
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 09 June 2023
Sec. Marine Conservation and Sustainability
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1192024
Perna perna (Linnaeus, 1758) is one of the most commercialized mollusk species in Brazil. The individuals with a shell length of at least 50 mm are considered to be adults and suitable for commercialization and human consumption. However, the lack of control over the size of extracted mussels is a recurrent and worrisome issue, which may compromise the long-term survival of natural stocks. The present study evaluated the potential to differentiate juvenile brown mussels from adult individuals by using Near Infrared Spectroscopy (NIRS). A total of 176 mussels were obtained from Jurujuba and Vermelha beaches, both located in Guanabara Bay, in the state of Rio de Janeiro, Brazil. Spectra were obtained from the muscle group and a specific part of the shell, with the specimens being separated by size (shell length < 50 mm or > 50 mm), following the current Brazilian legislation. The classification of the muscles by mussel size obtained a hit rate of 66.03%, while the hit rate of the shells was 78%. The two groups were also distinguished efficiently when the two localities were analyzed separately. The results reflect the influence of environmental factors on the chemical composition of the P. perna adults from different areas, with a 85.71% hit rate. The results indicate that NIRS is a potentially effective diagnostic tool for the monitoring and management of the commercial exploitation of natural P. perna stocks.
Worldwide, mussel farming produces approximately two million tons of this mollusk per annum (FAO, 2017). In Brazil, the farming of bivalves focuses primarily on mussels, oysters, and scallops. In 2019, the production of these mollusks reached 15,200 tons, which represents 1.34% of the country’s total aquaculture production (IBGE, 2020). The brown mussel, Perna perna (Linnaeus, 1758), is the most widely-cultivated mollusk species in Brazil (Ferreira and Magalhães, 2004). Mussels are sold in the shell for US$ 2.25–3.75 per kilo in Brazil, with prices varying among different regions (Lage and Jablonski, 2008; Alves et al., 2020).
The commercial harvesting of P. perna represents a significant subsistence resources and source of income, whether complementary or full-time, for many fishing communities in Brazil. Mussels are a nutritious food with high concentrations of proteins and minerals (Resgalla et al., 2008). Despite being found primarily in Africa, P. perna is relatively abundant on the coast of southern and southeastern Brazil (Marques, 1998; Resgalla et al., 2008).
The Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA, 2006) establishes a minimum total shell length of 50 mm for the commercial exploitation of natural stocks of P. perna. The growth of bivalves is normally assessed by shell length, rather than weight, which may be unreliable due to the presence of water inside the shell (Marques, 1998). Specimens of P. perna with a total shell length of 20–30 mm are classified as juveniles by IBAMA (2006), while individuals with a shell length of at least 50 mm are considered to be adults.
The unregulated harvesting of P. perna may compromise the long-term survival of natural stocks (Casarini and Henriques, 2011). In this context, IBAMA (2006) established an off season for the harvesting and sale of P. perna mussels from natural stocks (at any stage of the life cycle) in southern and southeastern Brazil between September 1st and December 31st. A number of Brazilian studies have evaluated the effects of the unregulated harvesting of mussels. Lage and Jablonski (2008) interviewed fishers from different locations within Guanabara Bay, in Rio de Janeiro, and found the widespread avoidance of the constant harvesting of mussels from a given area with the intention of preserving the natural stocks, as well as the rejection of individuals of small size, based on a visual assessment. Casarini and Henriques (2011) concluded that the difference between the available adult mussel stocks in Santos Bay, in the Brazilian state of São Paulo, and the harvest extracted by local fishers was due to the systematic exploitation of individuals with a shell length of less than 50 mm. This lack of any regulation of the size of the mussels harvested was also emphasized by Sodré et al. (2008), who identified this problem as a factor determining low population densities, and even local extinction on some rocky shores of the Brazilian state of Espírito Santo. Rech and Scherer (2020) also reported a decline in the P. perna stocks, in terms of both the size and abundance of the mussels, on the rocky coastline of Santa Catarina state.
Dishes prepared with mussels are common in coastal restaurants in Brazil, where they appeal to tourists, although seafood is not a common ingredient in everyday Brazilian cuisine (Ferreira and Magalhães, 2004; Sodré et al., 2008). In local restaurants, mussels are typically served cooked, without the shell (Lage and Jablonski, 2008; Sodré et al., 2008), which impedes the reliable identification of juveniles and adults. An additional problem is the lack of adequate sanitary controls and inspection of seafood destined for human consumption (Ferreira and Magalhães, 2004; Lage and Jablonski, 2008).
Due to the difficulty of identifying the origin and life stage (juvenile or adult) of a harvested mussel, the development of new methods will be needed to inspection in food quality and minimize the damage to the environment caused by the commercial exploitation of juvenile mussels and individuals harvested from contaminated areas. The consumption of contaminated foods can cause adverse effects on human health (Stankovic et al., 2012). Near Infrared Spectroscopy (NIRS) is one of the tools that has been used to estimate food quality parameters and energy storage and consumption in bivalves (Fluckiger et al., 2011; Bartlett et al., 2018), as well as monitoring marine toxins (Lopes et al., 2018), and differentiating mollusk species obtained from both the field and captive stocks (Valladares et al., 2021). The spectroscopic technology of NIRS evaluates molecular vibrations based on the combination of covalent bonds, such as -CH, -NH, -OH, and -SH, referring to the chemical composition of the material, being able to differentiate among apparently identical samples. The physical composition, for example shape and size, also influences the analysis, affecting the way that the energy is reflected from the sample (Siesler et al., 2008; Lopes et al., 2018; Williams et al., 2019). A NIRS spectrum thus provides by a computer both qualitative and quantitative data that can be used to compile the chemical phenotype of a sample. The application of NIRS requires no pre-treatment of samples, is rapid, and non-destructive (Siesler et al., 2008; Valladares et al., 2021). Aspects such as comprehensive sample selection and assembly, reproducible and accurate reference analysis, great spectral precision, and effective sample presentation to the equipment are fundamental to the successful application of this technique. The wavelengths of absorption bands can vary according to the principal constituents of the analyzed materials (Williams et al., 2019).
