- Postgraduate Program in Ecology and Evolution (PPGEE), Rio de Janeiro State University (UERJ), Rio de Janeiro, Brazil
Animal behavior represents the connection between individuals and their environment. Environmental conditions on sandy beaches determine the presence, demographic characteristics, and life history of benthic macrofaunal species. The expression of agile behaviors on sandy beaches is a key factor given the rapid environmental changes. Atlantorchestoidea brasiliensis is a sandhopper crustacean, a bioindicator for sandy beach quality, which usually builds burrows along the intersections of wet and dry sand. This study aimed to evaluate differences in the burial depth of A. brasiliensis in regions with and without anthropogenic interactions. We hypothesize that the sandhopper buries further deep on beaches with greater human interference as a behavioral adaptation to avoid direct human interaction. Samples of the A. brasiliensis burrows and data on their burial depths were collected from four beaches in Rio de Janeiro, two with free access to people and two with restricted access. Environmental variables were determined and their influence on burial depth was tested using a Generalized Linear Model. Generalized Additive Models were used to test the occurrence of individuals at each burial depth in the two beach situations. No clear relationship was observed between the environmental variables and burial depth whereas, deeper burials were observed only on free access beaches. The results obtained substantiate the initial hypothesis. The present study is thus an initial step towards further research on changes in the pattern of animal behavior induced by direct anthropogenic pressures on sandy beaches.
1 Introduction
Animal behavior represents the connection between individuals and their environment (Breed and Moore, 2022), a phenotypic response arising from the interactions between genotype and environmental conditions. Thus, the potential of certain behaviors of an individual will only be verified in terms of the characteristics of the environment (Sene, 2016). Innate and instinctive behaviors inherent to the species are not learned and are explained by heredity (Templeton, 2006; Sene, 2016). This explains similar behavioral patterns of different populations of the same species.
Instinctive behavior of crustaceans from sandy beaches can be verified by their ability to burrow, swim, and hop, as observed, respectively, in the beach crustaceans Emerita brasiliensis Schmitt, 1935, Excirolana braziliensis Richardson, 1912, and Atlantorchestoidea brasiliensis (Dana, 1853). Behaviors range from agile movements to very discreet ones, such as ammonia excretion (Zhang et al., 2020), the release of pheromones by marine crustacean species (Ryan, 1966), and color change to camouflage in marine shrimp such as, Hippolyte obliquimanus Dana, 1852 (Duarte et al., 2016).
Patterns of certain behaviors can be modified in the face of natural selective pressures, in which organisms must find ways to survive. Basic behaviors conferring to survival and maintenance of primary vital functions on exposed sandy beaches (McLachlan, 1980) mainly involve searching for shelter, feeding, and reproduction (Brown, 1996; Scapini, 2014). Three key factors of beach ecosystem determining the presence of benthic macrofaunal species namely: mean grain size, slope of the beach, and wave climate (McLachlan and Defeo, 2018), along with their demographic characteristics and life history, body size and biomass, reproduction rates, ecological interactions, and behaviors involved in feeding and foraging (McLachlan and Defeo, 2018).
The availability of food plays a crucial role in shaping the structure of macrofauna on sandy beaches. These ecosystems rely on a variety of organic matter sources including, phytoplankton from the surf zone, carrion, and wrack (McLachlan and Defeo, 2018). However, the inputs of these food sources are not constant and exhibit spatiotemporal variations (Bergamino et al., 2016; Quillien et al., 2016), directly influencing the composition and organization of the macrofaunal communities. In these ecosystems, the success of species depends on their spatiotemporal responses to environmental factors, requiring an appropriate orientation in choosing periods to leave their burrows to feed and foraging sites.
The expression of agile behaviors on sandy beaches is a key factor given the rapid environmental changes in these ecosystems (Scapini, 2014). Burying is common among all species inhabiting intertidal beaches, and involves a penetration phase followed by excavation movements, the success of which, in terms of depth and stability, is related to the physical properties of the substrates, including the mean grain size, compactability, and penetrability of sand (McLachlan and Defeo, 2018).
Atlantorchestoidea brasiliensis (synonymized/unaccepted names: Orchestia brasiliensis, Orchestoidea brasiliensis, Pseudorchestoidea brasiliensis) is a sandhopper crustacean of the Talitridae family (Malacostraca: Amphipoda) with an aggregated distribution pattern, forming visible banks of individuals and records of occurrence on beaches exposed to the action of high-energy waves in Brazil and Uruguay (Cardoso, 2002; Defeo and Gómez, 2005; Veloso et al., 2010; Gómez and Defeo, 2012; Costa and Zalmon, 2019; Pennafirme and Soares-Gomes, 2022).
The ability to jump (to hop) is a differential between talitrid amphipods and other benthic macrofaunal groups that inhabit sandy beaches; this is attributed to a body-catapult mechanism and configures the possibility of a quick escape from threats from the environment (Wan and Gorb, 2021). A. brasiliensis populations usually occupy the regions along the intersections of wet and dry sand in which individuals exhibit burrowing behavior up to an average depth of 15 cm (Cardoso, 2002; Veloso et al., 2010). Homotype and homochromy (Sene, 2016) are traits verified in A. brasiliensis; homotype is evident from the shape and size of individuals, often visually confused with grains of sand, whereas the yellowish-white color of homochromia disguises them from the beach environment. A. brasiliensis individuals have an average length of 8 mm and a lifespan of approximately 16 months, with continuous annual reproduction (Cardoso and Veloso, 1996; Gómez and Defeo, 1999; Pennafirme and Soares-Gomes, 2022). The semi-terrestrial A. brasiliensis presents the ability to orient towards the sea using solar orientation (Bessa et al., 2017) with ideal periods for feeding, including greater intensity at dawn and nightfall, probably because of lower environmental stress (Cardoso, 2002; Veloso et al., 2012).
This species has been identified as a bioindicator for the environmental quality of sandy beaches, given that its density decreases in more urbanized sectors and beaches (Veloso et al., 2010; Cardoso et al., 2016; Costa et al., 2017; Suciu et al., 2018) because of human trampling (Veloso et al., 2010). The influence of human pressure on the morphology and behavior of beach organisms has been evaluated in previous studies. Costa et al. (2022) reported negative effects of urbanization on Callichiridae Callichirus major (Say, 1818), affecting its body size and fecundity, and Afghan et al. (2020) highlighted that the bivalve Donacidae Donax serra Röding, 1798, can bury themselves further deep to avoid trampling.
Anthropogenic activities daily affect the beaches global on spatiotemporal scales. Most beaches in Rio de Janeiro experience frequent human interference in form of leisure, tourism, commerce, and recreational activities (Andreatta et al., 2009), causing trampling over benthic fauna, the local disposal of human solid waste left by visitors, and the consequent cleaning procedures, manual or mechanized, to remove them (Abude et al., 2021). Most beaches in the city are in the urban context with coastal armoring, further attracting tourists, and thus reducing the banks and population of A. brasiliensis (Veloso et al., 2010). A. brasiliensis burrows and their activity have however been observed along beaches less frequented. Sarongs, beach chairs, and umbrellas also have adverse effects on the A. brasiliensis burrows and communities (in the middle of the beach). On beaches with some degree of conservation and less direct human pressure, it is possible to observe extensive banks and intense activity of A. brasiliensis.
Given this context, the primary objective of this study was to investigate whether the direct human interference on beach is capable of affecting the burying behavior of A. brasiliensis. This study thus aims to expand the knowledge on the burial behavior and its plasticity of the Talitridea bioindicator for beach environmental quality, as a response mechanism to direct human pressures. We hypothesize that on beaches frequented by humans, trampling, and use of heavy cleaning machinery, talitrids bury themselves deeper. To verify this hypothesis, differences in the depth of burial and individual characteristics, such as sex and age group, were evaluated for beaches frequented and trampled, and beaches without direct human interference.
2 Materials and methods
2.1 Study area
Rio de Janeiro has a littoral area of more than 600 km, with at least 15 ocean beach arches. Irrespective of whether the beach is free or restricted access, especially the ones possible only by trails crossing mountains, they are an important leisure and recreational attraction for residents and tourists of the city.
This study focused on beaches not subjected to intense human pressure, to ensure the occurrence of A. brasiliensis (Cardoso et al., 2016). Some city beaches are legally protected by Conservation Unites, which favors their environmental quality in the face of human pressures and allows the presence of A. brasiliensis. Other beaches are practically deserted, owned by the military authorities, thus restricting the civilian. Although Conservation Units reduce the incidence of anthropic impacts, beaches in the prior category are frequented by people and regular mechanized cleaning procedures. Thus, despite guaranteeing a certain level of environmental quality, these beaches are characterized as more impacted environments than those in military areas, where there is no regular presence of people or mechanized cleaning procedures.
Four beaches were chosen in this study, two with free access and two with restricted access: Grumari (23° 2′ S, 43° 31′ W) and Reserva (23° 00′ S, 43° 24′ W) beaches, located in Conservation Units (free access and trampled beaches), and Marambaia (23° 03′ S, 43° 30′ W) and Fora (22° 57′ S, 43° 11′ W) beaches, located in military areas (restricted access and no trampled beaches) (Figure 1). The Urbanization Index proposed by González et al. (2014) was used to characterize beaches in relation to human interventions (Table S1).
