- 1Key Laboratory of Environment Controlled Aquaculture, Ministry of Education, Dalian, China
- 2College of Marine Technology and Environment, Dalian Ocean University, Dalian, China
- 3College of Fisheries and Life Science, Dalian Ocean University, Dalian, China
Light and temperature are necessary conditions for migratory fish. The assessment of fish physiology and behavior is important for identifying fish welfare, but also for the assessment of the optimal setting of recirculating aquaculture systems (RASs). This study aimed to explore the interactive effect of photoperiod and temperature on steelhead trout culture. Four treatments were set up with specific settings were as follows: a LP-LT group treated with 16L:8D and 12°C, a LP-HT group treated with 16L:8D and 16°C, a SP-LT group treated with 12L:12D and 12°C, and a SP-HT group treated with 12L:12D and 16°C. Growth performance, behavioral and physiological parameters were measured. Two indexes, locomotor activity and social interaction were used for behavioral analysis, and the results were applied to interpret the behavioral responses to the photoperiod and temperature stimulation in juveniles. The growth performances were significantly lower in treatments LP-LT and SP-LT. The treatment LP-HT had significantly higher growth performance than the other treatments, but no significant differences were noted in survival rate and coefficient of variation. The results of fish behavior indicated that the movement of juveniles should be primarily monitored at high temperatures or long photoperiods, and the state parameters should be primarily monitored at low temperatures or short photoperiods. The results of the physiological parameters showed that the recovery time from stress varied among different treatments. After 60 days of the experiment, superoxide dismutase and alanine aminotransferase dropped back to their initial level. The results of Na+-K+-ATPase showed that although the combined effect of photoperiod and temperature could advance the time of smoltification, it may result in poorer salt tolerance. Our findings underscore the importance of the interaction of photoperiod and temperature on steelhead trout culture. The outcome could provide guidance for the development of effective aquaculture systems.
Introduction
Rainbow trout (Oncorhychus mykiss) is a popular salmonidae for aquaculture, with a global production volume of more than 814,090 tons in 2016 (FAO, 2016; Pauly and Zeller, 2017; Yu et al., 2021; Liu et al., 2021). The two types of rainbow trout are non-migratory and migratory. The migratory type, known as steelhead trout in China, has a life cycle that includes freshwater and saltwater stages. With the development of aquaculture technology, the hatching and smolting processes of steelhead trout could be accomplished in land-based recirculating aquaculture systems (RASs). Under natural conditions, the length of the pre-smolt stage of juveniles depends on the latitude and longitude of their place of residence, i.e., the natural variation of light and temperature (Bowden et al., 2004; Rosengren et al., 2017; Archer et al., 2019). RAS is treated using artificial lighting and temperature control to promote fish growth and inhibit their gonadal development and maturation (Pauly and Zeller, 2017; Guraslan et al., 2017; Churova et al., 2020). Developing different light manipulation strategies (including constant light or darkness) to promote fish growth and counter-seasonal reproduction is common depending on the local environment at each site (Guraslan et al., 2017; Sievers et al., 2018).
The design and development of effective aquaculture systems are essential for successful aquaculture practice. The conditions of the freshwater stage could significantly affect the smolting processes and the viability in the seawater stage (del Villar-Guerra et al., 2019). Much research is devoted to reducing the duration of the freshwater stage of salmonids with low technology and low cost while ensuring high survival and growth rates in seawater (Rosengren et al., 2017; Sievers et al., 2018; Hines et al., 2019; Nemova et al., 2020; Suzuki et al., 2020). The culture of Salmonidae has considerably developed in terms of scale and distribution (Albert et al., 2019; Barrett et al., 2020; Hvas et al., 2021). Although the condition control of culture has been very mature in RAS and many studies about the environmental effects on fish performance have been conducted, only few studies have been reported on steelhead trout compared with other fish species (Ge et al., 2021; Salinas et al., 2022). The optimal environmental factor varies among species and developmental stages but is also related to requirements and mastery of farmers’ skill degree. Thus, understanding how these key environmental factors affect fish’s performance is important to reduce production costs (Pauly and Zeller, 2017; Hvas et al., 2021).
Migration time and success in Salmonidae are related to physiological basis, such as energy metabolic, osmolarity, and anti-oxidative stress (Höglund et al., 2008; Archer et al., 2019; Shry et al., 2019). However, a decrease or increase in physiological parameters as a response to increased stress makes it difficult to veridically predict the body state (Birnie-Gauvin et al., 2019). With the exception of morphology and physiology, the behavior of steelhead trout varies significantly during the transition from freshwater to saltwater. A search summarized 39 relevant studies on fish behavior applied to improve commercial aquaculture production processes or fish welfare between 1993 and 2020 (Macaulay et al., 2021). Understanding the features of fish behavior could help farmers clarify how they move, what diets they choose, how much they eat, and how they respond to potential stress (acute or chronic stress) (Huntingford et al., 2012; Damsgård et al., 2019). In this respect, fish behavior is used to provide necessary bioengineering information for feeding strategy (Conallin et al., 2016), system optimization (Dempster et al., 2019), and decreased labor consumption (Cogliati et al., 2019). However, the behavioral manifestations of successful migration and its underlying mechanisms remain poorly understood.
On the basis of the authors’ previous work, the present study attempted to explore the interactive effect of photoperiod and temperature on steelhead trout culture from the aspects of growth, behavior and physiological performance, specifically whether the smoltification window could directly be judged by specific behavioral characteristics. In this study, the following questions were explored: 1) Is there an interaction between light and water temperature on growth performance of steelhead trout juveniles? 2) Could the environmental stimulation improve the regulation of osmolarity? 3) What are the key behavioral characteristics of steelhead trout during the smoltification window?
Materials and methods
Experimental animal and source
Steelhead trout juveniles were purchased from Wanzefeng Fishery Company (Rizhao, China). The experiment was conducted in 2021 at the Key Laboratory of Environment Controlled Aquaculture (AET), Dalian City, Liaoning Province, China. Juveniles with a mean initial body length of 3.32 ± 0.02 cm and a body weight of 4.40 ± 0.20 g were kept temporarily. The dissolved oxygen content was more than 7.0 mg/L, ammonia nitrogen was lower than 0.10 mg/L, and pH was adjusted at 7.50 ± 0.50; all were measured using a YSI 6920 multi-parameter water quality instrument (YSI Inc., Ohio, USA).
Experimental design
The experiment lasted for eight weeks. Based on the actual operations in aquaculture, the experimental conditions involved two photoperiods (L, 12L:12D and 16L:8D) and two temperatures (T, 12°C and 16°C), for a total of four treatments. Each treatment was represented by three replicates. The specific settings were as follows: long photoperiod, low water-temperature group treated with 16L:8D and 12°C (treatment LP-LT); long photoperiod, high water-temperature group treated with 16L:8D and 16°C (treatment LP-HT); short photoperiod, low water-temperature group treated with 12L:12D and 12°C (treatment SP-LT); and short photoperiod, high water-temperature group treated with 12L:12D and 16°C (treatment SP-HT).
A total of 12 randomly arranged plastic tanks were used, and the volume of freshwater was about 300 L (diameter of 0.8 m, water depth of 0.6 m) in each tank. All tanks were designed as a semi-closed recirculation system with a water outlet in the center. Dechlorinated freshwater temperature was controlled within ± 0.5°C with a customized temperature control system. The different treatments were insulated from one another by silver shading material. In total, 480 juveniles were used in this experiment and forty juveniles were used as biological replicates in each tank (n=40) and fed with commercial feed pellets (Qihao Aquatech Co., Ltd., China). All tanks were thoroughly cleaned by scrubbing the walls and bottom after each weekly sampling to avoid unnecessary disturbances.
