- 1Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, Sanya Tropical Fisheries Research Institute, Sanya, China
- 2Shenzhen Base of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shenzhen, China
- 3College of Fisheries and Life Science, Dalian Ocean University, Dalian, China
- 4South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences/Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, Guangzhou, China
In recent years, as the marine environment has been deteriorated, the aquaculture water environment has also been negatively affected to varying degrees. Negative environmental factors make extremely damaging to organisms, resulting in stress-induced diseases and high mortality rates of cultured shrimp. Therefore, stress resistance breeding of Litopenaeus vannamei and evaluating the stress tolerance of the breeding population are urgently needed now. Litopenaeus vannamei collected from Thailand (T) and the United States (M) were used as parents, while four progeny populations of TT, MM, TM, and MT were constructed by diallel cross, with a total of 20 families. The tolerance of young shrimp to high ammonia-N, high pH, and low salt was compared through a 96-h acute toxicity test; the heterosis of each mating combination was analyzed; and the tolerance of parents and offspring was evaluated. Here we show that under 96 h of high ammonia-N, high pH, and low salt stress, the mortality rates of each family were 19.52%–92.22%, 23.33%–92.22%, and 19.33%–80.00%, respectively. There were significant differences in the tolerance of different families to ammonia-N, pH, and salinity stress (P < 0.05). The population with a female parent from the United States had stronger tolerance to ammonia-N, pH, and low salt stress than the population with a female parent from Thailand. The population with a male parent from Thailand had weaker tolerance to pH and low salt stress than the population with a male parent from the United States, but it was superior to the population with a male parent from the United States under ammonia-N stress. The heterosis rates of the hybrid population TM in acute high ammonia-N, high pH and low salinity were 81.67%, 44.58% and −10.13%, respectively; However, the heterosis rates of the MT population were 14.89%, 38.89%, and −8.96%, respectively. The overall resistance of the four populations showed MM > MT > TT > TM. The population TM had obvious heterosis in high ammonia-N and high pH tolerance traits, and the family MM7 had a strong low salt tolerance, so it can be considered a candidate family for subsequent breeding work. Moreover, variability in stress resistance and heterosis of stress resistance of different populations obtained by family selection was discussed in this paper. The experimental results provide a basis for screening new strains of vannamei shrimp with strong stress resistance through family breeding.
Introduction
The Pacific white shrimp, Litopenaeus vannamei, is naturally distributed in tropical waters off the Pacific Ocean from Peru to Mexico, from the Gulf of Mexico to central Peru, especially close to Ecuador (Zhang et al., 1990). It benefits from small head and chest armor, high meat content, strong stress resistance, fast growth, a long breeding period, high density and low salinity tolerance for aquaculture, and simple transportation of live shrimp. Along with Fenneropenaeus chinensis and Penaeus monodon, it is listed as one of the world’s three most widely cultivated shrimp species and is crucial to both shrimp fisheries and aquaculture (Wang et al., 2004; Zhang et al., 2009).
One of the most important methods for genetic improvement in aquatic animals is cross-breeding (Zhang et al., 2019). Their offspring can outperform their parents in terms of production performance, environmental tolerance, and meat quality by crossing individuals of different strains or genotypes (Piferrer et al., 2009). Because they can quantify the level of heterosis obtained after crossing different lines and reflect the matching effect of target traits, heterosis and combining ability are very important references in breeding. Zhou (Zhou et al., 2021) studied the heterosis and combining ability of body mass traits of three populations of Penaeus monodon through a complete diallel cross design. Sun (Sun et al., 2011) discovered significant differences in high ammonia-N tolerance among various families of Penaeus monodon, and He (He et al., 2008) found that there were significant differences in the resistance of juvenile shrimp seedlings among Chinese shrimp families. Different families have significant differences in tolerance to ammonia-N and pH, indicating significant selection potential. Fang (Fang et al., 2000) discovered that as pH increased, fewer juvenile Chinese shrimp survived. Acute salinity stress has been shown to affect the growth and survival rate of L. vannamei larvae in previous studies. Pan (Pan et al., 2007) reported these findings; Lin (Lin et al., 2010) compared the tolerance of different families of L. vannamei to freshwater and obtained the basic population of excellent freshwater tolerant families; and Wang (Wang et al., 2022) discovered that both growth and comprehensive stress tolerance traits can be genetically improved through breeding, and growth traits can also be manipulated. This study aims to evaluate the stress tolerance of the breeding population, access the mortality rates of two introduced self-bred and hybrid families of L. vannamei under 96 h of high ammonia-N, high pH, and low salt stress, provide candidate materials for further family selection, and provide reference data for stress resistance breeding of L. vannamei.
