
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 07 December 2023
Sec. Aquatic Physiology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1286789
 Jirawat Saetan1
Jirawat Saetan1 Supawadee Duangprom2
Supawadee Duangprom2 Sineenart Songkoomkrong2
Sineenart Songkoomkrong2 Prateep Amonruttanapun2
Prateep Amonruttanapun2 Teva Phanaksri2
Teva Phanaksri2 Piyaporn Surinlert2
Piyaporn Surinlert2 Chompunut Samhuay3
Chompunut Samhuay3 Montakan Tamtin4
Montakan Tamtin4 Saowaros Suwansa-Ard5
Saowaros Suwansa-Ard5 Scott F. Cummins5,6
Scott F. Cummins5,6 Prasert Sobhon7
Prasert Sobhon7 Napamanee Kornthong2*
Napamanee Kornthong2*The mud crab Scylla olivacea is widely cultured for its economic value, but reproduction issues limit its production. Vitellogenesis-inhibiting hormone (VIH), serotonin (5-HT), and gonadotropin-releasing hormone (GnRH) are important neurohormones that control reproduction in crustaceans. Mimicking crab hormone stimulation during reproduction has scarcely been reported. Comparison of the single hormone and multiple hormone approaches to crab hormonal control in S. olivacea is limited. In situ hybridization showed that injection of dsRNA-VIH could abolish its gene expression in neuronal clusters of female S. olivacea eyestalks, potentially reducing its inhibitory effects on ovarian maturation. This was confirmed by assessing the ovarian gonadosomatic index (GSI), hemolymph vitellogenin (Vg), an indicator of vitellogenesis, and gonad histology using dsRNA-VIH and 5-HT/GnRH combinations. Based on our findings, we demonstrated that administration of dsRNA-VIH significantly increased the gonadosomatic index (GSI) on days 14 and 28 post-treatment. The combination cocktail, however, consisting of 5-HT + GnRH + dsRNA-VIH on days 14 and 28, and GnRH + dsRNA-VIH on day 28, was the most efficacious in increasing GSI and enhancing crab ovarian maturation. Upregulation of hemolymph Vg levels was observed solely on the 28th day following treatment with dsRNA-VIH, 5-HT + GnRH + dsRNA-VIH, and GnRH + dsRNA-VIH. Differential gene expression analysis using quantitative RNA-sequencing of the neural tissues (brain and ventral nerve cord), revealed a significant upregulation of certain receptors (5-HTR, GnRHR, LHR, and FSHR), neuropeptides (sNPF, NPF1, NPF2, SIFamide, AKH/Crz, CHH, and RPCH), downstream reproductive-related genes (FAMeT, ESULT, progesterone-like protein), and prostanoid-related genes (phospholipase A and C, COX, Thromboxane A synthase, prostaglandin D, E, and F synthases) following treatment, particularly dsRNA-VIH + GnRH and/or 5-HT-injected individuals. Upregulation of prostaglandin E synthase and estrogen sulfotransferase genes was confirmed by real-time PCR. Since the construction and propagation of dsRNA-VIH is costly, its lower dose application supplemented with synthetic GnRH and/or 5-HT may be an alternative approach to ensure that female S. olivacea attain sufficient reproductive fecundity in aquaculture. Furthermore, we propose that the administration of multiple hormones in crabs may better emulate the physiological conditions of crustaceans in their natural habitat.
Comparative transcriptome analysis of the nervous system and gonads of aquaculture crustaceans at different reproductive stages has been beneficial for gaining a better understanding of their associated molecular components (Uawisetwathana et al., 2011; Suwansa-Ard et al., 2016). With regard to the neuroendocrine control of reproduction, several neurohormones and neurohormone-like molecules have been identified, which may now be explored to manipulate crustaceans in aquaculture (Alfaro-Montoya et al., 2019). However, a defined neuroendocrine pathway that facilitates crustacean reproduction has not yet been established.
The mud crab, Scylla olivacea, is one of the most valuable crustacean species, with high prices and demand in international markets, and lives mostly in the mangrove ecosystem of tropical and subtropical waters of the Indo Pacific region. Considering the high market demand for this species, the culture system must prioritize the sustainable long-term well-being of its population. In general, economic crustacean aquaculture practices always seek a more efficient approach to either enhance offspring production or sustainably retain captive broodstock. The technique of eyestalk ablation has been used to accelerate ovarian maturation in various crustacean species, including the Pacific white shrimp (Sultana et al., 2023), ridgetail white prawn (Jia et al., 2023), and mud crab (Muhd-Farouk et al., 2019); however, this practice has been criticized for being unethical (Tinikul et al., 2017). Recent practices have used the aforementioned key reproductive molecules, which are thought to trigger pathways that stimulate reproduction (Jayasankar et al., 2020). This study used the mud crab S. olivacea, an economically important crab in many areas of the world, including Thailand, as a model to demonstrate the effects of selected neurotransmitters and neurohormones (e.g., serotonin, gonadotropin-releasing hormone), as well as double-stranded RNA against the vitellogenin-inhibiting hormone (dsRNA-VIH). These outcomes were proposed to enhance the reproduction of mud crab aquaculture.
Serotonin (5-hydroxytryptamine; 5-HT) is considered a crucial neurotransmitter because substantial evidence indicates its role in promoting the reproduction of crustaceans. The presence of 5-HT in the central nervous system and/or gonads has been reported in S. olivacea (Khornchatri et al., 2015), Penaeus monodon (Wongprasert et al., 2006), Macrobrachium rosenbergii (Soonthornsumrith et al., 2018), and Portunus pelagicus (Nakeim et al., 2020). Administration of 5-HT to numerous species of crustaceans, both in vivo and in vitro, demonstrated ovarian maturation (Meeratana et al., 2006; Wongprasert et al., 2006; Soonthornsumrith et al., 2018) and stimulation of ovarian steroid release (Soonthornsumrith et al., 2018). In addition, 5-HT positively modulates the mRNA expression of the red pigment concentrating hormone (RPCH) and crustacean hyperglycemic hormone (CHH) in S. olivacea (Kornthong et al., 2013; Duangprom et al., 2017).
A gonadotropin-releasing hormone (GnRH)-like peptide in crustaceans is difficult to identify; however, a single putative GnRH-like peptide was found in M. rosenbergii following de novo transcriptome assembly and analysis (Suwansa-Ard et al., 2016). Its mature peptide sequence contains 14 amino acids (pQQHFRTSHFRPDNVamide), which is distinct from other known GnRH core peptides. In addition, this GnRH had no effect on ovarian maturation when injected into prawns whereas the lamprey (l) GnRH-III injection stimulated ovarian maturation in M. rosenbergii (Ngernsoungnern et al., 2009; Suwansa-Ard et al., 2016). In crayfish, Procambarus clarkii, it was reported that GnRH contains 11 amino acids (pQSYHFSLGWKP-amide), and its effects were increases in the gonadosomatic index (GSI) and ovarian vitellin (Guan et al., 2014). In addition, a GnRH-like peptide was predicted in numerous species of crustaceans by immunohistochemistry using antibodies generated against tunicate (t) GnRH-I-, lGnRH-III-, and octopus (oct) GnRH-like peptides (Ngernsoungnern et al., 2008; Tinikul et al., 2011; Saetan et al., 2013). In addition, the administration of synthetic lGnRH-I, lGnRH-III, and octGnRH to various crustacean species has been positively correlated with ovarian maturation (Ngernsoungnern et al., 2009; Saetan et al., 2013; Tinikul et al., 2014). However, to date, there is no evidence of GnRH function in S. olivacea.
Crustacean VIH is known to inhibit reproduction. In P. monodon, immunohistochemical localization of a PemVIH-like peptide was observed in the brain, ventral nerve cord, and commissural ganglion, whereas dsRNA-PemVIH promoted ovarian vitellogenesis, suggesting that PemVIH plays an inhibitory role in shrimp. The lvVIH identified in Litopenaeus vannamei belongs to the family of crustacean hyperglycemic hormones (CHHs) and is expressed in the brain and eyestalk. lvVIH suppresses the expression of vitellogenin in the hepatopancreas and eliminates the eyestalk ablation-induced vitellogenesis via the GC/cGMP-MAPK pathway (Chen et al., 2014; Chen et al., 2018). VIH was successfully identified in S. olivacea (Scyol-VIH). This hormone is produced in the eyestalk X-organ as well as in neuronal clusters of the brain and ventral nerve cord, as revealed by RT-PCR and in situ hybridization; its expression increases in response to dopamine administration (Kornthong et al., 2019). Recently, dsRNA-Scyol-VIH was successfully developed, as it effectively suppressed Scyol-VIH mRNA expression in the eyestalk (Duangprom et al., 2022). In addition, the administration of Scyol-VIH significantly enhanced both ovarian maturation and reproduction-related gene expression (Duangprom et al., 2022).
In the present study, we demonstrated the combined effects of 5-HT, GnRH, and VIH on vitellogenesis in S. olivacea. As this mud crab is considered an economically significant species, the application of this cocktail in aquaculture could lead to the development of a sustainable technique to increase S. olivacea production.
Serotonin (5-HT) was purchased from Sigma-Aldrich (USA). Synthetic gonadotropin-releasing hormone (GnRH: pQHWSHDWKPG-amide) lamprey III, Petromyzon marinus, GenBank accession no. P30948.1 was produced with 98% purity (GenScript, USA). S. olivacea double-stranded RNA-VIH (dsRNA-VIH) was synthesized using the method from Feijó et al. (2016) and Duangprom et al. (2022). Briefly, recombinant plasmids encoding the Scyol-VIH gene (GenBank accession no. MH882453) were linearized using XhoI and used as a template. dsRNAs were then generated using the SP6 RiboMAX™ Express Large-Scale RNA Production System (Promega, USA) according to the manufacturer’s protocol. The remaining portion of the template was eliminated with RQ1 RNase-free DNase (Promega, USA), and dsRNA was extracted using TriPure™ Isolation reagent (Roche, Germany). The integrity of dsRNA was evaluated by using agarose gel electrophoresis, and the concentration was measured using a NanoDrop2000 spectrophotometer (Thermo Scientific, USA).
