
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 13 March 2023
Sec. Marine Biogeochemistry
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1132851
This article is part of the Research TopicOxygen Decline in Coastal Waters: Its Cause, Present Situation and Future ProjectionView all 7 articles
Global warming is affecting the composition, structure, and function of marine ecosystems. The increase in hypoxic regions due to stratification is a major environmental problem worldwide. Off the southern coast of Korea, hypoxia occurs frequently in summer, and the area of water affected is gradually expanding. In this study, we investigated the effects of hypoxia on the eggs of copepods in the order Calanoida. Data on the distribution and abundance of eggs in benthic sediments were collected from 17 stations, using a piston core sampler (64 mm internal diameter, 50 cm length), from August 1 to 7, 2012. Significant variations in the distribution of calanoid eggs and the occurrence of abnormalities in egg development were found between stations. The abundance of eggs found in the sediments ranged from 0.004 to 2.389 × 106 eggs·m−2, with higher abundances identified in hypoxic than in normoxic areas. The proportion of abnormal eggs ranged from 0 to 92.7%. In particular, there were significantly more abnormal than normal eggs in areas where hypoxia occurred (p < 0.01). These results show that hypoxia can have a lethal effect on calanoid eggs and further affect population and community dynamics.
Worldwide, the concentration of dissolved oxygen in coastal waters has changed dramatically over recent decades, and widespread anthropogenic eutrophication-induced hypoxia is a major environmental problem in coastal systems (Diaz and Rosenberg, 2008; Rabalais et al., 2010; Kodama and Horiguchi, 2011). Hypoxia can be a serious stressor to marine organisms and ecosystems, and low dissolved oxygen concentrations can reduce the range of organisms and suitability of habitats, and even further accelerate community change (Rabalais et al., 2001; Breitburg, 2002; Lai et al., 2022). Vaquer-Sunyer and Duarte (2008) showed that the number of coastal areas where hypoxia has been reported has increased at a rate of 5.5%·year−1. Examples of marine regions with permanent, seasonal, periodic, or episodic hypoxia include the Baltic Sea, Black Sea, Gulf of Mexico, Chesapeake Bay, Yangtze River Estuary, Masan Bay, and Gamak Bay (Hagy et al., 2004; Conley et al., 2009; Rabalais et al., 2010; Chen et al., 2015; Choi et al., 2016; Jessen et al., 2017; Du et al., 2018; Choi et al., 2021).
Most marine species of the order Calanoida lay their eggs freely in the water column; relatively few species place them in egg sacs (Hansen, 2019). Marine calanoids may produce subitaneous eggs, which can develop without delay, or diapause eggs, which enter an obligatory refractory phase during which they cannot hatch (Grice and Marcus, 1981; Baumgartner and Tarrant, 2017; Belmonte and Rubino, 2019). Hatching of diapause eggs replenishes the population of copepods in the water column for portions of the year, and the presence of subitaneous eggs is important for maintaining the population during active seasons (Marcus, 1979; Marcus, 1996). Many studies have been reported that the onset of adverse conditions induce quiescence (subitaneous), whereas diapausal eggs are produced during normal conditions (Belmonte, 1992; Onoue et al., 2004; Tachibana et al., 2019; Takayama and Toda, 2019). It has been confirmed that before population biomass declines, females release diapause eggs that can survive for a long time in anoxic sediments (Marcus, 1984; Katajisto, 1996).
Calanoid egg abundances are high in sediments, varying between 104 and 107 eggs·m-2 (Belmonte et al., 1995; Marcus, 1995; Uriarte and Villate, 2006; Choi et al., 2021); high egg abundance is found in bays or estuaries rather than in the open ocean (Masero and Villate, 2004; Glippa et al., 2011). The eggs of marine calanoids sink because they are denser than the surrounding seawater (Tang et al., 1998). The accumulation of a large number of eggs contributes to the recruitment of nauplii (larvae), as well as serves as an “egg bank” for long-term persistence of the species (Marcus, 1984; Marcus et al., 1997; Katajisto, 2006). Most of the sunk calanoid eggs spend weeks to years on the seabed during their benthic resting phase (Marcus and Boero, 1998). Therefore, eggs that quickly sink to the seabed in shallow water habitats can be buried by sedimentation processes and may be exposed to stressful conditions, such as hypoxia and anoxia.
Uye and Fleminger (1976) demonstrated the importance of oxygen in calanoid embryo development, as eggs did not hatch in deoxygenated water, even under favorable temperature conditions. Choi et al. (2021) showed that long-term exposure of calanoid eggs to hypoxic conditions reduces hatching rates and increases the relative proportion of abnormal eggs (unhatched egg or missing egg contents). Marcus et al. (2004) suggested that exposure to hypoxia could substantially reduce egg hatching, which in turn could have considerable impacts on population and community dynamics in coastal systems, which may be exacerbated by prolonged exposure to low oxygen or hypoxic conditions.
The IPCC Assessment Report (AR6) shows that, by the end of the 21st century, the global average sea surface temperature will rise by 1.4–3.7 °C compared to current conditions. Global warming can increase sea level rise, temperature, precipitation and increase coastal hypoxia (Altieri and Gedan, 2015). In the Southern Sea of Korea, there are continuous inputs of industrial wastewater and domestic sewage; consequently, low oxygen or hypoxic conditions arise in summer in some semi-closed bays where the rate of seawater exchange is relatively low (Kim et al., 2006; Lee et al., 2019). Such conditions are expected to have a significant impact on calanoid copepod eggs that form near the bottom.
The study area in South Korea is affected by various ocean currents that change seasonally. These relatively shallow coastal waters are important as spawning grounds for various species of fish and shellfish (Kim and Pang, 2005; Baek et al., 2010; Ko et al., 2010). However, the area is surrounded by populated cities in the southeastern (Masan, Changwon, and Jinhae) and south–central (Yeosu, Namhae) parts of the country, extending up to the east coast, with the establishment of the Imhae Industrial Complex along the Namhae coast (Lee and Min, 1990; Lee and Kim, 2008). As a result, constant hypoxia or anoxia occurs in summer (July to September) in semi-closed bays (Gamak Bay, Jinhae Bay), where the rate of seawater exchange is low.
The response and abundance of mesozooplankton to hypoxia have been the focus of many studies, but there have been relatively few investigations of the relationship between calanoid eggs and hypoxia. Calanoid egg abundance is a key factor in nauplii recruitment and drives the continuation of active populations (Marcus, 1984; Belmonte and Pati, 2007). The occurrence of hypoxic conditions associated with climate change may threaten the existence of zooplankton, which have an important position in food webs. Accordingly, the objectives of this study were to indirectly evaluate the effects of hypoxia on (1) the distribution characteristics of normal and abnormal eggs, and (2) the abundance of calanoid eggs in the Southern Sea of Korea, where hypoxia occurs frequently in summer.
Data were collected from a total of 17 stations along the southern coast of South Korea. Four stations (S1 to S4) were located in Jinhae Bay in the north of Geoje, and three stations (S5 to S7) were west of Geoje (Figure 1). Two stations were located in Jinju Bay (S8, S9), north of Namhae. Station S10 was located within Gwangyang Bay, and stations S11 and S12 were located outside Gwangyang Bay. Stations S13 and S14 were located in Gamak Bay, S15 was within the inner bay, S16 in the center, and S17 further out, in Yeoja Bay. The water depth of the survey stations varied from 3 m (S15) to 39 m (S6).
The environmental variables (water temperature, salinity, chlorophyll-a fluorescence, and dissolved oxygen [DO] concentration) were measured at these 17 sites, with vertical profiles measures in the field, using a water quality multi-meter (Model 6600; Xylem Inc., Yellow Springs, OH, USA).