Materials and methods
Test materials
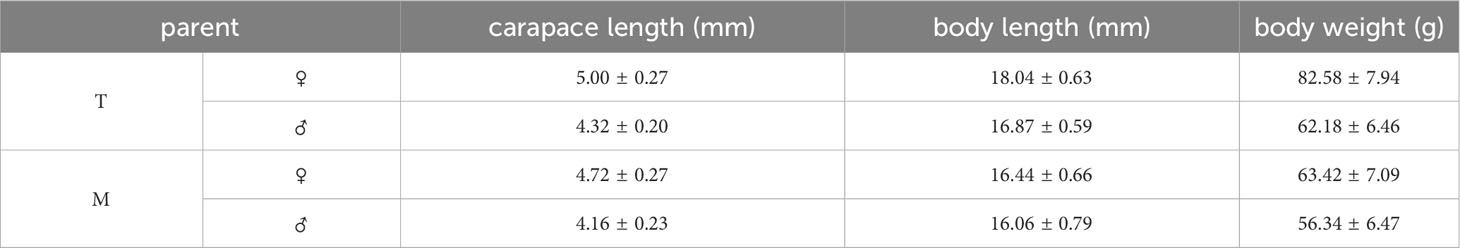
The experiment was conducted at Hainan Lutai Marine Biotechnology Co., Ltd., using two introduced populations of L. vannamei as parent shrimp, namely the Dingfeng strain (T) from Thailand and the Daynight Express strain (M) from the United States. Each strain of parent shrimp is raised separately, with each female shrimp corresponding to an eye label. After the parent shrimp is temporarily raised and stabilized, the female shrimp undergoes unilateral eye stalk removal and is fed with frozen squid, oysters, and fresh sand silkworms to promote maturation. In order to maximize the success rate of mating, the female shrimp with large orange ovaries and the male shrimp with mature gonads were selected as parent shrimp. Four self-propagation and hybrid populations were successfully established within 10 days by selecting mature gonadal parent shrimp and using diallel natural mating: T♀ × T♂ (TT)、M♀ × M♂(MM)、T♀ × M♂(TM) and M♀ × T♂(MT), see supplementary (Table 1) for detailed parental information, a total of 20 families. The inseminated female had been moved back to individual spawning tanks before the spawned eggs were incubated in the spawning tank until hatching, with 2000 nauplii selected from each family, and their larval development was tracked and observed. Each self-breeding and hybrid population was cultured in a standardized manner until the larvae were hatched. After cultivation to P15, 1000 nauplii were randomly selected and transferred to the cement pool of the standard coarse workshop to maintain consistent cultivation conditions at all stages, and reduce the impact of differences in environmental conditions (mainly including salinity, temperature, larval density, feed, and inflation of water at different stages) on growth and development. When the body length of the juvenile shrimp had reached at 4-5 cm, larvae of similar sizes were randomly picked from the cages of each line to carry out the experiment. Three parallel shrimp were placed in each group, and 50 shrimp were placed in each parallel. Before the experiment, the juvenile shrimp specifications were: TT body length (5.11 ± 0.65) cm, body weight (1.34 ± 0.45) g/tail; MM has a body length of (4.99 ± 0.60) cm and a weight of (1.30 ± 0.44) g/tail; TM has a body length of (4.47 ± 0.50) cm and a weight of (0.90 ± 0.30) g/tail; MT body length (4.66 ± 0.55) cm, body weight (1.02 ± 0.37) g/tail. During the feeding period, the larvae were supplied every 2 h with a diet of microalgae, rotifer, Artemia salina, and compound feed (8.32% moisture, 48.65% crude protein, 5.70% crude fat, and 11.89% ash) until 24 h before treatment.
Test method
Before the start of the formal experiment, a 96-h tolerance pre-experiment was conducted to obtain the 96-h half-lethal mass concentration of L. vannamei, which includes high ammonia-N, high pH, and low salt stress concentrations of 130mg·L–1, 9.7, and 2 ppt, respectively. The water in the experimental group was regulated with NH4CL (analytically pure), NaOH, and fully aerated fresh water: temperature was maintained at 28 ± 1.5℃, dissolved oxygen content ≥ 5mg/L. 150 uniformly sized juvenile shrimp were selected from each family in the standard coarse pool for fluorescence labeling and temporarily raised for 24 h. Then 150 individuals were randomly divided into 3 groups, with 50 individuals in each group. They were mixed in three 7.3m × 4.3m × 2m cement ponds, respectively, and stress tests were carried out with high ammonia-N, pH, and low salt. During the experiment, there was no inflation, and individual deaths were observed at regular intervals. Dead individuals and feces were promptly removed, and the number of deaths within 96 h was accurately recorded.
Statistical analyses
Excel 2010 was used to calculate the average value, standard deviation, and heterosis of mortality for each family; SPSS26.0 was used to perform one-way ANOVA on the data; and Duncan’s multiple comparison method was used to analyze the significance of differences between different combinations for traits with significant differences (P< 0.05). The results are expressed as mean ± standard deviation (mean ± SD).
We used the following heterosis formula for combined stress tolerance traits (Cruz and Ibarra, 1997; Lu et al., 2016):
Where , , and represent the first generation of reciprocal offspring of parent 1 and parent 2, the mean value of inbred offspring of parent 1, and the mean value of the representative type of inbred offspring of parent 2, respectively; H (%) is the reciprocal offspring heterosis rate.
