- 1Environmental Studies Program, Hamilton College, Clinton, NY, United States
- 2Natural Science Division, Pepperdine University, Malibu, CA, United States
Macroalgae form important coastal ecosystems and are considered to be highly productive, yet individual macrophyte carbon uptake rates are poorly documented and methodologies for in situ assessments of productivity are not well developed. In this study, we employ a 13C enrichment method in benthic chambers to calculate carbon uptake rates and assess δ13C signatures of a large stock of nearshore benthic macroalgae varying in taxa and morphology in Southern California. Our objectives are to 1) identify the variability of carbon uptake and inorganic carbon use among individuals of the same species or morphology, and 2) establish accurate and accessible carbon uptake procedures for coastal benthic primary producers. We found no significant relationship between the observed ranges of environmental factors such as nutrient concentrations, PAR, temperature, conductivity, and productivity rates, suggesting that unique physiological complexions underpin the high variability of carbon uptake and δ13C in studied macrophyte samples. We consider three reasons our experimental carbon uptake rates are 3–4 orders of magnitude lower than existing literature, which reports carbon uptake in the same units despite using different methods: 1) underrepresentation of Pmax, 2) incomplete carbon fractionation corrections, and 3) reduced hydrodynamics within the benthic chambers.
1 Introduction
Photosynthesis provides the primary source of organic matter in all aquatic ecosystems. (Falkowski and Raven, 2013; Krause-Jensen et al., 2016). As a result, the rate of photosynthesis places an upper limit on the biomass and productivity of ecosystems, making the study of primary productivity key to understanding the constraints of the biological flow of energy as well as the regulation of inorganic carbon and nutrient regimes and the greater global carbon cycle (Falkowski and Raven, 2013). Macroalgae are the predominate primary producers on nearly one-third of global coastlines (Krause-Jensen et al., 2016; Queiros et al., 2023; Duarte et al., 2022), serve as a foundation species, and provide a variety of ecosystem services to local biota and humans (Pfister et al., 2019; Ruhamak et al., 2019; James et al., 2022). Studying the productivity of macroalgae is therefore essential to understanding the physical, chemical, and ecological functioning of nearshore marine environments, particularly under environmental shifts associated with climate change.
Benthic macroalgae assemblages rank among the most productive habitats on earth (Mann, 1973; Tait and Schiel, 2018), with a net primary productivity (NPP) up to ten times higher than that of temperate coastal phytoplankton (Pessarrodona et al., 2022) and comparable to that of tropical rainforests by area (Pace and Lovett, 2013; Duarte et al., 2022). Individual macrophyte productivity rates, however, are poorly defined (Dolliver and O’Connor, 2022), leading to uncertain carbon sequestration estimations and community biomass limits, and obscuring the effectiveness of algae aquaculture and ocean-based carbon dioxide removal (CDR) practices that employ macroalgae (Krause-Jensen et al., 2016).
Oxygen evolution and carbon assimilation measurements are the two most traditional methods of resolving the productivity of macrophytes (Peterson, 1980; Miller and Dunton, 2007; Pace and Lovett, 2013). In recent decades, the vast majority of macroalgae productivity assessments (Littler and Murray, 1974; Tait and Schiel, 2018; James et al., 2022) have employed the oxygen evolution technique, first described by Strickland (1960). The oxygen evolution technique uses the quantity of dissolved oxygen produced in light vs. dark incubations as a metric for NPP and community respiration (Littler and Murray, 1974; Mateo et al., 2001; Spector and Edwards, 2020).
The growing importance of climate-related research and applied carbon accounting, however, has encouraged calculating productivity in carbon units. This is inherently problematic for the oxygen evolution technique, and variable and often inconsistent conversion factors from oxygen evolved to carbon assimilated prohibit reliable translations of productivity (Arístegui et al., 1996; Cornwall and Hurd, 2019). This conversion relies on a photosynthetic quotient (PQ) which may vary depending on the physiological state and nutrient status of the alga (Miller and Dunton, 2007; Cornwall and Hurd, 2019). Additional uncertainties with the oxygen evolution method lie in the supposition that light and dark respiration is equivalent (Pace and Lovett, 2013) and that only a negligible quantity of energy is used by other concurrent metabolic processes such as nitrate and nitrite reduction (Miller and Dunton, 2007), producing potentially inaccurate representations of raw productivity (Peterson, 1980).
Our study uses the stable isotope 13C (in the form of bicarbonate NaH13CO3) as a label for a fraction of photosynthetically incorporated carbon within macroalgae and a direct measurement of carbon uptake. Using 13C enrichment to calculate carbon uptake is a measurement of gross primary productivity (GPP) when deployed on short (i.e., hours) time scales because of the time lag between when labeled carbon is acquired by macrophyte tissue and when it becomes accessible to respiratory cycles (Miller and Dunton, 2007). Using 13C in enrichments is preferred to other forms of labeled carbon, such as 14C-CO2, because it avoids the environmental and procedural hazards associated with handling radioactive material (Miller and Dunton, 2007) and can be used in tandem with other isotope analyses such as 15N (Mateo et al., 2001).
This study examines the carbon uptake rates of macroalgal species from all three major taxa (i.e., Rhodophyta, Phaeophyta, and Chlorophyta) and spans five functional groups (e.g., articulated corallines, filamentous, kelps and fucoids, fleshy branched, and tubular or sheet) with diverse morphological traits and physiological mechanisms. We pair carbon uptake measurements with assessments of macroalgal δ13C signatures to identify the variability of carbon uptake and inorganic carbon use among individuals of the same species and morphology (Cornwall et al., 2015). Our use of field-based measurements complements the existing breadth of laboratory and culture-based experiments to directly evaluate the varying contribution of macroalgae species to coastal primary productivity. In situ procedures are difficult to execute due to the logistical difficulties of working in the marine environment (Raven et al., 2002) but are often favored because they more accurately reproduce environmental and physiological conditions than laboratory settings (Miller and Dunton, 2007; Carvalho et al., 2009b).
We hypothesize that macrophyte carbon uptake will vary according to morphology such that species with greater surface area to volume ratios will yield higher carbon uptake rates (form-function hypothesis). Additionally, we anticipate that species with high carbon uptake rates will have lower isotopic carbon discrimination in the carbon acquisition process and therefore greater δ13C signatures (impartial carbon acquisition hypothesis). A concluding methodological comparison between our study and results from previous literature is designed to present the advantages of and considerations with using the 13C method and establish accurate and accessible carbon uptake procedures for coastal benthic primary producers. To our knowledge, this study presents the first assessments of macroalgal productivity via a 13C enrichment method in situ using benthic chambers on the Pacific Coast of North America. The results from this study can be applied to understand the potential responses of macrophyte productivity to changing ocean chemistry associated with climate change.
2 Materials and methods
2.1 Study site description and experimental design
This study was conducted at the Latigo Point intertidal zone located in Malibu, Southern California (Figure 1). Our specific study area is a 15 by 15 meter tide pool that forms at low tides (< 0 m) on a rugged rocky substrate. This region is dominated by vegetation, with 50% cover of macroalgae and 14% cover of seagrass. Diffuse, high salinity (~34 ppt), and high nutrient submarine groundwater discharge (SGD) containing an average (± standard deviation) of 33.75 ± 18.89 µmol L-1 NO3-, 1.65 ± 0.52 µmol L-1 PO43+, 33.1 ± 8.15 µmol L-1 silicate, and 6.85 ± 7.0 µmol L-1 NH4+ seeps from the sandy beach above as close as 25 meters from the study site. A small pipe 50 meters from the study area dispenses storm drainage runoff from nearby paved roads and residential housing complexes directly onto the beach. Latigo Canyon Creek, the principal tributary of the Latigo Canyon watershed, empties into the ocean 350 meters from the sampling site.

Figure 1 (A) Aerial view of the study site and inlaid map of study location in Southern California. (B) Photo of the intertidal zone at Latigo Point.
In situ benthic chambers are designed to isolate specimens and evaluate their response to manipulation in their natural setting and environmental conditions. Ten benthic chamber experiments were executed at low tides ranging from -0.02 meters to -0.3 meters at peak daylight hours (i.e., 9:00−14:00) from May to June. These methods closely follow those of La Valle et al. (2023) for benthic chambers. Benthic chambers were constructed from flexible clear six mil polyethylene bags (Polyethylene Greenhouse film, 93% PAR transparency) that allowed for movement of the chamber with water flow and had a sealable sampling port. Benthic chambers were placed around a rock containing a variety of macroalgal species and sealed to the hard substrate using multiple ring-shaped sandbags at the base with additional weighted chains placed on top of the sandbags. Each benthic chamber was designed to hold about 20 L of seawater and encompassed a 0.25 by 0.25 m area of benthos at its base. No air was permitted inside the benthic chamber after it was sealed to the benthos. Benthic chambers were then enriched with approximately 0.3 g of 98 at. % (atomic percent) NaH13CO3 at the start of the experiment and subsequently incubated for 1 hour.
