- 1Marine Science Program, Biological and Environmental Science and Engineering Division (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
- 2Red Sea Research Center (RSRC), King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
- 3Computational Bioscience Research Center (CBRC), King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
- 4OceanX, New York, NY, United States
- 5National Center for Wildlife (NCW), Riyadh, Saudi Arabia
Observations are essential to explore and discover the ocean. The rapid advancements in technology have revolutionized our capacity to document the ocean and its diverse array of species, pushing the boundaries of our understanding further than ever before. The central Red Sea was exposed as part of the Red Sea Decade Expedition, which took place from 04 February to 18 June 2022 aboard the R/V OceanXplorer, using underwater submersibles. Here, for the first time in the Red Sea, we reported three observations of living Firoloida desmarestia specimens, one female and two male specimens, a heteropod from the Pterotracheidae family. This shell-less mollusk has been observed in the epipelagic zone of the world’s oceans, with the exception of polar regions, suggesting a global distribution for these observations. The two males were observed swimming in the water column, while the female was close to the seabed. All three observations were detected during the morning, raising the question if these organisms use vertical migrations to reach deep-sea waters during the daytime. However, no ROV or submersible dives were conducted at night. Our results show a depth range expansion for observations of this species in the Red Sea. Furthermore, as far as we know, no adults of F. desmarestia have been reported until now in the Red Sea. During the expedition, all three specimens were found at similar depths (350, 400, and 464.5 m depth), with the female being the deepest, thus confirming the eurybathic distribution of this species. The emerging technology is progressively enhancing our understanding of these enigmatic creatures and expanding our knowledge of their fascinating adaptations and ecological roles. In addition to our fieldwork, a literature search was performed to uncover any pre-existing observational records of this species to understand its global distribution and ecological significance.
Introduction
Heteropods are the only holoplanktonic prosobranch mollusks and are of particular interest because of their modifications for a pelagic life (Lalli and Gilmer, 1989). They show remarkable modifications of the basic prosobranch body plan and lifestyle (Keen, 1971). They are planktonic, voracious carnivores, whose shell is reduced or absent in the adult life form and whose foot is a thin, undulating paddle that propels the animals through the water (Pechenik, 2005). The body is completely transparent, with the exception of the viscera, an excellent adaptation for inconspicuous water movement. The visceral mass contains the digestive tract, heart, nephridia, and much of the reproductive system (Tesch, 1949). Among its most important characteristics are the presence of a pair of large spherical eyes and the modification of the foot, which is laterally compressed and elongated, giving rise to swimming fins (Lalli and Gilmer, 1989).
Heteropods swim in an upside position propelled by the swift movements of their upwardly held swimming fin in the water column (Lalli and Gilmer, 1989). They are active swimmers and solitary living prey-hunters. The anatomical transformations displayed by holoplanktonic gastropods are often so remarkable that they were initially misidentified by early researchers, who classified them as planktonic worms, ctenophores, and even fish, failing to recognize them as mollusks. However, recent advancements in research submersibles have provided valuable insights into these species and their behavior, particularly in mid-deep water that was previously inaccessible to SCUBA diving. The three families, Atlantidae, Carinariidae, and Pterotracheidae, are represented in a clear phylogenetic line when their shell structure is taken into consideration (Van der Spoel, 1976). Some animals live encased in the shell (Atlantidae); in others, the shell is much reduced (Carinariidae), while in pterotracheids, it is absent (Tesch, 1949; Thiriot-Quiévreux, 1973).
The Pterotracheoidea is a taxonomic superfamily of sea snails or sea slugs, marine gastropod mollusks in the clade Littorinimorpha (Bouchet and Rocroi, 2005). Among the heteropods, the family Pterotracheidae stands out as the most active and mobile, featuring a cylindrical body that is exceptionally elongated, flexible, and streamlined in comparison to the other two families (Seapy et al., 2003). The genus Firoloida Lesueur, 1817 consists of a single species, Firoloida desmarestia Lesueur, 1817, making it a monotypic genus. Within the family Pterotracheidae, F. desmarestia stands out as the smallest and most transparent species. Its morphology reveals several adaptations to its pelagic lifestyle. These include a transparent, elongate, and cylindrical body; the absence of shell and operculum; gill branches protruding from the body; and a dorsal visceral mass situated behind the ventral swimming fin (Owre, 1964; Lalli and Gilmer, 1989). Furthermore, the species possesses well-developed eyes, indicating its role as a visual predator (Land, 1982), and primarily feeds on gelatinous zooplankton (Lalli and Gilmer, 1989). Firoloida desmarestia has a proboscis, thereby its vernacular name of sea elephants, and an opaque visceral nucleus, a mass that includes the heart, gonad, liver, kidneys, and sexual glands at the distal end (Lalli and Gilmer, 1989). The long trunk of heteropods terminates with the presence of a visceral nucleus, followed by a short ventral tail. In females, a permanent egg string trails behind them, while in males, a tail filament is observed instead. Male heteropods are characterized by the presence of prominent tentacles, positioned anterior to the eyes, and a fin sucker located on the front edge of the fin. However, these structures are absent in females (Fagetti, 1958). Females of this species typically achieve larger sizes than males. Their maturation has been observed to occur within a length range of 10 to 40 mm (Tesch, 1949). In laboratory conditions, the developmental process from fertilized egg to free-swimming larva was found to take approximately 48 h (Owre, 1964). However, there is a scarcity of information regarding the reproductive season for this species (Lemus-Santana et al., 2015). A reproductive advantage has been proposed for the scarce F. desmarestia population, attributed to the presence of a seminal receptacle in females, indicating that only one successful mate encounter may be necessary for fertilizing a female (Thiriot-Quiévreux, 1973: Lemus-Santana et al., 2015). The presence of numerous eggs in various stages of development within the egg filaments has been documented, further supporting the notion that the reproductive cycle of F. desmarestia is continuous (Owre, 1964: Lemus-Santana et al., 2015). Moreover, the formation of swarms, an aggregation of numerous individuals, is believed to serve a significant reproductive function. These congregations likely facilitate the exchange of gametes, enhance the chances of successful fertilization, and promote genetic diversity within the population. The main goal of this study is to report and document the first-ever observation of living F. desmarestia specimens in the Red Sea.