Female S. olivacea were cultivated in Samut Sakhon, Thailand, at Samut Sakhon Coastal Aquaculture Research and Development Center under the Protocol Number 011/2562 approved by the Animal Care and Use Committee of Thammasat University, National Research Council of Thailand (NRCT), to ensure that animal suffering was kept to a minimum. To investigate the stimulation of ovarian maturation using a combination of hormones, experiments were carried out on mature female mud crabs during the intermolt stage (Kuballa et al., 2011; Xu et al., 2020). All crabs with an average weight of 135 g–145 g, gonad somatic index (GSI) <0.2, and a distance of 85 mm–92 mm between the two tips of the ninth spine of the anterolateral carapace (Amin-Safwan et al., 2016; Ghazali et al., 2017) were used to investigate the effect of hormone administration on ovarian maturation and reproductive gene expression.
A total of 210 female S. olivacea were randomly assigned to seven groups, with each group comprising 30 individuals. Crabs were added to 100 µl of the solution. The doses of the tested molecules were selected based on previous studies (Saetan et al., 2013; Siangcham et al., 2013; Duangprom et al., 2022; and Tinikul et al., 2009; Tinikul et al., 2014). The treatment groups consisted of five different conditions, comprising (T1) dsRNA-VIH at 0.6 µg/g BW; (T2) 5-HT (3 µg/g BW) + dsRNA-VIH (0.2 µg/g BW); (T3) 5-HT (3 µg/g BW) + GnRH (50 ng/g BW); (T4) 5-HT (1.5 µg/g BW) + GnRH (25 ng/g BW) + dsRNA-VIH (0.1 µg/g BW), and (T5) GnRH (50 ng/g BW) + dsRNA-VIH (0.2 µg/g BW), respectively. The two control groups were administered (C1) 0.9% normal saline (NSS) and (C2) dsRNA-EGFP at 0.6 µg/g BW. The crabs were injected on days 0 and 14 post-injection, and on days 0, 14, and 28, five crabs from each group were randomly selected for hemolymph collection and subsequently sacrificed for further analysis. Following sacrifice, the central nervous system (CNS) and ovaries of each crab were obtained, and the GSI was determined based on the proportion of ovarian mass to body mass.
The brain and ventral nerve cord (VNC) were collected and frozen at −80°C for subsequent use in differential RNA-seq and quantitative real-time PCR. The eyestalk and ovary were fixed for 12 h–16 h in Davidson’s fixative, rinsed, and then stored in 70% ethanol for in situ hybridization and histological analysis, respectively. Finally, hemolymph was obtained to quantify the vitellogenin (Vg) levels, as outlined in the subsequent section on Vg level measurement.
To determine the GSI: [ovary weight/crab body weight (BW)] ∗ 100, the weight of each crab and ovary was measured and recorded on days 0, 14, and 28 post-injections (Saetan et al., 2013; Kang et al., 2019). Routine histology was used to examine gonadal development cocktail hormone administration. Following sacrifice, the ovaries were fixed in Davidson’s fixative for 12 h–16 h, washed, and processed using paraffin. Ovarian tissues were sectioned to a thickness of 7 µm and subsequently stained using hematoxylin (H) and eosin (E) solution (Saetan et al., 2017). To validate the histological differences between the groups, the number of oogonia (Og) and the four stages of oocyte development [oocyte step 1 (Oc1)–oocyte step 4 (Oc4)] from ovary samples collected on days 0, 14, and 28 were randomly enumerated from four fields of non-consecutive sections (at ×200 magnification) taken from each crab (n = 5). The sections were examined and photographed using a Leica compound microscope connected to a digital camera (Leica, Germany).
Following the treatment, the eyestalk was chosen as the site of interest to assess the inhibitory potential of dsRNA-VIH. The experimental design involved the selection of two groups, including the control group (NSS) and dsRNA-VIH group, to conduct in situ hybridization (ISH). To conduct a qualitative comparison of Scyol-VIH expression in the eyestalk of the dsRNA-VIH- and NSS-injected groups, Scyol-VIH mRNA was detected in consecutive sections. Sense and antisense probe labeling were performed using a DIG-oligonucleotide labeling kit SP6/T7 (Roche, Germany). The sections were imaged using Leica compound microscope equipped with a digital camera (Leica, Germany). The study referred to the protocol of in situ hybridization (ISH) in a comprehensive manner, as outlined by Duangprom et al. (2017); Duangprom et al. (2018); Kornthong et al. (2021).
In this study, the level of vitellogenin (Vg) in the hemolymph was measured using vitellin (Vn) antiserum and anti-M. rosenbergii Vn (anti-MrVn) using an indirect enzyme-linked immunosorbent assay (ELISA) as described previously (Saetan et al., 2017; Tinikul et al., 2017; Duangprom et al., 2022). The specificity test for anti-MrVn was performed with S. olivacea ovaries using western blot analysis (Duangprom et al., 2022). Prior to sacrifice following the treatments, hemolymph samples from seven groups (T1–T5 and C1–C2) (n = 5 per group) were collected and mixed in equal volume ratio with an anticoagulant solution (0.45 M NaCl, 0.1 glucose, 30 mM tri-sodium citrate, 26 mM citric acid, and 10 mM EDTA at a pH of 4.6). The mixed samples were centrifuged at 7,000×g at 4°C, and the supernatant was obtained to determine protein concentration using the BCA assay (Thermo Fisher, USA). Three replicates of 10 μg/ml of protein (100 μl) from each sample were loaded into 96-well plates containing bicarbonate/carbonate-coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and incubated at 4°C overnight. Following washing, the plates were blocked with a solution consisting of 5% skim milk in Tris-buffered saline with 0.1% Tween™ 20 (TBST) for 1 h at room temperature. Subsequently, 100 μl of primary antibody: rabbit anti-MrVn antibody solution (1:1,000) (Duangprom et al., 2022) was applied to each well and incubated for an additional 2 h at 37°C. After thorough rinsing, 100 μl of secondary antibody:HRP-conjugated goat anti-rabbit IgG (Abcam, UK) at a dilution of 1:5,000 was applied to each well and incubated for 1 h at room temperature. The plates were washed and developed with 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate (Sigma, USA), and the absorbance at 492 nm was measured using a microplate reader (Thermo Fisher, USA). The hemolymph Vg concentration in each well was determined using a known standard Vn concentration, as outlined in the preparation section described by Duangprom et al. (2022).
To investigate the effect of hormone potency on mRNA expression, five S. olivacea were randomly selected from each group (T1–T5 and C1) and sacrificed on day 14 following the hormone cocktail injections. Pooled brain and VNC samples were obtained and subjected to RNA isolation using the TriPure™ Isolation reagent (Roche, Germany). The concentration and purity of each sample were determined using a NanoDrop2000 spectrophotometer (Thermo Scientific, USA). RNA samples were sent to BGI (Wuhan) for the evaluation of the RNA integrity number (RIN) using an Agilent 2100 Bioanalyzer (Agilent, CA, USA) and mRNA-seq using the BGISEQ-500 sequencing platform. Following read filtering, a de novo assembly was conducted using Trinity (Grabherr et al., 2011) on a set of clean reads. The TGICL software tool (Pertea et al., 2003) was employed to cluster transcripts, thereby eliminating abundance and generating unigenes for subsequent analyses.
Bowtie2 software (Langmead and Salzberg, 2012) was used to map all clean reads from each sample to de novo assembled unigenes. Gene expression levels were calculated and normalized to fragments per kilobase per transcript per million mapped reads (FPKM) using RSEM (Li and Dewey, 2011) based on the assembly results. Differentially expressed genes (DEGs) between the samples were identified based on gene expression levels. Bioinformatics service of BGI determined the significance of the DEGs using DEseq2 and PossionDis, with the criteria of log2 fold-change ≥1.00, p-value <0.05, and FDR ≤0.001 (Audic and Claverie, 1997; Love et al., 2014).
The brain and VNC of S. olivacea (n = 5) were obtained on days 0 and 14 of the experimental period. The tissues were homogenized using TriPure™ Isolation Reagent (Roche, Germany) and subsequently stored at −80°C, in accordance with the guidelines provided by the manufacturer. Each crab RNA sample (2 µg), was treated with DNase-I treatment to eliminate genomic DNA. Subsequently, cDNA synthesis was performed using a QuantiNova Reverse Transcription Kit (QIAGEN, United States) in accordance with the manufacturer’s instructions. The investigation carried out real-time quantitative polymerase chain reaction (qPCR) was used to analyze the expression levels of prostaglandin E synthase (Scyol-PGES) and estrogen sulfotransferase (Scyol-ESULT) in the crab tissue samples collected on days 0 and 14. qPCR was conducted using specific Scyol-PGES primers (forward primer: 5’ GCTGCTCGGTGTGGGTTTCGGT 3’ and reverse primer: 5’ GCAGCAAAATGGGCATGTCTGGTAC 3’) and specific Scyol-ESULT primers (forward primer: 5’ GCGTGGCAGAAGAGGCACCA3’ and reverse primer: 5’ TCCAGTCTCCCGTCTTGCCCT 3’). The β-actin gene was used as an internal control (forward primer: 5’ GAGCGAGAAATCGTTCGTGACAT 3’ and reverse primer: 5’ CCGATGGTGATGACCTGGCCGT 3’). PCR conditions used in this study were based on the methodology described by Duangprom et al. (2022). The 2−▵▵ct approach was employed to analyze the qPCR data and determine the relative expression levels of the two genes in relation to β-actin, as outlined by Livak and Schmittgen (2001).
The application of statistical methodologies was utilized in the assessment of the GSI value, hemolymph Vg, ovarian histological analysis, and the relative expression of Scyol-PGES and Scyol-ESULT genes in relation to the reference gene, β-actin. Graphs represent the mean values and standard error of the mean (SEM). One-way ANOVA with Tukey’s post hoc analysis was employed to investigate disparities in mean values between the experimental and control groups. Statistical analysis was performed using Prism 9.5.1 software for Windows (GraphPad Software, USA). Statistical significance was determined at a significance level ≤0.05, as denoted by the p-value (Duangprom et al., 2022).
