
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 24 February 2023
Sec. Marine Conservation and Sustainability
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1116837
This article is part of the Research TopicAquatic One Health—The Intersection of Marine Wildlife Health, Public Health, and Our OceansView all 10 articles
Dinoflagellates belonging to the Perkinsus genus are OIE (World Organization for animal Health)-listed pathogens extremely virulent for clams and oysters in many marine ecosystems throughout the world. During the monitoring activities of the Mediterranean mussel (Mytilus galloprovincialis) in Campania region (Italy), the presence of typical trophozoites of Perkinsus sp. was observed in mussels from farms and natural banks. Simultaneously, following mussel mortality in the Spanish waters of Catalonia, histopathological studies revealed the presence of the same parasite. Although perkinsosis is an endemic disease in clams in Italy (with prevalence from 40 to 80%), there are no reports to date of its presence in Mediterranean mussels and of the effect on this species. For this study, histopathology, Ray’s Fluid Thioglycollate Medium (RFTM), and molecular diagnostics with conventional Polimerase Chain Reaction (PCR) and qPCR were performed. In samples from Italy, histopathology in the mussel from one farm revealed a prevalence of 26% in February 2019, 40% in February 2020, 16% in November 2020, and 23% in April 2021. In a natural bank, Perkinsus was also detected in May 2020 but in lower prevalence. In Spain, in July 2020, the presence of the parasite was 20% in one site and 10% in a second site and related to animal mortality. In both areas, Perkinsus sp. elicited multiple inflammatory capsules of different size or infiltrates at the level of the digestive gland and gonad. Molecular diagnostics of the Internal Transcriber Spacer (ITS) region of the rDNA (ITS1, 5.8S, and ITS2) showed a 97% similarity of P. olseni from Italy with samples from New Zealand, Australia, and Uruguay and in bivalves such as Pitar rostrata, Astrovenus sp., and Haliotis sp., whereas in Spain the identity was 99% samples from South Korean venerids such as Anadara granosa. Phylogenetic analysis group together P. olseni from Italian and Spanish mussels but place them distant from other P. olseni described in the clams from Europe (Italy, France, and Spain). Direct impact of transboundary animal diseases in aquaculture constitutes a serious consequence for export living animals and their products, as well for international trade. This compromises food security, also causing a high socioeconomic impact on aquaculture exporting nations. P. olseni is a generalist pathogen able to infect different bivalve species, possibly passing from clams to oysters and mussels. Recognized international organizations should take this into account in the view of possible cross-infection. Other studies are needed to define pathogen virulence in this species.
In the past years, infectious diseases are emerging significantly in marine and freshwater environments. The elements involved in this emergence are different. Cultivated animals are involved in the global trading, facilitating the introduction of serious infectious diseases, called transboundary diseases (TD). Several transboundary aquatic animal diseases (TAADS) have swept regions over the past 30 years causing massive economic and social losses, responsible for the introduction, establishment, and spread of pathogens into new geographic areas. Nowadays, there are several international codes of practice and guidelines to reduce the risk of introducing pathogens. WOHA (World Organization of Animal Health) has developed recommendations and protocols in the International Aquatic Animal Health Code, which deals with the health surveillance of aquatic animals (OIE Listed Diseases, 2021).
Emerging disease in mussels has been reported repeatedly in the past years, as in other bivalve species. Recently, the potentially zoonotic bacteria Nocardia crassotreae were reported in the Mediterranean mussel M. galloprovincialis (Carella et al., 2013; De Vico and Carella, 2019), the OIE listed parasite Marteilia refringens have been observed in the area (Carella et al., 2010), and many other emerging disease conditions have been also reported in other bivalve species in the same area (Carella et al., 2013; Carella et al., 2018).
Perkinsosis is an important disease that has been reported worldwide in bivalves and gastropods. Perkinsus pathogens can infect a wide range of hosts and possibly are responsible for mortality events for their extensive invasive ability and virulence. Nowadays, seven species within the genus Perkinsus have been reported including P. marinus, P. olseni, P. qugwadi, P. chesapeaki, P. mediterraneus, P. honshuensis, and P. beihaiensis (Ramilo et al., 2015) with only P. olseni and P. marinus listed notifiable parasites listed by OIE (OIE Listed Diseases, 2021).
Pathogens can display a highly flexible ranges of hosts, called multi-host or generalist pathogens, or can infect only one or a few related species and called specialist pathogens. P. olseni has been reported in 30 mollusc species, bivalves, and gastropods over a wide range of geographical locations (Itoïz et al., 2022). It is generally associated with mass mortality of clams such as Manila clams Ruditapes philippinarum in Europe, the venerid clam R. philippinarumin Asia, the cockle Austrovenus stutchburyi in New Zealand (Dungan et al., 2007), and in the abalone Haliotis spp in Australia. Recently, reports of perkinsosis in mussels have been increasing; Itoh et al. (2019) reported P. beihaiensis infection in the invasive M. galloprovincialis in Japan, whereas Vazquez et al. (2022) reported P. olseni in M. chilensis in Argentina.
In this study, we report, for the first time, the presence of the parasite Perkinsus sp. in the Mediterranean mussel M. galloprovincialis in Europe. First detection was in mussels from Italy, in Campania region, in mussel farms from 2019 to 2021 and later in natural beds in 2020. During the study, we also observed the presence of Perkinsus sp. like cells in mussel samples from Catalonia (Spain) following a mussel episode of mortality. Within the past few years, more data on the genetic variation within some of the Perkinsus species have become available, and many ITS (internal transcribed spaces) regions now described have that allowed to assess intraspecific variation and to compare the dissimilarity of sequence with the differences observed among the Perkinsus species. During the surveys, we conducted phylogenetic analyses to estimate the relationships with the group of Perkinsus spp. in mussels from Italy and Spain and haplotype characterization along with animal histopathology to define host response and possible Perkinsus pathogenicity.
During 2018–2021, a field survey targeting infectious agents of the Mediterranean mussel Mytilus galloprovincialis was conducted in which mussels were manually collected in one mussel farm and one natural bank on the north coast of the Campania region (Italy).
