
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 27 September 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1252969
 Cristina Gioia Di Camillo1,2*
Cristina Gioia Di Camillo1,2* Massimo Ponti2,3,4,5*
Massimo Ponti2,3,4,5* Annalisa Storari1
Annalisa Storari1 Clarissa Scarpa1
Clarissa Scarpa1 Camilla Roveta1
Camilla Roveta1 Torcuato Pulido Mantas1
Torcuato Pulido Mantas1 Martina Coppari1
Martina Coppari1 Carlo Cerrano1,2
Carlo Cerrano1,2There is an urgent need to better understand the stressors, namely heatwaves, changes in thermohaline circulation and mucilage events, that are rapidly re-shaping bioconstructions, such as coralligenous assemblages. This calls for increased monitoring efforts in these invaluable habitats that will improve our understanding of the resistance and resilience of bioconstructions. Since 2009, 16 indexes have been designed to assess the ecological quality of Mediterranean coralligenous reefs. The main objective of this work is to propose a framework to support the development of a shared, cost-effective, and practical index to assess the status of the coralligenous biocenosis. To achieve this, studies conceiving these 16 indexes were reviewed: comparing their objectives, metrics, and applied methodologies. A standardized nomenclature of anthropogenic pressures is supplied, using, when possible, definitions from the European Habitat Directive, Marine Strategy Framework Directive and Water Framework Directive. Additionally, given the unprecedented climatic conditions, we highlight that a common index should give particular attention to the response of the coralligenous to thermal stress and mucilage. A list of priority anthropogenic pressures/environmental stressors and relative indicators and metrics are suggested. This review stresses the urgency to align the methodologies at basin scale and highlights the pros and cons of the preexisting indexes that must be considered in the design of a new, shared procedure to evaluate the status of coralligenous assemblages.
Coralligenous reefs are one of the most diversified biogenic habitat typologies in the Mediterranean Sea that depend on a fragile equilibrium between bioconstruction and bioerosion and other physical processes (UNE/MAP, 2017). They create a mosaic of unique seascapes supporting over 1600 species (Ballesteros, 2006; Bertolino et al., 2013), providing valuable ecosystem services such as food and bioactive compounds, and an attraction for scuba diving (Ballesteros, 2006; Chimienti et al., 2017). Coralligenous concretions are produced by the slow accumulation of encrusting algal thalli and by calcareous animal skeletons (Ingrosso et al., 2018; Casoli et al., 2020). They reach the highest degree of complexity when covered with large, long-living organisms such as gorgonians (Gómez‐Gras et al., 2021) which allow the consolidation of a rich and diversified understorey (Ponti et al., 2018) and the establishment of coevolutionary relationships among organisms (Ponti et al., 2016; Cerrano et al., 2019).
The synergistic effects of mechanical damage (e.g., fishing activities, lost fishing gear, anchoring, scuba diving; Di Franco et al., 2009; Linares et al., 2012; Ferrigno et al., 2018), heatwaves and consequent mass mortality events (Cerrano et al., 2000; Garrabou et al., 2009; Garrabou et al., 2022a), pollution and eutrophication (Danovaro et al., 2009), are leading to the depletion of habitat-formers and to a general over-simplification of the coralligenous three-dimensional architecture (Rossi, 2013; Cerrano et al., 2019), with consequent loss in biodiversity, ecosystem functions and aesthetic value (Gómez‐Gras et al., 2021).
According to the Marine Strategy Framework Directive (MSFD, 2008/56/EC) and the Action Plan for the conservation of the coralligenous and other calcareous bio-concretions in the Mediterranean Sea (UNEP-MAP-RAC/SPA, 2017), the scientific community has been developing biotic indexes to assess the coralligenous environmental quality status (Bavestrello et al., 2016). These will help verify to what extent the habitat is providing its services, and to identify the need to take action to limit a shift in habitat functional identity (Gómez‐Gras et al., 2021).
Since 2009, at least 16 indexes have been designed (Table 1), but none of them have been officially adopted by the Regional Activity Centre for Specially Protected Areas (RAC/SPA) to estimate the quality of the coralligenous habitats. The main objective of this work is to propose a framework to orient the scientific community towards the development of a shared, cost-effective and practical index to assess the status of the coralligenous biocenosis.
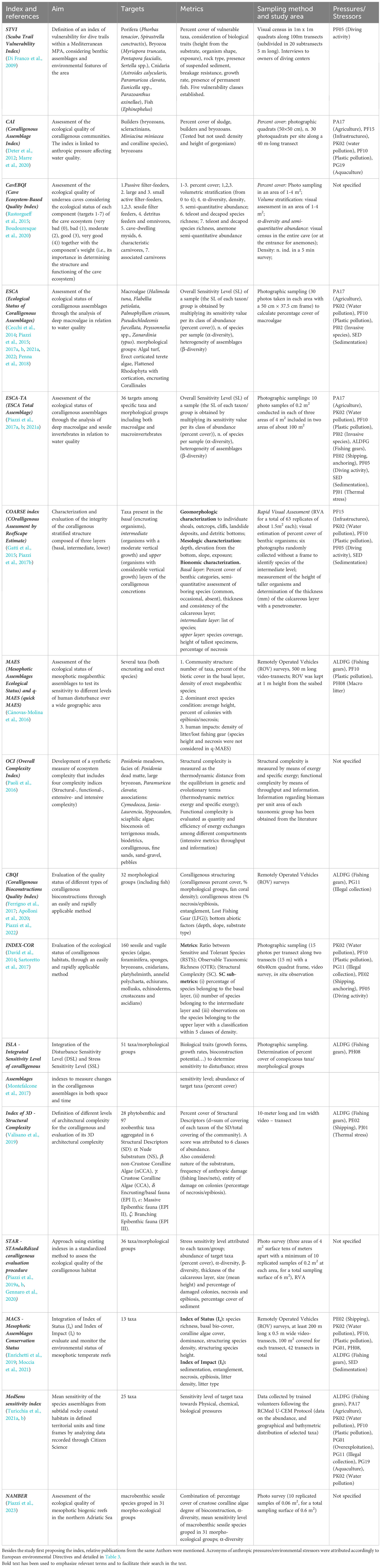
Table 1 Summary of indexes (in chronological order) to assess the ecological status of coralligenous reefs.
We reviewed the studies conceiving these indexes, comparing their objectives, metrics and applied methodologies to:
- detect the most suitable sampling methodologies for data collection on the repercussions of pressures/stressors on the three-dimensional architecture of the coralligenous habitats;
- highlight major issues hindering the creation of a common approach to assess the ecological quality of coralligenous habitats;
- supply a list of priority anthropic pressures/environmental stressors, indicators, and metrics as a starting point to forge a common index;
- standardize nomenclature of anthropogenic pressures using, when possible, definitions from main European Directives on marine conservation;
- supply a process flow summarizing steps towards the consolidation of a multimetric biotic index to assess the quality of coralligenous in the Mediterranean Sea.
To retrieve papers about indexes designed to estimate the quality of coralligenous habitats, a literature search was conducted on the Web of Science (WoS) platform. The search was carried out using the string “Mediterranean AND coralligenous AND (index OR indexes OR indices)” in all the fields of the WoS Core Collection (last search 20/04/2023). In the first web-search, 60 documents were found. After screening the retrieved documents, by reading titles and abstracts, 23 papers were retained and considered relevant for this review. Five more articles (Di Franco et al., 2009; David et al., 2014; Rastorgueff et al., 2015; Paoli et al., 2016; Gennaro et al., 2020) were added after checking reference lists within the retrieved articles. The papers cover the development and implementation of 16 distinct indexes. For comparative purposes, the objectives, methodologies, considered pressures, and main characteristics of each analyzed index are detailed in Table 1 and summarized in Table 2.
