- Key Laboratory of East China Sea Fishery Resources Exploitation, Ministry of Agriculture and Rural Affairs, East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai, China
Dietary intake is an essential source of energy and nutrients, and plays an irreplaceable role in the breeding of S. paramamosain seedlings. In this study, live Artemia nauplii (LA), compound feeds (CF), frozen copepods (FC) and frozen adult Artemia (FA) were utilized as feed during the megalopa stage. To determine the impact that diet has on the metabolism of crablets that underwent metamorphosis from the megalopa stage, analyses of both metabolomics and fatty acid content were conducted. In the LC-MS-based metabolomics analysis, a total of 104, 205 and 83 significantly different metabolites (SDMs) were identified after being fed with FC, FA, and CF, respectively, as compared to the LA group. Furthermore, significant differences in KEGG compounds among the three comparisons exhibited similarity and were mainly associated with categories such as “Lipids”, “Hormones and transmitters”, and “Peptides”. The fatty acid content analysis indicated that the ΣMUFA was significantly higher in the LA and CF groups compared to the other two groups. In contrast, the highest level of ΣPUFA was found in the LA group. In addition, the CF group showed significantly higher expression levels of the fatty acid synthesis genes, FAS and ACC. Conversely, the expression level of the fatty acid decomposition-related gene CPT1 was the highest in the LA group. In comparison with the FA group, the expression level of FABP3 was significantly decreased in the LA and CF groups. In summary, there were significant differences observed in the metabolic profiles of crablets that metamorphosed from the megalopa under different diets. Our experimental results suggested that LA is more advantageous in the cultivation of the S. paramamosain megalopa compared to the other three diets. While it remains a diet that cannot be entirely substituted at present, LA has the potential to improve the culture performance of the S. paramamosain megalopa. The current study could provide valuable data into the development of artificial diets necessary for the future of the mud crab seedling breeding industry.
1 Introduction
The mud crab, Scylla paramamosain (Crustacea, Decapoda, Portunidae, Scylla), an important commercial crustacean, is widely distributed throughout the Indo-Western Pacific and South-East Asia regions (Le Vay et al., 2007; Ma et al., 2011). Due to its rapid growth, strong adaptability, appealing taste, and rich nutrition (Le Vay et al., 2007; Meng et al., 2017; Zheng et al., 2020), this species has become a vital to crab aquaculture over the past decade. In China, the aquaculture and fishing yield of S. paramamosain exceeded 220 thousand tons in 2022 (Bureau of Fisheries and Fishery Management, 2023). However, the current increasing production of S. paramamosain still remains insufficient to meet the growing consumer demand (Shi et al., 2019; Zhang et al., 2022). In order to meet the demand, the development of intensive aquaculture is a potential approach (Zhang et al., 2022). Supplying a large quantity of high-quality S. paramamosain crablets (juvenile I crabs) can aid in cost reduction for breeding while also mitigating the rapid escalation in price. S. paramamosain aquaculture is based on larval breeding (Li et al., 2021). Unfortunately, consistent success in S. paramamosain larval rearing remains a challenge (Syafaat et al., 2020), owing to variations in diet composition, salinity level, water temperature, and other unknown factors. Consequently, it is necessary to investigate the effects of diet and aquatic environment on the larval development mechanism so as to build up the efficiency of S. paramamosain larval aquaculture.
Like most crabs (Tang et al., 2020), S. paramamosain has four distinct life stages: zoea, megalopa, juvenile and adult. Each developmental period is accompanied by molting. Molting, an essential and recurring process in the development of all crustaceans, involves the periodic shedding of the old rigid carapace, which is replaced by a newly formed softened exoskeleton (Hamasaki, 2003; Xu et al., 2017). Compared to the five early molts from the zoea I to megalopa stages, the megalopa goes through a longer period to metamorphose into the juvenile. After the metamorphosis, the juvenile is already morphologically crab-like, and behaviorally adapted to benthic life. The transition from megalopa to juvenile through molting represents one of the pivotal stages in artificial larval breeding, significantly influencing the success of S. paramamosain culture.
The provision of daily diets is crucial element for seedling breeding. Due to the correlation between developmental stages and varying feed size requirements, live Artemia nauplii (LA) and compound feeds (CF) are currently the most common food choices for post-seedling breeding in S. paramamosain (Holme et al., 2009). Numerous studies have been conducted to optimize dietary conditions for post-larval culture in crustaceans (Ut et al., 2007; Rasdi and Qin, 2016; Zhao et al., 2016; Tang et al., 2020). For instance, the use of frozen adult Artemia (FA) and frozen copepods (FC) has been explored due to their cost-effectiveness and ready availability (Tang et al., 2020). However, the potential effects of utilizing FA, FC, or even CF as diet compositions during the stages from megalopa to juvenile of S. paramamosain remain largely unknown.
Metabolism is a series of complexly and orderly chemical reactions that enable living organisms to grow, develop, reproduce, and respond to changes in the external environment (Johnson et al., 2016; Li et al., 2021). Due to its high throughput and sensitivity, metabolomics is an excellent approach for elucidating small-molecule metabolite profiles and understanding the complexity of metabolic networks (Johnson et al., 2016). To date, there has been an increasing number of reports on the application of metabolomics in crabs (Ma et al., 2017; Tang et al., 2020; Yu et al., 2020; Zhang et al., 2021; Fu et al., 2022; Zhu et al., 2022). For example, metabolomics revealed that a water salinity of 4‰ is not suitable for S. paramamosain overwintering, as it led to an accelerated consumption of amino acids, carbohydrate and lipid metabolisms (Zhou et al., 2021). Notably, the study of metabolomics often involves changes in metabolic patterns and pathways associated with energy production and consumption (Zhang et al., 2021; Fu et al., 2022). Hence, elucidating these changes in various metabolites will provide a better understanding of the effects of daily diets on the seedling breeding of S. paramamosain.
Data on the dietary requirements for the transition from megalopa to the juvenile stages of S. paramamosain is extremely limited. In this experiment, metabolomics technology was used to analyze the metabolite differences among four diets, namely, LA, CF, FC and FA. The fatty acid content was quantified, and the expression analysis of marker genes associated with fatty acid metabolism was performed. The aim of the current study was to evaluate the effects of different commonly used feed on megalopa cultivation, and to provide valuable data for determining the nutrient ratio of artificial food required for the seedling breeding industry of S. paramamosain in the future.
2 Materials and methods
2.1 Experiment animals and sample collection
The full-sibling megalopa of S. paramamosain was sourced from Ninghai Research Center of Chinese Academy of Fishery Sciences, Zhejiang Province. LA were hatched from dormant eggs, which were aerated and incubated in 29 ± 1 °C water with a salinity of 30‰. FA, FC and CF, were purchased from Ningbo Yifeng Aquaculture Co., LTD. The particle size of CF was 0.30-0.45 mm according to the potential palatability of S. paramamosain megalopa (Qiao et al., 2023).
The dietary treatment experiment was carried out in 400 L cylindrical polyethylene tanks. The water volume was consistently maintained at 360 L throughout the experiment. According to the experimental design, four treated groups were established, consisting of the LA, FA, FC, and CF groups, respectively. Each group was tested in triplicate. A total of 1000 ± 30 newly metamorphosed megalopa were stocked in each experimental tank. All tanks were exposed to natural sunlight, and the water temperature was maintained at 28 ± 1°C. During the experiment, the megalopa were fed with each of the four tested diets twice daily in equal proportions. For the metamorphosis from the megalopa to juvenile, it took eight days for the LA group, ten days for the FA and FC groups, and 11 days for the CF group. Compared to the other groups, the metamorphosis survival rate was the highest (47.1%) in the LA group whilst it was the lowest (25.4%) in the CF group. The metamorphosis survival rate was 39.6% and 35.4% in the FA and FC group, respectively (Qiao et al., 2023). Following metamorphosis to the juvenile stage, approximately 4 g wet weight (~500 individuals) of crablets (juvenile I crabs) were sampled and stored in liquid nitrogen for subsequent analyses, including metabolomics, fatty acid content and gene expression.
2.2 Metabolite extraction and LC-MS analysis
Samples of 30 crablets were pooled in each group and used for untargeted LC-MS-based metabolomics experiment. Three replicates were used for each group. After homogenization, 100 μL samples were accurately measured, and extracted with 400 μL of extraction liquid. The solution was homogenized and subjected to ultrasonic treatment, followed by incubation at -20°C for 30 min to precipitate proteins. After centrifugation at 13000 g for 15 min, the supernatant was carefully transferred for the subsequent LC-MS analysis. Using a UHPLC-Q Exactive HF platform (Waters, USA), 2 μL of the samples were injected into C18 chromatographic column for chromatography separation. The column temperature was maintained at 30°C and a flow rate of 0.3 mL/min was used. The mobile phase consisted of phase A (0.1% formic acid in water) and phase B (pure acetonitrile). Mass spectrometric data were collected using UHPLC-Q Exactive HF Mass Spectrometer (Thermo, USA) equipped with an electrospray ionization (ESI) source operating in either positive or negative ion mode. Briefly, the positive and negative ion spray voltages were set at 3.5 kV and 2.8 kV, respectively. The aus gas heater temperature was 40°C. The resolution ratio of MS1 and MS2 was 70000 and 17500, respectively.
Meanwhile, a mixture of equal volumes from all samples was prepared to serve as quality control (QC) samples for system conditioning. The QC samples were subjected to the same analytical procedure as the test samples, with one QC sample added after every three samples to monitor the analysis’s stability.
