
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 19 May 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1176767
This article is part of the Research Topic The Biology and Conservation of Elasmobranchs and Chimaeras View all 12 articles
 Brooke N. Anderson1*
Brooke N. Anderson1* Juliana Kaloczi1
Juliana Kaloczi1 Courtney Holden1
Courtney Holden1 Amanda Einig1
Amanda Einig1 Linda Donaldson1
Linda Donaldson1 Hunter Malone1
Hunter Malone1 Michelle S. Passerotti2
Michelle S. Passerotti2 Lisa J. Natanson2†
Lisa J. Natanson2† Heather D. Bowlby3
Heather D. Bowlby3 James A. Sulikowski1
James A. Sulikowski1While lethal sampling can be the most effective technique to collect critical reproductive data for elasmobranchs, non-lethal techniques need to be validated for future use. Concentrations of reproductive hormones in plasma and muscle have been found to correlate to sexual maturity and/or reproductive cycles in oviparous as well as yolk-sac, placental, and histotrophic viviparous elasmobranchs, offering a potentially non-lethal technique to study reproduction. However, reproductive hormone analysis is scant for oophagous sharks. This study utilized muscle tissues from porbeagles Lamna nasus that were dissected for other life history studies and were stored frozen for up to 37 years to quantify testosterone (T) and estradiol (E2) concentrations in relation to previously-known maturity and reproductive stage. A total of 207 samples (92 males, 115 females) from porbeagles ranging in size from 80 to 256.5 cm fork length were analyzed. Muscle T and E2 concentrations were related to maturity and reproductive stage in porbeagles, with the highest T concentrations found in mature males during the spermatogenic season (summer) and the highest E2 concentrations found in gravid females. These results suggest muscle hormone concentrations have the potential to serve as a non-lethal proxy of reproductive stage in oophagous sharks. This study also demonstrates the value of specimen sharing and the potential for continued use of stored vertebral muscle tissue for reproductive hormone analysis in order to optimize the amount of data gained from biological samples. Future use of these methods would be particularly valuable for threatened species for which lethal sampling is restricted.
Effective conservation and management of elasmobranchs requires a comprehensive understanding of a species life history, including age and size at sexual maturity, reproductive cycles, and habitats used for reproduction (Walker, 2005; Awruch, 2013). Such information can be used to determine sustainable harvesting rates, understand a population’s potential for timely recovery, and/or develop protected areas or strategies for bycatch avoidance (Awruch, 2013). Although lethal sampling of a relatively large number of specimens has historically been the approach for studying the reproductive biology of elasmobranchs (Heupel and Simpfendorfer, 2010), sacrificing threatened species is in direct opposition to the conservation and management goals science is intending to support (Hammerschlag and Sulikowski, 2011). Given this nuance, non-lethal alternatives for collecting reproductive data should be validated and prioritized whenever possible (Hammerschlag and Sulikowski, 2011). One promising technique that can be used to study the reproduction (reproductive cycles, maturity, reproductive habitats) of elasmobranchs is the quantification of sex steroids (reproductive hormones) in muscle tissues (Prohaska et al., 2013; Verkamp et al., 2021). This technique may be most practical for large elasmobranch species for which other non-lethal sampling collections (i.e., a blood sample for plasma hormone analysis) are logistically challenging, as muscle samples can be collected from free-swimming animals (Prohaska et al., 2013; Verkamp et al., 2021). Muscle reproductive hormone concentrations have been found to correlate to plasma concentrations as well as dissection-verified reproductive stage (preovulatory, early, mid, late gestation) in an oviparous (little skate Leucoraja erinacea), yolk-sac viviparous (spiny dogfish Squalus acanthias) and placental viviparous (Atlantic sharpnose shark Rhizoprionodon terraenovae) species (Prohaska et al., 2013). Muscle hormone concentrations were also used non-lethally (not verified by dissection) to gain preliminary insight into the possible reproductive role of an aggregation site for white sharks Carcharodon carcharias (Verkamp et al., 2021). Most studies conducting reproductive hormone analysis for elasmobranchs have quantified estradiol (E2), progesterone (P4) and/or testosterone (T) (Becerril-Garcia et al., 2020). In general, in female elasmobranchs E2 has primarily been related to vitellogenesis and maturation, while P4 has been related to ovulation and the maintenance of early pregnancy (Awruch, 2013). In males, T is associated with spermatogenesis (Awruch, 2013).
The Northwest Atlantic (NWA) porbeagle Lamna nasus is overfished and is a population of conservation concern (ICCAT, 2020). This population’s life history is characterized by late age (8 and 13 years for males and females, respectively; Natanson et al., 2002) and large size (162-185 cm fork length (FL) for males and 210-230 cm FL for females; Jensen et al., 2002) at maturity as well as low fecundity (average of 4 pups; Jensen et al., 2002). The population was originally thought to have an annual reproductive cycle (Jensen et al., 2002) but more recent evidence suggests at least a portion of the population reproduces biennially (Natanson et al., 2019). The embryos are nourished by consuming unfertilized eggs ovulated by the mother throughout much of gestation (oophagy; Jensen et al., 2002). Mating occurs in September through November and pupping from April through June (Jensen et al., 2002).
While the life history of the NWA porbeagle is relatively well studied, information on reproductive hormones is absent for this species and is scant for all oophagous sharks, particularly for mature females (Tribuzio, 2004; Sulikowski et al., 2012; Verkamp et al., 2021). Most information of shark reproductive endocrinology is provided by direct assessment of plasma hormone concentrations. However, because blood samples are logistically difficult to obtain in the large specimens of porbeagles, and currently are unavailable, the assessment of reproductive hormone concentrations in muscle tissue could be a useful method to evaluate reproductive endocrinology in this species, providing further the possibility of a non-lethal methodology. In this sense, a large collection of stored vertebral specimens or muscle tissues from NWA porbeagles that were dissected between 1985 and 2019 for other life history studies (Natanson et al., 2002) offers the opportunity to have available samples to assess reproductive endocrinology in the species. The objectives of this study were therefore to 1) determine if reproductive hormones (T, E2) could be quantified from shark muscle tissue that was stored frozen for up to 37 years, 2) determine if muscle T and E2 concentrations were related to size or maturity in the NWA porbeagle, and 3) determine if muscle T and E2 concentrations were related to reproductive stage in the NWA porbeagle.
