
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 14 August 2023
Sec. Marine Ecosystem Ecology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1221922
This article is part of the Research TopicICYMARE - Early Career Researchers in Marine ScienceView all 32 articles
Coral aquaculture techniques have been developed for many species based on a well-documented understanding of the factors that affect coral growth and fitness. However, there is limited knowledge about the composition and structure of ectosymbiotic communities associated with cultured coral colonies. To address this gap, we conducted a study of Pocillopora verrucosa colonies reared from fragments in fixed nurseries and exposed for 6 and 12 months, as well as natural colonies in the same location. Our analysis focused on the species composition, species richness, and density of obligate and facultative ectosymbionts associated with the colonies. Obtained results indicate that the community associated with nursery-reared colonies was highly diverse, comprising 25 ectosymbionts, including 9 obligate and 16 facultative species. The prevalence, species richness, and density of the communities associated with natural colonies were significantly higher than those associated with reared ones. We also observed differences in the communities associated with reared colonies of different exposures, but we were able to group them as stages of the same community based on the size and exposure of the colonies. The differences between communities associated with reared and natural colonies may be related to the microhabitat properties of the symbiotic communities, as the former were elevated above the substrate, while the latter were attached to it. The age of natural colonies was also estimated to be more than three times higher than that of reared colonies of the same size, contributing to the differences. Our study clearly demonstrates that coral nurseries not only serve as a means of propagating corals but also offer artificial habitats for the maintenance and conservation of associated fauna. These findings have important implications for the management and conservation of coral reefs.
The cultivation of scleractinian corals has been practiced for several decades both in natural conditions and ex situ (Alcala et al., 1982; Auberson, 1982; Borneman and Lowrie, 2001). Reared colonies are used both for restoring damaged coral reefs and for the aquarium trade (Delbeek, 2001; Boch and Morse, 2012; Barton et al., 2017; O'Donnell et al., 2017). Active reef rehabilitation based predominantly on in situ culture of corals has become a routine conservation and management tool. Usually, corals are cultivated from small fragments in the nurseries, which can be commonly classified into “fixed” and “floating” (Shaish et al., 2008). There are many variations of constructions used for coral rearing, but irrespective of the design, the corals in the nurseries are usually elevated above the bottom and are in conditions that differ from their natural habitat.
The techniques of coral aquaculture are now well established for many species and locations (Shafir et al., 2006), and environmental and biological parameters affecting coral fragment growth and fitness have been well-documented (Shaish et al., 2010; Koval et al., 2020). Little is known, however, about the symbionts associated with cultivated coral fragments (Wee et al., 2019), despite their critical role in the functioning of natural coral ecosystems (Enochs, 2012).
All coral species harbor diverse fauna of fish and invertebrates, which is especially rich in branching corals belonging to the Pocilloporidae and Acroporidae families (Patton, 1974; Stella et al., 2010). In particular, the total number of symbiont species living on corals of the genus Pocillopora exceeds 260 species, of which 67 species are obligate symbionts (Stella et al., 2011), while the diversity of symbionts associated with the species of morphologically simpler mushroom corals rarely exceeds 20 species (Hoeksema et al., 2012). Each coral colony of Pocillopora is inhabited by several species and dozens of individuals, forming a well-integrated symbiotic infracommunity (Stella et al., 2010; Britayev et al., 2017). For example, a single coral species Pocillopora verrucosa in the Red Sea can host from two to 14 species and from three to 85 individuals of ectosymbionts among its colony branches (Britayev et al., 2017).
All symbionts interact with their host corals, receiving reliable shelter, food, a place of reproduction, and rearing offspring (Knudsen, 1967; Stella et al., 2011). Some of them have a negative impact on the host by causing coral injuries (Hoeksema et al., 2019; Hoeksema et al., 2022a; Hoeksema et al., 2022b) or acting as parasites (Rotjan and Lewis, 2008; Potkamp et al., 2017), predators (Robertson, 1970; Moerland et al., 2016), destructors (Clark and Morton, 1999; Smith, 2011), or disease vectors (Sussman et al., 2003; Montano et al., 2022). In contrast, other generally ectosymbiotic decapods have established mutually beneficial relationships with corals, providing them with various services, such as protecting host from attacks by predatory starfish and mollusks (Glynn, 1980; DeVantier et al., 1986; Pratchett, 2001; Rouzé et al., 2014), aerating corals by high-frequency fin motions (Goldshmid et al., 2004), providing nutrients necessary for the reproduction of symbiotic zooxanthellae and accelerating coral growth (Liberman et al., 1995; Mokady et al., 1998), and removing sediment, bacterial lesions, and fouling algae from coral colonies (Stachowicz and Hay, 1999; Stewart et al., 2006). It has been experimentally proven that the removal of ectosymbionts reduces the fitness of corals and increases their mortality rates (Stewart et al., 2006). Therefore, the presence of ectosymbionts is essential for the survival of coral fragments transplanted into natural coral habitats from nurseries.
However, knowledge of the establishment of an association between corals and ectosymbionts is relatively scarce. Existing literature suggests that symbionts settle on the host during the early stages of colony formation (Rouzé et al., 2019). Recent research has shown that relatively rich and abundant symbiotic fauna can be found in colonies of Pocillopora acuta, Platygyra sinensis, and Echinopora lamellosa just 5 months after their nursery rearing (Wee et al., 2019). This finding implies that coral farms, by establishing new microhabitats, can increase local biodiversity and contribute to the creation of new links in local food webs.
Despite potential importance of ectosymbionts for understanding the ecological role of coral farms in natural ecosystems and in coral aquaculture management, little is known about their species composition and abundance in nursery-reared coral colonies (Wee et al., 2019). It is also unclear whether the species composition and abundance of symbionts on reared and natural coral colonies are similar.
Our study aims to determine whether the species composition and structure of ectosymbiotic communities associated with nursery-reared colonies of the coral P. verrucosa are similar to those of natural ones. To answer this question, we examined the diversity, species richness, and density of ectosymbionts from transplanted fragments exposed for 12 months in the nursery and natural ones in the same locality and season, and compared them. Additionally, we investigated whether exposure (age) affects the structure of symbiotic communities by comparing the diversity, species richness, and density of nursery-reared colonies exposed for 6 and 12 months.
For this study, we used morphologically similar coral colonies belonging to the P. verrucosa–P. meandrina group (hereafter referred to as P. verrucosa) without attempting to precisely identify the species used. P. verrucosa was chosen as the object of study because it is a fast-growing species commonly used in aquaculture (Combillet et al., 2022). Furthermore, Pocillopora spp. host the richest known fauna of obligate and facultative ectosymbionts, which have been well studied in several regions of the tropical Indo-Pacific (Patton, 1966; Patton, 1974; Coles, 1980; Edwards and Emberton, 1980; Tkachenko et al., 2022), including the area where our research was conducted (Marin et al., 2005; Marin and Spiridonov, 2007; Britayev and Mikheev, 2013). Moreover, there is a natural population of P. verrucosa where the coral nurseries are located, meaning that both transplanted and natural coral colonies experience similar hydrological conditions, enabling an adequate comparison of their ectosymbiotic faunas.
The experiment was conducted at the Dam Bay research station of the Marine Branch of the Joint Russian-Vietnamese Tropical Research and Technological Center (Tropical Center), located on Tre Island, Nha Trang Bay (Figure 1). Coral fragments were transplanted and reared, and then the reared colonies were collected and processed in accordance with the methodology developed earlier (Britayev et al., 2023). Distal branch fragments measuring 5–8 cm in length and 3–6 cm in diameter were used in the experiment (Figure 2). These fragments were taken from large donor colonies cultivated in a local nursery. The fragments were relieved of any symbionts before planting, as detailed in the methodology below. In April 2012, the fragments were attached by divers to fixed triangular iron frames that had been installed on coral bioherms at a depth of 2–3 m (Figure 3A). The frames were elevated above the ground to a height of 40 cm. The bioherms were separated from each other by a sandy bottom. In order to eliminate the influence of other possible factors, natural colonies of P. verrucosa were sampled in the same localities (bioherms) during the same season and at the same depths as the nurseries with transplanted fragments (Figure 3B). This allowed us to compare the structure of symbiotic communities solely based on the location of the colonies (raised above or located on the substrate) and the age of the colonies.
