- 1Aquaculture Industry Division, West Sea Fisheries Research Institute, Incheon, Republic of Korea
- 2Department of Marine Biology, Pukyong National University, Busan, Republic of Korea
Polychlorinated biphenyls (PCBs) are endocrine-disrupting chemicals that mimic estrogen in fish. Among the various PCBs, 3,3’,4,4’,5-pentachlorobiphenyl (PCB126), considered an estrogen antagonist, has been reported to elicit estrogenic activity in fish. We investigated the estrogenic potency of PCB126 in in vitro oocyte maturation in dusky tripletooth goby, Tridentiger obscurus. In this study, we quantified steroid metabolites following exposure to PCB126 and estradiol-17β (E2). Vitellogenic ovarian follicles were incubated, in vitro, with 10 and 100 ng/mL PCB126 or E2 with [3H]17α-hydroxyprogesterone as a precursor. Testosterone (T) and E2, were identified using thin-layer chromatography, high-performance liquid chromatography, and gas chromatography-mass spectrometry. Both PCB126 and E2 increased T and E2 metabolite production. Further, vitellogenic ovarian follicles were exposed to PCB126 (1, 10 and 100 ng/mL) or E2 (0.01, 0.1 and 1 ng/mL) in vitro, and T and E2 from the incubation media were measured. PCB126 (100 ng/mL) inhibited T production and increased E2 production. Furthermore, we investigated the effects of PCB126 on the final oocyte maturation process. Germinal vesicle migration ovarian follicles were in vitro incubated with 0.1, 1, 10 and 100 ng/mL of PCB126 or E2. High doses of PCB126 (10 and 100 ng/mL) inhibited germinal vesicle breakdown. These results suggest that PCB126 has an estrogenic potency in oocyte maturation in T. obscurus.
1 Introduction
Considerable investigations have been carried out on various chemicals that can disrupt the endocrine system of humans and other animals (Colborn et al., 1993). These endocrine-disrupting chemicals (EDCs) include diverse chemicals such as pesticides, industrial detergents, sewage effluents, and polychlorinated biphenyls (PCBs). Several types of EDCs have the potential to mimic the estrogens of vertebrates, including fish (Scholz and Mayer, 2008; Diamanti-Kandarakis et al., 2009). Estrogens regulate physiological processes during vertebrate reproduction. In fish, vitellogenin production from the liver controlled by estradiol-17β (E2), has been a powerful method for detecting the estrogenicity of EDCs (Jobling and Tyler, 2003; Navas and Segner, 2006). After vitellogenesis, progestins such as 17α,20β-dihydroxy-4-pregnen-3-one (17α20βP) and 17α,20β,21-trihydroxy-4-pregnen-3-one, induce final oocyte maturation, including germinal vesicle breakdown (GVBD) and ovulation (Nagahama et al., 1994; Das and Thomas, 1999). E2 and estrogenic EDCs inhibit in vitro progesterone–induced GVBD in amphibian ovarian follicles Baulieu et al., 1978; Lin and Schuetz, 1983; Pickford and Morris, 1999). In addition, inhibition of progestin-induced GVBD by EDCs has been reported in fish oocytes (Ghosh and Thomas, 1995; Tokumoto et al., 2005; Hwang et al., 2010).
PCBs, including 209 congeners, have been used extensively in the industry as insulating materials, flame retardants, diluents, and detergents (Safe, 1994). PCBs have been reported to be environmental pollutants because of their high stability and bioaccumulation with lipophilic characteristics (Bonefeld-Jørgensen et al., 2001; Guruge et al., 2001). Among the various PCBs, 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) is a coplanar congener and is known to be the most toxic PCB congener with dioxin-like properties (Safe, 1994). PCB126 is also an estrogen antagonist in mammals and fish (Lind et al., 1999; Wojtowicz et al., 2000; Muto et al., 2002; Vaccaro et al., 2005). In contrast, PCB 126 has been suggested to elicit estrogenic activity in fish (Gregoraszczuk et al., 2003a; Liu et al., 2006; Matthews et al., 2007; Mortensen and Arukwe, 2008a; Mortensen and Arukwe, 2008b). We reported that PCB126 does not have estrogenic or anti-estrogenic effects on in vitro steroid production from oocytes of the red lip mullet, Chelon haematocheilus (Baek et al., 2011). Gregoraszczuk et al., (2003b) reported that PCB126 has both estrogenic and anti-estrogenic effects in fish, depending on the time of exposure and developmental stage of follicles.
Despite the above contradictory studies, there are few studies with endocrine disrupting profiling of PCB126 on the maturation process of fish oocytes. Therefore, in the present study, we aimed to investigate the estrogenic potency of PCB126 on in vitro steroidogenesis in vitellogenic ovarian follicles of the dusky tripletooth goby, Tridentiger obscurus. Furthermore, we compared the estrogenic potency of PCB126 with that of exogenous E2 treatment. We conducted a GVBD assay, liquid chromatography, and steroid hormone quantification to confirm the direct estrogenic potency of PCB126. Gobiid fish are appropriate subjects for investigating the effects of EDCs due to their small size, ease of handling, and strong tolerance even in the extreme environmental condition (Robinson et al., 2007). Therefore, we used the dusky tripletooth goby Tridentiger obscurus, an ideal model organism (Kaneko and Hanyu, 1985; Hwang et al., 2010), in the present study.
2 Materials and methods
2.1 Chemicals
Authentic steroids were purchased from Sigma Chemical (St. Louis, Missouri, USA) or Steraloids, Inc. (Wilton, NH, USA). Stock solutions (mg/mL) were prepared by dissolving the authentic steroids in pure ethanol. PCB126 (Aldrich Chemical, Milwaukee, WI, USA) was prepared as a stock solution (mg/mL) by diluting in ethanol. The ethanol concentration in the incubation medium was maintained at less than 0.1%. Testosterone (T) and E2 were purchased from Sigma Chemical (St. Louis, MO, USA) or Steraloids, Inc. (Wilton, NH, USA). The antiserum for T was purchased from Sigma Chemical, and that for E2 was kindly donated by Dr. Alexis Fostier (INRAE, Paris, France). Radioactive [3H]-17α-hydroxyprogesterone ([3H]-17αP), [3H]-T, and [3H]-E2 were obtained from Amersham Life Sciences (London, England).
2.2 Experimental fish and histological observation of ovarian follicles
The dusky tripletooth goby used in this study were captured in coastal waters off Jeju Island, South Korea, during the spawning season (May–June). We isolated ovaries from 10 fishes. The ovaries were dissected manually into individual ovarian follicles using fine forceps. The ovarian follicles were separated in accordance with oocyte diameter of 0.53 and 0.78 mm from three different fish, respectively. And then we used these ovarian follicles for separated incubation experiment with duplicate (steroid metabolites analysis) or triplicate (RIA and GVBD assay). To determine the developmental stage of ovarian follicles, we conducted histological analysis. Gonadal fragments were processed by Midway and Scharf (2012). The paraffin-embedded samples were cut at 5-6 μm and sections were stained using Mayer’s hematoxylin and eosin and observed under a light microscope (BX50, Olympus, Japan).