Results
Effect of stress tolerance traits on mortality of different L. vannamei families
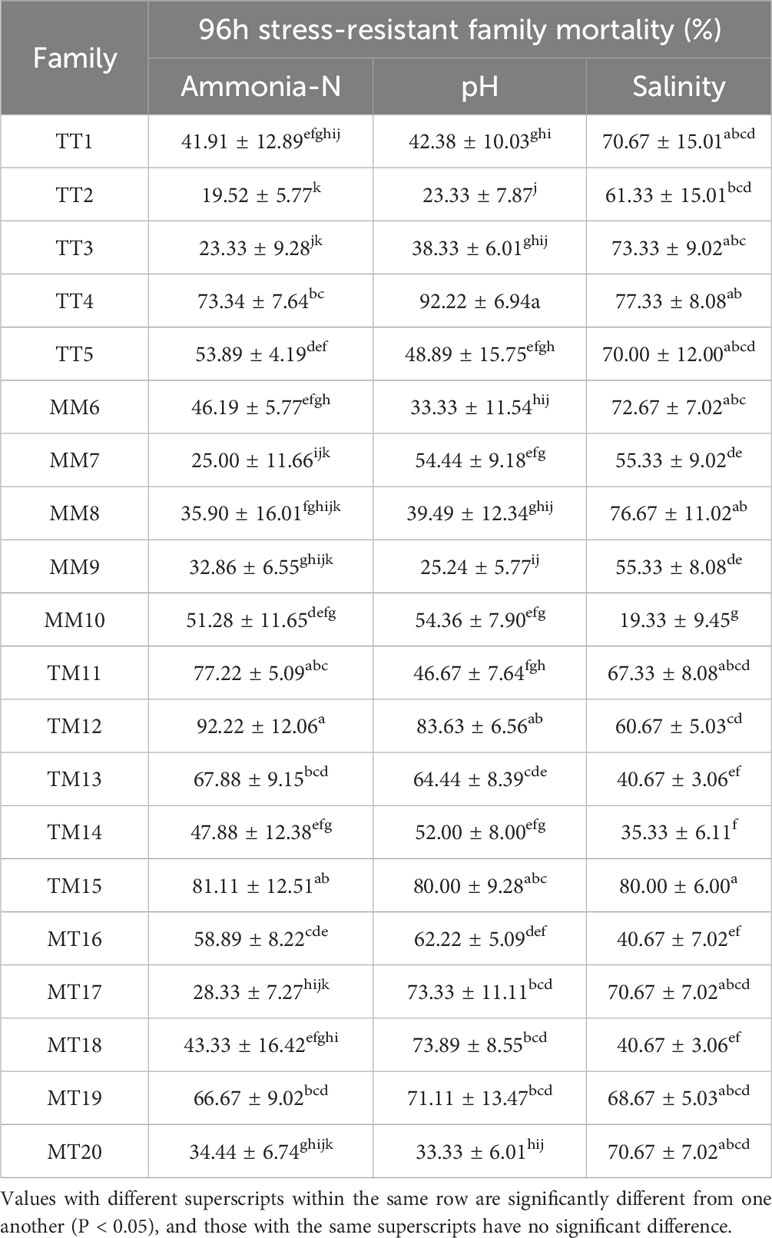
The survival rates of 20 F1 generation populations of juvenile shrimp under different ammonia-N, pH, and salinity stresses for 96 h are shown in Table 2. A one-way analysis of variance was conducted on the ammonia-N tolerance of each family, and the results showed that there were differences in the tolerance of different families to 96-h stress (P< 0.05). The range of acute stress tolerance mortality rates for 20 families under ammonia-N stress for 96 h is 19.52% - 92.22%. The mortality rate of the TT2 family under ammonia-N stress for 96 h is the lowest, at 19.52% ± 5.77%, while the TM12 family has the highest, at 92.22% ± 12.06%. The range of tolerance mortality rate for acute stress families at pH 96 h is 23.33% - 92.22%, with family TT4 having the lowest survival rate and family TT2 having the highest survival rate; The MM10 family had the lowest acute stress tolerance death rate after 96 h of low salt tolerance, which was 19.33% ± 9.45%. The TM15 family had the highest mortality rate, which was 80.00% ± 6.00%. Under ammonia-N and pH stress, the TT2 family has the strongest tolerance. Some families also overlap in tolerance under pH and low salt stress. If the average mortality rate of a family is less than 30%, it is classified as a high tolerance family; 30% - 60% is a moderate tolerance family; and more than 60% is classified as a weak tolerance family; then the family coefficients of strong, medium, and weak ammonia-N tolerance are 4, 10, and 6, respectively. There are 2, 10, and 8 pH values, respectively; There are 1, 6, and 13 low salt levels, respectively.
Comparison of tolerance between different paternal and maternal lineages
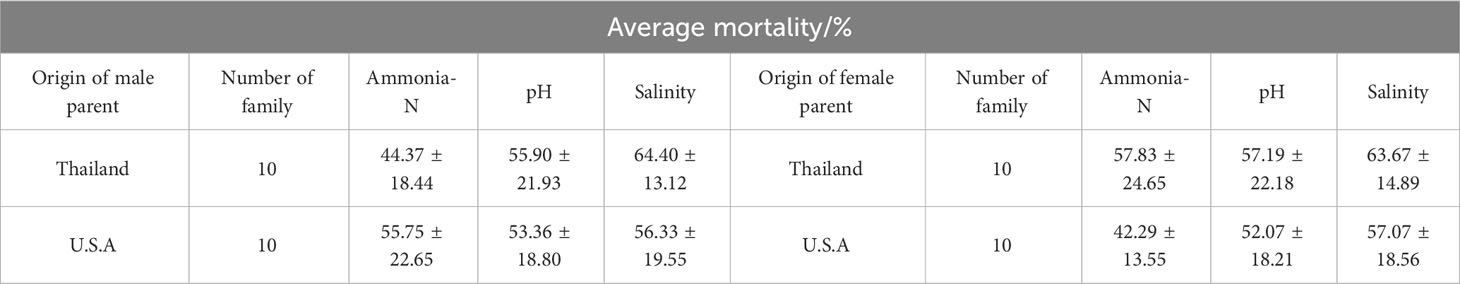
Compare the mortality rates of families with different parental and maternal origins under different stress conditions at 96 h (Table 3). The results showed that under ammonia-N stress, the average mortality rate of families with a female parent from the United States was the lowest (42.29 ± 13.55%), but there was no significant difference compared to families with a male parent from Thailand or the United States (P > 0.05); Under pH stress, there was no significant difference in tolerance among families whose parents were originally from Thailand or the United States (P > 0.05), with the family with the strongest tolerance being the parent from the United States (52.07 ± 18.21%); Under low salt stress, the mortality rate of families with American parents was lower than that of families with American parents, while the mortality rate of families with Thai parents was lower than that of families with Thai parents, but there was no significant difference (P > 0.05).