In Italy, collections were made in a mussel farm in Campania Region (Naples Bay) in February 2019 (N = 20), February 2020 (N = 20), November 2020 (N = 30), and April 2021 (N = 30). A sampling was performed in a close natural bank in May 2020 (N = 30). Alfacs Bay, located in the south of the Ebro delta (Western Mediterranean), is a shellfish growing area where mussels and oysters are grown in ropes hanging from rafts. Sampling in Alfacs Bay was conducted on 21 July 2020, 2 weeks after the beginning of the mortality event on 6 July 2020 as reported by the mussel farmers. Sampling in Alfacs Bay was conducted in two sites, A (40°37’15.28’’N; 0°39’14.58’’E) and B (40°37’1.98”N; 0°37’51.66”E) (Figure 1). The mussels were transported to the laboratory alive in isothermal boxes. Prior to processing, the animals were measured for animal shell length, total weight, and meat weight (MW) according to Galtsoff (1964).
For animal histopathology, from each animal, a transverse section including digestive tissue, gill, foot, and gonad was obtained and fixed in Davidson’s solution for 72–96h. Fixed tissues were embedded in paraffin blocks and sectioned at 3 µm with a rotary microtome (Bioptica, Italy). Tissue sections were deparaffinised, stained with Carazzi haematoxylin and eosin and a special stain such as Mallory’s trichrome (Mazzi, 1977). Digital images and measurements were obtained using an integrated Axioscope A1 (Zeiss, Germany) and camera Axiocam 208.
From each specimen, 25–30 mg of gonad, digestive tissue and gills, preserved in TE buffer at −20°C, was taken for total genomic DNA extraction using QIAamp DNA Mini Kit (QIAGEN, Germany), according to the manufacturer’s instructions (tissue protocol). The DNA quality and quantity were measured with the use of a Nanodrop spectrophotometer (Thermo Fisher Scientific) and stored at −80°C for long-term preservation. Primers used in the study are listed in Table 1. More in detail, PCR assays using the generic primers PerkITS750/PerkITS85 (Casas et al., 2002) were carried out first to detect Perkinsus spp. in all sampled mussels. PCR reactions were carried out in 50 μl of final volume using the Mastermix GoTaq polymerase (Promega) following the instructions of the manufacturer. A positive control provided by IRTA institute constituted by a clam infected P. olseni was included in each reaction along with a negative control (master mix with no DNA). Amplification parameters were performed as follows: An initial denaturation of 4 min at 94°C followed by 35 cycle amplifications (1 min at 94°C, 1 min at 53°C, and 3 min at 68°C) and a final extension of 5 min at 68°C. The resulting PCR products were purified and sent to an external sequencing facility (Eurofins Genomics, Germany).
To better define pathogen presence in the Italian samples, a more sensitive procedure of real-time quantitative PCR (qPCR) was also performed using primers Perk-ITS-qF1/Perk-ITS-qR2 (Ríos et al., 2020) that amplifies the internal transcribed spacer region (ITS-1 and ITS-2) of the gene complex that codes for ribosomal RNAs in P. olseni. Wells were filled to a final volume of 10 µl, using 1 µl of DNA, 5 µl of Taq Universal SYBR green mix (Applied Biosystem), 0.5 µl of each primer (10 µM), and 3 µl of distilled water. Amplification was performed under the following conditions: denaturation for 10 min at 95°C, amplification by 40 cycles of 15 s at 95°C and 60 s at 60°C, melting curve evaluation 1 min at 95°C, and increase of 0.5°C each 30 s starting in 60°C, end at 95°C for 15 s. All reactions were performed using two technical replicates.
The resulting ITS chromatograms (648 bp) were analyzed using BioEdit software (v. 7.2). All generated sequences were searched for identity using BLAST (Basic Local Alignment Search Tool) through web servers of the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). The sequences were also aligned with the available sequences for Perkinsus spp. found in the GenBank database using the MUSCLE algorithm. Maximum likelihood (ML) analysis was conducted using MEGA version X software (Kumar et al., 2004) with 1,000 replicates for calculating bootstrap values.
For the haplotype network analysis, ITS1 sequences of Perkinsus obtained from Campania samples (n = 2) and Catalonia (n = 2) and 163 ITS1 sequences of P. olseni with geographic information deposited in GenBank were used. Using MEGA X software, sequence data were aligned by CLUSTAL W (Thompson et al., 1994) at default settings. The haplotype network among 163 ITS1 sequences of P. olseni was constructed with the TCS network method (Clement et al., 2000) using PopART (Leigh and Bryant, 2015).
RFTM has been considered the best assay for Perkinsus diagnosis (Ray, 1963). In the mussel farm from Italy, starting from the samples of 2020, aseptically excised small pieces (3–5 mm) of digestive gland, gill, mantle, and muscle were in RFTM supplemented with Chloramphenicol 2,5% w/v and Nystatin (4000 U ml−1) in the dark, at for 6 days at 26°C. After incubations, the tissues were placed on a glass slide, covered by a drop of Lugol’s iodine solution, cover-slipped and examined under a light microscope (Zeiss Axioscope 5) at different magnification (4×, 10×, and 20× objectives). The sample resulted positive when blue–black hypnospores were observed.
Perkinsosis prevalence in mussels from Italy was variable over the years and seasons by light microscopy (Table 2). The highest prevalence was detected in April 2021 with the 23% of the affected individuals.
