
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 03 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1183438
This article is part of the Research Topic Functional Feed Additives: Current Trends View all 8 articles
Chinese seabass (Lateolabrax maculatus) is a popular carnivorous fish with delicious taste. Although the feed value of condensed tannins has been well documented for L. maculatus, information about the effects of hydrolyzable tannins (HTs) on the growth and health of L. maculatus is limited. This study was conducted to assess the effects of dietary HTs on growth performance, body composition, intestinal digestive enzyme activities, serum metabolites, antioxidant and immune response, and intestine and liver morphology of L. maculatus. A total of 640 fish were randomly divided into four groups with four replicates per group and 40 fish per replicate. Four diets were prepared to contain 0 (G0), 1 (G1), 2 (G2), and 4 (G4) g/kg of HTs. Fish were fed to apparent satiation twice a day during the 56-day feeding trial. Results showed that the final body weight, weight gain rate, specific growth rate, and feed intake were linearly decreased (p< 0.001) as dietary HTs increased. All fish had similar (p > 0.05) whole body compositions. Fish fed G2 and G4 had lower (p< 0.05) intestinal trypsin and lipase activities than those fed G0 and G1, whereas G4 had higher (p< 0.05) aspartate aminotransferase and alanine aminotransferase activities than G0. Serum total antioxidant capacity and lysozyme were linearly decreased (p< 0.01), but the malondialdehyde concentration was linearly increased (p< 0.01) as dietary HTs increased. Intestinal villi in G2 and G4 showed increased deformation, and the vacuolation of liver cells began to appear in G1 and was aggravated as dietary HTs increased. This study showed that HTs should be used with caution due to their growth-inhibiting effect, and the dietary HT level for L. maculatus is recommended to be less than 1 g/kg.
Hydrolyzable tannins (HTs), a group of plant secondary metabolites widely existing in nature, are commonly composed of gallic acid (3,4,5-trihydroxybenzoic acid) with a polyol core (usually d-glucose) through ester bonds (Huang et al., 2018). It has been reported that HTs may have the potential as a promising alternative to in-feed antibiotics (Zhou et al., 2020). However, relevant research and application of HTs in aquatic animals are limited (Guo et al., 2019; Yao, 2020; Chen et al., 2021; Novriadi et al., 2021; Zhu et al., 2021).
The biological activity of tannins depends on their dietary concentration and animal species. For instance, Zhu et al. (2021) reported that 0.1%–0.15% of dietary HTs improved growth performance and intestinal histomorphology of pearl gentian grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). However, Yao (2020) indicated that supplementation of HTs exceeding 0.75% in grass carp (Ctenopharyngodon idellus) diets significantly increased the feed coefficient, induced oxidative stress, and caused structural damage to the intestine and liver. In contrast, Chen et al. (2021) suggested that the inclusion of chestnut tannins up to 0.9 g/kg in Litopenaeus vannamei diets did not affect growth performance and digestive enzyme activities.
Chinese seabass (Lateolabrax maculatus) is a popular carnivorous fish with delicious taste and high economic value. Our previous studies suggested that dietary condensed tannins less than 1 g/kg did not alter growth and digestion but increased antioxidant and immune capacities, improved intestinal health, and enhanced the anti-stress capacity of Lateolabrax japonicus (Peng et al., 2020a; Peng et al., 2020b; Peng et al., 2021a). However, the effects of HTs on the growth and health of L. maculatus have not been evaluated so far. This study was conducted to assess the effects of HTs on growth performance, whole body compositions, intestinal digestive enzyme activities, serum metabolites, antioxidant and immune response, and intestine and liver morphology of L. maculatus.
The ingredients and analyzed nutrient compositions of the experimental diets are shown in Table 1. Four iso-nitrogenic and iso-lipid diets were prepared to contain 0 (G0), 1 (G1), 2 (G2), and 4 (G4) g/kg of HTs (from Rhus chinensis, purity ≥ 96%) provided by Guangzhou Xingyu Biotechnology Co., Ltd. (Guangzhou, China). HTs were dissolved in distilled water prior to being added to the diet. All ingredients were ground by a pulverizer (Changzhou Qungan Drying Equipment Co., Ltd., Changzhou, China) to pass through a 60-mesh sieve, mixed thoroughly by a mixer (YHJ-50B, Valva Mechanical Equipment Co., Ltd., Guangzhou, China), extruded into 2-mm pellets by an F75 twin-screw extruder (South China University of Technology Machinery Factory), dried at 55°C, and stored at −20°C until use. Actual HT concentrations in the experimental diets of G0, G1, G2, and G4 were 0, 1.0, 2.1, and 3.9 g/kg, respectively, which were determined using the standard method of spectrophotometry (National Standard of China, 2011).
