- 1Ocean Sunfish Research Trust, Auckland, New Zealand
- 2Ichthyology Department, California Academy of Sciences, San Francisco, CA, United States
- 3Coral Triangle Center, Sanur, Bali, Indonesia
Photo ID is a common tool in ecology, but has not previously been attempted for the ocean sunfishes (Mola spp., Molidae; ‘molids’). The technique, based on body patterns, could potentially be informative for studying the seasonal occurrence of giant sunfish (Mola alexandrini) on the Bali reefs (Indonesia), where this species is an important drawcard for the local SCUBA diving tourism. However, molids are capable of rapid physiological colouration change, which may complicate the application of the method. Our study aimed to determine if photo ID is nevertheless achievable and informative. To test this, we created the citizen-science platform ‘Match My Mola’ for crowd-sourcing imagery (photos and video) of M. alexandrini in Bali, and undertook trial matching (n=1,098 submissions). The submitted imagery revealed a wide range of pattern clarity, from fish with no pattern to bold displays. Video confirmed physiological colouration change can occur in seconds in this species from low to high contrast, and cause individuals to look very different between moments. However, individual patterns appear to be stable although at least some parts can become inconspicuous during low contrast displays. Despite of this, photo ID is possible, including in some instances, where only partial patterns are visible on one image compared with another. However, true negatives (confirming two fish are not the same) can be challenging. Most identified matches were of fish photographed by different divers on the same day. Only a small number (n=9) were found with resighting durations ≥1 day (1 – 2,652 days). These matches demonstrate that at least some individuals return to the same reefs both within and between seasons, with the resighting duration of 7.2 years constituting the longest known example of molid site fidelity. Comparing body morphology between resightings of > 1 year (n=6) revealed limited indications of growth, contradicting the current understanding of rapid growth in captive molids (Mola mola), and highlighting the knowledge gap regarding growth in the wild. Continued photo ID in the Bali area could provide valuable complementary information to future growth studies using other methods as well as provide further insights into molid site fidelity.
1 Introduction
Photo identification of individual animals has become an established method in population ecology and is used across a wide range of taxa, including marine megafauna such as elasmobranchs, reptiles and mammals (Blount et al., 2022; Petso et al., 2022). The method has also been applied to teleosts (e.g., Giglio et al., 2014; Chaves et al., 2016; Couffer, 2017; Mucientes et al., 2019; Pedersen and Mohammed, 2021; Sèbe, 2021), but has to date not been applied to the ocean sunfishes (‘molids’). The family comprises five species (Fricke et al., 2022), including the world’s heaviest bony fish, giant sunfish Mola alexandrini (Ranzani, 1839) sensu (Sawai et al., 2018), capable of reaching at least 2.7 ton in body weight and at least 3.3 m in total length (TL) (Gomes-Pereira et al., 2022; Sawai and Nyegaard, 2022).
A main challenge to photo identification of individual molids (hereafter termed photo ID) lies in obtaining sufficient images. Encounter rates are generally low globally, and molid tourism is rare (Nyegaard, 2018; Thys et al., 2020). The reefs of the Nusa Penida Marine Protected Area (MPA) in Bali, Indonesia (Figure 1) provide an exception. Here, M. alexandrini appear seasonally to solicit parasite cleaning services from reef fish, and serve as an important draw card for local SCUBA diving tourism (Konow et al., 2006; Thys et al., 2016). While these molids occur in the area year round from the surface to well below the recreational SCUBA diving limit (40 m), SCUBA divers most commonly encounter individuals between c. July/August and November/December, locally known as the “sunfish season” (Nyegaard, 2018). Such encounters yield numerous opportunities for molid photography.
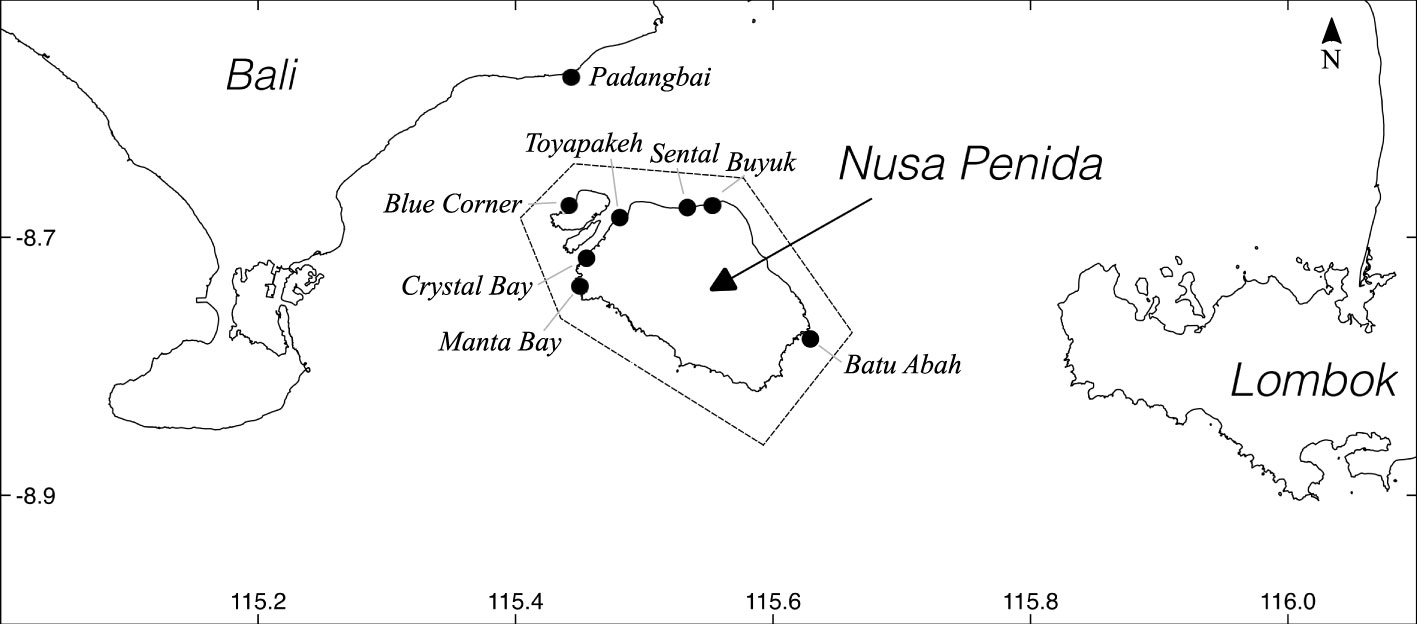
Figure 1 The Nusa Penida Island group in the Lombok Strait between the Indonesian Islands of Bali and Lombok. Hashed line shows the boundary of the Nusa Penida Marine Protected Area (Ruchimat et al., 2013), and black circles indicate popular SCUBA dive sites.
Despite the local popularity of the molids, their seasonal occurrence is not well understood. The fish are locally referred to as ‘mola-mola’, but have been morphologically and genetically identified as M. alexandrini (Thys et al., 2016; Nyegaard, 2018; see also Riawan et al., 2019), although we note not everyone agrees on the nomenclature (Britz, 2022). The size range of individuals has not been studied in the Nusa Penida MPA, but most fish appear comparable in length to the height of a human diver. Our approximation is c. 130 – 200 cm TL, based on personal in situ observations and review of imagery [see Nyegaard (2018) and Supplemental Material Figure 1 for examples]. It is not known if individuals of this size are sexually mature, but for the close relative Mola mola (Linnaeus, 1758) maturation occurs around 150 – 160 cm TL (Forsgren et al., 2020). Further, it is not known if M. alexandrini are year-round residents in the area, or if they are seasonal visitors whose presence may be linked to seasonal cold water upwelling along the southern coastline of Bali and neighbouring islands (Thys et al., 2016; Nyegaard, 2018). While photo ID could be informative, not all taxa are suitable for this method, as it relies on clear, individual markings that are stable over time (Marshall and Pierce, 2012; Urian et al., 2015).
1.1 Individual markings
The elaborate body patterns on M. alexandrini consist of dusky-white spots, stripes and irregular shapes against a dark reddish-brown colour dorsally and laterally, merging into a dusky-white area ventrally (Sawai et al., 2018) (Figure 2A). The high degree of pattern intricacy and variation between individuals points to a well-suited taxa for photo ID, as also suggested by Kushimoto et al. (2022) for M. mola and sharptail sunfish Masturus lanceolatus (Liénard, 1840). However, the boldness of these skin patterns can change rapidly in all Mola species (Sawai, 2017; Nyegaard et al., 2018; Sawai et al., 2018; Thys et al., 2020; Kushimoto et al., 2022). Such ‘physiological colouration change’ is not uncommon among teleosts, and has been reported for numerous taxa (Kodric-Brown, 1998; Rosenthal and Marshall, 2011; Sköld et al., 2016). It is achieved through aggregation, dispersal and interaction of various light-absorbing and light reflectance pigment granules within dermal chromatophore units (Thurman, 1998; Sugimoto, 2002; Ligon and Mccartney, 2016; Sköld et al., 2016).
