- Department of Estuarine and Delta Systems, Netherlands Institute for Sea Research and Utrecht University, Yerseke, Netherlands
Introduction
The ways living forms develop in the biosphere are the same everywhere: growing and surviving for an ultimate reproductive success. In this achievement, organisms need to cope with various environmental constraints, but they have found solutions over evolutionary time by combining differently life history traits. Studying these adaptation processes has been in the heart of functional ecology, with a growing research endeavour in the marine benthos, particularly well suited given its presence in habitats of highly variable spatio-temporal dynamics. The marine benthos is subject to a particularly appealing research interest as, next to its diversity of life cycles, it ensures crucial ecosystem functions. This has led to numerous compilations of biological trait data sets in which very different functional information can be found. In recent years, trait-based benthic ecology has been strongly fostered by functional diversity assessments (Weigel et al., 2016; Breine et al., 2018; Llanos et al., 2020; Murillo et al., 2020; Sutton et al., 2020; Dreujou et al., 2021; Zhulay et al., 2021; Gusmao et al., 2022; Robinson et al., 2022; Festjens et al., 2023). Nowadays, benthic ecologists dispose of sophisticated analytical tools that can process various sets of traits to generate functional diversity indices (FD). However, FD assessments have been done in various contexts with mixed types of traits, often without specifying the theoretical links between traits and FD, which brings the meaning of FD subject to debate. In this opinion piece, I point out important issues regarding FD assessment in the marine benthos in the context of ecosystem functioning.
A major trait dichotomy
A multiplicity of traits can be described in a species, but as numerous as they are, they belong to only two types that lead ultimately to only two general questions (Lavorel and Garnier, 2002). The first type, related to life history, is called “response trait”, referring to species response and adaptation to environmental constraints by investing energy into survival at the juvenile or adult stage (Kindsvater et al., 2016). As pure descriptors of Darwinian fitness, typical response traits include age at sexual maturity, life span, reproductive frequency, fecundity and offspring aspects (type, size and development duration). From a fundamental perspective, the use of these traits leads to life strategies as evolutionary convergences resulting from universal energetic allocation trade-offs, generally three or four, depending on the considered taxocenosis (Greenslade, 1983; Southwood, 1988; Kindsvater et al., 2016): stress-resistant and disturbance-resilient (A- and r-strategists, respectively, both short-lived), and long-lived to favour adult survival (K-strategists, including “episodic” and “survivor” types). From an applied perspective, response traits inform on species ability to withstand a human pressure mimicking natural stress or disturbance by sorting vulnerable species (slow-growing, long-lived) from lowly vulnerable ones (fast-growing, short-lived). However, response traits do not say if removing a species is detrimental to ecosystem functioning as they do not directly express ecosystem function (Violle et al., 2007; Schmera et al., 2016). Rather, they are species properties acquired over evolutionary time as a result of the optimization of the fitness components.
The loss of a species is always critical, but the loss of species that ensure important or rare functions in the ecosystem is even more critical. Thus, “effect traits” represent the second type of traits that express species abilities to contribute in various ways to fluxes of energy and material in the ecosystem (i.e. ecosystem function; Díaz and Cabido, 2001) beyond their ultimate reproductive achievement (Lavorel and Garnier, 2002). Effect traits are particularly prominent in the marine benthos through ecosystem engineering such as habitat creation (Ballesteros, 2006) and substratum alteration (sediment mixing type, bioirrigation, biostabilisation, bioersion; Pearson, 2001; Kristensen et al., 2012). Traits related to trophic aspects like feeding type can also account for ecosystem functioning (Fauchald and Jumars, 1979; Woodin and Jackson, 1979), yet simple trait modality attribution per species may not relevantly express complex fluxes of energy and material as food web analysis does (i.e. species controlling one another).
Hence, this leads to consider a fundamental dichotomy: response traits as descriptors of species requirements for reproductive success, and effect traits that express ecosystem functions as side effects; in simple terms, what species are on the one hand, and what species do on the other hand. Although a single trait can express both aspects, its consideration in a data set must be theoretically justified in order to support its functional significance (de Bello et al., 2021).
