
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 04 October 2023
Sec. Marine Pollution
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1182213
This article is part of the Research TopicBenthic Organisms as Indicators of Marine Pollution: Community, Biomarkers, and Biological ResponsesView all 6 articles
The thermal discharge of Daya Bay Nuclear Power Plant has a certain impact on the ecological environment of the adjacent waters. In order to understand and evaluate changes in the fish egg community structure in the adjacent waters of Daya Bay Nuclear Power Plant and their relationship with environmental factors, four surveys were conducted to investigate fish eggs in January (winter), March (spring), August (summer) and November (autumn) of 2020. A total of 100,985 fish eggs were collected and 17 taxa were identified, belonging to five orders, 14 families and 17 genera. Among them, Perciformes and Clupeiformes were the main contributing taxa to fish egg species and abundance in the waters adjacent to Daya Bay Nuclear Power Plant. The number of fish egg species ranged from high to low was summer, spring, autumn and winter; the average abundance ranged from high to low was spring, summer, winter and autumn. The results showed that except in winter, the average abundance of fish eggs was greater in northeast area than in southwest area. The Shannon-Weiner species diversity index (H’), Pielou evenness index (J’) and Margalef richness index (d) were significantly different between seasons, but none were significantly different between areas. The results of NMDS analysis showed that there were significant differences in fish egg communities between seasons. Surface seawater temperature the average abundance of fish eggs was positively correlated in the results of all four seasons. Although the entrainment effect of nuclear power plant water intake and thermal pollution of partial waters owing to thermal discharge can cause some loss of fish eggs, fish resources can still be effectively maintained.
Daya Bay is a typical subtropical semi-enclosed bay in the northern South China Sea, covering an area of approximately 600 km², with a winding coastline, many islands, diverse habitats and subtropical characteristics, and is an important breeding ground for fish. In 1983, Daya Bay was designated as a provincial aquatic resources breeding reserve by the People’s Government of Guangdong Province (Wang et al., 2003). With rapid economic development, Daya Bay and the surrounding areas were also classified as an important economic development zone in Guangdong Province. Petrochemical, plastic, printing and other industries have been established in the area (Song et al., 2004). In addition, the demand for electricity in the areas surrounding Daya Bay is high. Daya Bay Nuclear Power Plant (DNPP, comprising two nine hundred-thousand-kilowatt class units) and Ling’ao Nuclear Power Plant (LNPP) Phase I and II (four-million-kilowatt class units) have been built on the north shore at Dapeng’ao in the southwest of Daya Bay Reserve. The discharge of used once-through cooling seawater is up to approximately 315 m3/s. The tidal difference in Daya Bay is small and the water exchange conditions are at the lower to middle level. In recent years, marine fishery resources have been at risk of decline owing to increased human activities and climate change (Lotze et al., 2006; Stelzenmüller et al., 2010; Dahlke et al., 2020). The establishment of the DNPP and LNPP is certain to have an impact on the ecological environment of the adjacent waters (Wu and Wang, 2007). Therefore, studying the effects of thermal discharge from DNPP on early fish resources is helpful to understand the response of the fish egg communities in terms of spatial and temporal distribution in the adjacent waters of DNPP.
The early stages of fish development are divided into three periods: fish egg, larva and juvenile (Zhang et al., 1985); individual fish at this stage are referred to as early fish resources (Cao et al., 2007). As the early developmental stages in the life history of fish, fish eggs, larvae and juveniles are of great significance in energy transfer in the marine ecosystem. Fish eggs, larvae and juvenile fish are not only major prey, but also important primary consumers. They play an important role in connecting the food web of the marine ecosystem (Wan and Jiang, 1998; Xiao et al., 2017). Fish eggs comprise the initial stage of fish development with no movement ability, relying only on external forces such as water flow and wind for movement. The influence of environmental changes on eggs is obvious (Rakocinski et al., 1996; Chambers and Trippel, 1997), with temperature and salinity the main influencing factors. Water temperature dominates the life history of fish (Selleslagh and Amara, 2008), affecting the number, distribution and population structure of fish eggs by influencing adult gonad development and reproductive migration (Xiao et al., 2010), as well as having significant effects on fish egg metamorphosis and incubation rate (Liu et al., 2015). Salinity affects the distribution of fish eggs by influencing hatching and reproduction processes (Song et al., 2016; Long et al., 2021). At present, there are few studies on the effects of DNPP thermal discharge on the spatial and temporal distribution of the fish egg communities, thus it is of great importance to carry out relevant investigations in Daya Bay to maintain the balance of the marine ecosystem and enable sustainable utilization of fishery resources.
There were 19 stations in this study. The adjacent waters of Daya Bay Nuclear Power Plant were divided into the southwest area (SWA, control area) and the northeast area (NEA, thermal discharge impact area) (Figure 1). The number of sampling stations in winter, spring, summer and autumn was 10, 13, 11 and 12, respectively. Samples were collected from stations in both the SWA and NEA in each season. The sampling range was 114.5319° E–114.6295° E, 22.5287° N–22.6635° N. In January, March, August and November 2020, four sampling trips were conducted to investigate the early fish resources in the waters adjacent to DNPP. As shown in Figure 1, S3 is the area of the thermal discharge outfall from the nuclear power plant.
Surface seawater salinity (SSS), surface seawater temperature (SST) and depth (Dep) were measured at each station using a conductivity, temperature and depth (CTD) instrument (SonTek CastAway, Xylem Analytics, Beijing, China). Surface seawater was collected with a water sampler, dissolved oxygen (DO) was measured using a YSI ProPlus (Xylem Analytics). Suspended solids (SS) were measured by weight method and Chlorophyll-a (Chl-a) was measured by spectrophotometric method. Suspended solids and Chlorophyll-a were measured according to The specification for marine monitoring (GB 17378.4-2007) and Specifications for oceanographic survey (GB/T 12763.6-2007), respectively.
Two large plankton nets (net length 280 cm, inner diameter of net mouth 80 cm, net mouth area 0.5 m2) one placed on each side of the boat, were used to collect fish eggs, by trawling horizontally for 10 minutes at a towing speed of approximately 1.5 kn, with the net mouth submerged below the sea surface (Lin et al., 2010). The fish eggs were mainly distributed in the surface layer, so the horizontal trawl produced a better result in collecting a more representative sample of eggs.
