- 1Departamento de Ecología y Geología, Instituto Andaluz de Biotecnología y Desarrollo Azul (IBYDA), Universidad de Málaga, Malaga, Spain
- 2Department of Biology & Biomedical Sciences, Salve Regina University, Newport, RI, United States
- 3State Key Laboratory of Marine Environmental Science and College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
Editorial on the Research Topic
Macroalgal blooms in a global change context
Following the Second World War, the development of industrial agriculture and medical advances allowed for an exponential increase in the human population. During this period, the concept of mass consumption society was also encouraged in developed and developing countries, increasing the demand for resources (Cherubini et al., 2018; Gaulin and Le Billon, 2020; Elmqvist et al., 2021). The associated expansion and intensification of human activities necessary to cover the needs of a larger and more demanding population led to important changes in the earth system, making humankind one of the most important drivers of global change (e.g., ocean acidification, climate change, eutrophication, biological invasions). All of these human-induced changes in environmental conditions have produced important alterations and imbalances in the structure and functioning of ecosystems, especially in aquatic systems (Lotze et al., 2006; James et al., 2023).
In coastal and estuarine waters, one of the most evident signs of the impact of human activities is the development of macroalgal blooms. Macroalgal blooms are accumulations of fast-growing opportunistic species, which can lead to anoxic events and release nuisance or toxic compounds during the degradation of the biomass (Fletcher, 1996; Valiela et al., 1997; Green-Gavrielidis et al., 2018). These blooms alter ecosystem functioning of nearshore environments and limit the services these areas provide (Fletcher, 1996; Gonzales et al., 2013). Macroalgal blooms became more frequent and larger in the 1970s, especially in industrialized countries. Since then, the number of reports from new locations and the magnitude of these blooms have continued to increase (Smetacek and