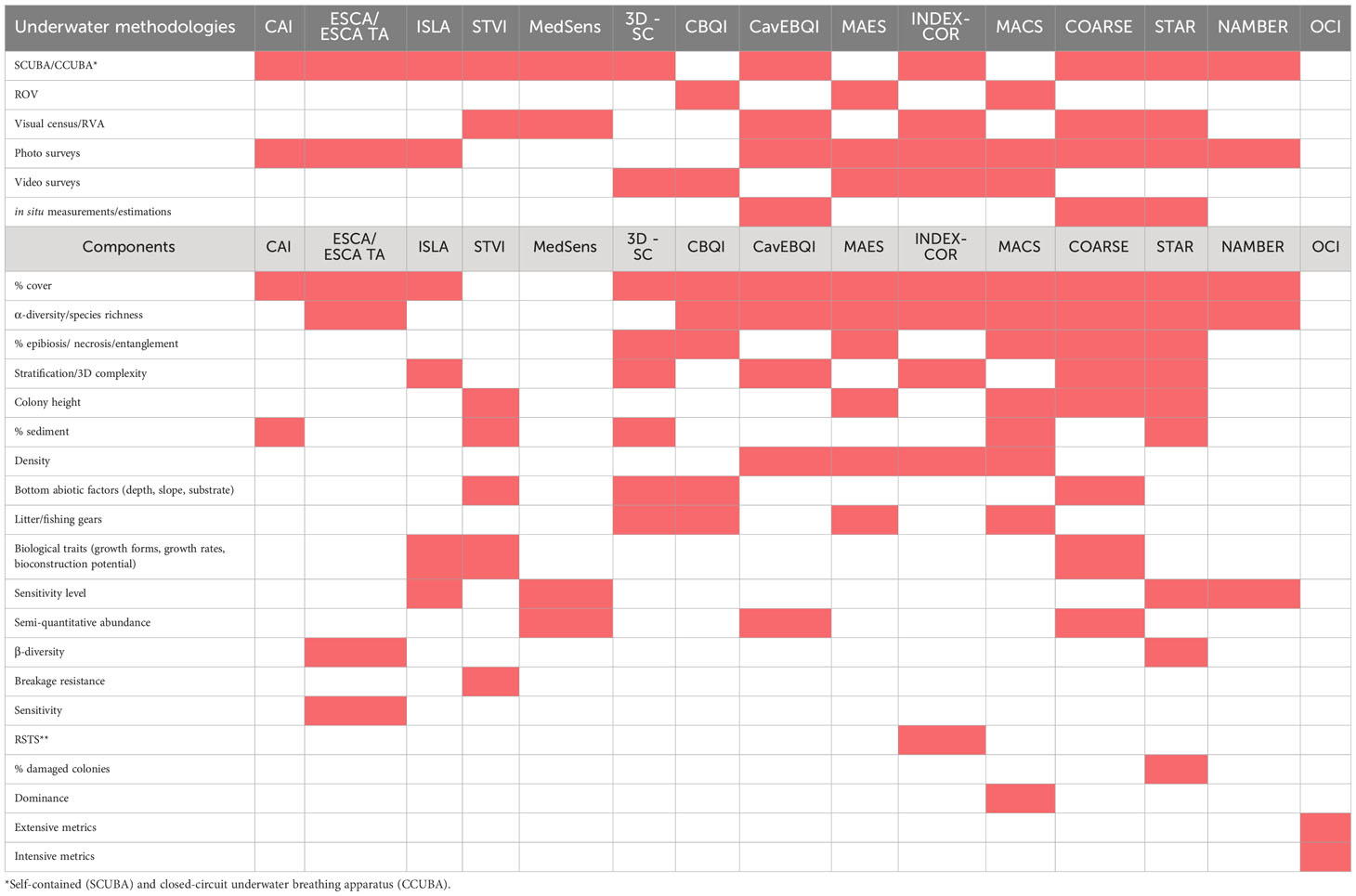
Table 2 Comparison of the underwater scientific methodologies applied by the considered studies to collect data and the components (i. e., metrics and parameters useful to obtain metrics, such as abundance) to compute each index.
The STVI (Scuba Trail Vulnerability Index, Di Franco et al., 2009) is based on a census of vulnerable benthic species populations that can potentially be affected by the direct (mechanical) or indirect (sediment resuspension) impacts of diving activity on the coralligenous. The suggested protocol is easy to be applied, however its usefulness is limited to narrow scuba trails where divers are in close proximity to walls/ceiling/substrates (i.e., submerged caves, tunnels, arcs, or canyons) and the damage caused can be reliably attributed to divers.
The CAI (Coralligenous Assemblage Index, (Deter et al., 2012)) was designed considering three metrics (i.e., the percentage coverage of sludge, builder taxa and bryozoans) correlated with anthropogenic pressures affecting water quality. The ‘builders’ of the coralligenous framework considered include coralline algae, bryozoans, scleractinians, and the foraminifera Miniacina miniacea (Pallas, 1766). Other animal builders such as serpulids polychaetas or sponges (Ballesteros, 2006) are not considered. Metrics referring to gorgonian demography (colony height and necrosis) are discarded because injuries to these long-living organisms may be related to ancient stress, such as heat waves (Garrabou et al., 2022a), and not to more recent anthropogenic pressures. Other indexes (i.e., MAES, CBQI, 3D complexity, STAR, MACS, see ahead) addressed this issue by linking the level of epibiosis to the age of the impact.
Biocenosis of semi-dark caves (at least at the entrance) can overlap with those of the coralligenous (Bellan-Santini et al., 2015), therefore the CavEBQI (Cave Ecosystem-Based Quality Index, Rastorgueff et al., 2015) was considered in this review. The index aims to evaluate the ecological quality of marine submerged caves by adapting the approach used to gauge the ecological quality of Posidonia oceanica meadows (Personnic et al., 2014). The data collection combines photo sampling, underwater estimations (volume stratification) and visual census to evaluate the status of seven ecological cave components (see details in Table 1). However, there is inherent risk associated with applying these complex protocols in an entire (accessible) underwater cave. Moreover, occurrence, abundance, and spatial distribution of vagile components (for example, cave-dwelling mysids) inside a cave may vary in relation to season, abiotic factors and reproductive behavior (Ribes et al., 1996), leading to inaccuracies when applying the index. Passive filter feeders are considered as the most valuable target given their sensitivity to pressures, but this component includes taxa that differ greatly in morphology, tolerance to disturbance and lifespan (e.g., hydrozoans and gorgonians). In these dark habitats, the geological structure of the caves, their rock composition and the possible occurrence of freshwater springs or hydrothermal emissions can greatly affect the composition of the benthic assemblage, which influences the outcomes of the index and how they are interpreted.
The ESCA (Ecological Status of Coralligenous Assemblages, Cecchi et al., 2014) index considers deep macroalgae (ten taxa/morphological groups) and three metrics to evaluate coralligenous status. The index is expressed as Ecological Quality Ratio (EQR) i.e., the ratio between the measured value for each metric (or Ecology Quality Value, EQV) and its expected value in the absence of major human disturbance (Reference Condition (RC), Water Frame Directive (WFD), 2000/60/EC).
Sessile macroinvertebrates are considered along with seaweeds to compute the ESCA-TA (Piazzi et al., 2017a) and to evaluate the response to disturbance of the “Total Assemblage”. The application of this last index highlighted that macroalgae are more suitable to detect pressures such as, the increase in nutrients or water turbidity, while the macroinvertebrates are more sensitive to the effects of water warming or mechanical damage, as reported previously (see Coma et al., 2006; Cerrano and Bavestrello., 2009; de la Nuez-Hernández et al., 2014). In general, the methodology is simple to apply and easy to replicate. However, reference conditions are not available for all sites (Ponti et al., 2009; Piazzi et al., 2021a) and may be difficult to define (Bouleau and Pont, 2015), especially if there is scarce knowledge of the possible exploitation of the study area.
The COARSE (COralligenous Assessment by ReefScape Estimate) index, (Gatti et al., 2015) evaluates the coralligenous integrity as a descriptor of the marine environmental status (as required by the MSFD) rather than water quality (as per WFD). Here, the vulnerability towards physical stress and mechanical damage of the multi-layer architecture of this habitat is considered. The COARSE index is based in part on a Rapid Visual Assessment (RVA), providing a time and cost-effective methodology, which allows for the direct collection of a sufficient amount of data with a congruent diving effort (Gatti et al., 2012). However, visual estimations of percentage cover (Meese and Tomich, 1992) and species identification (Thompson and Mapstone, 1997) may be inaccurate at 30 m or deeper, given the limited dive time and need to collect other underwater measurements.
The combination of two indexes (COARSE and ESCA) was tested in the same area obtaining different but complementary information (Piazzi et al., 2017b) given the different objectives of the two indexes. However, the implementation of two or more underwater protocols is more demanding in terms of time and cost.
The INDEX-COR (Sartoretto et al., 2017) is based on in situ observation/estimations, combined with photographic sampling. The metrics Ratio between Sensitive and Tolerant Species (RSTS), Observable Taxonomic Richness (OTR), and Structural Complexity (SC) seems to be related to human pressures defined by expert judgment; however, the authors themselves stated that a more objective impact index is needed to correlate the structural complexity with anthropogenic pressures at a lower scale.