Zooplankton and sediment samples were collected from August 1 to 7, 2012. Zooplankton samples were collected vertically, from the near-bottom water to the surface layer, using a conical net (mouth opening diameter 45 cm, mesh size 200 µm) to filter an adequate water volume. Zooplankton samples were immediately fixed to a final concentration of 5%, using neutralized formalin solution in situ. The water volume was measured by attaching a flow meter (model 438115; Hydro-Bios, Altenholz, Germany) to the net mouth and measuring the amount of filtered seawater that passed through the net. Zooplankton were counted using a Bogorov counting chamber using a stereomicroscope (Nikon SMZ 1000; Nikon, Japan), and identified using a high-magnification optical microscope (Nikon ECLIPSE 80i; Nikon, Japan). Calanoid abundance was converted to the number of individuals per cubic meter (individuals·m-3). Only adults were counted.
The distribution and abundance of calanoid eggs in the sediments, and the ratio of normal and abnormal eggs, were determined at each sediment core collected, using a piston core sampler (64 mm internal diameter, 50 cm length), from August 1 to 7, 2012 (Figure 1). The sediment samples were placed in a dark-treated icebox and immediately transferred to the laboratory. The sediment obtained by cutting the upper 1 cm of each core sediment sample was washed through a 40 µm mesh, and the remaining eggs were recovered from the mesh, fixed in 5% formalin solution, and placed in a conical 50 mL tube (SPL Life Science Co., Ltd., GyunggiDo, Korea). The potential impact of the egg isolation method on egg morphology cannot be ruled out, as it could induce physical and osmotic stresses that may cause abnormal egg shapes. The common sugar floating isolation method, which has been shown to have a direct effect on egg morphology (Lukic et al., 2016), was not used in this study. Therefore, it is important to acknowledge the limitations of the egg isolation method used in this study and its potential impact on the results. After placing 1 mL of the concentrated sample in the Bogorov counting chamber and diluting it with filtered seawater, calanoid eggs were counted as the average of three replicates under a dissecting microscope (Olympus, SZX7, Tokyo, Japan). The egg abundance was converted to eggs per unit area (eggs·m−2), and the proportion of normal and abnormal eggs was simultaneously confirmed while counting the eggs.
Calanoid eggs were identified based on published descriptions (Kasahara et al., 1974; Belmonte et al., 1997). Normal and abnormal calanoid eggs were identified following the procedures of Poulet et al. (1995); Ban et al. (2000) and Choi et al. (2021), and the eggs were photographed using a high-magnification optical microscope (Nikon ECLIPSE 80i; Nikon, Japan). In this study, abnormal eggs were sorted into various categories based on particular characteristics, such as eggs with unusual shapes and leaking egg contents, eggs that failed to develop and hatch, eggs that produced deformed nauplii, and eggs with no cracks but missing egg contents (Figure 2).
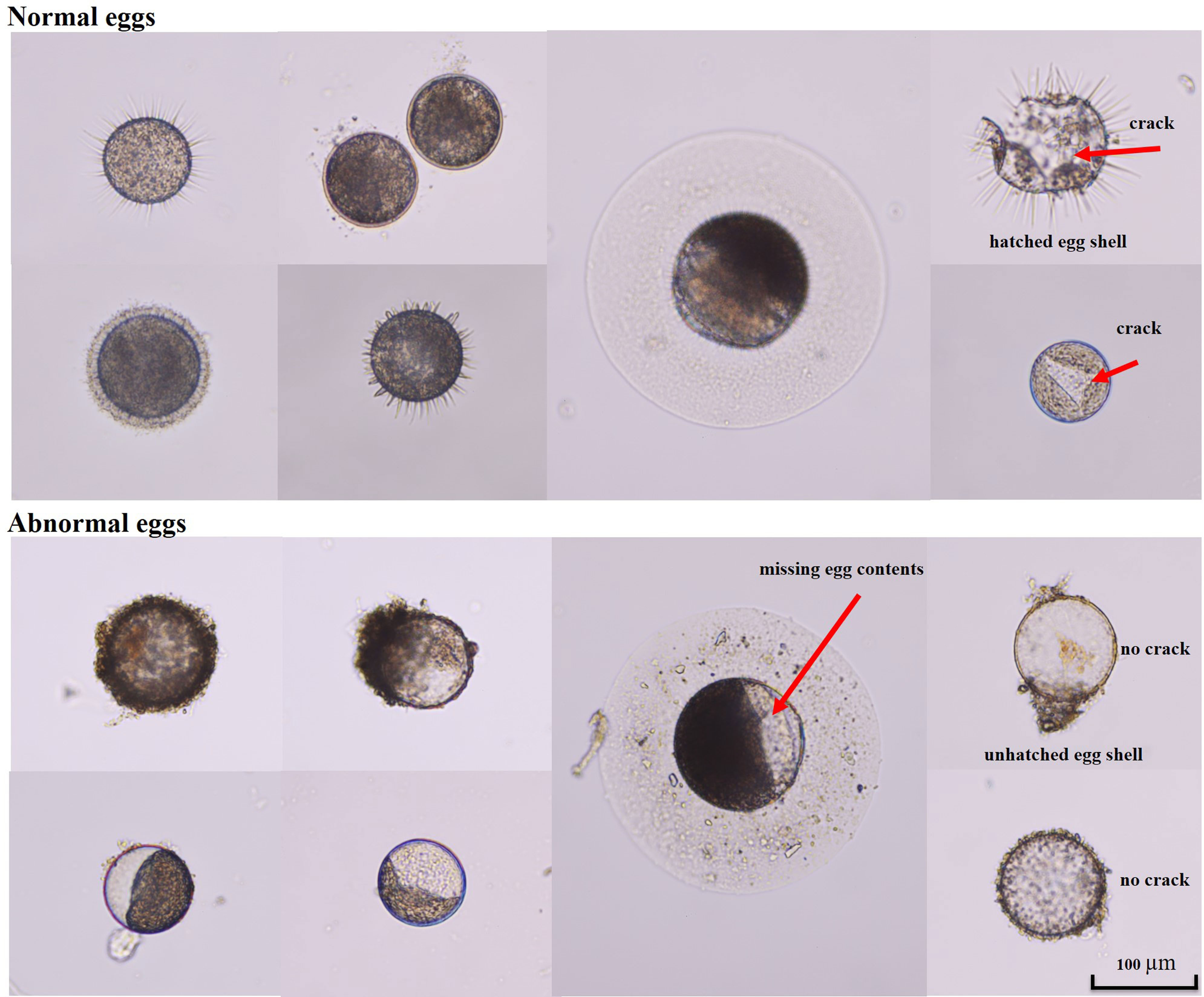
Figure 2 Normal and abnormal eggs of calanoid copepod, collected from benthic sediments in the Southern Sea of Korea in summer.
We performed Pearson’s test to determine the correlation between the ratio of normal to abnormal eggs and the following parameters: DO concentration, egg abundance, and chlorophyll-a concentration. The confidence interval was 95% for each of the correlations. All statistical tests were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), with a significance level of p < 0.05.
The water temperature in the survey area ranged from 24.3 to 31.1 °C in the surface layer and 15.8 to 30.3 °C in the near-bottom water (Figures 3A, B). Based on measurements at Namhae, the water temperature of the eastern stations (S1 to S7) at the bottom was lower than that of the western stations (S8 to S17), by more than 5 °C on average. The surface and bottom salinities were 29.4 to 32.7 and 29.6 to 34.1, and the average salinity was 31.3 and 32.2, respectively (Figures 3C, D). At S3, located in Jinhae Bay, the salinity of the surface and near-bottom water was significantly different. Salinity of less than 30 was observed in the surface and near-bottom water in S15, the inner station of Yeoja Bay. The surface layer chlorophyll-a concentration in the study area ranged from 1.4 to 26.2 μg·L-1, and that of the near-bottom water ranged from 1.6 to 10.8 μg·L-1 (Figures 4A, B). The highest surface and bottom chlorophyll-a concentrations were found in S1 (26.2 μg·L-1) and S15 (10.8 μg·L-1), respectively. The range of dissolved oxygen in the surface layer varied between 3.71 and 7.72 mg·L-1, and in the bottom ranged from 0.55 to 5.58 mg·L-1 (Figures 4C, D). A low DO concentration of 2 mg·L-1 or less was observed at some stations (S1, S2, and S3) in Jinhae Bay.
