- 1Laboratory of Ichthyology, Department of Zoology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Institute of Marine Biological Resources and Inland Waters, Hellenic Centre for Marine Research, Attika, Greece
Greek waters are the recipient of several alien species, mainly through natural dispersal following invasion and establishment of non-indigenous species (NIS) in neighboring areas, making their monitoring and mitigating their effects of paramount importance. The European Union legislation framework toward alien species invasions considers risk assessments as the top of the spear for a first assessment of NIS and their potential to become invasive or not. The Union List has already included top priority species, with very few marine species. Golani’s round herring (Etrumeus golanii) is a species of round herrings in the family Dussumieriidae, a Lessepsian migrant and belonging to a group of NIS in the Mediterranean basin that are less studied. Its distribution range is mainly limited in the southeastern Mediterranean Sea, while in the Greek seas, it has not yet been observed in the north Aegean and Ionian seas, probably due to temperature and oceanographical reasons. Its presence in the basin is recorded by commercial fisheries landings in several countries (especially purse-seiners), indicating a potentially positive effect on commercial fisheries. A risk assessment of E. golanii in Greek waters was carried out in this work, based on the Risk Assessment Scheme developed by the GB Non-Native Species Secretariat (GB Non-Native Risk Assessment—GBNNRA). An overall semi-quantitative summary of risk, in terms of likelihood of events and magnitude of impacts, was facilitated for several attributors, including confidence levels for each one. The assessment highlighted a very likely possibility of introduction in the Greek seas from neighboring countries, as well as successful establishments of populations with high confidence levels. A moderate magnitude of impact regarding its further spread was deemed, while a minor one was indicated in terms of native species pressure and a minimal one in terms of economic costs and public health. Overall, E. golanii was not characterized as an invasive alien species (IAS) and local communities could benefit from its presence (commercial fisheries); however, further studies focusing on its reproduction and spawning grounds should be implemented.
1 Introduction
The Mediterranean Sea is under threat from many anthropogenic pressures with significant impacts on its diversified ecosystems and their biodiversity (Carmezim et al., 2022). The introduction of non-indigenous species (NIS) and especially of invasive alien species (IAS), which are harmful ecologically and socio-economically, is considered to be listed high among the most important threats in the basin (Tsirintanis et al., 2022). The high dispersal rate of NIS in the Mediterranean has been accelerated by the rapid development of technology, especially in the last decades, the intense exploitation of natural resources, and globalization (Simberloff et al., 2013; Zenetos et al., 2022a). Thus, many species have the potential to move beyond their distribution limits and establish successful populations in their non-native habitats (Seebens et al., 2017). The Mediterranean Sea is considered to be the most invaded marine region in the world with more than 1,000 validated NIS, most of which are regarded established (759) rather than casual (Zenetos et al., 2022a; Zenetos et al., 2022b). The eastern part of the basin is particularly vulnerable to alien species’ introduction due to its proximity to the Suez Canal, the latter being a significant pathway for NIS (Korpinen et al., 2019). Many scientific projects regarding alien species mitigation measures have been implemented in the Mediterranean Sea, incorporating citizen science as a useful tool in a wide extent, from new species detections and monitoring to complete surveys and management initiatives (Bodilis et al., 2014; Naasan Aga Spyridopoulou et al., 2020; Tiralongo et al., 2020). Greek waters, and especially the Aegean Sea, are the recipient of many NIS that can later spread out in other parts of the basin. An informative and collaborative network has been established in Greece, namely, the Ellenic Network of Aquatic Invasive Species (ELNAIS), aiming to collect and report spatial data on NIS in the Greek seas (Zenetos et al., 2015), which, alongside the recent rise of projects considering citizen science in Greece (Zenetos et al., 2013; Giovos et al., 2019), is the first line of defense towards IAS management and mitigation. Taking into account the difficulties of monitoring and numbering NIS, the latest update estimates the existence of 242 NIS and 64 cryptogenic species in Greek waters, with the Aegean Sea having the lion’s share compared to the Ionian Sea (Zenetos et al., 2020).
The European Union, following globally mandated approaches for NIS and IAS management, has established a legislation framework (EU Regulation No 1143/2014; EC, 2014), aimed at preventing and managing the introduction and spread of IAS. With numerous invasive species being confirmed in the Mediterranean Sea, as a rule of thumb, the regulation prioritizes its measures towards IAS that are considered of major biological, ecological, socio-economic, and human-health concerns. As general provisions, the regulation provides some steps towards NIS management, including identification, prevention, detection, and eradication. For IAS to be considered of EU concern, they must meet three criteria, which are provided from the results of research works and project implementations: (1) the name of the studied species, (2) a complete and thorough risk assessment, and (3) scientific evidence of IAS origins, population establishment, and impact on the native environment. Many Mediterranean IAS are right now in the “Union List” with the implementation of successful risk assessments. According to the EU Regulation No. 1143/2014 (EC, 2014), a risk assessment is considered successful when it satisfies eight descriptors related to (1) identity, (2) reproduction, (3) introduction, (4) establishment and spread, (5) presence in neighboring countries, (6) adverse impact on biodiversity, (7) costs of damage, and (8) potential social and economic exploitation. In the Mediterranean Sea, risk assessments that mainly concern fish IAS are available in the literature, including Plotosus lineatus (Galanidi et al., 2019), Pterois miles (Filiz et al., 2017; Kleitou et al., 2021), Siganus rivulatus (Shapiro Goldberg et al., 2021), Siganus luridus (D’Amen and Azzurro, 2020), and Lagocephalus sceleratus (Filiz et al., 2017; Galanidi and Zenetos, 2019). These species have well-established populations in the Greek waters, having great impacts on ecosystems, native species, and fisheries (Katsanevakis et al., 2020).
Etrumeus golanii (DiBattista et al., 2012) is a species of round herring belonging to the family Dussumieriidae, a Lessepsian migrant in the Mediterranean Sea and the only one recorded from this family in Greek waters (Figure 1). The species can be easily misidentified with similar pelagic species of high commercial interest, like the European anchovy (Engraulis encrasicolus) and the European pilchard (Sardina pilchardus). However, it can be distinguished from other Mediterranean species of the order Clupeiformes, due to its smooth abdomen and the position of the pelvic fin, which is behind the dorsal (Golani, 2000). Its native range is the western Indian Ocean up to the northern Red Sea, while more specifically native populations of the species can be found in Egypt (Sanders and Morgan, 1989), Eritrea (DiBattista et al., 2012), Israel (Whitehead, 1985), and Saudi Arabia (DiBattista et al., 2012). In 2019, a risk assessment for Lessepsian migrants entering the basin from the Suez Canal was carried out on the southwest coast of Turkey, including E. golanii (Bilge et al., 2019). The assessment highlighted that the species was not an IAS and the possibility of its introduction, establishment, and spread was characterized as moderate risk (Bilge et al., 2019). The results of this study might be indicative of the species in eastern Mediterranean Sea; however, the assessment was not species-specific. Recently, a scientific project with the acronym 4ALIEN (www.4alien.gr) was carried out in the Greek seas, aiming to study the biology, ecology, and potential exploitation of four IAS, including E. golanii. Moreover, in the frame of the project, risk assessments, habitat mappings, and potential exploitation analyses were also conducted.

Figure 1 Picture of Etrumeus golanii (DiBattista et al., 2012; photo credits: PK Karachle).
The aims of the present work are to (1) present part of the results of the 4ALIEN project, focusing on the biology and economic exploitation prospects of E. golanii from the Greek seas; (2) provide a complete risk assessment of E. golanii, a comparatively less-studied NIS in the Mediterranean Sea; and (3) focus on the commercial benefits of exploiting this species that could be helpful in mitigating the effects of NIS invasions in the basin.
2 Invasive history of Etrumeus golanii in the Mediterranean Sea and species characteristics
Etrumeus golanii is a Lessepsian migrant, native to the western Indian Ocean and most common in the Red Sea (Golani and Fricke, 2005). Its presence in the Mediterranean Sea was first recorded in Lebanon (Gruvel, 1931), as Klupea kowal (Rüppell, 1837). This observation was followed by continuous records of the species in the eastern Mediterranean Sea, where populations seem to have been successfully established (Golani, 2000; Golani, 2005). In Greek waters, E. golanii has been recorded since 2003 in Rhodes (Corsini et al., 2005), since 2004 in the Cyclades Islands (Kallianiotis and Lekkas, 2005), and since 2005 in Crete (Kasapidis et al., 2007). The species continued to spread westward with the first record in the central Mediterranean, reported from the island of Lampedusa (Falautano et al., 2006). Until then, the species was recorded under the name Etrumeus teres (DeKay 1842). In 2012, it was found by DiBattista et al. (2012) that the specimens in the Mediterranean Sea identified as the Lessepsian immigrant E. teres are actually a totally new species, i.e., E. golanii. Its spread continued in the western Mediterranean with new records in Tunisia (Boussellaa et al., 2016; Rafrafi-Nouira et al., 2017), Libya (Shakman et al., 2017), Algeria (Stamouli et al., 2018), and Morocco (Tamsouri et al., 2019). Despite its small size, its spread from the eastern to the western Mediterranean Sea occurred in high rates, while new populations were established. Its rapid spread was possibly based on some characteristics of the species, such as its reproductive biology, its prolonged spawning season and the fact that it takes advantage of seasonal zooplankton reproduction for its nutritional needs (Somarakis et al., 2021). The chronological spread of E. golanii in the Mediterranean is depicted in Figure 2.