The Index of 3D - Structural Complexity (Valisano et al., 2019) aims to assess the status of coralligenous assemblages by aggregating taxa according to their morpho-functional role in the multi-layer structure of the habitat. A different weight is assigned to each cluster reflecting its relevance in the bioconstruction/bioerosion equilibrium and as ecosystem engineers building animal forests (Cerrano et al., 2019). The index assesses the 3D architectural complexity of coralligenous assemblages.
The STAR (STAndaRdized coralligenous evaluation procedure, Piazzi et al., 2019a) is the first attempt to standardize metrics and protocols to collect and to interpret data on the status of the coralligenous. Findings of a literature review revealed April-June to be the best period to carry out sampling activities according to seasonal dynamics of native and invasive macroalgae; while 35 m and 85–90 m were considered as optimal depth and slope, respectively to carry out photo-surveys. The authors also suggested carrying out sampling with high replication at small scales (i.e., tens of meters) and less replicates at intermediate or large scales (i.e., hundreds of meters to kilometers). The ideal sampling design should consider three areas of 4 m2, tens of meters apart, with at least 10 replicates of 0.2 m2 at each area, for a total sampling surface of 6 m2.
The ISLA Index (Integrated Sensitivity Level of coralligenous Assemblages, Montefalcone et al., 2017), allows for the change experienced by the coralligenous assemblages to be estimated in both space and time by multiplying abundance (i.e., percentage cover) of several target taxa/morphological groups per an integrated index that considers both sensitivity to disturbance and sensitivity to stress. Disturbance causing mortality or physical damage (subtraction of biomass) is evaluated considering the biological traits of the target taxa. Stress causing physiological alteration (reduction in productivity) and sensitivity to stress is estimated based on expert judgment.
The MedSens sensitivity index (Turicchia et al., 2021a) is a biotic index developed to provide information on the environmental status of subtidal rocky coastal habitats, and above all, coralligenous reefs. It uses data collected by trained volunteers (scuba divers, free divers, snorkelers) which record the abundance and depth ranges of the searched taxa through a protocol of simple application (The Reef Check Med Underwater Coastal Environment Monitoring (RCMed U-CEM) Protocol, Turicchia et al., 2021b). Despite the need for rigorous participant training (subject to learning tests) and steps to assure data quality, the protocol is easy to learn and provides a large amount of timely, up to date geo referenced data (Turicchia et al., 2021b, c) which is the greatest benefit of this approach. The sensitivity level to physical, chemical, and biological pressures is attributed to selected taxa according to the Marine Evidence-based Sensitivity Assessment (MarESA, Tyler-Walters et al., 2018). The index calculates the mean sensitivity of the various taxa considered weighted for the abundance class of the taxa.
Three indexes were based on photography and video footage collected by Remotely Operated Vehicle (ROV): the CBQI (Coralligenous Bioconstructions Quality Index, Ferrigno et al., 2017) focused on fishing pressure, the MAES (Mesophotic Assemblages Ecological Status, Cánovas-Molina et al., 2016), and the MACS (Mesophotic Assemblages Conservation Status, Enrichetti et al., 2019) to monitor the environmental status of mesophotic temperate reefs. ROVs allow exploration of deep reefs (Jones et al., 2007; Enrichetti et al., 2019) providing sufficient levels of detail and a large amount of recorded information (Smith and Rumohr, 2005).
The NAMBER Index (North Adriatic Mesophotic BiogEnic Reefs, Piazzi et al., 2023), draws inspiration from the STAR and ESCA-TA indices, by selecting the metrics and adapting the interpretation scales to the ecological and operational conditions encountered when investigating the mesophotic coralligenous banks in the northern Adriatic Sea. Starting with a close-up photographic sampling strategy, the degree of bioconstruction (expressed as percentage cover of crustose coralline algae), α diversity (expressed as the mean number of taxa), and the degree of sensitivity to human disturbances and climate changes (based on literature data and expert judgement) of the benthic assemblages are combined in the index, in terms of ecological quality ratios (sensu WFD) using as a reference the best values that the three metrics can currently achieve in the studied region.
Lastly, the OCI (Overall Complexity Index, Paoli et al., 2016) aims to measure habitat structural and functional complexity through a thermodynamic analysis, based on exergy and specific exergy, and a network analysis. The index was tested in an MPA where the most complex habitats were found to be the seagrass meadows and the coralligenous reefs. The information about environmental quality and natural capital was already available for the study area, but a larger scale application of this index would require considerable data collection and a sound baseline.
Table 2 summarizes and compares the underwater scientific methodologies applied in the studies considered to collect data and the components (i.e., metrics and parameters useful to obtain metrics, such as abundance) to calculate the indexes. All the proposed approaches (Tables 1, 2) are based on non-destructive methods but, when considered, the thickness of the calcareous layer is measured in a destructive way (insertion of a tool marked with a millimetric scale in the biogenic concretion, Piazzi et al., 2019a). However, the damage caused is limited.
Three indexes (CBQI, MAES, MACS), use ROV exploration, one (CAI) uses CCUBA (Closed Circuit Underwater Breathing Apparatus) to sample down to 84 m, while the others use SCUBA diving. According to Gennaro et al. (2020), sampling within a depth of 40 m should be carried out via SCUBA divers while, for safety reasons, below this depth the coralligenous should be explored using ROVs. However, trimix diving (either open or closed breathing circuits) by well-trained operators could be useful to conduct the surveys with high levels of safety down to 90-100 m depth.
Higher performing ROV systems, intended for > 80 m depth, are more expensive, and often require specialized operators to be maneuvered and large boats to be transported.
Photo surveys are the preferred method for underwater data collection (10 indexes), followed by visual census (6) and video surveys (5). Percent cover estimates from photo samples is the most applied procedure to estimate benthic species’ abundance.
The same index could be calculated using more than one underwater sampling method, for example photo/video surveys could be carried out via SCUBA diving or an ROV.
Video-surveys and photo quadrats appear to be the best methods to collect data on the coralligenous. Video transects randomly conducted in georeferenced repeatable stations are the most efficient, fastest, and simplest method to detect general changes in the habitat architecture. Besides supplying permanent records about density and size of habitat-formers (i.e., gorgonians, Savalia savaglia (Bertoloni, 1819), erect sponges…), the videos contain information about the geomorphological features of the site, occurrence of macro-invasive species and lost fishing gears. Photo-surveys provide a better estimate of the percentage coverage of organisms with a low elevation from the substrate (e.g., encrusting, massive or little branched phyto/zoobenthos).
Despite their accuracy, the complexity of some approaches renders them unsuitable for the citizen science context (a helpful strategy fostering the continuous monitoring of the effects of thermal stress and anthropic pressures on a larger spatial and temporal scale (Krželj et al., 2020)).
Even if citizen science cannot override professional monitoring activities, with the number of target species often being lower than the taxa considered by scientists, an easy-to-apply citizen science-based index for the Mediterranean Sea, providing a community-based monitoring strategy, would be a valuable tool to gather a constant flux of data on benthic communities (Kelly et al., 2020). Examples of citizen science projects engaging divers in the study and monitoring of Mediterranean coralligenous assemblages are the CIGESMED SeasEra (ERAnet) (Gerovasileiou et al., 2016), the Reef Check Mediterranean Underwater Coastal Environment Monitoring Protocol (Ponti et al., 2021; Turicchia et al., 2021b; Turicchia et al., 2021c), and the derived MedSens index.
Establishing an unambiguous list of anthropic pressure and environmental stressors on the coralligenous habitat is fundamental to assess its environmental and ecological quality. Given that different descriptions of pressures were used in the reviewed papers, here, a standardized nomenclature for 15 forms of pressure/stressors on the coralligenous has been devised (Table 3). Attempts were made to align the pressures with those defined by EU regulations (the Habitat Directive (Council Directive 92/43/EEC), the MSFD, and the EU Water Frame Directive (WFD, Directive 2000/60/EC), according to Article 17 report formats of the Habitats Directive (https://cdr.eionet.europa.eu/last updated: 24/01/2023)).
