- 1Institute of Marine Sciences (ISMAR), Italian National Research Council (CNR), Venezia, Italy
- 2Department of Biology, University of Padova, Padova, Italy
The study of the relationships between growth patterns, energy reserves and reproduction, and their interdependency with environmental variables is crucial to increase the knowledge of the physiological processes regulating the recruitment and survival of commercially exploited bivalve species. In the present study, the biochemical profile and gametogenic cycle of the striped Venus clam Chamelea gallina monthly sampled in fishing grounds of the Chioggia district in 2001 and 2010 were investigated to identify possible variations that occurred after 10 years and to analyze inter-relationships among the biological parameters with changing environmental conditions. Our results indicated that higher phytoplankton availability and increased water temperature recorded from 2001 to 2010 resulted in higher reserve accumulation, mostly in the form of proteins, carbohydrates and glycogen. An increase was also observed in the calcification rate of the shells, which consequently became heavier in clams collected in 2010. Moreover, in 2010, as for the reproductive conditions, a prolonged spawning period was observed with respect to 2002. Overall results highlighted clear-cut differences in the physiological performances of clams collected in 2001 and 2010, suggesting the importance to monitor changes over time to unveil potential pros and cons for single populations of commercial interest.
1 Introduction
The striped venus clam Chamelea gallina is an infaunal and filter-feeding bivalve species, occurring in the infralittoral zone in habitats normally showing limited variations in environmental parameters such as temperature and salinity, and peculiar conditions in sediment grain size and oxygenation (Stella and Rodinò, 1986; Brooks et al., 1991; Moschino and Marin, 2006). C. gallina is distributed in temperate coastal waters of British and Ireland islands, the Iberian Peninsula, Morocco, Madeira, and the Canary Islands. It is also a common species in the Mediterranean and the Black Sea, particularly along the central and northern Adriatic coasts of Italy, where nutrients and organic matter are abundant, mainly due to the Po River inputs (Orban et al., 2006). This clam lives in a wide variety of sediment types (sand, sandy mud, and mud), but preferentially in the coastal well-sorted fine sand between 3 and 12 m depth (Baeta et al., 2021). It is characterized by high growth rates and a short life span (about 4 years), therefore, population size is strongly dependent on the success of spawning and recruitment (Baeta et al., 2021).
C. gallina has considerable economic importance, being commercially exploited in the Mediterranean, particularly in the inshore waters of Italy, Spain, Turkey and Morocco (Ramón and Richardson, 1992). Commercial landings of C. gallina along the Adriatic coasts of Italy reached a peak value of about 100,000 tons per year in the early 1980s following the introduction of modern hydraulic dredges, which led to constant and consistent exploitation of the resource, ultimately resulting in overfishing conditions (Romanelli et al., 2009). More recently, the fishery of C. gallina experienced a progressive decrease in catches up to approximately 10,000 tons per year (DGPEMAC, 2019). Similar declines in C. gallina populations have been reported over the last decades also in other fishing grounds, such as in the Gulf of Cádiz and the Ebro Delta in Spain (Delgado et al., 2013; Baeta et al., 2021) and along the Andalusia-Algarve coast (Joaquim et al., 2014). The observed declines in C. gallina abundance might be associated with multiple synergistic stressors, such as changes in environmental parameters (temperature, phytoplankton blooms), infections, pollutants and poor management of the fishery resource, eventually resulting in several mass mortality outbreaks (Romanelli et al., 2009; Delgado et al., 2013; Milan et al., 2019; Baeta et al., 2021).
Genetic homogeneity and strong connectivity among populations inhabiting different geographical areas were recently described in C. gallina from the Mediterranean (Papetti et al., 2018; Oztürk and Altınok, 2021). In particular, in the northern-central Adriatic, a weak genetic differentiation among size classes at the local and temporal scale was found, with a low number of breeders responsible for the recruitment (Papetti et al., 2018). Since the maintenance of genetic diversity is essential to ensure long-term reproductive potential, this condition could promote negative effects at the population level under increasing environmental impacts, resulting in decreased clam abundance.
The study of the relationships among changes in growth patterns (both shell and meat weights, energy reserves and reproduction, and their interdependency with environmental variables is crucial to deepen the knowledge of the physiological processes regulating the recruitment and survival of commercially exploited bivalve species. This is particularly relevant for the Adriatic Sea, where multiple and overlapping human-made pressures (such as high urbanization, shipping traffic, aquaculture activities, chemical pollution), synergistically interact with climate-related drivers (e.g. rising sea levels, increased sea temperatures and ocean acidification) (Furlan et al., 2019).
Some studies were performed in C. gallina beds along the Adriatic coasts to determine the biochemical composition and reproductive characteristics of the species. In particular, Orban et al. (2006) studied the seasonal variability of the biochemical composition, but from a nutritional and commercial quality point of view. More recently, Bargione et al. (2021) addressed some aspects of the reproductive biology of C. gallina, such as the gametogenic cycle, first sexual maturity and partial fecundity, in central Adriatic (Ancona district), with fishery management purposes.
The aim of the present study is to evaluate the physiological and reproductive performances of clams in the Northern Adriatic and analyze them over the span of a decade. In particular, the specific aims are to: 1) determine the biochemical profile and gametogenic cycle of the striped Venus clam C. gallina monthly sampled in fishing grounds of the Chioggia district in 2001 and 2010; 2) identify the possible variations that occurred in the biochemical profile and gametogenic cycle after 10 years and 3) analyze inter-relationships among the biological parameters measured and correlate their variations with changes occurred in environmental parameters. To our knowledge, this is the first fully comprehensive study performed on clam fishing grounds of the Northern Adriatic, where Chioggia represents one of the most important maritime compartments in Italy.
2 Materials and methods
2.1 Environmental parameters
To achieve an overall picture of the environmental parameters’ patterns over time, data were analyzed throughout the whole ten-year period.
Monthly data of seawater temperature, salinity and pH were provided by the Hydrobiological Station of Chioggia (University of Padova), located close to the southern inlet connecting the lagoon to the open sea (45.22446, 12.28435), which collects daily data of water parameters at about 0.5 m depth during the incoming tide. Daily data were averaged as monthly data and then as annual values. The detected minimum and maximum monthly values within each year are reported as well.
Data of chlorophyll a and phytoplankton abundance were courtesy provided by ARPA Veneto (Veneto Regional Agency for Environmental Protection). Samples were collected in front of Pellestrina – Ca Roman (Chioggia, Venice), at 500 m from the coastline (45.23870, 12.29903). Chlorophyll a was spectrophotometrically measured according to Parsons et al. (1984) and phytoplankton abundance was determined following the procedure reported in ICRAM-ANPA (2001).
2.2 Clam sampling
C. gallina specimens (25 ± 2 mm in length) were sampled monthly from January 2001 to December 2001 and from January 2010 to December 2010. The clams used for the study of the gametogenic cycle were collected from November 2001 to November 2002 and from November 2009 to November 2010. Clams were collected by professional fishers in fishing grounds ranging from 3 to 10 m depth along the coast off Pellestrina and Chioggia. After collection, samples were immediately transported to the laboratory in refrigerated boxes and then processed. The analyses performed in the two periods were carried out in the same laboratories applying the same protocols.
2.3 Weight determinations and condition index
Ash-free dry weight of meat (AFDW) and dry weight of shell (DSW) were determined on 30 clams for each sample. Soft body and shell from individual clams were put separately in an oven at 60° C and weighed after 48 h. Soft tissues were then placed in a muffle furnace at 450°C for 6 h and re-weighed to quantify ash content. Ash-free dry weight was calculated as the difference between dry weight and ash weight of meat. The use of ash-free dry weight of meat is more precise, since ash, like water content, has been known to increase under unfavorable physiological conditions, thus resulting in a less accurate evaluation of the physiological state of the organism (Lucas and Beninger, 1985). All measurements were expressed as grams. Weight data were normalized according to the shell length (L) in cm for each individual clam.
The condition index (CI) was then determined following the method of Walne and Mann (1975):
(Ash-free dry weight of meat/Dry weight of shell) * 100.
2.4 Biochemical analyses
Clams were split up into six groups of 8–10 individuals, and soft tissues were excised, frozen in liquid nitrogen and then stored at – 80° C until analysis. When possible, depending on gonad maturation, clams were distinguished according to sex by microscopical observation of gonad smears. Two pools of males, two of females and two of randomly chosen clams were set up.
Before analyses, samples were lyophilized and powdered. Protein content was determined following the method of Lowry et al. (1951), and carbohydrate and glycogen contents according to Dubois et al. (1956). Lipids were extracted following the method of Mann and Gallagher (1985) and then quantified through the colorimetric method of Pande et al. (1963). All samples were analyzed in triplicate. The results were expressed as micrograms per milligram of ash-free dry weight (AFDW); the ash proportion was quantified by incinerating aliquots of lyophilized tissue in a muffle furnace at 450°C for 6 h.
The caloric contents of carbohydrates, proteins and lipids in tissues were calculated to obtain the energy values, in J, using the following conversion factors:
- 17.2 kJ g-1 for carbohydrates (Paine, 1971);
- 17.9 kJ g-1 for proteins (Beukema and De Bruin, 1979);
- 33 kJ g-1 for lipids (Beninger and Lucas, 1984).