This study was conducted in an indoor recirculating aquaculture system located in the Aquatic Research Center of the Guangdong Academy of Agricultural Sciences. A total of 640 L. maculatus with an initial average body weight of 10.9 g were selected and randomly divided into four groups with four replicates per group and 40 fish per replicate. Fish were fed to apparent satiation (about 5% of body weight per day) twice (07:00 and 19:00) daily. During the 56-day feeding trial, the water temperature was maintained at 25.5°C–28.5°C, dissolved oxygen concentration >5 mg/L, pH 7.7 ± 0.3, and ammonia and nitrite concentration<0.01 mg/L. The protocol (no. GDAASSC 2021-020) of this study was ethically approved by the Animal Care and Use Committee of the Guangdong Academy of Agricultural Sciences (Guangdong, China).
At the end of the feeding trial, all fish were fasted for 24 h and were anesthetized with 40 mg/L of MS-222 (Sigma, USA) (Peng et al., 2022a) before sampling. The weight and number of fish in each tank were recorded to calculate the final body weight, weight gain rate, survival rate, specific growth rate, and feed coefficient. Feed intake was calculated as the gravimetric difference between offered and residual diets. Uneaten feeds were collected from the tank by siphoning and dried for the calculation of feed intake.
Six fish per tank were randomly selected to measure the body weight and body length. The livers and intestines of fish were separated and weighed to calculate the condition factor, viscerosomatic index, hepatosomatic index, and intestinesomatic index.
Three fish per tank were collected to determine the whole body compositions of dry matter, crude protein, crude lipid, and ash.
The intestines from three randomly selected fish per tank were obtained and stored at −80°C for subsequent determination of digestive enzyme activities.
Six fish per tank were randomly selected to collect blood from the tail vein of the fish. Blood samples were kept at room temperature for 4 h and centrifuged at 3,500 r/min for 10 min. The resultant serum was stored at −20°C for analyses of serum metabolites and antioxidant and immune parameters.
The intestines and livers of three selected fish per tank were sampled, washed with 4°C normal saline, and fixed in the 4% paraformaldehyde solution. Samples were sectioned into 5-μm slices, stained with hematoxylin and eosin using standard histological techniques, and examined under light microscopy equipped with images analysis software (HALO v2.3).
After sampling, all of the remaining fish were euthanized under approved animal care and use protocol.
Nutrient compositions of the diets and fish, such as dry matter, crude protein, crude lipid, and ash, were determined using the Association of Official Analytical Chemists (AOAC, 1999) methods. Serum metabolites were measured using an automatic biochemical analyzer (Hitachi 7180, Tokyo, Japan).
Commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to analyze the trypsin (no. A080-2-2), lipase (no. A054-1-1), amylase (no. C016-1-1), total antioxidant capacity (no. A015-1), catalase (no. A007-1-1), superoxide dismutase (no. A001-1-2), glutathione peroxidase (no. A005-1-2), malondialdehyde (no. A003-1-2), alkaline phosphatase (no. A059-2-2), immunoglobulin M (E025-1-1), and lysozyme (no. A050-1-1), following the corresponding manufacturer’s instructions.
Growth performance parameters were calculated according to the formulas described by Peng et al. (2020a). The calculation formulas are as follows:
Data were analyzed by one-way ANOVA using SPSS 17.0 with tank as the statistical unit. Duncan’s method was used for multiple comparisons when the data met the homogeneity of variance, whereas Dunnett’s T3 test method was utilized for multiple comparisons when the homogeneity of variance was not satisfied. A polynomial comparison was used to analyze the linear and quadratic responses to the HT concentrations. Significant was declared at p< 0.05, and the significant trend was set at 0.05< p< 0.10.
All fish had similar (p > 0.05) survival rates, initial body weight, feed coefficient, condition factor, and viscerosomatic index (Table 2). The final body weight, weight gain rate, specific growth rate, feed intake, and intestinesomatic index were linearly decreased (p< 0.05), but the hepatosomatic index was linearly increased (p = 0.046) as dietary HT concentrations increased. Compared with G0 and G1, G2 and G4 had lower (p< 0.05) final body weight, weight gain rate, specific growth rate, feed intake, and intestinesomatic index but higher (p< 0.05) hepatosomatic index. All fish had similar (p > 0.05) whole body compositions of dry matter, crude protein, crude lipid, and ash (Table 3).
