- 1Department of Veterinary Sciences, University of Messina, Messina, Italy
- 2Department of Chemical, Biological, Pharmaceutical, and Environmental Sciences, University of Messina, Messina, Italy
The genus Stephanostomum consists of digenean trematodes found in many marine teleosts. In this study, Stephanostomum cesticillus (Molin, 1858) Looss, 1899 metacercariae were identified in European hake (Merluccius merluccius, Linnaeus, 1758) caught in the Tyrrhenian Sea. The metacercariae were found encapsulated in the muscle, close to the spine and gills. Out of 131 specimens, 111 (P = 84.7%, mI = 25.1 mA = 21.3) were infected by digenean trematode metacercariae. Morphological and histological evaluations were carried out. The members of this genus are characterised by a double crown of spines close to the oral sucker. For identification of the parasite, molecular analysis was performed via 28S and 18S ribosomal DNA (rDNA) genes. Partial rDNA sequences of Stephanostomum highly matched to S. cesticillus for the percentage of similar identity from the nucleotide database of BLAST. The present survey reports the presence of S. cesticillus metacercariae in M. merluccius for the first time. Our results improve current knowledge on hake parasites to better understand the distribution of S. cesticillus in M. merluccius caught in the Mediterranean Sea and shed light on the life cycle of the parasite adding other possible hosts.
1 Introduction
European hake (Merluccius merluccius, Linnaeus, 1758) is one of the most important and widespread fish species in Western European fisheries (Casey and Pereiro, 1995) and in the Mediterranean Sea (Oliver and Massutí, 1995). Its fishing range extends from Mauritania to off the western coast of Norway (between 21°N and 62°N) and the south of Iceland (Casey and Pereiro, 1995), being more extensive between the British Isles and the south of Spain (ICES, 2011). M. merluccius is a demersal teleost, found mainly between 70 and 370 m depth, although it can be caught from coastal waters (30 m) to deep waters (1,000 m) (Lloris and Matallanas, 2003). The existence of different stocks in European waters is based on a multi-methodological approach: North-East Atlantic and Mediterranean Sea populations of M. merluccius are considered separate stocks (Mattiucci et al., 2004; Cimmaruta et al., 2005; Milano et al., 2014). Moreover, Atlantic and Mediterranean hake populations show significant differences, such as growth rate, size at maturity, recruitment patterns, and spawning season (Froese and Pauly, 2013). This species is the most important demersal species caught in Western Europe. Host–parasite coevolution shows that fish and their parasites are in a dynamic equilibrium (Ferrer-Maza et al., 2014), yet parasitism is frequently neglected in fish health assessments. Several fish parasites cause behavioural and morphological changes in their hosts, and some parasitic organisms may become dangerous and even lethal in systemic infections for both fish and humans (Simões et al., 2010; Hajipour et al., 2022). Parasites can also influence community structure and modulate host population dynamics (Marcogliese, 2005). Stephanostomum is a genus of digenean trematode belonging to the family Acanthocolpidae and includes more than 100 species (Ngamniyom et al., 2020), and its life cycle is considered a model for the study of all genera belonging to Acanthocolpidae (Bray et al., 2005). The first intermediate hosts are gastropod molluscs, within which the parasites develop to the rediae stage. The second intermediate hosts are characterised by many seawater teleosts, in which the parasites develop as metacercariae, mainly encysted in the skeletal muscle. Definitive hosts are large piscivorous teleosts that become infested after ingestion of second intermediate hosts (MacKenzie and Liversidge, 1975; Køie, 1978; Bray and Cribb, 2003). Since 2000, Stephanostomum sp. metacercariae have also been found in some species of bivalve molluscs (Perez-Urbiola and Martinez-Diaz, 2001).
Many studies have reported teleosts from marine and brackish water as hosts of Stephanostomum sp., for example, Stephanostomum cloacum (Srivastava, 1938) found in Triacanthus biaculeatus (Bloch, 1786) and Stephanostomum bicoronatum (Stossich, 1883) described in Sciaena umbra (Madhavi and Shameem, 1993; Bray and Cribb, 2003; Bray and Reimer, 2004). Among M. merluccius digenean trematode infections, Aporocotyle spinosicanalis (Williams, 1958), Hemiurus communis, Hemipera magnaprostatica, and Lecithochirium musculus adult specimens have been found in the stomach of M. merluccius caught in the Mediterranean Sea (Odhner, 1905; Looss, 1907; Gaevskaya and Aleshkina, 1995). There is a high prevalence (up to 100%) of Bucephaloides gracilenses metacercariae retrieved in the brain and spinal nerves of M. merluccius caught in the Irish Sea (Northern Ireland) (Crofton and Eraser, 1954; Johnston and Halton, 1981).