Comparison of tolerance for families of different mate combinations
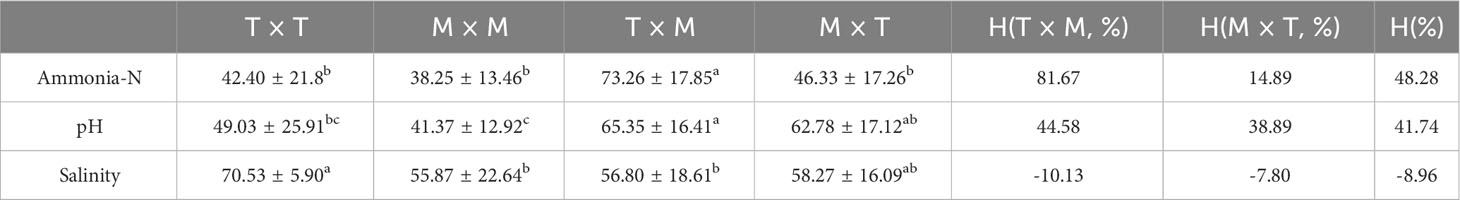
Duncan’s multiple comparison method was used to perform variance analysis on the significant differences in mortality rates among four hybrid combinations under stress (Table 4). The results showed that under ammonia-N stress, the ammonia-N tolerance of the TM combination population was significantly lower than that of the other three combination populations (P< 0.01), and there was no significant difference in ammonia-N tolerance among the other three populations (P > 0.05); Under pH stress, the tolerance of self-pollinated population MM to pH was significantly higher than that of hybrid population TM and MT combination population (P< 0.05), while the tolerance of hybrid population TM was significantly lower than that of self-pollinated population, and there was no significant difference in pH tolerance between self-pollinated population and MT combination population (P > 0.05). Under low salt stress, there was a significant difference (P< 0.05) in the tolerance to low salt between the TT combination population and the MM and TM combination population, while there was no significant difference (P > 0.05) between the TT combination population and the MT combination population. The survival rate of the MM combination population was the highest under all three types of stress. The heterosis analysis of the mortality rate of inbred combinations and hybrid combination families from Thailand and the United States under stress resistance is shown in Table 4. The results show that the average heterosis rate of stress resistance of most combinations is positive, and all family combinations show different degrees of heterosis (14.89% - 81.67%) under Ammonia-N and pH stress, of which the TM combination shows high heterosis (81.67%, 44.58%) for Ammonia-N and pH tolerance. The hybrid generation showed a certain degree of hybridization disadvantage (H (%)< 0) in low salt tolerance.
Survival rates of different populations under acute stress
Figure 1A showed that under ammonia-N stress, the survival rates of all four populations showed a significant downward trend at 48 h (P< 0.05), but the downward trend of the survival rates of the four populations was significantly different. Within 24 h of stress, the survival rate slowly decreased, and after 48 h, the TM population showed a rapid decline trend compared to the other three populations. Figure 1B showed that under pH stress, the survival rates of the four populations significantly reached a low point at 36 h (P< 0.05), and the decline rate slowed down thereafter. The self-crossing populations TT and MM, as well as the hybrid populations TM and MT, maintained a consistent downward trend; Figure 1C showed that under low salt stress, the survival rate of the four populations showed a rapid decline at 12 h, and thereafter, the decline trend of the four populations slowed down, and there was no significant difference in survival rate between the four populations (P > 0.05).