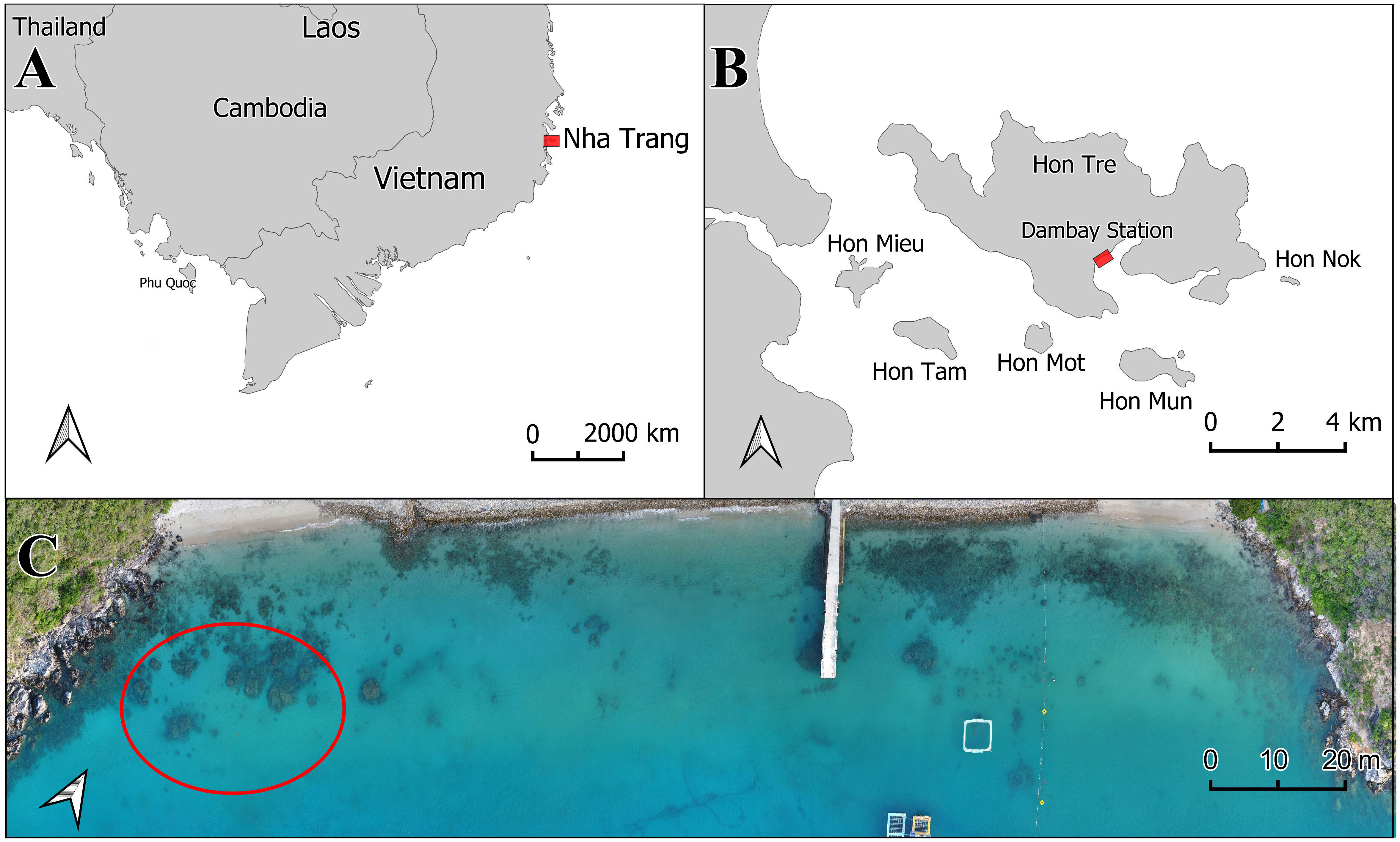
Figure 1 Study area location. (A) Location of Nha Trang Bay along the Vietnam coast; (B) Location of Dam Bay station within Nha Trang Bay. (C) Drone image of the shoreline at Dam Bay station, with the sampling area for planted and natural colonies of Pocillopora verrucosa circled in red.

Figure 2 Coral propagation. Branch fragments from Pocillopora verrucosa donor colonies that were applied for rearing coral colonies in the experiment. Scale bar is 5 cm.
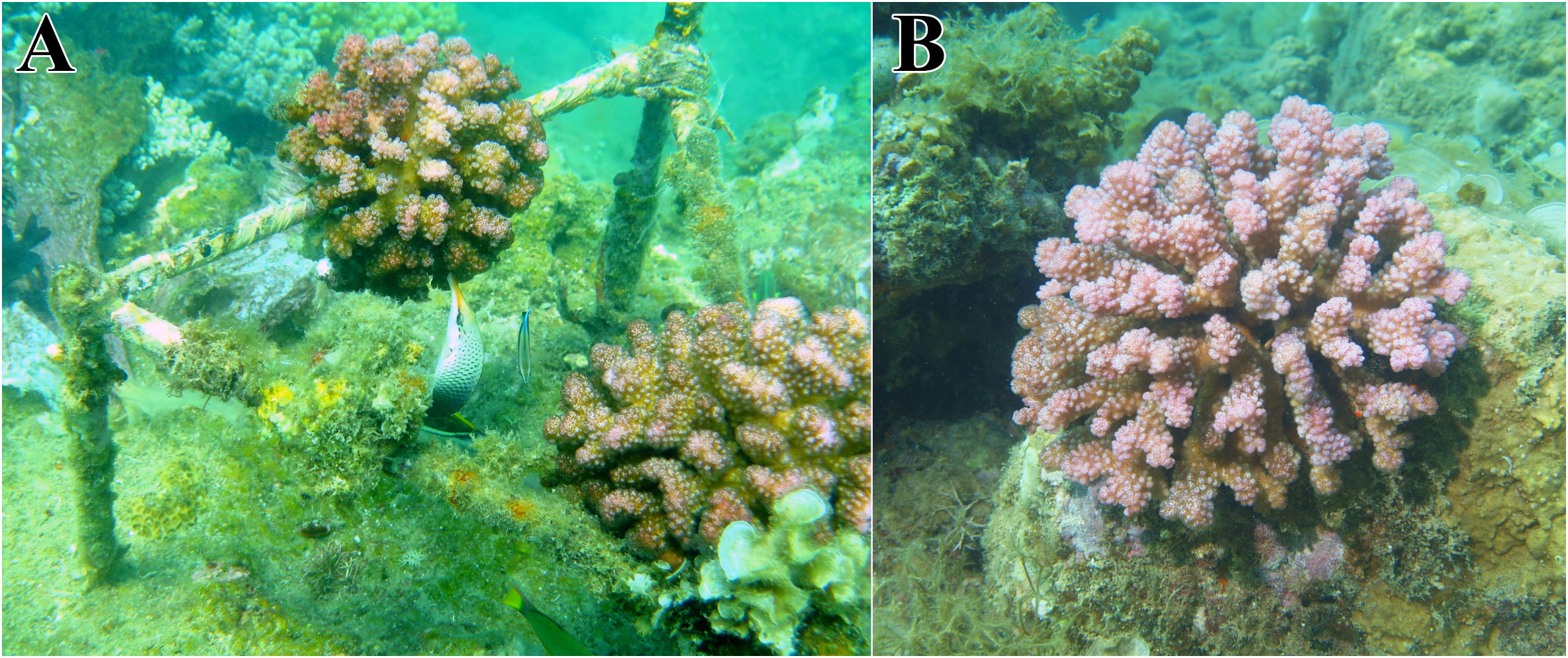
Figure 3 Planted and natural colonies of Pocillopora verrucosa. (A) Planted colonies elevated above the substrate on a triangular construction are examined by the wrasse fish Gomphosus varius; (B) Natural colony attached to a bioherm.
In recent studies on coral symbiotic communities, divers carried out the identification and abundance assessment of symbiotic animals in situ without removing coral colonies (e.g., Counsell et al., 2018). Although this approach prevents the destruction of target coral colonies, it does not enable a complete inventory of symbionts and their precise identification. However, the traditional approach of removing coral heads from the sea (Abele and Patton, 1976; Austin et al., 1980) allows obtaining more accurate information on the species composition and abundance of symbionts but is harmful to the coral population and the reef in the broader sense. In our study, we used reared colonies, which allowed us to combine the advantages of both approaches: precise counting of symbionts and avoiding the destruction of colonies in the natural population.
For comparison of symbiotic communities, we used 41 reared colonies exposed for 12 months on the frames in the natural environment and 10 natural coral colonies of unknown age, both collected in spring 2013 (April and March respectively). To minimize the impact on the local coral population, the number of natural colonies was limited to 10 heads. To investigate whether the structure of infracommunities depends on the age (exposure) of colonies, an additional 49 colonies with a 6-month exposure, transplanted at the same time as the colonies of the first group in April 2012, were also utilized.
The sampling of P. verrucosa colonies was conducted through scuba diving. Each colony was covered with a fine mesh to prevent the escape of ectosymbionts. Subsequently, the colonies were carefully detached from the nursery or natural substrate, placed in individual zip-lock plastic bags, and promptly transported to a coastal laboratory. The volume between coral branches is a major factor influencing the number of animals inhabiting a coral colony. This volume exhibits a strong correlation with the total coral colony volume, which is calculated by multiplying the maximum diameter, perpendicular diameter, and height of the colony (Austin et al., 1980). Therefore, in this study, we measured these three parameters to determine the volume of the colonies, as they were considered the most suitable for processing numerous colonies.
Each colony was immersed in a clove oil emulsion (15 drops per liter of seawater) for 3 min and intensively washed to immobilize and remove symbionts. The resulting solution was filtered through a 1 × 1-mm mesh to collect the washed-off animals, excluding those that were smaller than 1 mm in length. The sample processing method we used does not allow us to obtain quantitative data on endosymbionts; therefore, we did not take them into account in this study. After being washed, the colonies were carefully inspected, and all found ectosymbionts were collected with tweezers. The collected animals were then photographed to capture their coloration and preserved in 70% or 100% alcohol for further examination. Finally, the treated coral colonies were planted in a coral nursery for subsequent use in the coral reef restoration program of the Tropical Center.