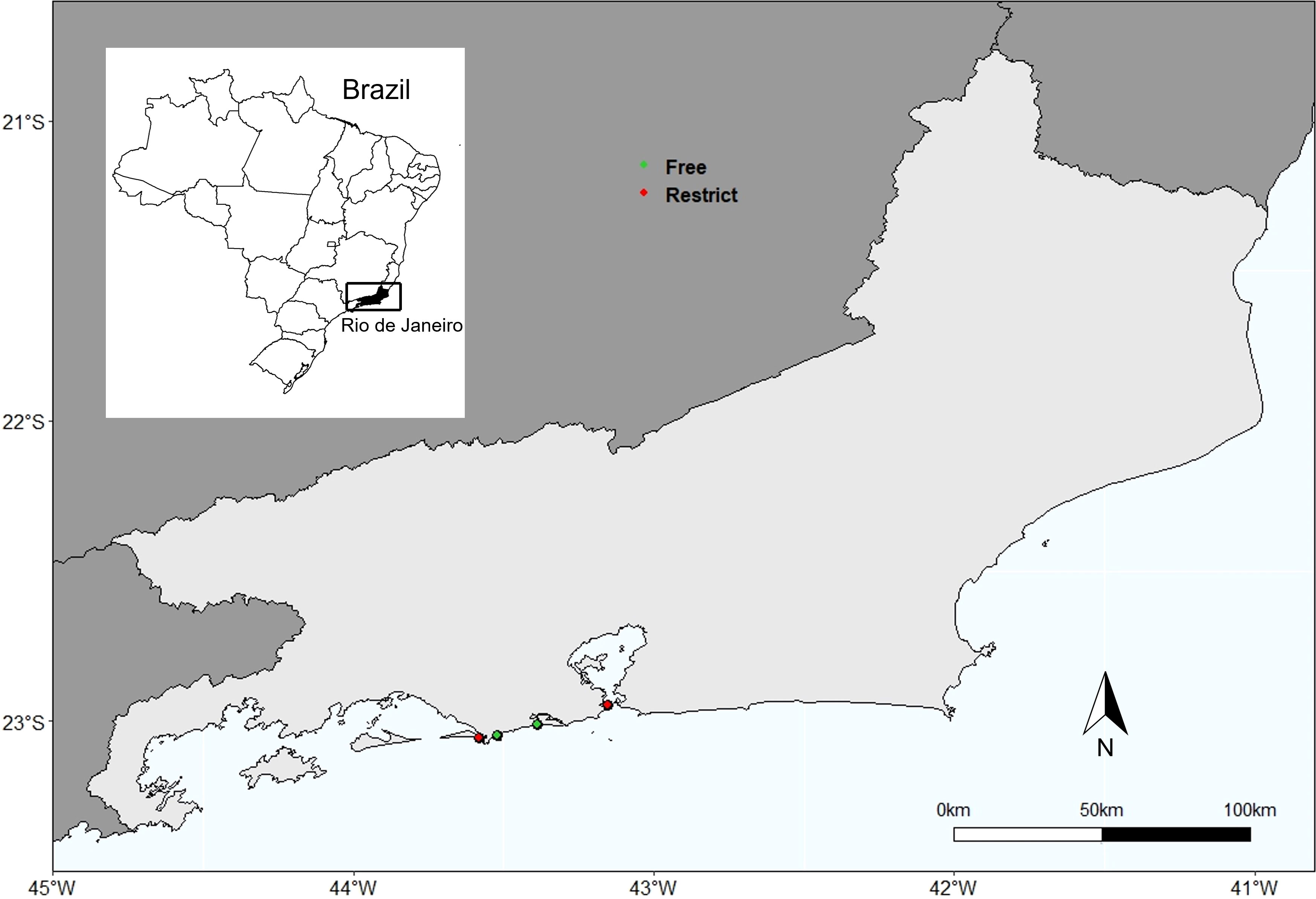
Figure 1 Study Area. Highlighting the city of Rio de Janeiro, southeastern Brazil. Green dots: free access beach; red dots: restricted access beaches.
Grumari is a ~2 km long beach arc legally protected by the Grumari Environmental Protection Area and Grumari Municipal Natural Park. Reserva beach is situated in the ~18 km long Barra da Tijuca beach arc, with a stretch of ~8 km located on the edge of Marapendi Municipal Natural Park and Barra da Tijuca Municipal Natural Park Nelson Mandela. The stretch of the Reserva beach has a well-preserved area of lagoons and coastal vegetation. Like Grumari, the Reserva region has extensive sandbanks and a well-preserved backshore, with no housing settlements in the surroundings and some kiosks and restaurants on the seafront. These beaches are popular for leisure, tourism, sports, and surfing. The two beaches are not easily accessible by public transport, thus limiting the number of visitors. This limitation is especially important in summer and sunny weekends when beaches are more frequented. Daily, these beaches receive regular mechanized cleaning procedures using tractors and heavy machinery.
Marambaia represents the largest beach arc in Rio de Janeiro (~48 km), including the limits of three municipalities: Rio de Janeiro, Itaguaí, and Mangaratiba. In this study, we investigated the Rio de Janeiro’s first kilometer stretch under the authority of the Brazilian Army. Owing to restrictions on access, this beach is free from direct human interference, making it an ideal model of a well-preserved beach ecosystem. There is well-preserved coastal vegetation and no housing settlement in the immediate vicinity. The beach is directly influenced by neighborhoods adjacent to Sepetiba Bay, which transport human solid waste to the beach (Abude et al., 2021). However, there are no mechanized or regular manual cleaning procedures in place, resulting in accumulation of a significant amount of solid waste in the upper littoral area.
Fora is an ~800 m pocket beach situated under the jurisdiction of the Brazilian Army. Despite restricted access, the beach has a seafront boardwalk and no coastal vegetation. The area is home to an important Physical Education school. Similar to Marambaia, few people are seen on this beach, frequented only by some residents of the military area, mostly for waterside walks. Sports practices are prohibited on beaches. Fora beach receives manual cleaning procedures daily for the removal of solid waste brought by the waters of Guanabara Bay.
2.2 Sampling procedures
Samples were collected in the winter and summer of 2021, during low spring tide periods. Three sampling areas were established on each beach over the visible banks of A. brasiliensis. Sampling was conducted for 1 h on each area, accounting for a total of 3 h on the beach (maximum possible time to avoid rising tide). For sampling, a corer-type cylindrical sampler, 5 cm in diameter and 25 cm in height, subdivided into five sections, each of 5 cm depth (5, 10, 15, 20, 25 cm) was used. In each area, twenty replicates from individual burrows were randomly sampled, once each.
The sampler was introduced into the sediment to a maximum depth (25 cm) for each sampling. The sampled sediment was removed, and each 5 cm section was subsequently separated from the other by a physical barrier. In the field, the material was screened by eliminating the physical barrier from each section one at a time. The presence and frequency of individuals at each depth range were recorded. The collected individuals were separated by area, replica, and depth and preserved in 70% alcohol for further analysis.
Key physical beach variables were also sampled (McLachlan and Defeo, 2018). On each area per beach, the slope was estimated according to Emery (1961) (Cardoso et al., 2016). Fifteen sediment samples per area and beach were collected and replicated per season at varying depths (5, 10, 15, 20, 25 cm), using a corer-type cylindrical sampler 3.5 cm in diameter, for granulometric analysis and measurement of the organic matter in the sediments. Moreover, sediment penetrability in each of the sampled areas was obtained using an analog penetrometer (Herrick and Jones, 2001), and sediment temperature was measured using a thermometer.
2.3 Laboratory analysis
Individual A. brasiliensis were measured from the beginning of the cephalon to the end of the telson in millimeters with a digital caliper and ocular lens and were classified by age group (adults and juveniles) (Cardoso and Veloso, 1996). Those smaller than 4 mm and without oostegites were classified as juveniles. Adult males were identified by a developed second gnathopod, females were identified by the presence of oostegites and without a developed second gnathopod, and ovigerous females with oostegites, without developed second gnathopod and the presence of eggs (Cardoso and Veloso, 1996).
Sediment samples were dried in a stove at 70°C for 24 h. Following this, approximately 10 g of each sample was burned in a muffle furnace at 600°C for 4 h. The weight difference before and after burning was used as a proxy for the organic matter content in the sediment (Greiser and Faubel, 1988). For granulometric characterization, approximately 30 g of sediment was sieved by agitation as per method specified by to Suguio (1973). The average value of all replicates from each beach was used to classify the beaches’ sand according to Wentworth’s classification (1934), adapted from Suguio (1973).
2.4 Statistical analysis
A Generalized Linear Model with Binomial distribution and logit link function (Zuur et al., 2009) was used to test the occurrence of adult individuals, juveniles, and ovigerous females on all the study areas, considering the various burial depths and the interaction between these two factors. A Wald Chi-square test (Type II Wald chi-square test) was applied to each term and interaction to obtain the Analysis of Deviance Table (R “car” package). The significance of pairwise differences between levels of significant effects was tested using Tukey’s HSD test with Bonferroni correction of p values (R ‘multcomp’ package).
The influence of physical environmental variables (organic matter content, mean grain size, sediment temperature, beach profile slope, and sand surface penetrability) on the occurrence of adults, juveniles, and ovigerous females were initially tested using Additive Models Generalized (GAM; R “mgcv” package), with Binomial distribution (link=logit). For the application of the models, the explanatory variables were submitted to a collinearity test (VIF; R ‘HH’ package). The penetrability variables were not considered in the models because they presented VIF = 3.92, which is higher than the cutoff value (VIF > 3) proposed by Zuur et al. (2009), collinear with the grain size variable.
The smoothing spline of the explanatory variables was applied using the cubic regression spline method (cubic regression spline), and optimal smoothing was estimated using cross-validation (Zuur et al., 2009). The explanatory variables were evaluated for the need for a smoothing spline using the chi-square test applied to the effective degrees of freedom. Since smoothing was found to be unnecessary (if not significant) for any variable, a Generalized Linear Model (GLM) with Binomial distribution (link=logit) was applied. The GLM model applied to the occurrence of ovigerous females included variable penetrability, aiming at a better fit and validation of the model.
The most parsimonious model was selected from the complete model using the lowest Akaike Information Criterion (AIC) and analysis of deviations from the residuals (Likelihood Ratio Test compared to a chi-square distribution), where each explanatory variable was removed by time before the test. Deviation analysis tests the null hypothesis that the regression parameter of the removed variable does not differ from zero (β=0); therefore, its removal will not cause a significant loss to the balance between the precision and explanation of the model, measured by the AIC (Zuur et al., 2009).
The most parsimonious models were validated through the graphical analysis of the quantiles of the randomized residuals (R “DHARMa” package) (Dunn and Smyth, 1996; Gelman and Hill, 2006). The graphical result of each model was displayed graphically through the predicted values (± CI) for each predictor variable (R “ggeffects” package).
All analysis and graphs were performed in an R environment (R Core Team, 2022) using the packages ‘HH’ (Heiberger and Holland, 2004), ‘mgcv’ (Wood, 2004), and ‘DHARMa’.