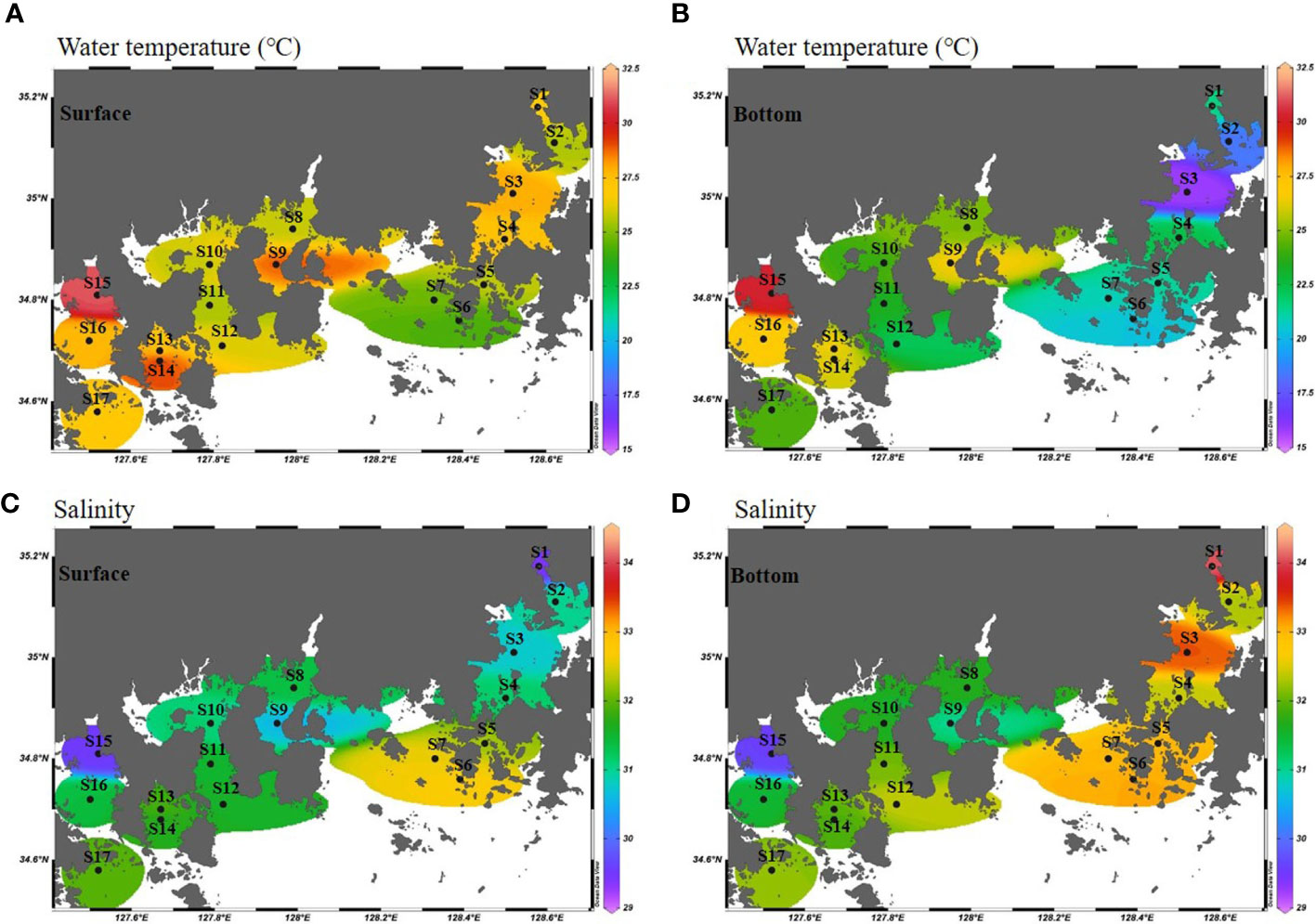
Figure 3 Horizontal distribution of water temperature (°C) (A, B) and salinity (C, D) between the surface (0 m) and near-bottom water (B-1 m) of the Southern Sea of Korea in summer.
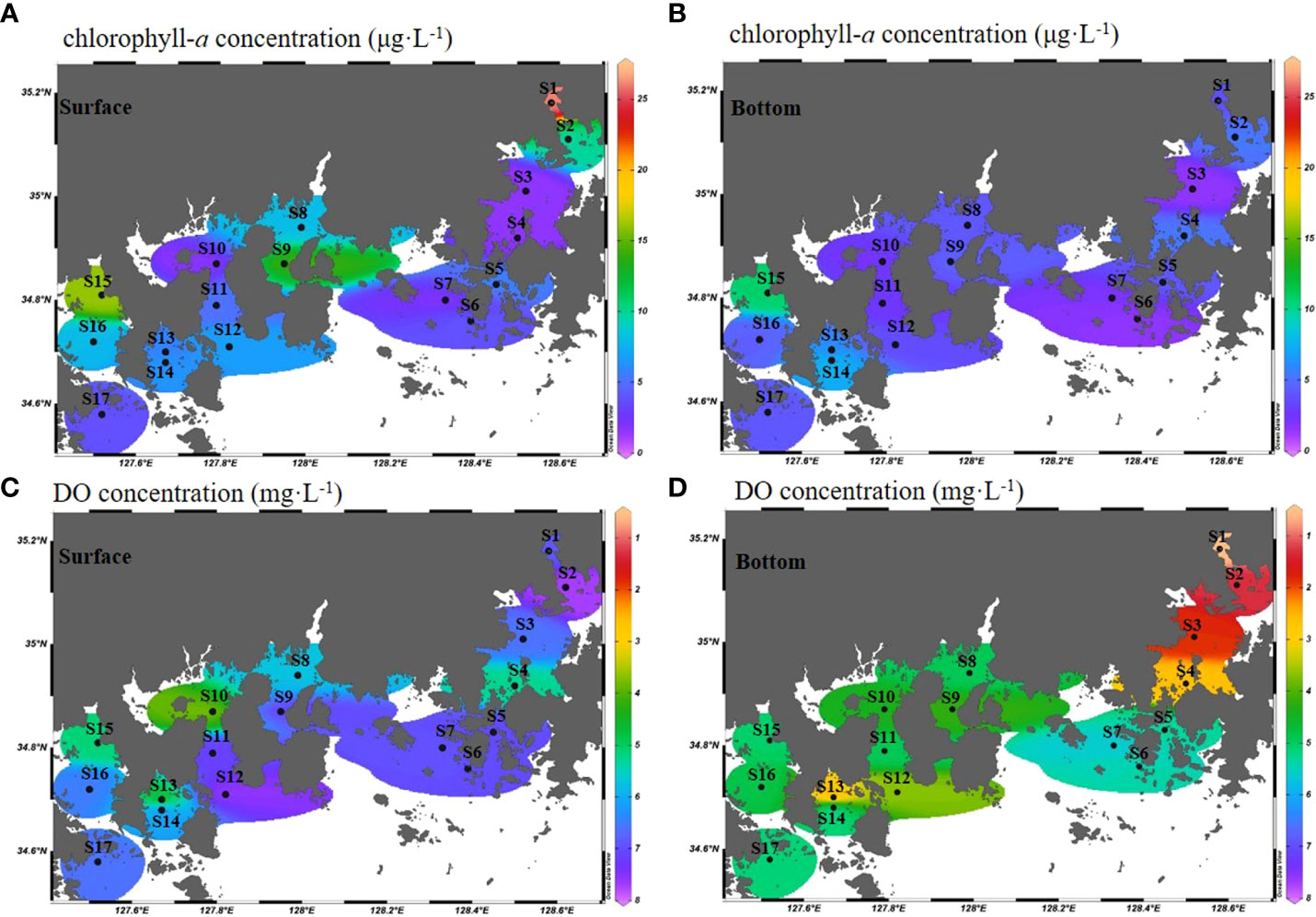
Figure 4 Horizontal distribution of chlorophyll-a concentrations (μg·L-1) (A, B) and dissolved oxygen concentrations (mg·L-1) (C, D) between the surface (0 m) near-bottom water (B-1 m) of the Southern Sea of Korea in summer.