Mussels are an important economic and subsistence resource in Brazil and many other parts of the world, which means that the unregulated exploitation of natural stocks may impact both these stocks and the environments they inhabit. Given this, we evaluate the potential use of NIRS to differentiate juvenile and adult mussels obtained from two localities in the Brazilian state of Rio de Janeiro, based on their chemical phenotypes.
A total of 176 mussel specimens were collected from Guanabara Bay, in Rio de Janeiro state (Brazil) between 2019 and 2021 for analysis in the present study. These specimens included 92 samples obtained from Jurujuba Beach, in the municipality of Niterói (22°55’53” S, 43°06’35” W) and 84 from Vermelha Beach, in the municipality of Rio de Janeiro (22°57’15” S, 43°09’51” W). The two beaches are located on opposite sides of the entrance to Guanabara Bay, to the east (Jurujuba) and west (Vermelha). The mussels were collected from rocky shores, although some specimens were collected from a mussel farming in Jurujuba. The samples were packed in polystyrene boxes containing ice and seawater for transportation to the laboratory, where they were processed. In the laboratory, the mussels were weighed (in their shells) and the total shell length was recorded. As no standard classification has been published for mussels 30–50 mm in length, the specimens collected in this size range were classified as juveniles here for analysis. The valves were opened using a robust blade to remove the muscle group, which was weighed. The muscle group includes the anterior, median, and posterior byssal retractor muscles, the adductor muscle, and the retractor muscle of the foot (Ferreira and Magalhães, 2004). The sex and the immaturity of the specimens were determined through the macroscopic and microscopic examination of the mantle and gonads, following Lunetta (1969). Fragments of mussel tissues were collected and frozen for molecular analysis. All dissection tools were sanitized with 70% ethanol prior to the processing of a new individual.
To confirm the species of the mussels analyzed in the present study, genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s recommendations. The ITS1, 5.8S and ITS2 rDNA regions were amplified using the BD1 (5’-GTCGTAACAAGGTTTCCGTA-3’) and BD2 (5’-TATGCTTAARTTCAGCGGGT-3’) primers (Luton et al., 1992). The mitochondrial cytochrome oxidase c subunit 1 gene (COI) was amplified using the LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’) primers (Folmer et al., 1994). The Polymerase Chain Reactions (PCR) were run using the cycling parameters described by the respective authors. The PCR products were analyzed by electrophoresis in 1.5% agarose/Tris-borate Ethylenediamine tetraacetic acid (EDTA) gels stained with SybrGreen DNA gel stain (Invitrogen, Eugene, Oregon, USA) and photographed under ultraviolet transillumination. The PCR amplicons were purified using the ExoSap-IT (USB® Affymetrix Products Inc., Cleveland, Ohio, USA) and the DNA sequencing reactions were run using the BigDye Terminator v.3.1 kit (Applied Biosystems, Foster City, CA, USA). Automated sequencing was run in the sequencing platform at the Oswaldo Cruz Foundation (PDTIS/Fiocruz) in Rio de Janeiro. The resulting sequences were verified and edited using the MEGA software version X (Kumar et al., 2018), and compared to those available in the GenBank database using the BLAST program available on the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/BLAST) (Altschul et al., 1990).
An ABB Boomem MB 3600 near-infrared spectrophotometer with the Fourier transformation (NIR-FT) was used to collect the spectra of the fresh muscle and shell. The shells were washed to remove any possible residue and placed on absorbent paper at room temperature until they had dried out completely. The excess moisture in the muscle group was also removed using absorbent paper. The methods used here were adapted from Valladares et al. (2021), with some minor modifications. The samples were analyzed individually and in a random sequence. For analysis, the muscle group was placed in transparent 20-mL glass vials from Thermo Fisher Scientific, while the shell spectra were obtained from the area of the scar of the posterior adductor muscle of the right valve (Figures 1A, B). This area is the widest part of the shell, which fills the entire window of the spectrophotometer. The inner part of the shell was selected for this analysis to avoid potential interference from other organisms, such as algae or barnacles, which are often found attached to the external surface of mussel shells. A total of 50 spectra of the same area were obtained from each specimen, measured as absorbance, with a mean spectrum being calculated for each individual at a resolution of 16 cm-1. The spectra formed were due to the interaction of the electromagnetic wave with the sample within a wavelength range 750-2500 nm. A 20-mL Thermo Fisher glass vial containing spectralon was used as the spectroscopic reference for all the analyses.