2.5 Histology of gonadal tissues
Visceral mass from 20 individuals per sample was excised and fixed in Baker’s formol for 24 h at 4°C.
Tissues were dehydrated in a series of increasing concentrations of ethyl alcohol solutions, then clarified in Clearene@ and embedded in paraffin wax (Paraplast@ at 56°C). Sections (5-6 µm thick) were cut in the mid-region of the gonad, and the resulting slides were stained using Papanicolaou’s method. Microscopic examination under the light microscope was aimed at determining the stages of the gametogenic cycle. Gonadal stages were classified according to Valli et al. (1996) with slight modifications: stage 0, sexual inactivity; stage I, early developing; stage II, late developing; stage III, ripe; stage IV, spawning; stage V, spent.
The gonadal index (Seed, 1980) was then calculated for each sample as follows: the number of clams at each stage was multiplied by the numerical score attributed to that stage (stage 0 = score 0, stages 1 and 5 = score 1, stages 2 and 4 = score 2, stage 3 = score 3), the products were added, and the result divided by the total number of individuals examined. The gonadal index ranged from 0 (all individuals in the sample were in resting or spent stages) to 3 (all individuals were ripe).
2.6 Statistical analysis
The correlation coefficient r was calculated for each environmental parameter, and the linear relationship was analyzed through linear correlation analysis.
Data were checked for normal distribution and homogeneity of variance before each statistical analysis, Mann-Whitney tests for two independent samples were used to compare the biochemical data, as they did not fulfil the assumptions for the parametric tests (Sokal and Rohlf, 1981). For all the other analyses, the ANOVA test was applied followed by Fisher-LSD post-hoc tests. Spearman’s correlation coefficients were calculated to investigate the statistical relationship between biochemical components, weight determinations, CI and the mean values of the environmental parameters recorded in the corresponding month.
A multivariate analysis, Principal Component Analysis (PCA), was performed to investigate potential differences in biochemical components between females and males, on a data matrix including protein, lipid, carbohydrate and glycogen mean values. A second PCA was carried out with the whole data set of biochemical components, CI, normalized AFDW, normalized DSW, GI and environmental variables to investigate the differences between 2001 and 2010 samples. STATISTICA 7.0 software (StatSoft) was used for all statistical processing.
3 Results
3.1 Environmental parameters
Annual variations of water temperature, salinity and pH from 2000 to 2011 are shown in Figure 1; monthly variations of the environmental parameters detected in 2001 and 2010 are listed in Table S1. An increase in water temperatures was observed over time (Figure 1A). In particular, the main changes were observed for mean values and minima, when comparing the data recorded from 2001 to 2006 with those from 2007 to 2011 (significant correlation coefficient r for p<0.01). An opposite behavior was observed for salinity, which decreased over time (Figure 1B). The pH exhibited slight variations in mean and maximum values, whereas the minimum decreased from 2001 to 2011 (Figure 1C).

Figure 1 Minimum, maximum and mean annual values of temperature (A), salinity (B) and pH (C) recorded in front of Chioggia from 2000 to 2011. Trendline, equation and R2 value (p<0.01 **) are shown for mean and minimum temperature values.
Monthly values of chlorophyll a showed a slight decrease from 2001 to 2010, whereas the total phytoplankton increased, with a significant value of the correlation coefficient r (p<0.05) (Figures 2A, B).
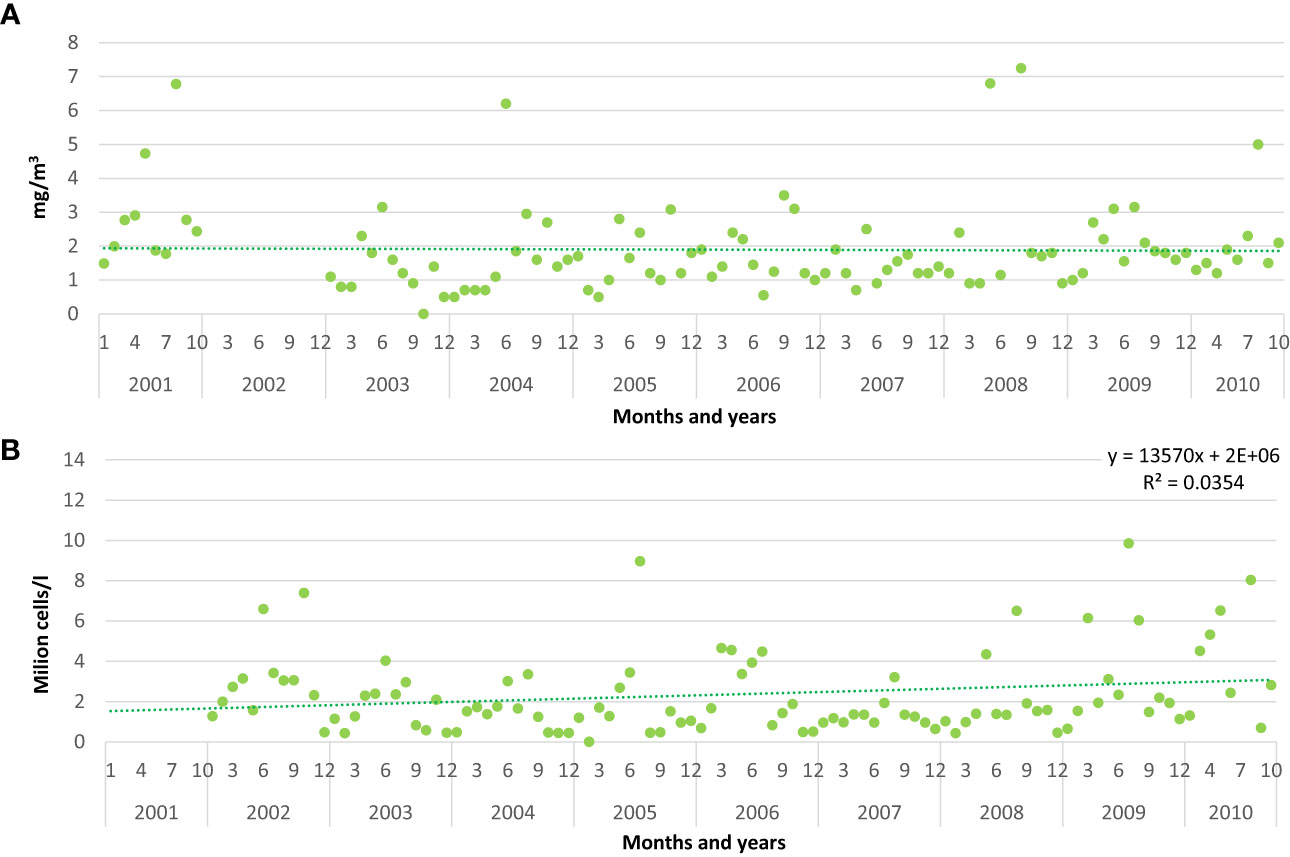
Figure 2 Monthly values of chlorophyll a (A) and total phytoplankton abundance in million cells/l (B) recorded in front of Pellestrina – Ca Roman. Trendline, equation and R2 value (p<0.05 *) are shown for total phytoplankton abundance.
3.2 Weight determinations and condition index
In 2001, AFDW normalized on shell length values decreased until March, when a minimum was observed (0.027 g/cm); then a peak was recorded in April, (0.080 g/cm). From May onward, values were quite stable ranging from 0.032 g/cm in August to 0.053 g/cm in November. In 2010, values were less variable in most of the year ranging from 0.042 g/cm in June to 0.056 in February; a peak was observed in April and May (0.083 and 0.086, respectively) (Figure 3A). As for the normalized DSW, in 2001 values ranged from 0.87 g/cm in March to 1.17 g/cm in January, whereas in 2010 values were higher and less variable ranging from 1.20 g/cm in December to 1.33 g/cm in October (Figure 3B). CI showed quite constant values over both 2001 (from 3.0 to 4.5) and 2010 (from 3.3 to 4.4), except for the peak observed in spring, in April 2001 (7.1) and in April-May 2010 (6.5 and 6.7, respectively) (Figure 4).
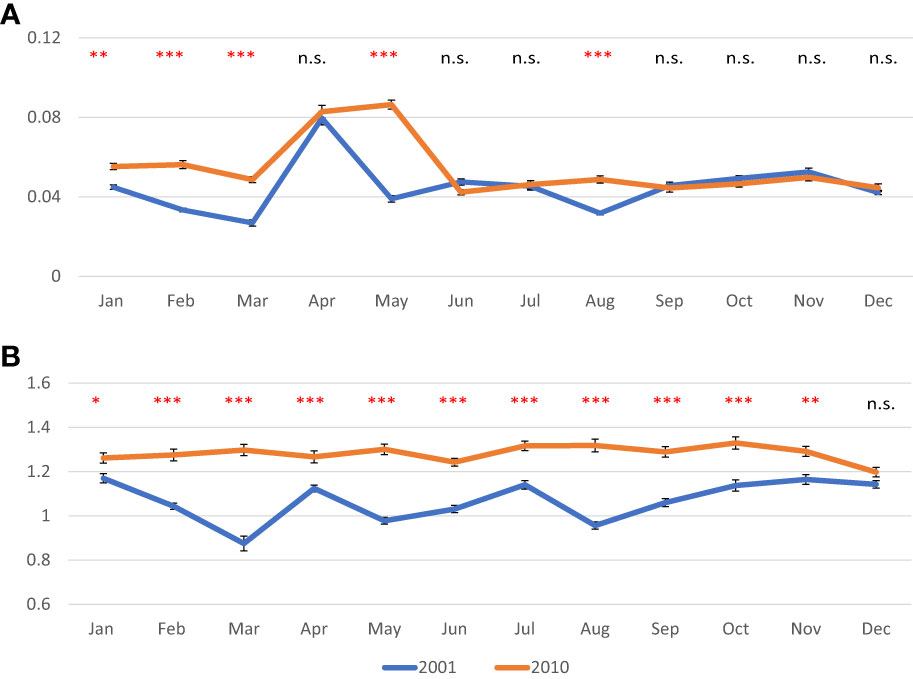
Figure 3 Monthly mean values in g of normalized ash free dry weight of meat (A) and normalized shell dry weight (B) in the clam C. gallina collected in 2001 and 2010. Mean ± s.e., n=30. ANOVA: p<0.05 *, p<0.01 **, p<0.001 ***. Significantly higher values observed in 2010 samples are shown in red.
