the introduction of regulations to reduce the fishing effort (e.g., EU Regulation 1967/2006) among the EU countries through fishing capacity and effort limitations, regulation of mesh size, and spatial/temporal closures, the Mediterranean fisheries resources did not show signs of recovery (Cardinale and Scarcella, 2017).
The main fishing ground for blue whiting is located in the Pomo/Jabuka Pits, characterized by muddy seafloors sloping down to 270 m and delimiting the largest continental shelf in the Mediterranean and Black Sea (Marini et al., 2016). As one of the most important fishing grounds within the basin, this area has been exploited by both Italian and Croatian fisheries (mainly bottom trawlers) targeting the Norway lobster (Nephrops norvegicus) and European hake (Merluccius merluccius). Stable bottom temperatures (12−15°C) and weak bottom currents make this area one of the main nursery grounds of the Mediterranean Sea for many species (Druon et al., 2015) and, thanks to the presence of ideal conditions for fish spawning, feeding and/or growth to maturity, it has been identified as an essential fish habitat (de Juan and Lleonart, 2010). Consequently, after a long discussion about the establishment of a fishing ban in the area, the first partial closure applied toward trawling activities was approved in July 2015, followed by other spatial and temporal regulations (Ministerial Decrees 03/07/2015 and 20/07/2016; Ministerial Decrees 19/10/2016 and 01/06/2017). A Fishery Restricted Area was established in 2018, identifying a fishery-ban zone and two buffer zones where trawling is limited to a small number of authorized vessels with a working-time limit (FAO-GFCM, 2017; MIPAAF, 2017). Although previous studies attempted to investigate the biology of blue whiting in this area (Froglia and Gramitto, 1981), several aspects of the life history of this species are still unknown. Moreover, considering the introduction of management measures, there is a critical need to evaluate the effectiveness of these measures on the species inhabiting the area (Chiarini et al., 2022).
Based on these premises, this study aims at 1) determining whether differences are present in the age structure and growth performance between samples of blue whiting collected in the Pomo/Jabuka Pits area with a time interval of 35 years; 2) updating and providing new insights into the biological cycle of blue whiting in the Adriatic Sea; 3) discussing the potential effects of fishing and climate change on this species.
2 Materials and methods
2.1 Sampling
The sampling activity was carried out in the central Adriatic Sea at depths between 100 and 250 m around the western side of the Pomo/Jabuka Pits. Samples were collected in 2020–21 from landings of the San Benedetto Del Tronto and Giulianova fishing fleets (Figure 1) (Italian-type bottom otter trawl, 40 mm mesh size at the codend) and in 1985–86 from research cruises on board the research vessel S. Lo Bianco (Italian-type bottom otter trawl, 30 mm mesh size at the codend). The former sampling was carried out in July, October, November, December 1985, and July 1986; the latter in November 2020, February, March, April, July, and December 2021, and aimed to obtain at least 40–60 specimens per sample (representative of the whole size range). Samples were stored in cooling containers and transferred to the laboratory for further processing. In April 2021, an extra sampling activity was performed in the same area onboard the research vessel G. Dallaporta during the “Monitoraggio Pomo 2021” survey (Martinelli et al., 2021) to collect exclusively juveniles coming from the latest reproductive event, the so-called young-of-the-year (YOY), using an experimental bottom otter trawl (12 mm mesh size at the codend).
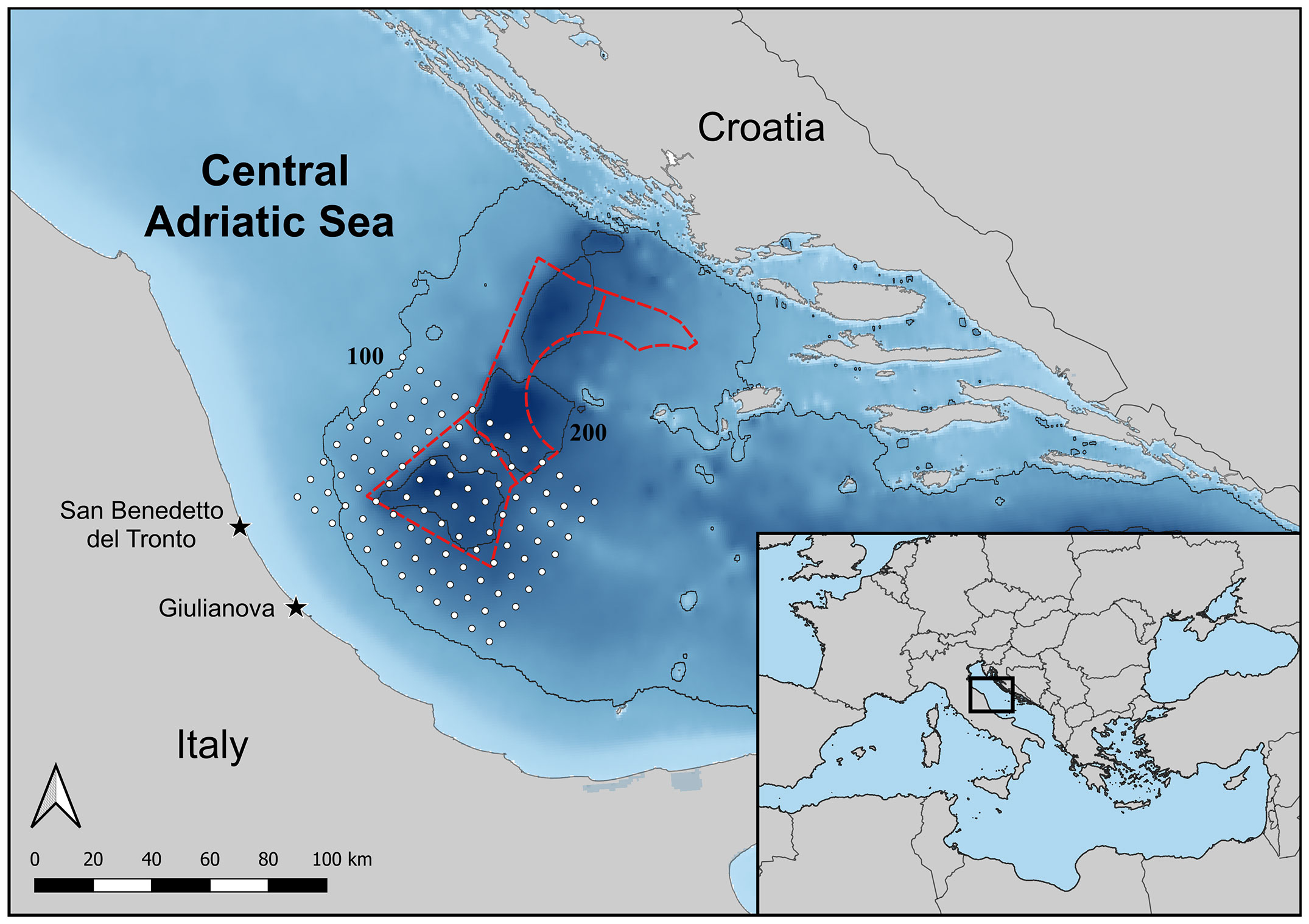
Figure 1 Map of the Central Adriatic Sea with bathymetric lines (100 and 200 meters), showing the location of the Pomo/Jabuka Pits Fishery Regulated Area (red dotted line) and blue whiting fishing ground (grey dotted area).
Total length (TL) to the nearest mm, total weight (TW) to the nearest 0.1 g, and gonad weight (GW, only in 2020–21) to the nearest 0.01 g were recorded for each specimen. The sagittal otoliths were removed, cleaned, and stored dry in vials for aging purposes. Sex was assigned macroscopically in specimens ≥16 cm TL.