This study reports the first finding of Stephanostomum cesticillus infecting M. merluccius from the southern Tyrrhenian Sea (FAO 37.1.3). Identification of the parasite was performed via 28S and 18S ribosomal DNA sequencing. In addition, histological examination and surface recognition were carried out by scanning electron microscopy (SEM).
2 Material and methods
2.1 Fish and parasite sampling
Between June and December 2020, 131 fish, caught in the southern Tyrrhenian Sea (FAO 37.1.3), were sent from some Sicilian fisheries to the Centre of Experimental Fish Pathology (Centro di Ittiopatologia Sperimentale della Sicilia (CISS)) at the University of Messina. Biological indices of body weight (BW) and total length (TL) were recorded for each specimen, mean + standard deviation (range) for length (MW) and weight (MW) were calculated, and fish gender was recorded. Epidemiological indices of infection as prevalence (P%), mean abundance (mA), and mean intensity (mI) were calculated according to Bush et al. (1997). Spearman’s correlation coefficient and chi-square test were used, respectively, to evaluate the relationship between the biometric data (body weight and total length) and gender of M. merluccius and S. cesticillus cyst abundance. The level of significance was set at p-value < 0.05. Statistical analyses were performed using GraphPad Prism version 9.0 software (GraphPad Software, San Diego, CA, USA).
The fish were initially subjected to an external examination to assess any external lesions; a necropsy was later conducted. The metacercarial cysts were gently separated from the fish muscle using needles and scalpel blades under stereomicroscopy (SteREO Discovery.V12, Zeiss, Jena, Germany) and then opened. All parasites were clarified in glycerine for 24 h, mounted, and then identified with keys suggested by Bartoli and Bray (2001). Cysts were sampled in vials and stored at −80°C for molecular analysis.
2.2 Histological analysis
Tissue samples positive to encysted metacercariae were fixed in a 10% buffered formalin (Histoline, Milan, Italy), washed in tap water, dehydrated by increasing alcohol, clarified in xylene, and finally paraffin-embedded. Sections of 3-μm thickness were obtained with a microtome (EG11504 Leica Biosystems, Wetzlar, Germany). Sections were stained with haematoxylin and eosin (H&E) and observed under an optical microscope (Leica DM6B).
2.3 Scanning electron microscopy
Sample preparation was carried out as described by Iaria et al. (2021). Briefly, five metacercariae were dehydrated in increasing alcoholic solutions, dried according to the critical point method, and sputtered with a palladium gold layer (20 nm ± 5%) for observation with SEM (EVO MA10, Zeiss, Jena, Germany).
2.4 Molecular identification
The samples were washed three times in phosphate buffered saline (PBS) for 20 min. Total genomic DNA was extracted from 20 metacercariae cysts using a Wizard SV Genomic DNA Purification System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Subsequently, the concentration and purity of the extracted DNA were verified using the Implen Nanophotometer N50. PCR was carried out using a GoTaq® Colorless Master Mix (Promega, Madison, WI, USA), pairing four different primers: 28S and 18S. The 28S DNA forward primer 5′ GTCCGATAGCGAACAAGTACCGT 3′ and reverse primer 5′ AGCATAGTTCACCATCTTTCGGGTCTCAA 3′ (Mladineo et al., 2010) were used to investigate 18S DNA, and the primer sets Trematodes C-For 5′ ATGGCTCATTAAATCAGCTAT 3′ and Trematodes A-Rev 5′ TGCTTTGAGCACTCAAATTTG 3′ were used, which were found to be specific for trematodes due to the higher polymorphism among trematode species (Routtu et al., 2014). The PCR condition used for 28S was as follows: initial denaturation at 94°C for 30 s, followed by 35 cycles of denaturation at 94°C for 30 s, and annealing 30 s at 58°C, elongation at 72°C for 60 s, and the final extension step was set at 72°C for 10 min. The cycling conditions for 18S were the following: denaturation at 94°C for 2 min once, denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and elongation at 72°C for 60 s repeated for 30 cycles, followed by a final 1-min elongation at 72°C. The positivity of the sample was assessed by electrophoresis on 1% (w/v) agarose gel. Gel extraction was carried out using the Promega kit Wizard® SV Gel and PCR Clean-Up System, and samples were sequenced by Genechron Biotech (Rome, Italy) in both forward and reverse directions using the same primers used for amplification. The resulting sequences were aligned using the ClustalW algorithm (https://www.genome.jp/tools-bin/clustalw) and analysed using the nucleotide BLAST search (BLAST, http://blast.ncbi.nlm.nih.gov/) against the National Center for Biotechnology Information (NCBI; https://blast.ncbi.nlm.nih.gov/Blast.cgi) to calculate the statistical significance of the match.