2.3 Cell culture
Three separate experiments were performed. Prior to culture, ovarian follicles were separated in an ice-cold balanced salt solution (132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4·7H2O, 0.98 mM MgCl2·6H2O, 3.40 mM CaCl2·6H2O, and 3.65 mM HEPES). Twenty ovarian follicles were incubated in 24-well culture plates containing 1 ml of Leibovitz L15 medium (Gibco, Grand Island, NY, USA).
To assess estrogenic potency of PCB126 on steroidogenic metabolism of ovarian follicles, we quantified steroid metabolites following exposure to PCB126 and exogenous E2 in the presence of precursor. We incubated vitellogenic ovarian follicles with 10 and 100 ng/mL PCB126 or E2 in the presence of [3H]-17αP (55 kBq) as a precursor in two separate experiments. To evaluate estrogenic potency of PCB126 on steroid production from vitellogenic ovarian follicles, we quantified T and E2 levels following exposure to PCB126 and exogenous E2. The vitellogenic ovarian follicles were incubated with only 1, 10 and 100 ng/mL of PCB126 or 0.01, 0.1 and 1 ng/mL E2 in three separate experiments. For evaluation of estrogenic potency of PCB126 on final maturation process, we conducted GVBD assay. The fully vitellogenic ovarian follicles were separated and incubated with 0.1, 1, 10 and 100 ng/mL of PCB126 or E2 in three separate experiments.
The plates were then incubated for 24 h at 18°C with gentle shaking. The pH and osmolality of the medium were adjusted to the plasma values of this species, 7.62 and 290 mOsm/kg, respectively.
2.4 Steroid metabolites
At the end of incubation with the radiolabeled precursor, the incubation media, and ovarian follicles were collected and stored at -80°C. Steroids were extracted three times from the medium and ovarian follicles, using 4 mL of dichloromethane. The extracts were concentrated and applied to a thin-layer chromatography (TLC) plate (60F254; Merck, Darmstadt, Germany) with non-radioactive standard steroids as carrier steroids and then developed in a mixture of benzene:acetone (4:1) and benzene:ethyl acetate (4:1). Radioactive steroid metabolites were analyzed using a BAS 1500 bio-imaging analyzer (Fuji Film, Tokyo, Japan), and standards E1 and E2 were visualized by exposure to iodine vapor. Other standard steroids were detected using UV absorption at 254 nm. The migration zones corresponding to the carrier steroids, T and E2, were eluted twice from the bands of the silica plates with 5 ml of dichloromethane:methanol (90:10). Following centrifugation at 1000 × g for 10 min, the supernatants were vacuum-dried before being dissolved in 20 μl of acetonitrile. The extracts were then analyzed using reverse-phase high-performance liquid chromatography (HPLC; Table 1). After HPLC analysis, metabolites with the same retention time as the standard were recovered and identified using gas chromatography-mass spectrometry (GC-MS, Table 2).
Table 2 Analytical conditions of gas chromatography-mass spectrometry for identification of steroid metabolites.
2.5 Radioimmunoassay
After incubation, steroids were extracted twice from aliquots of the medium, using ethyl acetate and cyclohexane. Then, T and E2 levels were measured by radioimmunoassay (RIA), following Kobayashi et al., 1987. The intra-assay coefficients of variance were 2.5% (n=3) and 3.2% (n=3) for the T and E2 assays, respectively, and the respective inter-assay coefficients of variance were 12.1% (n=5) and 11.3% (n=5). The minimum detectable limits were 10.5 and 12.2 pg/mL for T and E2, respectively.
2.6 GVBD assay
For the GVBD assay, the incubated ovarian follicles were fixed with a clearing solution (ethanol:formalin:glacial acetic acid, 6:3:1) after 24 h of incubation. The location of the germinal vesicle (GV = nucleus) was observed under low-power magnification using a dissection microscope. The number of ovarian follicles completing GVBD, that is, the dissolution of the nucleus, was counted in each well and calculated as a percentage.
2.7 Statistics
The data from RIA and GVBD were expressed as mean with a standard error of the mean (SEM) from three separate experiments (n =3). These data were analyzed by Kolmogorov-Smirnov and Levene’s test to verify whether the variance fulfilled the conditions of normality and homogeneity, respectively. The results were not satisfactory fit to the normal distribution and were therefore conducted in the non-parametric test. Differences between control and each treatment were analyzed by Kruskal-Wallis test followed by Bonferroni adjustment. Statistical significance was set at p < 0.05 using SPSS 11.0 for Windows (SPSS, Inc., Chicago, IL, USA).
3 Results
3.1 Histological observations of the ovarian follicles
In 0.53-mm-diameter ovarian follicles, yolk globules (Yg) were accumulated into the cytoplasm, the oil droplets (Ods) were distributed over the cytoplasm, and the nucleus was present in the center of oocytes (Figure 1A). In 0.78-mm-diameter oocytes, Yg and Ods continued to accumulate to the cytoplasm (Figure 1B). In these oocytes, one or two large Ods (40–50 μm) were observed around the nucleus, and the nucleus began to migrate.