2.3 Metabolomics data analysis
Raw metabolomics data were filtered and processed by Progenesis QI (Waters Corporation, Milford, USA). Metabolites were then identified and annotated with HMDB (http://www.hmdb.ca/), KEGG (http://www.genome.jp/kegg/) and Metlin (http://metlin.scripps.edu) databases. In general, metabolic features that were detected in at least 80% of any sample were retained for the subsequent analysis. After normalization, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed to ensure the repeatability and reliability of data within the groups. In addition, model validity and potential over-fitting of the PLS-DA model were checked by performing 200 permutation tests. Significant difference (P < 0.05) and potential biomarkers between groups were defined based on model variable importance in projection (VIP) > 1.5, and fold change (FC) ≥ 1. Subsequently, KEGG database was utilized for metabolic pathway enrichment analysis. The identification of statistically significant enriched pathways was performed with Fisher’s exact test using the scipy.stats module (https://docs.scipy.org/doc/scipy/).
2.4 Determination of fatty acid content
The determination of the fatty acid content in the crablets from the four diet groups was carried out using an Agilent 7890 GC gas chromatograph system (Agilent, USA), equipped with a DB-5MS capillary column (J&W Scientific, USA). Briefly, the total lipid content was methylated using a boron trichloride-methanol solution. Subsequently, all samples were treated on a rotary evaporator to achieve the test concentration for determination of fatty acid content. In general, 1 μL aliquot of derivatized sample was injected to run in a splitless mode. The front inlet purge flow was set at 3 mL/min, and the constant gas flow rate was maintained at 20 mL/min. The energy used in the electron impact mode was -70 eV.
2.5 RNA extraction and cDNA preparation
Total RNA from the juveniles was isolated using RNAiso Plus reagent (TaKaRa, Japan) according to the manufacturer’s instruction. The integrity of total RNA was verified by observing distinct bands corresponding to 18S and 28S ribosomal RNA on a 2% agarose gel. RNA concentration and purity were determined using a NanoDrop 2000c Spectrophotometer (Thermo, USA). Total RNA was treated with RQ1 RNase-free DNase (Promega, USA) to thoroughly remove the residual DNA. Then, cDNA was synthesized from 0.75 μg total RNA using a PrimeScript RT-PCR Kit (TaKaRa) according to the manufacturer’s protocol.
2.6 Quantitative real-time PCR and statistical analysis
To learn more about the effects that the diet has on the fatty acid metabolism in S. paramamosain, we selected four important marker genes related to fatty acid metabolism: fatty acid synthetase gene (FAS), acetyl-CoA carboxylase gene (ACC), carnitine palmitoyl transferase I (CPT1) and fatty acid binding protein 3 gene (FABP3). The expression profiles of these genes were determined through qPCR analysis. The gene-specific primers used for qPCR were selected according to previously published articles (Wang et al., 2021). The qPCR was performed on the ABI 7500 system (Applied Biosystems, USA) using a 20 μL reaction volume. The qPCR program consisted of an initial denaturation step at 95°C for 5 min, followed by 40 cycles of 95 °C for 20 s, 60 °C for 15 s, and 72°C for 20 s. The expression levels were calculated using the 2−ΔΔCT method. The 18S rRNA gene was used as an endogenous control. A negative control without cDNA was included. The triplicate fluorescence intensities of each sample, as measured by the crossing-point (Ct) values, were compared and converted to fold differences using the relative quantification method. Statistical analysis was performed using SPSS 23.0, employing one-way ANOVA followed by Duncan’s test to identify significant differences. The qPCR data were presented as the mean ± standard error (Johnson et al., 2016). In addition, P < 0.05 was considered statistically significant and represented by different letters.
3 Results
3.1 Metabolic profiles of S. paramamosain juvenile I crab
LC-MS-based metabolomics experiments were carried out in positive/negative-ion modes to maximize the coverage of metabolites. The results of PCA and PLS-DA showed that QC samples were tightly clustered, indicating that the group separations were associated with biological variations (Figures 1A, B). As observed, the PCA of the metabolic profiles of the juveniles displayed distinct separation among the four groups, while no outstanding separation was observed among the biological samples within each group (Figure 1A). In the PCA model, the R2X value which represents the explained variance, was 0.792. To further elucidate the differences in metabolic profiles resulting from different treatments, PLS-DA was employed. Similar to the PCA model, the PLS-DA scatter plots clearly distinguished the four treated groups, with the cumulative interpretation rate values, R2X and R2Y, of 0.833 and 0.991, respectively. After 200 permutations, the intercept values of R2 and Q2 were 0.4026 and -0.586, respectively, indicating that the PLS-DA model was stable and reliable without overfitting (Figure 1C). As shown in Figure 1D, a stronger correlation was observed among biological samples in each group, indicating higher similarity in metabolic composition and abundance within these samples. However, the correlation was relatively weaker between groups, particularly between the CF group and the other three groups. Evidently, the samples of LA group and FA group formed a clade first, then clustered with the FC group, and finally with the CF group. Therefore, the analytical system was considered stable and reliable, and discernible differences in metabolic profiles were evident among the four groups.
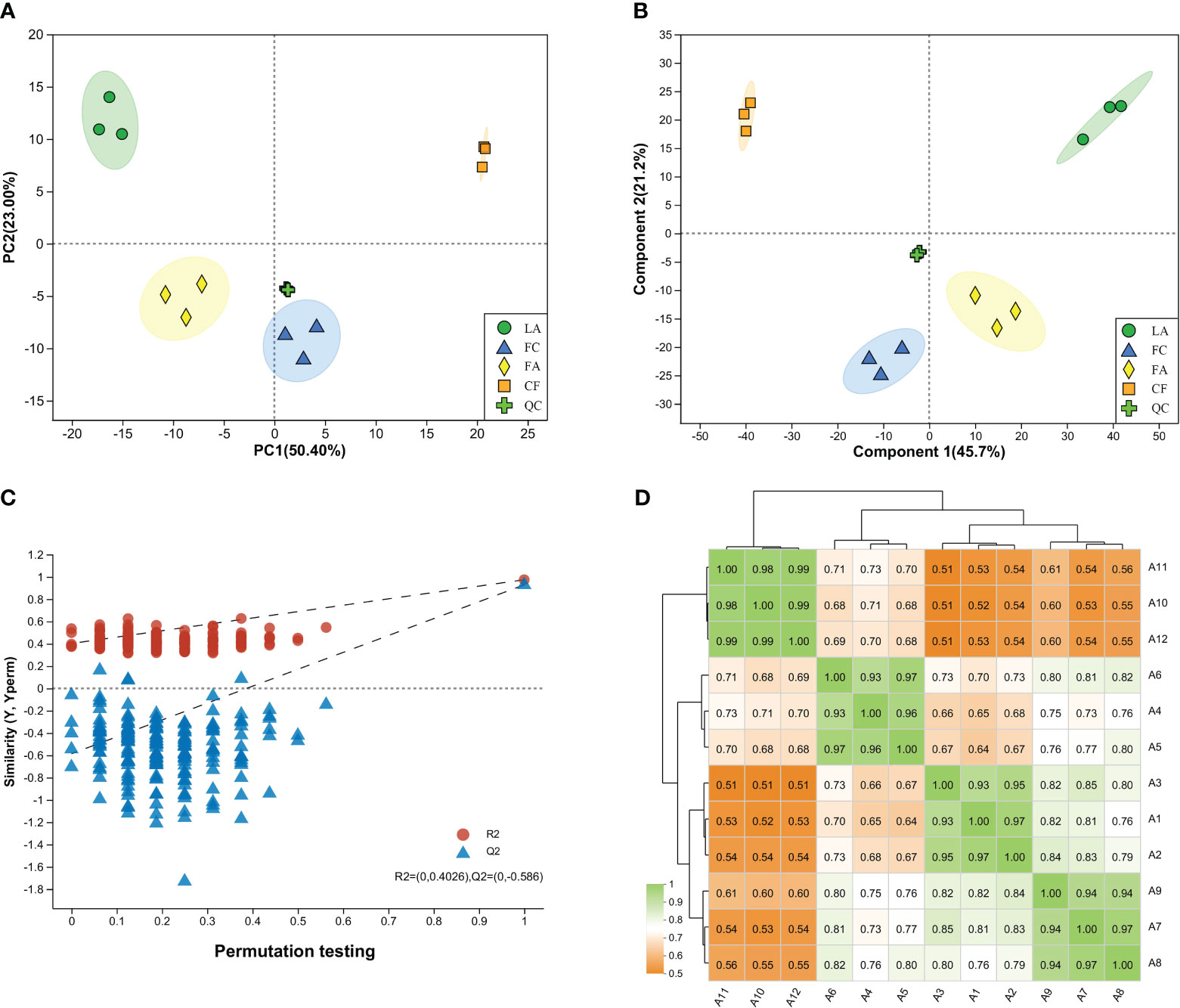
Figure 1 Characterization of LC-MS based metabolite data. (A) PCA analysis of metabolite profiles of all the samples. PC1, principal component 1; PC2, principal component 2. The ellipse in the score plots constituted Hotelling’s T-squared ellipse with a 95% confidence interval; (B) PLS-DA analysis of metabolite profiles of all the samples. LA, live Artemia nauplii group; FC, frozen copepods group; FA, frozen adult Artemia group; CF, compound feed group; QC, quality control; (C) Permutation tests to confirm the quality of PLS-DA model. The intercept of the regression line for R2 and Q2 were 0.4026 and -0.586, respectively; (D) Heat map correlation matrix among different groups of three biological samples. Correlation was calculated upon the Pearson correlation algorithm. Darker green represented stronger correlation between samples. A1-A3, A4-A6, A7-A9, and A10-A12, were samples belonging to the live Artemia nauplii group, frozen copepods group, frozen adult Artemia group and compound feed group, respectively.