A total of 12 species of Calanoida were observed in the summer (early August) in the Southern Sea of Korea, and showed different prevalence characteristics depending on the station (Figures 5A, B). Paracalanus sp. accounted for more than 63% of the calanoid copepod population in the surveyed area and reached population abundances of more than 1,000 individuals·m-3 in S5, S8, and S16. Acartia erythraea was absent at three stations (S10, S12, and S17); in contrast, the abundance of this species was found to be greater than 1,000 individuals·m-3 at S13. Acartia erythraea showed high abundance, especially at the stations in the inner bay (Jinhae, Gamak and Yeoja Bay), and was almost ubiquitous. Acartia ohtsukai appeared at S8 and S9 located in Jinju Bay, and S10 located in Gwangwang Bay, and A. sinjiensis only appeared at S4, S5, and S6 located nearshore waters of Jinhae Bay. Tortanus forcipatus appeared at all of the western stations (S10 to S17), but only at S5 among the eastern stations. Acartia sp. accounted for more than 5% of the population of Calanoida in the surveyed area and represented more than 80% of the calanoid species at S1.
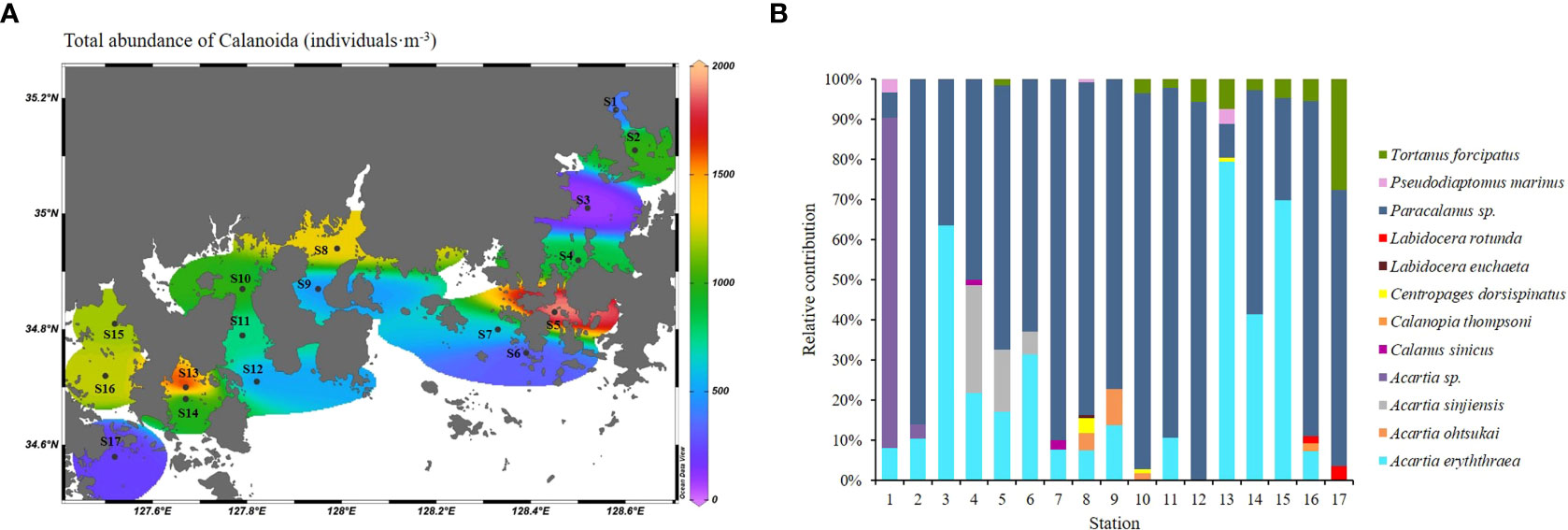
Figure 5 Horizontal distribution of Calanoida (A) and relative contribution (%) (B) of the Southern Sea of Korea in summer.
Overall, the abundance of calanoid eggs was higher at the nearshore stations located in the bay (Figure 6A). Egg abundance ranged from 0.004 to 2.389 × 106 eggs·m−2, being highest at S1 and lowest at S6. An egg abundance of 0.2 × 106 eggs·m−2 or more was confirmed at a total of five stations (S1, S9, S13, S15, and S17).

Figure 6 The abundance of calanoid eggs (A) (y-axis is divided into two parts, each with its own linear scale) and relative composition of normal and abnormal eggs (B) in sediments of the Southern Sea of Korea in summer.
The ratio of abnormal eggs ranged from 0 to 92.7% (Figure 6B). At S1, S2, and S3, the proportion of abnormal eggs accounted for more than 80%. Approximately 54% of eggs collected at station S13, in the nearshore waters of Gamak Bay, were abnormal. At stations located to the west of Geoje (S5, S6, and S7), and the stations located in Gwangyang Bay (S10, S11, and S12), the collected eggs were 100% normal. We found an increasing incidence of abnormal eggs in areas with decreasing DO concentrations (r2 = 0.734, p < 0.01) (Figure 7A), indicating a clearly negative correlation between the proportion of abnormal eggs and the DO concentration. The abundance of calanoid eggs was positively correlated with chlorophyll-a concentration (r2 = 0.485, p < 0.05) (Figure 7B).
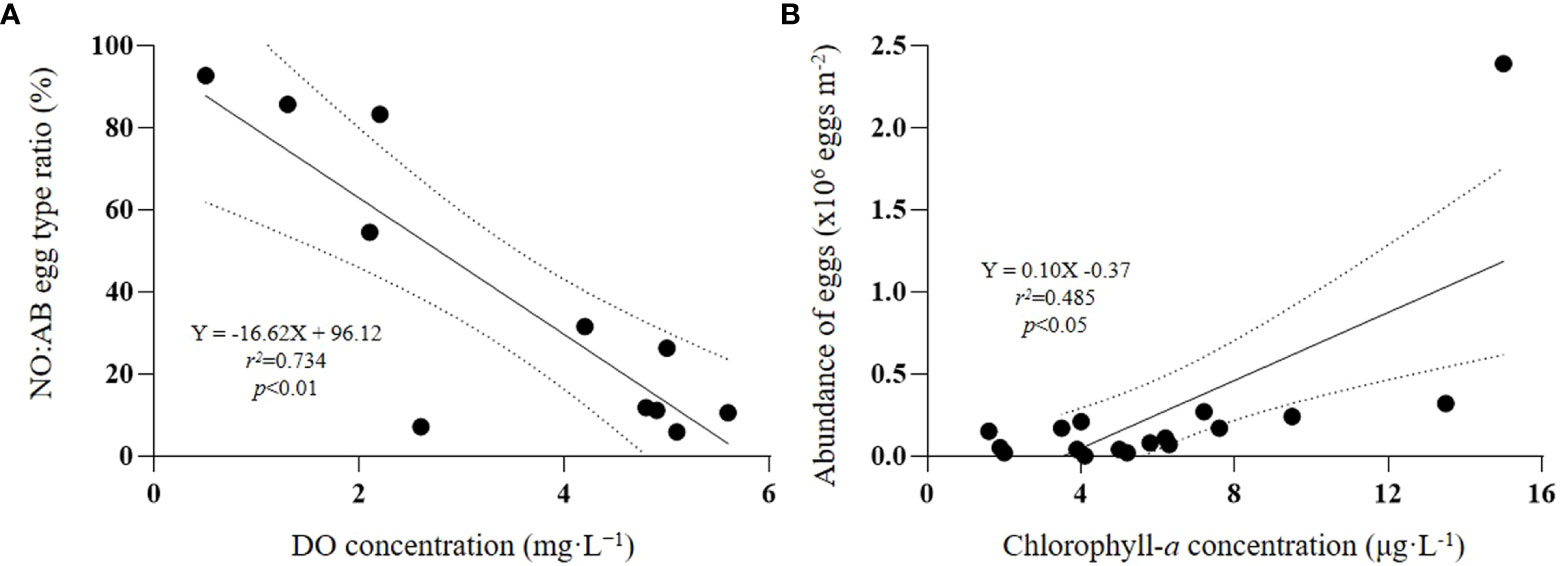
Figure 7 Linear regression between normal: Abnormal egg type ratio and near-bottom DO concentration (A) and abundance of calanoid eggs and chlorophyll-a concentration (B), including 95% confidence intervals (dashed).