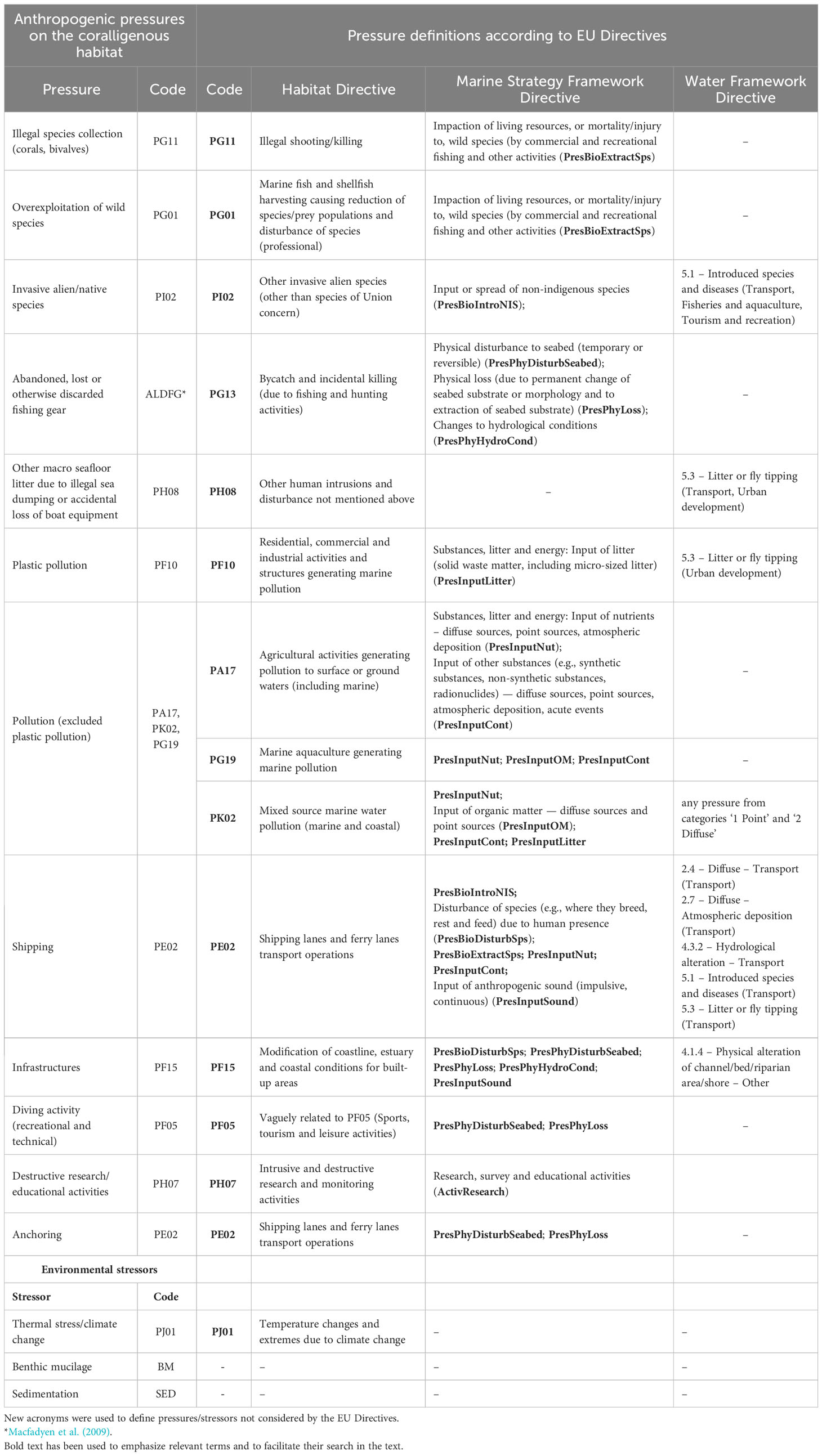
Table 3 List of main anthropogenic pressures/environmental stressors on the coralligenous habitat and eventual corresponding descriptions/codes from the main environmental EU Directives according to the Article 17 report formats of the Habitats Directive (https://cdr.eionet.europa.eu/help/habitats_art17 last updated: 24/01/2023).
Pollution is the most considered pressure in the indexes (47%) reviewed, followed by abandoned, lost or discarded fishing gear (40%, ALDFG, Macfadyen et al., 2009).
Some pressures/stressors on coralligenous habitat are easy to detect and quantify due to the presence of long-lasting macroscopic signs (such as presence of marine litter, lost fishing gear, benthic mucilage, invasive and non-indigenous macrozoo- and phytobenthos, high sedimentation, collection of date mussels). On the contrary, it could be tough to attribute signs of damage (breakages, necrosis/epibiosis) to intangible and temporary pressures/stressors (liquid waste, contacts of divers, heatwaves, storms), above all in case of cumulative impacts. In the next paragraph we better analysed these issues and explored some solutions.
The indexes can produce inaccurate assessments of the environmental status where the low diversity is related to intrinsic traits of the site (water movements, light, geomorphology, food availability) rather than human disturbance (Enrichetti et al., 2019; Valisano et al., 2019; Pierdomenico et al., 2021), such as the assemblage of the coralligenous developed on granitic substrates (Canessa et al., 2020). Another issue detected in this review is how to define intermediate environmental status classes (i.e., good, moderate, poor) if historical data about biodiversity and pressures are unavailable and the pristine conditions are unknown (Ponti et al., 2009 and references therein). However, these obstacles will be overcome once periodic and continuous monitoring campaigns are established to detect the evolution of benthic communities and to differentiate among fluctuations, disease events and human pressure (Franz et al., 2019). As for other monitoring programs of the MSFD, the establishment of fixed stations (García-Gómez et al., 2020) will be indispensable for observing the dynamics of benthic communities and to assess their environmental status. These could be in known coralligenous hotspots (Martin et al., 2014; Di Camillo et al., 2018a), independent of their level of protection, within which random sampling could be conducted.
To establish the causes of biodiversity loss that are unrelated to mechanical damage, trends in physical and chemical parameters should be considered. Funding should be provided to increase the analysis of water/biota and to provide the fixed stations with benthic temperature loggers placed at different depths and multisensory buoys transmitting real-time data to a monitoring network. The analysis of the collected data would be valuable in helping link the transformation occurring in the coralligenous to atmospheric, oceanographic, and biogeochemical conditions (Bahamon et al., 2020; Garrabou et al., 2022b).
One of the major issues in designing an index to define the ecological status of a habitat is the attribution of a sensitivity level to taxa/morphological groups. The sensitivity level may be available in the literature, or expert judgement may be used (Piazzi et al., 2017a and references therein). However, sometimes it is unclear why some taxa are more sensitive than others; for example, Eunicella cavolini and Cystoseira spp. are considered more sensitive than Paramuricea clavata by the ESCA, while P. clavata was the most sensitive taxon for the MedSens index (Turicchia et al., 2021a) because of its sensitivity to physical, chemical, and biological pressures. According to Turicchia et al. (2021a), most sensitive taxa are long-living cnidarians. Since these species reflects the highest complexity level of the coralligenous, it would be crucial to assign one of the largest weights in the design of a new index.
To evaluate the status of coralligenous health, it is crucial to know its successional stages. The accretion rate of the coralligenous is very low (0.006–0.83 mm yr–1) and variable depending on depth and period (Sartoretto, 1994; Turicchia et al., 2022), suggesting that dynamics of this environment are difficult to study on a short-term timescale (Garrabou and Harmelin, 2002). Mortality events drive a shift from ecosystem engineering (e.g., Paramuricea clavata, Corallium rubrum) to fast-growing and photophilous species (among which are encrusting organisms, serpulids, hydrozoans, turf-forming algae (Ponti et al., 2014; Cerrano et al., 2019; Gómez-Gras et al., 2021)). In some cases, bryozoans recolonize bare coralligenous after a severe impact in a few years (Cocito and Sgorbini, 2014; Casoli et al., 2020, and personal observations), but also other patterns are documented (Piazzi et al., 2021b). On the contrary, re-settlement and growth of gorgonians or red coral may be very slow (Garrabou and Harmelin, 2002; Cerrano et al., 2005; Cupido et al., 2009; Huete-Stauffer et al., 2013); in some cases, no signs of recovery have been registered even after a ten-year period (Ruffaldi Santori et al., 2021). Information about recovery of habitat-formers after mortality is scant and highlights a knowledge gap that needs to be filled urgently.
Heatwaves, changes in thermohaline circulation, and mucilage events are rapidly re-shaping bioconstructions in the Mediterranean basin (Cramer et al., 2018; Bevilacqua et al., 2021; Garrabou et al., 2022a); therefore, in these unprecedent critical conditions, the index design should focus first on the response of the coralligenous to these stressors.
Considering that heatwaves and mucilage events occur from late spring to late autumn (Garrabou et al., 2009), data collection should be carried out in early spring (April or May) and autumn (October) to have a better understanding of the effects of these pressures and the resistance and resilience of coralligenous bioconstructions (Ponti et al., 2014).