3.2 Identification of Metabolites with significant differences
A total of 1294 metabolites were identified in the positive and negative-ion modes of the four treated groups. The significantly different metabolites (SDMs) were compared between the LA group and the other three groups (FA, FC, and CF) (Table 1). Among them, 104 different metabolites were identified in the positive and negative ion mode between the FC group and the LA group. Among these metabolites, 74 were significantly increased and 30 were significantly decreased. In the comparison between the FA group and the LA group, a total of 205 significantly different metabolites were identified, of which 178 were up-regulated and 27 were down-regulated. In addition, 83 different metabolites were found between the CF group and the LA group, of which 38 showed an increase and 45 showed a decrease (Table 1).
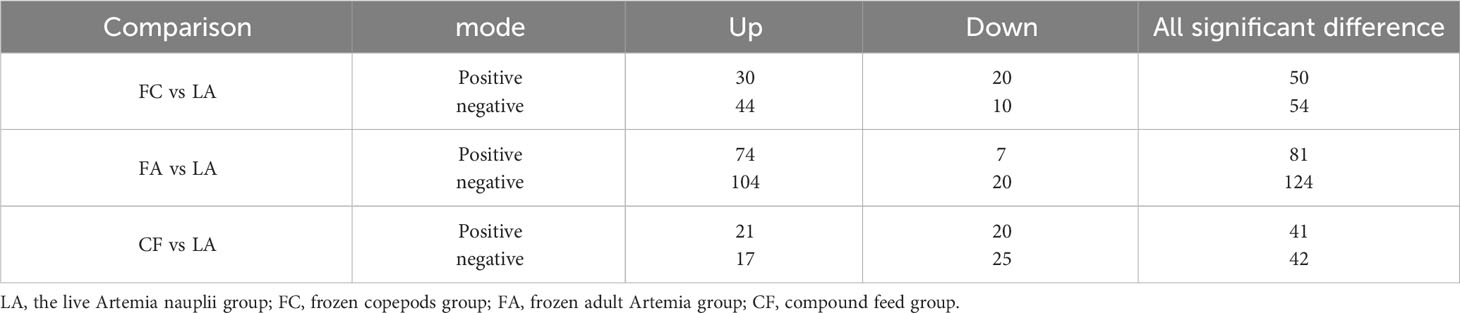
Table 1 Number of significantly different metabolites in different comparisons in S. paramamosain juvenile I crabs.
3.3 KEGG pathway analysis
All annotated metabolites were found to be enriched in six main KEGG pathways, namely, “metabolism”, “organismal systems”, “human diseases”, “environmental information processing”, “genetic information processing”, and “cellular processes” (Figure 2). Notably, the highest enrichment was observed in the “metabolism” pathway, accounting for 43.8% of the total. Subsequently, these metabolites were further classified into 35 KEGG secondary pathway terms. In the “metabolism” pathway, the top term was “lipid metabolism”, followed by “amino acid metabolism” and “metabolism of cofactors and vitamins”. In the “organismal systems” pathway, the top terms were “digestive system”, “nervous system”, and “endocrine system”. The top term in the “human diseases” pathway was “cancer overview”, and in the “environmental information processing” pathway, it was “membrane transport”. While the number of enriched terms in “genetic information processing” and “cellular processes” was less than 15 metabolites.
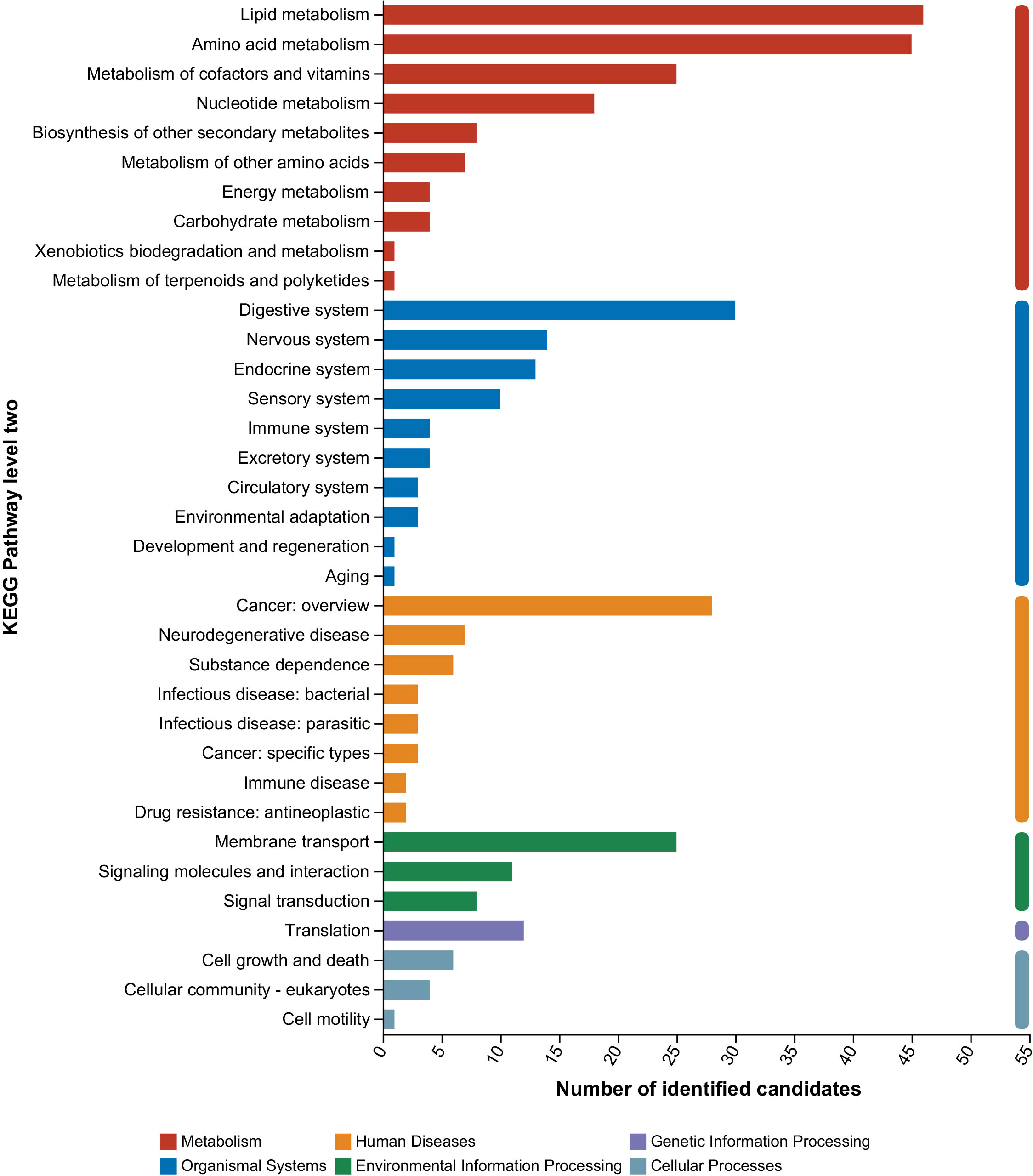
Figure 2 KEGG pathway classification of annotated metabolites. The x-axis and y-axis represented level 2 terms and the number of identified metabolites, respectively.
In addition, pathway enrichment analysis was conducted for the differentially expressed metabolites (DEMs) (Figure 3). The results showed that “cAMP signaling pathway”, “Phenylalanine metabolism”, “Biosynthesis of unsaturated fatty acids”, and “Bile secretion” were the most enriched pathways of DEMs (P < 0.05) between the FA group and LA group (Figure 3A). As shown in Figure 3B, the comparison of DEMs between the FA group and the LA group revealed that “Neuroactive ligand-receptor interaction” and “Biosynthesis of unsaturated fatty acids” were the most enriched pathways. This was followed by “cAMP signaling pathway”, “Retinol metabolism” and “Linoleic acid metabolism”, all of which had a P value of less than 0.01. In contrast, in the comparison between the CF group and LA group, the “Retinol metabolism” pathway was found to be the most enriched (Figure 3C).
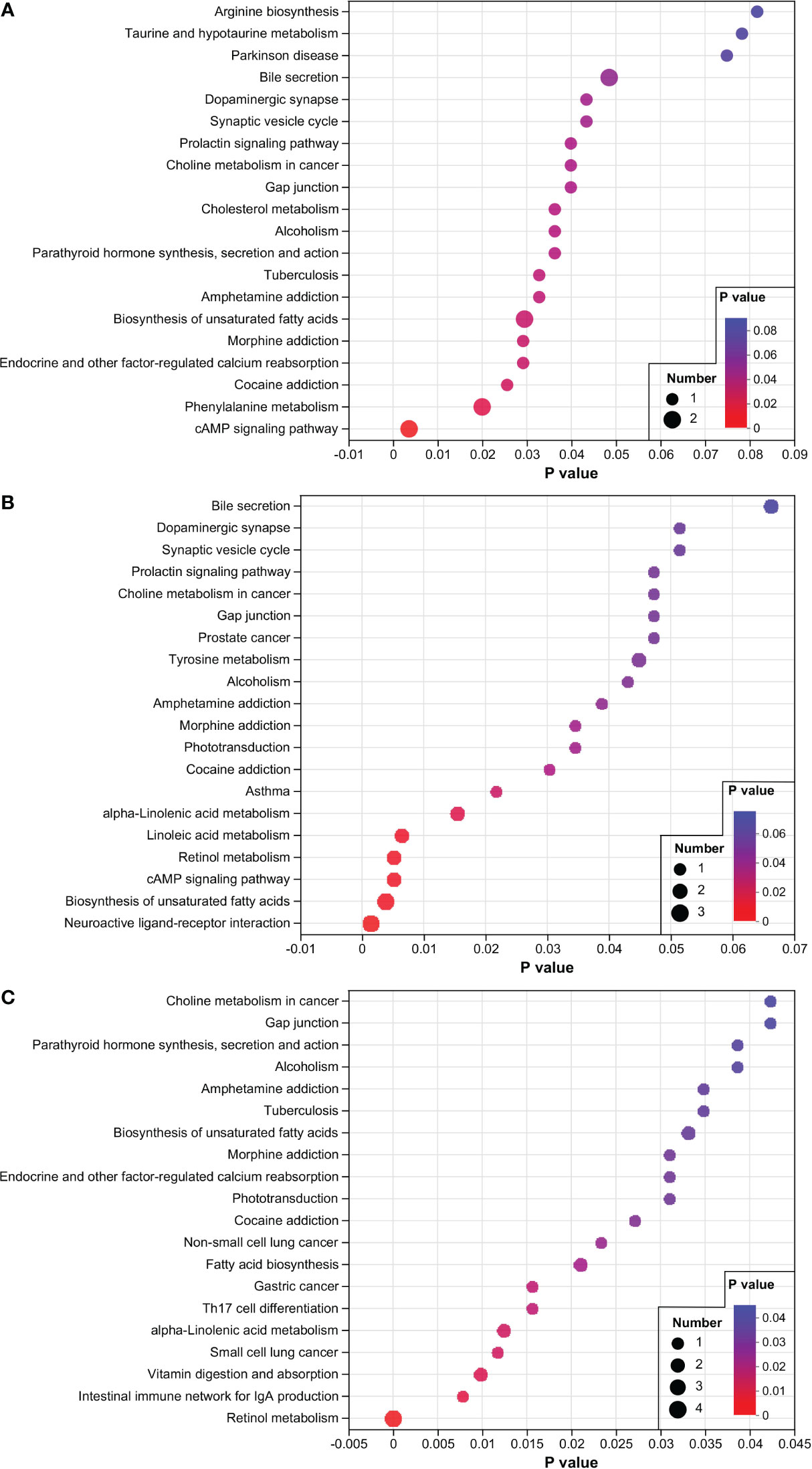
Figure 3 KEGG enrichment of different metabolites. (A) FC vs LA; (B) FA vs LA; (C) CF vs LA. The number of different metabolites enriched in the corresponding pathways were represented by the size of the dots. LA, live Artemia nauplii group; FC, frozen copepods group; FA, frozen adult Artemia group; CF, compound feed group.