Samples of macroalgae were picked from the specific location of the benthic chamber deployment site before sealing it to the substrate and after the hour-long incubation with NaH13CO3. To achieve an unbiased sampling protocol, specific species were not targeted in benthic chamber deployments; rather, suitable substrates with high macrophyte diversity were selected. Due to the diversity and spatial variability of macroalgae species at the study site, each benthic chamber contained a different array of species. All macroalgae were identified to the lowest possible taxonomic level with the naked eye or scope in the laboratory after the experiment had concluded by cross-referencing morphology with identifications from established literature. Species that occurred more than once were included in data analysis performed in R.
2.2 Isotope analyses
Macroalgae samples were cleaned of sediments and epiphytes in the laboratory directly after the experiment and dried in aluminum tins at 60°C for 48-72 hours. Dried macrophyte tissue was ground in a Wig-L-Bug and packed in a standard-weight tin-pressed capsule according to methods described by Teichberg et al. (2008) and de los Santos et al. (2022). Samples were sent to the Center for Stable Isotope Biogeochemistry at the University of California at Berkeley to determine δ13C, δ15N, and total C and N by continuous flow (CF) dual isotope analysis using a CHNOS Elemental Analyzer interfaced to an IsoPrime100 mass spectrometer (Carbon and Nitrogen Center for Stable Isotope Biogeochemistry). Long-term external precision for C and N isotope determinations is ± 0.10‰ and ± 0.20‰, respectively.
2.3 Water chemistry and physical parameter measurements
Conductivity, temperature, and PAR: Salinity (Odyssey Temperature and Conductivity loggers, 3 to 60 mS cm-1), temperature (Onset TidbiT v2 Water Temperature Data Logger), and photosynthetically active radiation (PAR) (Odyssey Submersible Photosynthetically Active Radiation logger) were monitored continuously at 1-minute intervals throughout the experiment. One set of sensors was placed within the benthic chamber and another set directly outside the benthic chamber in order to account for any environmental differences influencing the study. La Valle et al. (2023) showed that this method does not significantly affect the PAR, temperature, and flow within the chamber compared to surrounding waters in coral reef systems.
Dissolved inorganic nutrients: Water samples for dissolved inorganic nutrient analysis were collected from the benthic chamber before the NaH13CO3 enrichment and after the hour-long experiment had concluded. Nutrient samples were also collected during the experiment from the local SGD, the storm drainage runoff, the Latigo Canyon Creek tributary, and 200 meters offshore. These samples were collected to quantify sources and ranges of nutrient concentrations at each location with respect to the study site. 60 mL water samples were filtered through 0.2µM glass fiber filters (Whatman), frozen at< 0°C, and sent for analysis at Scripps Institute of Oceanography Shipboard Technical Support/Oceanographic Data Facility. Samples were thawed in a 50°C heating bath, brought to room temperature, and analyzed with a Seal Analytical continuous-flow AutoAnalyzer 3 (AA3). The final concentrations of nutrients were calculated using SEAL Analytical AACE software. Water samples were analyzed for soluble reactive phosphate (PO43-) ammonium (NH4+), nitrate + nitrite (N + N; NO2- + NO3-), and silicate (SiO4) using methods described by Atlas et al. (1971); Hager et al. (1972); Gordon et al. (1992), and Becker et al. (2019).
Dissolved inorganic carbon (DIC): 250 mL of water sample for DIC analysis was drawn from within the benthic chamber using a 500 mL syringe before the experiment, directly after the NaH13CO3 enrichment, and at the end of the hour-long incubation. DIC samples were stored in borosilicate bottles in dark-out bags and fixed with 20 μl HgCl2 per 250 mL seawater within 2 hours of collection. Samples were sent to the Center for Stable Isotope Biogeochemistry at the University of California at Berkeley for δ13C analysis according to procedures described by (Torres et al., 2005).
2.4 Numerical procedures and calculation of carbon uptake
Calculating carbon uptake requires defining the atomic % of 13C within the sample, determined from the 13C/12C of the sample based on the deviation of δ13C within the sample from the universal 13C V-PDB (Vienna PeeDee Belemnite) standard designated by Craig (1957). All isotopic calculations are taken from La Valle et al. (2023) and Hayes (2004), outlined below, and described in detail in Supplemental Table 1. Macroalgal carbon uptake rates were calculated using equation 1 below, derived from Hama et al. (1983) and used in Mateo et al. (2001) and La Valle et al. (2023). Similar calculations are also specified in Cornelisen and Thomas (2002).
Where atamb is the atomic % 13C in the ambient macrophyte tissue, atenr is the atomic % 13C of the macrophyte tissue 1 hour after enrichment, and atDIC is the atomic % 13C of DIC within the incubation directly after enrichment. C (mg) is the total carbon content of the sample, dw refers to the dry weight (g) of the sample after 48-72 hours of drying, and t (h) is the length of the experiment in hours. Alpha (α) refers to the fractionation coefficient and is described below. Phytoplankton uptake rates were determined using a carbon uptake rate equation similar to that found in Hama et al. (1983).
POC refers to the particulate organic carbon (ug C L-1) in the incubated sample. To account for isotopic discrimination, a fractionation coefficient, α, is calculated on an individual alga basis based on the following equation by Miller and Dunton (2007) and applied directly to raw carbon uptake rates.
Where δ13CDIC refers to the δ13C of ambient DIC, and δ13Camb refers to the δ13C of ambient macrophyte tissue.
3 Results
A total of 47 samples of macroalgae, spanning 12 species in all three major phyla, were collected and analyzed for carbon uptake rates and δ13C signatures. Samples were partitioned into functional groups which were determined by organizing macrophytes based on surface area to volume ratios (SA:V) according to the categorizations of Littler and Arnold (1982), amended by Murray and Bray (1993), and are synonymous with algal morphological groups. The most common morphology is the tubular or sheet group, comprised of 13 samples across 4 species. All four tubular or sheet species in our study have a thin and flat quality. The next most common morphologies are the articulated corallines (2 species, n = 9), characterized by their CaCO3 structure, the fleshy branched alga (2 species, n = 9) of feathery composition, and the kelps and fucoids (2 species, n = 8) with large branching fronds. Finally, the filamentous alga (2 species, n = 7) have distinct threadlike thalli.
Figure 2 reveals that carbon uptake varied greatly on individual macrophyte and functional group scales. There were significant differences between species (F11,34 = 2.846, p = 0.009) and between functional groups (F4, 41 = 6.493, p = 0.0004) as indicated by a one-way ANOVA. Carbon uptake rates for sampled macroalgae ranged from an average of 2.05 × 10-4 mgC g dry wt-1 h-1 (J. macmillanii) to 1.66 × 10-3 mgC g dry wt-1 h-1 (U. californica). The articulated coralline functional group exhibited the lowest average carbon uptake among functional groups (2.02 × 10-4 ± 1.47 × 10-4 mgC g dry wt-1 h-1), followed by the filamentous group (4.38 × 10-4 ± 2.28 × 10-4 mgC g dry wt-1 h-1), the kelps and fucoids (8.02 × 10-4 ± 6.71 × 10-4 mgC g dry wt-1 h-1), the fleshy branched group (8.03 × 10-4 ± 4.85 × 10-4 mgC g dry wt-1 h-1), and finally, the tubular or sheet group yielded the highest average carbon uptake (1.13 × 10-3 ± 7.25 × 10-4 mgC g dry wt-1 h-1). The homogeneity and normality of residuals prompted a log transformation of carbon uptake rates for statistical analysis. Tukey post-hoc analysis indicated significant differences between the articulated coralline group and the fleshy branched, tubular or sheet, and kelps and fucoids groups (p = 0.0039, p = 0.0002, p = 0.034 respectively). Significant differences were also found between species J. macmillanii and U. californica (p = 0.0028). Relationships between C. officinalis and U. californica and between J. macmillanii and P. capillacea approached significance (p = 0.056 and p = 0.084 respectively). In all cases, the alpha error rate was taken to be 5%. In terms of taxa, the Chlorophyta had the highest average carbon uptake rates (1.66 × 10-3 ± 0.65 × 10-3 mgC g dry wt-1 h-1), followed by the Phaeophyta (7.77 × 10-4 ± 6.02 × 10-4 mgC g dry wt-1 h-1), and finally the Rhodophyta (5.2 × 10-4 ± 4.45 × 10-4 mgC g dry wt-1 h-1).