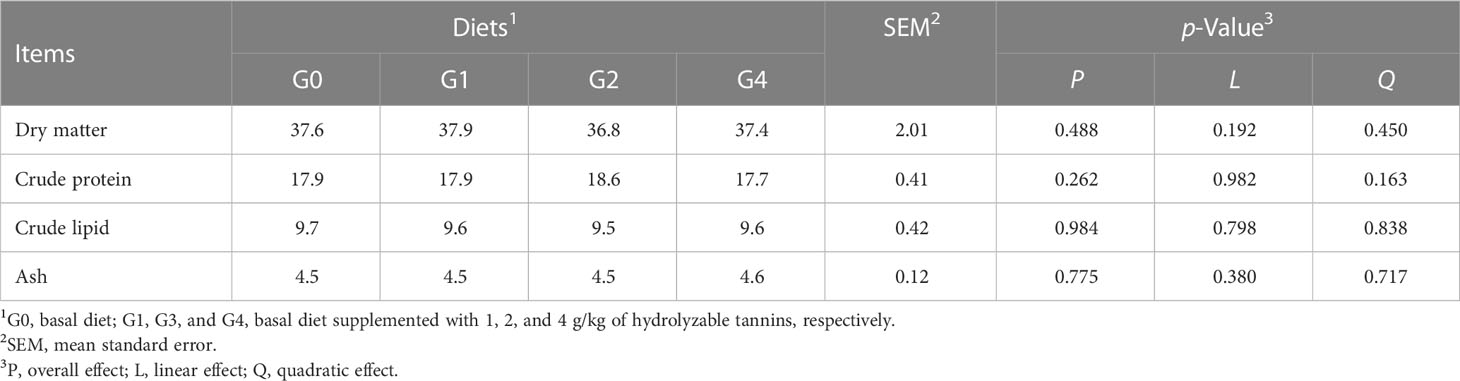
Table 3 Effect of dietary hydrolyzable tannins on the whole body composition (g/100 g body weight) of Lateolabrax maculatus.
Fish in G2 and G4 had lower (p< 0.05) intestinal trypsin and lipase activities than those of fish in G0 and G1 (Table 4). All fish had similar (p > 0.05) intestinal amylase activity.
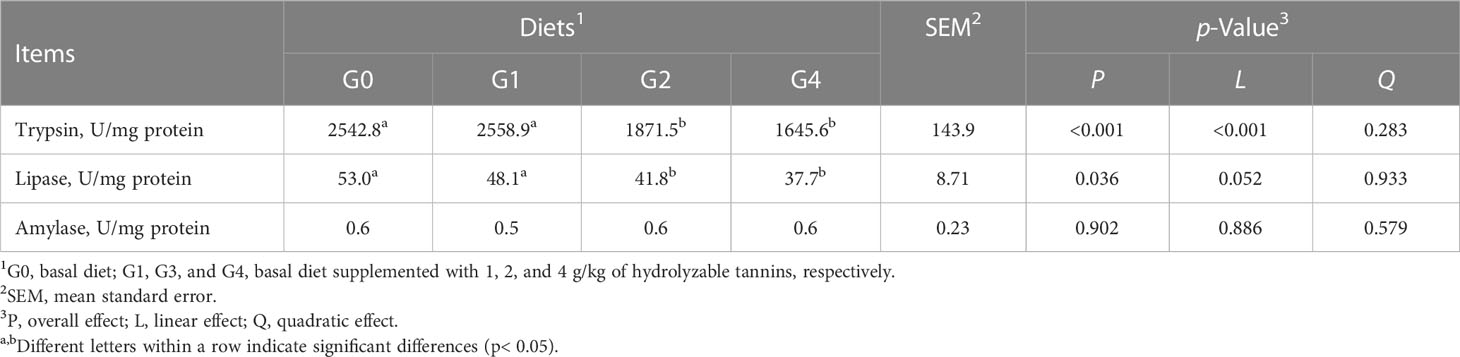
Table 4 Effect of dietary hydrolyzable tannins on the intestinal digestive enzyme activities of Lateolabrax maculatus.
Serum aspartate aminotransferase and alanine aminotransferase activities were increased (p< 0.05) as dietary HT concentrations increased from 0 to 4 g/kg and reached significance at the level of 4 g/kg (G4) (Table 5). Compared with G0 and G1, G2 and G4 had higher (p< 0.05) high-density lipoprotein cholesterol concentrations. All fish had similar (p > 0.05) albumin, globulin, total cholesterol, triacylglycerol, blood urea nitrogen, glucose, and low-density lipoprotein cholesterol among groups.
The total antioxidant capacity was linearly increased (p< 0.001), but malondialdehyde content was linearly decreased (p = 0.008) as dietary HT concentrations increased from 0 to 4 g/kg and reached significance at the levels of 2 and 4 g/kg (G2 and G4) (Table 6). The G4 had higher (p< 0.05) lysozyme activity than G0. All fish had similar (p > 0.05) catalase, superoxide dismutase, glutathione peroxidase, alkaline phosphatase activities, and immune globulin M content irrespective of the treatments.