Each animal was identified to the lowest possible taxonomic level using specialized literature (Banner and Banner, 1982; Bruce, 1993; Bruce, 1998; Castro et al., 2004; Marin et al., 2005; Marin and Spiridonov, 2007). Small animals like polychaetes, amphipods, and copepods were not identified and excluded from the analysis. The species associated with the colonies of P. verrucosa were grouped into two main categories, “obligatory ectosymbionts” and “facultative ectosymbionts”, according to the existing literature (Coles, 1980; Black and Prince, 1983; Stella et al., 2011; Britayev and Mikheev, 2013). The latter group includes both well-known facultative ectosymbiont species and poorly studied species with uncertain status. Juvenile Trapezia spp. crabs, which could not be identified at the species level, were used only for comparing the density of main groups of symbiotic species. All identified material has been deposited at the Institute of Ecology and Evolution Russian Academy of Sciences (IPEE RAS), Moscow, Russia.
The diversity (total number of obligate and facultative species), species richness (median number of species per 1 dm3 of colony volume of infested hosts), density (median number of individuals per 1 dm3 of colony volume of infested hosts), and prevalence (ratio between number of infested and total number of hosts) were compared between communities associated with planted and natural coral colonies. Typically, when comparing symbiotic communities, species richness and average abundance are calculated per host (Bush et al., 1997). However, to adjust for the correlation between the number of associated species and their abundance with the volume of the coral colony (Abele and Patton, 1976; Austin et al., 1980), the number of species and individuals per 1 dm3 were calculated instead of per colony (Britayev and Mikheev, 2013). Therefore, we used a more accurate term “density” instead of “average abundance”. These terms were employed with minor changes following Bush et al. (1997). The assemblage formed by all ectosymbionts from different species harbored by the same host individual is known as an infracommunity, while all infracommunities from a target host population are considered as the component community (Holmes and Price, 1986).
Each infracommunity associated with a given coral colony was characterized by the number of obligate and facultative species and the number of their individuals. These data and colony volume were tested for normality, using the Shapiro–Wilk test (Shapiro et al., 1968), with variables considered normal when both W and P were greater than 0.05.
Since none of the variables met this criterion, the Kruskal–Wallis H test was employed to compare differences between natural and transplanted colonies, as well as between transplanted colonies with 6 and 12 months of exposure. The analyzed parameters had non-normal distribution, so the median was used as the central tendency descriptor instead of the mean.
All tests were performed using Statistica v. 12.0 software (TIBCO Inc., Palo Alto, CA, USA).
A total of 37 taxa, including 11 obligate and 26 facultative species, were found in both reared and natural colonies (Table 1). Thirty species belonged to the crustacean order Decapoda, while other higher-order taxa, Polycladida, Sipuncula, Gastropoda, Ophiurida, and Osteichthyes, were represented by only one or two species each (Table 1).
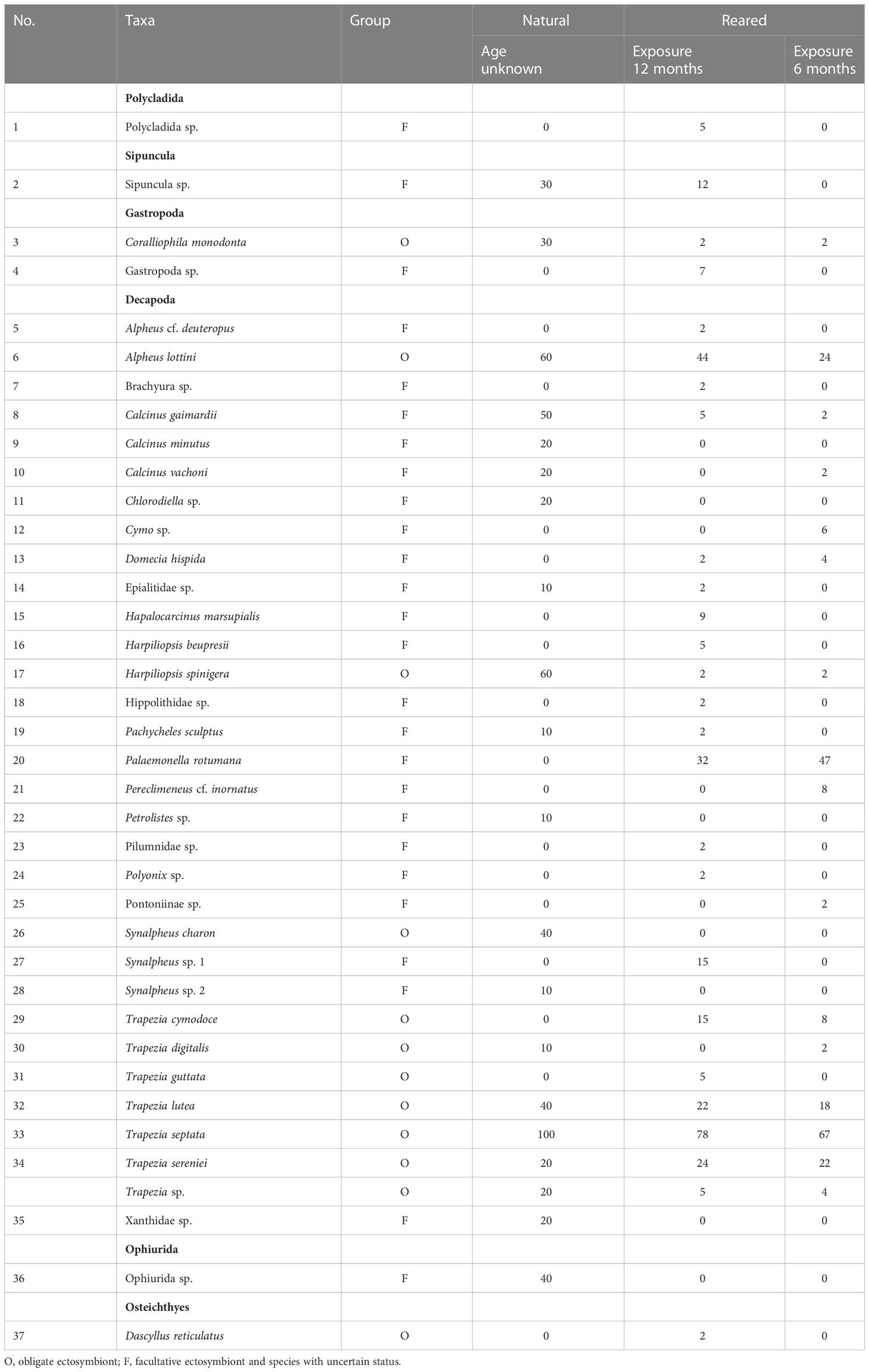
Table 1 Taxa associated with nursery-reared colonies during 12 and 6 months and natural colonies of Pocillopora verrucosa in Dam Bay (Tre Is., Nha Trang Bay, Vietnam) and their prevalence (%).
The reared colonies with 12-month exposure exhibited a higher total diversity, as well as a higher diversity of obligate and facultative species (25, 9, and 16 species), compared to the natural colonies (19, 8, and 11 species; Table 1). However, the species accumulation curve was higher for symbionts from natural colonies, indicating potentially higher diversity (Figure 4). Only 11 species were shared between planted and natural colonies (seven obligate and four facultative), 15 species were found exclusively on reared colonies (three obligate and 12 facultative), and nine species were found only on natural ones (two obligate and seven facultative). The ratio of obligate to facultative species was somewhat higher in natural than reared colonies with 12-month exposure (0.73 and 0.56, respectively).
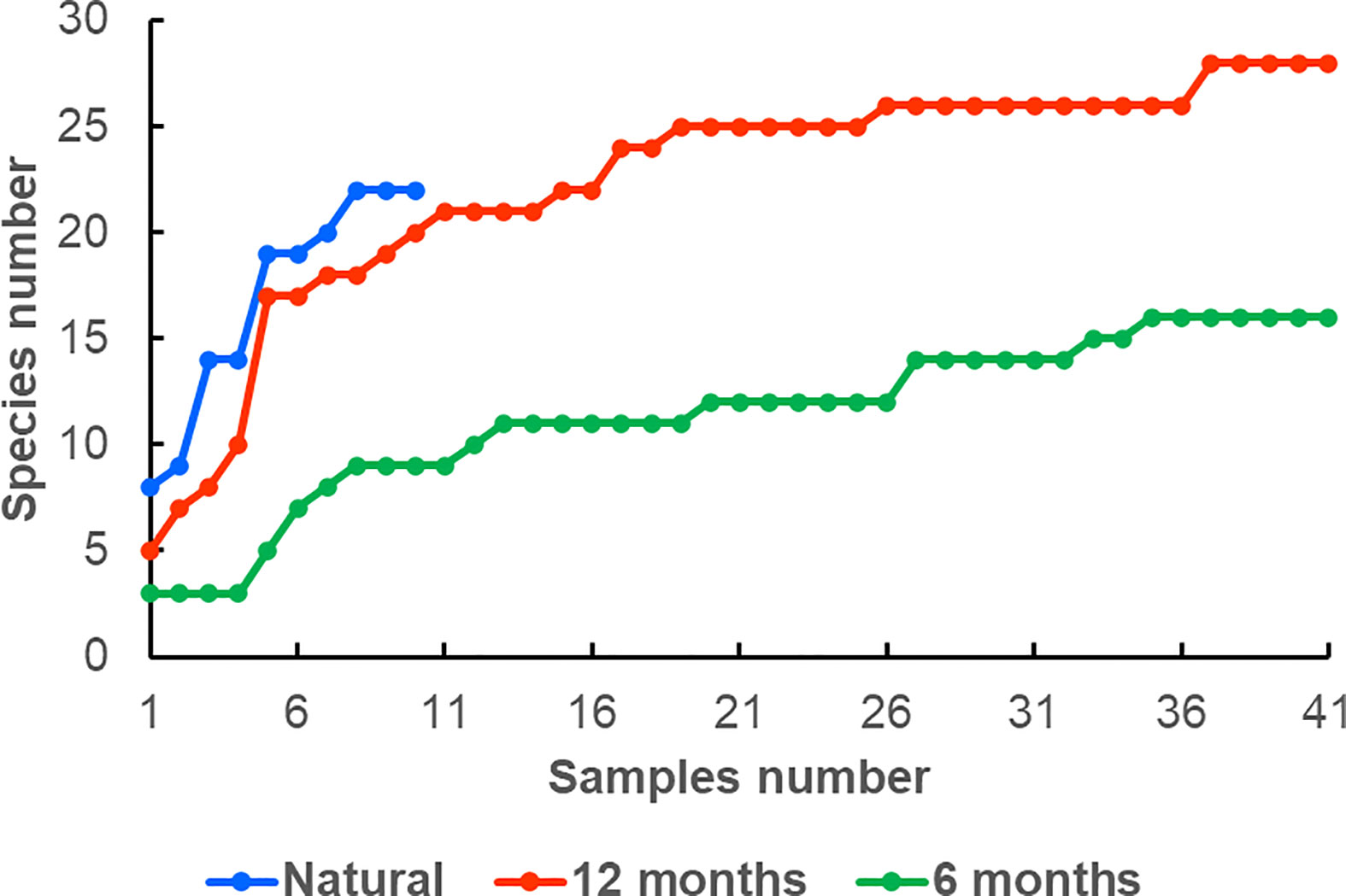
Figure 4 Species accumulation curves of Pocillopora verrucosa ectosymbiont communities in natural and reared colonies with 6 and 12 month exposures.