In comparison to the LA group, the other three groups showed similar significant differences in the KEGG compounds, with the majority concentrated in the “Lipids” category, followed by “Hormones and transmitters” and “Peptides” (Figure 4). The detailed information regarding the most significantly affected metabolites, involved in the differential expression of KEGG compounds, was shown in Table 2. When compared with the LA group, several metabolites such as L-arginine, hypoxanthine, and 2, 6-dihydroxypurine, showed significant down-regulation, while Lysphosphatidylcholine (LPC) (22:5) and LPC (22:4) showed significant up-regulation after feeding with FC (Table 2). Similarly, in the comparison of the FA group vs LA group, L-arginine was down-regulated, and LPC (22:5) and LPC (22:4) were up-regulated. Moreover, in the comparison of the CF group vs LA group, the expression of 2-(3, 4-dihydroxyphenyl) ethylamine was significantly decreased, and LPC (22:5), LPC (22:4) and LPC (18:0) were significantly increased. Notably, these prominent metabolites are primarily associated with glycerophospholipid metabolism, amino acid synthesis, and purine metabolism (Table 2).
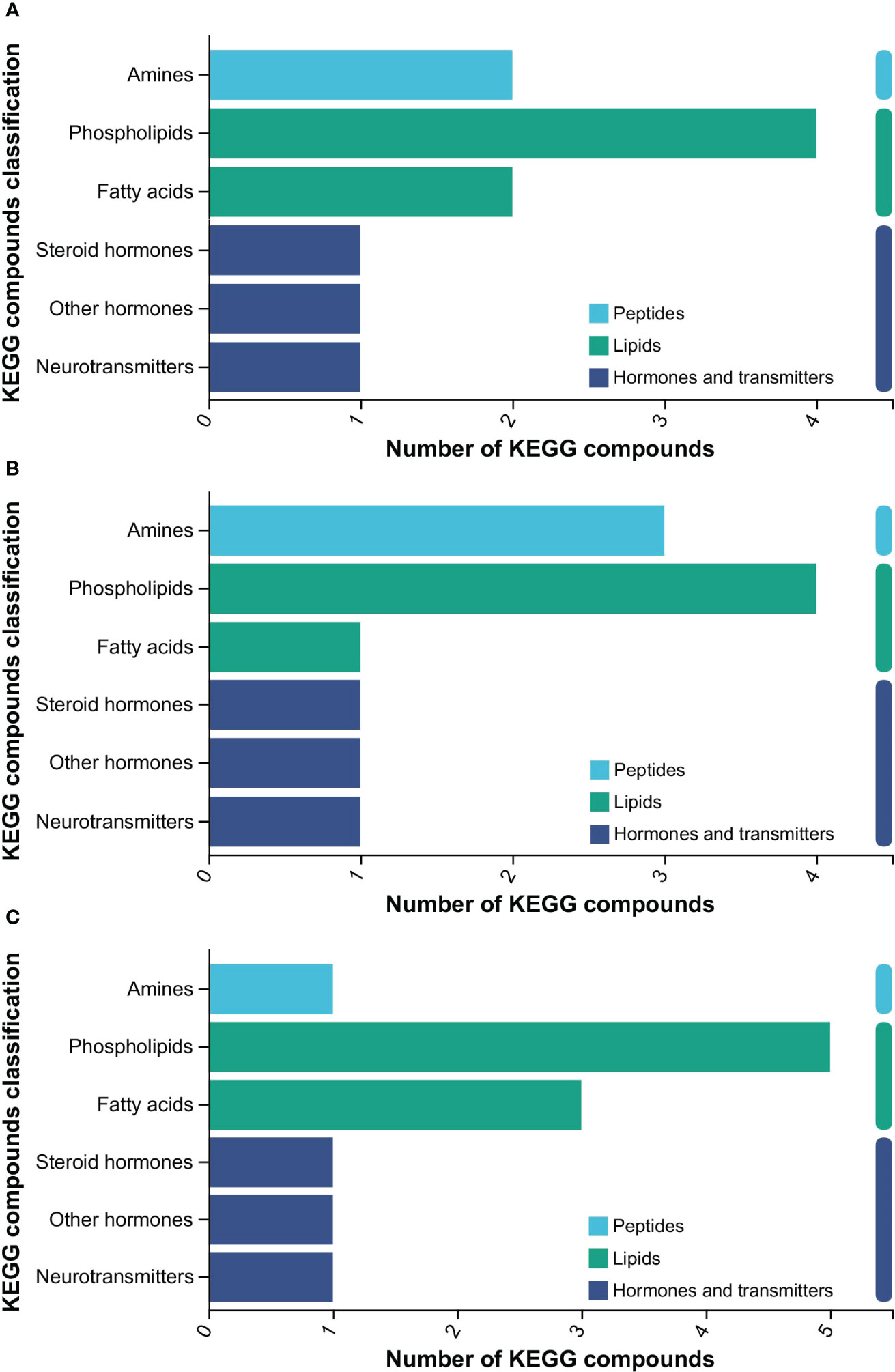
Figure 4 KEGG compounds of significantly different metabolites (P < 0.05). (A) FC vs LA; (B) FA vs LA; (C) CF vs LA. LA, live Artemia nauplii group; FC, frozen copepods group; FA, frozen adult Artemia group; CF, compound feed group.
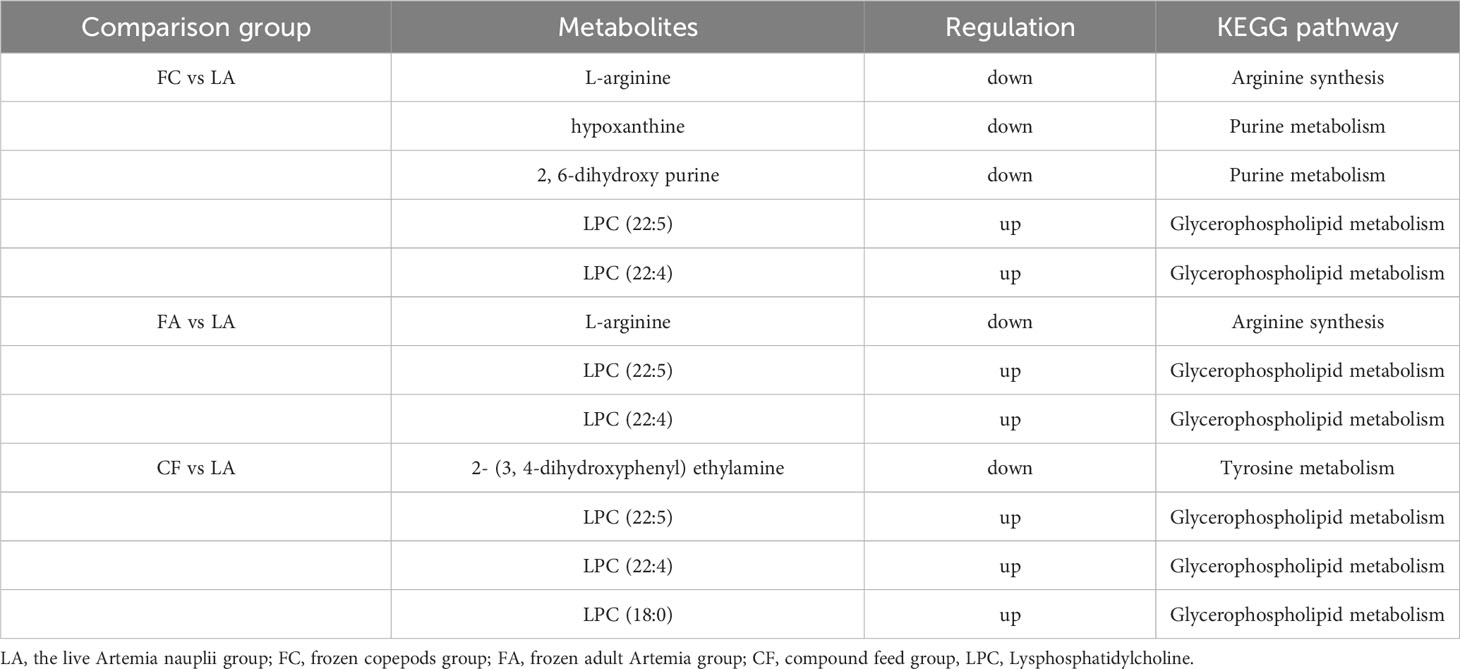
Table 2 Significantly different metabolites in KEGG compounds between comparisons in S. paramamosain juvenile I crabs.
