
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 21 June 2023
Sec. Aquatic Physiology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1167400
This article is part of the Research Topic Functional Feed Additives and Intestinal Health in Aquatic Animals View all 11 articles
Fucoidan with its excellent biological activities such as growth promotion, antioxidant and strong immunity, is widely used in animal production. The present study was conducted to investigate the influences of feeding fucoidan on growth performance, biochemical indices, immunity, the antibacterial ability of plasma, the digestive enzyme activity of the intestine, antioxidant capacity, and the histological structure of liver in juvenile common carp. Five experimental diets added with 0 (Diet 1), 500 (Diet 2), 1,000 (Diet 3), 1,500 (Diet 4), and 2,000 (Diet 5) mg/kg fucoidan were fed to triplicate groups of 30 fish (35.83 ± 0.24 g) respectively for 8 weeks. The results showed that fish fed diets with a fucoidan supplementation of 1,666.67–1,757 mg/kg might have the best growth performance (p< 0.05). The levels of plasma total protein (TP) and albumin (ALB) in Diet 3, Diet 4, and Diet 5 were higher than those in Diet 1 and Diet 2 (p< 0.05). Moreover, the contents of plasma C3, LYZ, and IgM; the antibacterial ability of serum; and the activity of SOD, CAT, POD, and GPX in the liver, and ACP, AKP, LPS, AMS, and TRY in the intestine significantly improved; the contents of LPO and MDA in the liver were notably decreased in diets with fucoidan supplement (p< 0.05). Furthermore, the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the contents of total bilirubin (TB) and glucose (Glu) in Diet 5 were the highest among the groups. Meanwhile, proinflammatory factors (plasma IL-6 and IL-1β) had a higher expression, but anti-inflammatory factors (plasma IL-1) had a lower expression in Diet 5 (p > 0.05). It indicated that a higher dose (2,000 mg/kg) of fucoidan may induce inflammation and metabolic disorders. Interestingly, histological results of liver also indicated that dietary fucoidan intake in certain amounts (500–1,500 mg/kg) could ameliorate hepatic morphology, but the high dosage (2,000 mg/kg) probably damaged the liver. To the best of our knowledge, this is the first study on the application of fucoidan as a functional additive to juvenile common carp. The results of the present study can be used to guide the application of fucoidan in healthy aquaculture and can further reveal the effect and mechanism of fucoidan on the nutritional physiology of aquatic animals.
Feed supplements are usually used in the aquaculture industry to improve fish growth rate and production. Fucoidan is a type of natural active polysaccharide mainly extracted from marine brown algae (Pomin, 2015) containing sufficient L-fucose and sulfate eater groups (Li et al., 2008). Fucoidan has been found to be effective at growth promotion (Dawood et al., 2018; Fazio et al., 2019) and gastrointestinal function regulation (Cui et al., 2020; Liu et al., 2020), and to have anti-inflammatory (Kirindage et al., 2022; Wang et al., 2022), anti-oxidation (Sony et al., 2019; Abdel-Daim et al., 2020; Mahgoub et al., 2020), anti-tumor (Han et al., 2015a; Han et al., 2015b; Jin et al., 2022), anti-bacterial (Yu et al., 2015; Zhu et al., 2021), and immunomodulatory (Wang et al., 2019; Ikeda-Ohtsubo et al., 2020) properties. Recently, fucoidan has attracted the attention of aquatic animal researchers. However, an extreme limitation is that fucoidan research on aquatic animals has mainly focused on a few species, such as Penaeus monodon (Chotigeat et al., 2004; Sivagnanavelmurugan et al., 2014), Marsupenaeus japonicus (Traifalgar et al., 2010), Pelteobagrus fulvidraco (Yang et al., 2014), Litopenaeus vannamei (Sinurat et al., 2016), Labeo rohita (Mir et al., 2017; Adnan et al., 2018), Pagrus major (Sony et al., 2019), Carassius auratus (Cui et al., 2020), and Oreochromis niloticus (Abdel et al., 2021), and it has been found to improve the growth rate and stimulate the immune system. Therefore, fucoidan is considered as a promising feed additive to enhance the growth and immunity in aquatic animals.
As a freshwater economic fish, common carp (Cyprinus carpio), is one of the key aquaculture species in the world. Common carp production provides an economical, tasty, hyperproteic, healthy food supplement for people around the world. Hence, researchers have focused on improving the growth (Mohammadian et al., 2021; Khorshidi et al., 2022; Zhang et al., 2022), disease resistance (Ahmadifar et al., 2022; Ghafarifarsani et al., 2022) and stress resistance (Banaee et al., 2022; Dawood et al., 2022; Xue et al., 2022b) of common carp. As mentioned earlier, fucoidan as feed additive has remarkable effects on improving growth and immunity, but its application in fish feed research is limited. To the best of our knowledge, there is no available information about the effect of fucoidan on common carp.
Therefore, the present study was conducted to determine the dietary fucoidan effects on growth performance, biochemical parameters, specific and non-specific immunity, antibacterial ability, antioxidant capacity, digestive enzyme activity, and the histological structure of the liver in juvenile common carp, and the results of the study help to determine the optimal amount of fucoidan that could be effectively used in carp culture.
The basal diet was based on soybean meal as carbohydrate sources and fish meal as protein sources. Five isonitrogenous (38%) and isolipidic (6.6%) experimental diets (Table 1) were formulated and supplemented with 0 (Diet 1), 500 (Diet 2), 1,000 (Diet 3), 1,500 (Diet 4), and 2,000 (Diet 5) mg/kg fucoidan (purity ≥98%, Xi ‘an Risen Biotechnology Co., LTD., China). The selected dosages of fucoidan were based on the findings of earlier studies (Sony et al., 2019; Cui et al., 2020). All feed ingredients were crushed and sifted by a 60-mesh sieve, weighed, and mixed by a stepwise expansion method to make pellet feed with a diameter of 1 mm, which was dried naturally outside in a drafty place that is not exposed to direct sunlight and then stored at −20°C until feeding.
Common carp juveniles were purchased from an aquafarm (Chongqing, China) and acclimated for 14 days in a cement breeding pond (6 m × 6 m × 0.8 m) feeding with the commercial feed (Tongwei Co. LTD., China) twice a day. After acclimation to the trial conditions, 450 healthy fish (35.83 ± 0.24 g) were randomly assigned to 15 cement breeding ponds (2.1 m × 1.2 m × 0.8 m), each with 30 fish species. The feeding trial was conducted outdoors for 8 weeks in the aquaculture base of Southwest University (Chongqing, China). Fish in triplicate groups were fed the respective experimental diets to apparent satiation twice daily at 8:00 and 17:00. Meanwhile, the feed intake and mortality of fish in each pond were recorded. During the trial, the dissolved oxygen was ≥6.5 mg/L and the water temperature ranged from 25 to 28°C. The total ammonia nitrogen and nitrites remained< 0.05 mg/L, and the pH was kept at 7.4–8.2. The natural photoperiod was applied.
At the end of the trial, carps were starved for 24 h, weighed and dissected, and then counted to calculate growth index. Twelve fish were caught randomly from each pond, and anesthetized by applying eugenol for sampling. Among them, six fish were selected for blood collection purposes; their body length and weight (body, viscera, and liver) were then recorded for the purpose of computing the somatic indices. The blood of three fish was drawn from their caudal vein using sterile disposable syringes (2 ml), rinsed with heparin sodium solution. After the centrifugation (3,500 rpm, 15 min, 4°C) of the sample, the plasma was separated and stored at −80°C for biochemical index detection. Non-heparinized blood of 3 fish was centrifuged (3,500 rpm, 15 min, 4°C) to obtain the serum for antibacterial activity test. Three fish from each pond were sacrificed to obtain liver and intestine collected in an ultralow-temperature refrigerator for analysis of enzyme activity. Moreover, three hepatic tissues from each pond were placed in 2-ml centrifuge tubes containing Bouin’s solution for micromorphological analysis. After 24 h of fixation, the samples were washed and stored using 70% ethanol solution until the preparation of tissue section.