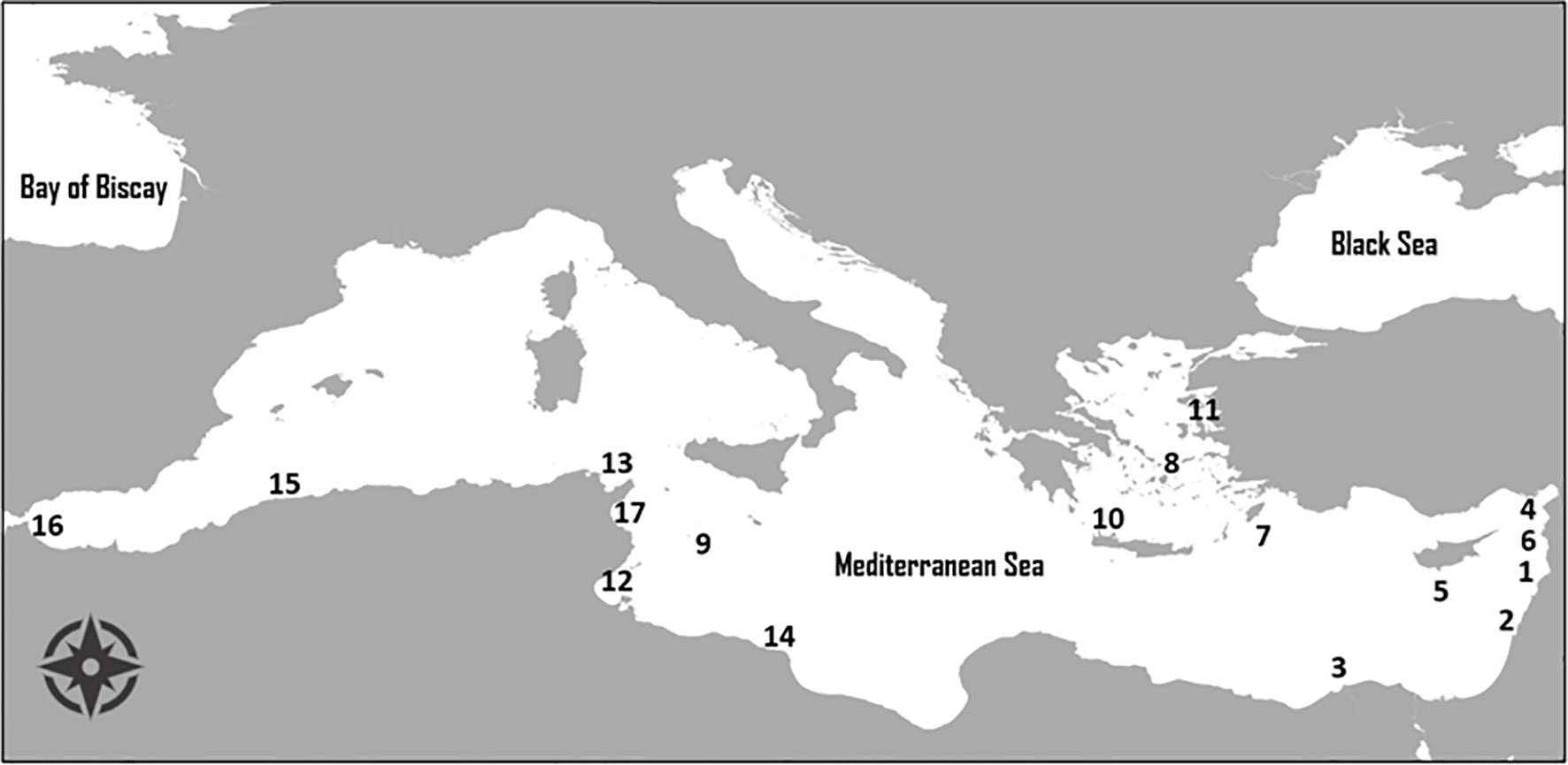
Figure 2 Map of the Mediterranean Sea depicting the distribution of Etrumeus golanii in chronological order; 1: Lebanon in 1931 (Gruvel, 1931), 2: Haifa Bay, Israel in 1961 (Whitehead, 1963), 3: Mediterranean Egypt in 1992 (El-Sayed, 1994), 4: Iskenderun Bay, Turkey in 1994 (Basusta et al., 1997), 5: Limassol, Cyprus in 1999 (Golani, 2000), 6: Syria in 2001 (Saad, 2002), 7: Rhodes Island, Greece in 2003 (Corsini et al., 2005), 8: Cyclades Islands, Greece in 2004 (Kallianiotis and Lekkas, 2005), 9: Lampedusa Island, Italy in 2005 (Falautano et al., 2006), 10: Crete, Greece in 2005 (Kasapidis et al., 2007), 11: Dikili, Turkey in 2009 (Yarmaz et al., 2010), 12: Gulf of Gabes, Tunisia in 2014 (Boussellaa et al., 2016), 13: Ras Jebel, Tunisia in 2017 (Rafrafi-Nouira et al., 2017), 14: Misrata, Libya in 2017 (Shakman et al., 2017), 15: Cherchell, Algeria in 2017 (Stamouli et al., 2018), 16: Gulf of Fnideq, Morocco in 2018 (Tamsouri et al., 2019), 17: Gulf of Hammamet, Tunisia (Mili et al., 2020).
Regarding EU member states, it has been recorded in Cyprus (Golani, 2005), Greece (Corsini et al., 2005), Malta, and Italy (Falautano et al., 2006), while it has established populations in Cyprus and Greece (Azzurro et al., 2014). In Greek waters, to date, its distribution has been limited in the central and southeastern Aegean Sea and more specifically in Crete, the Dodecanese Islands, the Cyclades Islands, and Saronikos Gulf (Figure 3). Although it has a slower spreading rate compared to other IAS (e.g., Lagocephalus sceleratus, Pterois miles, Siganus luridus, Siganus rivulatus), it is considered as established in these regions, contributing to the commercial fisheries (Zenetos et al., 2009), especially in purse-seiners, as it is active in the neritic zone, up to 50 m (Mehanna and El-Gammal, 2005). Etrumeus golanii is considered to be omnivorous, exhibiting trophic preferences for animal organisms (Karachle and Stergiou, 2017), such as small crustaceans, larvae, and small molluscs (Osman et al., 2013), whereas in the Aegean Sea, it preys mainly on Malacostraca Crustacea (Andrianopoulos et al., 2022).
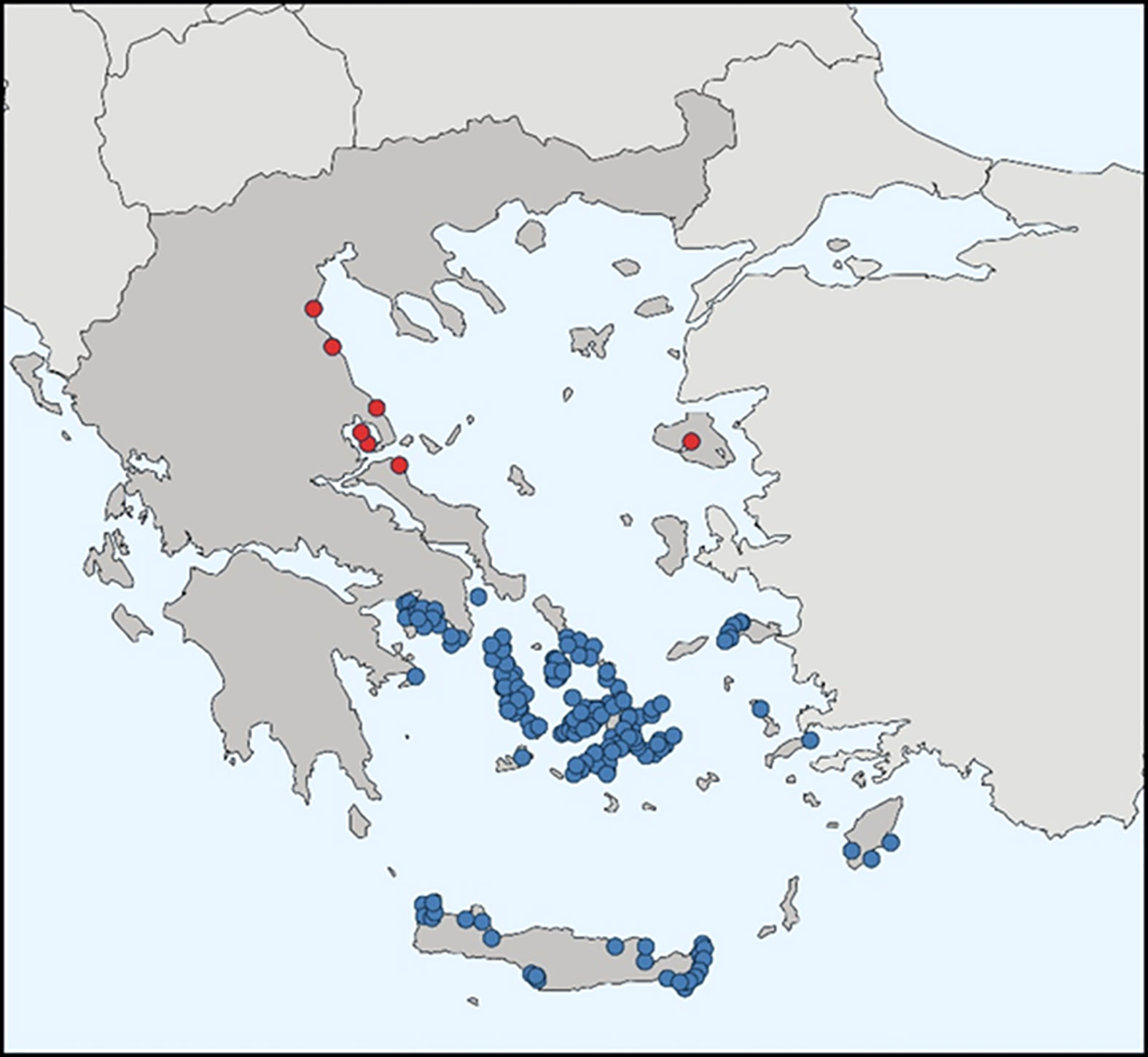
Figure 3 Distribution of Etrumeus golanii in Greek waters. Red dots indicate sporadic observations, whereas blue dots indicate established populations (modified from Karachle et al., 2021; map date October 2021).