The samples collected at each station were divided into two bottles, then one was fixed in 5% formalin solution for morphological identification in the laboratory and the other fixed in 95% alcohol for species identification by molecular biological means. The fish egg specimens in the samples were picked, identified and counted in the laboratory. Identification was mainly based on the morphological characteristics of the fish eggs, with reference to the spawning period of the fish (Wan et al., 2010). The samples with a large number of fish eggs were sampled to count. Fish egg density was calculated by dividing the number of eggs harvested per net by the volume of water filtered by the trawl. The volume of water filtered = trawl speed × trawl time × net mouth area. The general sequence for morphological identification of fish eggs is to observe the shape of the fish eggs first. The round eggs were more, and then they were distinguished by size, the presence or absence of special structures of the egg membrane, the development of embryos, the position and number of oil globules, and the shape and position of the pigment. DNA barcoding was the sequencing analysis of PCR and product amplification using a standard DNA sequence to enable accurate and rapid species identification. Fish egg specimens were first identified by cytochrome C oxidase subunit I (COI) to achieve the lowest level of taxonomic. Using a local DNA barcode library (Hou et al., 2018) and the Barcode of Life Data (BOLD) system, as well as a search tool similar to that used by Hubert et al. (2015) based on Basic Local Alignment Search Tool (BLAST), 15 eggs and larvae were randomly selected for the first DNA extraction and amplification in each horizontal and vertical trawl. If the number of specimens in a sample was greater than 60, perform 2 times. Extraction and amplification were stopped when the success rate of DNA extraction and sequence amplification dropped below 20% (Hou et al., 2021).
The species number and abundance of each type of fish egg were calculated for each sample. The abundance is expressed as the number of fish eggs (ind.) per 1,000 m3, calculated according to the following formula:
where S is the area of the cone net mouth (m2), Ci is the difference between the indicated number of revolutions from the mechanical Flow Meter counter at the beginning and end of sampling at the ith collection, Di is the average abundance of fish eggs collected at the ith collection (ind./1,000 m3), Ni is the number of fish eggs collected at the ith collection (ind.) and 0.3 is the formula coefficient for calculating the volume of water filtered.
The Shannon-Weiner species diversity index (H’) (Shannon and Weaver, 1998), Pielou evenness index (J’) (Pielou, 1969), Margalef richness index (d) (Jiang et al., 2011) and dominance (Y) were used to analyze fish egg communities in this study, using the following formula:
where Pi is the ratio of the ith fish egg abundance to the total abundance in the sampling site, S is the total number of species in the sampling site, N is the total abundance in the sampling site, ni is the ith fish egg abundance and fi is the occurrence frequency of the ith fish egg. The criterion for dominant species is Y ≥ 0.02 (Xu and Chen, 1989).
ArcGIS 10.3 (Esri, Redlands, CA, USA) and Origin 2021 (OriginLab Corporation, Northampton, MA, USA) and R 4.1.0 (R software, the R foundation for statistical computing, https://www.r-project.org/foundation/) were used for plotting graphs. One-way ANOVA, independent-samples t-test and Spearman’s correlation analysis was performed using SPSS 26 (IBM SPSS Statistics for Windows, Armonk, NY, USA). Nonmetric multidimensional scaling (NMDS) analysis was performed on the fish egg community structure in each of the four seasons using R 4.1.0.To avoid the influence of occasional/rare species, the analysis of fish egg community structure did not include species that occurred less than three times in four seasons (Rao et al., 2020). In this study, fish egg communities was analyzed using R 4.1.0. First, the fish egg abundance was transformed by Hellinger transformation and environmental factors were standardized, and detrended correspondence analysis (DCA) was performed on the fish egg abundance in each of the four seasons. All the first gradient lengths were less than four, so redundancy analysis (RDA) was used.
One-way ANOVA and independent-samples t-tests were performed on the environmental factors between seasons and between areas, respectively (Table 1). With the exception of depth, other environmental factors were significantly different between seasons, but none of the environmental factors differed significantly between areas. The station with highest SST in winter, spring and summer was S3, while in autumn was S7, both of which were in and near the thermal discharge outfall. SSS was highest in spring and lowest in summer, and the station with the highest SSS was S3 both in winter and summer. DO was highest in autumn and lowest in summer. The station with the highest concentration of SS in winter, spring and autumn was S3, and in summer was S17. Chl-a concentration was highest in autumn and lowest in summer.
A total of 100,985 fish eggs were collected in this survey. Among them, 17 taxa of eggs were identified, belonging to five orders, 14 families and 17 genera, 12 of which were identified to species level (Table 2). Perciformes accounted for the highest proportion of the total species, with eight species, accounting for 47.1%. Clupeiformes had four species, accounting for 23.5%. Scorpaeniformes and Pleuronectiformes had two species each, accounting for 11.8%. Anguilliformes had one species, accounting for 5.9%. The highest number of species was found in summer with nine, followed by spring and autumn with eight and seven, respectively; and the lowest number of species in winter with four (Figure 2). There were three, five, two and five dominant species in winter, spring, summer and autumn, respectively. The dominant fish egg species (Y ≥ 0.02) showed significant seasonal variation. Among them, Nematalosa japonica was the dominant species in three seasons. In winter, fish eggs community was dominated by Acanthopagrus schlegelii, Nematalosa japonica and Solea ovata. Acanthopagrus schlegelii and Nematalosa japonica were still dominant in spring, accompanied by three new dominant species — Leiognathus sp., Scorpaena sp. and Stolephorus sp. In summer, there were two new dominant species, Photopectoralis bindus and Stolephorus commersonnii. Five species were defined as dominant species in autumn, and the most dominant species was Stolephorus commersonnii (Table 3).