An anatomical assessment of the eyestalk was conducted using H&E staining (Figures 1A, D), which showed three clusters containing clusters 1, 2, and 3. Individuals injected with NSS exhibited the presence of Scyol-VIH-positive neurons within cluster 2 and X-organ (XO) (Figures 1B, E, H). Individuals that received dsRNA-VIH did not exhibit any neurons with positive results, indicating complete inhibition of Scyol-VIH mRNA production (Figures 1C, F, I). Finally, the control sections (using a sense riboprobe) exhibited no indication of positive staining (Figure 1G).

Figure 1 Localization of Scyol-VIH mRNA in the eyestalks of S. olivacea using in situ hybridization. (A) Sagittal section of the eyestalk stained with H&E showing the location of neuronal clusters (1, 2, 3) and neuropils with higher magnification shown in (D). (B) The NSS-injected group exhibited Scyol-VIH mRNA in neuronal clusters 2 and XO, as illustrated in (E, H) at a higher magnification. (C) The dsRNA-VIH-injected group exhibited no Scyol-VIH mRNA expression at higher magnifications (F, I). (G) Negative control sections probed with a DIG-labeled sense-strand Scyol-VIH riboprobe did not exhibit a positive signal. Abbreviations: LG, lamina ganglionaris; ME, medulla externa; MI, medulla interna; MT, medulla terminalis; XO, X-organ; HB, hemielipsoid body; Och, outer optic chiasm; Ich, inner optic chiasm, 1, Cluster1; 2, Cluster 2; and 3, Cluster 3.
Injections of dsRNA-VIH (7.14 ± 0.92) and the combination of 5-HT + GnRH + dsRNA-VIH (8.01 ± 0.66) significantly increased S. olivacea GSI, compared to the control, NSS-injected group (3.48 ± 1.41) on day 14 post-injection. However, on day 28 post-injection, all groups except for the 5-HT + GnRH group exhibited a significant increase in the GSI value of S. olivacea in comparison to the control groups (Figure 2).
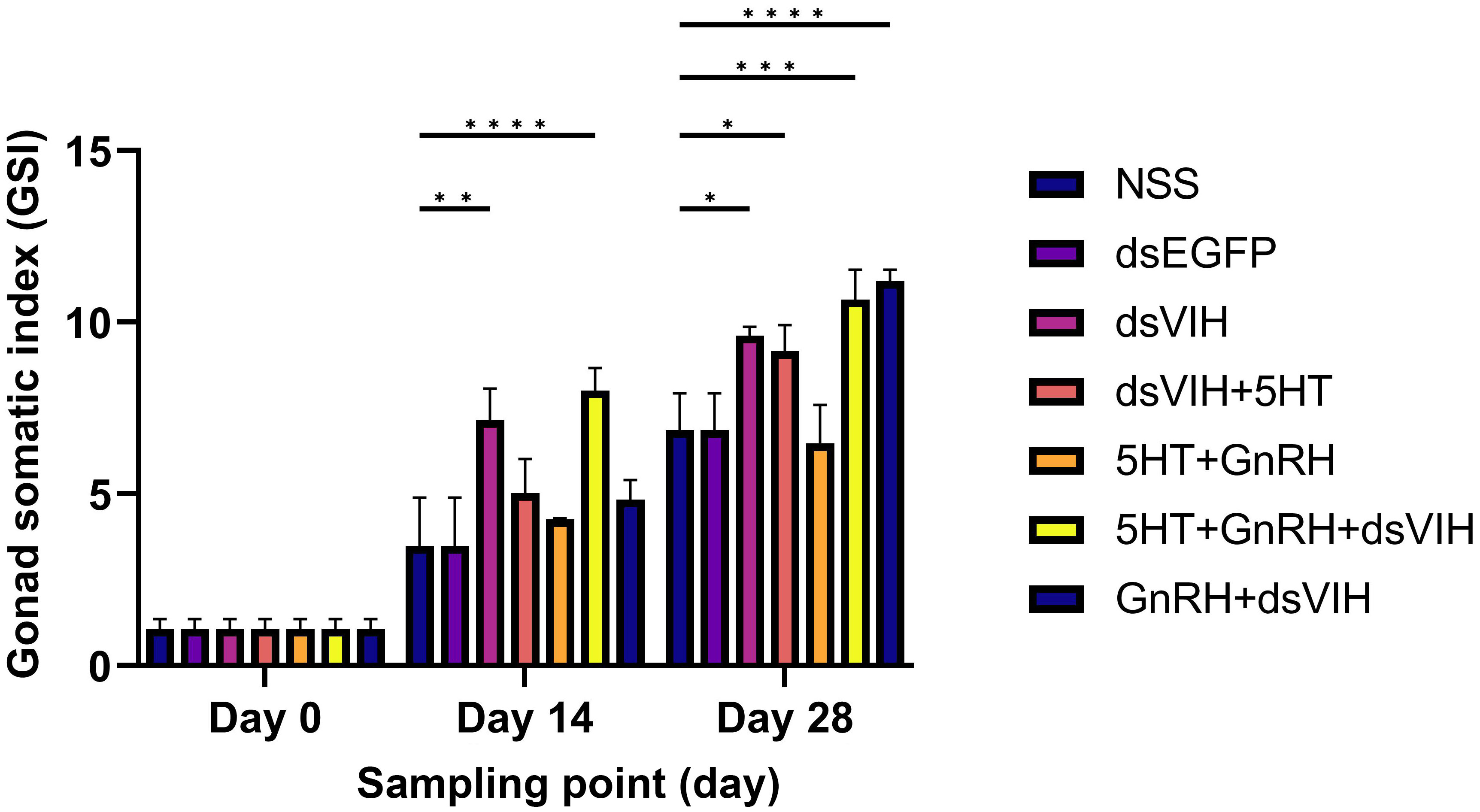
Figure 2 Graph showing the effect of treatments on female S. olivacea GSI on days 0, 14, and 28 post-injections. Bars indicate standard error of the mean (SEM). *p <0.05, **p <0.005, ***p <0.001, and ****p <0.0001 denoted level of significance.
The hemolymph Vg levels of crabs were measured on days 0, 14, and 28 of the experiment (Figure 3). No significant increase in Vg levels was observed in the hemolymph of any of the groups on days 0 and 14 post-injection. However, dsRNA-VIH (1,428.170 ± 124.010), GnRH + dsRNA-VIH (1,274.19 ± 89.27), and 5-HT + GnRH + dsRNA-VIH (1,197.128 ± 37.95)-injected groups exhibited a significant increase in hemolymph Vg compared to the NSS-injected group (364.00 ± 52.68).
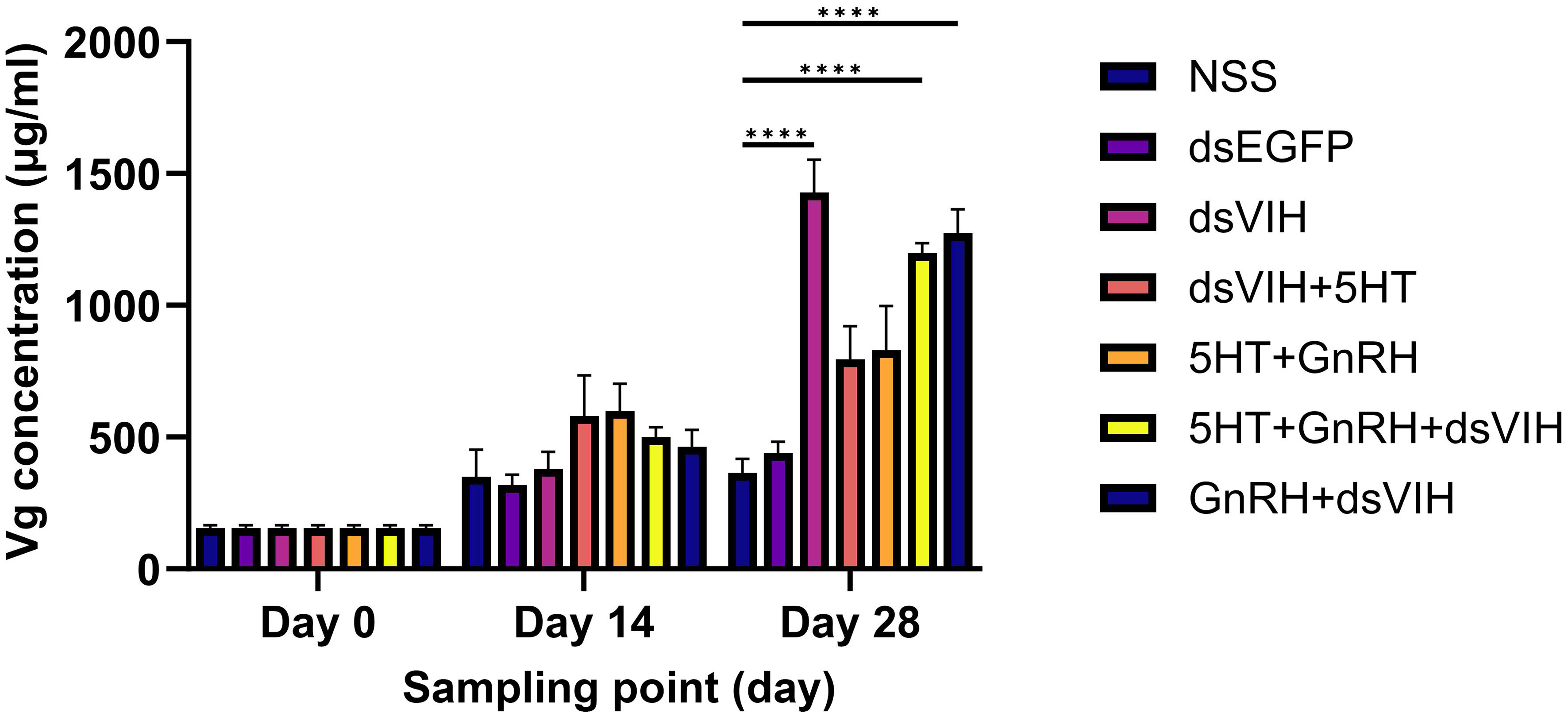
Figure 3 Hemolymph vitellogenin (Vg) levels in female S. olivacea on days 0, 14, and 28 post-injections. Values are presented as the term of mean ± SEM and ****p <0.0001 denotes the level of significance.