What functional diversity should express
FD indices have been designed to express trait information in a synthetic way (Mouillot et al., 2013). They are calculated in the Euclidean space of trait variables where species are positioned. The structural properties of a species community can be derived from the volume that species occupy in the space. The volume itself is a first index that represents the range of trait variations (“functional richness”; Cornwell et al., 2006; Villéger et al., 2008). Then, within the volume, other indices describe species distributions and related functional meanings such as aggregation, redundancy or divergence (Villéger et al., 2008; Mouillot et al., 2013).
Very often, multiple traits are used to ultimately quantify FD, especially if the aim is to identify areas of conservation interest. Some indices concisely express the multiplicity of functions of a community of which a large extent in the trait space should raise more attention for conservation: range of variation (functional richness) or, more based on species dissimilarity, Rao’s quadratic entropy and functional dispersion (Laliberté and Legendre, 2010). Although this assessment can be laudable from a data analytical perspective, a given value of FD is not necessarily meaningful in the absence of theoretical clarification on the type of traits used. The use of response traits inexorably leads to life strategies so a large FD means that the community is composed of a mix of resistant/resilient and vulnerable species whereas a low FD indicates a homogeneous assemblage of the one or the other type. Hence, from a conservational perspective, based on response traits, a low FD can be as critical as a high FD depending on the proportion of vulnerable species in the assemblage. In contrast, a large FD derived from effect traits means multiple and non-redundant ecosystem functions, so the larger the FD, the more critical a species loss, especially in cases where communities are composed of vulnerable species (Figure 1).
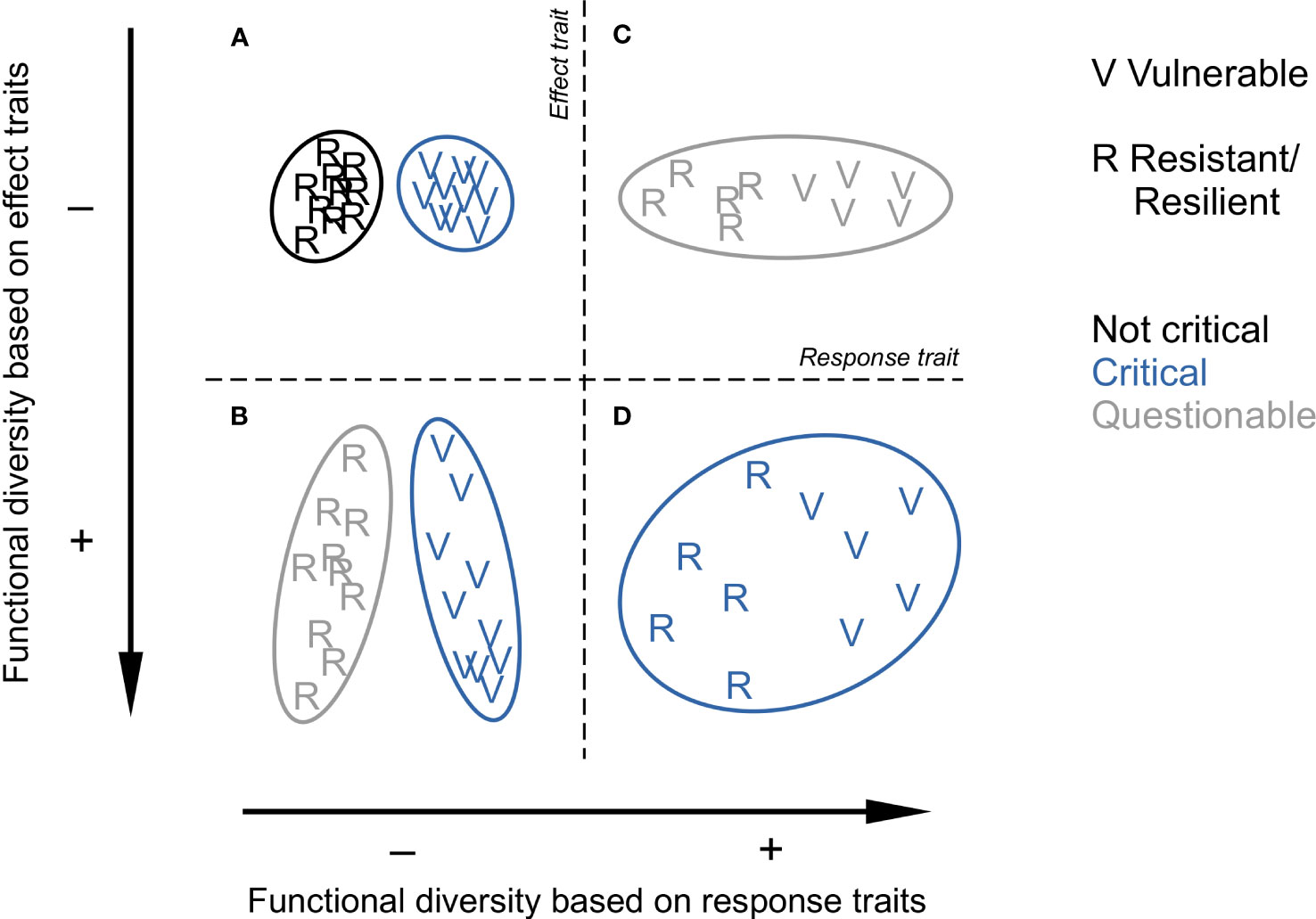
Figure 1 Conceptual confrontation of benthic functional diversities derived from response traits (horizontally) and effect traits (vertically). From (A-D), each panel represents one (C, D) or two (A, B) species (letters) communities in a space defined by a synthetic response trait separating resistant or resilient life strategies (R) from vulnerable (V) ones (horizontally) and a synthetic effect trait positioning species according to their ecosystem functions (vertically). What matters is that the surface area (ellipse size) of the community represents the extent of functional diversity from a species dissimilarity point of view. Colours indicate if species removal following disturbance could be critical for ecosystem functioning. The figure questions where research efforts should be put from a perspective of ecosystem functioning preservation. Note that these variations are independent of species richness (10 species in every case), as underlined by Cadotte et al. (2011).