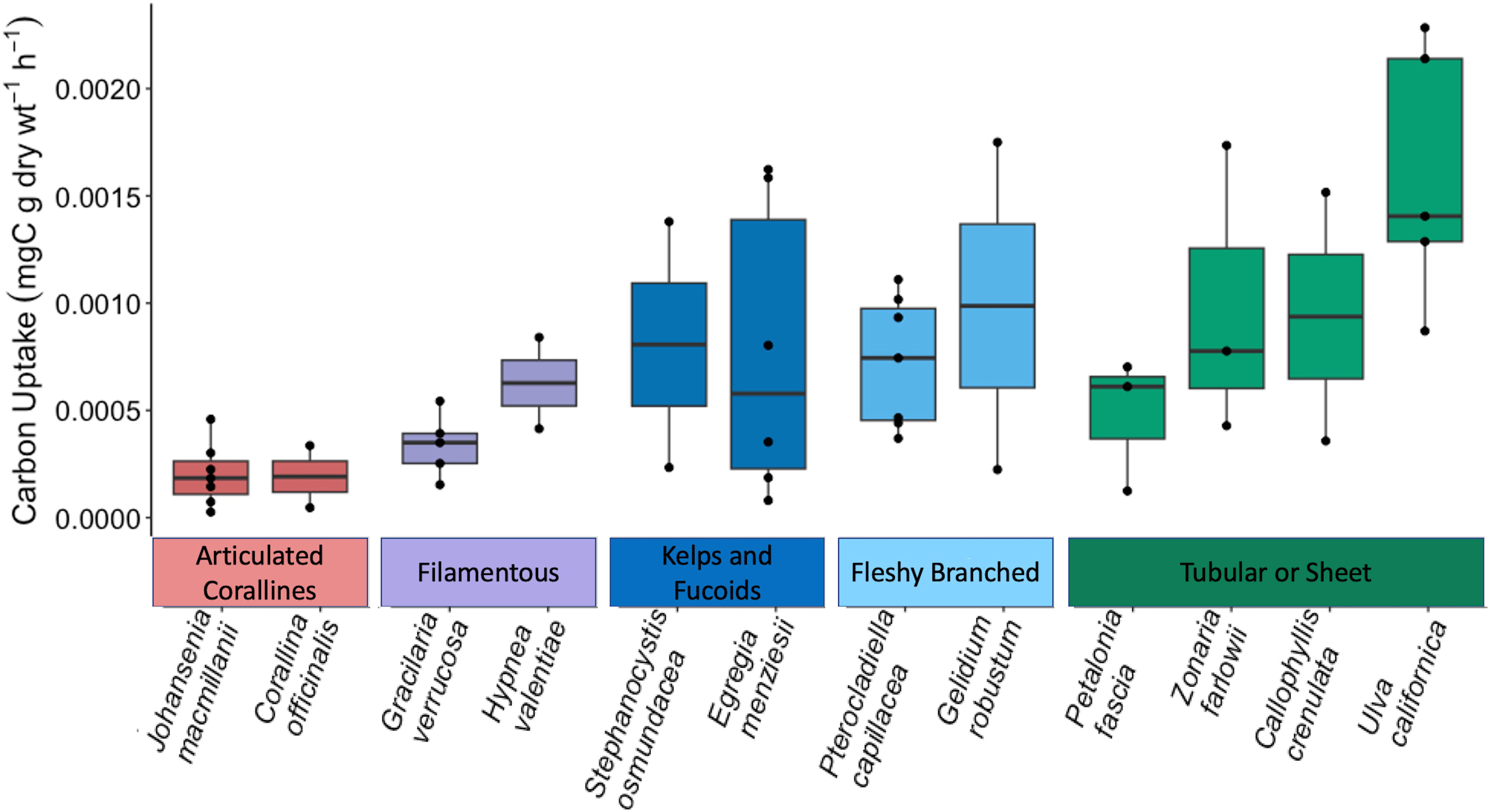
Figure 2 Boxplot displaying the carbon uptake rates of each macroalgae species. Species are displayed within their functional group from least productive (left) to most productive (right). Functional groups are also displayed from least productive (left: articulated corallines) to most productive (right: tubular or sheet).
δ13C signatures ranged across all species from -20.17‰ (C. crenulata) to -5.53‰ (J. macmillanii). This also represents the range of δ13C signatures of the Rhodophyta while the Chlorophyta δ13C ranged from -17.15‰ to -12.65‰ and the Phaeophyta ranged from -18.21‰ to -11.51‰. A Kruskal-Wallis test with Dunn post-hoc analysis confirmed high variability of δ13C and revealed significant differences between functional groups (Chi square = 17.2, p = 0.002, df = 4). The articulated corallines had the highest average δ13C (-9.9‰ ± 2.76‰), followed by the fleshy branched group (-13.61‰ ± 4.47‰), the kelps and fucoids (-15.35‰ ± 2.14‰), the filamentous group (-15.70‰ ± 2.58‰), and finally the tubular or sheet group (-16.03‰ ± 2.50‰). The articulated coralline functional group was determined to be significantly different in δ13C compared to the tubular or sheet, kelps and fucoids, and filamentous groups (p = 0.001, p = 0.026, p = 0.026 respectively). The Kruskal-Wallis test with Dunn post-hoc analysis also revealed significant differences between species (Chi squared = 29.98, p = 0.002, df = 11) such that J. macmillanii was significantly greater in δ13C compared to C. crenulata, E. menziesii, and H. valentiae (p = 0.016, p = 0.031, p = 0.04 respectively) and Z. farlowii was significantly lower in δ13C than P. capillacea (p = 0.04). A complete list δ13C values from all 12 species and 5 functional groups is provided in Table 1.
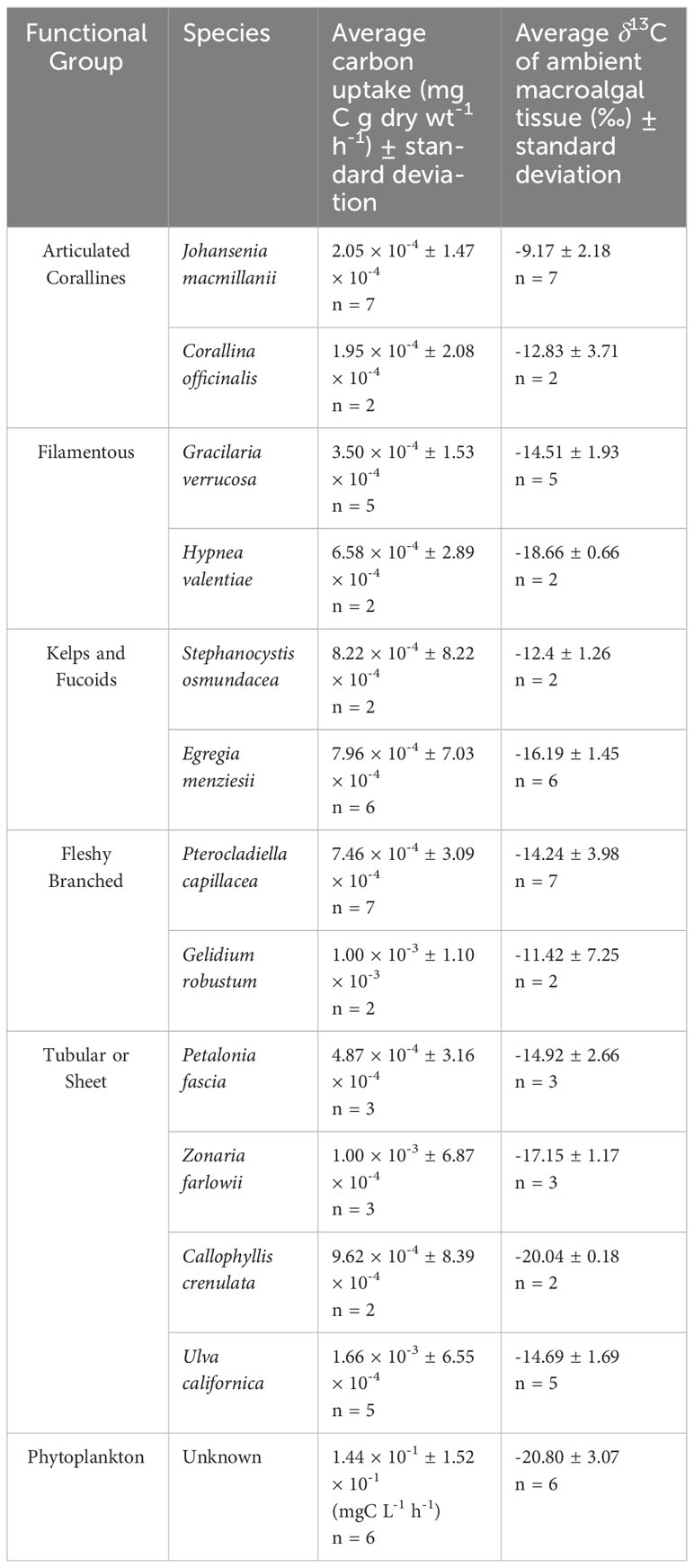
Table 1 Species-specific carbon uptake rates and ambient δ13C values of macrophyte tissue, including phytoplankton within the benthic chamber.