Behavior observations
Only 120 juveniles were tagged with visible implant elastomer (VIE) tags (Northwest Marine Technology, Shaw Island, WA, USA) allowing for individual identification throughout the experiment. The tagged fish were then randomly placed into each tank gently, that is, only 10 fish were tagged per tank to respect their welfare. The entire experiment area was surrounded by a black curtain to prevent external interferences and shadow rejection. The behavior of juveniles was recorded and tracked using an infrared camera mounted above the central arena during feeding. Each video lasted 10 minutes, and all the behavioral indicators were calculated using the Noldus EthoVision XT (version 12.0; Noldus Information Technology, Netherlands). Coordinates were set for each video to ensure that the observation area and fish movement exactly matched. The total observation area was divided into two subzones: the feeding zone and the remaining zone. The tracking data were additionally checked by all-occurrence recording method to ensure the accuracy of the results.
Sampling and analysis method
Growth performance
The number of dead fish was recorded and removed daily to calculate the survival rate (SR). Body length and body weight were measured every 10 days and nine fish were randomly selected from each treatment to determine growth parameters. The calculation formula for specific growth rate (SGR) and the coefficient of variation (CV) was as follows:
where W1. the initial body weight, W2. the final body weight, t1. the initial sampling day, t2. the sampling day, SD is the standard deviation of body weight, M is the average body weight.
Physiological parameters
After the end of the experiment, all tagged fish (a total of 120 juveniles) and random equal numbers of untagged fish were euthanized with an overdose of tricaine methanesulfonate (MS-222, Sigma), and tissues were sampled for physiological analysis. The liver, blood, and gill tissues were collected, immediately frozen in liquid nitrogen, and stored at −80°C. Superoxide dismutase (SOD) was detected by WST-1 method according to the SOD assay kit instructions. Alanine aminotransferase (ALT) was determined using a microplate reader using colorimetric detection at 570 nm as per kit instructions. Na+-K+-ATPase (NKA) was measured by Bonting method according to the NKA activity kit. The cortisol level wase determined using an ELISA kit. All measurements were performed at least three times and all assay kits were obtained from the Nanjing Jiancheng Bioengineering Institute, China.
Behavioral indicators
The assessment of fish behavior consisted of two aspects: locomotor activity and social interaction. Six behavioral indicators, including total distance moved (cm), maximum acceleration (cm/s2), acceleration state (%), residence time in feeding zone (s), distance to central point (cm), and the distance between fish (cm), were measured and calculated every 10 days. The total distance moved was used to provide a general measure of activity, which was also used as the basis for calculating other parameters, such as acceleration. Moreover, acceleration was obtained by calculating the difference in velocity with the unit time and used to mark bursts of rapid movement. The activity state depends on the total pixel change within the analysis area per unit time of the fish. The residence time in feeding zone represented the discrete state of fish during feeding, which was a standard variable for the usage of space. Distance to central point indicated the radius of fish distribution during feeding (cluster radius). Distance between fish was used to characterize the distance between two individuals. The total distance moved, maximum acceleration, and acceleration state were used as indicators of movement quality. The other three behavioral indicators were used to evaluate the state of fish, such as area preference and social interaction.
Data analysis
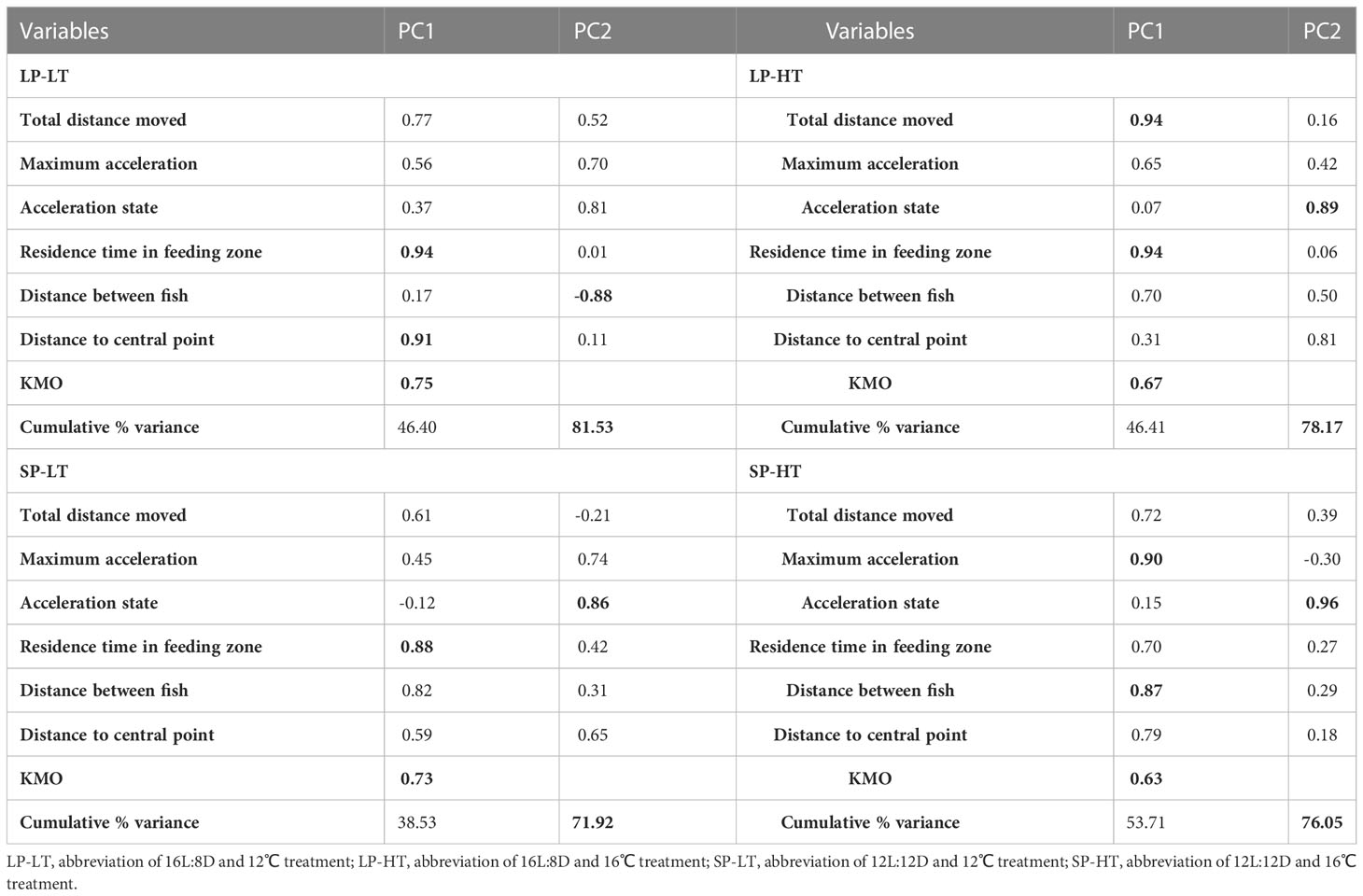
In accordance with the study’s purpose, different statistical methods were used. A completely randomized ANOVA was used to compare the differences in growth performance between groups. A generalized linear mixed model (GLMM) was constructed to assess the influence of the different experimental conditions on fish behavior, including a mixture of fixed and random effects. The individual ID of fish was included in the models as random effects. Statistical analysis was performed using R version 3.5.3. All statistical results were presented as the mean ± standard deviation (mean ± SD). The level of significance was set at P< 0.05. Multivariate statistical analysis, namely, principal component analysis (PCA), was used to obtain a low-dimensional representation of high-dimensional data to exact the key behavioral parameters in fish. The Kaiser-Mayer-Olkin (KMO) index and Bartlett’s test of sphericity assessed the adaptive validity of PCA.