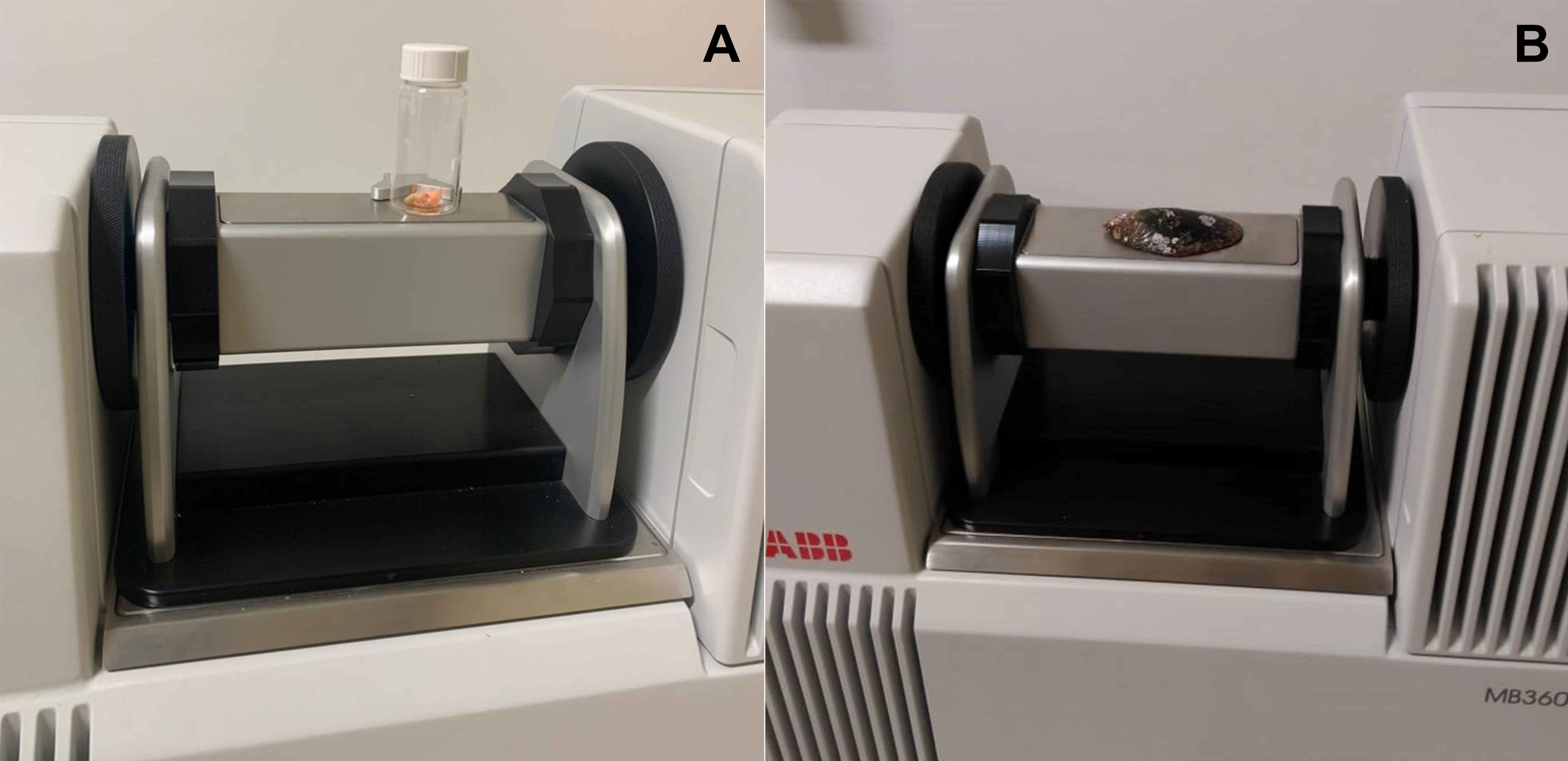
Figure 1 Acquisition of spectra by near infrared spectrophotometer from the Perna perna mussel. (A) Spectra obtained from the muscles. (B) Spectra obtained from the right valve.
The chemometric analyses were run in Unscrambler X, version 10.4, with the raw spectra being pre-processed using the Savitzky-Golay smoothing procedure (31-point window and 2nd order polynomial) and the 1st Savitzky-Golay derivative (15 point window and 2nd order polynomial) to minimize possible variation in noise. A Principal Component Analysis (PCA) was used to verify the presence of groupings in the samples, and a classification model was then built using a Linear Discriminant Analysis (LDA) to identify the most discriminative features, in which the samples were allocated to a specific class (Ferreira, 2015).
The statistical analyses were run in Graphpad Prism, version 9. The normality of the muscle weight data was first verified by the Shapiro-Wilk test, and as a non-normal distribution was identified, the data were analyzed using a Mann-Whitney test with a 5% significance level. The test was used to compare juvenile and adult mussels, and those from the two study beaches.
A lack of regulatory monitoring of the size of P. perna harvested from natural stocks has been recorded in a number of different Brazilian states (Sodré et al., 2008; Casarini and Henriques, 2011; Rech and Scherer, 2020). In order to avoid potential environmental impacts, which may compromise the long-term survival of natural stocks, it is necessary to implement stricter monitoring procedures and better enforce regulations on the commercial harvesting of mussels.
Most of the mussels analyzed had completely brown shells, although some shells had an underlying greenish tinge. The DNA sequences obtained from both study areas were consistent with those of P. perna and new sequences of the ITS1, 5.8S and ITS2, and COI regions were deposited in GenBank under accession numbers OM333735 and OM333930 (Jurujuba Beach), and OM333736 and OM333931 (Vermelha Beach). Despite the minor variation in shell color, the molecular analyses confirmed that all the mussels belonged to the same species, P. perna.