3 Results
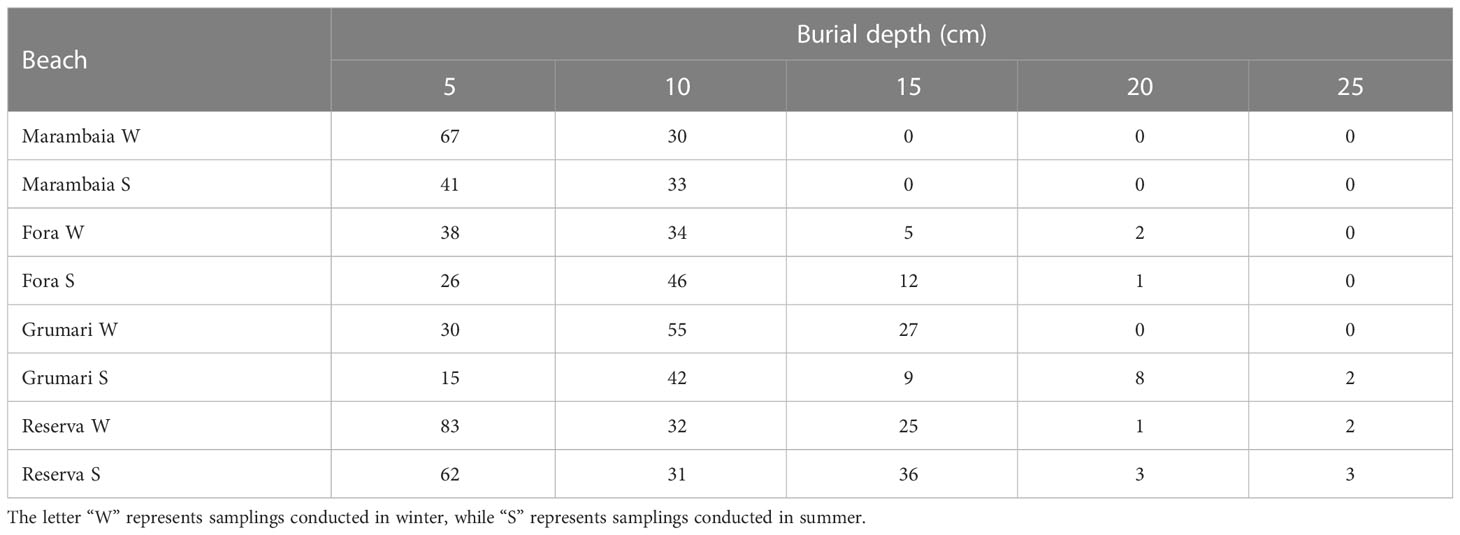
Of the total sampled A. brasiliensis, 8% were found to be juveniles and 92% adults. Among the adults, 32% were male and 68% were female, of which 60% were ovigerous. The total density of organisms collected at each burial depth for all beaches has been presented on Table 1. Depths of 5 cm, also known as the main layer, were identified as the preferred burial depths, and had 57% of the frequency of individual on free access beaches, and 60% on the restricted beaches, followed by depths of 10 cm, 15 cm, and 20 cm. Unlike the free access (trampled) beaches where individuals were reported from all burial depths, none were observed at depths of 25 cm from the restricted beaches. For the depth of 20 cm, the free access beaches recorded a total abundance of A. brasiliensis of 12%, whereas the restricted beaches recorded mere 1% (Figure 2).
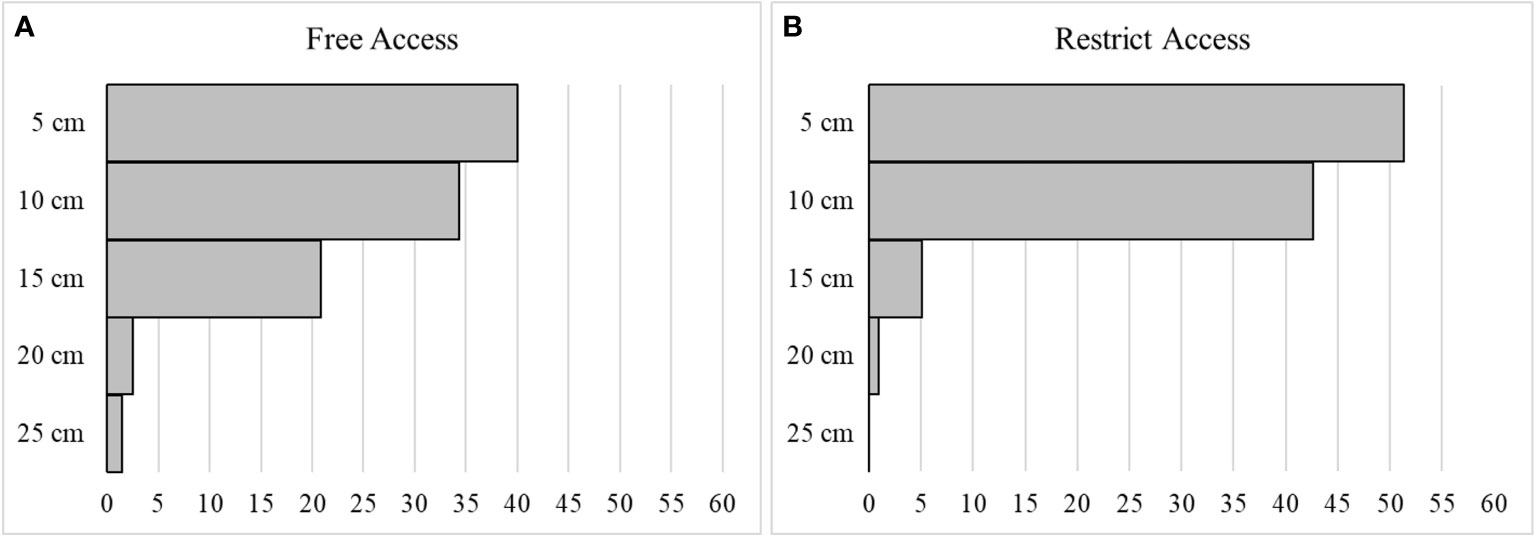
Figure 2 The horizontal, frequency, represented as a percentage (from 0 to 60%), of Atlantorchestoidea brasiliensis in each depth layers (5, 10, 15, 20 and 25 cm), for both free access beaches (A) and restricted access beaches (B).
At a 5 cm depth, adults registered a frequency of ~60% whereas, the juveniles were predominant with a frequency of 90% on free access and 85% on restricted access beaches. Overall, irrespective of whether females were ovigerous or not, they showed a higher occurrence than males at depth of 10 cm, which were found distributed more at 10 and 15 cm depths.
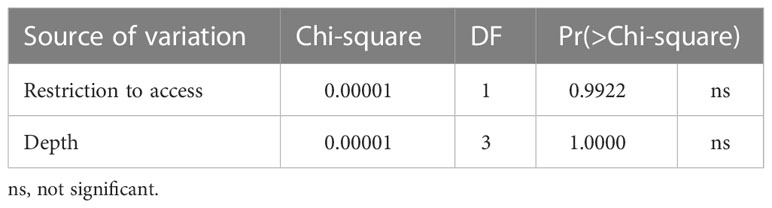
According to the model selection, the best model (GLM Binomial) to evaluate the occurrence of adult individuals in the two access situations and the different burial depths was the one without interaction between the factors (AICc = 101.7, weight = 0.955) (Table S2, Figure S1). However, according to Wald’s Chi-square test, the occurrence of adults was the same in both beach situations and at different depths (Table 2 and Figure 3).
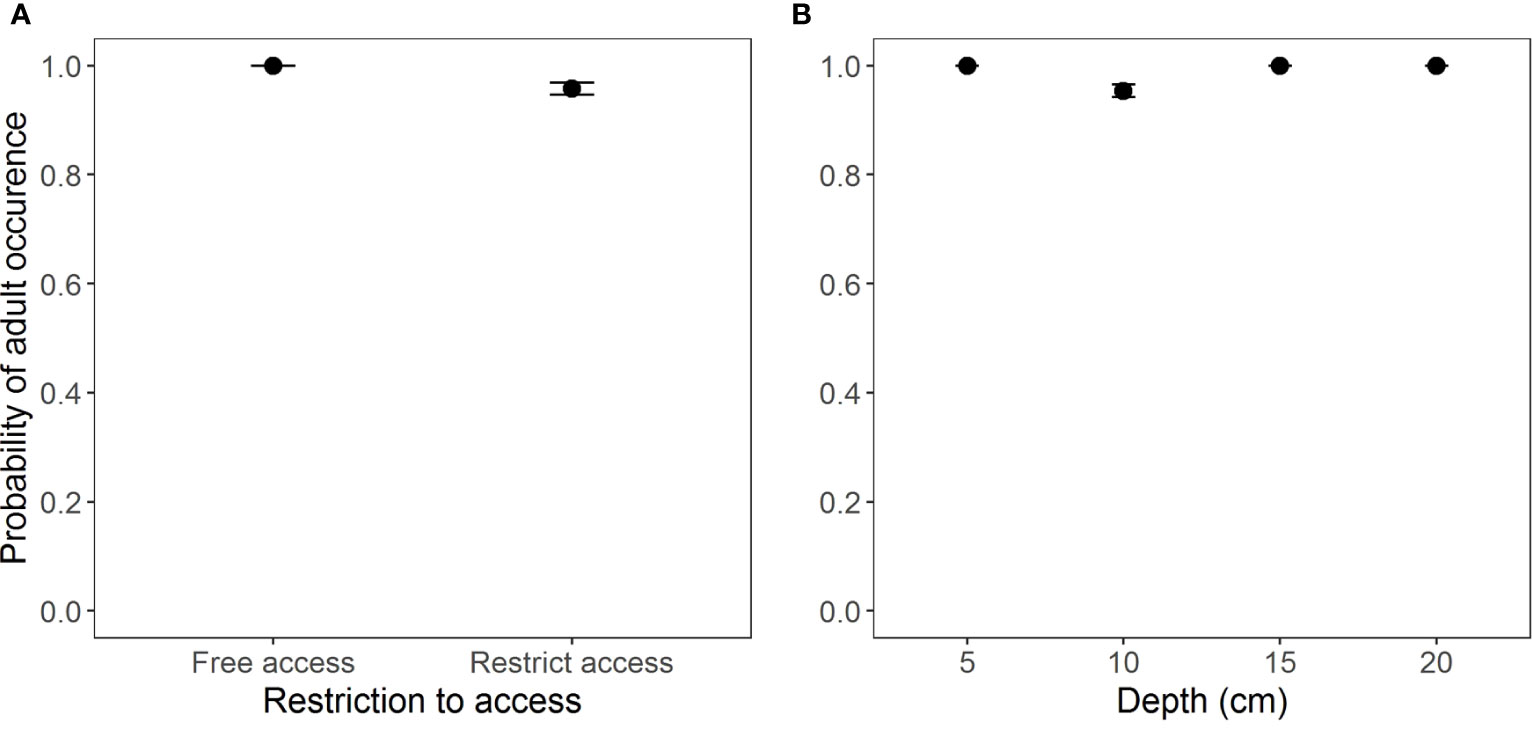
Figure 3 Occurrence of adults of Atlantorchestoidea brasiliensis in relation to (A) restricted access to beaches and (B) burial depth.
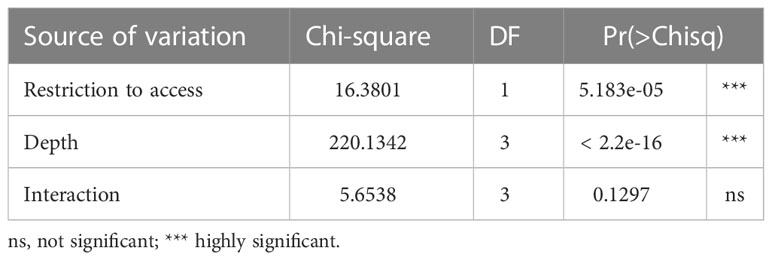
The most complete model containing the interaction between the two access situations and depth (AICc = 759.5, weight = 0.741, Table S3, Figure S2) exhibited the best fit for the occurrence of juveniles. However, the interaction was non-significant according to Wald’s Chi-square test (Table 3). Beaches with free access showed a higher occurrence of juveniles (Z = -4.69, p = 2.73e-06) (Figure 4) at a depth of 5 cm as compared to other depths (Figure 4B and Table 4).