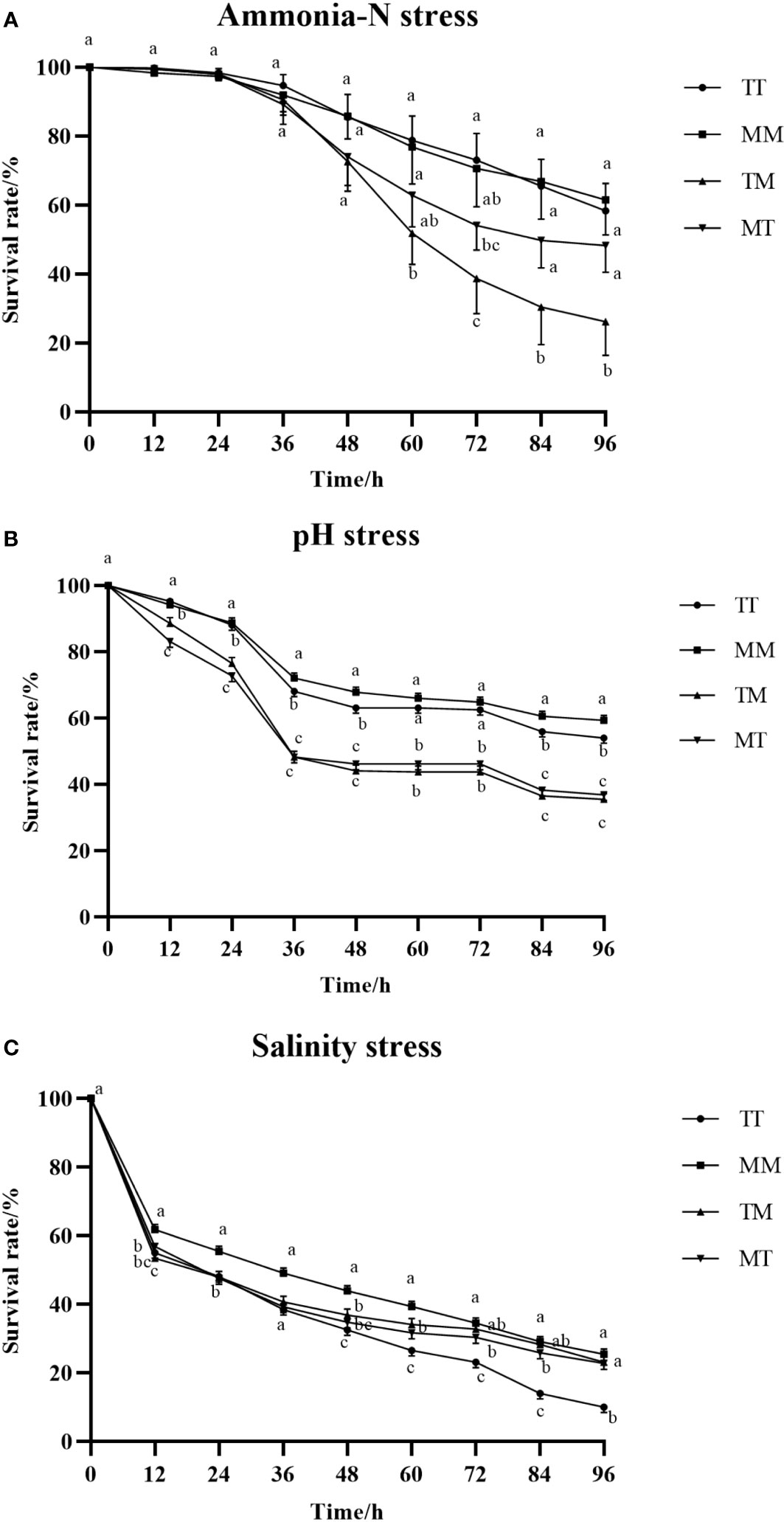
Figure 1 Survival rates of different populations under acute stress (Ammonia-N stress (A), pH (B) stress, Salinity stress (C)). The lowercase letters represent each of the line graphs in the figure.
Discussions
The genetic basis, disease resistance and pathogenicity of pathogenic microorganisms of mariculture animals are significantly impacted by adverse water quality factors in the aquaculture environment, such as abnormal temperature, salinity, ammonia-N content, pH, nitrite, and other stress factors (He et al., 2008). The main pollutant in the environment for shrimp farming is Ammonia-N, which is primarily found in the water body as non ionized ammonia (NH3) and ionic ammonia (NH4+), both of which are in a dynamic equilibrium. Because NH3 is not charged and has high fat solubility, it can penetrate cell membranes and negatively affect shrimp growth, molting, oxygen consumption, immunity, ammonia-N excretion, osmotic pressure regulation and disease resistance (Lei and Chen, 2003; Huang et al., 2012; Cui et al., 2017). pH is an important ecological factor that affects the survival status of aquaculture organisms and can reflect the quality of the water. Due to their extreme sensitivity to acidification and alkalization, shrimp and crab species can be somewhat impacted by high or low pH levels in terms of growth, immunity, ion balance, etc. The osmotic regulation of crustaceans is closely related to environmental salinity. Acute salinity stress will have an impact on the osmotic pressure of the internal environment, the level of respiratory metabolism, and immune function (Camacho-Jiménez et al., 2018; Joseph and Philip, 2020). Breeding work based on family lines, such as Pinctada martensii, Argopectenirradians, Patinopectenyessoensis, Paralichthys olivaceus, etc., has gradually become more popular in recent years (He et al., 2007; Qin et al., 2007; Chen et al., 2008; Zhang et al., 2008). When compared to population selection, family selection has the advantages of fast speed, obvious effects, high work efficiency, flexible operation, and outstanding excellent traits. The family’s genetic composition is relatively uniform, making it simple to determine the genotypes of the parents and offspring. As a result, family selection has begun to be widely applied in the breeding of superior aquatic animal varieties. Another important advantage of family selection is the ability to estimate the breeding values of some difficult to measure traits of the parents by observing the phenotypic traits of whole or half sib families. This enables the selection of quantitative traits through family selection, such as growth rate and disease resistance (Wu et al., 2011).
