- 1South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Fishery Ecology and Environment, Guangzhou, China
- 3Sanya Tropical Fisheries Research Institute, Sanya, China
The favorable natural conditions and variety of habitats in the Beibu Gulf provide a basis for harboring a high diversity of marine organisms. Sustainable coastal ecosystem management can be benefited from a comprehensive assessment of species diversity. In this study, we analyzed the seasonal changes in nektonic phylogenetic and community structures in the waters of Weizhou Island in the northern Beibu Gulf. The results showed that both the nektonic phylogenetic diversity and community structure in the northern Beibu Gulf exhibited strong seasonal differences between spring and autumn. The catch density was 291.9 kg per km2 in spring and 1081.1 kg per km2 in autumn. Phylogenetic diversity of nektonic communities obviously increased from spring to autumn, while phylogenetic patterns changed from clustering to overdispersion. The seasonal patterns of nektonic communities were mainly related to the different fishing intensities in spring and autumn. Summer fishing intensity in the Beibu Gulf was effectively controlled by a mid-summer fishing moratorium, during which nektonic diversity and fish stocks rapidly recovered from the larval pool. Our study revealed that fishing intensity had a greater impact on nektonic communities at smaller spatial scales, and even exceeded the effects of environmental factors.
Introduction
Studying species composition dynamics is key to understanding community ecology. Clarifying the causes of temporal and spatial variation in species composition has long been a major issue for ecologists (Palmer, 1994; Vellend and Agrawal, 2010). In the last 30 years, there have been two main theories regarding the formation of species composition and biodiversity of a local community. One is the niche-based theory, which emphasizes environmental filtration as a main driving force of community structuring. Environmental filtration includes the effects of the abiotic environment on organisms and the interactions between organisms; that is, environmental selection (Chase and Leibold, 2003). The other theory is the community neutral theory (Hubbell, 2001), which is a generalization of neutral theory of molecular evolution at the macro level (Kimura, 1968). The community neutral theory ignores interspecific difference or assumes that interspecific difference has little effect on species coexistence, and instead emphasizes the influence of stochastic processes on the formation of community composition (Hubbell, 2005). The balance between assembly and dispersal of ecological communities is caused by ecological drift; this is the core of the community neutral theory, similar to genetic drift in population genetics (Bell, 2001).
Ecologists have recognized that niche-based theory and community neutral theory are not diametrically opposed, and a community is likely determined by the interplay of the two processes (Tilman, 2004; Gewin, 2006; Adler et al., 2007). However, the species composition of a community does not sufficiently reflect the pattern of diversity and assembly process of the community. For example, although two communities may have the same number of species, their evolutionary origins and community construction processes may be completely different.
Since 2000, it has become increasingly common to use phylogenetic relatedness to investigate the origin and history of species within a community and to understand the influence of ecological and historical factors on assembly process of the community (Webb, 2000; Cavender-Bares et al., 2009; Mouquet et al., 2012). Given known phylogenetic relatedness and rates of evolution of functional traits, different patterns of phylogenetic community structure are expected depending on whether competitive exclusion or environmental filtering was the primary driver that influenced community historical assembly (Webb et al., 2002). If competitive exclusion drives community assembly, we expect phylogenetic overdispersion because closely related species tend to compete more intensely for the same resources than distantly related species. Conversely, if environmental filtering plays a driving role in community assembly, selection caused by environmental filtration often results in a higher degree of co-occurrence of closely related species, leading to phylogenetic clustering (Weiher and Keddy, 1999; Cavender-Bares et al., 2009).
Recently, several examples from different ecosystems worldwide have extended the use of phylogenetic approaches to investigate patterns in community structure. D’agata et al. (2014) reported that anthropogenic activities have significantly reduced the functional and phylogenetic diversity of a crucial fish family (Scaridae and Chaetodontidae) in the coral reefs of Pacific. Winter et al. (2009) analyzed the large-scale effects of native species extinctions and the introduction of alien species on taxonomic and phylogenetic diversity of floras across Europe; their findings revealed that plant invasions exceeded extinctions in the last 500 years. Jiang et al. (2019) revealed that the influences of human activities since 1960 were sufficient to obscure the phylogenetic distinctiveness in the nearly 5 million years of evolutionary history of freshwater fish from isolated plateau lakes in southwestern China. Xu et al. (2021a) studied the nektonic communities of two bays in the South China Sea; they demonstrated that differentiation of spatial and temporal patterns in both phylogenetic and community structure was primarily related to stochastic processes in structuring nektonic communities and fishing intensity differences between the two bays.
The Beibu Gulf (17°–21°45′N, 105°–110°10′E) (as known as the Gulf of Tonkin) is in the northwest of the South China Sea and has a long coastline that belongs to both Vietnam and China. As a natural semi-closed gulf, the Beibu Gulf covers a water area of approximately 128,000 km2 with an average water depth approximately 39 m and a maximum of 100 m. It extends from the Hainan Island and Leizhou Peninsula in the east to the coast of northern Vietnam in the west, and reaches the coast of Guangxi Zhuang Autonomous Region in the north (Xu et al., 2021b). The climate around the Beibu Gulf is tropical subtropical monsoon climate, moving northeast in spring and southwest in autumn; this transports warm sea water through ocean currents and circulation throughout the year. Moreover, the Beibu Gulf has a flat seabed and diverse habitats, including coral reefs, mangrove forests, and numerous estuaries, such as Nanliu River in China and Red River in Vietnam, from which rivers discharge sufficient nutrients (Ma et al., 2010). These favorable natural conditions and variety of habitats provide a basis for harboring a high diversity of marine organisms, and this gulf attracts thousands of species of fish to feed, breed, and spawn.
According to a previous report, more than 900 fish species that belong to 475 genera and 162 families inhabit the Beibu Gulf (Ma et al., 2008; Zhang et al., 2022). The Beibu Gulf is one of the most diverse and productive water-based ecosystems in the South China Sea. More than 60 commercially important fish, squid, and shrimp taxa support substantial fishing efforts, such as conger pike (Muraenesox cinereus), round scad (Decapterus maruadsi), hairtail (Trichiurus lepturus), blood snapper (Lutjanus sanguineus), Japanese horse mackerel (Trachurus japonicus), and longtail tuna (Thunnus tonggol) (Qiu et al., 2010; Wang et al., 2012). Consequently, the Beibu Gulf is one of the four famous fishing grounds in China, and it is important for the livelihoods of millions of fishermen along the coasts of China and Vietnam, and plays an increasingly important role in employment, food security, and the local economy (Chen et al., 2009). However, with the rapid increase in the number of marine fishing vessels in the Beibu Gulf since the 1970s, catch rates and fishing efforts have rapidly increased.
Fish community structure has been transformed from high-value species to lower-value species; for example, large yellow croaker (Larimichthys crocea) and crimson snapper (Lutjanus erythropterus) were replaced by finespot goby (Chaeturichthys stigmatias)(Wang et al., 2012; Zhang et al., 2022). Abrupt decreases in fish density occurred in 1993 and 1998, and overexploitation eventually depleted fisheries stocks in the late 1990s, especially those of demersal species. In addition to overexploitation, various other anthropogenic activities, such as coastal mangrove damage, illegal fishing, mariculture pollution, and habitat degradation, also pose substantial threats to the fish stocks and marine ecosystem of the Beibu Gulf (Qiu et al., 2010; Shen and Heino, 2014; Wang et al., 2019). Previous studies on the fishery resources and coastal environment of the Beibu Gulf mainly focused on the species diversity, quantity, population dynamic change, and spatiotemporal distribution of fishery resources (Chen et al., 2009; Wang et al., 2012; Wang et al., 2019; Hou et al., 2022; Tian et al., 2022).
A comprehensive assessment of species diversity is urgently needed to avoid permanent damage to ecosystems and the loss of valuable marine resources. Very little is known about the phylogenetic relationships among nektonic species of the Beibu Gulf, and the temporal distribution of phylogenetic diversity remains unclear. Therefore, the specific purposes of this study were to investigate nektonic community seasonal variation and factors affecting their phylogenetic diversity in the Beibu Gulf. We hypothesized that there would be obvious temporal variation of nektonic community phylogenetic diversity and composition in the Beibu Gulf.