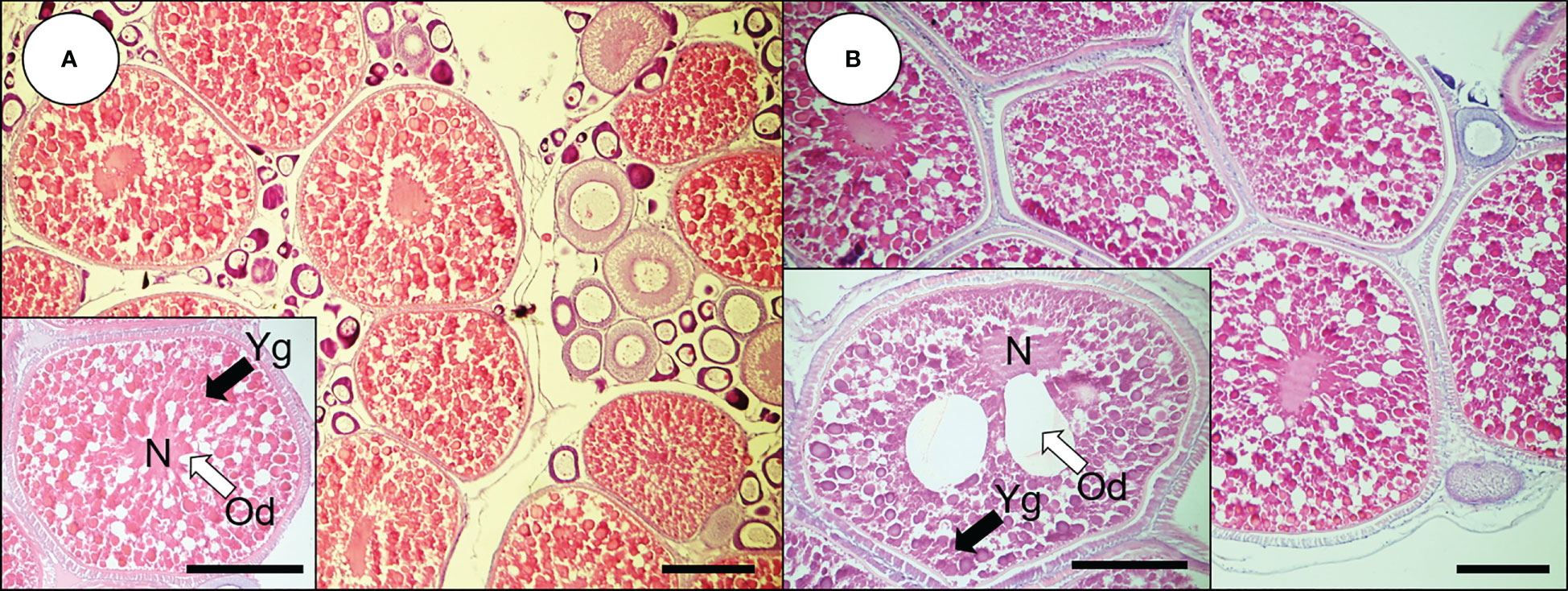
Figure 1 Histological observations of oocytes from dusky tripletooth goby. (A) oocytes of 0.53 mm; (B) oocytes of 0.76 mm. Scale bars indicate 200 μm. N, nucleus; Od, oil droplet; Yg, yolk granule.
3.2 Effects of PCB126 and E2 on steroid metabolites from [3H]-17αP
When vitellogenic ovarian follicles (0.53 mm in diameter) were incubated with [3H]-17αP, the two major metabolites were separated and identified as T and E2 by TLC, HPLC, and GC/MS (Figures 2, 3). Fractions of two progestins, co-migrated with standard 17α20βP and 17α,20α-dihydroxy-4-pregnen-3-one (17α20αP), were not identified because of their low levels. Steroid metabolites produced from [3H]-17αP in the presence of PCB126 and E2 were compared with the values obtained from photo-stimulated luminescence (PSL) autoradiography (Figure 4).
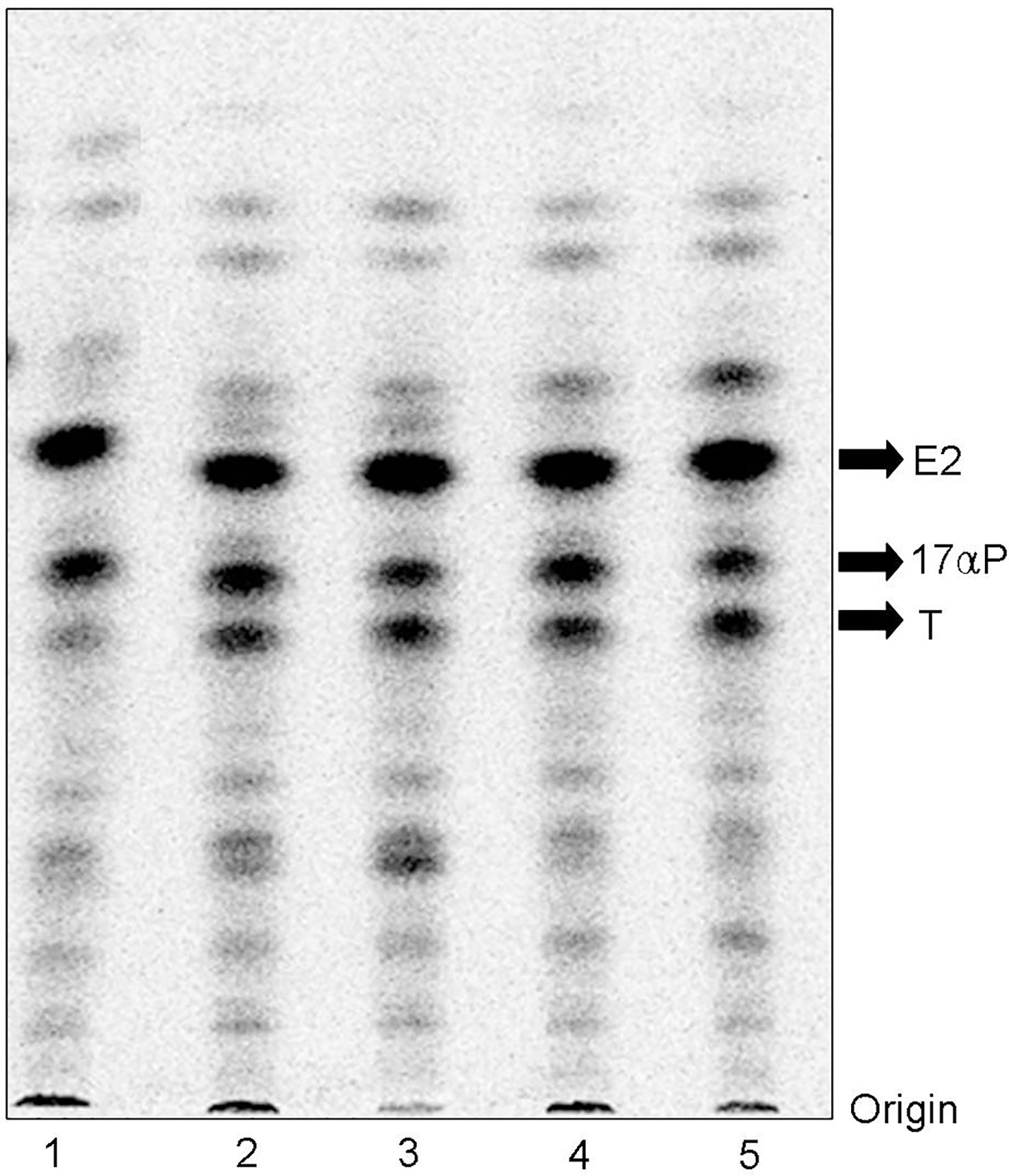
Figure 2 Autoradiograms of steroid metabolites incubated with [3H]-17αP from dusky tripletooth goby oocytes. T and E2 were separated by thin layer chromatography developed with a benzene: acetone (4: 1) and benzene: ethyl acetate (4: 1) mixture. 1, Control; 2, E2 10 ng/mL; 3, E2 100 ng/mL; 4, PCB126 10 ng/mL; 5, PCB126 100 ng/mL.