Results
Growth performance
Table 1 provides the growth performance of steelhead trout juveniles in different treatments. No deaths occurred during the entire experimental period. The results of ANOVA are presented in Table 1. The growth performance of juveniles was significantly influenced by photoperiod and temperature and their interaction (P = 0.000 < 0.01). No statistical differences (P > 0.05) were observed among treatments LP-LT, LP-HT, and SP-HT in terms of final body length, which was significantly higher than that in treatment SP-LT (P < 0.05). The final weight in treatment SP-HT was significantly lower than that in treatment LP-HT and significantly higher than that in treatments LP-LT and SP-LT (P < 0.05). The analysis results of SGR showed the same results as final weight. Regarding the consistency of fish growth rate, no significant differences were found in the CV level among the treatments (P > 0.05).
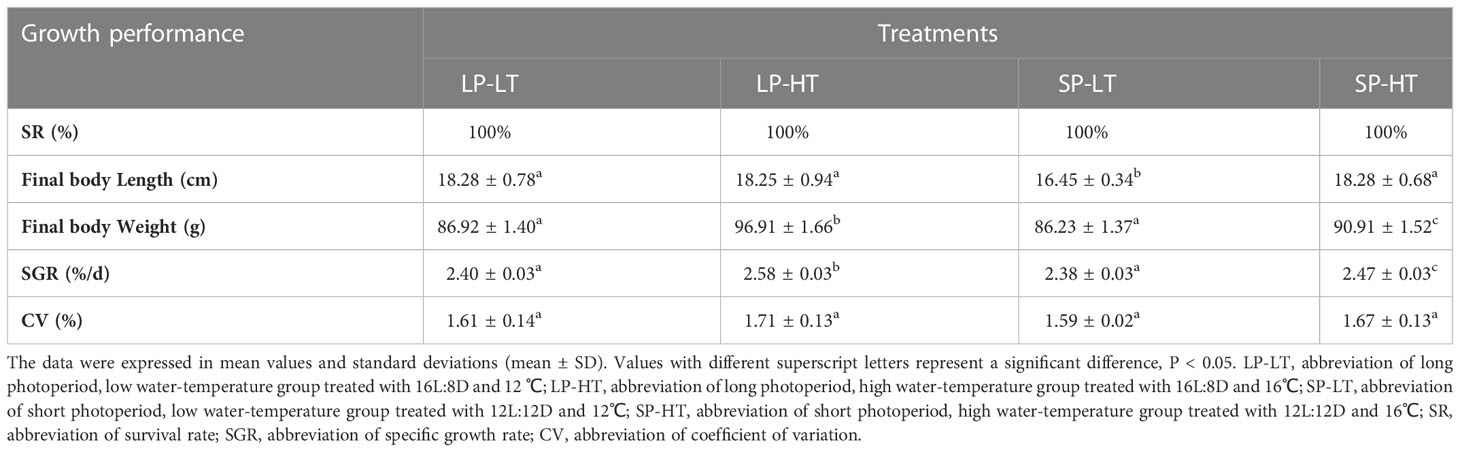
Table 1 Survival rate and growth performance of the different treatments at the end of the experiment.
Post-hoc comparisons revealed significant differences in growth performance at different levels of conditions (P = 0.000 < 0.01) (Table 2). Photoperiod and temperature had no significant effect on CV values, but a significant effect was found in their interaction (Table 2). Simple effect analysis revealed no significant differences in final body length between the two photoperiod levels at 16°C (P > 0.05) and significant differences at 12°C (P = 0.000 < 0.01). For final body weight, the differences were significant between all levels, except for the 16°C groups (B and D) (P = 0.000 < 0.01). SGR also showed the same results as final body weight.
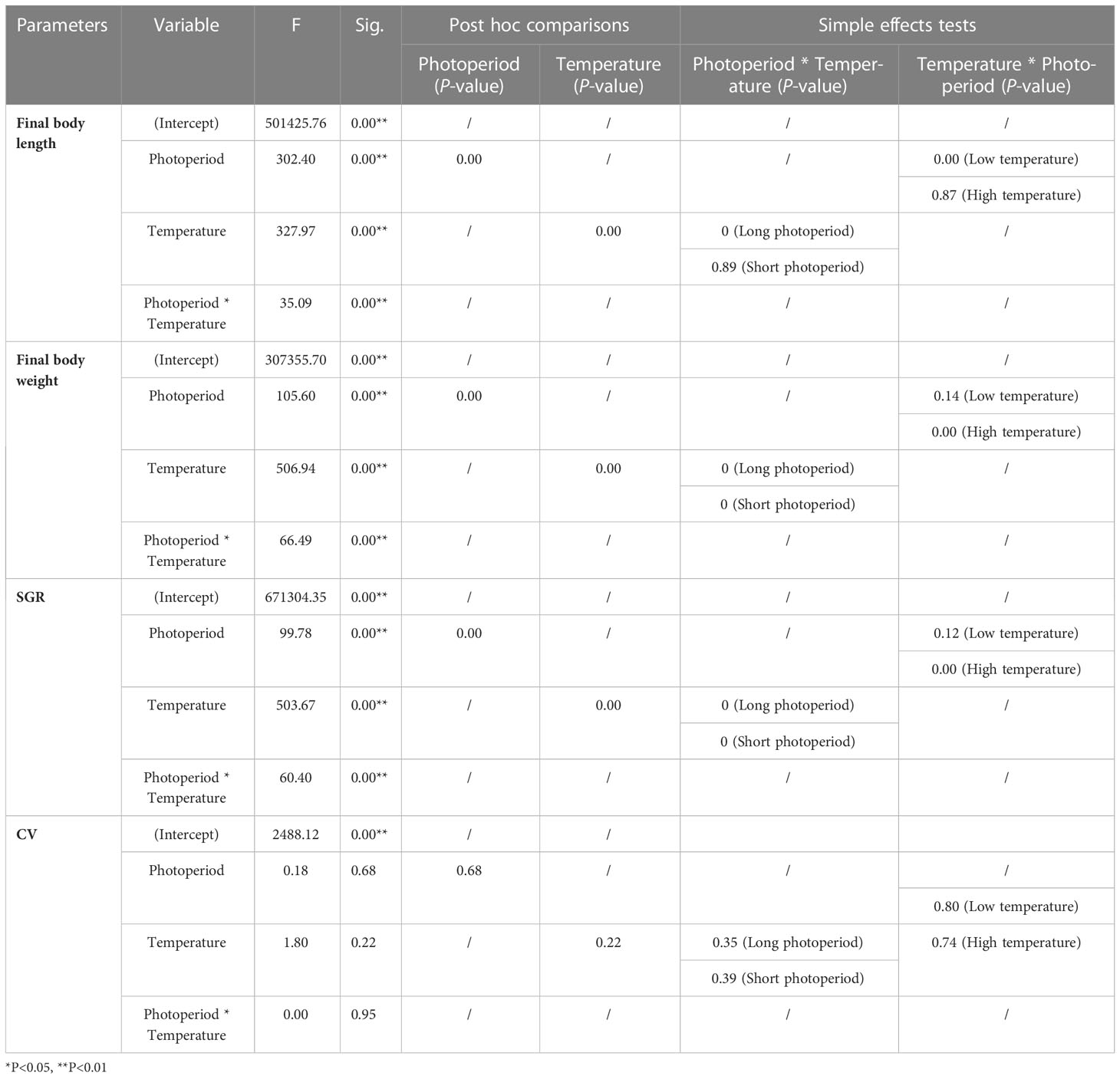
Table 2 The Bonferroni post hoc test was used for multiple comparisons of growth performance among groups.