Materials and methods
Study area
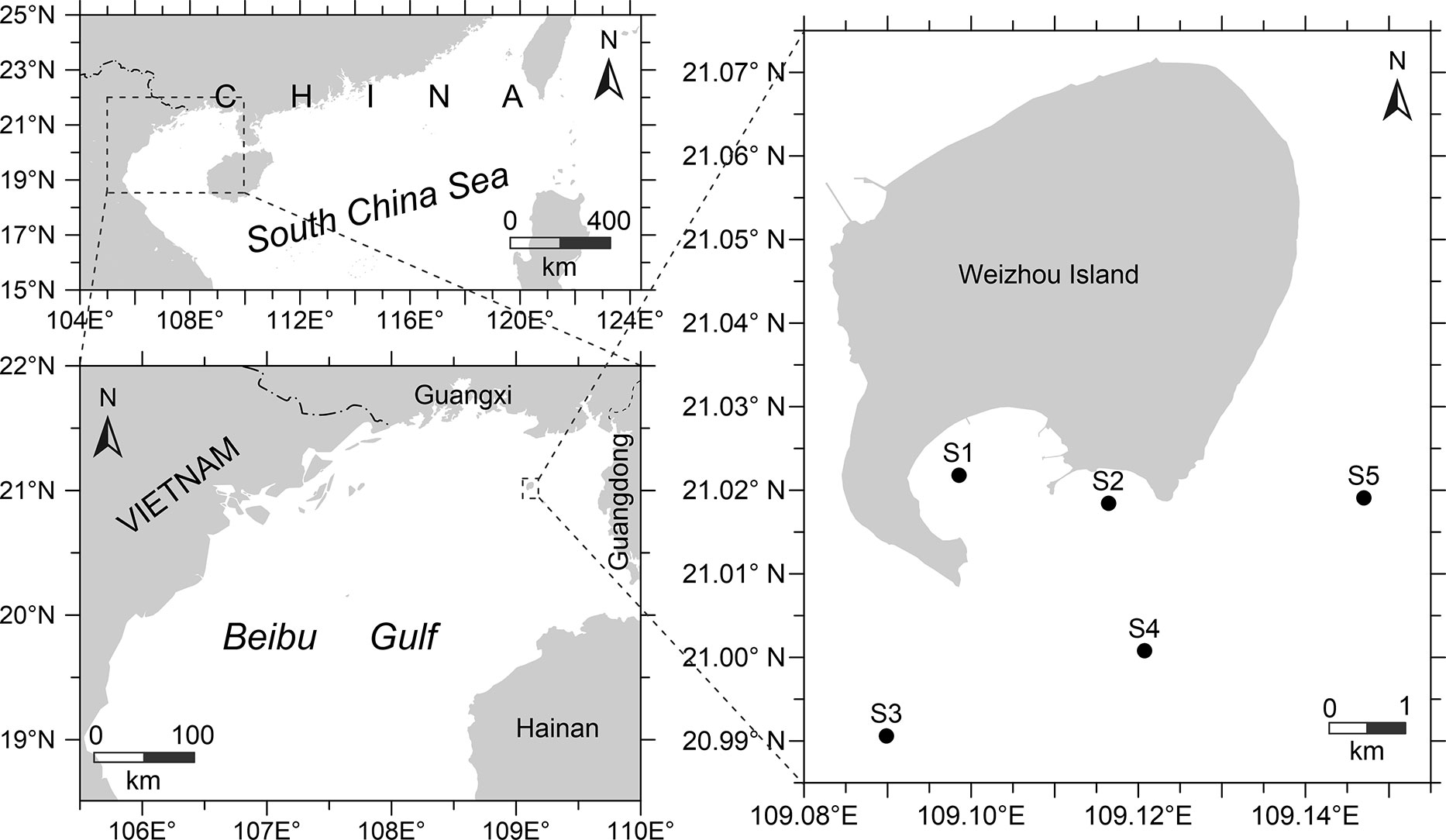
Weizhou Island (21°–21°5′N, 109°–109°10′E) is in the northern part of the Beibu Gulf, approximately 36 miles from the mainland. Weizhou Island is the youngest and biggest island in the Beibu Gulf. It is an inhabited volcanic island that covers an area of approximately 25 km2. The annual average water surface temperature is about 24°C, and ranges from 19°C to 30°C. The annual average seawater salinity is 32‰, pH of seawater ranges from 8.0 to 8.23, and seawater transparency ranges from 3 m to 10 m (Yu et al., 2019). Weizhou Island is the northernmost coral reef ecosystem in the South China Sea. It is also a popular tourist destination, attracting approximately 600,000 visitors annually in recent years, and it is heavily influenced by anthropogenic activities (Yu, 2012; Chen et al., 2013).
Sample collection
Two fishery surveys were conducted in the waters of Weizhou Island in the northern Beibu Gulf by the South China Sea Fisheries Research Institute in the spring (April 8–10) and autumn (September 22–24) of 2022. Nektonic communities were collected by the 135 kW commercial fishing vessel “Haiyu60087” using bottom trawl nets and pair trawl nets from five sampling sites in this area. At each sampling site, the trawl was towed for 30 min at an average speed of 3 kn (Figure 1).
The following data were recorded for each trawl: speed, GPS position, towing distance, depth and duration time. All collected samples were first identified to species by morphology, and then weighed and counted. Species identification and nomenclature follow the previous taxonomic literature and available documented diagnostic morphological characters for marine species from FishBase (http://www.fishbase.org). Conductivity, water temperature, pH, and dissolved oxygen were determined during the surveys using a handheld multiparameter meter (YSI Pro Plus). Water samples were taken with a 5-L plexiglass deep-water sampler from the bottom layer at each site for analysis of chlorophyll a.
Phylogenetic tree
For each identified species, we downloaded the sequences of cytochrome c oxidase subunit 1 gene from public database (GenBank or BOLD, see Table S1). All downloaded sequences were assembled and examined in BioEdit, and aligned under default options (Hall, 1999). Bayesian inference was used to reconstruct the phylogenetic tree with the COI dataset in BEAST 1.8 (Drummond et al., 2012). The BEAST parameters were set in BEAUti 1.8, and the sequences were further manually edited. A generalized time reversible substitution model with gamma distribution was set for the entire aligned sequence dataset. An uncorrelated relaxed molecular clock and a coalescent model with a constant population size were selected. Markov chain Monte Carlo (MCMC) chains were run for 200,000,000 iterations. The maximum clade credibility consensus tree was constructed in TreeAnnotator 1.8, with the first million generations discarded as burn-in. FigTree 1.4.0 was used to display the final consensus tree.
Statistical analysis
Catch density (kg·km−2) of each site was expressed as catch rate (catch per unit effort), according to Sparre and Venema (1998). Seasonal changes in the nekton species composition and community structure were analyzed by principal coordination analysis (PCoA) based on Bray–Curtis distance (Legendre and Anderson, 1999). We used the abundance data to analyze species composition and community structure. The differences of species composition and community structure between autumn and spring were assessed by permutational multivariate analysis of variance (Per-MANOVA) also based on Bray–Curtis distance (Anderson, 2017). The level of statistical significance was set to P<0.05. PCoA and Per-MANOVA were performed with R statistical software R 4.0 (R Development Core Team, 2021) using the cmdscale and adonis functions in the package of vegan (Oksanen et al., 2019).
Phylogenetic structures of the nektonic communities in the waters of Weizhou Island were separately analyzed for spring and autumn dataset. First, we calculated the phylogenetic diversity of nektonic communities in the waters of Weizhou Island and examined the differences between the two seasons. Then, the net relatedness index (NRI) was used as a standardized index to evaluate the average phylogenetic distance between pairs of taxa in the sample. This index was used to estimate the overall phylogenetic relatedness of a nektonic community, and quantify the total clustering of taxa in a phylogenetic tree. Positive NRI values indicate phylogenetic relatedness exhibiting clustering, whereas negative values indicate phylogenetic relatedness exhibiting overdispersion (Faith, 1992; Webb, 2000; Webb et al., 2002).