3 Results
3.1 Necroscopy and histological analysis
Following necropsy, in 111 out of 131 M. merluccius specimens, 2,791 disseminated encysted digenean trematode metacercariae were found in the muscle tissue, close to the spine and gills (P = 84.7%, mI = 25.1 ± 26.54, mA = 21.3 ± 25.54) (Figures 1A, B). All examined fish had a mean length of 30.7 ± 4.7 cm (17.5–49.0 cm) and a mean weight of 287.6 ± 136.3 g (87.0–925.0 g). Moreover, 36.6% were male and 63.4% female.
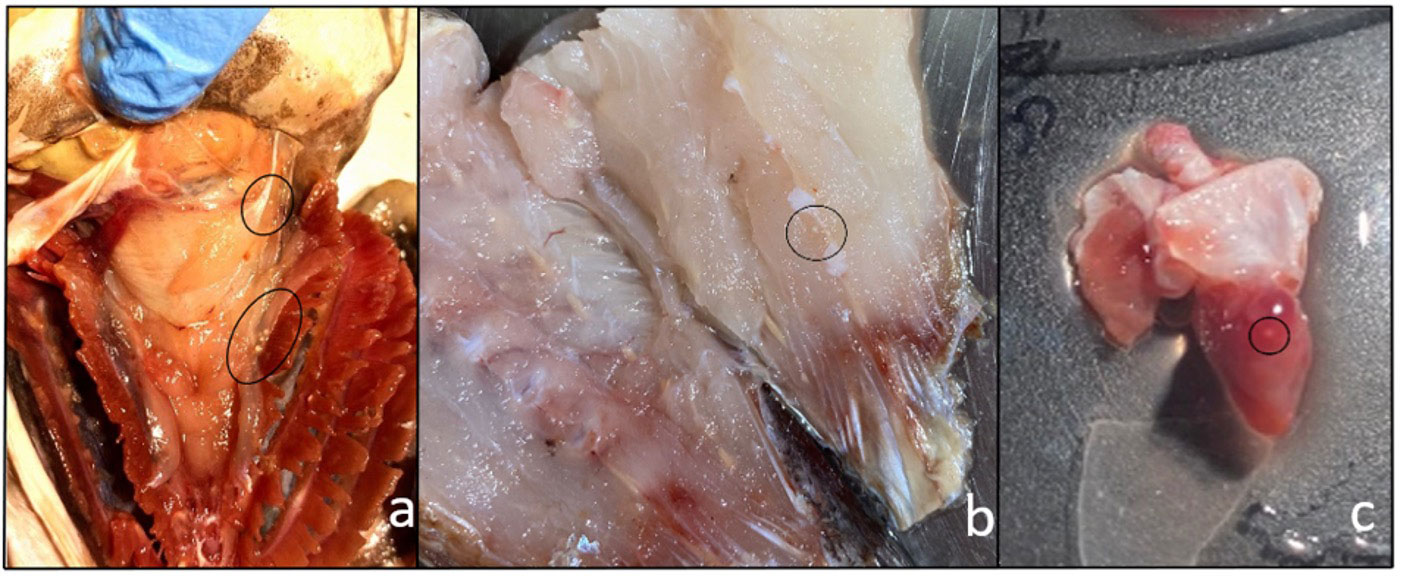
Figure 1 Metacercarial cysts of Stephanostomum cesticillus in different tissues and organs: (A) crowns of cysts underneath gills; (B) cysts lodged in muscle; (C) metacercariae encysted in heart.
No statistically significant differences between S. cesticillus metacercarial abundance and biometric data, including body weight (r = 0.0099; p = 0.9107), total length (r = 0.0951; p = 0.2801), and gender (χ2 = 1.691, df:1; p = 0.1934) of M. merluccius investigated in the current survey, were observed.