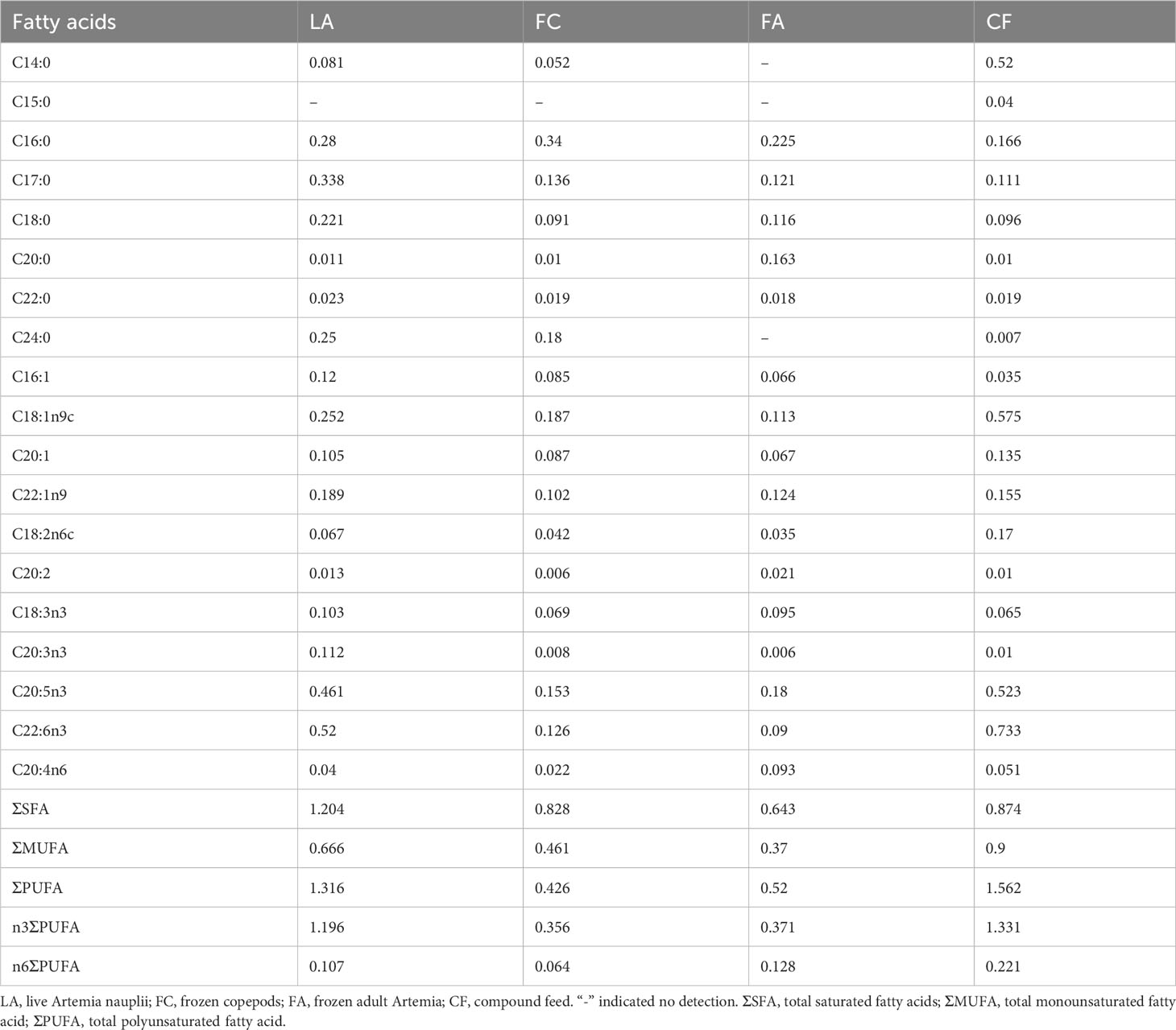
3.4 Fatty acid content of four diets
The LA group had the highest total saturated fatty acid (ΣSFA) content, whilst the FA group had the lowest ΣSFA content (Table 3). Among the four diets, the CF group had the highest levels of total monounsaturated fatty acids (ΣMUFA) and total polyunsaturated fatty acids (ΣPUFA), which were 0.9 g/100 g and 1.562 g/100 g, respectively. This was in contrast with the FA group, which had the lowest amounts of ΣMUFA and ΣPUFA, approximately accounting for one-third of those found in the CF group (Table 3). The CF and LA groups were also rich in DHA (C22:6n3) and EPA (C20:5n3). Among the four diets, the FA group had the highest levels of ARA (C20:4n6), containing more than twice the amount in the LA group (Table 3).
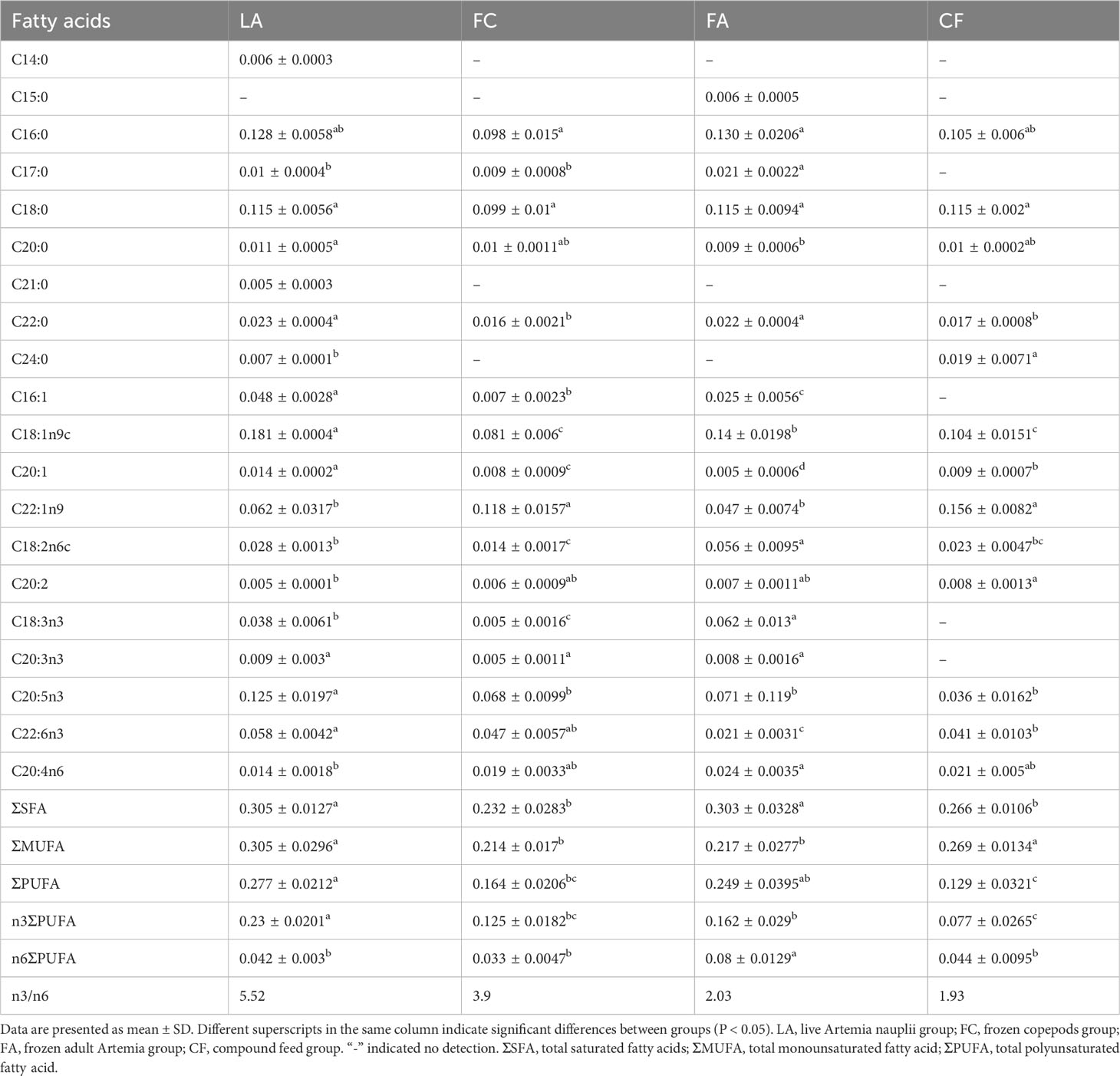
3.5 Fatty acid content of S. paramamosain juvenile I
In terms of ΣSFA, C14:0 and C21:0 fatty acids were only detected in the LA group (Table 4). The ΣSFA content in the LA and FA groups was significantly higher than that in the FC and CF groups. Additionally, the LA group exhibited the highest level of ΣMUFA compared to the other treated groups, including C16:1, C18:1n9c and C20:1 (P < 0.05). Moreover, the ΣMUFA content was significantly higher in the LA and CF groups compared to the other two groups (Table 4). The LA group showed the highest contents of DHA and EPA (P < 0.05), but the lowest content of ARA among the four groups (Table 4). Overall, the ΣPUFA content was the highest in the LA group, and significantly higher than that in the FC and CF groups. Meanwhile, the LA group presented the highest content of n3 ΣPUFA, while the n6 ΣPUFA content in the LA group was significantly lower than that in the FA group.