Of course, these remarks do not deprive FD analyses solely based on response traits from interest. The combination of indices expressing community extent, species aggregation and divergence enables very insightful investigations on species niche and co-existence in the frameworks of environmental filtering and limiting similarity (Weiher and Keddy, 1995).
Discussion
Although raised long ago (Lavorel and Garnier, 2002), the response-effect trait dichotomy does not seem to have been well assimilated in marine benthic studies, yet it is at the basis of hypothesis formulation when using traits in community ecology (de Bello et al., 2021). Whereas many marine trait studies considered the concept of ecosystem function and functional diversity, only a few ones focused specifically on effect traits to quantify more adequately benthic ecosystem functions (Norling et al., 2007; Hewitt et al., 2008; Lam-Gordillo et al., 2021). In contrast, the use of response traits or mixes of both response and effect traits dominates the literature (Beauchard et al., 2017) in spite of the poor ability of response traits to express ecosystem function.
The striving towards ecological indicator development, more relevantly addressed with response traits, may have prevented a more rational knowledge build-up in marine benthic ecology within the response-effect trait framework. Long ago, evolutionary convergences of traits responding to environmental forces had been a major focus in limnology (Statzner et al., 1994; Statzner et al., 1997; Townsend et al., 1997), which triggered the advent of trait studies in the marine benthos (Bremner et al., 2003). Until now, and curiously, this fundamental research aspect has raised only little interest in marine benthic studies whereas it represents the theoretical support of ecological indicator development (Sutton et al., 2021; Beauchard et al., 2022).
An ultimate issue required to assess ecosystem resistance/resilience to disturbance is to know which species, among vulnerable and invulnerable ones, provide the most important ecosystem functions. It is not intuitive as species evolve to achieve reproductive success, not ecosystem functions which are contingent upon ecological opportunities met or not over evolutionary time. As a consequence, species that ensure important functions, exhibit variable degrees of vulnerability (Beauchard et al., 2023). From this perspective, we should not protect species only for what they are in terms of evolutionary achievement (e.g. slow-growing; Rijnsdorp et al., 2018), but also for what they do with respect to generating opportunities for each other. The four theoretical contexts in Figure 1 illustrate this point and deserve some discussion in the context of ecosystem functioning, disturbance and recovery. They represent real cases reported in marine benthic ecology.