Porbeagles were sampled between 1985 and 2019 onboard commercial and research longline vessels in U.S. and Canadian waters between Massachusetts and the Grand Banks. The majority of samples were collected after 1990 and came from vertebral columns used to study age and growth in the U.S. (Natanson et al., 2002). Additional samples were collected during a Canadian fishery-dependent porbeagle survey in 2017. Capture date, geographic location, and sex was recorded and over the body fork length (FL; cm) was measured and recorded to the nearest mm. All Canadian survey samples had associated information on sexual maturity and reproductive stage and reproductive data were also taken from U.S. samples when possible (74% of U.S. samples). Determination of sexual maturity status and reproductive stage from both the U.S. and Canadian sampling followed established methodology based on morphometric measurements (including: clasper length, testes length and width, oviducal gland width, uterus length and width) and observations of the reproductive tract (including: ovary, uteri, vaginal membrane, testes, clasper calcification, etc.; Jensen et al., 2002; Natanson et al., 2019). In brief, males were grouped into immature, transitional (immature but maturing), and mature, with transitional males exhibiting lengthening claspers and initial development of the rhipidion (Jensen et al., 2002). Females were grouped into immature, transitional (immature but maturing), mature non-gravid, and mature gravid, with transitional females exhibiting the presence of a vaginal membrane and a thin tubular uterus indicating no previous mating but an ovary similar in appearance to mature non-gravid females (Natanson et al., 2019). While it is important to note that transitional sharks are technically classified as immature, they were separated from other immature sharks for this study due to predicted changes in reproductive hormone concentrations associated with the maturation process that may begin prior to reaching maturity (Barnett et al., 2009). For the U.S. samples, vertebral columns were stored frozen and muscle tissues used for reproductive hormone analysis were scraped from these stored vertebral columns in 2021. For samples collected during the 2017 Canadian survey, muscle tissues were excised from vertebral columns of porbeagles immediately upon dissection after capture. All muscle tissues were stored frozen and were shipped on ice to Arizona State University, where they were kept frozen at -20°C until processing.
Hormones were extracted from the muscle tissue following ether extraction protocols modified from Verkamp et al. (2021). Protocol modifications included adjusting the phosphate buffered saline (PBS) to muscle ratio and adding an additional extraction (as described below). Samples were thawed on ice and muscle was excised, weighed, and transferred to a conical centrifuge tube. Phosphate buffered saline (PBS) was added in a ratio ranging from 1 g:1 ml to 1 g:4 ml of muscle to PBS. While most samples (69%) were resuspended in a 1 g: 1 ml of muscle to PBS following Verkamp et al. (2021), initially processed samples and drier samples (31%) were resuspended in a higher ratio of PBS. Samples were homogenized using a Kinematica Polytron PT 10-35. Approximately 0.5 g of homogenate was transferred to a borosilicate tube in duplicate (if sample size allowed) and spiked with approximately 1000 counts min-1 of the appropriate tritiated hormone (1, 2, 6, 7, 3H-T for males or 2, 4, 6, 7, 16, 17, 3H-E2 for females; Perkin Elmer Life Sciences) in order to calculate the percent recovery of hormone during the extraction process. The ~0.5 g spiked homogenate aliquots were then extracted 4-5 times with 5 ml of diethyl ether (ACS grade) and snap frozen in a dry ice and acetone (ACS grade) bath. A fifth extraction with diethyl ether was added during the study to improve hormone recovery during the extraction procedure. The ether phase was decanted into a second borosilicate tube and the diethyl ether evaporated at 37°C under a stream of nitrogen. Dried isolated hormones were reconstituted in 250 µl of PBS with 0.1% gelatin (PBSG) and stored at 4°C.
T and E2 concentrations were quantified for males and females, respectively, following radioimmunoassay methods described in Prohaska et al. (2013). Antibodies (provided by Dr. Gordon Niswender, Colorado State, Fort Collins, CO) used to bind hormones for quantification were diluted in PBSG to final concentrations of 1:24,200 and 1:54,000 for T and E2, respectively. A Tri-Carb 4910TR liquid scintillation counter (Perkin Elmer Life Sciences) was used to quantify radioactivity. Final concentrations were corrected for procedural loss using individual sample hormone extraction recoveries. Any sample that had a hormone concentration below the detection limit (6.25 pg g-1 for T, 5 pg g-1 for E2) of our assay was assigned the minimum detection limit for that hormone. The inter-assay coefficients of variation were 15% and 9%, and the average intra-assay coefficients of variation were 7% and 6% for T and E2, respectively.
Muscle T and E2 concentrations were grouped by sampling season (summer = June-August; fall = September-December; spring = March-May) and then plotted by FL for males and females, respectively. Muscle T concentrations were then grouped and boxplots were plotted by reproductive stage (immature, transitional, and mature) for males and average inner clasper lengths (mm) were also plotted for a subset of these individuals for which reproductive measurements were available (n = 26). For males that were not formally assessed for maturity based on internal reproductive morphology, maturity was predicted based on size at 50% maturity (174 cm FL; Jensen et al., 2002). Mature males were further divided by reproductive seasonality, with mature males sampled in the summer (June-August; predicted to be undergoing spermatogenesis) separated from those sampled during the other times of year (September-May; predicted to not be undergoing spermatogenesis) based on previously established timing of spermatogenesis in male sharks (i.e., Manire and Rasmussen, 1997; Verkamp et al., 2022). Average (± standard error) muscle T concentrations were also plotted by month of the year for males grouped by reproductive stage to evaluate the relationship with reproductive seasonality. For this reproductive seasonality plot, males that were not formally assessed for maturity were plotted as separate groups based on whether they were smaller or larger than the size at 50% maturity. For females, muscle E2 concentrations were grouped and boxplots were plotted by reproductive stage (immature, transitional, mature non-gravid, and gravid) for only the females that were formally assessed for maturity and pregnancy. While many internal reproductive characteristics have been found to relate to maturity in this species (Jensen et al., 2002), average oviducal gland widths (mm) were plotted for a subset of these individuals given this was the morphological measurement with the largest sample size available (n = 19). One oviducal gland measurement from a gravid porbeagle that did not have a muscle sampled to analyze for E2 was included for reference. Average (± standard error) muscle E2 concentrations were also plotted by month of the year for females grouped by reproductive stage to evaluate the relationship with reproductive seasonality. For this reproductive seasonality plot, females that were not formally assessed for maturity were plotted as separate groups based on whether they were smaller or larger than the size at 50% maturity. Muscle T and E2 concentrations were tested for correlations with FL using Kendall ‘s tau rank correlation tests given data violated assumptions of parametric regression even following transformation. Muscle T and E2 concentrations were compared between different reproductive stages by Kruskal Wallis tests followed by pairwise Dunn tests with a Bonferroni correction for multiple testing. All tests were considered significant at α = 0.05.