Zingone, 2013). Important research efforts have been developed in order to understand the causes and mechanisms underlying these phenomena, which have demonstrated the key role nutrient over-enrichment and reduced herbivory play in explaining the occurrence of macroalgal blooms (Pedersen and Borum, 1996; Teichberg et al., 2012; Bermejo et al., 2022). Despite the critical role of nutrient over-enrichment in the occurrence of seaweed tides, additional abiotic and biotic factors such as light (Vergara et al., 1997), temperature (Gao et al., 2017), CO2 (Xu et al., 2017), local hydrodynamic conditions (Salomonsen et al., 1999), grazing (Nelson et al., 2008), precipitation changes (Green-Gavrielidis and Thornber, 2022), propagule bank size (Lotze et al., 2000), and local species pool (Bermejo et al., 2023) can be critical in explaining bloom development.
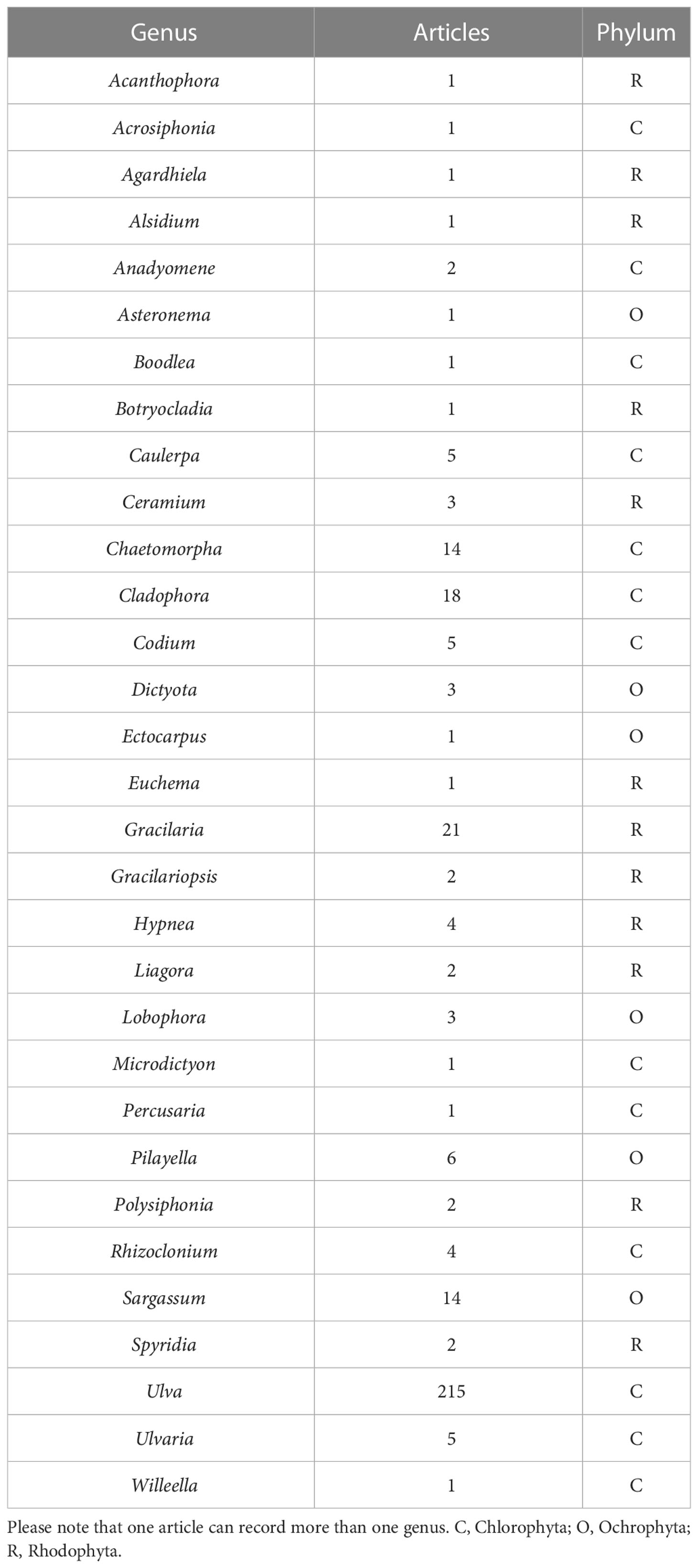
A quick search in Scopus using the term “seaweed tide” that only considered original research, written in English, in the marine environment published until 2022 identified 265 original research articles that were mostly conducted in developed regions (Figure 1A). Species of more than 30 macroalgal genera were reported as responsible for the occurrence of macroalgal blooms. Macroalgal blooms dominated by green seaweeds (especially Ulva; Figure 1B; Table 1) were the most studied, although in the last year there has been an increasing interest in golden tides, mostly related to the occurrence of Sargassum blooms (Figure 1C). Although these figures can be biased by differential research efforts in different regions or the use of other terms to define macroalgal blooms (e.g., “bioinvasion”), all these figures give an idea of the complexity and global nature of macroalgal blooms.
Figure 1 Number of mentions in eligible original research articles found in SCOPUS sorted by geographical region -the area is proportional to the number of mentions- (A), type of macroalgal bloom (B), and by type of bloom (green-triangles; golden-circles; red -squares) and year (C). In total, 265 original research articles published until 2022 were considered in the analysis.