3 Materials and methods
This research utilized the risk assessment protocol derived from the EU commissioned program ENV.B2.ETU/2016/0013 (Galanidi et al., 2019). The protocol was specifically designed to adhere to the EU Regulation (No.1143/2014; EC, 2018) and fully comply with the 13 minimum standards for risk assessment outlined by Roy et al. (2017). The risk assessment (RA) of E. golanii in Greek waters that is documented in this work was based on the Risk Assessment Scheme developed by the GB Non-Native Species Secretariat (GB Non-Native Risk Assessment—GBNNRA). The RA consists of different sections presenting basic organism information, describing the distribution of the organism, both current and potential (under current and future climate conditions), and addressing with a series of questions the four main aspects of the invasion process: (1) introduction, (2) establishment, (3) spread, and (4) impacts. Directly elicited estimates of entry, establishment, spread, and impact, with measures of assessor confidence, give an overall semi-quantitative summary of risk, in terms of likelihood (Table S1) and magnitude (Table S2). The primary considerations for evaluating the likelihood of the species’ introduction encompass various factors. These factors include the commodities it could be associated with the species’ reproductive strategy (i.e., the number of propagules capable of traveling along each pathway within a year), the survival rate of transportation along each pathway, and the successful establishment in suitable habitats in the recipient regions. Additionally, the efficacy of current management practices in detecting the species or influencing its survival is considered. Similarly, the risk of spread is assessed using a comparable set of factors, which also considers the documented spread of the species in the invaded area. Concerning the potential for establishment, particular emphasis was placed on understanding the physiological requirements of the species and how its reproductive strategy aligns with the environmental conditions present in the assessment area. Regarding the magnitude of impacts, various sets of inquiries were directed towards examining the environmental consequences, effects on ecosystem services, economic ramifications, and impacts on social wellbeing and human health. A confidence-level system proposed by Bacher et al. (2018) was also used in order to classify the socio-economic impacts of E. golanii (Table S3). The RA area was defined as the Greek seas, divided into three biogeographic sub-regions: South Aegean and Cretan Seas, North Aegean Sea, and Ionian Sea (Figure S1).
4 Risk assessment results and detailed evaluation of E. golanii in the Greek seas
4.1 Risk of introduction and associate pathways
The main introduction pathway of E. golanii in the Mediterranean Sea is through the Suez Canal, while the main countries in which the species has invaded are the southeastern Mediterranean countries (Golani, 2000). In countries where E. golanii has not yet been recorded, spread from neighboring countries with established populations is considered as a secondary introduction pathway. The risk assessment in the Greek seas indicated that both the Suez Canal and unaided introduction from neighboring countries (e.g., Turkey) are very likely introduction pathways (Table 1). Introduction from aquaria escapes can be considered as a third pathway, but in the case of E. golanii, it was deemed as very unlikely due to the species characteristics and also due to the fact that it is not an ornamental-aquarium species. The fecundity ratio of E. golanii seems to be low, ranging from 56 to 157 eggs/g, also depending on the size of individuals (Somarakis et al., 2021). In the Aegean Sea, its absolute fecundity has been estimated to 13,801 ± (standard error) 5,595 eggs, showing an increasing trend up to a total length of approximately 23 cm (Vagenas et al., 2022). Elsewhere, a relative fecundity of 278 to 645 eggs has been recorded for individuals from 14 to 22 cm (Osman et al., 2011). Although fecundity of the species is relatively low, it has a prolonged spawning season, from December to May for females, extending until July for males (Osman et al., 2011). Considering the water circulation patterns and higher salinity levels of the Red Sea, the Suez Canal has facilitated the migration of the species from the Red Sea to the Mediterranean, throughout the year, except for the summer months when the Mediterranean waters flow to the Red Sea (Bianchi, 2007), making the movement of planktonic organisms difficult due to the opposing water flow and currents. Adults can pass through the Suez Canal all year round, and since the spawning season begins in December and is completed by late spring or early summer, larvae are able to cross the canal by June or early July; however, their crossing is halted until September, when the direction of the currents favors their movement (Zakaria, 2015). Furthermore, recruitment of new individuals is seasonal, and individuals are reproductively mature at the age of two (Sanders and Kedidi, 1984). The eggs of E. golanii appear to be dispersed near the coastal zone, at depths between 20 and 30 m, while larvae surviving at depths between 50 and 70 m (Uehara and Mitani, 2002; Peristeraki et al., 2006). The spawning season of E. golanii is concluded by summer and the fry is found near the coast. Thus, it is likely that large numbers of individuals and/or eggs will enter through this pathway and establish local populations in the dispersal area. The occurring environmental and spatial conditions of the channel (i.e., successive expansions) are suitable for the survival of both eggs/larvae and adults (Katsanevakis et al., 2013). Furthermore, there are currently no management practices that would prevent the survival of marine alien species during their migration through the Suez Canal (Galil et al., 2017).
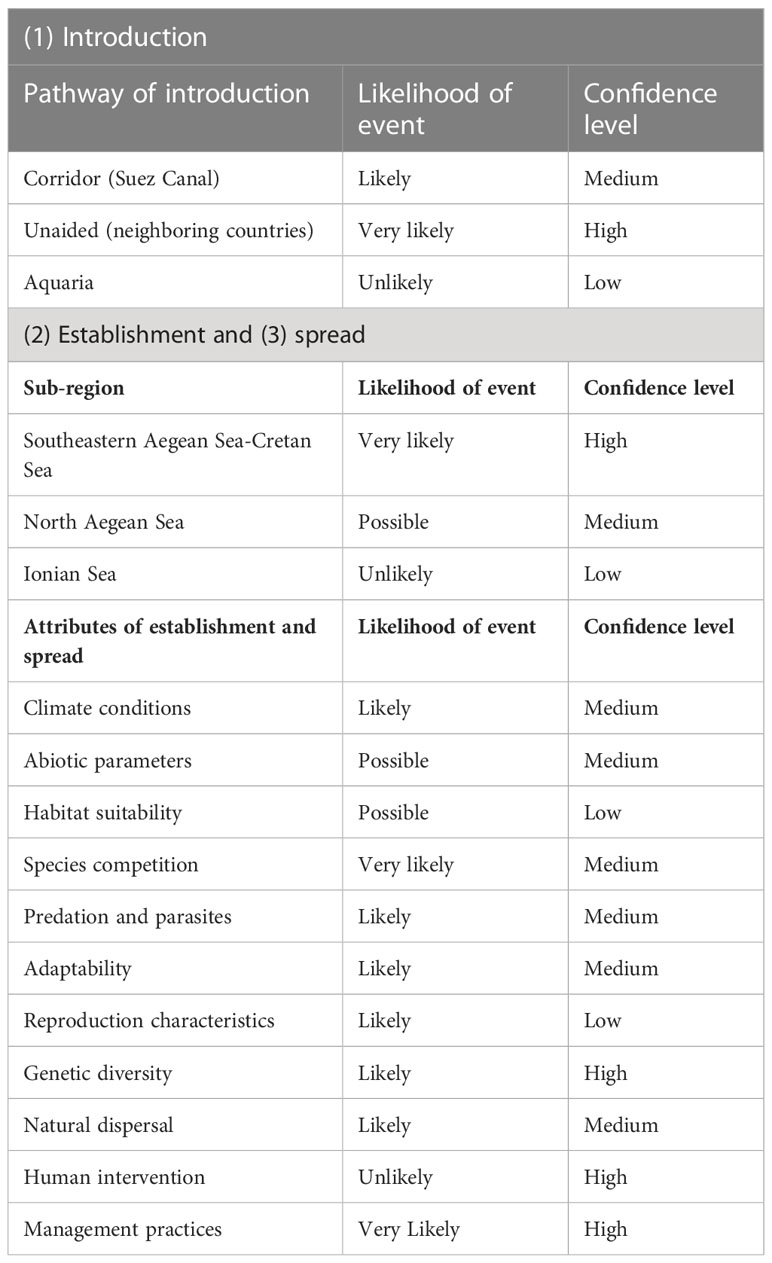
Table 1 Risk assessment summary results of E. golanii in the Greek seas regarding introduction, establishment, and spread, according to the likelihood of event and the corresponding confidence level.