Physiological parameters
The changes in physiological parameters are presented in Figure 1. Photoperiod and water temperature could affect the level of antioxidant enzymes in steelhead trout. The SOD levels behaved a general trend of increasing first and then decreasing, but the maximum concentration of SOD in the different treatments appeared at different timepoints (Figure 1A). The highest SOD levels for treatments LP-LT and SP-HT were at approximately day 30, whereas treatments LP-HT and SP-LT reached the highest levels at approximately day 15. After 30 days, significantly higher and lower SOD contents were observed in treatments SP-HT (P < 0.05 ) and LP-LT (P< 0.05), respectively. Similar to SOD, ALT mainly revealed an upward trend in the first 30 days and a downward trend after 30 days (Figure 1B). The ALT content dropped back to the initial level in all other groups except for treatment LP-HT after 60 days of the experiment. For treatment LP-HT, no significant difference was observed in the ALT content compared with other timepoints after 45 days (P > 0.05).
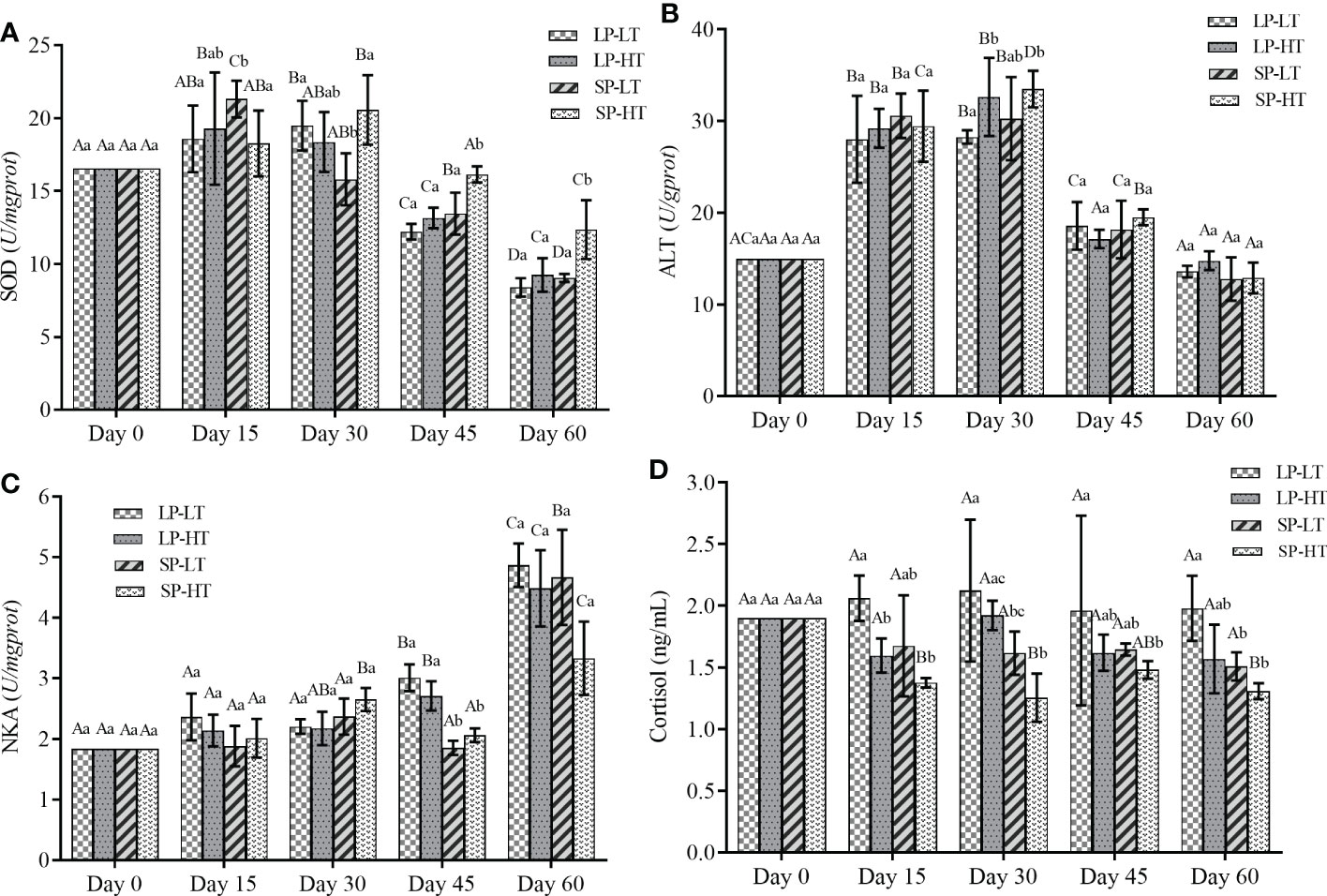
Figure 1 Effects of different treatments on different physiology parameters of steelhead trout juveniles. (A) SOD, (B) ALT, (C) NKA and (D) Cortisol content. The data were expressed in mean values and standard deviations (mean ± SD) (n=30 per treatment). Different capital letters represent significant differences between different treatments at the same time, different lowercase letters represent significant differences between different times of the same treatment (P < 0.05). LP-LT, abbreviation of long photoperiod, low water-temperature group treated with 16L:8D and 12 °C; LP-HT, abbreviation of long photoperiod, high water-temperature group treated with 16L:8D and 16°C; SP-LT, abbreviation of short photoperiod, low water-temperature group treated with 12L:12D and 12°C; SP-HT, abbreviation of short photoperiod, high water-temperature group treated with 12L:12D and 16°C. SOD, Superoxide dismutase; ALT, Alanine aminotransferase; NKA, Na+-K+-ATPase.
In general, the NKA activity increased with time in all groups (Figure 1C). It significantly increased after 45 and 60 days in the treatments with long and short photoperiods’ (P < 0.05), respectively. No statistical differences (P > 0.05) were observed in NKA among different treatments at different timepoints, and its activity was significantly higher than that in treatments SP-LT and SP-HT at 45 days (P < 0.05). The cortisol levels did not show clear trends compared with the other parameters. The cortisol levels in treatment SP-HT were significantly lower than those in other treatments throughout the experiment (P < 0.05), but no significant differences were observed among the three other treatments (P > 0.05).
Behavioral indicators
GLMM results are presented in Table 3. The fixed effects results revealed that both acceleration state and distance between fish were significantly affected by not only experimental condition but also the interaction of experimental condition and time. Experimental time had significant effects on the distance between fish. No significant main effects of body length and experimental time were found for all behavioral indicators except for the distance between fish. There was a significant effect of body weight on the total distance moved but not on the other behavioral indicators.