The near-bed strata of the water column DO concentration was in the range of 0.55 to 5.58 mg·L-1, and hypoxia was evident at several stations, with a range similar to that previously reported (Choi et al., 2005; Kim et al., 2006). Dead zones created by the depletion of dissolved oxygen in coastal waters are one of the most widespread and harmful anthropogenic threats to marine ecosystems worldwide (Gooday et al., 2009; Rabalais et al., 2010). The decrease in DO concentration in summer and hypoxia of the near-bed water on the southern coast of Korea affected the ratio of normal to abnormal eggs. Previous studies have shown that eggs exhibit varying responses depending on the duration of exposure to low oxygen or anoxic conditions (Invidia et al., 2004; Katajisto, 2004; Nielsen et al., 2006). In laboratory experiments, low DO concentrations have been shown to negatively affect the hatching success of non-diapause calanoid eggs (Marcus and Lutz, 1994; Marcus et al., 1994). Furthermore, Marcus (2001) reported that diapause eggs were able to withstand significant periods of exposure to anoxic conditions and toxic levels of hydrogen sulfide. Only calanoid eggs (as a stage) were considered in the present study. Calanoid eggs can be functionally different: diapausal and quiescent (subitaneous). Thus, a limitation of this study was that subitaneous eggs were not differentiated from diapausal ones; therefore, we cannot draw conclusions on how the functional status of the eggs (subitaneous vs. diapausal) might be correlated with egg abundance and/or abnormalities.
The egg abundance (0.004–2.389 × 106 eggs·m−2) recorded at the south coast stations was similar to that reported in other studies of marine and estuarine systems (Table 1). There were differences in egg abundance between stations, probably due to differences in sediment heterogeneity. Glippa et al. (2014) also noted high inter-station and replicate variability, which may be a problem of sediment heterogeneity that characterizes the study area. Although the particle size of the sediments was not measured in this study, the particle size of the sediments in Jinhae Bay corresponded to the “very fine” grade, in which 92% of the samples had an average grain size of 8 µm or less. Previous studies confirmed that the average grain size of Jinju Bay was 7–8 µm (Kim et al., 1988; Cho and Lee, 2012). The average grain size of the sediments in was in the range of 7–9 µm in Gwangyang Bay (Ryu et al., 2003), 7.0–8.8 μm in Gamak Bay (Kim et al., 2012), and 8.46 µm in Yeoja Bay (Choi et al., 2007).
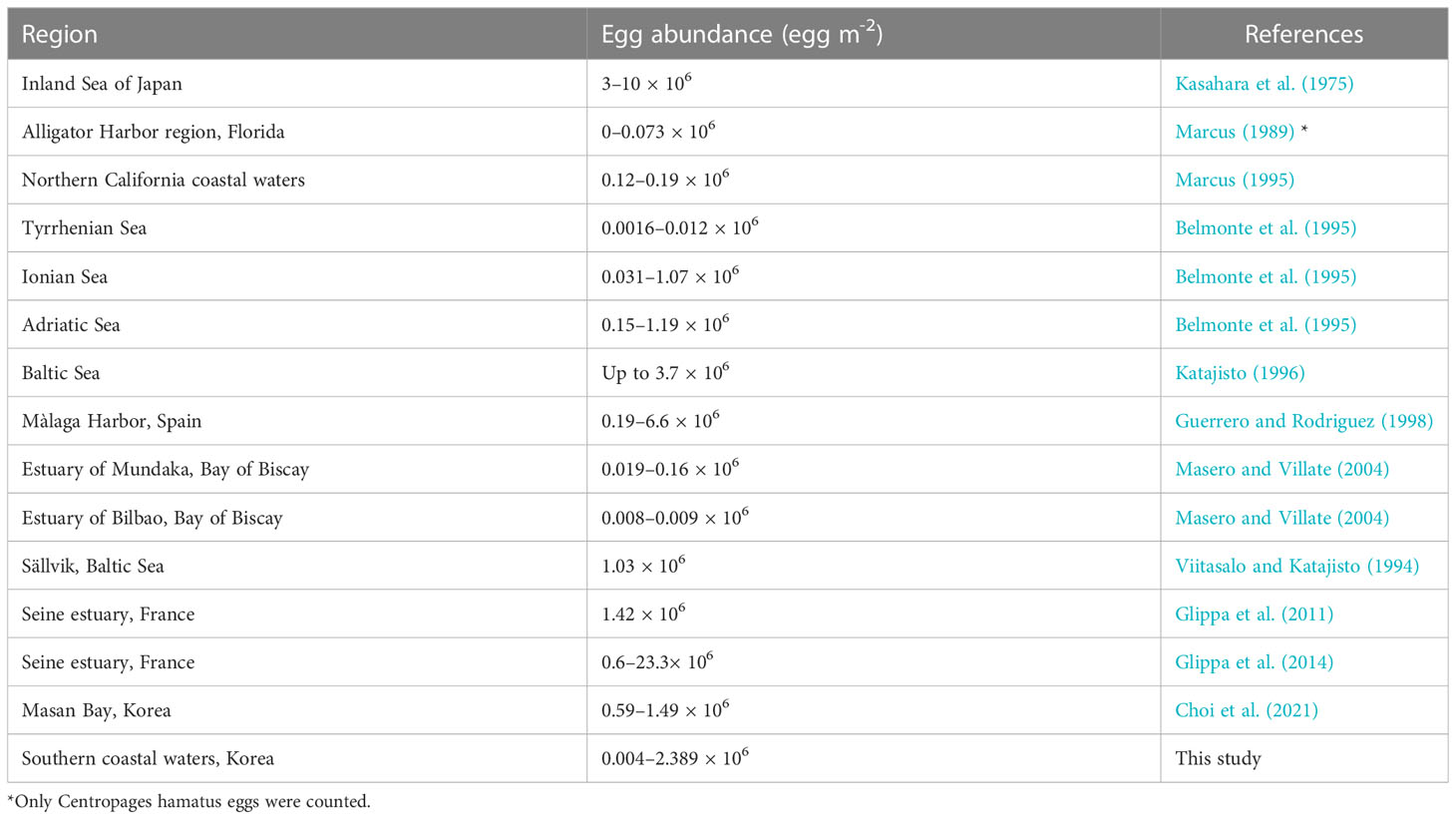
Table 1 Comparison of calanoid egg abundance in benthic sediments from various locations around the world.
We did not identify calanoid eggs at the species level, but a significant number of eggs are believed to originate from Calanoida in the study area. A total of 12 species of marine calanoids have been identified in the plankton along the southern coast of Korea; the predominant species were Paracalanus sp., Acartia spp. (A. erythraea, A. ohtsukai, A. sinjiensis, and Acartia sp. indet.), and Tortanus forcipatus. However, not all species eggs are likely to be observed in the sediment samples. For example, Paracalanus parvus may not produce dormant eggs (Næss, 1996) and some may have eggs that are too fragile to withstand sediment abrasion (Marcus, 1991). Conversely, it was reported that the eggs of T. forcipatus were found to have the strongest chorions compared to other calanoids (Uye et al., 1984). Therefore, the abundance of eggs in the sediments may not reflect the actual abundance of calanoid species in the water column, as the eggs of some species may not be present in the sediment samples.
Low DO conditions have a negative effect on calanoid egg production (Sedlacek and Marcus, 2005), and may cause growth retardation (Richmond et al., 2006). Acartia tonsa exposed to hypoxia showed ecologically adaptable behavior by reducing feeding (Elliott et al., 2013). Metabolic activity and respiration in Calanoida decrease significantly with body size (Hirst and Sheader, 1997; Mauchline, 1998). Decreased DO concentrations can lead to decreased metabolism in a variety of zooplankton, as reported in various studies. Our data did not directly show this effect. In our results, egg abundance was positively correlated with the chlorophyll concentration at the hypoxic stations. This is most likely due to the fact that phytoplankton generated in the water column sink to the bottom and are decomposed by microorganisms, thus promoting oxygen consumption (Hoegh-Guldberg and Bruno, 2010). However, it is difficult to describe distribution characteristics by associating only specific variables in situ. Marcus et al. (2004) proposed a method to accurately predict the DO reduction effect, considering the interaction effect of temperature and food concentration.