Based on its distribution history in the Mediterranean and while knowing the invasion of the species and its characteristics, the chances of new invasions in Greek waters going unnoticed are reduced. At present, early detection systems operate through formal and informal networks of expert scientists and local stakeholders and through official competent bodies (e.g., ELNAIS). Since the first record of the species in the basin occurred in Lebanon in 1931 (Gruvel, 1931) and in Greece between 2003 and 2005 (Corsini et al., 2005; Kallianiotis and Lekkas, 2005; Kasapidis et al., 2007), it may be assumed that E. golanii was introduced in Greek waters from neighboring countries rather than directly from the Suez Canal, although this cannot be excluded. Both introduction pathways are expected to be affected by climate change (Cramer et al., 2018), mainly due to changes in sea surface temperature (Bellard et al., 2018). Future rising temperatures are expected to increase the likelihood of introduction (and spread) in Mediterranean areas that offer less favorable climatic conditions for winter survival and summer spawning such as the Adriatic and Balearic Seas and the North Aegean Sea (Corrales et al., 2018; Azzuro et al., 2019).
4.2 Risk of establishment
Etrumeus golanii has already established populations in Greece, mainly in central and southeastern Aegean regions (Dodecanese Islands, Cyclades Islands, Crete) and its range of distribution seems to be constantly increasing. Temperature conditions seem to contribute and favor this pattern (Badran, 2001; Corsini-Foka et al., 2010). The species has not yet been established in the North Aegean Sea probably due to the winter isotherm (from Evvoia to Chios) of 15°C and the prevailing colder waters in the northern parts of the Aegean (Zervakis et al., 2019). In recent years, however, the isotherm seems to be changing its location and Lessepsian migrants, including E. golanii, are being recorded in northern parts of the Aegean Sea, signaling possible spreads (Corsini-Foka et al., 2010). The Eastern Mediterranean is characterized by the presence of oligotrophic ecosystems with similarities to the Red Sea (Reich et al., 2021). In terms of bacterial productivity, the two areas are similar but differ in phytoplankton production (Qurban et al., 2017). However, although primary productivity in the Red Sea is higher and lasts longer, the seasonal dynamics of phytoplankton indicate the effect of water mixing (Reich et al., 2021). During the winter, the waters of the Red Sea move towards the Mediterranean, thus increasing the primary productivity in the light zone, together with the temperature and salinity (Zakaria, 2015). This phenomenon helped the establishment of E. golanii in the southeastern Aegean Sea (Corsini-Foka and Economidis, 2007), with a possible likelihood of establishment due to abiotic parameters. As far as habitat suitability is concerned, a possible likelihood of establishment in all habitats was also assessed.
Regarding genetic analyses, a study has been implemented in three different populations of E. golanii in Turkey, with the results demonstrating high genetic diversity, both within the populations and among them, based on haplotype diversity values (Çiftçi and Bardakci, 2021). Each population had unique mtDNA haplotypes (70 total haplotypes, with 2 common among populations) and unique genetic structure. This indicates that populations had migrated from the Red Sea to the Mediterranean in different shoals, at different time intervals, independently of each other, increasing the genetic diversity (Çiftçi and Bardakci, 2021); thus, a likely establishment due to genetic diversity was deemed. Overall, a very likely possibility of establishment was assessed in South Aegean and Cretan Seas, possible in the North Aegean Sea, and unlikely in the Ionian Sea (Table 1).
4.3 Risk of spread
Etrumeus golanii has established populations in Crete, the Dodecanese islands, and the Cyclades Islands where the coastal zone is relatively small (Panayotidis et al., 2020) and constitutes an ideal environment for its reproduction and formation of thriving populations (Osman et al., 2013). On the other hand, the North Aegean has a steep continental shelf far from the coast forming great depths (Panayotidis et al., 2020), which makes it difficult for the species to spread there. Also, oceanographically, the Aegean Sea resembles the Mediterranean on a small scale: the gradients of temperature, salinity, and nutrients, from north to south, are comparable to those of the western to eastern Mediterranean (Emeis et al., 2000). As far as the Ionian Sea is concerned, it is characterized by deep waters and strong currents, which can make it difficult for certain alien species to survive and establish themselves, and together with the Adriatic Sea, they have the fewest observed NIS (Katsanevakis et al., 2013). The presence of the E. golanii as recorded in the ELNAIS network (Figure 3) seems to have information gaps, hence the need to update its distribution data (Zenetos et al., 2015). Moreover, its distribution seems to overlap with some Natura 2000 areas (Spiliopoulou et al., 2021).
Regarding species competition, the spread of E. golanii was deemed as very likely due to low pressure on other species for food resources (Bilge et al., 2019). Indeed, the other small pelagic species that co-occur with E. golanii in the Aegean Sea and might compete for food are the European anchovy and the European pilchard. Based on the observed feeding habits of E. golanii, it mainly preys on small crustaceans like Isopoda, Decapoda, and Mysida (Andrianopoulos et al., 2022). In contrast, E. encrasicolus and S. pilchardus are primarily zooplanktivorous species preying mainly on Copepoda (e.g., Nikolioudakis et al., 2012; Karachle and Stergiou, 2014). As prey, species of the genus Etrumeus have been recorded to be predated by tuna (Shimose et al., 2013), mackerel (Jarre-Teichmann et al., 1998), and dolphins (Niño-Torres et al., 2006). Nevertheless, they are not a basic component of their diet, and most presumably, E. golanii is not likely to be subjected to strong pressure by these predators in the Greek waters; however, since the species can reach high densities, this hypothesis should be handled with caution, with possible future dietary regime shifts (Brand et al., 2019). Regarding the presence of E. golanii parasites, four species have been recorded in the Mediterranean, namely, nematodes of the genus Anisakis, the trematode Lecithochirium jaffense, the copepod Mitrapus oblongus, and isopods of the genus Gnathia (El-Rashidy and Boxshall, 2010; Boussellaa et al., 2016). Mitrapus oblongus is considered to have entered in the region as a Lessepsian immigrant, since it has not been observed to parasitize in native species (El-Rashidy and Boxshall, 2010), while Anisakis spp. is found in native species, but there is a high possibility that it has also entered with the introduction of NIS (Boussellaa et al., 2016). Lecithochirium jaffense and Gnathia spp. are native in the Mediterranean and infect several pelagic species. As far as predators and parasites are concerned, a likely likelihood of spread was deemed.
Etrumeus golanii’s characteristics and reproduction period (Somarakis et al., 2021), combined with the environmental and climate conditions, allowed its spread throughout the southern Mediterranean Sea, with a moderate magnitude of impact. Regarding human impacts, E. golanii is a species that spreads naturally across neighboring areas and human intervention does not seem to have contributed to its spread (e.g., releases from aquariums) with a minimal scoring. From 1994 onwards, its records began to increase rapidly, and in recent years, new sightings are recorded every year (Galil et al., 2018). Naturally dispersed species are very difficult to contain (Çinar et al., 2021). This is particularly true for E. golanii due to its already widespread and abundant populations, long breeding season, pelagic nature, and frequent recruitment (Golani, 2000; Osman et al., 2013). Currently, there are no management measures that could affect the ability of the species to spread in the Greek waters. Early detection systems that operate through formal and informal networks of experts are not capable of preventing establishment and spread (Castro et al., 2021). If a management practice, such as intensive and/or targeted fishing, is to be implemented, especially during the spawning season, a reduction in the probability of survival could be achieved, but the expected reduction is difficult to quantify and highly uncertain (Kopf et al., 2017). Overall, the risk assessment indicated a moderate potential rate of spread in the biogeographic sub-regions of the Greek seas with medium confidence levels (Table 1).
4.4 Magnitude of impacts
Since its detection in the Mediterranean Sea, E. golanii has spread gradually in the southern Mediterranean, forming local populations and constituting an important part of fish fauna, which is also reflected in the commercial fisheries catches of Libya, Egypt, and Turkey (Osman et al., 2011; Shakman et al., 2019; Çinar et al., 2021). No records of the species exerting strong competitive pressure on native species have been documented, possibly due to the lack of long-term and reliable fishing data (Coll et al., 2010; Corrales et al., 2017). Thus, a minimal magnitude of impact regarding competition with native species, a minor one regarding competition for food and shelter, and a moderate one regarding predation and dietary preferences were assumed. With the ongoing migration rates and since E. golanii can form large schools and reach high densities in favorable conditions, these impacts on biodiversity parameters are expected to reach higher scores in the future (Table 2). Although the negative effects of IAS on marine ecosystems and fisheries are known, there have been no major economic consequences due to E. golanii invasion until now (Haubrock et al., 2022). For example, in Israel, although the percentage of recorded specimens of E. golanii found in purse-seiners’ catches has increased in the 2008–2011 period compared to the 1990–1994 period, indicating an increasing trend of its populations, there are no apparent signs of strong competitive pressure on natural populations (e.g., on European anchovy and/or pilchard) or the ecosystem, as it is difficult to identify a direct correlation between native stocks and the invasion of E. golanii (Arndt et al., 2018). Thus, regarding ecosystem functioning and services, a moderate magnitude of impact was assessed; however, in the future with the continuous inclusion of the species in commercial fisheries catches, this score can be mitigated.