3.6 Expression profile of fatty acid metabolism-related marker genes
The CF group exhibited the highest expression levels of fatty acid synthesis genes FAS and ACC, significantly surpassing those in the other three groups (Figure 5). In juvenile I crabs, the relative expression level of FAS significantly increased by 1.5 times in those fed with CF compared to those fed with LA. However, no significant difference was observed in the expression levels of FAS and ACC among the LA, FC, and FA groups (P > 0.05). In contrast with the expression level of FAS in the juveniles, the levels of the fatty acid decomposition-related gene CPT1 were the highest in the LA group (P < 0.05), while it was the lowest in the FA group (Figure 5). However, after treatment, the opposite was observed in the expression levels of the fatty acid transport-related genes FABP3. The expression levels of FABP3 were lower in the FC and CF groups compared to the FA group. Additionally, the LA group exhibited the lowest expression level of FABP3 (P < 0.05) (Figure 5).
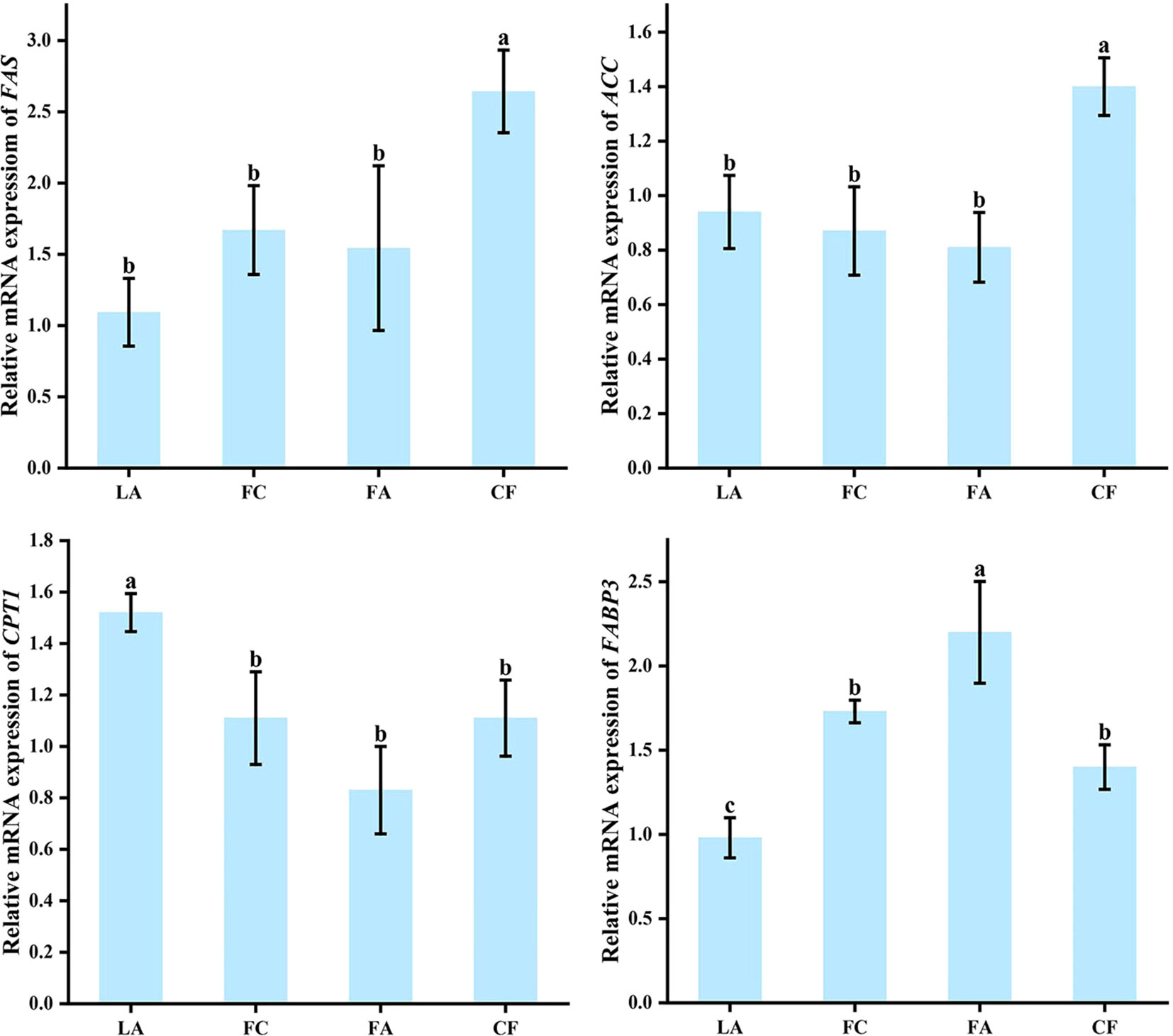
Figure 5 The expression levels of fatty acid metabolism-related marker genes in juvenile I crabs. Different letters denoted statistical significance (P < 0.05).
4 Discussion
After feeding with the four diets, the PCA and PLS-DA analyses of the metabolomics of S. paramamosain juveniles showed clear separation between the groups, implying that daily diets could significantly influence the metabolic products during megalopa metamorphosis. Purine metabolism is an important metabolic pathway responsible for eliminating nitrogen-containing compounds from the organism, and its proper functioning plays an important role in maintaining the balance and stability of the body (Zhang et al., 2009). The significant down-regulation of hypoxanthine and 2, 6-dihydroxypurine may indicate the imbalance of purine metabolism in the juveniles in the FC group, which reduced the ability to remove nitrogen-containing waste from the crab body. Arginine is an essential amino acid in animal cells, and its metabolites play a positive role in nutritional regulation (Wan et al., 2006). The significant down-regulation of L-arginine in the FC and FA groups suggested a decreased ability to synthesize arginine, possibly due to nutritional deficiency of diet intake. A previous study demonstrated significant down-regulation of L-arginine under starvation stress in Xiphophorus hellerii (Zhang et al., 2021), which is consistent with the findings of this study. Nutrition is closely related to immunity, and inadequate nutrition can lead to the down-regulation of the organism’s immune capacity (Humphrey and Klasing, 2004). This may affect the immune response of juvenile crabs in the FC and FA groups, resulting in poor growth performance and reduced survival rate (Qiao et al., 2023). Phospholipids are essential components of animal biofilms and play a critical role in maintaining cellular homeostasis. Among them, lysophosphatidylcholine (LPC) is an intermediate product of lecithin metabolism in organisms (Nishikimi et al., 2022). Notably, the levels of LPC were up-regulated in the FA, FC and CF groups in our study. Previous research showed that elevated levels of LPC may disrupt cellular homeostasis and lead to an increase risk of diseases (Ni, 2021). Therefore, our findings suggested that feeding with LA could potentially reduce the risk of diseases and enhance the disease resistance of juvenile S. paramamosain crabs.
Previous studies have shown that larval nutrition was the major bottleneck for crustacean larval breeding, and that dietary supplementations with specific PUFA could significantly contribute to larval development (Cheng et al., 1998; Wu and Xu, 2006; Zhao et al., 2013). Decapods can synthesize a limited amount of EPA and DHA endogenously, but the majority of these essential fatty acids are acquired from their diet (Chen et al., 2000). EPA and DHA play an important role in the growth, molting, metamorphosis rate and survival of crabs, thus, they are crucial fatty acids used for assessing the nutritional quality of diets (Zhao et al., 2013). During decapods larval culture, the survival rate and molting cycle of larvae were also significantly influenced by the EPA and DHA contents (Wu and Xu, 2006). Therefore, the selection of diets is an important factor in the successful larval rearing of S. paramamosain. In our experiments, significant differences were observed in the fatty acid content in juvenile I crabs among the four treatments. Among the three natural diets, a trend was observed where higher levels of EPA, DHA, and PUFA content correlated with elevated levels of corresponding fatty acids in juvenile I crabs. This observation aligns with prior findings in E. sinensis (Tang et al., 2020). Furthermore, our study showed that the juveniles subjected to the four diet treatments had a higher n3 PUFA content compared to n6, suggesting that the S. paramamosain megalopa might require more dietary n3 PUFA for metamorphosis.
Although the CF group showed notably higher levels of EPA and DHA compared to the other three natural diets, the concentrations of EPA and DHA in the juvenile I crabs fed with CF were significantly lower than those in crabs fed with LA, indicating that a preference of the S. paramamosain megalopa for live food over the CF used in this experiment. During the removal of the residual feed, it was observed that the CF group had the most remaining food, suggesting that the megalopa in this group may not have consumed adequate nutrients, potentially due to reduced diet palatability. This aligned with earlier findings of extended developmental days from the megalopa to juvenile I within the CF group (Qiao et al., 2023).
Furthermore, the levels of DHA and EPA in the FC and FA groups were observed to be lower compared to those in the LA group. It was evident that relying solely on these diets may not fully meet the nutritional demands. However, taking into account factors such as operational convenience and the cost of seedling cultivation (Tang et al., 2020), the partial substitution of LA could be considered a viable strategy during the culture of S. paramamosain megalopa. In terms of the present artificial cultivation of seedlings, live diets remain the preferred choice for the S. paramamosain larvae. CF offers many advantages, including high levels of DHA and EPA, and possesses the potential of replacing live diets, although its palatability needs to be improved. Modifications to the feeding strategy during artificial cultivation of seedlings of the S. paramamosain larvae could also be considered. Currently, there is very limited literature on the feeding patterns of S. paramamosain larvae, which directly influences feeding strategies, including feeding time and feeding frequency, and subsequently impacts feed efficiency and aquaculture environment of aquaculture systems (Jia et al., 2018). The current experiment offers fundamental insights into nutrient balance, while further studies and optimization of feeding strategies are essential to advance the sustainable development of S. paramamosain larvae culture.
