- 1School of Marine Sciences, Ningbo University, Ningbo, China
- 2Ningbo Institute of Oceanography, Ningbo, Zhejiang, China
- 3College of Marine Science and Engineering, Qingdao Agricultural University, Qingdao, China
Providing shelters is considered an effective method to prevent self-harm and improve the survival rate of Scylla paramamosain. In this study, four shelter groups—the shelters of which included fine sand, arched tiles, PVC pipes, and trapezoidal net cages—and one group without shelters were established to investigate the effects of shelters on S. paramamosain by calculating their feeding rate, molting rate, survival rate, and growth index. The results showed that the feeding rates of the fine sand and PVC pipe groups were greater than 12% and that the feeding rates of the arched tile and trapezoidal net cage groups were greater than 9%, while the feeding rate of the group without shelters was only 6.7%. The food conversion rates of both the fine sand and PVC pipe groups exceeded 21%. In contrast, the food conversion rate of the non-shelter group was the lowest at 14%. The molting rates of the fine sand and PVC pipe groups were the highest, reaching 29% in the later stages of the experiment, while the molting rate of the non-shelter group was only 19%. There was no significant difference in survival rates among the groups during the first 20 days of the experiment. However, on the 60th day, the survival rate of the fine sand group was 92%, while the non-shelter group had a survival rate of 79%. The experimental results showed that the incidence of cheliped injuries in the fine sand group was 16%, while it was 25% in the non-shelter group. During the daytime, the occupancy rate of burrows by the four shelter groups was 60–70%, while during the night, the occupancy rate of each shelter decreased to 40–50%, and there was no significant difference in occupancy rates among the shelter groups during the night. The weight and full carapace width of S. paramamosain in the fine sand and PVC pipe groups were significantly higher than those in the other groups (P< 0.05), and the weight gain rate and specific growth rate of the fine sand and PVC pipe groups were significantly higher than those of the other groups (P< 0.05). Research has shown that fine sand and PVC pipes, as shelters for S. paramamosain, can effectively prevent cannibalism, increase feeding rates and survival rates, and promote growth and molting. This study provides scientific guidance for the proper selection of shelters in the farming process of S. paramamosain.
1 Introduction
Mud crabs (Scylla paramamosain) are spread throughout the tropical and warm temperate regions of the Pacific, providing support for lucrative offshore fisheries in many countries (Keenan, 1999; Le Vay, 2001). In southern China, S. paramamosain has been widely cultivated (Zhao et al., 2015) and has become one of the most popular mud crab species. However, the output of S. paramamosain is far from meeting the increasing market demand; the market price is continuously high, and many key scientific and technological problems facing mud crab aquaculture have not been effectively solved. Therefore, it is urgent to increase the output of S. paramamosain by improving cultivation technology.
Intraspecific agonistic behavior-induced killing is one of the main causes of death in crustacean aquaculture, especially in the early larval and adult stages, due to the very high stocking densities in aquaculture ponds (Ye et al., 2011). Intraspecific self-harm in crustaceans may also be caused by a number of other factors, including living substrates and shelters (Kurihara and Okamoto, 1987; Moksnes et al., 1998). A crucial behavioral strategy for crustaceans is the selection of a suitable shelter for feeding and enemy defense (Nelson and Vance, 1979). After long-term environmental adaptation and evolution, crustaceans have developed certain behavioral characteristics, including a nocturnal lifestyle and the use of shelters (Tidau and Briffa, 2016). Therefore, shelter is critical for the survival and life activities of S. paramamosain.
Previous studies have indicated that shelters can effectively prevent aquatic organisms from killing each other (Moksnes et al., 1997; Catacutan, 2002). As a critical feature of aquaculture ponds, shelters not only provide protection for aquaculture animals but also serve as biological bait, thereby playing an instrumental role in the survival and growth of aquaculture animals (Douglas, 1976; Sloan and Bodungen, 1980). There are several common types of crustacean shelters, including substrates (e.g., rocks, sand, and bricks), burrows (e.g., cracks, holes, pipes, and perforated bricks), macrophytes, and sheets (e.g., onion bags and fiber cement) (Moksnes et al., 1998; Sáez-Royuela et al., 2001; González et al., 2011). In wild aquatic environments, seaweeds, filamentous algae, and shells are used by crabs as natural shelters, and these shelters help prevent crabs from killing each other (Heck and Thoman, 1981; Perkins-Visser et al., 1996).
The survival rate of mud crabs is higher when fine sand is used as a refuge compared to when there is no refuge (Fatihah et al., 2017). Additionally, Mirera and Moksnes (2013) found that using fine sand as a refuge for crabs can reduce intraspecific aggression by 50%. Studies have also shown that lobsters prefer to dwell in fine sand (Cobb, 1971). Using tiles as shelters can increase the abundance of crab populations (Sheehan et al., 2008). Moreover, related studies have found that placing tiles can alter the behavior of purple shore crabs, resulting in their higher aggregation (McGaw, 2001). After placing PVC pipes as shelters, the molting rate of crabs increased fivefold, and the spawning rate of female crabs also doubled (Beck, 1995). Additionally, Supriyono et al. (2017) found that placing PVC pipes can increase the survival rate and yield of lobsters. Utilizing net cages for rearing post-larval Macrobrachium rosenbergii can enhance the survival rate of the larvae (Avillanosa et al., 2019). Rodriguez et al. (2007) also found that the survival rate of mud crabs reared in net cages is significantly higher than those in earthen ponds.
In this study, fine sand, arch-shaped tiles, PVC pipes, and trapezoidal net cages were selected as shelters for the indoor cultivation of S. paramamosain. The effects of each shelter on the survival, molting, feeding, and growth performance of S. paramamosain were compared. The research findings of this paper will provide practical information for aquaculture. Farmers can use the simple and practical techniques described in this paper to reduce the mortality rate of S. paramamosain and thereby increase their production.
2 Materials and methods
2.1 Animal collection and maintenance
S. paramamosain specimens were procured from Ningbo Huada Haichang Aquatic Science and Technology Co., Ltd. (N:29°21′, E:121°54′) on May 31, 2021. A total of 450 crabs were acquired, boasting an average weight of 20 grams each. Subsequently, these crabs were transported to the indoor cultivation ponds of Shandong Haihong Industrial Group Co., Ltd. (N:37°54′, E:118°89′). The facility was equipped with 15 experimental ponds and three reservoirs. Each pond measured 6 × 3 × 1.5 m3 in volume, accommodating 30 crabs per experimental pond.