Muscle samples were analyzed from a total of 207 porbeagles (92 males, 115 females), ranging in size from 85 to 246 cm FL for males and 80 to 256.5 cm FL for females (Table S1). Average hormone recoveries from muscle tissue during the extraction process were 67.0% and 48.6% for T and E2, respectively. Hormone recovery (%) during the extraction process was not impacted by the age of the sample (Figure S1) nor the muscle to PBS ratio (Figure S2). For males, muscle T concentrations ranged from 6.25 to 735.15 pg g-1. For females, muscle E2 concentrations ranged from 5 to 954.32 pg g-1. Hormone concentrations were not impacted by the age of the sample (Figure S3) nor the muscle to PBS ratio (Figure S4) when considering the additional factor of reproductive stage.
Muscle T concentrations in males generally increased with increasing shark size (Figure 1), yet the overall range was greater in larger or mature males (6.25-735.15 pg g-1) compared to smaller, immature males (6.25- 427.32 pg g-1; Figure 1). When considering the time of year of sampling, the increase in T concentration with increasing shark size was most visible for sharks sampled in the summer (June-August), with less consistent trends for sharks sampled in the fall (September-December) and spring (March-May; Figure 1). Overall however, muscle T was not significantly correlated with FL in males (Kendall’s tau; z = -0.653; p = 0.514). In regards to reproductive stage, immature male porbeagles had T concentrations ranging from 6.25 to 427.32 pg g-1 (average = 163.09 pg g-1) and inner clasper lengths ranging from 82 to 167 mm (average = 137.3 mm; Figure 2A). Males that were considered to be in a transitional state had T concentrations ranging from 6.25 to 308.88 pg g-1 (average = 120.17 pg g-1) and inner clasper lengths ranging from 29.5 to 305 mm (average = 217.4 mm). Mature males that were sampled in seasons porbeagles are predicted to not be undergoing spermatogenesis (fall through spring) had T concentrations ranging from 6.25 to 439.89 pg g-1 (average = 81.89 pg g-1) and there were no clasper measurements available for this group. Finally, mature males that were sampled in the predicted spermatogenic season (summer) had T concentrations ranging from 96.68 to 735.15 pg g-1 (average = 360.70 pg g-1) and had inner clasper lengths ranging from 199 to 363 mm (average = 322.3 mm). Muscle T concentrations were significantly different between reproductive stages (Kruskal Wallis; χ2 = 28.026, p < 0.001; Figure 2A). Pairwise comparisons indicated mature males sampled during the summer had T concentrations that were significantly higher than concentrations in transitional