Being an important gene associated with fatty acid synthesis in organisms, FAS mainly catalyzes the synthesis of acetyl CoA and malonyl CoA into long-chain fatty acids (Chirala and Wakil, 2004). A previous study showed that the in vivo expression of FAS was affected by the fatty acid content in diet (Xiong, 1999). Furthermore, PUFA was found to suppress FAS expression, with the n3 series exhibiting a more potent inhibitory effect compared to the n6 series (Chen et al., 2005). The reduced expression of S. paramamosain FAS in the LA group could be attributed to the elevated levels of n3 series PUFA, which was consistent with the previous study (Chen et al., 2005). Nevertheless, it was worth noting that the expression level of FAS was significantly up-regulated in the CF group, despite the fact that the n3 series PUFA content was the highest among the four diets (Table 2). We speculated that the low cibation bias towards CF could be an essential underlying reason for this phenomenon. With regards to crustacean larvae, some of the current CF may still be insufficient in terms of feeding convenience and palatability (Li, 2015), resulting in reduced survival rates and limited access to adequate daily diets during subsequent developmental stages for some individuals (Qiao et al., 2023). Throughout the metamorphosis phase from the megalopa to crablets of S. paramamosain, individuals that managed to survive might have up-regulated the expression of genes involved in fatty acid synthesis in response to potential nutrient deficiencies. ACC is an important enzyme in the first step of fat synthesis, and the expression of ACC directly controls the activity of the ACC enzyme and the rate of body fat synthesis (McGarry et al., 1978). As the contents of various fatty acids were elevated in the CF group compared to the biological diets, the expression level of ACC in juveniles from the CF group was significantly higher than in the other three groups. This observation was consistent with previous reports on the function of ACC (Yuan, 2015). The juveniles of S. paramamosain seem to fulfil their fatty acid requirements by up-regulating ACC expression for necessary fat synthesis.
Dietary sources not only affect the expression of genes related to fatty acid synthesis, but also regulate the expression of fatty acid decomposition-related gene CPT1. CPT1 is a mitochondrial enzyme responsible for the formation of acylcarnitine by catalyzing the acyl group of long-chain fat acyl-CoA, which is a rate-limiting step in fatty acid oxidation (McGarry and Brown, 1997). The expression level of CPT1 in the LA group were significantly higher compared to those in the FA and FC groups, suggesting that the up-regulation of the CPT1 expression was related to the increased PUFA content in diets (Li et al., 2021). Noticeably, while the CF group showed the highest PUFA level, the expression of CPT1 was significantly down-regulated compared to the LA group. This could potentially be caused by issues with diet palatability. In this experiment, the juveniles were adverse towards the CF, as well as the FC and the FA, resulting in inadequate intake of PUFA. When PUFA intake is insufficient, juveniles may down-regulate the expression of genes associated with fatty acid decomposition, consequently reducing the corresponding enzyme activity as a response to the nutritional deficiencies caused by the diets.
The fatty acid transport-related genes FABPs play an important role in the cellular uptake of fatty acids and their intracellular transport (Fu et al., 2000; Cheng et al., 2017; Sui and Zhang, 2019). Previous studies have shown an increase in the FABP3 gene expression in the liver of Atlantic salmon during periods of starvation, indicating that there were increased levels of fatty acids entering the mitochondria for oxidation within the liver when subjected to food scarcity (Jordal et al., 2006). This process is crucial for sustaining the energy provision of liver cells (Ai et al., 2016). As a member of FABPs, the S. paramamosain FABP3 has also been sequenced in our previous transcriptome (unpublished). The results of this study showed a significant up-regulation of FABP3 expression within the three treatment groups as compared to the LA group, implying potential insufficiency in energy intake and the increased reliance on the transport and utilization of fatty acids when subjected to the other three dietary regimens. The lack of adequate energy supply could result in a disruption of lipid metabolism, potentially leading to elevated mortality rates during the metamorphosis transition from megalopa to juvenile stages of S. paramamosain (Qiao et al., 2023). Certainly, further studies need to be carried out to elucidate this process.
5 Conclusion
This is the first study to analyze the effects of four different diets on the metabolism of megalopa metamorphosis of S. paramamosain. Through the comparative metabolomics screening, the SDMs within KEGG compounds were mainly classified into “Arginine synthesis”, “Purine metabolism”, “Glycerophospholipid metabolism” and “Tyrosine metabolism”. This suggested that use of CF, FC and FA feeds could potentially lead to nutritional deficiencies and an elevated risk of disease incidence. The analysis of fatty acid content indicated that the LA group contained the highest levels of DHA and EPA, which play an important role in the crab molting, metamorphosis rate and survival. In addition, dietary sources also affect the expression of selected genes related to fatty acid metabolism. Given a comprehensive understanding of diets influencing the metabolism of S. paramamosain megalopa metamorphosis, more nutrients, such as amino acids, should be measured. Moreover, integration of “omics” analyses could also be performed to investigate the complex metabolic process. In terms of the current nutrient content and palatability, LA is currently the best choice for the S. paramamosain megalopa culture. However, further studies are needed to optimize the current feeding strategy, encompassing factors such as the cost-effectiveness, ease of operation, nutritional completeness, and palatability. These efforts will be integral in advancing the sustainability of the S. paramamosain seedling breeding industry in the future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: MetaboLights, MTBLS8409.
Ethics statement
The animal study was approved by East China Sea Fisheries Research Institute. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KM: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Methodology. ZL: Data curation, Methodology, Writing – original draft. GQ: Methodology, Writing – original draft. LM: Funding acquisition, Writing – review & editing. FZ: Project administration, Writing – review & editing. MZ: Writing – review & editing, Software. CM: Funding acquisition, Writing – review & editing, Conceptualization. WW: Funding acquisition, Resources, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (Project number: 2021Z03), the earmarked fund for China Agriculture Research System (CARS) (Project number: CARS-48), the Special Program on Agricultural Aspect of Science and Technology Commission of Ningbo (Project number: 2019B10010) and the National Infrastructure of Fishery Germplasm Resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai Q., Yan J., Mai K. (2016). Research progresses of lipids and fatty acids transport in fish. Acta Hydrobiol Sin. 4, 859–868. doi: 10.7541/2016.111
Bureau of Fisheries and Fishery Management (2023). China fishery Statistical Yearbook (Beijing, China: China Agriculture Press), 22–58.
Chen L. Q., Ai C. X., Wen X. B., Liu X. L., Jiang H. B. (2005). Studies on vitamin C requirements of the juvenile crab (Eriocheir sinensis). Acta Oceanol Sin. 27, 130–136. doi: 10.3321/j.issn:0253-4193.2005.01.019
Chen L. Q., Jiang H. B., Zhou Z. L., Yang J. X., Zhu J., Zhang D. N. (2000). Effects of ω-3 HUFA on survival rate and body fatty acids composition of Eriocheir sinensis larvae. J. Fish China 24, 448–453.
Cheng Y. X., Yan S. L., Wang W., Shi Z. F., Tan Y. J. (1998). Effects of dietary polyunsaturated fatty acids, phospholipids on the survival and growth of Eriocheir Sinensis from the megalopa to the juvenile. J. Fish China 22, 9–15.
Cheng Y., Zheng S., Li J., Wang H., Li J. (2017). Effects of different fatty acids on the expression of 3 types of FABPs in hepatopancreas of Chinese mitten crab (Eriocheir sinensis). Genomics Appl. Biol. 10, 4100–4107.
Chirala S. S., Wakil S. J. (2004). Structure and function of animal fatty acid synthase. Lipids 39, 1045–1053. doi: 10.1007/s11745-004-1329-9
Fu Y. C., Luo N. L., Lopes-Virella M. F. (2000). Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J. Lipid Res. 41, 2017–2023. doi: 10.1089/107999000750053807
Fu Y., Zhang F., Ma C., Wang W., Liu Z., Chen W., et al. (2022). Comparative metabolomics and lipidomics of four juvenoids application to Scylla paramamosain hepatopancreas: implications of lipid metabolism during ovarian maturation. Front. Endocrinol. 13. doi: 10.3389/fendo.2022.886351
Hamasaki K. (2003). Effects of temperature on the egg incubation period, survival and developmental period of larvae of the mud crab Scylla serrata (Forskal) (Brachyura : Portunidae) reared in the laboratory. Aquaculture 219, 561–572. doi: 10.1016/S0044-8486(02)00662-2
Holme M. H., Zeng C., Southgate P. C. (2009). A review of recent progress toward development of a formulated microbound diet for mud crab, Scylla serrata, larvae and their nutritional requirements. Aquaculture 286, 164–175. doi: 10.1016/j.aquaculture.2008.09.021
Humphrey B. D., Klasing K. C. (2004). Modulation of nutrient metabolism and homeostasis by the immune system. Worlds Poult Sci. J. 60, 90–100. doi: 10.1079/WPS20037
Jia E., Yan M., Lai Q., Luo W., Guangzhen J., Wenbin L., et al. (2018). Feeding rhythm of the Chinese mitten crab (Eriocheir sinensis). J. Fish Sci. China 25, 546–554. doi: 10.3724/SP.J.1118.2018.17444
Johnson C. H., Ivanisevic J., Siuzdak G. (2016). Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459. doi: 10.1038/nrm.2016.25
Jordal A. E. O., Hordvik I., Pelsers M., Bemlohr D. A., Torstensen B. E. (2006). FABP3 and FABP10 in Atlantic salmon (Salmo salar L.)-General effects of dietary fatty acid composition and life cycle variations. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 145, 147–158. doi: 10.1016/j.cbpb.2006.05.007
Le Vay L., Ut V. N., Walton M. (2007). Population ecology of the mud crab Scylla paramamosain (Estampador) in an estuarine mangrove system: a mark-recapture study. Mar. Biol. 151, 1127–1135. doi: 10.1007/s00227-006-0553-4
Li B. N. (2015). Optimize the technological process of Litopenaeus vannamei nursery and research on the problems of temporary culture feeding on live prey (Qingdao, China: Ocean University of China). Master Dissertation.