2.2 Experimental design
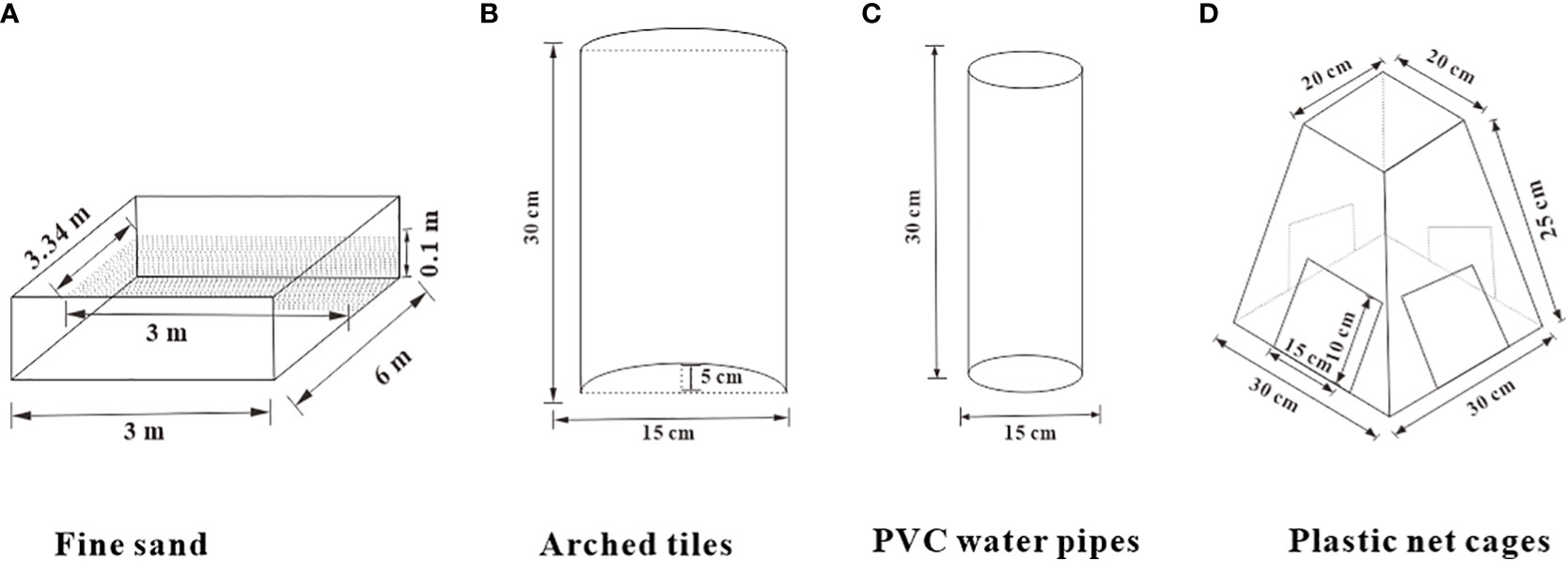
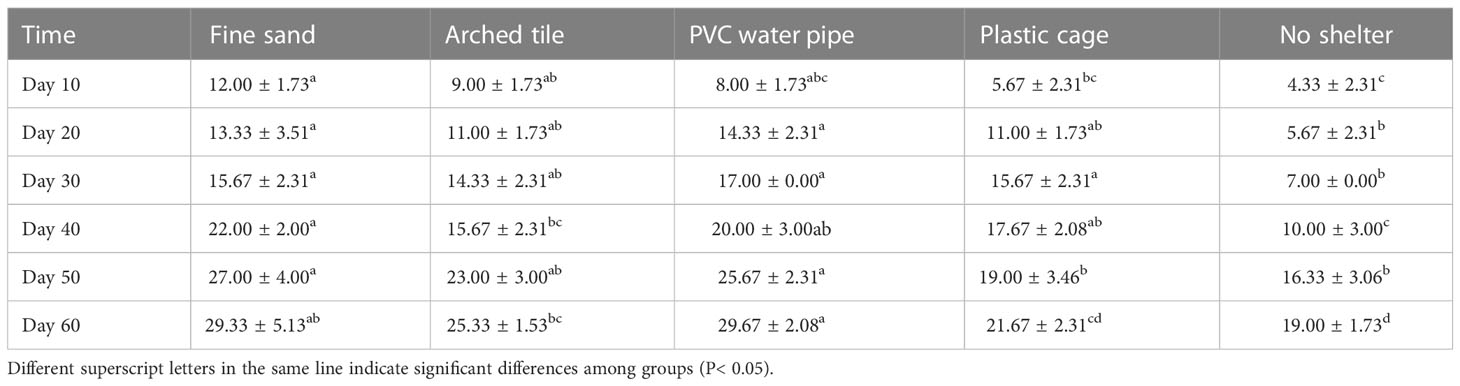
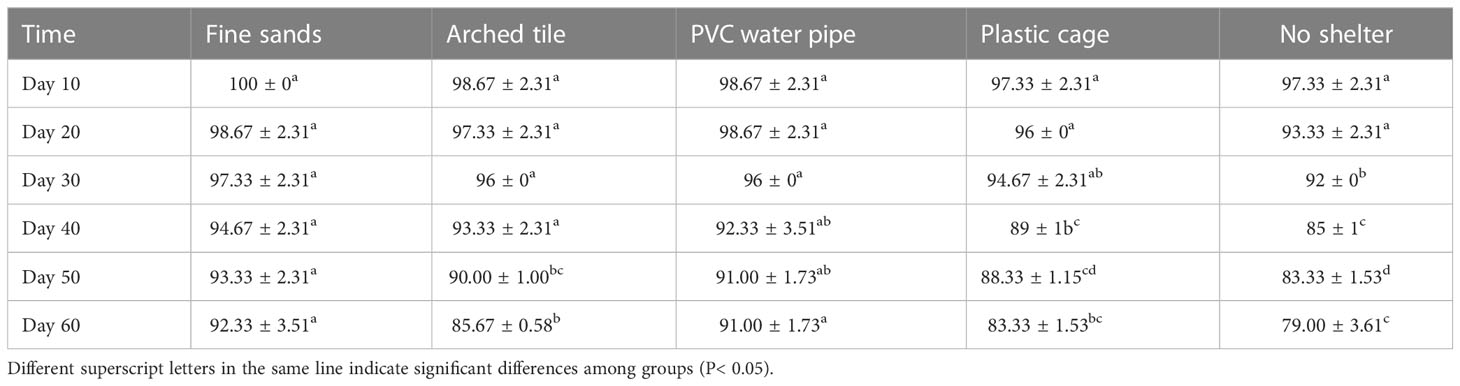
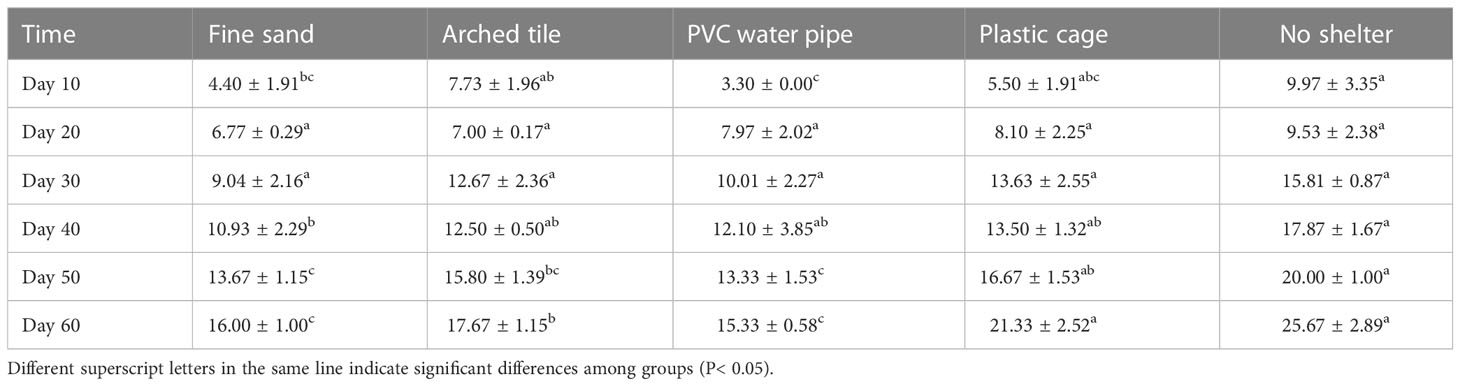
There were a total of five experimental groups: fine sand, arched tiles, PVC water pipes, plastic net cages, and a control group with no shelter. Each group had three parallel replicates, with each replicate being a single pond. The volume of fine sand particles was less than 0.5 mm, and a 10 m2 section of each pond was covered with fine sand to a depth of 10 cm. The remaining 8 m2 of each pond was designated for bait feeding and sewage discharge. The arched tiles (30 × 15 × 5 cm) were arranged in a parabolic shape, and 40 tiles were placed in each pond. PVC pipes (15 cm in diameter and 30 cm in length) were also used, with 40 pipes placed in each pond. The plastic mesh cage was trapezoidal in shape, with a wider bottom and a narrower top, and it had the following dimensions: a bottom length × width of 30 × 30 cm2, a top length × width of 20 × 20 cm2, and a height of 25 cm. The trapezoid had openings measuring 15 × 10 cm on all four sides (Figure 1). There were 40 mesh cages in each pond. The experiment lasted for 60 days, from June 1 to July 30, 2021. Every 10 days, the weight and growth indices of S. paramamosain were measured, and data on molting rate, survival rate, cheliped injury rate, shelter occupancy rate, and other indices were collected and analyzed.
2.3 Aquaculture management
The S. paramamosain in all ponds were provided with an artificial compound bait feed (manufactured by Tianbang Food Co., Ltd.) at 8:00 a.m. and 5:00 p.m. Each pond received an equal amount of feed (25 g) during each feeding session. Additionally, six oxygen-infused stones were placed in each pond to ensure a continuous oxygen supply. Water was replaced every three days, any remaining bait was promptly removed, and water quality was consistently monitored.
2.4 Index measurement
Before weighing, the moisture was gently wiped off the body surface with a dry towel. Then, each crab was weighed with an electronic balance (accurate to 0.01 g), and the growth index was measured with a vernier caliper. The following formulas were used to calculate daily food intake rate (FI), food conversion efficiency (FCE), molting rate (MR), survival rate (SR), cheliped injury rate (CIR), shelter occupancy rate (SO), specific growth rate (SGR), weight gain rate (WGR), and condition factor (CF) (Holland et al., 1971; Breteler, 1975; Fotheringham, 1976; Ennis, 1984; Snyder and Chang, 1986; Savolainen et al., 2004; Mazumder et al., 2016):