Seasonal factors, and the reproductive stage and sex of the individual may affect the biochemical makeup of a mussel (Resgalla et al., 2008). In Brazil, the reproduction of P. perna peaks in January, April, May, June, and September (Marques, 1998). The samples analyzed in the present study were thus collected both during one of these peaks (in January) and during periods of less intense breeding (February, March, July, and November). In November, the mussels were collected from a farm. Perna perna grows more slowly in natural environments than in aquaculture ponds, taking approximately 14 months to reach a shell length of 50 mm (Henriques et al., 2001). A number of different factors may influence growth times, including the amount and quality of available food, and the temperature of the water and its salinity. The biometric parameters (shell length, sex, and weight) of the mussels collected in the present study are shown in Table 1. Male and female specimens were collected in both areas, although the sex of some individuals could not be determined in all the groups, except for the juveniles from Vermelha Beach. The gonads of P. perna present a characteristic coloration, in particular during the breeding period, with a creamy white mantle in the males, which is orange in the females (Lunetta, 1969; Ferreira and Magalhães, 2004). The sex of individuals with a transparent mantle, which is probably associated with the emptying of the gonadal follicles (Lunetta, 1969), could not be determined. Overall, then, the sample encompassed individuals of different sexes and reproductive stages (Table 1).
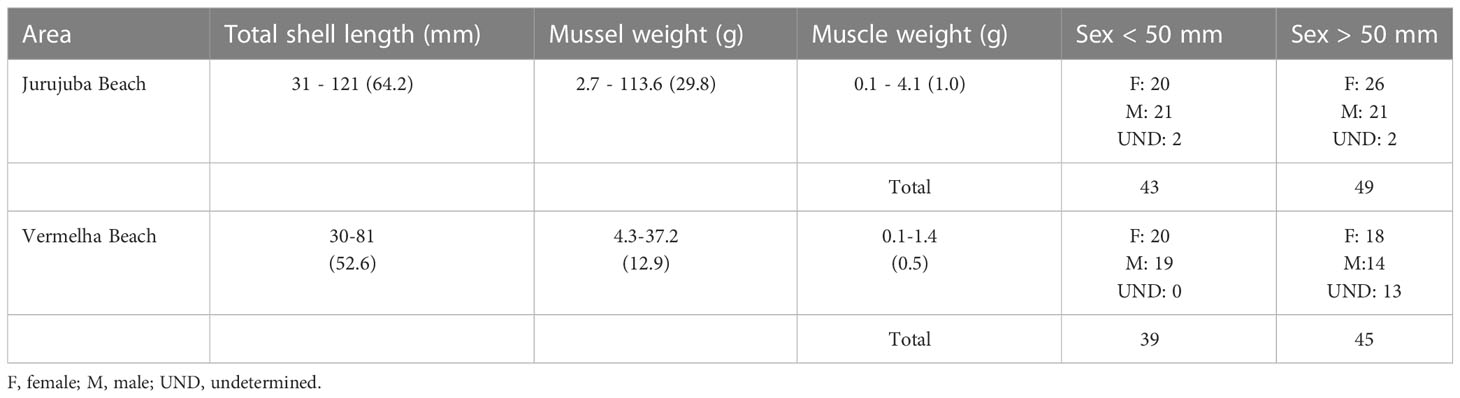
Table 1 Biometric data of the mussels analyzed by sampling area. Data are expressed as minimum-maximum (means).
In the PCA of the NIRS data on the muscle group of the specimens collected at the two study beaches as a function of mussel size, there was a clear separation (88%) of the larger (> 50 mm) and smaller (< 50 mm) specimens on the principal component 1 (PC-1) axis (Figure 2A). The percentage of hits obtained by LDA was 66.03%, with most of the errors were recorded in the small mussels (< 50 mm). The same analysis was performed between all shell samples from the two locations, which indicated a possible separation of the scores (18%) as a function of the principal component 2 (PC-2) axis (Figure 2B). In the case of the shells, the percentage of hits in the LDA classification was 78%. Despite the potential influence of the multiple variables mentioned above, these results demonstrate the feasibility of differentiating juvenile and adult P. perna by NIRS. It is interesting to note here that, while the shell records the environmental and metabolic conditions experienced by the organism over its lifetime, the composition of the soft tissue reflects its more recent period of life (Resgalla et al., 2008; Beierlein et al., 2015). This may account for the difference between the results obtained for the shell and muscle group, given that the shell is in more direct contact with the environment.