males (Dunn test; z = 2.80, p = 0.031) and mature males sampled in predicted non-spermatogenic seasons (Dunn test; z = 5.24, p < 0.001). However, T concentrations were not significantly different between any other groups (p > 0.05). When reproductive seasonality was considered for males (Figure 2B), a seasonal trend was clear for mature males (including males that were not formally assessed for maturity but were larger than the size 50% maturity). Average muscle T concentrations for mature males were low to moderate during the spring (March through May), increased and were highest during June and July, and then decreased for the remainder of the year. Lowest concentrations occurred during the mating season of September through November, while lowest variability in muscle T concentrations occurred in October through December (Figure 2B). Seasonal trends were less consistent among immature, transitional, and males that were not formally assessed for maturity but were smaller than the size at 50% maturity.
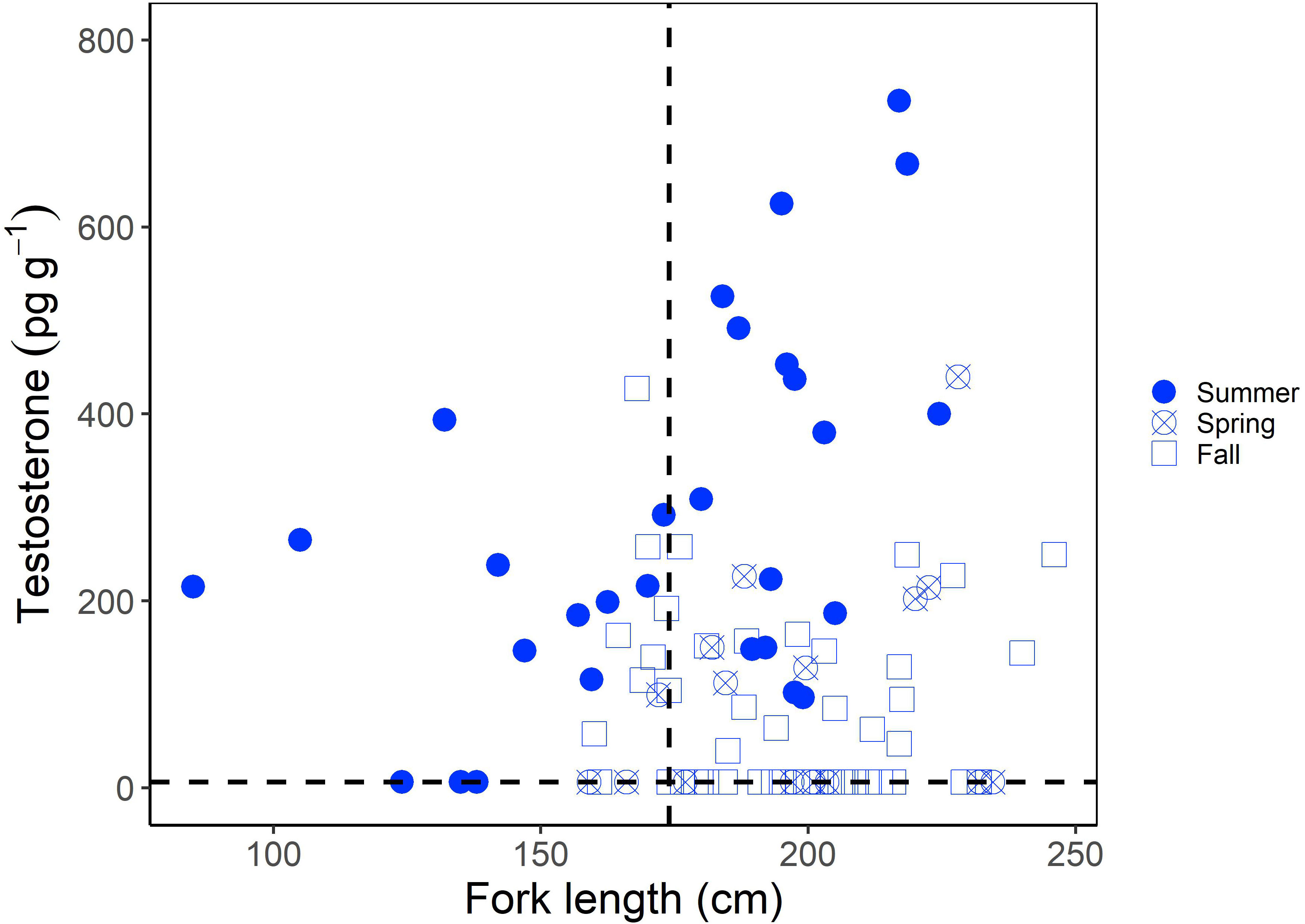
Figure 1 Muscle testosterone (T) concentration (pg g-1) as a function of fork length (FL; cm) in male porbeagles. The shape represents the sampling season with seasons grouped into summer (June-August), spring (March-May), and Fall (September-December). The size at 50% maturity (174 cm FL; Jensen et al., 2002) is identified by the vertical dashed line and the minimum detection limit of the assay (6.25 pg g-1) by the horizontal dashed line.
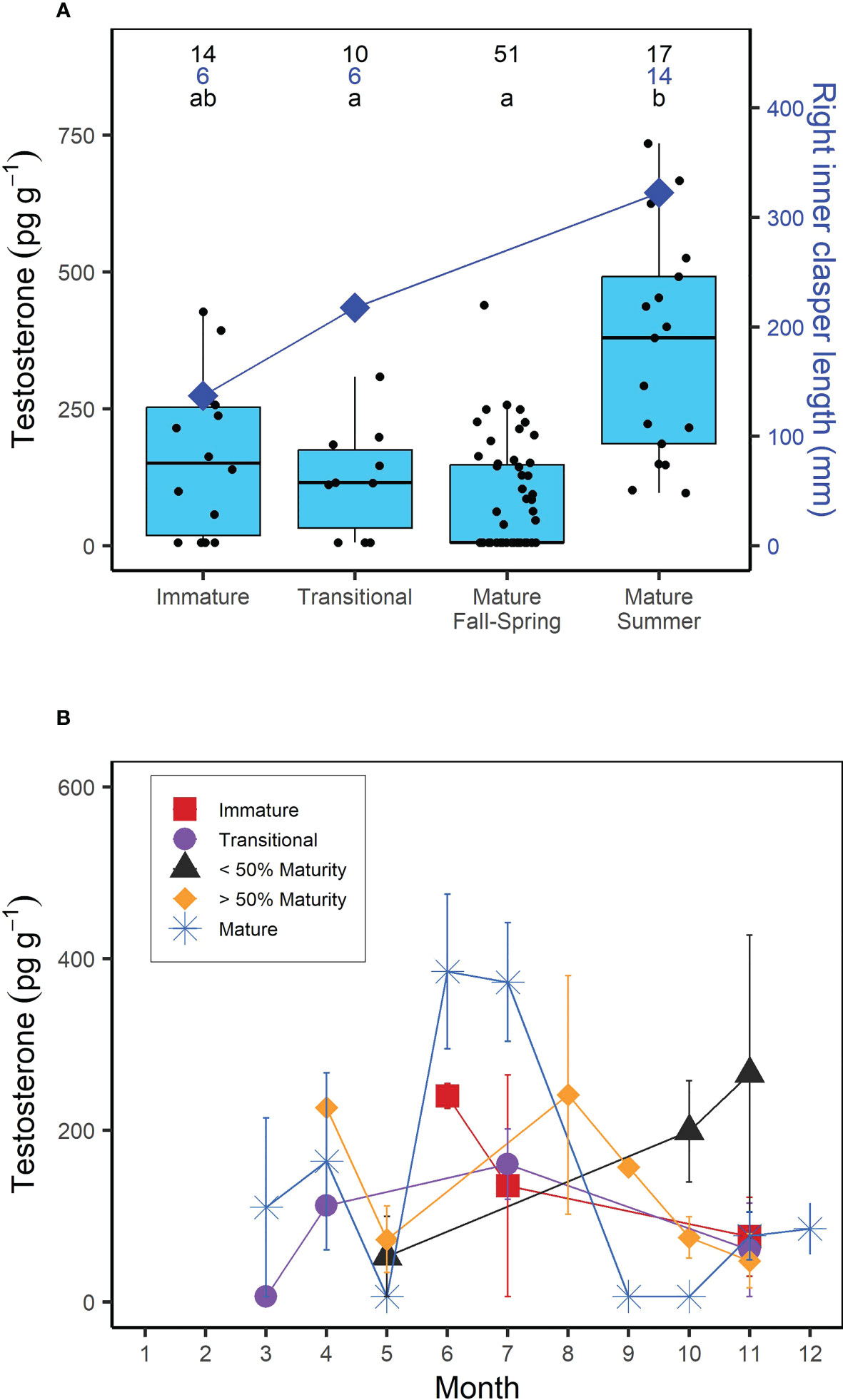
Figure 2 (A) Boxplots of muscle testosterone (T) concentration (pg g-1) and average right inner clasper length (mm) as a function of reproductive stage in male porbeagles. For males that were not formally assessed for maturity based on reproductive morphology, reproductive stage was predicted based on size at 50% maturity (174 cm FL; Jensen et al., 2002). Mature males were further divided into summer (June-August; predicted to be undergoing spermatogenesis) or Fall-Spring (September-May; predicted to not be undergoing spermatogenesis) based on previously established timing of spermatogenesis in male sharks (i.e., Manire and Rasmussen, 1997; Verkamp et al., 2022). Numbers represent sample size for T concentrations (black) and right inner clasper length (blue) and letters identify significant differences in T concentrations among groups. (B) Average muscle T concentration by month in male porbeagles grouped by reproductive stage. Sharks that were not formally assessed for maturity were grouped based on whether they were smaller or larger than the size at 50% maturity. Error bars represent standard error.