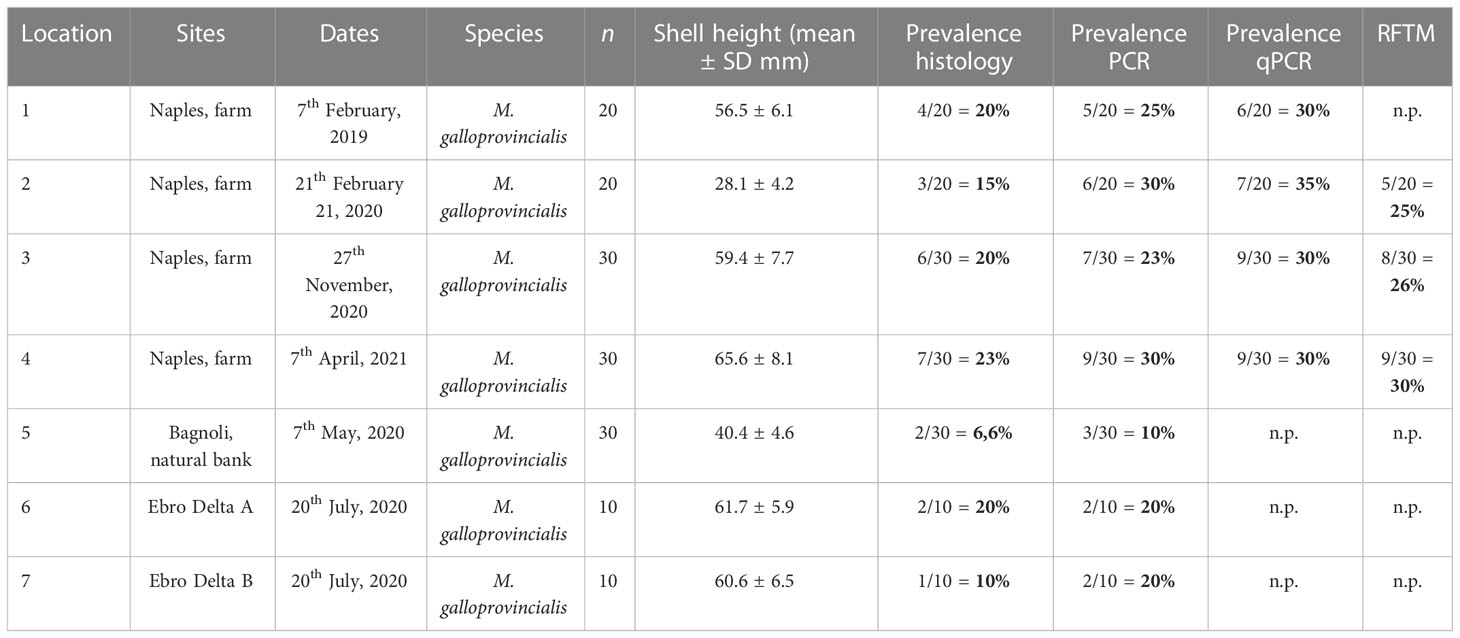
Table 2 Survey results of Perkinsus infection in the blue mussel, M. galloprovincialis collected from Campania region and Ebro Delta. n.p.: analysis not performed.
In Italy, typical features of Perkinsus-like cells with a trophozoite characterized by a large vacuole and eccentric nucleus were generally visible, accompanied by the typical production of many inflammatory capsules of different dimensions and with an infection intensity from moderate to high (Figures 2A–D). Chronic lesions, underlined by the involvement of fibroblast, were observed in samples from April 2021. The parasite developed in the connective tissue in the digestive gland, between the tubules, in the connective stroma in the gonad, and in few cases in the muscle of the foot. Within the capsule were visible haemocytes phagocytising at different phases of development were visible, from multinucleate schizonts from four to eight cells. Apoptotic haemocytes were also visible along with the production of yellowish granules (Figures 2A, C, E). In Spain, sites A and B showed animal mortality, 69% in site A and 17% in the site B. The infection was visible through histopathology in two individuals (2/10) in the site A and in one individual in site B. The inflammatory lesion was intense and infiltrative, spreading in all the digestive tissue, with total disappearance of tissue architecture. Moreover, differently from animals in Italy, trophozoites of P. olseni were also observed in the gill, a typical Perkinsus spp. tropism, and in the haemocyte vessels (Figure 2F). Clusters of trophozoites were encapsulated in well-circumscribed walls forming a cyst-like structure.
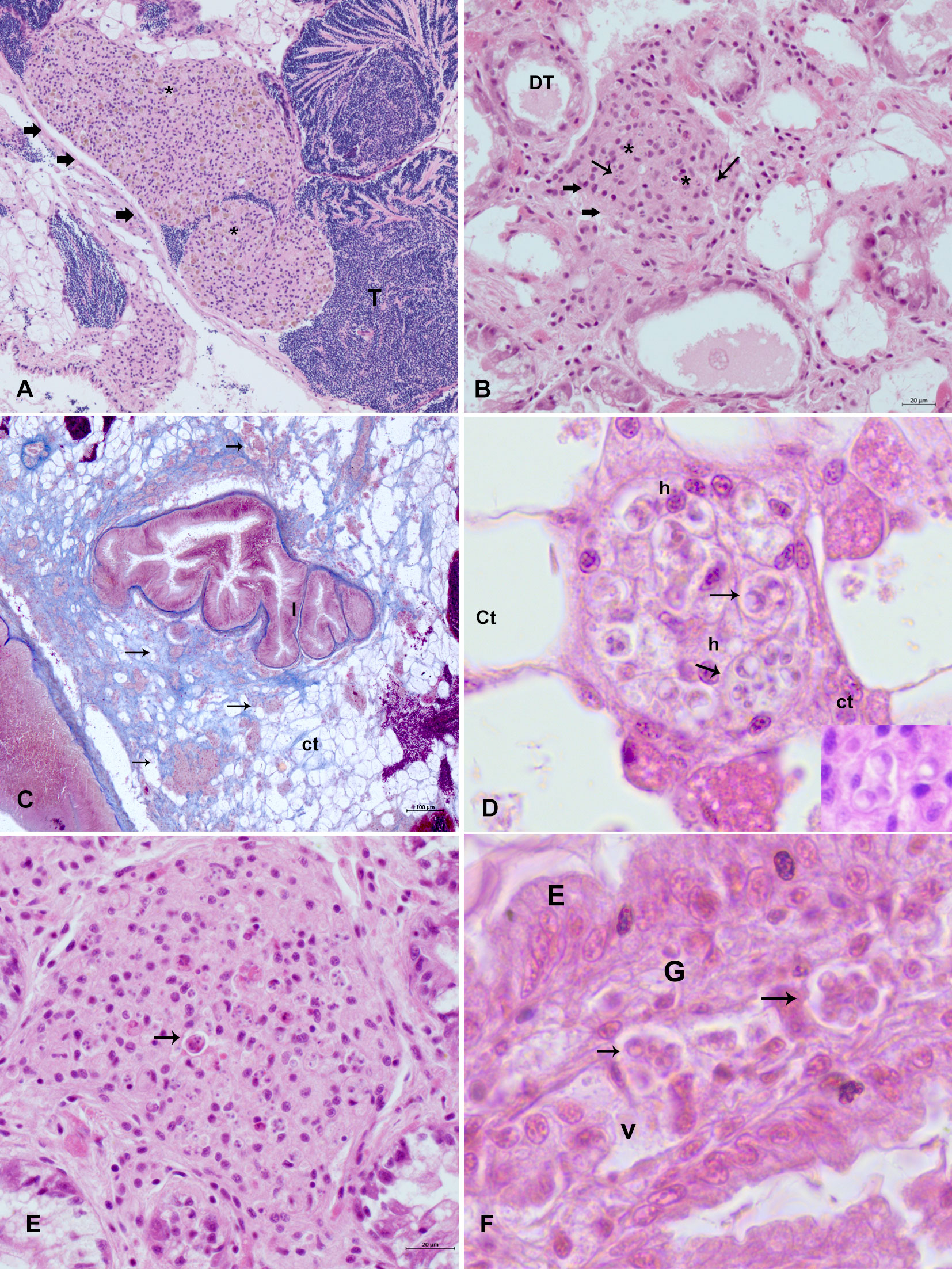
Figure 2 Histopathology of mussels affected by Perkinsosis in mussels (M. galloprovincialis) in Italy and Spain. (A, B) typical feature of the inflammatory lesion (big arrows) with haemocytes (*) related to Perkinsus (small arrows): haemocytes nodulation in gonadal follicle (A) and the interstitial space of digestive tubules (B) big arrows. (C) detail of the reactive connective tissue, underlined by Mallory Trichrome in light blue with inflammatory capsules (arrows); (D) detail of a capsule in the connective tissue space with haemocyte (h) phagocyting trophozoite of Perkinsus (arrow); (E) inflammatory capsule displaying apoptotic haemocytes (arrow) with visible trophozoite. (F) Perkinsus (arrows) in the gill (G) haemal vessel in samples from Spain; E, epithelium.
