- 1Department of Life and Environmental Science, Polytechnic University of Marche, Ancona, Italy
- 2Institute for Marine Biological Resources and Biotechnology, National Research Council, Ancona, Italy
- 3Department of Biological, Geological and Environmental Science, University of Catania, Catania, Italy
The ecological and economic relevance of the European sardine (Sardina pilchardus) in the Adriatic area is well established. High exploitation rates and instability of environmental parameters could potentially impair the reproductive physiology of this species, compromising the stock’s stability. To guarantee efficient stock management, there is a need to fill the lack of updated information regarding the structure, sex ratio and reproductive season of the sardine population in the Adriatic Sea. The present study provides new data on the distribution of females’ maturation phases, sex ratio, age frequency and seasonality of the reproductive period in the middle-western Adriatic Sea. Sardine specimens were collected monthly, from April 2021 to March 2022 in the Adriatic waters off the coast of Ancona. Size, weight and sex were determined for a sub-sample of almost 144 specimens during each sampling period. Through otolith analysis and ovary characterization, population age and females’ maturation phase were estimated respectively. The results obtained highlighted a general reduction in size (15.5cm the highest size class) and age (maximum age 2+, greater than 2 years old but less than 3 years) of the population that was characterized by the predominance of males. Also, an interesting scarcity of small female specimens (< 13 cm length) was observed. The reproductive period seemed to occur between October and June as suggested by ovarian characterization, GSI (0.50, 0.30 and 0.62 respectively) and Fulton’s condition factor (0.73, 0.74 and 0.74 respectively) analysis that showed the lowest values in July, August and September corresponding to the rest period.
1 Introduction
Small pelagic species play a strategic role in marine ecosystems as the predominant biomass of mid-trophic levels (Coll et al., 2007). They relocate energy from lower to higher trophic levels by modulating the abundances of top predators and limiting primary production rates by foraging on phytoplankton species (Cury et al., 2000; Fréon et al., 2005). Therefore, significant fluctuations in their abundance can affect both ecosystem’s structure and functioning (Daskalov, 2002). The European sardine (Sardina pilchardus) is one of the most abundant small pelagic species in the Mediterranean basin and the main exploited species in the Adriatic Sea (64,900 tons, 42.5% of total landings), with Italy dominating the catches (FAO, 2022). Since the second half of the ‘90s, Adriatic sardines biomass has experienced growth phases alternated to periods of stock decline (Santojanni et al., 2005; Morello and Arneri, 2009; Vilibić et al., 2016). This oscillating trend has occurred as a result of strong exploitation rates combined with fluctuations in environmental parameters by affecting food quantity and quality, annual recruitment, growth and health status of the stock (Basilone et al., 2017). The high susceptibility of sardines to environmental variations is attributable to their short life span, prolonged spawning season and the production of large quantities of pelagic eggs (Palomera et al., 2007; Giannoulaki et al., 2011; Patti et al., 2020). However, the above-mentioned features represent a common strategy adopted by small pelagic species to guarantee stock growth/survival and compensate for their short life fecundity (Ganias, 2014). Therefore, also reproduction is undoubtedly affected by the variation of environmental parameters. For example by regulating the availability of food (zooplankton and phytoplankton) before the reproductive season. Indeed, both ovarian maturation and fish fecundity depend to the extent of energy reserves, and hence food supply, to produce such a considerable number of eggs within a long reproductive period (Somarakis et al., 2004). Sardines reach sexual maturity approximately at the end of the first year of life and they are multiple-batch spawners which means they release several batches of pelagic eggs during an extended spawning season (Ganias et al., 2003). According to the latest data, in the Adriatic, the spawning season occurs between October and May with one or two peaks of spawning (in winter and early spring), whose timing greatly depends on the area and environmental factors (Zorica et al., 2020).
In this regard, monitoring studies conducted in the Adriatic Sea mainly concern the eastern spawning areas. The Croatian coast has been well-studied in this aspect (Sinovčić et al., 2008; Zorica et al., 2017; Zorica et al., 2019). One of the last data collections based on gonadosomatic index (GSI) values confirmed similar ranges of the spawning season (from early autumn to early spring) as observed in previous year. However, differences were observed concerning the peak of spawning which more likely depended on both food availability, temperature and salinity (Zorica et al., 2017).
Regarding the western side, there is less consistent data available about sardine spawning, although the western coastal waters are also suitable habitats for reproduction. Spawning grounds were identified in the Gulf of Trieste, the Po River Delta, the northern and central Italian coastlines and the northern Gargano coast (Zorica et al., 2020).
In the current scenario of irreversible climate changes and growing fishing efforts at global scale, scientists need to constantly update their knowledge concerning the reproductive physiology of marine species and how it may be affected by this additional environmental pressure. Stock management should consider the ripple effects of drastic and ever more frequent atmospheric events on nutrient availability, primary productivity, prey abundance and consequently health and reproduction in all areas of interest (Oliver et al., 2018).
This study aims to evaluate the actual state of sardines’ reproduction in the middle-western Adriatic Sea. Age-frequency, ovaries maturation phase, sex ratio and seasonality of the reproductive period were analyzed to highlight possible variation in the pattern observed in previous studies.
2 Materials and methods
2.1 Ethical statement
The present research was performed on fish caught by fishermen for commercial purposes, and since the procedures did not include animal experimentation, National ethics approval was not necessary according to the Italian legislation (D.L. 4 of March 2014, n. 26, art. 2).
2.2 Samples collection
1439 Sardina pilchardus specimens were collected once a month from April 2021 to March 2022 (~145 per month) in the Adriatic waters off the coast of Ancona (FAO Major Fishing Area 37, Subarea 37.2.1, between 7 and 36 miles from the coast). Sardines were caught by the Ancona’s “volanti” fleet consisting of mid-water pelagic trawl nets towed by two vessels, mostly operated in the northern and central areas of the Adriatic. Samplings were not performed in June and October 2021 due to the fishing stop as stated by the Italian government (D.M. 25th January 2016, art. 2).
From all specimens and weight and total length (tl) were individually measured to determine size class distribution and to calculate Fulton’s condition (in females). Based on the individual total length, fish were classified into 10 size classes with a range of 0.5 cm per class. 11 was the smallest size class (including fish with a tl between 11.0 and 11.4 cm) 15.5 was the greatest size class (including fish with a tl between 15.5 and 15.9 cm). The sex of all specimens was determined to calculate the sex ratio. At each sampling time, the ovary of at least 5 specimens per size class, (when present) was collected (for a total of 160 specimens), weighted for the GSI calculation and properly stored to perform histological analysis.