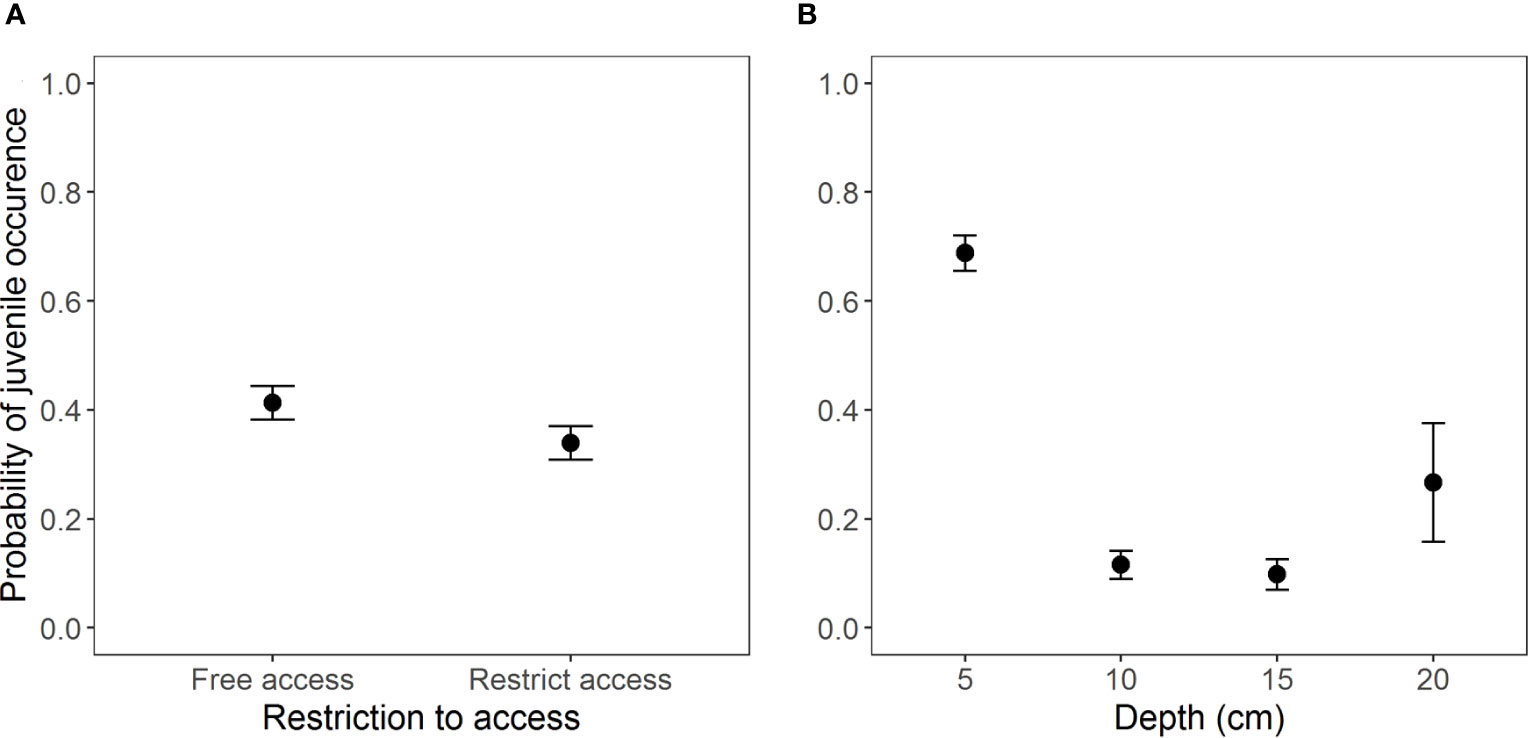
Figure 4 Occurrence of juveniles of Atlantorchestoidea brasiliensis in relation to (A) restricted access to beaches and (B) burial depth.
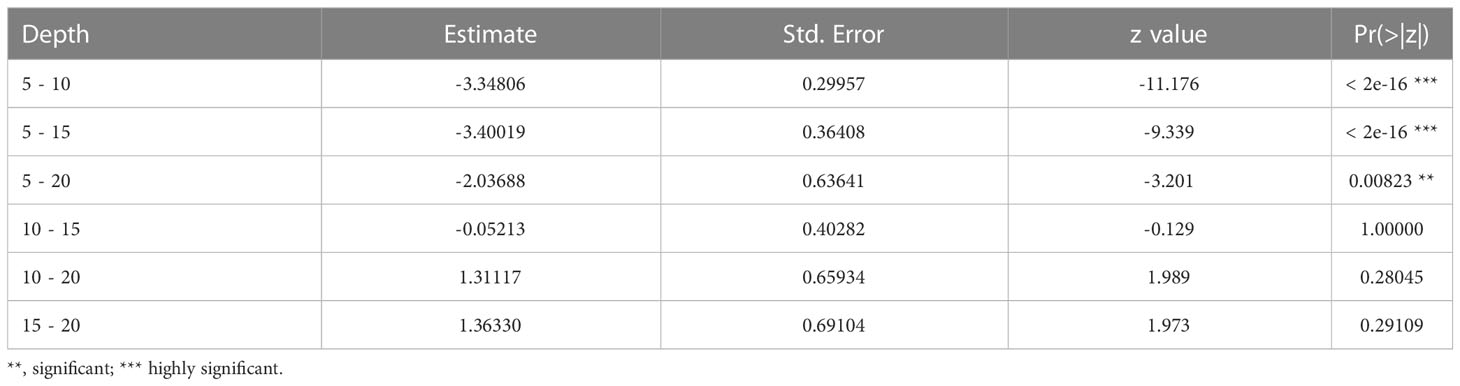
Table 4 Result of Tukey’s HSD test for pairwise comparisons between levels of depth for the significant effect of depth on the probability of occurrence of juveniles (e.g., the first line refers to the comparison between 5 cm and 10 cm in depth).
The occurrence of ovigerous females presented similar results as for the juveniles in regards to the selection of the model (AICc = 190.3, weight = 0.989) (Table S4, Figure S3). This interaction was found insignificant for the free access beaches (Table 5 and Figure 5A). According to the model selection, ovigerous females were more likely to occur at depths from 5 to 15 cm (Figure 5B and Table 6).
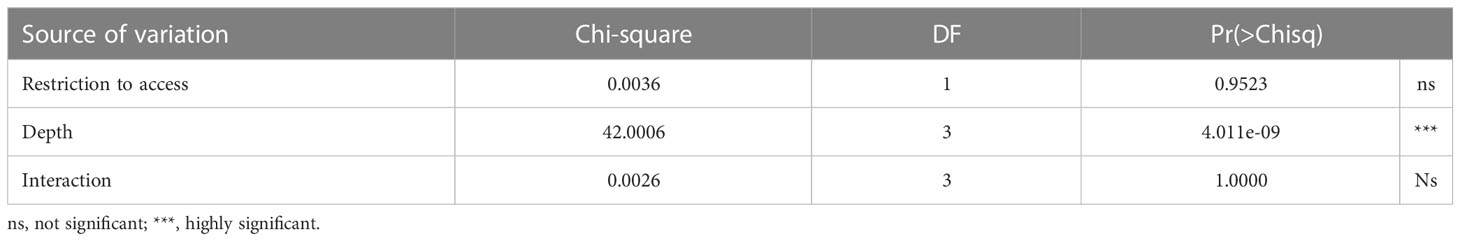
Table 5 Analysis of Deviance Table (Type II Wald chisquare test) for occurrence of ovigerous females.
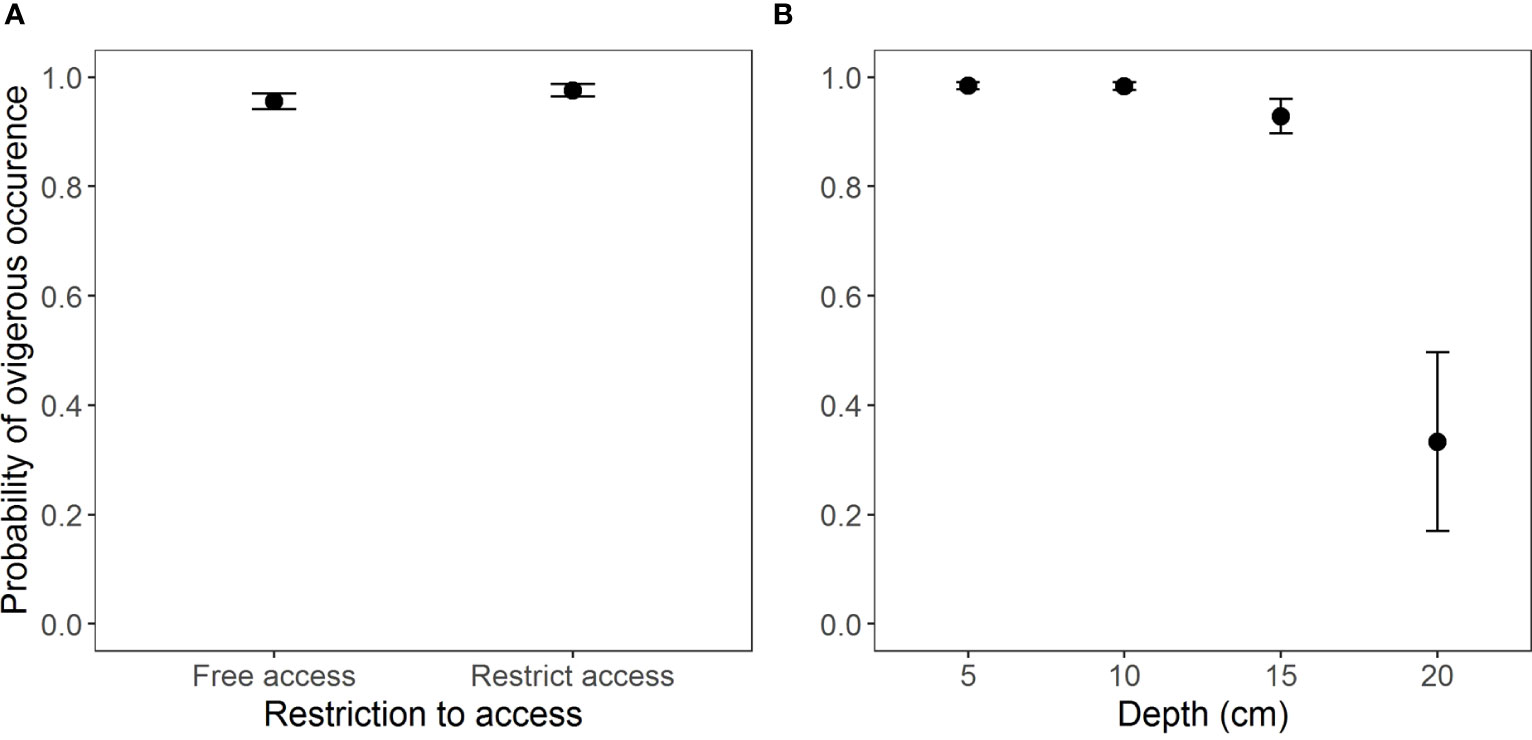
Figure 5 Occurrence of ovigerous females of Atlantorchestoidea brasiliensis in relation to (A) restricted access to beaches and (B) burial depth.