After 6 days, RFTM-cultivated infected mussel stained with Lugol’s iodine exhibited dark blue/black hypnospores in the digestive gland and mantle tissue and rarely in the gill (Figures 3A, B). In mantle, muscle, and digestive gland, hypnospores were observed as clusters when the intensity was low or completely dispersed. Comparison with PCR, qPCR, and cultivation methods in the two areas showed variable prevalence. In Italy, qualitative PCR reported values from 10 to 30% while detection was higher using qPCR values between 30 and 35%. RFTM detection was comparable with the qPCR although always more efficient than PCR from Casas et al. (2002). In Spain, PCR found positive samples in 13.30% of the animals in the two areas.
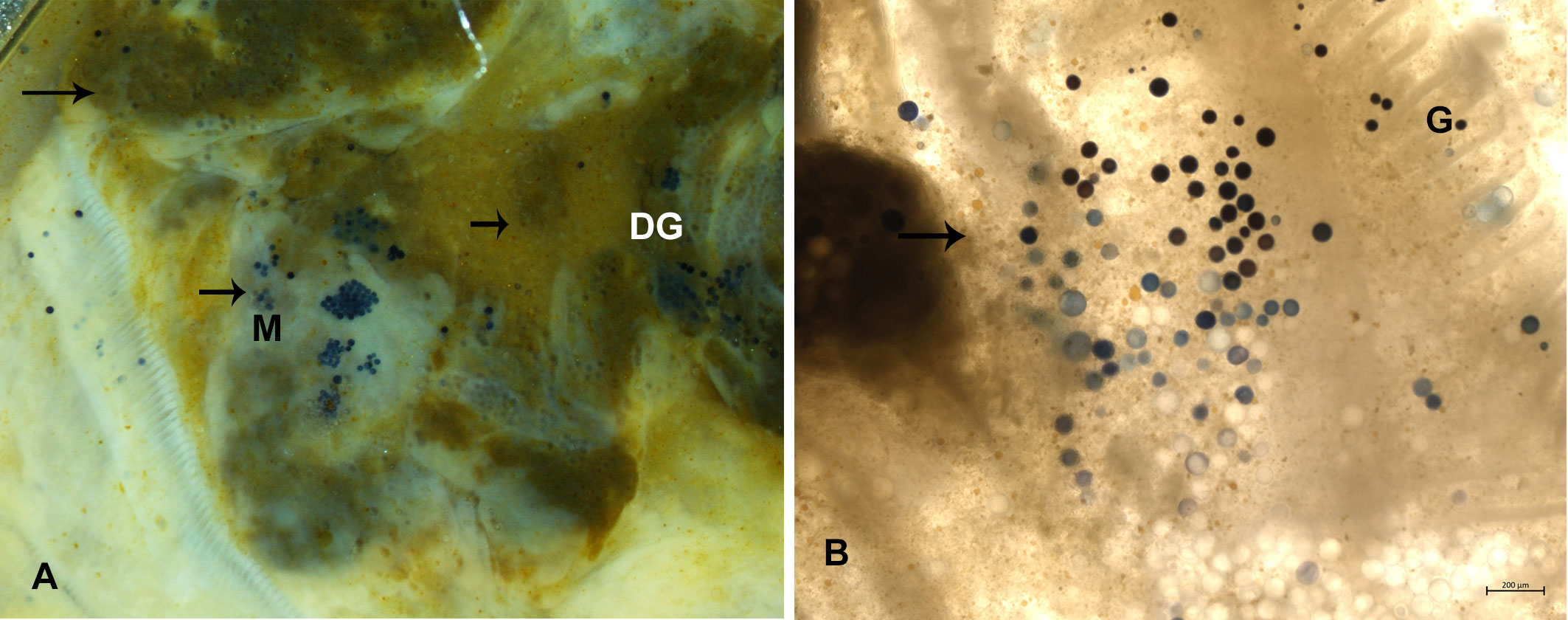
Figure 3 Ray’s fluid thioglycolate medium (RFTM) assay. (A) Perkinsus hypnospores in very heavy infection in M. galloprovincialis in Campania in digestive tissue (DG), muscle (M) and mantle (A) and connective tissue close to the gills (G) (B).
The infected animals resulted in positive detection for the genus-specific primers for Perkinsus with a 648 bp amplicon. The species identity was checked by sequencing followed by BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). In samples from Italy, BLAST analysis showed a 96% similarity of P. olseni with samples from New Zealand, Australia, and Uruguay and in bivalves such as Pitar rostrata, Astrovenus sp., and Haliotis sp., whereas in Spain, the identity was 99% with samples from South Korea such as Anadara granosa (GenBank Accession number: OP961719-OP961722).