Materials and methods
Observations were conducted as part of the Red Sea Decade Expedition (RSDE), which took place from 04 February to 18 June 2022 aboard the R/V OceanXplorer. The expedition in the Red Sea Saudi Arabian waters covered the coastal areas to the limit of the EEZ (16.560N to 29.303N). We conducted 114 ROV dives and 88 submersible dives (Neptune and Nadir, Triton 3300/3 Submersibles from ABS A1 Submersible Cayman Islands), along the Saudi coast of the Red Sea covering from surface waters down to 2,414 m depth (for ROV dives) and from the surface down to 686 m depth (for submarine dives). Recordings conducted using the ROV ranged from 2.5 to 11 h per session, accumulating a total of over 600 h. Similarly, recordings with the submersibles spanned between 3 and 8 h per dive, accumulating almost 300 h in total. Altogether, the cumulative recording time is approximately 900 h. Videos were recorded with an Olympus M.Zuiko 12–50 mm f/3.5–6.3 camera on the Neptune submersible (Supplementary Video S1), iPhone 12 Pro Max (Supplementary Video S2), Vivo Y70 (Supplementary Video S3), and a Nikon ED 70–180 mm F4.5–5.6D on the Nadir submersible (Supplementary Videos S4–S6). Videos were processed using QuickTime Player (version 10.5), to cut videos to short sequences and reduce the size of the files.
The specimens were identified by analyzing high-resolution pictures and video recordings, as referenced in Supplementary Videos S1–S6. Physiological characteristics were then compared with the descriptions available in the literature (Seapy, 2008; Seapy, 2009). Regrettably, due to logistical constraints, live specimens could not be collected during the campaign.
To find existing records and observations of the target species, we performed a literature search using Google Scholar (https://www.scholar.google.com/). The species was searched under the names “Firoloida desmarestia,” “Firoloida desmaresti,” and “Firoloida demarestia.” Additionally, we obtained data from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org; last search date for both databases: 12 December 2022; DOI: https://doi.org/10.15468/dl.eha4zf).
Results and discussion
During the RSDE, we observed one female and two male F. desmarestia specimens on 15 May 2022 and 17 May 2022 in the central Red Sea using the two submersible vehicles (Table 1; Figure 1) at a depth between 350 and 464.5 m. Specimens were identified as Firoloida as they match the typical characteristics of the genus (Lesueur, 1817: Seapy, 2009). They show a transparent, elongated, and cylindrical body and two eyes. They have a long trunk and a short and ventral tail, with a visceral nucleus visible terminal on the trunk. One swimming (dorsal) fin is round and on the back of the trunk between the eyes and the nucleus. No warts were observed around the swimming fin. Additionally, the female specimen exhibits the distinctive long egg string characteristic of this species.

Figure 1 Images and illustrations from the three different specimens recorded during the RSDE. Male NTN0155-Firoloida (A, B; also see Supplementary Videos S1, S2, and C; detailed illustration with key taxonomical features), male NTN0156-Firoloida2 (D, E; Supplementary Video S3, and F; detailed illustration) and female NDR0902-Firoloida3 (G, H; Supplementary Videos S4–S6, and I; detailed illustration).