In the above equations, F represents daily food intake (g), FA signifies feeding amount (g), S denotes the dissolution rate of residual bait, R corresponds to the weight of surplus feed (g), Nq indicates the number of S. paramamosain molts, Nf represents the final count of S. paramamosain, and Ni indicates the initial number of S. paramamosain. Furthermore, CI represents the count of lost or damaged S. paramamosain chelipeds, TIN signifies the number of surviving S. paramamosain on the assessment day, SN denotes the number of S. paramamosain in the shelter, Wf corresponds to the final weight of S. paramamosain (g), Wi indicates the initial weight of S. paramamosain (g), T represents the duration of the culture period (days), W denotes the body mass of S. paramamosain (g), and L indicates the width of S. paramamosain (cm).
2.5 Data analysis
The experimental data were statistically analyzed using Excel 2010 and SPSS 22.0. All experimental data were expressed as mean values ± SD (mean values ± standard deviation). The normality of the data was tested using the Levene test in SPSS 22.0 software. One-way analysis of variance (ANOVA) was performed on all data using SPSS 22.0 software. After testing for the homogeneity of variances, the differences between means were assessed using independent samples t-test, with a P-value less than 0.05 considered statistically significant.
3 Results
3.1 Daily food intake rate and food conversion rate
A significant difference in food intake rate was observed between the shelter group and the non-shelter group (P< 0.05). Furthermore, there were significant differences among the shelter groups (P< 0.05; see Figure 2). In particular, the feeding rates exceeded 12% in the fine sand and PVC water pipe groups, while the arch tile and plastic mesh box groups exhibited feeding rates exceeding 9%. However, the non-shelter group had a feeding rate of only 6.7%. The food conversion rates of the fine sand and PVC water pipe groups were significantly higher than those of other groups (P< 0.05), with rates exceeding 21%. In contrast, the non-shelter group had the lowest food conversion rate, at only 14%.