At each sampling time, otoliths (sagittae) were removed from at least 10 specimens per size class (when present) (for a total of 317 specimens), cleaned, dried and stored dry in vials for aging purposes.
2.3 Sex determination and sex ratio
The sex of each specimen (n=1439) was determined macroscopically by the observation of the gonads. The sex ratio (total number of males/total number of females) was then calculated considering all sampled specimens excluding the unidentified fish (n = 88) for which it was impossible to macroscopically identified the sex (n = 1351).
2.4 Somatic indices
Fulton’s condition factor (K)) was calculated individually for all females collected during the entire sampling period using the formula below:
where W is the individual total weight and L refers to the individual total length.
Gonadosomatic index (GSI) was calculated individually for females using the formula below:
where GW is the individual gonadal weight and BW corresponds to the individual body weight.
2.5 Histological analysis
Ovary samples were properly stored in a formaldehyde/glutaraldehyde solution (NaH2PO4–H2O + NaOH + Formaldehyde (36.5%) + Glutaraldehyde (25%) + H2O) at 4°C were processed as proposed by Chemello et al. (2023) to the final samples embedding in paraffin (Bio-Optica). Successively, samples embedded in paraffin (Bio-Optica) were cut into 5 µm sections using a microtome (Leica RM2125 RTS, Nussloch, Germany) and then stained with hematoxylin and eosin Y stain (Merck KGaA). Sections were observed using an optical microscope (Zeiss Axio Imager.A2, Oberkochen, Germany) and images were acquired through a combined color digital camera Axiocam 503 (Zeiss, Oberkochen, Germany).
2.6 Ovary maturation phase determination
The histological analysis was performed to determine the maturation phase of ovaries following the classification proposed by Brown-Peterson et al. (2011). Fish were classified into the following categories: immature (specimens that have not reached sexual maturity characterized by oogonia and primary growth oocytes); developing (presence of cortical alveola oocytes and few vitellogenic oocytes); spawning-capable (Vtg3 oocytes appearance, possible presence of post-ovulatory follicles (POFs) in multiple batch spawners as evidence of the previous spawning); spawning active (defined by the presence of late germinal vesicle migration, germinal vesicle breakdown, hydrated oocytes, ovulation, or newly collapsed POFs); regressing (which is characterized by atresia, POFs, and few, if any, healthy vitellogenic oocytes); and regenerating (primary growth (PG) oocytes, late-stage atresia and a thicker ovarian wall).
2.7 Age estimation
The total number of analyzed specimens for the age readings was 317 (153 males, 164 females), only out of 17 individuals it was not possible to assign the age.
One otolith from each pair (usually the left one) was immersed in 70% ethanol to be determined. Otoliths were analyzed by the binocular microscope with reflected light on a black background, at 25x magnification, connected to a digitized video computer system (Leica Application Suite 4.3.0.). The main conventions for reading age were the periodicity of otolith ring formation (one year equals one consecutive opaque and translucent ring) and date of birth (considered January 1) (Morales-Nin, 1992). All otoliths were read twice by a single reader and the age estimate was obtained from the average of the two readings. To assess the accuracy of aging between readings, the mean percent error index (APE) (Beamish and Fournier, 1981) and the mean coefficient of variation (CV) (Chang, 1982) were calculated. A bias plot was then calculated to assess systematic differences between the readings (Campana et al., 1995).
The length-mass relationship was estimated for each sex through the function TM = aTlb, where TM is the total body mass (g) and Tl is the total length (mm). The starting point of isometric growth (i.e. b = 3) was tested using a Student’s t-test. An F test has been performed to compare the allometric indices (b) calculated for males and females.
2.8 Statistical analysis
Fulton’s condition factor and gonadosomatic index data were analyzed using One-way ANOVA analysis and Tukey’s post-test following the assessment of data normality by the Shapiro-Wilk test using the GraphPad Software Prism8 for Windows. The sex ratio significance was calculated through Pearson’s chi-squared test. In all tests, the significance was set at p ≤ 0.05.
3 Results
3.1 Size class, age and sex ratio distribution
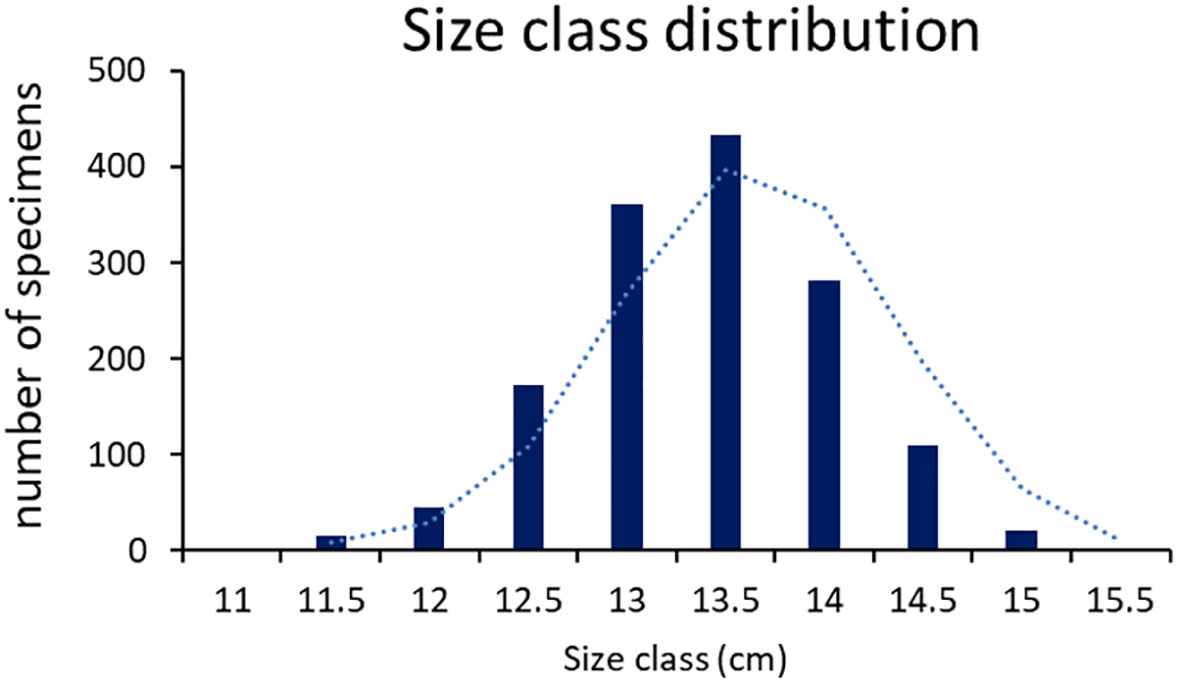
Size class distribution (Figure 1) followed a bell-like curve, the three intermediate size classes were the most numerous (362, 433 and 281 specimens in 13, 13.5 and 14 size classes respectively). While the number of fish in the smaller and higher classes notably decreased. Only 2 and 1 fish fell in the smallest (11) and highest (15.5) size classes respectively.