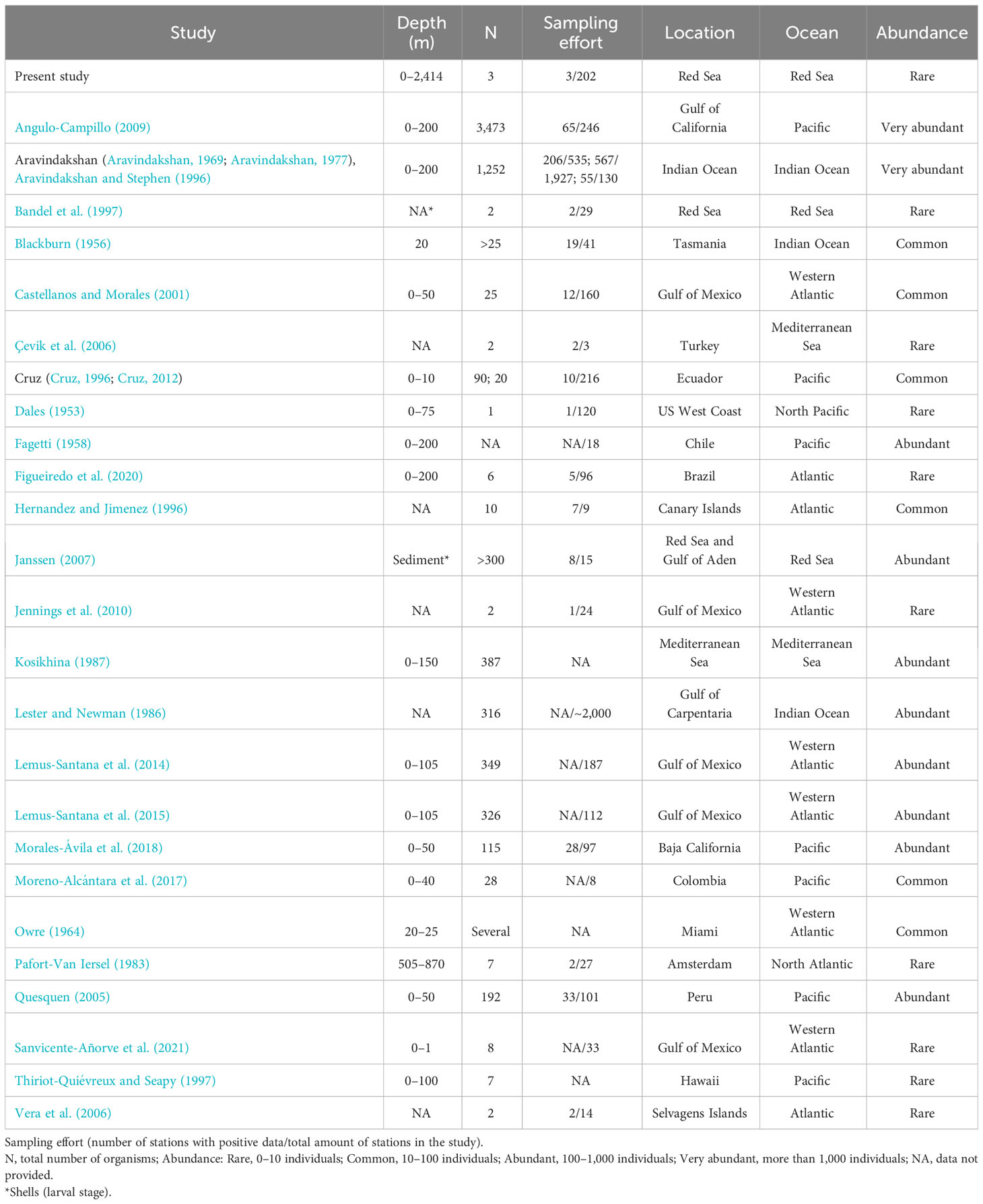
Based on the limited number of observations conducted alongside the extensive sampling effort, F. desmarestia could be considered a rare species in the Red Sea. This observation represents the second deepest record for this species, with the only prior record being reported in the mid-North Atlantic for three male specimens during a single haul from 505 to 870 m depth (Pafort-Van Iersel, 1983). Previous studies have mainly recorded this species within the depth range of 0–200 m (Fagetti, 1958; Angulo-Campillo, 2009; Figueiredo et al., 2020; Sanvicente-Añorve et al., 2021), with no literature records of a living organism beyond this depth (Table 2). The three specimens found in the expedition were at similar depths, 400, 464.5, and 350 m depth, confirming the eurybathic distribution of this species. Moreover, the three observations of F. desmarestia were recorded during the morning (09:48, 10:52, and 11:19 a.m. Arabic Standard Time), suggesting a potential association with vertical migrations to reach high depths during the daytime. However, it is crucial to approach this interpretation with caution, given the relatively limited number of detections in our study. Additionally, it is worth noting that no ROV or submersible dives were conducted at night, which could have implications for understanding the species’ behavior and distribution patterns. To the best of our knowledge, our observations are the only living adults recorded in the Red Sea until now (Figure 2).