All coral colonies were found to be populated by obligate ectosymbionts, resulting in 100% prevalence, while the prevalence for facultative ones was lower at 80.0% and 53.7% for natural and reared colonies with 12-month exposure, respectively (Figure 5A).
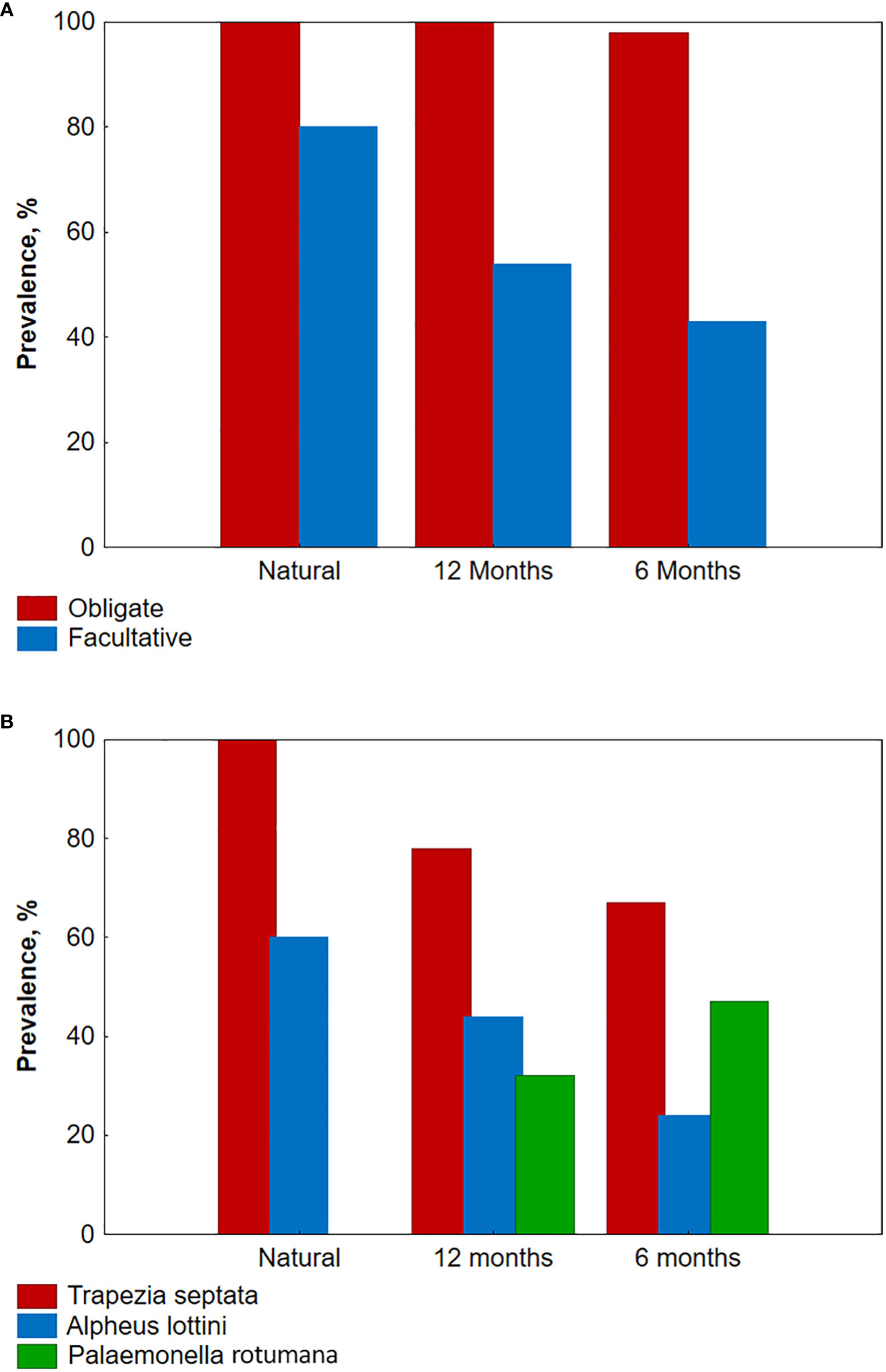
Figure 5 Prevalence of obligate, facultative, and dominant species in the three studied groups of Pocillopora verrucosa colonies. (A) Prevalence of obligate and facultative species; (B) Prevalence of the three dominant species.
The most common species in all samples were obligate ectosymbionts, the crab Trapezia septata, and the shrimp Alpheus lottini (Table 1; Figures 5B, 6A, C). Several obligate species, such as the shrimps Harpiliopsis spinigera (Figure 6B) and Synalpheus charon, the gastropod mollusk Coralliophila monodonta, and the facultative symbiont Calcinus gaimardii, which were common in natural colonies, were rare or absent in planted colonies with 12-month exposure. Conversely, one facultative symbiont, the shrimp Palaemonella rotumana (Figures 5A, 6D), was quite frequent in planted colonies but absent in natural ones.

Figure 6 The most frequently encountered obligate (A–C) and facultative (D) ectosymbionts of Pocillopora verrucosa in Nha Trang Bay. (A) Alpheus lottini; (B) Harpiliopsis spinigera; (C) Trapezia septata; (D) Palaemonella rotumana. Scale bars are 10 mm.
The diversity and abundance increased with colony volume in both natural and reared colonies with 12-month exposure in all ecological groups of symbionts (Figure S1).
Natural colonies exhibited significantly higher species richness than reared ones for all, obligate, and facultative species (Table 2; Figures 7A–C). These differences were significant in all cases (Mann–Whitney U test, p ≤ 0.0001, p ≤ 0.001, and p ≤ 0.05; Table S1).
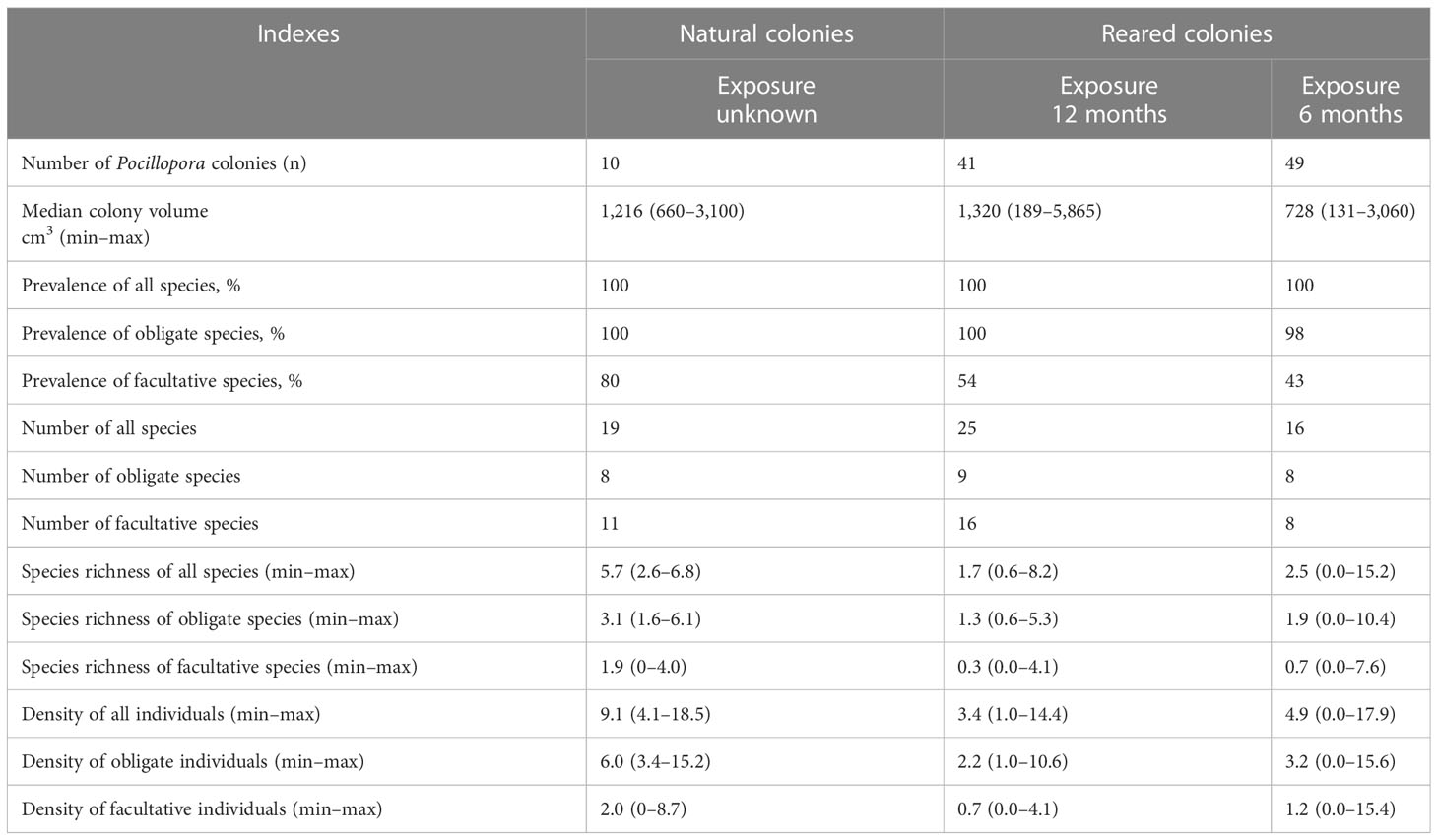
Table 2 Host characteristics, diversity, species richness, and density of obligate and facultative ectosymbionts associated with natural and nursery-reared colonies.