We compared our carbon uptake rates to those of Littler and Arnold (1982) because of their integral work in establishing productivity measurements for marine macrophytes and because of the use of different methodologies to achieve productivity rates with the same units (mg C g dry wt-1 h-1). In contrast with our methods, Littler and Arnold (1982) incubated individual macrophytes away from the benthos and observed oxygen evolution in light and dark bottles according to methods first described by Littler and Murray (1974). The quantity of produced oxygen was then converted to carbon uptake to estimate NPP via a photosynthetic quotient which was assumed to be a constant PQ = 1 (Littler and Arnold, 1982). Experimental carbon uptake rates are 3–4 orders of magnitude lower than those of Littler and Arnold (1982). Comparative data is listed in Table 2. The comparison between these methodologies is important because they represent the two most fundamental ways to measure primary productivity.
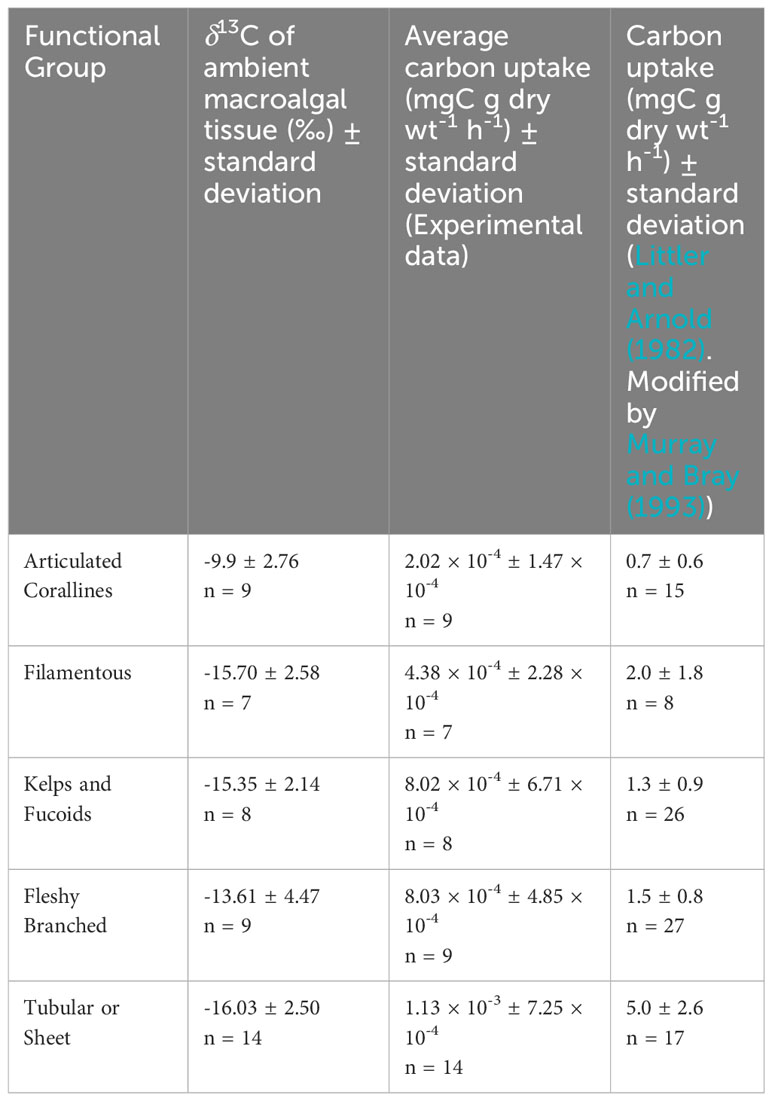
Table 2 Ambient δ13C signatures and carbon uptake rates for each functional group from experimental data and Littler and Arnold (1982).
4 Discussion
4.1 Carbon uptake rates and the form-function hypothesis
To our knowledge, this is the first study to calculate carbon uptake rates of macroalgae in situ using a 13C enrichment method in benthic chambers on the Pacific Coast of North America. Our study reveals carbon uptake rates vary among functional groups such that articulated corallines have the lowest average productivity rates and tubular or sheet forms have the highest, differing significantly (p = 0.0002) (Figure 2). This supports the form and function hypothesis developed by Littler and Arnold (1982), describing the physiological tradeoffs between surface area (SA) and volume (V) as it relates to the distribution of energy between structural development and photosynthetic production. Stewart and Carpenter (2003) agree that growth strategies designed to limit damage to the thallus are a principal driver of morphological plasticity in macrophytes and might directly reduce their ability to reach maximum photosynthetic capacity. Considering this tradeoff, we can attribute the lowest uptake rates of the articulated corallines to a prioritization of building robust geometrically complex and calcified structures (Littler and Littler, 1981; Williamson et al., 2017; Ryznar et al., 2021), and the high uptake rates of the tubular or sheet forms to high SA:V ratios which imply an emphasis on photosynthetic productivity (Tsubaki et al., 2020).
The three other functional groups identified in our study (i.e., filamentous, kelps and fucoids, and fleshy branched) have carbon uptake rates between the previously mentioned classifications, are not statistically different, and do not directly follow the form-function hypothesis (Figure 3). While the form-function hypothesis based on SA:V ratios explains the extremes of macroalgae productivity in our samples, the comparative productivity of macrophytes cannot solely be predicted by morphology. Additionally, carbon uptake rates do not show significant relationships with environmental factors including ambient nutrient concentrations, PAR, conductivity, temperature, and DIC (Supplementary Table 2, Supplementary Table 3). The lack of correlations between observed ranges of exterior environmental factors and carbon uptake position individual macrophyte physiology at the core of the variability in carbon uptake in our study. As a result, we caution against making general estimations of community-level productivity based on the productivity rates of individuals.
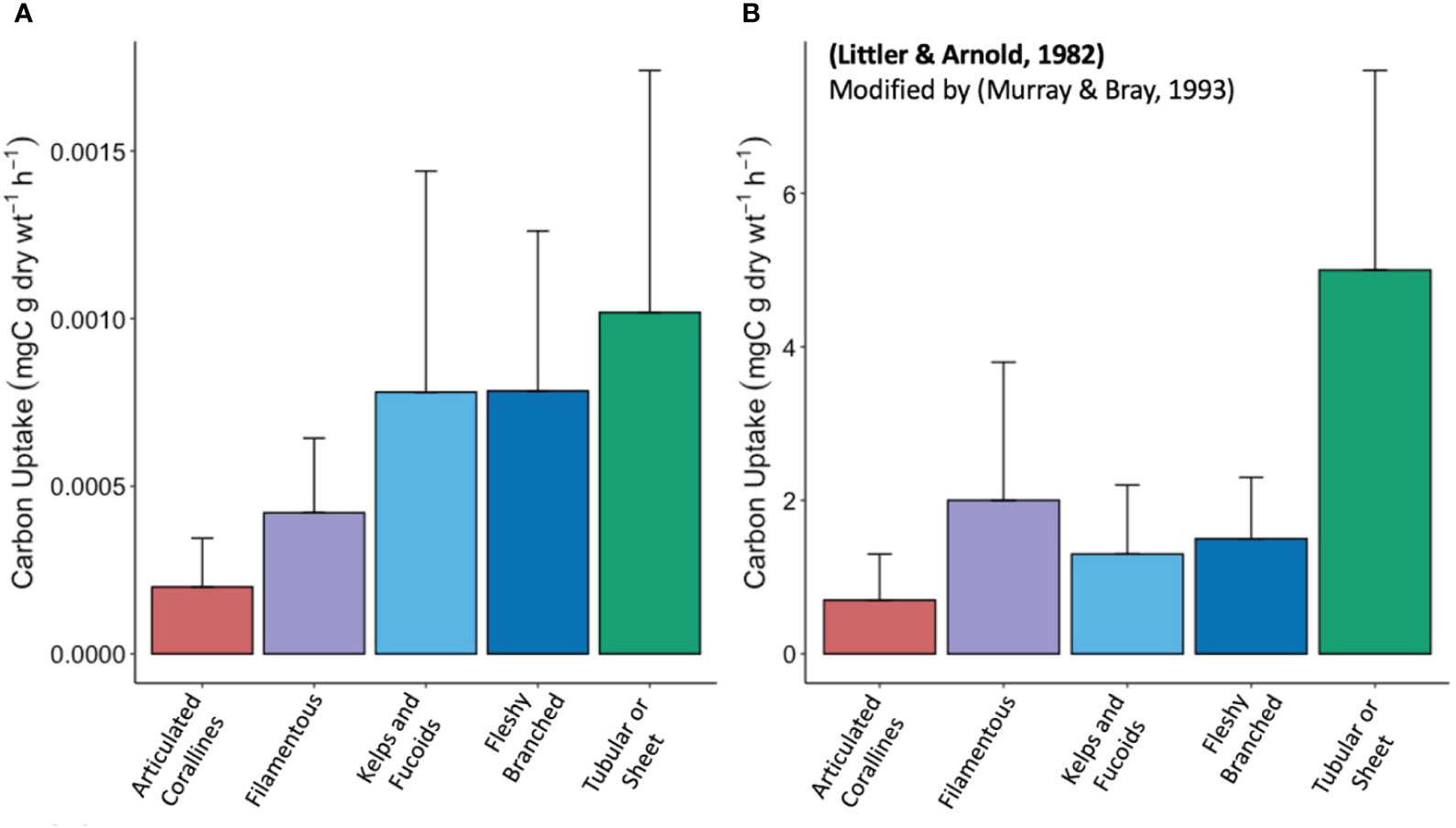
Figure 3 (A) Barplot of carbon uptake rates of functional groups from experimental data including standard deviations (B) Barplot of carbon uptake rates of functional groups from Littler and Arnold (1982) including standard deviations. Number of samples for each functional group in (A), experimental data, is articulated corallines (n = 9), filamentous (n = 7), kelps and fucoids (n = 8), fleshy branched (n = 9), and tubular or sheet (n = 13). The number of samples for each functional group in panel B, from Littler and Arnold (1982), is articulated corallines (n = 15), filamentous (n = 8), kelps and fucoids (n = 26), fleshy branched (n = 27), and tubular or sheet (n = 17). Different sample sizes between the studies are a result of our selection of study sites with random macrophyte diversity.