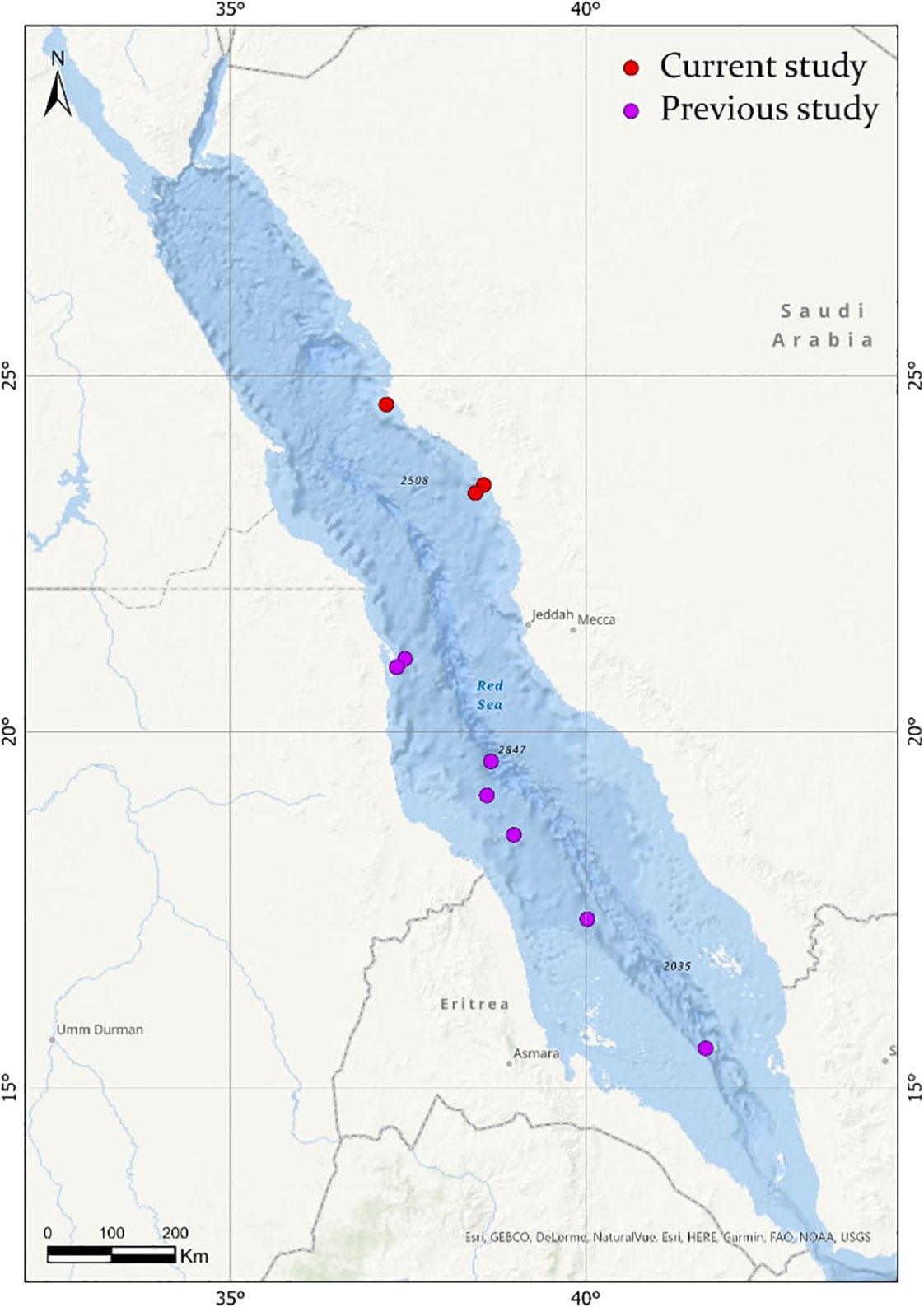
Figure 2 Map showing the distribution of Firoloida desmarestia in the Red Sea (red circles), compared with observations found in the literature (purple circles). References for observations in previous studies are listed in Tables 1, 2.
The different videos show individual adult specimens slowly swimming in front of the submersible (see Supplementary Videos S1–S6, cf. data availability section for the full dataset). The movement came mainly from the compressed fins while swimming in an upward position. One of the specimens (see Figure 1E) had a long string, indicating that this was the female individual carrying the eggs (see Supplementary Videos S4–S6). The two males (NTN0155-Firoloida1 and NTN0156-Firoloida2) were found in the water column, while the female (NDR0902-Firoloida3) was observed swimming close to the bottom.
The species F. desmarestia exhibits a cosmopolitan distribution and is found globally except in polar regions (Seapy et al., 2003) (see Table 2; Figure 3). However, there is a significant inconsistency between the data reported in the literature and the records obtained from GBIF. While many of the GBIF records are not sourced from the primary literature, we found that several data from various publications were not included in the database. This disparity is particularly evident in the Indian Ocean, where F. desmarestia is abundant and widely distributed (Aravindakshan, 1969; Aravindakshan, 1977; Aravindakshan and Stephen, 1996); however, these occurrences are not reported in the GBIF database. To address this disparity, we gathered available data from both primary literature and the biodiversity database (see Table 2; Supplementary Table S1). We aim to bridge the existing gap in the GBIF records (Figure 3). Unfortunately, certain entries suffer from a lack or mismatch of information, such as date, depth, location, coordinates, sampling method, type and number of samples collected (e.g., adult, larvae, or shell), and inaccessible data files.