Twenty families from 4 populations of 2 introduced populations of L. vannamei were used as experimental subjects in this study through a combination of self-breeding and hybridization. Seven families with strong tolerance were initially selected using ammonia-N, pH, and salinity tolerance as indicators. The optimal families under ammonia-N and pH stress overlapped (TT2), and some families overlapped under low salt and pH stress. Chinese shrimp larvae from different families were subjected to acute toxicity tests, and He (He et al., 2008) discovered that there were significant differences in ammonia-N tolerance and high pH traits, indicating significant selection potential. Chen (Chen et al., 2017) tested the low salt tolerance of 11 families of Penaeus monodon and found significant differences in each family’s survival rate at half-lethal time. In order to select strains of shrimp with stress resistance and resolve the paradox between environmental degradation and high yield in the later stages of shrimp breeding from the perspective of genetic improvement of breeding varieties, comparative analysis of physical differences between different populations, further analysis of heterosis of stress resistance, and comparison of genetic differences between different mating combinations are necessary (Camacho-Jiménez et al., 2018). In this experiment, a study on the mortality rate of families constructed from the same source of maternal and paternal parents found that although the mortality rates of families were different, there was no significant difference (P > 0.05). The progeny populations whose male parent were from the Thai and American populations had higher survival rates than those whose female parent were from the Thai and American populations under ammonia nitrogen and pH stresses, but the opposite results were observed under low salt stresses. The overall tolerance of different groups of families to ammonia-N, pH, and low salt is generally higher than that of Thai sources. The American strain (MM) and a few hybrid strains (MT) performed better under ammonia-N, pH, and low salt stress, whereas the Thai strain (TT) and a few Thai American hybrid families performed worse, indicating the American strain’s advantages in stress resistance. It can be assumed that this advantage was brought about by the American strain’s strong capacity for stress resistance. The Thai strain is relatively weak, and another possibility is that the advantage of stress resistance is determined jointly by both hybrid strains rather than by the advantage of a single strain. The possibility needs to be further investigated in future generations. The analysis showed that the hybrid families of Thailand and the United States had heterosis for ammonia-N and pH tolerance, and that, with the exception of low salt stress, the average heterosis value of growth traits of hybrid combinations was positive (14.89% to 81.67%). L. vannamei’s growth traits have been the subject of many studies both domestically and abroad, and it has been discovered that hybrid combinations generally show heterosis in growth performance (Sui et al., 2016; Lu et al., 2017). For ammonia-N and pH traits, TM and MT populations showed different levels of heterosis, but overall, the heterosis of TM was better than that of MT, and the average heterosis rate of the TM population for ammonia-N was the highest, 81.67%. In low salt tolerance, the hybrid combinations showed a certain degree of hybridization disadvantage (−10.13% to −7.80%), which was weaker than the median of both parents. Ji (Ji et al., 2018) compared the stress resistance and growth traits of the first generation of shrimp in different populations of L. vannamei, and found that the results were consistent. The survival rates of the four populations under acute stress showed that the stress resistance of the MM and MT populations was slightly higher than that of the TT and TM populations, and that the inheritance of the maternal parent was dominant. The hybrid MT population demonstrated significantly greater stress resistance than that of the TM population. Different cross combinations show different heterosis, and heterosis of different characters also varies. Heterosis is a complex biological phenomenon. According to some studies, the stronger the complementarity and the more obvious the heterosis, the greater the genetic difference between hybrid parents (Pillai et al., 2011). In this experiment, the hybrid population showed poor performance in low salt stress resistance, which may be due to the enrichment of harmful recessive genes at different loci in parents with different genetic backgrounds. The probability of growth or stress resistance decline in the hybrid offspring will increase if the harmful recessive genes in the chromosomes provided by the parents of the hybrid offspring happen to be alleles (Ma et al., 2005). Selection of parents is crucial in the hybrid breeding process because improper parent selection could result in the regression of certain important economic traits in hybrid offspring.
Stress resistance is a threshold trait that multiple genes control and refers to an organism’s ability to adapt to harsh environments. It is generally believed that heritability is relatively low and that it is relatively difficult to improve traits through breeding (Bulmer, 1980). The research on the stress resistance of Penaeus monodon (Huang et al., 2012), Macrobrachium rosenbergii (Thanh et al., 2010) and Penaeus chinensis (Li et al., 2005) shows that the stress resistance of some aquatic animals has high heritability and can be improved through breeding. This study screened out superior populations (MM and MT), as well as high ammonia-N and high pH families (TT2) and low salt families (MM10) using the average mortality rate and hybrid advantage of families under high ammonia-N, high pH, and low salt stress for 96 h. The results of this study provide two candidate dominant families for the breeding of L. vannamei families. The next step is to preserve the selected families with strong stress resistance and improve the stress resistance of the families through continuous breeding and purification. In addition, the genetic structure of different populations and families will be analyzed at the molecular level, and molecular marker-assisted breeding will be carried out for further verification and selection in future breeding work. To provide reference data for breeding a new strain of L. vannamei that is fast-growing and stress-resistant and has significantly improved target economic traits. to.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the South China Sea Fisheries Research Institute.
Author contributions
MS, SJ, YL, and FZ: conceptualization. MS, SJ, SGJ, YL, and QY: methodology. MS and SJ: data curation. MS: writing—original draft preparation. FZ: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Project of Sanya Yazhou Bay Science and Technology City (SCKJ-JYRC-2022-01), the Youth Fund of Hainan Natural Science Foundation (321QN351), the earmarked fund for CARS-48, Central Public-Interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2023TD34, 2021SD13), Special fund project for scientific and technological innovation and industrial development in Dapeng New Area (KJYF202101-08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bulmer M. G. (1980). The mathematical theory of quantitative genetics. (New York: Oxford University Press).