The assay of plasma biochemical indices in this study comprised alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), total bilirubin (TB), urea nitrogen (UN), creatinine (Cr), glucose (Glu), triglycerides (TG), and total cholesterol (TC), which were all measured by using a fully automated biochemical analyzer (Chemray 240, Shenzhen, China).
The content of interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-10 (IL-10), complement 3 (C3), lysozyme (LYZ), and immunoglobulin M (IgM) in the plasma; the activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione peroxidase (GPX), lipid peroxide (LPO), and malonaldehyde (MDA) in the liver; and the activities of acid phosphatase (ACP), alkaline phosphatase (AKP), lipase (LPS), amylase (AMS), and trypsin (TRY) in the intestine were determined by utilizing the commercial kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The above kits were carried out in accordance with the manufacturer’s specifications.
The serum from non-heparinized blood was used for antibacterial activity test in vitro. For this purpose, Aeromonas hydrophila (BBAh1) stored in the Key Laboratory of Freshwater Fish Reproduction and Development (Ministry of Education) (Li et al., 2019) was grown in tryptic soy broth for 24 h at 30°C. Bacterial cells were centrifuged and suspended in sterile PBS and adjusted to an optical density of 0.5 at 546 nm. The diluted bacterial suspension was mixed with serum homogenates for incubation at 37°C for 1 h. After incubation, the number of viable bacterial cells was counted (Eslami et al., 2022).
Hepatic samples were fixed in Bouin’s solution. Then, the fish tissues were placed in plastic cassettes and processed (gradual dehydration in 70%–100% alcohol, clearing in xylene, and paraffin wax embedding). Five-micron-thick sections were cut on a microtome and then stained with hematoxylin and eosin (HE). Slides were examined with a light microscope (Nikon DXM1200).
The growth performance parameters were calculated as follows (Geetanjali et al., 2020; Jayant et al., 2021):
Initial body weight (IW, g);
Final body weight (FW, g);
Final body length (FL, cm);
All of the data were expressed as the mean ± SEM. The data were analyzed by one-way analysis of variance and Tukey’s multiple range test using SPSS 19.0 (IBM, Chicago, USA). Differences were considered significant at p< 0.05. The optimal dietary requirement of fucoidan for juvenile carp was calculated by using broken line regression analysis (Robbins et al., 2006).
The growth performance, feed utilization, and somatic indices of juvenile common carp fed the experimental diets containing different levels of fucoidan were presented in Table 2. At the end of the 8-week culturing trial, Diet 3, Diet 4, and Diet 5 were higher in FW, WG, and SGR than Diet 1 and Diet 2 (p< 0.05). Moreover, FCR in Diet 3 and Diet 4 was significantly lower than in the other diets (p< 0.05). VSI in Diet 3 and Diet 4 was significantly higher than in Diet 1, and fish fed diet 1,500 had a higher VSI than fish fed diet 500. Nevertheless, there were nearly no significant changes in HI, CF, and SR among all the treatments (p > 0.05). Furthermore, the regression analysis between fucoidan supplementation and WG showed that the optimal dietary content of fucoidan was 1,757 mg/kg (Figure 1), and the regression analysis between fucoidan content and SGR showed that the optimal dietary supplementation of fucoidan was 1,666.67 mg/kg for juvenile common carp (Figure 2). Meanwhile, the survival rate of common carp fed with dietary fucoidan remained unaffected compared to the control group, which was 100% in all diets.
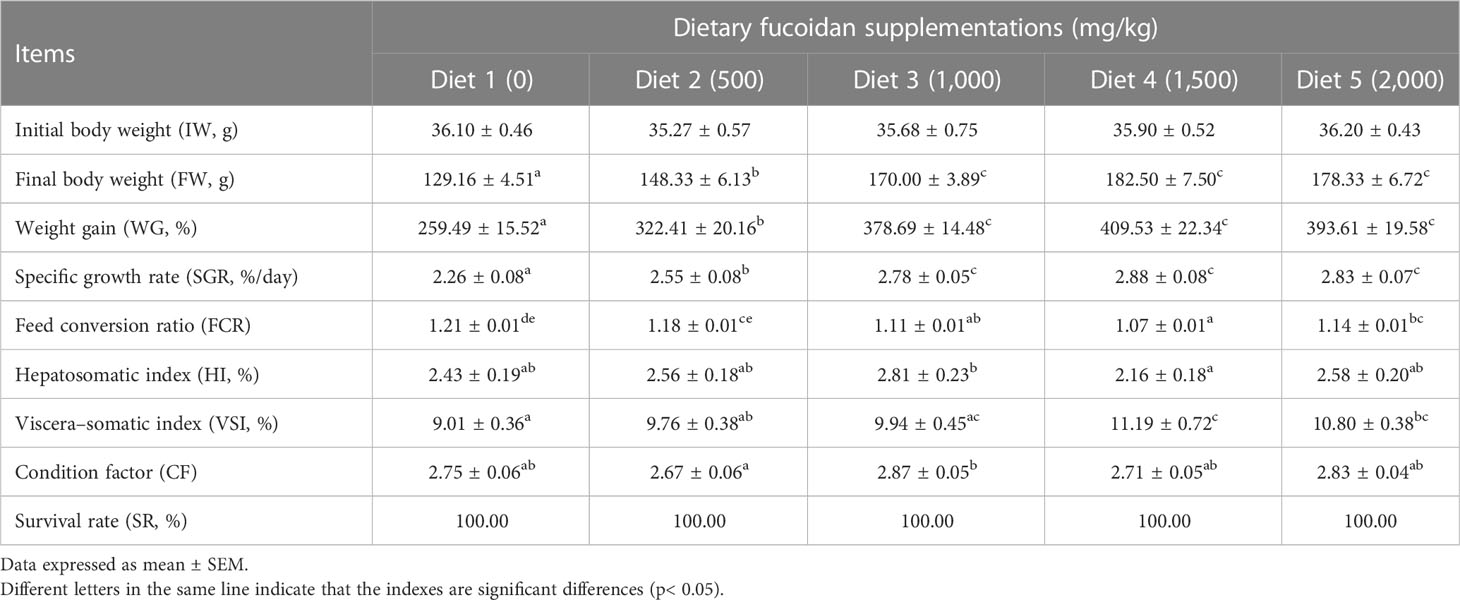
Table 2 Effect of dietary fucoidan supplementations on growth performance, feed utilization, and somatic indices of juvenile Cyprinus carpio (N = 18).
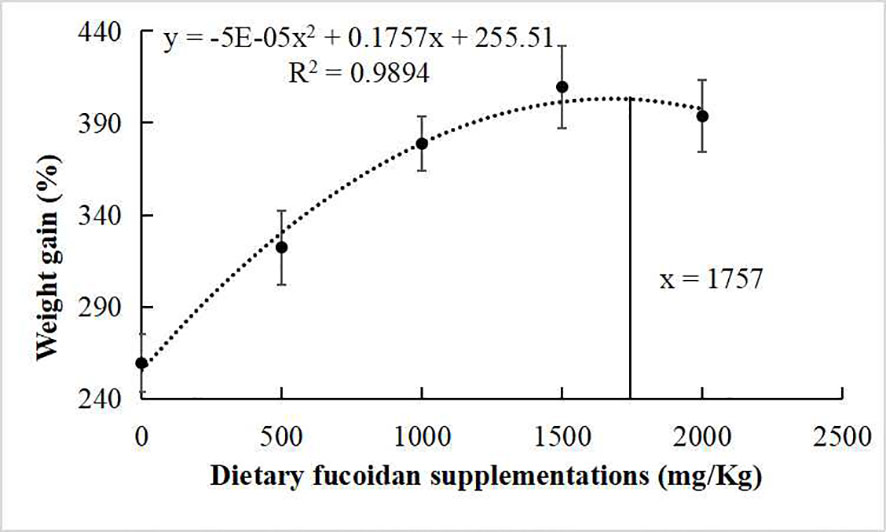
Figure 1 Broken line regression analysis between dietary fucoidan supplementation and weight gain of juvenile Cyprinus carpio.