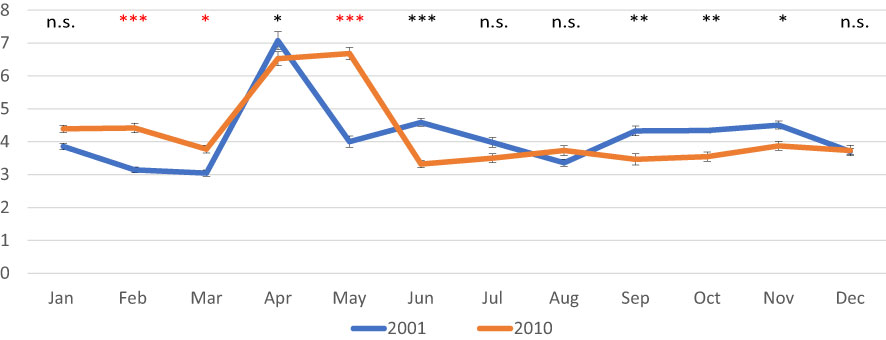
Figure 4 Monthly mean values of the condition Index in the clam C. gallina collected in 2001 and 2010. Mean ± s.e., n=30. ANOVA: p<0.05 *, p<0.01 **, p<0.001 ***. Significantly higher values observed in the 2010 samples are shown in red.
Normalized AFDW values were significantly higher in 2010 from January to March, then in May and August, whereas monthly values of normalized DSW were always higher in clams collected in 2010 with respect to those collected in 2001, except in December. CI showed higher variability with significantly higher values sometimes recorded in 2001, and others in 2010.
3.3 Biochemical composition
Proteins showed high variability during 2001, with higher values detected in February (497.4 µg/mg AFDW) and then from July to September (from 472.8 µg/mg AFDW to 477.8 µg/mg AFDW); lowest values were detected in January (350.6 µg/mg AFDW) and from October onward (from 349.3 µg/mg AFDW to 377.7 µg/mg AFDW). Proteins were less variable in 2010, and showed always higher values compared to 2001, ranging from 497.4 µg/mg AFDW in January to 563.4 µg/mg AFDW in March (Figure 5A).
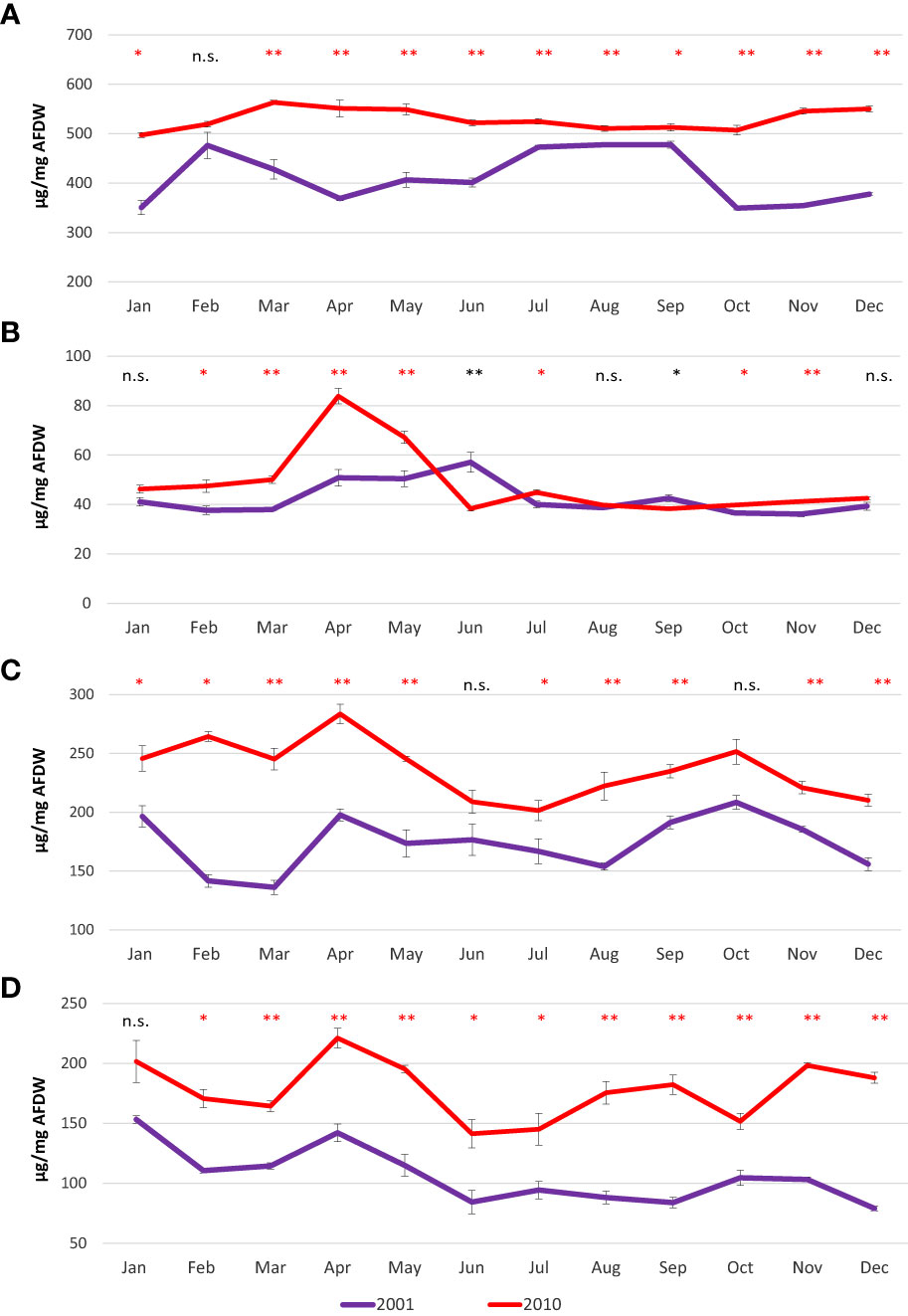
Figure 5 Mean monthly values of proteins (A), lipids (B), carbohydrates (C) and glycogen (D) in the clam C. gallina collected in 2001 and 2010. Mean ± s.e., n=6. Mann Whitney test: p<0.05 *, p<0.01 **. Significantly higher values observed in the 2010 samples are shown in red.
In 2001, a slight increase in lipid content was observed from April to June (50.8 and 57.1 µg/mg AFDW, respectively), whereas in 2010, a peak was recorded in April (83.8 µg/mg AFDW) (Figure 5B).
Carbohydrates showed similar behaviors when comparing 2001 and 2010. The patterns were characterized by three peaks observed in January/February (196.4 µg/mg AFDW in 2001 and 264.5 µg/mg AFDW in 2010), in April (197.8 µg/mg AFDW in 2001 and 283.6 µg/mg AFDW in 2010) and October (208.5 µg/mg AFDW in 2001 and 251.6 µg/mg AFDW in 2010) (Figure 5C). Glycogen showed a similar pattern to carbohydrates, with peaks and minima observed in the same periods in both years. Indeed, glycogen peaked twice during the year, once in January (153.3 µg/mg AFDW in 2001 and 201.7 µg/mg AFDW in 2010) and the other in April (142.2 µg/mg AFDW in 2001 and 221.2 µg/mg AFDW in 2010)(Figure 5D).
Total energy content showed slight variations, ranging from 10.7 kJ/g in November-December to 13.2 kJ/g in September in 2001 and from 14.2 kJ/g in June to 17.5 kJ/g in April in 2010 (Figure 6).
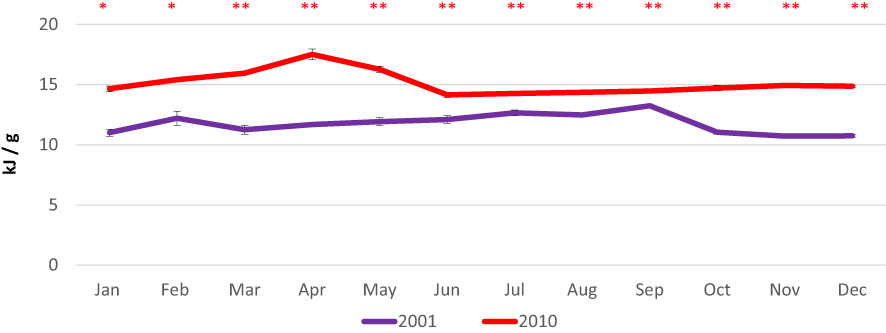
Figure 6 Monthly variations in total energy contents in the clam C. gallina collected in 2001 and 2010. Mean ± s.e., n=6. Mann Whitney test: p<0.05 *, p<0.01 **. Significantly higher values observed in the 2010 samples are shown in red.