Figure 3 Mass spectra of steroid metabolized from dusky tripletooth goby oocytes were identified as T and E2. (A) authentic T; (B) metabolized T; (C) authentic E2; (D) metabolized E2.
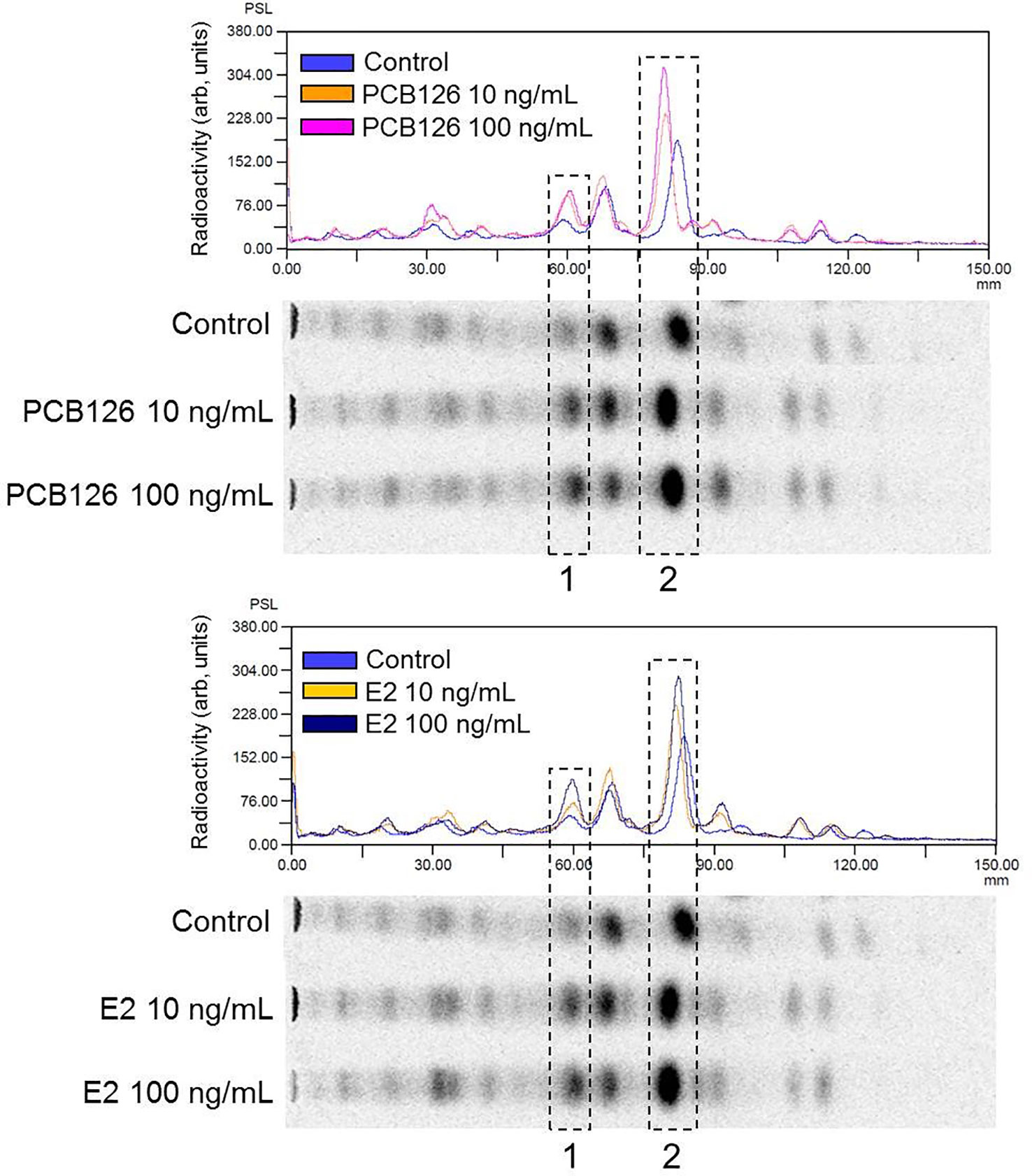
Figure 4 Comparison of radioactivity distribution on the TLC plate by digital photo-stimulated luminescence (PSL) autoradiography. The chromatograms are normalized to the maximum peak intensity in each chromatogram. 1, fraction of T metabolite; 2, fraction of E2 metabolite.
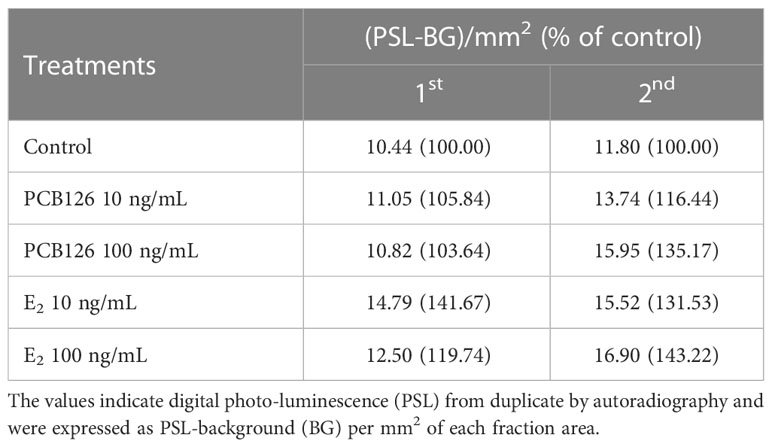
In T metabolites (Table 3), both 10 and 100 ng/ml PCB126 stimulated T synthesis (11.05, 13.74 and 10.82, 15.95 (PSL-BG)/mm2, respectively), compared with the control (10.44 and 11.80 (PSL-BG)/mm2). Exogenous E2 treatment with 10 and 100 ng/ml also stimulated T synthesis (14.79, 15.52 and 12.50, 16.90 (PSL-BG)/mm2, respectively) compared with the control.