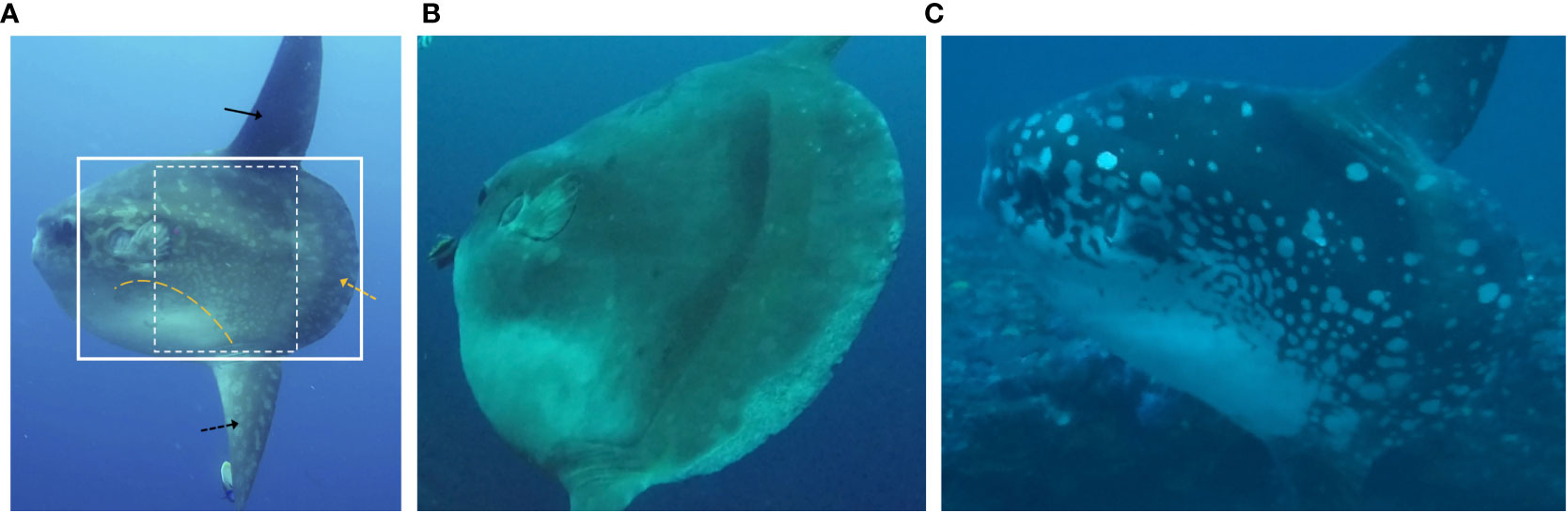
Figure 2 Three different Mola alexandrini individuals in the Nusa Penida Marine Protected Area. (A) Intricate patterns across the body and clavus (yellow hashed arrow); the pattern on both dorsal and anal fins (black solid and hashed arrows, respectively) are often obscured by shadow effects (e.g., dorsal fin here). White box: target area for photo identification; yellow hashed line: confluence of darker dorsal and lighter ventral colouration. (B) Inconspicuous and (C) highly conspicuous patterns during low contrast and bold displays, respectively. Cropped stills from unmanipulated Gopro video, natural light, orange filter. (C) Image by Richard Horner, printed with permission.
Physiological colouration change has not been formally documented in wild molids, but has been reported at least as far back as Duhamel du Monceau (1777). In captivity, it is known that M. mola can produce striking shifts in body patterns during feeding and handling [M. Howard, pers comm in Thys et al. (2020)], and that such changes can occur in less than a minute (Kushimoto et al., 2022). These changes to pattern boldness can temporally impact individual ‘distinctiveness’ and thereby complicate photo ID (e.g., Urian et al., 2015). The challenge of photo ID in taxa capable of physiological colouration change has indeed been noted by other researchers, including for dusky grouper [Epinephelus marginatus (Lowe, 1834)] (Lelong, 1999), manta rays (Mobula spp.) (Ari, 2014), goliath grouper [Epinephelus itajara (Lichtenstein, 1822)] (Giglio et al., 2014) and small-spotted catshark [Scyliorhinus canicula (Linnaeus, 1758)] (Navarro et al., 2018).
1.2 Pattern stability
Skin pattern development and stability has not been investigated for M. alexandrini, but recent research documented stable and recognizable skin patterns in a captive M. mola individual over a 4.5 year period (Kushimoto et al., 2022). During this time the fish grew from c. 90 to c. 154 cm TL, with the images presented in Kushimoto et al. (2022) seemingly indicating that a slight ‘stretching’ may have occurred, but that the patterns were otherwise identical. However, given the large size spectrum of Mola spp., it is possible that new patterns could potentially develop with growth, akin to Turing’s reaction-diffusion model (Turing, 1952) of pattern formation in biological systems (for examples in teleosts see Kondo and Asai, 1995; Rosenthal and Marshall, 2011; Delcourt et al., 2018). This is particularly pertinent for M. alexandrini due to the large growth spectrum and changes in morphology with size (Sawai et al., 2017; Sawai et al., 2018; Gomes-Pereira et al., 2022; Sawai and Nyegaard, 2022). Most notably, M. alexandrini develops a large head bump, a large chin bump, and bulging lateral ridges with growth, and as for all Mola species, the dorsal and anal fins become shorter and broader relative to fish length (Watanabe and Sato, 2008; Nyegaard et al., 2018; Phillips et al., 2018; Sawai et al., 2018) (Figure 3).

Figure 3 Generalised body morphology of giant sunfish (Mola alexandrini) of (A) 1 m (est. 48 kg), (B) 2 m (est. 484 kg), and (C) 3 m total length (est. 1,864 kg) (see Supplemental Material Figure 2 for examples). Head bump (black arrow), chin bump (grey arrow) and bulging lateral ridges (hashed arrows) develop with size. Estimated weights are based on the length-weight relationship for M. alexandrini in (Sawai and Nyegaard, 2022). Drawings by Robin Ljungfeldt Bryhni.
The growth rate is unknown for M. alexandrini, however, rapid growth has been documented for M. mola in captivity, including an extreme case where an individual increased in mass from 57 to 880 kg in 15 months (Powell, 2001). Another study estimated that M. mola in captivity may reach c. 3 m in length in 20 – 23 years (Nakatsubo and Hirose, 2007), equating to a weight gain of c. 1,500 – 1,750 kg (Nakatsubo and Hirose, 2007; Phillips et al., 2018). If such rapid growth is representative for wild M. alexandrini, and if skin patterns change beyond recognition with growth, long-term photo identification could prove a significant challenge for this species.
This study aims to determine if individual photo ID based on skin patterns is possible and informative for M. alexandrini. To test this, we created the citizen-science platform ‘Match My Mola’ for crowd-sourcing imagery (photos and video) of M. alexandrini in Bali. We undertook trial Photo ID matching to explore if this is achievable despite the species’ capacity for rapid physiological colouration change. We further compared the body morphology of matched individuals for indications of growth between re-sightings. Lastly, we discuss challenges, restrictions and possible applications of photo ID for this and other molid species.
2 Methods
2.1 Image submission
The ongoing citizen science project ‘Match My Mola’ was launched in 2013 to collect and curate new and existing M. alexandrini imagery (photos and video) from the Bali area. Imagery can be submitted via an online platform, email, through social media or (at times) directly to a local project representative. The submitter is invited to name the fish. Imagery is also mined from internet sites Flickr, YouTube, and iNaturalist, as well as local tour operators’ social media accounts (Facebook and Instagram). In addition, images obtained during related molid research dives in the Nusa Penida MPA (Nyegaard, 2018) were added. The term ‘Photo Event’ here refers to one or more images of the same fish taken by the same photographer during the same dive and logged with ‘Match My Mola’.
2.2 Metadata processing
Photo Event metadata are collated in an off-line Photo Event Database built with Claris Filemaker Pro. Metadata includes date, time, location, temperature and depth of the Photo Event, date and platform of submission (or mining), photographer and submitter (or person posting), and all associated communication and links to online footprints. For mined Photo Events, the internet upload date is noted, and the authenticity of mined images and associated metadata are verified by contacting the photographer or person posting. All metadata (submitted and mined) are carefully scrutinised including comparisons of purported date of the Photo Event with image file date stamp (when available), date of submission/mining, and all digital and online footprints. Where information (provided or mined) is ambiguous or appears unreliable for any reason, the metadata resolution is reduced to the lowest verifiable level, such as general area in lieu of a dive site, or month and/or year in lieu of an exact date etc. Where the photographer or submitter cannot be contacted, the metadata is treated as ‘unverified’. For match pairs, all metadata are re-examined, rigorously scrutinised, the divers and background habitat in the images are compared and the photographers/submitters are contacted again if deemed necessary, to gain full confidence in the results.
2.3 Image processing
Photo Events are added to the database as follows: Up to three images (photos and/or video stills) are selected from each side of the fish to overall represent the lateral side of the body posterior of the eye (Figure 2A). As the dorsal and anal fins are used for propulsion in Molidae (Watanabe and Sato, 2008), their patterns are often obscured by shadow effects, and the images are therefore cropped around the body of the fish (Figure 2A). Images showing faint body patterns are digitally enhanced using the application “Photos” by Apple Inc. Following editing, images with indiscernible skin patterns are omitted (e.g., blurry, low resolution, sunfish in silhouette, grossly over- or under-exposed, distant sunfish, sunfish displaying no patterns). However, poor quality images, where skin patterns are nevertheless discernible, are included according to a subjective expectation of the possibility of matching. Each photo event, consisting of either images of the left, right, or both sides of the molid, is assigned a unique Photo Event number and filed in a left and right Photo Event Catalogue.
2.4 Matching
At the time of this study, 1,098 Photo Events from the Bali area were available from ‘Match My Mola’. Matching of Photo Events was undertaken by three observers for the left (n=606) and right (n=633) sides separately. Observer 1 matched all left and right side Photo Events, while observers 2 and 3 each matched a subset of these (Observer 2: n=358 left side, Observer 3: n=354 right side). The observers compared images side-by-side in a random, pairwise manner, using the skin patterns for matching. A particularly useful area for initiating a match check was found to be the confluence of the darker dorsal area and lighter ventral area (Figure 2A). All Match Events (i.e., two Photo Events deemed to depict the same fish) were verified by a fourth observer, who checked all visible patterns across the fish for similarity. Any physical marks such as clavus damage, skin irregularities, scars and injuries were used as secondary verification.