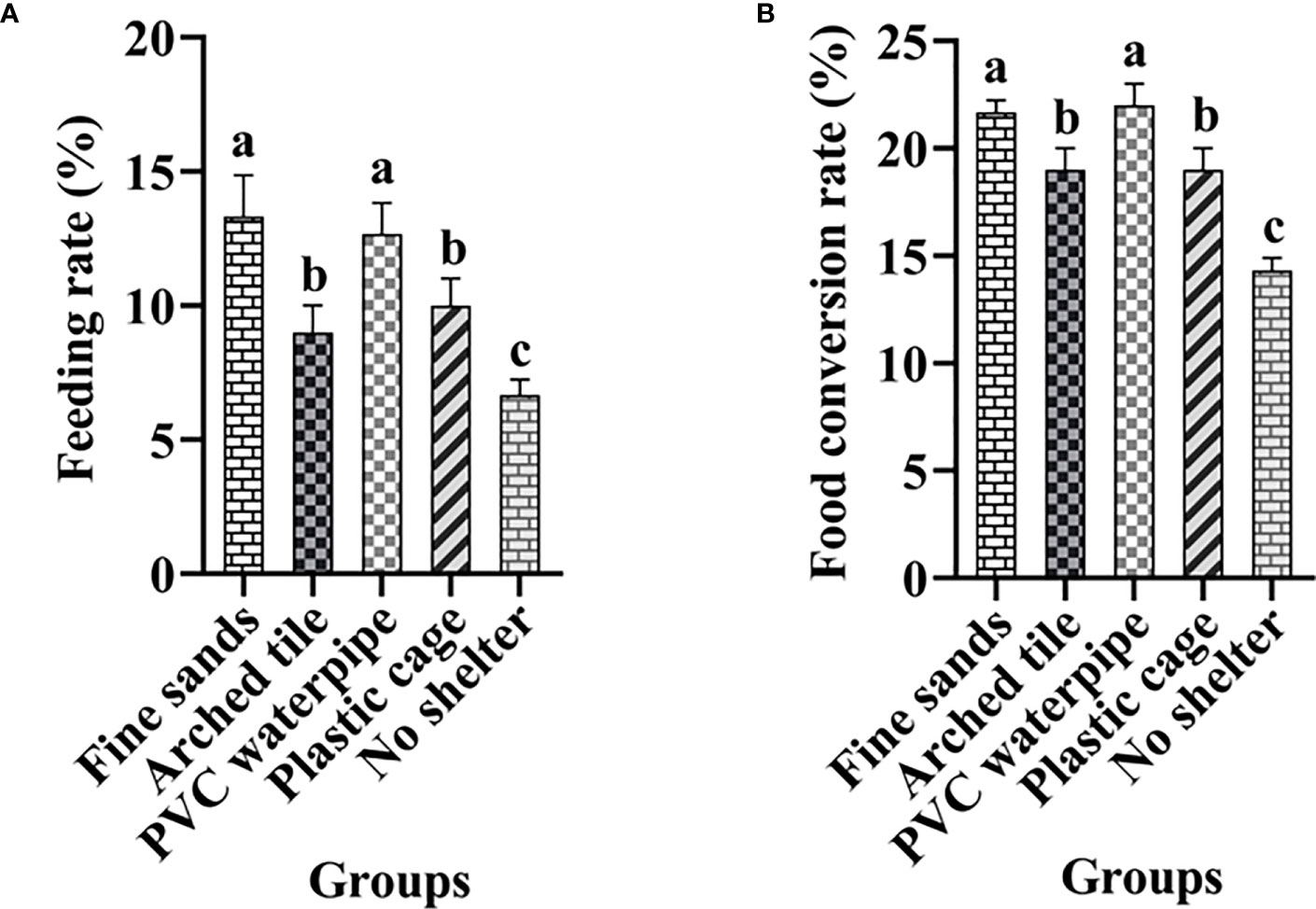
Figure 2 Analysis of the feeding rate (A) and the food conversion rate (B) of Scylla paramamosain under different shelter conditions (mean ± SD; different letters indicate significant differences among groups).
3.2 Molting
Throughout the experiment, we collected data every 10 days and observed a significant difference in molting rates between S. paramamosain with and without shelter (P< 0.05). Notably, the molting rates of S. paramamosain in the fine sand and PVC water pipe groups were significantly higher than those in the arch tile and plastic net cage groups (P< 0.05). At each sampling point, the fine sand group exhibited the highest molting rates, while the group without shelter had the lowest. Furthermore, we found that the longer the culture period, the greater the molting rate of S. paramamosain, as shown in Table 1.
3.3 Survival and cheliped injury rates
During the first 20 days of the trial (as shown in Table 2), we observed no statistically significant differences in survival rates among the groups. However, as the trial period extended, differences in survival rates among the groups became more pronounced. At the conclusion of the 60-day trial, we found that the S. paramamosain survival rate was highest in the fine sand group, followed by the PVC water pipe group. In contrast, the group without shelter exhibited the lowest survival rate.
Throughout the experiment, we observed an increase in the cheliped injury rate of S. paramamosain across all groups, as shown in Table 3. Specifically, between the 10th and 30th day of the trial period, we found no significant difference in cheliped injury rates among the groups. However, from day 30 to day 60, we observed the highest S. paramamosain cheliped injury rate in the non-shelter group, followed by the plastic cage group. In contrast, the fine sand and PVC water pipe groups exhibited the lowest cheliped injury rates.
3.4 Shelter occupancy
The behavior of S. paramamosain, specifically their movement in and out of shelters, was meticulously documented and examined during both daytime and nighttime throughout the trial duration. At the conclusion of the study, the pertinent data were gathered and analyzed. The findings revealed that S. paramamosain exhibited a shelter-occupancy rate of 60–70% during daylight hours, whereas the occupancy rate at night fell to 40–50%. Notably, significant differences in daytime occupancy rates were observed among various shelters (P< 0.05), while no such significant disparities were detected in nighttime occupancy rates (Figure 3).