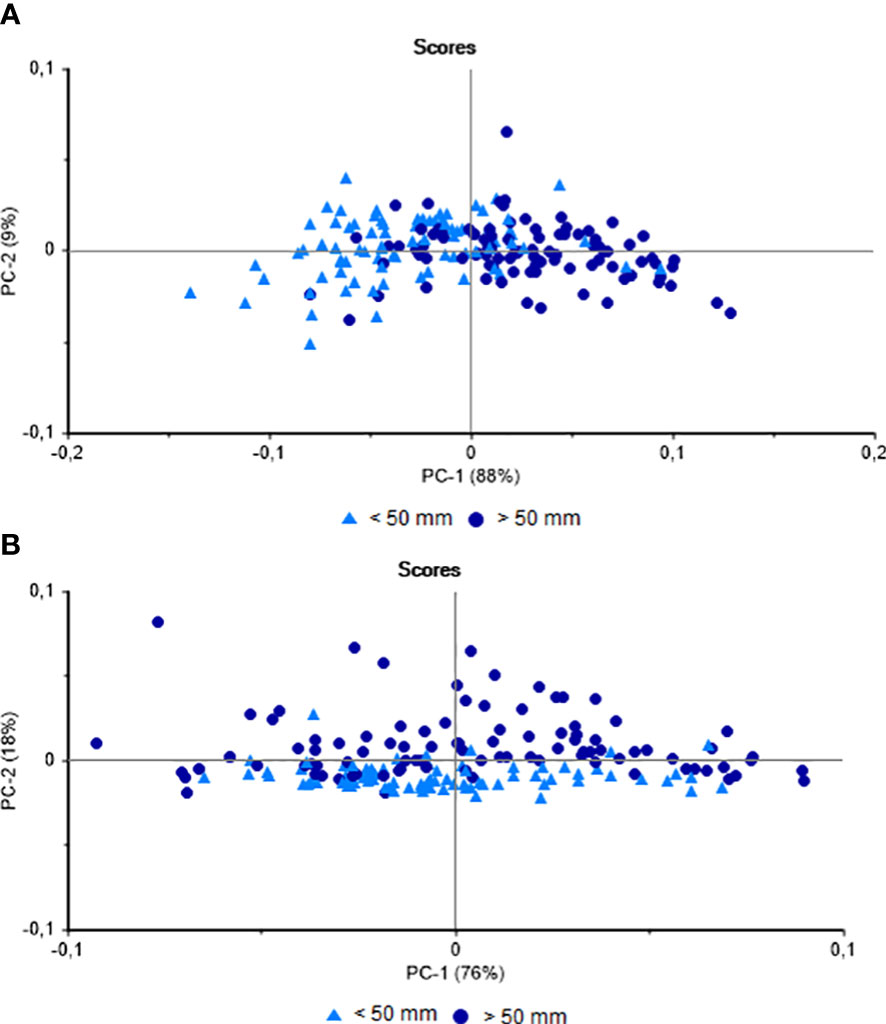
Figure 2 Principal Component Analysis of the Perna perna mussels collected from Jurujuba and Vermelha beaches. (A) NIR spectra obtained from the muscles. (B) NIR spectra obtained from the shells.
As the weight of the muscle did not vary significantly (p = 0.1199) between the two study beaches, the differences found by NIRS were likely metabolic. The highly significant differences (p < 0.0001) found between the muscle group of the juvenile and adult mussels, both overall and on each beach (when analyzed separately), points to a clear difference in the metabolism between the larger (total length > 50 mm) and smaller (< 50 mm) individuals. Xião et al. (2014) observed different effects on bivalve metabolism correlated with the body size and suggested that smaller individuals were more metabolically active than the large ones. A similar result was reported by Basova et al. (2012), that identified a decline in the energetically costly production of antioxidant enzymes as the bivalves increased in size. These authors associated this fact due to a reduction in free radical generation by the mitochondria. Pandey et al. (2014) and Smaal et al. (1997) detected differences in the biochemical and the physiological energetics, respectively, of bivalves of different size classes. Although the present study did not perform biochemical analyses in the mussels analyzed, the studies mentioned above demonstrated that there are differences in metabolism between specimens of different sizes.
The PCA of the muscle profiles from Vermelha Beach revealed a separation of the larger (> 50 mm) and smaller (< 50 mm) mussels, although many scores overlapped, whereas the profiles from Jurujuba Beach were separated more clearly. The first PCA axis encompassed 85% of the variation in the spectral data from Vermelha Beach and 90% from Jurujuba Beach (Figures 3A, B). The accuracy of the LDA for the classification of the mussels from Vermelha Beach was 68%, while it was 78.6% for Jurujuba Beach. Most of the errors in the classification of the muscle samples were, once again, from smaller individuals (< 50 mm). The size of shellfish at first sexual maturity can be influenced by the pressure of the constant extraction of immature individuals from the stock (Lasiak, 1991). Souza et al. (2019) identified geographic differences in the size at first maturity of P. perna and associated this variation with the different levels of anthropogenic pressure found at each site. Although the sexual maturity of the mussels was not assessed here, it is an important question, given that the lack of regulation of the size of the mussels harvested from natural stocks may influence the chemical phenotype of the smaller individuals (< 50 mm) by provoking precocial maturity. As this hypothesis cannot be confirmed in the present study, further research will be required, based on the application of techniques that are complementary to NIRS.