3 Results
3.1 Pattern clarity and physiological colouration change
The clarity of molid skin patterns varied markedly across Photo Events, ranging from fish with no or vague patterns (Figure 2B), to fish with intricate patterns in black and white across most of the body, clavus and fins (Figure 2C). Pattern clarity was clearly influenced by the physiological state of the patterns themselves (i.e., the result of physiological colouration change), as well as by external factors (e.g., light conditions, depth/light attenuation, water clarity, angle and distance between camera and fish, use of artificial light) combined with image quality (e.g., pixel resolution, focus, exposure, shutter speed). To distinguish between the true state of patterns versus the appearance on images, ‘boldness’ herein refers to the physiological state, while ‘conspicuity’ refers to the patterns as they appear on images, i.e., the result of the interplay between boldness, external factors and image quality. This distinction was made to acknowledge the inherent difficulty in determining true pattern boldness on arbitrary underwater images.
A total of n=363 Photo Events included video. Changes in pattern conspicuity was evident on more than a third of these, but most occurred during movements of molid and/or camera. In other words, it was typically not possible to establish that physiological colouration change had indeed occurred, rather than a sudden change in light conditions causing the patterns to appear more conspicuous. Only in a small number of cases could the changes confidently be attributed to physiological colouration change. Combined, these indicated that 1) pattern boldening can happen rapidly in M. alexandrini, e.g., in <10 seconds (Figure 4), and can render the same fish looking very different between moments (Figure 5); 2) at least some parts of the body patterns can become inconspicuous during low contrast, but once ‘emerged’, the patterns remain stable during further physiological colouration change (Figure 6); 3) bold displays appear to be a result of ‘contrast enhancement’ seemingly due to both darkening dark areas, and lightening light areas of the underlying pattern. It is unclear, however, if M. alexandrini can conceal the skin patterns entirely, or if uniformly grey individuals are just ‘missing’ their patterns as an artefact of low quality images and/or suboptimal light conditions (e.g., Figures 4A, B) although high quality images of uniform grey individuals seemingly indicate the former (e.g., Figure 2B). Note here that ‘grey’ refers to the typical colour on underwater images, which may not reflect the true colour of the fish.

Figure 4 A giant sunfish (Mola alexandrini) during rapid physiological colouration change from low contrast to bold display. Unmanipulated stills in two second (s) intervals from digital video (natural light, red filter) by Amin Kancil, Nusa Penida Marine Protected Area, Indonesia (2019). Printed with permission. Video available at https://www.facebook.com/amin.kancil/videos/2776134922397013/.

Figure 5 A Mola alexandrini individual in the Nusa Penida Marine Protected Area, Indonesia, revealing dots and patterns not evident seconds (s) prior. Arrows point to clavus damage, evident during both pattern states. Unmanipulated stills from video (natural light) by Sandra Clopp, printed with permission.
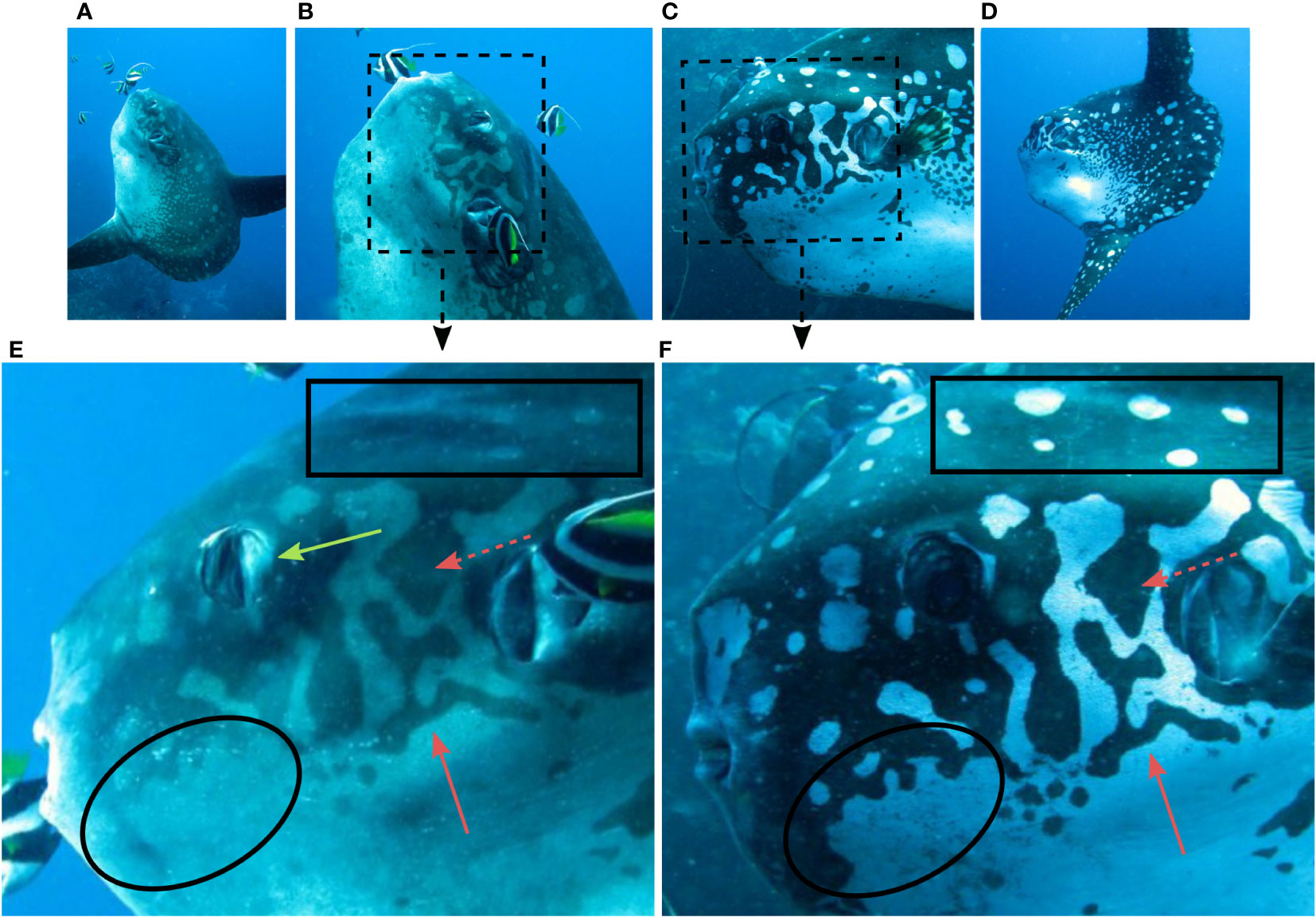
Figure 6 A Mola alexandrini, individual, Nusa Penida Marine Protected Area, Indonesia. (A) The fish was initially in cleaning position (head angled upwards, nictitating membranes covering eyes). As the photographer moved closer (B), the fish righted itself (C), withdrew the nictitating membranes and displayed bold skin patterns before (D) moving away. Inserts (E, F): The skin patterns were stable during the change (compare red arrows), but some patterns were inconspicuous or vague during low contrast (compare black squares and circles). Yellow arrow: nictitating membrane covering the eye. Unmanipulated photos (except (E, F), which were cropped). The images were taken within 60 seconds of each other by Roberto Piazza, 2012, printed with permission.
3.2 Matching
Observer 1 – 3 reported that matching was straight forward when pattern conspicuity was high (e.g., Figure 7), but was more challenging for fish with low pattern conspicuity, especially when image quality was low and/or differing angles between fish required mental geometric rotation. Not all observers found all matches; the subset of Photo Events matched by two observers yielded at total of 20 (left) and 22 (right) unique Match Events. Of these, Observer 1 found 80% (left) and 91% (right), Observer 2 found 100% (left) and Observer 3 found 59% (right).