2.2 Age estimation
To enhance the contrast between translucent and opaque zones, otoliths were soaked in fresh water for at least 12 hours before reading. Both sagittae of each specimen were analyzed immersed in water over a black surface under reflected light. The sulcus acusticus was set facing downwards and the axis from the nucleus to the posterior margin of the otolith was used to count the annual rings. Readings were performed using a stereomicroscope (Leica MZ6) at low magnification (10x) connected to an image analysis system (Leica Flexacam C3 camera and Leica Application Suite software). Since the presence of up to two false rings in the sagittal otoliths within the first year of life was reported in this species (ICES, 2017), the diameter of the first annual ring and the diameter of false inner rings (when present) were measured, using the criterion proposed elsewhere (Mir-Arguimbau et al., 2020) to identify them and considering as true rings the ones between 8.3 and 9.3 mm. Age was estimated as the number of completely formed annuli, consisting of one opaque and one neighboring translucent ring (Figure 2). Samples were blindly read twice and in random order by two operators independently, without any indication of the date of capture, sex, or size of the specimens. The age and edge type (translucent or opaque) of each otolith sample were recorded and, in case of disagreement between readers, the sample was excluded. The edge condition (percentage of otoliths with an opaque margin for each month available) was analyzed to determine the periodicity and timing of ring formation, to verify the annual formation of the rings. To evaluate the aging precision (Campana, 2001), the index of average percent error (APE) (Beamish and Fournier, 1981) and the mean coefficient of variation (CV) (Chang, 1982) were calculated by comparing readings between readers. The age was estimated in years, establishing a conventional common birth date (1st February) according to the reproductive period of this species, from December to March (Serrat et al., 2019), and considering the capture date and the edge type. Since we observed only the opaque deposition in fish smaller than 12 cm, they were considered Young of the Year (YOY), and their age was calculated as a year fraction taking into account only the month of capture.

Figure 2 Micromesistius poutassou sagittal otoliths (distal side up) under reflected light, showing four completely formed annuli (black dots). Female specimen of 26 cm TL captured in April 2021. D, dorsal axis; A, anterior axis; V, ventral axis; P, posterior axis.
The von Bertalanffy growth function (VBGF) was used to describe the growth of the population:
where TL is the length-at-age t, L∞ is the asymptotic total length, k is the so-called Brody growth rate coefficient which determines how fast the fish approaches L∞, and t0 is the theoretical age at which length is zero. The von Bertalanffy growth function was fitted to the estimated age-length data set and the VBGF parameters were estimated for males, females, and sex combined. Unsexed juveniles smaller than 16 cm TL were included in both female and male growth curves to improve the model fit in the first period of growth. Differences in growth between sampling periods and sexes were tested by applying the Likelihood-ratio test (Kimura, 1980), while mean length-at-age was compared using the Wilcoxon signed-rank test (Wilcoxon, 1945). The Growth Performance Index (ɸ’ = logk + 2logL∞) was calculated to allow comparison among the growth parameters estimated in different populations of blue whiting and other gadids (Froese and Pauly, 2022). Growth statistical analyses were conducted using R (R Core Team, 2022), RStudio (Posit Team, 2022), and the packages FSA and car (Fox and Weisberg, 2019; Ogle et al., 2022).
2.3 Reproductive cycle
Reproductive traits were investigated only from the 2020–21 samples as these data were not available from the 1985–86 samples. The gonad maturity stage was evaluated using the standard ICES six-point scale proposed for whiting (M. merlangus) (ICES, 2008): 1) Immature; 2) Maturing; 3) Spawning; 4) Spent; 5) Resting/Skip of spawning; 6) Abnormal. The GW was used to calculate the gonadosomatic index (GSI = GW/TW × 100), to assess the reproductive investment and its temporal trend. The size-at-first sexual maturity L50, i.e. the size at which 50% of individuals are mature, was estimated by considering 173 females and 261 males collected between November (when individuals in early maturation gonadal stages were observed) and March (when still a few spawning individuals were detected among post-spawner ones). Data on size and maturity stage were coupled to fit a predicted proportion of mature individuals (namely at stages 3 and 4) at size using a logistic model using R (R Core Team, 2022), RStudio (Posit Team, 2022), and the package FSA (Ogle et al., 2022). The Condition Factor (CF) was calculated using the following relationship, CF = 102 (TW/TLb), where b is 3 or ≠ 3 in the case of isometric or allometric growth, respectively (Bolger and Connolly, 1989). Differences in CF values between sexes and sampling periods were tested by the Mann-Whitney test.
3 Results
3.1 Fish samples
The size ranges of the samples were between 12–38 cm TL and 6–32 cm TL in 1985–86 and 2020–21, respectively. The total number of specimens analyzed was 193 (83 females, 85 males, 25 unsexed) in 1985–86 and 453 (173 females, 261 males, 19 unsexed) in 2020–21. The length-frequency distributions (LFDs) were different in the two sampling periods (two samples Kolmogorov–Smirnov test, p<0.05), showing a higher proportion of individuals larger than 28 cm TL in 1985–86 (Figure 3A). In 2020–21 the modal class was comparable between males and females at around 22 cm TL (Figure 3B). Comparing the mean CF calculated for the same size classes (in cm) in both sexes and sampling periods, CF was higher in the 1985–86 sample (mean ± SD = 1.04 ± 0.13) compared to 2020–21 (mean ± SD = 0.98 ± 0.07) (Mann-Whitney test, p<0.05), while no difference was found between sexes (Mann-Whitney test, p = 0.29) in both samplings.
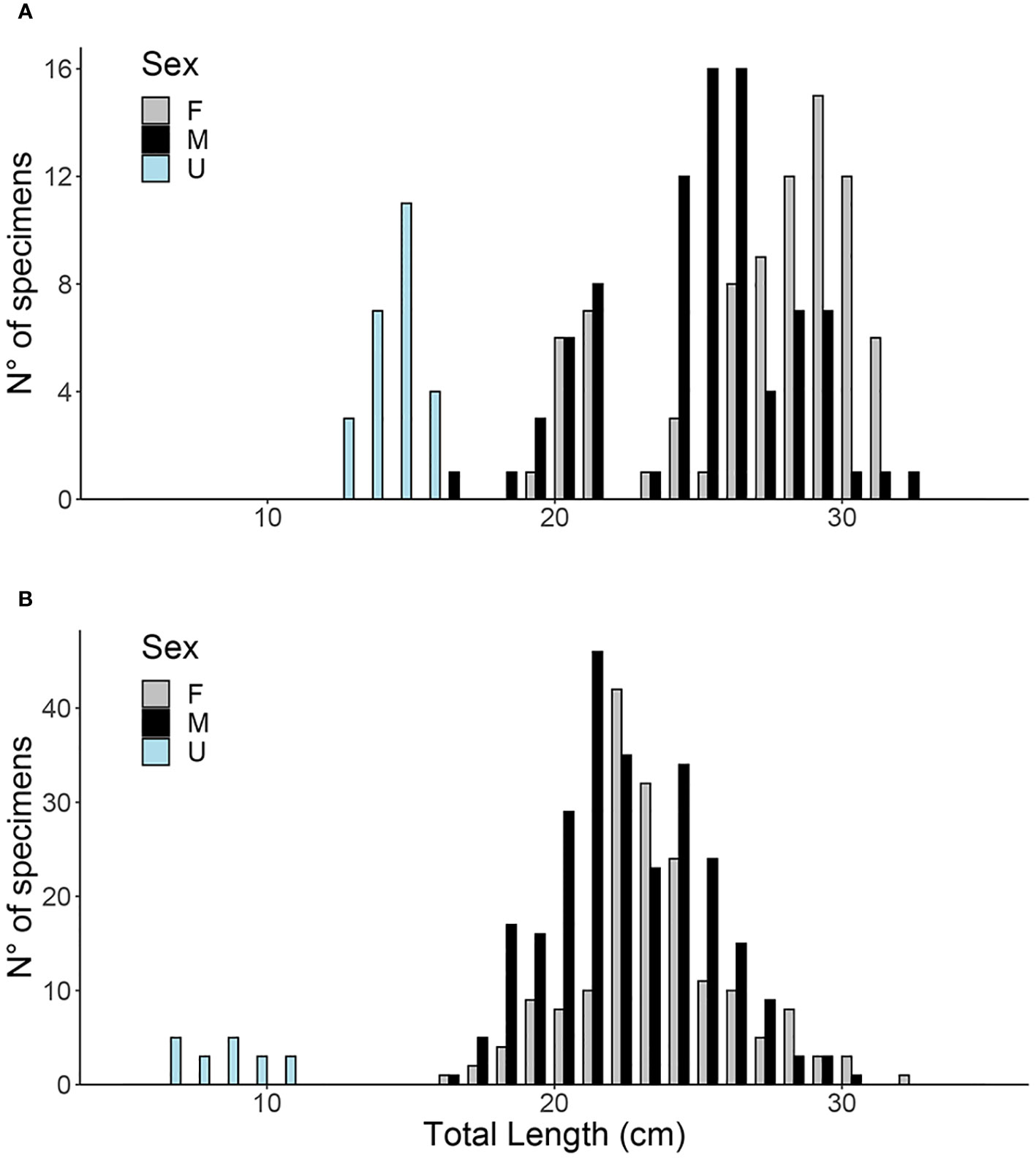
Figure 3 Length-frequency distribution (LFD) of Micromesistius poutassou from the Pomo/Jabuka Pits. F, females; M, males; U, Unsexed. (A) 1985–86; (B) 2020–21.