Regarding this Research Topic, the scientific contributions published cover a wide geographical range with contributions from Asia, Europe, North America, and South America. Two contributions focused on green tides [Ulva lactuca (Reidenbach et al.) and U. prolifera (Cai et al.)], two on golden tides [Sargassum horneri (Xu et al.) and Rugulopteryx okamurae (Roca et al.)] and one on a red seaweed bloom [Trichogloeopsis pedicelata (Gavio et al.)]. The factors explaining the development of the studied macroalgal blooms are diverse and include the occurrence of extreme climatic events [T. pedicelata (Gavio et al.)], biological invasions [R. okamurae (Roca et al.)], eutrophication (Ulva spp. and S. horneri) and changes in oceanic currents (S. horneri). Three articles are laboratory-based ecophysiological experiments, two of them (Ulva spp.) aimed to understand the effects of acidification in two contexts of nutrient enrichment (high and normal) (Reidenbach et al.; Cai et al.), and one investigated the effects of ultraviolet radiation on S. horneri (Xu et al.). The two laboratory-based studies focused on Ulva spp. provide useful information for the forecast and management of green tides in a global change context (i.e., ocean acidification), under different scenarios of nutrient management (Reidenbach et al.; Cai et al.). The contribution assessing the effect of an enhanced light condition (including ultraviolet radiation) as a consequence of a change in the habitat of S. horneri (i.e., anchored vs floating) contributes to a better understanding of the factors explaining the development of golden tides in East China and Yellow Seas (Xu et al.). The other two contributions were field-based studies. The study from the southwestern Caribbean Sea describes the occurrence of a red macroalgal bloom dominated by the rhodophyte T. pedicellata following a hurricane (Gavio et al.). To assess and describe the effects of climatic extremes (e.g., hurricanes, heat waves) on marine ecosystem becomes specifically relevant as an increase in the occurrence of these events is expected. The other field-based study developed a methodology for monitoring a non-native species causing extensive macroalgal blooms in southern Spain using Unmanned Aerial Vehicles (UAV) (Roca et al.). Using Earth observation technologies (e.g., UAV and satellites) combined with field surveys will be key for reducing the costs of the necessary environmental monitoring required, while increasing coverage, data reliability and effectiveness (Karki et al., 2021). The wide geographical range covered by the presented contributions and their diversity in taxa, environmental situations, and methodologies reflects the interest and the global concerns around macroalgal blooms in the current context of global change.
Author contributions
Conceptualization: RB, GG, LG-G Data curation: GG, RB. Methodology: RB. Writing and original draft: RB. Writing and review & editing: LG-G, GG. All authors contributed to the article and approved the submitted version.
Funding
RB was supported by 2014-2020 EPA Research Strategy (Environmental Protection Agency, Ireland; project no: 2018-W-MS-32 – the MACRO-MAN Project). LG-G was supported partially by the Rhode Island Institutional Development Award (IDeA) Network of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health (grant no: P20GM103430). GG research was supported by the MEL Internal Research Program (MELRI2304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bermejo R., Galindo-Ponce M., Golden N., Linderhoff C., Heesch S., Hernández I., et al. (2023). Two bloom-forming species of ulva (Chlorophyta) show different responses to seawater temperature and no antagonistic interaction. J. Phycol. 59, 167–178. doi: 10.1111/jpy.13302
Bermejo R., Golden N., Schrofner E., Knöller K., Fenton O., Serrão E., et al. (2022). Biomass and nutrient dynamics of major green tides in Ireland: implications for biomonitoring. Mar. pollut. Bull. 175, 113318. doi: 10.1016/j.marpolbul.2021.113318
Cherubini F., Huang B., Hu X., Tolle M. H., Hammer Stromman A. (2018). Quantifying the climate response to extreme land cover changes in Europe with a regional model. Environ. Res. Lett. 13, 1–12. doi: 10.1088/1748-9326/aac794
Elmqvist T., Andersson E., McPhearson T., Bai X., Bettencourt L., Brondizio E., et al. (2021). Urbanization in and for the anthropocene. NPJ Urban Sustain. 1, 1–6. doi: 10.1038/s42949-021-00018-w
Fletcher R. L. (1996). “The occurrence of “Green tides” - a review,” in Marine benthic vegetation: recent changes and the effects of eutrophication. Eds. Schramm W., Nienhuis P. H. (Springer), 7–43.