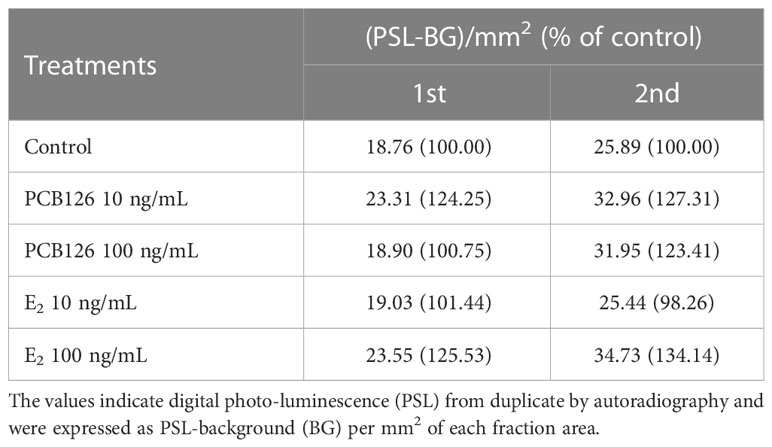
In E2 metabolites (Table 4), both 10 and 100 ng/ml PCB126 stimulated E2 synthesis (23.31, 32.96 and 18.90, 31.95 (PSL-BG)/mm2, respectively) compared with the control (18.76 and 25.89 (PSL-BG)/mm2). Exogenous E2 treatment with 100 ng/ml also stimulated E2 synthesis (23.55 and 34.73 (PSL-BG)/mm2); however, the second value in the treatment with 10 ng/ml (25.44 (PSL-BG)/mm2) was decreased slightly compared to controls.
3.3 Effects of PCB126 and E2 on steroid production
PCB126 treatment with 100 ng/mL decreased T production (152.60 ± 16.49 pg/mL) compared to the control (212.93 ± 7.31 pg/mL, p < 0.05), although there were no significant effects at 1 and 10 ng/mL of PCB126 treatment (Figure 5). In the exogenous E2 treatment, E2 decreased T production in a dose-dependent manner; however, the effect was not significant. E2 treatment (1 ng/mL) increased E2 production (3353.85 ± 385.93 pg/mL) compared with the control. (1228.91 ± 136.15 pg/mL, p < 0.05). Lower concentrations of E2 (0.01 and 0.1 ng/mL) increased E2 production slightly, although there was no significant effect.
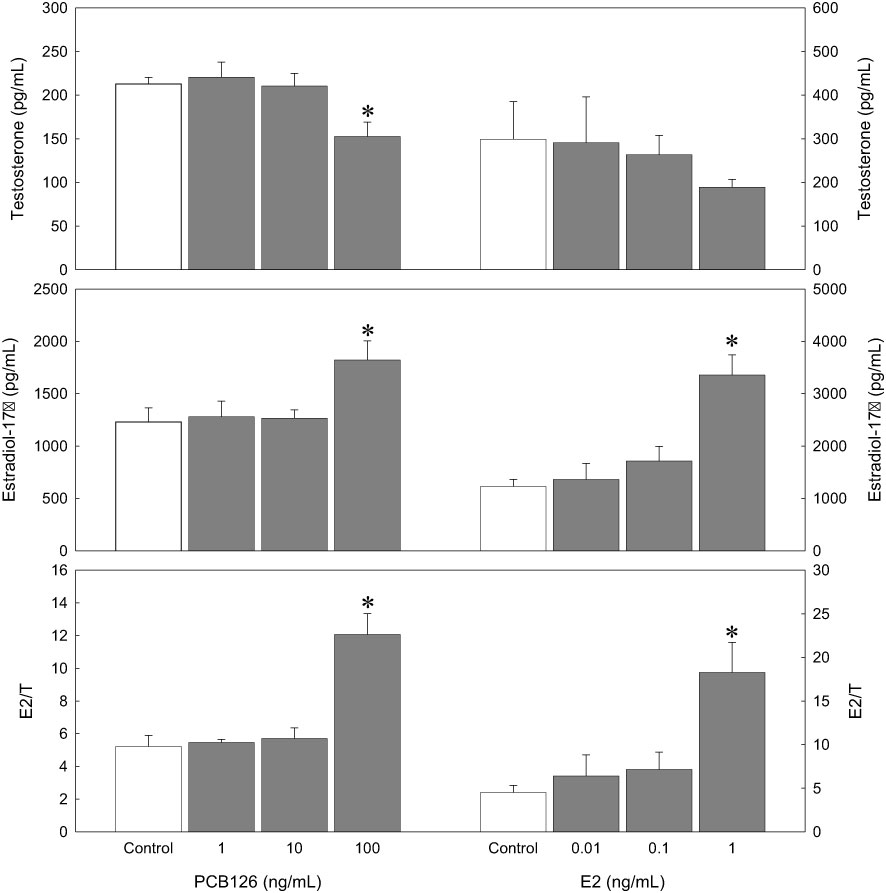
Figure 5 Effects of PCB126 on in vitro steroid production in dusky tripletooth goby oocytes (oocyte diameter=0.53 mm) after a 24 h incubation. Values are the mean ± SE of each steroid in three replicate wells with 20 oocytes/well. Data were analyzed using the Kruskal–Wallis test followed by the Bonferroni adjustment. Asterisks show significant differences from controls (p < 0.05).
In the ratio of E2/T, as a sensitive biomarker of sex-steroid concentrations (Bevans et al., 1996; Folmar et al., 1996), PCB126 increased E2/T significantly at the highest dose (12.07 ± 1.28) compared to the control (5.22 ± 0.68, p < 0.05). Exogenous E2 treatment also increased E2/T significantly at the highest dose (18.27 ± 3.44) compared to the control (4.52 ± 0.80, p < 0.05).
3.4 Effects of PCB126 and E2 on GVBD
We incubated germinal vesicle migratory (GVM) ovarian follicles (0.78 mm diameter) with PCB126 or exogenous E2. PCB126 decreased GVBD in all treatments, and 10 and 100 ng/mL PCB126 resulted in a significant decrease in GVBD (0.94 ± 0.77 and 0%) compared with the control (12.12 ± 1.52%, p < 0.05). In the exogenous E2 treatment, 0.1, 10, and 100 ng/mL E2 resulted in a decrease in GVBD, although there were no significant differences (Figure 6).