3.2 Growth and age structure
The otolith edge-zone analysis over the months covered by both sampling periods revealed that the opaque zone was laid during the warmer months, while the hyaline zone was observed during winter and early spring (Supplementary Figure 1), confirming the annual deposition of one opaque ring plus one translucent ring. The highest percentage of opaque deposition was observed in July, reaching values above 90% in both samplings (but it should be noticed that there were no samples in May-June and August-September). The average percentage error (APE) and the coefficient of variation (CV) were low, respectively 0.7% and 1.0%, indicating high precision of age readings. The age classes obtained from otolith readings of the selected subsamples were seven and 10 in 1985–86 (age range 0–8) and 2020–21 (age range 0–9), respectively. Significant differences were found in the mean length-at-age calculated for the two populations in both sexes, particularly in ages classes between 0 and 3 years (Wilcoxon signed-rank test; Z= 2.02, p<0.1), with the 1985–86 samples showing higher values than 2020–21 across the groups (Supplementary Figure 2). The age-length keys of males, females, and unsexed specimens are reported in Tables 1 and 2.
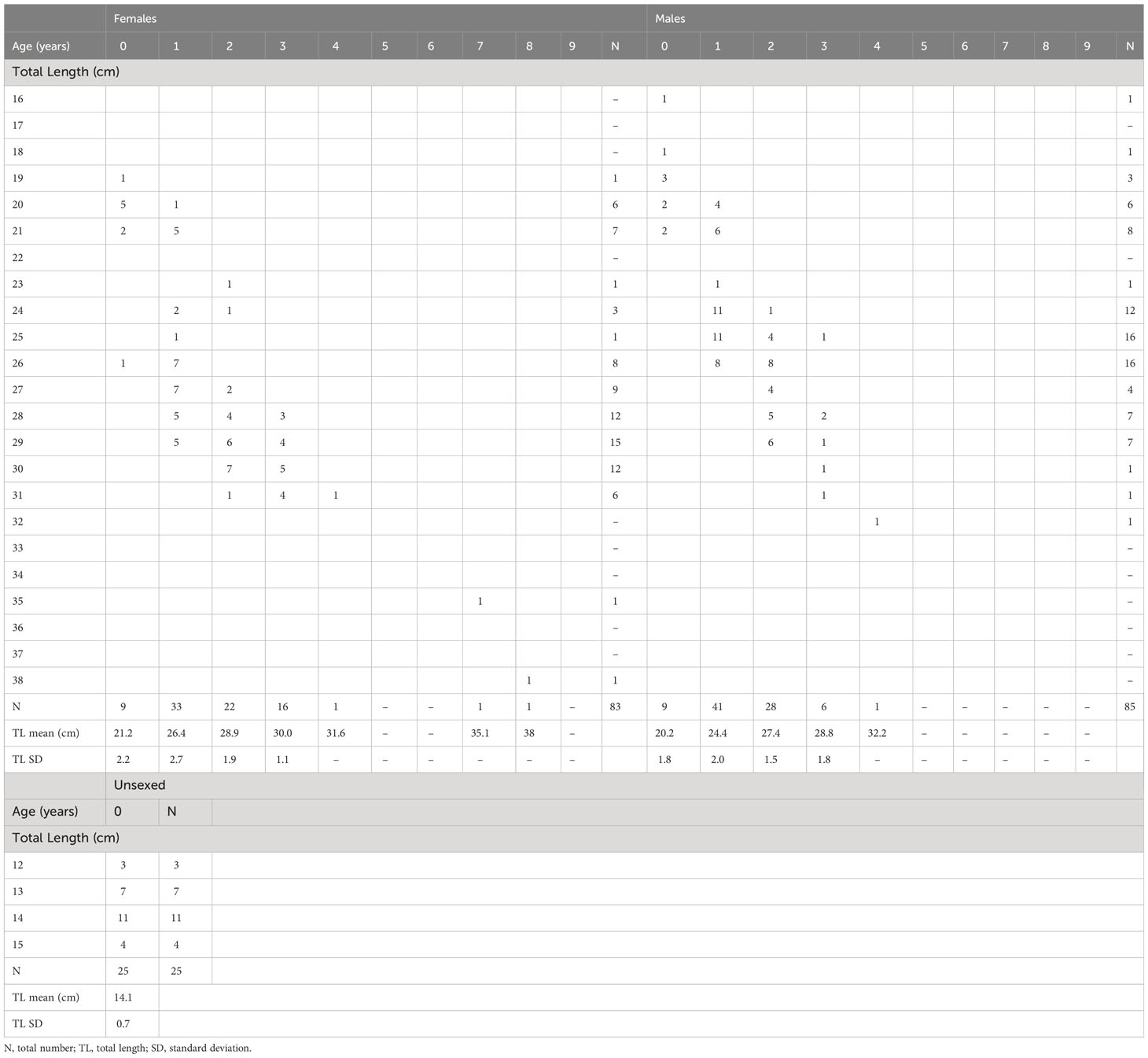
Table 1 Age-length key for females, males, and unsexed specimens based on otolith readings of Micromesistius poutassou sampled in 1985–86 from the Pomo/Jabuka Pits.