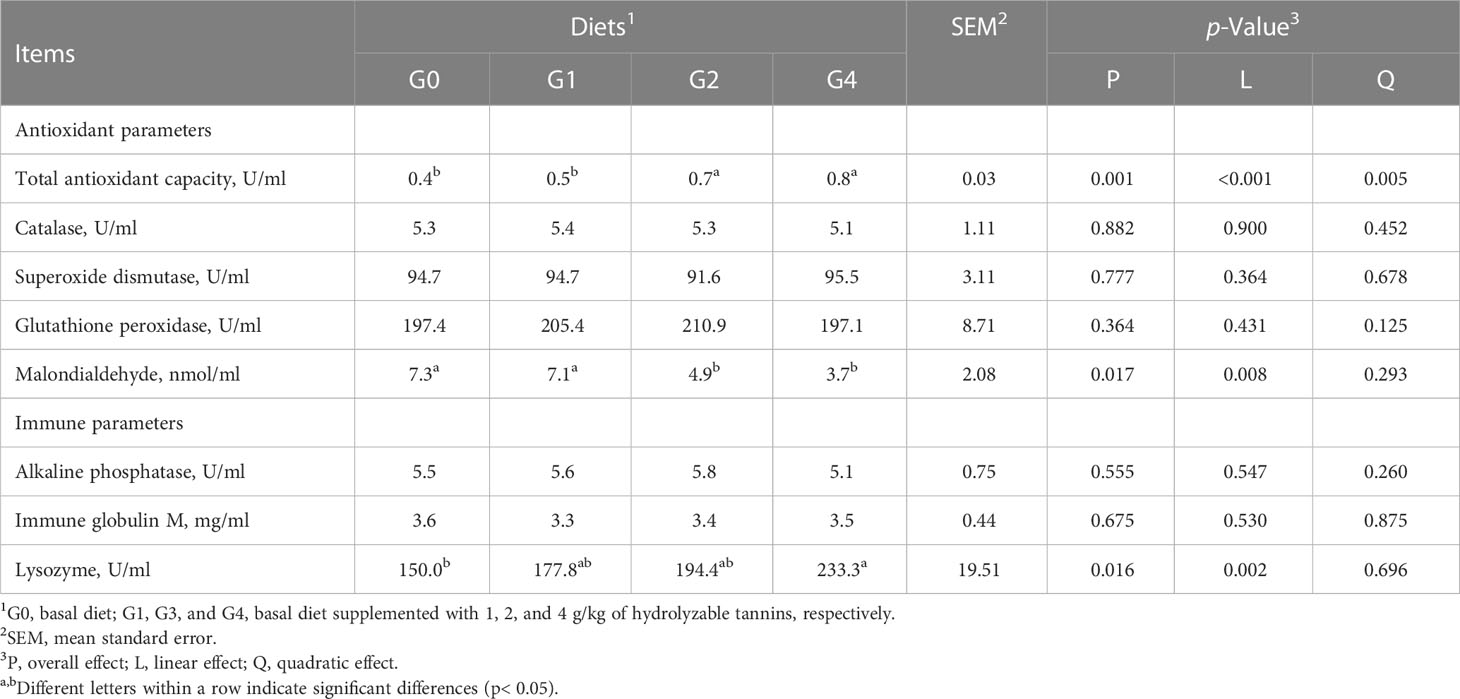
Table 6 Effect of dietary hydrolyzable tannins on the serum antioxidant and immune response of Lateolabrax maculatus.
The intestinal villi in G0 and G1 were observed to be arranged orderly with no obvious deformation and degeneration (Figure 1), whereas the intestinal villi in G2 and G4 were shown to have different degrees of deformation as reflected by the atrophic villus. Liver cells in G0 and G1 were evenly distributed with obvious nuclei (Figure 2), but the vacuolation of liver cells appeared in G1 and was aggravated in G4.

Figure 1 Effect of dietary hydrolyzable tannins on the intestinal histological appearance (×100) of Lateolabrax maculatus. G0, basal diet; G1, G3, and G4, basal diet supplemented with 1, 2, and 4 g/kg of hydrolyzable tannins, respectively.