The phylogenetic tree resulting from Maximum likelihood is shown in Figure 4. All Perkinsus spp. sequences from this study groups together P. olseni from Italian and Spanish mussels but places them more distant from other P. olseni described in the clams from Europe such as clams from Italy, France, and Spain. In Spain, in particular, they are distant from Perkinsus sp. such as those from Galicia and from sequences previously described from clams from the Ebro Delta. The genetic distance between these isolates and other isolates of P. olseni from different geographic locations ranged from 0.20 to 0.31. The pairwise genetic distance between the P. olseni isolates from Campania with those of Spain varied from 0. 31 to 0.27, respectively (Supplementary Table 3).
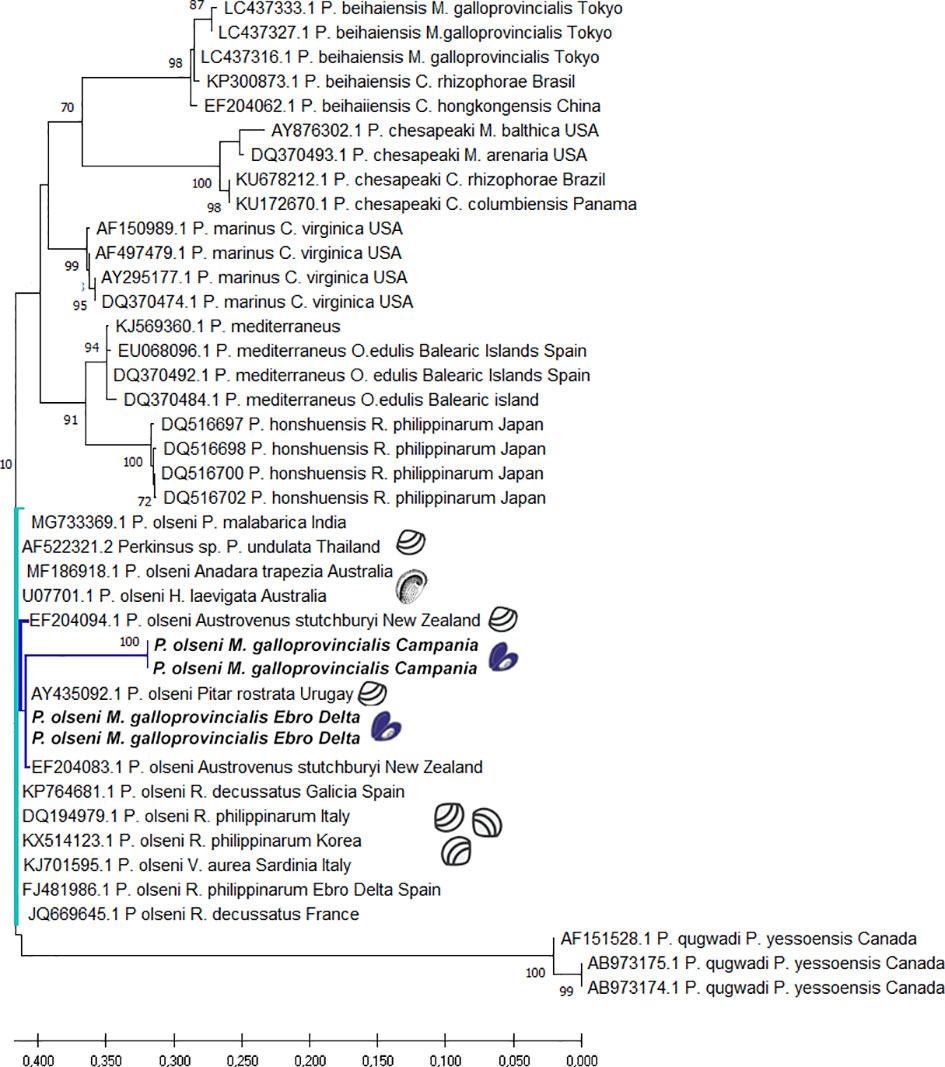
Figure 4 Evolutionary analysis by Maximum Likelihood method of Perkinsus spp. ITS sequences. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 41 nucleotide sequences. There was a total of 794 positions in the final dataset.
We found 20 different ITS haplotypes among 163 ITS sequences (Figure 5). One of the biggest haplogroups was composed of haplotypes from Asian countries (Korea, Thailand, and Japan), Europe (Spain, Italy, and France), and few sequences from Brazil. The other haplotypes were divided into the two major haplogroups, which were separated by at least three nucleotide differences. Perkinsus sp. in mussels from Aflacs Bay was grouped in another haplogroup containing sequences of bivalves from Europe, New Zealand, Japan, and Korea. The sequences from New Zealand belonged to the New Zealand cockle Austrovenus stutchburyi. P. olseni in Naples Bay belonged to an isolated haplogroup close to the Aflacs Bay haplogroup.