Ovary histology was investigated at days 0, 14, and 28 post-injection (Figure 4A). Comparative analysis of oocyte counts at various developmental stages was conducted across seven groups. On day 0, the ovaries of the treatment and control groups exhibited Og and Oc1–Oc3, as shown in Figures 4A, B. On day 14, the ovaries of the NSS-injected and dsRNA-EGFP-injected groups exhibited Oc1–Oc3, whereas the dsRNA-VIH, 5-HT + GnRH + dsRNA-VIH. and GnRH + dsRNA-VIH-injected groups predominantly contained Oc4 (Figures 4A, C). The 5-HT + GnRH-injected group exhibited a statistically significant increase in Oc3 expression (Figures 4A, C). On day 28, all the groups displayed predominantly mature ovaries. Oocyte counts revealed that dsRNA-VIH (96.12 ± 1.97), 5-HT + GnRH + dsRNA-VIH (97.25 ± 0.64), and GnRH + dsRNA-VIH (98.93 ± 1.06)-injected groups had significantly greater numbers of Oc4 than the NSS-injected group (85.07 ± 4.90) (Figures 4A, D). However, the 5-HT + dsRNA-VIH- and 5-HT + GnRH-injected groups also exhibited a high number of Oc4, which was not significantly different from the NSS-injected group (Figures 4A, D).
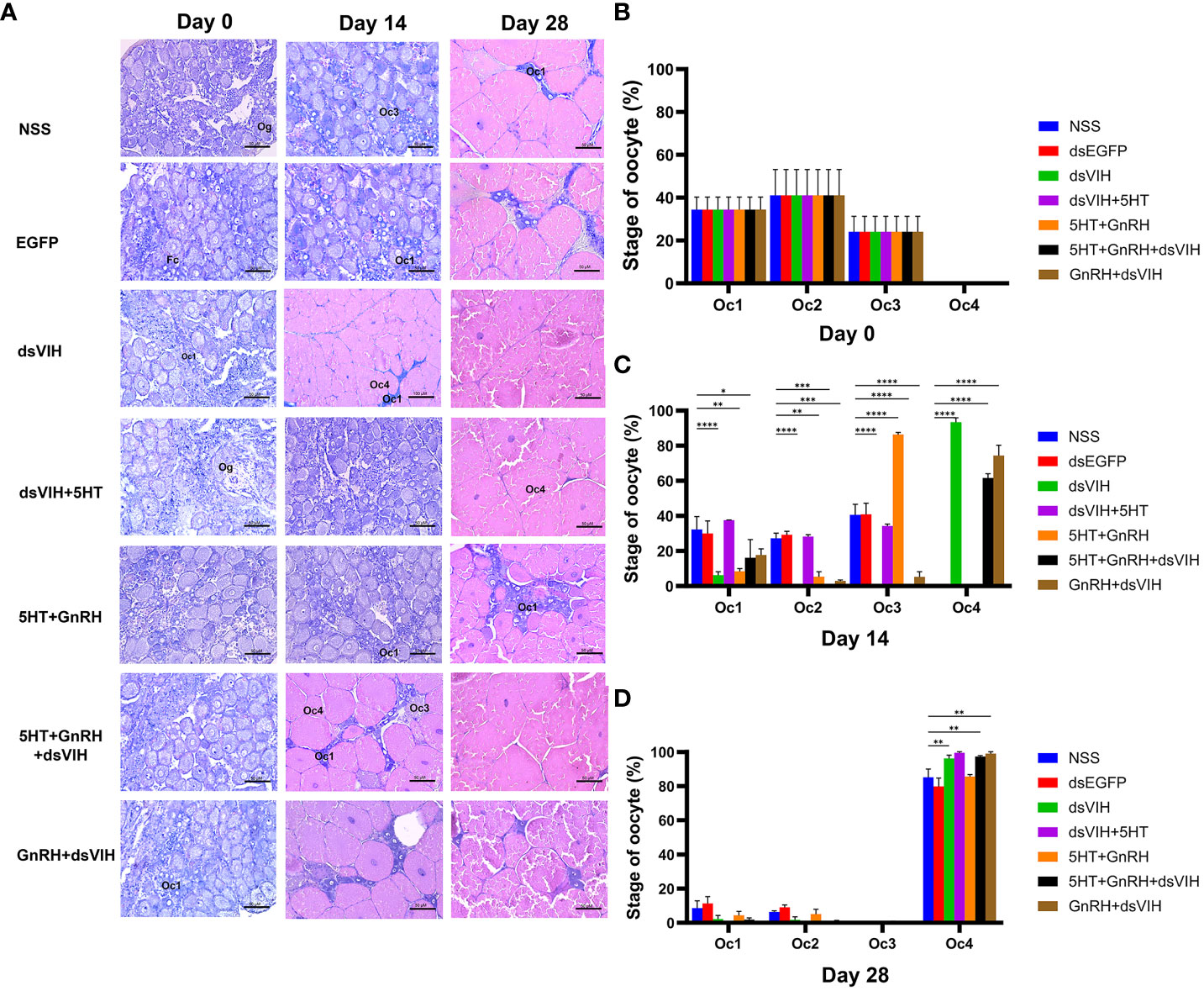
Figure 4 Histological examination and quantification of S. olivacea ovaries after treatment. (A) Representative images from each group on days 0, 14, and 28. On day 0, histological examination of the ovaries in all groups revealed a comparable number of Oc1–Oc3. On day 14, the ovaries of the dsRNA-VIH-HT + GnRH + dsRNA-VIH and GnRH + dsRNA-VIH-injected groups exhibited greater maturity, as evidenced by the presence of a higher number of Oc4, in contrast to the other groups that still displayed Oc1–Oc3. On day 28, the ovaries of all the groups reached maturity, exhibiting a higher quantity of Oc4. (B–D) Quantification of the oocyte on days 0, 14, and 28. *p <0.05, **p <0.005, ***p <0.001, and **** p <0.0001 denoted level of significance. Abbreviations: Oc, oocyte; Og, oogania; N, nucleus.
The availability of data and materials Raw RNA-Seq reads of all treatments are accessible through the NCBI (Sequence Read Archive-SRA) (BioProject ID: PRJNA1006859 https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1006859). DEG analysis of the pooled fbrain and VNC of female S. olivacea was performed on day 14 post-treatment, showing that 30 genes were significantly upregulated and two genes were significantly downregulated. Hormone-related genes were targeted and classified into four distinct groups based on whether they encoded receptors, neuropeptides, downstream hormone-related, or prostanoid-related genes (Tables 1–4). Significant upregulation of 5-HT1 and GnRH receptors was observed following all treatments. The lutropin-choriogonadotropic hormone (LH) receptor was upregulated in the dsRNA-VIH, 5-HT + GnRH + dsRNA-VIH, and GnRH + dsRNA-VIH groups. Additionally, the follicle-stimulating hormone (FSH) receptor was upregulated in all groups except the dsRNA-VIH + 5-HT group (Table 1). Transcriptomic profiles revealed significant upregulation of several neuropeptide hormone genes, such as red pigment-concentrating hormone precursor (RPCH), SIFamide (FMRFamide-related neuropeptides), and short neuropeptide F (sNPF) across all treatments. The expression levels of adipokinetic hormone/corazonin-related peptide (AKH/Crz) and neuropeptide F (NPF-2) were upregulated in all treatments except for the dsRNA-VIH + 5-HT and 5-HT + GnRH treatments, respectively. The expression of CHH was upregulated in the dsRNA-VIH + 5-HT and 5-HT + GnRH groups but downregulated in the 5-HT + GnRH + dsRNA-VIH group. In contrast, NPF-F1 was upregulated only in the 5-HT+GnRH+ dsRNA-VIH group (Table 2).
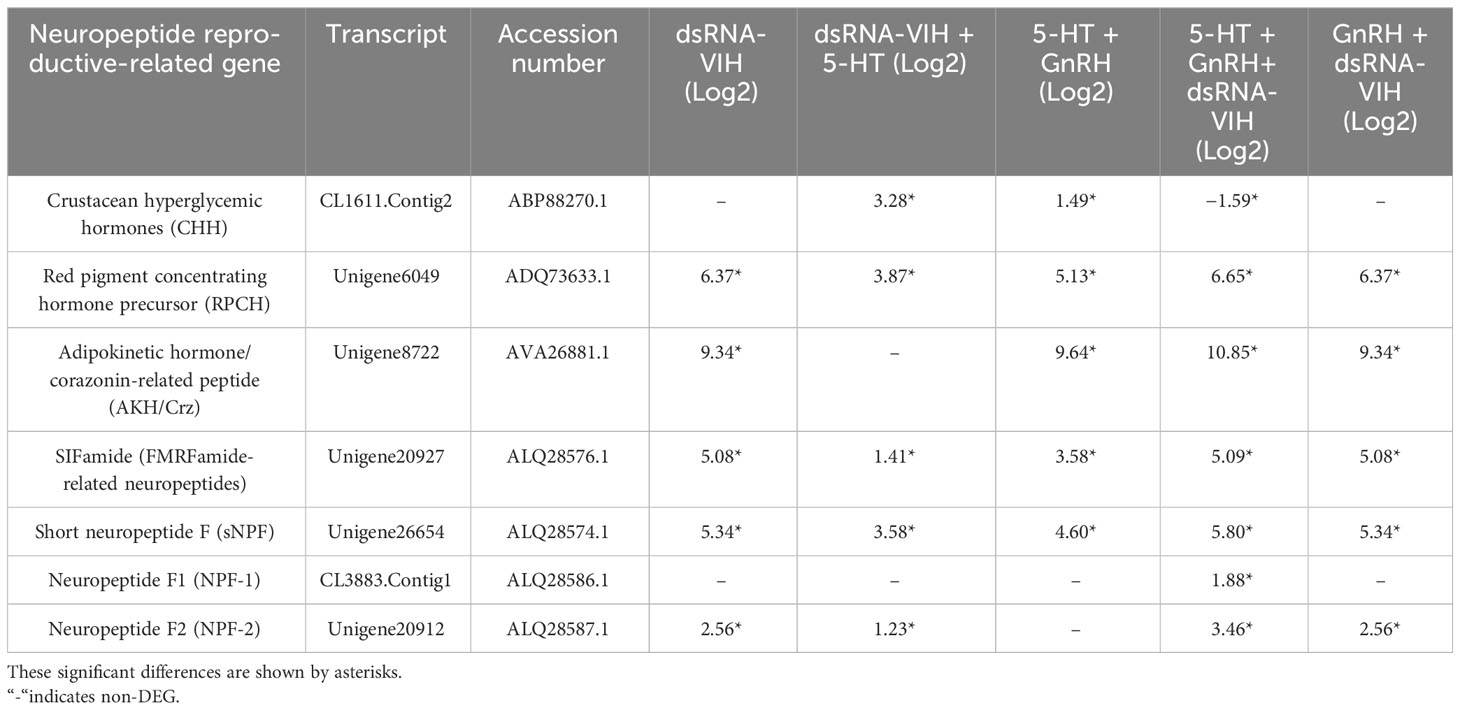
Table 2 Summary of differential expression of neuropeptide reproductive-related genes compared with the control.