Five specimens out of 131 (3.8%) were positive for encysted metacercariae in the heart (Figure 1C). Histological analysis showed the presence of encysted metacercariae in muscle tissue, encapsulated by connective tissue sheets within the host musculature (Figure 2).
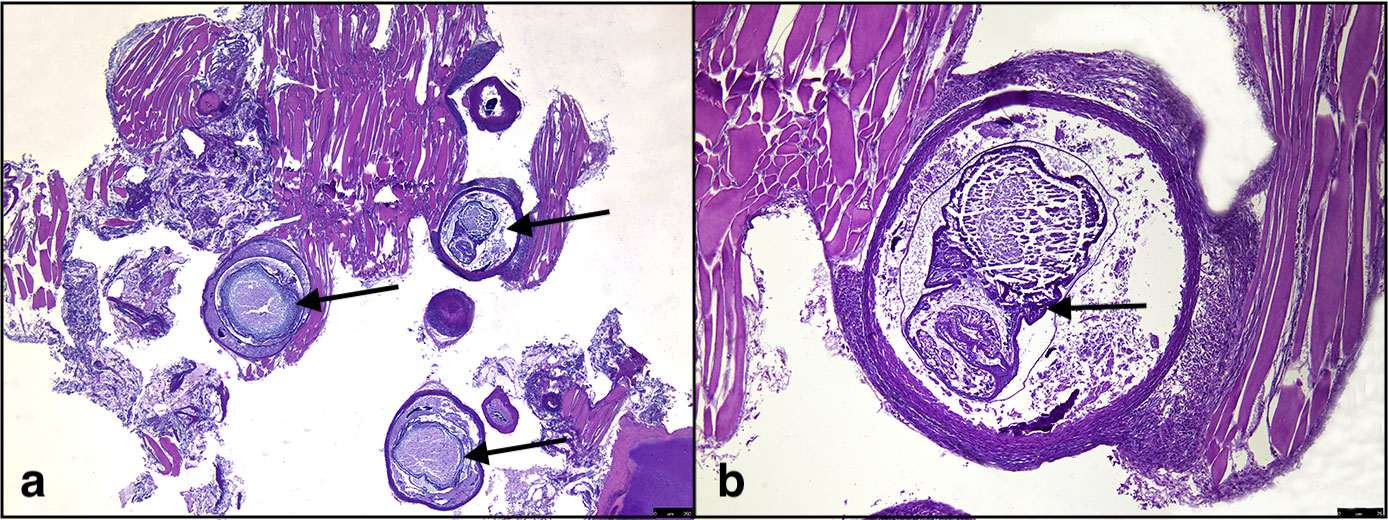
Figure 2 Histological evidence of Stephanostomum cesticillus metacercariae; (A) S. cesticillus metacerciae encysted in muscle tissue (arrows); (B) higher magnification of encysted S. cesticillus metacercaria (arrow).
3.2 Morphological evaluation
The examined spherical cysts were 130 µm in size and whitish in colour. Diaphonised cysts showed that the parasite’s body was curved within a thick cyst wall. Morphological metacercarial evaluation of the cephalic region showed a double crown of spines, composed of approximately 34 spines, around the oral sucker. The entire parasite body was covered with small rod-shaped spines (Figures 3A, B). The ventral sucker was larger than the oral sucker and localised in the middle of the body. Due to the early-stage metacercaria and cyst size, no other morphological characteristics were highlighted by diaphonisation (Figure 4). Morphological characteristics, compared to the keys, allowed us to identify the retrieved parasite as a member of the genus Stephanostomum. For this reason, to better describe the parasite species, also molecular evaluation was performed.