4.2 δ13C and implications for carbon acquisition strategies
Bicarbonate is the preferred medium to introduce labeled DIC (13C) and directly measure carbon uptake for marine macrophytes due to its natural abundance in marine ecosystems and the high concentration of carbon concentrating mechanisms (CCMs) in macroalgae (Cornwall et al., 2015; Zweng et al. 2018). While the passive diffusion of CO2 into tissue is the least energetically expensive route of carbon acquisition for macrophytes (Cornwall et al. 2012; Diaz-Pulido et al., 2016), CO2 only accounts for 1% (~ 10–20 µM) of seawater DIC (James et al., 2022; Capó-Bauçá et al., 2023) and diffuses into photosynthetic tissue 10,000 times slower in water than in air (Bergstrom et al., 2020; Capó-Bauçá et al., 2023). This has prompted the selection of CCMs in macroalgae that exploit the more abundant bicarbonate, accounting for 92% - 98% of seawater DIC (~ 1700–2100 µM) (Rautenberger et al., 2015; James et al., 2022) by converting HCO3- to CO2 intracellularly at the site of Rubisco where it is fixed for photosynthesis (Raven et al., 1995; Miller and Dunton, 2007; Ji and Gao, 2021).
Since macroalgae utilize the lighter and more abundant 12C quicker than 13C as a result of fractionation during carbon assimilation, the carbon uptake mechanism (i.e., CCM or non-CCM) of an individual macrophyte can be inferred from its δ13C signature (Lovelock et al., 2020; Velázquez-Ochoa et al., 2022). This is because each species of DIC in seawater has a specific affinity for 13C with CO2, HCO3-, and CO32- having average δ13C signatures of -10 ‰, -0.5 ‰, and 2 ‰, respectively (Kroopnick, 1985; Velázquez-Ochoa et al., 2022). Accordingly, species that employ CCMs are expected to have proportionally higher δ13C values within their tissue than their non-CCM counterparts (Raven et al., 2002).
Macrophytes can be categorized into three approximate strategies according to their use of CCMs: (1) entirely active HCO3- diffusion (δ13C > -10 ‰), (2) utilizing both passive CO2 and active HCO3- diffusion (-10 ‰ > δ13C > -30 ‰), and (3) exclusive diffusive entry of CO2 (δ13C < -30 ‰) (Cornwall et al., 2015; Diaz-Pulido et al., 2016; Velázquez-Ochoa et al., 2022). Macroalgae samples in the present study show high δ13C variability across taxa and morphological groups. All macroalgae in this study have a δ13C > -30 ‰ (Table 1), indicating that their carbon requirements are satisfied, either partially or fully, by bicarbonate (Raven et al., 2002; Giordano et al., 2005; Hepburn et al., 2011). The majority of our samples (86%) utilize varying degrees of HCO3- and CO2, and only 14% of our total samples have δ13C > -10 ‰ and solely rely on bicarbonate during carbon acquisition (Figure 4).
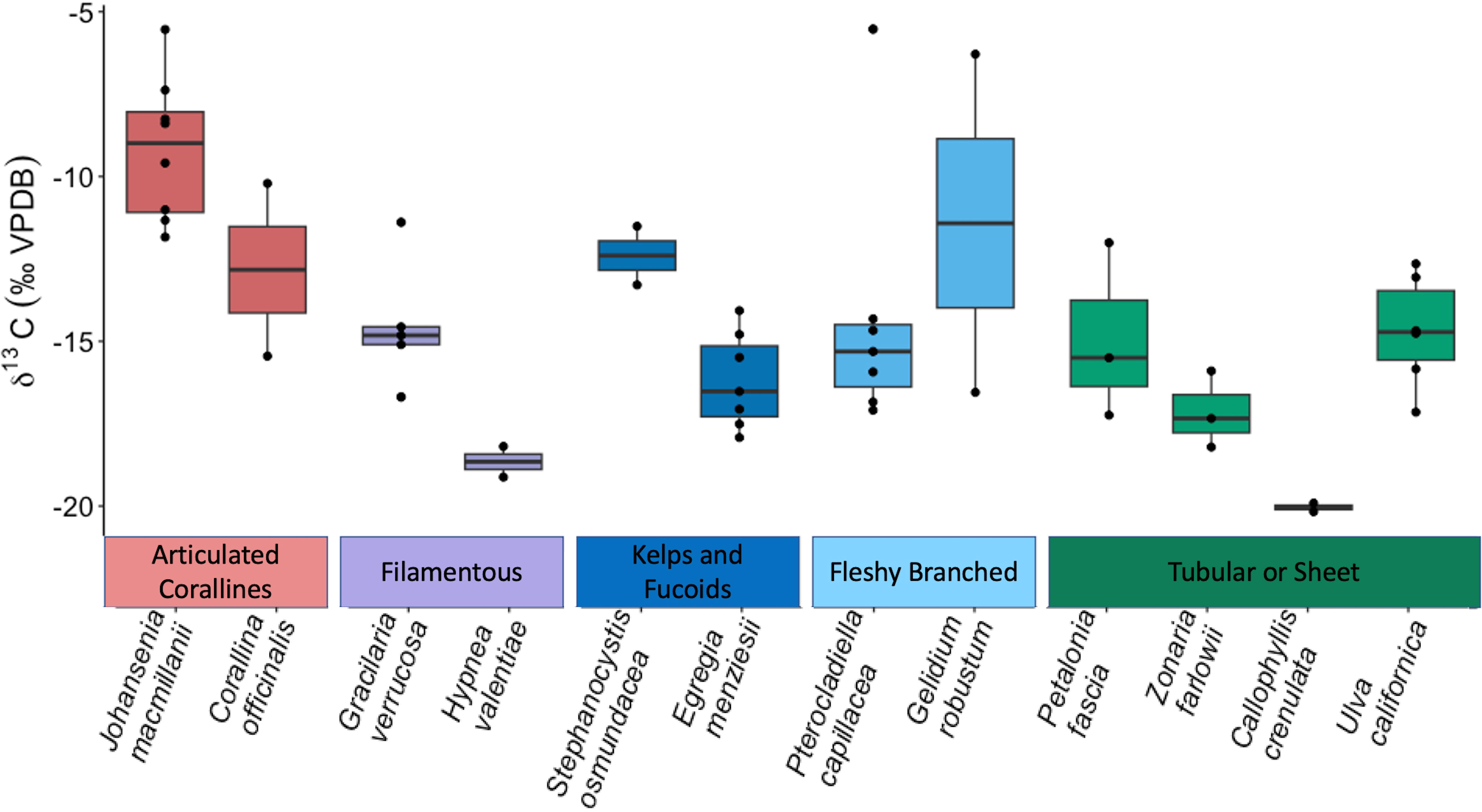
Figure 4 Boxplot displaying δ13C in ambient macrophyte tissue across species and among functional groups. Species are not ranked by δ13C signature, rather the display order follows the ranking of the carbon uptake rates as described in the Figure 2 caption.
The Rhodophyta have the greatest range of δ13C values (-20.17 ‰ to -5.53 ‰), suggesting the use of a diverse array of carbon uptake strategies. The articulated coralline functional group, belonging exclusively to the Rhodophyta, has the highest average δ13C (-9.9 ‰ ± 2.76 ‰), significantly lower than the tubular or sheet, kelps and fucoids, and filamentous groups (p = 0.001, p = 0.026, p = 0.026 respectively) (Table 2), implying a preference for bicarbonate (Carvalho et al., 2009b). This can be explained by the integral role of bicarbonate assimilation in meeting the high energetic requirements of calcification via chemical buffering to low pH (Hepburn et al., 2011; Cornwall et al., 2017). Therefore, bicarbonate assimilation for the articulated corallines serves the dual function as a substrate for photosynthesis and maintenance of complex and energetically expensive morphology.