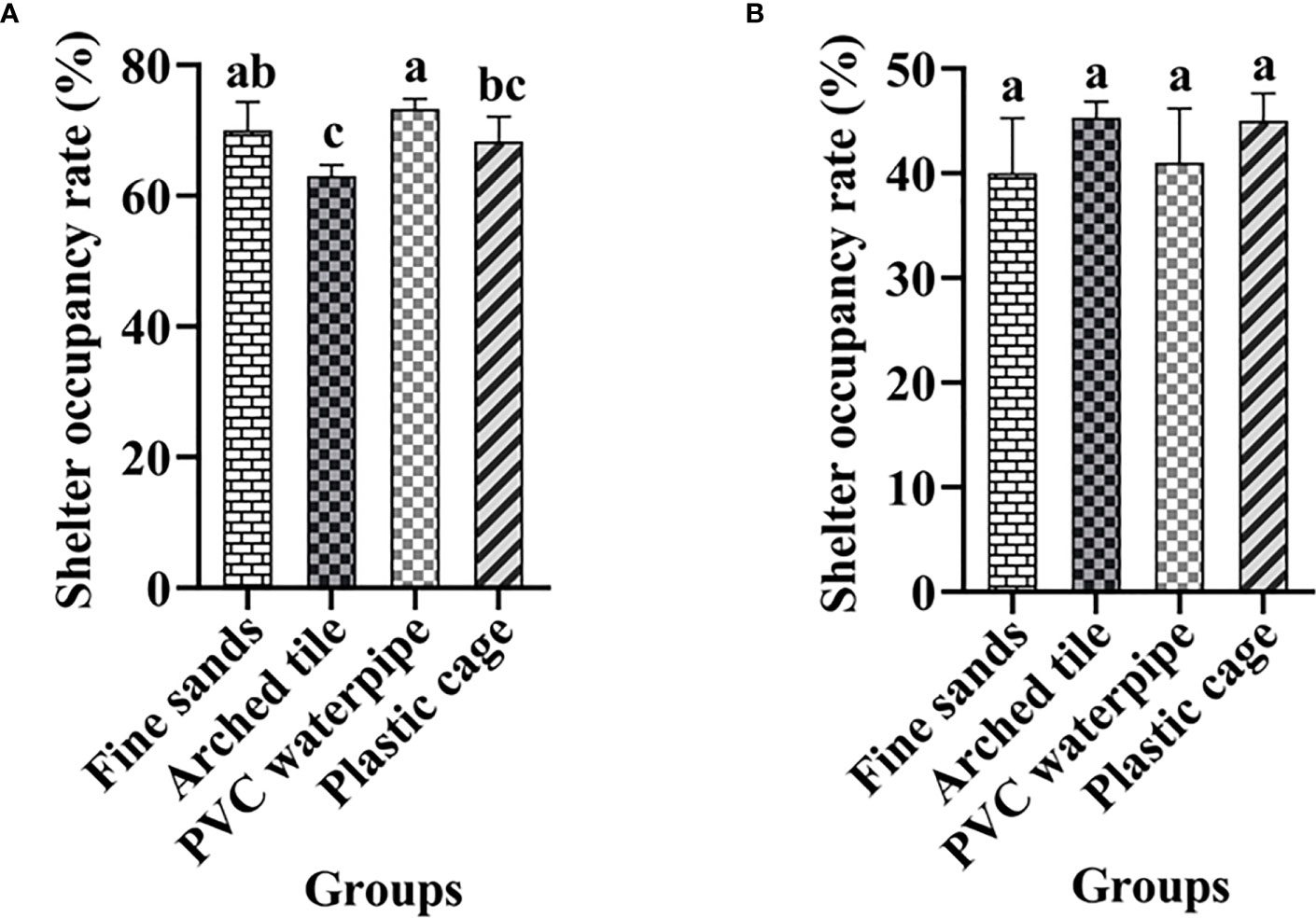
Figure 3 Artificial shelter occupancy (%) of S. paramamosain during the daytime (A) and nighttime (B) over 60 days (mean ± SD; Different letters indicate significant differences among groups).
3.5 Growth
After the experiment, we examined the growth indices of S. paramamosain. Our findings showed significant differences in weight between the shelter and non-shelter groups, as well as among different types of shelters (P< 0.05). Specifically, S. paramamosain weight was highest in the fine sand group (122 g) and the PVC water pipe group (120 g), while the group without shelter had the lowest weight (only 100 g). Additionally, we observed significant differences in full carapace width, carapace width, carapace length, and body height among the different groups (P< 0.05) (refer to Figure 4).
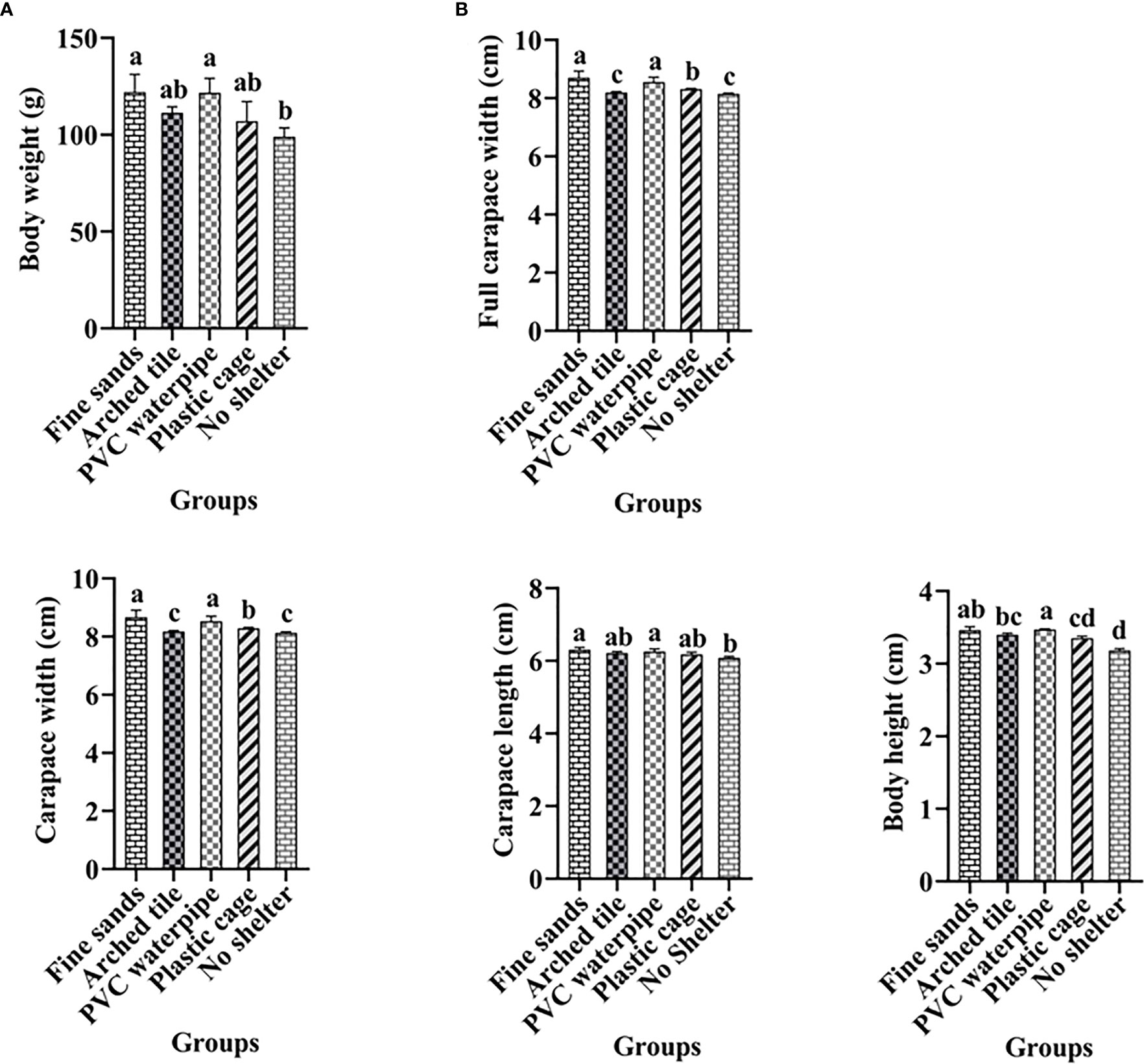
Figure 4 Analysis of growth indices of S. paramamosain under different shelter conditions after the experiment (mean ± SD; Different letters indicate significant differences between groups).