For females, muscle E2 concentrations were significantly correlated with shark size (Kendall’s tau; z = 6.233; p < 0.0001); E2 showed a clear increase in sharks above the size at 50% maturity (218 cm FL; Jensen et al., 2002) (Figure 3). However, similar to males, there was a greater range in muscle E2 concentrations in larger or mature females (5-954.32 pg g-1) compared to smaller, immature females (5-90.11 pg g-1) (Figure 3). When considering the time of year sharks were sampled, E2 was elevated in females sampled in the fall (September-December; Figure 3). In regards to reproductive stage, all immature females, except for one individual (which had an E2 concentration of 90.11 pg g-1), had muscle E2 concentrations below the minimum detection limit (5 pg g-1, average = 6.81 pg g-1) and oviducal gland widths ranging from 2.9 to 46 mm (average = 9.6 mm; Figure 4A). Females that were considered to be in a transitional stage had E2 concentrations ranging from 5 to 152.78 pg g-1 (average = 54.26 pg g-1), yet there were no oviducal gland measurements available for this group. Females that were confirmed to be mature but were non-gravid had E2 concentrations ranging from 5 to 152.18 pg g-1 (average = 49.60 pg g-1) and oviducal gland widths ranging from 21 to 37 mm (average = 29 mm). Finally, females that were confirmed to be mature and gravid had the highest E2 concentrations, ranging from 5 to 954.32 pg g-1 (average = 196.47 pg g-1). A gravid porbeagle that did not have associated hormone data had an oviducal gland width of 42.2 mm. Muscle E2 concentrations were significantly different between reproductive stages (Kruskal Wallis; χ2 = 58.248; p < 0.001; Figure 4A). Pairwise comparisons indicated that E2 concentrations were significantly higher in gravid females compared to immature females (Dunn test; z = 7.6, p < 0.001). However, E2 concentrations were not significantly different between any other groups (p > 0.05). There were no trends in reproductive seasonality for immature, transitional, or sharks that were not formally assessed for maturity (Figure 4B). Samples for females that were confirmed to be mature but were non-gravid were limited to the months of July, September, and November. E2 was low in these females that were sampled in July and September but was elevated in the single mature non-gravid female that was sampled in November. Samples for gravid females were limited to October through December. E2 concentrations increased from October to December for gravid female samples.
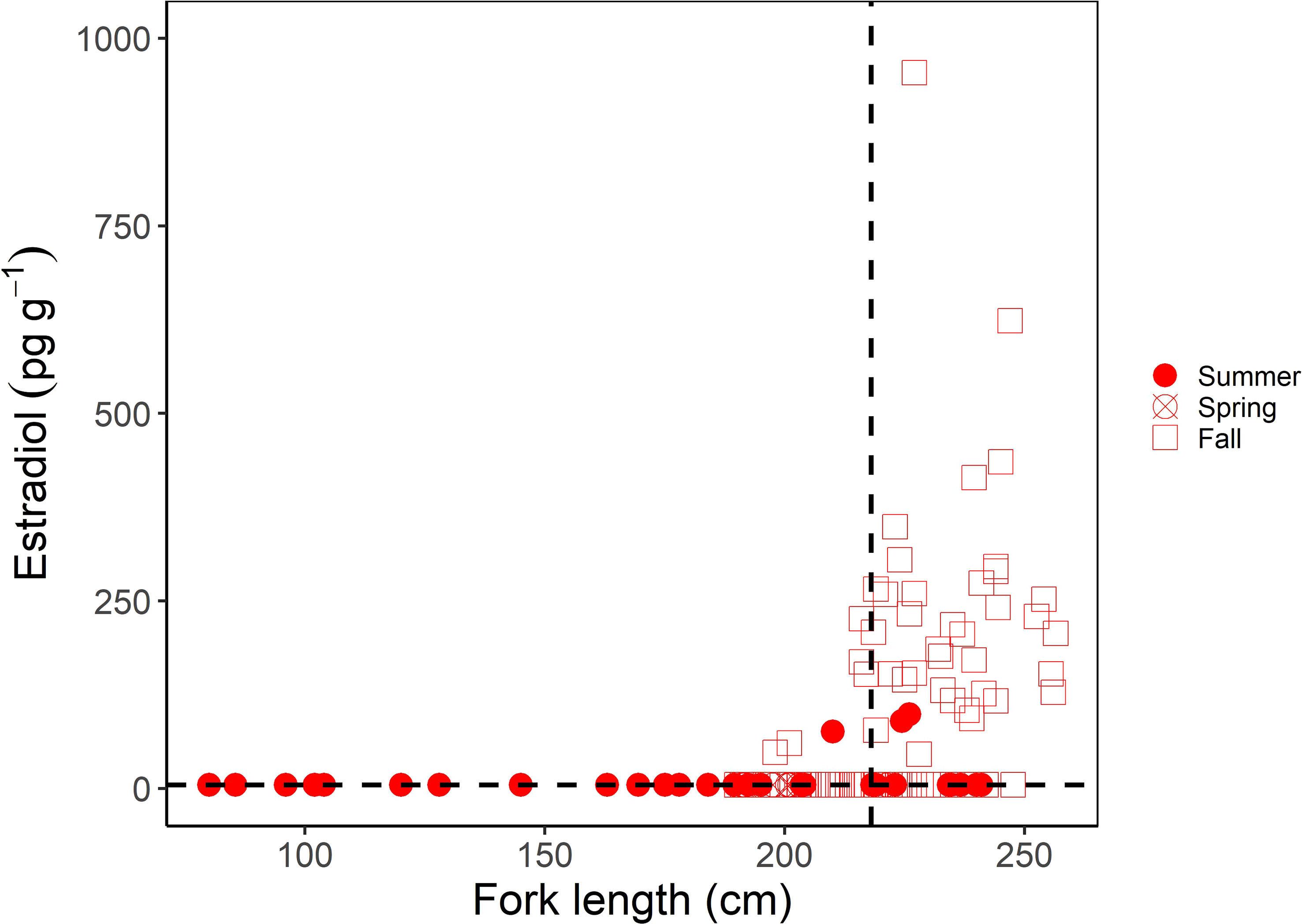
Figure 3 Muscle estradiol (E2) concentration (pg g-1) as a function of fork length (FL; cm) in female porbeagles. The shape represents the sampling season with seasons grouped into summer (June-August), spring (March-May), and Fall (September-December). The size at 50% maturity (218 cm FL; Jensen et al., 2002) is identified by the vertical dashed line and the minimum detection limit of the assay (5 pg g-1) by the horizontal dashed line.