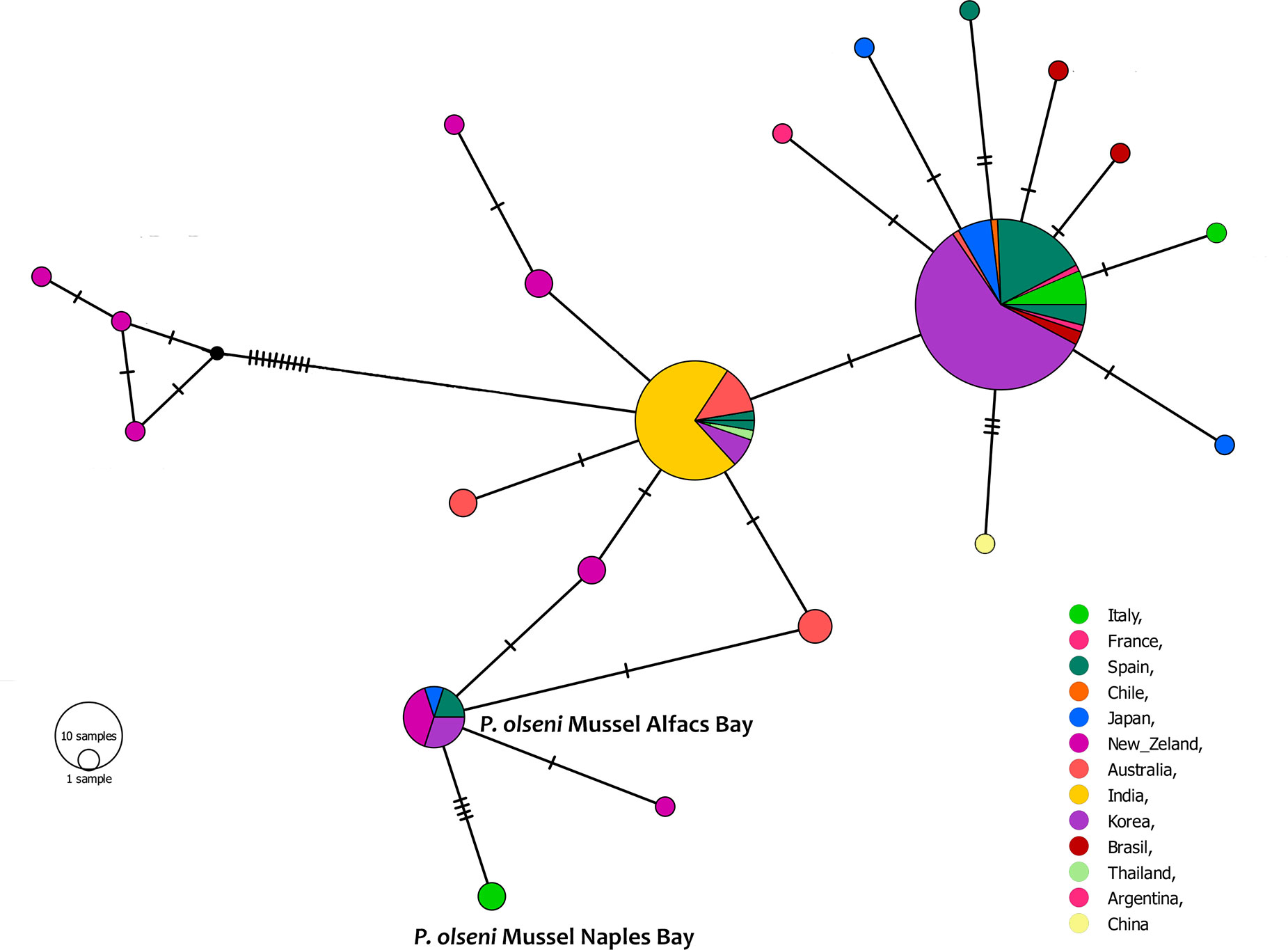
Figure 5 TCS network of 163 ITS1 haplotypes of Perkinsus olseni estimated by PopART. Each line between haplotypes indicates a single nucleotide substitution. The size of each circle is proportional to the absolute haplotype frequency, small black circles represent missing haplotypes, and color shows localities where each haplotype was observed.
This is the first report of P. olseni in Mytilus galloprovincialis in the Mediterranean Sea. Perkinsosis due to Perkinsus olseni in an OIE-listed disease impacts on the health and fitness of populations of many bivalve species (Villalba et al., 2004). Previous works reported the presence of Perkinsus species in other mytilidae group, in Japan and Argentina, also describing strong inflammatory lesions connected to the pathogen presence. P. marinus and P. olseni are the most devastating species and associated with massive mortalities and economic loss. P. olseni typically parasitizes Manila clams in Europe and Asia, the carpet shell R. decussatus in Europe as well as a the gastropod Haliotis ruber in the South of Australia (Lester and Davis, 1981).
Recent advancement of the in vitro culture of Perkinsus spp. improved the understanding of the biology of the parasite, its pathogenicity and virulence and the impact on the host like clams and abalone (La Peyre, 1993; Soudant et al., 2013; Ruan et al., 2019). On the other side, literature report that mussels could be less affected by infectious agents compared with other bivalves’ species (Auguste et al., 2020; Moreira et al., 2020). Because of the scarce knowledge on mussel–Perkinsus relationship, it is critical to define the potential negative effect on this new host and consider pathogen prevalence, intensity, and aspects of the inflammatory response. Perkinsosis is a parasitic disease that develops essentially on an inflammatory basis. In clams, the parasite destroys the epithelia and damages the basal membranes of digestive tissues and gills. It is distributed in different tissues and organs, causing an intense inflammation characterized by the formation of infiltrates, nodules, and capsules, to surround and destroy the pathogen, incorporated in an abundant visible PAS-positive substance, a lectin, secreted by the haemocytes (Montes et al., 1995; Montes et al., 1996; Kim et al., 2006; Soudant et al., 2013). In mussels from this study, P. olseni showed different tropisms, rarely present in the epithelia, a feature observed in other bivalve species such as the blood cockle Anadara kagoshimensis from Korea (Cho et al., 2022) and in other mussel species (Itoh et al., 2019; Vazquez et al., 2022). In the Mediterranean mussel, Perkinsus spp. trophozoites were mainly present into haemal spaces in the vesicular connective tissues that surround mussel digestive and reproductive organs, extending into the mantle. Haemocytes, probably granulocytes, perform phagocytosis accompanied by the formation of a capsule to guarantee pathogen elimination. In many cases, recruited granulocytes go through apoptosis, a mechanism that can be part of the mussel immune response or that could be performed by the parasite to evade host immune defence (Soudant et al., 2013). For example, Perkinsus marinus was found to modulate apoptosis of eastern oyster haemocytes challenged in vitro (Hughes et al., 2010).
Pathogen preference for specific tissues can have an impact on its life cycle and expansion into the host. For example, it may promote its persistence into the tissue, amplify transmission potential, and it can be associated with key virulent phenotypes. P. olseni is defined as BHR, as other Perkinus species such as P. mediterraneaus, P. chesapeaki, and P. beihaiensis presenting a Broad Host Range possibility, similarly to the trend observed for and Marteilia refringens that can affect both oysters and mussels (Itoïz et al., 2022; Le Roux et al., 2001; Carella et al., 2010; Carrasco et al., 2012; Arzul et al., 2014; Guo and Ford, 2016). Direct impact of transboundary animal diseases in aquaculture is a significant limitation for living animal’s international trade, causing a high socio-economic impact on aquaculture exporting nations. Considering Perkinsus plasticity to many bivalve species, like other mollusc’s pathogens, recognized international organizations should take this into account in the view of possible cross infection.