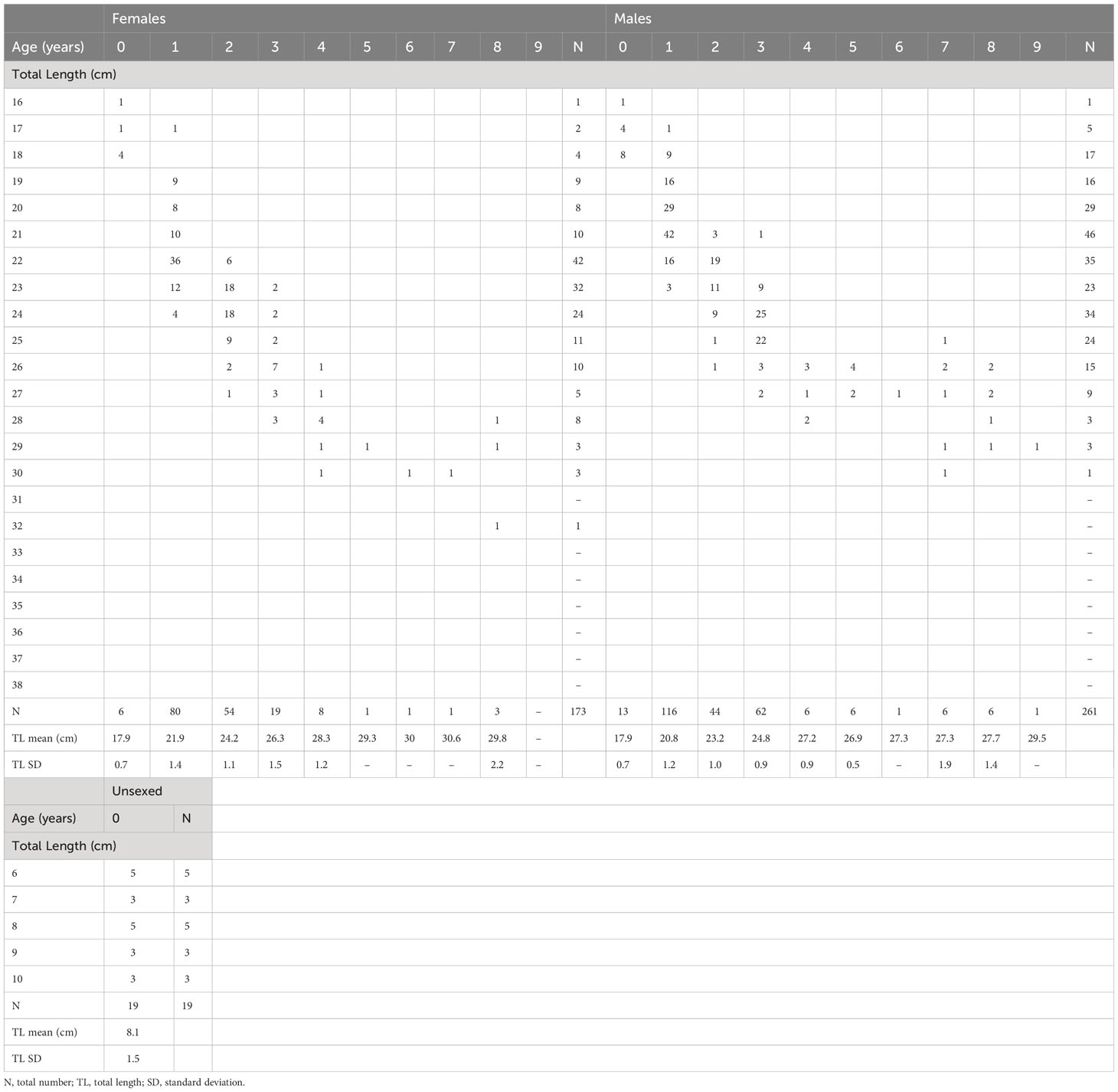
Table 2 Age-length key for females, males, and unsexed specimens based on otolith readings of Micromesistius poutassou sampled in 2020–21 from the Pomo/Jabuka Pits.
Based on the likelihood ratio test on the estimated VBGF parameters, the 1985–86 sample showed higher values of L∞ and lower values of k compared to 2020–21 (Table 3 and Supplementary Table 1; Figure 4). Males attained lower L∞ than females in both populations, as expected considering the sexual dimorphism in size, which was barely noticeable in the 2020–21 sample compared to 1985–86 (Table 3; Figure 4).

Table 3 Von Bertalanffy growth parameters estimates and their standard errors (SE) of Micromesistius poutassou from the Pomo/Jabuka Pits and growth performance index (φ’) for combined sexes, females and males of both sampling periods.
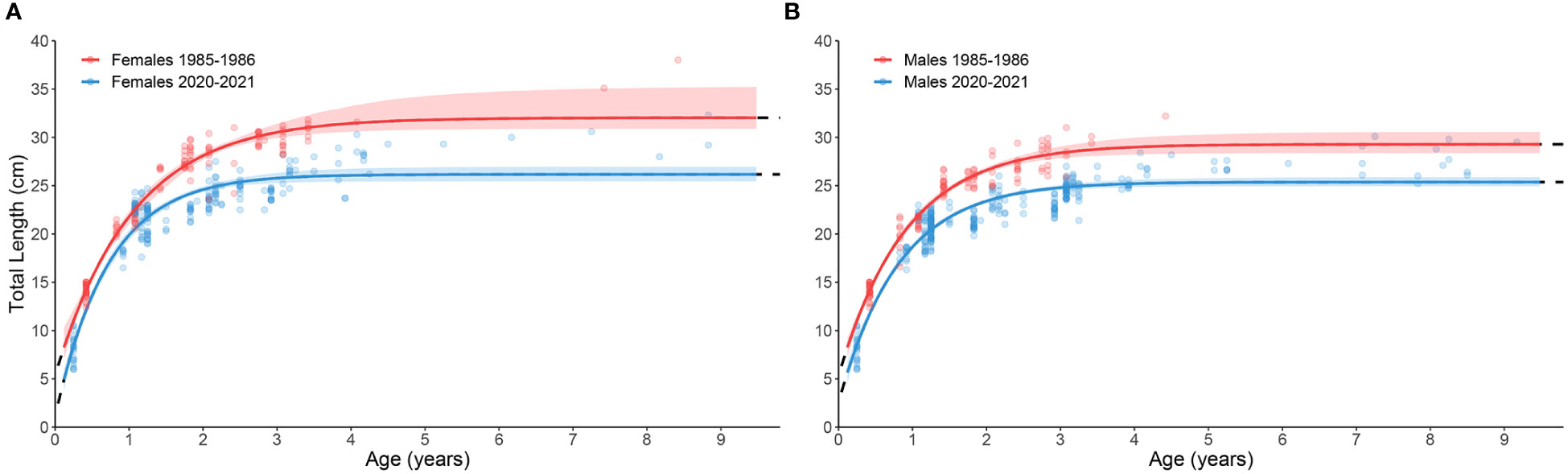
Figure 4 Von Bertalanffy growth curves for Micromesistius poutassou females (A) and males (B) sampled in 1985–86 (red) and 2020–21 (blue).
3.3 Reproductive cycle
Based on the macroscopic observation of gonad maturation and GSI monthly pattern, the spawning season of blue whiting in the study area took place from December to March, with a peak in February. The maximum GSI values were 8% and 3% in females and males, respectively, showing a marked difference in reproductive efforts between the sexes (Figure 5). Fitting the logistic model to the proportion of sexually mature specimens, size-at-first maturity (L50) was estimated to be 18.9 ± 0.1 cm TL for males and 19.8 ± 0.3 cm TL for females (Supplementary Figure 3).

Figure 5 Temporal trend of the gonadosomatic index (GSI) for Micromesistius poutassou females (A) and males (B). Note the different scales in y-axes.
4 Discussion
The present study provides a detailed and updated picture of important life history traits of blue whiting in the central Adriatic Sea. Based on growth rates reflected in the samples collected over two sampling periods from the Pomo/Jabuka Pits, we highlight differences between the present and the late 1980s.
4.1 Biological parameters
Overall, the size ranges of blue whiting were smaller than previously observed in the same area (Froglia and Gramitto, 1980) and in comparison with other studies carried out in the Mediterranean Sea (Orsi-Relini and Peirano, 1985; Sartor, 1995; Serrat et al., 2019; Mir-Arguimbau et al., 2020) (Table 4). The possible explanations for the size reduction are discussed in the next section. As already reported for other gadids, the maximum size in the Mediterranean Sea is much smaller than in the North Atlantic (Mahe et al., 2016; Gonçalves et al., 2017; Mir-Arguimbau et al., 2020) (Table 4). To our knowledge, this study provides for the first time the VBGF parameters estimated through otolith readings in the Adriatic Sea. The maximum age estimated in the 2020–21 sample was far higher than that estimated in previous studies in the Adriatic Sea, which used LFDs to estimate VBGF parameters and reported only three age classes (Froglia and Gramitto, 1980). Conversely, our estimates of maximum lifespan were similar to those based on otolith readings reported from other areas of the Mediterranean Sea (Ligurian Sea, Orsi-Relini and Peirano, 1985, and Gulf of Lions, Mir-Arguimbau et al., 2020), but smaller than those reported from the North Atlantic (Raitt, 1968; Post et al., 2019) (Table 4). The high proportion of opaque otolith margins observed in July of both sampling periods confirms that opaque deposition is associated with the fast-growing season, which occurs during warmer months. Although our samples did not cover the whole year, the analysis of the edge condition agrees with previous studies in the Mediterranean Sea (ICES, 2017; Mir-Arguimbau et al., 2020), allowing us to corroborate the annual ring formation. It is worth noting that the estimated k values were the highest and t0 the closest to 0 ever reported from the Mediterranean Sea (Maiorano et al., 2010; Mannini and Lanteri, 2017; Mir-Arguimbau et al., 2020) and North Atlantic (Bailey, 1982; Monstad, 1990; Magnussen, 2007; Froese and Pauly, 2022) (Table 4). The presence of juveniles caught in early spring-summer allowed a good fitting of the VBGF at the origin and contributed to the estimation of more accurate values of these parameters. Usually, data regarding the length-at-age during the early life stages (when growth is fastest) is not available. Lack of data may lead to an underestimation of k values and could explain the discrepancies in growth parameter estimates between the present and previous studies (Supplementary Figure 4).