Proteins, carbohydrates, glycogen and total energy values were significantly higher in 2010 than in 2001, with rare exceptions. Lipids showed higher variability with significantly higher values sometimes recorded in 2001 and other times in 2010.
When comparing biochemical components in male and female clams, proteins and lipids showed similar trends and values in both sampling periods. Conversely, both carbohydrate and glycogen contents were always higher in males from June to September (Table S2). These observations are confirmed by the PCA results. Indeed, the PCA performed to detect gender differences in the biochemical components highlighted no differences in male and female spatial distribution, evidencing a similar pattern of the various biochemical components. Factor 1 and 2 explained over 80% of the variance; Factor 1 explained 61.9% of the total variance and showed significant negative loadings of the variables carbohydrates (-0.87) and glycogen (-0.91) (Figure 7).
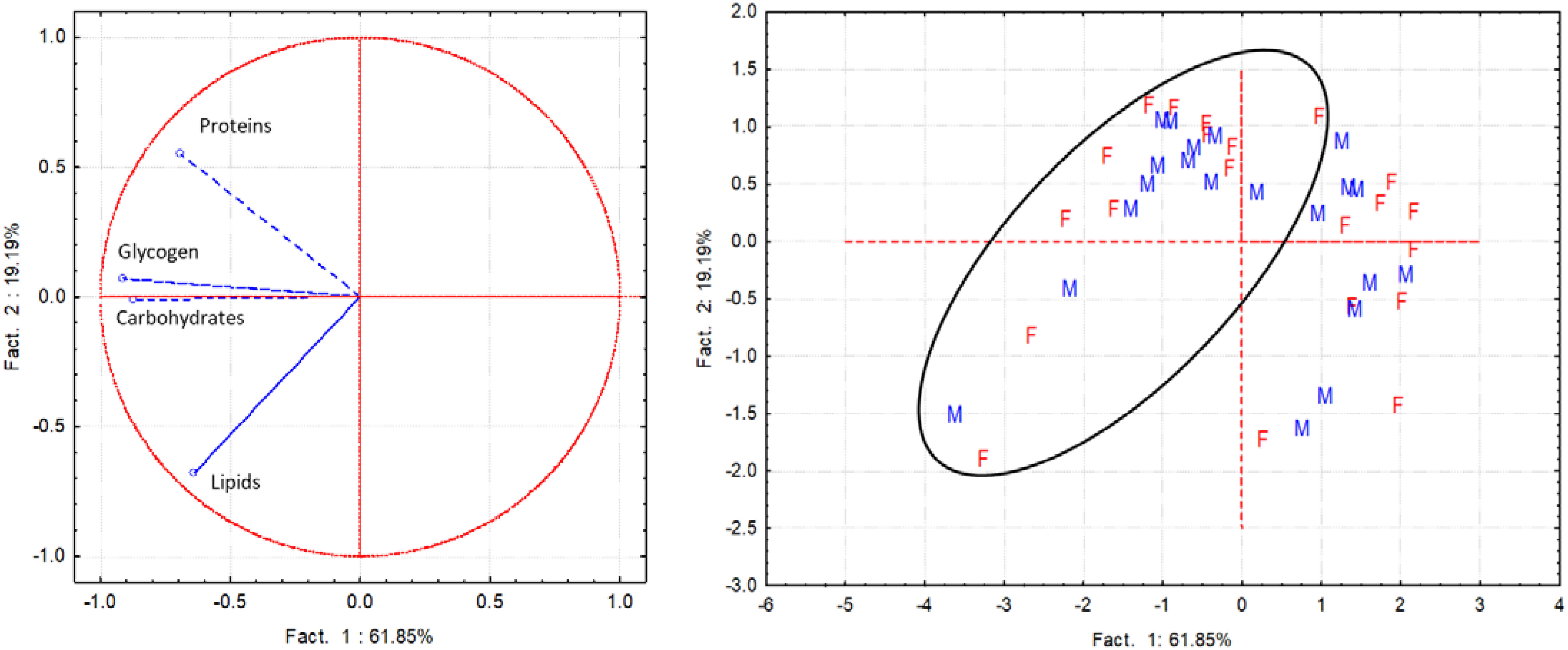
Figure 7 PCA performed with the whole data set of biochemical components (proteins, lipids, carbohydrates, glycogen) assessed separately in female (F) and male (M) of C. gallina during the sampling campaigns performed in 2001 and 2010 (samples within the circle).
3.4 Gametogenic cycle
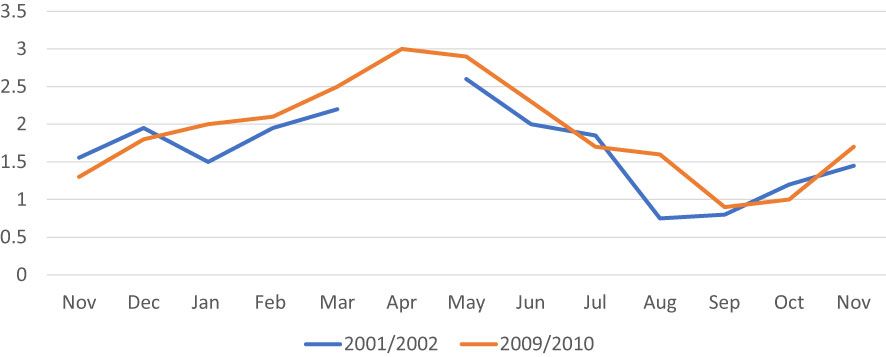
The general pattern of the gametogenic cycle appeared to be similar when comparing clams collected in 2001-2002 with those collected in 2009-2010 (Figure 8). In both surveys, from November to February most individuals were in the developing stages (I and II). From February onward, individuals at sexual maturity (stage III) started to be observed, peaking in April and May. Spawning (stage IV) occurred from May to July in 2002 and from May to August in 2010. Spent clams (stage V) were found in the period July – November in 2002, and in the period July – September in 2010. In 2002, the first resting organisms (stage 0) were also observed in July, whereas in 2010 this stage was shifted forward appearing in September. In October-November, most organisms were at early developing and late developing stages.

Figure 8 Stages of reproductive cycles of C. gallina collected in the 2001/2002 and 2009/2010. Percentages of clams corresponding to each gonad stage are identified by different bar patterns. Legend: 0, resting; I, early developing stage; II, late developing stage; III, ripe stage; IV, spawning stage; V, spent stage.
The monthly gonadal index, which briefly describes the maturation level reached by the population,
peaked in April and/or May in both 2002 and 2010, fitting the period of sexual maturity; in summer, it decreased sharply to reach minimum values in August 2002 and September 2010. From October onwards, the index rose, indicating the end of sexual rest and the gradual onset of gametogenesis (Figure 9).
3.5 Spearman correlation and PCA results
Spearman’s correlation coefficients between the different biological parameters and between measurements performed in clams and environmental parameters are shown in Tables 1 and 2. Generally, a higher number of significant correlations between biological parameters were observed in 2010 compared to 2001 (Table 1). Only occasionally, the correlations between biological parameters and the environmental variables were statistically significant (Table 2).