Based on these reflections, a list of priority anthropic pressures and environmental stressors and relative indicators are suggested below (Table 4).
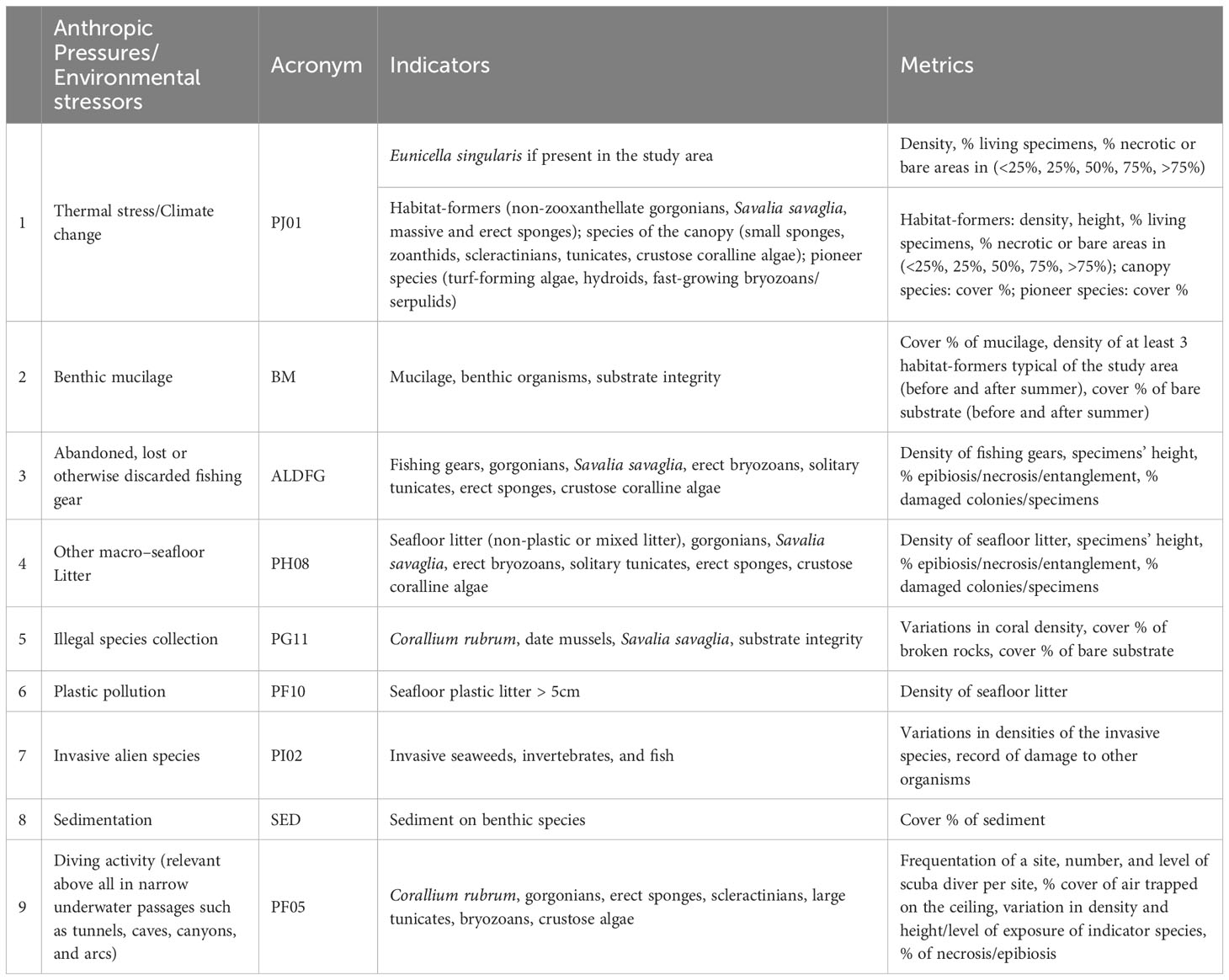
Table 4 List of priority anthropic pressures/environmental stressors, indicators, and metrics as a starting point to define a shared index to assess the environmental quality of the coralligenous habitats.
This work highlights the urgent need to align the methodologies to assess the status of the coralligenous habitat at basin scale. The steps needed to develop a unified index are illustrated in the framework below (Figure 1) and detailed here:
1. Adoption of a standardized method of data acquisition (regardless of the underwater exploration technique). Darwin Core glossary terms should be used as the biodiversity data standard (Wieczorek et al., 2012; Di Camillo et al., 2018b). When possible, data should be integrated through Marine Citizen Science (MCS) protocols, Local Ecological Knowledge (LEK, Azzurro et al., 2019), and Web Ecological Knowledge (WEK, Di Camillo et al., 2018a).
2. Definition of a scale of scores correspondent to different classes of coralligenous quality.
3. Set up of a user-friendly digital tool that could support the collection of data and its visualization and improve the applicability of the index. The new tool should both calculate the index (as per the software for assessing the quality of soft-bottom benthic macroinvertebrate communities (AMBI, Borja et al., 2000)) and allow the spatial and temporal visualization of the scores through geographic information systems (as per the plugin to compute the MedSens index (Turicchia et al., 2021b).
4. Consideration of FAIR principles (Findability, Accessibility, Interoperability and Reusability (Tanhua et al., 2019) to make protocols, digital support tools and collected data more readily available.

Figure 1 Main stages proposed to develop a unified index to assess the health status of the coralligenous habitat. MCS, Marine Citizen Science; LEK, Local Ecological Knowledge; WEK, Web Ecological Knowledge; FAIR, Findability, Accessibility, Interoperability and Reusability.
Until now, the only open access data is the Reef Check Med dataset on key Mediterranean marine species (Turicchia et al., 2021c), hosted by the European Marine Observation and Data Network (EMODnet; Martín Míguez et al., 2019), and periodically updated on the dedicated webpage (https://www.reefcheckmed.org).
Open science is a priority for the European Commission (EC), and data obtained from the application of a monitoring protocol and the relative index should respect the FAIR principles (Findability, Accessibility, Interoperability and Reusability (Tanhua et al., 2019); therefore, data should be shared for their re-use in an EU repository (e.g., the European Marine Observation and Data Network (EMODnet, https://emodnet.ec.europa.eu), Copernicus, https://www.copernicus.eu/among others).
All data are available as tables embedded in this study.
CDC conceived the idea, and wrote the original draft. MP contributed to writing the original draft. AS, CS, CR, TM, MC, CC contributed to searching articles and preparing tables. All authors contributed to the article and approved the submitted version.
This research was supported by the Università Politecnica delle Marche (Ricerca Scientifica di Ateneo, RSA).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Apolloni L., Ferrigno F., Russo G. F., Sandulli R. (2020). β-Diversity of morphological groups as indicator of coralligenous community quality status. Ecol. Indic. 109, 105840. doi: 10.1016/j.ecolind.2019.105840
Article 17 report formats of the Habitats Directive. Available at: https://cdr.eionet.europa.eu/help/habitats_art17/2013-2018 (Accessed 01/01/2023).
Azzurro E., Sbragaglia V., Cerri J., Bariche M., Bolognini L., Ben Souissi J., et al. (2019). Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: A large-scale survey based on local ecological knowledge. Glob. Change Biol. 25, 2779–2792. doi: 10.1111/gcb.14670
Bahamon N., Aguzzi J., Ahumada-Sempoal M.Á., Bernardello R., Reuschel C., Company J. B., et al. (2020). Stepped coastal water warming revealed by multiparametric monitoring at NW Mediterranean fixed stations. Sensors 20 (9), 2658. doi: 10.3390/s20092658
Ballesteros E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr. Mar. Biol.: Ann. Rev. 44, 123–195. doi: 10.1201/9781420006391.ch4
Bavestrello G., Bertolino M., Betti F., Bianchi C. N., Cattaneo-Vietti R., Montefalcone M., et al. (2016). New perspectives in the study of Mediterranean coralligenous assemblages. Biol. Mar. Mediterr. 23 (1), 170–173.
Bellan-Santini D., Bellan G., Bitar G., Harmelin J.-G., Pergent G. (2015). Handbook for interpreting types of marine habitat for the selection of sites to be included in the national inventories of natural sites of conservation interest. Action Plan for the Mediterranean Sea. United Nations Environment Programme. Regional Activity Centre for Specially Protected Areas (RAC/SPA) (Tunis, Tunisia).