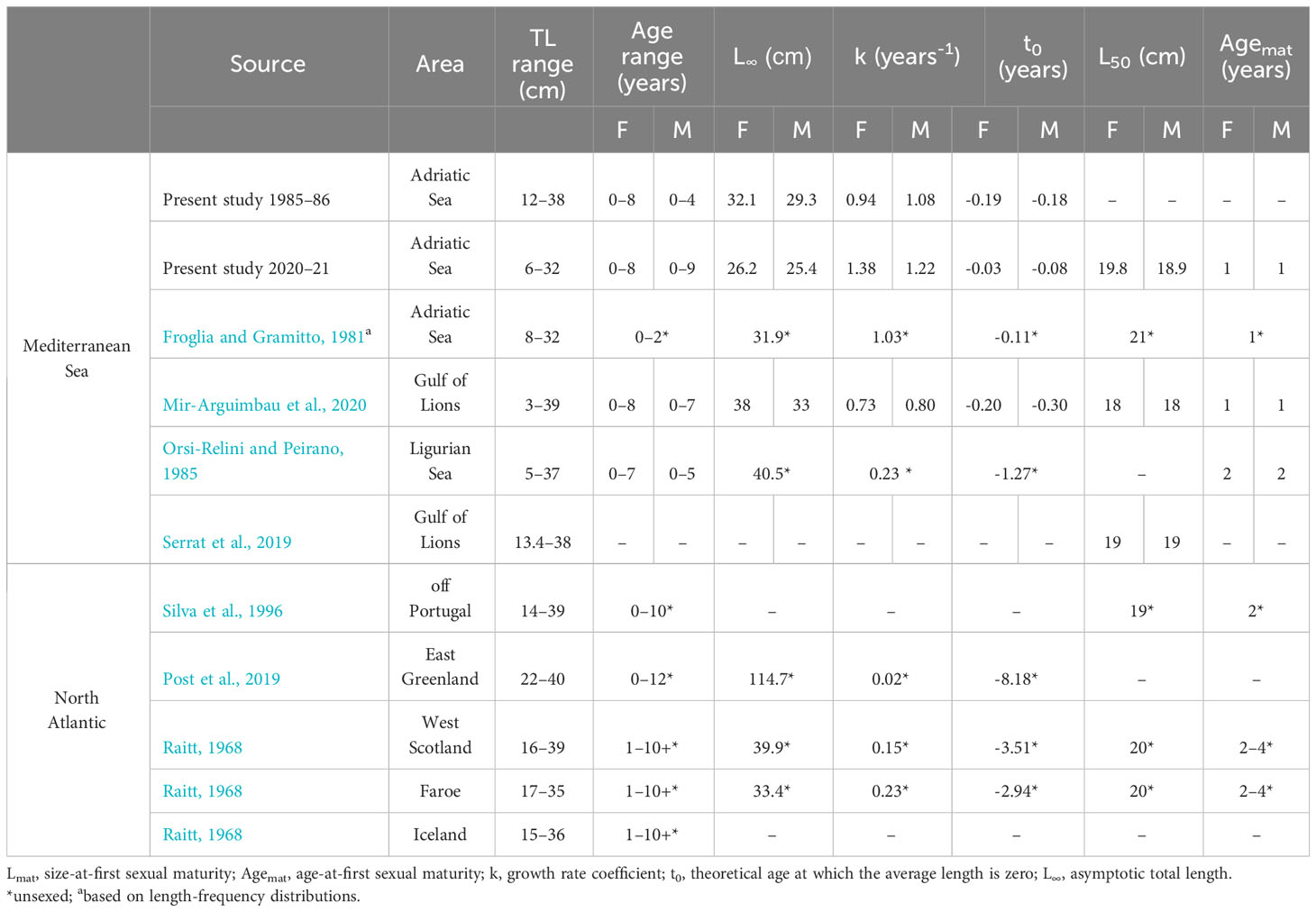
Table 4 Population parameters of Micromesistius poutassou from different areas of the Mediterranean Sea and North Atlantic.
According to the analysis of samples from 2020–21, sexual maturity is reached within the first year of life in males and females, at 18.9 and 19.8 cm TL, respectively. Our estimates agree with previous observations carried out in the Adriatic Sea (Froglia and Gramitto, 1981) and are slightly higher compared to those of other areas of the Mediterranean Sea (Silva et al., 1996; Serrat et al., 2019; Mir-Arguimbau et al., 2020) (Table 4). As expected, they are lower than those estimated in the North Atlantic (Bailey, 1982; ICES, 2004). The difference observed in the comparison of maturity size with other Mediterranean areas could be related to a bias in the sampling strategy, as we were not able to collect samples during the spawning peak in January and we also had a limited number of specimens <20 cm TL. Macroscopic observations on gonads and GSI trends indicated clearly that spawning took place in winter, in agreement with the pattern observed in general in the Mediterranean Sea (Froglia and Gramitto, 1981; Serrat et al., 2019; Mir-Arguimbau et al., 2020). The highest mean values of GSI were reached in February, 5% for females and 1% for males, confirming the different reproductive efforts between sexes (Serrat et al., 2019).
4.2 Historical comparison
The historical comparison highlighted some differences in the growth performance and age structure between the 1985–86 and 2020–21 samples. In particular, the 2020–21 sample was characterized by specimens with a lower mean length-at-age across the age classes (Figure 5), and a higher proportion of older age classes (9% was 4 or more years old), compared to 1985–86. Comparing the age-length key, a stronger slowdown in growth seemed to occur after the Year Class four in 2020–21. The lack of old specimens in the 1985–86 sampling prevented us from making robust assumptions for the comparison over the entire lifespan of this species. However, the length-at-age data of the only two specimens older than four years from the 1985–86 sample allows us to hypothesize a possible decrease in the growth potential of blue whiting over the total investigated period. It is worth noting that the age readings of both sampling periods were performed by the same expert readers, making data comparison reliable and maximizing the reduction of methodological biases. The decrease in growth efficiency is further supported by comparing the CF between the sampling periods, indicating a higher energetic investment in body growth in 1985–86. A growing body of knowledge has linked a wide range of biological responses of marine organisms to climate change/environmental anomalies and fishing (Engelhard et al., 2014; Poloczanska et al., 2016; Diaz Pauli and Sih, 2017; Charbonneau et al., 2019) and it is well-known in literature that they played a primary role in the recent interdecadal dynamics of the Adriatic resources (Coll et al., 2009; Conversi et al., 2009; Barausse et al., 2011; Lotze et al., 2011; Fortibuoni et al., 2015; Fortibuoni et al., 2017; Sguotti et al., 2022). Body condition in harvested fish is known to be affected by the combined effects of environmental features, population density and fishing impact, with a species-specific and ontogenetic variation (Rueda et al., 2015). Similarly, the growth potential of a population may be strongly influenced by environmental factors, such as food supply and temperature (Magnussen, 2007), and/or by fishing exploitation (e.g., through the removal of larger/older individuals or the selection of fast-growing and early maturing specimens) (Bianchi et al., 2000; Shin et al., 2005; Hidalgo et al., 2012). Although the historical comparison is limited just to two sampling periods, our findings nevertheless raise intriguing questions regarding the nature of the changes in growth patterns observed, as they could be explained by one or a combination of the above-mentioned factors.