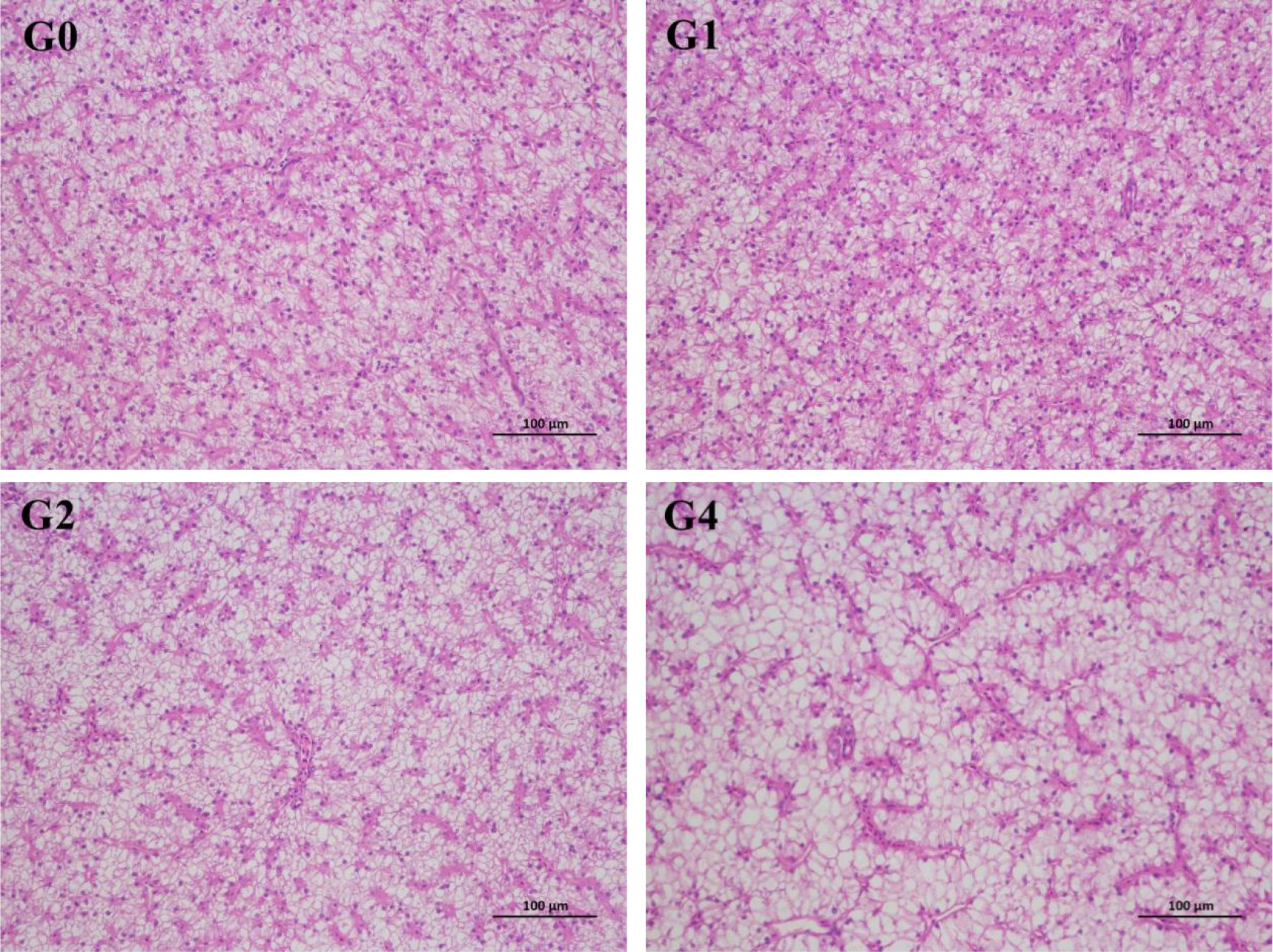
Figure 2 Effect of dietary hydrolyzable tannins on the liver histological appearance (×200) of Lateolabrax maculatus. G0, basal diet; G1, G3, and G4, basal diet supplemented with 1, 2, and 4 g/kg of hydrolyzable tannins, respectively.
Currently, the effects of dietary HTs on the growth performance of aquatic animals have been evaluated in a few species, e.g., L. vannamei (Guo et al., 2019; Novriadi et al., 2021), pearl gentian grouper (Zhu et al., 2021), obscure puffer (Takifugu fasciatus) and C. idellus (Yao, 2020), and Oreochromis niloticus (Buyukcapar et al., 2011). However, limited information is available about the effects of HTs on the growth performance of L. maculatus. This study showed that dietary HTs exceeding 2 g/kg had negative effects on the growth of L. maculatus. This was consistent with the observation by Yao (2020) in that 0.75% of HTs did not alter the growth performance of C. idellus but that 1.75% of HTs significantly increased the feed coefficient of fish. Yao (2020) also documented that T. fasciatus could tolerate 0.75% dietary HTs without influencing the growth performance of fish, but 1.25% of HTs significantly impaired digestion and metabolism of protein. Similarly, Buyukcapar et al. (2011) reported that supplementation of 5 g/kg of HTs in the O. niloticus diet did not alter growth performance, but 15 and 25 g/kg of HTs had an adverse effect on the growth performance of fish. In this study, the growth-inhibiting effect of HTs on L. maculatus is likely attributable to the poor palatability and depressed digestion induced by dietary HTs. Tannins were regarded as anti-nutritional factors owing to their astringent taste and digestive inhibition effect due to their capacity to bind and precipitate proteins (Peng et al., 2018). Thus, the effects of HTs on the growth of fish may partly depend on their dietary concentrations. However, it is interesting to find that the same dietary concentration of HTs had different biological activities on the growth performance of aquatic animals. For instance, this study showed that 2 g/kg of HTs reduced the growth of L. maculatus, whereas Zhu et al. (2021) indicated that 2 g/kg of HTs did not alter the growth of pearl gentian grouper. On the contrary, Guo et al. (2019) reported that 2 g/kg of HTs increased the growth rate of L. vannamei. This information indicates that the effects of HTs on the growth performance of aquatic animals depended on both dietary HT concentrations and aquatic animal species.