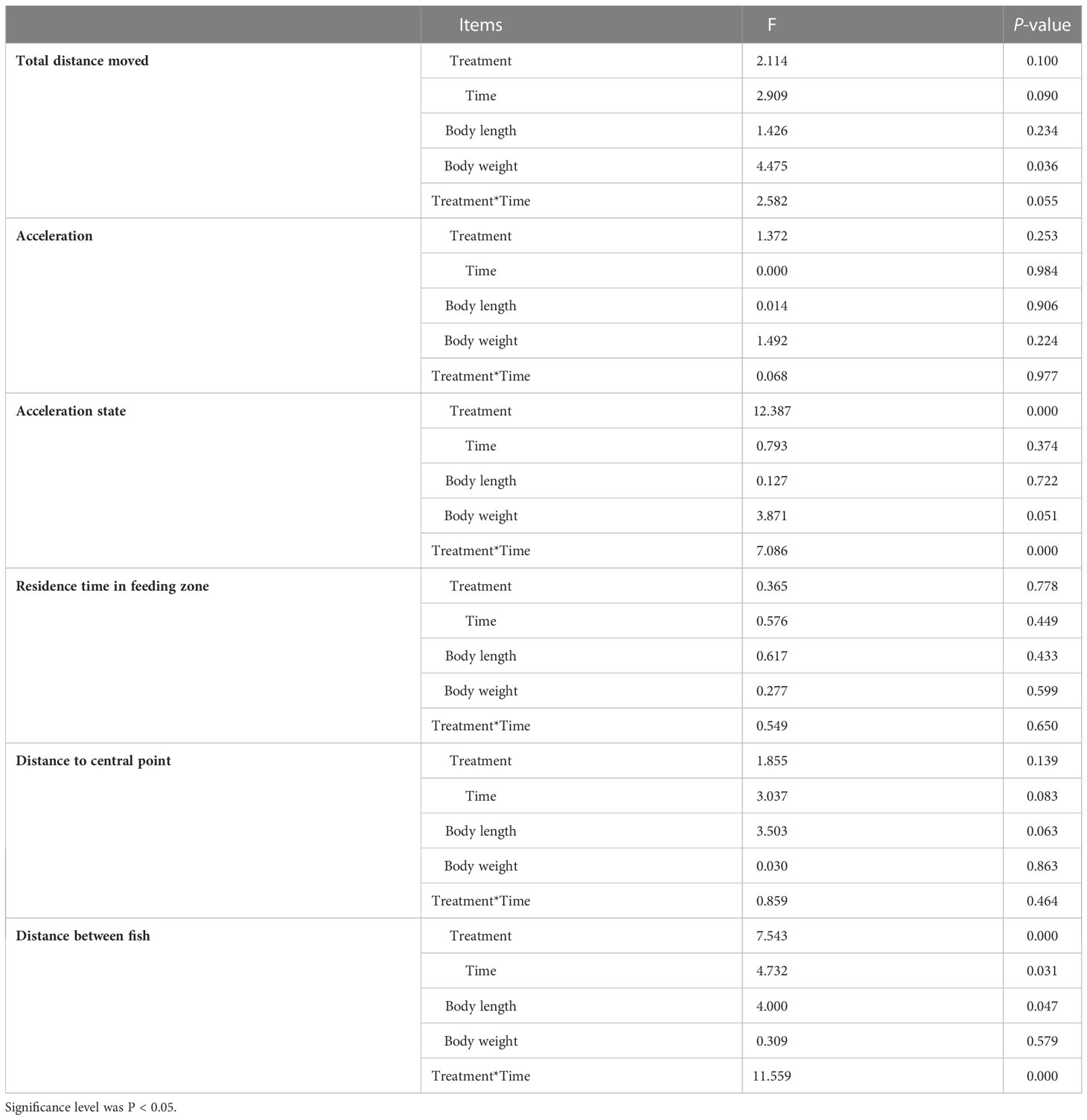
Table 3 Repeated measures of behavioral indicators were analyzed using a generalized linear mixed model (GLMM) approach, considering treatment, time, body length, body weight, and treatment*time as fixed effects and individuals within the groups as random.
For the long photoperiod groups (treatments SP-LT and SP-HT), the mean total distance moved was significantly higher than the short photoperiod groups (treatments SP-LT and SP-HT) (P < 0.05) (Table 4). No significant difference was noted in the total distance moved at different temperatures for the same photoperiod (P > 0.05). For the low water-temperature groups (treatments LP-LT and SP-LT), the maximum acceleration was significantly higher than the high water-temperature groups (treatments LP-HT and SP-HT) (P < 0.05). When the water temperature was the same, there was no significant difference in the maximum acceleration between different photoperiod groups (P > 0.05). The acceleration state of treatment LP-LT was significantly higher than that of the other treatments (P < 0.05), but no difference was found between the other treatments (P > 0.05). Moreover, no significant differences were observed among all treatments in terms of residence time in the feeding zone (P > 0.05). The distance to central point was significantly lower in treatment LP-LT than in treatment SP-HT (P < 0.05). For the high-temperature groups, the distance to central point was significantly higher than the low-temperature group (P < 0.05). The distance between fish was significantly lower than that of the other treatments (P < 0.05), but no difference was found between the other treatments (P > 0.05).
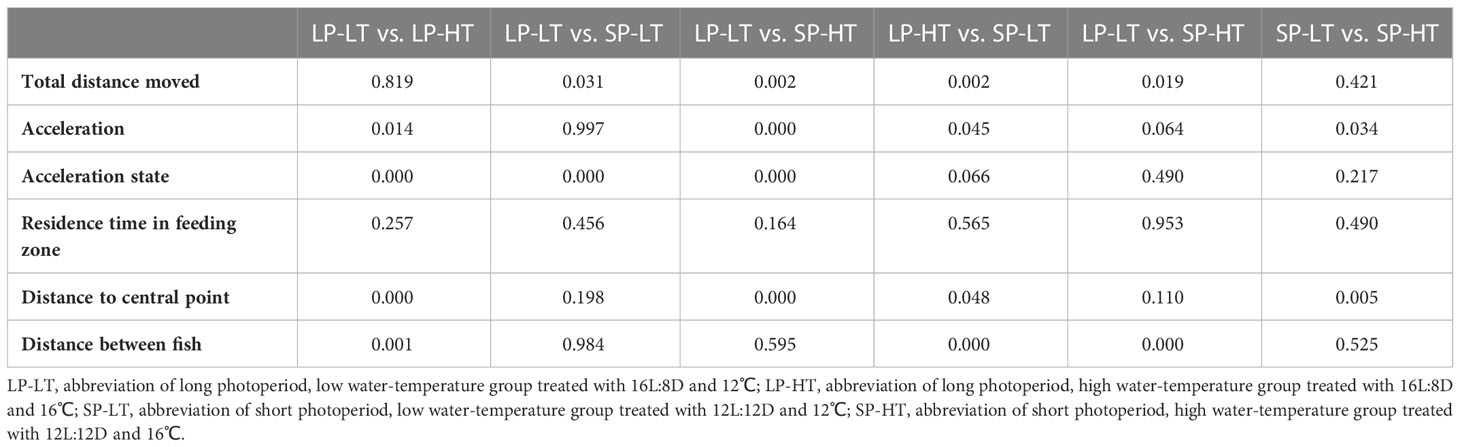
Table 4 The significance differences with P < 0.05 (P-value) in the pair-wise comparisons of behavioral traits in all treatment groups.