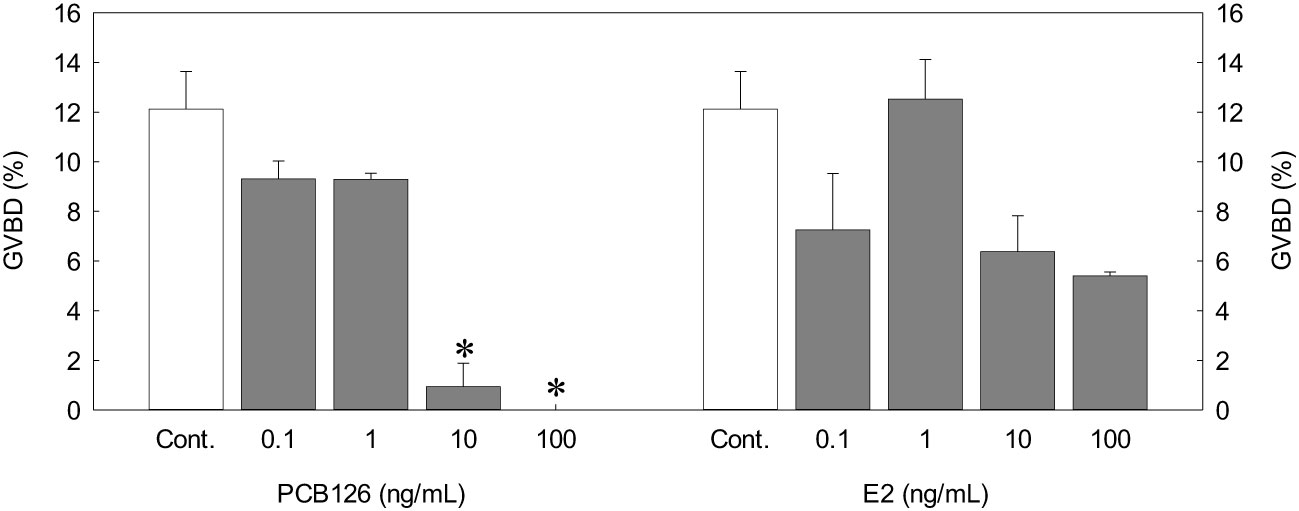
Figure 6 Effects of different PCB126 concentrations on in vitro GVBD in dusky tripletooth goby oocytes (oocyte diameter = 0.78 mm) after a 24 h incubation. Values are means ± SEM of the percentage of GVBD in three replicate wells with 20 oocytes/well. Controls were incubated with only incubation medium. Data were analyzed using the Kruskal-Wallis test followed by Bonferroni adjustment. Asterisks show significant differences from controls (P < 0.05).
4 Discussion
In the present study, we investigated the estrogenic potency of PCB126 on in vitro steroid synthesis in vitellogenic ovarian follicles with an exogenously labeled precursor. In addition, we compared estrogenic potency in the final oocyte maturation process with that in GVM ovarian follicles. There have been controversies regarding PCB126 whether its effects are estrogenic or anti-estrogenic, although it is the most toxic PCB congener (Safe, 1994; Lind et al., 1999; Wojtowicz et al., 2000; Gregoraszczuk et al., 2003a, b; Vaccaro et al., 2005; Mortensen and Arukwe, 2008a, b; Gregoraszczuk et al., 2008; Sechman et al., 2016). The different effects may be caused by the diverse actions of the aryl hydrocarbon receptor (AhR) (Calo et al., 2010). In our previous study, PCB126 had no significant effect on in vitro E2 production from the ovarian follicles of red lip mullet (Baek et al., 2011). Coupled with prior studies, Gjernes et al. (2012) hypothesized that PCB126 has estrogenicity via estrogen receptor (ER)-hijacking (modulating estrogenic responses with activated AhR in the absence of an ER agonist). Recent studies have also reported that PCB126 exhibits estrogenic activity (Pinto et al., 2018; Licata et al., 2019).
In the present study, we separated and identified two major metabolites, T and E2. The metabolites were scanned and the data were converted as a PSL value. According to Kamarainen et al. (2006), PSL provides high sensitivity and resolution and superior linearity compared with the other methods such as radioactivity scanning and film autoradiography. Our results indicate that PCB126 has estrogenic potency in 0.53 mm diameter ovarian follicles; it stimulated not only E2 metabolites but also E2 production, in vitro. Since aromatase is the key enzyme for converting androgen to estrogen (Nagahama et al., 1994), we considered that PCB126 may stimulate aromatase activity, resulting in a decrease in T production and an increase in E2 production from RIA results. These were remarkably analogous to the exogenous E2 treatment. However, PCB126 and exogenous E2 stimulated T metabolite biosynthesis in the presence of the labeled precursor. This difference may be due to different steroidogenic pathways and/or activities due to the absence or presence of the exogenous precursor, [3H]-17αP.
Coupled with the results of the increase in E2 metabolites, however, we could not identify two progestins that co-migrated with 17α20αP and 17α20βP in our LC system due to their low activity (data not shown). To date, maturation inducing steroid (MIS) of the dusky tripletooth goby has not yet been identified, although 17α20βP, the representative progestin, has been reported as a MIS in various teleost species (Nagahama and Yamashita, 2008). Previous studies have reported that 17α20αP may act as an MIS in several fish species (Canario and Scott, 1990; Hwang and Baek, 2021). The identification of these progestins, their maturational capacity, and the effects of PCB126 on progestin synthesis would provide more information about the estrogenic potency of PCB126.
PCB126 may act as an estrogen or anti-estrogen depending on the time and dose of exposure and different physiological conditions, even in different stages of ovarian development (Wojtowicz et al., 2000; Gregoraszczuk et al., 2003a; b; Mortensen and Arukwe, 2008b). Therefore, we studied the estrogenic potency of PCB126 in the final oocyte maturation process in GVM ovarian follicles and hypothesized that PCB126 may also indicate estrogenic potency analogous to exogenous E2 at this stage. Our results clearly indicate that PCB126 inhibits GVBD, similar to exogenous E2 treatment. The inhibitory effect of high doses of PCB126 on GVBD was stronger than that of the exogenous E2 treatment. However, we could not quantify steroid levels from the incubation media of 0.78-mm ovarian follicles due to problems with collecting media. We suspect that PCB126 may disrupt the activity of steroidogenic enzymes, increase E2 levels and decrease progestogen levels such as 17α20βP, simultaneously. Prior to the maturation stage, there is a dramatic shift in the steroidogenic pattern; an increase in the formation of progestins concurrently with a decline in E2 level (Nagahama et al., 1994). The shift in the steroidogenic pattern from the synthesis of estrogens and androgens toward the synthesis of progestins involves a decrease in 17-20 lyase activity and a rise in that of 20β-hydroxysteroid dehydrogenase (HSD). An inhibitory mechanism of PCBs on mammalian oocyte maturation was reported; PCBs acted as toxicants and thus inhibited maturation (Pocar et al., 2006). However, we need to consider the estrogenic potency of PCB126: it stimulates E2 production directly so that inhibition of GVBD by its estrogenic effect rather than toxicity.
The estrogenic effect or estrogenicity defined by previous study (Scholz and Mayer, 2008) refers to (1) mimicking the action of estrogens and activation of responsive genes, or (2) elevation of endogenous plasma estrogen levels caused by competitive binding of EDCs to sex steroid-binding globulins. Although we did not conduct these experiments, our results suggest that PCB126 has an estrogenic potency, stimulates the biosynthesis of E2 from vitellogenic ovarian follicles, and inhibits GVBD from GVM ovarian follicles with effects analogous to exogenous E2.