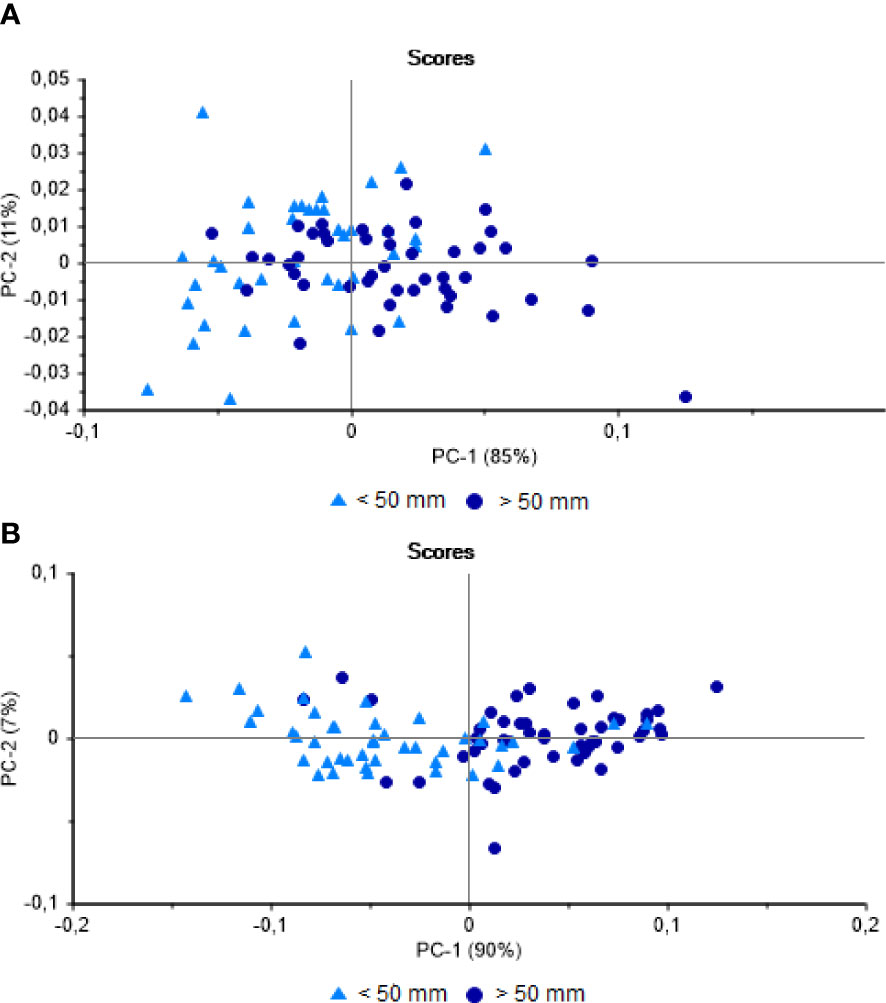
Figure 3 Principal Component Analysis of the Perna perna muscles. (A) NIR spectra obtained from mussels from Vermelha Beach. (B) NIR spectra obtained from mussels from Jurujuba Beach.
Bivalves are filter feeders that may accumulate contaminants present in their local environment and the exposure of these organisms to specific stressors may provoke shifts in biochemical, histological, physiological, and genetic parameters (Resgalla et al., 2008). Thus, the variable environmental conditions that mussels find during their growth affect in the chemical composition of these animals (Orban et al., 2002). When only adult (> 50 mm) mussels were analyzed, specimens from the two study beaches were well distinguished (85%) on the PC-1 axis (Figure 4), with a classification hit rate of 85.7%. These results indicate that the environment influences the metabolism of the mussels, given that all the individuals were adults, and that these data can be used as markers for the identification of the origin of the individuals.
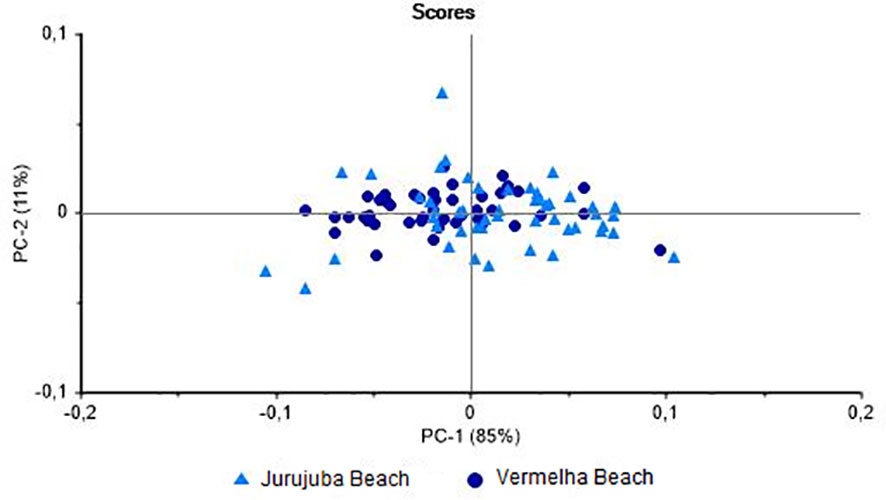
Figure 4 Principal Component Analysis of muscles of Perna perna specimens larger than 50 mm total shell length. Differentiation between specimens collected at Jurujuba and Vermelha beaches.
The results shown here are in line with previous studies. The NIRS successfully discriminated Mytilus galloprovincialis Lamarck, 1819 from six different European areas (Puleo et al., 2022). Fuentes et al. (2009) evidenced differences in the chemical composition of the M. galloprovincialis from three different Spanish origins. According to both studies, the differences found would have an influence on the quality of the molluscs, which can be a determining factor for the purchase by the consumers. In a NIRS analysis, Valladares et al. (2021) found that the field and laboratory environments had differential effects on the metabolism of freshwater snails of the same species. Using the same technique, Bartlett et al. (2018) demonstrated the effects of stress on marine bivalves through natural energetic changes and metal contamination. While Vermelha and Jurujuba beaches are located within the same bay, they are affected by distinct environmental impacts. Vermelha Beach is rocky and exposed directly to oceanic waves, and is open to the public, whereas Jurujuba Beach is more sheltered and calm. Oliveira et al. (2022) evaluated elemental contamination in P. perna from Jurujuba Beach, and concluded that the ingestion of these mussels by human consumers may have potential risks, especially for the local population, which may consume this shellfish relatively frequently, given that the concentrations of some chemical elements exceed the levels recommended for human consumption by international and Brazilian regulatory agencies. The potential for the identification of adult mussels harvested from different areas by NIRS is a valuable finding, given that it may contribute to the assessment of the quality of the environment and, in turn, of the animals themselves. In particular, the data presented here can be used to calibrate NIRS models for the analysis of P. perna samples from other areas of the coast of Rio de Janeiro.