Table 2 Magnitude of impacts of E. golanii in the Greek seas in environmental and socio-economic attributes, regarding their score (Sc) and corresponding confidence levels (Cl) for current and future state.
Etrumeus species are of high economic importance in the Red Sea, contributing up to 11.5% of the region’s total purse-seiners production (Mehanna, 2004). In addition, they constitute 25% of total catch in Red Sea Egypt (Mehanna and El-Gammal, 2005), and 16% of total catch in Mediterranean Egypt (Akel, 2009). Etrumeus golanii alone constitutes 6.9% of the catch of the Clupeiformes order in Mediterranean Turkey (Sakinan, 2014) and 10.93% of the total fish catch in Mediterranean Egypt. Considering that E. golanii could be of high economic value in those regions, a positive impact of Lessepsian migration can be traced in Mediterranean Egypt (Farrag et al., 2014), Tunisia (Mili et al., 2020), and Mediterranean Morocco (Tamsouri et al., 2019). In the Greek seas, E. golanii has become established in the southeastern Aegean, contributing to the fisheries of Crete, the Dodecanese, and the Cyclades Islands (Zenetos et al., 2009). Since Mediterranean purse-seine fleets share many similarities (Kleitou et al., 2022), it can be an important species for commercial fisheries, thus having a positive impact on the Greek fisheries sector. Its population in these areas is constantly increasing, being part of the local fish fauna, without knowing, however, the exact abundance of the species and therefore its impact on the native stocks.
Coastal marine substrates with vegetation used as spawning habitats by E. golanii (mainly Posidonia oceanica) in the Mediterranean sublittoral zone are considered vulnerable (Galil and Goren, 2014). Thus, the risk assessment indicated a minor magnitude of impact on habitats (soft sediments, hard substrates, and seagrass beds), which is expected to grow with the increase of the species’ populations in the future. Globally, there have been no records of individuals of the genus Etrumeus negatively affecting the ecosystem and its components (Galil et al., 2019). Because of its small size, morphology, and biology, which do not pose a risk to public health, and the fact that it exploits seasonal planktonic cycles for its diet, it currently does not seem to have a negative impact on the native habitats of the study area.
Economic costs or losses are associated with impacts on commercial and recreational fisheries in terms of damage to fishing gear, increased labor demand, and predation of commercial fisheries target species (Cuthbert et al., 2021). Etrumeus golanii does not appear to be costly to commercial fisheries as it does not damage fishing gear, injure personnel, or cause a reduction in native fish stocks (Tamsouri et al., 2019), yet fishers in Greece consider that it reduces the overall economic turnover of the catches due to its high abundances and the low commercial price as there is a minimal market demand (Karachle, personal communication). A minimal magnitude of impact was documented regarding damage of fishing gear, with a same score also deemed for the future. As far as high discard rates and change of fishing places are concerned, a moderate magnitude of impact was assessed, since local fisheries communities tend to still prefer to harvest native pelagic stocks. However, this score is expected to be lower in the future, with the increased consumers’ familiarity and market trends and sustainability awareness campaigns (Penca et al., 2021). Estimates of economic losses in EU Member States are not yet assessed, while further studies are needed to be implemented in order to estimate the abundance of the species in Greek waters and to have sufficient data for a holistic and correct assessment (Kourantidou et al., 2022). Until now, it is not known to which extent it functions territorially and competitively with commercial small pelagic species such as the European anchovy and European pilchard (Vorsatz et al., 2019). However, it does not appear to have negative impacts on these stocks and currently no population management practices are in place (Akyol and Ulaş, 2016). The risk assessment indicated a minimal magnitude of impact regarding economic costs and an efficient management will require population dynamics studies with particular emphasis on its breeding grounds (Hirai et al., 2017; Nyuji and Takasuka, 2017).
Regarding public health, E. golanii does not appear to have a negative impact, as it is very small, is not aggressive, and does not carry poisonous substances or sharp spines like other alien species (e.g., Lagocephalus sceleratus and Pterois miles), which could impact human health. Although species of the genus Etrumeus have been recorded to be predated by larger pelagic species and dolphins, no official cases of predation of E. golanii have been recorded yet. However, and in any case, they are not a major component of their diet and are therefore not likely to exert a strong pressure on them. Regarding the parasites that have been identified in E. golanii, they do not seem to be a limiting factor for the growth of its population (Boussellaa et al., 2016). Overall, the risk assessment regarding the impacts of E. golanii in several attributes revealed that it cannot be characterized yet as an IAS, and further analyses and studies might be implemented to demonstrate the full aspects of this NIS in the Mediterranean Sea (Pyšek et al., 2020).
5 Conclusions
Etrumeus golanii has entered in the Mediterranean Sea via the Suez Canal, a pathway that is still active and will likely provide opportunities for new introduction events. In the Greek seas, a natural spread through neighboring countries has resulted in successfully established populations in the central and southeastern Aegean territories. It is likely that, in the future, it will be introduced in other Greek marine areas that it has been absent until now; however, it seems not to spread rapidly towards the northern Aegean and Ionian Sea due to environmental constraints such as temperature. Etrumeus golanii is a pelagic species with a long reproductive season, short and frequent recruitment periods, and high adaptability. These characteristics, alongside with the current environmental conditions, have allowed its gradual spread in the Mediterranean Sea. In the southeastern Aegean, E. golanii has established itself forming local populations, as shown by commercial fisheries catches, without having estimated the exact abundance of the species. It is relatively small in size and feeds on animal organisms such as small crustaceans, without exerting competitive pressure on native species. Finally, it does not seem to have a negative impact on fish stocks and public health; thus, it is not classified as invasive by this assessment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
IK collected the data, performed the risk assessment and wrote the manuscript. PK conceived the study. AT, AZ, and PK reviewed the manuscript. AT and PK supervised the manuscript and acquired funding. All authors contributed to the article and approved the submitted version.
Funding
The present work was supported by the NSRF 2014–2020 funded project “4ALIEN: Biology and the potential economic exploitation of four alien species in the Hellenic Seas”, grant number MIS: 5049511, focusing on the biology and ecology of four alien fish species and the effects of their expansion on fisheries, biodiversity, and ecosystem services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1220318/full#supplementary-material
References
Akel E. H. K. (2009). Fisheries of experimental purse seine net using light and population dynamics of Sardinella aurita (Family Clupeidae) east of Alexandria, Egypt. Egyptian J. Aquat. Biol. Fisheries 13 (1), 55–77. doi: 10.21608/ejabf.2009.2023
Akyol O., Ulaş A. (2016). The second record of Lessepsian migrant Etrumeus golanii from the north-eastern Aegean Sea (Izmir Bay, Turkey). Annales: Ser. Hist. Naturalis 26 (1), 25–28. doi: 10.19233/ASHN.2016.4
Andrianopoulos I, Karachle P. K., Dogrammatzi A., Michaloudi E. (2022). The feeding habits of the non-indigenous Etrumeus golanii from the Aegean Sea. Book of Abstracts, Joint ESENIAS and DIAS Scientific Conference 2022 and 11th ESENIAS Workshop ‘Invasive alien species under conditions of global crisis’, 13-15 November 2022, GDFA, MFRPTI, IBER-BAS, ESENIAS, Demre, Antalya, Türkiye, 109pp.
Arndt E., Givan O., Edelist D., Sonin O., Belmaker J. (2018). Shifts in eastern Mediterranean fish communities: Abundance changes, trait overlap, and possible competition between native and non-native species. Fishes 3 (2), 19. doi: 10.3390/fishes3020019
Azzuro E., Sbragaglia V., Cerri J., Bariche M., Bolognini L., Ben Souissi J., et al. (2019). Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: A large-scale survey based on local ecological knowledge. Global Change Biol. 25 (8), 2779–2792. doi: 10.1111/gcb.14670
Azzurro E, Tuset V. M., Lombarte A., Maynou F., Simberloff D., Rodríguez-Pérez A., et al. (2014). External morphology explains the success of biological invasions. Ecol. Lett. 17, 1455–1463. doi: 10.1111/ele.12351
Bacher S., Blackburn T. M., Essl F., Genovesi P., Heikkilä J., Jeschke J. M., et al. (2018). Socio-economic impact classification of alien taxa (SEICAT). Methods Ecol. Evol. 9 (1), 159–168. doi: 10.1111/2041-210X.12844
Badran M. I. (2001). Dissolved oxygen, chlorophyll a and nutrients: Seasonal cycles in waters of the Gulf of Aquaba, Red Sea. Aquat. Ecosystem Health Manage. 4 (2), 139–150. doi: 10.1080/14634980127711
Basusta N., Erdem U., Mater S. (1997). A new Lessepsian immigrant fish species in Iskenderun Bay: red-eyed sardine Etrumeus teres (DeKay 1842). Mediterr. fisheries congress, Izmir 921–924.