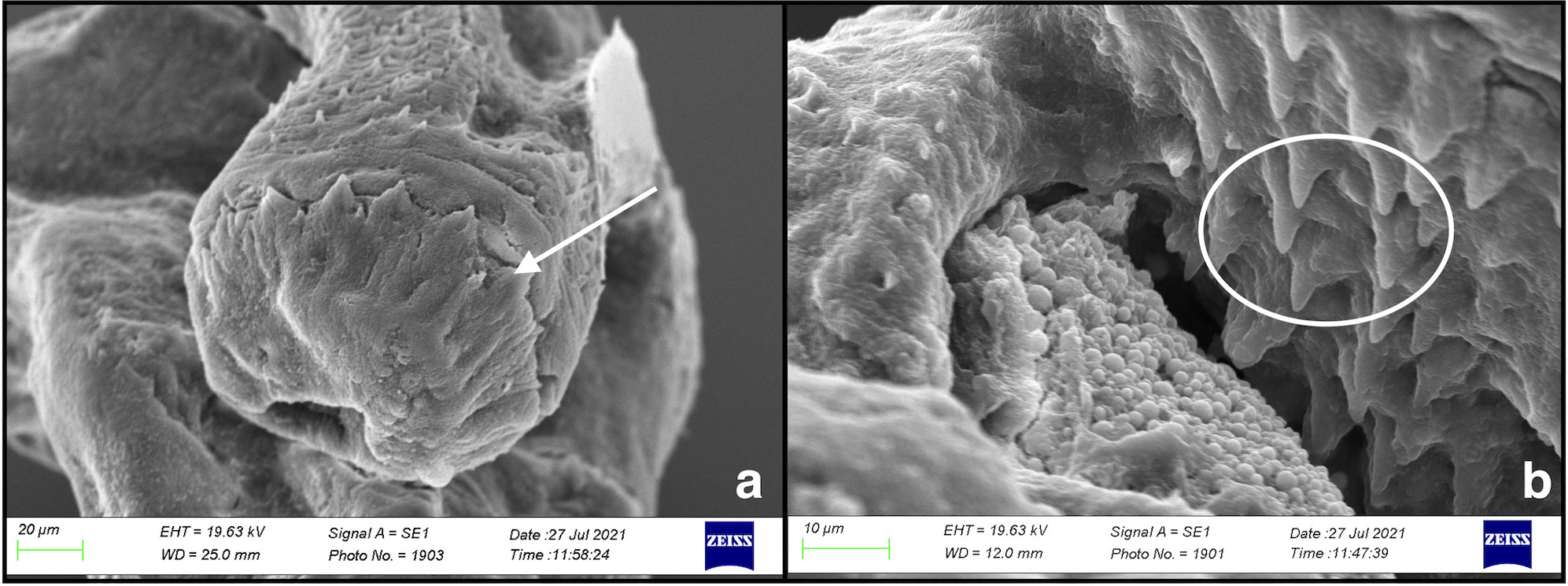
Figure 3 Scanning electron microscopy of Stephanostomum cesticillus metacercaria: (A) circum-oral spines (arrow); (B) small rod-shaped spines on the metacercarial body surface (circle).

Figure 4 Stephanostomum cesticillus metacercaria after glycerin diaphonisation. Oral sucker (arrowhead); ventral sucker (arrow).
3.3 Molecular analysis
The nucleotide sequences of the amplicons (792 and 844 bp for 18S rDNA and 28S rDNA, respectively) obtained from both primer sets were identical among biological replicates. BLAST (NCBI) analysis of the sequences showed a similarity of 100% for S. cesticillus, for both 18S rDNA and 28S rDNA markers (DQ248214.1 and DQ248227.1, respectively), E value of 0.0, and Query cover of 100%. The representative DNA sequences for both small and large subunit ribosomal RNA genes were submitted to GenBank (accession numbers OQ283820 and OQ283821, respectively).
4 Discussion and conclusion
Trematode metacercariae are recorded continuously in many coastal fish species native to the Mediterranean Sea; several authors reported the presence of Stephanostomum spp. infecting various organs and tissues of red mullet (Mullus surmuletus, Linnaeus, 1758) caught in the central Mediterranean Sea (Bottari et al., 2020) and of other teleost species from the Western Mediterranean Sea (Bartoli and Bray, 2001). Among them, Stephanostomum kovalevae in monkfish (Lophius vomerinus, Valenciennes, 1837) intestine, Stephanostomum murielae in bumpnose trevally (Carangoides hedlandensis, Whitley, 1934) digestive tract, and Stephanostomum sp. in orange-spotted trevally (Carangoides bajad) body cavity were reported (Bray and Reimer, 2004; Al-Zubaidy, 2011; Bray and Justine, 2011). Moreover, a significantly high prevalence of encysted Stephanostomum baccatum (Nicoll, 1907) and Stephanostomum tenue (Linton, 1898) metacercariae has been reported in fins, skin, muscle, gills, pericardium, and spleen of fish species (Gibson, 1984). The prevalence data reported confirm the high impact of this parasite in wild demersal teleosts. The highlighted morphological characteristics such as size, number of spines, and body surface analysis all identified metacercariae as a member of the genus Stephanostomum, following characteristic features described by Bartoli and Bray (2001). Identifying trematodes can be difficult when using only morphology due to an early developmental stage or when the sample is found inside the host tissue. Therefore, we carried out molecular identification with a tool that selectively amplifies only trematode DNA and at the same time is universal for trematodes and able to differentiate between trematode species. Selective PCR primer sets for 18S rDNA made it possible to distinguish between different trematodes. The chosen oligonucleotide primer set C-for and A-rev amplified a sequence that allowed the species to be classified into clear clusters. Many authors showed that morphology is not always a reliable indicator of species divergence; morphologically similar trematodes may be genetically diverse (Bray and Cribb, 2003; Routtu et al., 2014). We can conclude that PCR amplification and direct sequencing of 18S and 28S ribosomal DNA (rDNA) products allowed for correct taxonomic assignment.