When analyzing the mussels by locality, the results obtained for the shells were similar to those recorded for the muscle group. As only part of the shell was analyzed in the NIRS, the LDA classification of the misses by size returned a 62.5% hit rate for the specimens from Vermelha Beach and 92.3% for those from Jurujuba Beach. However, the principal advantage of applying NIRS to the muscle tissue, rather than the shell, is the fact that the mussels are often sold for consumption without the shell.
Near Infrared Spectroscopy proved to be a potentially rapid and valuable tool for the inspection of the size of P. perna harvested commercially from natural stocks. The analysis produced differentiated chemical phenotypes of mussels in the two shell length classes (< 50 mm and > 50 mm), which were also associated with the different environments inhabited by these individuals. These findings indicate the potential for the use of this technique for the identification of juvenile and adult specimens, which can be a valuable tool for the management of harvesting practises and natural stocks. The technique is effective for the analysis of the muscle group, without the need for the whole animal, in particular, the shell. This diagnostic technique thus represents a potentially valuable tool for the monitoring and inspection of the commercial exploitation of natural mussel stocks, and the enforcement of the fishery regulations that aim to guarantee the sustainability of these stocks.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OM333735, https://www.ncbi.nlm.nih.gov/genbank/, OM333930 https://www.ncbi.nlm.nih.gov/genbank/, OM333736, https://www.ncbi.nlm.nih.gov/genbank/, OM333931.
This study was authorized by the Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA, license nos. 68263–1, 68263–2, and 68263–3) and the National Genetic Heritage and Associated Traditional Knowledge Management System (SISGEN no. A20BC45) and was approved by the Animal Ethics Committee of the Oswaldo Cruz Foundation (CEUA, Fiocruz no. L-008/2018 and L-020/2018) in accordance with the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
AO, CS and CM-S led the conceptualisation and writing of the manuscript. AO collected samples and conducted experiments. AO and VV analysed data. CS and CM-S supervised. All authors contributed to the article and approved the submitted version.
The present study was supported financially by the Oswaldo Cruz Foundation (PAEF no. IOC-023-FIO-18-2-4). This study is part of the doctoral dissertation of the first author in the Graduate Program in Biodiversity and Health at the Oswaldo Cruz Foundation and the Brazilian Coordination for Higher Education Personnel Training (CAPES code 001), and. C. P. Santos was supported by a fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Alves J. L., Galvão M. S. N., Garcia C. F., Marques H. L. A. (2020). Productive performance of brown mussels Perna perna (Linnaeu) cultivated on ropes at low densities in caraguatatuba, Brazil. Aquac. Res. 51, 3297–3304. doi: 10.1111/are.14665
Bartlett J. K., Maher W. A., Purss M. B. J. (2018). Cellular energy allocation analysis of multiple marine bivalves using near infrared spectroscopy. Ecol. Indic. 90, 247–256. doi: 10.1016/j.ecolind.2018.03.007
Basova L., Begum S., Strahl J., Sukhotin A., Brey T., Philipp E., et al. (2012). Age-dependent patterns of antioxidants in Arctica islandica from six regionally separate populations with different lifespans. Aquat. Biol. 14, 141–152. doi: 10.3354/ab00387
Beierlein L., Nehrke G., Trofimova T., Brey T. (2015). “Bivalve shells–unique high-resolution archives of the environmental past,” in Towards an interdisciplinary approach in earth system science. Eds. Lohmann G., Meggers H., Unnithan V., Wolf-Gladrow D., Notholt J., Bracher A. (Switzerland: Springer), 173–182.
Casarini L. M., Henriques M. B. (2011). Estimativa de estoque do mexilhão Perna perna e da espécie invasora Isognomon bicolor em bancos naturais da baía de santos, são paulo, brasil. Bol. Inst. Pesca. 37, 1–11.
FAO (2017) Limited trade in bivalves. Available at: http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/522564/ (Accessed March 14, 2021).
Ferreira M. M. C. (2015). “Preparação dos dados para análise,” in Quimiometria: conceitos, métodos e aplicações. Ed. Ferreira M. M. C. (Campinas: Editora Unicamp), 29–107.
Ferreira J. F., Magalhães A. R. M. (2004). “Cultivo de mexilhões,” in Aquicultura: experiências brasileiras. Eds. Poli C. R., Poli A. T. B., Andreatta E., Beltrame E. A. (Florianópolis: Multitarefa), 221–250.
Fluckiger M., Brown M. R., Ward L. R., Moltschaniwskyj N. A. (2011). Predicting glycogen concentration in the foot muscle of abalone using near infrared reflectance spectroscopy (NIRS). Food Chem. 126, 1817–1820. doi: 10.1016/j.foodchem.2010.12.078
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. (1994). DNA Primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 5, 294–299.
Fuentes A., Fernández-Segovia I., Escriche I., Serra J. A. (2009). Comparison of physico-chemical parameters and composition of mussels (Mytilus galloprovincialis lmk.) from different Spanish origins. Food Chem. 112 (2), 295–302. doi: 10.1016/j.foodchem.2008.05.064
Henriques M. B., Marques H. L. A., Barrella W., Pereira O. M. (2001). Estimativa do tempo de recuperação de um banco natural do mexilhão Perna perna (Linnaeu) na baía de santos, estado de são paulo. HOLOS Environment. 2, 85–100. doi: 10.14295/holos.v1i2.1619
IBGE (2020) Pesquisa da pecuária municipal: tabela 3940 - produção da aquicultura, por tipo de produto. Available at: https://sidra.ibge.gov.br/tabela/3940 (Accessed March 10, 2021).