Following the 60-day trial period, substantial disparities in S. paramamosain weight gain and specific growth rate were observed between the shelter and non-shelter groups, as well as among various shelter types (P< 0.05). The weight gain rate and specific growth rate of S. paramamosain reached their peak in the PVC water pipe group, while the lowest rates for weight gain and specific growth were recorded in the non-shelter group. No significant differences were detected in the condition factor between the shelter and non-shelter groups or among the different shelter types (Table 4).
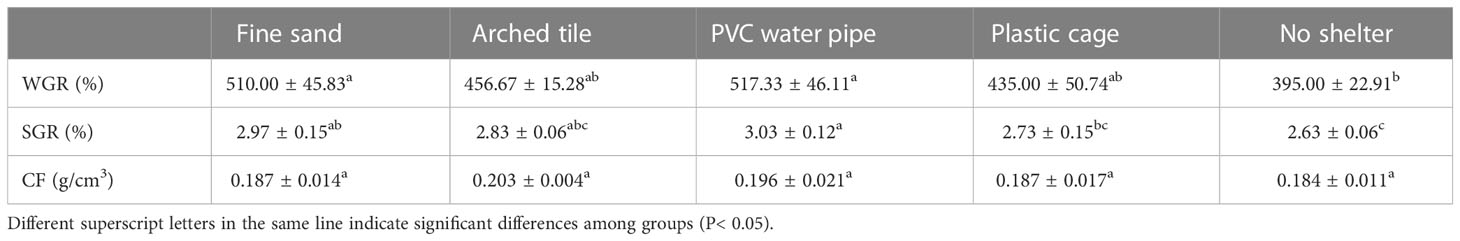
Table 4 Analysis of weight gain rate (WGR), specific growth rate (SGR), and fatness index (CF) of S. paramamosain under different shelter conditions.
4 Discussion
Owing to its delectable flavor, abundant nutritional content, and favorable economic impact, S. paramamosain has emerged as a crucial aquaculture species in China (Xu et al., 2020). Although the S. paramamosain industry has experienced rapid growth in recent years, it has faced numerous challenges that demand immediate attention, such as high self-injury rates and low survival rates during breeding (Ut et al., 2007). In this study, we aimed to tackle the high rate of self-harm during the S. paramamosain breeding process. To this end, we established four shelter groups and one group without shelter to ascertain if shelter availability mitigated self-harm rates. Prior research has indicated that shelters offer camouflage and refuge for S. paramamosain, consequently preventing self-harm and predation (Ye et al., 2011). In this study, we observed that S. paramamosain rapidly retreats to its shelter to feed upon capturing prey, thereby preventing intraspecific food competition. Moreover, the feeding rates of the shelter groups were significantly higher than that of the non-shelter group, indicating that shelters played a critical role in promoting S. paramamosain feeding. Therefore, shelters not only reduce conflict among S. paramamosain but also decrease the energy expenditure required for vital activities, ultimately increasing the energy available for molting and growth.
Shelter is among the most critical ecological factors affecting the living conditions of aquatic animals, as it offers a suitable habitat and an escape mechanism from natural enemies (Moksnes et al., 1998). Crustaceans, known for their territorial and aggressive behavior, frequently engage in combat (Dingle and Caldwell, 1969). Numerous studies have shown that cheliped injuries significantly affect the physiological ecology of crustaceans, impacting aspects such as food intake, survival, immunity, growth, reproduction, and resistance to enemy damage (Smith, 1992; Frisch and Hobbs, 2011; Quinitio and Estepa, 2011). During or immediately after molting, shrimps and crabs are soft and possess limited mobility, rendering them susceptible to intraspecific attacks (Polis, 1981). Consequently, shrimps and crabs tend to molt in relatively concealed locations (Moksnes et al., 1997). Our experimental data indeed revealed that S. paramamosain molt more swiftly in indoor pools with shelter compared to those without shelter, and that molting rates are higher in pools offering better concealment. For instance, as observed in this study, fine sand shelters enable the crab to bury its entire body. Fine sand grains are small yet non-sticky, providing high air permeability and a loose texture that facilitates easy hiding for the crab. This aligns with prior studies demonstrating that fine sand can serve as a shelter for crustaceans, and that fine-sand shelters enhance crustacean safety due to their potent concealment properties (Richards, 1992; Mirera and Moksnes, 2013). Thus, fine sand, as a shelter for S. paramamosain, is characterized by strong concealment, high air permeability, and elevated comfort. Numerous studies have examined the use of PVC water pipes as shelters for crustaceans and fish (Supriyono et al., 2017; Rahman et al., 2020), showing that PVC water pipes significantly improve crustacean molting rates, growth rates, and fecundity (Beck, 1995; Sáez-Royuela et al., 2001; Balasundaram et al., 2004). The PVC water pipes employed in this study were of suitable length and height for adult S. paramamosain crabs, offering excellent concealment and ample space for movement. S. paramamosain moved freely within the PVC water pipes and were relatively safe. Thus, the advantages of residing in a refuge include the following: first, the crab can hide under the refuge during molting, minimizing the risk of intraspecific attack; and second, as the crab typically restricts its activity to its own territory and adjacent areas for effective territorial defense, the frequency of intraspecific contact, and consequently intraspecific conflict, is reduced.
The survival rates and yields of S. paramamosain were enhanced by providing artificial shelters in aquaculture ponds. Our experimental data revealed that the survival rates of all four shelter groups exceeded 80%. In fact, the presence of shelters considerably reduced fighting and cannibalism among S. paramamosain compared to the group without shelters. However, among the four shelter groups, the survival rates of the fine sand group and the PVC water pipe group were significantly higher than those of the tile group and the plastic cage group. The chelate injury rate was also substantially lower in the fine sand and PVC water pipe groups, suggesting that these shelter types more effectively decreased the disability rate and improved S. paramamosain survival rates. Moreover, we observed that S. paramamosain autonomously entered the shelter, especially during daytime, and remained concealed under or within the shelter for extended periods. When confronted with an invading conspecific, S. paramamosain exhibited a threatening posture (elevating the body and extending the double cheliped) to deter and repel the intruder. Similar conspicuous territory-defense behaviors have also been observed in other aquatic animals (Tian et al., 2012; Guo et al., 2015).
Throughout the trial, the shelter occupancy rates of S. paramamosain were analyzed. We discovered that over 70% of S. paramamosain remained in the shelter during the day. However, only 40% of S. paramamosain entered the shelter at night. A similar behavioral pattern (i.e., hiding during the daytime and emerging at night) was also reported in Portunus trituberculatus (He et al., 2017). S. paramamosain occupancy rates during the daytime varied significantly among the shelter types. The fine sand and PVC water pipe groups exhibited the highest occupancy rates during the day, whereas the arch tiles demonstrated the lowest occupancy rates. During the nighttime, there were no significant differences in occupancy rates among the groups. This may be attributed to S. paramamosain typically emerging for feeding at night.