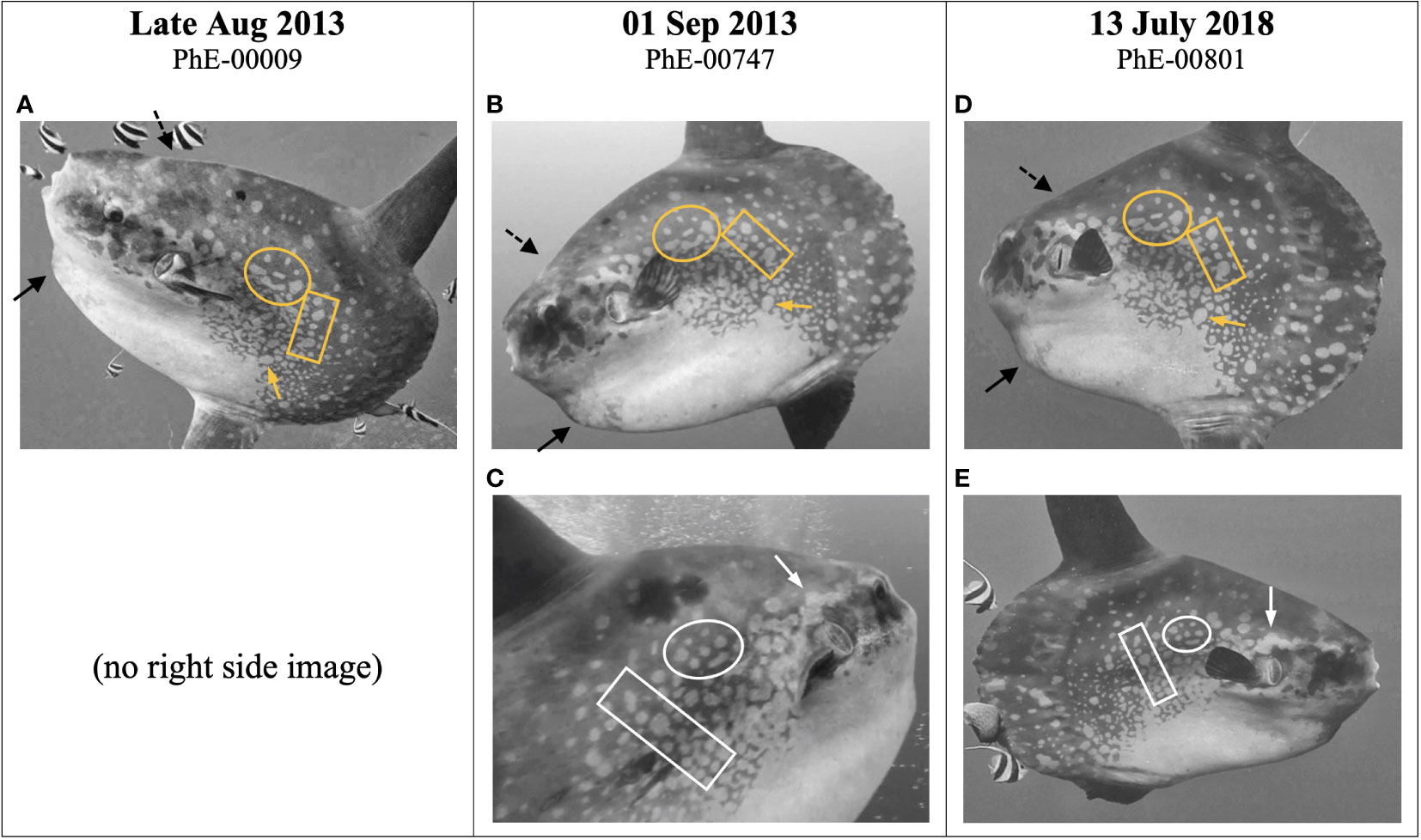
Figure 7 Three separate Photo Events of the same Mola alexandrini individual (“Concept”) at dive site Crystal Bay, Nusa Penida Marine Protected Area, Indonesia (Figure 1). See Supplemental Material Table 1 for full metadata details. Coloured arrows and boxes indicate examples of matching patterns. Black arrows point to chin bump (solid) and head bump (hashed). ‘PhE’ is the Photo Event number. (A) Photo by Sabrina Si Sadi; stills from video by (B, C) Michael Shark and (D, E) Niamh Lynch, printed with permission. All images were converted to black and white and the contrast was adjusted to enhance the patterns.
Combined across all Photo Events (i.e., matched by one or two observers), the three observers found a total of 107 unique Match Events (60 left, 47 right). All Match Events were confirmed by the fourth observer, with no false matches identified. Approximately half of the Match Events (52% left, 54% right) were secondarily verified based on physical marks, or from matching based on the other side of the fish (e.g., Figure 7).
Most Match Events consisted of image pairs with similar skin pattern extents (e.g., Figure 7). A small number (n=17) consisted of images where only a subset of patterns was visible on one Photo Event compared with the other. All pairs were highly similar in body morphology (i.e. appeared to be the same fish), with n=6 pairs secondarily verified via physical marks. The differences in pattern extent appeared to be caused by a combination of low contrast display by the fish and poor image quality, which rendered parts of the patterns on one image indiscernible compared with the other (see Supplemental Material Figure 3 for an example).
The 107 unique Match Events mostly comprised same-day (n=32) or likely same-day Photo Events (incomplete/unverified data) (n=50), i.e., individual fish which had been photographed on the same day by different photographers and submitted independently of each other. A further n=9 Match Events had unverified and/or insufficient metadata to determine the duration between resightings. The remaining Match Events (n=15) comprised nine individuals, which had been photographed with intervals of 1–2,652 days (Figure 8). The high quality of the extensively scrutinised metadata for the associated Photo Events boosted confidence in the results (see Supplemental Material Table 1 for details).
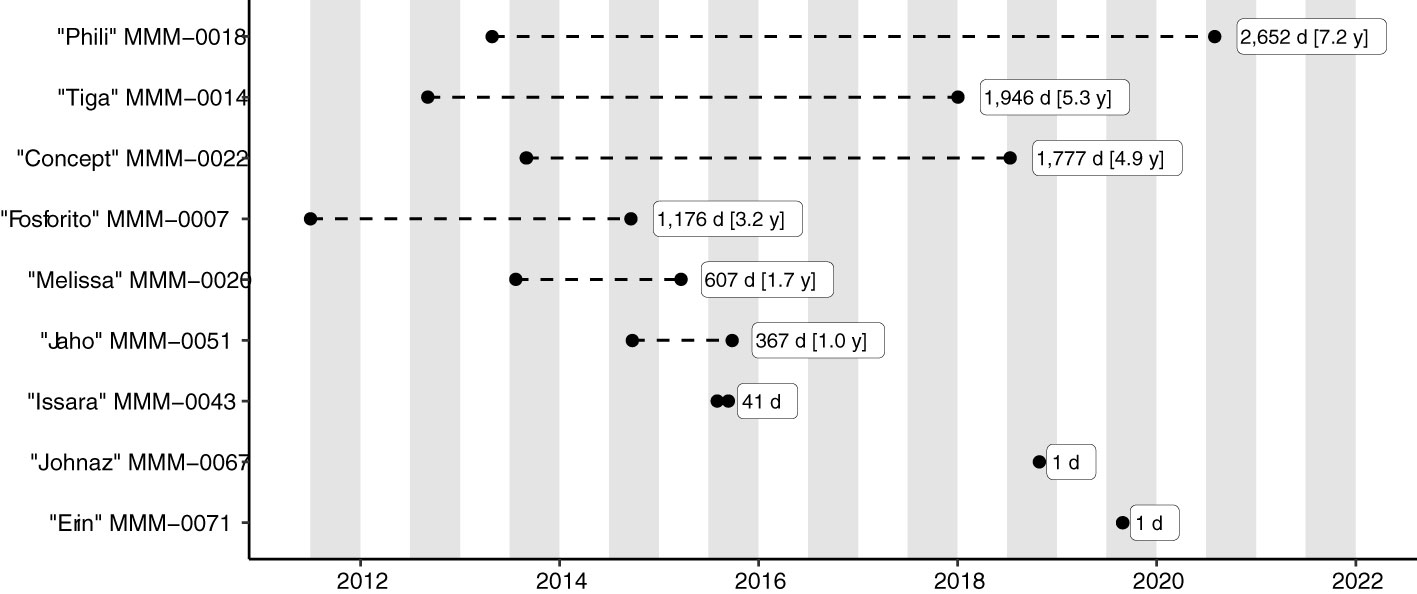
Figure 8 Photo Events (black circles) of individual Mola alexandrini in the Nusa Penida Marine Protected Area, Indonesia, with resighting intervals ≥ 1 day (d). Grey bars indicate ‘sunfish seasons’ (approx. Jul/Aug – Nov/Dec; see text for details). The MMM-number is the Match My Mola unique identifier for individual fish, here given together with the submitters’ fish name.
3.3 Fish size
Remarkably, all matched individuals had similar body morphologies between Photo Events despite some being separated by several years (Figures 7, 9A–E). For example, Fish “Fosforito” was photographed with 3.2 years interval with no indication of morphological changes (and thereby growth) during this time. Generally, no size estimates are available for Match My Mola submissions, however, an accurate size estimate was available for “Fosforito” (c. 160–180 cm TL) from the second Photo Event, where first author M. Nyegaard was able to compare her own height to the length of the fish during in-water biopsy sampling. Back-casting using the Von Bertalanffy growth curve for captive M. mola (Nakatsubo and Hirose, 2007), “Fosforito” would have been c. 63 – 95 cm TL at the first Photo Event 3.2 years earlier (PhE-00007; Figure 9C). Using the length-weight relationship for M. alexandrini (Sawai and Nyegaard, 2022) the difference in body mass would have been substantial; c. 10 – 41 kg (first Photo Event), and c. 231 – 341 kg (second Photo Event). However, such a small size during the first Photo Event is incompatible with both the body morphology and the in-water observations by the experienced dive guide who submitted the photograph (K. Belykh, pers com 2013). Instead, the comparable body morphologies indicate limited growth between the two Photo Events.

Figure 9 Five different individuals of Mola alexandrini, photographed in the Nusa Penida Marine Protected Area, with 1.0 – 7.2 years interval. ‘PhE’ is the Photo Event number. See Supplemental Material Table 1 for full metadata details, and Supplemental Material Figure 4 and Supplemental Material Figure 5 for detailed comparison of skin patterns between Photo Events. Photos or stills from video by: (A) (left) Choocart Treetavekul; (B) (left) Emanuel Groult; (B) (right) Marianne Nyegaard; (B) (right) Ben Coffey; (C) (left) Lembongan Dive Adventure; (C) (right) Jonathan Andersen; (D) (left) Tomoki Ueda; (D) (right) Benny Gunawan; (E) (left) Aquatic Alliance; (E) (right) Keith Mash. All images printed with permission.
Similar theoretical calculations and hypothesized body sizes between Photo Events become increasingly unrealistic for other Match Events with longer re-sighting durations (Figure 7, Figures 9D, E). For example, the individual labelled “Phili” was re-sighted after 7.2 years (Figure 8). This fish had remarkably similar body morphology during both Photo Events and was also similar in size relative to the surrounding cleaner fish (Figure 9E) indicating limited growth between re-sightings. For comparison, during 7.2 years in captivity, M. mola is estimated to grow from the ‘size at age 0’ (Mola spp. hatch at c. 2 mm; Martin and Drewry, 1978) to c. 209 cm TL, corresponding to an estimated weight gain of 493 – 572 kg (Nakatsubo and Hirose, 2007; Phillips et al., 2018).