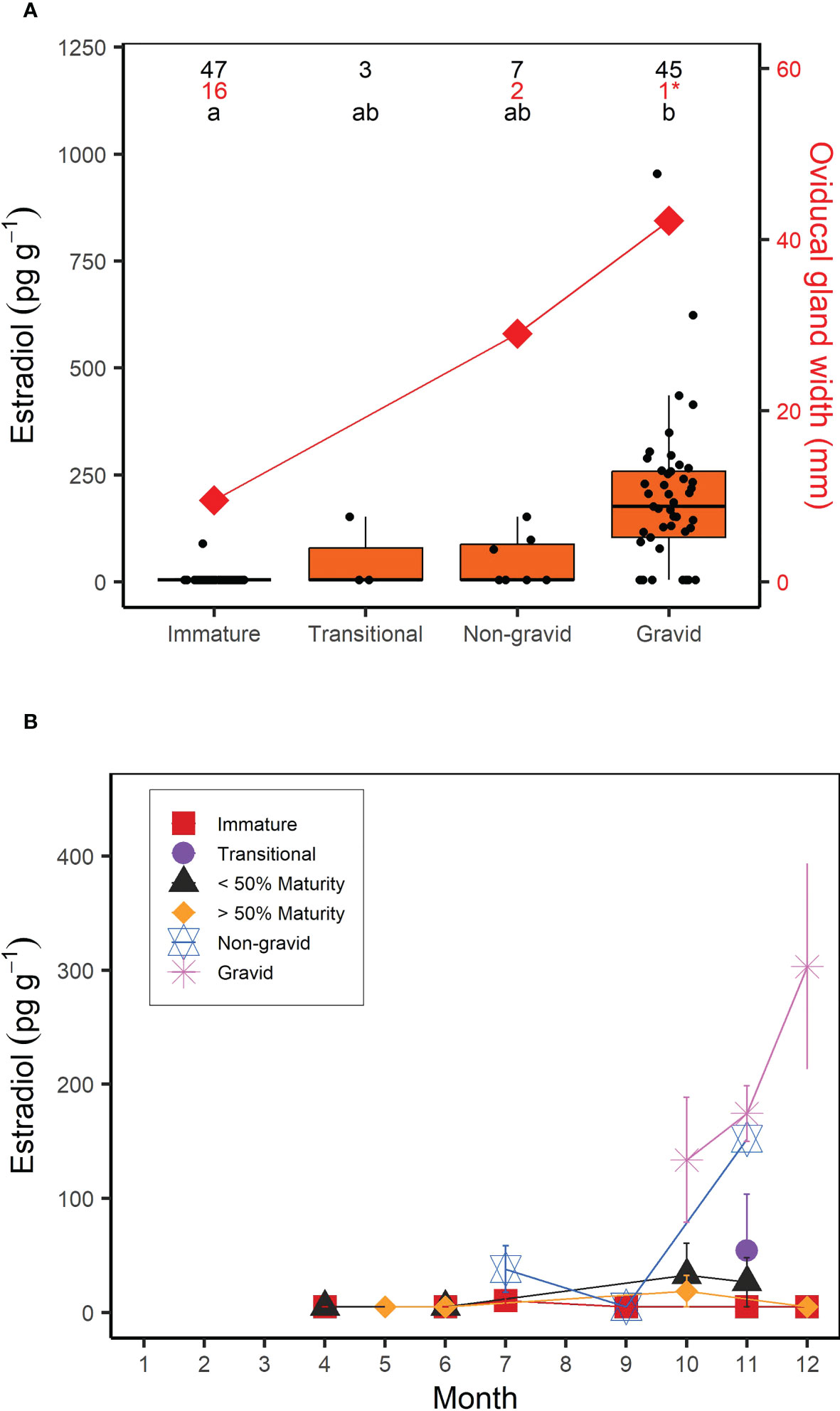
Figure 4 (A) Boxplots of muscle estradiol (E2) concentration (pg g-1) and average oviducal gland width (mm) as a function of reproductive stage in female porbeagles that were formally assessed for maturity and pregnancy. Numbers represent the sample size for E2 concentrations (black) and oviducal gland width (red) and letters identify significant differences in E2 concentrations among groups. *Indicates an oviducal gland width measurement taken from a gravid porbeagle that was not analyzed for muscle E2 but was included for reference. (B) Average muscle E2 concentration by month in female porbeagles grouped by reproductive stage. Sharks that were not formally assessed for maturity were grouped based on whether they were smaller or larger than the size at 50% maturity. Error bars represent standard error.