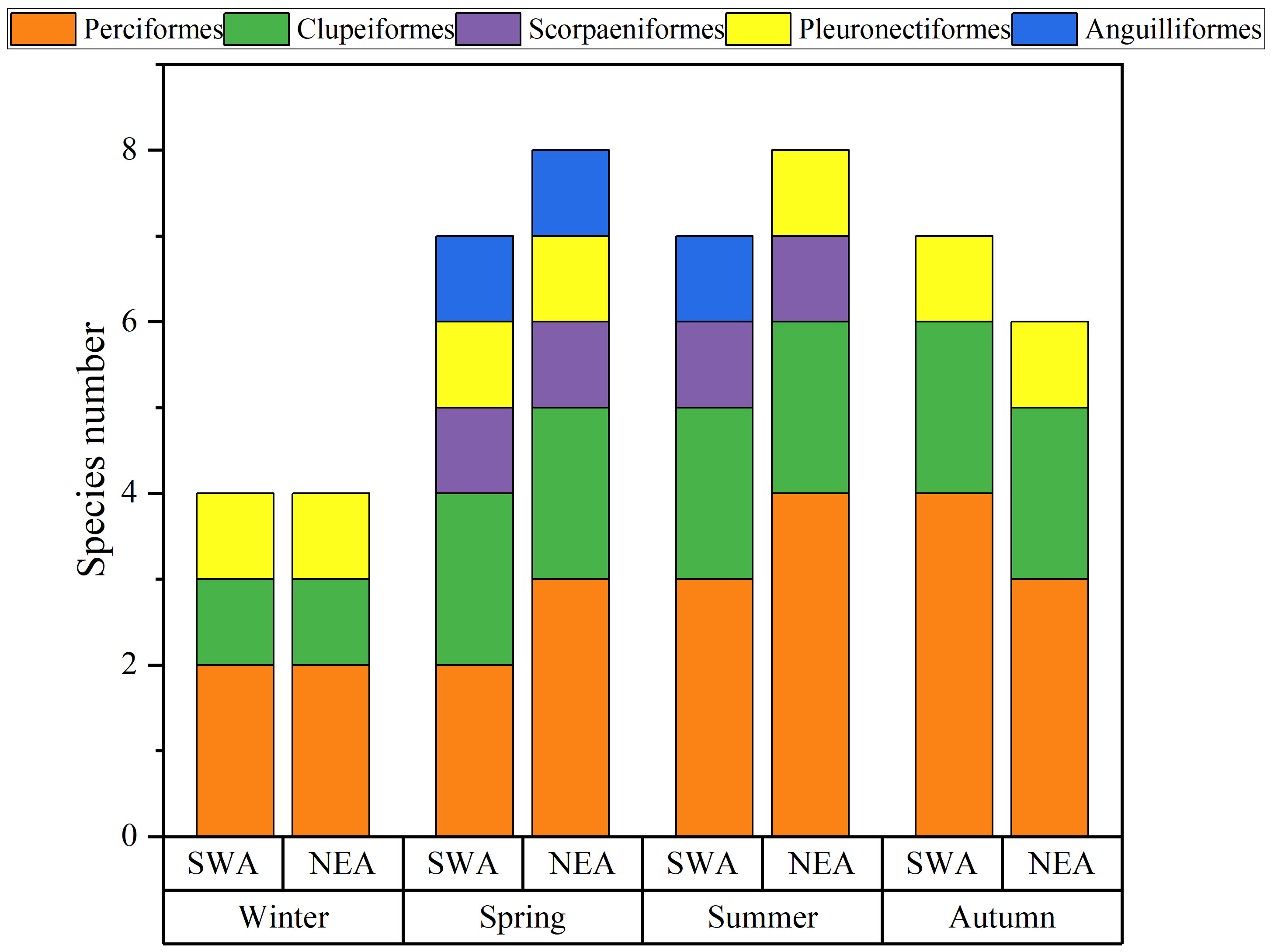
Figure 2 Seasonal and spatial variation of the species number of fish egg in the adjacent waters of DNPP.
The average abundance of fish eggs in the adjacent waters of DNPP in the four seasons was 7,686 ± 22,637.26 ind./1,000 m3. Among them, the average abundance of Perciformes was the highest (6,859.07 ± 20,904.55 ind./1,000 m3, 86.8%), followed by Clupeiformes (470.66 ± 1033.22 ind./1000 m3, 6.0%), Scorpaeniformes (382.27 ± 1,205.42 ind./1,000 m3, 4.8%), Pleuronectiformes (90.82 ± 258.09 ind./1,000 m3, 1.1%) and Anguilliformes (36.09 ± 151.78 ind./1,000 m3, 0.5%), with the remainder being unidentified species (64.74 ± 190.87 ind./1,000 m3, 0.8%). The seasonal and spatial distributions of fish egg abundance in the adjacent waters of DNPP are shown in Figure 3. The highest average abundance was in spring (19,674.02 ± 39,046.91 ind./1000 m3), followed by summer (6,378.37 ± 13,167.76 ind./1000 m3), winter (2,354.03 ± 4831.58 ind./1000 m3) and the lowest in autumn (343.53 ± 404.26 ind./1000 m3).
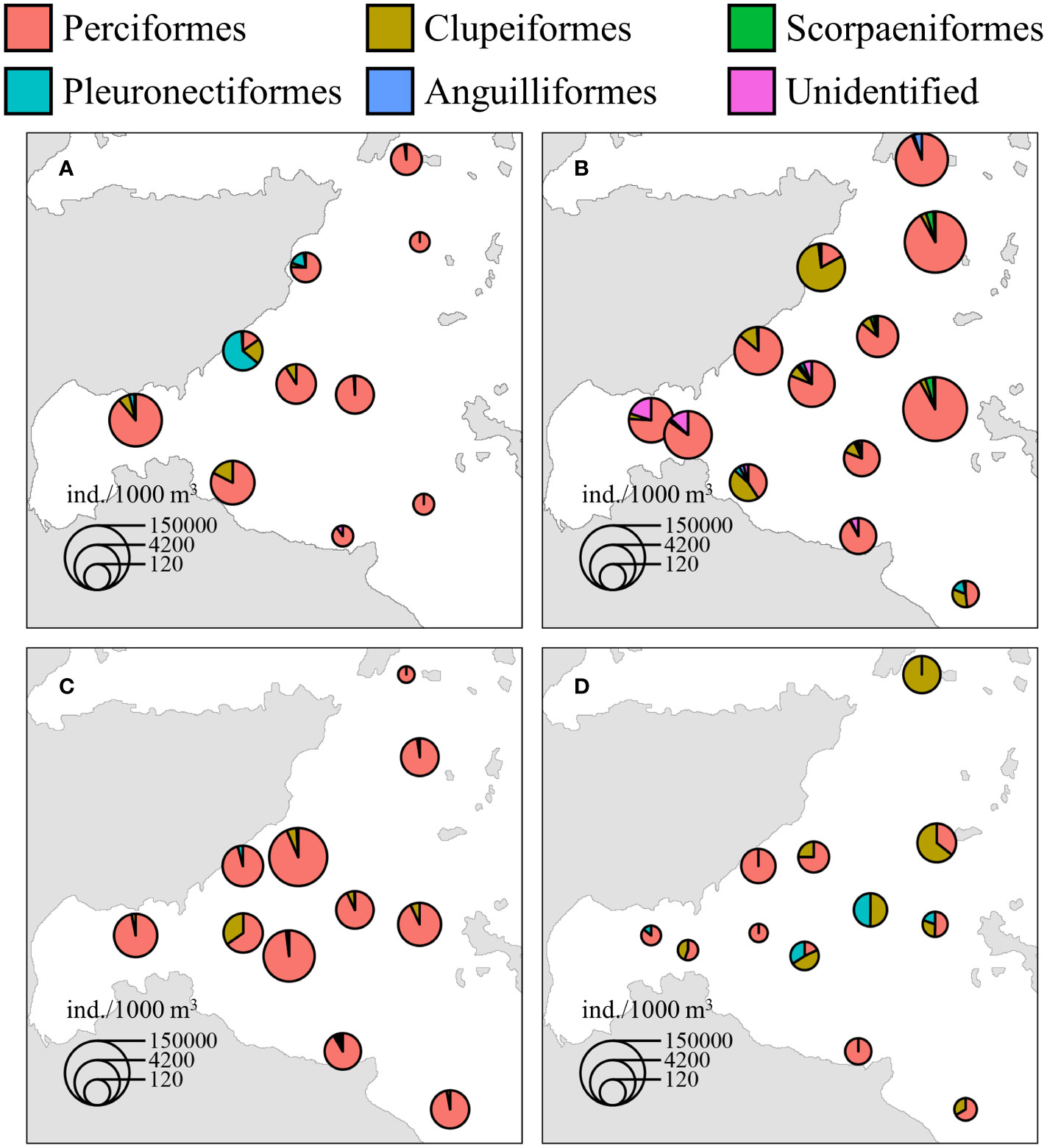
Figure 3 Seasonal and spatial distribution of the abundance of fish egg in the adjacent waters of DNPP (A: Winter; B: Spring; C: Summer; D: Autumn).