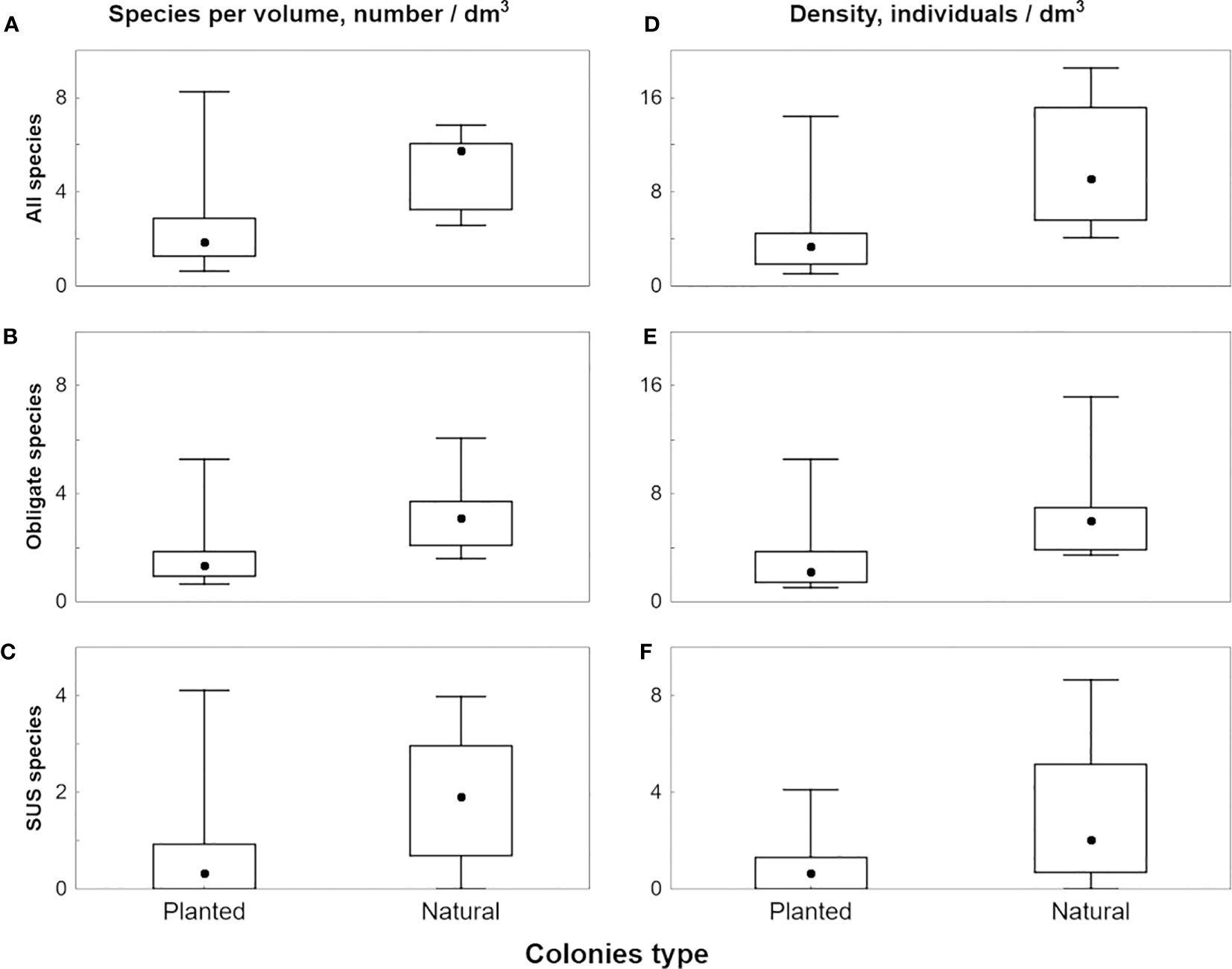
Figure 7 Median values of species richness (A–C) and density (D–F) of ectosymbionts in planted and natural colonies, box stands for 2-3 quartile range, whiskers illustrate full non outlier range. (A, D) all species; (B, E) obligate species; (D, F) facultative species.
Density in natural colonies was 2.7 times higher for all and obligate species and nearly three times higher for facultative ectosymbionts than in reared ones (Table 2; Figures 7D–F). These differences were also significant in all cases (p ≤ 0.0001, p ≤ 0.001, and p ≤ 0.05; Table S1).
The reared colonies with 12-month exposure exhibited a higher total diversity, as well as a higher diversity of facultative species (25 versus 16, and 16 versus 8 species, respectively). The number of obligate ectosymbionts was the same in both cases, with eight species (Table 2). Although the diversity of ectosymbionts was close to saturation in both samples, the asymptote was substantially higher in 12-month exposure colonies, indicating an increase in biodiversity with exposure (Figures 4, S1). We identified 10 species that were shared between two samples, with seven obligate and three facultative. Fifteen species were found only in 12-month exposure colonies (two obligate and 13 facultative) and five species only in 6-month ones (one obligate and four facultative. The ratio of obligate to facultative species was higher in 6-month exposure colonies than in 12-month exposure colonies (1.0 and 0.56, respectively).
All planted coral colonies were inhabited by ectosymbionts and almost all by obligate ectosymbionts. However, the prevalence of facultative ectosymbionts was higher in 12-month exposure colonies than in 6-month exposure colonies (53.7% and 42.8%, respectively; Table 1; Figure 5A).
The most common obligate species, the crab T. septata and the shrimp A. lottini, and the most common facultative ectosymbiont, the shrimp P. rotumana, were the same in both samples. However, the prevalence of T. septata and A. lottini was higher in 12-month exposure colonies, while the prevalence of facultative symbiont P. rotumana was lower compared to that in 6-month exposure colonies (Table 1; Figure 5B).
The diversity and abundance increase with the volume of colonies, and the regression lines are almost identical for all ecological groups of symbionts in colonies with 6- and 12-month exposure (except for facultative symbionts in colonies with 6-month exposure; Figure S1).
Species richness was lower in 12-month exposure colonies compared to colonies with 6-month exposure for all, obligate, and facultative ectosymbionts (Table 2; Figures 8A–C). These differences were significant for all species and obligate species, but not for facultative ectosymbionts (Table S1).