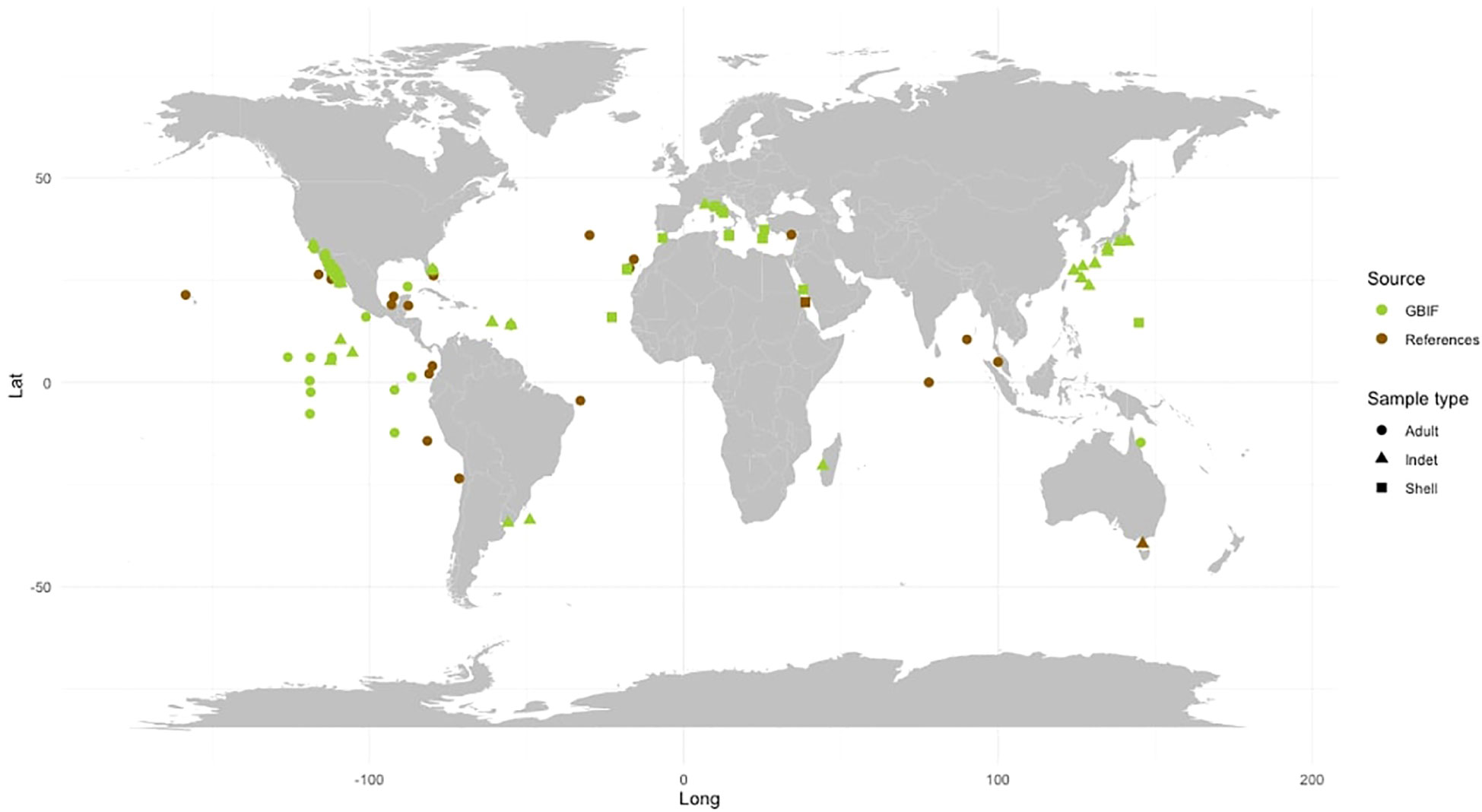
Figure 3 Global distribution of Firoloida desmarestia found in the GBIF database (green) versus those found in the peer-reviewed literature (brown). Observations were recorded from adult individuals (dot), indet (triangle), and shell (quadrat). References for observations in peer-reviewed literature are listed in Tables 1, 2.
A total of 28 studies, comprising research papers, books, Ph.D. thesis, and expedition reports, presented data on the abundance and distribution of F. desmarestia. Based on these literature, some authors describe this species as “rare” (Bandel et al., 1997; Figueiredo et al., 2020), while others call it “extremely abundant” (Aravindakshan, 1969; Aravindakshan, 1977; Angulo-Campillo, 2009). Sometimes swarms of specimens were recorded, forming scattering layers (Tesch, 1949). The abundance of F. desmarestia reported in previous studies ranged as low as 1 or 2 specimens (Dales, 1953; Jennings et al., 2010) to over 3,000 individuals observed (Angulo-Campillo, 2009). However, it is not possible to make direct comparisons due to variations in sampling efforts across these studies (Table 2). Other authors point to a more or less coast-bound distribution (Pafort-Van Iersel, 1983). The occurrence of F. desmarestia primarily, if not exclusively, in the slope region and along the continental shelf provides compelling evidence of its oceanic origin. Many records are derived from the Arabian Sea and the equatorial zone (Aravindakshan, 1969; Aravindakshan, 1977; Aravindakshan and Stephen, 1996). It has not been found south of 20°S East of the Agulhas Stream. Larval shells of F. desmarestia were found to be exceptionally rare in plankton hauls conducted in the Red Sea, with only two deceased larvae discovered across 29 stations (Bandel et al., 1997), employing various plankton gears. In contrast, they exhibited high abundance in the sediment of the southwestern region of the Red Sea and the Gulf of Aden, where 15 box core samples were collected by Janssen (2007). These cores, representing approximately 0.5 m of sea bottom sediment, cover a temporal span of approximately 6,000 years, adding further complexity to the analysis. Notably, the distinct sampling methods used in these studies present challenges for direct comparison. Intriguingly, despite this extended temporal coverage, no detections of F. desmarestia were found in the northern half of the Red Sea (up to 21°N latitude). Importantly, to the best of our knowledge, there have been no previous reports of adult F. desmarestia in the Red Sea until now.