Bellard C., Jeschke J. M., Leroy B., Mace G. M. (2018). Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 8 (11), 5688–5700. doi: 10.1002/ece3.4098
Bianchi C. N. (2007). Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 580, 7–21. doi: 10.1007/s10750-006-0469-5
Bilge G., Filiz H., Yapici S., Tarkan S. A., Vilizzi L. (2019). A risk screening study on the potential invasiveness of Lessepsian fishes in the south-western coasts of Anatolia. Acta Ichthyologica Piscatoria 49 (1), 23–31. doi: 10.3750/AIEP/02422
Bodilis P., Louisy P., Draman M., Arceo H. O., Francour P. (2014). Can citizen science survey non-indigenous fish species in the Eastern Mediterranean Sea? Environ. Manage. 53, 172–180. doi: 10.1007/s00267-013-0171-0
Boussellaa W., Boudaya L., Derbel H., Neifar L. (2016). A new record of the Lessepsian fish Etrumeus golanii (Teleostei: Clupeidae) in the Gulf of Gabes, Tunisia, with notes on its parasites. Cahiers Biologie Mar. 57, 389–395.
Brand D., Edelist D., Goffman O., Hadar N., Scheinin A., Kerem D. (2019). Common dolphins, common in neritic waters off southern Israel, demonstrate uncommon dietary habits. Aquat. Conservation: Mar. Freshw. Ecosyst. 31 (S1), 15–21. doi: 10.1002/aqc.3165
Carmezim J., Pennino M. G., Martínez-Minaya J., Conesa D., Coll M. (2022). A mesoscale analysis of relations between fish species richness and environmental and anthropogenic pressures in the Mediterranean Sea. Mar. Environ. Res. 180, 105702. doi: 10.1016/j.marenvres.2022.105702
Castro K. L., Battini N., Giachetti C. B., Trovant B., Abelando M., Basso N. G., et al. (2021). Early detection of marine invasive species following the deployment of an artificial reef: Integrating tools to assist the decision-making process. J. Environ. Manage. 297, 113333. doi: 10.1016/j.jenvman.2021.113333
Çiftçi S. E., Bardakci F. (2021). A Lessepsian invader round herring (Etrumeus golanii) with high genetic diversity without bottlenecking in the northeastern Mediterranean Sea. Turkish J. Zool 45, 102–107. doi: 10.3906/zoo-2011-28
Çinar M. E., Bilecenoğlu M., Yokeş M. B., Öztürk B., Taşkin E., Bakir K., et al. (2021). Current status (as of end of 2020) of marine alien species in Turkey. PloS One 16 (5), e0251086. doi: 10.1371/journal.pone.0251086
Coll M., Piroddi C., Steenbeek J., Kaschner K., Lasram F. B. R., Aguzzi J., et al. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PloS One 5 (8), e11842. doi: 10.1371/journal.pone.0011842
Corrales X., Coll M., Ofir E., Heymans J. J., Steenbeek J., Goren M., et al. (2018). Future scenarios of marine resources and ecosystem conditions in the Eastern Mediterranean under the impacts of fishing, alien species and sea warming. Sci. Rep. 8, 14284. doi: 10.1038/s41598-018-32666-x
Corrales X., Ofir E., Coll M., Goren M., Edelist D., Heymans J. J., et al. (2017). Modeling the role and impact of alien species and fisheries on the Israeli marine continental shelf ecosystem. J. Mar. Syst. 170, 88–102. doi: 10.1016/j.jmarsys.2017.02.004
Corsini M., Margies P., Kondilatos G., Economidis P. S. (2005). Lessepsian migration of fishes to the Aegean Sea: First record of Tylerius spinosissimus (Tetraodontidae) from the Mediterranean and six more fish records from Rhodes. Cybium 29 (4), 347–354.
Corsini-Foka M., Economidis P. S. (2007). Allochthonous and vagrant ichthyofauna in Hellenic marine and estuarine waters. Mediterr. Mar. Sci. 8 (1), 67–90. doi: 10.12681/mms.163
Corsini-Foka M., Pancucci-Papadopoulou M.-A., Kalogirou S. (2010). Is the Lessepsian Province in expansion? The Aegean Sea experience. Report of the Sub-Regional Technical meeting on the Lessepsian migration and its impact on Eastern Mediterranean fishery, GCP/INT/041/EC – GRE – ITA/TD-04, 138pp.
Cramer W., Guiot J., Fader M., Garrabou J., Gattuso J.-P., Iglesias A., et al. (2018). Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Climate Change 8, 972–980. doi: 10.1038/s41558-018-0299-2
Cuthbert R. N., Pattison Z., Taylor N. G., Verbrugge L., Diagne C., Ahmed D. A., et al. (2021). Global economic costs of aquatic invasive alien species. Sci. Total Environ. 775, 145238. doi: 10.1016/j.scitotenv.2021.145238
D’Amen M., Azzurro E. (2020). Lessepsian fish invasion in Mediterranean marine protected areas: a risk assessment under climate change scenarios. ICES J. Mar. Sci. 77 (1), 388–397. doi: 10.1093/icesjms/fsz207
DiBattista J. D., Randall J. E., Bowen B. W. (2012). Review of the round herrings of the genus Etrumeus (Clupeidae: Dussumieriinae) of Africa, with descriptions of two new species. Cybium 36 (3), 447–460.
EC. (2014). “Regulation No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species,” in Secondary regulation no 1143/2014 of the european parliament and of the council of 22 october 2014 on the prevention and management of the introduction and spread of invasive alien species (Strasbourg, France: European Commission).
EC. (2018). Study on invasive alien species. Development of risk assessments to tackle priority species and enhance prevention: final report (Brussels: Publications Office of the European Union).
El-Rashidy H., Boxshall G. A. (2010). Parasitic copepods on immigrant and native clupeid fishes caught in Egyptian coastal waters off Alexandria. Syst Parasitol. 76, 19–38. doi: 10.1007/s11230-010-9230-6
El-Sayed R. S. (1994). Checklist of Egyptian mediterranean fishes (Alexandria, Egypt: National Institute of Oceanography and Fisheries), 1–77.
Emeis K.-C., Struck U., Schulz H.-M., Rosenberg R., Bernasconi S., Erlenkeuser H., et al. (2000). Temperature and salinity variations of Mediterranean Sea surface waters over the last 16,000 years from records of planktonic stable oxygen isotopes and alkenone unsaturation ratios. Palaeogeogr Palaeoclimatol Palaeoecol. 158 (3-4), 259–280. doi: 10.1016/S0031-0182(00)00053-5
Falautano M., Castriota L., Andaloro F. (2006). First record of Etrumeus teres (Clupeidae) in the central Mediterranean Sea. Cybium 30 (3), 287–288.
Farrag M. M. S., Osman A. G. M., Akel E. H. K., Moustafa M. A. (2014). Catch and effort of night purse seine with emphasize to Age and Growth of lessepsian Etrumeus teres (Dekay 1842), mediterranean sea, Egypt. Egyptian J. Aquat. Res. 40 (2), 181–190. doi: 10.1016/j.ejar.2014.06.003
Filiz H., Tarkan S. A., Bilge G., Yapici S. (2017). Assessment of invasiveness potential of Pterois miles by the Aquatic Species Invasiveness Screening Kit. J. Black Sea/Mediterranean Environ. 23 (1), 17–37.
Galanidi M., Turan C., Öztürk B., Zenetos A. (2019). Europen Union (EU) risk assessment of Plotosus lineatus (Thunberg 1787); a summary and information update. J. Black Sea/Mediterranean Environ. 25 (2), 210–231.
Galanidi M., Zenetos A. (2019). “EU Risk Assessment of Lagocephalus sceleratus. State of knowledge, evaluation and criteria, data needs/formats and management,” in Proceedings of the 1st Mediterranean Symposium on the Non-Indigenous Species, 45–51 (Antalya, Turkey: UNEP/MAP – SPA/RAC).
Galil B. S., Danovaro R., Rothman S. B. S., Goren M. (2019). Invasive biota in the deep-sea Mediterranean: an emerging issue in marine conservation and management. Biol. Invasions 21, 281–288. doi: 10.1007/s10530-018-1826-9
Galil B. S., Goren M. (2014). “Metamorphoses: bioinvasions in the mediterranean sea,” in The mediterranean sea. Eds. Goffredo S., Dubinsky Z. (Dordrecht: Springer).
Galil B. S., Marchini A., Occhipinti-Ambrogi A. (2018). East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuarine Coast. Shelf Sci. 201, 7–16. doi: 10.1016/j.ecss.2015.12.021
Galil B., Marchini A, Occhipinti-Ambrogi A, Ojaveer H (2017). The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manage. Biol. Invasions 8 (2), 141–152. doi: 10.3391/mbi.2017.8.2.02
Giovos I., Kleitou P., Poursanidis D., Batjakas I., Bernardi G., Crocetta F., et al. (2019). Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean. Biol. Invasions 21, 3707–3721. doi: 10.1007/s10530-019-02083-w
Golani D. (2000). The Lessepsian migrant, the Red-eye Round Herring, Etrumeus teres (DeKay 1842), a new record from Cyprus. Zool Middle East 20 (1), 61–64. doi: 10.1080/09397140.2000.10637813
Golani D. (2005). Checklist of the mediterranean fishes of Israel. Zootaxa 947, 1–200. doi: 10.11646/zootaxa.947.1.1
Golani D., Fricke R. (2005). Checklist of the Red Sea Fishes with delineation of the Gulf of Suez, Gulf of Aqaba, endemism and Lessepsian migrants. Zootaxa 4509 (1), 1–215. doi: 10.11646/ZOOTAXA.4509.1.1
Gruvel A. (1931). Les Etats de Syrie. Richesses marines et fluviales, exportation actuelle - Avenir (Paris: Société d’Edition Geographiques, Maritimes et Coloniales), 453.