This study identified upregulation of farnesoic acid O-methyltransferase (FAMeT) long and short isoforms, estrogen sulfotransferase (ESULT), and progesterone-like protein 2 across all treatments. The upregulation of estradiol 17-beta-dehydrogenase 8 was observed exclusively in the dsRNA-VIH+5-HT group, whereas upregulation of progesterone-like protein 1 was observed in both dsRNA-VIH and GnRH + dsRNA-VIH groups. In contrast, oxytocin/vasopressin-like peptide was significantly upregulated in the 5-HT + GnRH + dsRNA-VIH and GnRH + dsRNA-VIH groups (Table 3). Prostanoid-related genes have been shown to regulate reproductive capacity. Our results indicate that prostaglandin E synthase (PGES) was upregulated in all treatments, whereas prostaglandin F synthase (PGFS) was upregulated in all treatments, except for the dsRNA-VIH + 5-HT group (Table 4). In addition, the supplementary data included the results of the BLAST search for each of the selected transcripts, as well as contig and unigene IDs, KEGG, KOG, InterPro, and GO annotation (Supplementary File S1).
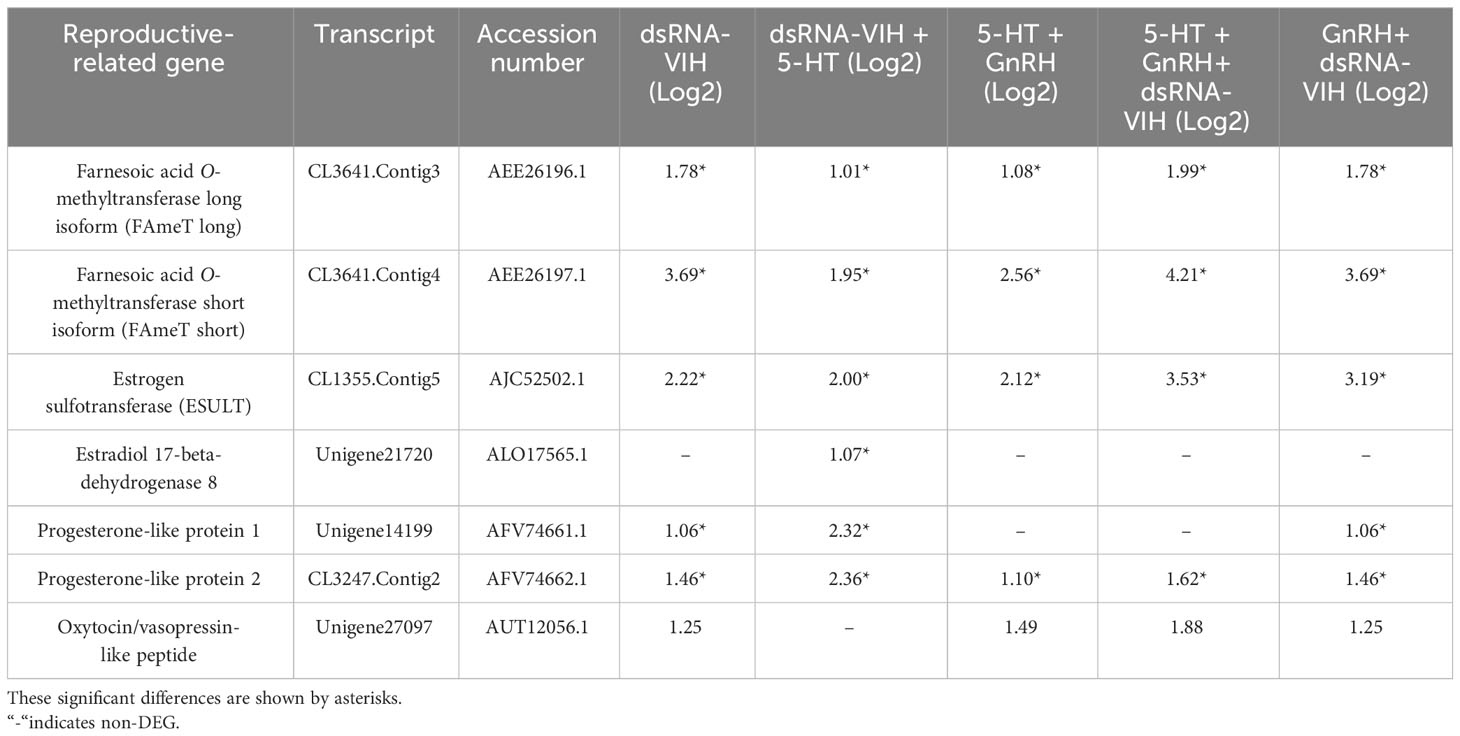
Table 3 Summary of the differential expression of downstream reproductive-related genes compared with the control.

Table 4 Summary of the differential expression of prostanoid-related genes compared with the control.
Changes in the expression of Scyol-estrogen sulfotransferase (ESULT) and Scyol-prostaglandin E synthase (PGES) in the brain and VNC were determined between days 0 and 14 post-treatment. The administration of dsRNA-VIH and the combination of 5-HT + GnRH + dsRNA-VIH resulted in significant upregulation of Scyol-ESULT expression in the brain on day 14. In contrast, injection of dsRNA-VIH + 5-HT and 5-HT + GnRH exhibited a tendency to increase Scyol-ESULT expression, but the effect was not statistically significant. Only the combination of 5-HT+ GnRH + dsRNA-VIH increased Scyol-ESULT expression in the VNC (Figures 5A, B). For Scyol-PGES, only the combination of 5-HT + GnRH + dsRNA-VIH injection significantly increased expression in both the brain and VNC on day 14 post-treatment (Figures 5C, D).
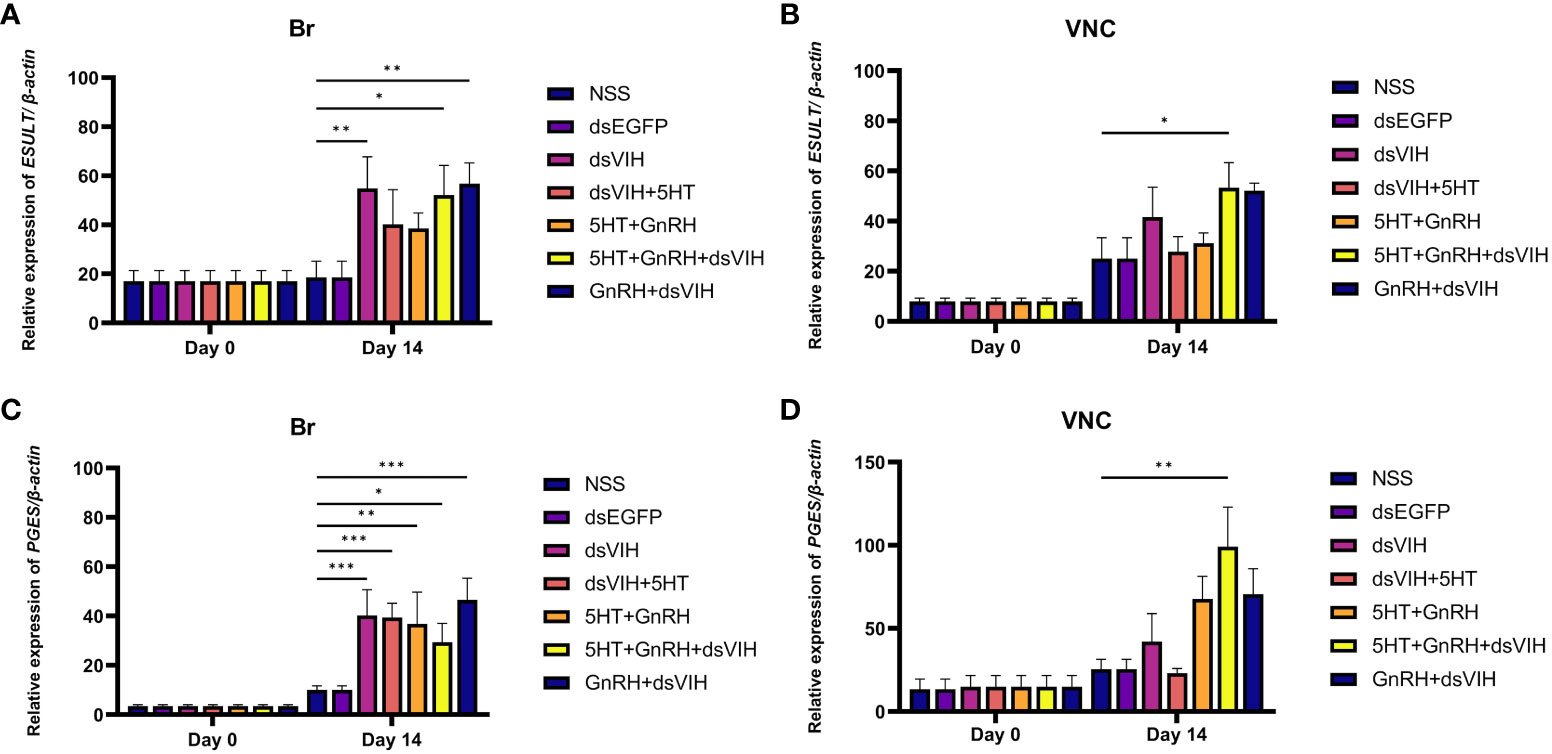
Figure 5 The effect of hormones on the expression of Scyol-ESULT and Scyol-PGES in the brain (Br) and ventral nerve cord (VNC) using quantitative RT-PCR. (A, B) Histograms showing the expression levels of Scyol-ESULT in the Br and VNC groups on days 0 and 14 post-treatment. (C, D) Histograms showing the expression levels of Scyol-PGES in the Br and VNC groups on days 0 and 14 post treatment. Bars represent standard error of the mean (SEM). *p <0.05, **p <0.005, and ***p <0.001 denoted level of significance.