No consistent differences were observed concerning the size distribution in inshore and offshore sampling areas (Figure 2).
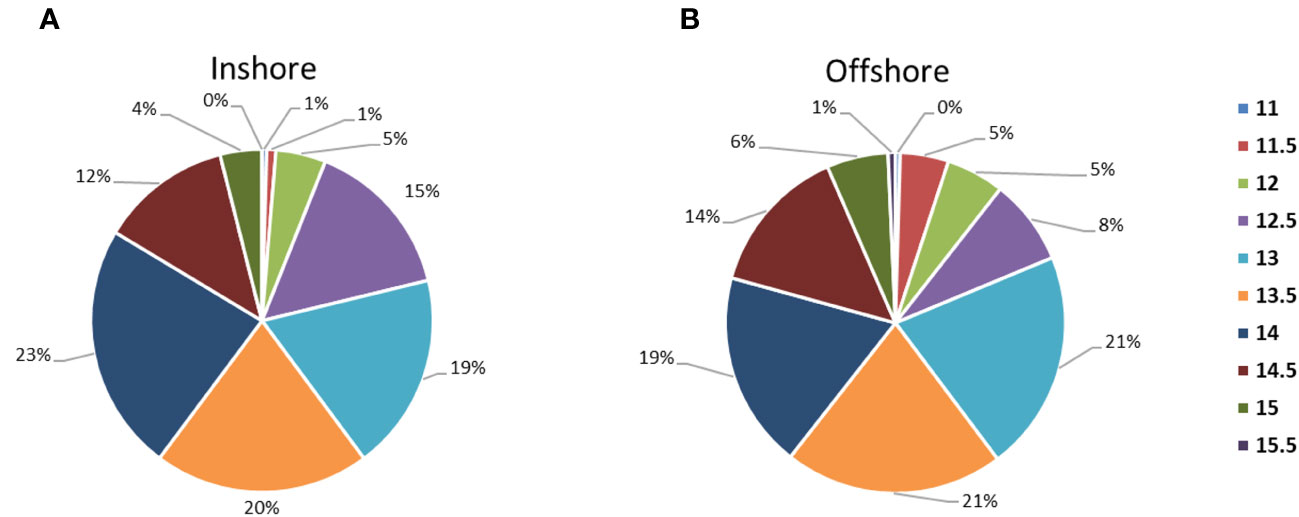
Figure 2 Size class (cm) percentage frequency between (A), inshore (7-20 miles from the coast) and (B), offshore (25-36 from the coast) sampling areas.
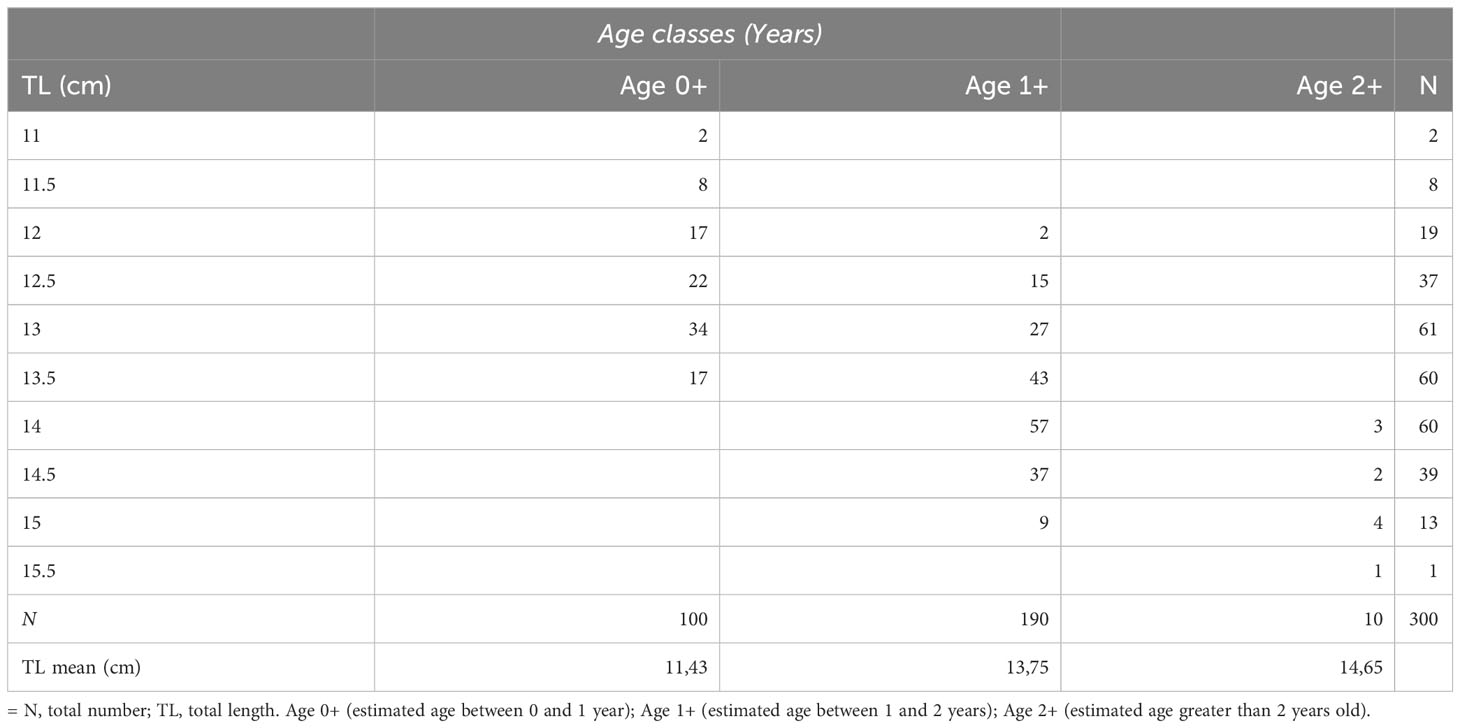
The age-length key is summarized in Table 1. Indices of aging precision APE and CV were relatively low (23% and 16%, respectively), indicating the goodness of the aging procedure adopted and a good consistency or reproducibility among readings. No bias between readings was present across the whole age range estimated. The age classes obtained from otolith readings of the selected subsamples were three (age 0+, 1+, 2+). Most fish analyzed were at age 1+ followed by age 0+ and age 2+ (100 and 10 specimens respectively).