Species with high photosynthetic activity have high carbon requirements and it has been proposed that these species have lower isotopic carbon discrimination (Carvalho et al., 2010; Rautenberger et al., 2015; Velázquez-Ochoa et al., 2022). This is not the case in our results and contradicts our impartial carbon acquisition hypothesis as highly productive species such as U. californica and C. crenulata have among the highest discrimination values, and the articulated coralline species with low carbon uptake rates discriminate the least against 13C. Strong variation of macrophyte δ13C signatures among macrophytes of the same species implies a range of inorganic carbon use. This makes it difficult to quantify how macrophytes of a specific species will tolerate and perform under different ambient inorganic carbon regimes. As a result, more information than solely the degree of inorganic carbon species use is required to make general predictions about fitness in anticipated future ocean chemistries associated with climate change (van der Loos et al. 2018).
4.3 Considerations for variability in carbon uptake rates
Considerable variation is evident in both carbon uptake rates and δ13C for macroalgae and it is important to consider the reality of such “snapshot” (Raven et al., 1995) measurements, representing the algal physiology and the seawater chemistry at a specific point in time. E. menziesii, for example, has a small range of δ13C values (-17.92 ‰ to -14.07 ‰) but the greatest range of any species in carbon uptake rates (8.17 × 10-5 to 1.65 × 10-3 mgC g dry wt-1 h-1), supporting that individuals of the same species and carbon complexion can express very different carbon uptake rates, likely resulting from unique physiological complexions. Varying anatomical location (e.g., thallus or holdfast), sample age, morphology, surrounding community structure, nutrient regimes, light acclimation, and desiccation (Raven et al., 1995; Lovelock et al., 2020; Velázquez-Ochoa et al., 2022) can all impact the photosynthetic performance and chemical composition of an alga. Additional sources of variability in carbon uptake among macrophytes can arise from seasonal variability (Raven et al., 1995), daily periodicity effects on production (Littler and Murray, 1974), and the geographic range from which the specimen originates (Maberly et al., 1992), but these factors are unlikely contributing to discrepancies within our data due to concentrated sampling protocols by location and time. It does, however, mean that these productivity rates may not be representative of other specimens collected at different locations or during a different season.
The most pronounced difference between experimentally derived carbon uptake rates and those of Littler and Arnold (1982), is that our experimental values are 3–4 orders of magnitude lower. Low experimental carbon uptake does not reflect the productivity rates of highly productive vegetation which we expected of our specimens of macroalgae. These comparatively low values, however, are most likely a result of discrepancies between data gathered with the oxygen evolution method and the 13C enrichment method. The remainder of this discussion will be dedicated to exploring how our experimental procedures using 13C may have resulted in an underestimation of carbon uptake rates. We theorize three potential reasons for this underestimation: 1) underrepresentation of maximum photosynthetic rates, 2) incomplete consideration of fractionation in calculations, and 3) reduced water flow within the incubations.
4.3.1 Underrepresentation of maximum photosynthetic rates
Firstly, implicit in methods of in situ sampling is the underrepresentation of the absolute maximum photosynthetic rate (Pmax). Miller and Dunton (2007), using similar 13C assimilation methods, estimated their experimental carbon uptake rate to be <10% Pmax with media at. % 13C to be >50% in 1.5-hour incubations, showing that there are potentially high disparities between experimental and actual carbon uptake rates despite considerable introduction of 13C. Comparatively, in our 1-hour incubation, at. % 13C never reached above 20%, providing a possible explanation for low carbon uptake rates. Additionally, Mateo et al. (2001) found that an increase of seawater δ13C of 34‰ is needed to achieve a 1% change in carbon uptake rates. These suggest that a substantial increase in DIC is required to induce measurable changes in at. % 13C of macroalgae tissue. This is a potential drawback of using the 13C enrichment method (Miller and Dunton, 2007).
4.3.2 Degrees of fractionation
Another fundamental consideration of using isotopes in biological systems is fractionation; in this case, the kinetic fractionation in carbon assimilation of the carbonic-anhydrase-catalyzed conversion of HCO3- to CO2 in macroalgae (Raven et al., 1995). The average fractionation correction (α), based on the equation by Miller and Dunton (2007) and described in methods, of our macroalgae samples is 1.027 ± 0.02 and agrees with the widely used average from Hama et al. (1983) of 1.025, referring to discrimination in phytoplankton. There are two matters, however, that this fractionation correction equation does not account for that are potentially significant drivers of 13C assimilation in algal tissue being the significant change in seawater chemistry after 13C enrichment, and the high variability of ambient seawater δ13C at our study site.
The fractionation coefficient is highly sensitive to the δ13C of ambient seawater such that an increase of 50‰ δ13C will increase the fractionation correction by a near-equal percentage (49%). This can be problematic because the fractionation coefficient is based on ambient δ13C and the seawater medium δ13C within our incubations changes dramatically after 13C enrichment. The fractionation coefficient, a snapshot estimation of algal physiology before the experiment, might not represent the carbon assimilation strategies employed under different regimes of δ13C which vary significantly throughout the incubation period.
This variability in fractionation is supported by highly variable ambient seawater δ13C at our site. δ13C has deviated from the reasonably uniform global seawater average of 1.07‰ (Kroopnick, 1985; Tsubaki et al., 2020) such that our site has an average δ13C (± standard deviation) of 2.42‰ ± 2.45‰, and on consecutive days we have sampled values from 0.69‰ to 4.93‰, supporting that not only in our incubations but on a day-to-day basis, macrophytes at our study site are exposed to highly variable δ13C. It is possible that this frequent variation has resulted in the development of malleable carbon acquisition mechanisms that are not implicit in the snapshot fractionation coefficient calculation. This discrimination plasticity has been explored by others including Young and Beardall (2005); Carvalho et al. (2009a); Carvalho et al. (2009b); Carvalho et al. (2010), and Bergstrom et al. (2020) but existing research to support carbon fractionation variation on such short temporal scales is presently absent.
4.3.3 Hydrodynamics
Two factors indicate that slow tracer diffusion is another potential influence on calculated carbon uptake rates. Back-calculations of benthic chamber volume and estimations of the percent by which we enriched our incubations can be inconsistent based on post-enrichment DIC concentrations. This suggests that at the time of post-enrichment DIC sampling, our introduced carbon could be poorly mixed, the post-enrichment DIC sample could be an underrepresentation of total carbon introduced, and our carbon uptake rates calculated with this value could be a misrepresentation of actual carbon uptake in our experiments (Yacobi et al., 2007). This highlights the importance of hydrodynamics for mixing the incubation media in benthic chambers (Thomas and Cornelisen, 2003; La Valle et al., 2019).
Hydrodynamics is also integral for the exchange of assimilated and respired compounds to and from the macrophyte tissue (Houlihan et al., 2020). The interaction between the biological activity of the macrophyte and the surrounding seawater produces a diffusive boundary layer (DBL) at the algal-seawater interface (Cornwall et al., 2013), ranging from μm to cm thick (Houlihan et al., 2020), that is chemically unique from the surrounding seawater. DBLs can prevent the assimilation of inorganic carbon and nutrients under stagnant flow conditions (Hurd, 2000; Cornwall et al., 2015; James et al., 2022). Algal morphology and surrounding water velocity determine the thickness and influence of the DBL (Raven and Hurd, 2012) and therefore affect photosynthetic rates (James et al., 2022). If slow tracer diffusion is present within the incubations, it is likely that reduced hydrodynamics within our benthic chambers play a role in limiting carbon uptake and underestimating true photosynthetic rates.
In summary, this study is an important step toward better understanding the carbon uptake of macrophytes by using novel in situ procedures of 13C enrichments. The lack of correlation between observed ranges of environmental factors and carbon uptake suggests that unique macrophyte physiologies underpin the high variability of productivity rates and δ13C signatures in our study. As such, assumptions about macrophyte productivity and responses of macroalgae to changing ocean chemistry regimes cannot be generalized and require the acknowledgment of complex differences among individuals of the same species and origin. Repetition of in situ productivity assessments is needed to better comprehend the contribution of individual macrophytes to community-level primary productivity and to monitor changes in productivity associated with shifting ocean chemistry.
We suggest future research using the 13C enrichment method to address the potential reasons for underestimation of carbon uptake, answering key remaining questions such as, does carbon fractionation exhibit plasticity on short time scales in macrophytes? Does the physiology responsible for carbon uptake change at different saturations of carbon in seawater? Subsequent iterations of field-based benthic chambers are strongly encouraged with the underlying considerations of hydrodynamics and an emphasis on properly mixing introduced material. These field-based measurements are essential contributions to phycology research by providing direct estimations of carbon-based productivity rates.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Our data and metadata was submitted to Pepperdine Digital Commons and will be submitted to the US National Science Foundation Biological and Chemical Oceanography Data Management Office (BCO-DMO) repository. Data is publicly available at the following link: https://digitalcommons.pepperdine.edu/surb/29
Author contributions
JJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. LH: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. FL: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Boyajian Fund, NSF DBI 1950350, NSF DEB 2312723, and Pepperdine University’s Seaver Dean’s Office and Division of Natural Sciences.