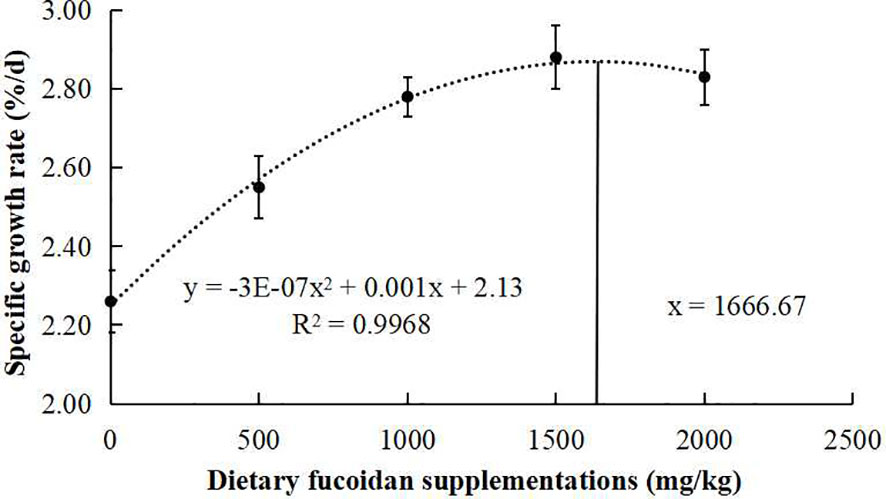
Figure 2 Broken line regression analysis between dietary fucoidan content and specific growth rate of juvenile Cyprinus carpio.
The effects of dietary fucoidan on plasma biochemical parameters of juvenile common carp are presented in Table 3. The highest levels of plasma ALT, AST, TB, and Glu were found in Diet 5, which were statistically higher than other diets (p< 0.05). Additionally, plasma TP and ALB in Diet 3, Diet 4, and Diet 5 were higher than that in Diet 1 and Diet 2 (p< 0.05), whereas nearly no statistically significant differences were observed in UN, Cr, TG, and TC for any of the treatment (p > 0.05).
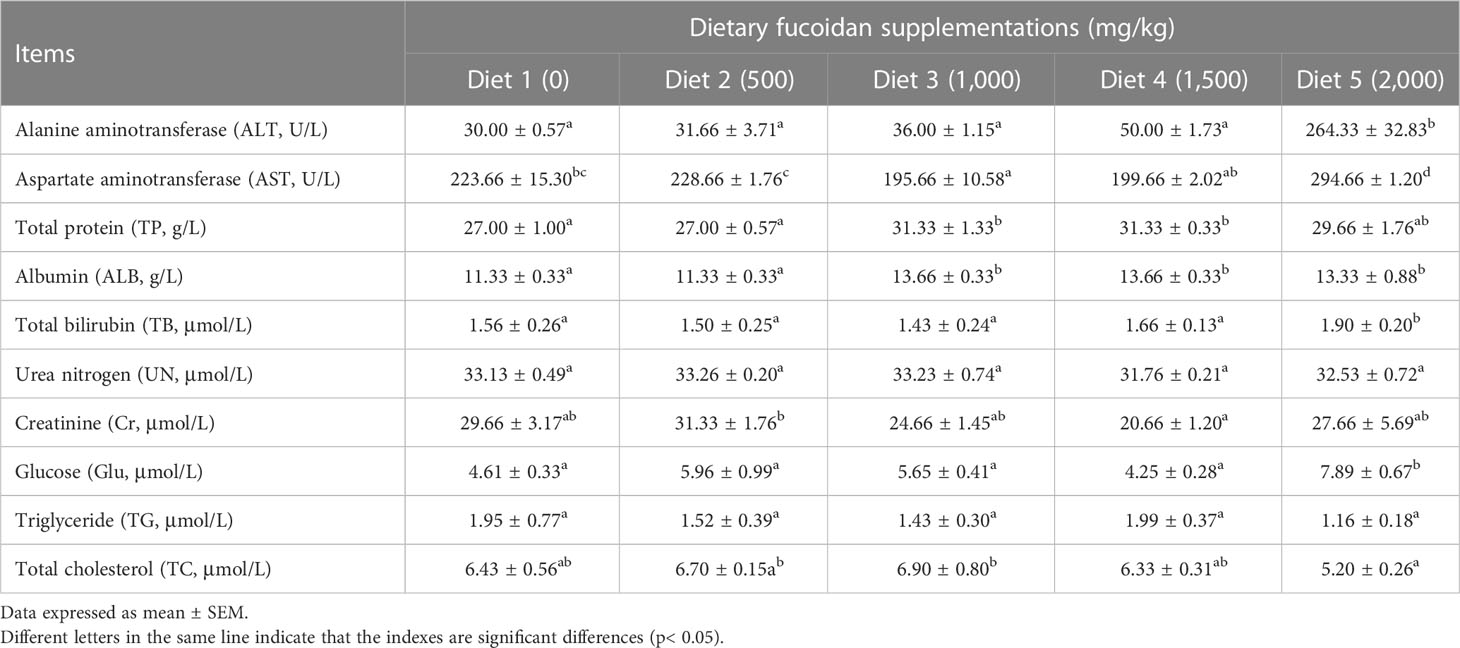
Table 3 Effect of dietary fucoidan supplementations on plasma biochemical indexes of juvenile Cyprinus carpio (N = 9).
Table 4 shows that dietary supplementation with fucoidan containing different levels in common carp impacted the immune parameters in plasma. The values of proinflammatory cytokines IL-6 and IL-1β showed a decreasing trend in Diet 2, Diet 3, and Diet 4, and an increasing trend in Diet 5 (p< 0.05). Conversely, the values of anti-inflammatory cytokine IL-10 showed an increasing trend in Diet 2 and Diet 3, and a decreasing trend in Diet 4 and Diet 5 (p< 0.05). Furthermore, compared with Diet 1, dietary addition of fucoidan significantly improved the C3, LYZ, and IgM content (p< 0.05). Moreover, the highest value of C3 and LYZ appeared in Diet 3, and the highest value of IgM appeared in Diet 4. Quite noticeably, the number of A. hydrophila colonies notably diminished (p< 0.05) in the fish serum feeding Diet 3 (cfu = 226 ± 21), Diet 4 (cfu = 301 ± 12), and Diet 5 (cfu = 309 ± 18) compared to Diet 1 (cfu = 362 ± 17) and Diet 2 (cfu = 343 ± 12) (Figure 3).
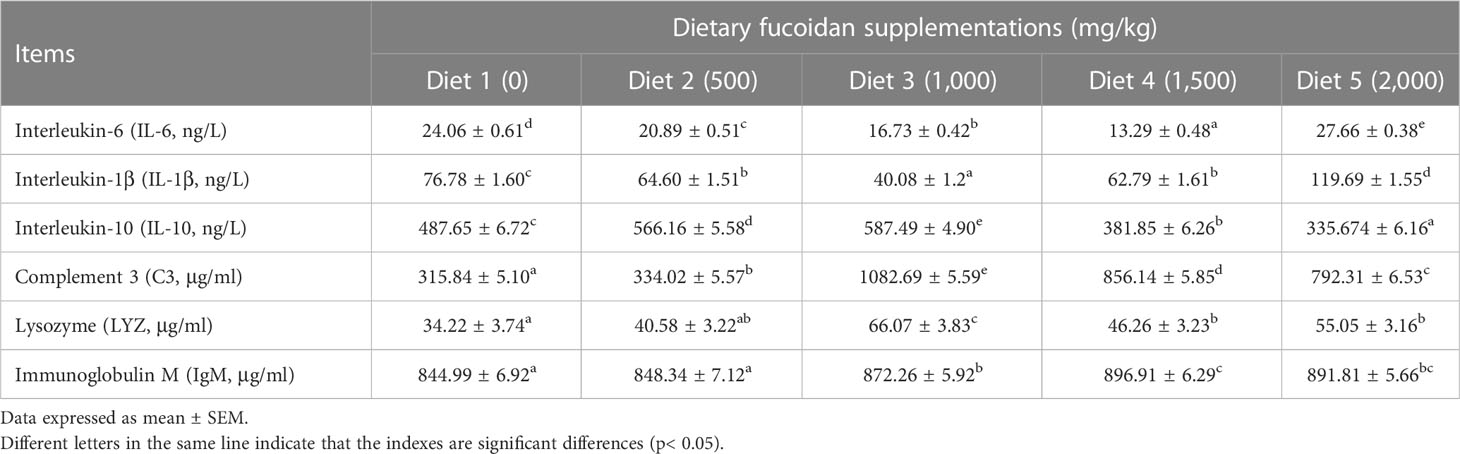
Table 4 Effect of dietary fucoidan supplementations on plasma immune parameters of juvenile Cyprinus carpio (N = 9).