This shell-less mollusk is found globally except in polar waters in the epipelagic zone of the world’s oceans (Figure 3; Table 2). Several authors emphasize that the habitat of heteropod species is situated in the upper photic layers of the sea (Tesch, 1949; Van der Spoel, 1976; Aravindakshan, 1977), with the predominant depth above 200 m (Table 2; Supplementary Table S1). When found in samples from meso- or bathypelagic waters from vertical hauls, the validity of the observations is usually doubted, as such specimens are supposed to have entered the net on their way up to the surface and not at the maximum depth of the haul. Whereas diurnal vertical movements are usually discarded for heteropods, their vertical distribution shows that they are not restricted to the photic zone and diurnal vertical migration occurs among the larger species (Van der Spoel, 1976; Pafort-Van Iersel, 1983; Clark et al., 2021). The literature reports adult specimens found in upper levels (0–18 m) and young specimens mainly found between 45 and 105 m (Lemus-Santana et al., 2015). Nevertheless, conflicting findings have arisen from numerous studies, as certain species demonstrate nocturnal migration toward the surface while others display no discernible migration patterns. Oberwimmer (1898) was the first to propose that heteropods, similar to pteropods, inhabit deeper depths during daylight and undertake upward migration toward the surface during darkness. Data from the literature and collections show two patterns of migration behavior: 1) small species that reside in shallow water at all times and 2) larger species that make diurnal migrations from the surface at night to deep waters during the daytime (Seapy, 1990).
Richter (1974) discovered a correlation between larger eyes and deeper habitat preferences in heteropod species, potentially linked to the reduction in light intensity at greater depths. Lalli and Gilmer (1989) and Seapy (1990) proposed that the complex eyes are used to detect prey in low-light conditions during the nighttime, with a possibility of feeding on bioluminescent organisms. Collectively, these findings highlight the complexity of vertical migration in heteropods, which are influenced by factors such as seasonality, species variation, and sampling methods. The process of comparing published data poses challenges due to inherent difficulties in obtaining representative samples, observing specimens in their natural habitat, and conducting accurate studies in laboratory settings and the presence of completely different sampling methods and sampling efforts across studies. It is likely that novel techniques enabling direct observations of the oceans (e.g., ROV, manned submersibles, SCUBA observations) and improved sampling methodologies are required. This emerging technology is progressively enhancing our understanding of these enigmatic creatures and expanding our knowledge of their fascinating adaptations and ecological roles.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.gbif.org. High-quality videos are available upon request to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements.
Author contributions
MQ, VP, and CD designed the expedition. AS made the first observation of the specimen and had the initial idea for the paper. CA-P, AS, SA, and CD conceptualized the study. CA-P, AS, and IA designed/structured the study. CA-P prepared the first draft of the manuscript, with contributions from AS, IA, and CD. CA-P, AS, and IA prepared the display figures, tables, and Supplementary Materials. All authors approved the final version of the manuscript.
Acknowledgments
We thank the National Center of Wildlife (NCW) for the invitation to participate in the Red Sea Decade Expedition. We also thank the crew of the R/V OceanXplorer for the logistic support. We want to thank Mario Tadinac and Collin Williams for the video recording, Carly Tarricone for the help with the metadata of video footage, and Fabio Marchese for the help with video processing. We also want to extend our gratitude to Anastasiia Martynova for helping us to extract data from a Russian source. Finally, we are grateful for the invaluable feedback provided by the two reviewers, which has significantly enhanced the quality of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1215195/full#supplementary-material
References
Angulo-Campillo J. (2009). Taxocenosis de moluscos Holoplanctónicos (Mollusca: Gastropoda) y su relación biogeográfica en el Golfo de California. PhD diss (La Paz (Baja California Sur): Instituto Politecnico Nacional. Centro Interdisciplinario de Ciencias Marinas).
Aravindakshan P. N. (1969). Preliminary report on the geographical distribution of the species of Carinariidae and Pterotracheidae (Heteropoda, Mollusca) from the international Indian Ocean expedition. Bull. Natl. Inst. Sci. India 38, 575–585. Available at: http://drs.nio.org/drs/handle/2264/5978.
Aravindakshan P. N. (1977). “Pterotracheidae (Heteropoda, Mollusca) of the Indian Ocean from the international Indian Ocean expedition,” in Proceedings of the Symposium on Warm Water Zooplankton (Goa, India: National Institute of Oceanography), 137–145.
Aravindakshan P. N., Stephen R. (1996). "Composition of heteropods in the Andaman Sea." in Proceedings of the Second Workshop on Scientific Results of FORV Sagar Sampada Eds. Pillai V. K., Abidi S. A. H., Ravindran V., Balachandran K. K., Agadi V. V.. 193–196.
Bandel K., Riedel F., Weikert H. (1997). Planktonic gastropod larvae from the red sea: A synopsis. Ophelia 47 (3), 151–202. doi: 10.1080/00785236.1997.10428670
Bouchet P., Rocroi J. P. (2005). Classification and nomenclator of gastropod families. Int. J. Malacol. 47 (1–2).