Haubrock P. J., Bernery C., Cuthbert R. N., Liu C., Kourantidou M., Leroy B., et al. (2022). Knowledge gaps in economic costs of invasive alien fish worldwide. Sci. Total Environ. 803, 149875. doi: 10.1016/j.scitotenv.2021.149875
Hellenic Navy Hydrographic Service (2017). “National report of Greece,” in 20th Meeting of Mediterranean and Black Seas Hydrographic Commission (MBSHC), 4-6 July 2017(Herceg Novi, Montenegro).
Hirai J., Hidaka K., Nagai S., Ichikawa T. (2017). Molecular-based diet analysis of the early post−larvae of Japanese sardine Sardinops melanostictus and Pacific round herring Etrumeus teres. Mar. Ecol. Prog. Ser. 564, 99–113. doi: 10.3354/meps12008
Jarre-Teichmann A, Shannon L. J., Moloney C. L., Wickens P. A. (1998). Comparing trophic flows in the southern Benguela to those in other upwelling ecosystems. Afr. J. Mar. Sci. 19, 391–414. doi: 10.2989/025776198784127024
Kallianiotis A., Lekkas V. (2005). First documented report on the Lessepsian migrant Etrumeus teres De Kay 1842 (Pisces: clupeidae) in the greek seas. J. Biol. Res. 4, 225–229.
Karachle P. K., Dogrammatzi A., Apostolopoulos G., Konida K., Nalmpanti M., Tsikliras A. C., et al. (2021). Enriching the ELNAIS database through Citizen Science with information on the distribution of four invasive alien marine species: input from the scientific project 4ALIEN. Book of Abstracts, Joint ESENIAS and DIAS Scientific Conference 2021 and 10th ESENIAS Workshop ‘Ten years of cooperation and networking on invasive alien species in East and South Europe’, 07-09 December 2022, GDFA, MFRPTI, IBER-BAS, ESENIAS, Virtual Conference.
Karachle P. K., Stergiou K. I. (2014). Feeding and ecomorphology of three clupeoids in the N Aegean Sea. Mediterr. Mar. Sci. 15 (1), 9–26. doi: 10.12681/mms.350
Karachle P. K., Stergiou K. I. (2017). An update on the feeding habits of fish in the Mediterranean Sea, (2002-2015). Mediterr. Mar. Sci. 18 (1), 43–52. doi: 10.12681/mms.1968
Kasapidis P., Peristeraki P., Tserpes G., Magoulas A. (2007). A new record of the Lessepsian invasive fish Etrumeus teres (Osteichthyes: Clupeidae) in the Mediterranean Sea (Aegean, Greece). Aquat. Invasions 2 (2), 152–154. doi: 10.3391/ai.2007.2.2.12
Katsanevakis S., Zenetos A., Belchior C., Cardoso A. C. (2013). Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manage. 76, 64–74. doi: 10.1016/j.ocecoaman.2013.02.024
Katsanevakis S., Zenetos A., Corsini-Foka M., Tsiamis K. (2020). “Biological invasions in the Aegean Sea: temporal Trends, pathways, and impacts,” In The handbook of environmental chemistry. (Berlin, Heidelberg: Springer) doi: 10.1007/698_2020_642
Kleitou P., Hall-Spencer J. M., Savva I., Kletou D., Hadjistylli M., Azzuro E., et al. (2021). The case of lionfish (Pterois miles) in the Mediterranean Sea demonstrates limitations in EU legislation to address marine biological invasions. J. Mar. Sci. Eng. 9, 325. doi: 10.3390/jmse9030325
Kleitou P., Moutopoulos D. K., Giovos I., Kletou D., Savva I., Cai L. L., et al. (2022). Conflicting interests and growing importance of non-indigenous species in commercial and recreational fisheries of the Mediterranean Sea. Fisheries Manage. Ecol. 29 (2), 169–182. doi: 10.1111/fme.12531
Kopf R. K., Nimmo D. G., Humphries P., Baumgartner L. J., Bode M., Bond N. R., et al. (2017). Confronting the risks of large-scale invasive species control. Nat. Ecol. Evol. 1, 0172. doi: 10.1038/s41559-017-0172
Korpinen S., Klančnik K., Peterlin M., Nurmi M., Laamanen L., Zupančič G., et al. (2019). Multiple pressures and their combined effects in Europe’s seas (ETC/ICM Technical Report 4/2019: European Topic Centre on Inland, Coastal and Marine waters), 164.
Kourantidou M., Haubrock P. J., Cuthbert R. N., Bodey T. W., Lenzner B., Gozlan R. E., et al. (2022). Invasive alien species as simultaneous benefits and burdens: trends, stakeholder perceptions and management. Biol. Invasions 24, 1905–1926. doi: 10.1007/s10530-021-02727-w
Mehanna S. F. (2004). Maximum sustainable yield of the round herring, Etrumeus teres and slimy mackerel, Scomber japonicus in the Gulf of Suez. Egyptian J. Aquat. Res. 30 (B), 322–325.
Mehanna S. F., El-Gammal F. I. (2005). Stock assessment of the round herring, Etrumeus teres (De Kay 1842) in the Egyptian sector of red sea. Indian J. Fisheries 52 (4), 377–383.
Mili S., Ennouri R., Rafrafi-Nouira S., Capapé C. (2020). Additional record of Golani roud herring, Etrumeus golanii (Osteichthyes: Dussumieriidae) from Tunisian waters with comments on its distribution in the Mediterranean Sea. Annales: Ser. Hist. Naturalis 30 (1), 105–110. doi: 10.19233/ASHN.2020.13
Naasan Aga Spyridopoulou R., Langeneck J., Bouziotis D., Giovos I., Kleitou P., Kalogirou S. (2020). Filling the gap of data-limited fish species in the Eastern Mediterranean Sea: A contribution by citizen science. J. Mar. Sci. Eng. 8 (2), 107. doi: 10.3390/jmse8020107
Nikolioudakis N., Isari S., Pitta P., Somarakis S. (2012). Diet of sardine Sardina pilchardus: an ‘end-to-end’ field study. Mar. Ecol. Prog. Ser. 453, 173–188. doi: 10.3354/meps09656
Niño-Torres C. A., Gallo-Reynoso J. P., Galván-Magaña F., Escobar-Briones E., Macko S. A. (2006). Isotopic analysis of δ13C, δ15N, and δ34S “A feeding tale” in teeth of the longbeaked common dolphin, Delphinus capensis. Mar. Mammal Sci. 22 (4), 831–846. doi: 10.1111/j.1748-7692.2006.00065.x
Nyuji M., Takasuka A. (2017). Spawning cycle and fecundity of a multiple spawner round herring Etrumeus teres off southern Japan: Oocyte growth and maturation analysis. J. Sea Res. 122, 11–18. doi: 10.1016/j.seares.2017.02.009
Osman A. G. M., Akel E. H. K., Farrag M. M. S., Moustafa M. A. (2011). Reproductive biology of round herring etrumeus teres (Dekay 1842) from the Egyptian mediterranean water at alexandria. Int. Scholarly Res. Network 2011, 215950. doi: 10.5402/2011/215950
Osman A. G. M., Farrag M. M. S., Akel E. H. K., Moustafa M. A. (2013). Feeding behavior of lessepsian fish Etrumeus teres (Dekay 1842) from the mediterranean waters, Egypt. Egyptian J. Aquat. Res. 39 (4), 275–282. doi: 10.1016/j.ejar.2013.12.004
Panayotidis P., Orfanidis S., Gerakaris V., Papathanasiou V. (2020). Coastal habitats in the Aegean Sea: soft bottom, seagrasses, and hard bottom. In: The Handbook of Environmental Chemistry (Berlin, Heidelberg: Springer).
Penca J., Said A., Cavallé M., Pita C., Libralato S. (2021). Sustainable small-scale fisheries markets in the Mediterranean: weaknesses and opportunities. Maritime Stud. 20, 141–155. doi: 10.1007/s40152-021-00222-5
Peristeraki P., Lazarakis G., Skarvelis C., Georgiadis M., Tserpes G. (2006). Additional records on the occurrence of alien fish species in the eastern Mediterranean Sea. Med. Mar. Sci. 7 (2), 61–66. doi: 10.12681/mms.170
Pyšek P., Hulme P. E., Simberloff D., Bacher S., Blackburn T. M., Carlton J. T., et al. (2020). Scientists' warning on invasive alien species. Biol. Rev. 95 (6), 1511–1534. doi: 10.1111/brv.12627
Qurban M. A., Wafar M., Jyothibabu R., Manikandan K. P. (2017). Patterns of primary production in the Red Sea. J. Mar. Syst. 169, 87–98. doi: 10.1016/j.jmarsys.2016.12.008
Rafrafi-Nouira S., Ounifi-Ben Amor K., Ben Amor M. M., Capapé C. (2017). Abundant records of red-eye round herring Etrumeus golanii (Osteichthyes: Clupeidae) from the Tunisian coast (Central Mediterranean). Annales: Ser. Hist. Naturalis 27 (1), 65–68. doi: 10.19233/ASHN.2017.09
Reich T., Ben-Ezra T., Belkin N., Tsemel A., Aharonovich D., Roth-Rosenberg D., et al. (2021). A year in the life of the Eastern Mediterranean: Monthly dynamics of phytoplankton and bacterioplankton in an ultra-oligotrophic sea. Deep Sea Res. Part I: Oceanographic Res. Papers 182, 103720. doi: 10.1016/j.dsr.2022.103720
Roy H. E., Rabitsch W., Scalera R., Stewart A., Gallardo B., Genovesi P., et al. (2017). Developing a framework of minimum standards for the risk assessment of alien species. J. Appl. Ecol. 55 (2), 526–538. doi: 10.1111/1365-2664.13025
Saad A. (2002). “Characterization of Lessepsian migrant fish at Syrian sea waters,” in Mediterranean vermitid terrace and migratory/ invasive organisms (Beirut/Lebanon: INOC and SNRSL).