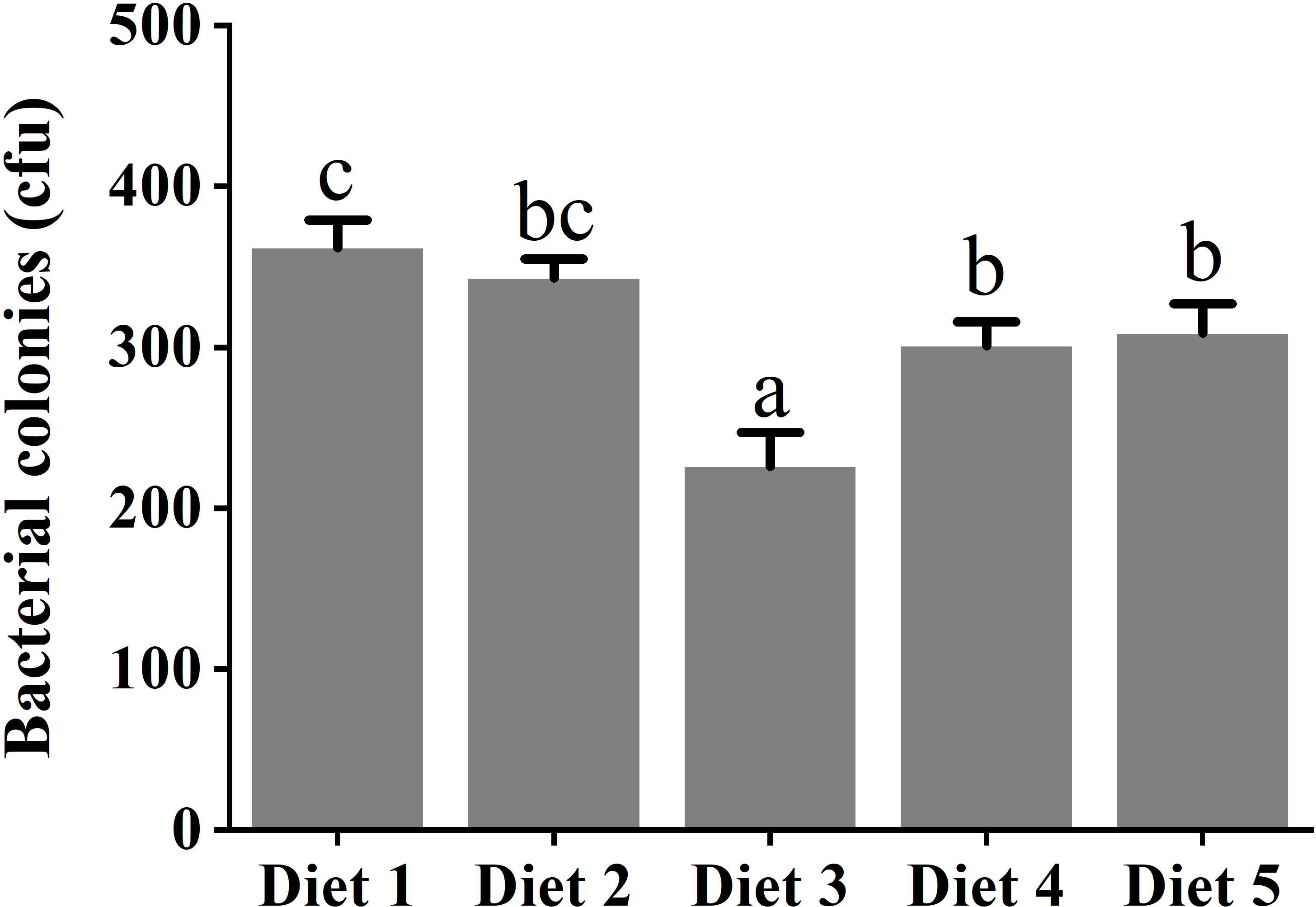
Figure 3 Challenge with Aeromonas hydrophila in serum of juvenile Cyprinus carpio fed dietary fucoidan. Different superscript letters indicate marked differences among the groups (p< 0.05). Diet 1, Diet 2, Diet 3, Diet 4, and Diet 5 refer to fish fed diets with varying levels of fucoidan (0, 500, 1,000, 1,500, and 2,000 mg/kg).
As displayed in Table 5, antioxidative parameters in the liver were also affected significantly by dietary fucoidan supplementation. Distinctly, the activity of enzymes in antioxidant systems SOD (in Diet 3 and Diet 4), CAT (in Diet 2 to Diet 5), and POD and GPX (in Diet 3 to Diet 5) was significantly increased by feeding fucoidan (p< 0.05). Meanwhile, the content of the peroxidation product LPO (in Diet 3 to Diet 5) and MDA (in Diet 2 to Diet 5) was dramatically decreased by the supplementation of fucoidan (p< 0.05).
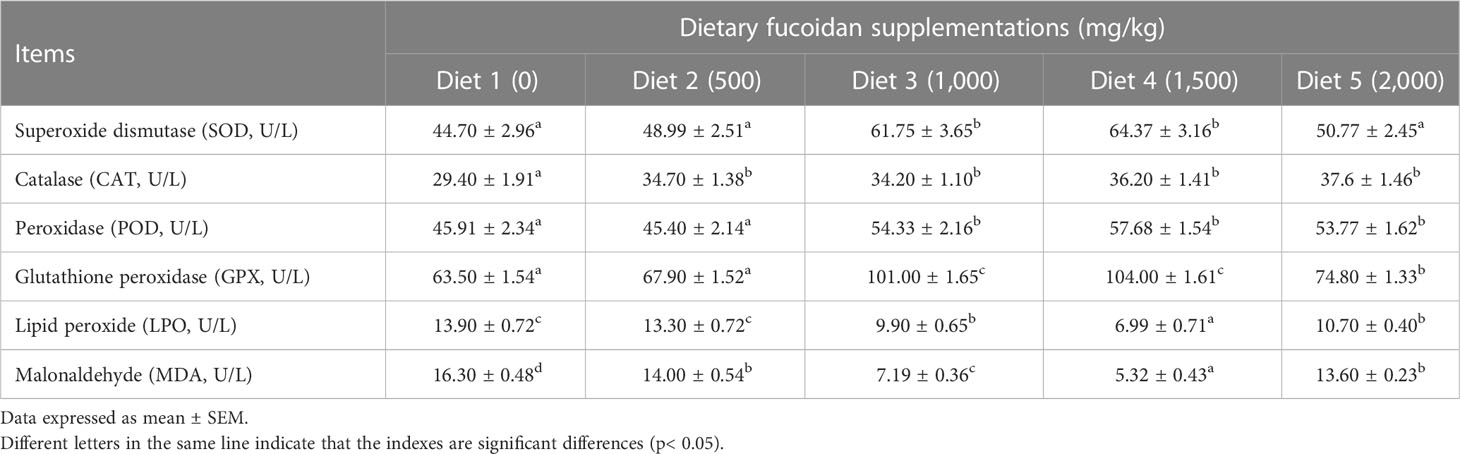
Table 5 Effect of dietary fucoidan supplementations on liver antioxidant activities and oxidative stress of juvenile Cyprinus carpio (N = 9).