Castellanos I., Morales E. S. (2001). Heteropod molluscs (Carinariidae and Pterotracheidae) of the Gulf of Mexico and western Caribbean Sea. Anales del Instituto Biología. Serie Zool. 72 (2), 221–232. Available at: http://www.redalyc.org/articulo.oa?id=45872206.
Çevik C., Kideys A., Toklu B., Ergüden D., Sarihan E. (2006). New pelagic Gastropoda species encountered on the Turkish coast of the Levant Sea. Turkish J. Vet. Anim. Sci. 30 (2), 151–157. Available at: https://journals.tubitak.gov.tr/veterinary.
Clark K. A., Vecchione M., Seibel B. A., Judkins H. L. (2021). Species abundance, spatial and vertical distributions, and eye-size trends of large heteropods (Pterotracheidae and Carinariidae) in the Northern Gulf of Mexico. Am. Malacol. Bull. 38 (2), 34–43. doi: 10.4003/006.038.0201
Cruz M. (1996). Pterópodos tecosomados y heterópodos (Gasterópodos) como bioindicadores del evento “El Niño”1992, en la estación fija “La Libertad”, Ecuador. Acta Oceanogr. del Pacifico 8 (1), 51–66. Available at: http://hdl.handle.net/1834/2190.
Cruz M. (2012). Preferencia y rangos de tolerancia a la temperatura y salinidad de los pterópodos y heterópodos frente a la costa ecuatoriana. Acta Oceanográfica del Pacífico 17 (1), 93–125. Available at: http://hdl.handle.net/1834/4706.
Dales R. P. (1953). The distribution of some heteropod molluscs off the Pacific coast of North America. Proc. Zool. Soc. London 122 (4), 1007–1016. doi: 10.1111/j.1096-3642.1953.tb00359.x
Fagetti E. (1958). Dos especies de moluscos planctónicos (Heteropoda) encontrados frente a la costa de Chile. Rev. Biol. Marina VIII, 143–147.
Figueiredo G. G. A. A., Schwamborn R., Bertrand A., Lira S. M. A. (2020). New records of the mollusk Firoloida desmarestia Lesueur 1817 (Gastropoda: Pterotracheidae) off Fernando de Noronha Archipelago and Northeastern Brazilian continental slope, Tropical Atlantic. Trop. Oceanogr. 48 (1), 39–47.
Hernandez F., Jimenez S. (1996). Nota sobre moluscos pelágicos de la Gomera (campaña TFMCBM/92). Rev. Academia Canaria Cienc. VIII (2,3,4), 161–171.
Janssen A. W. (2007). Holoplanktonic mollusca (Gastropoda) from the gulf of aqaba, red sea and gulf of aden (Late holocene-recent). Veliger 49 (3), 140–195.
Jennings R. M., Bucklin A., Ossenbrügger H., Hopcroft R. R. (2010). Species diversity of planktonic gastropods (Pteropoda and Heteropoda) from six ocean regions based on DNA barcode analysis. Deep Sea Res. Part II: Topical Stud. Oceanogr. 57 (24-26), 2199–2210. doi: 10.1016/j.dsr2.2010.09.022
Keen M. (1971). Sea shells of tropical west America; marine mollusks from Baja California to Peru (Stanford University Press), 1–1064.
Kosikhina O. V. (1987). The way of nutrition and quantitative indices of food consumption by mediterranean Heteropods Firoloida desmarestia and Atlanta guoyana. Экология моря 25, 70–75.
Lalli C., Gilmer R. (1989). Pelagic snails: the biology of holoplanktonic gastropod mollusks (Stanford University Press).
Land M. F. (1982). Scanning eye movements in A heteropod mollusc. J. Exp. Biol. 96, 427–430. doi: 10.1242/jeb.96.1.427
Lemus-Santana E., Sanvicente-Añorve L., Alatorre-Mendieta M., Flores-Coto C. (2015). Population structure and mating encounter rates in a marine pelagic invertebrate, Firoloida desmarestia (Mollusca). Sexual. Early Dev. Aquat. Organ. 1 (2), 163–173. doi: 10.3354/sedao00015
Lemus-Santana E., Sanvicente-Anorve L., Hermoso-Salazar M., Flores-Coto C. (2014). The holoplanktonic Mollusca from the southern Gulf of Mexico. Part 1: heteropods. Cahiers Biol. Mar. 55, 229–239.
Lester R. J. G., Newman L. J. (1986). First rediae and cercariae to be described from heteropods. J. Parasitol. 72 (1), 195–197. doi: 10.2307/3281823
Lesueur C. A.. (1817). Characters of a new genus, and descriptions of three new species upon which it is formed: discovered in the Atlantic ocean in the months of March and April, 1816; lat. 22° 9. J. Acad. Nat. Sci. Phila. 1 (1), 37–41.