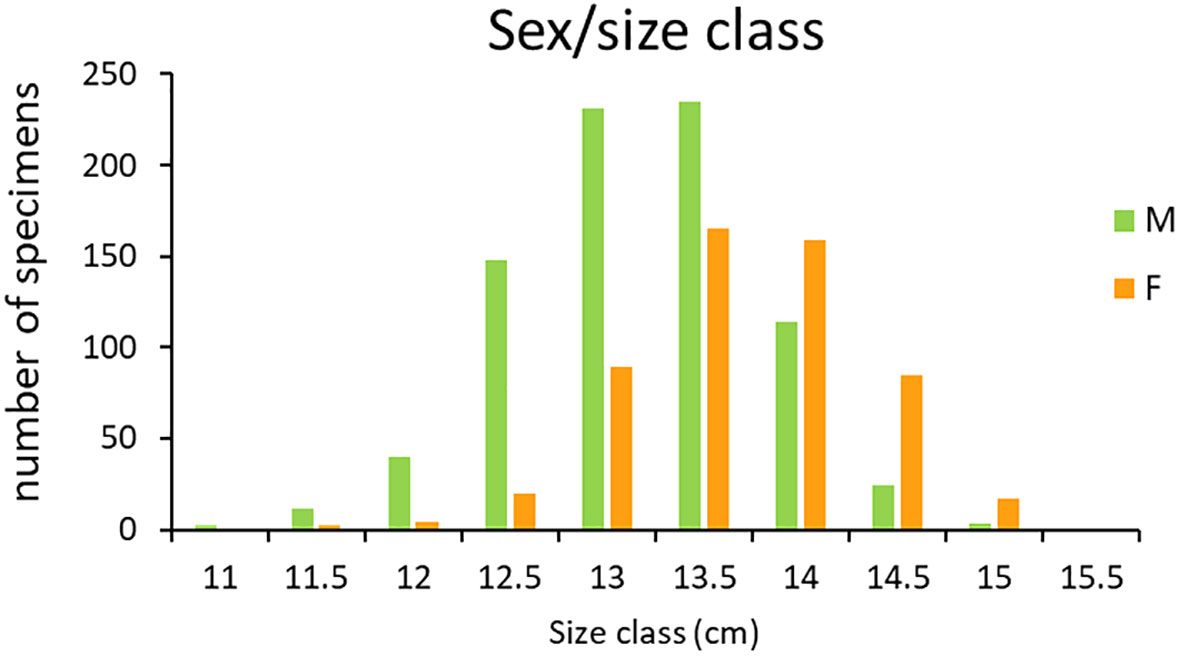
The sex ratio within each size class is presented in Figure 3. In smaller size classes the number of males was always higher than the number of females. Conversely, from the intermediate size class (13.5) to the largest one the opposite trend was observed with more females than males observed. In the smallest and highest size classes (11 and 15.5) only 2 and 1 specimen detected respectively were all males. The overall sex ratio (m/f = 1.5) deviated significantly from the hypothetical distribution of 1:1 (χ2 = 53.561, df = 1, p < 0.0001), as more males were observed than females among all the samples sexually determined (810 and 541 and respectively).
3.2 Histological analysis
3.2.1 Ovary maturation phase distribution
The histological analysis highlighted the total absence of immature females and the presence of all five ovarian maturation phases within the females’ subsample considered (Figure 4). European sardine as an intermittent batch spawner showed specific ovary development. Ovaries at the developing phase (Figure 4A) were characterized by the presence of oocytes at different stages, primary growth oocytes (PG), primary vitellogenic oocytes and secondary vitellogenic oocytes (Vtg1 and Vtg2 respectively). The subsequently spawning capable phase (Figure 4B) was characterized by the presence of tertiary vitellogenic oocytes (Vtg3) bigger in size respect to Vtg2 and Vtg1 oocytes still present in the gonad. Figure 4C, showed an ovary at the spawning active phase characterized by the progress of oocytes development through the breakdown of germinal vesicle (GVBD) and the coalescence of vitellogenic granules. The regressing phase (Figure 4D) is identified by the presence of atretic oocytes and POFs. Finally, in the regenerating phase ovaries (Figure 4E) only primary growth oocytes were present and interstitial tissue became clearly visible. Regenerating and regressing phases were the only two phases observed in July, August and September (Figure 5A). During the other sampling months, the majority of females were at the developing phase while a smaller number of specimens were at spawning active and spawning capable phases. A different situation was observed in May when no regenerating females were detected, and six females were at the regressing phase. Finally, one female was observed at the regenerating phase in both February and March (Figure 5A). In Figure 5B ovary phases were distributed per size class, the developing phase was the most abundant in all size classes. Within the size class range from 13 to 15, all the other reproductive phases were identified but at a lower percentage. In the 12 size class, only a small percentage of spawning capable females were identified together with the most abundant developmental phase.