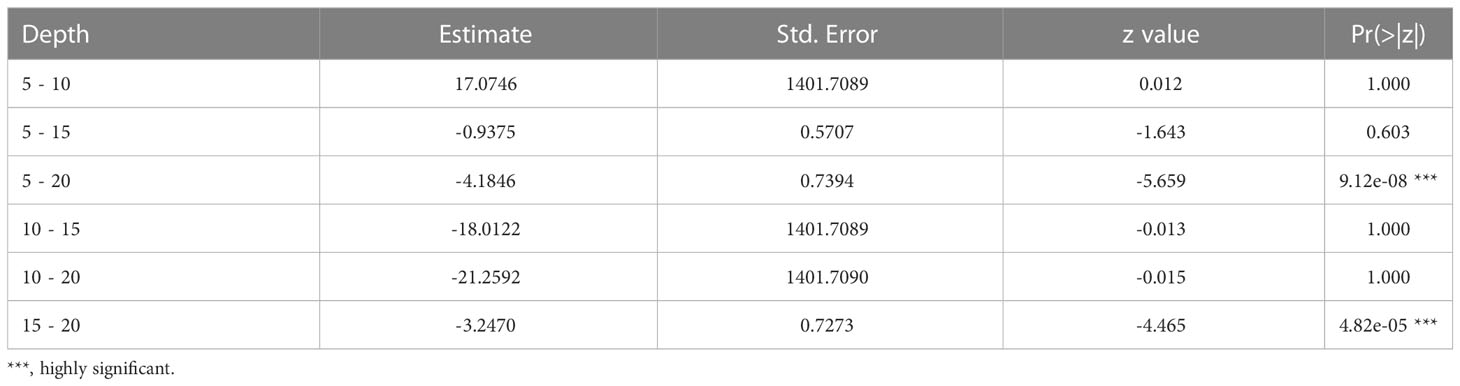
Table 6 Result of Tukey’s HSD test for pairwise comparisons between levels of depth, for the significant effect of depth on the probability of occurrence of ovigerous females. (e.g., the first line refers to the comparison between 5 cm and 10 cm in depth).
Regarding the physical environmental variables, the mean grain size varied among the beaches; however (Table S5), the model selection showed no influence of the environmental variables on the burial depth.
For each class of individuals (juveniles, adults, males, females, and ovigerous females), the physical environmental variables showed different effects. The model with the best fit and the most parsimonious (AICc = 141.5, weight = 0.328) (Table S6, Figure S4) showed that the occurrence of adults decreased with increasing organic matter content (intercept = 4.6668, slope = -1.6606, std. error = 0.7846, z = -2.117, p = 0.0343) (Figure 6). For juveniles, the relationship with physical environmental variables was better adjusted by the model that considered the organic matter content, temperature, and slope (AICc = 971.7, weight = 0.729) (Table S7, Figure S5). The occurrence of juveniles increased when the organic matter content was higher and decreased when the temperature and slope were higher (Table 7 and Figures 7A-C). For ovigerous females, the model with the best fit when temperature, slope, and penetrability were considered (AICc = 250.01, weight = 0.515) (Table S8, Figure S6). The occurrence of ovigerous females increased with increasing beach slope and penetrability (Table 8 and Figures 7D-F). Despite being considered in the selected model, temperature was found unrelated to the occurrence of ovigerous females.
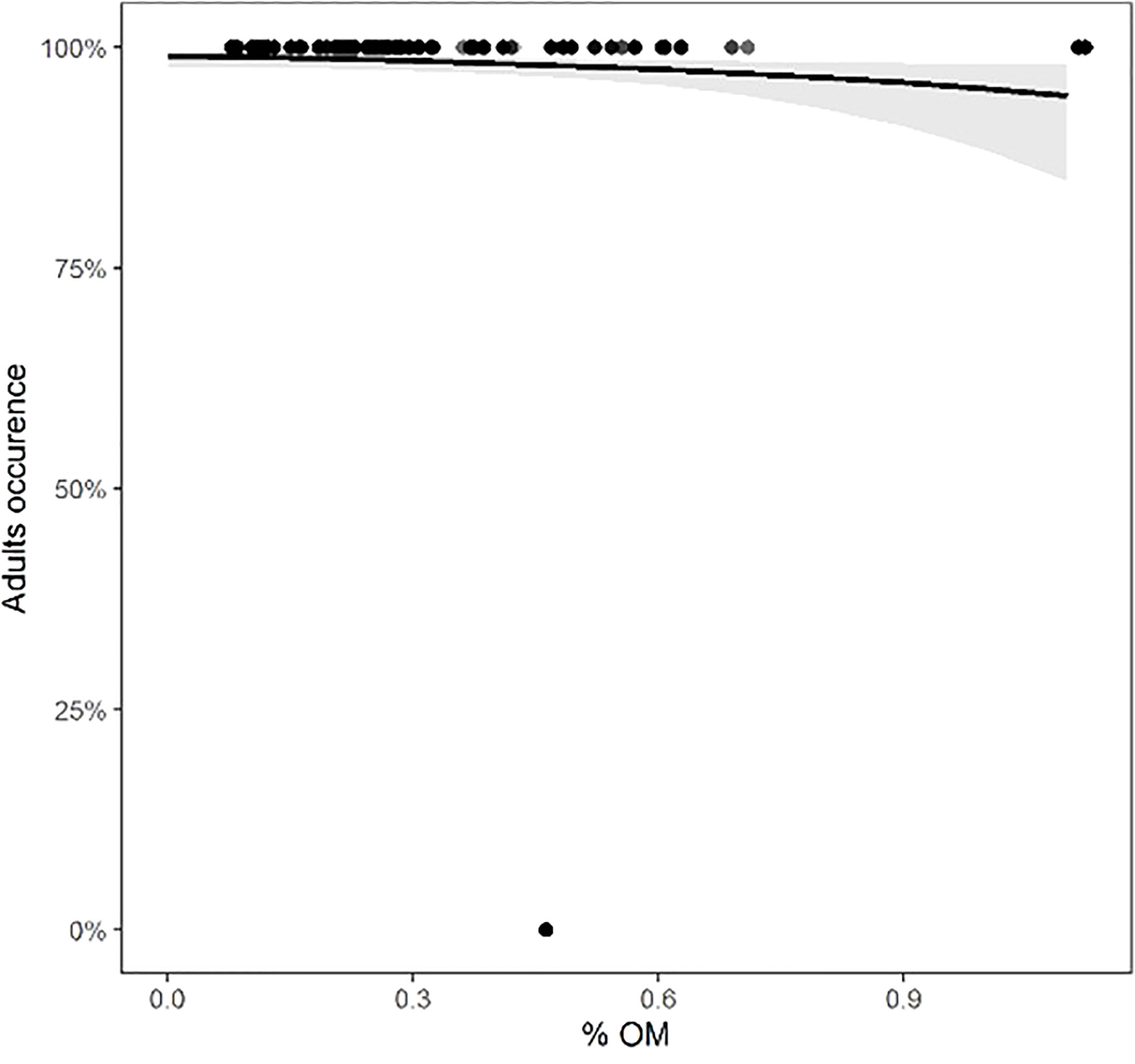
Figure 6 Occurrence of adults of Atlantorchestoidea brasiliensis in relation to content organic matter.
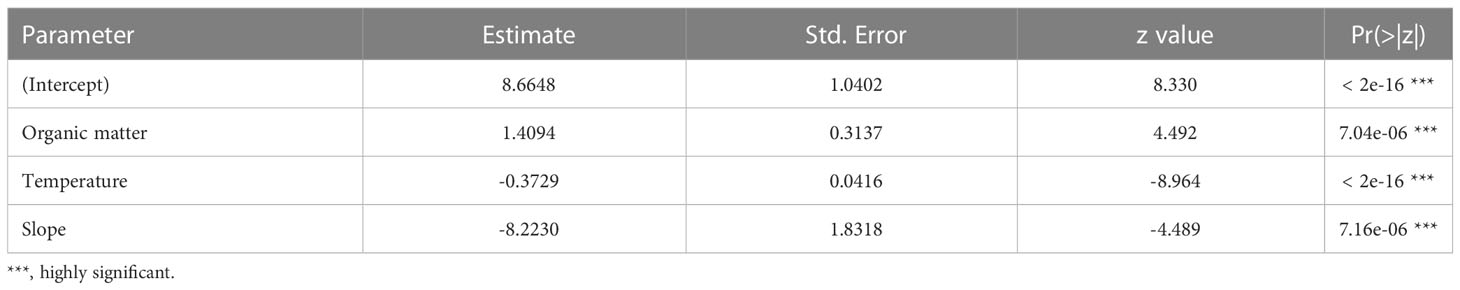
Table 7 Estimated parameters of the model with the best fit for the relationship between the probability of occurrence of juveniles and environmental descriptors.