Environmental fluctuations, such as hypoxia and anoxia, occur frequently in the southern coast of Korea. In the present study, in Jinhae Bay and Gamak Bay, where hypoxia occurred, water quality has severely deteriorated; massive algal blooms occurr every year, and hypoxia has increased every year in the near bottom sediment during summer (June-September) (Kim et al., 2006; Lim et al., 2006; Lee et al., 2009). Thus, the population of Calanoida in these two regions (Jinhae and Gamak Bay) may experience higher mortality rates and may show more significant nauplii recruitment from sediment than in other regions investigated in the study. The high egg abundance of calanoids in hypoxic regions identified in this study may be one key for population maintenance under adverse conditions. Despite the high egg abundance observed in hypoxic regions in Jinhae Bay and Gamak Bay, there was no correlation found between the abundance of mature calanoids and egg abundance. This may be due to the presence of diapausal eggs, which are known to accumulate in an egg bank and can persist for multiple seasons or years (Marcus et al., 1994; Marcus, 1996). As such, the high egg numbers found in these hypoxic regions could be a result of diapausal eggs produced in previous years rather than a direct relationship with calanoid abundance.
In the present study, we found that the proportion of abnormal eggs was higher at hypoxic stations, indicating the potential impact of long-term exposure to hypoxia on egg abnormalities (Choi et al., 2021). This is consistent with previous research that has shown that exposure to hypoxic or anoxic conditions can affect the eggs of Calanoida (Katajisto, 2004; Richmond et al., 2006). Marine calanoids lay two types of eggs: subitaneous eggs, which can hatch within hours to days after spawning, and diapause eggs, which must complete a dormancy (refractory) period before hatching (Grice and Marcus, 1981; Glippa et al., 2014; Belmonte and Rubino, 2019). Subitaneous eggs have higher metabolic demands and are unable to tolerate prolonged exposure to hypoxia, unlike diapause eggs, which can survive in deeper layers of sediment (Dahms et al., 2006; Hansen and Drillet, 2013; Roman et al., 2019). Short-term exposure to anoxia did not significantly affect egg hatching success of subitaneous eggs in Acartia tonsa; however, hatching generally decreased with increasing exposure time (Nielsen et al., 2006). As the anoxic exposure time increased, egg viability of subitaneous eggs in A. tonsa decreased after incubation periods of 15 and 32 days (Invidia et al., 2004). The origin of the eggs in present study was not investigated, and further experiments are needed to determine how the type of eggs affects survival when exposed to long-term anoxic conditions in a laboratory. Nevertheless, the findings of this study demonstrate the potential for hypoxia to cause egg abnormalities in Calanoida.
In conclusion, we confirmed that the abundance of calanoid eggs on the southern coast of Korea is similar to what it has been found in other estuaries and coastal waters. In addition, the high rate of abnormal eggs suggested a negative effect of hypoxia and changes in egg morphology due to long-term exposure to hypoxic conditions. Eggs in the sediment may experience strong hypoxic conditions over many years, eventually changing the structure and function of ecosystems and plankton communities. The viability of eggs decreases with increasing exposure to hypoxia, and eggs may not hatch, or may hatch into deformed nauplii, as has also been emphasized by Choi et al. (2021). These changes in eggs have considerable potential to serve as indicators of quality in marine ecosystems, such as hypoxic conditions due to summer climate change. The high prevalence of abnormal eggs can be used as a tool to detect DO stress in situ.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SYC conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft. HYS conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft. KS conceived and performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft. SWJ conceived and performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft. MCJ contributed to funding acquisition, conceptualization of the experiments, data interpretation and discussion, authored or reviewed drafts of the paper, and approved the final draft. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Korea Institute of Ocean Science and Technology (PEA0111), and the ‘Techniques development for management and evaluation of biofouling on ship hulls (20210651)’, funded by the Ministry of Oceans and Fisheries, Korea.
We thank the reviewers for their valuable comments and suggestions to improve the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altieri A. H., Gedan K. B. (2015). Climate change and dead zones. Glob. Change Biol. 21, 1395–1406. doi: 10.1111/gcb.12754
Baek S. H., Shin K. S., Hyun B. G., Jang P. G., Kim H. S., Hwang O. M. (2010). Distribution characteristics and community structure of phytoplankton in the different water masses during early summer of southern sea of Korea. Ocean Polar Res. 32, 1–13. doi: 10.4217/OPR.2010.32.1.001
Ban S., Lee H. W., Shinada A., Toda T. (2000). In situ egg production and hatching success of the marine copepod Pseudocalanus newmani in funka bay and adjacent waters off southwestern Hokkaido, Japan: Associated to diatom bloom. J. Plankton Res. 22, 907–922. doi: 10.1093/plankt/22.5.907
Baumgartner M. F., Tarrant A. M. (2017). The physiology and ecology of diapause in marine copepods. Ann. Rev. Mar. Sci. 9, 387–411. doi: 10.1146/annurev-marine-010816-060505
Belmonte G. (1992). Diapause egg production in Acartia (Paracartia) latisetosa (Crustacea, copepoda, calanoida). Ital. J. Zool. 59, 363–366. doi: 10.1080/11250009209386694
Belmonte G., Castello P., Piccinni M. R., Quarta S., Rubino F., Geraci S., et al. (1995). “Resting stages in marine sediments off the Italian coast,” in Biology and ecology of shallow coastal waters. Eds. Elefteriou A., Ansel A. D., Smith C. J. (Olsen and Olsen: Fredensborg), 53–58.
Belmonte G., Miglietta A., Rubino F., Boero F. (1997). Morphological convergence of resting stages of planktonic organisms: A review. Hydrobiologia 355, 159–165. doi: 10.1023/A:1003071205424
Belmonte G., Pati A. C. (2007). Hatching rate and diapause duration in eggs of Paracartia latisetosa (Copepoda: Calanoida). J. Plankton Res. 29, i39–i47. doi: 10.1093/plankt/fbl064
Belmonte G., Rubino F. (2019). Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. 57, 1–88. doi: 10.1201/9780429026379-1
Breitburg D. (2002). Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries Coast. 25, 767–781. doi: 10.1007/BF02804904
Chen X., Shen Z., Li Y., Yang Y. (2015). Physical controls of hypoxia in waters adjacent to the Yangtze estuary: A numerical modeling study. Mar. pollut. Bull. 97, 349–364. doi: 10.1016/j.marpolbul.2015.05.067
Cho Y. G., Lee C. B. (2012). Heavy metal contamination in surface sediments from masan and jinhae bay, southeast coast of Korea. J. Korean Soc Mar. Environ. Energy 15, 302–313. doi: 10.7846/JKOSMEE.2012.15.4.302
Choi S. Y., Hyun B., Jang P. G., Shin K., Soh H. Y., Kang J. H., et al. (2021). Effects of hypoxia on the distribution of calanoid copepod eggs in the seabed sediments of the eutrophic masan bay, Korea. Water 13, 3116. doi: 10.3390/w13213116
Choi K. H., Jang M. C., Shin H. H., Lee W. J., Shin K. (2016). In situ hatching success of calanoid copepod eggs in hypoxic sediments of a coastal bay. J. Coast. Res. 32, 333–338. doi: 10.2112/JCOASTRES-D-14-00096.1
Choi J. W., Seo J. Y., Lee C. H., Ryu T. K., Sung C. G., Han G. M., et al. (2005). Spatial distribution patterns of macrobenthic communities during winter and summer in the masan bay special management area, southern coast of Korea. Ocean Polar Res. 27, 381–395. doi: 10.4217/OPR.2005.27.4.381
Choi J. M., Woo H. J., Lee Y. G. (2007). Suspended sediments influx and variation of surface sediments composition in semi-enclosed bay-spring season in yeoja bay south coast of Korea. J. Korean Soc Mar. Environ. Energy 10, 1–12.