The RFTM assay was effective in detecting Perkinsus infection, and qPCR was the most sensitive in define pathogen presence. Both methods can be advised to detect a new infection and its prevalence in a given area. The result of phylogeny strongly suggests parasite transfer from clams of Asia and Australia, providing evidence that P. olseni from Italian and Spanish mussels are grouped together, but are genetically distant from other P. olseni described in Europe (Italy, France, and Spain). Moreover, haplotype network analysis revealed one haplogroup for mussels in Italy and another haplogroup for mussels in Spain, strictly linked to clams from Asia, America, and New Zealand.
The One Health approach acknowledges the connection of human, animal, and ecosystem health. TAADs are highly transmissible, spread very quickly through national borders, causing serious socio-economic consequences. More research is necessary for biosecurity and protection of valuable marine food resources for a growing human population. In the absence of proper surveillance of stocks, the movement of mussels could increase the risk of introduction into other areas and in the local natural population. In Europe, surveillance efforts regarding mollusc diseases is different between Member States and partly depends on the amount and the diversity of the shellfish production. Developing an environmentally friendly and competitive farming practice is a priority objective of the EU to reach high standards in terms of animal/human health and consumer protection. Mussel aquaculture accounts for 15% of the global bivalve production. In Europe, Italy is the second main producer after Spain, with about 64,000 tonnes produced per year and considered one of the largest European markets with an average consumption of 120,000 tonnes per year (FAO Fisheries and Aquaculture Circular, 2020; European Market Observatory for Fisheries and Aquaculture Products (EUMOFA), 2019).
In our study, P. olseni prevalence of infection showed a slight pattern of seasonality, as it was higher in warmer seasons than in coldest. Literature reports that this tendency could be due to seasonal seawater temperature changes, as relatively higher temperature during warmer seasons may stimulate P. olseni proliferation.
The complex outcome of host–parasite interactions is regulated by different aspects including host biology and immune defence, pathogen virulence, and abiotic factors. Unfortunately, due to the lack of feedback from mussel farmers in Naples Bay, we have no data on possible mortality episodes in the area and how the presence of Perkinsosis could potentially be involved in the fluctuations of the population. Data of mortality are only present from Alfacs Bay, but we cannot conclude that its presence was the trigger of the event. Other studies are needed to define pathogen biology and virulence. P. olseni presence in mussels in more than one region of Europe raises possible concerns, considering the high economical value of mussels for the local aquaculture sector in both Italy and Spain.
The data presented in the study are deposited in the NCBI repository, accession number : OP961719-OP961722.
Conceptualization: FC. Data curation: FC, MF-T and KA. Formal analysis: FC and GD. Methodology: FC, GV, KA. Writing review and editing; FC, KA, GD, MF-T. All authors contributed to the article and approved the submitted version.
UNINA GRANT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1116837/full#supplementary-material
Arzul I., Chollet B., Boyer S., Bonnet D., Gaillard J., Baldi Y., et al. (2014). Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens. Parasitology 141, 227–240. doi: 10.1017/S0031182013001418
Auguste M., Balbi T., Ciacci C., Canonico B., Papa S., Borello A., et al. (2020). Shift in immune parameters after repeated exposure to nanoplastics in the marine bivalve Mytilus. front. Immunol 11. doi: 10.3389/fimmu.2020.00426
Carella F., Aceto S., Marrone R., Maiolino P., De Vico G. (2010). Marteilia refringens infection in cultured and natural beds of mussels (Mytilus galloprovincialis) along the campanian coast (Tirrenian sea, south of Italy). EAFP Bull. 30, 189–196.
Carella F., Carrasco N., Andree K. B., Lacuesta B., Furones D., De Vico G. (2013). Nocardiosis in Mediterranean bivalves: First detection of Nocardia crassostreae in a new host mytilus galloprovincialis and in ostrea edulis from the gulf of Naples (Italy). J. Invertebrate Pathol. 114, 324–328. doi: 10.1016/j.jip.2013.10.001
Carella F., Culurgioni J Aceto S., Fichi G., Pretto T., Luise D., Gustinelli A., et al. (2018). Postmonorchis sp. inq. (Digenea: Monorchiidae) metacercariae infecting natural beds of wedge clam Donax trunculus in Italy. Dis. Aquat Organ 106 (2), 163–172. doi: 10.3354/dao02650
Carrasco N., Andree K. B., Lacuesta B., Roque A., Rodgers C., Furones M. D. (2012). Molecular characterization of the Marteilia parasite infecting the common edible cockle Cerastoderma edule in the Spanish Mediterranean coast. Aquaculture 324–325, 20–26. doi: 10.1016/j.aquaculture.2011.10.017
Casas S. M., Villalba A., Reece K. S. (2002). Study of perkinsosis in the carpet shell clam Tapes decussatus in Galicia (NW spain). i. identification of the aetiological agent and in vitro modulation of zoosporulation by temperature and salinity. Dis. Aquat Org 50, 51–65. doi: 10.3354/dao050051
Cho Y.-G., Lee H.-M., Hwang J. Y., Jang G. I., Kwon M. G., Kim B. S., et al. (2022). Molecular and histological identification of the protozoan parasite Perkinsus olseni in the blood cockle Anadara kagoshimensis (Tokunaga 1906) occurring on the south coast of Korea. Aquaculture 561, 738721. doi: 10.1016/j.aquaculture.2022.738721
Clement M., Posada D., Crandall K. A. (2000). TCS: A computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x
De Vico, Carella (2019). Nocardiosis and mycobacteriosis of bivalves: “Yet-to-emerge” zoonoses of public concern. Zoonoses Public Health 66 (6), 559–561. doi: 10.1111/zph.12614
Dungan C. F., Reece K. S., Moss J. A., Hamilton R. M., Diggles B. K. (2007). Perkinsus olseni in vitro isolates from the new Zealand clam Austrovenus stutchburyi. J. Eukaryot. Microbiol. 54, 263–270. doi: 10.1111/j.1550-7408.2007.00265.x
European Market Observatory for Fisheries and Aquaculture Products (EUMOFA) (2019) Fresh mussel in the UE: price structure in the supply chain. Available at: https://www.eumofa.eu/documents/20178/151118/PTAT+Fresh+Mussel_EN.pdf.