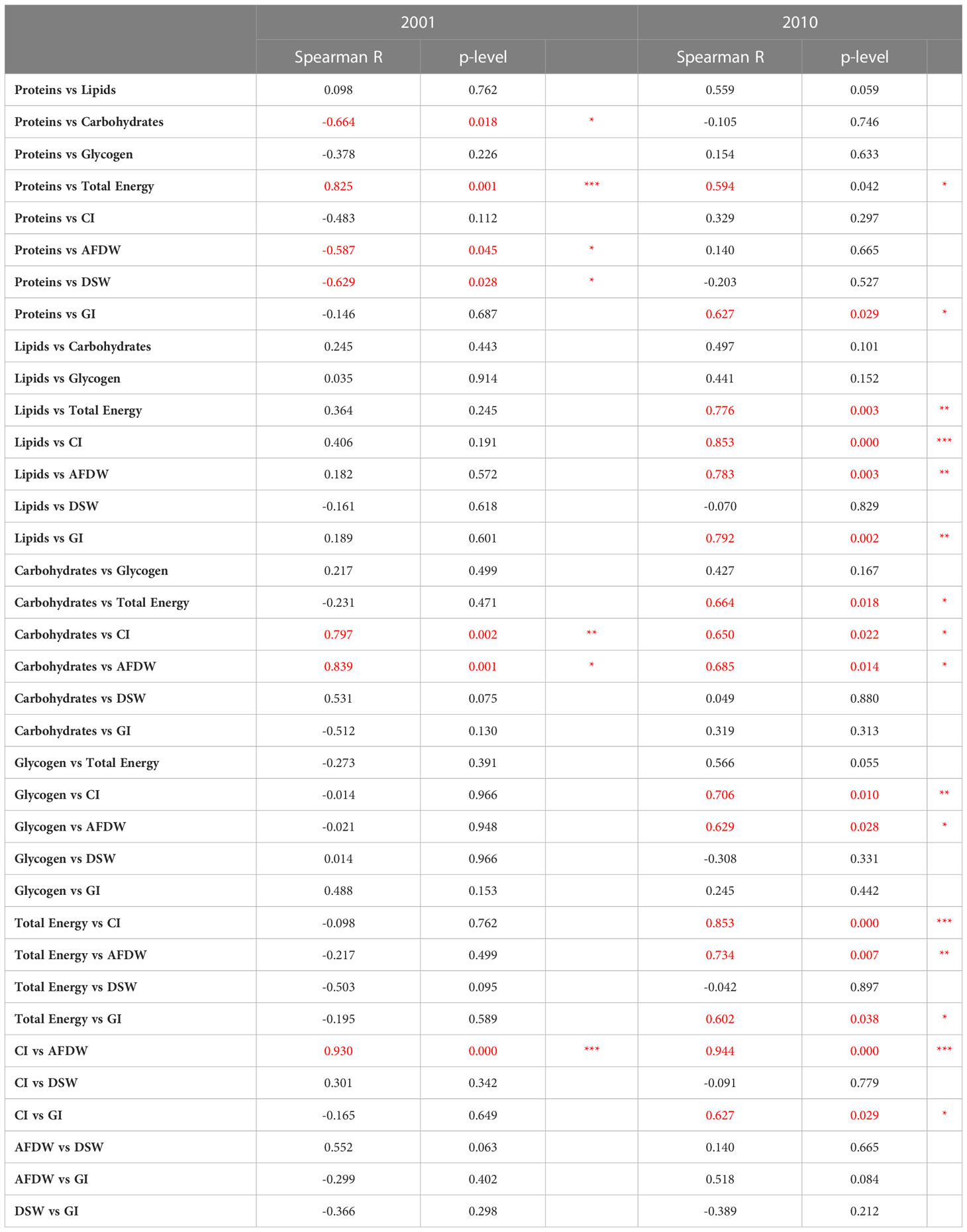
Table 1 Spearman’s correlation coefficients between the biological parameters evaluated in C. gallina in 2001 and 2010, and related significance (*p< 0.05; **p < 0.01; *** p<0.001 in red).
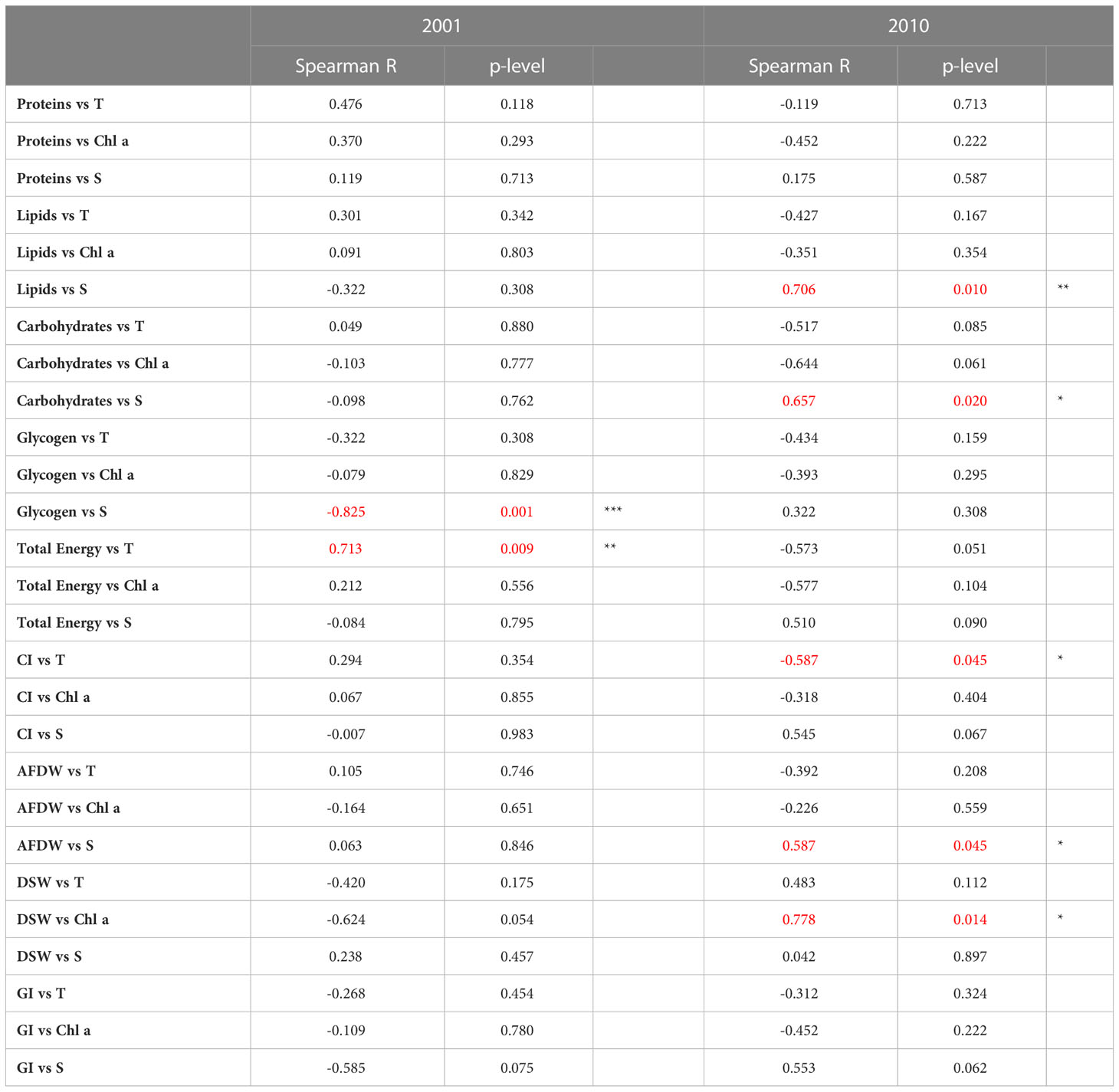
Table 2 Spearman’s correlation coefficients between the biological parameters evaluated in C. gallina and environmental variables detected in 2001 and 2010, and related significance (*p< 0.05; **p < 0.01; *** p<0.001 in red).
Using the multivariate analysis the whole dataset of monthly biological and environmental measurements was analyzed. Factors 1 and 2 explained over 67% of the variance; factor 1 explained 50,2% of the total variance and showed significant positive loadings of the variables lipids (0.74), carbohydrates (0.89), glycogen (0.89), total energy (0.92), CI (0.72), AFDW (0.89) and DSW (0.78); Factor 2 showed a significant negative loading of the variable salinity (-0.73) (Figure 10), this environmental variable contributing mainly to the variability of CI and lipids, based on a positive correlation. A quite clear spatial separation of the monthly surveys performed in 2001 and 2010 emerged, particularly as regards April and May 2010.
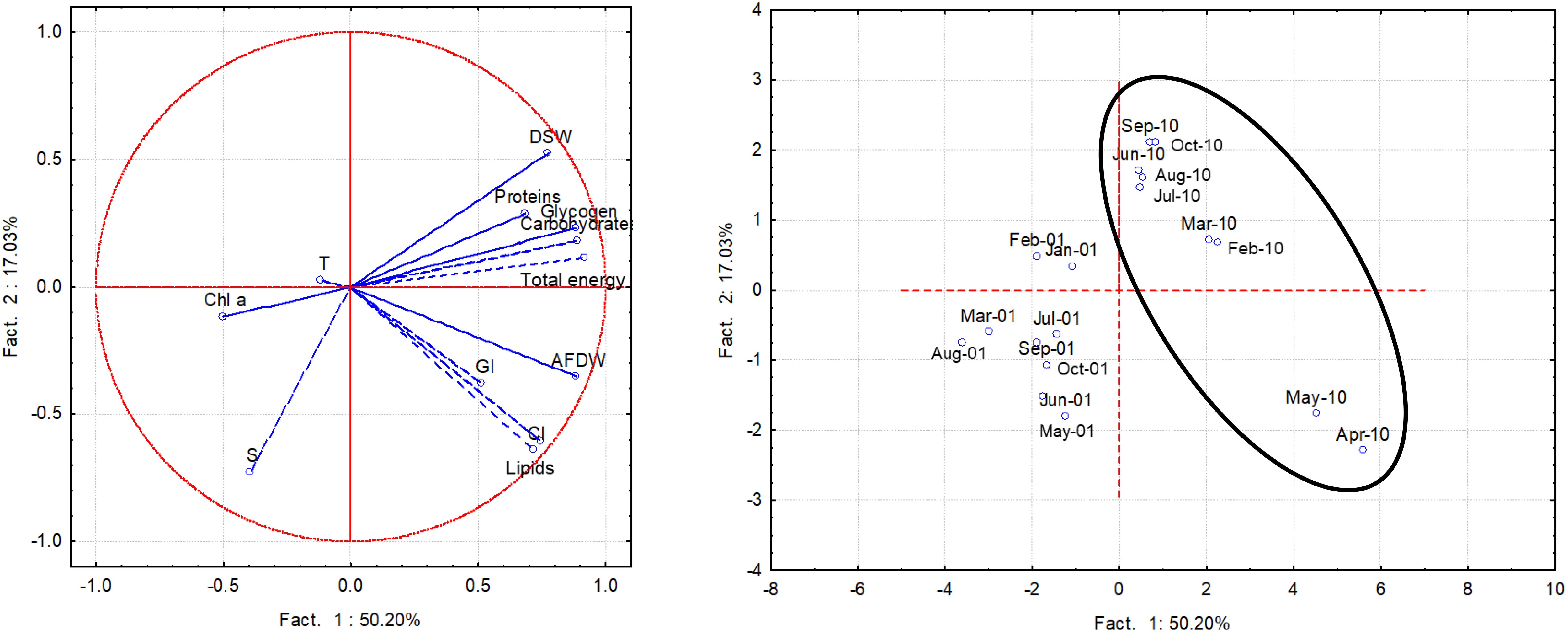
Figure 10 PCA performed with the whole data set of biochemical components, CI, AFDW/L, DSW/L, GI and environmental variables in 2001 and 2010 (samples within the circle).