Conley D. J., Björck S., Bonsdorff E., Carstensen J., Destouni G., Gustafsson B. G., et al. (2009). Hypoxia-related processes in the Baltic Sea. Environ. Sci. Technol. 43, 3412–3420. doi: 10.1021/es802762a
Dahms H. U., Li X., Zhang G., Qian P. Y. (2006). Resting stages of Tortanus forcipatus (Crustacea, calanoida) in sediments of Victoria harbor, Hong Kong. Estuar. Coast. Shelf Sci. 67, 562–568. doi: 10.1016/j.ecss.2005.12.011
Diaz R. J., Rosenberg R. (2008). Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. doi: 10.1126/science.1156401
Du J., Shen J., Park K., Wang Y. P., Yu X. (2018). Worsened physical condition due to climate change contributes to the increasing hypoxia in Chesapeake bay. Sci. Total Environ. 630, 707–717. doi: 10.1016/j.scitotenv.2018.02.265
Elliott D. T., Pierson J. J., Roman M. R. (2013). Predicting the effects of coastal hypoxia on vital rates of the planktonic copepod Acartia tonsa Dana. PloS One 8, e63987. doi: 10.1371/journal.pone.0063987
Glippa O., Denis L., Lesourd S., Souissi S. (2014). Seasonal fluctuations of the copepod resting egg bank in the middle seine estuary, France: impact on the nauplii recruitment. Estuar. Coast. Shelf Sci. 142, 60–67. doi: 10.1016/j.ecss.2014.03.008
Glippa O., Souissi S., Denis L., Lesourd S. (2011). Calanoid copepod resting egg abundance and hatching success in the sediment of the seine estuary (France). Estuar. Coast. Shelf Sci. 92, 255–262. doi: 10.1016/j.ecss.2010.12.032
Gooday A. J., Jorissen F., Levin L. A., Middelburg J. J., Naqvi S. W. A., Rabalais N. N., et al. (2009). Historical records of coastal eutrophication-induced hypoxia. Biogeosciences 6, 1707–1745. doi: 10.5194/bg-6-1707-2009
Grice G. D., Marcus N. H. (1981). Dormant eggs of marine copepods. Oceanogr. Mar. Biol. Annu. Rev. 19, 125–140.
Guerrero F., Rodriguez V. (1998). Existence and significance of e resting eggs (Copepoda: Calanoida) in sediments of a coastal station in the alboran Sea (SE Spain). J. Plankton Res. 20, 305–314. doi: 10.1093/plankt/20.2.305
Hagy J. D., Boynton W. R., Keefe C. W., Wood K. V. (2004). Hypoxia in Chesapeake bay 1950–2001: long-term change in relation to nutrient loading and river flow. Estuaries Coast. 27, 634–658. doi: 10.1007/BF02907650
Hansen B. W., Drillet G. (2013). Comparative oxygen consumption rates of subitaneous and delayed hatching eggs of the calanoid copepod Acartia tonsa (Dana). J. Exp. Mar. Biol. Ecol. 442, 66–69. doi: 10.1016/j.jembe.2013.01.029
Hansen B. W. (2019). Copepod embryonic dormancy: “an egg is not just an egg”. Biol. Bull. 237, 145–169. doi: 10.1086/705546
Hirst A. G., Sheader M. (1997). Are in situ weight-specific growth rates body-size independent in marine planktonic copepods? A re-analysis of the global syntheses and a new empirical model. Mar. Ecol. Prog. Ser. 154, 155–165. doi: 10.3354/meps154155
Hoegh-Guldberg O., Bruno J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528. doi: 10.1126/science.1189930
Invidia M., Sei S., Gorbi G. (2004). Survival of the copepod Acartia tonsa following egg exposure to near anoxia and to sulfide at different pH values. Mar. Ecol. Prog. Ser. 276, 187–196. doi: 10.3354/meps276187
Jessen G. L., Lichtschlag A., Ramette A., Pantoja S., Rossel P. E., Schubert C. J., et al. (2017). Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Sci. Adv. 3, e1601897. doi: 10.1126/sciadv.1601897
Kasahara S., Uye S. I., Onbé T. (1974). Calanoid copepod eggs in sea-bottom muds. Mar. Biol. 26, 167–171. doi: 10.1007/BF00388886
Kasahara S., Uye S. I., Onbé T. (1975). Calanoid copepod eggs in sea-bottom muds. II. seasonal cycles of abundance in the populations of several species of copepods and their eggs in the inland Sea of Japan. Mar. Biol. 31, 25–29. doi: 10.1007/BF00390644
Katajisto T. (1996). Copepod eggs survive a decade in the sediments of the Baltic Sea. Hydrobiologia 320, 153–159. doi: 10.1007/BF00016816
Katajisto T. (2004). Effects of anoxia and hypoxia on the dormancy and survival of subitaneous eggs of Acartia bifilosa (Copepoda: Calanoida). Mar. Biol. 145, 751–757. doi: 10.1007/s00227-004-1361-3
Katajisto T. (2006). Benthic resting eggs in the life cycles of calanoid copepods in the northern Baltic Sea. PhD thesis. (Finland: University of Helsinki), 46 pp.
Kim D. C., Kim H. J., Song Y. S., Paik I. S., Park M. E., Chung S. Y., et al. (1988). Provenance of recent clay minerals of the jinju bay, southern coast of Korea. Korean. J. Fish. Aquat. Sci. 21, 246–258.
Kim J. B., Lee S. Y., Yu J., Choi Y. H., Jung C. S., Lee P. Y. (2006). The characteristics of oxygen deficient water mass in gamak bay. J. Korean Soc Mar. Environ. Energy 9, 216–224.
Kim P. J., Park S. Y., Kim S. S., Jang S. J., Jeon S. B., Ju J. S. (2012). Biogeochemistry of alkaline and alkaline earth elements in the surface sediment of the gamak bay. J. Korean Soc Mar. Environ. Saf. 18, 1–13. doi: 10.7837/kosomes.2012.18.1.001
Kim S. H., Pang I. C. (2005). Distribution and characteristic of transport mechanism of eggs and larvae of anchovy, Engraulis japonica, in the southwestern Sea of Korea in July and novembe. J. Korean Fish. Soc 38, 331–341. doi: 10.5657/kfas.2005.38.5.331
Ko J. C., Seo Y. L., Kim H. Y., Lee S. K., Cha H. K., Kim J. I. (2010). Distribution characteristics of eggs and larvae of the anchovy Engraulis japonica in the yeosu and tongyeong coastal waters of Korea. Korean J. Ichthyol. 22, 256–266.