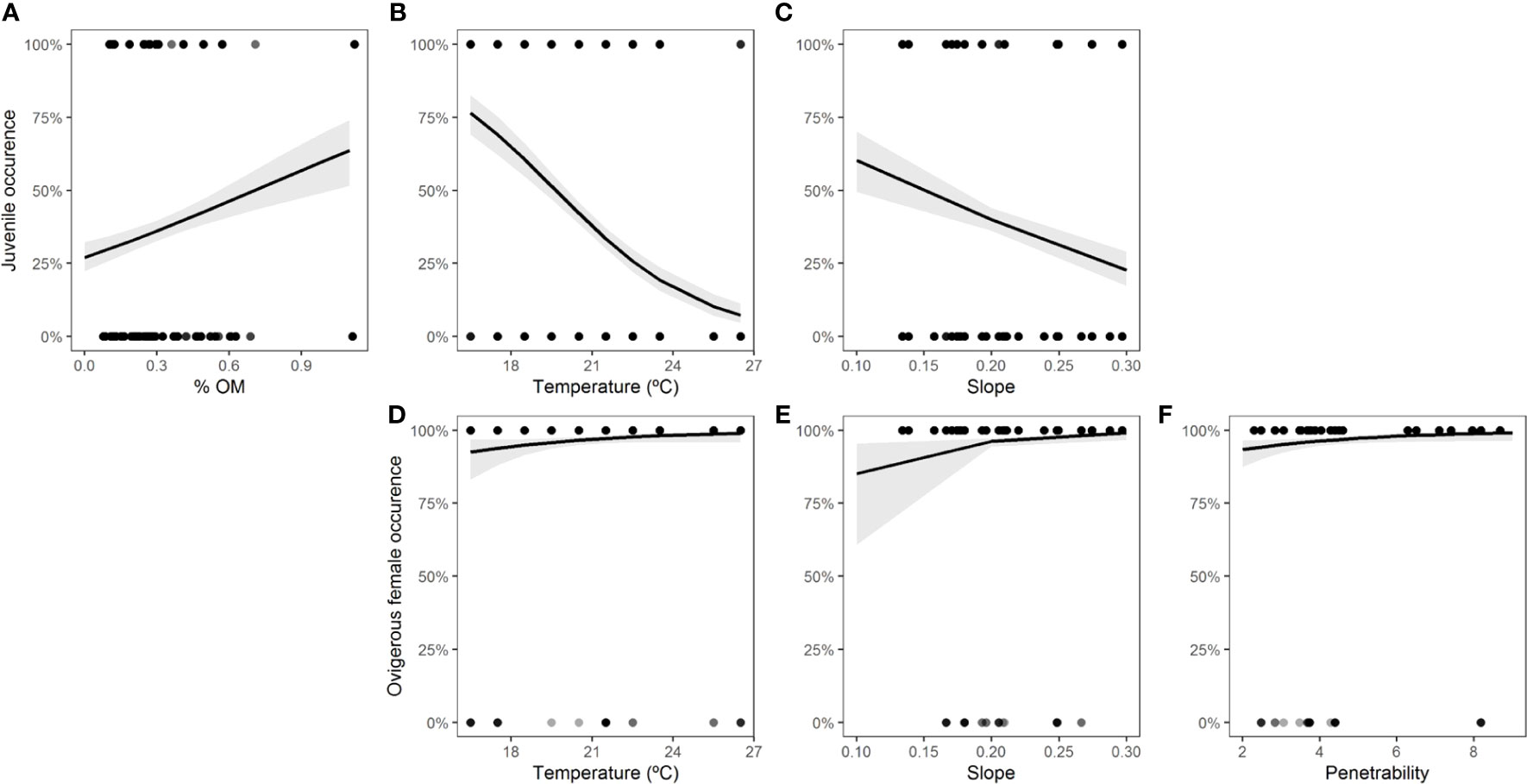
Figure 7 Occurrence of juveniles (A-C) and ovigerous females (D-F) of Atlantorchestoidea brasiliensis in relation to the physical environmental variables.
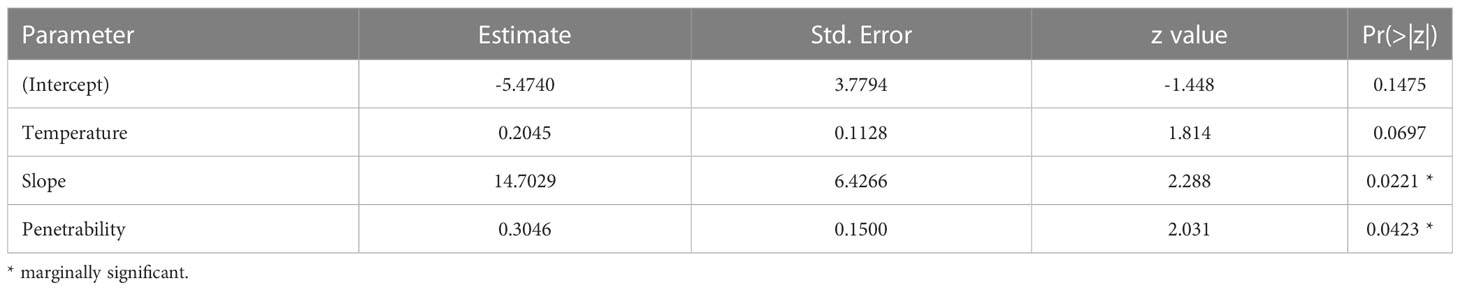
Table 8 Estimated parameters of the model with the best fit for the relationship between the probability of occurrence of ovigerous females and environmental descriptors.
4 Discussion
Recent studies have focused on the relationships between the absence and presence of bioindicators of environmental quality and the density or abundance of key species in order to assess the anthropogenic impacts on sandy beach species (Cardoso et al., 2016; Costa et al., 2020; Barboza et al., 2021). Few studies have investigated the morphological changes in organisms in response to anthropogenic impacts (Frota et al., 2019; Ragagnin and Turra, 2022). However, the interaction between anthropogenic stressors and adaptation strategies on sandy beaches yet remains to be fully elucidated (Costa et al., 2018). The limitations of studies on behavior driven by anthropic pressure may be because of the synergism and co-occurrence of stressors between environmental and anthropogenic forces to which sandy beaches are prone (Costa et al., 2022). Furthermore, there is a lack of deep knowledge of the behavioral aspects of the target species and the use of locations and temporal variations for strategic comparisons.
Studies conducted on amphipods from sandy beaches since the 2000s have assessed the resistance of organisms to anthropic stressors, focusing especially on their ability to survive certain impacts with regards to beach recreation and trampling (Veloso et al., 2006; Veloso et al., 2010; Bessa et al., 2017). A protein synthesis response at an individual level was recently observed by Lynn et al. (2022), who reported a higher level of hemocyanin protein in amphipods exposed to artificial light at night, and by Frota et al. (2019) who reported a higher level of body asymmetry for A. brasiliensis on more urbanized beaches. Body and phenotypic responses can unintentionally induce changes in individual behavior (Sene, 2016), potentially affecting the entire population (McLachlan and Defeo, 2018). Given the high importance of the ecology of sandy beaches, it is essential that amphipod talitrids assume new impact assessment metrics, considering their behavioral variation plasticity and pre-adaptive capabilities against mortality. The present study is the first to evaluate the burial depth of a sandy beach species as an adaptive strategy against human trampling and pressure. A. brasiliensis has great potential as a bio monitor for variations in animal behavior on sandy beaches driven by anthropogenic pressures (Market et al., 2003).
Scapini et al. (2015) found that seagrasses (food availability) are an important environmental determinant responsible for the stability of the behavioral adaptation of the talitrids’ population on sandy beaches of Mediterranean coasts. Food availability on beaches determines individual’s vigor and body size (Incera et al., 2006; McLachlan and Defeo, 2018), in addition to ensuring the survival and maintenance of animal species (Dugan et al., 2003). The effect of the importance of wrack supply on the ecology of beach environments was demonstrated by Barreiro et al. (2011), who pointed out that the wave exposure, coastal topography, and seasonality are important factors determining the arrival of algae on beaches. Therefore, the removal of this material by anthropic cleaning actions can be harmful to beach ecosystems, since it favors the food supply of animal species, especially detritivores (Robbe et al., 2021, Ulaski et al., 2023). Despite the beaches under study having different cleaning strategies, no relationships were observed between beaches and organic matter content that would support the different burial depths.
Although sandy beaches exposed to wave action have a low organic matter content in comparison to protected ones (McLachlan and Defeo, 2018), is possible that the loss of carbonates during the burning sand sample processes could lead to the overestimation of organic matter values. However, considering that all samples underwent the same analysis procedure, the results of the present study show that organic matter content was the only variable that affected the global occurrence of adults, juveniles, and ovigerous females. A. brasiliensis has a detritivorous feeding habit, constantly leaving their burrows to feed (Cardoso, 2002; Veloso et al., 2012); the organic matter content may thus be related to food availability (McLachlan and Defeo, 2018). The higher organic matter content thus seems to benefit the feeding habits of juveniles but not adults. Notably, only one sample unit did not register the occurrence of adults; despite this, unlike the juveniles, adults were observed at all depths. Juveniles potentially move less for food, thus occurring in areas with a higher organic matter content. Their reduced sizes, compared to adults, can also facilitate the assimilation of organic matter as food, as evident from the results for soft bottom macrofauna by Mavraki et al. (2020).
The water filtration process substantially controls the interstitial system (McLachlan and Defeo, 2018) in which, beaches with a higher mean grain size have a greater infiltration capacity and at a faster rate, thus oxygenating the environment. Excess organic input creates anaerobic conditions; hence, the balance between oxygen input and organic matter content is substantial for life in grains (McLachlan and Turner, 1994; Charbonnier et al., 2016). Comparison of the two beach situations showed that the organic matter content was not a determinant of the differences in burial depths.
Fanini et al. (2009) evaluated behavioral adaptations of A. brasiliensis for zonal orientation (horizontal) on the beach and tested predictions of the Habitat Safety Hypothesis (Defeo and Gómez, 2005). The results obtained suggested that on a reflective beach (coarser grains and steeper slope), the horizontal orientation of organisms is more precise than on a dissipative beach. Moreover, Scapini et al. (2015) demonstrated the effects of the physical environment (extension and morphology of beaches) on the population of different species of talitrids (Orchestia stephenseni, Orchestia montagu, and Talitrus saltator). The study indicated that populations from more sheltered locations showed greater precision of horizontal orientation than those from extensive beaches and were more subject to changes in coastal sedimentary transport. The mean grain size associated with the vertical orientation (burial) has been known to determine oxygen concentrations (Charbonnier et al., 2016), food availability (McLachlan and Turner, 1994), and the process of water filtration (McLachlan and Defeo, 2018). Beaches with coarser grains display less compaction between grains favoring a greater burial depth (Costa et al., 2022).
In the present study, depths of 5 and 10 cm registered a higher occurrence of individuals for free and restricted access beaches. This range appears optimal for the species, which provides individuals with ideal conditions for temperature and sediment humidity (Maurer et al., 1981; Veloso et al., 2010). Although individuals have been predominantly found at the mentioned depths on all beaches, a substantial population was also observed in the deeper sediment layers of the free access beaches (15, 20, and 25 cm) than on the restricted. Therefore, the present results indicate a possible response behavior to direct pressures suffered on free access beaches because of central paradigms of sandy beach ecology; any environmental variable evaluated here thus did not seem to affect the depth of burial of A. brasiliensis.
The results obtained demonstrate that morphodynamic conditions should not be considered exclusively as factors that affect the patterns of beach populations, thus substantiating the results of Defeo and Martinez (2003). Furthermore, in view of the advancement of human pressure on beach ecosystems, interweaving environmental and biotic factors with anthropogenic conditions is fundamental. Trampling and vehicle traffic on beaches are important stressors for crustaceans worldwide, causing individual mortality and reducing population densities (Moffett et al., 1998; Schlacher and Thompson, 2007, Schlacher et al., 2008; Veloso et al., 2010; Costa et al., 2018; Afghan et al., 2020; Costa et al., 2020). Therefore, they should be considered as anthropic factors affecting species on beaches.