Two indices were used to evaluate the variation of phylogenetic diversity at different phylogenetic levels. Mean pairwise distance (MPD) and mean nearest taxon distance (MNTD) represented the average pairwise distances of all taxa in the phylogenetic tree and the average distance between each taxon and its most closely related terminal taxon in the phylogenetic tree, respectively. Then, we used nonmetric multidimensional scaling ordination (nMDS) to reveal the differences in phylogenetic diversity among the sampling sites. For phylogenetic beta diversity (phylobeta diversity), we used mean phylogenetic dissimilarity between species (Dpw) in pairs of sampling sites and mean nearest taxon distance (Dnn) between the two sampling sites to capture deep and shallow phylogenetic variation, respectively (Webb et al., 2002; Swenson, 2011). Multiple regression on distance matrices (MRM) was used to reveal the effects of each environmental variable on both phylogenetic beta diversity indices (Dpw and Dnn) of the nektonic communities in the waters of Weizhou Island (Lichstein, 2007). All of the above analyses were carried out in R 4.0 with the packages splits, vegan, ape, picante, nlme, and ecodist (Goslee and Urban, 2007; Ezard et al., 2009; Kembel et al., 2010; Oksanen et al., 2019; R Development Core Team, 2021).
Results
Species composition and community structure
A total of 102 nektonic species were identified in our samples from the waters of Weizhou Island in the northern Beibu Gulf, including 8 cephalopods, 31 crustaceans, and 63 fish species, respectively. In the spring survey, we identified 6, 17, and 31 species associated with cephalopods, crustaceans, and fish, respectively; in the autumn survey, we identified 7, 21, and 31 species associated with cephalopods, crustaceans, and fish, respectively.
Fish, crustacean, and cephalopod catch densities of each sampling site in the waters of Weizhou Island are shown in Table S2. Maximum total catch density of Weizhou Island was found at S5 in the autumn survey (2346.81 kg·km−2), whereas minimum total catch density was found at S2 in the spring survey (169.75 kg·km−2). The total catch density as well as densities of fish and invertebrates in the autumn survey were significantly higher than those in the spring survey (Figure 2). The PCoA showed that all the samples in autumn were well separated from those in spring (Figure 3). The Per-MANOVA test showed that species composition and community structure were both significantly different between autumn and spring (p <0.05) (Table 1).

Table 1 The statistics of the Per-MANOVA test based on the Bray-Curtis distances of community structure between autumn and spring.
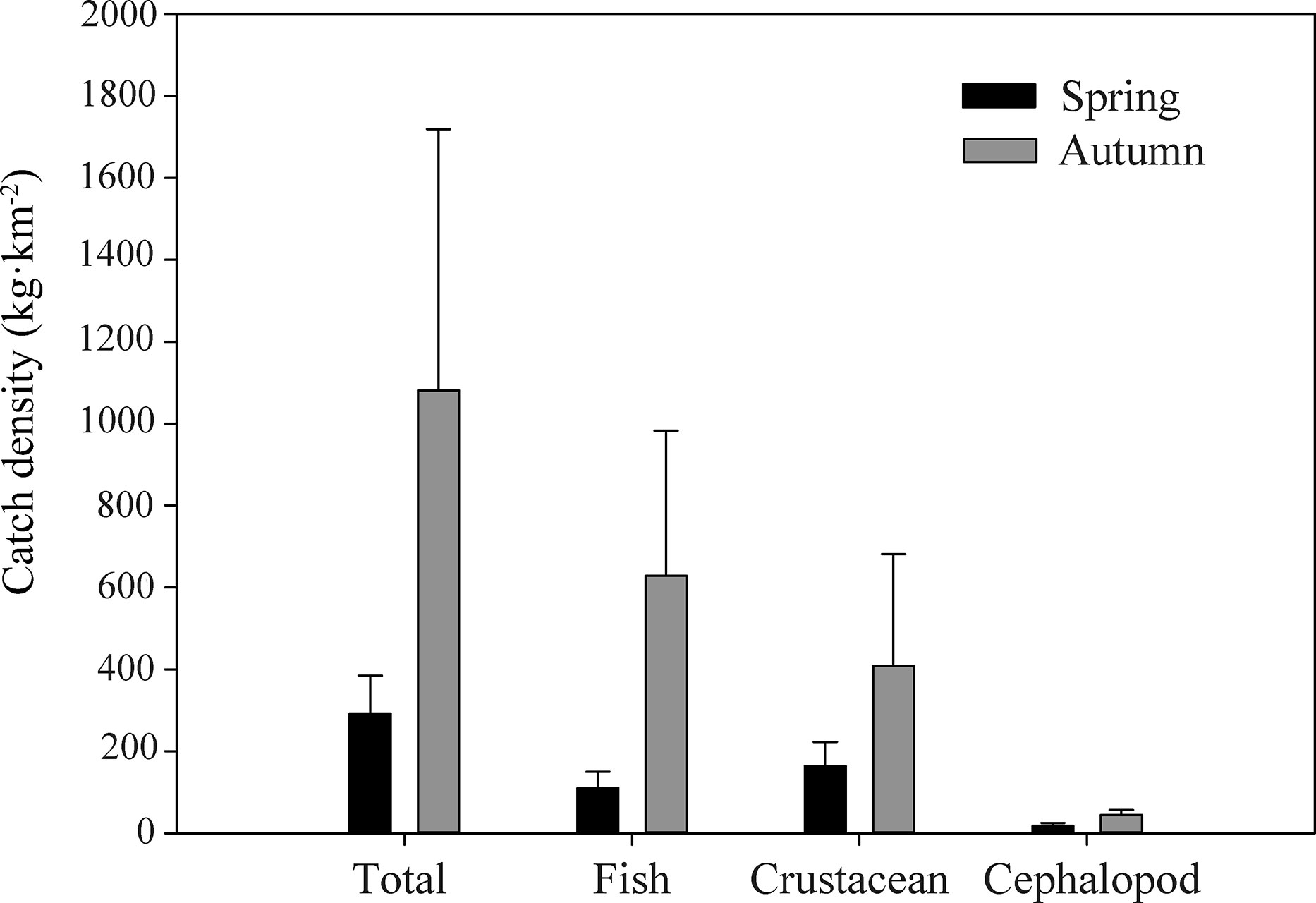
Figure 2 Comparison of catch density of fish and benthic invertebrates in Weizhou Island of Beibu Gulf in spring and autumn.
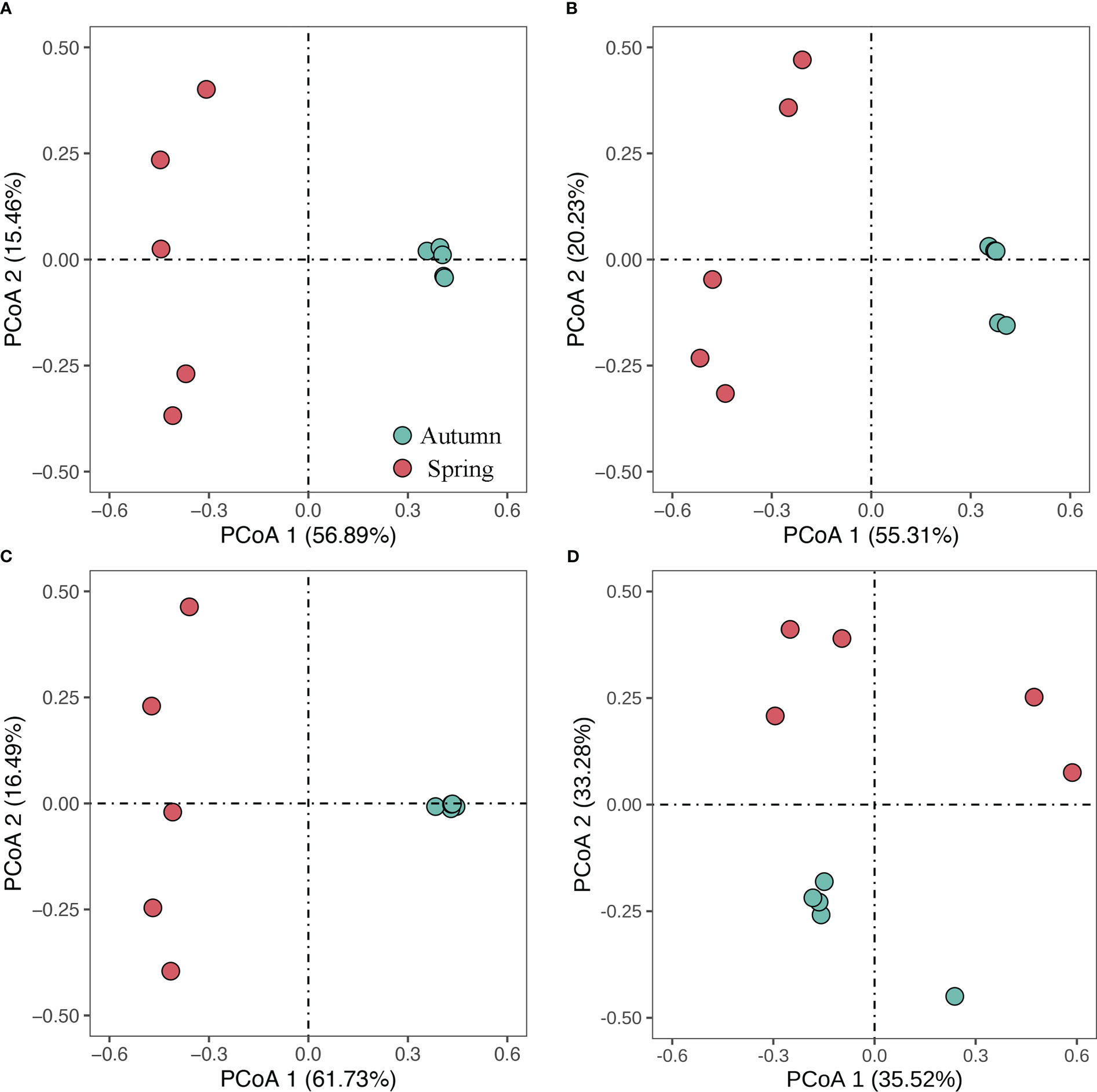
Figure 3 Biplot of principle coordination analysis showing the Bray-Curtis distance of community structure of (A) all species, (B) fish, (C) crustacean and (D) cephalopod between spring and autumn.