Acknowledgments
This project was funded by the Boyajian Fund, NSF DBI 1950350, NSF DEB 2312723, and Pepperdine University’s Seaver Dean’s Office and Division of Natural Sciences. Field and lab support was provided by S. Zummo, M. Sprute, C. Dao, J. Fuqua, R. Ang, J. Moser, M. Sadecki, H. Vaidya.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1290054/full#supplementary-material
References
Arístegui J., Montero M., Ballesteros S., Basterretxea G., Van Lenning K. (1996). Planktonic primary production and microbial respiration measured by 14C assimilation and dissolved oxygen changes in coastal waters of the Antarctic Peninsula during austral summer:implications for carbon flux studies. Mar. Ecol. Prog. Ser. 132, 191–201. doi: 10.3354/meps132191
Atlas E. L., Hager S. W., Gordon L. I., Park P. K. (1971). A Practical Manual for Use of the Technicon AutoAnalyzer in Seawater Nutrient Analyses Revised. Technical Report. 215 (Oregon State University, Department of Oceanography), 71–22.
Becker S., Aoyama M., Woodward E. M. S., Bakker K., Coverly S., Mahaffey C., et al. (2019). “The precise and accurate determination of dissolved inorganic nutrients in seawater; Continuous Flow Analysis methods and laboratory practices,” in IOCCP Report No. 14. GO-SHIP Repeat Hydrography Manual: A Collection of Expert Reports and Guidelines (ICPO Publication Series No. 134).
Bergstrom E., Ordoñez A., Ho M., Hurd C., Fry B., Diaz-Pulido G. (2020). Inorganic carbon uptake strategies in coralline algae: Plasticity across evolutionary lineages under ocean acidification and warming. Mar. Environ. Res. 161, 105107. doi: 10.1016/j.marenvres.2020.105107
Capó-Bauçà S., Galmés J., Aguiló-Nicolau P., Ramis-Pozuelo S., Iñiguez C. (2023). Carbon assimilation in upper subtidal macroalgae is determined by an inverse correlation between Rubisco carboxylation efficiency and CO2 concentrating mechanism effectiveness. New Phytol. 237, 2027–2038. doi: 10.1111/nph.18623
Carbon and Nitrogen Center for Stable Isotope Biogeochemistry. Available at: https://nature.berkeley.edu/stableisotopelab/analyses/organic-analysis/13c-15n-organic-analysis/ (Accessed June 21, 2023).
Carvalho M. C., Hayashizaki K., Ogawa H. (2009a). Carbon stable isotope discrimination: a possible growth index for the kelp Undaria pinnatifida. Mar. Ecol. Prog. Ser. 381, 71–82. doi: 10.3354/meps07948
Carvalho M. C., Hayashizaki K.-I., Ogawa H. (2009b). Short-term measurement of carbon stable isotope discrimination in photosynthesis and respiration by Aquatic macrophytes, with marine macroalgal examples1. J. Phycol. 45, 761–770. doi: 10.1111/j.1529-8817.2009.00685.x
Carvalho M. C., Hayashizaki K., Ogawa H. (2010). Temperature effect on carbon isotopic discrimination by Undaria pinnatifida (phaeophyta) in a closed experimental system1. J. Phycol. 46, 1180–1186. doi: 10.1111/j.1529-8817.2010.00895.x
Cornelisen C. D., Thomas F. I. M. (2002). Ammonium uptake by seagrass epiphytes: Isolation of the effects of water velocity using an isotope label. Limnol. Oceanogr. 47, 1223–1229. doi: 10.4319/lo.2002.47.4.1223
Cornwall C. E., Hepburn C. D., Pilditch C. A., Hurd C. L. (2013). Concentration boundary layers around complex assemblages of macroalgae: Implications for the effects of ocean acidification on understory coralline algae. Limnol. Oceanogr. 58, 121–130. doi: 10.4319/lo.2013.58.1.0121
Cornwall, Hepburn, Pritchard (2012). CARBON-USE STRATEGIES IN MACROALGAE: DIFFERENTIAL RESPONSES TO LOWERED PH AND IMPLICATIONS FOR OCEAN ACIDIFICATION. J. Phycol. 48, 137–144. doi: 10.1111/j.1529-8817.2011.01085.x
Cornwall C. E., Hurd C. L. (2019). Variability in the benefits of ocean acidification to photosynthetic rates of macroalgae without CO2-concentrating mechanisms. Mar. Freshw. Res. 71, 275–280. doi: 10.1071/MF19134
Cornwall C. E., Revill A. T., Hall-Spencer J. M., Milazzo M., Raven J. A., Hurd C. L. (2017). Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci. Rep. 7, 46297. doi: 10.1038/srep46297
Cornwall C. E., Revill A. T., Hurd C. L. (2015). High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynth. Res. 124, 181–190. doi: 10.1007/s11120-015-0114-0
Craig H. (1957). Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochimica Cosmochimica Acta 12, 133–149. doi: 10.1016/0016-7037(57)90024-8
de los Santos C. B., Egea L. G., Martins M., Santos R., Masqué P., Peralta G., et al. (2022). Sedimentary organic carbon and nitrogen sequestration across a vertical gradient on a temperate wetland seascape including salt marshes, seagrass meadows and rhizophytic macroalgae beds. Ecosystems. 26, 826–842. doi: 10.1007/s10021-022-00801-5
Diaz-Pulido G., Cornwall C., Gartrell P., Hurd C., Tran D. V. (2016). Strategies of dissolved inorganic carbon use in macroalgae across a gradient of terrestrial influence: implications for the Great Barrier Reef in the context of ocean acidification. Coral Reefs 35, 1327–1341. doi: 10.1007/s00338-016-1481-5
Dolliver J., O’Connor N. (2022). Whole system analysis is required to determine the fate of macroalgal carbon: A systematic review. J. Phycol. 58, 364–376. doi: 10.1111/jpy.13251
Duarte C. M., Gattuso J.-P., Hancke K., Gundersen H., Filbee-Dexter K., Pedersen M. F., et al. (2022). Global estimates of the extent and production of macroalgal forests. Global Ecol. Biogeogr. 31, 1422–1439. doi: 10.1111/geb.13515
Falkowski P. G., Raven J. A. (2013). Aquatic Photosynthesis. 2nd ed. (Elsevier, Princeton University Press).
Giordano M., Beardall J., Raven J. A. (2005). CO2 CONCENTRATING MECHANISMS IN ALGAE: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 56, 99–131. doi: 10.1146/annurev.arplant.56.032604.144052
Gordon L. I., Jennings J. C., Ross A. A., Krest J. M. (1992). A suggested protocol for continuous flow automated analysis of seawater nutrients in the WOCE hydrographic program and the joint global ocean fluxes study. Grp. Tech Rpt 92-1 OSU Coll. Oceanography Descr. Chem. Oc.
Hager S. W., Atlas E. L., Gordon L. I., Mantyla A. W., Park P. K. (1972). A comparison at sea of manual and autoanalyzer analyses of phosphate, nitrate, and silicate. Limnol. Oceanogr. 17, 931–937. doi: 10.4319/lo.1972.17.6.0931
Hama T., Miyazaki T., Ogawa Y., Iwakuma T., Takahashi M., Otsuki A., et al. (1983). Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 73, 31–36. doi: 10.1007/BF00396282
Hayes J. (2004). An Introduction to Isotopic Calculations (Woods Hole Oceanographic Insitution). doi: 10.1575/1912/27058
Hepburn C. D., Pritchard D. W., Cornwall C. E., McLEOD R. J., Beardall J., Raven J. A., et al. (2011). Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biol. 17, 2488–2497. doi: 10.1111/j.1365-2486.2011.02411.x
Houlihan E. P., Espinel-Velasco N., Cornwall C. E., Pilditch C. A., Lamare M. D. (2020). Diffusive boundary layers and ocean acidification: implications for sea urchin settlement and growth. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.577562
Hurd C. L. (2000). Water motion, marine macroalgal physiology, and production. J. Phycol. 36, 453–472. doi: 10.1046/j.1529-8817.2000.99139.x
James R. K., Hepburn C. D., Pritchard D., Richards D. K., Hurd C. L. (2022). Water motion and pH jointly impact the availability of dissolved inorganic carbon to macroalgae. Sci. Rep. 12, 21947. doi: 10.1038/s41598-022-26517-z
Ji Y., Gao K. (2021). Effects of climate change factors on marine macroalgae: A review. Adv. Mar. Biol. (Elsevier) 88, 91–136. doi: 10.1016/bs.amb.2020.11.001
Krause-Jensen D., Marbà N., Sanz-Martin M., Hendriks I. E., Thyrring J., Carstensen J., et al. (2016). Long photoperiods sustain high pH in Arctic kelp forests. Sci. Adv. 2, e1501938. doi: 10.1126/sciadv.1501938
Kroopnick P. M. (1985). The distribution of 13C of ΣCO2 in the world oceans. Deep Sea Res. Part A. Oceanographic Res. Papers 32, 57–84. doi: 10.1016/0198-0149(85)90017-2
La Valle F. F., Jacobs J. M., Thomas F. I., Nelson C. E. (2023). Nutrient-rich submarine groundwater discharge increases algal carbon uptake in a tropical reef ecosystem. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1178550
La Valle F. F., Thomas F. I., Nelson C. E. (2019). Macroalgal biomass, growth rates, and diversity are influenced by submarine groundwater discharge and local hydrodynamics in tropical reefs. Mar. Ecol. Prog. Ser. 621, 51–67. doi: 10.3354/meps12992
Littler M. M., Arnold K. E. (1982). Primary productivity of marine macroalgal functional-form groups from southwestern North America1. J. Phycol. 18, 307–311. doi: 10.1111/j.1529-8817.1982.tb03188.x
Littler M. M., Littler D. S. (1981). Intertidal macrophyte communities from Pacific Baja California and the upper gulf of California: relatively constant vs. Environmentally fluctuating systems. Mar. Ecol. Prog. Ser. 4, 145–158.