4 Discussion
We undertook trial Photo ID matching of giant sunfish (M. alexandrini) based on 1,098 citizen science imagery submissions from the Bali area. Matching was possible despite the capacity of M. alexandrini to rapidly (within seconds) change the boldness of their skin patterns, and consequently look very different between moments. Most matches were same-day photos by different divers, with a small number (n=9) with longer resighting durations (1–2,652 days). For these, the body morphology was virtually identical between matches, suggesting limited growth, starkly contradicting the current understanding of rapid growth in captive molids (M. mola).
4.1 Matching
Our study confirmed that physiological colouration change in M. alexandrini from low contrast to bold patterns can occur in seconds, and that the same individual can look very different between moments, corroborating observations of captive M. mola (Kushimoto et al., 2022). This ability clearly influences pattern conspicuity on images and thereby the ‘distinctiveness’ of individual between moments (e.g., Urian et al., 2015).
Our study confirmed that true positive matches (correctly determining that two individuals are the same) is nevertheless possible, as also suggested for M. mola (Kushimoto et al., 2022). Our study furthermore confirmed that it is possible to match individuals where the skin pattern extent differs between images. However, determining true negative matches (correctly determining that two individuals are not the same) is challenging for fish in low contrast display, in particular where image quality is low. Aggressive image grading to exclude fish with little or vague patterns could solve this issue, but would require development of a ‘cut-off’ point (non-trivial), and would reduce the number of images available for positive matches – a significant issue due to the time consuming task of crowd sourcing images and reliable metadata.
4.2 Physiological colouration change
The biological function of rapidly changing displays in Mola spp. remains unclear. Physiological colouration change in fish is complex and can serve both as intra- and inter-specific communication, as well as crypsis and means of predator avoidance (Rosenthal and Marshall, 2011; Sköld et al., 2016; Figon and Casas, 2018). As the bold skin patterns in M. alexandrini render individuals striking and highly visible, it presumably serves as a signal rather than camouflage [although the visual ability of the receiver ultimately determines how a display is perceived (Stuart-Fox and Moussalli, 2009; Marshall et al., 2019)].
In some taxa, bold displays are associated with aposematism (signaling toxicity) (e.g., Figon and Casas, 2018), however this is doubtful for M. alexandrini. Despite previous beliefs that Mola spp. contain tetrodotoxin and is toxic to marine mammals (e.g., Halstead 1968 in Gladstone, 1988) this remains unsubstantiated (Baptista et al., 2020; Baptista et al., 2022) and seems unlikely as molids fall prey to both orca (Visser et al., 2023) and sealions (Powell, 2001; Nyegaard et al., 2019).
In other teleost taxa, boldening of patterns and/or darkening body colours is a fright response (Potts, 1974; Beeching, 1995), and in yet others is associated with intra-specific aggression and/or dominance (Hamilton and Peterman, 1971; Dawkins and Guilford, 1993; Delcourt et al., 2018). Although quantitative data is lacking, the boldening of skin patterns in M. alexandrini in the Nusa Penida MPA appears to be associated with disturbance during cleaner fish interactions (M. Nyegaard pers obs). While a freight response cannot be ruled out, the bold patterns may be a warning signal to intimidate intruding divers by accentuating the molid’s large body size. Indeed, harassed M. alexandrini individuals sometimes ‘glide’ slowly past the intruding divers rather than swimming off, seemingly ‘show-casing’ both the large body size and vibrant lateral display (M Nyegaard, pers obs). In turn, M. alexandrini engaged in cleaning are typically displaying low contrast patterns (M Nyegaard pers obs). This may be a neutral pattern state, or could be a component of cleaner-client signaling, perhaps serving to render skin parasites more visible to the cleaner fish, as seen in some teleost taxa (Hobson, 1965; Hobson, 1969; Hobson, 1971; Caves et al., 2018).
If boldening of skin patterns in M. alexandrini is indeed a response to diver disturbance then any behavioral differences between individual M. alexandrini (akin to the bold-shy trait continuum; Hulthén et al., 2014) could potentially introduce bias in photo identification towards individuals (or perhaps sex)? which more readily display bold patterns. Bias within the same individual could even arise over time if molids develop a tolerance to diver disturbances (e.g. Titus et al., 2015), or shy individuals become more bold (e.g., Hulthén et al., 2014), influencing their propensity to display bold patterns. Such challenges and biases must be considered in relation to individual research questions where photo ID is applied, including for creation of discovery curves and application of capture-recapture models (e.g. Germanov et al., 2019).
4.3 Within-season Match Events
The findings of several Match Events consisting of same-day Photo Events submitted by different photographers was not unexpected as molids in the Nusa Penida MPA are frequently surrounded by large numbers of divers (Nyegaard, 2018; M. Nyegaard, T. Thys, J. Karmy, M. Welly pers. obs.). In contrast, the low number of multi-day matches within the same sunfish season was unexpected, as telemetry studies have found that at least some individual M. alexandrini exhibit seasonal site fidelity to the area within sunfish seasons (Thys et al., 2016; Nyegaard, 2018). During the 2015 sunfish season, four individuals tagged with high-accuracy Fast-loc satellite tags appeared to utilise the Lombok Strait to forage, with occasional visits very close to shore, both off Nusa Penida and Bali (Nyegaard, 2018) – presumably to seek cleaner fish interactions. However, only four within-season Match Events were found in the current study, with two of these being on consecutive days. It is unclear if our results reflect low within-season re-visitation rates by individuals, or if re-visitations occur more frequently, but were not captured by the relatively low number of Photo Events per season in this study. Or, if once photographed, fish avoid re-entering areas replete with piscine paparazzi, akin to hook-shy fish avoiding hooks for some time after having been hooked once (e.g., Brown, 2015).
4.4 Inter-annual Match Events
The number of inter-annual Match Events (n=6), while low, provided unexpected results with regards to the limited indications of growth over the 1.0 – 7.2 year duration between Photo Events. One possible explanation is that the six re-sighted individuals were outliers, which had simply failed to grow, allowing them to be matched due to their unchanged body patterns. However, captive M. mola individuals retain stable body patterns which are recognisable over at least 4.5 years and across a growth span of at least c. 90 – c. 156 cm (Kushimoto et al., 2022). Consequently, we would expect to find inter-annual matches of M. alexandrini exhibiting at least some evidence of growth, e.g., with increased size of the head bump and chin bump, increased swelling of the lateral ridges, and decreased aspect ratios of the dorsal and anal fins, however, no such matches were found in this study.
Another possibility is that all matched individuals were males at or near their maximum size, exhibiting slow growth. In M. alexandrini females appear to grow larger than males (Sawai and Nyegaard, 2022), similar to both M. mola (Sawai et al., 2011; Sawai et al., 2018) and Ma. lanceolatus (Liu et al., 2009). The maximum size of M. alexandrini males is not known, but is at least 197 cm TL (Vogelnest, 2003; see Supplemental Material Figure 6). An all-male Match Event scenario at Nusa Penida, however, still does not account for the lack of inter-seasonal matches of individuals exhibiting at least some evidence of growth (i.e., females) – unless only males frequent the Nusa Penida MPA. As external morphological sexual dimorphism has not been established for M. alexandrini (apart from size) the latter cannot currently be explored based on ‘Match My Mola’ imagery.
It is also possible our findings reflect markedly slower growth in wild M. alexandrini compared with captive M. mola. Captivity indeed appears to influence life history traits in M. mola. Nakatsubo and Hirose (2007) found that both body weight and gonadosomatic index were higher relative to TL in captive versus wild M. mola. Further, the diet of captive M. mola must be carefully controlled to avoid obesity (Howard et al., 2020). Currently, the only study on growth in wild Molidae is for Ma. lanceolatus. Based on vertebrae growth ring chronology, Liu et al. (2009) estimated longevities (i.e., years to reach 99% of the estimated maximum size) of 105 and 82 years for female and male Ma. lanceolatus, respectively. While this lends credibility to a slow-growth scenario for wild M. alexandrini, it is currently unknown if the findings of Liu et al. (2009) are representative of wild Mola spp. In summary, our findings highlight the current knowledge gaps in age and growth in wild Mola spp. It is essentially unclear whether these unusual fishes are short-lived and exhibit fast growth (akin to captive M. mola), or are long(er)-lived with slow(er) growth (as reported in wild Ma. lanceolatus).
Molid age and growth information is critical to gauge resilience to anthropogenic pressures such as interactions with commercial fisheries, and to establish effective conservation management plans (Liu et al., 2015; Forsgren et al., 2020; Nyegaard et al., 2020). Mola spp. otoliths are small and granular (Nolf and Tyler, 2006) and inadequate for age estimates, so other approaches are needed (Hays et al., 2020). Eye lens nuclei chronology (e.g., Nielsen et al., 2016), vertebrae chronology (e.g., Liu et al., 2009), or epigenetics (e.g., Mayne et al., 2021) may potentially be informative, and if growth is slow then bomb radiocarbon dating (e.g., Allen and Andrews, 2012) could be relevant for very large M. alexandrini individuals. Photo ID catalogues such as ‘Match My Mola’ could potentially provide important supportive information. Specifically, by establishing relative morphometrics (for example dorsal fin aspect ratio relative to fish length) body size and growth between Photo Events could potentially be estimated. However, further research is needed to establish such relative morphometrics for M. alexandrini, and to explore the robustness of such an approach. Relative morphometrics could be obtained underwater from live molids using photogrammetry (Deakos, 2010) and from preserved museum specimens (Nyegaard and Sawai, 2018).
4.5 Population indicators
Very few multi-day matches were found in our study. While a direct comparison is not possible due to differing methods, a photo ID study of manta rays [Mobula alfredi (Krefft, 1868)] in the Nusa Penida MPA recently found 624 uniquely identified individuals (6,087 images; June 2004–April 2018), with 82% of these sighted more than once, 29% sighted more than 10 times, and 5% sighted 31–99 times (Germanov et al., 2019).