Not only can reproductive hormones be quantified from muscle samples stored frozen for up to 37 years, new insights into hormonal control of reproduction are evident for this oophagous shark species. Although validated through relatively few samples, this study indicates measurable relationships between hormones and sexual maturity and reproductive stage in porbeagles. These data indicate muscle T concentrations could most easily differentiate between reproductive stages in the summer, with mature males sampled during the summer having higher T than transitional males and mature males sampled in other times of the year. Muscle E2 concentrations could most easily differentiate between gravid females and immature females. However, it is important to note that there was overlap in hormone concentrations among stages for both sexes, suggesting there is potential for misclassification of individuals if muscle hormone concentrations are used as the sole predictor of reproductive stage. This overlap is likely associated with individual level variation in hormone concentrations (i.e., Verkamp et al., 2022) rather than the age (Prohaska et al., 2018; Figure S3) or dryness of the sample (Figure S4) given concentrations were found to be variable (from below the detection limit to hundreds of pg g-1) among samples that were collected from animals in the same reproductive stage, in the same year, and homogenized with the same ratio of PBS.
Muscle T concentrations appeared to be higher in many larger male porbeagles compared to smaller conspecifics, suggesting a possible role of T in the maturation of the male reproductive tract in this species. Moreover, although sample size was small and did not include mature individuals sampled during seasons in which male porbeagles are not predicted to be undergoing spermatogenesis, T appeared to be related to reproductive morphology, as T concentrations were highest in males that had the largest claspers. A relationship between plasma T concentration and size or maturity has been documented in males of other shark species (Awruch et al., 2008; Awruch et al., 2014). However, it is important to note that there was a much larger range in T concentrations in larger or mature males compared to immature males. This variability in T concentrations among mature males of the same size was suggested to be attributed to sharks being sampled at varying stages of the reproductive cycle (Awruch et al., 2008). For example, muscle T concentrations were highest in mature males sampled in June through August, which likely corresponds to when individuals actively undergo spermatogenesis in the months prior to mating (Manire and Rasmussen, 1997; Verkamp et al., 2022). Muscle T concentrations began to drop and were lowest in mature males sampled during the known mating season (September through November; Jensen et al., 2002) when these sharks are likely undergoing testicular regression (Manire and Rasmussen, 1997; Verkamp et al., 2022). Collectively, these findings suggest that male porbeagle muscle T concentrations are related to the combined influences of maturity and reproductive seasonality. This highlights the importance of considering reproductive seasonality relative to when samples were collected when predicting sexual maturity based on T concentrations, as has been suggested in previous work on male elasmobranchs (Awruch et al., 2008). In the case of porbeagles, predicting sexual maturity based on T concentrations would be most applicable during the summer months when mature males undergo spermatogenesis.
Female muscle E2 concentrations had a more distinct relationship with maturity in the porbeagle. All females (with the exception of one individual) that were confirmed to be immature based on internal morphology had E2 concentrations below the detection limit of our assay and immature females had the smallest average oviducal gland width. This finding was expected given undetectable or very low E2 concentrations are commonly observed in immature female sharks of other species (Awruch, 2013; Verkamp et al., 2021), including the related oophagous white shark (Verkamp et al., 2021). In mature female sharks, E2 is associated with the follicular phase and vitellogenesis and is thus typically found to be elevated prior to ovulation (i.e., Awruch, 2013). However, our study found that gravid female porbeagles had elevated E2 compared to mature non-gravid females. This finding may be related to multiple factors. First, elevated E2 in gravid compared to non-gravid females is likely unique to oophagous species that continue follicular development and ovulation throughout gestation (Gilmore, 1993; Tribuzio, 2004). Second, it is possible that the non-gravid females analyzed in this study, most of which had E2 concentrations comparable to females in a transitional stage, were post-partum (if sampled in summer) or in a resting phase of the reproductive cycle. These non-gravid mature females analyzed in this study could be part of the portion of female porbeagles that reproduce biennially in the NWA (Natanson et al., 2019). Low E2 concentrations have been observed in other sharks during resting phases when not actively undergoing vitellogenesis (Tribuzio, 2004; Prohaska et al., 2013), including the closely related, oophagous, salmon shark L. ditropis (Tribuzio, 2004). These conclusions are consistent with our limited data on oviducal gland widths. Mature non-gravid females had oviducal gland widths comparable to porbeagles found to be in a post-partum or resting phase (Natanson et al., 2019), while the gravid female had a larger oviducal gland, comparable to other gravid porbeagles (Jensen et al., 2002).
Overall, the hormonal trends observed in this study are consistent with the current understanding of the role of reproductive hormones during sexual maturation and reproductive stages in sharks. It appears muscle reproductive hormones have the potential to serve as a non-lethal proxy of reproductive stage in the porbeagle and potentially other oophagous sharks, albeit with limitations regarding overlap in concentrations among reproductive stages. It is possible that this limitation may be overcome by including additional non-lethal assessments of reproductive stage, such as examinations of claspers for males and ultrasonography for females. Moreover, while the size of muscle samples available for this opportunistic study precluded the quantification of additional reproductive hormones (i.e., P4, 11-ketotestosterone), it is possible that the inclusion of other hormones may improve the differentiation of reproductive stages, and this is a potential avenue of future research. Another limitation of this study was the lack of samples available from mature females in the winter and spring, which precluded the ability to assess changes in reproductive hormones across the entirety of the reproductive cycle, such as late gestation. Nevertheless, the success of this work has wide-reaching applications for elasmobranch reproductive research. We demonstrated for the first time that reproductive hormones could be successfully extracted and quantified from shark muscle tissues that have been stored frozen for decades and found no impact of sample age or dryness on hormone recovery or concentration. Therefore, similar work could be done for other species that have archived biological samples in order to increase the amount of scientific information gathered from lethal sampling. This would be especially relevant for threatened species for which lethal sampling is restricted and reproductive data is especially needed for conservation and management decisions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Arizona State University Institutional Animal Care and Use Committee.
Study design was led by JAS, LJN, MSP, and HDB. Sample collection was led by LJN, MSP, and HDB. Laboratory analyses were completed by BNA, JK, CH, AE, LD, and HM. Data analyses were completed by BNA and JK. Manuscript was written by BNA and edited by JAS, LJN, MSP, HDB, JK, CH, AE, LD, and HM. All authors contributed to the article and approved the submitted version.
This work was supported by the National Oceanic and Atmospheric Administration International Science Fund, Award #1333MF20PNFFM0087.