Ovarian maturation in crustaceans requires the cooperation of molecules from multiple pathways (Rotllant et al., 2018; Jayasankar et al., 2020). Captivity-induced stress in marine invertebrate broodstock, such as handling, overcrowding, diseases, and other inappropriate culture conditions, can interfere with these ovarian maturation-related pathways, which is problematic for their sustainable culture (Gianasi, 2017). In some cultured crustaceans, reproductive dysfunction may be overcome by eyestalk ablation, which stimulates maturation, but it is associated with a high mortality rate (Gianasi, 2017; Jin et al., 2021; Asmat-Ullah et al., 2023; Jia et al., 2023). The use of dsRNA-VIH and reproduction-associated neurohormones has shown promise as a replacement for eyestalk ablation, and is highly effective in enhancing reproductive performance in several crustaceans (Cohen et al., 2021; Kang et al., 2021; Duangprom et al., 2022). In this study, we initially confirmed the role of dsRNA-VIH in the female crab S. olivacea, and showed that combinatorial administration of dsRNA-VIH, 5-HT, and GnRH, may provide the most efficient approach for manipulating reproductive maturation in captive broodstock.
Scyol-VIH is expressed in the X organ of the eyestalk, brain, and VNC of S. olivacea (Kornthong et al., 2019). Its expression in the eyestalk was significantly promoted by dopamine, a potent reproductive inhibitor; however, no significant effect was noted for Scyol-VIH in the brain and VNC (Kornthong et al., 2019). Therefore, we expected the eyestalk Scyol-VIH to be the target of our dsRNA-VIH. In this study, this transcript was in the X-organ cluster and its expression was abolished by dsRNA-VIH treatment, as shown by in situ hybridization which corresponded to our previous dsRNA-VIH efficiency shown by RT-PCR (Duangprom et al., 2022). As described elsewhere, dsRNA-VIH potentiates reproductive enhancement in S. olivacea and other crustaceans. Crustacean GnRH-like molecules have been successfully identified in P. clarkii (Guan et al., 2014) and M. rosenbergii (Suwansa-Ard et al., 2016). LGnRH-III and 5-HT have long been known to promote reproduction in crustaceans (Ngernsoungnern et al., 2008; Tinikul et al., 2009; Poljaroen et al., 2011; Saetan et al., 2013; Siangcham et al., 2013). However, the effect of GnRH on the reproduction of S. olivacea has not yet been reported. In this study, we combined various doses of dsRNA-VIH, GnRH, and 5-HT to lower them compared to the previous successful doses reported to promote reproduction (Poljaroen et al., 2011; Saetan et al., 2013; Duangprom et al., 2022). We believe that these three molecules might work together, and this might be closer to their physiological conditions in mud crabs. Our present study provided evidence that either dsRNA-VIH itself (T1) or a combination of dsRNA-VIH and GnRH with or without 5-HT (T4 and T5) significantly increased the GSI and hemolymph Vg, and possessed more advanced stage of ovary maturation than the control group. This indicated the importance of dsRNA-VIH as the key molecule to promote reproduction, whereas GnRH and 5-HT probably showed supportive effects, especially in the presence of low concentrations of dsRNA-VIH. Duangprom et al. (2022) reported that 0.6 μg/g BW dsRNA-VIH was most effective at abolishing brain and eyestalk Scyol-VIH expression, while dosages below this concentration partially inhibited brain expression but not eyestalk expression. This could explain our results that a combination of GnRH and 5-HT with a low dose of dsRNA-VIH acceptably promoted mud crab reproduction at the same level as that seen in the 0.6 μg/g BW dsRNA-VIH. Noticeably, the low dose of dsRNA-VIH, 5-HT (T2), GnRH, and 5-HT (T3) did not strongly elevate the hemolymph Vg, and their ovarian histology progressed slowly to meet maturation as seen on day 14. This corresponds with evidence in P. indicus that lower 5-HT efficiency involving reproduction was reported in the presence of VIH (eyestalk-intact animals) (Tomy et al., 2016). Therefore, we suggested the combination of 5-HT and GnRH with low dose of dsRNA-VIH (not lower than 0.1 μg/g BW) was recommended for mud crab reproduction stimulation.
Differential gene expression analysis of the mixed brain and VNC of female crabs following individual or combined dsRNA-VIH, 5-HT, and GnRH treatments, led to the identification of molecular factors that may explain their role in ovarian development. The upregulation of 5-HTR and GnRHR could be attributed to the increased abundance of their ligands. In addition, due to peptide structure similarity, we propose that injected GnRH (lGnRHR-III) could activate crab adipokinetic hormone/corazonin-related peptide (ACP) receptor expression. ACP has been identified in M. rosenbergii and postulated to play a role in lipid metabolism (Suwansa-Ard et al., 2016). Similarly, 5-HT treatment stimulated higher levels of RPCH, CHH, and FAMeT expression, as previously described (Kornthong et al., 2013; Kornthong et al., 2014; Duangprom et al., 2017).
Our study is the first to report the potential of VIH, 5-HT, and GnRH in upregulating the expression of short neuropeptide F (sNPF), NPF-1, NPF-2, and SIFamide. In M. rosenbergii, both short and long NPFs have been reported to be involved in spermatogenesis and ovarian maturation (Tinikul et al., 2017; Thongrod et al., 2019). In addition, the expression of NPF-1, NPF-2, sNPF, and SIFamide transcripts in the cerebral ganglia of the crab Scylla paramamosain was high in pre- and early vitellogenic individuals (Bao et al., 2015), suggesting a possible role in reproduction. Oxytocin/vasopressin-like peptide was not upregulated following our treatments, correlating with the non-reproductive function of this peptide reported in P. pelagicus (Saetan et al., 2018) and S. paramamosain (Lin et al., 2020).
In vertebrates, the LH, FSH, and their cognate receptors contribute to the reproductive steroid hormone activation (Casarini and Crépieux, 2019). In this study, LHR and FSHR gene upregulation in hormonally injected groups correlated with the upregulation of steroid-related genes, progesterone-like protein, and ESULT, the latter of which was confirmed by real-time qPCR. However, the association of LHR/FSHR with the steroid pathway or reproduction has not been reported in crustaceans. A possible relevance to mammalian LH and FSH is the glycoproteins GPA2/GPB5 identified together with its receptor, LGR1, in invertebrates, including crustaceans (Rocco and Paluzzi, 2016; Wahl et al., 2022). The GPA2/GPB5 heterodimer neither activated FSHR nor LHR (Rocco and Paluzzi, 2016), and it served as a gonad inhibiting factor in the female M. rosenbergii (Wahl et al., 2022). The pathway associated with the prostanoid-related gene product has been described in the transcriptome data of M. rosenbergii (Thongbuakaew et al., 2021), and it has also been suggested that this gene is regulated in terms of reproductive potential (Di Costanzo et al., 2019). Upregulation of phospholipase A and C, COX, and prostaglandin D, E, and F synthases was detected after treatment, and real-time qPCR confirmed the upregulation of Scyol-PGES. Numerous studies have indicated that these genes are involved in reproduction of crustaceans. For example, in M. rosenbergii, the immunoreactivities of PGE2, COX1, and PGES and the level of PGE2 were abundant in early vitellogenic ovaries and the effects of PGE2 positively stimulated several reproductive parameters (Sumpownon et al., 2015). This gene is upregulated following eyestalk ablation, which correlates with the progression of ovarian development (Thongbuakaew et al., 2021). In addition, 5-HT injection increased PGFS and ESULT expression in the CNS and ovaries of S. olivacea (Kornthong et al., 2014).
Most reproductive-related genes associated with GSI and hemolymph Vg levels in this study were stimulated by either the injection of dsRNA-VIH (0.6 µg/g BW) or lower doses of dsRNA-VIH (0.2 µg/g and 0.1 µg/g BW) combined with GnRH and/or 5-HT. However, the receptor-related reproductive genes (5-HTR, GnRHR, LHR, and FSHR) were significantly more highly expressed in the dsRNA-VIH (T1), 5-HT + GnRH + dsRNA-VIH (T4), and GnRH + dsRNA-VIH (T5)-injected groups. The peptide hormone genes sNPF, NPF1, NPF2, ACP, CHH, and RPCH were highly expressed in the cocktail hormone-injected groups. Finally, other downstream reproductive-related genes, such as FAMeT, steroid-, and prostanoid-related genes, were shown to be more abundant in the multiple hormonal treatment groups (especially T4) than in the dsRNA-VIH-treated group. However, the effects of the combined hormonal treatment were significantly more powerful in resolving aquaculture issues. As discussed, dsRNA-VIH, GnRH, and/or 5-HT significantly modified the expression of numerous reproduction-related genes, inducing ovarian maturation and vitellogenesis. The precise signaling pathways activated by these hormones and dsRNA-VIH in crustaceans are not yet known and require further investigation. In contrast to the use of synthetic lGnRH-III and 5-HT, dsRNA-VIH production is expensive (Duangprom et al., 2022). Therefore, we propose that a combination of low-dose dsRNA-VIH with synthetic GnRH and/or 5-HT would be an effective solution for enhancing reproduction in captive broodstocks.
In conclusion, this study demonstrated the effects of dsRNA-VIH, GnRH, and 5-HT on the reproduction of the mud crab, S. olivacea. These three molecules are known to stimulate reproduction in crustaceans. In the case of mud crab, S. olivacea, we suggested a combination of the dsRNA-VIH at dose not lower than 0.1 µg/g BW with either lGnRH-III at dose of 25 ng/g–50 ng/g BW or 5-HT at dose of 1.5 μg/g BW, or with both molecules to effectively boost ovarian development.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was approved by The Animal Care and Use Committee of Thammasat University, National Research Council of Thailand (NRCT) which ensured that animal suffering was minimal under authorized Protocol Number 011/2562. The study was conducted in accordance with local legislation and institutional requirements.