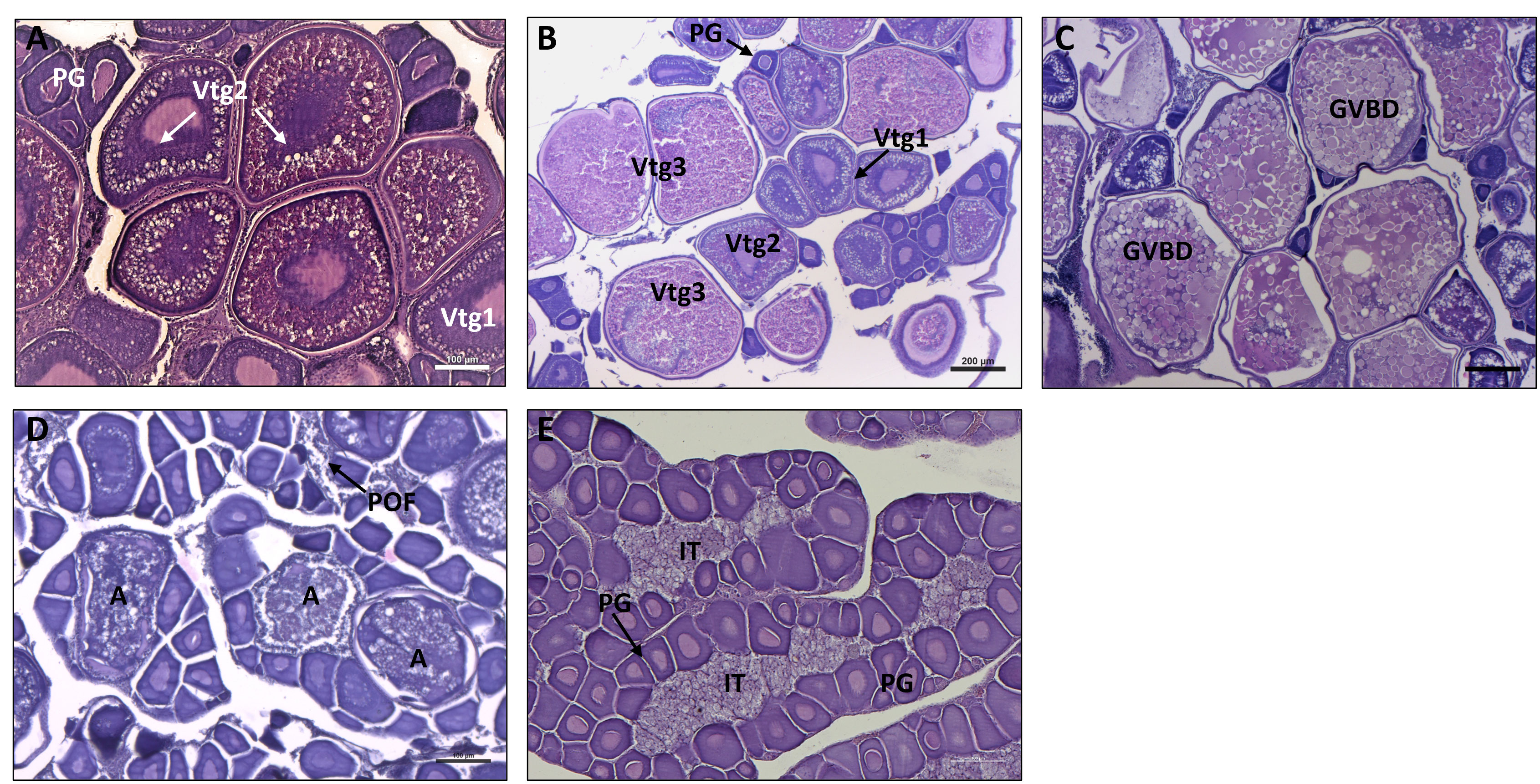
Figure 4 Histological sections stained with haematoxylin & eosin stain, explicative of the five different ovarian maturation phases observed. (A), developing phase; (B), spawning capable; (C), spawning active; (D), regressing; (E), regenerating. PG, primary growth oocyte; Vtg1 primary vitellogenic oocyte Vtg2, secondary vitellogenic oocyte; Vtg3, tertiary vitellogenic oocyte; GVBD, germinal vesicle breakdown; (A), atretic oocyte; POF, post-ovulatory follicle.
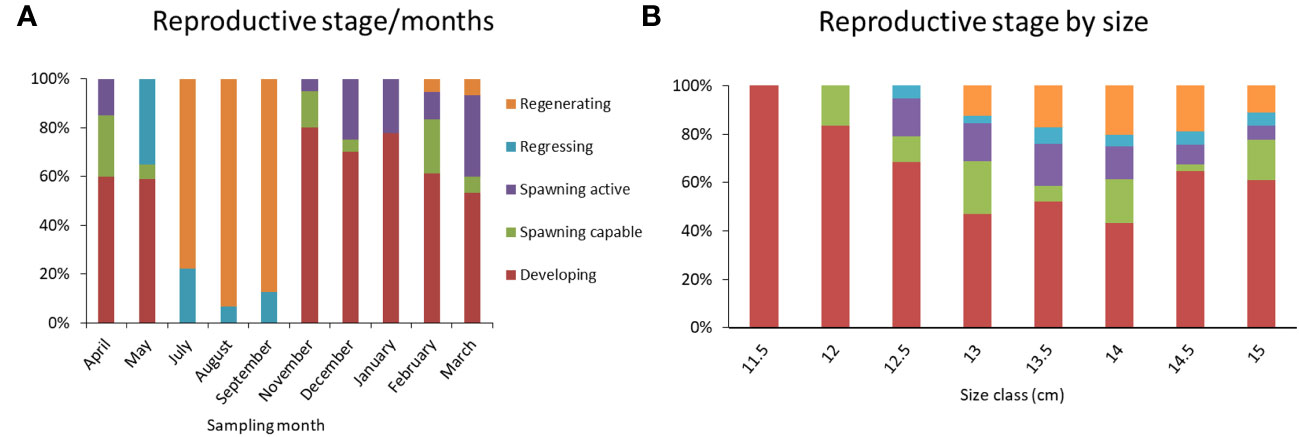
Figure 5 Ovarian reproductive phase distribution among sampling months (n = 160) (A) and size class (cm) (B).
3.3 Somatic indices
The gonadosomatic index calculated for female specimens in July, August and September was significantly lower than values obtained during the other months (Figure 6A). A decreasing trend, although not significant, could be observed from April to July. Whereas an opposite (but not significant) trend characterized the GSI values from November to March. Fulton’s condition factor (K) was significantly higher within the period from July to December with respect to the other months considered (Figure 6B).