Camacho-Jiménez L., Díaz F., Sánchez-Castrejón E., Ponce-Rivas E. (2018). Effects of the recombinant crustacean hyperglycemic hormones rCHH-B1 and rCHH-B2 on the osmo-ionic regulation of the shrimp Litopenaeus vannamei exposed to acute salinity stress. J. Comp. Physiol. B. 188, 1–15. doi: 10.1007/s00360-018-1151-8
Chen J. C., Lin M. N., Ting Y. Y., Lin J. N. (1995). Survival, haemolymph osmolality and tissue water of Penaeus chinensis juveniles acclimated to different salinity and temperature levels. Comp. Biochem. Physiol. Part A: Physiol. 110, 253–8. doi: 10.1016/0300-9629(94)00164-O
Chen J. S., Zhou F. L., Jiang S. G., Huang J. H., Yang Q. B., Ma Z. H. (2017). Salinity tolerance of Penaeus monodon across different breeding families. J. Fisheries China. 41, 687–93. doi: 10.11964/jfc.20160510392
Chen S. M., Chen J. C. (2003). Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 218, 613–23. doi: 10.1016/S0044-8486(02)00265-X
Chen S., Tian Y. S., Xu T. J., Deng H., Liu S. T., Liu B. W., et al. (2008). Development and characterization for growth rate and disease resistance of disease-resistance population and family in Japanese flounder (Paralichthys olivaceus). J. Fisheries China 5, 665–73. doi: 10.3321/j.issn:1000-0615.2008.05.002
Cruz P., Ibarra A. M. (1997). Larval growth and survival of two catarina scallop (Argopecten circularis, Sowerby 1835) populations and their reciprocal crosses. J. Exp. Mar. Biol. Ecology. 212, 95–110. doi: 10.1016/S0022-0981(96)02742-6
Cui Y. T., Ren X. Y., Li J., Zhai Q. Q., Feng Y. Y., Xu Y., et al. (2017). Effects of ammonia-N stress on metabolic and immune function via the neuroendocrine system in Litopenaeus vannamei. Fish Shellfish Immunol. 64, 270–5. doi: 10.1016/j.fsi.2017.03.028
Dunier M., Siwicki A. K. (1993). Effects of pesticides and other organic pollutants in the aquatic environment on immunity of fish: a review. Fish Shellfish Immunol. 3, 423–38. doi: 10.1006/fsim.1993.1042
Fang W. H., Wang H., Lai Q. F. (2000). Toxicity of carbonate–alkalinity and pH to larval Penaeus chinensis. J. Fishery Sci. China. 4, 78–81. doi: 10.3321/j.issn:1005-8737.2000.04.018
He M. X., Guan Y. Y., Lin Y. G., Huang L. M. (2007). Growth comparison between families of pearl oyster Pinctada martensii Dunker. J. Trop. Oceanography 26, 39–43. doi: 10.3969/j.issn.1009-5470.2007.01.007
He Y. Y., Li J., Liu P., Huang F. Y., Wang Q. Y. (2008). Comparison of the resistance to pH value and ammonia in Chinese shrimp (Fenneropenaeus chinensis) families. Periodical Ocean Univ. China 5, 761–765. doi: 10.16441/j.cnki.hdxb.2008.05.012
Hu Z. G., Liu J. Y., Yuan R. P., Zhang J. C. (2016). Analysis of combining ability of survival of imported Litopenaeus vannamei populations under temperature and salinity stress. Mar. Sci. 40, 25–31. doi: 10.11759/hykx20141009003
Huang J. H., Li Y., Yang Q. B., Su T. F., Zhu C. Y., Jiang S. G. (2012). Comparison of tolerance to ammonia-N in Penaeus monodon families. South China Fisheries Sci. 8, 37–43. doi: 10.3969/j.issn.2095–0780.2012.06.006
Ji D. W., Hu. L. H., Yan M. C., Luo K., Chen X. F., Zhang M. (2018). A comparative study on stress resistance and growth among the inbred and hybrid offsprings of wild population F1 and inbreeding population of Litopenaeus vannamei. J. Fishery Sci. China 25, 1227–35. doi: 10.3724/SP.J.1118.2018.18037
Joseph A., Philip R. (2020). Immunocompetence of Penaeus monodon under acute salinity stress and pathogenicity of Vibrio harveyi with respect to ambient salinity. Fish Shellfish Immunol. 106, 555–62. doi: 10.1016/j.fsi.2020.07.067
Lei Y. Z., Chen Y. (2003). Aquaculture environmental chemistry (Beijing: China Agriculture Press), 117–24.