Except in winter, the average abundance of fish eggs in the SWA was lower than that in the NEA in all other seasons. The average abundance of fish eggs in the SWA was highest in winter and in the NEA was highest in spring, and was lowest in autumn in both the SWA and NEA. According to analysis of the average abundance over four seasons, the average abundance of fish eggs in the SWA (2,426.23 ± 4,265.67 ind./1,000 m3) was lower than that in the NEA (12,105.45 ± 30,018.65 ind./1,000 m3).
One-way ANOVA and independent-samples t-test were respectively performed on the Shannon-Weiner diversity index (H'), Pielou evenness index (J') and Margalef richness index (d) between seasons and between areas (Table 4). All species diversity indexes (H', J', d) were significantly different between seasons but none were significantly different between areas. In particular, H’ was significantly higher in spring than in summer and winter (P ≤ 0.021), J' was significantly higher in autumn than in spring, summer and winter (P ≤ 0.002), and d was significantly higher in spring than in summer and winter (P ≤ 0.037). The species diversity indexes (H', J', d) was higher in spring and autumn than in summer and winter.
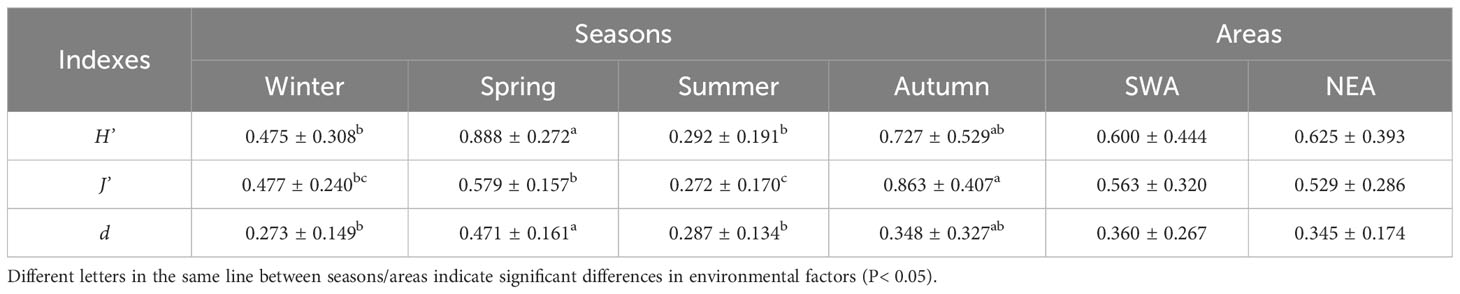
Table 4 Mean and standard deviation of Shannon-Weiner diversity index (H’), Pielou evenness index (J’) and Margalef richness index (d) in the adjacent waters of DNPP.
NMDS analysis based on the Bray-Curtis distance was performed for the fish egg community structure in the four seasons by adding confidence ellipses with 95% confidence intervals. The analysis did not include species that occurred less than three times in the four seasons of the survey combined and a quadratic root transformation was performed on fish egg abundance. The stress value was 0.059, indicating the validity of NMDS analysis (Figure 4). The results showed that the fish egg communities were approximately divided into four groups, corresponding to the four seasons, indicating significant differences between seasons.
RDA was performed on fish egg abundance and environmental factors after transformation in each of the four seasons (Figure 5). In winter, the first axis was mainly related to depth, SST, SSS and Chl-a concentration, and the second axis was mainly related to DO and SS. The average abundance of Acanthopagrus schlegelii was negatively correlated with SST and SSS, and in contrast to Solea ovata, the abundance of Nematalosa japonica was high where Chl-a concentration was high. In spring, the first axis was mainly related to DO, and the second axis was mainly related to depth, SST and SSS. The abundance of Leiognathus sp. was high in deep water and high DO and that of Acanthopagrus schlegelii was positively correlated with SST in spring, in contrast to winter. In summer, the first axis was mainly related to SST, and the second axis was mainly related to SSS. The abundance of Photopectoralis bindus was higher where SST and Chl-a concentrations were high, while the opposite was true for Stolephorus commersonnii. In autumn, the first axis was mainly related to SS, and the second axis was mainly related to depth and Chl-a concentration. The abundance of Photopectoralis bindus and Nematalosa japonica was high in shallow water and high Chl-a, while the opposite was true for Stolephorus commersonnii and Cynoglossus sp.