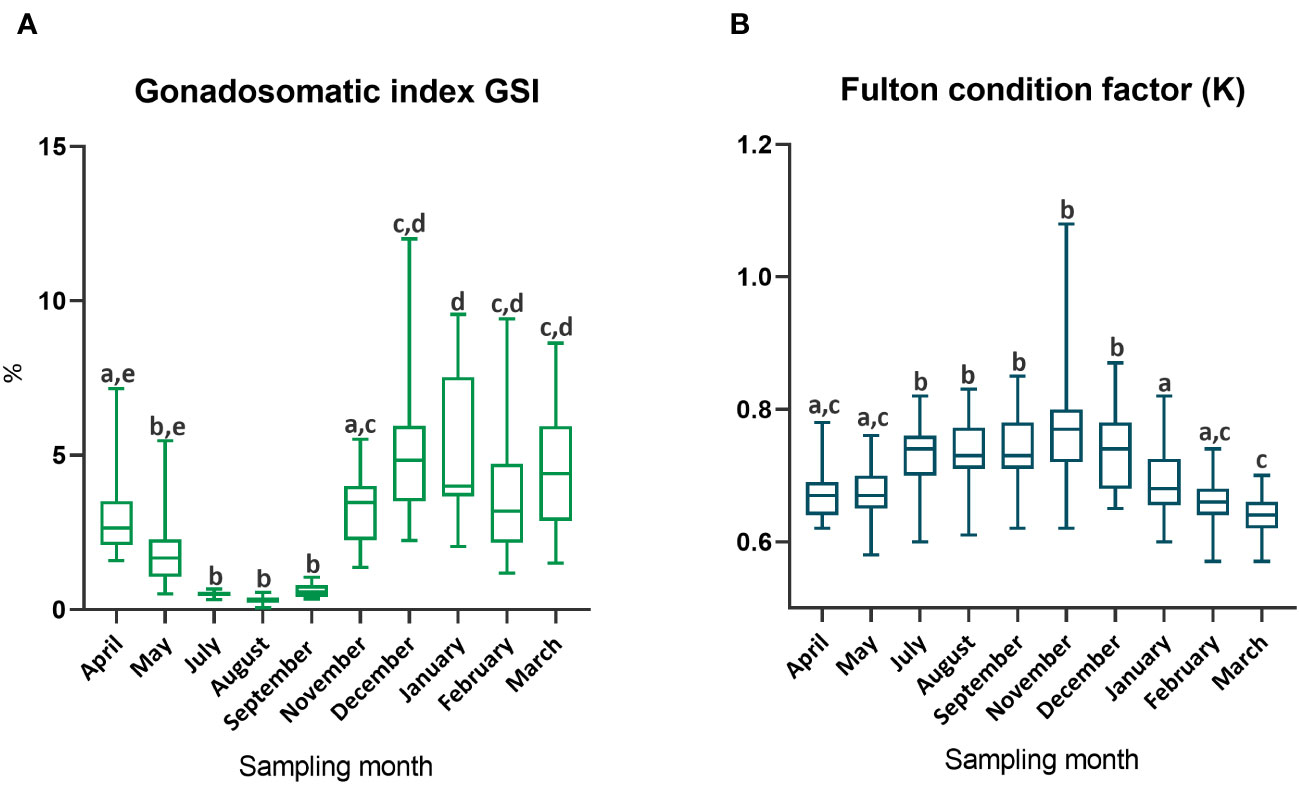
Figure 6 (A), Gonadosomatic index and (B), Fulton condition factor calculated for female specimens during each sampling month. Different lowercase letters represent significant differences (p< 0.05).
4 Discussion
Fluctuation of small pelagic biomasses commonly occurs in different Mediterranean and oceanic areas (Azzali et al., 2002; Van Beveren et al., 2014; Coll et al., 2019). Overfishing and alteration of environmental conditions represent the main events inducing stock variability which is usually characterized by an oscillation between population decrease and recovery. However, when an event of biomass decrease is not properly recognized, high fishing rates can have tragic consequences as occurred for the Peruvian anchoveta stock in the 1970’s with severe effects also on fishery (Clark, 1976).
In light of such or similar events, it is evident that monitoring studies should be performed with continuity also considering the extent of the environmental changes in the actual global scenario.
In this study, a predominance of sardines’ biomass at the intermediate size classes (from 13 to 14.4 cm in total length) was observed at the expense of smaller and higher sizes. Excluding fish below 11 cm that cannot be caught as stated by the European legislation (EC, 2006), the lack of specimens under 13 cm may be explained by considering different environments populated by juveniles and adults. Indeed, sardine juveniles usually live in inshore coastal waters, bays and river estuaries (Giannoulaki et al., 2011). However, there were no differences in the size frequency of fish sampled inshore and offshore therefore, a possible explanation of this scenario could be related to the reduction of spawning success during the previous year and a consequent absence of younger specimens.
Whereas the absence of large-size specimens contradicted the data gathered in recent studies regarding the eastern and northern part of the Adriatic where sardines’ total length was observed up to 21.0 cm and 17.0 cm respectively (Zorica et al., 2019; Hure and Mustać, 2020; Caballero-Huertas et al., 2022). The decrease of maximum length in sardines’ stock usually involved different factors such as adverse changes in the environmental parameters, reduction of food availability, parasites infection and overfishing. Though, the latter could be excluded, in this specific case, considering the decrease in fishing activities related to the COVID pandemic in 2020 when both large and small-scale fisheries were forced to stop (Bennett et al., 2020). Since the majority of sardines collected were between 0+ and 1+ years followed by 2+ years old sardines while no specimen had more than 3 years, the combined effects of environmental variables might be responsible not only for the reduction of sardines’ average size but also for their lifespan, at least in the mid-west part of the Adriatic Sea. Indeed, a decline of sardines together with a decrease in size and age was already observed in the Gulf of Lion (Van Beveren et al., 2014). Reports of sardines reaching 9 years date back to the end of the twentieth century and do not reflect the current stock situation more probably due to overfishing (Morello and Arneri, 2009). This drastic reduction of sardine lifespan together with the high fishing rates in the Adriatic Sea needs an urgent action to preserve the fishery resources. The sardines population has been already identified to be at greatest risk of overexploitation in the Adriatic geographical subarea GSA 17 and 18 (ICES, 2019; GFCM_SRC-AS, 2023).
Another interesting aspect concerns the overall sex ratio which was observed in favor of males that predominated from the lowest to the 13 size classes while females were more abundant in the upper size classes. It is not surprising that previous studies have produced conflicting results since sardines’ sex ratio may vary with depth, time of the year, maturation and area (Morello and Arneri, 2009).
As stated by Turner et al. (1983) and accepted by the scientific community, variations of the sex ratio at different sizes could be related to unequal rates of growth and mortality. Generally, a greater number of females is considered advantageous for the population since a high number of females have a greater rate of reproduction (Mustać and Sinovčić, 2010). However, the lack of females in the lower-size classes is not justified by a different spatial distribution of small specimens than bigger ones and neither between mature nor immature females since all sampled females appeared to be mature. Factors influencing this peculiar sex frequency should be investigated more thoroughly.
In small pelagic species, another aspect affected by the changes in environmental conditions is the plasticity of the reproductive period. Current literature states that sardines reproduce from autumn/winter to spring in different areas of the Mediterranean with a month difference in the duration of the reproductive period (Zorica et al., 2020). The present study confirmed this statement. through the lowest values of GSI observed during July, August and September concurrently with the observation of ovaries at the regressing and resting phase exclusively. These two gonadal phases are typical of females during the resting period (Pešić et al., 2010). Consistent results were also provided by the highest values of the Fulton’s condition factor in the no reproductive period. Fulton’s condition reflects the health status of the fish and its decrease during the months of reproduction has been related to the different allocation of energy greatly applied in the reproduction process. Indeed, the Fulton’s condition factor indicates the energy available for fish growth and usually decreases during reproduction due to the high energy investment required (Lambert and Dutil, 1997).