Given the positive correlation between primary productivity and forage fish abundance in the Adriatic Sea (Santojanni et al., 2006), as well as the gradual decrease in nutrient inputs over the last four decades (Marini and Grilli, 2023), a possible explanation for the decrease in growth performance could be related to a decrease in prey availability for blue whiting, which feeds on zooplankton and small planktivorous fish (Sartor, 1995). Another environmental change driver is represented by the rise in sea temperature in the Adriatic Sea (Supplementary Figure 5), which could have had direct negative effects by creating unfavorable thermal conditions that interfere with the metabolic functions of this cold-water species, as reported by Trenkel et al. (2015). This mechanism has recently been linked to a decrease in landings and abundance of blue whiting in other Mediterranean areas (Sbrana et al., 2019). Finally, the possible effect of fishing activity must be considered when explaining the reduction of length-at-age and specimens larger than 28 cm TL across our study-period (Supplementary Figure 2 and Figure 3), taking into account the long history of exploitation of the area as a fishing ground for the European hake and Norway lobster (Chiarini et al., 2022). Fishing is a size-selective process, determining higher mortality rates for the larger/older specimens and modifying the size structure of fish assemblages (Bianchi et al., 2000; Shin et al., 2005). As a result, it can determine evolutionary responses in harvested populations towards favoring a smaller maximum size and size/age at maturity, and therefore a higher probability of reproducing before being caught (Trippel, 1995).
As stated, these data must be interpreted with caution because we used two punctual sampling periods, that are not representative of a real trend over the last decades. Indeed, the adaptive phenotypic plasticity may determine changes in population life history traits even on a short-term scale, depending on the environmental context (Hidalgo et al., 2014). Such natural oscillations occur not only in biological parameters but also in abundance as a result of environmental variations and fishing exploitation (Martin et al., 2016; Mir-Arguimbau et al., 2022). Another limitation of this study is represented by the small sample size of the 1985–86 sample (not fully representative of the population) and the different methodologies used in the two periods. The different selectivity of the net due to different mesh sizes represents a limit to properly compare the LFDs and size structure of the samples, even if we can assume a comparable selectivity on adult specimens since it was reported that juveniles smaller than 20 cm TL account for a large proportion of catches in otter bottom trawler deploying a 40 mm mesh size at the codend (Mir-Arguimbau et al., 2022).
Despite the noted constraints, the decrease of length-at-age and asymptotic length together with the decline observed in the landing trend in the Adriatic Sea (Supplementary Figure 6) support the idea of a poor health status for the blue whiting population in the Adriatic Sea, which is likely due to fishing exploitation and/or environmental constraints and is worthy of closer attention. Despite the restrictive measures established in 2015 in the Pomo/Jabuka Pits, there has been no evidence of a positive effect on the abundance of blue whiting (Chiarini et al., 2022). It could be possible that unfavorable environmental factors hamper the recovery of the stock and could threaten its long-term maintenance. Nevertheless, the presence in the 2020–21 sample of specimens close to the known maximum age for Mediterranean Sea populations (Fiorentino et al., 2003; Tsikliras and Stergiou, 2015) could represent an early signal of a recovery process made very slow by unfavorable environmental conditions, but further studies are needed to confirm or dismiss this hypothesis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because biological samples were obtained from landings of commercial fishing vessels.
Author contributions
FC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft. ML: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing – review & editing. FD: Methodology, Validation, Writing – review & editing. CM: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing – review & editing. MM: Resources, Writing – review & editing. AS: Resources, Writing – review & editing, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from Ministero dell’Università e della Ricerca (MUR) to CM and from Direzione Generale della Pesca Marittima e dell’Acquacoltura of the Italian Ministry of Agricultural, Food and Forestry Policies (MASAF) to AS (grant number CUP 2017-2020 J82F17 000 000 007). FC was supported by a scholarship from the International PhD Program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources” (https://www.fishmed-phd.org, accessed June 1, 2023).
Acknowledgments
We thank all of the staff of the National Research Council (CNR) and the Hydrobiological Station Umberto D’Ancona (University of Padova), as well as the fishers involved for their essential contribution to the data collection and interpretation. We wish also to thank Dr. Carlo Froglia for his work in collecting data and otoliths that made the historical comparison possible and Jennifer Pytka for the English editing. We especially thank Aluizio Junior Gomes Filho and Luca Giaccaglia (EcoTechSystems), for kindly providing precious support in collecting the samples at landing sites. The research leading to these results was conceived under the International Ph.D. program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources” (www.FishMed-PhD.org, accessed July 10, 2023). This study represents partial fulfillment of the requirements for the Ph.D. thesis of Federico Calì.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer, AS, declared a past co-authorship with author, CM.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1291173/full#supplementary-material
Supplementary Figure 1 | Monthly proportion of Micromesistius poutassou individuals showing otoliths with opaque (O; grey) or hyaline margin (H; black).
Supplementary Figure 2 | Boxplots of length-at-age of Micromesistius poutassou females (A) and males (B) sampled in 1985–86 (red) and 2020–21 (blue).
Supplementary Figure 3 | Sexual maturity ogives of males (A) and females (B) of Micromesistius poutassou. The dotted vertical blue line intercepting the x-axis indicates the size at which 50% of individuals are mature (TL50).
Supplementary Figure 4 | Auximetric plot for Micromesistius poutassou allowing to compare the asymptotic length (L∞) and growth rate coefficient (k) parameters estimated in this study (black) with other estimates obtained for the same species (red), other gadids (green), and other bony fish (yellow). Modified from Froese and Pauly, 2022.
Supplementary Figure 5 | Temporal trend of the mean annual (black) and winter (grey; between January and March) values of surface temperature (SST) in the Pomo/Jabuka Pits area from 1982 to 2021. Satellite data from Copernicus Marine Service, Mediterranean Sea - High Resolution L4 Sea Surface Temperature Reprocessed. https://doi.org/10.48670/moi-00173.
Supplementary Figure 6 | Time series of landings of Micromesistius poutassou from the Adriatic Sea, obtained from FAO official landings (FishStatJ).
References
Bailey R. S. (1982). The population biology of blue whiting in the North Atlantic. Adv. Mar. Biol. 19, 257–355. doi: 10.1016/S0065-2881(08)60089-9
Barausse A., Michieli A., Riginella E., Palmeri L., Mazzoldi C. (2011). Long-term changes in community composition and life-history traits in a highly exploited basin (northern Adriatic Sea): The role of environment and anthropogenic pressures. J. Fish Biol. 79, 1453–1486. doi: 10.1111/j.1095-8649.2011.03139.x
Beamish R. J., Fournier D. A. (1981). A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 38, 982–983.
Bianchi G., Gislason H., Graham K., Hill L., Jin X., Koranteng K., et al. (2000). Impact of fishing on size composition and diversity of demersal fish communities. ICES J. Mar. Sci. 57, 558–571. doi: 10.1006/jmsc.2000.0727
Bolger T., Connolly P. L. (1989). The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 34, 171–182. doi: 10.1111/j.1095-8649.1989.tb03300.x
Campana S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59, 197–242. doi: 10.1006/jfbi.2001.1668
Cardinale M., Scarcella G. (2017). Mediterranean Sea: A failure of the European fisheries management system. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00072
Chang W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 39, 1208–1210.
Charbonneau J. A., Keith D. M., Hutchings J. A. (2019). Trends in the size and age structure of marine fishes. ICES J. Mar. Sci. 76, 938–945. doi: 10.1093/icesjms/fsy180
Chiarini M., Guicciardi S., Zacchetti L., Domenichetti F., Canduci G., Angelini S., et al. (2022). Looking for a simple assessment tool for a complex task: Short-term evaluation of changes in fisheries management measures in the Pomo/Jabuka Pits area (Central Adriatic Sea). Sustainability 14, 7742. doi: 10.3390/su14137742
Cohen D. M., Inada T., Iwamoto T., Scialabba N. (1990). FAO species catalogue. Vol. 10. Gadiform fishes of the world (Order gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fish. Syn. 125, 319–327.