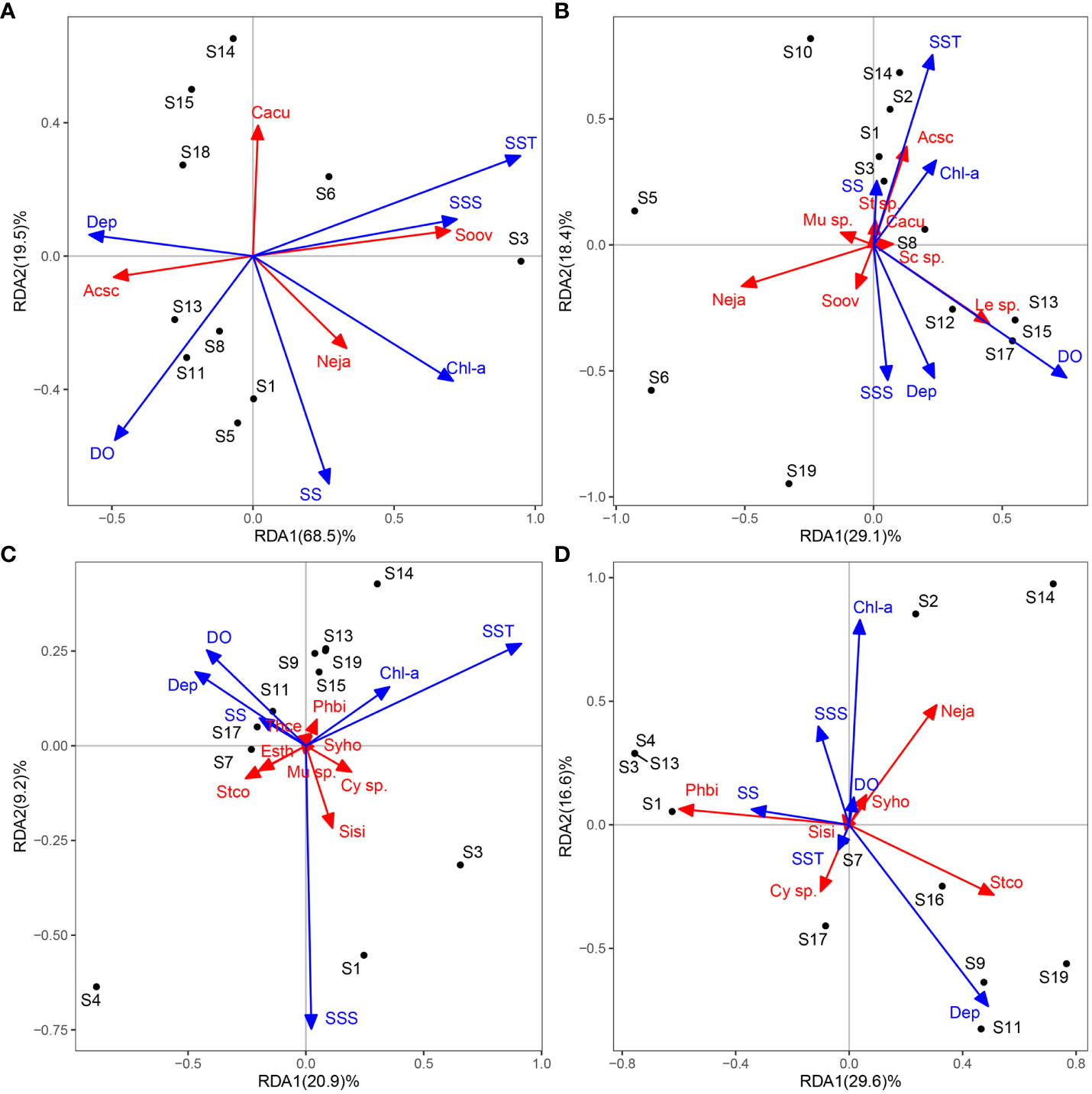
Figure 5 RDA ordination plot of the relationship between fish egg communities and environmental factors (A: Winter; B:Spring; C: Summer; D: Autumn).
Spearman correlation analysis was performed between species diversity indexes (H', J', d), fish egg abundance (N) and environmental factors (Table 5). The results showed that the same environmental factors had different effects on H', J', d and fish egg abundance in different seasons. In winter, H', J' and d were all significantly and positively correlated with SST. In spring, H' was significantly and negatively correlated with Chl-a concentration; d was significantly and positively correlated with depth; and fish egg abundance was significantly and positively correlated with SS. In summer, H', J', d and fish egg abundance were not significantly correlated with environmental factors. In autumn, fish egg abundance was highly significantly and positively correlated with SSS. In the results of all four seasons, H' was highly significantly and positively correlated with SSS, and significantly and positively correlated with DO; J' was highly and positively correlated with DO and SS.
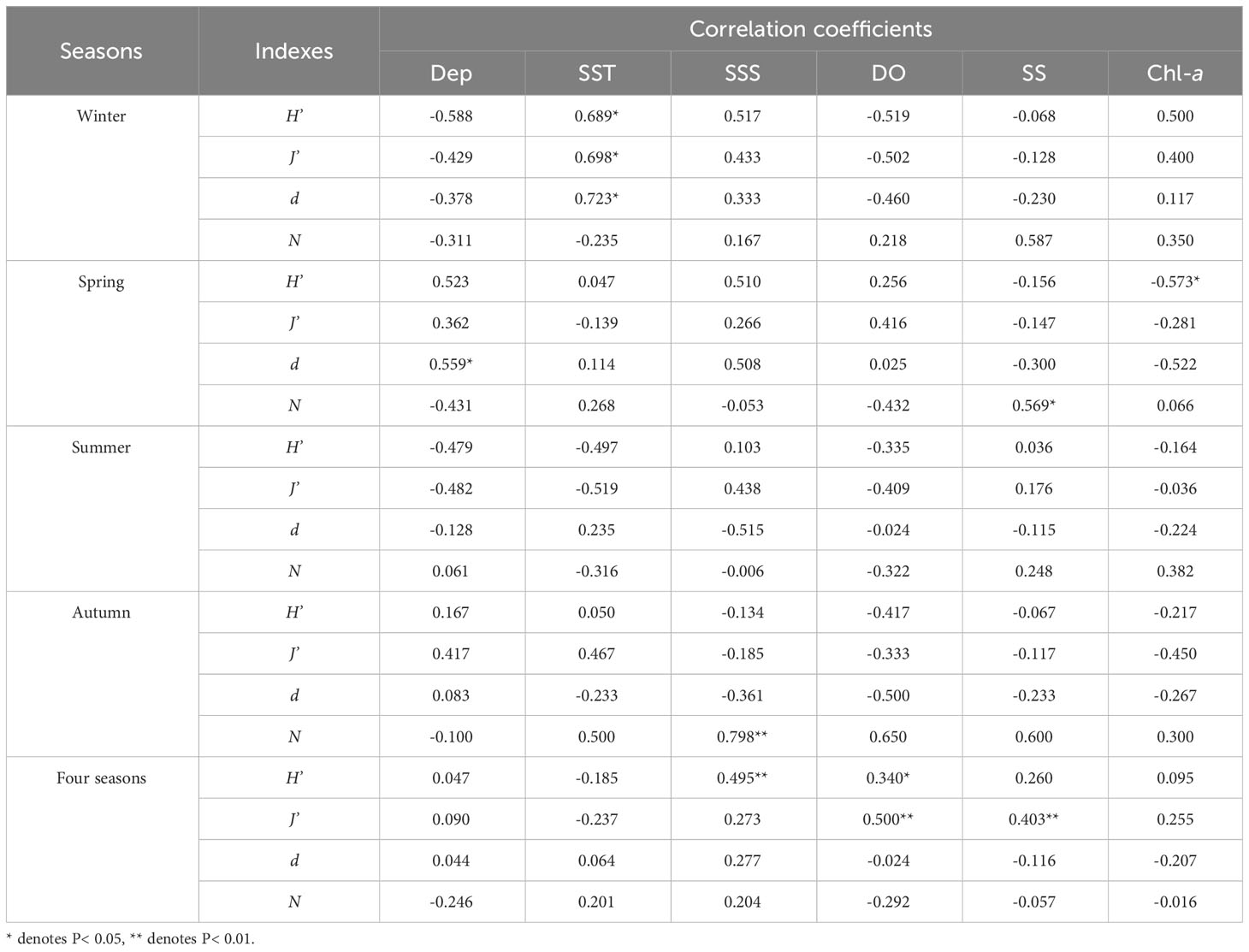
Table 5 Spearman correlation analysis between species diversity indexes (H’, J’, d), fish egg abundance (N) and environmental factors.