The PCA in all groups extracted two principal components (PCs), which accounted for more than 70% of the total variance (Table 5). Between the two PCs obtained in treatment LP-LT, PC1 accounted for 46.41% of the total variance, and it had a strong positive loading on residence time in the feeding zone and distance between fish (> 0.85). PC2 accounted for 35.13% of the total variance, and it had a strong negative loading on distance to point. For treatment LP-HT, PC1 accounted for 46.41% of the total variance, with a strong positive loading on total distance moved and residence time in the feeding zone. PC2 accounted for 31.76% of the total variance, with a strong positive loading on acceleration state. For treatment SP-LT, PC1 accounted for 38.53% of the total variance, and it had a strong positive loading on residence time in the feeding zone. PC2 accounted for 33.39% of the total variance, and it had a strong positive loading on acceleration state. For treatment SP-HT, PC1 accounted for 53.71% of the total variance, with a strong positive loading on maximum acceleration and distance to point. PC2 accounted for 22.34% of the total variance, with a strong positive loading on acceleration state.
Discussion
Benefiting from the current application of technologies in RAS, growing Salmonidae (salmon and trout) under full light conditions (24:0 h photoperiod) for maximal growth performance is common (Hines et al., 2019; Churova et al., 2020). As shown in Table 1, a considerable variation could be found in the growth performance under different photoperiod conditions at the same temperature conditions. However, the 24:0 h photoperiod is not a natural photoperiod for most salmonids, and it may be stressful to fish. For example, continuous light exposure induces abnormal gonadal development or/and lack of synchronization of smoltification processes (Sievers et al., 2018; Churova et al., 2020). With the increasing attention on fish welfare, less extreme photoperiods, such as 12:12 or 16:8 h, are being used instead in aquaculture (Ge et al., 2021; Macaulay et al., 2021). However, systematic studies that determine the photoperiod conditions for optimal growth and minimal stimulation are still lacking (Hvas et al., 2021). One of the difficulties in undertaking such research is the limited knowledge on the synergistic and antagonistic effects between different environmental factors.
In general, increased temperature results in increased body weight (Nisembaum et al., 2020). Within the suitable temperature interval, high temperature could lead to early smoltification date and large fish size (Rosengren et al., 2017; Bernard et al., 2020). Therefore, temperature could be viewed as a regulatory factor, especially in controlling (accelerating or delaying) the growth rate. The results of the present study also confirmed this information. Photoperiod is an important environmental signal to initiate complex changes that coincide with marine migration for migratory fish (Nemova et al., 2020; van Rijn et al., 2020). Once the fish size reaches a critical number, photoperiod and temperature could play important roles in controlling the growth rate and the synchronization process of smoltification. The results also confirmed the first hypothesis that photoperiod and temperature have measurable interactions on growth performance in steelhead trout juvenile under indoor aquaculture condition. The results further showed that photoperiod promoted increased body length and temperature promoted a significant increase in the weight of fish. Several studies have noted an overall lack of synchrony in the absence of photoperiodic regulation, although smoltification characteristics still appear in trout (Nisembaum et al., 2020). No meaningful differences were observed among the groups in terms of CV values, suggesting that the two photoperiods in this experiment were appropriate for smoltification synchrony. However, as mentioned earlier, whether such conditions make the juvenile comfortable is unclear.
The response of fish to a stressor could be monitored by physiological changes, which are commonly used as markers of stress level and fish welfare (Vindas et al., 2018; Cogliati et al., 2019). Increasing evidence has suggested a direct link between oxidative stress and different ecological phenomena (Archer et al., 2019; Shry et al., 2019). A complete and balanced antioxidant system normally exists in fish. When the fish’s body is in a state of stress, many oxygen-free radicals are produced in a short time, subsequently damaging the body’s metabolic balance and self-repair functions. SOD is the predominant defensive enzyme in the antioxidant system of the body, and it could directly affect the antioxidant capacity (Liu et al., 2021; Yu et al., 2021). ALT is central in protein metabolism, and it could be measured to determine organ damage (Suzuki et al., 2020). Figure 1 shows that the SOD and ALT levels were significantly higher in treatment LP-LT at the beginning of the experiment, indicating that the juvenile fish had different degrees of stress response. Fish reduce their stress response to a stimulus through repeated exposure over time, a natural process often referred to as habituation (Bratland et al., 2010; Barrett et al., 2020). This phenomenon was manifested in the present study as the SOD and ALT levels showed a trend of descending sometime after the start of the experiment. However, differences in the time required for injury repair were found among the four treatments. The SOD and ALT levels in treatments LP-LT and SP-HT returned to initial level after 30 days, whereas it took only 15 days in treatments LP-HT and SP-LT. One possible reason is that lower temperatures lead to a slowing of immune mechanisms leading to increased disease susceptibility, which has been proven previously in other fish species (Martin et al., 2008; Valero et al., 2014). In addition, a shorter time required for injury repair was observed in treatment LP-HT, which is strong evidence that photoperiod and temperature could have significant interaction effects on physiological stress responses in steelhead trout juveniles.
Cortisol is considered a biomarker of stress (Birnie-Gauvin et al., 2019). The cortisol level is shown in Figure 1, and no significant difference could be found among treatments in the whole experimental period. Cortisol may be influenced by many factors, including the state of fish at sampling and the high inter-dividual variability (Gaudio et al., 2018). The difference was not significant, probably due to the time of sampling interval being longer than that of cortisol clearance from the body. The “Smolt window” refers to a specific stage in which Salmonidae could significantly improve seawater tolerance and adapt to seawater environment due to the synergistic action of environmental factors. NKA plays an important role in the response to alterations in environmental salinity. The increased NKA in branchial chloride cells is beneficial to fish growth, swimming, and survival after smoltification (Bernard et al., 2020; Ge et al., 2021). All treatments showed an increase in the NKA level with increasing treatment time but to different degrees. Many studies have been conducted to identify the relationship between the success of migration and NKA concentration (Archer et al., 2019; Bernard et al., 2020; Ge et al., 2021). The higher the NKA concentrations were, the better the ability of migratory fish to adapt to seawater to a certain extent (Bernard et al., 2020). Moreover, increased cortisol level during smoltification was previously found coincident with increased gill NKA activity (Birnie-Gauvin et al., 2019; Shry et al., 2019). This finding was also demonstrated in the present study. Treatment LP-LT had higher cortisol and NKA level, indicating better readiness to enter seawater. A notable detail that relying solely on cortisol to determine the timing of migration is risky, as higher cortisol levels may also be due to individuals being under chronic stress and leaving to escape the stress (Damsgård et al., 2019). The role of temperature on smoltification has been controversial, with studies showing that elevated temperature alone could not play a driving role in smoltification (McCormick et al., 2000; Hines et al., 2019; Nisembaum et al., 2020). From the results presented in this paper, juveniles under long photoperiod treatments did have higher osmoregulatory capacity, but the difference was not significant. Furthermore, temperature had a regulatory role on smoltification, as the ability to regulate osmolality did not exhibit any significant differences among the groups under different photoperiods. These results showed that the combined effect of photoperiod and temperature could advance the time of smoltification, but may result in poorer salt tolerance.