The digestive enzyme activity in the intestine was also affected by feeding fucoidan (Table 6). Obviously, all the digestive enzyme activity in Diet 3, Diet 4, and Diet 5 was significantly elevated compared to Diet 1 (p< 0.05). Notably, the activity of LPS in Diet 5 was the highest (p< 0.05), and the activity of AMY and TRY in Diet 3 and Diet 4 was higher than the other Diets (p< 0.05).
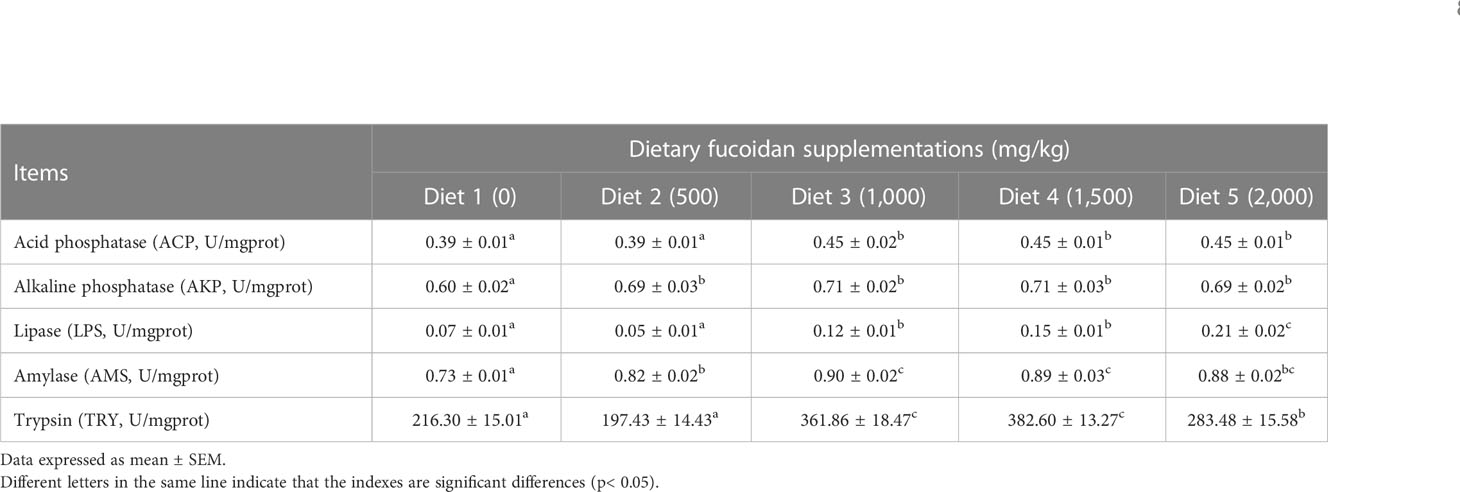
Table 6 Effect of dietary fucoidan supplementations on digestive enzyme activity in the anterior intestine of juvenile Cyprinus carpio (N = 9).
The effect of dietary supplementation of fucoidan on the hepatic morphology of juvenile common carp is presented in Figure 4. Compared to Diet 1 and Diet 5, the hepatic tissues of fish feeding Diet 2, Diet 3, and Diet 4 were obviously more intact: hepatocytes were arranged neatly, with a clear cellular boundary and the nucleus mainly in the cell center, and slight vacuolization was observed. In Diet 5, hepatocytes were arranged irregularly, with a blurred cellular boundary and the nucleus located at the cellular periphery (or even disappeared), cellular swelling, severe vacuolization, and even melanin macrophage and lymphocyte infiltration.
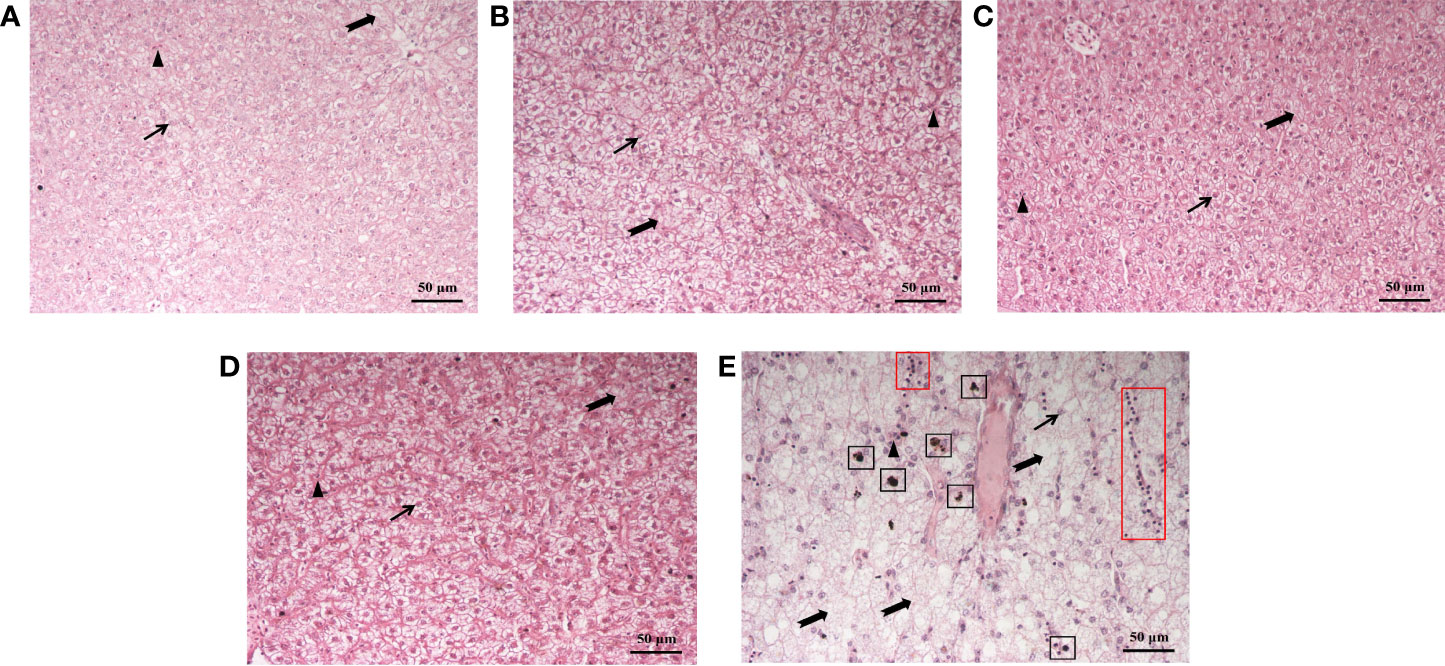
Figure 4 Histological structure of the liver of juvenile Cyprinus carpio fed experimental diets supplemented with different levels of fucoidan after the 8-week feeding trial. (A) Diet 1 (0 mg/kg); (B) Diet 2 (500 mg/kg); (C) Diet 3 (1,000 mg/kg); (D) Diet 4 (1,500 mg/kg); (E) Diet 5 (2,000 mg/kg). Scale bar = 50 μm. HE staining. Thin arrow: hepatic nucleus; triangle: hepatic sinusoid; dovetail arrow: hepatocyte vacuolation; black box: melanin macrophages; red box: lymphocytes.
Fucoidan as a functional supplement with various benefits, such as improved growth rate, feed utilization, immunity, and antioxidant capacity, was used in aquatic animals (Pomin, 2015; Yu et al., 2015). In the current study, the effect of fucoidan, a type of sulfated polysaccharide extracted from brown algae, as a feed additive in the soy protein-based diet for juvenile common carp has been evaluated.