(A) Based on response traits, a low FD (i.e. small extent in the trait space due to low species dissimilarity) necessarily indicates that the community is composed of species of similar life strategies. A homogeneous community of resistant/resilient species represents one of the least ambiguous situations: at worst, its brief absence following disturbance is not critical to ecosystem functioning as encountered in wave-disturbed communities (Barry, 1989). However, when most of the species are vulnerable, their long recovery may deprive the ecosystem from biomass for a substantial duration. This case could be questionable if the community would not ensure various ecosystem functions, but the nature of the functions also matters. Coral reefs, although mainly homogeneous habitat builders, are known to be of major importance for the marine ecosystem.
(B) Vulnerable or not, both communities ensure various functions (i.e. high species dissimilarity). The vulnerable community represents an unambiguous situation: when disturbed, its FD strongly declines, critically affecting ecosystem functioning; deep sea communities, composed of species that generally exhibit longer life spans, are typical of this case (Montero-Serra et al., 2018). When composed of resilient species (e.g. many estuarine communities), a relevant issue related to disturbance emerges: beyond which disturbance frequency the temporal removal of functions becomes detrimental to ecosystem functioning?
(C) A large FD based on response traits is necessarily due to co-occurring life strategies. Disturbance in this context is questionable as it entails a loss of species, at least for some time before recovery; the situation will become likely critical with increasing disturbance frequency. Such communities can be found in shallow rocky habitats where resilient (e.g. short-lived crustaceans and epibionts) and slow-growing species (e.g. mussels, oysters, corals) whose ecosystem functions are limited to habitat creation co-occur.
(D) A large FD due to different co-occurring strategists ensuring dissimilar ecosystem functions. Although this situation is more likely to be encountered with increasing spatial scale (Beauchard et al., 2022), shallow muddy habitats are potentially typical as they host both epi- and endobenthic engineering species (Beauchard et al., 2023). The issues highlighted in the three other cases are combined: loss of vulnerable species (A) that ensure dissimilar ecosystem functions (B) and of which the removal can be critical depending on disturbance frequency (B and C).
Through this simplified confrontation of response versus effect trait-based FD, we can see that FD may be critical in most of cases from a perspective of ecosystem function and service preservation. This does not call into question the relevance of FD assessment for this purpose, on the contrary, this bolsters it in showing that a theoretically-sound manipulation of traits can bring a clear mechanistic understanding of complex FD patterns.
In conclusion, the use of traits without theoretical considerations risks to drive the concept of FD to become devoid of meaning. The consequences can be critical from a perspective of ecosystem functioning preservation in case of irrelevant trait selection to characterise ecosystem functions. It is conspicuous that assessing FD solely from response traits or mixed with effect ones returns a blurred image of ecosystem functions. At least, the dual use of both types of traits in any analytical exercise should preliminarily proceed by separate analyses to disentangle life strategies from ecosystem functions. By this way, management efforts could be ensured by coherent combinations of vulnerability (what species are) and functions (what species do). Hence, the remarks in this paper advocate for (1) strengthening the theoretical bases of life history in marine benthic synecology, (2) more relevant uses of traits regarding the life strategy-ecosystem function dichotomy and (3) more adequate uses of both response and effect traits for meaningful FD assessments that better take account for critical aspects of ecosystem functioning.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Financial support was provided by the Dutch Research Council through the BFIAT project (Bottom Fishing Assessment Tool; NWO 18523) and the European Union through the NECCTON project (New Copernicus Capability for Tropic Ocean Networks; grant agreement 101081273).