Li X., Yuan Y., Jin M., Hu X., Zhao M., Luo J., et al. (2021). Growth performance, antioxidant capacity, tissue fatty acid composition and lipid metabolism of juvenile green mud crab Scylla paramamosain in response to different dietary n-3 PUFA lipid sources. Aquacult Rep. 19, 100599. doi: 10.1016/j.aqrep.2021.100599
Li N., Zhou J., He C., Fu Y., Wang C., Liu L., et al. (2021). GC-MS-based metabolomics reveal that light intensity during indoor overwintering affects the metabolism of Scylla paramamosain. Aquacult Res. 52, 1013–1025. doi: 10.1111/are.14956
Ma Q. Q., Chen Q., Shen Z. H., Li D. L., Han T., Qin J. G., et al. (2017). The metabolomics responses of Chinese mitten-hand crab (Eriocheir sinensis) to different dietary oils. Aquaculture 479, 188–199. doi: 10.1016/j.aquaculture.2017.05.032
Ma H. Y., Ma C. Y., Ma L. B. (2011). Population genetic diversity of mud crab (Scylla paramamosain) in Hainan Island of China based on mitochondrial DNA. Biochem. Syst. Ecol. 39, 434–440. doi: 10.1016/j.bse.2011.06.005
McGarry J. D., Brown N. F. (1997). The mitochondrial carnitine palmitoyltransferase system: From concept to molecular analysis. Eur. J. Biochem. 244, 1–14. doi: 10.1111/j.1432-1033.1997.00001.x
McGarry J. D., Takabayashi Y., Foster D. W. (1978). The role of malonyl-CoA in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J. Biol. Chem. 253, 8294–8300. doi: 10.1016/S0021-9258(17)34395-8
Meng F., Gao H., Tang X., Wang A., Yao X., Liu C., et al. (2017). Biochemical composition of pond-cultured vs. wild gravid female mud crab Scylla paramamosain in Hainan, China: Evaluating the nutritional value of cultured mud crab. J. Shellfish Res. 36, 445–452. doi: 10.2983/035.036.0216
Ni D. K. (2021). Effects of snails and formulated feed on growth, metabolomics and gut microbiotas of Chinese mitten crab (Eriocheir sinensis) (Master Dissertation, Ocean University of China).
Nishikimi M., Yagi T., Shoaib M., Takegawa R., Rasul R., Hayashida K., et al. (2022). Phospholipid screening postcardiac arrest detects decreased plasma lysophosphatidylcholine: Supplementation as a new therapeutic approach. Crit. Care Med. 50, E199–E208. doi: 10.1097/CCM.0000000000005180
Qiao G., Ma C., Zhang F., Wang W., Zhao M., Chen W., et al. (2023). Effects of different baits on metamorphosis of megalopa, Scylla paramamosain. Fish Sci.
Rasdi N. W., Qin J. G. (2016). Improvement of copepod nutritional quality as live food for aquaculture: a review. Aquacult Res. 47, 1–20. doi: 10.1111/are.12471
Shi X., Lu J., Wu Q., Waiho K., Aweya J. J., Fazhan H., et al. (2019). Comparative analysis of growth performance between female and male mud crab Scylla paramamosain crablets: Evidences from a four-month successive growth experiment. Aquaculture 505, 351–362. doi: 10.1016/j.aquaculture.2019.02.062
Sui X., Zhang Y. (2019). Research progress of fatty acid binding proteins in fish :a review. Fish Sci. 4, 563–574. doi: 10.16378/j.cnki.1003-1111.2019.04.019
Syafaat M. N., Mohammad S., Azra M. N., Ma H., Abol-Munafi A. B., Ikhwanuddin M. (2020). Effect of water temperature on survival, growth and molting cycle during early crablet instar of mud crab, Scylla paramamosain (Estampado). Thalassas 36, 543–551. doi: 10.1007/s41208-020-00233-9
Tang M., Wu R., Jiang X., Deng D., Xiang C., Cheng Y., et al. (2020). Effects of four natural diets on the culture performance and biochemical composition of megalopa of Eriocheir sinensis during desalination period. Aquacult Res. 51, 2831–2841. doi: 10.1111/are.14622
Ut V. N., Le Vay L., Nghia T. T., Hanh T. T. H. (2007). Development of nursery culture techniques for the mud crab Scylla paramamosain (Estampador). Aquacult Res. 38, 1563–1568. doi: 10.1111/j.1365-2109.2006.01608.x
Wan J. L., Mai K. S., Ai Q. H. (2006). The recent advance on arginine nutritional physiology in fish. J. Fish Sci. China 13, 679–685.
Wang X., Jin M., Cheng X., Hu X., Zhao M., Yuan Y., et al. (2021). Dietary DHA/EPA ratio affects growth, tissue fatty acid profiles and expression of genes involved in lipid metabolism in mud crab Scylla paramamosain supplied with appropriate n-3 LC-PUFA at two lipid levels. Aquaculture 532, 736028. doi: 10.1016/j.aquaculture.2020.736028
Wu G. T., Xu Z. W. (2006). Research progress of hatch feed for aquatic animals. Feed Ind. 27, 28–31.
Xiong W. Z. (1999). Effects of fatty acid in feedstuff on lipid synthetases and their gene expression. Acta Zoonutrimenta Sin. 11, 12–18.
Xu B. P., Tu D. D., Yan M. C., Shu M. A., Shao Q. J. (2017). Molecular characterization of a cDNA encoding Na+/K+/2Cl(-) cotransporter in the gill of mud crab (Scylla paramamosain) during the molt cycle: Implication of its function in osmoregulation. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 203, 115–125. doi: 10.1016/j.cbpa.2016.08.019
Yu D., Peng X., Ji C., Li F., Wu H. (2020). Metal pollution and its biological effects in swimming crab Portunus trituberculatus by NMR-based metabolomics. Mar. pollut. Bull. 157, 111307. doi: 10.1016/j.marpolbul.2020.111307
Yuan Y. H. (2015). Effects of dietary lipid and arachidonic acid level on growth performance, fatty acid composition and genes expression of metabolism related enzymes of larval half-smooth tongue sole, Cynoglossus semilaevis. (Qingdao, China: Ocean University of China). Master Dissertation.
Zhang Y., Cong Y., Zhang S. C. (2009). The genes encoding purine metabolism enzymes in amphioxus: Structure and evolution. Period Ocean Univ. China 39, 96–104.
Zhang L., Guo L., Mu C., Ye Y., Wang C. (2021). Postmortem metabolite profile changes of mud crab (Scylla paramamosain) under different storage conditions. J. Ocean Univ. China 20, 608–618. doi: 10.1007/s11802-021-4558-x
Zhang Y., Huang Z., Zhou Y., Ma H., Saqib H. S. A., Su Q., et al. (2022). The effects of different diet, salinity and light condition on growth performance and moulting cycle of juvenile mud crab, Scylla paramamosain. Aquacult Res. 53, 6333–6342. doi: 10.1111/are.16106
Zhang Y. K., Yang B. K., Li H. Y., Hu L. Y., Zhao X. Q., Xu S. X. (2021). Metabolomics of Xiphophorus helleri liver under starvation stress. Sichuan J. Zool. 40, 611–621. doi: 10.7524/AJE.1673-5897.20210401001
Zhao J., Wen X., Li S., Zhu D., Li Y. (2016). Effects of different dietary lipid sources on tissue fatty acid composition, serum biochemical parameters and fatty acid synthase of juvenile mud crab Scylla paramamosain (Estampador 1949). Aquacult Res. 47, 887–899. doi: 10.1111/are.12547
Zhao Y. T., Wu X. G., Chang G. L., Qiu R. J., Cheng Y. X. (2013). Effects of dietary DHA levels on growth, lipid composition and hypoxia stress of juvenile Chinese mitten crab Eriocheir Sinensis. Acta Hydrobiol Sin. 37, 1133–1144. doi: 10.7541/2013.154
Zheng P., Han T., Li X., Wang J., Su H., Xu H., et al. (2020). Dietary protein requirement of juvenile mud crab Scylla paramamosain. Aquaculture 518, 734852. doi: 10.1016/j.aquaculture.2019.734852
Zhou J., Li N., Fu Y., He C., Meng K., Li Y., et al. (2021). GC-MS-based metabolomics reveals metabolic changes in overwintering Scylla paramamosain at two different salinities. Aquacult Res. 52, 3110–3123. doi: 10.1111/are.15157
Keywords: crustacean, Scylla paramamosain, megalopa, metamorphosis, metabolomics, fatty acid content
Citation: Ma K, Liu Z, Qiao G, Ma L, Zhang F, Zhao M, Ma C and Wang W (2023) Effects of four diets on the metabolism of megalopa metamorphosis of the mud crab, Scylla paramamosain. Front. Mar. Sci. 10:1276717. doi: 10.3389/fmars.2023.1276717
Received: 12 August 2023; Accepted: 04 October 2023;
Published: 17 October 2023.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
Huan Wang, Ningbo University, ChinaLijun Ning, South China Agricultural University, China
Copyright © 2023 Ma, Liu, Qiao, Ma, Zhang, Zhao, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Ma, bWFjeUBlY3NmLmFjLmNu; Wei Wang, d2FuZ3dAZWNzZi5hYy5jbg==
†These authors have contributed equally to this work
 Keyi Ma
Keyi Ma Zhiqiang Liu
Zhiqiang Liu Guangde Qiao
Guangde Qiao Lingbo Ma
Lingbo Ma