The low re-sightings rate in our study may be linked to the limited overlap between molids and SCUBA divers in the Bali area. Here, M. alexandrini forage in deep water of several hundred meters, spend little time near the surface (Nyegaard, 2018), and generally only overlap with SCUBA divers when seeking cleaner fish interactions on reefs of <40 m water depth (with a frequency currently unknown). In contrast, manta rays can be seen off Nusa Penida during a wider spectrum of behaviours, including cleaner fish interactions, foraging, courtship and cruising (Germanov et al., 2019). Individual manta ray observations and interactions furthermore occur over much longer periods, in much shallower waters and in much less challenging current conditions compared with M. alexandrini (Nyegaard pers obs). Overall, this provides more frequent and better photo opportunities for manta rays by both SCUBA divers and snorkelers, and allows for faster acquisition of high quality images for photo ID, which overall increases the chances of resighting the same animal when it is in the area.
Our low re-sightings rate may also reflect differences in site fidelity to the Nusa Penida area between manta rays and molids. Mobula alfredi individuals are generally sighted multiple times over several years (Germanov et al., 2019). In turn, at least some M. alexandrini individuals move widely (at least hundreds of km) both within and outside the sunfish season (Thys et al., 2016; Nyegaard, 2018), with unknown visitation rates to the Nusa Penida reefs both within, outside and between seasons.
In comparison, both small-scale site fidelity and wide movements have been recorded elsewhere for M. alexandrini. Acoustic telemetry in the Galapagos Islands found a high degree of site fidelity to a “sunfish hotspot” (Punta Vicente Roca), whereby three of five tagged M. alexandrini were detected nearly year-round over a duration of 202–733 days (Thys et al., 2017). However, another individual tagged with a satellite tag first remained local for c. one month, then moved 2,700 km westward in c. two weeks (Thys et al., 2017). Similarly, some individuals tagged in the Nusa Penida MPA have been tracked moving away hundreds of km, both within and outside the sunfish season (Thys et al., 2016; Nyegaard, 2018), and most recently, four M. alexandrini tagged in Taiwan moved between 552–6,952 km; two travelled north (one reached Japan after 178 days), while two travelled south and crossed the equator, with one reaching New Caledonia after 240 days (Chang et al., 2021).
It is not clear if M. alexandrini in the Bali area belong to a large and/or open population, to what extent individuals remain in the area outside the sunfish season, nor if some/all undertake large scale movements of hundreds or thousands of kilometers. However, our study demonstrates that among a relatively limited number of Photo Events from the small area of Nusa Penida, we nevertheless resighted a small number of individuals with several years interval (longest of 7.2 years). This is to the best of our knowledge the longest example of site fidelity in any molid species, and the first confirmation that the same individual may return to the Nusa Penida MPA reefs between years. This site fidelity may be further explored through continued photo ID, in particular if combined with acoustic telemetry within the Nusa Penida MPA. Satellite tracking and population genetics could shed light on wider movements and connectivity with M. alexandrini elsewhere, including in the waters of Taiwan and Japan, where molids are fished commercially for human consumption (Nyegaard et al., 2020).
4.6 Other applications
Common uses of curated Photo-ID image databases include determining population parameters such as sex ratios and maturity based on external morphology, however, neither is currently possible for M. alexandrini (or other molids) as external morphological sexual dimorphism and size at maturity is unknown (Hays et al., 2020; Nyegaard et al., 2020). However, if relative morphometrics were established, population size distributions within and between seasons could be estimated and compared between seasons. Other potential applications include assessing injury rates from boat strikes and entanglement in fishing gear, which would be informative in understanding impacts from human marine activities within the Nusa Penida MPA.
To date, ‘Match My Mola’ has confirmed that molids seen by SCUBA divers on Nusa Penida and Bali reefs are almost exclusively M. alexandrini (Nyegaard, 2018). Recently, SLKT et al. (2021) also reported M. mola in the Nusa Penida MPA, however, examination of images provided to us by one of the authors points to M. alexandrini (Nyegaard pers obs). To date, the only confirmed records known to us of other species in the area are three videos of Ma. lanceolatus lodged with ‘Match My Mola’. These were recorded by: 1) snorkelers near the surface at Toyapakeh, Nusa Penida, on 06 July 2017; 2) SUBA divers at 40 m off Nusa Penida on 31 October 2018; and 3) SCUBA divers off Padangbai, east coast of Bali, on 03 October 2019 (Supplemental Material Figure 7). The videos appear to be of three different individuals, all swimming, and none interacting with cleaner fish. Masturus lanceolatus is known to occur in the Bali area from stranding events (M Nyegaard unpublished data) and is also listed as a species occurring in commercial and/or artisanal fisheries and/or at fish markets in southern Indonesia (White et al., 2013).
4.7 Automated recognition
As for other taxa, undertaking manual matching of M. alexandrini images is extremely time consuming. Computerised recognition based on algorithms and application of machine learning has been developed for numerous taxa (e.g., https://www.wildme.org; Cheema and Anand, 2017; Miele et al., 2020; Blount et al., 2022) and can significantly ease the matching task. However, these approaches typically require sizeable image datasets to train and test the matching algorithms. Obtaining sufficiently large datasets for elusive species such as molids can be challenging. An alternative approach that shows promising preliminary results for M. alexandrini combines traditional use of algorithms and machine learning, without the need for prior training (Pedersen et al., 2022, Pedersen et al., 2023). Further development of this method could increase match capacity, and be applied to other molid hotspots such as the Galapagos Islands (www.oceansunfish.org), and perhaps aid in photo identification of other elusive taxa.
Data availability statement
By default, the citizen science images submitted to Match My Mola are not shared or published unless explicit permission is provided by the photographer or submitter. The generated match data can be shared upon request. Requests to access the datasets should be directed to bW55ZWdhYXJkQG9jZWFuc3VuZmlzaHJlc2VhcmNoLm9yZw==.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee, Murdoch University, Western Australia (permit R2542/12).
Author contributions
MN founded the photo ID catalogue in 2013 and manages the database, JK and LM assisted in obtaining images, curating and verifying metadata and undertook image matching; MN undertook match checks, did summary statistics and wrote the manuscript, TT critically reviewed the manuscript, RD and MW provided field support to MN and scientific input in the initial stages of the initiative, provided assistance with obtaining Indonesian research permits, shared local knowledge and networks, and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The ‘Match My Mola’ initiative was initially supported by an Australian Postgraduate Award and a Prime Ministers’ Endeavour fellowship to MN at Murdoch University. The majority of work was undertaken on a volunteer basis by the authors and numerous volunteers through the Ocean Sunfish Research Trust, with financial support to the Adopt a Sunfish Project from the Wanderlust Fund, Andy and Steffanie Smith and Charlotte Williams.
Acknowledgments
We are grateful for the help and support of Jonathan Anderson (field work), Olivia Duncan (field work and image matching), the dive community on Nusa Lembongan and on Bali for continued photo submissions and support of the project; numerous volunteers for chasing photos, and countless SCUBA divers and underwater photographers, who continue to submit their photos, answer questions and provide metadata to Match My Mola. We are also grateful to Ching-Tsun (Joyce) Chang, Sven Faust, Etsuro Sawai and Larry Vogelnest for sharing invaluable information, to all photographers, who allowed their images to be reproduced, and to Robin Ljungfeldt Bryhni for creating molid drawings.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1179467/full#supplementary-material
References
Allen L. G., Andrews A. H. (2012). Bomb radiocarbon dating and estimated longevity of giant Sea bass (Stereolepis gigas). Bull. South. Calif. Acad. Sci. 111, 1–14. doi: 10.3160/0038-3872-111.1.1
Ari C. (2014). Rapid coloration changes of manta rays (Mobulidae). Biol. J. Linn. Soc 113, 180–193. doi: 10.1111/bij.12321
Baptista M., Braga A. C., Rosa R., Costa P. R. (2022). Does ocean sunfish Mola spp. (Tetraodontiformes: Molidae) represent a risk for tetrodotoxin poisoning in the Portuguese coast? Mar. Drugs 20, 594. doi: 10.3390/md20100594
Baptista M., Figueiredo C., Lopes C., Costa P. R., Dutton J., Adams D. H., et al. (2020). “Biotoxins, trace elements, and microplastics in the ocean sunfishes (Molidae),” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. D. R. (Boca Raton: CRC Press), 186–215. doi: 10.1201/9780429343360-11
Beeching S. C. (1995). Colour pattern and inhibition of aggression in the cichlid fish Astronotus ocellatus. J. Fish Biol. 47, 50–58. doi: 10.1111/j.1095-8649.1995.tb01872.x
Blount D., Gero S., Van Oast J., Parham J., Kingen C., Scheiner B., et al. (2022). Flukebook: an open-source AI platform for cetacean photo identification. Mamm. Biol. (Special Issue) 102, 1005–1023. doi: 10.1007/s42991-021-00221-3
Britz R. (2022). Comments on the holotype of Orthragoriscus alexandrini, Ranzani 1839 (Teleostei: Molidae). Zootaxa 5195, 391–392. doi: 10.11646/zootaxa.5195.4.6
Brown C. (2015). Fish intelligence, sentience and ethics. Anim. Cogn. 18, 1–17. doi: 10.1007/s10071-014-0761-0
Caves E. M., Green P. A., Johnsen S. (2018). Mutual visual signaling between the cleaner shrimp Ancylomenes pedersoni and its client fish. Proc. R. Soc B Biol. Sci. 285, 20180800. doi: 10.1098/rspb.2018.0800
Chang C. T., Chiang W. C., Musyl M. K., Popp B. N., Lam C. H., Lin S. J., et al. (2021). Water column structure influences long-distance latitudinal migration patterns and habitat use of bumphead sunfish Mola alexandrini in the Pacific Ocean. Sci. Rep. 11, 21934. doi: 10.1038/s41598-021-01110-y
Chaves L. C. T., Hall J., Feitosa J. L. L., Côté I. M. (2016). Photo-identification as a simple tool for studying invasive lionfish Pterois volitans populations. J. Fish Biol. 88, 800–804. doi: 10.1111/jfb.12857
Cheema G. S., Anand S. (2017). Automatic detection and recognition of individuals in patterned species. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 10536, 27–38. doi: 10.1007/978-3-319-71273-4_3
Couffer M. C. (2017). Individually-unique spot patterns of young-of-the-year giant sea bass (Stereolepis gigas) in captive-raised fish. Bull. South. Calif. Acad. Sci. 116, 98–109. doi: 10.3160/soca-116-02-98-109.1
Dawkins M. S., Guilford T. (1993). Colour and pattern in relation to sexual and aggressive behaviour in the bluehead wrasse Thalassoma bifasciatum. Behav. Processes 30, 245–251. doi: 10.1016/0376-6357(93)90136-F
Deakos M. H. (2010). Paired-laser photogrammetry as a simple and accurate system for measuring the body size of free-ranging manta rays Manta alfredi. Aquat. Biol. 10, 1–10. doi: 10.3354/ab00258
Delcourt J., Ovidio M., Denoël M., Muller M., Pendeville H., Deneubourg J. L., et al. (2018). Individual identification and marking techniques for zebrafish. Rev. Fish Biol. Fish. 28, 839–864. doi: 10.1007/s11160-018-9537-y
Duhamel du Monceau H. (1777). Traité général, des pesches, et histoire des poissons qu’elles fournissent, tant pour la sub- sistance des hommes, que pour plusieurs autres usages qui ont rapport aux arts et au commerce (Paris: Saillant & Nyon; Veuve Desaint).