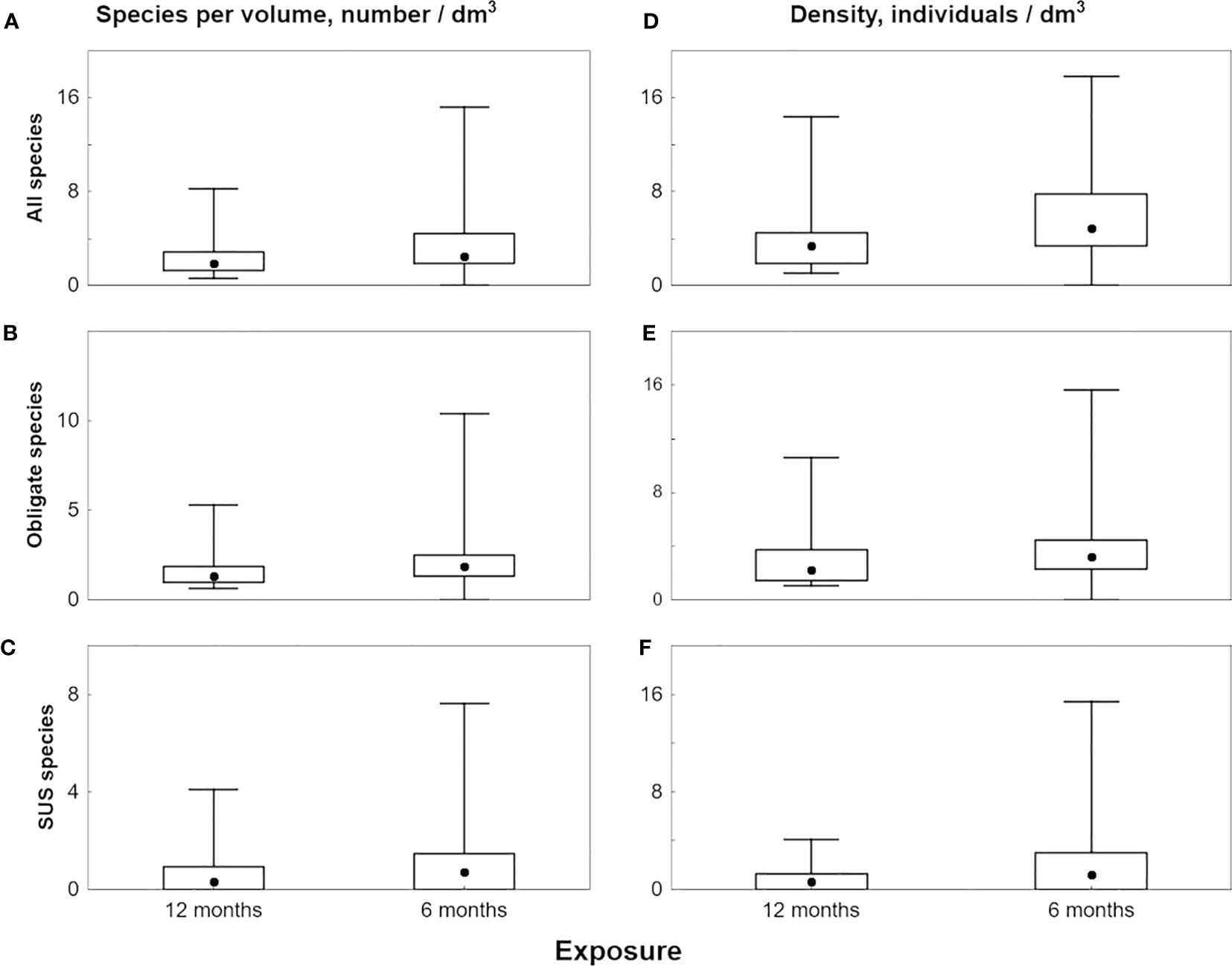
Figure 8 Median values of species richness (A–C) and density (D–F) of ectosymbionts in planted colonies of 6-month and 12-month exposure, box stands for 2-3 quartile range, whiskers illustrate full non outlier range. (A, D) all species; (B, E) obligate species; (D, F) facultative species.
Density was also lower in 12-month exposure colonies than in colonies with 6-month exposure for all, obligate, and facultative symbionts (Table 2; Figures 8D–F). These differences were significant for all species and obligate species, but not for facultative ectosymbionts (Table S1).
The Indo-Pacific pocilloporid-associated communities are known for their high species diversity, ranging from 36 to 127 species depending on geographical location (Britayev et al., 2017). However, the majority of species forming coral-associated communities are non-specialized facultative ectosymbionts, while the diversity of obligate species is much lower and ranges just from nine to 19 species (Britayev et al., 2017). The overall diversity of Poсillopora-associated species in Dam Bay is relatively low, with only 36 species, which is comparable to the diversity in the Red Sea (36 species, Britayev et al., 2017). However, the diversity of obligate ectosymbionts, which is 11 species, is very close to the numbers recorded in symbiotic communities associated with pocilloporids in other regions of the tropical Indo-Pacific (8–15 species; Patton, 1966; Black and Prince, 1983; Stella et al., 2010). In fact, it is even higher than values reported for Southeastern Australia and the Pacific coast of Panama (Abele and Patton, 1976; Black and Prince, 1983).
It is well established in the literature that the diversity of coral ectosymbionts increases with sample size (Austin et al., 1980; our data, Figure 3) and the size of coral heads (Abele and Patton, 1976; Austin et al., 1980; Edwards and Emberton, 1980). However, the number of natural colonies in our study was relatively low, and we only used relatively small coral heads. Therefore, we can assume that the diversity we identified in this study is only a small fraction of the overall diversity of Pocillopora-associated fauna of Nha Trang Bay. Increasing the sample size of natural colonies and examining larger coral heads would undoubtedly lead to a higher diversity of both facultative and, to a lesser extent, obligate coral ectosymbionts. In this study, we documented rather rich fauna of invertebrates and fish (30 species, including 10 obligate and 20 facultative ectosymbionts), associated with colonies of P. verrucosa reared in an in situ coral nursery (both 12-month and 6-month exposure). In fact, this is one of the first data on the actual diversity of ectosymbiotic fauna associated with reared colonies. Earlier studies showed the presence of several ectosymbionts on reared corals (Shaish et al., 2010). Later, Wee et al. (2019) found 63 taxa of symbiont species on alive and dead colonies of three species of reared corals, including one species of Pocillopora, based on the limited number of sampled colonies. Our study, based on large sample sizes, confirms the existence of a rich and abundant fauna of obligate and facultative ectosymbionts on reared corals and significantly expands our knowledge of their diversity. Furthermore, these findings highlight the growing importance of coral farms in not only propagating corals but also conserving the diversity of animals closely associated with them in the face of global coral reef degradation.
The comparison of the diversity of communities associated with natural and reared coral colonies revealed a low level of similarity between them. Out of the 33 ectosymbiotic species, only 10 (most of them obligate symbionts) were shared between natural and nursery-reared colonies. Although the total number of species was higher in reared colonies, the position of the species accumulation curves indicates a higher potential diversity of the symbiont fauna in natural colonies. We also registered substantial differences in the structure of symbiotic communities between natural and reared coral colonies. Species richness and density indices were higher for natural colonies. Although all natural and reared colonies were populated with ectosymbionts, the prevalence of facultative symbionts and the dominant species of obligate symbionts were higher for natural colonies. These results raise the question of why such dissimilarities exist.
The environmental conditions for these two groups of coral colonies were similar. However, there were two main differences in the habitat properties of communities on reared and natural colonies that can affect their structure. First, there was a difference in location, with reared colonies being elevated above the substrate, while natural ones are attached to a hard substrate. Second, the exposure (age) of the colonies is unknown for natural colonies and may differ from that of the reared colonies at similar sizes.
(1) The location of colonies above the substrate has two main ecological consequences for ectosymbionts. Symbionts living on natural colonies attached to the substrate are only accessible to predators (mainly coral fish) from above. The space between the substrate and the coral head provides protection against predator attacks and serves as a safe zone for symbionts (Figure 2B). In contrast, ectosymbionts living in reared colonies lack this refuge, making them more vulnerable to predators (Figure 2A).
Migration of post-settled juvenile or adult ectosymbionts from one host individual to another is a fairly common phenomenon in marine ecosystems, playing an important role in shaping symbiotic communities (e.g., Wirtz and Diesel, 1983; Bell, 1984; Yanagisawa and Hamaishi, 1986; Thiel et al., 2003; Dgebuadze et al., 2012). Such migrations were also observed in the obligate symbiont of pocilloporid corals, the crab Trapezia bidentata (as Trapezia ferruginea, Castro, 1978). Obligatory ectosymbionts are poorly adapted to survive outside their hosts, making host-to-host migrations risky due to high levels of predation pressure (Castro, 1978). It can be assumed that the movement of ectosymbionts between colonies located in the water column is riskier than migration between colonies located on the substrate (Mekhova et al., 2015), which could reduce the recruitment of symbiont populations and adversely affect the species richness and density of symbiotic communities in aquaculture.
Both of these factors may contribute to a decrease in species richness, density, and prevalence of dominant species, especially of obligate ectosymbionts, in symbiotic communities in reared colonies.
Another potential predictor of species richness and abundance is the extent of partial coral colony mortality (Stella et al., 2010; Britayev et al., 2017). Although we lack information on the proportion of dead tissue, our subsequent observations of natural and reared colonies at the same site (Britayev et al., unpublished) suggest that natural colonies exhibit a higher incidence of partial mortality. This characteristic could lead to an increase in diversity, species richness, and abundance, particularly of facultative ectosymbionts, in natural colonies (Stella et al., 2010; Counsell et al., 2018). However, further research is needed to test this hypothesis.
(2) It is important to consider that the exposure (age) of natural colonies may be significantly longer than that of nursery-reared colonies of the same size. The size of P. verrucosa fragments used for planting roughly corresponded to, or even exceed, the size of 2-year-old colonies formed from settled larvae (Zakaria, 2004). According to this, we assume that the exposure (age) of natural colonies in our samples could be at least 3 years. Consequently, the succession of communities on natural colonies likely lasted at least three times longer compared to reared ones. This extended time frame could also account for the significant differences observed in the composition and structure between communities of natural and reared colonies.
The development of symbiotic communities in scleractinian corals is a poorly understood process (Rouzé et al., 2019) and is completely unknown in Pocillopora species. In order to investigate the effect of exposure on symbiotic communities, we compared reared colonies exposed for 6 and 12 months.