Lin et al. (2010) concluded that the dominant species in Daya Bay were mainly Perciformes and Clupeiformes. Similarly, Wang et al. (2010) conducted four fisheries resource surveys and found that Perciformes were dominant in Daya Bay. Based on data from bottom trawl surveys of four cruises, Guo et al. (2018) conducted in Daya Bay between 2015 and 2016, showed that Perciformes were overwhelmingly dominant. Our study had similar results that the Perciformes and Clupeiformes had the highest number of species and the highest average abundance.
The annual average number of fish eggs in Daya Bay fluctuates greatly from year to year. From March 1994 to February 1995, Lin and Zhan, (2000) conducted a survey of early fish resources in the adjacent waters of DNPP, and the annual average number of eggs was 236.0 eggs/net, and the annual average number of eggs harvested from 1984 to 1993 ranged from 46.9 to 660.1 eggs/net. The DNPP was established in 1994, and the average number of fish eggs harvested before and after its establishment did not exceed the historical fluctuation range, but was within the fluctuation range, indicating that the number of eggs in the adjacent waters of DNPP remained normal even though it was affected by thermal discharge. The average abundance of fish egg in 2020 was at the higher level of the fluctuating range of historical surveys. The increase of fish eggs in Daya Bay did not represent an improvement in fish stocks, but rather a result of a change in the structure of the species, because the proportion of Leiognathidae eggs increased, while the proportion of economic fish decreased.
Numerous studies have shown that the number and abundance of fish eggs are both higher in spring and summer than in autumn and winter, and the results of this study were consistent with previous studies (Xiao et al., 2013; Li et al., 2014; Mota et al., 2017). In this investigation, jellyfish were present in all four seasons. The abundance of fish eggs, larvae and juveniles was negatively correlated with the abundance of jellyfish owing to the predation and competition between them. The average abundance of jellyfish was highest and the average abundance of fish eggs was lowest in winter, but the average abundance of fish eggs was mainly related to the different spawning periods and the optimum environmental conditions for the fish. A number of studies have demonstrated that jellyfish predate on fish eggs, larvae and juveniles, forming a predatory relationship (Underwood and Seymour, 2007; Riascos et al., 2014). Meanwhile, jellyfish, fish larvae and juveniles have similar feeding habits, forming a competitive relationship (Ajiboye et al., 2011; Nagata and Morandini, 2018).
Acanthopagrus schlegelii were mainly found in deeper water in winter, but mainly in shallower water near the shore in spring, probably related to the fact that Acanthopagrus schlegelii spawned near the shore in spring. The study by Lin et al. (2010) showed that Sparidae eggs in Daya Bay were overwhelmingly dominant in winter and were also present in March, while none were found in May, which is consistent with the results of this study, and similar results were found in the geographically close Hong Kong and its adjacent waters (Deng et al., 2001; Law and Sadovy de Mitcheson, 2017).
In this survey, Leiognathidae were present in spring, summer and autumn. In this study, the average abundance of Leiognathidae eggs was 54.57%, 91.99% and 24.87% in spring, summer and autumn, respectively, and the species was dominant in all three seasons. The results of Lin et al. (2010) showed that in spring, summer and autumn of 2004, the number of eggs of Leiognathidae was 71.7%, 73.1% and 86.0%, respectively, with an overwhelming dominance of number. The increase of Leiognathidae populations was related to environmental changes, such as warming of water due to thermal discharge, which may facilitated the reproduction of some small pelagic fishes (Lin and Zhan, 2000).
The average abundance of Stolephorus commersonnii in the NEA was higher, probably in higher salinity waters, fish eggs can float, absorbing oxygen and increasing the hatching rate of eggs (Lu et al., 2020). The environment in which Stolephorus commersonnii occur was dominated by higher temperature and salinity (Tian et al., 2017), which was similar to the results of this study.
The Sillago sihama are mainly found in estuaries and nearshore waters (Qiu et al., 2020). In our study, the stations with high abundance of Sillago sihama were mainly in the shallow inshore waters on the western side of Daya Bay. The inshore waters of Daya Bay are shallow, rich in nutrient salts and abundant in bait, which is conducive to the growth of Sillago sihama.
The once-through cooling system in nuclear power plant operation needs to extract a large amount of seawater as circulating cooling water. When cooling seawater enters the cooling system, larger marine organisms (generally larger than 3 mm) carried into the cooling system will be intercepted by the filter screen and other devices, while smaller marine organisms (generally smaller than 3 mm) will be carried into the cooling water system pipeline with the seawater, resulting in a certain loss of marine biological resources. The total circulating water volume of the existing six units of DNPP and LNPP is about 315 m3/s. Approximately 125.9 m3/s of new circulating water will be added after the completion and operation of the LNPP Phase III project. The total cooling water intake and discharge capacity for the operation of the eight nuclear power plant units will then be approximately 440 m3/s, which will increase the entrainment effect on marine organisms in Daya Bay reserve and partially weaken the supplemental function of aquatic resources in the waters adjacent to the nuclear power plant.
The results of this survey showed that the number of fish eggs was at a high level of normal fluctuation in the historical survey of Daya Bay. Even in S3, which is the closest to the nuclear power plant thermal discharge outlet, we were able to collect a number of fish eggs with an average abundance up to 2,603.65 ind./1,000 m3. In addition, this survey also found that fish tend to spawn in the thermal discharge plume area (0.5–3°C temperature rise). Areas S6-S7-S8-S9-S10-S11 (temperature rise 1–3 °C) and S14-S15-S16-S17 (temperature rise< 1 °C) showed dense distribution areas of fish eggs, with average abundance up to 6,806.90 ind./1,000 m3 and 22,720.74 ind./1,000 m3, respectively. The effect of thermal discharge on fish is a complex process, and different fish species have different abilities to adapt to and perceive temperature differences, but generally fishes prefer a slightly higher temperature for spawning than their usual environment (Evans et al., 1986; Lin and Zhan, 2000; Jiang et al., 2016). The temperature rise frontal area of thermal discharge is a mixed area of cold and warm water, where plankton is more abundant. As a result, fishes are abundant and also prefer to spawn here.