Figon F., Casas J. (2018). Morphological and physiological colour changes in the animal kingdom. eLS 1–11. doi: 10.1002/9780470015902.a0028065
Forsgren K., McBride R. S., Nakatsubo T., Thys T. M., Carson C. D., Tholke E. K., et al. (2020). “Reproductive biology of the ocean sunfishes,” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. D. R. (Boca Raton: CRC Press), 87–104. doi: 10.1201/9780429343360-6
Fricke R., Eschmeyer W. N., van der Laan R. (2022) Eschmeyer’s catalog of fishes -version of 4 Oct 2022. Available at: https://www.calacademy.org/scientists/projects/eschmeyers-catalog-of-fishes (Accessed October 23, 2022).
Germanov E. S., Bejder L., Chabanne D. B., Dharmadi D., Hendrawan I. G., Marshall A. D., et al. (2019). Contrasting habitat use and population dynamics of reef manta rays within the Nusa Penida Marine Protected Area, Indonesia. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00215
Giglio V. J., Adelir-Alves J., Bertoncini A. A. (2014). Using scars to photo-identify the goliath grouper, Epinephelus itajara. Mar. Biodivers. Rec. 7. doi: 10.1017/S1755267214001080
Gladstone W. (1988). Killer whale feeding observed underwater. J. Mammal. 69, 629–630. doi: 10.2307/1381360
Gomes-Pereira J., Pham C., Catarino D., Miodonsky J., Santos M. A. R., Dionisio G., et al. (2022). The heaviest bony fish in the world: a 2744 kg giant sunfish Mola alexandrini (Ranzani, 1839) from the North Atlantic. J. Fish Biol. 102, 1–4. doi: 10.1111/jfb.15244
Hamilton W. J., Peterman R. M. (1971). Countershading in the colourful reef fish Chaetodon lunula: concealment, communication or both? Anim. Behav. 19, 357–364. doi: 10.1016/S0003-3472(71)80017-9
Hays G. C., Houghton J. D. R., Thys T. M., Adams D. H., Ahuir-Baraja A. E., Alvarez J., et al. (2020). “Unresolved questions about ocean sunfishes, Molidae: a family comprising some of the world’s largest teleosts,” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. D. R. (Boca Raton: CRC Press), 280–296. doi: 10.1201/9780429343360-15
Hobson E. S. (1969). Comments on certain recent generalizations regarding cleaning symbiosis in fishes. Pacific Sci. 23, 35–39.
Howard M. J., Nakatsubo T., Correira J. P., Batista H., Baylina N., Taura C., et al. (2020). “Sunfish on display. Husbandry of the ocean sunfish Mola Mola,” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. R. (Boca Raton: CRC Press), 242–261. doi: 10.1201/9780429343360-11
Hulthén K., Chapman B. B., Nilsson P. A., Hollander J., Brönmark C. (2014). Express yourself: bold individuals induce enhanced Morphological defences. Proc. R. Soc B Biol. Sci. 281, 20132703. doi: 10.1098/rspb.2013.2703
Kodric-Brown A. (1998). Sexual dichromatism and temporary color changes in the reproduction of fishes. Amer zool 38, 70–81. doi: 10.1093/icb/38.1.70
Kondo S., Asai R. (1995). A reaction-diffusion wave on the skin of the marine angelfish Pomacanthus. Nature 376, 765–768. doi: 10.1038/376765a0
Konow N., Fitzpatrick R., Barnett A. (2006). Adult emperor angelfish (Pomacanthus imperator) clean giant sunfishes (Mola Mola) at Nusa Lembongan, Indonesia. Coral Reefs 25, 208. doi: 10.1007/s00338-006-0086-9
Kushimoto T., Kakino A., Shimomura N. (2022). Possible individual identifications by the body surface marking patterns in the ocean sunfish Mola Mola and the sharptail sunfish Masturus lanceolatus (Molidae). Ichthy Nat. Hist. Fishes Japan 19, 1–7. doi: 10.34583/ichthy.19.0_1
Lelong P. (1999). Individual identification of dusky grouper, Epinephelus marginatus (Lowe 1834) by cephalic blotches. Mar. Life 9, 29–35.
Ligon R. A., Mccartney K. L. (2016). Biochemical regulation of pigment motility in vertebrate chromatophores: a review of physiological color change mechanisms. Curr. Zool. 62, 237–252. doi: 10.1093/cz/zow051
Liu K. M., Lee M. L., Joung S. J., Chang Y. C. (2009). Age and growth estimates of the sharptail Mola, Masturus lanceolatus, in waters of eastern Taiwan. Fish. Res. 95, 154–160. doi: 10.1016/j.fishres.2008.08.013
Liu J., Zapfe G., Shao K. T., Leis J., Matsuura K., Hardy G., et al. (2015) Mola Mola (errata version published in 2016). The IUCN red list of threatened species 2015: e.T190422A97667070. Available at: https://www.iucnredlist.org/search?query=MolaMola&searchType=species.
Marshall N. J., Cortesi F., de Busserolles F., Siebeck U. E., Cheney K. L. (2019). Colours and colour vision in reef fishes: past, present and future research directions. J. Fish Biol. 95, 5–38. doi: 10.1111/jfb.13849
Marshall A. D., Pierce S. J. (2012). The use and abuse of photographic identification in sharks and rays. J. Fish Biol. 80, 1361–1379. doi: 10.1111/j.1095-8649.2012.03244.x
Martin F. D., Drewry G. E. (1978). Development of fishes of the mid-Atlantic bight: an atlas of egg, larval, and juvenile stages, vol VI - stromateidae through ogcocephalidae. Fort Collins.
Mayne B., Espinoza T., Roberts D., Butler G. L., Brooks S., Korbie D., et al. (2021). Nonlethal age estimation of three threatened fish species using DNA methylation: Australian lungfish, Murray cod and Mary river cod. Mol. Ecol. Resour 21, 2324–2332. doi: 10.1111/1755-0998.13440
Miele V, Dussert G, Spataro B, Chamaillé-Jammes S, Allainé D, Bonenfant C (2020). Revisiting animal photo-identification using deep metric learning and network analysis. Methods Ecol Evol. 12, 863–873. doi: 10.1111/2041-210X.13577
Mucientes G., Irisarri J., Villegas-Ríos D. (2019). Interannual fine-scale site fidelity of male ballan wrasse Labrus bergylta revealed by photo-identification and tagging. J. Fish Biol. 95, 1151–1155. doi: 10.1111/jfb.14111
Nakatsubo T., Hirose H. (2007). Growth of captive ocean sunfish, Mola Mola. Aquac. Sci. 55, 403–407. doi: 10.11233/aquaculturesci1953.55.403
Navarro J., Perezgrueso A., Barría C., Coll M. (2018). Photo-identification as a tool to study small-spotted catshark Scyliorhinus canicula. J. Fish Biol. 92, 1657–1662. doi: 10.1111/jfb.13609
Nielsen J., Hedeholm R. B., Heinemeier J., Bushnell P. G., Christiansen J. S., Olsen J., et al. (2016). Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Sci 353, 702–704. doi: 10.1126/science.aaf1703
Nolf D., Tyler J. C. (2006). Otolith evidence concerning interrelationships of caproid, zeiform and tetraodontiform fishes. Bull. l’Institut R. Des. Sci. Nat. Belgique 76, 147–189.
Nyegaard M. (2018) There be giants! The importance of taxonomic clarity of the large ocean sunfishes (genus Mola, family Molidae) for assessing sunfish vulnerability to anthropogenic pressures. Available at: https://researchrepository.murdoch.edu.au/id/eprint/41666/ (Accessed 01 March 2023).