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 6, 1547–1549. doi: 10.1093/molbev/msy096
Lage H., Jablonski S. (2008). Mussel Perna perna extraction and commercialization in guanabara bay, Brazil. Atlântica. 30, 161–169.
Lasiak T. (1991). The susceptibility and/or resilience of rocky littoral molluscs to stock depletion by the indigenous coastal people of transkei, southern Africa. Biol. Conserv. 56, 245–264. doi: 10.1016/0006-3207(91)90060-M
Lopes M. B., Amorim A., Calado C., Costa P. R. (2018). Determination of cell abundances and paralytic shellfish toxins in cultures of the dinoflagellate Gymnodinium catenatum by Fourier transform near infrared spectroscopy. J. Mar. Sci. Eng. 6, 147. doi: 10.3390/jmse6040147
Lunetta J. E. (1969). Fisiologia da reprodução dos mexilhões (Mytilus perna - Mollusca lamellibranchia). Boletim Zoologia e Biol. Marinha. 26, 33–111. doi: 10.11606/bffcluspzoobm.v26i26.121175
Luton K., Walker D., Blair D. (1992). Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: trematoda: digenea). Mol. Biochem. Parasitol. 56, 323–328. doi: 10.1016/0166-6851(92)90181-I
Oliveira A. G. L., Rocha R. C. C., Saint’Pierre T. D., Hauser-Davis R. A., Mello-Silva C. C., Santos C. P. (2022). Elemental contamination in brown mussels (Perna perna) marketed in southeastern Brazil. Biol. Trace Elem. Res. 200, 402–412. doi: 10.1007/s12011-021-02644-y
Orban E., Di Lena G., Nevigato T., Casini I., Marzetti A., Caproni R. (2002). Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 77 (1), 57–65. doi: 10.1016/S0308-8146(01)00322-3
Pandey A., Singh A., Srivastava A., Datta S. N. (2014). Biochemical analysis of cultivated Lamellidens marginalis of different size group from pond environment in punjab. Ecoscan. 8, 127–130.
Puleo S., Monaco R. D., Langellotti A. L., Masi P. (2022). The origin of mussels (Mytilus galloprovincialis): NIRS explanatory identification and the effect on consumers. Food Chem.: X. 16, 100497. doi: 10.1016/j.fochx.2022.100497
Rech T. F., Scherer M. E. G. (2020). Entre a pedra e a onda: legislação e percepções da extração de Perna perna dos costões da ilha de Santa catarina. Rev. Costas. 2, 87–106. doi: 10.26359/costas.1102
Resgalla C. Jr., Weber L. I., Conceição M. B. (2008). O Mexilhão perna perna (L.): biologia, ecologia e aplicações (Rio de Janeiro: Interciência).
Siesler H. W., Ozaki Y., Kawata S., Heise H. M. (2008). Near-infrared spectroscopy: principles, instruments, applications (Weinheim: WILEY-VCH).
Smaal A. C., Vonck A. P. M. A., Bakker M. (1997). Seasonal variation in physiological energetics of Mytilus edulis and Cerastoderma edule of different size classes. J. Mar. Biol. Ass. U.K. 77 (3), 817–838. doi: 10.1017/S0025315400036213
Sodré F. N. G. A. S., Freitas R. R., Rezende V. L. F. M. (2008). Histórico e desenvolvimento da maricultura no estado do espírito Santo, brasil. Rev. Bras. Agroecologia. 3, 36–46.
Souza T. B., Silva B. R., Pereira R. M., Aride P. H. R., Oliveira A. T., Souza A. B., et al. (2019). Artificial selection and size at first sexual maturity of Perna perna mussels (Linnaeu) in southeastern Brazil. J. Shellfish Res. 38, 63–69. doi: 10.2983/035.038.0106
Stankovic S., Jovic M., Stankovic A. R., Katsikas L. (2012). “Heavy metals in seafood mussels. risks for human health,” in Environmental chemistry for a sustainable world - volume 1: nanotechnology and health risk. Eds. Lichtfouse E., Schwarzbauer J., Robert D. (Switzerland: Spring), 311–373.
Valladares V., Pasquini C., Thiengo S. C., Fernandez M. A., Mello-Silva C. C. (2021). Field application of NIR spectroscopy for the discrimination of the Biomphalaria species that are intermediate hosts of Schistosoma mansoni in Brazil. Front. Public Health 9. doi: 10.3389/fpubh.2021.636206
Williams P., Antoniszyn J., Manley M. (2019). Near infrared technology: getting the best out of light (South Africa: African Sun Media).
Keywords: mussel harvesting, size classes, NIRS, chemical phenotype, legal controls
Citation: de Oliveira AGL, Valladares V, Santos CP and Mello-Silva CC (2023) Near infrared spectroscopy: a method for the monitoring and management of the commercial exploitation of the brown mussel (Perna perna) in southeastern Brazil. Front. Mar. Sci. 10:1192024. doi: 10.3389/fmars.2023.1192024
Received: 22 March 2023; Accepted: 25 May 2023;
Published: 09 June 2023.
Edited by:
James Scott Maki, Marquette University, United StatesReviewed by:
Kwang-Sik Albert Choi, Jeju National University, Republic of KoreaCopyright © 2023 de Oliveira, Valladares, Santos and Mello-Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa Valladares, dmFuZXNzYS52YWxsYWRhcmVzY21AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.