Intestinal digestive enzyme activity is an important index reflecting the physiological digestive function of animals. Activities of the digestive enzymes directly affect the digestibility of nutrients and therefore influence the growth of aquatic animals. This study showed that a high dose of HTs inhibited the digestion of protein and lipids. Tannins are known to bind or interfere with protein and digestive enzymes, forming tannin–protein complexes and thereby reducing protein digestibility and digestive enzyme activities (Yao et al., 2019). It has also been reported that tannins bind to digestive enzymes by altering their spatial structure and inhibiting their activity (Yao, 2020). Al-Mamary et al. (2001) observed that 0.79% of dietary tannin significantly reduced intestinal trypsin and lipase activities of rabbits. In this study, the decreased activities of trypsin and lipase may also be attributed to the injured intestine as reflected by the intestinal morphology analysis of L. maculatus because intestinal damage induced by dietary factors could directly result in decreased activities of trypsin and lipase (Zhang et al., 2012). Conversely, some studies have shown that dietary HTs at low dietary concentrations increased the activities of some intestinal digestive enzymes. For instance, Li et al. (2021) indicated that 0.2 and 0.4 g/kg of HTs in broilers diets increased intestinal trypsin and lipase activities. Sun et al. (2014) and Liu et al. (2020) reported that 0.1% of HTs increased the digestive enzyme activities of piglets. Yao et al. (2019) documented that 0.75%–1.75% of dietary HTs increased the intestinal trypsin activity of C. idellus. Moreover, Chen et al. (2021) suggested that dietary HTs from chestnuts up to 0.09% did not alter intestinal digestive enzyme activities.
Serum metabolites reflect the physiological state of animals, and their concentrations are indicative of animals’ health status. Increased activities of serum aspartate aminotransferase and alanine aminotransferase are generally related to liver damage (Peng et al., 2022a; Peng et al., 2022b). This study showed that 2 and 4 g/kg of HTs caused liver damage of L maculatus. This was also reflected by the vacuolation of liver cells in the morphological analysis of fish. High-density lipoprotein cholesterol is responsible for transporting cholesterol from extrahepatic tissues to the liver and promoting the metabolism of blood lipids. In this study, dietary HTs at 2 and 4 g/kg promoted the metabolism of blood lipids. A similar observation was also reported by Cong et al. (2021) that 1 g/kg of HTs significantly increased serum high-density lipoprotein cholesterol concentrations of broilers.
Total antioxidant capacity is the sum of the antioxidant capacities of individual bioactive substances. Malondialdehyde is the product of polyunsaturated fatty acid peroxidation, and therefore, the serum concentration of malondialdehyde directly reflects the level of lipid peroxidation and the degree of endogenous oxidative damage (Abdel-Latif et al., 2023). Lysozyme is an important immune factor, and it has been considered one of the basic biochemical characteristics in assessing the immune state of fish. In this study, HTs enhanced the antioxidant and immune response of L. maculatus. Zhu et al. (2021) reported that 0.1% and 0.15% of HTs improved the antioxidant and immune capacities of pearl gentian grouper. Novriadi et al. (2021) also indicated that 0.2% of HTs increased the serum lysozyme activity of L. vannamei. Tannins possess various biological activities including antimicrobial, antioxidant, and immunomodulatory (Peng et al., 2020b) and hence could be used to partly activate the antioxidant and immune systems.
The observation of tissue morphology directly reflects the histological changes caused by adverse factors (Shi et al., 2017). This study showed that supplementation of 2 and 4 g/kg of HTs in L. maculatus diets resulted in vacuolation of the liver and atrophy of the intestinal villi. Similarly, Peng et al. (2021b) reported that dietary tannins at 1–4 g/kg induced intestinal villus damage of L. vannamei. Peng et al. (2022a) also suggested that 1 and 2 g/kg of tannins caused liver damage of L. maculatus as reflected by the obvious vacuolar degeneration and inflammatory cell infiltration. In this study, the negative effect of HTs on intestinal morphology may have affected intestinal development because 2 and 4 g/kg of HTs reduced intestinesomatic index of L. maculatus.