In this study, we describe S. cesticillus infection in M. merluccius collected from the Mediterranean area. Parasite infection assessment in fish plays a key role in several studies. Moreover, the assessment is important to gather further information on intermediate and definitive hosts, improving current knowledge on host–parasite interactions and trematode parasitic disease spread. The variability of parasites between populations in different geographical areas is a crucial factor in stock assessment and has been demonstrated and thoroughly investigated by several authors (Espínola-Novelo and Oliva, 2021).
The data here reported add information on the possibility of using S. cesticillus as a biological tag since it has never been described in Mediterranean hake; thus, reports of these parasites could add information on the distribution of this fish species. Although a positive correlation between biological indices and parasite abundance has been reported in freshwater and seawater fish (Poulin and Morand, 2005; Cruz and Alexander, 2013; Lisnerová et al., 2022), in our study, no correlation was observed, confirming a homogeneous distribution and a perfect interaction between S. cesticillus and M. merluccius. Furthermore, as reported in several interim studies, the data support and improve knowledge on disease transmission and the prevalence of these parasites. This study represents the first report of S. cesticillus infection in muscle tissue of Mediterranean hake. Despite the high prevalence reported, this parasite species, even if highly similar to other digenean trematodes that are considered zoonotic agents (e.g., Heterophyidae and Diplostomidae), is not characterised by zoonotic potential and does not represent a risk for humans.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI database https://www.ncbi.nlm.nih.gov/nuccore/ accession numbers OQ283820 - OQ283821.
Author contributions
Conceptualisation: CG and GB. Data curation: SN and KR. Formal analysis: GB, SN, and RF. Funding acquisition: CI. Investigation: CG, GB, and SN. Methodology: JA, RF, KR, and DP. Supervision: CI. Roles/writing—original draft: CG, GB, and CI. Writing—review and editing: GB and CI. All authors contributed to the article and approved the submitted version.
Funding
Research funds were provided by PO FEAMP 2014-2020 2.56 FISH PATH NET, grant no. G47B18000130009 Project code SIPA 01/MS/18.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Zubaidy A. B. (2011). Digenetic trematodes (Acanthocolpidae lühe 1906: genus Stephanostomum looss 1899) from red Sea fishes, Yemen coast. J. King Abdulaziz Univ. 22, 65. doi: 10.4197/Mar.22-1.5
Bartoli P., Bray R. A. (2001). Contribution to the knowledge of species of the genus Stephanostomum looss 1899 (Digenea: acanthocolpidae) from teleosts of the Western Mediterranean, with the description of S. gaidropsari n. sp. Syst. Parasitol. 49, 159–188. doi: 10.1023/A:1010676806117
Bottari T., Gaglio G., Mobilia V., Garofalo G., Iaria C., Fiorentino F. (2020). Discrimination of red mullet populations (Teleostean, mullidae) off the Sicilian coasts (Mediterranean Sea) on the basis of metazoan parasites. Thalassas: Int. J. Mar. Sci. 36, 357–363. doi: 10.1007/s41208-020-00211-1
Bray R. A., Cribb T. H. (2003). Species of Stephanostomum loos (Digenea: acanthocolpidae) from fishes of Australian and south pacific waters, including five new species. Syst. Parasitol. 55, 159–197. doi: 10.1023/a:1024655818783
Bray R. A., Justine J.-L. (2011). Acanthocolpidae (Digenea) of marine fishes off new Caledonia, with the descriptions of two new species. Folia Parasitol. (Praha) 58, 35. doi: 10.14411/fp.2011.004
Bray R. A., Reimer L. W. (2004). Two species of Stephanostomum looss 1899 (Digenea: acanthocolpidae) from marine fishes off Namibia, including S. beukelaardori n. sp. Syst. Parasitol. 58, 209–216. doi: 10.1023/b:sypa.0000032931.23060.d3
Bray R. A., Webster B. L., Bartoli P., Littlewood D. T. J. (2005). Relationships within the acanthocolpidae lüh And their place among the digenea. Acta Parasitol. 50, 281–291.