Gao G., Clare A. S., Rose C., Caldwell G. S. (2017). Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. pollut. Bull. 114: 439-447. doi: 10.1016/j.marpolbul.2016.10.003
Gaulin N., Le Billon P. (2020). Climate change and fossil fuel production cuts: assessing global supply-side constraints and policy implications. Clim. Policy. 20, 1–14. doi: 10.1080/14693062.2020.1725409
Gonzalez D. J., Smyth A. R., Piehler M. F., Mcglathery K. J. (2013). Mats of the nonnative macroalga, gracilaria vermiculophylla, alter net denitrification rates and nutrient fluxes on intertidal mudflats. Limnol. Oceanogr. 58, 2101–2108. doi: 10.4319/lo.2013.58.6.2101
Green-Gavrielidis L. A., MacKechnie F., Thornber C. S., Gomez-Chiarri M. (2018). Bloom-forming macroalgae (Ulva spp.) inhibit the growth of co-occurring macroalgae and decrease eastern oyster larval survival. Mar. Ecol. Prog. Ser. 595, 27–37. doi: 10.3354/meps12556
Green-Gavrielidis L. A., Thornber C. S. (2022). Will climate change enhance algal blooms? the individual and interactive effects of temperature and rain on the macroalgae Ulva. Estuaries Coasts 45, 1688–1700. doi: 10.1007/s12237-022-01048-y
James R. K., Keyzer L. M., van de Velde S. J., Herman P. M. J., van Katwijk M. M., Bouma T. J. (2023). Climate change mitigation by coral reefs and seagrass beds at risk: how global change compromises coastal ecosystem services. Sci. Total Environ. 857, 159576. doi: 10.1016/j.scitotenv.2022.159576
Karki S., Bermejo R., Wilkes R., Monagail M. M., Daly E., Healy M., et al. (2021). Mapping spatial distribution and biomass of intertidal Ulva blooms using machine learning and earth observation. Front. Mar. Sci. 8, 633128. doi: 10.3389/fmars.2021.633128
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. doi: 10.1126/science.1128035
Lotze H. K., Worm B., Sommer U. (2000). Propagule banks, herbivory and nutrient supply control population development and dominance patterns in macroalgal blooms. Oikos 89, 46–58. doi: 10.1034/j.1600-0706.2000.890106.x
Nelson T. A., Haberlin K., Nelson A. V., Ribarich H., Hotchkiss R., Alstyne K. L., et al. (2008). Ecological and physiological controls of species composition in green macroalgal blooms. Ecology 89, 1287–1298. doi: 10.1890/07-0494.1
Pedersen M. F., Borum J. (1996). Nutrient control of algal growth in estuarine waters. nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar. Ecol. Prog. Ser. 142, 261–272. doi: 10.3354/meps142261
Salomonsen J., Flindt M., Geertz-Hansen O., Johansen C. (1999). Modelling advective transport of ulva lactuca (L) in the sheltered bay, mollekrogen, roskilde fjord, Denmark. Hydrobiologia 397, 241–252. doi: 10.1023/A:1003790625535
Smetacek V., Zingone A. (2013). Green and golden seaweed tides on the rise. Nature 504, 84–88. doi: 10.1038/nature12860
Teichberg M., Martinetto P., Fox S. E. (2012). “Bottom-up versus top-down control of macroalgal blooms,” in Seaweed biology. Eds. Wiencke C., Bischof K. (Berlin, Heidelberg: Springer Berlin Heidelberg).
Valiela I., Mcclelland J., Hauxwell J., Behr P. J., Hersh D., Foreman K. (1997). Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr. 42, 1105–1118. doi: 10.4319/lo.1997.42.5_part_2.1105
Vergara J. J., Lucas J. P., Peralta G., Hernandez I., Niell F. X. (1997). Seasonal variation of photosynthetic performance and light attenuation in ulva canopies from palmones river estuary. J. Phycol. 779, 773–779. doi: 10.1111/j.0022-3646.1997.00773.x
Keywords: macroalgal bloom, seaweed tide, global change, green tide, golden tide, red seaweed bloom, invasive species
Citation: Bermejo R, Green-Gavrielidis L and Gao G (2023) Editorial: Macroalgal blooms in a global change context. Front. Mar. Sci. 10:1204117. doi: 10.3389/fmars.2023.1204117
Received: 11 April 2023; Accepted: 19 April 2023;
Published: 28 April 2023.
Edited and Reviewed by:
Stelios Katsanevakis, University of the Aegean, GreeceCopyright © 2023 Bermejo, Green-Gavrielidis and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ricardo Bermejo, cmljYXJkby5iZXJtZWpvQHVtYS5lcw==
 Ricardo Bermejo
Ricardo Bermejo Lindsay Green-Gavrielidis
Lindsay Green-Gavrielidis Guang Gao
Guang Gao