Acknowledgments
The author is grateful to Emil de Borger and Karline Soetaert for useful comments. The paper also benefited from a valuable feedback from the ICES Working Group on Biodiversity Science (WGBIODIV). The author thanks the reviewer for constructive comments on an earlier version of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ballesteros E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr Mar. Biol. Annu. Rev. 44, 123–195. doi: 10.1201/9781420006391
Barry J. P. (1989). Reproductive response of a marine annelid to winter storms: an analog to fire adaptation in plants? Mar. Ecol. Prog. Ser. 54, 99–107. doi: 10.3354/MEPS054099
Beauchard O., Mestdagh S., Koop L., Ysebaert T., Herman P. M. J. (2022). Benthic synecology in a soft sediment shelf: habitat contrasts and assembly rules of life strategies. Mar. Ecol. Prog. Ser. 682, 31–50. doi: 10.3354/meps13928
Beauchard O., Thompson M. S. A., Ellingsen K., Piet G. J., Laffargue P., Soetaert K. (2023). Quantifying sea floor functional biodiversity and vulnerability. Mar. Ecol. Prog. Ser. 708, 21–43. doi: 10.3354/meps14270
Beauchard O., Veríssimo H., Queirós A. M., Herman P. M. J. (2017). The use of multiple biological traits in marine community ecology and its potential in ecological indicator development. Ecol. Indic 76, 81–96. doi: 10.1016/j.ecolind.2017.01.011
Breine N. T., De Backer A., Van Colen C., Moens T., Hostens K., Van Hoey G. (2018). Structural and FD of soft-bottom macrobenthic communities in the Southern North Sea. Estuar. Coast. Shelf Sci. 214, 173–184. doi: 10.1016/j.ecss.2018.09.012
Cadotte M. W., Carscadden K., Mirotchnick N. (2011). Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087. doi: 10.1111/j.1365-2664.2011.02048.x
Cornwell W. K., Schwilk D. W., Ackerly D. D. (2006). A trait-based test for habitat filtering: convex hull volume. Ecology 87, 1465–1471. doi: 10.1890/0012-9658(2006)87[1465:ATTFHF]2.0.CO;2
de Bello F., Carmona C. P., Dias A. T. C., Götzenberger L., Moretti M., Berg M. P. (2021). Handbook of trait-based ecology. From theory to R tools (Cambridge: Cambridge University Press).
Díaz S., Cabido M. (2001). Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. doi: 10.1016/S0169-5347(01)02283-2
Dreujou E., Desroy N., Carrière J., Tréau de Coeli L., McKindsey C. W., Archambault P. (2021). Determining the ecological status of benthic coastal communities: a case in an anthropized sub-Arctic area. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.637546
Fauchald K., Jumars P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanogr Mar. Biol. Annu. Rev. 17, 193–284.
Festjens F., Buyse J., De Backer A., Hostens K., Lefaible N., Vanaverbeke J., et al. (2023). Functional trait responses to diffe rent anthropogenic pressures. Ecol. Indic. 146, 109854. doi: 10.1016/j.ecolind.2022.109854
Greenslade P. J. M. (1983). Adversity selection and the habitat templet. Am. Nat. 122, 352–365. doi: 10.1086/284140
Gusmao J. B., Thieltges D. W., Dekker R., Govers L. L., Meijer K. J., Eriksson B. K. (2022). Comparing taxonomic and functional trait diversity in marine macrozoobenthos along sediment texture gradients. Ecol. Indic 145, 109718. doi: 10.1016/j.ecolind.2022.109718
Hewitt J. E., Thrush S. F., Dayton P. D. (2008). Habitat variation, species diversity and ecological functioning in a marine system. J. Exp. Mar. Biol. Ecol. 366, 116–122. doi: 10.1016/j.jembe.2008.07.016
Kindsvater H. K., Mangel M., Reynolds J. D., Dulvy N. K. (2016). Ten principles from evolutionary ecology essential for effective marine conservation. Ecol. Evol. 6, 2125–2138. doi: 10.1002/ece3.2012
Kristensen E., Penha-Lopes G., Delefosse M., Valdemarsen T., Quintana C. O., Banta G. T. (2012). What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 446, 285–302. doi: 10.3354/meps09506
Laliberté E., Legendre P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Lam-Gordillo O., Baring R., Dittmann S. (2021). Taxonomic and functional patterns of benthic communities in southern temperate tidal flats. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.723749
Lavorel S., Garnier E. (2002). Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct. Ecol. 16, 545–556. doi: 10.1046/j.1365-2435.2002.00664.x
Llanos E. N., Saracho Bottero M. A., Lourdes Jaubet M., Elías R., Garaffo G. V. (2020). Functional diversity in the intertidal macrobenthic community at sewage-affected shores from southwestern Atlantic. Mar. pollut. Bull. 157, 111365. doi: 10.1016/j.marpolbul.2020.111365
Montero-Serra I., Linares C., Doak D. F., Ledoux J. B., Garrabou J. (2018). Strong linkages between depth, longevity and demographic stability across marine sessile species. Proc. R. Soc B 285, 20172688. doi: 10.1098/rspb.2017.2688
Mouillot D., Graham N. A. J., Villéger S., Mason N. W. H., Bellwood D. R. (2013). A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. doi: 10.1016/j.tree.2012.10.004
Murillo F. J., Weigel B., Bouchard Marmen M., Kenchington E. (2020). Marine epibenthic FD on Flemish cap (north-west atlantic)–identifying trait responses to the environment and mapping ecosystem functions. Divers. Distrib. 26, 460–478. doi: 10.1111/ddi.13026
Norling K., Rosenberg R., Hulth S., Grémare A., Bonsdorff E. (2007). Importance of functional biodiversity and species-specific traits of benthic fauna for ecosystem functions in marine sediment. Mar. Ecol. Prog. Ser. 332, 11–23. doi: 10.3354/meps332011
Pearson T. H. (2001). Functional group ecology in soft-sediment marine benthos: the role of bioturbation. Oceanogr Mar. Biol. Annu. Rev. 39, 233–267.