Based on experiments, Reyes-Martínez et al. (2015) reported that the Amphipod Bathyporeia pelagica appeared to be highly sensitive to human trampling pressure, and Ugolini et al. (2008) observed a reduction in the population density of the sandhopper Talitrus saltator due to increased human presence. At the behavioral level, Lucrezi et al. (2009) observed that burrows of ghost crabs (Ocypode spp.) were sensitive to disturbances caused by short-term trampling, with recovery during the night when there was no human presence and restoration the next day. Costa et al. (2018) observed that Ocypode quadrata avoided building burrows in plots trampled by people and modify their immersion time, suggesting changes in the species’ behavior according to human presence. In a review study on the orientation of A. brasiliensis, Scapini (2006) observed that the first response of the species to potential disturbances is to escape as a reaction behavior to avoid stress, demonstrating that A. brasiliensis has an instinctive behavior of self-preservation. In laboratory experiments, Maurer et al. (1981) demonstrated the capability of the amphipod Parahaustorius longimerus to migrate vertically and reach greater depths in stressful situations that demand survival strategies. Thus, burying behavior can be characterized as a phenotype with considerable adaptive potential (Brown, 1996). Therefore, if A. brasiliensis has behavioral learning (plasticity), populations would learn to bury themselves further to avoid mortality by trampling, in the long term, on the most trampled beaches. Therefore, the deeper burial of A. brasiliensis observed can be seen as behavioral plasticity and a long-term change in trampled beaches.
The behavioral patterns in relation to stressful situations of the species need to be better understood for enhanced effective management and research on the conservation of the species and/or its use as a bio monitor of animal behavior driven by anthropic pressures (Primack and Rodrigues, 2001). Therefore, the present study is an initial step toward further research on changes in the patterns of animal behavior induced by direct anthropogenic pressures on sandy beaches.
The results obtained in this study corroborate the initial hypothesis as greater burial depths were observed on beaches with a higher incidence of trampling (free access) and with the low influence of environmental variables, indicating a possible strategic behavior of preservation against mortality by organisms on the most trampled beaches. However, the synergistic effects of physical, biological, and anthropogenic forces must be considered to better understand this subject.
A more philosophical approach demanding long-term studies, arising from our guiding question, can be thought upon in context of future studies: Do organisms bury themselves further deep as a survival mechanism on the most impacted beaches or only the organisms genetically capable (pre-adapted) of surviving bury themselves deeper?
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
RA: Data curation, investigation, conceptualization, formal analysis, visualization, writing – original draft. YR: Data curation, project administration, visualization. MA: Data curation, investigation, visualization, writing – review & editing. TC: Resources, investigation, supervision, conceptualization, project administration, formal analysis, writing – review & editing, validation. All authors contributed to the article and approved the submitted version.
Funding
RA was supported by Coordination for the Improvement of Higher Education Personnel (CAPES) post-graduate research scholarships. YR was supported by Brazilian National Council for Scientific and Technological Development (CNPq). MA was supported by Federal University of the State of Rio de Janeiro (UNIRIO) graduate research scholarships. TC was supported by FAPERJ (E-26/010.001576/2019).
Acknowledgments
The authors express their deepest gratitude to all fieldwork participants. We also would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1167049/full#supplementary-material
References
Abude R. R. S., Augusto M., Cardoso R. S., Cabrini T. M. B. (2021). Spatiotemporal variability of solid waste on sandy beaches with different access restrictions. Mar. pollut. Bull. 171, 112743. doi: 10.1016/j.marpolbul.2021.112743
Afghan A., Cerrano C., Luzi G., Calcinai B., Puce S., Pulido Mantas T., et al. (2020). Main anthropogenic impacts on benthic macrofauna of sandy beaches: a review. J. Mar. Sci. Eng. 8, 405. doi: 10.1016/j.biocon.2021.108972
Andreatta V., Chiavari M. P., Rego H. (2009). O Rio De Janeiro e a sua orla: história, projetos e identidade (Brazil: SMU/Prefeitura da Cidade do Rio de Janeiro).
Barboza C., Mattos G., Soares-Gomes A., Zalmon I., Costa L. (2021). Low densities of the ghost crab Ocypode quadrata related to large scale human modification of sandy shores. Front. Mar. Sci. 8, 589542. doi: 10.3389/fmars.2021.589542
Barreiro F., Gómez M., Lastra M., López J., de la Huz R. (2011). Annual cycle of wrack supply to sandy beaches: effect of the physical environment. "Mar. Ecol. Prog. Ser. 433, 65–74. doi: 10.3354/meps09130
Bergamino L., Martínez A., Han E., Lercari D., Defeo O. (2016). Trophic niche shifts driven by phytoplankton in sandy beach ecosystems. Estuar. Coast. Shelf Sci. 180, 33–40. doi: 10.1016/j.ecss.2016.06.023
Bessa F., Scapini F., Cabrini T. M. B., Cardoso R. S. (2017). Behavioural responses of talitrid amphipods to recreational pressures on oceanic tropical beaches with contrasting extension. J. Exp. Mar. Biol. 486, 170–177. doi: 10.1016/j.jembe.2016.10.007
Breed M. D., Moore J. (2022). Animal behavior. 3rd Edition (USA: Elsevier, Academic Press). doi: 10.1016/C2018-0-03842-6
Brown A. C. (1996). Behavioural plasticity as a key factor in the survival and evolution of the macrofauna on exposed sandy beaches. Rev. Chil. Hist. Natural. 69, 469–474.
Cardoso R. S. (2002). Behavioural strategies and surface activity of the sandhopper Pseudorchestoidea brasiliensis (Amphipoda: talitridae) on a Brazilian beach. Mar. Biol. 141 (1), 167–173. doi: 10.1007/s00227-002-0784-y
Cardoso R. S., Barboza C. A. M., Skinner V. B., Cabrini T. M. B. (2016). Crustaceans as ecological indicators of metropolitan sandy beaches health. Ecol. Indic 62, 154–162. doi: 10.1016/j.ecolind.2015.11.039
Cardoso R. S., Veloso V. G. (1996). Population biology and secondary production of the sandhopper Pseudorchestoidea brasiliensis (Amphipoda: talitridae) at prainha beach, Brazil. Mar. Ecol. Prog. Ser. 142, 11–119. doi: 10.3354/meps142111
Charbonnier C., Anschutz P., Deflandre B., Bujan S., Lecroart P. (2016). Measuring pore water oxygen of a high-energy beach using buried probes. Estuar. Coast. Shelf Sci. 179, 66–78. doi: 10.1016/j.ecss.2015.12.004
Costa L. L., Constantino W. D., Ferreira A. K., Zalmon I. R., Soares-Gomes A. (2022). Sandy beach ecology in the anthropocene: ghost shrimp (Crustacea: callianassidae) as a study model. Estuar. Coast. Shelf Sci. 275, 107999. doi: 10.1016/j.ecss.2022.107999
Costa L. L., Fanini L., Zalmon I. R., Defeo O. (2020). Macroinvertebrates as indicators of human disturbances on sandy beaches: a global review. Ecol. Indic. 118, 106764. doi: 10.1016/j.ecolind.2020.106764
Costa L. L., Madureira J. F., Zalmon I. R. (2018). Changes in the behaviour of Ocypode quadrata (Fabricius 1787) after experimental trampling. J. Mar. Biol. Assoc. UK 1, 1135–1140. doi: 10.1017/S0025315418001030
Costa L. L., Tavares D. C., Suciu M. C., Rangel D. F., Zalmon I. R. (2017). Human-induced changes in the trophic functioning of sandy beaches. Ecol. Indic. 82, 304. doi: 10.1016/j.ecolind.2017.07.016
Costa L. L., Zalmon I. R. (2019). Issues to consider when sampling the sandhopper Atlantorchestoidea brasiliensis (Dana 1853) for impact assessments on sandy beaches. Mar. Biol. Res. 15, 8–9. doi: 10.1080/17451000.2019.1680848
Dana J. D. (1852). “Conspectus crustaceorum, &c. conspectus of the Crustacea of the exploring expedition under Capt. c. Wilkes, U.S.N. macroura,” in Proceedings of the academy of natural sciences of Philadelphia, vol. 6. , 10–28.
Dana J. D. (1853). “Crustacea,” in Part II. united states exploring expedition. during the years 1838, 1839, 1840, 1841, 1842.
Defeo O., Gómez J. (2005). Morphodynamics and habitat safety in sandy beaches: life history adaptations in a supralittoral amphipod. Mar. Ecol. Prog. Ser. 293, 143–153. doi: 10.3354/meps293143
Defeo O., Martinez G. (2003). The habitat harshness hypothesis revisited: life history of the isopod Excirolana braziliensis in sandy beaches with contrasting morphodynamics. J. Mar. Biol. Assoc. UK 83 (2), 331–340. doi: 10.1017/s0025315403007161h
Duarte R. C., Flores A. A. V., Stevens M. (2016). Morph-specific habitat and sex distribution in the caridean shrimp hippolyte obliquimanus. Philos. Trans. R. Soc B. 372, 0342. doi: 10.1017/s0025315416000230
Dugan J. E., Hubbard D. M., McCrary M. D., Pierson M. O. (2003). The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beaches of southern California. Estuar. Coast. Shelf Sci. 58S, 25–40. doi: 10.1016/S0272-7714(03)00045-3
Dunn P. K., Smyth G. K. (1996). Randomized quantile residuals. J. Comput. Graph Stat. 5 (3), 236. doi: 10.2307/1390802
Emery K. O. (1961). A simple method of measuring beach profiles. Limnol. Oceanogr 6 (1), 90–93. doi: 10.4319/lo.1961.6.1.0090
Fanini L., Defeo O., Santos C. D., Scapini F. (2009). Testing the habitat safety hypothesis with behavioural field experiments: amphipod orientation on sandy beaches with contrasting morphodynamics. Mar. Ecol. Prog. Ser. 392, 133–141. doi: 10.3354/meps08219
Frota G. P., Cabrini T. M. B., Cardoso R. S. (2019). Fluctuating asymmetry of two crustacean species on fourteen sandy beaches of Rio de Janeiro state. Estuar. Coast. Shelf Sci. 223, 138–146. doi: 10.1016/j.ecss.2019.03.013
Gelman A., Hill J. (2006). Data analysis using regression and Multilevel/Hierarchical models (New York: Cambridge University Press).
Gómez J., Defeo O. (1999). Life history of the sandhopper Pseudorchestoidea brasiliensis (Amphipoda) in sandy beaches with contrasting morphodynamics. Mar. Ecol. Prog. Ser. 182, 209–220. doi: 10.3354/meps182209
Gómez J., Defeo O. (2012). Predictive distribution modeling of the sandy-beach supralittoral amphipod Atlantorchestoidea brasiliensis along a macroscale estuarine gradient. Estuar. Coast. Shelf Sci. 98, 84–93. doi: 10.1016/j.ecss.2011.12.008
González S. A., Yáñez-Navea K., Muñoz M. (2014). Effect of coastal urbanization on sandy beach coleoptera phaleria maculata (Kulze) in northern Chile. Mar. pollut. Bull. 83 (1), 265–274. doi: 10.1016/j.marpolbul.2014.03.0
Greiser N., Faubel A. (1988). “Biotic factors,” in Introduction to the study of meiofauna. Eds. Higgins R. P., Thiel H. (USA: Smithsonian Inst. Press), 79–114.