As expected, communities with different exposure times differed in ectosymbiont diversity and abundance, which increased with longer exposure and in accordance with the increase of the colony size (Figure S1). Although almost all colonies in both exposure groups were populated with ectosymbionts, the prevalence of facultative symbionts and the dominant species of obligate symbionts was higher in the 12-month exposure colonies (Table 2; Figure 5B). The regression lines between the number of species and individuals of symbionts and the volume of colonies were almost identical in corals with different exposure (Figure S1). These findings suggest that communities in 6- and 12-month-old colonies are not different communities but rather different stages of succession in the same community.
On the contrary, the species richness and density of symbionts in 6-month exposure colonies were significantly higher than in colonies exposed for 12 months (Table 2). This finding may be attributed to the territorial behavior of coral ectosymbionts. Previous studies have shown that pairs of obligate shrimp A. lottini and crabs of Trapezia spp., which settle in the early stages of colony formation, continue to control the entire coral head as it grows, excluding individuals of both their own species (Patton, 1974; Abele and Patton, 1976) and other species (Preston, 1973). Therefore, the decrease in species richness and density of symbionts in 12-month exposure colonies can be attributed to the expected territorial defense by resident symbionts. As the coral grows, resident symbionts expand their territory, preventing the invasion of new individuals. However, to distinguish the contribution of each factor to the formation of symbiotic communities, further experimental studies with planted coral colonies are needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements.
TB: Experimental design, data collection, writing of the MS, supervising the project. SZ: Data collection, data processing, writing of the MS. FL: Data processing, writing of the MS. YD: Data collection, data processing, writing of the MS. All authors contributed to the article and approved the submitted version.
This work was supported by the grant of the Russian Science Foundation, RSF 22-24-00836. Open access publication fees were covered by the state of Bremen.
We would like to express our sincere appreciation to Doctor Nguyen Thi Hai Thanh and all our colleagues from Russian-Vietnamese Tropical Center for their organizational and technical assistance throughout this project. We are also grateful to Marin I.N. and Ogurtsov A. for their valuable contributions to coral rearing and sampling. Additionally, we would like to thank S. Gorin for the images of living shrimps used in this study. This work was supported by a grant from the Russian Science Foundation, RSF 22-24-00836. Open-access publication fees were covered by the state of Bremen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1221922/full#supplementary-material
Abele L. G., Patton K. (1976). The size of coral heads and the community biology of associated decapod crustaceans. J. Biogeogr. 3, 35–47. doi: 10.2307/3038097
Alcala A. C., Gomez E. D., Alcala L. C. (1982). Survival and growth of coral transplants in Central Philippines. Kalikasan Philipp. J. Bioi. 11, 136–147.
Auberson B. (1982). Coral transplantation: an approach to the re-establishment of damaged reefs. Kalikasan Philipp. J. Biol. 11 (1), 158–172.
Austin A., Austin S. A., Sale P. F. (1980). Community structure of the fauna associated with the coral Pocillopora damicomis (L) on the Great Barrier Reef. Aust. J. Mar. Fresh. Res. 31, 163–174. doi: 10.1071/MF9800163
Banner D. M., Banner A. B. (1982). The alpheid shrimp of Australia Part III: The remaining alpheids, principally the genus Alpheus, and the family Ogyrididae. Rec. Aust. Mus. 34, 1–357. doi: 10.3853/j.0067-1975.34.1982.434
Barton J. A., Willis B. L., Hutson K. S. (2017). Coral propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquaculture 9, 238–256. doi: 10.1111/raq.12135
Bell J. L. (1984). Changing residence: dynamics of the symbiotic relationship between Dissodactylus mellitae Rathbun (Pinnotheridae) and Mellita quinquiesperforata (Leske) (Echinodermata). J. Exp. Mar. Biol. Ecol. 82, 101–115. doi: 10.1016/0022-0981(84)90097-2
Black R., Prince J. (1983). Fauna associated with the coral Pocillopora damicornis at the southern limit of its distribution in Western Australia. J. Biogeogr. 10 (2), 135–152. doi: 10.2307/2844624
Boch C. A., Morse A. N. C. (2012). Testing the effectiveness of direct propagation techniques for coral restoration of Acropora spp. Ecol. Eng. 40, 11–17. doi: 10.1016/j.ecoleng.2011.12.026
Borneman E., Lowrie J. (2001). Advances in captive husbandry and propagation: an easily utilized reef replenishment means from the private sector? Bull. Mar. Sci. 69, 897–913.
Britayev T. A., Mekhova E., Deart Y., Martin D. (2017). Do syntopic host species harbour similar symbiotic communities? The case of Chaetopterus spp. (Annelida: Chaetopteridae). PeerJ 5, e2930. doi: 10.7717/peerj.2930
Britayev T. A., Mikheev V. N. (2013). Clumped spatial distribution of scleractinian corals influences the structure of their symbiotic associations. Dokl. Biol. Sci. 448, 45–48. doi: 10.1134/S0012496613010146
Britayev T. A., Petrochenko R. A., Burmistrova Y. A., Nguyen T. H., Lishchenko F. V. (2023). Density and bleaching of corals and their relationship to the coral symbiotic community. Diversity 15, 456. doi: 10.3390/d15030456
Bruce A. J. (1998). New keys for the identification of Indo-West Pacific coral associated pontoniine shrimps, with observations on their ecology: (Crustacea: Decapoda: Palaemonidae). Ophelia 49, 29–46. doi: 10.1080/00785326.1998.10409371
Bush A. O., Lafferty K. D., Lotz J. M., Shostak A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83, 575–583. doi: 10.2307/3284227
Castro P. (1978). Movements between coral colonies in Trapezia ferruginea (Crustacea: brachyura), an obligate symbiont of scleractinian corals. Mar. Biol. 46, 237–245. doi: 10.1007/BF00390685
Castro P., Ng P. K. L., Ahyong S. T. (2004). Phylogeny and systematics of the Trapeziidae Miers 1886 (Crustacea: Brachyura), with the description of a new family. Zootaxa 643, 1–70. doi: 10.11646/zootaxa.643.1.1
Clark T., Morton B. (1999). Relative roles of bioerosion and typhoon-induced disturbance on the dynamics of a high latitude scleractinian coral community. J. Mar. Biol. Assoc. U.K. 79, 803–820. doi: 10.1017/S0025315498000988
Coles S. (1980). Species diversity of decapods associated with living and dead reef coral Pocillopora meandrina. Mar. Ecol. Prog. Ser. 2, 281–291. doi: 10.3354/meps002281
Combillet L., Fabregat-Malé S., Mena S., Marín-Moraga J. A., Gutierrez M., Alvarado J. J. (2022). Pocillopora spp. growth analysis on restoration structures in an Eastern Tropical Pacific upwelling area. PeerJ 10, e13248. doi: 10.7717/peerj.13248
Counsell C. W., Donahue M. J., Edwards K. F., Franklin E. C., Hixon M. A. (2018). Variation in coral-associated cryptofaunal communities across spatial scales and environmental gradients. Coral Reefs 37, 827–840. doi: 10.1007/s00338-018-1709-7
Delbeek J. C. (2001). Coral farming: past, present and future trends. Aquarium Sci. Conserv. 3, 171–181. doi: 10.1023/A:1011306125934
DeVantier M., Reichelt R. E., Bradbury R. H. (1986). Does Spirobranchus giganteus protect host Porites from predation by Acanthaster planci: predator pressure as a mechanism of coevolution? Mar. Ecol. Prog. Ser. 32, 307–310. doi: 10.3354/meps032307
Dgebuadze P. Y., Mehova E. S., Britayev T. A. (2012). Recolonization of the Himerometra robustipinna (Himerometridae, Crinoidea) by macrosymbionts: an in situ experiment. Symbiosis 58, 253–258. doi: 10.1007/s13199-013-0227-1
Edwards A., Emberton H. (1980). Crustacea associated with the scleractinian coral, Stylophora pistillata (Esper.) in the Sudanese Red Sea. J. Exp. Mar. Biol. Ecol. 42, 225–240. doi: 10.1016/0022-0981(80)90178-1
Enochs I. C. (2012). Motile cryptofauna associated with live and dead coral substrates: implications for coral mortality and framework erosion. Mar. Biol. 159, 709–722. doi: 10.1007/s00227-011-1848-7
Glynn P. W. (1980). Defense by symbiotic crustacea of host corals elicited by chemical cues from predator. Oecologia 47, 287–290. doi: 10.1007/BF00398518
Goldshmid R., Holzman R., Weihs D., Genin A. (2004). Aeration of corals by sleep swimming fish. Limnol. Oceanogr. 49, 1832–1839. doi: 10.4319/lo.2004.49.5.1832
Hoeksema B. W., Harper C. E., Langdon-Down S. J., van der Schoot R. J., Smith-Moorhouse A., Spaargaren R., et al. (2022a). Host range of the coral-associated worm snail Petaloconchus sp. (Gastropoda: Vermetidae), a newly discovered cryptogenic pest species in the southern Caribbean. Diversity 14, 196. doi: 10.3390/d14030196
Hoeksema B. W., Timmerman R. F., Spaargaren R., Smith-Moorhouse A., van der Schoot R. J., Langdon-Down S. J., et al. (2022b). Morphological modifications and injuries of corals caused by symbiotic feather duster worms (Sabellidae) in the Caribbean. Diversity 14, 332. doi: 10.3390/d14050332
Hoeksema B., van der Meij S., Fransen C. (2012). The mushroom coral as a habitat. J. Mar. Biol. Assoc. U. K. 92 (4), 647–663. doi: 10.1017/S0025315411001445
Hoeksema B. W., Wels D., van der Schoot R. J., ten Hove H. A. (2019). Coral injuries caused by Spirobranchus opercula with and without epibiotic turf algae at Curaçao. Mar. Biol. 166, 60. doi: 10.1007/s00227-019-3504-6
Holmes J.С., Priсe P. W. (1986). Parasite communities: the roles of phylogeny and ecology. Syst. Zool. 29, 203–213. doi: 10.1093/sysbio/29.2.203
Knudsen J. (1967). Trapezia and Tetralia (Decapoda, Brachyura, Xanthidae) as obligate ectoparasites of pocilloporid and acroporid corals. Pac. Sci. 21, 51–57.