Rijnsdorp A. D., Bolam S. G., Garcia C., Hiddink J. G., Hintzen N. T., van Denderen P. D., et al. (2018). Estimating sensitivity of seabed habitats to disturbance by bottom trawling based on the longevity of benthic fauna. Ecol. Appl. 28, 1302–1312. doi: 10.1002/eap.1731
Robinson B. J. O., Barnes D. K. A., Grange L. J., Morley S. A. (2022). The extremes of disturbance reduce functional redundancy: functional trait assessment of the shallow Antarctic benthos. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.797112
Schmera D., Heino J., Podani J., Erős T., Dolédec S. (2016). FD: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 787, 27–44. doi: 10.1007/s10750-016-2974-5
Statzner B., Hoppenhaus K., Arens M. F., Richoux P. (1997). Reproductive traits, habitat use and templet theory: synthesis on world-wide data on aquatic insects. Freshw. Biol. 38, 109–135. doi: 10.1046/j.1365-2427.1997.00195.x
Statzner B., Resh V. H., Roux L. (1994). The synthesis of long term ecological research in the context of currently developed ecological theory: design of a research strategy for the upper rhône river and its floodplain. Freshw. Biol. 31, 253–263. doi: 10.1111/j.1365-2427.1994.tb01739.x
Sutton L., Iken K., Bluhm B. A., Mueter F. J. (2020). Comparison of FD of two alaskan Arctic shelf epibenthic communities. Mar. Ecol. Prog. Ser. 651, 1–21. doi: 10.3354/meps13478
Sutton L., Mueter F. J., Bluhm B. A., Iken K. (2021). Environmental filtering influences functional community assembly of epibenthic communities. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.736917
Townsend C. R., Dolédec S., Scarsbrook M. R. (1997). Species traits in relation to temporal and spatial heterogeneity in streams: a test of habitat templet theory. Freshw. Biol. 37, 367–387. doi: 10.1046/j.1365-2427.1997.00166.x
Villéger S., Mason N. W. H., Mouillot D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
Violle C., Navas M. L., Vile D., Kazakou E., Fortunel C., Hummel I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Weigel B., Blenckner T., Bonsdorff E. (2016). Maintained functional diversity in benthic communities in spite of diverging functional identities. Oikos 125, 1421–1433. doi: 10.1111/oik.02894
Weiher E., Keddy P. A. (1995). Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74, 159–164. doi: 10.2307/3545686
Woodin S. A., Jackson J. B. C. (1979). Interphyletic competition among marine benthos. Amer Zool. 19, 1029–1043. doi: 10.1093/icb/19.4.1029
Keywords: benthic invertebrate, response trait, effect trait, life strategy, ecosystem functioning, functional diversity
Citation: Beauchard O (2023) The importance of trait selection on the meaning of functional diversity in benthic studies. Front. Mar. Sci. 10:1195595. doi: 10.3389/fmars.2023.1195595
Received: 28 March 2023; Accepted: 09 June 2023;
Published: 22 June 2023.
Edited by:
Federica Nasi, National Institute of Oceanography and Applied Geophysics - OGS, ItalyReviewed by:
Erik Bonsdorff, Åbo Akademi University, FinlandCopyright © 2023 Beauchard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Beauchard, b2xpdmllci5iZWF1Y2hhcmRAbmlvei5ubA==
 Olivier Beauchard
Olivier Beauchard