Authors gratefully acknowledge undergraduate and graduate students of the Sulikowski laboratory at Arizona State University who assisted in this project. We thank K. Viducic for the preparation of U.S. muscle samples from vertebral columns and H. Verkamp for advice on muscle hormone analysis techniques. We would also like to thank all the fishermen who allowed us access to their catch.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1176767/full#supplementary-material
Awruch C. A. (2013). Reproductive endocrinology in chondrichthyans: the present and the future. Gen. Comp. Endocrinol. 192, 60–70. doi: 10.1016/j.ygcen.2013.05.021
Awruch C. A., Frusher S. D., Pankhurst N. W., Stevens J. D. (2008). Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar. Ecol. Prog. Ser. 355, 277–285. doi: 10.3354/meps07227
Awruch C. A., Jones S. M., Asorey M. G., Barnett A. (2014). Non-lethal assessment of the reproductive status of broadnose sevengill sharks (Notorynchus cepedianus) to determine the significant of habitat use in coastal areas. Conserv. Physiol. 2. doi: 10.1093/conphys/cou013
Barnett L. A. K., Earley R. L., Ebert D. A., Cailliet G. M. (2009). Maturity, fecundity, and reproductive cycle of the spotted ratfish, Hydrolagus colliei. Mar. Biol 156, 301–316. doi: 10.1007/s00227-008-1084-y
Becerril-Garcia E. E., Arellano-Martinez M., Bernot-Simon D., Hoyos-Padilla E. M., Galvan-Magana F., Godard-Codding C. (2020). Steroid hormones and chondrichthyan reproduction: physiological functions, scientific research, and implications for conservation. PeerJ 8, e9686. doi: 10.7717/peerj.9686
Gilmore R. G. (1993). Reproductive biology of lamnoid sharks. Environ. Biol. Fish. 38, 95–114. doi: 10.1007/BF00842907
Hammerschlag N., Sulikowski J. (2011). Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endang. Species. Res. 14, 135–140. doi: 10.3354/esr00354
Heupel M. R., Simpfendorfer C. A. (2010). Science or slaughter: need for lethal sampling of sharks. Conserv. Biol. 24, 1212–1218. doi: 10.1111/j.1523-1739.2010.01491.x
ICCAT (2020). Report of the 2020 porbeagle shark stock assessment meeting. Collect. Vol. Sci. Pap. ICCAT 77, 1–88.
Jensen C. F., Natanson L. J., Pratt H. L. Jr., Kohler N., Campana S. E. (2002). The reproductive biology of the porbeagle shark (Lamna nasus) in the western north Atlantic ocean. Fish. Bull. 100 (4), 727–738.
Manire C. A., Rasmussen L. E. L. (1997). Serum concentrations of steroid hormones in the mature male bonnethead shark, Sphyrna tiburo. Gen. Comp. Endocrinol. 107, 414–420. doi: 10.1006/gcen.1997.6937
Natanson L. J., Deacy B. M., Joyce W., Sulikowski J. (2019). Presence of resting population of female porbeagles (Lamna nasus), indicating a biennial reproductive cycle, in the western north Atlantic ocean. Fish. Bull. 117 (1-2), 70–77. doi: 10.7755/FB.117.1-2.8
Natanson L. J., Mello J. J., Campana S. E. (2002). Validated age and growth of the porbeagle shark (Lamna nasus) in the western north Atlantic ocean. Fish. Bull. 100, 266–278.
Prohaska B. K., Tsang P. C. W., Driggers W. B., Hoffmayer E. R., Wheeler C. R., Brown A. C., et al. (2013). Assessing reproductive status in elasmobranch fishes using steroid hormones extracted from skeletal muscle tissue. Conserv. Physiol. 1, cot028. doi: 10.1093/conphys/cot028
Prohaska B. K., Tsang P. C. W., Driggers W. B., Hoffmayer E. R., Wheeler C. R., Sulikowski J. A. (2018). Effects of delayed phlebotomy on plasma steroid hormone concentrations in two elasmobranch species. J. Appl. Ichthyol. 34, 861–866. doi: 10.1111/jai.13700
Sulikowski J. A., Williams L. J., Domeier M. L. (2012). “The use of a nonlethal technique to assess the reproductive biology of the white shark, Carcharodon carcharias,” in Global perspectives on the biology and life history of the white shark. Ed. Domeier M. L. (Boca Raton, FL: CRC Press), 467–476.
Tribuzio C. A. (2004). An investigation of the reproductive physiology of two north pacific shark species: spiny dogfish (Squalus acanthias) and salmon shark (Lamna ditropis) (Seattle, WA: University of Washington).
Verkamp H. J., Hammerschlag N., Quinlan J., Langan J. A., Sulikowski J. A. (2022). Preliminary investigation of reproductive hormone profiles in the blacktip shark (Carcharhinus limbatus), a placental viviparous species, in southern Florida. Mar. Freshw. Res. 73 (4), 520–527. doi: 10.1071/MF21235
Verkamp H. J., Skomal G., Winton M., Sulikowski J. A. (2021). Using reproductive hormone concentrations from the muscle of white sharks Carcharodon carcharias to evaluate reproductive status in the Northwest Atlantic ocean. Endang. Species. Res. 44, 231–236. doi: 10.3354/esr01109
Keywords: oophagy, reproduction, shark, testosterone, estradiol
Citation: Anderson BN, Kaloczi J, Holden C, Einig A, Donaldson L, Malone H, Passerotti MS, Natanson LJ, Bowlby HD and Sulikowski JA (2023) Using reproductive hormones extracted from archived muscle tissue to assess maturity and reproductive status in porbeagles Lamna nasus. Front. Mar. Sci. 10:1176767. doi: 10.3389/fmars.2023.1176767
Received: 28 February 2023; Accepted: 28 April 2023;
Published: 19 May 2023.
Edited by:
Elizabeth Grace Tunka Bengil, University of Kyrenia, CyprusReviewed by:
Mariano Elisio, Ganadería y Pesca, ArgentinaCopyright © 2023 Anderson, Kaloczi, Holden, Einig, Donaldson, Malone, Passerotti, Natanson, Bowlby and Sulikowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brooke N. Anderson, Ym5hbmRlcnNvQGdtYWlsLmNvbQ==
†RetiredFritz Creek, AK, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.