Morales-Ávila J. R., Saldierna-Martínez R. J., Moreno-Alcántara M., Violante-González J. (2018). New insights on the role of the holoplanktonic mollusk Firoloida desmarestia (Gastropoda: Pterotracheidae) as host for digenetic trematodes. Parasitol. Res. 117, 2149–2158. doi: 10.1007/s00436-018-5902-y
Moreno-Alcántara M., Giraldo A., Aceves-Medina G. (2017). Heteropods (Gastropoda: Pterotracheoidea) identified along a coastal-oceanic transect in the Colombian Pacific. Boletin Investigaciones Marinas y Costeras 46 (2), 175–181. doi: 10.25268/bimc.invemar.2017.46.2.733
Oberwimmer A. (1898). Mollusken II. (Heteropoden und Pteropoden, Sinusigera): Gesammelt von SM Schiff “Pola” 1890-1894 (KK Hof-und Staatsdruckerei).
Owre H. B. (1964). Observations on development of the Heteropod molluscs Pterotrachea hippocampus and Firoloida desmaresti. Bull. Mar. Sci. Gulf Caribbean 14 (4), 529–538.
Pafort-Van Iersel T. (1983). Distribution and variation of carinariidae and pterotracheidae (Heteropoda, gastropoda) of the amsterdam mid North Atlantic plankton expedition. Beaufortia 33 (6), 73–96.
Pechenik J. A. (2005). Biology of the Invertebrates (United Kingdom: McGraw-Hill, Higher Education), 207–284.
Quesquen R. C. (2005). Moluscos holoplanctónicos Heteropoda y Pteropoda colectados en Noviembre y Diciembre de 1996 en el mar Peruano (Lima, Peru: Universidad Ricardo Palma).
Richter G. (1974). Die Heteropoden der Meteor Expedition in den Indischen Ozean 1964/65. Meteor Forschungsergebnisse 17 (1974), 55–78.
Sanvicente-Añorve L., Rubio-Sandoval K., Lemus-Santana E., Alatorre-Mendieta M. (2021). Species richness of holoplanktonic mollusks from Mahahual, Mexican Caribbean Biosphere Reserve. Rev. mexicana biodiversidad 92, 1–7. doi: 10.22201/IB.20078706E.2021.92.3427
Seapy R. R. (1990). Patterns of vertical distribution in epipelagic heteropod molluscs off Hawaii. Mar. Ecol. Prog. series. Oldendorf 60 (3), 235–246. doi: 10.3354/meps060235
Seapy R. R. (2008). Firoloida desmarestia Lesueur 1817. Version 08. Available at: http://tolweb.org/Firoloida_desmarestia/28736/2008.10.08.
Seapy R. R. (2009). Pterotracheoidea Rafinesque 1814. Heteropoda Lamarck 1812, heteropods, sea elephants. Available at: http://tolweb.org/Pterotracheoidea/27801/2009.10.09.
Seapy R. R., Lalli C. M., Wells F. E. (2003). “Heteropoda from western Australian waters,” in The Marine Flora and Fauna of Dampier, Western Australia (Perth: Western Australian Museum), 513–546.
Tesch J. J. (1949). Heteropoda. The Carlsberg Foundation’s Oceanographical Expedition round the world 1928–30 and previous ‘Dana’ expeditions. Dana Report No. 34 (Copenhagen).
Thiriot-Quiévreux C. (1973). Heteropoda. Oceanogr. Mar. Biol. Annu. Rev. 11, 237–261. Available at: https://archimer.ifremer.fr/doc/00000/5117/.
Thiriot-Quiévreux C., Seapy R. R. (1997). Chromosome studies of three families of pelagic heteropod molluscs (Atlantidae, Carinariidae, and Pterotracheidae) from Hawaiian water. Can. J. Zool. 75, 237–244. doi: 10.1139/z97-030
Keywords: sea elephant, mollusc, deep-sea, pelagic, plankton, videos, observation
Citation: Angulo-Preckler C, Steckbauer A, Armelles I, Agustí S, Rodrigue M, Pieribone V, Qurban M and Duarte CM (2023) First record of a live adult heteropod Firoloida desmarestia in the Red Sea. Front. Mar. Sci. 10:1215195. doi: 10.3389/fmars.2023.1215195
Received: 01 May 2023; Accepted: 21 September 2023;
Published: 12 October 2023.
Edited by:
Oliver Nicholas Shipley, University of New Mexico, United StatesReviewed by:
Laura Sanvicente-Añorve, National Autonomous University of Mexico, MexicoAnna Maria Addamo, Nord University, Norway
Copyright © 2023 Angulo-Preckler, Steckbauer, Armelles, Agustí, Rodrigue, Pieribone, Qurban and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Angulo-Preckler, Y2FybG9zLnByZWNrbGVyQGthdXN0LmVkdS5zYQ==
†ORCID: Carlos Angulo-Preckler, orcid.org/0000-0001-9028-274X
Alexandra Steckbauer, orcid.org/0000-0002-8477-2256
Carlos M. Duarte, orcid.org/0000-0002-1213-1361
 Carlos Angulo-Preckler
Carlos Angulo-Preckler Alexandra Steckbauer
Alexandra Steckbauer Isabel Armelles
Isabel Armelles Susana Agustí
Susana Agustí Mattie Rodrigue
Mattie Rodrigue Vincent Pieribone4
Vincent Pieribone4 Carlos M. Duarte
Carlos M. Duarte