Phylogenetic diversity
The phylogenetic tree represented by all sample species collected in this study is shown in Supplementary Figures S1, S2. The average phylogenetic diversity of spring nektonic communities was 2.1, but reached 2.9 in autumn nektonic communities. There were clear significant differences in phylogenetic diversity between spring and autumn nektonic communities (p < 0.001); (Figure 4A). Positive NRI values in spring nektonic communities indicated phylogenetic clustering, whereas negative NRI values in autumn nektonic communities indicated phylogenetic overdispersion (Figure 4B). Nonmetric multidimensional scaling detected obvious ordination differentiations of phylogenetic alpha diversity at deep and shallow phylogenetic levels (MPD and MNTD) in the nektonic communities of Weizhou Island (Figure 5), showing a clear trend of seasonal separation.
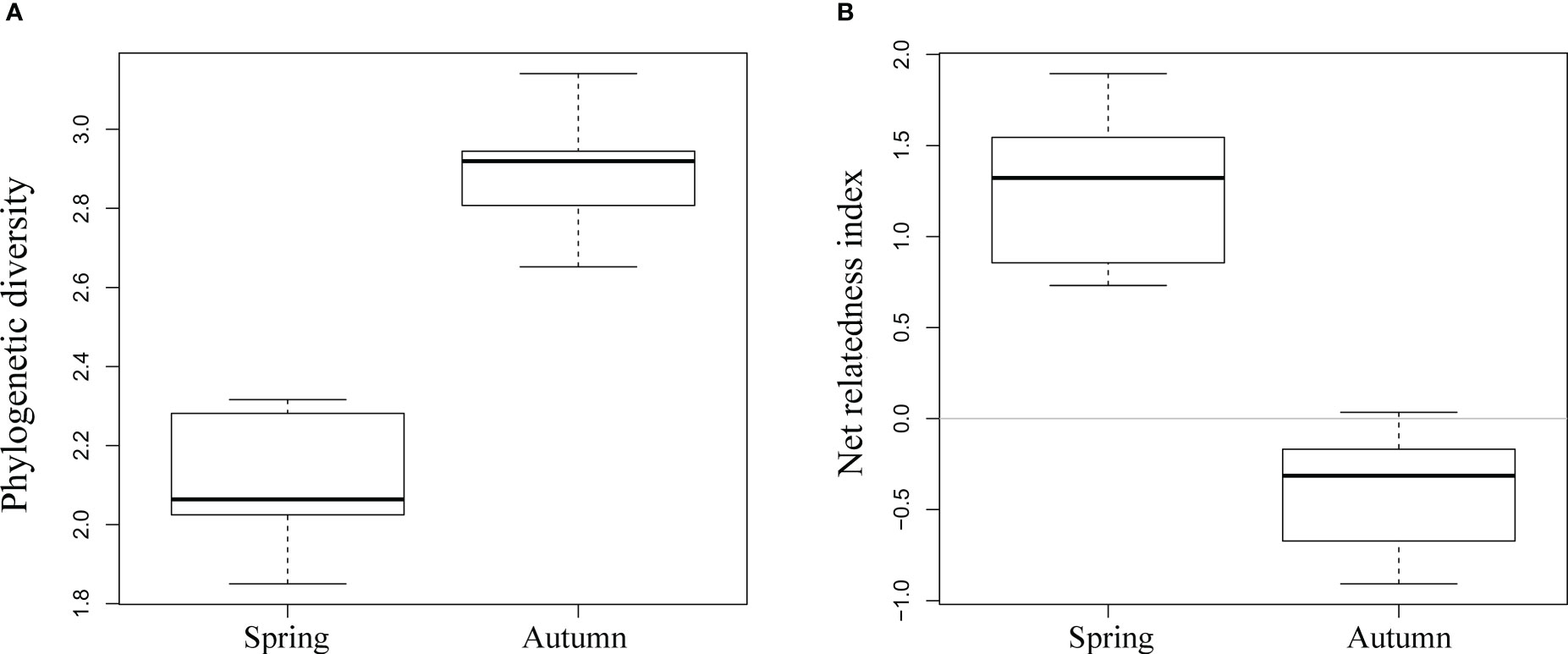
Figure 4 Variance analysis with permutation tests for values of phylogenetic diversity (A) and net relatedness index (B) in nektonic communities of Weizhou Island in spring and autumn.
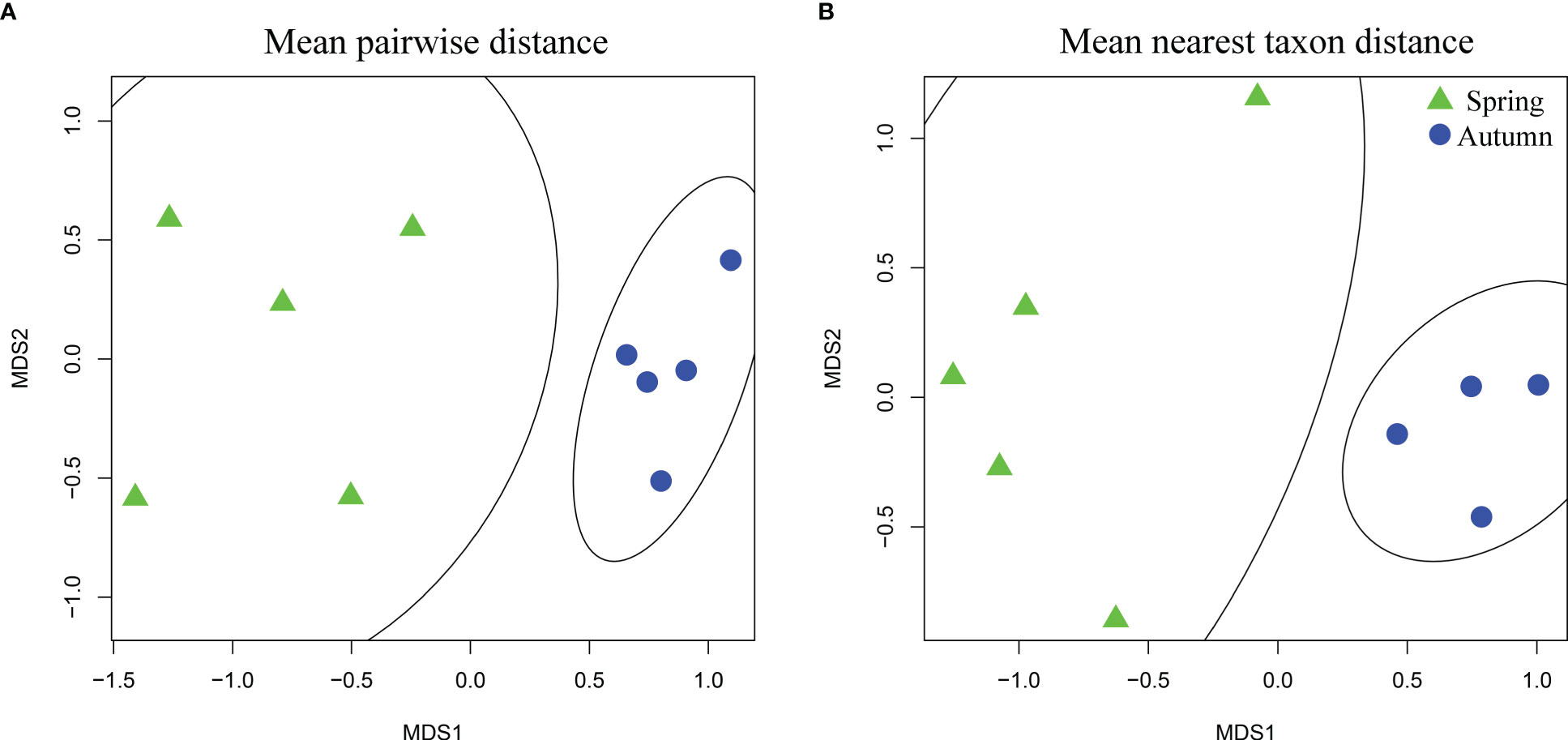
Figure 5 Nonmetric multidimensional scaling ordination plots of phylogenetic alpha diversity of nektonic communities occurring in spring and autumn based on mean pairwise distance (A) and mean nearest taxon distance (B).