The quantification analysis of fish behavior is extremely important for the design and operation of aquaculture system. Herbert et al. (2011) used light rings to induce optimal swimming motility in Atlantic salmon smolts and improve productivity. With the smoltification process, a series of behavior changes occurs in juvenile steelhead trout, in addition to changes in physiology (Macaulay et al., 2021). Table 3 shows that the total distance moved in treatments LP-LT and LP-HT was significantly lower than that in treatments SP-LT and SP-HT possibly because juvenile has higher willingness to search for food in the long photoperiod treatments (Stewart et al., 2012; Ye et al., 2016). In general, the higher the water temperature is, the greater the burst swimming speed (Ma et al., 2020). The results presented here showed that the acceleration status of treatment LP-LT was significantly higher than that of other treatments. This may be because the fish in this experiment were cold-water fish. The radius of treatments LP-HT and SP-HT was significantly larger than that of treatments LP-LT and SP-LT, indicating that lower temperature was more favorable for fish shoaling (Barrett et al., 2020; Bratland et al., 2010). The radius of fish distribution presented no significant difference between the treatments with same temperature, suggesting that temperature may be the main factor affecting fish feeding behavior compared with photoperiod. The organizational structure and individual interactions of fish are usually described by distance between them, which is influenced by photoperiod and temperature (Brakstad et al., 2018; Cogliati et al., 2019). In fact, the present and previous results showed that fish form a relatively stable structure regardless of the culture environmental condition. This structure is also significantly affected by the species type, population size, and individual interactions (Lei et al., 2020).
Fish are constantly moving and changing, and monitoring multiple fish behavior parameters simultaneously is difficult (Ye et al., 2016; Macaulay et al., 2021). The smaller the number of monitoring parameters, the higher the monitoring efficiency. Different management procedures may be necessary in fish that are under different culture conditions, such as more aggressive treatment in some cases. Therefore, feature extraction of fish behavior becomes crucial. Moreover, identifying the key behavioral indicators during smoltification process is a priority for future research (Dempster et al., 2019; Hvas et al., 2021). PCA is an effective tool to reduce the dimensionality of datasets in multivariate problems. It is capable of preserving the majority of its statistical information and identifying the most significant variations. The KMO values for all the four treatments were above 0.5, indicating that these treatments are suitable for PCA analysis. The PCA results showed that the main parameters (strong loadings) differed among the four treatments, proving the effectiveness and necessity of using PCA to identify key parameters in complex datasets. Except for treatment SP-HT, the residence time in the feeding zone was identified as the main component in all treatments. This finding is consistent with the findings of previous studies, as juvenile trout are stationary predators, whereas silvered trout are cruising predators (Folkedal et al., 2012b). The acceleration state was extracted as the main component, suggesting that the experimental conditions produced pressure on the movement smoothness of juvenile steelhead trout.
In summary, the fish status could be evaluated by continuously identifying and quantifying the key parameters of fish behavior. Although fish behavior is complex, it could be integrated into aquaculture management as an additional resource and pave the way for new approaches to fish farming. Future production of finfish continues to increase and optimizing production and improving fish welfare could become increasingly important. Indeed, incorporating fish behavior into production management still has a long way to go. However, with the increasing application of advanced technologies in aquaculture, such as computer vision and modern behavioral science, fish behavior × environment interactions could be widely used in aquaculture management in the near future (Barrett et al., 2020; Macaulay et al., 2021).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Dalian Ocean University (GBT 35892-2018).
Author contributions
ZM designed and performed the experiments, analyzed the data, and prepared the draft manuscript. LG provided suggestions in experiment design and manuscript preparation. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was supported by Key Area Research Plan of Guangdong (2021B0202030001), the National Natural Science Foundation of China (32102753), the Fund of the Scientific and Technical Innovation of Dalian (2021JJ13SN73, 2021JJ13SN72), the Liaoning Applied Basic Research Program (2022JH2/101300140), Modern Agro-industry Technology Research System (CARS-49), Innovation Support Program for High-level Talents of Dalian City (2019RD12), and the Science & Technology Program of Liaoning Province (2021JH2/10200011).
Acknowledgments
We thank all laboratory members for their constructive suggestions and discussions. Special thanks are also given to the reviewers for their constructive comments regarding the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albert K. D., Frogg N., Stefansson S. O., Reynolds P. (2019). Improving sea lice grazing of lumpfish (Cyclopterus lumpus l.) by feeding live feeds prior to transfer to Atlantic salmon (Salmo salar l.) net-pens. Aquaculture 511, 734224. doi: 10.1016/j.aquaculture.2019.734224
Archer L. C., Hutton S. A., Harman L., O'Grady M. N., Kerry J. P., Poole W. R., et al. (2019). The interplay between extrinsic and intrinsic factors in determining migration decisions in brown trout (Salmo trutta): An experimental study. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00222
Barrett L. T., Oppedal F., Robinson N., Dempster T. T. (2020). Prevention not cure: a review of methods to avoid sea lice infestations in salmon aquaculture. Rev. Aquacult., 12 (4), 2527–2543. doi: 10.1111/raq.12456
Bernard B., Leguen I., Mandiki S. N. M., Corne V., Redivo B., Kestemon P. (2020). Impact of temperature shift on gill physiology during smoltification of Atlantic salmon smolts (Salmo salar l.). Com. Biochem. Phys. A 244, 110685. doi: 10.1016/j.cbpa.2020.110685
Birnie-Gauvin K., Flávio H., Kristensen M. L., Walton-Rabideau S., Cooke S. J., Willmore W. G., et al. (2019). Cortisol predicts migration timing and success in both Atlantic salmon and sea trout kelts. Sci. Rep. 9 (1). doi: 10.1038/s41598-019-39153-x
Bowden T. J., Butler R., Bricknell I. R. (2004). Seasonal variation of serum lysozyme levels in Atlantic halibut (Hippoglossus hippoglossus l.). Fish Shell. Immun. 17 (2), 129–135. doi: 10.1016/j.fsi.2003.12.001
Brakstad O. M., Hagspiel V., Lavrutich M. N., Matanovic D. (2018). Optimal investment decisions in lice-fighting technologies: A case study in Norway. Aquaculture 504, 300–313. doi: 10.1016/j.aquaculture.2019.01.040
Bratland S., Stien L. H., Braithwaite V. A., Juell J. E., Folkedal O., Nilsson J., et al. (2010). From fright to anticipation: Using aversive light stimuli to investigate reward conditioning in large groups of Atlantic salmon (Salmo salar). Aquacult. Int. 18, 991–1001. doi: 10.1007/s10499-009-9317-8
Churova M. V., Shulgina N., Kuritsyn A., Krupnova M. Y., Nemova N. N. (2020). Muscle-specific gene expression and metabolic enzyme activities in Atlantic salmon Salmo salar l. fry reared under different photoperiod regimes. Com. Biochem. Phys. B 239, 110330. doi: 10.1016/j.cbpb.2019.110330
Cogliati K. M., Herron C. L., Noakes D. L. G., Schreck C. B. (2019). Reduced stress response in juvenile Chinook salmon reared with structure. Aquaculture 504, 96–101. doi: 10.1016/j.aquaculture.2019.01.056
Conallin A. J., Smith B. B., Thwaites L. A., Walker K. F., Gillanders B. M. (2016). Exploiting the innate behaviour of common carp, Cyprinus carpio, to limit invasion and spawning in wetlands of the river Murray, Australia. Fish. Manage. Ecol. 23, 431–449. doi: 10.1111/fme.12184
Damsgård B., Evensen T. H., Øverli Ø., Gorissen M., Ebbesson L. O. E., Rey S., et al. (2019). Proactive avoidance behaviour and pace-of-life syndrome in Atlantic salmon. R. Soc Open Sci. 6 (3), 181859. doi: 10.1098/rsos.181859
del Villar-Guerra D., Larsen M. H., Baktoft H., Koed A., Aarestrup K. (2019). The influence of initial developmental status on the life-history of sea trout (Salmo trutta). Sci. Rep. 9, 13468. doi: 10.1038/s41598-019-49175-0
Dempster T., Bui S., Overton K., Oppedal F. (2019). Jumping to treat sea lice: harnessing salmon behaviour to enable surfacebased chemotherapeutant application. Aquaculture 512, 734318. doi: 10.1016/j.aquaculture.2019.734318
FAO (2016)Food outlook. In: Biannual report on global food markets. food outlook (Accessed October 2016).