Bush A. O., Lafferty K. D., Lotz J. M., Shostak A. W. (1997). Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 83, 575–583. doi: 10.2307/3284227
Casey J., Pereiro J. (1995). European Hake (M. merluccius) in the north-east Atlantic. Hake: Biology fisheries markets 15, 125–147. doi: 10.1007/978-94-011-1300-7_5
Cimmaruta R., Bondanelli P., Nascetti G. (2005). Genetic structure and environmental heterogeneity in the European hake (Merluccius merluccius). Mol. Ecol. 14, 2577–2591. doi: 10.1111/j.1365-294X.2005.02595.x
Crofton H. D., Eraser P. G. (1954). The mode of infection of the hake, Merluccius merluccius (L.) by the trematode Bucephalopsis gracilescens (Rud.). Proc. Zoological Soc. London 124, 105–109. doi: 10.1111/j.1096-3642.1954.tb01482.x
Cruz M. G., Alexander M. E. (2013). Uncertainty associated with model predictions of surface and crown fire rates of spread. Environ. Model. Software 47, 16–28. doi: 10.1016/j.envsoft.2013.04.004
Espínola-Novelo J. F., Oliva M. E. (2021). Spatial and temporal variability of parasite communities: implications for fish stock identification. Fishes 6, 71. doi: 10.3390/fishes6040071
Ferrer-Maza D., Lloret J., Munoz M., Faliex E., Vila S., Sasal P. (2014). Parasitism, condition and reproduction of the European hake (Merluccius merluccius) in the northwestern Mediterranean Sea. ICES J. Mar. Sci. 71, 1088–1099. doi: 10.1093/icesjms/fst217
Froese R., Pauly D. (2013). Fish stocks in Levin S ed Encyclopedia of biodiversity, 2nd ed. (Waltham M.A.: Elsevier) 3, 477–487.
Gaevskaya A. V., Aleshkina L. D. (1995). New species of Hemipera (Trematoda: derogenidae) from the Atlantic fishes. Parazitologiya 29, 321–323.
Hajipour E., Khavari F., Hajiaghapour-Moghimi M., Hosseini K. A., Vakilian M. (2022). An economic evaluation framework for cryptocurrency mining operation in microgrids. Int. J. Electrical Power Energy Syst. 142, 108329. doi: 10.1016/j.ijepes.2022.108329
Iaria C., Spanò N., Smeriglio A., Capparucci F., De Benedetto G., Lanteri G., et al. (2021). Massive infection of Cystidicoloides ephemeridarum in brown trout Salmo trutta with skeletal deformities. Dis. Aquat Organ 143, 159–168. doi: 10.3354/dao03559
ICES (2011). Report of the working group on the assessment of southern shelf stocks of hake, monk and megrim (WGHMM).
Johnston B. R., Halton D. W. (1981). Occurrence of Bucephaloides gracilescens metacercariae in three species of gadoid fish. J. Fish Biol. 18, 685–691. doi: 10.1111/j.1095-8649.1981.tb03810.x
Køie M. (1978). On the morphology and life-history of Stephanostomum caducum (Looss 1901) manter 1934 (Trematoda, acanthocolpidae). Ophelia 17, 121–133. doi: 10.1080/00785326.1978.10425476
Lisnerová M., Lisner A., Cantatore D. M. P., Schaeffner B. C., Pecková H., Tyml T., et al. (2022). Correlated evolution of fish host length and parasite spore size: a tale from myxosporeans inhabiting elasmobranchs. Int. J. Parasitol. 52, 97–110. doi: 10.1016/j.ijpara.2021.05.008
Lloris D., Matallanas J. (2003). Merluzas del mundo (Familia merlucciidae): catálogo comentado e ilustrado de las merluzas conocidas (Food & Agriculture Org) 2, 57.