Coll M., Santojanni A., Palomera I., Arneri E. (2009). Food-web changes in the Adriatic Sea over the last three decades. Mar. Ecol. Prog. Ser. 381, 17–37. doi: 10.3354/meps07944
Colloca F., Scarcella G., Libralato S. (2017). Recent trends and impacts of fisheries exploitation on Mediterranean stocks and ecosystems. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00244
Conversi A., Peluso T., Fonda-Umani S. (2009). Gulf of Trieste: A changing ecosystem. J. Geophys. Res. Ocean. 114, 1–10. doi: 10.1029/2008JC004763
de Juan S., Lleonart J. (2010). A conceptual framework for the protection of vulnerable habitats impacted by fishing activities in the Mediterranean high seas. Ocean Coast. Manage. 53, 717–723. doi: 10.1016/j.ocecoaman.2010.10.005
Diaz Pauli B., Sih A. (2017). Behavioural responses to human-induced change: Why fishing should not be ignored. Evol. Appl. 10, 231–240. doi: 10.1111/eva.12456
Druon J. N., Fiorentino F., Murenu M., Knittweis L., Colloca F., Osio C., et al. (2015). Modelling of European hake nurseries in the Mediterranean Sea: An ecological niche approach. Prog. Oceanogr. 130, 188–204. doi: 10.1016/j.pocean.2014.11.005
Engelhard G. H., Righton D. A., Pinnegar J. K. (2014). Climate change and fishing: A century of shifting distribution in North Sea cod. Glob. Change Biol. 20, 2473–2483. doi: 10.1111/gcb.12513
EU. (2006). Council Regulation (EC) No 1967/2006 of 21 December 2006 Concerning Management Measures for the Sustainable Exploitation of Fishery Resources in the Mediterranean Sea, Amending Regulation (EEC) No 2847/93 and Repealing Regulation (EC) No 1626/94 (EU), pp. 11–85. Available at: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC068324/.
FAO. (2011). “Review of the state of world marine fishery resources,” in FAO fisheries and aquaculture technical paper no. 569 (Rome, Italy: FAO).
FAO. (2020). FishStatJ - Universal software for fishery statistical time series. Available at: https://www.fao.org/fishery/en/topic/166235/en.
FAO-GFCM. (2017). Recommendation GFCM/41/2017/3 on the establishment of a fisheries restricted area in the Jabuka/Pomo Pit in the Adriatic Sea. Available at: https://www.fao.org/gfcm/decisions/en/.
FAO-GFCM. (2020). The State of Mediterranean and Black Sea Fisheries 2020 (Rome, Italy: General Fisheries Commission for the Mediterranean). doi: 10.4060/cb2429en.
Fiorentino F., Gancitano S., Giusto G. B., Rizzo P. (2003). Stima della longevità in gadiformi dello stretto di sicilia sulla base della lettura di otoliti. Biol. Mar. Mediterr. 10, 814–818.
Fortibuoni T., Aldighieri F., Giovanardi O., Pranovi F., Zucchetta M. (2015). Climate impact on Italian fisheries (Mediterranean Sea). Reg. Environ. Change 15, 931–937. doi: 10.1007/s10113-015-0781-6
Fortibuoni T., Giovanardi O., Pranovi F., Raicevich S., Solidoro C., Libralato S. (2017). Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Front. Mar. Sci. 4. doi: 10.3389/FMARS.2017.00033
Fox J., Weisberg S. (2019) An R Companion to Applied Regression. Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Free C. M., Thorson J. T., Pinsky M. L., Oken K. L., Wiedenmann J., Jensen O. P. (2019). Impacts of historical warming on marine fisheries production. Science 365, 979–983. doi: 10.1126/science.aax5721
Froese R., Pauly D. (2022) FishBase (World Wide Web Electron. Publ). Available at: https://fishbase.mnhn.fr/popdyn/PopGrowthList.php?ID=29&Genus-Name=Micromesistius&SpeciesName=poutassou&fc=183 (Accessed 9 December 2022).
Froglia C., Gramitto M. E. (1980). “Observations on growth of Micromesistius poutassou (Risso) (Pisces, Gadidae) in the central Adriatic Sea,” in XXVIIe Congres - Assemblee pleniere CIESM, Comite Vertebres marins et Cephalopodes, Cagliari, Italy.
Froglia C., Gramitto M. E. (1981). Summary of biological parameters on Micromesistius poutassou (Risso) in the Adriatic. FAO Fish. Rep. 253, 93–94.
Gonçalves P., Ávila De Melo A., Murta A. G., Cabral H. N. (2017). Blue whiting (Micromesistius poutassou) sex ratio, size distribution and condition patterns off Portugal. Aquat. Living Resour. 30, 24. doi: 10.1051/alr/2017019
Hidalgo M., El-Haweet A. E., Tsikliras A. C., Tirasin E. M., Fortibuoni T., Ronchi F., et al. (2022). Risks and adaptation options for the Mediterranean fisheries in the face of multiple climate change drivers and impacts. ICES J. Mar. Sci. 79, 2473–2488. doi: 10.1093/icesjms/fsac185
Hidalgo M., Olsen E. M., Ohlberger J., Saborido-Rey F., Murua H., Piñeiro C., et al. (2014). Contrasting evolutionary demography induced by fishing: The role of adaptive phenotypic plasticity. Ecol. Appl. 24, 1101–1114. doi: 10.1890/12-1777.1
Hidalgo M., Rouyer T., Bartolino V., Cerviño S., Ciannelli L., Massutí E., et al. (2012). Context-dependent interplays between truncated demographies and climate variation shape the population growth rate of a harvested species. Ecography 35, 637–649. doi: 10.1111/j.1600-0587.2011.07314.x
ICES. (2004). “Report of the northern Pelagic and blue whiting fisheries working group (WGNPBW),” in ICES Expert Gr. Reports (until 2018). (Copenhagen: ICES), 241. 241pp. doi: 10.17895/ices.pub.19266569.v1%0A%0A.
ICES. (2008). Report of the Workshop on Sexual Maturity Staging of Cod, Whiting, Haddock and Saithe (WKMSCWHS). doi: 10.17895/ices.pub.19280246.
ICES. (2017). Workshop on age estimation of Blue Whiting (Micromesistius poutassou) WKARBLUE2, 6-9 June 2017. Lisbon, Portugal: ICES CM 2017/ SSGIEOM:22.
Kimura D. K. (1980). Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 77, 765–776.
Lloret J., Serrat A., Thordarson G., Helle K., Jadaud A., Bruno I., et al. (2021). The poor health of deep-water species in the context of fishing activity and a warming climate: will populations of Molva species rebuild or collapse? J. Fish Biol. 98, 1572–1584. doi: 10.1111/jfb.14347
Lotze H. K., Coll M., Dunne J. A. (2011). Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 14, 198–222. doi: 10.1007/s10021-010-9404-8
Magnussen E. (2007). Interpopulation comparison of growth patterns of 14 fish species on Faroe Bank: Are all fishes on the bank fast-growing? J. Fish Biol. 71, 453–475. doi: 10.1111/j.1095-8649.2007.01502.x
Mahe K., Oudard C., Mille T., Keating J., Gonҫalves P., Clausen L. W., et al. (2016). Identifying blue whiting (Micromesistius poutassou) stock structure in the Northeast Atlantic by otolith shape analysis. Can. J. Fish. Aquat. Sci. 73, 1363–1371. doi: 10.1139/cjfas-2015-0332
Maiorano P., Sion L., Carlucci R., Capezzuto F., Giove A., Costantino G., et al. (2010). The demersal faunal assemblage of the north-western Ionian Sea (central Mediterranean): Current knowledge and perspectives. Chem. Ecol. 26, 219–240. doi: 10.1080/02757541003693987
Mannini A., Lanteri L. (2017). “Micromesistius poutassou,” in Sintesi delle conoscenze di biologia, ecologia e pesca delle specie ittiche dei mari italiani / Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas, vol. Suppl. 1). Eds. Sartor P., Mannini A., Carlucci R., Massaro E., Queirolo S., Sabatini A., et al., Biol. Mar. Medirerr. 24, 304–310.