5 Conclusion
In fish, the function of estrogens associated with reproductive characteristics (single or multiple spawning, vitellogenic or maturation stage, and so on) varies, and may regulate many other physiological processes (Nelson and Habibi, 2013). Up to date, PCB126, the most toxic PCB congener may act as an estrogenic agonist or antagonist in various species. The present study demonstrated that PCB126 has an estrogenic potency; (1) increase in E2 synthesis and (2) decrease in GVBD in vitro during the oocyte maturation process of T. obscurus. Future studies with authentic steroidogenic pathway, changes in progestin levels and the related steroidogenic enzymes during this period would be conducted for more comprehension in estrogenic mechanism of PCB126.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of Pukyong National University (PKNU; Regulation No. 554).
Author contributions
IH: conceptualization, methodology, formal analysis, investigation, writing – original draft, visualization. HB: conceptualization, writing – review & editing, visualization, supervision, project administration, funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Korea (R2023016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baek H. J., Hwang I. J., Lee Y. D., Kim H. B. (2011). Effects of nonylphenol and 3,3’4,4’5-pentachlorobiphenyl on in vitro oocyte steroidogenesis in redlip mullet, Chelon haematocheilus. Anim. Cells Syst. 15, 189–196. doi: 10.1080/19768354.2011.604938
Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. (1978). Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature 275, 593–598. doi: 10.1038/275593a0
Bevans H. E., Goodbred S. L., Miesner J. F., Watkins S. A., Gross T. S., Denslow N. D., et al. (1996). Synthetic organic compounds and carp endocrinology and histology in Las Vegas Wash and Las Vegas and Callville Bays of Lake Mead, Nevada 1992 and 1995. In Water Resources Investigations Report 96-4266. Carson City, NV, USA: US Department of the Interior-US Geological Survey, Vol. 12.
Bonefeld-Jørgensen E. C., Andersen H. R., Rasmussen T. H., Vinggaard A. M. (2001). Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158, 141–153. doi: 10.1016/S0300-483X(00)00368-1
Calo M., Alberghina D., Bitto A., Lauriano E. R., Cascio P. L. (2010). Estrogenic followed by anti-estrogenic effects of PCBs exposure in juvenile fish (Spaurus aurata). Food Chem.Toxicol. 48, 2458–2463. doi: 10.1016/j.fct.2010.06.013
Canario A. V. M., Scott A. P. (1990). Plasma levels of ovarian steroids, including 17α,20α- dihydroxy-4-pregnen-3-one and 3β,17α,20α-trihydroxy-5β-pregnane, in female dabs (Limanda limanda) - marine flatfish - induced to mature and ovulate with human chorionic gonadotrophin. Gen. Comp. Endocrinol. 77, 177–191. doi: 10.1016/0016-6480(90)90302-3
Colborn T., Vom Saal F. S., Soto A. M. (1993). Developmental effects of endocrine disrupting chemicals in wildlife and humans. Environ. Health Perspect. 101, 378–384. doi: 10.1289/ehp.93101378
Das S., Thomas P. (1999). Pesticides interfere with nongenomic action of a progestogen on meiotic maturation by binding to its plasma membrane receptor on fish oocytes. Endocrinology 140, 1953–1956. doi: 10.1210/endo.140.4.6781
Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M. R., et al. (2009). Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr. Rev. 30, 293–342. doi: 10.1210/er.2009-0002
Folmar L. C., Denslow N. D., Rao V., Chow M., Crain D. A., Enblom J., et al. (1996). Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ. Health Perspect. 104, 1096–1101. doi: 10.1289/ehp.961041096
Ghosh S., Thomas P. (1995). Antagonistic effects of xenobiotics on steroid-induced final maturation of Atlantic croaker oocytes in vitro. Mar. Environ. Res. 39, 159–163. doi: 10.1016/0141-1136(94)00036-O
Gjernes M. H., Schlenk D., Arukwe A. (2012). Estrogen receptor-hijacking by dioxin-like 3,3’4,4’,5-pentachlorobiphenyl (PCBC126) in salmon hepatocytes involves both receptor activation and receptor protein stability. Aquat. Toxicol. 124-125, 197–208. doi: 10.1016/j.aquatox.2012.08.015
Gregoraszczuk E. L., Grochowalski A., Chrzaszcz R., Wegiel M. (2003a). Congener-specific accumulation of polychlorinated biphenyls in ovarian follicular wall follows repeated exposure to PCB126 and PCB153. comparison of tissue levels of PCB and biological changes. Chemosphere 50, 481–488. doi: 10.1016/S0045-6535(02)00637-9
Gregoraszczuk E. L., Sowa M., Kajta M., Ptak A., Wójtowicz A. (2003b). Effect of PCB126 and PCB153 on incidence of apoptosis in cultured theca and granulosa cells collected from small, medium and large preovulatory follicles. Reprod. Toxicol. 17, 465–471. doi: 10.1016/S0890-6238(03)00042-x
Gregoraszczuk E. L., Rak A., Ludewig G., Gasin’ska A. (2008). Effects of estradiol, PCB3 and their hydroxylated metabolites on proliferation, cell cycle, and apoptosis of human breast cancer cells. Environ. Toxicol. Pharmacol. 25, 227–233. doi: 10.1016/j.etap.2007.10.004
Guruge K. S., Watanabe M., Tanaka H., Tanabe S. (2001). Accumulation status of persistent organochlorines in albatrosses from the north pacific and the southern ocean. Environ. Pollut. 114, 389–398. doi: 10.1016/S0269-7491(00)00234-7
Hwang I. J., Baek H. J. (2021). In vitro Sex steroid metabolism in red spotted grouper, Epinephelus akaara during oocyte maturation. Dev. Reprod. 25, 75–82. doi: 10.12717/DR.2021.25.2.75
Hwang I. J., Kim H. W., Kim J. K., Lee Y. D., Baek H. J. (2010). Estrogenicity of 4-nonylphenol and diethylstilbestrol on in vitro oocyte maturation of the dusky tripletooth goby, Tridentiger obscurus. Anim. Cells Syst. 14, 161–167. doi: 10.1080/19768354.2010.504339
Jobling S., Tyler C. R. (2003). Endocrine disruption, parasites and pollutants in wild freshwater fish. Parasitology 126, S103–S108. doi: 10.1017/S0031182003003652
Kamarainen E., Haaparanta M., Siitari-Kauppi M., Koivula T., Lipponen T., Solin O. (2006). Analysis of 18F-labelled synthesis products on TLC plates: comparison of radioactivity scanning, film autoradiography, and a phosphoimaging technique. Appl. Radiat. Isot. 64, 1043–1047. doi: 10.1016/j.apradiso.2006.04.012