The multiple regression on distance matrices significantly predicted the influence of environmental variables on the phylobeta diversity at both the deep and shallow phylogenetic levels for nekton community. The MRM models total explained 68.7% (p = 0.006) and 80.5% (p = 0.006) of environmental variance in phylobeta diversity at both deep (Dpw) and shallow (Dnn) phylogenetic levels, respectively. Salinity (p = 0.014), water temperature (p = 0.027), and transparency (p = 0.022) significantly explained the variation in phylobeta diversity at deep phylogenetic levels for nekton community, whereas salinity (p = 0.011), water temperature (p = 0.006) and depth (p = 0.043) were significantly related to the variation in phylobeta diversity at shallow phylogenetic levels (Table 2).
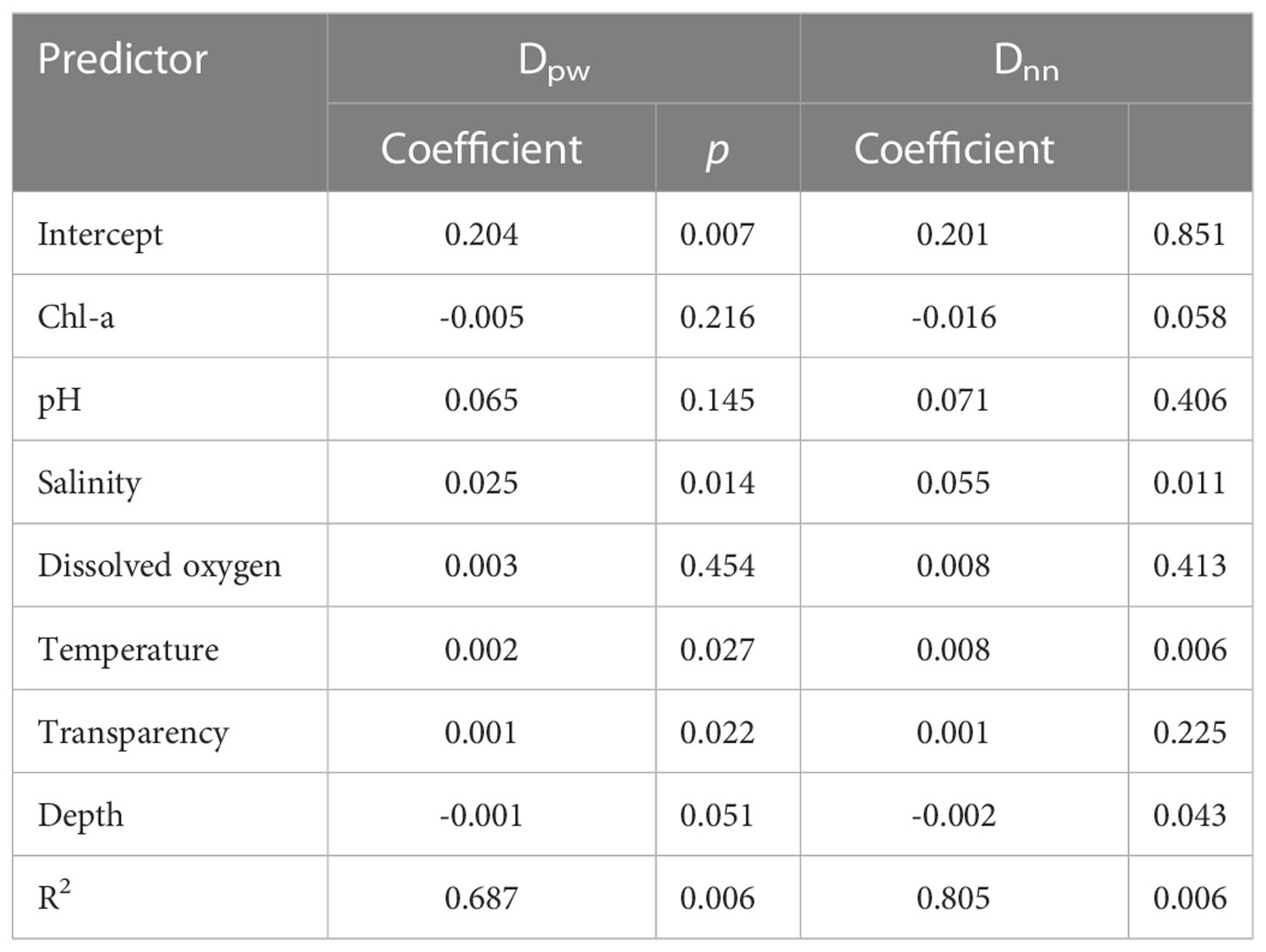
Table 2 Results of multiple regression on distance matrices (MRM) analyses for the effect of environmental variables in phylogenetic beta diversity both at deep (Dpw) and shallow (Dnn) phylogenetic levels from spring and autumn nektonic communities in the waters of Weizhou Island.
Discussion
Our results showed significant seasonal patterns in average catch density, community structure and phylogenetic diversity. The total catch density in spring was only 1/3 of that in autumn, which is approximately 1/7 of the fishery resource density in the 1960s and approximately 1/4 of that surveyed at the end of the 1970s. Phylogenetic diversity of nekton community increased appreciably from spring to autumn, while phylogenetic patterns changed from clustering to overdispersion. As a highly productive area with rich fish diversity and fertile fishery resources, fisheries, marine ecosystem dynamics, oceanography, and marine policy have been intensively studied in the Beibu Gulf (Yu and Mu, 2006; Wang et al., 2012; Gao et al., 2015; Wang et al., 2019; Xu et al., 2019). Detailed investigation of fishery resources in the Beibu Gulf can be traced back to the China and Vietnam comprehensive oceanographic survey of the Beibu Gulf in the late 1950s and early 1960s. A total of 1519 fish species have been recorded in the Beibu Gulf since the first comprehensive oceanographic survey, of which 107 are currently listed as endangered by the IUCN.
Previous studies have shown that the total fishing density in the Beibu Gulf has decreased by more than 60% in recent years, and the catch density of 12 traditional commercial demersal fishes has decreased by an even greater 85%. Indeed, the average stock density of many important fishes is only 10% of what it was in the 1960s (Zhang et al., 2021; Su et al., 2022). According to Yuan (1995), fishery resource density along the coast of Beibu Gulf was approximately 5 tons per km2 in the 1960s, but was 3 ton per km2 by the end of the 1970s. In our investigation, the average catch density in Weizhou Island in the northeast part of the Beibu Gulf was 686 kg per km2, indicates that the fishery resources in the northeast of the Beibu Gulf are in serious decline.