JS: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing, Writing – original draft. SD: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SS: Formal analysis, Investigation, Methodology, Writing – original draft. PA: Formal analysis, Investigation, Methodology, Writing – original draft. TP: Formal analysis, Investigation, Methodology, Writing – original draft. PSu: Formal analysis, Investigation, Methodology, Writing – original draft. CS: Formal analysis, Investigation, Methodology, Writing – original draft. MT: Formal analysis, Investigation, Methodology, Writing – original draft. SS-A: Supervision, Writing – review & editing. SC: Supervision, Writing – review & editing. PSo: Supervision, Writing – review & editing. NK: Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Thammasat University Research Unit in Biotechnology and its application of aquatic animals. The authors also gratefully acknowledge the financial support provided by Thailand Science Research and Innovation Fundamental Fund (TUFF 60/2565).
The authors are especially grateful to the Thammasat University Research Unit in Biotechnology and its application to aquatic animals, the Thailand Science Research and Innovation Fundamental Fund. The authors also gratefully thank the Center of Scientific Equipment for Advanced Research, Office of Advanced Science and Technology, Thammasat University, and the Coastal Aquaculture Research and Development Center, Samut Sakorn, Thailand, for supporting the research instrument and crab culture area in this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1286789/full#supplementary-material
Alfaro-Montoya J., Braga A., Umaña-Castro R. (2019). Research frontiers in penaeid shrimp reproduction: Future trends to improve commercial production. Aquaculture 503, 70–87. doi: 10.1016/j.aquaculture.2018.12.068
Amin-Safwan A., Muhd-Farouk H., Nadirah M., Ikhwanuddin M. (2016). Effect of water salinity on the external morphology of ovarian maturation stages of orange mud crab, scylla olivacea (Herbst 1796) in captivity. Pak. J. Biol. Sci. 19 (5), 219–226. doi: 10.3923/pjbs.2016.219.226
Asmat-Ullah M., Rozaimi R., Fazhan H., Shu-Chien A. C., Wang Y., Waiho K. (2023). Eyestalk ablation to increase ovarian maturation in mud crabs. J. Vis. Exp. (193), e65039. doi: 10.3791/65039
Audic S., Claverie J. M. (1997). The significance of digital gene expression profiles. Genome Res. 7 (10), 986–995. doi: 10.1101/gr.7.10.986
Bao C., Yang Y., Huang H., Ye H. (2015). Neuropeptides in the cerebral ganglia of the mud crab, Scylla paramamosain: transcriptomic analysis and expression profiles during vitellogenesis. Sci. Rep. 5, 17055. doi: 10.1038/srep17055
Casarini L., Crépieux P. (2019). Molecular mechanisms of action of FSH. Front. Endocrin. 10. doi: 10.3389/fendo.2019.00305
Chen T., Ren C., Jiang X., Zhang L., Li H., Huang W., et al. (2018). Mechanisms for type-II vitellogenesis-inhibiting hormone suppression of vitellogenin transcription in shrimp hepatopancreas: Crosstalk of GC/cGMP pathway with different MAPK-dependent cascades. PloS One 13 (3), e0194459. doi: 10.1371/journal.pone.0194459
Chen T., Zhang L. P., Wong N. K., Zhong M., Ren C. H., Hu C. Q. (2014). Pacific white shrimp (Litopenaeus vannamei) vitellogenesis-inhibiting hormone (VIH) is predominantly expressed in the brain and negatively regulates hepatopancreatic vitellogenin (VTG) gene expression. Biol. Reprod. 90 (3), 47. doi: 10.1095/biolreprod.113.115030
Cohen S., Ilouz O., Manor R., Sagi A., Khalaila I. (2021). Transcriptional silencing of vitellogenesis-inhibiting and molt-inhibiting hormones in the giant freshwater prawn, Macrobrachium rosenbergii, and evaluation of the associated effects on ovarian development. Aquaculture 538, 736540. doi: 10.1016/j.aquaculture.2021.736540
Di Costanzo F., Di Dato V., Ianora A., Romano G. (2019). Prostaglandins in marine organisms: A review. Mar. Drugs 17 (7), 428. doi: 10.3390/md17070428
Duangprom S., Ampansri W., Suwansa-Ard S., Chotwiwatthanakun C., Sobhon P., Kornthong N. (2018). Identification and expression of prostaglandin E synthase (PGES) gene in the central nervous system and ovary during ovarian maturation of the female mud crab, Scylla olivacea. Anim. Reprod. Sci. 198, 220–232. doi: 10.1016/j.anireprosci.2018.09.022
Duangprom S., Kornthong N., Suwansa-Ard S., Srikawnawan W., Chotwiwatthanakun C., Sobhon P. (2017). Distribution of crustacean hyperglycemic hormones (CHH) in the mud crab (Scylla olivacea) and their differential expression following serotonin stimulation. Aquaculture 468, 481–488. doi: 10.1016/j.aquaculture.2016.11.008
Duangprom S., Saetan J., Phanaksri T., Songkoomkrong S., Surinlert P., Tamtin M., et al. (2022). Acceleration of ovarian maturation in the female mud crab with RNA interference of the vitellogenesis-inhibiting hormone (VIH). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.880235
Feijó R. G., Braga A. L., Figueiredo M. A., Romano L. A., Klosterhoff M. C., Lanes C. F. C., et al. (2016). Silencing of gonad-inhibiting hormone transcripts in Litopenaeusvannamei females by use of the RNA interference technology. Mar. Biotechnol. (NY) 18(1), 117–23. doi: 10.1007/s10126-015-9676-2
Ghazali A., Noordin N. M., Abol-Munafi A. B., Azra M. N., Ikhwanuddin M. (2017). Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malays. 46 (12), 2273–2280. doi: 10.17576/jsm-2017-4612-03
Gianasi B. (2017). Marine invertebrate broodstock management: breakthroughs, challenges and future perspectives. World Aquac. 48, 63–67.
Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29 (7), 644–652. doi: 10.1038/nbt.1883
Guan Z.-B., Shui Y., Liao X.-R., Xu Z.-H., Zhou X. (2014). Primary structure of a novel gonadotropin-releasing hormone (GnRH) in the ovary of red swamp crayfish Procambarus clarkii. Aquaculture 418-419, 67–71. doi: 10.1016/j.aquaculture.2013.10.010
Jayasankar V., Tomy S., Wilder M. N. (2020). Insights on molecular mechanisms of ovarian development in decapod crustacea: focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. 11. doi: 10.3389/fendo.2020.577925
Jia S., Li J., Lv J., Ren X., Wang J., Wang Q., et al. (2023). Molecular characterization related to ovary early development mechanisms after eyestalk ablation in Exopalaemon carinicauda. Biology 12 (4), 596. doi: 10.3390/biology12040596
Jin S., Fu Y., Hu Y., Fu H., Jiang S., Xiong Y., et al. (2021). Transcriptome profiling analysis of the testis after eyestalk ablation for selection of the candidate genes involved in the male sexual development in macrobrachium nipponense. Front. Genet. 12. doi: 10.3389/fgene.2021.675928
Kang B. J., Bae S.-H., Suzuki T., Niitsu S., Wilder M. N. (2019). Transcriptional silencing of vitellogenesis-inhibiting hormone (VIH) subtype-I in the whiteleg shrimp, Litopenaeus vannamei. Aquaculture 506, 119–126. doi: 10.1016/j.aquaculture.2019.03.028
Kang B. J., Sultana Z., Wilder M. N. (2021). Assessment of the effects of double-stranded RNAs corresponding to multiple vitellogenesis-inhibiting hormone subtype I peptides in subadult female whiteleg shrimp, litopenaeus vannamei. Front. Endocrinol. 12. doi: 10.3389/fendo.2021.594001
Khornchatri K., Kornthong N., Saetan J., Tinikul Y., Chotwiwatthanakun C., Cummins S. F., et al. (2015). Distribution of serotonin and dopamine in the central nervous system of the female mud crab, Scylla olivacea (Herbst). Acta Histochem. 117 (2), 196–204. doi: 10.1016/j.acthis.2014.12.006
Kornthong N., Chotwiwatthanakun C., Chansela P., Tinikul Y., Cummins S. F., Hanna P. J., et al. (2013). Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. Gen. Comp. Endocrinol. 185, 28–36. doi: 10.1016/j.ygcen.2013.01.011
Kornthong N., Cummins S. F., Chotwiwatthanakun C., Khornchatri K., Engsusophon A., Hanna P. J., et al. (2014). Identification of genes associated with reproduction in the mud crab (Scylla olivacea) and their differential expression following serotonin stimulation. PloS One 9 (12), e115867. doi: 10.1371/journal.pone.0115867
Kornthong N., Duangprom S., Suwansa-Ard S., Saetan J., Phanaksri T., Songkoomkrong S., et al. (2019). Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Anim. Reprod. Sci. 208, 106122. doi: 10.1016/j.anireprosci.2019.106122
Kornthong N., Phanaksri T., Saetan J., Duangprom S., Lekskul B., Vivattanasarn T., et al. (2021). Identification and localization of growth factor genes in the sea cucumber, Holothuria scabra. Heliyon 7 (11), e08370. doi: 10.1016/j.heliyon.2021.e08370
Kuballa A. V., Holton T. A., Paterson B., Elizur A. (2011). Moult cycle specific differential gene expression profiling of the crab Portunus pelagicus. BMC Genomics 12, 147. doi: 10.1186/1471-2164-12-147
Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 (4), 357–359. doi: 10.1038/nmeth.1923
Li B., Dewey C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12, 323. doi: 10.1186/1471-2105-12-323
Lin D., Wei Y., Ye H. (2020). Role of oxytocin/vasopressin-like peptide and its receptor in vitellogenesis of mud crab. Int. J. Mol. Sci. 21 (7), 2297. doi: 10.3390/ijms21072297
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550. doi: 10.1186/s13059-014-0550-8