Kaneko T., Hanyu I. (1985). Annual reproductive cycle of the chichibu goby, Tridentiger obscurus. Bull. Jap. Soc Sci. Fish. 51, 1645–1650. doi: 10.2331/suisan.51.1645
Kobayashi M., Aida K., Sakai H., Kaneko T., Asahina K., Hanyu I., et al. (1987). Radioimmunoassay for salmon gonadotropin. Nippon Suisan Gakk. 53, 995–1003. doi: 10.2331/suisan.53.995
Licata P., Tardugno R., Pergolizzi S., Capillo G., Aragona M., Colombo A., et al. (2019). In vivo Effects of PCB-126 and genistein on vitellogenin expression in zebrafish. Nat. Prod. Res. 17, 2507–2514. doi: 10.1080/14786419.2018.1455048
Lin Y. W. P., Schuetz A. W. (1983). In vitro Estrogen modulation of pituitary and progesterone-induced oocyte maturation in Rana pipiens. J. Exp. Zool. 97, 281–292. doi: 10.1002/jez.1402260214
Lind P. M., Eriksen E. F., Sahlin L., Edlund M., Orberg J. (1999). Effects of the antiestrogenic environmental pollutant 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) in rat bone and uterus: diverging effects in ovariectomized and intact animals. Toxicol. Appl. Pharmacol. 154, 236–244. doi: 10.1006/taap.1998.8568
Liu S., Abdelrahim M., Khan S., Ariazi E., Jordan V. C., Safe S. (2006). Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells. Biol. Chem. 387, 1209–1213. doi: 10.1515/BC.2006.149
Matthews J., Wihlen B., Heldring N., MacPherson L., Helguero L., Treuter E., et al. (2007). Coplanar 3,3’,4,4’,5-pentachlorinated biphenyl and non-coplanar 2,2’,4,6,6’-pentachlorinated biphenyl differentially induce recruitment of estrogen receptor alpha to aryl hydrocarbon receptor target genes. Biochem. J. 406, 343–353. doi: 10.1042/BJ20070585
Midway S. R., Scharf F. S. (2012). Histological analysis reveals larger size at maturity for southern flounder with implications for biological reference points. Mar. Coast. Fish. 4, 628–638. doi: 10.1080/19425120.2012.717524
Mortensen A. S., Arukwe A. (2008a). Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Mar. Environ. Res. 66, 119–120. doi: 10.1016/j.marenvres.2008.02.041
Mortensen A. S., Arukwe A. (2008b). Activation of estrogen receptor signaling by the dioxin-like aryl hydrocarbon receptor agonist, 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) in salmon in vitro system. Toxicol. Appl. Pharmacol. 227 (2), 313–324. doi: 10.1016/j.taap.2007.11.003
Muto T., Wakui S., Imano N., Nakaaki K., Takahashi H., Hano H., et al. (2002). Mammary gland differentiation in female rats after prenatal exposure to 3,3’,4,4’,5-pentachlorobiphenyl. Toxicology 177, 197–205. doi: 10.1016/S0300-483X(02)00224-X
Nagahama Y., Yamashita M. (2008). Regulation of oocyte maturation in fish. Dev. Growth Differ. 50, S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x
Nagahama Y., Yoshikuni M., Yamashita M., Tanaka M. (1994). “Regulation of oocyte maturation in fish,” in Fish physiology. V13. molecular endocrinology of fish. Eds. Sherwood N. M., Hew C. L. (London: Academic Press), 393–439.
Navas J. M., Segner H. (2006). Vitellogenin synthesis in primary cultures of fish liver cells as endpoint for in vitro screening of the (anti)estrogenic activity of chemical substances. Aquat. Toxicol. 80, 1–22. doi: 10.1016/j.aquatox.2006.07.013
Nelson E. R., Habibi H. R. (2013). Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 192, 15–24. doi: 10.1016/j.ygcen.2013.03.032
Pickford D. B., Morris I. D. (1999). Effects of endocrine-disrupting contaminants on amphibian oogenesis: methoxychlor inhibits progesterone-induced maturation of Xenopus laevis oocytes in vitro. Environ. Health Perspect. 107, 285–292. doi: 10.1289/ehp.99107285
Pinto C. L., Markey K., Dix D., Browne P. (2018). Identification of candidate reference chemicals for in vitro steroidogenesis. toxicol. In Vitro 47, 103–119. doi: 10.1016/j.tiv.2017.11.003
Pocar P., Brevini T. A. L., Antonini S., Gandolfi F. (2006). Cellular and molecular mechanisms mediating the effect of polychlorinated biphenyls on oocyte in vitro maturation. Reprod. Toxicol. 22, 242–249. doi: 10.1016/j.reprotox.2006.04.023
Robinson C. D., Brown E., John A. C., Davies I. M., Megginson C., Miller C., et al. (2007). Bioindicators and reproductive effects of prolonged 17β-oestradiol exposure in a marine fish, the sand goby (Pomatoschistus minutus). Aquat. Toxicol. 81, 397–408. doi: 10.1016/j.aquatox.2006.12.020
Safe S. (1994). Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 24, 87–149. doi: 10.3109/10408449409049308
Scholz S., Mayer I. (2008). Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 293, 57–70. doi: 10.1016/j.mce.2008.06.008
Sechman A., Batoryna M., Antos P. A., Hrabia A. (2016). Effects of PCB 126 and PCB 153 on secretion of steroid hormones and mRNA expression of steroidogenic genes (STAR, HSD3B, CYP19A1) and estrogen receptors (ERα, ERβ) in prehierarchical chicken ovarian follicles. Toxicol. Lett. 264, 29–37. doi: 10.1016/j.toxlet.2016.11.001
Tokumoto T., Tokumoto M., Nagahama Y. (2005). Induction and inhibition of oocyte maturation by EDCs in zebrafish. Reprod. Biol. Endocrinol. 3, 69. doi: 10.1186/1477-7827-3-69
Vaccaro E., Meucci V., Intorre L., Soldani G., Di Bello D., Longo V., et al. (2005). Effects of 17β-estradiol, 4-nonylphenol and PCB126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax). Aquat. Toxicol. 75, 293–305. doi: 10.1016/j.aquatox.2005.08.009
Keywords: estrogen, 3,3’,4,4’,5-pentachlorobiphenyl, ovarian follicles, maturation, dusky triple tooth goby
Citation: Hwang IJ and Baek HJ (2023) Effects of 3,3’,4,4’,5-pentachlorobiphenyl on in vitro oocyte maturation in dusky tripletooth goby, Tridentiger obscurus: an implication of estrogenic potency. Front. Mar. Sci. 10:1159560. doi: 10.3389/fmars.2023.1159560
Received: 08 February 2023; Accepted: 18 April 2023;
Published: 16 May 2023.
Edited by:
Gongliang Yu, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Jian Han, Chinese Academy of Sciences (CAS), ChinaIrfan Ahmad Bhat, University of Iceland, Iceland
Copyright © 2023 Hwang and Baek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hea Ja Baek, aGpiYWVrQHBrbnUuYWMua3I=
 In Joon Hwang
In Joon Hwang Hea Ja Baek
Hea Ja Baek