Koval G., Rivas N., D’Alessandro M., Hesley D., Santos R., Lirman D. (2020). Fish predation hinders the success of coral restoration efforts using fragmented massive corals. PeerJ 8, e9978. doi: 10.7717/peerj.9978
Liberman T., Genin A., Loya Y. (1995). Effects on growth and reproduction of the coral Stylophora pistillata by the mutualistic damselfish Dascyllus marginatus. Mar. Biol. 121, 741–746. doi: 10.1007/BF00349310
Marin I. N., Britayev T. A., Anker A. (2005). Pontoniine shrimps associated with cnidarians: new records and list of species from coastal waters of Viet Nam. Arthropoda Selecta 13 (4), 199–218.
Marin I. N., Spiridonov V. A. (2007). “Coral-associated crabs (Decapoda: Domecidae, Trapeziidae, Tetraliidae, Xanthidae: Cymoinae) from Nhatrang Bay,” in Benthic Fauna of the Bay of Nhatrang. Eds. Britayev T. A., Pavlov D. S. (Moscow: KMK Scientific Press), 209–234.
Mekhova E. S., Dgebuadze P. Y., Mikheev V. N., Britayev T. A. (2015). Colonization of depopulated crinoids by symbionts: who comes from the bottom and who from the water column? J. Mar. Biol. Assoc. U.K. 95 (8), 1607–1612. doi: 10.1017/S0025315415000600
Moerland M. S., Scott C. M., Hoeksema B. W. (2016). Prey selection of corallivorous muricids at Koh Tao (Gulf of Thailand) four years after a major coral bleaching event. Contributions to Zool. 85, 291–309. doi: 10.1163/18759866-08503003
Mokady O., Loya Y., Lazar B. (1998). Ammonium contribution from boring bivalves to their coral host—a mutualistic symbiosis? Mar. Ecol. Prog. Ser. 169, 295–301. doi: 10.3354/meps169295
Montano S., Aeby G., Galli P., Hoeksema B. W. (2022). Feeding behavior of Coralliophila sp. on corals affected by Caribbean ciliate infection (CCI): a new possible vector? Diversity 14, 363. doi: 10.3390/d14050363
O'Donnell K. E., Lohr K. E., Bartels E., Patterson J. T. (2017). Evaluation of staghorn coral (Acropora cervicornis, Lamarck 1816) production techniques in an ocean-based nursery with consideration of coral genotype. J. Exp. Mar. Biol. Ecol. 487, 53–58. doi: 10.1016/j.jembe.2016.11.013
Patton W. K. (1966). Decapod Crustacea commensal with Queensland branching corals. Crustaceana 10, 271–295. doi: 10.1163/156854066X00180
Patton W. K. (1974). “Community structure among the animals inhabiting the coral Pocillopora damicomis at Heron Island, Australia,” in Symbiosis in the sea. Ed. Vernberg W. B. (Columbia: University of South Carolina Press), 219–243.
Potkamp G., Vermeij M. J. A., Hoeksema B. W. (2017). Genetic and morphological variation in corallivorous snails (Coralliophila spp.) living on different host corals at Curaçao, southern Caribbean. Contributions to Zool. 86, 111–144. doi: 10.1163/18759866-08602002
Pratchett M. (2001). Influence of coral symbionts on feeding preferences of crown-of-thorns starfish Acanthaster planci in the western Pacific. Mar. Ecol. Prog. Ser. 214, 111–119. doi: 10.3354/meps214111
Preston E. M. (1973). A computer simulation of competition among five sympatric congeneric species of xanthid crabs. Ecology 54, 469–483. doi: 10.2307/1935333
Robertson R. (1970). Review of the predators and parasites of stony corals, with special reference to symbiotic prosobranch gastropods. Pacific Sci. 24, 43–54.
Rotjan R. D., Lewis S. M. (2008). Impact of coral predators on tropical reefs. Mar. Ecol. Prog. Ser. 367, 73–91. doi: 10.3354/meps07531
Rouzé H., Lecellier G., Mills S. C., Planes S., Berteaux-Lecellier V., Stewart H. (2014). Juvenile Trapezia spp. crabs can increase juvenile host coral survival by protection from predation. Mar. Ecol. Prog. Ser. 515, 151–159. doi: 10.3354/meps10970
Rouzé H., SinNiger F., Harii S. (2019). Onset of symbiosis between Tetralia crabs and Acropora corals revealing the smallest host habitat size. Galaxea J. Coral Reef Stud. 21, 1–2. doi: 10.3755/galaxea.21.1_1
Shafir S., Van Rijn J., Rinkevich B. (2006). Coral nubbins as source material for coral biological research: A prospectus. Aquaculture 259, 444–448. doi: 10.1016/j.aquaculture.2006.05.026
Shaish L., Levy G., Gomez E., Rinkevich B. (2008). Fixed and suspended coral nurseries in the Philippines: Establishing the first step in the “gardening concept” of reef restoration. J. Exp. Mar. Biol. Ecol. 358, 86–97. doi: 10.1016/j.jembe.2008.01.024
Shaish L., Levy G., Katzir G., Rinkevich B. (2010). Coral reef restoration (Bolinao, Philippines) in the face of frequent natural catastrophes. Restor. Ecol. 18 (3), 285–299. doi: 10.1111/j.1526-100X.2009.00647.x
Shapiro S. S., Wilk M. B., Chen H. J. (1968). A comparative study of various tests for normality. J. Am. Stat. Assoc. 63 (324), 1343–1372. doi: 10.1080/01621459.1968.10480932
Smith S. D. A. (2011). Densities of the endolithic bivalve Lithophaga lessepsiana (Vaillant 1865) in Pocillopora damicornis, Solitary Islands Marine Park, northern NSW, Australia. Molluscan Res. 31 (1), 42–46.
Stachowicz J. J., Hay M. E. (1999). Mutualism and coral persistence: the role of herbivore resistance to algal chemical defence. Ecology 80, 2085–2101. doi: 10.1890/0012-9658(1999)080[2085:MACPTR]2.0.CO;2
Stella J. S., Jones G. P., Pratchett M. S. (2010). Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs. 29 (4), 957–973. doi: 10.1007/s00338-010-0648-8
Stella J. S., Pratchett M. S., Hutchings P. A., Jones G. P. (2011). Coral-associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. Annu. Rev. 49, 43–104. doi: 10.1201/b11009-3
Stewart H. L., Holbrook S. J., Schmitt R. J., Brooks A. J. (2006). Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs. 25 (4), 609–615. doi: 10.1007/s00338-006-0132-7
Sussman M., Loya Y., Fine M., Rosenberg E. (2003). The marine fireworm Hermodice carunculata is a winter reservoir and springsummer vector for the coral-bleaching pathogen Vibrio shiloi. Environ. Microbiol. 2003 (5), 250–255. doi: 10.1046/j.1462-2920.2003.00424.x
Thiel M., Zander A., Valdivia N., Baeza J. A., Rueffler C. (2003). Host fidelity of a symbiotic porcellanid crab: the importance of host characteristics. J. Zool. 261 (4), 353–362. doi: 10.1017/S0952836903004333
Tkachenko K. S., Soong K., Deart Y. V., Britayev T. A. (2022). Coral symbiotic communities from different environments of an isolated atoll: reef lagoon versus forereef. Invertebr. Zool. 19 (1), 78–90. doi: 10.15298/invertzool.19.1.08
Wee S. Y. C., Sam S. Q., Sim W. T., Ng C. S. L., Taira D., Afiq-Rosli L., et al. (2019). The role of in situ coral nurseries in supporting mobile invertebrate epifauna. J. Nat. Conserv. 50, 125710. doi: 10.1016/j.jnc.2019.125710
Wirtz P., Diesel R. (1983). The Social Structure of Inachus phalangium, a Spider Crab Associated with the Sea Anemone Anemonia sulcata. Ethology 62, 209–234. doi: 10.1111/j.1439-0310.1983.tb02152.x
Yanagisawa Y., Hamaishi A. (1986). Mate acquisition by a solitary crab Zebrida adamsii, a symbiont of the sea urchin. J. Ethol. 4, 153–162. doi: 10.1007/BF02348117
Keywords: ectosymbionts, obligate, facultative, aquaculture, coral nurseries, Pocillopora verrucosa
Citation: Britayev TA, Zvonareva SS, Lishchenko FV and Deart YV (2023) Symbiotic communities associated with nursery-reared and natural corals: are they similar? Front. Mar. Sci. 10:1221922. doi: 10.3389/fmars.2023.1221922
Received: 13 May 2023; Accepted: 24 July 2023;
Published: 14 August 2023.
Edited by:
Simon Jungblut, University of Bremen, GermanyReviewed by:
Bert W. Hoeksema, Naturalis Biodiversity Center, NetherlandsCopyright © 2023 Britayev, Zvonareva, Lishchenko and Deart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. A. Britayev, YnJpdGF5ZXZAeWFuZGV4LnJ1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.