Bertolino M., Cerrano C., Bavestrello G., Carella M., Pansini M., Calcinai B. (2013). Diversity of Porifera in the Mediterranean coralligenous accretions, with description of a new species. ZooKeys 336, 1–37. doi: 10.3897/zookeys.336.5139
Bevilacqua S., Airoldi L., Ballesteros E., Benedetti-Cecchi L., Boero F., Bulleri F., et al. (2021). Mediterranean rocky reefs in the Anthropocene: Present status and future concerns. Adv. Mar. Biol. 89, 1–51. doi: 10.1016/bs.amb.2021.08.001
Borja A., Franco J., Pérez V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 40 (12), pp.1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Boudouresque C. F., Astruch P., Bănaru D., Blanchot J., Blanfuné A., Carlotti F., et al. (2020). “The Management of Mediterranean Coastal Habitats: A Plea for a Socio-ecosystem-Based Approach,” in Evolution of Marine Coastal Ecosystems under the Pressure of Global Changes (Cham: Springer), 297–320.
Bouleau G., Pont D. (2015). Did you say reference conditions? Ecological and socio-economic perspectives on the European Water Framework Directive. Environ. Sci. Policy. 4, 32–41. doi: 10.1016/j.envsci.2014.10.012
Canessa M., Bavestrello G., Bo M., Trainito E., Panzalis P., Navone A., et al. (2020). Coralligenous assemblages differ between limestone and granite: a case study at the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Mediterranean Sea). Regional Stud. Mar. Sci. 35, 101159. doi: 10.1016/j.rsma.2020.101159
Cánovas-Molina A., Montefalcone M., Bavestrello G., Cau A., Bianchi C. N., Morri C., et al. (2016). A new ecological index for the status of mesophotic megabenthic assemblages in the Mediterranean based on ROV photography and video footage. Continental Shelf Res. 121, 13–20. doi: 10.1016/j.csr.2016.01.008
Casoli E., Piazzi L., Nicoletti L., Jona-Lasinio G., Cecchi E., Mancini G., et al. (2020). Ecology, distribution and demography of erect bryozoans in Mediterranean coralligenous reefs. Estuarine Coast. Shelf Sci. 235, 106573. doi: 10.1016/j.ecss.2019.106573
Cecchi E., Gennaro P., Piazzi L., Ricevuto E., Serena F. (2014). Development of a new biotic index for ecological status assessment of Italian coastal waters based on coralligenous macroalgal assemblages. Eur. J. Phycology 49 (3), 298–312. doi: 10.1080/09670262.2014.918657
Cerrano C., Arillo A., Azzini F., Calcinai B., Castellano L., Muti L., et al. (2005). Gorgonian population recovery after a mass mortality event. Aquat. Conservation: Mar. Freshw. Ecosyst. 15 (2), 147–157. doi: 10.1002/aqc.661
Cerrano C., Bastari A., Calcinai B., Di Camillo C., Pica D., Puce S., et al. (2019). Temperate mesophotic ecosystems: gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur. Zoological J. 86 (1), 370–388. doi: 10.1080/24750263.2019.1677790
Cerrano C., Bavestrello G. (2009). “Mass mortalities and extinctions,” in Marine hard bottom communities (Berlin, Heidelberg: Springer), 295–307.
Cerrano C., Bavestrello G., Bianchi C. N., Cattaneo-Vietti R., Bava S., Morganti C., et al. (2000). A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol. Lett. 3 (4), 284–293. doi: 10.1046/j.1461-0248.2000.00152.x
Chimienti G., Stithou M., Mura I. D., Mastrototaro F., D’Onghia G., Tursi A., et al. (2017). An explorative assessment of the importance of mediterranean coralligenous habitat to local economy: The case of recreational diving. J. Environ. Account. Manage. 5, 315–325. doi: 10.5890/JEAM.2017.12.004
Cocito S., Sgorbini S. (2014). Long-term trend in substratum occupation by a clonal, carbonate bryozoan in a temperate rocky reef in times of thermal anomalies. Mar. Biol. 161 (1), 17–27. doi: 10.1007/s00227-013-2310-9
Coma R., Linares C., Ribes M., Diaz D., Garrabou J., Ballesteros E. (2006). Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). Mar. Ecol. Prog. Ser. 327, 51–60. doi: 10.3354/meps327051
Cramer W., Guiot J., Fader M., Garrabou J., Gattuso J. P., Igliesas A., et al. (2018). Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Climate Change 8 (11), 972–980. doi: 10.1038/s41558-018-0299-2
Cupido R., Cocito S., Barsanti M., Sgorbini S., Peirano A., Santangelo G. (2009). Unexpected long-term population dynamics in a canopy-forming gorgonian coral following mass mortality. Mar. Ecol. Prog. Ser. 394, 195–200. doi: 10.3354/meps08260
Danovaro R., Fonda Umani S., Pusceddu A. (2009). Climate change and the potential spreading of marine mucilage and microbial pathogens in the Mediterranean Sea. PloS One 4 (9), e7006. doi: 10.1371/journal.pone.0007006
David R., Dubois S., Erga Z., Guillemain D., de Ville d’Avray L. T., Arvanitidis C., et al. (2014). “CIGESMED’s protocol and network (Coralligenous based Indicators to evaluate and monitor the “Good Environmental Status” of Mediterranean coastal waters),” in Proceedings of 5th International Symposium MONITORING OF MEDITERRANEAN COASTAL AREAS: PROBLEMS AND MEASUREMENT TECHNIQUES Edition: Livorno (Italy) 17-18-19 June 2014. CNR-IBIMET Florence (Italy), Benincasa F. ed, 828.
de la Nuez-Hernández D., Valle C., Forcada A., Correa J. M. G., Torquemada Y. F. (2014). Assessing the erect bryozoan Myriapora truncata (Palla) as indicator of recreational diving impact on coralligenous reef communities. Ecol. Indic. 46, 193–200. doi: 10.1016/j.ecolind.2014.05.035
Deter J., Descamp P., Ballesta L., Boissery P., Holon F. (2012). A preliminary study toward an index based on coralligenous assemblages for the ecological status assessment of Mediterranean French coastal waters. Ecol. Indic. 20, 345–352. doi: 10.1016/j.ecolind.2012.03.001
Di Camillo C. G., Gravili C., De Vito D., Pica D., Piraino S., Puce S., et al. (2018b). The importance of applying Standardised Integrative Taxonomy when describing marine benthic organisms and collecting ecological data. Invertebrate Systematics 32 (4), 794–802. doi: 10.1071/IS17067
Di Camillo C. G., Ponti M., Bavestrello G., Krželj M., Cerrano C. (2018a). Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodiversity Conserv. 27 (5), 1257–1276. doi: 10.1007/s10531-017-1492-8
Di Franco A., Milazzo M., Baiata P., Tomasello A., Chemello R. (2009). Scuba diver behaviour and its effects on the biota of a Mediterranean marine protected area. Environ. Conserv. 36 (1), 32–40. doi: 10.1017/S0376892909005426
Enrichetti F., Bo M., Morri C., Montefalcone M., Toma M., Bavestrello G., et al. (2019). Assessing the environmental status of temperate mesophotic reefs: A new, integrated methodological approach. Ecol. Indic. 102, 218–229. doi: 10.1016/j.ecolind.2019.02.028
Ferrigno F., Appolloni L., Russo G. F., Sandulli R. (2018). Impact of fishing activities on different coralligenous assemblages of Gulf of Naples (Italy). J. Mar. Biol. Assoc. United Kingdom 98 (1), 41–50. doi: 10.1017/S0025315417001096
Ferrigno F., Russo G. F., Sandulli R. (2017). Coralligenous Bioconstructions Quality Index (CBQI): A synthetic indicator to assess the status of different types of coralligenous habitats. Ecol. Indic. 82, 271–279. doi: 10.1016/j.ecolind.2017.07.020
Franz M., Barboza F. R., Hinrichsen H. H., Lehmann A., Scotti M., Hiebenthal C., et al. (2019). Long-term records of hard-bottom communities in the southwestern Baltic Sea reveal the decline of a foundation species. Estuarine Coast. Shelf Sci. 219, 242–251. doi: 10.1016/j.ecss.2019.02.029
García-Gómez J. C., González A. R., Maestre M. J., Espinosa F. (2020). Detect coastal disturbances and climate change effects in coralligenous community through sentinel stations. PloS One 15 (5), e0231641. doi: 10.1371/journal.pone.0231641
Garrabou J., Bensoussan N., Di Franco A., Boada J., Cebrian E., Santamaria J., et al. (2022b). Monitoring Climate-related responses in Mediterranean Marine Protected Areas and beyond: ELEVEN STANDARD PROTOCOLS. 74 pp. Edited by: Institute of Marine Sciences, Spanish Research Council ICM-CSIC, Passeig Marítim de la Barceloneta 37-49 (Barcelona, Spain).