Dietary HTs at 2 and 4 g/kg reduced feed intake, inhibited intestinal trypsin and lipase activities, induced damage to the intestine and liver, and therefore decreased the growth performance of L. maculatus. Supplementation of HTs did not alter the whole body composition but rather improved the antioxidant and immune response of fish. HTs should be used with caution due to their growth-inhibiting effect, and the appropriate dietary HT levels for L. maculatus are recommended to be less than 1 g/kg.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences.
KP conceived and designed the experiment. JQ, WH, JC, HZ, BC, and LJ-Y performed the experiments. JQ analyzed the nutrient compositions of the experimental diets. JQ and KP analyzed the data. KP and JQ wrote the paper. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (31902388), National Foreign Expert Program (G2022030077L), Natural Science Foundation of Guangdong Province of China (2021A1515010850, 2022A1515010545), Science and Technology Planning Project of Guangzhou (202002030378), and Special Fund for Scientific Innovation Strategy-Construction of High-Level Academy of Agriculture Science (R2021PY-QY001).
The authors would like to thank Mr. Haomin Wu in the Institute of Animal Science, Guangdong Academy of Agricultural Sciences, for his technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Latif H., Shukry M., Noreldin A. E., Ahmed H. A., El-Bahrawy A., Ghetas H. A., et al. (2023). Milk thistle (Silybum marianum) extract improves growth, immunity, serum biochemical indices, antioxidant state, hepatic histoarchitecture, and intestinal histomorphometry of striped catfish, Pangasianodon hypophthalmus. Aquaculture 562, 738761. doi: 10.1016/J.AQUACULTURE.2022.738761
Al-Mamary M., Al-Habori M., Al-Aghbari A., Al-Obeidi A. (2001). In vivo effects of dietary sorghum tannins on rabbit digestive enzymes and mineral absorption. Nutr. Res. 21, 1393–1401. doi: 10.1016/S0271-5317(01)00334-7
AOAC (1999). Official methods of analysis of the association of official agricultural chemists. 16th ed (Gaithersburg: AOAC International, 5th rev).
Buyukcapar H. M., Atalay A. I., Kamalak A. (2011). Growth performance of Nile tilapia (Oreochromis niloticus) fed with diets containing different levels of hydrolysable and condensed tannin. J. Agr. Sci. Tech. 13, 1045–1051. doi: 10.1007/s10460-011-9341-y
Chen Q. K., Liu X. W., Xie R. T., Zhang H. T., Feng Z. Z., Xu D. (2021). Effect of chestnut tannins on growth performance, digestive enzyme, antioxidase and intestinal microflora in Litopenaeus vannamei. Feed Res. 44 (20), 33–37. doi: 10.13557/j.cnki.issn1002-2813.2021.20.008
Cong G., Xiao Y., Zhang Q., Wu S., Zhang L., Yan L., et al. (2021). Effects of dietary Quercus acutissima carruth tannin on growth performance, immune organ indexes and plasma biochemical indexes of broilers. Chin. J. Anim. Nutr. 33 (5), 2652–2660. doi: 10.3969/j.issn.1006-267x.2021.05.025
Guo H., Zhu X., Chen J., Li G., Zhu C. (2019). Effects of hydrolyzable tannins on growth performance and intestinal microflora in Litopenaeus vannamei. J. Fish. Sci. China 26 (5), 883–892. doi: 10.3724/SP.J.1118.2019.18425
Huang Q., Liu X., Zhao G., Hu T., Wang Y. (2018). Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 4 (2), 137–150. doi: 10.1016/j.aninu.2017.09.004
Li J., Xie Y., Zhang S., Li M., Li S., Xu Q., et al. (2021). Effects of dietary hydrolyzed tannic acid on growth performance and intestinal health of broilers. Chin. J. Anim. Nutr. 33 (5), 2642–2651. doi: 10.3969/j.issn.1006-267x.2021.05.024
Liu H., Hu J., Mahfuz S., Piao X. (2020). Effects of hydrolysable tannins as zinc oxide substitutes on antioxidant status, immune function, intestinal morphology, and digestive enzyme activities in weaned piglets. Animals 10, 757. doi: 10.3390/ani10050757
National Standard of China (2011). Determination of tannin in feeds-spectrophotometry. (Beijing, China: General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China), Standard No. GB/T 27985.