Furthermore, the traditional commercial fish and dominant species changed from larger, higher value, and higher trophic level species to smaller species with a shorter life span, lower value, and lower trophic level. For example, golden threadfin bream (Nemipterus virgatus), lizardfish (Trachinocephalus myops), and humphead snapper (Lutjanus sanguineus) were replaced by round scad (Decapterus maruadsi), cardinalfish (Apogonichthys ellioti), and horse mackerel (Trachurus japonicus) (Wang et al., 2012; Wang et al., 2019; Su et al., 2022). At the end of the 1960s, the number of fishing boats and their horsepower increased with the introduction and promotion of motorboats, and fishing techniques (fish-finders and navigators) continuously improved; this dramatically increased the fishing efforts and catches. Overfishing is one of the main reasons for the decline of fish biodiversity and fishery resources in the Beibu Gulf even the South China Sea (Chen et al., 2011; Zhang et al., 2021; Su et al., 2022).
Numerous studies have revealed the importance of seasonal variations in explaining the structure and distribution of coastal nektonic communities (Hoff and Ibara, 1977; Lazzari et al., 1999; Raposa et al., 2003; Pattrick and Strydom, 2008; Ramos-Miranda et al., 2008). The results of our study further support that the environmental heterogeneity caused by seasonal variation contributes to the coastal nekton community assembly and phylogenetic diversity in the Beibu Gulf. There was distinct seasonal differentiation in both community structure and phylogenetic diversity of coastal nekton communities. A total of 64 nekton species were caught in spring, including 31, 6, and 17 species associated with fish, cephalopods, and crustaceans, respectively. Alternatively, a total of 76 nekton species were found in autumn, including 48, 7, and 31 species associated with fish, cephalopods, and crustaceans, respectively.
The fishes in the middle and northern part of the Beibu Gulf were mainly tropical and carnivorous. The changes of fish community structure in this area were mainly related to the seasonal variation of the bottom water temperature (Wang et al., 2020; Feng et al., 2021). Water temperature is a key factor that affects the distribution of fish communities because it directly affects the growth and development of fish and their traits, such as feeding, reproduction, and winter migration. Therefore, seasonal changes of water temperature resulted in seasonal differences in fish community composition. When the water temperature was low in winter, the tropical seasonal migratory fish left the coastal area of the Beibu Gulf and migrated to the deep-water area of the open sea, which resulted in the presence of mainly sedentary fish species in the Beibu Gulf in winter. In spring, the water temperature gradually warmed up, and the coastal current turned from weak to strong; this brought abundant nutrients to the coastal area, and the migratory fish began to swim to the coastal area for spawning and breeding, thus enriching the composition of fish species in the northern waters of the Beibu Gulf (Yuan, 1995; Hou et al., 2008; Wang et al., 2012; Zhang et al., 2014; Xu et al., 2021b).
In addition, the seasonal variation of fish communities in the northern part of the Beibu Gulf may also be related to the monsoon, tidal flow, coastal current, and the habitats. Upwelling along the coast of the Beibu Gulf can transport nutrient-rich bottom seawater to the middle and upper layers; this provides sufficient nutrients for the growth and reproduction of bait organisms, and thus affects the seasonal changes of fish community structure. Under the influence of the summer monsoon, there was obvious cyclonic circulation in the northern waters of the Beibu Gulf, which formed in June and reached maturity for one month. This drove the migration of fish community to the southeast (Gao et al., 2017). In spring, the coastal water force was relatively weak, which created favorable conditions for the propagation of plankton, thus driving fish to migrate to the shore. In autumn, the fish community migrated to the southeastern sea area under the influence of the anticyclonic circulation in the southern Beibu Gulf (Gao et al., 2015). In winter, when the water temperature drops, the seasonal migratory fish migrated to the open sea, and fish richness decreased. The inshore sea area was dominated by sedentary fish.
In general, the large-scale distribution patterns of fish or nektonic communities are primarily influenced by oceanographic factors, such as circulation, tidal flow, and coastal currents, which can greatly affect on larval dispersal distance and bait organism distribution (Norcross and Shaw, 1984; McClanahan and Arthur, 2001). However, at small spatial or regional scales, such as coastal, coral reef, or estuarine ecosystems, the distribution and composition of nektonic communities are also associated with stochasticity in nektonic community structuring (Letourneur et al., 2003; La Mesa et al., 2011; Xu et al., 2021a). Our results showed that the average phylogenetic diversity of autumn nektonic communities was significantly higher than that of spring nektonic communities. Positive NRI values in spring nektonic communities indicated phylogenetic clustering, whereas negative NRI values in autumn nektonic communities indicated phylogenetic overdispersion. These results indicated that stochastic processes play a strong role in structuring nektonic communities of the northern waters of the Beibu Gulf.
In late spring, fishing intensity becomes stronger in the northern waters of the gulf as fishermen from both China and Vietnam try to catch as many fish as possible, especially some commercial species, in a shorter period of time using more efficient equipment and methods (such as a lot of artisanal shrimp trawlers) (Yu and Mu, 2006; Vu, 2013; Tian et al., 2022). In our case, the total catch density declined to 291.9 kg per km2 in spring, which was only approximately 1/3 of the fishery resource density in autumn. Fishing intensity acted as an environmental filter, leading to coexistence of closely related species, and remarkable phylogenetic clustering was found in nektonic communities of this area. Therefore, fishing intensity had a greater impact on nektonic community, and even exceeded the effect of environmental factors.
The northern Beibu Gulf, like other coastal areas of China, faces overexploitation and depletion of fishery resources. Because of the combined effects of marine and coastal resource overexploitation and marine pollution, many highly productive coastal and offshore fisheries have disappeared or relocated far from the country’s coastlines (Zhong and Power, 1997). To improve this situation, the Chinese government implemented a series of corresponding management regulations and protection policies. First, they introduced the fishing license system. The Chinese government recognized that depletion of coastal and offshore fishery resources was due to a lack of effective management and overcapacity in fishing vessels. Since 1979, the government has controlled the national fishing capacity through the fishing license system. All fishing vessels were required to obtain licenses from the government before fishing. Second, the government introduced a mid-summer fishing moratorium. China’s Ministry of Agriculture has instituted a fishing ban from May to August every year since 1998 across a large area of the South China Sea north of latitude 12°N. The annual fishing ban covers the spawning season for most fish and invertebrates, mainly in the summer, and is therefore known as the ‘‘mid-summer fishing moratorium” (Yu and Yu, 2008). Third, they signed agreements for fisheries management with neighboring countries. For the Beibu Gulf, China and Vietnam signed a fishing agreement to peacefully settle their fishing disputes and maintain lasting stability of the fishing communities (Yu and Mu, 2006). Under comprehensive management, the summer fishing intensity in the Beibu Gulf was controlled to the maximum extent possible, and nektonic diversity and fish stocks were effectively recovered and recruited from the larval pool. However, we still have a long way to go to restore the fishery to levels of 1970s even 1960s. This can be achieved through demonstrated management and restoration methods and experiences from others cases around the world (Moland et al., 2021; Kemp et al., 2023).
Conclusion
The nektonic communities in the waters of Weizhou Island in the northern Beibu Gulf showed significant seasonal patterns in average catch density, community structure and phylogenetic diversity. Phylogenetic diversity of nekton community obviously increased from spring to autumn, while phylogenetic patterns changed from clustering to overdispersion. The seasonal patterns of nektonic communities were mainly related to the different fishing intensities in the two seasons. Nektonic communities benefited from the mid-summer fishing moratorium, when the fish stocks rapidly recovered. We propose that fishing intensity had a greater impact on nektonic community at small spatial scales, even exceeding the effect of environmental factors. Our research also provided insights into effective future fishery management practices in the Beibu Gulf.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences.
Author contributions
LX: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review and editing. QT, LW, JN, DH, YL, and SL: Methodology, Formal analysis. XW and FD: Funding acquisition, Project administration, Resources. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Hainan Provincial Natural Science Foundation of China (422MS156), Science and Technology Basic Resources Investigation Program of China (2018FY100105, 2017FY201405).