The distribution of fish eggs is influenced not only by the spawning behavior of brood stocks and various environmental and ecological factors, but also by physical factors such as sea currents (Zhang, 1996; Lu et al., 2020). The temperature rise frontal area formed by the DNPP thermal discharge is in the central spawning and breeding ground of Daya Bay, which has the characteristic of a topographic vortex due to the influence of the thermal discharge plume and the central islands. Both fish eggs and larvae are better able to remain in the vortex to develop and grow, and are less likely to be entrained in the west side of the water intake. Thus it can be seen that there is no obvious effect of nuclear power plant thermal discharge on fish spawning and breeding.
In this study, differences in environmental factors, average fish egg abundance and species diversity indexes (H', J' and d, respectively) were all more significant between seasons than between areas. The results of NMDS analysis also revealed significant differences in the fish egg communities between seasons. Fish eggs of the dominant species (Y ≥ 0.02) showed significant seasonal variation. Perciformes and Clupeiformes were the main contributing taxa to fish egg species and abundance in the adjacent waters of DNPP. The results of RDA and Spearman’s correlation analysis demonstrated that SST had different effects on the fish egg communities in different seasons. Areas of thermal discharge at the temperature rise front tend to form spawning grounds. In addition, the flow field created by the nuclear power plant thermal discharge plume and the central islands make it less likely that fish eggs will be entrained into the water intake. Although the entrainment effect of the nuclear power plant water intake and thermal pollution of partial waters owing to thermal discharge can cause some loss of fish eggs, the temperature front formed by the dispersion of thermal discharge is conducive to the formation of fish spawning grounds. The function of supplement of fish resources in the waters adjacent to nuclear power plant can still be effectively maintained.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HH (corresponding author) provided ideas and guidance, obtained fundings. ZT carried the data analysis and wrote the paper. FW, YR, CP, were responsible for field and laboratory work. GH conducted the identification of fish eggs and proposed some valuable suggestions in revising this paper. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (2018YFC1407501), Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO.2023TD15), Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (2021SD03).
We would like to extend our sincere gratitude to the editors and all reviewers of Frontiers in Marine Science for their work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BL declared a shared affiliation with the author ZT to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ajiboye O. O., Yakubu A. F., Adams T. E., Olaji E. D., Nwogu N. A. (2011). A review of the use of copepods in marine fish larviculture. Rev. Fish Biol. Fisheries 21 (2), 225–246. doi: 10.1007/s11160-010-9169-3
Cao W. X., Chang J. B., Qiao Y. (2007). Fish Resources of Early Life History Stages in Yangtze River (Beijing: China Water Power Press).
Chambers R. C., Trippel E. A. (1997). Early life history and recruitment in fish populations (Springer Science & Business Media).
Dahlke F. T., Wohlrab S., Butzin M., Pörtner H. O. (2020). Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369 (6499), 65–70.
Deng L., Zhang W. M., Lin H. R., Cheng C. H. K. (2001). Seasonal variations of serum growth hormone levels and growth hormone receptors in Sparus macrocephalus. J. Fisheries China 25 (3), 203–208.
Evans M. S., Warren G. J., Page D. I. (1986). The effects of power plant passage on zooplankton mortalities: Eight years of study at the Donald C. Cook nuclear plant. Water Res. 20 (6), 725–734.
Guo J. Z., Chen Z. Z., Xu Y. W., Xu S., Huang Z., Li C. (2018). The effects of anthropogenic activities on the diversity and succession of fish community in Daya Bay. J. Fishery Sci. China 25 (3), 595–607. doi: 10.3724/SP.J.1118.2018.17277
Hou G., Chen W. T., Lu H. S., et al. (2018). Developing a DNA barcode library for perciform fishes in the South China Sea: Species identification, accuracy and cryptic diversity. Mol. Ecol. Resour. 18 (1), 137–146. doi: 10.1111/1755-0998.12718
Hou G., Wang J., Liu L., Chen Y., Pan C., Lin J., et al. (2021). Assemblage structure of the ichthyoplankton and its relationship with environmental factors in spring and autumn off the Pearl River Estuary. Front. Mar. Sci. 8, 732970. doi: 10.3389/fmars.2021.732970
Hubert N., Espiau B., Meyer C., Planes S. (2015). Identifying the ichthyoplankton of a coral reef using DNA barcodes. Mol. Ecol. Resour. 15 (1), 57–67. doi: 10.1111/1755-0998.12293
Jiang C. P., Xu Z. L., Chen J. J., Sun L. F., Que J. L. (2016). Effects of the thermal discharge from Qinshan Nuclear Plant on the distribution pattern of fish. J. Fishery Sci. China 23 (02), 478–488.
Jiang Y., Xu H., Hu X., Zhu M., Al-Rasheid K. A., Warren A. (2011). An approach to analyzing spatial patterns of planktonic ciliate communities for monitoring water quality in Jiaozhou Bay, northern China. Mar. pollut. Bull. 62 (2), 227–235. doi: 10.1016/j.marpolbul.2010.11.008
Law C. S. W., Sadovy de Mitcheson Y. (2017). Reproductive biology of black seabream Acanthopagrus schlegelii, threadfin porgy Evynnis cardinalis and red pargo Pagrus major in the northern South China Sea with consideration of fishery status and management needs. J. fish Biol. 91 (1), 101–125. doi: 10.1111/jfb.13331
Li K., Yin J., Huang L., Lin Z. (2014). Seasonal variations in diversity and abundance of surface ichthyoplankton in the northern South China Sea. Acta Oceanologica Sin. 33 (12), 145–154. doi: 10.1007/s13131-014-0533-3
Lin Z., Wang X., Jiang Y. (2010). Distribution and species composition of fish eggs in Daya Bay. J. Fishery Sci. China 17 (03), 543–550.
Lin Z., Zhan H. (2000). Effects of thermal effluent on fish eggs and larvae in waters near Daya Bay Nuclear Plant. J. Trop. Oceanography 01), 44–51.
Liu S. H., Wang J. H., Liu C. C., Qin Y. T., Liu Z. G., Deng B. P. (2015). Inter-annual variation in pelagic fish egg, larval, and juvenile assemblages during summer in the Yangtze River Estuary, China. Acta Ecologica Sin. 35 (21), 7190–7197.
Long X., Wan R., Li Z., Ren Y., Song P., Tian Y., et al. (2021). Spatio-temporal distribution of Konosirus punctatus spawning and nursing ground in the South Yellow Sea[J]. Acta Oceanologica Sin. 40 (8), 133–144. doi: 10.1007/s13131-021-1790-6
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas[J]. Science 312 (5781), 1806–1809.