Kodama K., Horiguchi T. (2011). Effects of hypoxia on benthic organisms in Tokyo bay, Japan: A review. Mar. pollut. Bull. 63, 215–220. doi: 10.1016/j.marpolbul.2011.04.022
Lai Y., Jia Z., Xie Z., Li S., Hu J. (2022). Water quality changes and shift in mechanisms controlling hypoxia in response to pollutant load reductions: A case study for shiziyang bay, southern china. Sci. Total Environ. 842, 156774. doi: 10.1016/j.scitotenv.2022.156774
Lee M. O., Kim J. K. (2008). Characteristics of algal blooms in the southern coastal waters of Korea. Mar. Environ. Res. 65, 128–147. doi: 10.1016/j.marenvres.2007.09.006
Lee M., Kim B., Kwon Y., Kim J. (2009). Characteristics of the marine environment and algal blooms in gamak bay. Fish. Sci. 75, 401–411. doi: 10.1007/s12562-009-0056-6
Lee T., Kim H. C., Son Y. B. (2019). Sediment oxygen consumption and hydrogen sulfide release in hypoxic areas of gamak bay, Korea. Appl. Ecol. Environ. Res. 17, 3199–3214. doi: 10.15666/aeer/1702_31993214
Lee C. W., Min B. Y. (1990). Pollution in masan bay, a matter of concern in south Korea. Mar. pollut. Bull. 21, 226–229. doi: 10.1016/0025-326X(90)90338-9
Lim H. S., Diaz R. J., Hong J. S., Schaffner L. C. (2006). Hypoxia and benthic community recovery in Korean coastal waters. Mar. pollut. Bull. 52, 1517–1526. doi: 10.1016/j.marpolbul.2006.05.013
Lukic D., Vad C. F., Horvath Z. (2016). Isolation by sugar flotation has no direct effect on the hatching success of zooplankton resting eggs. J. Limnol. 75, 415–241. doi: 10.4081/jlimnol.2016.1385
Marcus N. H. (1979). On the population biology and nature of diapause of Labidocera aestiva (Copepoda: Calanoida). Biol. Bull. 157, 297–305. doi: 10.2307/1541056
Marcus N. H. (1984). Recruitment of copepod nauplii into the plankton: importance of diapause eggs and benthic processes. Mar. Ecol. Prog. Ser. 15, 47–54. doi: 10.3354/meps015047
Marcus N. H. (1989). Abundance in bottom sediments and hatching requirements of eggs of Centropages hamatus (Copepoda: Calanoida) from the alligator harbor region, Florida. Biol. Bull. 176, 142–146. doi: 10.2307/1541581
Marcus N. H. (1991). Planktonic copepods in a sub-tropical estuary: seasonal patterns in the abundance of adults, copepodites, nauplii, and eggs in the sea bed. Biol. Bull. 181, 269–274. doi: 10.2307/1542098
Marcus N. H. (1995). Seasonal study of planktonic copepods and their benthic resting eggs in northern California coastal waters. Mar. Biol. 123, 459–465. doi: 10.1007/BF00349225
Marcus N. H. (1996). Ecological and evolutionary significance of resting eggs in marine copepods: past, present, and future studies. Hydrobiologia 320, 141–152. doi: 10.1007/BF00016815
Marcus N. H. (2001). ”Zooplankton: responses to and consequences of hypoxia“, in Coastal hypoxia: consequences for living resources and ecosystems Eds. Rabalais N.N., Turner R.E.(Washington, DC: American Geophysical Union). doi: 10.1029/CE058p0049
Marcus N. H., Boero F. (1998). The importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnol. Oceanogr. 43, 763–768. doi: 10.4319/lo.1998.43.5.0763
Marcus N. H., Lutz R. V. (1994). Effects of anoxia on the viability of subitaneous eggs of planktonic copepods. Mar. Biol. 121, 83–87. doi: 10.1007/BF00349476
Marcus N. H., Lutz R., Burnett W., Cable P. (1994). Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank. Limnol. Oceanogr. 39, 154–158. doi: 10.4319/lo.1994.39.1.0154
Marcus N. H., Lutz R. V., Chanton J. P. (1997). Impact of anoxia and sulfide on the viability of eggs of three planktonic copepods. Mar. Ecol. Prog. Ser. 146, 291–295. doi: 10.3354/meps146291
Marcus N. H., Richmond C., Sedlacek C., Miller G. A., Oppert C. (2004). Impact of hypoxia on the survival, egg production and population dynamics of Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 301, 111–128. doi: 10.1016/j.jembe.2003.09.016
Masero R., Villate F. (2004). Composition, vertical distribution and age of zooplankton benthic eggs in the sediments of two contrasting estuaries of the bay of Biscay. Hydrobiologia 518, 201–212. doi: 10.1023/B:HYDR.0000025063.22926.67
Næss T. (1996). Benthic resting eggs of calanoid copepods in Norwegian enclosures used in mariculture: Abundance, species composition and hatching. Hydrobiologia 320, 161–168. doi: 10.1007/BF00016817
Nielsen P., Mortensen J., Vismann B., Hansen B. W. (2006). Physiological tolerance of marine calanoid copepod eggs to sulphide. Mar. Ecol. Prog. Ser. 328, 171–182. doi: 10.3354/meps328171
Onoue Y., Toda T., Ban S. (2004). Morphological features and hatching patterns of eggs in Acartia steueri (Crustacea, copepoda) from sagami bay, Japan. Hydrobiologia 511, 17–25. doi: 10.1023/B:HYDR.0000014013.37891.46
Poulet S. A., Laabir M., Ianora A., Miralto A. (1995). Reproductive response of Calanus helgolandicus. i. abnormal embryonic and naupliar development. Mar. Ecol. Prog. Ser. 129, 85–95. doi: 10.3354/meps129085
Rabalais N. N., Díaz R. J., Levin L. A., Turner R. E., Gilbert D., Zhang J. (2010). Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7, 585–619. doi: 10.5194/bg-7-585-2010
Rabalais N. N., Harper D. E. Jr., Turner R. E. (2001). “Responses of nekton and demersal and benthic fauna to decreasing oxygen concentrations,” in Coastal hypoxia: Consequences for living resources and ecosystems. Eds. Rabalais N. N., Turner R. E. (Washington, DC: American Geophysical Union). doi: 10.1029/CE058p0115
Richmond C., Marcus N. H., Sedlacek C., Miller G. A., Oppert C. (2006). Hypoxia and seasonal temperature: short-term effects and long-term implications for Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 328, 177–196. doi: 10.1016/j.jembe.2005.07.004
.Roman M. R., Brandt S. B., Houde E. D., Pierson J. J. (2019). Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front. Mar. Sci 6, 139. doi: 10.3389/fmars.2019.00139
Ryu S. O., Kim J. Y., Lee H. J., Cho Y. G., Ahn S. M. (2003). Seasonal changes of tidal-flat sediments: Kwangyang bay, south coast of Korea. J. Korean Soc Oceanogr. 8, 349–356.
Sedlacek C., Marcus N. H. (2005). Egg production of the copepod Acartia tonsa: the influence of hypoxia and food concentration. J. Exp. Mar. Biol. Ecol. 318, 183–190. doi: 10.1016/j.jembe.2004.12.012
Tachibana A., Nomura H., Ishimaru T. (2019). Impacts of long-term environmental variability on diapause phenology of coastal copepods in Tokyo bay, Japan. Limnol. Oceanogr. 64, S273–S283. doi: 10.1002/lno.11030
Takayama Y., Toda T. (2019). Switch from production of subitaneous to delayed-hatching and diapause eggs in acartia japonica mori 1940(Copepoda: Calanoida) from sagami bay, Japan. Reg. Stud. Mar. Sci. 29, 100673. doi: 10.1016/j.rsma.2019.100673
Tang K. W., Dam H. G., Feinberg L. R. (1998). The relative importance of egg production rate, hatching success, hatching duration and egg sinking in population recruitment of two species of marine copepods. J. Plankton Res. 20, 1971–1987. doi: 10.1093/plankt/20.10.1971
Uriarte I., Villate F. (2006). First evidences of Acartia bifilosa resting eggs in sediments of the urdaibai estuary (Bay of biscay): Abundance and hatching success. Sci. Mar. 70, 565–572. doi: 10.3989/scimar.2006.70n4565
Uye S. I., Fleminger A. (1976). Effects of various environmental factors on egg development of several species of Acartia in southern California. Mar. Biol. 38, 253–262. doi: 10.1007/BF00388938
Uye S., Yoshiya M., Ueda K., Kasahara S. (1984). The effect of organic sea-bottom pollution on survivability of resting eggs of neritic calanoids. Crustaceana suppl. 7, 390–403.
Vaquer-Sunyer R., Duarte C. M. (2008). Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. U. S. A. 105, 15452–15457. doi: 10.1073/pnas.0803833105
Keywords: abnormal development, marine copepods, oxygen, sediment egg bank, chlorophyll
Citation: Choi SY, Soh HY, Shin K, Jung SW and Jang M-C (2023) Effects of hypoxia on benthic eggs of calanoid copepods in the Southern Sea of Korea. Front. Mar. Sci. 10:1132851. doi: 10.3389/fmars.2023.1132851
Received: 28 December 2022; Accepted: 28 February 2023;
Published: 13 March 2023.
Edited by:
Tsuneo Ono, Japan Fisheries Research and Education Agency (FRA), JapanReviewed by:
James J. Pierson, University of Maryland, College Park, United StatesCopyright © 2023 Choi, Soh, Shin, Jung and Jang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min-Chul Jang, bWNqYW5nQGtpb3N0LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.