Novriadi R., Fadhilah R., Wahyudi A. E., Trullàs C. (2021). Effects of hydrolysable tannins on the growth performance, total haemocyte counts and lysozyme activity of pacific white leg shrimp Litopenaeus vannamei. Aquacult. Rep. 21, 100796. doi: 10.1016/J.AQREP.2021.100796
Peng K., Chen B., Zhao H., Wang Y., Huang W. (2022a). Condensed tannins improve glycolipid metabolism but induce liver injury of Chinese seabass (Lateolabrax maculatus). Front. Mar. Sci. 9. doi: 10.3389/FMARS.2022.902633
Peng K., Chen B., Zhao H., Zheng C., Wang Y., Luo C., et al. (2022b). Condensed tannins alleviate aflatoxin B1-induced injury in Chinese Sea bass (Lateolabrax maculatus). Aquaculture 552, 738029. doi: 10.1016/J.AQUACULTURE.2022.738029
Peng K., Huang Q., Xu Z., McAllister T. A., Acharya S., Mueller-Harvey I., et al. (2018). Characterization of condensed tannins from purple prairie clover (Dalea purpurea vent.) conserved as either freeze-dried forage, sun-cured hay or silage. Molecules 23, 586. doi: 10.3390/molecules23030586
Peng K., Huang W., Zhao H., Sun Y., Chen B. (2021b). Dietary condensed tannins improved growth performance and antioxidant function but impaired intestinal morphology of Litopenaeus vannamei. Aquacult. Rep. 21, 100853. doi: 10.1016/J.AQREP.2021.100853
Peng K., Wang G., Wang Y., Chen B., Sun Y., Mo W., et al. (2020a). Condensed tannins enhanced antioxidant capacity and hypoxic stress survivability but not growth performance and fatty acid profile of juvenile Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 269, 114671. doi: 10.1016/j.anifeedsci.2020.114671
Peng K., Zhao H., Wang G., Chen B., Mo W., Huang Y. (2021a). Effects of condensed tannins on growth performance, intestinal immune capacity and bacterial microbiomes of Lateolabrax japonicus. Aquacult. Res. 52, 5321–5331. doi: 10.1111/ARE.15402
Peng K., Zhou Y., Wang Y., Wang G., Huang Y., Cao J. (2020b). Inclusion of condensed tannins in Lateolabrax japonicus diets: effects on growth, nutrient digestibility, antioxidant and immune capacity and copper sulphate stress resistance. Aquacult. Rep. 18, 100525. doi: 10.1016/j.aqrep.2020.100525
Shi X., Luo Z., Chen F., Wei C. C., Wu K., Zhu X. M., et al. (2017). Effect of fish meal replacement by chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquacult. Res. 48, 3244–3256. doi: 10.1111/are.13154
Sun Z., Li J., Chen B. (2014). Effects of tannic acid on growth performance, nutrient utilization and related digestive enzyme activity of piglets. Feed Res. 1, 46–49. doi: 10.13557/j.cnki.issn1002-2813.2014.01.010
Yao J. (2020). Effects of rapeseed meal and hydrolysable tannin on health and nutrition metabolism in obscure puffer (Takifugu fasciatus) and grass carp (Ctenopharyngodon idellus) (Shanghai: Shanghai Ocean University).
Yao J., Chen P., Apraku A., Zhang G., Huang Z., Hua X. (2019). Hydrolysable tannin supplementation alters digestibility and utilization of dietary protein, lipid, and carbohydrate in grass carp (Ctenopharyngodon idellus). Front. Nutr. 6. doi: 10.3389/fnut.2019.00183
Zhang F., Zhang W., Mai K., Sun R. (2012). Effects of replacement of dietary fish meal by soybean meal on growth, digestive enzyme activity and digestive tract histology of juvenile Large yellow croaker, Pseudosciaena crocea r. Period. Ocean Univ. China 42 (S1), 75–82. doi: 10.16441/j.cnki.hdxb.2012.s1.011
Zhou Y., Peng K., Wang G., Huang Y., Chen B., Mo W., et al. (2020). Biological activities and research progress in aquaculture of plant extract tannin. Feed Res. 43 (5), 117–122. doi: 10.13557/j.cnki.issn1002-2813.2020.05.033
Zhu X., Huang Y., Huang J., Li G., Zhu C. (2021). Effects of hydrolyzable tannins on growth performance, antioxidant capacity, intestinal morphology and bacterial diversity of pearl gentian grouper (Epinephelus lanceolatus♂×Epinephelus fuscoguttatus♀). Chin. J. Anim. Nutr. 33 (2), 1020–1035. doi: 10.3969/j.issn.1006-267x.2021.02.042
Keywords: hydrolysable tannins, Lateolabrax maculatus, histomorphology, growth, digestion
Citation: Qiu J, Huang W, Cao J, Zhao H, Chen B, Jiun-Yan L and Peng K (2023) Dietary hydrolyzable tannins reduce growth performance and induce histological damage of Chinese seabass (Lateolabrax maculatus). Front. Mar. Sci. 10:1183438. doi: 10.3389/fmars.2023.1183438
Received: 10 March 2023; Accepted: 19 April 2023;
Published: 03 May 2023.
Edited by:
Amina Zuberi, Quaid-i-Azam University, PakistanReviewed by:
Muhammad Kamran, University of Sialkot, PakistanCopyright © 2023 Qiu, Huang, Cao, Zhao, Chen, Jiun-Yan and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Peng, cGVuZ2thaTEwMTZAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.