The growth rate (FW, WG, and SGR) of juvenile common carp supplemented with fucoidan were enhanced compared with the control group in the present study. Similarly, fucoidan additive in the diets of rohu (L. rohita) (Mir et al., 2017; Adnan et al., 2018), red sea bream (P. major) (Sony et al., 2019), gible carp (C. auratus) (Cui et al., 2020), and Nile tilapia (O. niloticus) (Abdel et al., 2021) improved growth performance. Notably, the regression analysis between dietary fucoidan supplementation and SGR or WG showed that the optimal dietary content of fucoidan was 1,666.67 mg/kg or 1,757 mg/kg for the growth of juvenile common carp in this study. However, the best recipes of fucoidan in P. major (0.4%) (Sony et al., 2019), C. auratus (30 g/kg) (Cui et al., 2020), and L. rohita (2%) (Mir et al., 2017) are very different. Therefore, a study on the appropriate amount of fucoidan added to different fishes was very important and necessary.
In the present study, dietary fucoidan could significantly improve the immunity of juvenile common carp by downregulating the level of proinflammation factors (IL-6 and IL-1β) and enhancing the specific (IgM) and non-specific (lysozyme and C3) immunity. Analogously, immune cells’ activation and the high expression of IgM, IgG, and IgA in plasma were observed in mice with dietary fucoidan (Makoto et al., 2019). The results of cytokines in this study showed that IL-6 and IL-1b decreased in Diets 2, 3, and 4, and increased in Diet 5. On the other hand, IL-10 increased in Diets 2 and 3, but decreased in Diets 4 and 5. Therefore, we could speculate that fucoidan might regulate the level of inflammation and have a proinflammatory effect at a high dose, which means that the dosage of fucoidan added in the feed should not be that high; otherwise, it will have adverse effects. Obviously, fucoidan could regulate the expression of inflammatory cytokines by activating inflammation-related signaling pathways, such as the NF-kB and eNOS signaling pathways (Wang and Chen, 2015; Caroline et al., 2019). Moreover, the survival rate of fry of L. rohita infected with A. hydrophila was significantly improved, and the content of lysozyme in plasma was also markedly increased (Adnan et al., 2018). Interestingly, we found that plasma lysozyme activity was negatively correlated with serum antimicrobial capacity in this study, which means that lysozyme activity in plasma is an important index to measure the antibacterial ability of the body (Kord et al., 2021).
The antioxidant system consisted of enzymes (SOD, CAT, POD, and GPX) and non-enzyme (glutathione and vitamins E and C) components, which jointly regulated the antioxidant level and maintained homeostasis (Sony et al., 2019; Abdel-Daim et al., 2020). In addition, the lipid peroxidation product LPO and its decomposition product MDA could directly reflect the rate and degree of lipid peroxidation in tissues (Mahgoub et al., 2020). In this regard, we evaluated the antioxidant capacity of the liver tissue in juvenile common carp fed with dietary fucoidan. The results showed that feeding with fucoidan significantly increased the activities of SOD, CAT, POD and GPX, and decreased the levels of LPO and MDA in common carp. Similarly, P. fulvidraco fed with dietary fucoidan could increase SOD and CAT activities and decrease MDA content (Yang et al., 2014). It was found that the antioxidant activity of fucoidan usually played a role through molecular pathways, such as MAPK (Kim et al., 2011; 2012), Keap1-Nrf2-ARE (Zhang et al., 2012), PI3K-Akt (Han et al., 2015a; 2015b), and the TLR-NFkB signaling pathway (Asanka et al., 2019).
The liver function could be regulated by using proper feed supplements in aquafeeds (Hemre et al., 2002), which would be reflected in antioxidant levels (Mohammadi et al., 2021), inflammation index (Yang et al., 2021), and the histological structure of the liver (Cui et al., 2020). ALT and AST levels in liver were much higher than those in plasma, but liver cell necrosis could lead to ALT and AST levels that are two times higher than plasma. Therefore, plasma ALT and AST could serve as indices to evaluate the degree of hepatic damage (Josekutty et al., 2013). Previous studies have documented that the activities of plasma ALT and AST restored the normal level in mice fed the diet containing 100 mg/(kg·day) fucoidan after BCG vaccine and lipopolysaccharide-induced immunological liver injury; it suggested that fucoidan could significantly reduce the necrosis of hepatocytes and protect the liver (Xiao et al., 2017). Opposite results in the present study showed that the activity of plasma ALT and AST, and the content of TB and Glu were significantly increased in Diet 5 compared to other diets. It indicated that a higher dose (2,000 mg/kg) of fucoidan may induce liver injury and metabolic disorders. Moreover, hepatic micromorphology can be used to assess the healthy status of the liver. In our study, histological observation of the liver showed that the diet with 500–1,500 mg/kg fucoidan could ameliorate the morphology of hepatic tissue to a certain extent, such as reducing vacuolation, inflammatory response, and improving blood circulation in the liver. These findings clearly show that fucoidan may protect the liver, whose mechanism may be associated with the suppression of hepatocyte apoptosis, intrahepatic inflammation, and antioxidant activity (Ahmad et al., 2022; Xue et al., 2022a), whereas a high dosage of fucoidan, particularly at 2,000 mg/kg, led to the increase of plasma ALT and AST levels, as well as significant pathological changes in liver tissue. Thus, it indicated that the addition of low dosage of fucoidan could ameliorate the hepatic morphology of juvenile common carp, whereas the high dosage probably caused further damage to the liver.
ACP was one of the signature enzymes of lysosomes, and its activity could represent the intensity of intracellular digestion in the intestine (Maritza et al., 2012; Zacarias et al., 2013). AKP could directly participate in the transfer of phosphate groups and play a key regulatory role in metabolism. Furthermore, AKP was also an important immunoreactive enzyme, and its activity was often used as a key indicator to diagnose foreign pathogens and environmental pollution (Jean, 2020; Singh and Lin, 2021). In the present study, the activity of ACP, AKP LPS, AMS, and TRY in the intestine was notably enhanced in groups feeding fucoidan, which indicated that fucoidan could effectively improve the digestive ability of the intestine of juvenile common carp. Similar results were observed in C. auratus feeding fucoidan (Cui et al., 2020). Of course, the intestinal digestive function was closely related to intestinal microbial composition (Wu et al., 2019; Amenyogbe et al., 2022; Chang et al., 2023). Thus, future studies are needed to explore the effect of fucoidan on intestinal microorganisms of common carp.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Committee of Laboratory Animal Experimentation at Southwest University.
FL and HS conceived the ideas and designed the methodology. YL, DH, and CR caught and dissected fish individuals. FL and CZ collected data. FL and GL analyzed data. FL and HS led the writing of the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the Foundation and Advanced Research Project of Chongqing Science and Technology Commission (cstc2019jscx-gksbX0147).