MacKenzie K., Liversidge J. M. (1975). Some aspects of the biology of the cercaria and metacercaria of Stephanostomum baccatum (Nicoll 1907) manter 1934 (Digenea: acanthocolpidae). J. Fish Biol. 7, 247–256. doi: 10.1111/j.1095-8649.1975.tb04596.x
Madhavi R., Shameem U. (1993). Cercariae and metacercariae of Stephanostomum cloacum (Trematoda: acanthocolpidae). Int. J. Parasitol. 23, 341–347. doi: 10.1016/0020-7519(93)90009-n
Marcogliese D. J. (2005). Parasites of the superorganism: are they indicators of ecosystem health? Int. J. Parasitol. 35, 705–716. doi: 10.1016/j.ijpara.2005.01.015
Mattiucci S., Abaunza P., Ramadori L., Nascetti G. (2004). Genetic identification of Anisakis larvae in European hake from Atlantic and Mediterranean waters for stock recognition. J. Fish Biol. 65, 495–510. doi: 10.1111/j.0022-1112.2004.00465.x
Milano I., Babbucci M., Cariani A., Atanassova M., Bekkevold D., Carvalho G. R., et al. (2014). Outlier SNP markers reveal fine-scale genetic structuring across e uropean hake populations (Merluccius merluccius). Mol. Ecol. 23, 118–135. doi: 10.1111/mec.12568
Mladineo I., Bott N. J., Nowak B. F., Block B. A. (2010). Multilocus phylogenetic analyses reveal that habitat selection drives the speciation of didymozoidae (Digenea) parasitizing pacific and Atlantic bluefin tunas. Parasitology 137, 1013–1025. doi: 10.1017/S0031182009991703
Ngamniyom A., Sriyapai T., Sriyapai P., Panyarachun B. (2020). Introduction of encysted metacercarial Stephanostomum sp. in Javanese ricefish (Oryzias javanicus) and bacterial diversity of encysts from mangrove swamps of trang province, Thailand. Songklanakarin J. Sci. Technol. 42, 42–49.
Oliver P., Massutí E. (1995). Biology and fisheries of western Mediterranean hake (M. merluccius). Hake: Biology fisheries markets, 181–202. doi: 10.1007/978-94-011-1300-7_7
Perez-Urbiola J. C., Martinez-Diaz S. F. (2001). Stephanostomum sp.(Trematoda: acanthocolpidae), the cause of" pimientilla" disease in catarina scallop Argopecten ventricosus (circularis)(Sowerby II 1842) in Baja California sur, Mexico. J. Shellfish Res. 20, 107–109.
Routtu J., Grunberg D., Izhar R., Dagan Y., Guttel Y., Ucko M., et al. (2014). Selective and universal primers for trematode barcoding in freshwater snails. Parasitol. Res. 113, 2535–2540. doi: 10.1007/s00436-014-3903-z
Keywords: parasites, Acanthocolpidae, trematodes, Mediterranean Sea, European hake, Stephanostomum cesticillus
Citation: De Benedetto G, Gervasi C, Riolo K, Abbate JM, Natale S, Di Paola D, Falleti R and Iaria C (2023) First report of Stephanostomum cesticillus (Molin, 1858) Looss, 1899 in Merluccius merluccius (Linnaeus, 1758) from the Tyrrhenian Sea (Southern Italy). Front. Mar. Sci. 10:1178977. doi: 10.3389/fmars.2023.1178977
Received: 03 March 2023; Accepted: 02 May 2023;
Published: 22 May 2023.
Edited by:
Pedro Morais, Florida International University, United StatesReviewed by:
Maria João Santos, University of Porto, PortugalIlya Gordeev, All-Russian Research Institute Fisheries and Oceanography, Russia
Copyright © 2023 De Benedetto, Gervasi, Riolo, Abbate, Natale, Di Paola, Falleti and Iaria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabrina Natale, c25hdGFsZUB1bmltZS5pdA==
†These authors have contributed equally to this work
 Giovanni De Benedetto
Giovanni De Benedetto Claudio Gervasi
Claudio Gervasi Kristian Riolo
Kristian Riolo Jessica Maria Abbate
Jessica Maria Abbate Sabrina Natale
Sabrina Natale Rosa Falleti2
Rosa Falleti2 Carmelo Iaria
Carmelo Iaria