Acknowledgments
We thank all colleagues and students for their help with sampling. We thank Mallory Eckstut, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1133462/full#supplementary-material
References
Adler P. B., HilleRisLambers J., Levine J. M. (2007). A niche for neutrality. Ecol. Lett. 10 (2), 95–104. doi: 10.1111/j.1461-0248.2006.00996.x
Anderson M. J. (2017). “Permutational multivariate analysis of variance (PERMANOVA),” in Wiley StatsRef: Statistics reference online. Eds. Balakrishnan N., Colton T., Everitt B., Piegorsch W., Ruggeri F., Teugels J. L. (New York: Wiley), 1–15.
Bell G. (2001). Neutral macroecology. Science 293 (5539), 2413–2418. doi: 10.1126/science.293.5539.2413
Cavender-Bares J., Kozak K. H., Fine P. V. A., Kembel S. W. (2009). The merging of community ecology and phylogenetic biology. Ecol. Lett. 12 (7), 693–715. doi: 10.1111/j.1461-0248.2009.01314.x
Chase J. M., Leibold M. A. (2003). Ecological niches: Linking classical and contemporary approaches (Chicago: University of Chicago Press).
Chen T., Li S., Yu K., Zheng Z., Wang L., Chen T. (2013). Increasing temperature anomalies reduce coral growth in the weizhou island, northern south China Sea. Estuar. Coast. Shelf Sci. 130, 121–126. doi: 10.1016/j.ecss.2013.05.009
Chen Z., Qiu Y., Xu S. (2011). Changes in trophic flows and ecosystem properties of the beibu gulf ecosystem before and after the collapse of fish stocks. Ocean Coast. Manage. 54 (8), 601–611. doi: 10.1016/j.ocecoaman.2011.06.003
Chen Z., Xu S., Qiu Y., Lin Z., Jia X. (2009). Modeling the effects of fishery management and marine protected areas on the beibu gulf using spatial ecosystem simulation. Fish. Res. 100 (3), 222–229. doi: 10.1016/j.fishres.2009.08.001
D’agata S., Mouillot D., Kulbicki M., Andréfouët S., Bellwood, David R., et al. (2014). Human-mediated loss of phylogenetic and functional diversity in coral reef fishes. Curr. Biol. 24 (5), 555–560. doi: 10.1016/j.cub.2014.01.049
Drummond A. J., Suchard M. A., Xie D., Rambaut A. (2012). Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29 (8), 1969–1973. doi: 10.1093/molbev/mss075
Ezard T. H. G., Fujisawa T., Barraclough T. G. (2009). Splits: SPecies’ LImits (Threshold Statistics) Free R package.
Faith D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61 (1), 1–10. doi: 10.1016/0006-3207(92)91201-3
Feng Y., Shi H., Hou G., Zhao H., Dong C. (2021). Relationships between environmental variables and spatial and temporal distribution of jack mackerel (Trachurus japonicus) in the beibu gulf, south China Sea. PeerJ 9, e12337. doi: 10.7717/peerj.12337
Gao J., Chen B., Shi M. (2015). Summer circulation structure and formation mechanism in the beibu gulf. Sci. China Earth Sci. 58 (2), 286–299. doi: 10.1007/s11430-014-4916-2
Gao J., Wu G., Ya H. (2017). Review of the circulation in the beibu gulf, south China Sea. Cont. Shelf Res. 138, 106–119. doi: 10.1016/j.csr.2017.02.009
Gewin V. (2006). Beyond neutrality–ecology finds its niche. PloS Biol. 4 (8), e278. doi: 10.1371/journal.pbio.0040278
Goslee S. C., Urban D. L. (2007). The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Software 22 (7), 1–19. doi: 10.18637/jss.v022.i07
Hall T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98.
Hoff J. G., Ibara R. M. (1977). Factors affecting the seasonal abundance, composition and diversity of fishes in a southeastern new England estuary. Estuar. Coast. Mar. Sci. 5 (5), 665–678. doi: 10.1016/0302-3524(77)90091-3
Hou G., Chen Y., Wang J., Pan C., Lin J., Feng B., et al. (2022). Molecular identification of species diversity using pelagic fish eggs in spring and late autumn-winter in the Eastern beibu gulf, China. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.806208
Hou G., Feng B., Lu H., Zhu J. (2008). Age and growth characteristics of crimson sea bream paragyrops edita Tanaka in beibu gulf. J. Ocean Univ. China 7, 457–465. doi: 10.1007/s11802-008-0457-7
Hubbell S. P. (2001). The unified neutral theory of biogeography and biodiversity (Princeton: Princeton University Press).
Hubbell S. P. (2005). Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 19 (1), 166–172. doi: 10.1111/j.0269-8463.2005.00965.x
Jiang X., Ding C., Brosse S., Pan B., Lu Y., Xie Z. (2019). Local rise of phylogenetic diversity due to invasions and extirpations leads to a regional phylogenetic homogenization of fish fauna from Chinese isolated plateau lakes. Ecol. Indic. 101, 388–398. doi: 10.1016/j.ecolind.2019.01.041
Kembel S. W., Cowan P. D., Helmus M. R., Cornwell W. K., Morlon H., Ackerly D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26 (11), 1463–1464. doi: 10.1093/bioinformatics/btq166
Kemp P. S., Subbiah G., Barnes R., Boerder K., O’Leary B. C., Stewart B. D., et al. (2023). The future of marine fisheries management and conservation in the united kingdom: Lessons learnt from over 100 years of biased policy. Mar. Policy 147, 105075. doi: 10.1016/j.marpol.2022.105075
Kimura M. (1968). Evolutionary rate at the molecular level. Nature 217 (5129), 624–626. doi: 10.1038/217624a0
La Mesa G., Molinari A., Gambaccini S., Tunesi L. (2011). Spatial pattern of coastal fish assemblages in different habitats in north-western Mediterranean. Mar. Ecol. 32 (1), 104–114. doi: 10.1111/j.1439-0485.2010.00404.x
Lazzari M. A., Sherman S., Brown C. S., King J., Joule B. J., Chenoweth S. B., et al. (1999). Seasonal and annual variations in abundance and species composition of two nearshore fish communities in Maine. Estuaries 22 (3), 636–647. doi: 10.2307/1353051
Legendre P., Anderson M. J. (1999). Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 69 (1), 1–24. doi: 10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Letourneur Y., Ruitton S., Sartoretto S. (2003). Environmental and benthic habitat factors structuring the spatial distribution of a summer infralittoral fish assemblage in the north-western Mediterranean Sea. J. Mar. Biol. Assoc. U.K. 83 (1), 193–204. doi: 10.1017/S0025315403006970h
Lichstein J. W. (2007). Multiple regression on distance matrices: A multivariate spatial analysis tool. Plant Ecol. 188 (2), 117–131. doi: 10.1007/s11258-006-9126-3
Ma F., Wang Y., Li Y., Ye C., Xu Z., Zhang F. (2010). The application of geostatistics in grain size trend analysis: A case study of eastern beibu gulf. J. Geographical Sci. 20 (1), 77–90. doi: 10.1007/s11442-010-0077-1
Ma C., You K., Zhang M., Li F., Chen D. (2008). A preliminary study on the diversity of fish species and marine fish faunas of the south China Sea. J. Ocean Univ. China 7 (2), 210–214. doi: 10.1007/s11802-008-0210-2
McClanahan T. R., Arthur R. (2001). The effect of marine reserves and habitat on populations of East African coral reef fishes. Ecol. Appl. 11 (2), 559–569. doi: 10.2307/3060909
Moland E., Fernández-Chacón A., Sørdalen T. K., Villegas-Ríos D., Thorbjørnsen S. H., Halvorsen K. T., et al. (2021). Restoration of abundance and dynamics of coastal fish and lobster within northern marine protected areas across two decades. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.674756
Mouquet N., Devictor V., Meynard C. N., Munoz F., Bersier L.-F., Chave J., et al. (2012). Ecophylogenetics: advances and perspectives. Biol. Rev. 87 (4), 769–785. doi: 10.1111/j.1469-185X.2012.00224.x
Norcross B. L., Shaw R. F. (1984). Oceanic and estuarine transport of fish eggs and larvae: A review. Trans. Am. Fish. Soc 113 (2), 153–165. doi: 10.1577/1548-8659(1984)113<153
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2019). Vegan: Community ecology package. Free R package
Palmer M. (1994). Variation in species richness: Towards a unification of hypotheses. Folia Geobot. Phytotaxon 29, 511–530. doi: 10.1007/BF02883148
Pattrick P., Strydom N. A. (2008). Composition, abundance, distribution and seasonality of larval fishes in the shallow nearshore of the proposed greater addo marine reserve, algoa bay, south Africa. Estuar. Coast. Shelf Sci. 79 (2), 251–262. doi: 10.1016/j.ecss.2008.04.009
Qiu Y., Lin Z., Wang Y. (2010). Responses of fish production to fishing and climate variability in the northern south China Sea. Prog. Oceanogr. 85 (3), 197–212. doi: 10.1016/j.pocean.2010.02.011
Ramos-Miranda J., Mouillot D., Sosa-Lopez A., Do-Chi T., Flores-Hernández D. (2008). How much variation can be explained by seasonal, spatial and environmental effects in nekton assemblages of the terminos lagoon? Aquat. Conserv.: Mar. Freshwat. Ecosyst. 18 (5), 508–517. doi: 10.1002/aqc.873
Raposa K. B., Roman C. T., Heltshe J. F. (2003). Monitoring nekton as a bioindicator in shallow estuarine habitats. Environ. Monit. Assess. 81 (1), 239–255. doi: 10.1023/A:1021389327224
R Development Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Shen G., Heino M. (2014). An overview of marine fisheries management in China. Mar. Policy 44, 265–272. doi: 10.1016/j.marpol.2013.09.012
Sparre P., Venema S. C. (1998). Introduction to tropical fish stock assessment part i. manual (FAO Fisheries Technical Paper Rome: FAO).