4 Discussion
The biochemical composition of bivalves is typically characterized by periodic cycles of accumulation and utilization of the body reserves. In opportunistic species, such as the Venus clam C. gallina, gonadal development coincides with the period of maximum accumulation of energetic reserves, and the timing is mainly regulated by environmental parameters, in particular temperature and food supply (Bayne, 1976). Consequently, in temperate climates during spring, when phytoplankton availability is high enough, energy reserves increase and are then used for gonadal development and gametogenesis (Joaquim et al., 2014). Accumulated energy reserves are firstly spent for basic metabolism, any excess being then allocated to somatic growth of tissues and/or shell and to store glycogen, which is therefore available as an energy source for reproductive maturation (Bayne et al., 1982).
The study of the relationships between growth (both shell and meat), energy reserves and reproduction, and their interdependency with environmental variables are crucial to increase the knowledge of the physiological processes regulating the recruitment and survival of commercially exploited bivalve species. Some studies have already related changes in the biochemical composition of the Venus clam C. gallina with the reproductive cycle (Orban et al., 2006; Joaquim et al., 2014). The specific aim of the present study is to highlight differences in the physiological performance of clams monitored, ten years later, in the same fishing area of the northern Adriatic Sea, and to analyze them in the light of changing environmental conditions. Temporal trends and monthly mean values over the two one-year periods were generally similar for normalized AFDW, CI and lipid content. Total carbohydrates and glycogen showed similar trends, but were higher in 2010, whereas proteins and normalized DSW exhibited different patterns of variation and higher values in 2010. The main difference observed in the gametogenic cycle is the duration of the spawning stage, which was one month longer in 2010.
The environmental variables mostly influencing the general physiological condition of marine bivalves are temperature and food supply (Fernández-Reiriz et al., 2007), phytoplankton being their primary food source (Silva et al., 2021). For example, in laboratory experiments with the clam Ruditapes philippinarum, Fernández-Reiriz et al. (2007) observed that gonadal development and accumulation of reserves occurred both at low and high temperature conditions when the food was abundant, but they were faster and complete when clams were exposed to higher temperature.
In the present study, changes in water temperature and salinity, as well as in chlorophyll a and phytoplankton abundance were analyzed over the entire 10-year period, from January 2001 to December 2010. This approach allows us to better understand the influence of the temporal variation of environmental parameters on clam physiological performances. Over the 10-year period, an increase was observed for water temperature, mainly regarding the minimum values, and phytoplankton abundance. In particular, a constant increase in seawater temperature mean annual values was recorded, starting from 15.4°C in 2001 to 17.3 and 16.4°C in 2009 and 2010, respectively. An evident increase was also recorded for phytoplankton abundance mean values, starting from 3.1 million cells/l in 2002 (data are not available for 2001) to 5.2 million cells/l in 2010. The variation of these parameters could determine changes in the biochemical composition and reproductive performances of the clam C. gallina. Anacleto et al. (2014) clearly demonstrated that warming greatly affects the biochemical composition of two clam species (the native R. decussatus and the invasive R. philippinarum), particularly with regard to the fatty acid profile.
In this study, proteins are confirmed to be the main constituent of bivalve soft tissues, accounting for 65% of the biochemical components in both 2001 and 2010. Proteins can assume the role of energy providers during sexual maturation (Holland, 1978), functioning as structural and energy storage when glycogen levels are low (De Zwaan and Zandee, 1972; Pérez-Camacho et al., 2003). A negative significant correlation was observed between proteins and carbohydrates, meat and shell weights only in 2001, when phytoplankton was less abundant than in 2010. In this regard, when food supply and carbohydrates were lower, proteins could be used for soft tissue and shell growth. Proteins not only quantitatively constitute the largest fraction of soft tissues, but they are also used for shell formation. Shell matrix proteins are secreted by the mantle and then are exported to the shell matrix space (Addadi et al., 2006; Arivalagan et al., 2016). Moreover, the observed minimum in April 2001 could be related to the use of proteins in the gametogenic processes, in particular for oocyte production (Holland, 1978).
The overall lipid content in the clam C. gallina was low in comparison with carbohydrates and proteins, accounting for just 6% of the biochemical components in both 2001 and 2010. During the year 2001, lipid variability was quite low, with a slight increase recorded during the period of higher phytoplankton availability and when clams reached the maximum gonadal ripeness (from April to June), then a decrease occurred during spawning. In this year, no correlations were observed between lipids and the other analyzed parameters. A different situation was observed in 2010. Lipids started to increase from January, peaked in April and then decreased to values similar to those recorded in 2001. This trend was highly correlated with those of CI, normalized soft tissue weight and gonadal index. Lipids represent a nutritional reserve and are important components of bivalve oocytes (Holland, 1978; Beninger and Lucas, 1984). Lipids are synthetized de-novo during the gametogenesis, which takes place in spring for C. gallina, their maximum levels thus occurring in the pre-spawning period. The lipid content and trend of variation in 2010 were similar to those observed in C. gallina from the Algarve coast (southern Portugal), confirming a common behavior in temperate geographical areas when food supply is abundant (Joaquim et al., 2014).
In both 2001 and 2010, carbohydrate patterns were strongly related to those of glycogen, with glycogen accounting for 80% to 50% of total carbohydrates. These results indicated that, as already observed for other bivalve species (Marin et al., 2003; Pérez-Camacho et al., 2003; Matias et al., 2013; Silva et al., 2021), glycogen is the main polysaccharide in C. gallina and represents the main energy reserve in adult clams, being used during gametogenesis and in conditions of nutritional stress. Total carbohydrates and glycogen were always higher in 2010 than in 2001, with a mean increase of 26% for carbohydrates and 40% for glycogen. The highest carbohydrate and glycogen contents recorded in 2010 with respect to those in 2001 may be related to the increase in food availability observed through the 10-year period. Although Chl a content did not vary, the observed higher phytoplankton abundance is possibly related to the increased nanoplanktonic component and decreased presence of diatoms already reported in long-term studies in the northern Adriatic (Bernardi-Aubry et al., 2012; Mozetic et al., 2012; Flander-Putrle et al., 2022). This shift in the phytoplankton community can be beneficial to clams. Indeed, many bivalves are known to feed preferentially on nanophytoplankton (5-20 μm) size class (Shumway et al., 1985; Defossez and Hawkins, 1997).
The values of glycogen observed in this study are much higher than those recorded in C. gallina from the Algarve coast (southern Portugal) by Joaquim et al. (2014). This is probably due to the overall higher phytoplankton availability that characterized the North Adriatic, as evidenced by the differences in chlorophyll a recorded in the two geographical areas. Unlike monthly mean values, the pattern of variation in the two years was similar for both total carbohydrates and glycogen. In particular, both resources started to increase in March and peaked in April, when also phytoplankton concentrations increased. Then, they decreased throughout late spring and summer, as a consequence of maximum gonadal maturation and spawning. During the spent and resting periods, occurring from August to October, some glycogen reserves were maintained, probably reflecting joined conditions of reduced energy requirements and lower levels of food supply. Interestingly, carbohydrates and glycogen were the only biochemical components showing different accumulation in male and female clams. Higher values observed in males mostly during summer suggested that energy requirement for oogenesis is more relevant resulting in increased consumption of these resources. Another hypothesis may be that glycogen is to a greater extent accumulated in males, as also observed in R. decussatus, in which a high glycogen content was detected in sperms (Rodriguez- Moscoso and Arnaiz, 1998).