The authors thank Professor Z.J. Wang’s lab for providing site support and personnel help for this experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel W. A., Younis E., Alasgah N. A., Gewaily M. S., Eltonoby S. M., Dawood M. A. O. (2021). Role of fucoidan on the growth behavior and blood metabolites and toxic effects of atrazine in Nile tilapia Oreochromis niloticus (Linnaeus 1758). Animals 11, 1448. doi: 10.3390/ANI11051448
Abdel-Daim M. M., Dawood M. A. O., Aleya L., Alkahtani S. (2020). Effects of fucoidan on the hematic indicators and antioxidative responses of Nile tilapia (Oreochromis niloticus) fed diets contaminated with aflatoxin b1. Environ. Sci. Pollut. Res. 27, 12579–12586. doi: 10.1007/s11356-020-07854-w
Adnan H. G., Narottam P. S., Sujata S., Saima R., Showkat A. D., Irshad A., et al. (2018). Effect of dietary Sargassum wightii and its fucoidan-rich extract on growth, immunity, disease resistance and antimicrobial peptide gene expression in Labeo rohita. Int. Aqua. Res. 10, 115–131. doi: 10.1007/s40071-018-0193-6
Ahmad T., Ishaq M., Karpiniec S., Park A., Stringer D., Singh N., et al. (2022). Oral Macrocystis pyrifera fucoidan administration exhibits anti-inflammatory and antioxidant properties and improves DSS-induced colitis in C57BL/6J mice. Pharmaceutics 14, 2388. doi: 10.3390/PHARMACEUTICS14112383
Ahmadifar E., Mohammadzadeh S., Kalhor N., Yousefi M., Moghadam M. S., Naraballobh W., et al. (2022). Cornelian cherry (Cornus mas l.) fruit extract improves growth performance, disease resistance, and serum immune-and antioxidant-related gene expression of common carp (Cyprinus carpio). Aquaculture 558, 738372. doi: 10.1016/J.AQUACULTURE.2022.738372
Amenyogbe E., Luo J., Fu W., Abarike E. D., Wang Z., Huang J., et al. (2022). Effects of autochthonous strains mixture on gut microbiota and metabolic profile in cobia (Rachycentron canadum). Sci. Rep. 12, 17410. doi: 10.1038/S41598-022-19663-X
Asanka S. K. K., Jayawardena T. U., Kim H. S., Kim S. Y., Fernando S., Wang L., et al. (2019). Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-kB signal pathway. Carbohyd. Polym. 224, 115195. doi: 10.1016/j.carbpol.2019.115195
Banaee M., Sureda A., Faggio C. (2022). Protective effect of protexin concentrate in reducing the toxicity of chlorpyrifos in common carp (Cyprinus carpio). Environ. Toxicol. Phar. 94, 103918. doi: 10.1016/J.ETAP.2022.103918
Caroline R., Delma S., Thirugnanasambandan G. P. S., Nune R., Sunil K., Manna M. N., et al. (2019). Fucoidan from marine brown algae attenuates pancreatic cancer progression by regulating p53-NFkB crosstalk. Phytochemistry 167, 112078. doi: 10.1016/j.phytochem.2019.112078
Chang X., Kang M., Feng J., Zhang J., Wang X. (2023). Effects of BDE-209 exposure on growth performance, intestinal digestive enzymes, and intestinal microbiome in common carp (Cyprinus carpio l.). Aquac. Fish. 8, 33–41. doi: 10.1016/J.AAF.2021.05.005
Chotigeat W., Tongsupa S., Supamataya K., Phongdara A. (2004). Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 233, 23–30. doi: 10.1016/j.aquaculture.2003.09.025
Cui H., Wang Z., Liu J., Wang Y., Wang Z., Fu J., et al. (2020). Effects of a highly purified fucoidan from Undaria pinnatifida on growth performance and intestine health status of gibel carp Carassius auratus gibelio. Aquac. Nutr. 26, 47–59. doi: 10.1111/anu.12966
Dawood M. A. O., Alkafafy M., Sewilam H. (2022). The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 1–12. doi: 10.1007/S10695-022-01052-W
Dawood M. A., Koshio S., Esteban M. Á. (2018). Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev. Aquac. 10, 950–974. doi: 10.1111/raq.12209
Eslami M., Zaretabar A., Dawood M. A. O., Mohammadzadeh S., Ahmadifar E., Sheikhzadeh N., et al. (2022). Can dietary ethanolic extract of propolis alter growth performance, digestive enzyme activity, antioxidant, and immune indices in juvenile beluga sturgeon (Huso huso)? Aquaculture 552, 737939. doi: 10.1016/J.AQUACULTURE.2022.737939
Fazio F., Iaria C., Saoca C., Costa A., Piccione G., Spanò N. (2019). Effect of dietary vitamin c supplementation on the blood parameters of striped bass Morone saxatilis (walbaum 1752). Turk. J. Fish. Aquat. Sci. 20, 491–497. doi: 10.4194/1303-2712-V20_6_07
Geetanjali Y., Dharmendra K. M., Amiya K. S., Basanta K. D., Ramkrishna S. (2020). Effective valorization of microalgal biomass for the production of nutritional fish-feed supplements. J. Clean. Prod. 243, 118697. doi: 10.1016/j.jclepro.2019.118697
Ghafarifarsani H., Hoseinifar S. H., Sheikhlar A., Raissy M., Chaharmahali F. H., Maneepitaksanti W., et al. (2022). The effects of dietary thyme oil (Thymus vulgaris) essential oils for common carp (Cyprinus carpio): growth performance, digestive enzyme activity, antioxidant defense, tissue and mucus immune parameters, and resistance against Aeromonas hydrophila. Aquac. Nutr. 2022, 7942506. doi: 10.1155/2022/7942506
Han Y. S., Lee J. H., Lee S. H. (2015a). Antitumor effects of fucoidan on human colon cancer cells via activation of akt signaling. Biomol. Ther. 23, 225–232. doi: 10.4062/biomolther.2014.136
Han Y. S., Lee J. H., Lee S. H. (2015b). Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/ mechanistic target of rapamycin pathways. Mol. Med. Rep. 12, 3446–3452. doi: 10.3892/mmr.2015.3804
Hemre G. I., Mommsen T. P., Krogdahl A. (2002). Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 8, 175–194. doi: 10.1046/j.1365-2095.2002.00200.x
Ikeda-Ohtsubo W., López Nadal A., Zaccaria E., Iha M., Kitazawa H., Kleerebezem M., et al. (2020). Intestinal microbiota and immune modulation in zebrafish by fucoidan from Okinawa mozuku (Cladosiphon okamuranus). Front. Nutr. 7. doi: 10.3389/fnut.2020.00067
Jayant M., Sahu N. P., Deo A. D., Gupta S., Rajendran K. V., Garg C. K., et al. (2021). Effective valorization of bio-processed castor kernel meal based fish feed supplements concomitant with oil extraction processing industry: a prolific way towards greening of landscaping/environment. Environ. Technol. Inno. 21, 101320. doi: 10.1016/J.ETI.2020.101320
Jean L. (2020). Intestinal alkaline phosphatase in the gastrointestinal tract of fish: biology, ontogeny, and environmental and nutritional modulation. Rev. Aquac. 12, 555–581. doi: 10.1111/raq.12340
Jin J. O., Yadav D., Madhwani K., Puranik N., Chavda V., Song M. (2022). Seaweeds in the oncology arena: anti-cancer potential of fucoidan as a drug-a review. Molecules 27, 6032. doi: 10.3390/MOLECULES27186032
Josekutty J., Iqbal J., Iwawaki T., Kohno K., Hussain M. M. (2013). Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1α/cJun to enhance plasma ALT/AST. J. Biol. Chem. 288, 14372–14383. doi: 10.1074/jbc.M113.459602
Khorshidi Z., Paknejad H., Sodagarm M. D., Hajimoradloo A., Shekarabi S. P. H. (2022). Effect of a commercial multi-effect additive (Biotronic® Top3) on growth performance, digestive enzymes, and intestinal barrier gene expression in common carp (Cyprinus carpio). Aquaculture 560, 738588. doi: 10.1016/J.AQUACULTURE.2022.738588
Kim K. J., Lee O. H., Lee B. Y. (2011). Low-molecular-weight fucoidan regulates myogenic differentiation through the mitogen-activated protein kinase pathway in C2C12 cells. Br. J. Nutr. 106, 1836–1844. doi: 10.1017/S0007114511002534
Kim K. J., Lee O. H., Lee B. Y. (2012). Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 83, 1628–1635. doi: 10.1016/j.fitote.2012.09.014