The presence of females at the spawning capable phase in May suggested the possible extension of the reproductive period until June. Changes in the timing of reproduction and length at the first reproductive event represent a common strategy adopted by small pelagic species to overcome environmental alteration and overfishing (Basilone et al., 2006). In this regard, the presence of different reproductive phases in females of different size classes was in accordance with a previous study performed in the Adriatic Sea (Basilone et al., 2021). Indeed, the lower size class with mature females in the spawning capable phase was 12 around 0+ year of age.
Sardines’ size at first maturity, previously identified, ranged between 10.8-12.4 cm (total length) for females (Basilone et al., 2021). Records of mature females at 7.9 cm (total length) were also reported by Sinovčić et al. (2008).
5 Conclusion
The present study proposed a snapshot of the reproductive status of Sardina pilchardus in the middle-western Adriatic during 2021-2022. Data reflected the situation of the stock with an altered sex ratio and the progressive reduction of age and size with respect to the past. Moreover, it seemed that sardines are facing a modification in terms of the reproductive season probably due to environmental alterations. The results obtained so far could be useful for future monitoring studies on the status of sardine stock in the Adriatic Sea. We therefore stress the importance of monitoring commercial stocks over time, because, in the current scenario of climate changes, a dramatic alteration in the reproduction period and survival of eggs and larvae of relevant species could have more deleterious effects than in the past.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the present research was performed on fish caught by fishermen for commercial purposes, and since the procedures did not include animal experimentation, national ethics approval was not necessary according to the Italian legislation (D.L. 4 of March 2014, n. 26, art. 2).
Author contributions
GC: Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. GLC: Formal analysis, Investigation, Writing – review & editing. VT: Formal analysis, Investigation, Writing – review & editing. FD: Validation, Writing – original draft, Writing – review & editing. FT: Validation, Writing – review & editing. GG: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge local fishermen of the Ancona fish market for the collaboration and the Association “Federpesca (Federazione Nazionale delle Imprese di Pesca)” for the logistic support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Azzali M., De Felice A., Luna M., Parmiggiani G. C. (2002). The state of the adriatic sea centered on the small pelagic fish populations. Mar. Ecol. 23, 78–91. doi: 10.1111/j.1439-0485.2002.tb00009.x
Basilone G., Ferreri R., Aronica S., Mazzola S., Bonanno A., Gargano A., et al. (2021). Reproduction and sexual maturity of European sardine (Sardina pilchardus) in the central mediterranean sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.715846
Basilone G., Guisande C., Patti B., Mazzola S., Cuttitta A., Bonanno A., et al. (2006). Effect of habitat conditions on reproduction of the European anchovy (Engraulis encrasicolus) in the Strait of Sicily. Fish. Oceanogr. 15 (4), 271–280.
Basilone G. J., Mangano S. J., Pulizzi M., Fontana I., Giacalone G., Ferreri R. (2017). European anchovy (Engraulis encrasicolus) age structure and growth rate in two contrasted areas of the Mediterranean Sea: the paradox of faster growth in oligotrophic seas. Mediterr. Mar. 18, 504–516. doi: 10.12681/mms.2059
Beamish R. J., Fournier D. D. A. (1981). A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 38, 982–983. doi: 10.1139/f81-132
Bennett N. J., Finkbeiner E. M., Ban N. C., Belhabib D., Jupiter S. D., Kittinger J. N., et al. (2020). The COVID-19 pandemic, small-scale fisheries and coastal fishing communities. Coast. Manage. 48, 336–347. doi: 10.1080/08920753.2020.1766937
Brown-Peterson N. J., Wyanski D. M., Saborido-Rey F., Macewicz B. J., Lowerre-Barbieri S. K. (2011). A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 3, 52–70. doi: 10.1080/19425120.2011.555724
Caballero-Huertas M., Frigola-Tepe X., Viñas J., Muñoz M. (2022). Somatic condition and reproductive potential as a tandem in European sardine: an analysis with an environmental perspective in the northern adriatic (Gulf of trieste). Fishes 7, 105. doi: 10.3390/fishes7030105
Campana S. E., Annand M. C., McMillan J. I. (1995). Graphical and statistical methods for determining the consistency of age determinations. Trans. Am. Fish. Soc 124, 131–138. doi: 10.1577/1548-8659(1995)124<0131:gasmfd>2.3.co;2
Chang W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 39, 1208–1210. doi: 10.1139/f82-158
Chemello G., Faraoni V., Notarstefano V., Maradonna F., Carnevali O., Gioacchini G. (2023). First evidence of microplastics in the yolk and embryos of common cuttlefish (Sepia officinalis) from the central adriatic sea: evaluation of embryo and hatchling structural integrity and development. Animals 13, 95. doi: 10.3390/ani13010095
Clark W. G. (1976). “The lessons of the Peruvian anchoveta fishery,” in California Cooperative Oceanic Fisheries Investigations Reports, vol. 19. (Rome, Italy:Food and Agriculture Organization of the United Nations (FAO)), 57–63.
Coll M., Albo-Puigserver M., Navarro J., Palomera I., Dambacher J. M. (2019). Who is to blame? Plausible pressures on small pelagic fish population changes in the northwestern Mediterranean. Sea. Mar. Ecol. Prog. Ser. 617, 277–294. doi: 10.3354/meps12591
Coll M., Santojanni A., Palomera I., Tudela S., Arneri E. (2007). An ecological model of the Northern and Central Adriatic Sea: Analysis of ecosystem structure and fishing impacts. J. Mar. Syst. 67, 119–154. doi: 10.1016/J.JMARSYS.2006.10.002
Cury P., Bakun A., Crawford R. J. M., Jarre A., Quiñones R. A., Shannon L. J., et al. (2000). Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist“ ecosystems. ICES J. Mar. Sci. 57, 603–618. doi: 10.1006/JMSC.2000.0712
Daskalov G. M. (2002). Overfishing drives a trophic cascade in the Black Sea. Mar. Ecol. Prog. Ser. 225, 53–63. doi: 10.3354/MEPS225053
EC. (2006). Council regulation (EC) no 1967/2006 of 21 december 2006. Off. J. Eur. Union L409 11-85 L 409, 11–85.
FAO. (2022). The State of Mediterranean and Black Sea Fisheries 2022, The State of Mediterranean and Black Sea Fisheries 2022 (Rome: FAO). doi: 10.4060/CC3370EN
Fréon P., Cury P., Shannon L., Roy C. (2005). Sustainable exploitation of small pelagic fish stocks challenged by environmental and ecosystem changes: A review. Bull. Mar. Sci. Mar. Sci. 76, 385–462.