Nyegaard M., Andrzejaczek S., Jenner C. S., Jenner M. N. M. (2019). Tiger shark predation on large ocean sunfishes (Family Molidae) – two Australian observations. Environ. Biol. Fishes 102, 1559–1567. doi: 10.1007/s10641-019-00926-y
Nyegaard M., Garcia-Barcelona S., Phillips N. D., Sawai E. (2020). “Fisheries interactions, distribution modelling and conservation issues of the ocean sunfishes,” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. D. R. (Boca Raton: CRC Press), 216–242. doi: 10.1201/9780429343360
Nyegaard M., Sawai E. (2018). Species identification of sunfish specimens (Genera Mola and Masturus, family Molidae) from Australian and New Zealand natural history museum collections and other local sources. Data Br. 19, 2404–2415. doi: 10.1016/j.dib.2018.07.015
Nyegaard M., Sawai E., Gemmell N., Gillum J., Loneragan N. R., Yamanoue Y., et al. (2018). Hiding in broad daylight: molecular and Morphological data reveal a new ocean sunfish species (Tetraodontiformes: Molidae) that has eluded recognition. Zool. J. Linn. Soc 182, 631–658. doi: 10.1093/zoolinnean/zlx040
Pedersen M., Nyegaard M., Moeslund T.B. (2023). Finding Nemo’s Giant Cousin: Keypoint Matching for Robust Re-Identification of Giant Sunfish. J. Mar. Sci. Eng. 11, 889. doi: 10.3390/jmse11050889
Pedersen M., Haurum J. B., Moeslund T. B., Nyegaard M. (2022). Re-identification of giant sunfish using keypoint matching. Proc. Northern Lights Deep Learn. Workshop 2022 3. doi: 10.7557/18.6234
Pedersen M., Mohammed A. (2021). Photo identification of individual Salmo trutta based on deep learning. Appl. Sci. 11, 9039. doi: 10.3390/app11199039
Petso T., Jamisola R. S., Mpoeleng D. (2022). Review on methods used for wildlife species and individual identification. Eur. J. Wildl. Res. 68. doi: 10.1007/s10344-021-01549-4
Pedersen M., Nyegaard M., Moeslund T.B. (2022). Finding nemo’s giant cousin: Keypoint matching for robust re-identification of giant sunfish. J. Mar. Sci. Eng. 11, 889. doi: 10.3390/jmse11050889
Phillips N. D., Kubicek L., Payne N. L., Harrod C., Eagling L. E., Carson C. D., et al. (2018). Isometric growth in the world’s largest bony fishes (genus Mola)? Morphological insights from fisheries bycatch data. J. Morphol. 279, 1312–1320. doi: 10.1002/jmor.20872
Potts G. W. (1974). The colouration and its behavioural significance in the corkwing wrasse, Crenilabrus melops. J. Mar. Biol. Assoc. United Kingdom 54, 925–938. doi: 10.1017/S0025315400057659
Powell D. C. (2001). A fascination for fish: adventures of an underwater pioneer (Berkeley and Los Angeles: University of California Press).
Riawan I. M. O., Setiabudi G. I., Merdana I. M., Mariasa I. P. M., Wirasastra K. T. (2019). First molecular identification of sunfish in North Bali water. Adv. Trop. Biodivers. Environ. Sci. 3, 12. doi: 10.24843/atbes.2019.v03.i01.p04
Rosenthal G. G., Marshall N. J. (2011). Communication behavior: visual signals. Encycl. Fish Physiol. 1, 692–698. doi: 10.1016/B978-0-12-374553-8.00080-0
Ruchimat T., Basuki R., Welly M. (2013). Nusa Penida Marine Protected Area (MPA) Bali - Indonesia: why need to be protected? Transylvanian Rev. Syst. Ecol. Res. 15, 193–202. doi: 10.2478/trser-2013-0016
Sawai E. (2017). The mystery of ocean sunfishes [Manbou no himitsu] (Tokyo: Iwanami Shoten Publishers).
Sawai E., Nyegaard M. (2022). A review of giants: examining the species identities of the world’s heaviest extant bony fishes (ocean sunfishes, family Molidae). J. Fish Biol. 100, 1345–1364. doi: 10.1111/jfb.15039
Sawai E., Yamanoue Y., Jawad L., Al-Mamry J., Sakai Y. (2017). Molecular and Morphological identification of Mola sunfish specimens (Actinopterygii: Tetraodontiformes: Molidae) from the Indian Ocean. Species Divers. 22, 99–104. doi: 10.12782/sd.22_99
Sawai E., Yamanoue Y., Nyegaard M., Sakai Y. (2018). Redescription of the bump-head sunfish Mola alexandrini (Ranzani 1839), senior synonym of Mola ramsayi (Giglioli 1883), with designation of a neotype for Mola Mola (Linnaeus 1758) (Tetraodontiformes: Molidae). Ichthyol. Res. 65, 142–160. doi: 10.1007/s10228-017-0603-6
Sawai E., Yamanoue Y., Yoshita Y., Sakai Y., Hashimoto H. (2011). Seasonal occurrence patterns of Mola sunfishes (Mola spp. A and B; Molidae) in waters off the Sanriku region, eastern Japan. Japanese J. Ichthyol. 58, 181–187. doi: 10.11369/jji.58.181
Sèbe M. (2021). The use of photo-identification for moray eel monitoring. Cah. Biol. Mar. 62, 11–16. doi: 10.21411/CBM.A.F5F29BE0
Sköld H. N., Aspengren S., Cheney K. L., Wallin M. (2016). Fish chromatophores - from molecular motors to animal behavior. Int. Rev. Cell Mol. Biol. 321, 171–219. doi: 10.1016/bs.ircmb.2015.09.005
SLKT D., Kamal M. M., Tarsidin T., Yulianto G. (2021). Sunfish (Mola spp.) habitat characteristics on their appearance at dive tourism depths in Nusa Penida waters, Bali. J. Biol. Trop. 21, 149–156. doi: 10.29303/jbt.v21i1.2442
Stuart-Fox D., Moussalli A. (2009). Camouflage, communication and thermoregulation: lessons from colour changing organisms. Philos. Trans. R. Soc B Biol. Sci. 364, 463–470. doi: 10.1098/rstb.2008.0254
Sugimoto M. (2002). Morphological color changes in fish: regulation of pigment cell density and morphology. Microsc. Res. Tech. 58, 496–503. doi: 10.1002/jemt.10168
Thurman C. L. (1998). Rhythmic physiological colour change in Crustacea–a review. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 91, 171–185. doi: 10.1016/0742-8413(88)90184-3
Thys T. M., Hearn A. R., Weng K. C., Ryan J. P., Peñaherrera-Palma C. (2017). Satellite tracking and site fidelity of short ocean sunfish, Mola ramsayi, in the Galapagos Islands. J. Mar. Biol. 2017, Article ID 7097965. doi: 10.1155/2017/7097965
Thys T. M., Nyegaard M., Kubicek L. (2020). “Ocean sunfishes and society,” in The ocean sunfishes: evolution, biology and conservation. Eds. Thys T. M., Hays G. C., Houghton J. D. R. (Boca Raton: CRC Press), 263–279. doi: 10.1201/9780429343360-14
Thys T., Ryan J. P., Weng K. C., Erdmann M., Tresnati J. (2016). Tracking a marine ecotourism star: movements of the short ocean sunfish Mola ramsayi in Nusa Penida, Bali, Indonesia. J. Mar. Biol. 2016, Article ID 8750193. doi: 10.1155/2016/8750193
Titus B. M., Daly M., Exton D. A. (2015). Do reef fish habituate to diver presence? Evidence from two reef sites with contrasting historical levels of SCUBA intensity in the Bay Islands, Honduras. PloS One 10, e0119645. doi: 10.1371/journal.pone.0119645
Turing A. M. (1952). The chemical basis for morphogenesi. Phil. Trans. R. Soc Lond. B 237, 37–72. doi: 10.1098/rstb.1952.0012
Urian K., Gorgone A., Read A., Balmer B., Wells R. S., Berggren P., et al. (2015). Recommendations for photo-identification methods used in capture-recapture models with cetaceans. Mar. Mammal Sci. 31, 298–321. doi: 10.1111/mms.12141
Visser I., Nyegaard M., Fletcher L. (2023). Orca, Orcinus orca (Linnaeus 1758) (Mammalia cetacea), interactions with ocean sunfishes (Family Molidae, genus Mola kölreuter 1766 and Masturus gill 1884): a global review. Biodivers. J. 14, 61–164. doi: 10.31396/Biodiv.Jour.2023.14.1.61.164
Vogelnest L. (2003). “The tale of two ocean sunfishes (Mola Mola) - an unusual mortality in an unusual species,” in Wildlife Disease Association Australasian Section 2003 Annual Conference, Healesville, Victoria: Wildlife Disease Association. 30–32.
Watanabe Y., Sato K. (2008). Functional dorsoventral symmetry in relation to lift-based swimming in the ocean sunfish Mola Mola. PloS One 3, e3446. doi: 10.1371/journal.pone.0003446
Keywords: citizen science, skin pattern stability, bold display, growth, Nusa Penida Marine Protected Area, Bali, Indonesia
Citation: Nyegaard M, Karmy J, McBride L, Thys TM, Welly M and Djohani R (2023) Rapid physiological colouration change is a challenge - but not a hindrance - to successful photo identification of giant sunfish (Mola alexandrini, Molidae). Front. Mar. Sci. 10:1179467. doi: 10.3389/fmars.2023.1179467
Received: 04 March 2023; Accepted: 19 April 2023;
Published: 05 May 2023.
Edited by:
Nuno Queiroz, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalReviewed by:
José Pedro Andrade, University of Algarve, PortugalJorge Hernández-Urcera, Spanish National Research Council (CSIC), Spain
Copyright © 2023 Nyegaard, Karmy, McBride, Thys, Welly and Djohani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marianne Nyegaard, bW55ZWdhYXJkQG9jZWFuc3VuZmlzaHJlc2VhcmNoLm9yZw==
 Marianne Nyegaard
Marianne Nyegaard Jennifer Karmy
Jennifer Karmy Lauren McBride
Lauren McBride Tierney M. Thys
Tierney M. Thys Marthen Welly3
Marthen Welly3