Garrabou J., Coma R., Bensoussan N., Bally M., Chevaldonné P., Cigliano M., et al. (2009). Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Global Change Biol. 15 (5), 1090–1103. doi: 10.1111/j.1365-2486.2008.01823.x
Garrabou J., Gómez-Gras D., Medrano A., Cerrano C., Ponti M., Schlegel R., et al. (2022a). Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Global Change Biol. 28 (19), pp.5708–5725. doi: 10.1111/gcb.16301
Garrabou J., Harmelin J. G. (2002). A 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: insights into conservation and management needs. J. Anim. Ecol. 71 (6), 966–978. doi: 10.1046/j.1365-2656.2002.00661.x
Gatti G., Bianchi C. N., Morri C., Montefalcone M., Sartoretto S. (2015). Coralligenous reefs state along anthropized coasts: Application and validation of the COARSE index, based on a rapid visual assessment (RVA) approach. Ecol. Indic. 52, 567–576. doi: 10.1016/j.ecolind.2014.12.026
Gatti G., Montefalcone M., Rovere A., Parravicini V., Morri C., Albertelli G., et al. (2012). Seafloor integrity down the harbor waterfront: the coralligenous shoals off Vado Ligure (NW Mediterranean). Adv. Oceanography Limnology 3 (1), 51–67. doi: 10.4081/aiol.2012.5326
Gennaro P., Piazzi L., Cecchi E., Montefalcone M., Morri C., Bianchi C. N. (2020). Monitoraggio e valutazione dello stato ecologico dell’habitat a coralligeno. Il coralligeno di parete. ISPRA, Manuali e Linee Guida. Available at: https://www.isprambiente.gov.it/files2020/pubblicazioni/manuali-e-linee-guida/mlg-191-2020-ec.pdf.
Gerovasileiou V., Dailianis T., Panteri E., Michalakis N., Gatti G., Sini M., et al. (2016). CIGESMED for divers: Establishing a citizen science initiative for the mapping and monitoring of coralligenous assemblages in the Mediterranean Sea. Biodiversity Data J. 4, e8692. doi: 10.3897/BDJ.4.e8692
Gómez-Gras D., Linares C., Dornelas M., Madin J. S., Brambilla V., Ledoux J.-B., et al. (2021). Climate change transforms the functional identity of Mediterranean coralligenous assemblages. Ecol. Lett. 24 (5), 1038–1051. doi: 10.1111/ele.13718
Huete-Stauffer C., Previati M., Scinto A., Palma M., Pantaleo U., Cappanera V., et al. (2013). Long-term monitoring in a multi-stresses Paramuricea clavata population. Rapp. Commun. Int. Mer Médit 40, 654.
Ingrosso G., Abbiati M., Badalamenti F., Bavestrello G., Belmonte G., Cannas R., et al. (2018). Mediterranean bioconstructions along the Italian coast. Advances in marine biology 79, 61–136. doi: 10.1016/bs.amb.2018.05.001
Jones D. O. B., Wigham B. D., Hudson I. R., Bett B. J. (2007). Anthropogenic disturbance of deep-sea megabenthic assemblages: a study with remotely operated vehicles in the Faroe-Shetland Channel, NE Atlantic. Mar. Biol. 151, 1731–1741. doi: 10.1007/s00227-007-0606-3
Kelly R., Fleming A., Pecl G. T., von Gönner J., Bonn A. (2020). Citizen science and marine conservation: a global review. Philos. Trans. R. Soc. B 375 (1814), 20190461. doi: 10.1098/rstb.2019.0461
Krželj M., Cerrano C., Di Camillo C. G. (2020). Enhancing Diversity Knowledge through Marine Citizen Science and Social Platforms: The Case of Hermodice carunculata (Annelida, Polychaeta). Diversity 12 (8), 311. doi: 10.3390/d12080311
Linares C., Garrabou J., Hereu B., Diaz D., Marschal C., Sala E., et al. (2012). Assessing the effectiveness of marine reserves on unsustainably harvested long-lived sessile invertebrates. Conserv. Biol. 26 (1), 88–96. doi: 10.1111/j.1523-1739.2011.01795.x
Macfadyen G., Huntington T., Cappell R. (2009). Abandoned, lost or otherwise discarded fishing gear. UNEP Regional Seas Reports and Studies, No. 185 (Rome: FAO Fisheries and Aquaculture Technical Paper), 115. Available at: https://www.fao.org/3/i0620e/i0620e.pdf
Marre G., Braga C. D. A., Ienco D., Luque S., Holon F., Deter J. (2020). Deep convolutional neural networks to monitor coralligenous reefs: operationalizing biodiversity and ecological assessment. Ecol. Inf. 59, 1–26. doi: 10.1016/j.ecoinf.2020.101110
Martin C. S., Giannoulaki M., De Leo F., Scardi M., Salomidi M., Knittweis L., et al. (2014). Coralligenous and maërl habitats: predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 4 (1), 1–9. doi: 10.1038/srep05073
Martín Míguez B., Novellino A., Vinci M., Claus S., Calewaert J. B., Vallius H., et al. (2019). The European Marine Observation and Data Network (EMODnet): visions and roles of the gateway to marine data in Europe. Front. Mar. Sci. 6, 313. doi: 10.3389/fmars.2019.00313
Meese R. J., Tomich P. A. (1992). Dots on the rocks: a comparison of percent cover estimation methods. J. Exp. Mar. Biol. Ecol. 165 (1), pp.59–pp.73. doi: 10.1016/0022-0981(92)90289-M
Moccia D., Cau A., Carugati L. C. (2021). “October. Assessing the Environmental Status of five Sardinian black corals forests via Mesophotic Assemblages Conservation Status Index (MACS),” in 2021 International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea) (IEEE Institute of Electrical and Electronics Engineers), 204–208. doi: 10.1109/MetroSea52177.2021.9611628
Montefalcone M., Morri C., Bianchi C. N., Bavestrello G., Piazzi L. (2017). The two facets of species sensitivity: Stress and disturbance on coralligenous assemblages in space and time. Mar. Pollut. Bull. 117 (1-2), 229–238. doi: 10.1016/j.marpolbul.2017.01.072
Paoli C., Morten A., Bianchi C. N., Morri C., Fabiano M., Vassallo P. (2016). Capturing ecological complexity: OCI, a novel combination of ecological indexes as applied to benthic marine habitats. Ecol. Indic. 66, 86–102. doi: 10.1016/j.ecolind.2016.01.029
Penna M., Gennaro P., Bacci T., Trabucco B., Cecchi E., Mancusi C., et al. (2018). Multiple environmental descriptors to assess ecological status of sensitive habitats in the area affected by the Costa Concordia shipwreck (Giglio Island, Italy). J. Mar. Biol. Assoc. U. K. 98 (1), 51–59. doi: 10.1017/S0025315417001485
Personnic S., Boudouresque C. F., Astruch P., Ballesteros E., Blouet S., Bellan-Santini D., et al. (2014). An ecosystem-based approach to assess the status of a Mediterranean ecosystem, the Posidonia oceanica seagrass meadow. PloS One 9 (6), e98994. doi: 10.1371/journal.pone.0098994
Piazzi L., Atzori F., Cadoni N., Cinti M. F., Frau F., Pansini A, et al. (2021b). Animal forest mortality: Following the consequences of a gorgonian coral loss on a Mediterranean coralligenous assemblage. Diversity 13 (3), 133. doi: 10.3390/d13030133
Piazzi L., Bianchi C. N., Cecchi E., Gatti G., Guala I., Morri C., et al. (2017b). What’s in an index? Comparing the ecological information provided by two indexes to assess the status of coralligenous reefs in the NW Mediterranean Sea. Aquat. Conservation: Mar. Freshw. Ecosyst. 27 (6), 1091–1100. doi: 10.1002/aqc.2773
Piazzi L., Cecchi E., Cinti M. F., Stipcich P., Ceccherelli G. (2019b). Impact assessment of fish cages on coralligenous reefs through the use of the STAR sampling procedure. Mediterr. Mar. Sci. 20 (3), 627–635. doi: 10.12681/mms.20586
Piazzi L., Ferrigno F., Guala I., Cinti M. F., Conforti A., De Falco G., et al. (2022). Inconsistency in community structure and ecological quality between platform and cliff coralligenous assemblages. Ecol. Indic. 136, 108657. doi: 10.1016/j.ecolind.2022.108657