Li J., Liu P., He Y. Y., Song Q. S., Mu N. H., Wang Q. Y. (2005). Artificial selection in the new breed of Fenneropenaeus chinensis named “Yellow Sea1” based on fast growth trait. J. Fisheries China 1, 1–5. doi: 10.3321/j.issn:1000-0615.2005.01.001
Lin H. J., Zhang L. P., Shen Q., Hu C. Q. (2010). The comparison of tolerance to fresh water in 10 full-sib families of white leg shrimp, litopenaeus vannamei. Trans. Oceanology Limnology. 127, 143–8. doi: 10.13984/j.cnki.cn37–1141.2010.04.022
Lu X., Luan S., Cao B. X., Sui J., Dai P., Meng X. H., et al. (2017). Heterosis and heritability estimates for the survival of the Pacific white shrimp (Litopenaeus vannamei) under the commercial scale ponds. Acta Oceanologica Sinica. 36, 62–8. doi: 10.1007/s13131-016-0942-6
Lu X., Luan S., Luo K., Meng X. H., Li W. J., Sui J., et al. (2016). Genetic analysis of the pacific white shrimp (litopenaeus vannamei): Heterosis and heritability for harvest body weight. Aquaculture Res. 47, 3365–3375. doi: 10.1111/are.12820
Ma D. Y., Hu H. L., Kong J. (2005). Inbreeding and its impact on aquaculture. J. Fisheries China 06, 849–56. doi: 10.3321/j.issn:1000-0615.2005.06.019
Pan L. Q., Zhang L. J., Liu H. Y. (2007). Effects of salinity and pH on ion-transport enzyme activities, survival and growth of Litopenaeus vannamei postlarvae. Aquaculture. 273, 711–20. doi: 10.1016/j.aquaculture.2007.07.218
Piferrer F., Beaumont A., Falguière J. C., Flajšhans M., Haffray P., Colombo L. (2009). Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293, 125–56. doi: 10.1016/j.aquaculture.2009.04.036
Pillai B. R., Mahapatra K. D., Ponzoni R. W., Sahoo L., Lalrinsanga P. L., Nguyen N. H., et al. (2011). Genetic evaluation of a complete diallel cross involving three populations of freshwater prawn (Macrobrachium rosenbergii) from different geographical regions of India. Aquaculture. 319, 347–54. doi: 10.1016/j.aquaculture.2011.07.026
Qin Y. J., Liu X., Zhang H. B., Zhang G. F. (2007). Analysis on morphological characters in reciprocal-cross populations in bay scallop, Argopecten irradians irradians. Mar. Sci. 213, 22–7. doi: 10.3969/j.issn.1000-3096.2007.03.006
Sui J., Luan S., Luo K., Meng X. H., Lu X., Cao B. X., et al. (2016). Genetic parameters and response to selection for harvest body weight of pacific white shrimp, Litopenaeus vannamei. Aquaculture Res. 47, 2795–803. doi: 10.1111/are.12729
Sun M. M., Huang J. H., Yang Q. B., Zhou F. L., Wen W. G., Chen X., et al. (2011). Comparison on characteristics of growth and resistance to ammonia among 13 families of Penaeus monodon. Shanghai Ocean Univ. 20, 510–6.
Thanh N. M., Nguyen N. H., Ponzoni R. W., Vu N. T., Barnes A. C., Mather P. B. (2010). Estimates of strain additive and non-additive genetic effects for growth traits in a diallel cross of three strains of giant freshwater prawn (Macrobrachium rosenbergii) in Vietnam. Aquaculture 299, 30–6. doi: 10.1016/j.aquaculture.2009.12.011
Wang X. Q., Ma S., Dong S. L. (2004). studies on the biology and cultural ecology of litopenaeus vannamei: a review. Trans. Oceanology Limnology 30, 94–100. doi: 10.3969/j.issn.1003-6482.2004.04.016
Wang L., Wang C. Y., Liu J. Y. (2022). Evaluation of genetic parameters for growth and comprehensive stress tolerance traits of Litopenaeus vannamei. South China Fisheries Sci. 18, 95–102. doi: 10.12131/20210252
Wu L. F., Zhang L. P., Hu C. Q., Shen Q. (2011). Comparison on growth rates of two full-sib families of Litopenaeus vannamei in different salinities. J. Trop. Oceanography. 30, 152–8. doi: 10.3969/j.issn.1009-5470.2011.01.022
Zhang W. Q. (1990). Biological profile of important world aquaculture species-Penaeus vannamei. Mar. Sci. 14, 69–73.
Zhang L. P., Wu L. F., Shen Q., Du S. B., Hu C. Q. (2009). Full-sib families construction and their growth comparison of Pacific white leg shrimp, Litopenaeus vannamei. Fish China. 33, 932–9. doi: 10.3724/SP.J.00001
Zhang C. S., Yang X. G., Song J., Jiang S. G., Yin X. X. (2008). Establishment of families and their early growth of Japanese scallop (Patinopecten yessoensis). South China Fisheries Science. 5, 44–5. doi: 10.3969/j.issn.2095-0780.2008.05.007
Zhang W. (1990). Biological profile of important world aquaculture species-Penaeus vannamei. Marine Sciences 14 (3), 69–73.
Zhang X. J., Zhou L., Gui J. F. (2019). Biotechnological innovation in genetic breeding and sustainable green development in Chinese aquaculture. Scientia Sinica. 49, 1409–29.
Keywords: Litopenaeus vannamei, ammonia-N, pH, salinity, tolerance, heterosis
Citation: Shi M, Jiang S, Jiang S, Yang Q, Li Y and Zhou F (2023) Comparison of stress tolerance of hybrid and selfed offspring of two populations of Litopenaeus vannamei. Front. Mar. Sci. 10:1232937. doi: 10.3389/fmars.2023.1232937
Received: 06 June 2023; Accepted: 09 October 2023;
Published: 19 October 2023.
Edited by:
Amjad Balange, Central Institute of Fisheries Education (ICAR), IndiaReviewed by:
Shiming Peng, Chinese Academy of Fishery Sciences, ChinaChangkao Mu, Ningbo University, China
Copyright © 2023 Shi, Jiang, Jiang, Yang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Falin Zhou, emhvdWZhbGluQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
 Miao Shi
Miao Shi Song Jiang
Song Jiang Shigui Jiang
Shigui Jiang Qibin Yang1,2,3,4
Qibin Yang1,2,3,4 Yundong Li
Yundong Li