Sakinan S. (2014). Current distribution of the small pelagic fish populations in the Northeastern Levantine sea in relation to environmental conditions and predicting the impacts of temperature rise on their future distributions (Middle East Technical University), 182.
Sanders M. J., Kedidi S. M. (1984). Stock assessment for the round herring (Etrumeus teres) caught by purse seine in the Gulf of Suez.
Sanders M. J., Morgan G. R. (1989). Review of the fisheries resources of the Red Sea and Gulf of Aden. FAO Fisheries Tech. Paper 304, 138.
Seebens H., Blackburn T. M., Dyer E. E., Genovesi P., Hulme P. E., Jeschke J. M., et al. (2017). No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435. doi: 10.1038/ncomms14435
Shakman E. A., Ben Abdalha A., Talha F., Al-Faturi A., Bariche M. (2017). First records of seven marine organisms of different origins from Libya (Mediterranean Sea). Bioinvasions Records 6 (4), 377–382. doi: 10.3391/bir.2017.6.4.13
Shakman E., Eteayb K., Taboni I., Ben Abdalha A. (2019). Status of marine alien species along the Libyan coast. J. Black Sea/Mediterranean Environ. 25 (2), 188–209.
Shapiro Goldberg D., Rilov G., Villéger S., Belmaker J. (2021). Predation cues lead to reduced foraging of invasive Siganus rivulatus in the Mediterranean. Front. Mar. Sci. 8, 678848. doi: 10.3389/fmars.2021.678848
Shimose T., Watanabe H., Tanabe T., Kubodera T. (2013). Ontogenetic diet shift of age-0 year Pacific bluefin tuna Thunnus orientalis. J. Fish Biol. 82 (1), 263–276. doi: 10.1111/j.1095-8649.2012.03483.x
Simberloff D., Martin J.-L., Genovesi P., Maris V., Wardle D. A., Aronson J., et al. (2013). Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28 (1), 58–66. doi: 10.1016/j.tree.2012.07.013
Somarakis S., Giannoulaki M., Markakis K., Tsiaras K., Schismenou E., Peristeraki P. (2021). Ovarian dynamics, batch fecundity and spawning phenology of the lessepsian migrant Etrumeus golanii DiBattista, Randall & Bowen 2012 (Clupeidae: dussumieriinae). Mediterr. Mar. Sci. 22 (3), 466–479. doi: 10.12681/mms.27099
Spiliopoulou K., Dimitrakopoulos P. G., Brooks T. M., Kelaidi G., Paragamian K., Kati V., et al. (2021). The Natura 2000 network and the ranges of threatened species in Greece. Biodiversity Conserv. 30, 945–961. doi: 10.1007/s10531-021-02125-7
Stamouli C., Akel E. H. K., Azzurro E., Bakiu R., Bas A. A., Bitar G., et al. (2018). New Mediterranean biodiversity records (December 2017). Mediterr. Mar. Sci. 18 (3), 534–556. doi: 10.12681/mms.15823
Tamsouri M. N., Benchoucha S., Idhalla M., El Aamri F. (2019). Etrumeus golanii (Actinopterygii: Clupeiformes: Dussumieriidae) a new Lessepsian migrant recorded in Morocco, Alboran Sea (south-west Mediterranean). Acta Ichthyologica Piscatoria 49 (1), 43–47. doi: 10.3750/AIEP/02495
Tiralongo F., Crocetta F., Riginella E., Lillo A. O., Tondo E., Macali A., et al. (2020). Snapshot of rare, exotic and overlooked fish species in the Italian seas: A citizen science survey. J. Sea Res. 164, 101930. doi: 10.1016/j.seares.2020.101930
Tsirintanis K., Azzurro E., Crocetta F., Dimiza M., Froglia C., Gerovasileiou V., et al. (2022). Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 17 (3), 308–352. doi: 10.3391/ai.2022.17.3.01
Uehara S., Mitani T. (2002). Horizontal and diel vertical distribution of eggs and larvae of two clupeoid fish (Etrumeus teres and Sardinops melanostictus). Fisheries Sci. 68, 435–436. doi: 10.2331/fishsci.68.sup1_435
Vagenas G., Dogrammatzi A., Stoumboudi M., Karachle P. K. (2022). “Fecundity estimation of two Lessepsian migrants, Pterois miles and Etrumeus golanii, from the Eastern Mediterranean Sea (Aegean Sea, Greece),” in 2nd Southeast European Ichthyological Conference, Vol. 2. 44 (SEEIC).
Vorsatz L. D., van der Lingen C. D., Gibbons M. J. (2019). Observations on the biology and seasonal variation in feeding of the east coast round herring Etrumeus wongratanai (Clupeiformes), off Scottburgh, KwaZulu-Natal, South Africa. J. Fish Biol. 94 (3), 498–511. doi: 10.1111/jfb.13914
Whitehead P. J. P. (1963). A revision of the recent round herrings (Pisces: Dussumieriidae). Bull. Br. Museum Natural History (Zoology) 10, 305–380. doi: 10.5962/bhl.part.20529
Whitehead P. J. P. (1985). An annotated and illustrated catalogue of the Herrings, Sardines, Pilchards, Sprats, Shads, Anchovies and Wolf-Herrings. Part 2, Engraulidae (Rome: FAO Species Catalogue, Clupeoid Fishes of the World, FAO), 1–303.
Yarmaz A., Balaban C., Türkakın M., Türker- Çakır D. (2010). A new record of Lessepsian migrant Etrumeus teres (DeKay 1842) (Osteichthyes: clupeidae) from the northern aegean sea. J. Appl. Ichthyol. 26, 134–136. doi: 10.1111/j.1439-0426.2009.01365.x
Zakaria H. Y. (2015). Article Review: Lessepsian migration of zooplankton through Suez Canal and its impact on ecological system. Egyptian J. Aquat. Res. 41 (2), 129–144. doi: 10.1016/j.ejar.2015.04.001
Zenetos A., Albano P. G., López Garcia E., Stern N., Tsiamis K., Galanidi M. (2022a). Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 23 (1), 196–212. doi: 10.12681/mms.29106
Zenetos A., Albano P. G., López Garcia E., Stern N., Tsiamis K., Galanidi M. (2022b). Corrigendum to the Review Article (Medit. Mar. Sci. 23/1 2022, 196-212) Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 23 (4), 876–878. doi: 10.12681/mms.31523
Zenetos A., Arianoutsou M., Bazos I., Balopoulou S., Corsini-Foka M., Dimiza M., et al. (2015). ELNAIS: A collaborative network on Aquatic Alien Species in Hellas (Greece). Manage. Biol. Invasions 6 (2), 185–196. doi: 10.3391/mbi.2015.6.2.09
Zenetos A., Karachle P. K., Corsini-Foka M., Gerovasileiou V., Simboura N., Xentidis N.-J., et al. (2020). Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Mediterr. Mar. Sci. 21 (3), 775–793. doi: 10.12681/mms.25136
Zenetos A., Koutsogiannopoulos D., Ovalis P., Poursanidis D. (2013). The role played by citizen scientists in monitoring marine alien species in Greece. Cahiers Biologie Mar. 54 (3), 419–426.
Zenetos A., Pancucci-Papadopoulou M.-A., Zogaris S., Papastergiadou E., Vardakas L., Aligizaki K., et al. (2009). Aquatic alien species in Greece, (2009): tracking sources, patterns and effects on the ecosystem. J. Biol. Research-Thessaloniki 12, 135–172.
Keywords: risk assessment, Etrumeus golanii, alien species, Mediterranean Sea, Greek seas
Citation: Keramidas I, Tsikliras AC, Zenetos A and Karachle PK (2023) Risk assessment of Golani’s round herring (Etrumeus golanii) in the Greek seas (northeastern Mediterranean Sea). Front. Mar. Sci. 10:1220318. doi: 10.3389/fmars.2023.1220318
Received: 10 May 2023; Accepted: 31 July 2023;
Published: 21 August 2023.
Edited by:
Lorenzo Mari, Polytechnic University of Milan, ItalyReviewed by:
Francesco Tiralongo, University of Catania, ItalyMaria Flavia Gravina, University of Rome Tor Vergata, Italy
Copyright © 2023 Keramidas, Tsikliras, Zenetos and Karachle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Keramidas, a2VyYWlvYW5AYmlvLmF1dGguZ3I=
 Ioannis Keramidas
Ioannis Keramidas Athanassios C. Tsikliras
Athanassios C. Tsikliras Argyro Zenetos
Argyro Zenetos Paraskevi K. Karachle
Paraskevi K. Karachle