Littler M. M., Murray S. N. (1974). The primary productivity of marine macrophytes from a rocky intertidal community. Mar. Biol. 27, 131–135. doi: 10.1007/BF00389065
Lovelock C. E., Reef R., Raven J. A., Pandolfi J. M. (2020). Regional variation in δ13C of coral reef macroalgae. Limnol. Oceanogr. 65, 2291–2302. doi: 10.1002/lno.11453
Maberly S. C., Raven J. A., Johnston A. M. (1992). Discrimination between12C and13C by marine plants. Oecologia 91, 481–492. doi: 10.1007/BF00650320
Mann K. H. (1973). Seaweeds: their productivity and strategy for growth | Science. Science 182, 975 — 981. doi: 10.1126/science.182.4116.975
Mateo M. A., Renom P., Hemminga M. A., Peene J. (2001). Measurement of seagrass production using the 13C stable isotope compared with classical O2 and 14C methods. Mar. Ecol. Prog. Ser. 223, 157–165. doi: 10.3354/meps223157
Miller H. L., Dunton K. H. (2007). Stable isotope (13C) and O2 micro-optode alternatives for measuring photosythesis in seaweeds. Mar. Ecol. Prog. Ser. 329, 85–97. doi: 10.3354/meps329085
Murray S. N., Bray R. N. (1993). Benthic macrophytes. Ecology of the Southern California Bight: a synthesis and interpretation 304–368.
Pace M. L., Lovett G. M. (2013). “Chapter 2 - primary production: the foundation of ecosystems,” in Fundamentals of Ecosystem Science. Eds. Weathers K. C., Strayer D. L., Likens G. E. (Academic Press), 27–51. doi: 10.1016/B978-0-08-091680-4.00002-0
Pessarrodona A., Assis J., Filbee-Dexter K., Burrows M. T., Gattuso J.-P., Duarte C. M., et al. (2022). Global seaweed productivity. Sci. Adv. 8. doi: 10.1126/sciadv.abn2465
Peterson B. J. (1980). Aquatic primary productivity and the 14C-CO2 method: A history of the productivity problem. Annu. Rev. Ecol. Evol. Syst. 11, 359–385. doi: 10.1146/annurev.es.11.110180.002043
Pfister C. A., Altabet M. A., Weigel B. L. (2019). Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 100, e02798. doi: 10.1002/ecy.2798
Queirós A. M., Tait K., Clark J. R., Bedington M., Pascoe C., Torres R., et al. (2023). Identifying and protecting macroalgae detritus sinks toward climate change mitigation. Ecol. Appl. 33, e2798. doi: 10.1002/eap.2798
Rautenberger R., Fernández P. A., Strittmatter M., Heesch S., Cornwall C. E., Hurd C. L., et al. (2015). Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecol. Evol. 5, 874–888. doi: 10.1002/ece3.1382
Raven J. A., Hurd C. L. (2012). Ecophysiology of photosynthesis in macroalgae. Photosynth. Res. 113, 105–125. doi: 10.1007/s11120-012-9768-z
Raven J. A., Johnston A. M., Kübler J. E., Korb R., McInroy S. G., Handley L. L., et al. (2002). Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct. Plant Biol. 29, 355–378. doi: 10.1071/pp01201
Raven J., Walker D., Johnston A., Handley L., Kübler J. (1995). Implications of 13C natural abundance measurements for photosynthetic performance by marine macrophytes in their natural environment. Mar. Ecol. Prog. Ser. 123, 193–205. doi: 10.3354/meps123193
Ruhamak B. A., Hamdani H., Rostini I., Sahidin A. (2019). Macroalgae Association with Invertebrate Biota in Madasari Coast Vol. 7 (West Java, Indonesia).
Ryznar E. R., Fong P., Fong C. R. (2021). When form does not predict function: Empirical evidence violates functional form hypotheses for marine macroalgae. J. Ecol. 109, 833–846. doi: 10.1111/1365-2745.13509
Spector M., Edwards M. S. (2020). Species-specific biomass drives macroalgal benthic primary production on temperate rocky reefs. Algae 35, 237–252. doi: 10.4490/algae.2020.35.8.19
Stewart, Carpenter (2003). The effects of morphology and water flow on photosynthesis of marine macroalgae. Ecology 84, 2999–3012. doi: 10.1890/02-0092
Strickland J. D. (1960). Measuring the production of marine phytoplankton. Fish. Res. Bd. Canada Bull. 122, 172.
Tait L. W., Schiel D. R. (2018). Ecophysiology of layered macroalgal assemblages: importance of subcanopy species biodiversity in buffering primary production. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00444
Teichberg M., Fox S. E., Aguila C., Olsen Y. S., Valiela I. (2008). Macroalgal responses to experimental nutrient enrichment in shallow coastal waters: growth, internal nutrient pools, and isotopic signatures. Mar. Ecol. Prog. Ser. 368, 117–126. doi: 10.3354/meps07564
Thomas F. I. M., Cornelisen C. D. (2003). Ammonium uptake by seagrass communities: effects of oscillatory versus unidirectional flow. Mar. Ecol. Prog. Ser. 247, 51–57. doi: 10.3354/meps247051
Torres M. E., Mix A. C., Rugh W. D. (2005). Precise δ13C analysis of dissolved inorganic carbon in natural waters using automated headspace sampling and continuous-flow mass spectrometry. Limnol. Oceanogr.: Methods 3, 349–360. doi: 10.4319/lom.2005.3.349
Tsubaki S., Nishimura H., Imai T., Onda A., Hiraoka M. (2020). Probing rapid carbon fixation in fast-growing seaweed Ulva meridionalis using stable isotope 13C-labelling. Sci. Rep. 10, 20399. doi: 10.1038/s41598-020-77237-1
van der Loos L. M., Schmid M., Leal P. P., McGraw C. M., Britton D., Revill A. T., et al. (2018). Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol. Evol. 9, 125–140. doi: 10.1002/ece3.4679
Velázquez-Ochoa R., Ochoa-Izaguirre M. J., Soto-Jiménez M. F. (2022). An analysis of the variability in δ13C in macroalgae from the Gulf of California: indicative of carbon concentration mechanisms and isotope discrimination during carbon assimilation. Biogeosciences 19, 1–27. doi: 10.5194/bg-19-1-2022
Williamson C. J., Perkins R., Voller M., Yallop M. L., Brodie J. (2017). The regulation of coralline algal physiology, an in situ study of Corallina officinalis (Corallinales, Rhodophyta). Biogeosciences 14, 4485–4498. doi: 10.5194/bg-14-4485-2017
Yacobi Y. Z., Perel N., Barkan E., Luz B. (2007). Unexpected underestimation of primary productivity by 18O and 14C methods in a lake: Implications for slow diffusion of isotope tracers in and out of cells. Limnol. Oceanogr. 52, 329–337. doi: 10.4319/lo.2007.52.1.0329
Young E. B., Beardall J. (2005). Modulation of photosynthesis and inorganic carbon acquisition in a marine microalga by nitrogen, iron, and light availability. Can. J. Bot. 83, 917–928. doi: 10.1139/b05-081
Keywords: carbon uptake1, macroalgae2, primary productivity3, 13C4, isotope enrichment5, benthic chambers6, in situ7, marine algae8
Citation: Jacobs JM, Himes L and La Valle FF (2023) In situ carbon uptake of marine macrophytes is highly variable among species, taxa, and morphology. Front. Mar. Sci. 10:1290054. doi: 10.3389/fmars.2023.1290054
Received: 06 September 2023; Accepted: 03 November 2023;
Published: 22 November 2023.
Edited by:
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanSoumya Prasad Panda, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2023 Jacobs, Himes and La Valle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florybeth F. La Valle, ZmxvcnliZXRoLmxhdmFsbGVAcGVwcGVyZGluZS5lZHU=
 Julian M. Jacobs
Julian M. Jacobs Lucian Himes
Lucian Himes Florybeth F. La Valle
Florybeth F. La Valle