Lu Y., Yu J., Lin Z. J., Chen P. (2020). Environmental influence on the spatiotemporal variability of spawning grounds in the western Guangdong waters, South China Sea. J. Mar. Sci. Eng. 8 (8), 607. doi: 10.3390/jmse8080607
Mota E. M. T., Garcia T. M., Freitas J. E. P., Soares M. O. (2017). Composition and cross-shelf distribution of ichthyoplankton in the Tropical Southwestern Atlantic. Regional Stud. Mar. Sci. 14, 27–33. doi: 10.1016/j.rsma.2017.05.001
Nagata R. M., Morandini A. C. (2018). Diet, prey selection, and individual feeding rates of the jellyfish Lychnorhiza lucerna (Scyphozoa, Rhizostomeae). Mar. Biol. 165 (12), 1–17. doi: 10.1007/s00227-018-3445-5
Qiu B., Fang S., Ikhwanuddin M., Wong L., Ma H. (2020). Genome survey and development of polymorphic microsatellite loci for Sillago sihama based on Illumina sequencing technology. Mol. Biol. Rep. 47, 3011–3017. doi: 10.1007/s11033-020-05348-z
Rakocinski C. F., Lyczkowski-Shultz J., Richardson S. L. (1996). Ichthyoplankton assemblage structure in Mississippi Sound as revealed by canonical correspondence analysis. Estuarine Coast. Shelf Sci. 43 (2), 237–257. doi: 10.1006/ecss.1996.0067
Rao Y. Y., Cai L. Z., Chen B. W., Chen X., Zheng L., Lin S. (2020). How do spatial and environmental factors shape the structure of a coastal macrobenthic community and meroplanktonic larvae cohort? Evidence from Daya Bay. Mar. pollut. Bull. 157, 111242. doi: 10.1016/j.marpolbul.2020.111242
Riascos J. M., Villegas V., Pacheco A. S. (2014). Diet composition of the large scyphozoan jellyfish Chrysaora plocamia in a highly productive upwelling centre off northern Chile. Mar. Biol. Res. 10 (8), 791–798. doi: 10.1080/17451000.2013.863353
Selleslagh J., Amara R. (2008). Environmental factors structuring fish composition and assemblages in a small macrotidal estuary (eastern English Channel). Estuarine Coast. Shelf Sci. 79 (3), 507–517. doi: 10.1016/j.ecss.2008.05.006
Shannon C., Weaver W. (1998). The Mathematical Theory of Communication (Urbana-Champaign: University of Illinois Press).
Song X. Y., Huang L. M., Zhang J. L., Huang X., Zhang J., Yin J. (2004). Variation of phytoplankton biomass and primary production in Daya Bay during spring and summer. Mar. pollut. Bull. 49 (11-12), 1036–1044. doi: 10.1016/j.marpolbul.2004.07.008
Song C., Wang Y. T., Liu Z. L., Zhang H., Lin Y., Jiang Y. Z. (2016). Relationship between environmental factors and distribution of Scomberomorus niphonius eggs, larvae, and juveniles in Xiangshan Bay. J. Fishery Sci. China 23 (5), 1197–1204.
Stelzenmüller V., Ellis J. R., Rogers S. I. (2010). Towards a spatially explicit risk assessment for marine management: assessing the vulnerability of fish to aggregate extraction. Biol. Conserv. 143 (1), 230–238. doi: 10.1016/j.biocon.2009.10.007
Tian F. G., Zheng Y. J., Xiao Y. Z., Fang H. D. (2017). Spawning season and variation of the eggs of Stolephorus commersonii in Guanghaiwan Bay. J. Appl. Oceanography 36 (03), 395–402.
Underwood A. H., Seymour J. E. (2007). Venom ontogeny, diet and morphology in Carukia barnesi, a species of Australian box jellyfish that causes Irukandji syndrome. Toxicon 49 (8), 1073–1082. doi: 10.1016/j.toxicon.2007.01.014
Wan R. J., Jiang Y. W. (1998). The distribution and variation of eggs and larvae of osteichthyfs in the bohai sea. J. Fishery Sci. China 5 (1), 43–50.
Wan R., Zhou F., Shan X., Sun S. (2010). Impacts of variability of habitat factors on species composition of ichthyoplankton and distribution of fish spawning ground in the Changjiang River estuary and its adjacent waters. Acta Ecologica Sin. 30 (3), 155–165.
Wang X. H., Du F. Y., Qiu Y. S., Li C. H., Sun D. R., Jia X. P. (2010). Variations of fish species diversity, faunal assemblage, and abundances in Daya Bay in 1980-2007. Yingyong Shengtai Xuebao 21 (9).
Wang Z. D., Lian J. S., Hu J. X., Wei G. F. (2003). Characteristics of degraded ecosystem in Daya Bay China. Ecol. Sci. 04), 313–320.
Wu M. L., Wang Y. S. (2007). Using chemometrics to evaluate anthropogenic effects in Daya Bay, China. Estuarine Coast. Shelf Sci. 72 (4), 732–742. doi: 10.1016/j.ecss.2006.11.032
Xiao Y., Wang R., Ou Q., Fang H. (2010). Relationship between abundance distribution of fish eggs, larvae and juveniles and environmental factors in the Pearl River Estuary waters in spring. J. oceanography Taiwan Strait/Taiwan Haixia 29 (4).
Xiao Y. Z., Wang R., Zheng Y. J., He W. (2013). Species composition and abundance distribution of ichthyoplankton in the Pearl River Estuary. J. Trop. Oceanography 32 (6), 80–87.
Xiao H. H., Zhang C. L., Xue Y., Xu B. D., Yu H. Q., Ren Y. P. (2017). Community structure of ichthyoplankton from typical transects in Haizhou Bay and its adjacent waters during spring and summer. J. Fishery Sci. China 24 (5), 1079–1090. doi: 10.3724/SP.J.1118.2017.17017
Xu Z. L., Chen Y. Q. (1989). Aggregated intensity of dominant species of zooplankton in autumn in the East China Sea and Yellow Sea. J. Ecol. 8 (4), 13–15.
Zhang W. Z. (1996). The influences of thermal water from the nuclear power station on the distribution of fish eggs and larvae of Sparidae in Daya Bay. Tropic Oceanology 15 (4), 80–84.
Keywords: Daya Bay, thermal discharge, fish eggs, spatial and temporal distribution, community structure
Citation: Tan Z, Wu F, Rao Y, Pan C, Hou G and Huang H (2023) Spatial and temporal distribution of fish egg communities in the adjacent waters of Daya Bay nuclear power plant and their relationship with environmental factors. Front. Mar. Sci. 10:1182213. doi: 10.3389/fmars.2023.1182213
Received: 08 March 2023; Accepted: 21 September 2023;
Published: 04 October 2023.
Edited by:
Lihua Yang, Sun Yat-sen University, ChinaReviewed by:
Bilin Liu, Shanghai Ocean University, ChinaCopyright © 2023 Tan, Wu, Rao, Pan, Hou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honghui Huang, aHVhbmdoaEBzY3NmcmkuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.