Su L., Xu Y., Qiu Y., Sun M., Zhang K., Chen Z. (2022). Long-term change of a fish-based index of biotic integrity for a semi-enclosed bay in the beibu gulf. Fishes 7 (3), 124. doi: 10.3390/fishes7030124
Swenson N. G. (2011). Phylogenetic beta diversity metrics, trait evolution and inferring the functional beta diversity of communities. PloS One 6(6)(1932-6203 (6), e21264. doi: 10.1371/journal.pone.0021264
Tian H., Liu Y., Tian Y., Jing Y., Liu S., Liu X., et al. (2022). Advances in the use of nighttime light data to monitor and assess coastal fisheries under the impacts of human activities and climate and environmental changes: A case study in the beibu gulf. Mar. Policy 144, 105227. doi: 10.1016/j.marpol.2022.105227
Tilman D. (2004). Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. U. S. A. 101 (30), 10854–10861. doi: 10.1073/pnas.0403458101
Vellend M., Agrawal A. (2010). Conceptual synthesis in community ecology. Q. Rev. Biol. 85 (2), 183–206. doi: 10.1086/652373
Vu H. D. (2013). A bilateral network of marine protected areas between Vietnam and China: An alternative to the Chinese unilateral fishing ban in the south China Sea? Ocean Dev. Int. Law 44 (2), 145–169. doi: 10.1080/00908320.2013.750984
Wang X., Qiu Y., Du F., Lin Z., Sun D., Huang S. (2012). Population parameters and dynamic pool models of commercial fishes in the beibu gulf, northern south China Sea. Chin. J. Oceanol. Limnol. 30 (1), 105–117. doi: 10.1007/s00343-012-1017-y
Wang X., Qiu Y., Du F., Liu W., Sun D., Chen X., et al. (2019). Roles of fishing and climate change in long-term fish species succession and population dynamics in the outer beibu gulf, south China Sea. Acta Oceanologica Sin. 38 (10), 1–8. doi: 10.1007/s13131-019-1484-5
Wang Y., Yao L., Chen P., Yu J., Wu Q.E. (2020). Environmental influence on the spatiotemporal variability of fishing grounds in the beibu gulf, south China Sea. J. Mar. Sci. Eng. 8 (12), 957. doi: 10.3390/jmse8120957
Webb C. O. (2000). Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 156 (2), 145–155. doi: 10.1086/303378
Webb C. O., Ackerly D. D., McPeek M. A., Donoghue M. J. (2002). Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33 (1), 475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448
Weiher E., Keddy P. (1999). “Ecological assembly rules,” in Perspectives, advances, retreats (Cambridge: Cambridge University Press).
Winter M., Schweiger O., Klotz S., Nentwig W., Andriopoulos P., Arianoutsou M., et al. (2009). Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. U. S. A. 106 (51), 21721. doi: 10.1073/pnas.0907088106
Xu L., Wang X., Wang L., Ning J., Li Y., Huang D., et al. (2021b). Threadfin porgy (Evynnis cardinalis) haplotype pattern and genetic structure in beibu gulf, south China Sea. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.726533
Xu L., Wang L., Wang X., Van Damme K., Ning J., Li Y., et al. (2021a). Spatial and temporal variabilities of coastal nekton community structure and phylogenetic diversity in daya and dapeng bay, southern China. Ecol. Indic. 131, 108226. doi: 10.1016/j.ecolind.2021.108226
Xu Y., Zhang T., Zhou J. (2019). Historical occurrence of algal blooms in the northern beibu gulf of China and implications for future trends. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00451
Yu K. (2012). Coral reefs in the south China Sea: Their response to and records on past environmental changes. Sci. China Earth Sci. 55 (8), 1217–1229. doi: 10.1007/s11430-012-4449-5
Yuan W. (1995). Dynamics and succession of demersal resources in beibu gulf. J. fishery Sci. China 2 (2), 57–65.
Yu Y., Mu Y. (2006). The new institutional arrangements for fisheries management in beibu gulf. Mar. Policy 30 (3), 249–260. doi: 10.1016/j.marpol.2004.12.006
Yu W., Wang W., Yu K., Wang Y., Huang X., Huang R., et al. (2019). Rapid decline of a relatively high latitude coral assemblage at weizhou island, northern south China Sea. Biodivers. Conserv. 28 (14), 3925–3949. doi: 10.1007/s10531-019-01858-w
Yu H., Yu Y. (2008). Fishing capacity management in China: Theoretic and practical perspectives. Mar. Policy 32 (3), 351–359. doi: 10.1016/j.marpol.2007.07.004
Zhang Y., Dai C., Yan Y., Yang Y., Lu H. (2014). Feeding habits and trophic level of crimson sea bream,(Parargyrops edita tanaka)in the beibu gulf. J. Fisheries China 38, 265–273. doi: 10.3724/SP.J.1231.2014.48919
Zhang K., Geng P., Li J., Xu Y., Kalhoro M. A., Sun M., et al. (2022). Influences of fisheries management measures on biological characteristics of threadfin bream (Nemipterus virgatus) in the beibu gulf, south China Sea. Acta Oceanologica Sin. 41 (3), 24–33. doi: 10.1007/s13131-021-1925-9
Zhang K., Li J., Hou G., Huang Z., Shi D., Chen Z., et al. (2021). Length-based assessment of fish stocks in a data-poor, jointly exploited (China and Vietnam) fishing ground, northern south China Sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.718052
Keywords: phylogenetic diversity, nekton community, the Beibu Gulf, Weizhou Island, community structure
Citation: Xu L, Du F, Tang Q, Wang L, Ning J, Huang D, Li Y, Liu S and Wang X (2023) Seasonal variability of nektonic community structure and phylogenetic diversity in Weizhou Island, the Beibu Gulf. Front. Mar. Sci. 10:1133462. doi: 10.3389/fmars.2023.1133462
Received: 29 December 2022; Accepted: 31 January 2023;
Published: 09 February 2023.
Edited by:
Kit Yue Kwan, Beibu Gulf University, ChinaReviewed by:
Ying Xue, Ocean University of China, ChinaIsmael Aaron Kimirei, Tanzania Fisheries Research Institute, Tanzania
Copyright © 2023 Xu, Du, Tang, Wang, Ning, Huang, Li, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehui Wang, d3hoc2NzQDE2My5jb20=
 Lei Xu
Lei Xu Feiyan Du
Feiyan Du Quehui Tang1,2
Quehui Tang1,2 Delian Huang
Delian Huang Yafang Li
Yafang Li Xuehui Wang
Xuehui Wang