Meeratana P., Withyachumnarnkul B., Damrongphol P., Wongprasert K., Suseangtham A., Sobhon P. (2006). Serotonin induces ovarian maturation in giant freshwater prawn broodstock, Macrobrachium rosenbergii de Man. Aquaculture 260 (1), 315–325. doi: 10.1016/j.aquaculture.2006.06.010
Muhd-Farouk H., Nurul H. A., Abol-Munafi A. B., Mardhiyyah M. P., Hasyima-Ismail N., Manan H., et al. (2019). Development of ovarian maturations in orange mud crab, Scylla olivacea (Herbs) through induction of eyestalk ablation and methyl farnesoate. Arab. J. Basic Appl. Sci. 26 (1), 171–181. doi: 10.1080/25765299.2019.1588197
Nakeim J., Kornthong N., Saetan J., Duangprom S., Sobhon P., Sretarugsa P. (2020). Presence of serotonin and its receptor in the central nervous system and ovary and molecular cloning of the novel crab serotonin receptor of the blue swimming crab, Portunus pelagicus. Acta Histochem. 122 (1), 151457. doi: 10.1016/j.acthis.2019.151457
Ngernsoungnern P., Ngernsoungnern A., Sobhon P., Sretarugsa P. (2009). Gonadotropin-releasing hormone (GnRH) and a GnRH analog induce ovarian maturation in the giant freshwater prawn, Macrobrachium rosenbergii. Invertebr. Reprod. Dev. 53 (3), 125–135. doi: 10.1080/07924259.2009.9652298
Ngernsoungnern A., Ngernsoungnern P., Weerachatyanukul W., Chavadej J., Sobhon P., Sretarugsa P. (2008). The existence of gonadotropin-releasing hormone (GnRH) immunoreactivity in the ovary and the effects of GnRHs on the ovarian maturation in the black tiger shrimp Penaeus monodon. Aquaculture 279 (1), 197–203. doi: 10.1016/j.aquaculture.2008.04.018
Pertea G., Huang X., Liang F., Antonescu V., Sultana R., Karamycheva S., et al. (2003). TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics 19 (5), 651–652. doi: 10.1093/bioinformatics/btg034
Poljaroen J., Tinikul Y., Phoungpetchara I., Kankoun W., Suwansa-Ard S., Siangcham T., et al. (2011). The effects of biogenic amines, gonadotropin-releasing hormones and corazonin on spermatogenesis in sexually mature small giant freshwater prawns, Macrobrachium rosenbergii (De Ma). Aquaculture 321 (1), 121–129. doi: 10.1016/j.aquaculture.2011.08.022
Rocco D. A., Paluzzi J.-P. V. (2016). Functional role of the hererodimeric glycoprotein hormone, GPA2/GPB5, and its receptor, LGR1: An invertebrate perspective. Gen. Comp. Endocrinol. 234, 20–27. doi: 10.1016/j.ygcen.2015.12.011
Rotllant G., Nguyen T. V., Aizen J., Suwansa-Ard S., Ventura T. (2018). Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia 825 (1), 91–119. doi: 10.1007/s10750-017-3497-4
Saetan J., Kruangkum T., Phanthong P., Tipbunjong C., Udomuksorn W., Sobhon P., et al. (2018). Molecular cloning and distribution of oxytocin/vasopressin-like mRNA in the blue swimming crab, Portunus pelagicus, and its inhibitory effect on ovarian steroid release. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol., 218, 46–55. doi: 10.1016/j.cbpa.2018.01.012
Saetan J., Senarai T., Tamtin M., Weerachatyanukul W., Chavadej J., Hanna P. J., et al. (2013). Histological organization of the central nervous system and distribution of a gonadotropin-releasing hormone-like peptide in the blue crab, Portunus pelagicus. Cell Tissue Res. 353 (3), 493–510. doi: 10.1007/s00441-013-1650-6
Saetan J., Senarai T., Thongbuakaew T., Kruangkum T., Chansela P., Khornchatri K., et al. (2017). The presence of abalone egg-laying hormone-like peptide in the central nervous system and ovary of the blue swimming crab, Portunus pelagicus, and its effect on ovarian maturation. Aquaculture 479, 412–422. doi: 10.1016/j.aquaculture.2017.06.007
Siangcham T., Tinikul Y., Poljaroen J., Sroyraya M., Changklungmoa N., Phoungpetchara I., et al. (2013). The effects of serotonin, dopamine, gonadotropin-releasing hormones, and corazonin, on the androgenic gland of the giant freshwater prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 193, 10–18. doi: 10.1016/j.ygcen.2013.06.028
Soonthornsumrith B., Saetan J., Kruangkum T., Thongbuakaew T., Senarai T., Palasoon R., et al. (2018). Three-dimensional organization of the brain and distribution of serotonin in the brain and ovary, and its effects on ovarian steroidogenesis in the giant freshwater prawn, Macrobrachium rosenbergii. Invert. Neurosci. 18 (2), 5. doi: 10.1007/s10158-018-0209-3
Sultana Z., Kang B. J., Nohara S., Kinoshita S., Wilder M. (2023). Reproductive performance under laboratory conditions of non-ablated and unilaterally-ablated Pacific white shrimp Litopenaeus vannamei subjected to different feeding regimes. Nippon Suisan Gakkai Shi 89 (2), 127–136. doi: 10.2331/suisan.22-00039
Sumpownon C., Engsusophon A., Siangcham T., Sugiyama E., Soonklang N., Meeratana P., et al. (2015). Variation of prostaglandin E2 concentrations in ovaries and its effects on ovarian maturation and oocyte proliferation in the giant fresh water prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 223, 129–138. doi: 10.1016/j.ygcen.2015.04.019
Suwansa-Ard S., Zhao M., Thongbuakaew T., Chansela P., Ventura T., Cummins S. F., et al. (2016). Gonadotropin-releasing hormone and adipokinetic hormone/corazonin-related peptide in the female prawn. Gen. Comp. Endocrinol. 236, 70–82. doi: 10.1016/j.ygcen.2016.07.008
Thongbuakaew T., Sumpownon C., Engsusophon A., Kornthong N., Chotwiwatthanakun C., Meeratana P., et al. (2021). Characterization of prostanoid pathway and the control of its activity by the eyestalk optic ganglion in the female giant freshwater prawn, Macrobrachium rosenbergii. Heliyon 7 (1), e05898. doi: 10.1016/j.heliyon.2021.e05898
Thongrod S., Wanichanon C., Sobhon P. (2019). Distribution of neuropeptide F in the ventral nerve cord and its possible role on testicular development and germ cell proliferation in the giant freshwater prawn, Macrobrachium rosenbergii. Cell Tissue Res. 376 (3), 471–484. doi: 10.1007/s00441-019-02999-8
Tinikul Y., Engsusophon A., Kruangkum T., Thongrod S., Tinikul R., Sobhon P. (2017). Neuropeptide F stimulates ovarian development and spawning in the female giant freshwater prawn, Macrobrachium rosenbergii, and its expression in the ovary during ovarian maturation cycle. Aquaculture 469, 128–136. doi: 10.1016/j.aquaculture.2016.11.026
Tinikul Y., Poljaroen J., Nuurai P., Anuracpreeda P., Chotwiwatthanakun C., Phoungpetchara I., et al. (2011). Existence and distribution of gonadotropin-releasing hormone-like peptides in the central nervous system and ovary of the Pacific white shrimp, Litopenaeus vannamei. Cell Tissue Res. 343 (3), 579–593. doi: 10.1007/s00441-010-1112-3
Tinikul Y., Poljaroen J., Tinikul R., Anuracpreeda P., Chotwiwatthanakun C., Senin N., et al. (2014). Effects of gonadotropin-releasing hormones and dopamine on ovarian maturation in the Pacific white shrimp, Litopenaeus vannamei, and their presence in the ovary during ovarian development. Aquaculture 420–421, 79–88. doi: 10.1016/j.aquaculture.2013.10.036
Tinikul Y., Soonthornsumrith B., Phoungpetchara I., Meeratana P., Poljaroen J., Duangsuwan P., et al. (2009). Effects of serotonin, dopamine, octopamine, and spiperone on ovarian maturation and embryonic development in the giant freshwater prawn, Macrobrachium rosenbergii (De Man 1879). Crustaceana 82 (2), 1007–1022. doi: 10.1163/156854009X448844
Tomy S., Saikrithi P., James N., Balasubramanian C. P., Panigrahi A., Otta S. K., et al. (2016). Serotonin induced changes in the expression of ovarian gene network in the Indian white shrimp, Penaeus indicus. Aquaculture 452, 239–246. doi: 10.1016/j.aquaculture.2015.11.003
Uawisetwathana U., Leelatanawit R., Klanchui A., Prommoon J., Klinbunga S., Karoonuthaisiri N. (2011). Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp. PloS One 6 (9), e24427. doi: 10.1371/journal.pone.0024427
Wahl M., Levy T., Manor R., Aflalo E. D., Sagi A., Aizen J. (2022). Genes encoding the glycoprotein hormone GPA2/GPB5 and the receptor LGR1 in a female prawn. Front. Endocrinol. (Lausanne) 13. doi: 10.3389/fendo.2022.823818
Wongprasert K., Asuvapongpatana S., Poltana P., Tiensuwan M., Withyachumnarnkul B. (2006). Serotonin stimulates ovarian maturation and spawning in the black tiger shrimp Penaeus monodon. Aquaculture 261 (4), 1447–1454. doi: 10.1016/j.aquaculture.2006.08.044
Keywords: dsRNA-VIH, GnRH, 5-HT, ovarian maturation, S. olivacea, sustainable development goals (SDGs)
Citation: Saetan J, Duangprom S, Songkoomkrong S, Amonruttanapun P, Phanaksri T, Surinlert P, Samhuay C, Tamtin M, Suwansa-Ard S, Cummins SF, Sobhon P and Kornthong N (2023) Potent ovarian development as being stimulated by cocktail hormone in the female Scylla olivacea. Front. Mar. Sci. 10:1286789. doi: 10.3389/fmars.2023.1286789
Received: 31 August 2023; Accepted: 20 November 2023;
Published: 07 December 2023.
Edited by:
MD Saydur Rahman, The University of Texas Rio Grande Valley, United StatesReviewed by:
Joseph Aizen, Ruppin Academic Center, IsraelCopyright © 2023 Saetan, Duangprom, Songkoomkrong, Amonruttanapun, Phanaksri, Surinlert, Samhuay, Tamtin, Suwansa-Ard, Cummins, Sobhon and Kornthong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Napamanee Kornthong, bmFwYW1hbmVlbmF0dEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.