Heiberger R. M., Holland B. (2004). Statistical analysis and data display: An intermediate course with examples in s-plus, r, and SAS. doi: 10.1007/978-1-4757-4284-8
Herrick J. E., Jones T. L. (2001). A dynamic cone penetrometer for measuring soil penetration resistance. Soil Sci. Soc. Am. J. 66 (4), 1320. doi: 10.2136/sssaj2002.1320
Incera M., Lastra M., López J. (2006). Effect of swash climate and food availability on sandy beach macrofauna along the NW coast of the Iberian peninsula. Mar. Ecol. Prog. Ser. 314, 25–33. doi: 10.3354/meps314025
Lucrezi S., Schlacher T. A., Robinson W. (2009). Human disturbance as a cause of bias in ecological indicators for sandy beaches: experimental evidence for the effects of human trampling on ghost crabs (Ocypode spp.). Ecol. Indic 9 (5), 913–921. doi: 10.1016/j.ecolind.2008.10.013
Lynn K. D., Quintanilla-Ahumada D., Duarte C., Quijón P. A. (2022). Hemocyanin as a biological indicator of artificial light at night stress in sandy beach amphipods. Mar. pollut. Bull. 184, 114147. doi: 10.1016/j.marpolbul.2022.114147
Market B., Breure A., Zechmester H. G. (2003). Bioindicators and biomonitors (Amsterdan: Elsevier). doi: 10.1080/0021213X.1979.10676851
Maurer D., Keck R. T., Tinsman J. C., Leathem W. A. (1981). Vertical migration and mortality of benthos in dredged material: part II–crustacea. Mar. Environ. Res. 5 (4), 301–317. doi: 10.1016/0141-1136(81)90014-3
Mavraki N., Degraer S., Vanaverbeke J., Braeckman U. (2020). Organic matter assimilation by hard substrate fauna in an offshore wind farm area: a pulse-chase study. ICES J. Mar. Sci. 77, 7–8. doi: 10.1093/icesjms/fsaa133
McLachlan A. (1980). Exposed sandy beaches as semi-closedecosystems. Mar. Environ. Res. 4, 5963. doi: 10.1016/0141-1136(80)90059-8
McLachlan A., Defeo O. (2018). The ecology of sandy shores (USA: Elsevier, Academic Press). doi: 10.1016/B978-0-12-809467-9.00001-1
McLachlan A., Turner J. (1994). The interstitial environment of sandy beaches. PZNI Mar. Ecol. 15 (3-4), 177–212. doi: 10.1111/j.1439-0485.1994.tb00053.x
Moffett M. D., McLachlan A., Winter P. E. D., De Ruyck A. M. C. (1998). Impact of trampling on sandy beach macrofauna. J. Coast. Conserv. 4 (1), 87–90. doi: 10.1007/bf02806494
Pennafirme S., Soares-Gomes A. (2022). Population dynamics and reproductive biology of the sandhopper Atlantorchestoidea brasiliensis (Amphipoda: talitridae) of a sandy beach in southwestern Atlantic coast. J. Nat. Hist 56, 13. doi: 10.1080/00222933.2022.2101957
Quillien N., Nordström M. C., Schaal G., Bonsdorff E., Grall J. (2016). Opportunistic basal resource simplifies food web structure and functioning of a highly dynamic marine environment. J. Exp. Mar. Biol. Ecol. 477, 92–102. doi: 10.1016/j.jembe.2016.01.010
Ragagnin M. N., Turra A. (2022). Imposex incidence in the sandy beach snail Hastula cinerea reveals continued and widespread tributyltin contamination after its international ban. Reg. Stud. Mar. Sci. 49, 102118. doi: 10.1016/j.rsma.2021.102118
R Core Team (2022). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Reyes-Martínez M. J., Ruíz-Delgado M. C., Sánchez-Moyano J. E., García-García F. J. (2015). Response of intertidal sandy-beach macrofauna to human trampling: an urban vs. natural beach system approach. Mar. Environ. Res. 103, 36–45. doi: 10.1016/j.marenvres.2014.11.005
Richardson H. (1912). “Descriptions of a new genus of isopod crustaceans, and of two new species from south America,” in Proceedings of the United States National Museum, Vol. 43. 201–204.
Robbe E., Woelfel J., Balčiūnas A., Schernewski G. (2021). An impact assessment of beach wrack and litter on beach ecosystem services to support coastal management at the Baltic Sea. Environ. Manage. 68, 835–859. doi: 10.1007/s00267-021-01533-3
Röding P. F. (1798). “Museum boltenianum sive catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat joa. fried bolten, m. d. p. d. per XL. annos proto physicus hamburgensis,” in Pars secunda continens Conchylia sive Testacea univalvia, bivalvia & multivalvia, Trapp, Hamburg. viii + 199.
Ryan E. P. (1966). Pheromone: evidence in a decapod crustacean. Science 151 (3708), 340–341. doi: 10.1126/science.151.3708.340
Say T. (1817-1818). An account of the Crustacea of the united states. J. Acad. Natural Sci. Philadelphia. 1 (1), 57–63. 65-80 (pl.), 97-101, 155-169 (1817); 1(2): 235-253, 313-319, 374-401, 423-441 (1818).
Scapini F. (2006). Keynote papers on sandhopper orientation and navigation. Mar. Freshw. Behav. Physiol. 39 (1), 73–85. doi: 10.1080/10236240600563412
Scapini F. (2014). Behaviour of mobile macrofauna is a key factor in beach ecology as response to rapid environmental changes. Estuar. Coast. Shelf Sci. 150 (A), 36–44. doi: 10.1016/j.ecss.2013.11.001
Scapini F., Gambineri S., Rossano C., ElGtari M., Fanini L., Nourisson D. H. (2015). Talitrid orientation as bioindicator of shoreline stability: protected headland-bays versus exposed extended beaches. Ecol. Indic 53, 28–36. doi: 10.1016/j.ecolind.2015.01.012
Schlacher T. A., Richardson D., McLean I. (2008). Impacts of off-road vehicles (ORVs) on macrobenthic assemblages on sandy beaches. Environ. Manage 41 (6), 878–892. doi: 10.1007/s00267-008-9071-0
Schlacher T. A., Thompson L. M. C. (2007). Exposure of fauna to off-road vehicle (ORV) traffic on sandy beaches. Coast. Manage. 35 (5), 567–583. doi: 10.1080/08920750701593402
Schmitt W. L. (1935). “Crustacea Macrura and anomura of Porto Rico and the virgin islands,” in Scientific survey of Porto Rico and the virgin islands, vol. 15. (New York Academy of Sciences), 125–227.
Sene F. M. (2016). Cada caso, um caso… puro acaso: os processos da evolução biológica dos seres vivos. 2nd Edition (Ribeirão Preto: Brazilian Society of Genetics).
Suciu M. C., Tavares D. C., Zalmon I. R. (2018). Comparative evaluation of crustaceans as bioindicators of human impact on Brazilian sandy beaches. J. Crustac. Biol. 38 (4), 420–428. doi: 10.1093/jcbiol/ruy027
Templeton A. R. (2006). Population genetics and microevolutionary theory. 1st Edition (USA: Wiley-Liss).
Ugolini A., Ungherese G., Somigli S., Galanti G., Baroni D., Borghini F., et al. (2008). The amphipod Talitrus saltator as a bioindicator of human trampling on sandy beaches. Mar. Environ. Res. 65 (4), 349–357. doi: 10.1016/j.marenvres.2007.12.002
Ulaski B. P., Sikes D. S., Konar B. (2023). Beach-cast and drifting seaweed wrack is an important resource for marine and terrestrial macroinvertebrates in high latitudes. Mar. Environ. Res. 187, 105970. doi: 10.1016/j.marenvres.2023.105970
Veloso V. G., Sallorenzo I. A., Barros W. K. A., Neves G. (2012). Analysis of behavior and food consumption rate of Atlantorchestoidea brasiliensis (Dana 1953) (Crustacea: amphipoda: talitridae). Sci. Mar. 38 (4), 653–664. doi: 10.7773/cm.v38i4.2108
Veloso V. G., Sallorenzo I. A., Ferreira B. C. A., Souza G. N. (2010). Atlantorchestoidea brasiliensis (Crustacea: amphipoda) as an indicator of disturbance caused by urbanization of a beach ecosystem. Braz. J. Oceanogr 58 (1), 13–21. doi: 10.1590/s1679-87592010000100002
Veloso V. G., Silva E. S., Caetano C. H. S., Cardoso R. S. (2006). Comparison between the macroinfauna of urbanized and protected beaches in Rio de Janeiro state, Brazil. Biol. Conserv. 127 (4), 510–515. doi: 10.1016/j.biocon.2005.09.027
Wan C., Gorb S. N. (2021). Body-catapult mechanism of the sandhopper jump and its biomimetic implications. Acta Biomaterialia 124, 282–290. doi: 10.1016/j.actbio.2021.01.033
Wood S. N. (2004). “Stable and efficient multiple smoothing parameter estimation for generalized additive models.“. J. Am. Stat. Assoc. 99 (467), 673–686.
Zhang X., Pan L., Wei C., Tong R., Li Y., Ding M., et al. (2020). Crustacean hyperglycemic hormone (CHH) regulates the ammonia excretion and metabolism in white shrimp, litopenaeus vannamei under ammonia-n stress. Sci. Total Environ. 723, 138128. doi: 10.1016/j.scitotenv.2020.138128
Keywords: invertebrates, learning, adaptation, human impact, trampling, vertical zonation, Atlantorchestoidea brasiliensis
Citation: Abude RRS, Ribeiro YSF, Augusto M and Cabrini TMB (2023) Could anthropic pressures drive the burrowing behavior of sandy beaches species? An investigation on Atlantorchestoidea brasiliensis (Amphipoda: talitridae). Front. Mar. Sci. 10:1167049. doi: 10.3389/fmars.2023.1167049
Received: 15 February 2023; Accepted: 29 June 2023;
Published: 09 August 2023.
Edited by:
Lucia Fanini, University of Salento, ItalyReviewed by:
Felicita Scapini, University of Florence, ItalyMariano Lastra, University of Vigo, Spain
Copyright © 2023 Abude, Ribeiro, Augusto and Cabrini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rayane R. S. Abude, cmF5YW5lLnJvbWFvQGhvdG1haWwuY29t
 Rayane R. S. Abude
Rayane R. S. Abude Yuri S. F. Ribeiro
Yuri S. F. Ribeiro Matheus Augusto
Matheus Augusto Tatiana M. B. Cabrini
Tatiana M. B. Cabrini