FAO Fisheries and Aquaculture Circular (2020) REGIONAL REVIEW ON STATUS AND TRENDS IN AQUACULTURE DEVELOPMENT IN EUROPE. Available at: https://www.fao.org/3/cb7809en/cb7809en.pdf.
Galtsoff P. S. (1964). The american oyster, Crassostrea virginica gmelin fish. Bull. US 64 (1964) pp, 1–480. doi: 10.4319/lo.1966.11.2.0312
Guo X., Ford S. E. (2016). Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos Trans R Soc Lond B Biol Sci 371, 16.
Hughes J., Foster B., Grewal S., Sokolova I. M. (2010). Apoptosis as a host defense mechanism in crassostrea virginica and its modulation by Perkinsus marinus. Fish Shellfish Immunol. 29, 247–257. doi: 10.1016/j.fsi.2010.03.003
Itoh N., Komatsu Y., Maeda K., Hirase S., Yoshinaga T. (2019). First discovery of perkinsus beihaiensis in Mediterranean mussels (Mytilus galloprovincialis) in Tokyo bay, Japan. J. Invertebr. Pathol. 166, 107226. doi: 10.1016/j.jip.2019.107226
Itoïz S., Metz S., Derelle E., Reñé A., Garcés E., Bass D., et al. (2022). Emerging parasitic protists: The case of perkinsea. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.735815
Kim Y. M., Park K., Choi K., Alvarez R. A., Cummings R. D., Cho M. (2006). Lectin from the Manila clam Ruditapes philippinarum is induced upon infection with the protozoan parasite Perkinsus olseni. J. Biol.Chem. 281, 26854–26864. doi: 10.1074/jbc.M601251200
Kumar S, Tamura K, Nei M (2004). MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5 (2), 150–163.
La Peyre J. F. (1993). Studies on the oyster pathogen Perkinsus marinus (Apicomplexa): Interactions with host defenses of Crassostrea virginica and Crassostrea gigas, and in vitro propagation. Coll. William Mary Ph D Thesis p, 177.
Leigh J. W., Bryant D. (2015). POPART: full-feature software for haplotype network construction. Meth. Ecol. Evol. 6, 1110–1116. doi: 10.1111/2041-210X.12410
Le Roux F., Lorenzo G., Peyret P., Audemard C., Figueras A., Vivares C. (2001). Molecular evidence for the existence of two species of Marteilia in Europe. J. Eukaryot. Microbiol. 48, 449–454. doi: 10.1111/j.1550-7408.2001.tb00178.x
Lester R. J. G., Davis G. H. G. (1981). A new Perkinsus species (Apicomplexa, perkinsea) from the abalone haliotis ruber. J. Invertebr. Pathol. 37, 181–187. doi: 10.1016/0022-2011(81)90073-2
Montes J. F., Durfort M., García-Valero J. (1995). Cellular defence mechanism of the clam Tapes semidecussatus against infection by the protozoan perkinsus sp. Cell Tissue Res. 279, 529–538. doi: 10.1007/BF00318165
Montes J. F., Durfort M., García-Valero J. (1996). When the venerid clam Tapes decussatus is parasitized by the protozoan perkinsus sp. it synthesizes a defensive polypeptide that is closely related to p225. DAO 26, 149–157. doi: 10.3354/dao026149
Moreira R., Romero A., Rey-Campos M., Pereiro. P., Rosani U., Novoa B., et al. (2020). Stimulation of mytilus galloprovincialis hemocytes with different immune challenges induces differential transcriptomic, miRNomic, and functional responses. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00426
OIE Listed Diseases (2021) OIE - world organisation for animal health. Available at: https://www.woah.org/en/what-we-do/animal-health-and-welfare/animaldiseases/ (Accessed 10 October, 2022).
Ramilo A., Carrasco N., Reece K. S., Valencia J. M., Grau A., Aceituno P. (2015). Update of information on perkinsosis in NW Mediterranean coast: Identification of perkinsus spp. (Protista) in new locations and hosts. J. Invertebr. Pathol. 125, 37–41. doi: 10.1016/j.jip.2014.12.008
Ray S. M. (1963). A review of the culture method for detecting Dermocystidium marinum, with suggested modifications and precautions. Proc. Natl. Shellfish. Assoc. 54, 55–69.
Ríos R., Aranguren R., Gastaldelli M., Arcangeli G., Novoa B., Figueras A. (2020). Development and validation of a specific real-time PCR assay for the detection of the parasite Perkinsus olseni. J. Invertebr. Pathol. 169, 107301. doi: 10.1016/j.jip.2019.107301
Ruan L. ,. S., Yang X., Li M., Yang D. (2019). Immune recognition, antimicrobial and opsonic activities mediated by a sialic acid binding lectin from Ruditapes philippinarum. Fish Shellfish Immunol. 93, 66–72. doi: 10.1016/j.fsi.2019.07.027
Soudant P., Fu-Lin E., Volety A. (2013). Host–parasite interactions: Marine bivalve molluscs and protozoan parasites. Perkinsus species J. Invertebrate Pathol. 114 (2), 196–216. doi: 10.1016/j.jip.2013.06.001
Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matriX choice. Nucl. Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Vazquez N., Itoh N., Cremonte F. (2022). First record of Perkinsus olseni in cultured mussels (Mytilus chilensis) in the beagle channel. Southwestern Atlantic Ocean Aquaculture 550, 737893. doi: 10.1016/j.aquaculture.2022.737893
Keywords: Perkinsus, TAADs, mussel, dinoflagellate, emerging pathogen
Citation: Carella F, Fernandez Tejedor M, Villari G, Andree KB and De Vico G (2023) The endoparasite Perkinsus olseni affecting the Mediterranean mussels (Mytilus galloprovincialis) in the Italian and Spanish waters: A new possible threat for mussel aquaculture and wild animal population. Front. Mar. Sci. 10:1116837. doi: 10.3389/fmars.2023.1116837
Received: 05 December 2022; Accepted: 27 January 2023;
Published: 24 February 2023.
Edited by:
Claudia Venegas, VESO, ChileReviewed by:
Karin Berta Lohrmann, Catholic University of the North, Coquimbo, ChileCopyright © 2023 Carella, Fernandez Tejedor, Villari, Andree and De Vico. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Carella, ZnJhbmNlc2NhLmNhcmVsbGFAdW5pbmEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.