Kirindage K. G. I. S., Jayasinghe A. M. K., Cho N., Cho S. H., Yoo H. M., Fernando I. P. S., et al. (2022). Fine-Dust-Induced skin inflammation: low-Molecular-Weight fucoidan protects keratinocytes and underlying fibroblasts in an integrated culture model. Mar. Drugs 21, 12. doi: 10.3390/MD21010012
Kord M. I., Srour T. M., Omar E. A., Farag A. A., Nour A. A. M., Khalil H. S. (2021). The immunostimulatory effects of commercial deed additives on growth performance, non-specific immune response, antioxidants assay, and intestinal morphometry of Nile tilapia, Oreochromis niloticus. Front. Physiol. 12. doi: 10.3389/FPHYS.2021.627499
Li B., Lu F., Wei X., Zhao R. (2008). Fucoidan: structure and bioactivity. Molecules 13, 1671–1695. doi: 10.3390/molecules13081671
Li F., Wu D., Gu H. R., Yin M., Ge H. L., Liu X. H., et al. (2019). Aeromonas hydrophila and Aeromonas veronii cause motile Aeromonas septicaemia in the cultured Chinese sucker, Myxocyprinus asiaticus. aquac. Res. 50, 1515–1526. doi: 10.1111/are.14028
Liu W. C., Zhou S. H., Balasubramanian B., Zeng F. Y., Sun C. B., Pang H. Y. (2020). Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 104, 202–212. doi: 10.1016/j.fsi.2020.05.079
Mahgoub H. A., El-Adl M. A. M., Ghanem H. M., Martyniuk C. J. (2020). The effect of fucoidan or potassium permanganate on growth performance, intestinal pathology, and antioxidant status in Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 46, 2109–2131. doi: 10.1007/s10695-020-00858-w
Makoto T., Takeaki N., Tomofumi M., Masahiko I. (2019). Evaluation of the immunomodulatory effects of fucoidan derived from Cladosiphon okamuranus tokida in mice. Mar. Drugs 17, 547. doi: 10.3390/md17100547
Maritza A., Camilo P., Alejandro B., Delbert M. G. (2012). The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × m. saxatilis). Brit. J. Nutr. 109, 623–629. doi: 10.1017/S0007114512001754
Mir I. N., Sahu N. P., Pal A. K., Makesh M. (2017). Synergistic effect of l-methionine and fucoidan rich extract in eliciting growth and non-specific immune response of Labeo rohita fingerlings against Aeromonas hydrophila. Aquaculture 479, 396–403. doi: 10.1016/j.aquaculture.2017.06.001
Mohammadi G., Hafezieh M., Karimi A. A., Azra M. N., Van D. H., Tapingkae W., et al. (2021). The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 120, 304–313. doi: 10.1016/J.FSI.2021.11.028
Mohammadian T., Monjezi N., Peyghan R., Mohammadian B. (2021). Effects of dietary probiotic supplements on growth, digestive enzymes activity, intestinal histomorphology and innate immunity of common carp (Cyprinus carpio): a field study. Aquaculture 549, 37787. doi: 10.1016/J.AQUACULTURE.2021.737787
Pomin V. H. (2015). Sulfated glycans in inflammation. Eur. J. Med. Chem. 92, 353–369. doi: 10.1016/j.ejmech.2015.01.002
Robbins K. R., Saxton A. M., Southern L. L. (2006). Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 84, 155–165. doi: 10.2527/2006.8413_supplE155x
Singh S. B., Lin H. C. (2021). Role of intestinal alkaline phosphatase in innate immunity. Biomolecules 11, 1784. doi: 10.3390/BIOM11121784
Sinurat E., Saepudin E., Peranginangin R., Hudiyono S. (2016). Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vannamei. J. Appl. Pharm. Sci. 6, 82–91. doi: 10.7324/japs.2016.601011
Sivagnanavelmurugan M., Thaddaeus B. J., Palavesam A., Immanuel G. (2014). Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish Shellfish Immunol. 39, 439–449. doi: 10.1016/j.fsi.2014.05.037
Sony N. M., Ishikawa M., Hossain M. S., Koshio S., Yokoyama S. (2019). The effect of dietary fucoidan on growth, immune functions, blood characteristics and oxidative stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol. Biochem. 45, 439–454. doi: 10.1007/s10695-018-0575-0
Traifalgar R. F., Kira H., Tung H. T., Michael F. R., Yokoyama A. L. S., Ishikaw M. A., et al. (2010). Influence of dietary fucoidan supplementation on growth and immunological response of juvenile Marsupenaeus japonicus. J. World Aquac. Soc 41, 235–244. doi: 10.1111/j.1749-7345.2010.00363.x
Wang X. L., Chen M. H. (2015). Fucoidan inhibits cardiac remodeling after myocardial infarction in mice. Basic Clin. Med. 35, 959–962. doi: 10.16352/j.issn.1001-6325.2015.07.022
Wang L., Cui Y. R., Wang K., Fu X., Xu J., Gao X., et al. (2022). Anti-inflammatory effect of fucoidan isolated from fermented Sargassum fusiforme in in vitro and in vivo models. Int. J. Biol. Macromol. 222, 2065–2071. doi: 10.1016/J.IJBIOMAC.2022.10.005
Wang Y., Xing M., Cao Q., Ji A., Liang H., Song S. (2019). Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar. Drugs 17, 183. doi: 10.3390/md17030183
Wu P., Xie L. Y., Wu X. Z., Wang Y. L., Wu Y., Li N., et al. (2019). Effect of Rhodopseudomonas sphaeroides-treated wastewater on yield, digestive enzymes, antioxidants, nonspecific immunity, and intestinal microbiota of common carp. N. Am. J. Aquac. 81, 385–398. doi: 10.1002/naaq.10106
Xiao Y., Li X. F., Ding H. (2017). Protective effects of fucose on immunological liver injury in mice. Food Sci. 38, 155–159. doi: 10.7506/spkx1002-6630-201713026
Xue M. L., Tian Y. J., Sui Y. Z., Zhao H., Gao H. Q., Liang H., et al. (2022a). Protective effect of fucoidan against iron overload and ferroptosis-induced liver injury in rats exposed to alcohol. Biomed. Pharmacother. 153, 113402. doi: 10.1016/J.BIOPHA.2022.113402
Xue S., Xia B., Zou Y., Li L., Zhang B., Shen Z., et al. (2022b). Dandelion extract on growth performance, immunity, stress and infection resistance in common carp. Aquac. Rep. 26, 101330. doi: 10.1016/J.AQREP.2022.101330
Yang Q., Yang R., Li M., Zhou Q. C., Liang X. P., Zacharia C. E. (2014). Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 41, 264–270. doi: 10.1016/j.fsi.2014.09.003
Yang G., Yu R., Geng S., Xiong L., Yan Q., Kumar V., et al. (2021). Apple polyphenols modulates the antioxidant defense response and attenuates inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture 534, 736284. doi: 10.1016/J.AQUACULTURE.2020.736284
Yu S. H., Wu S. J., Wu J. Y., Wen D. Y., MiF. L. (2015). Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 126, 97–107. doi: 10.1016/j.carbpol.2015.02.068
Zacarias M., Barón-Sevilla B., Lazo J. P. (2013). Ontogeny and distribution of alkaline and acid phosphatases in the digestive system of California halibut larvae (Paralichthys californicus). Fish Physiol. Biochem. 39, 1331–1339. doi: 10.1007/s10695-013-9787-5
Zhang Y., Gao K., Ren Y., Zhang J., Lu R., Cao X., et al. (2022). Broken xinyang maojian tea supplementation in a high-fat diet improves the growth performance, flesh quality and lipid metabolism of yellow river carp (Cyprinus carpio). Aquac. Rep. 25, 101236. doi: 10.1016/J.AQREP.2022.101236
Zhang C. Y., Wu W. H., Wang J., Lan M. B. (2012). Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar. Drugs 10, 119–130. doi: 10.3390/md10010119
Keywords: fucoidan, common carp, growth, immunity, antioxidant
Citation: Li F, Sun H, Li Y, He D, Ren C, Zhu C and Lv G (2023) Effects of fucoidan on growth performance, immunity, antioxidant ability, digestive enzyme activity, and hepatic morphology in juvenile common carp (Cyprinus carpio). Front. Mar. Sci. 10:1167400. doi: 10.3389/fmars.2023.1167400
Received: 16 February 2023; Accepted: 09 May 2023;
Published: 21 June 2023.
Edited by:
Zhen Zhang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), IndiaCopyright © 2023 Li, Sun, Li, He, Ren, Zhu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanchang Sun, c3VuaGFuY2hhbmcxOTlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.