Piazzi L., Gennaro P., Cecchi E., Bianchi C. N., Cinti M. F., Gatti G., et al. (2021a). Ecological status of coralligenous assemblages: Ten years of application of the ESCA index from local to wide scale validation. Ecol. Indic. 121, 107077. doi: 10.1016/j.ecolind.2020.107077
Piazzi L., Gennaro P., Cecchi E., Serena F. (2015). Improvement of the ESCA index for the evaluation of ecological quality of coralligenous habitat under the European Framework Directives. Mediterr. Mar. Sci. 16 (2), 419–426. doi: 10.12681/mms.1029
Piazzi L., Gennaro P., Cecchi E., Serena F., Bianchi C. N., Gatti G., et al. (2017a). Integration of ESCA index through the use of sessile invertebrates. Sci. Mar. 81 (2), 283–290. doi: 10.3989/scimar.04565.01B
Piazzi L., Gennaro P., Montefalcone M., Bianchi C. N., Cecchi E., Morri C., et al. (2019a). STAR: An integrated and standardized procedure to evaluate the ecological status of coralligenous reefs. Aquat. Conservation: Mar. Freshw. Ecosyst. 29 (2), 189–201. doi: 10.1002/aqc.2983
Piazzi L., Turicchia E., Rindi F., Falace A., Gennaro P., Abbiati M., et al. (2023). NAMBER: A biotic index for assessing the ecological quality of mesophotic biogenic reefs in the northern Adriatic Sea. Aquat. Conservation: Mar. Freshw. Ecosyst. 33, 298–311. doi: 10.1002/aqc.3922
Pierdomenico M., Bonifazi A., Argenti L., Ingrassia M., Casalbore D., Aguzzi L., et al. (2021). Geomorphological characterization, spatial distribution and environmental status assessment of coralligenous reefs along the Latium continental shelf. Ecol. Indic. 131, 108219. doi: 10.1016/j.ecolind.2021.108219
Ponti M., Grech D., Mori M., Perlini R. A., Ventra V., Panzalis P. A., et al. (2016). The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar. Biol. 163, 1–14. doi: 10.1007/s00227-016-2897-8
Ponti M., Perlini R. A., Ventra V., Grech D., Abbiati M., Cerrano C. (2014). Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PloS One 9 (7), e102782. doi: 10.1371/journal.pone.0102782
Ponti M., Turicchia E., Ferro F., Cerrano C., Abbiati M. (2018). The understorey of gorgonian forests in mesophotic temperate reefs. Aquatic Conservation: Marine and Freshwater Ecosystems 28 (5), 1153–1166. doi: 10.1002/aqc.2928
Ponti M., Turicchia E., Rossi G., Cerrano C. (2021). Reef check med – key Mediterranean marine species 2001-2020. Dataset maintained by Reef Check Italia onlus. EMODnet Biol. Data Portal. doi: 10.14284/468
Ponti M., Vadrucci M. R., Orfanidis S., Pinna M. (2009). Biotic indexes for ecological status of transitional water ecosystems. Transit. Water. Bull. 3, 32–90. doi: 10.1285/i1825229Xv3n3p32
Rastorgueff P. A., Bellan-Santini D., Bianchi C. N., Bussotti S., Chevaldonné P., Guidetti P., et al. (2015). An ecosystem-based approach to evaluate the ecological quality of Mediterranean undersea caves. Ecol. Indic. 54, 137–152. doi: 10.1016/j.ecolind.2015.02.014
Ribes M., Coma R., Zabala M., Gili J. M. (1996). Small-scale spatial heterogeneity and seasonal variation in a population of a cave-dwelling Mediterranean mysid. J. plankton Res. 18 (5), 659–671. doi: 10.1093/plankt/18.5.659
Rossi S. (2013). The destruction of the A’nimal forests’ in the oceans: towards an over-simplification of the benthic ecosystems. Ocean Coast. Manage. 84, 77–85. doi: 10.1016/j.ocecoaman.2013.07.004
Ruffaldi Santori S., Benedetti M. C., Cocito S., Peirano A., Cupido R., Erra F., et al. (2021). After the fall: the demographic destiny of a gorgonian population stricken by catastrophic mortality. Oceans 2 (2), 337–350. doi: 10.3390/oceans2020020
Sartoretto S. (1994). Structure et dynamique d’un nouveau type de bioconstruction à Mesophyllum lichenoides (Ellis) Lemoine (Corallinales, Rhodophyta). Comptes rendus de l’Académie des sciences. Série 3 Sci. la vie 317 (2), 156–160.
Sartoretto S., Schohn T., Bianchi C. N., Morri C., Garrabou J., Ballesteros E., et al. (2017). An integrated method to evaluate and monitor the conservation state of coralligenous habitats: The INDEX-COR approach. Mar. pollut. Bull. 120 (1-2), 222–231. doi: 10.1016/j.marpolbul.2017.05.020
Smith C. J., Rumohr H. (2005). “Imaging techniques,” in Methods for the Study of Marine Benthos (Oxford, UK: Blackwell Science Ltd), 87–111. doi: 10.1002/9780470995129.ch3
Tanhua T., Pouliquen S., Hausman J., O’Brien K., Bricher P., De Bruin T., et al. (2019). Ocean FAIR data services. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00440
Thompson A. A., Mapstone B. D. (1997). Observer effects and training in underwater visual surveys of reef fishes. Mar. Ecol. Prog. Ser. 154, 53–63. doi: 10.3354/meps154053
Turicchia E., Abbiati M., Bettuzzi M., Calcinai B., Morigi M. P., Summers A. P., et al. (2022). Bioconstruction and bioerosion in the northern Adriatic coralligenous reefs quantified by X-ray computed tomography. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.790869
Turicchia E., Cerrano C., Ghetta M., Abbiati M., Ponti M. (2021a). MedSens index: The bridge between marine citizen science and coastal management. Ecol. Indic. 122, 107296. doi: 10.1016/j.ecolind.2020.107296
Turicchia E., Ponti M., Rossi G., Cerrano C. (2021c). The Reef Check Med dataset on key Mediterranean marine species 2001-2020. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.675574
Turicchia E., Ponti M., Rossi G., Milanese M., Di Camillo C. G., Cerrano C. (2021b). The Reef Check Mediterranean underwater coastal environment monitoring protocol. Front. Mar. Sci. 1086. doi: 10.3389/fmars.2021.620368
Tyler-Walters H., Tillin H. M., d’Avack E. A. S., Perry F., Stamp T. (2018). Marine Evidence-based Sensitivity Assessment (MarESA) – A Guide. Marine Life Information Network (MarLIN) (Plymouth: Marine Biological Association of the UK).
UNEP-MAP-RAC/SPA (2017). Action plan for the conservation of the coralligenous and other calcareous bio-concretions in the Mediterranean Sea (Tunis: UNEP MAP RAC-SPA publ.).
Valisano L., Palma M., Pantaleo U., Calcinai B., Cerrano C. (2019). Characterization of North–Western Mediterranean coralligenous assemblages by video surveys and evaluation of their structural complexity. Mar. Pollut. Bull. 148, 134–148. doi: 10.1016/j.marpolbul.2019.07.012
Keywords: mesophotic, temperate seas, bioconstructions, biogenic reefs, environmental status, Mediterranean
Citation: Di Camillo CG, Ponti M, Storari A, Scarpa C, Roveta C, Pulido Mantas T, Coppari M and Cerrano C (2023) Review of the indexes to assess the ecological quality of coralligenous reefs: towards a unified approach. Front. Mar. Sci. 10:1252969. doi: 10.3389/fmars.2023.1252969
Received: 04 July 2023; Accepted: 30 August 2023;
Published: 27 September 2023.
Edited by:
Alexander Ereskovsky, UMR7263 Institut Méditerranéen de Biodiversité et d’Écologie marine et continentale (IMBE), FranceReviewed by:
Giorgio Bavestrello, University of Genoa, ItalyCopyright © 2023 Di Camillo, Ponti, Storari, Scarpa, Roveta, Pulido Mantas, Coppari and Cerrano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Gioia Di Camillo, Yy5kaWNhbWlsbG9Ac3RhZmYudW5pdnBtLml0; Massimo Ponti, bWFzc2ltby5wb250aUB1bmliby5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.