Marini M., Grilli F. (2023). The role of nitrogen and phosphorus in Eutrophication of the northern Adriatic sea : history and future scenarios. Appl. Sci. 13, 9267. doi: 10.3390/app13169267
Marini M., Maselli V., Campanelli A., Foglini F., Grilli F. (2016). Role of the Mid-Adriatic deep in dense water interception and modification. Mar. Geol. 375, 5–14. doi: 10.1016/j.margeo.2015.08.015
Martin P., Maynou F., Recasens L., Sabatés A. (2016). Cyclic fluctuations of blue whiting (Micromesistius poutassou) linked to open-sea convection processes in the northwestern Mediterranean. Fish. Oceanogr. 25, 229–240. doi: 10.1111/fog.12147
Martinelli M., Angelini S., Belardinelli A., Canduci G., Chiarini M., Domenichetti F., et al. (2021). Accordo tra MIPAAF e CNR-IRBIM Ancona in merito alla proposta progettuale relativa alle attività di monitoraggio periodico delle fosse di Pomo e all’attuazione di misure che, nel rispetto dei piani di gestione, comportino il mantenimento delle condizioni ambientali idonee alla vita e all’accrescimento dei molluschi bivalvi, ponendo in essere misure supplementari tese a proteggere le diverse fasi del ciclo biologico delle specie interessate (CUP J41F19000080001). Parte Monitoraggio Fosse di Pomo periodo 2019-2020, esteso 2021. Report finale, Ottobre 2021.
MIPAAF. (2017). Misure per la pesca nella Fossa di Pomo. D.M. 01/06/2017. Rome: Ministero delle politiche agricole alimentari e forestali.
Mir-Arguimbau J., Balcells M., Raventós N., Martín P., Sabatés A. (2020). Growth, reproduction and their interplay in blue whiting (Micromesistius poutassou, Risso 1827) from the NW Mediterranean. Fish. Res. 227, 105540. doi: 10.1016/j.fishres.2020.105540
Mir-Arguimbau J., Martín P., Balcells M., Sala-Coromina J., Sabatés A. (2022). Fishery dynamics of blue whiting, Micromesistius poutassou, a highly discarded bycatch species in the NW Mediterranean Sea. Sci. Mar. 86, 1–12. doi: 10.3989/scimar.05240.025
Monstad T. (1990). “Distribution and growth of blue whiting in the North-East Atlantic 1980-1988. PINRO-IMR Symp," in Proceedings of the fourth Soviet-Norwegian Symposium; 1989 June 12-16; Bergen. 227–267.
Moullec F., Barrier N., Drira S., Guilhaumon F., Marsaleix P., Somot S., et al. (2019). An end-to-end model reveals losers and winners in a warming Mediterranean Sea. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00345
Ogle D. H., Doll J. C., Wheeler A. P., Dinno A. (2022) FSA: Simple Fisheries Stock Assessment Methods. Available at: https://cran.r-project.org/package=FSA.
Orsi-Relini L., Peirano A. (1985). Biological notes on the blue whiting, Micromesistius poutassou (Risso) of the Ligurian Sea. FAO Fish. Rep. 336, 113–117.
Pitcher C. R., Hiddink J. G., Jennings S., Collie J., Parma A. M., Amoroso R., et al. (2022). Trawl impacts on the relative status of biotic communities of seabed sedimentary habitats in 24 regions worldwide. Proc. Natl. Acad. Sci. U.S.A. 119, 2. doi: 10.1073/pnas.2109449119
Pollack M. J. (1951). The sources of the deep water of the eastern Mediterranean Sea. J. Mar. Res. 10, 128–152.
Poloczanska E. S., Burrows M. T., Brown C. J., Molinos J. G., Halpern B. S., Hoegh-Guldberg O., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00062
Posit Team. (2022). RStudio: Integrated Development Environment for R. Available at: http://www.posit.co/.
Post S., Fock H. O., Jansen T. (2019). Blue whiting distribution and migration in Greenland waters. Fish. Res. 212, 123–135. doi: 10.1016/j.fishres.2018.12.007
Raitt D. F. S. (1968). Synopsis of biological data on the blue whiting Micromesistius poutassou (Risso 1810). FAO Fish. Synopsis 54, 1–39.
R Core Team. (2022). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.r-project.org/.
Roether W., Manca B. B., Klein B., Bregant D., Georgopoulos D., Beitzel V., et al. (1996). Recent changes in eastern Mediterranean deep waters. Science 271, 333–335. doi: 10.1126/science.271.5247.333
Rueda L., Massutí E. M., Alvarez-Berastegu D., Hidalgo M. (2015). Effect of intra-specific competition, surface chlorophyll and fishing on spatial variation of gadoid’s body condition. Ecosphere 6.10, 1–17. doi: 10.1890/ES15-00087.1
Santojanni A., Arneri E., Bernardini V., Cingolani N., Di Marco M., Russo A. (2006). Effects of environmental variables on recruitment of anchovy in the Adriatic Sea. Clim. Res. 31, 181–193. doi: 10.3354/cr031181
Sartor P. (1995). Regime alimentare di osteitti gadiformi nel mar Tirreno Settentrionale. Atti Soc Tosc. Sci. Nat. Mem. Ser. B 102, 59–67.
Sbrana M., Fiorentino F., Musumeci C., Garofalo G., Lanteri L., Ligas A., et al. (2019). “Climate change, fishery and resources: the case of cold water species Micromesistius poutassou (Risso 1827) (Pisces, Gadidae),” in 50° Congresso della Società Italiana di Biologia Marina Livorno, 10-14 giugno 2019, Livorno, Italy.
Serrat A., Lloret J., Frigola-Tepe X., Muñoz M. (2019). Trade-offs between life-history traits in a coldwater fish in the Mediterranean Sea: the case of blue whiting Micromesistius poutassou. J. Fish Biol. 95, 428–443. doi: 10.1111/jfb.13993
Sguotti C., Bischoff A., Conversi A., Mazzoldi C., Möllmann C., Barausse A. (2022). Stable landings mask irreversible community reorganizations in an overexploited Mediterranean ecosystem. J. Anim. Ecol. 91, 2465–2479. doi: 10.1111/1365-2656.13831
Shin Y. J., Rochet M. J., Jennings S., Field J. G., Gislason H. (2005). Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 62, 384–396. doi: 10.1016/j.icesjms.2005.01.004
Silva A., Pestana G., Dias C., Godinho S. (1996). “Preliminary results on the distribution and spawning of blue whiting,” in Micromesistius poutassou, off the Portuguese coast. ICES C. 1996/H. ICES Council Meeting, vol. 16., 1–22. Available at: https://www.ices.dk/sites/pub/CM%20Doccuments/1996/H/1996_H16.pdf.
Trenkel V. M., Lorance P., Fässler S. M. M., Høines A. S. (2015). Effects of density dependence, zooplankton and temperature on blue whiting Micromesistius poutassou growth. J. Fish Biol. 87, 1019–1030. doi: 10.1111/jfb.12775
Tsikliras A. C., Stergiou K. I. (2015). Age at maturity of Mediterranean marine fishes. Mediterr. Mar. Sci. 16, 5–20. doi: 10.12681/mms.659
Keywords: Adriatic sea, Pomo Pits, climate change, growth, blue whiting, cold-water species
Citation: Calì F, La Mesa M, Donato F, Mazzoldi C, Martinelli M and Santojanni A (2023) Life history traits and historical comparison of blue whiting (Micromesistius poutassou) growth performance from the western Pomo/Jabuka Pits area (central Adriatic Sea). Front. Mar. Sci. 10:1291173. doi: 10.3389/fmars.2023.1291173
Received: 08 September 2023; Accepted: 02 November 2023;
Published: 27 November 2023.
Edited by:
Josipa Ferri, University of Split, CroatiaReviewed by:
Alen Soldo, University of Split, CroatiaTatjana Dobroslavic, University of Dubrovnik, Croatia
Mark C. Belk, Brigham Young University, United States
Copyright © 2023 Calì, La Mesa, Donato, Mazzoldi, Martinelli and Santojanni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Calì, ZmVkZXJpY28uY2FsaTJAc3R1ZGlvLnVuaWJvLml0
 Federico Calì
Federico Calì Mario La Mesa4
Mario La Mesa4 Fortunata Donato
Fortunata Donato Carlotta Mazzoldi
Carlotta Mazzoldi Michela Martinelli
Michela Martinelli Alberto Santojanni
Alberto Santojanni