The growth index of each S. paramamosain group was assessed after the 60-day trial period. S. paramamosain with shelter exhibited significantly higher growth indices compared to those without shelter. However, there were also notable differences in growth index among the shelter groups: the fine sand group and the PVC water pipe group displayed significantly higher growth indices than the arch tile group and the plastic net cage group. The weight gain rate and specific growth rate of S. paramamosain were also highest in the fine sand group and the PVC water pipe group. Nevertheless, there were no significant differences in the condition factor index among the groups. Our results demonstrated that access to shelter stimulated S. paramamosain feeding, enabling the crabs to accumulate more energy. With sufficient energy, S. paramamosain will molt and grow (He et al., 2017). Consequently, the presence of suitable shelters benefits S. paramamosain feeding and molting, ultimately promoting growth.
5 Conclusion
This study elucidates the effects of different types of shelters on the survival, molting, feeding, and growth performance of S. paramamosain, revealing that fine sand and PVC pipes are more conducive to enhancing these parameters. Therefore, during the cultivation of S. paramamosain, placing fine sand or PVC pipes as shelters can be beneficial in reducing aggression and increasing survival rates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DZ: Methodology, Software, Data curation, Writing-Original draft preparation. LL: Conceptualization, Methodology, Writing-Reviewing and Editing, Funding acquisition. XH: Formal analysis, Visualization, Investigation. WF: Investigation, Validation. YF: Software, Validation. YL: Supervision. CW: Project administration, Supervision.
Funding
This work was supported by the National Key R & D Program of China (grant number 2019YFD0900405), the Zhejiang Provincial Natural Science Foundation of China (grant number LY20C190005) and the K.C. Wong Magna Fund of Ningbo University.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Avillanosa A. L., Ecube K. A., Española M. D., Caipang. C. M. A., Palla. H. P., Becira. J. G. (2019). Effects of stocking density and artificial shelters during the nursery production of giant freshwater prawn, Macrobrachium rosenbergii (De Man 1879) in net cages. Int. J. @ Aquat. Sci. 10, 76–82.
Balasundaram C., Jeyachitra P., Balamurugan P. (2004). Shelter preference in Macrobrachium spp. with reference to aquaculture. Acta ethologica. 7, 95–101. doi: 10.1007/s10211-004-0090-4
Beck M. W. (1995). Size-specific shelter limitation in stone crabs: a test of the demographic bottleneck hypothesis. Ecology 76, 968–980. doi: 10.2307/1939360
Breteler W. K. (1975). Food consumption, growth and energy metabolism of juvenile shore crabs, Carcinus maenas. Netherlands J. Sea Res. 9, 255–272. doi: 10.1016/0077-7579(75)90002-2
Catacutan M. R. (2002). Growth and body composition of juvenile mud crab, Scylla serrata, fed different dietary protein and lipid levels and protein to energy ratios. Aquaculture 208, 113–123. doi: 10.1016/S0044-8486(01)00709-8
Cobb J. S. (1971). The shelter-related behavior of the losbter, homarus americanus. Ecology 52, 108–115. doi: 10.2307/1934741
Dingle H., Caldwell R. L. (1969). The aggressive and territorial behaviour of the mantis shrimp Gonodactylus bredini Manning (Crustacea: Stomatopoda). Behaviour 33, 115–136. doi: 10.2307/4533261
Douglas C. A. (1976). Availability of drift materials and the covering response of the sea urchin Strongylocentrotus purpuratus (Stimpson). Pacific Sci. 30, 83–89.
Ennis G. P. (1984). Territorial behavior of the American lobster Homarus americanus. Trans. Am. Fisheries Society. 113, 330–335. doi: 10.1577/1548-8659
Fatihah S. N., Julin H. T., Chen C. A. (2017). Survival, growth, and molting frequency of mud crab Scylla tranquebarica juveniles at different shelter conditions. Aquaculture Aquarium Conserv. Legislation. 10, 1581–1589.
Fotheringham N. (1976). Effects of shell stress on the growth of hermit crabs. J. Exp. Mar. Biol. Ecology. 23, 299–305. doi: 10.1016/0022-0981(76)90027-7
Frisch A. J., Hobbs J. P. A. (2011). Effects of autotomy on long-term survival and growth of painted spiny lobster (Panulirus versicolor) on the Great Barrier Reef, Australia. Mar. Biol. 158, 1645–1652. doi: 10.1007/s00227-011-1678-7
González R., Celada J. D., García Carral V. J.M., González A., Sáez-Royuela M. (2011). Shelter and lighting in the intensive rearing of juvenile crayfish (Pacifastacus leniusculus, Astacidae) from the onset of exogenous feeding[J]. Aquacult. Res. 42, 450–456. doi: 10.1111/j.1365-2109.2010.02641.x
Guo H., Zhang X., Gao T. (2015). Effects of artificial shelters and feeding frequency on growth and behavior of juvenile Sebastes schlegelii. J. Fishery Sci. China. 22, 319–331. doi: 10.3724/SP.J.1118.2015.14227
He J., Gao Y., Xu W., Yu F., Su Z., Xuan F. (2017). Effects of different shelters on the molting, growth and culture performance of Portunus trituberculatus. Aquaculture 481, 133–139. doi: 10.1016/j.aquaculture.2017.08.027
Heck J. K.L., Thoman T. A. (1981). Experiments on predator-prey interactions in vegetated aquatic habitats. J. Exp. Mar. Biol. Ecology. 53, 125–134. doi: 10.1016/0022-0981(81)90014-9
Holland J. S., Aldrich D. V., Strawn K. (1971). Effects of temperature and salinity on growth, food conversion, survival and temperature resistance of juvenile blue crabs. Texas A&M University ProQuest Dissertations Publishing 1971, 7205674.
Keenan C. P. (1999). “Aquaculture of the mud crab, genus Scylla-past, present and future,” in Aciar Proceedings (Australian Centre for International Agricultural) 78, 9–13.