The normalized shell dry weight was always higher in 2010 samples, however the normalized ash-free dry weight of meat was higher only from January to May. Condition index is a widely used parameter in ecological and physiological studies, as it is a rapid and practical method to evaluate health status, growth, meat yield, sexual maturity and effects of environmental stressors in commercially exploited bivalves and other mollusk species (Zeng and Yang, 2021). As a gravimetric measurement of the body components, CI in bivalves is based on the ratio of meat to total shell masses (Lucas and Beninger, 1985). Compared to 2001, in 2010 shell and soft tissue weights did not increase to the same extent thus not resulting in a higher condition index, in particular from June onward. Changes in the relationship between meat and shell weights, such as those observed, provided indirect evidence of changes in the growth patterns. The constant increase of shell weight observed in 2010 samples suggested that higher water temperature could favor the production of carbonate shells, being less expensive than in colder waters. Previous studies on C. gallina from the Adriatic Sea reported that net calcification rates of shells increased along a latitudinal gradient, with increasing solar radiation and water temperature, both in immature and mature clams (Mancuso et al., 2019). In particular, the authors suggested that the increase in net calcification rates with increasing temperature could be due to lower energetic costs of shell formation in warmer waters where the aragonite saturation state is higher In addition, enhanced calcification rate led to higher shell density, mostly in immature clams (< 18 mm), and increased linear extension rate in mature clams (Mancuso et al., 2019). More dense shells could reduce vulnerability to predation, which largely affects juvenile clams (Arsenault and Himmelman, 1996). In the Northern Adriatic, predation on C. gallina has been poorly studied: among predators, the allochthonous carnivorous gastropod Rapana venosa showed increased numbers in the first decade of the 2000s (Savini et al., 2004). However, similar to predators, hydraulic dredging used to catch clams impacts on shell integrity of animals, more severely in small size ones. All undersized clams captured by fishing gear are sieved and then rejected into the sea, contributing to restock natural populations. Discarded animals can suffer physical damage incurred during dredging or be more exposed to predators or diseases (Moschino et al., 2003). As highlighted by Law (2000), fishing can cause phenotypic evolution in fished populations through the selection of traits, which enhance survival, growth and fecundity. Also for C. gallina, we can hypothesize that intense fishing acted as a selective driver favoring clams with denser and more resistant shells at the expense of shell elongation.
As for the reproductive conditions, slight differences were recorded when comparing the gonadal index and development stages in 2001/2002 and 2009/2010. In 2010, C. gallina showed a prolonged spawning period, from May to August, instead of from May to July. Moreover, in 2010 the spent stage was shorter and with lower percentages of individuals. This observation confirmed the previous hypothesis that higher food availability and glycogen content observed in 2010 provided clams with higher energy reserves to counteract stressful conditions. The results obtained in 2010 were similar to those of Bargione et al. (2021), which took into consideration samples performed in the period 2018-2019. The spawning period started in June and ended in August, and ripe gonads were first observed in March.
The PCA performed with the whole dataset summarized all the above observations. A clear-cut separation was highlighted comparing 2001 and 2010 samples, with a further separation for April and May 2010, mainly related to higher contents in lipids, total carbohydrates and glycogen, as well as to higher values of CI, normalized AFDW and DSW. In both years, accumulation of the reserves was shown to correlate with salinity, although not univocally. This finding suggested that variations in precipitation patterns due to changing climate conditions could markedly affect growth and reproduction of clams.
5 Conclusions
Under a global change scenario, awareness increases about the need to consider long-term trends of variations in physiological performances and well-being of commercially exploited species. In this context, biometric changes (in both shell and meat), energy accumulation and reproduction, and their relations, as well as interdependence with environmental variables were evaluated in two annual surveys performed 10 years apart in the clam C. gallina from the Northern Adriatic Sea. Our results indicated that better food supply and increased water temperature recorded from 2001 to 2010 resulted in higher reserve accumulation, mostly in the form of proteins, carbohydrates and glycogen. Since heavier shells were found in clams collected in 2010, an increase in calcification rate can be hypothesized. Moreover, the reproductive cycle showed a longer spawning period in 2010 compared with the previous observation. The overall results of this study highlighted relevant changes in the physiological traits of C. gallina in the Northern Adriatic that occurred in a relatively short time interval, thus representing a baseline for future research. Moreover, our study stressed the importance to monitor changes in both abiotic and biotic ecosystem components, evaluating their interrelations to unveil potential pros and cons for single populations of interest. This approach could provide useful insights for the management of commercially relevant resources.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VM and MM conceived and wrote the manuscript. VM, GR, TM, and FC collected and analyzed the samples. VM and TM performed the statistical analysis. LD and MM supervised the study and acquired the funding. All the authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the project CLODIA funded to M.G. Marin by the Veneto Region (Italy) Law 15/2007 (DGR no 4069).
Acknowledgments
The authors wish to thank Co.Ge.Vo. Chioggia (Consortium for the Management and Protection of Bivalve Mollusc Fishery) for the support in clam samplings. Special thanks go to Marco Marangon and the crew of the hydraulic dredge “Matteo”. We are also grateful to ARPAV and Dr Anna Rita Zogno for providing chlorophyll and phytoplankton abundance data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1158327/full#supplementary-material
References
Addadi L., Joester D., Nudelman F., Weiner S. (2006). Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem. Eur. J. 12, 980–987. doi: 10.1002/chem.200500980
Anacleto P., Maulvault A. L., Bandarra N. M., Repolho T., Nunes M. L., Rosa R., et al. (2014). Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res. Int. 64, 439–445. doi: 10.1016/j.foodres.2014.07.023
Arivalagan J., Yarra T., Marie B., Sleight V. A., Duvernois-Berthet E., Clark M. S., et al. (2016). Insights from the shell proteome: biomineralization to adaptation. Mol. Biol. Evol. 34 (1), 66–77. doi: 10.1093/molbev/msw219
Arsenault D. J., Himmelman J. H. (1996). Size-related changes in vulnerability to predators and spatial refuge use by juvenile Iceland scallops Chlamys islandica. mar. Ecol. Prog. Ser. 140, 115–122. doi: 10.3354/meps140115
Baeta M., Solís M. A., Ballesteros M., Defeo O. (2021). Long-term trends in striped venus clam (Chamelea gallina) fisheries in the western Mediterranean Sea: the case of ebro delta (NE Spain). Mar. Policy 134, 104798. doi: 10.1016/j.marpol.2021.104798
Bargione G., Donato F., Barone G., Virgili M., Penna P., Lucchetti A., et al. (2021). Chamelea gallina reproductive biology and minimum conservation reference size: implications for fishery management in the Adriatic Sea. BMC Zool 6, 32. doi: 10.1186/s40850-021-00096-4
Bayne B. L. (1976). “Aspects of reproduction in bivalve molluscs,” in Estuarine processes, vol. 1. (New York: Academic Press), 432–448.
Bayne B. L., Bubel A., Gabbott P. A., Livingstone D. R., Lowe D. M., Moore M. N. (1982). Glycogen utilisation and gametogenesis in Mytilus edulis l. Mar. Biol. Lett. 3, 89–105.
Beninger P. G., Lucas A. (1984). Seasonal variation in condition, reproductive activity and gross biochemical composition of two species of adult clam reared in a common habitat: Tapes decussatus l. (Jeffreys) and Tapes philippinarum (Adam and reeve). J. Exp. Mar. Biol. Ecol. 79, 19–37. doi: 10.1016/0022-0981(84)90028-5
Bernardi Aubry F., Cossarini G., Acri F., Bastianini M., Bianchi F., Camatti E., et al. (2012). Plankton communities in the northern Adriatic Sea: Patterns and changes over the last 30 years. Estuar. Coast. Shelf Sci. 115, 125–137.
Beukema J. J., De Bruin W. (1979). Calorific values of the soft parts of the tellinid bivalve Macoma balthica (L.) as determined by two methods. J. Exp. Mar. Biol. Ecol. 37, 19–30.
Brooks S. P. J., de Zwaan A., van den Thillart G., Cattani O., Cortesi P., Storey K. B. (1991). Differential survival of Venus gallina and Scapharca inaequivalvis during anoxic stress: covalent modifications of phosphofructokinase and glycogen phosphorylase during anoxia. J. Comp. Physiol. 161B, 207–212. doi: 10.1007/BF00262885
Defossez J.-M., Hawkins A. J. S. (1997). Selective feeding in shellfish: size-dependent rejection of large particles within pseudofaeces from Mytilus edulis, Ruditapes philippinarum and Tapes decussatus. Mar. Biol. 129, 139–147. doi: 10.1007/s002270050154
Delgado M., Silva L., Juárez A. (2013). Aspects of reproduction of striped venus Chamelea gallina in the gulf of cádiz (SW spain): implications for fishery management. Fish. Res. 146, 86–95. doi: 10.1016/j.fishres.2013.04.005
De Zwaan A., Zandee D. I. (1972). Body distribution and seasonal changes in glycogen content of the common sea mussel Mytilus edulis. Comp. Biochem. Physiol. 43, 53–58. doi: 10.1016/0300-9629(72)90468-9
DGPEMAC (2019). The national management plan for fishing with hydraulic dredges and boat-operated shell-rakes as identified in the classification of fishing equipment use by mechanical dredges including mechanized dredges (HMD) and boat dredges (DRB) (Rome, Italy: Ministry for Agricultural, Food and Forestry Policies (MiPAAF).
Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Fernández-Reiriz M. J., Pérez-Camacho A., Delgado M., Labarta U. (2007). Dynamics of biochemical components, lipid classes and energy values on gonadal development of R. philippinarum associated with the temperature and ingestion rate. Comp. Biochem. Physiol. 147, 1053–1059. doi: 10.1016/j.cbpa.2007.03.018
Flander-Putrle V., Francé J., Mozetic P. (2022). Phytoplankton pigments reveal size structure and interannual variability of the coastal phytoplankton community (Adriatic Sea). Water 14, 23. doi: 10.3390/w14010023
Furlan E., Torresan S., Critto A., Lovato T., Solidoro C., Lazzari P., et al. (2019). Cumulative impact index for the Adriatic Sea: accounting for interactions among climate and anthropogenic pressures. Sci. Total Environ. 670, 379–397. doi: 10.1016/j.scitotenv.2019.03.021
Holland D. L. (1978). “Lipid reserves and energy metabolism in the larvae of benthic marine invertebrates,” in Biochemical and biophysical perspectives in marine biology. Eds. Malins P. L., Sargent J. R. (London: Academic Press), 85–123.
ICRAM-ANPA (2001). Ministero dell'Ambiente e della tutela del territorio – servizio difesa mare. programma di monitoraggio per il controllo dell'ambiente marino-costiero (triennio 2001-2003). metodologie analitiche di riferimento (ICRAM - ANPA). (Rome, Italy), 122 pp.
Joaquim S., Matias D., Matias A. M., Moura P., Roque C., Chícharo L., et al. (2014). Biochemical and energy dynamics throughout the reproductive cycle of the striped venus Chamelea gallina (Mollusca, bivalvia). Invertebr. Reprod. Dev. 58, 284–293. doi: 10.1080/07924259.2014.921646
Law R. (2000). Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 57, 659–669. doi: 10.1006/jmsc.2000.0731
Lowry O. H., Rosebrough N. J., Lewis Farr A., Randall R. J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. doi: 10.1016/S0021-9258(19)52451-6
Lucas A., Beninger P. G. (1985). The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44, 187–200. doi: 10.1016/0044-8486(85)90243-1
Mancuso A., Stagioni M., Prada F., Scarponi D., Piccinetti C., Goffredo S. (2019). Environmental influence on calcification of the bivalve Chamelea gallina along a latitudinal gradient in the Adriatic Sea. Sci. Rep. 9, 11198. doi: 10.1038/s41598-019-47538-1
Mann R., Gallagher S. M. (1985). Physiological and biochemical energetics of larvae of Teredo navalis l. and Bankia gouldi (Bartsch) (Bivalvia: teredinidae). J. Exp. Mar. Biol. Ecol. 85, 211–2228. doi: 10.1016/0022-0981(85)90159-5
Marin M. G., Moschino V., Deppieri M., Lucchetta L. (2003). Variations in gross biochemical composition, energy value and condition index of Tapes philippinarum from the lagoon of Venice. Aquaculture 219, 859–871. doi: 10.1016/S0044-8486(03)00035-8
Matias D., Joaquim S., Matias A. M., Moura P., Sousa J. T., Sobral P., et al. (2013). The reproductive cycle of the European clam Ruditapes decussatus (L. 1758) in two Portuguese populations: implications for management and aquaculture programs. Aquaculture 406–407, 52–61. doi: 10.1016/j.aquaculture.2013.04.030
Milan M., Smits M., Dalla Rovere G., Iori S., Zampieri A., Carraro L., et al. (2019). Host-microbiota interactions shed light on mortality events in the striped venus clam Chamelea gallina. Mol. Ecol. 28 (19), 4486–4499. doi: 10.1111/mec.15227
Moschino V., Deppieri M., Marin M. G. (2003). Evaluation of shell damage to the clam Chamelea gallina captured by hydraulic dredging in the Northern Adriatic Sea. ICES J. Mar. Sci.60, 393–401.
Moschino V., Marin M. G. (2006). Seasonal changes in physiological responses and evaluation of “well-being” in the Venus clam Chamelea gallina from the northern Adriatic Sea. Comp. Biochem. Physiol. 145, 433–440. doi: 10.1016/j.cbpa.2006.07.021
Mozetic P., Francé J., Kogovsek T., Talaber I., Malej A. (2012). Plankton trends and community changes in a coastal sea (northern adriatic): bottom-up vs. top-down control in relation to environmental drivers. Estuar. Coast. Shelf S. 115, 138–148. doi: 10.1016/j.ecss.2012.02.009
Orban E., Lena G., Nevigato T., Casini I., Caproni R., Santaroni G., et al. (2006). Nutritional and commercial quality of the striped venus clam, Chamelea gallina, from the Adriatic sea. Food Chem. 101, 1063–1070. doi: 10.1016/j.foodchem.2006.03.005
Oztürk R.Ç., Altınok I. (2021). Genetic population structure of the striped venus clam chamelea gallina across its range. Fish. Res. 234, 105758. doi: 10.1016/j.fishres.2020.105758
Paine R. T. (1971). The measurement and application of the calorie to ecological problems. Annu. Rev. Ecol. Syst. 2, 145–164. doi: 10.1146/annurev.es.02.110171.001045
Pande S. V., Parvin Khan R., Venkitasubramanian T. A. (1963). Microdetermination of lipids and serum total fatty acids. Anal. Biochem. 6, 415–423. doi: 10.1016/0003-2697(63)90094-0
Papetti C., Schiavon L., Milan M., Lucassen M., Caccavo J. A., Paterno M., et al. (2018). Genetic variability of the striped venus Chamelea gallina in the northern Adriatic Sea. Fish. Res. 201, 68–78. doi: 10.1016/j.fishres.2018.01.006
Parsons T. R., Maita Y., Lalli M. (1984). A manual of chemical and biological methods for seawater analysis (Elmsford, New York: Pergamon Press), 173.
Pérez-Camacho A., Delgado M., Fernández-Reiríz M. J., Labarta U. (2003). Energy balance, gonad development and biochemical composition in the clam Ruditapes decussatus (L.). Mar. Ecol. Prog. Ser. 258, 133–145. doi: 10.3354/meps258133
Ramón M., Richardson C. A. (1992). Age determination and shell growth of Chamelea gallina (Bivalvia: veneridae) in the western Mediterranean. Mar. Ecol. Prog. Ser. 89, 15–23. doi: 10.3354/meps089015
Rodriguez- Moscoso E., Arnaiz R. (1998). Gametogenesis and energy storage in a population of the grooved carpet-shell clam, Tapes decussatus (Linnè, 1787), in north-west Spain. Aquaculture 162, 291–299. doi: 10.1016/S0044-8486(98)00170-7
Romanelli M., Cordisco C. A., Giovanardi O. (2009). The long-term decline of the Chamelea gallina L.(Bivalvia: veneridae) clam fishery in the Adriatic Sea: is a synthesis possible. Acta Adriat. 50, 171–205.
Savini D., Castellazzi M., Favruzzo M., Occhipinti-Ambrogi A. (2004). The alien mollusc Rapana venosa (Valenciennes 1846; Gastropoda, muricidae) in the northern Adriatic Sea: population structure and shell morphology. Chem. Ecol. 20, S411–S424. doi: 10.1080/02757540310001629242
Seed R. (1980). Reproduction and growth in Anomia ephippium (L.) (Bivalvia, anomiidae) in strangford lough, northern Ireland. J. Conch. 30, 239–245.
Shumway S. E., Cucci T. L., Newell R. C., Yentsch C. M. (1985). Particle selection, ingestion and absorption in the filter feeding bivalves. J. Exp. Mar. Biol. Ecol. 91, 77–92. doi: 10.1016/0022-0981(85)90222-9
Silva D. C. C., Neto J. M., Nunes C., Gonçalves F. J. M., Coimbra M. A., Marques J. C., et al. (2021). Assessment of seasonal and spatial variations in the nutritional content of six edible marine bivalve species by the response of a set of integrated biomarkers. Ecol. Indic. 124, 107378. doi: 10.1016/j.ecolind.2021.107378
Stella P., Rodinò E. (1986). Ricerche sulla variabilità genetica del bivalve Chamelea (Venus) gallina (L.). Atti Ist. Veneto Sci. Lett. Arti, Cl. Sci. Fis. Mat. Nat 144, 49–62.
Valli G., Mazzolini D., Raimondi V. (1996). Ciclo riproduttivo e biometria in Tapes philippinarum (Adams and reeve 1850) dell’Alto adriatico durante un ciclo annuale. Ann. Hydrores 12 (13), 41–53.
Walne P. R., Mann R. (1975). “Growth and biochemical composition in ostrea edulis and crassostrea gigas,” in Proc. 9th eur. mar. biol. symp. Ed. Barnes H. (Aberdeen: Aberdeen Univ. Press), 587–607.
Keywords: Chamelea gallina, energy reserves, physiological performances, gross biochemical components, bivalves, gonadal and condition indices
Citation: Moschino V, Rizzo G, Marĉeta T, Cernigai F, Masiero L, Da Ros L and Marin MG (2023) Biochemical composition and reproductive cycle of the clam Chamelea gallina in the Northern Adriatic Sea: an after-10-year comparison of patterns and changes. Front. Mar. Sci. 10:1158327. doi: 10.3389/fmars.2023.1158327
Received: 03 February 2023; Accepted: 15 May 2023;
Published: 26 May 2023.
Edited by:
Nazli Demirel, Istanbul University, TürkiyeCopyright © 2023 Moschino, Rizzo, Marĉeta, Cernigai, Masiero, Da Ros and Marin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Gabriella Marin, bWdtYXJAYmlvLnVuaXBkLml0
 Vanessa Moschino
Vanessa Moschino Giulia Rizzo2
Giulia Rizzo2 Maria Gabriella Marin
Maria Gabriella Marin