Ganias K. (2014). Biology and ecology of sardines and anchovies, Biology and Ecology of Sardines and Anchovies (USA: CRC Press). doi: 10.1201/b16682
Ganias K., Somarakis S., Machias A., Theodorou A. J. (2003). Evaluation of spawning frequency in a Mediterranean sardine population (Sardina pilchardus sardina). Mar. Biol. 142, 1169–1179. doi: 10.1007/s00227-003-1028-5
GFCM_SRC-AS. (2023). Report of the Subregional Committee for the Adriatic Sea (SRC-AS). Available at: https://www.fao.org/gfcm/technical-meetings/detail/en/c/1642341.
Giannoulaki M., Pyrounaki M. M., Liorzou B., Leonori I., Valavanis V. D., Tsagarakis K., et al. (2011). Habitat suitability modelling for sardine juveniles (Sardina pilchardus) in the Mediterranean Sea. Fish. Oceanogr. 20, 367–382. doi: 10.1111/j.1365-2419.2011.00590.x
Hure M., Mustać B. (2020). Feeding ecology of Sardina pilchardus considering co-occurring small pelagic fish in the eastern Adriatic Sea. Mar. Biodivers. 50, 1–12. doi: 10.1007/s12526-020-01067-7
ICES (2019). Workshop on data-limited stocks of short-lived species (WKDLSSLS). ICES Sci. Rep. 1 (73), 166. doi: 10.17895/ices.pub.5549
Lambert Y., Dutil J. D. (1997). Condition and energy reserves of Atlantic cod (Gadus morhua) during the collapse of the northern Gulf of St. Lawrence stock. Can. J. Fish. Aquat. Sci. 54, 2388–2400. doi: 10.1139/f97-145
Morales-Nin B. (1992). Determination of Growth in Bony Fishes from Otolith Microstructure (Rome: Food & Agriculture Org).
Morello E. B., Arneri E. (2009). Anchovy and sardine in the adriatic sea - an ecological review. Oceanography Mar. Biol. 47, 209–256. doi: 10.1201/9781420094220-8
Mustać B., Sinovčić G. (2010). Reproduction, length-weight relationship and condition of sardine, Sardina pilchardus (Walbaum 1792), in the eastern middle adriatic sea (Croatia). Period. Biol. 112, 133–138.
Oliver E. C. J., Donat M. G., Burrows M. T., Moore P. J., Smale D. A., Alexander L. V., et al. (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9, 1–12. doi: 10.1038/s41467-018-03732-9
Palomera I., Olivar M. P., Salat J., Sabatés A., Coll M., García A., et al. (2007). Small pelagic fish in the NW Mediterranean Sea: An ecological review. Prog. Oceanogr 74, 377–396.
Patti B., Torri M., Cuttitta A. (2020). General surface circulation controls the interannual fluctuations of anchovy stock biomass in the Central Mediterranean Sea. Sci. Rep. 10 (1), 1554.
Pešić A., Durović M., Joksimović A., Regner S., Simonović P., Glamuzina B. (2010). Some reproductive patterns of the sardine, sardina pilchardus (Walb 1792), in boka kotorska bay (Montenegro, southern Adriatic Sea). Acta Adriat. 51, 159–168.
Santojanni A., Cingolani N., Arneri E., Kirkwood G., Belardinelli A., Giannetti G., et al. (2005). Stock assessment of sardine (Sardina pilchardus, Walb.) in the Adriatic Sea with an estimate of discards. Sci. Mar. 69, 603–617. doi: 10.3989/SCIMAR.2005.69N4603
Sinovčić G., Keč V.Č., Zorica B. (2008). Population structure, size at maturity and condition of sardine, Sardina pilchardus (Walb. 1792), in the nursery ground of the eastern Adriatic Sea (Krka River Estuary, Croatia). Estuar. Coast. Shelf Sci. 76, 739–744. doi: 10.1016/j.ecss.2007.07.037
Somarakis S., Ganias K., Tserpes G., Koutsikopoulos C. (2004). Ovarian allometry and the use of the gonosomatic index: A case study in the Mediterranean sardine, Sardina pilchardus. Mar. Biol. 146, 181–189. doi: 10.1007/s00227-004-1419-2
Turner S. C., Grimes C. B., Able K. W. (1983). Growth, mortality, and age/size structure of the fisheries for tilefish, Lopholatilus chamaeleonticeps, in the Middle Atlantic-Southern New England region. Fish. Bull. 81, 751–763.
Van Beveren E., Bonhommeau S., Fromentin J. M., Bigot J. L., Bourdeix J. H., Brosset P., et al. (2014). Rapid changes in growth, condition, size and age of small pelagic fish in the Mediterranean. Mar. Biol. 161, 1809–1822. doi: 10.1007/s00227-014-2463-1
Vilibić I., Čikeš Keč V., Zorica B., Šepić J., Matijević S., Džoić T. (2016). Hydrographic conditions driving sardine and anchovy populations in a land-locked sea. Mediterr. Mar. Sci. 17, 1–12. doi: 10.12681/mms.1120
Zorica B., Anđelić I., Keč V.Č. (2019). Sardine (Sardina pilchardus) spawning in the light of fat content analysis. Sci. Mar. 83, 207–213. doi: 10.3989/scimar.04898.07A
Zorica B., Čikeš Keč V., Vidjak O., Kraljević V., Brzulja G. (2017). Seasonal pattern of population dynamics, spawning activities, and diet composition of sardine (Sardina pilchardus Walbaum) in the eastern Adriatic Sea. Turkish J. Zool. 41, 892–900. doi: 10.3906/zoo-1609-27
Keywords: European sardine, reproduction, ovary maturation, oogenesis, sex ratio, age determination
Citation: Chemello G, Cerrone GL, Tavolazzi V, Donato F, Tiralongo F and Gioacchini G (2023) One year study on the reproductive biology, ovary characterization and age of the European sardine (Sardina pilchardus) in the middle-west Adriatic Sea. Front. Mar. Sci. 10:1266894. doi: 10.3389/fmars.2023.1266894
Received: 25 July 2023; Accepted: 16 October 2023;
Published: 27 October 2023.
Edited by:
Vitor H. Paiva, University of Coimbra, PortugalReviewed by:
Nadia Bouzzammit, Ibn Zohr University, MoroccoKaterina Charitonidou, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Chemello, Cerrone, Tavolazzi, Donato, Tiralongo and Gioacchini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgia Gioacchini, Z2lvcmdpYS5naW9hY2NoaW5pQHVuaXZwbS5pdA==
 Giulia Chemello1
Giulia Chemello1 Francesco Tiralongo
Francesco Tiralongo Giorgia Gioacchini
Giorgia Gioacchini