Kurihara Y., Okamoto K. (1987). Cannibalism in hemigrapsus. Mar. Ecol. Prog. Ser. 41, 123–127. doi: 10.3354/meps041123
Le Vay L. (2001). Ecology and management of mud crab Scylla spp. Asian Fisheries Sci. 14, 101–112. doi: 10.33997/j.afs.2001.14.2.001
Mazumder S. K., Das S. K., Bakar Y., Ghaffar M. A. (2016). Effects of temperature and diet on length-weight relationship and condition factor of the juvenile Malabar blood snapper (Lutjanus malabaricus Bloch & Schneider 1801). J. Zhejiang University. Science. B. 17, 580. doi: 10.1631/jzus.B1500251
McGaw I. J. (2001). Impacts of habitat complexity on physiology: purple shore crabs tolerate osmotic stress for shelter. Estuarine Coast. Shelf Science. 53, 865–876. doi: 10.1006/ecss.2001.0826
Mirera O. D., Moksnes P. O. (2013). Cannibalistic interactions of juvenile mud crabs Scylla serrata: the effect of shelter and crab size. African J. Mar. Sci. 35, 545–553. doi: 10.2989/1814232X.2013.865677
Moksnes P. O., Lipcius R. N., Pihl L., Van Montfrans J. (1997). Cannibal–prey dynamics in young juveniles and postlarvae of the blue crab. J. Exp. Mar. Biol. Ecology. 215, 157–187. doi: 10.1016/S0022-0981(97)00052-X
Moksnes P. O., Pihl L., van Montfrans J. (1998). Predation on postlarvae and juveniles of the shore crab Carcinus maenas: importance of shelter, size and cannibalism. Mar. Ecol. Prog. Series. 166, 211–225. doi: 10.3354/meps166211
Nelson B. V., Vance R. R. (1979). Diel foraging patterns of the sea urchin Centrostephanus coronatus as a predator avoidance strategy. Mar. Biol. 51, 251–258. doi: 10.1007/BF00386805
Perkins-Visser E., Wolcott T. G., Wolcott D. L. (1996). Nursery role of seagrass beds: enhanced growth of juvenile blue crabs (Callinectes sapidus Rathbun). J. Exp. Mar. Biol. Ecology. 198, 155–173. doi: 10.1016/0022-0981(96)00014-7
Polis G. A. (1981). The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Systematics. 12, 225–251. doi: 10.1146/annurev.es.12.110181.001301
Quinitio E. T., Estepa F. D. P. (2011). Survival and growth of mud crab, Scylla serrata, juveniles subjected to removal or trimming of chelipeds. Aquaculture 318, 229–234. doi: 10.1016/j.aquaculture.2011.05.034
Rahman M. M., Nur N., Mahmud-Al-Hasan M., Asaduzzaman S., Rouf M. A., Rahman S. M. (2020). Effects of light and artificial fish shelter (PVC pipe) on some phenotypic traits of stinging catfish (Heteropneustes fossilis Bloch 1794). Aquaculture Res. 51, 124–134. doi: 10.1111/are.14354
Richards R. A. (1992). Habitat selection and predator avoidance: ontogenetic shifts in habitat use by the Jonah crab Cancer borealis (Stimpson). J. Exp. Mar. Biol. Ecology. 156, 187–197. doi: 10.1016/0022-0981(92)90245-6
Rodriguez E. M., Parado-Estepa F. D., Quinitio E. T. (2007). Extension of nursery culture of Scylla serrata (Forsskål) juveniles in net cages and ponds. Aquaculture Res. 38, 1588–1592. doi: 10.1111/j.1365-2109.2007.01725.x
Sáez-Royuela M., Carral J. M., Celada J. D., Pérez J. R. (2001). Effects of shelter type and food supply frequency on survival and growth of stage-2 juvenile white-clawed crayfish (Austropotamobius pallipes Lereboullet) under laboratory conditions. Aquaculture Int. 9, 489–497. doi: 10.1023/A:1020509627870
Savolainen R., Ruohonen K., Railo E. (2004). Effect of stocking density on growth, survival and cheliped injuries of stage 2 juvenile signal crayfish Pasifastacus leniusculus Dana. Aquaculture 231, 237–248. doi: 10.1016/j.aquaculture.2003.09.045
Sheehan E. V., Thompson R. C., Coleman R. A., Attrill M. J. (2008). Positive feedback fishery: population consequences of ‘crab-tiling’on the green crab Carcinus maenas. J. Sea Res. 60, 303–309. doi: 10.1016/j.seares.2008.09.002
Sloan N. A., Von Bodungen B. (1980). Distribution and feeding of the sea cucumber Isostichopus badionotus in relation to shelter and sediment criteria of the Bermuda platform. Mar. Ecol. Prog. Series. 2, 257–264. doi: 10.3354/meps002257
Smith L. D. (1992). The impact of limb autotomy on mate competition in blue crabs Callinectes sapidus Rathbun. Oecologia 89, 494–501. doi: 10.1007/BF00317155
Snyder M. J., Chang E. S. (1986). Effects of eyestalk ablation on larval molting rates and morphological development of the American lobster, Homarus americanus. Biol. Bulletin. 170, 232–243. doi: 10.2307/1541805
Supriyono E., Prihardianto R. W., Nirmala K. (2017). The stress and growth responses of spiny lobster Panulirus homarus reared in recirculation system equipped by PVC shelter. Aquaculture Aquarium Conserv. Legislation. 10, 147–155.
Tian F., Tang Y. L., Tang M., Zhang P. P. (2012). The attractive effects of several artificial reefs on Pagrosomus major. Mar. Sci. 36, 85–89. doi: 10.1007/s11783-011-0280-z
Tidau S., Briffa M. (2016). “Review on behavioral impacts of aquatic noise on crustaceans,” in Fourth International Conference on the Effects of Noise on Aquatic Life, Vol. 27. 010028(Acoustical Society of America). doi: 10.1121/2.0000302
Ut V. N., Le Vay L., Nghia T. T., Hong Hanh T. T. (2007). Development of nursery culture techniques for the mud crab Scylla paramamosain (Estampador). Aquaculture Res. 38, 1563–1568. doi: 10.1111/j.1365-2109.2006.01608.x
Xu H., Han T., Li X., Wang J., Zheng P., Yin F., et al. (2020). Effects of dietary lipid levels on survival, growth performance, and antioxidant ability of the early juvenile Scylla paramamosain. Aquaculture 528, 735559. doi: 10.1016/j.aquaculture.2020.735559
Ye H., Tao Y., Wang G., Lin Q., Chen X., Li S. (2011). Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China. Aquaculture Int. 19, 313–321. doi: 10.1007/s10499-010-9399-3
Keywords: Scylla paramamosain, shelter, fine sand, arched tile, PVC pipe, plastic cage
Citation: Zhou D, Liu L, Huang X, Fang W, Fu Y, Li Y and Wang C (2023) Effects of different shelters on feeding, molting, survival, and growth of Scylla paramamosain. Front. Mar. Sci. 10:1191025. doi: 10.3389/fmars.2023.1191025
Received: 21 March 2023; Accepted: 13 July 2023;
Published: 02 August 2023.
Edited by:
Xiaotong Wang, Ludong University, ChinaReviewed by:
Xianliang Meng, Chinese Academy of Fishery Sciences (CAFS), ChinaLiang Junping, Henan Normal University, China
Kai Zhang, Nanjing Normal University, China
Copyright © 2023 Zhou, Liu, Huang, Fang, Fu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Liu, bGl1bGVpMUBuYnUuZWR1LmNu
 Dongping Zhou1
Dongping Zhou1 Lei Liu
Lei Liu