Folkedal O., Torgersen T., Olsen R. E., Fernö A., Nilsson J., Oppedal F., et al. (2012b). Duration of effects of acute environmental changes on food anticipatory behaviour, feed intake, oxygen consumption, and cortisol release in Atlantic salmon parr. Physiol. Behav. 105, 283–291. doi: 10.1016/j.physbeh.2011.07.015
Gaudio E., Bordin S., Lora I., Lora M., Massignani M., De Benedictis G. M. (2018). Leukocyte coping capacity chemiluminescence as an innovative tool for stress and pain assessment in calves undergoing ring castration. J. Anim. Sci. 96 (11), 4579–4589. doi: 10.1093/jas/sky342
Ge J., Huang M., Zhou Y., Deng Q., Liu R., Gao Q., et al. (2021). Effects of seawater acclimation at constant and diel cyclic temperatures on growth, osmoregulation and branchial phospholipid fatty acid composition in rainbow trout Oncorhynchus mykiss. J. Comp. Physiol. B 191 (2), 313–325. doi: 10.1007/s00360-020-01330-0
Guraslan C., Fach B. A., Oguz T. (2017). Understanding the impact of environmental variability on anchovy overwintering migration in the black sea and its implications for the fishing industry. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00275
Herbert N. A., Kadri S., Huntingford F. A. (2011). A moving light stimulus elicits a sustained swimming response in farmed Atlantic salmon, Salmo salar l. Fish Physiol. Biochem. 37, 317–325. doi: 10.1007/s10695-011-9499-7
Hines C. W., Fang Y., Chan V. K. S., Stiller K. T., Brauner C. J., Richards J. G. (2019). The effect of salinity and photoperiod on thermal tolerance of Atlantic and coho salmon reared from smolt to adult in recirculating aquaculture systems. Com. Biochem. Phys. A 230, 1–6. doi: 10.1016/j.cbpa.2018.12.008
Höglund E., Gjøen H. M., Pottinger T. G., Pottinger T. G., Øverli Ø. (2008). Parental stress-coping styles affect the behaviour of rainbow trout Oncorhynchus mykiss at early developmental stages. Comp. Biochem. Physiol. A 73 (7), 1764–1769. doi: 10.1016/j.cbpa.2018.12.008
Huntingford F., Jobling M., Kadri S. (2012). Aquaculture and behavior. In Tools for Studying the Behaviour of Farmed Fish (eds M. L. Bégout, S. Kadri, F. Huntingford and B. Damsgård) Oxford, UK; Blackwell Publishing Ltd., pp. 65–67. doi: 10.1002/9781444354614
Hvas M., Folkedal O., Oppedal F. (2021). Fish welfare in offshore salmon aquaculture. Rev. Aquacult. 13 (2), 836–852. doi: 10.1111/raq.12501
Lei L., Escobedo R., Sire C., Theraulaz G. (2020). Computational and robotic modeling reveal parsimonious combinations of interactions between individuals in schooling fish. PloS Comput. Biol. 16 (3), e1007194. doi: 10.1371/journal.pcbi.1007194
Liu Z. L., Zhao W., Hu W. S., Zhu B., Xie J. J., Liu Y. J., et al. (2021). Lipid metabolism, growth performance, antioxidant ability and intestinal morphology of rainbow trout (Oncorhynchus mykiss) under cage culture with flowing water were affected by dietary lipid levels. Aquacult. Rep. 19, 100593. doi: 10.1016/j.aqrep.2021.100593
Macaulay G., Bui S., Oppedal F., Dempster T. (2021). Challenges and benefits of applying fish behaviour to improve production and welfare in industrial aquaculture. Rev. Aquacult. 13 (2), 934–948. doi: 10.1111/raq.12505
Ma Z., Li H. X., Hu Y., Fan J. Z., Liu Y. (2020). Growth performance, physiological, and feeding behavior effect of Dicentrarchus labrax under different culture scales. Aquaculture 534 (3), 736291. doi: 10.1016/j.aquaculture.2020.736291
Martin L. B., Weil Z. M., Nelson R. J. (2008). Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc B 363 (1490), 321–339. doi: 10.1098/rstb.2007.2142
McCormick S. D., Moriyama S., Björnsson B. T. (2000). Low temperature limits photoperiod control of smolting in Atlantic salmon through endocrine mechanisms. Am. J. Physiol. 278, 1352–1361. doi: 10.1152/ajpregu.2000.278.5.R1352
Nemova N. N., Nefedova Z. A., Pekkoeva S. N., Voronin V. P., Shulgina N. S., Churova M. V., et al. (2020). The effect of the photoperiod on the fatty acid profile and weight in hatchery-reared underyearlings and yearlings of Atlantic salmon Salmo salar l. Biomolecules 10, 845. doi: 10.3390/biom10060845
Nisembaum L. G., Martin P., Fuentes M., Besseau L., Magnanou E., McCormick S. D., et al. (2020). Effects of a temperature rise on melatonin and thyroid hormones during smoltification of Atlantic salmon, Salmo salar. J. Comp. Physiol. B 190 (6), 731–748. doi: 10.1007/s00360-020-01304-2
Pauly D., Zeller D. (2017). Comments on FAOs state of world fisheries and aquaculture (SOFIA 2016). Mar. Policy 77, 176–181. doi: 10.1016/j.marpol.2017.01.006
Rosengren M., Thörnqvist P. O., Johnsson J. I., Sandblom E., Winberg S., Sundell K. (2017). High risk no gain-metabolic performance of hatchery reared Atlantic salmon smolts, effects of nest emergence time, hypoxia avoidance behaviour and size. Physiol. Behav. 175, 104–112. doi: 10.1016/j.physbeh.2017.03.028
Salinas P., Molina F., Hernández N., Sandoval C. (2022). Phenotypic response of male and neomale of O. mykiss parr subjected to 8° and 16 °C water temperature during early life stage. Aquacult. Rep. 22, 100996. doi: 10.1016/j.aqrep.2021.100996
Shry S. J., McCallum E. S., Alanärä A., Persson L., Hellström G. (2019). Energetic status modulates facultative migration in brown trout (Salmo trutta) differentially by age and spatial scale. Front. Ecol. Evol. 7, 411. doi: 10.3389/fevo.2019.00411
Sievers M., Korsøen Ø., Dempster T., Fjelldal P. G., Kristiansen T., Folkedal O., et al. (2018). Growth and welfare of submerged Atlantic salmon under continuous lighting. Aquacult. Env. Interac. 10, 501–510. doi: 10.3354/aei00289
Stewart A. M., Gaikwad S., Kyzar E., Kalueff A. V. (2012). Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 1451, 44–52. doi: 10.1016/j.brainres.2012.02.064
Suzuki S., Takahashi E., Nilsen T. O., Kaneko N., Urabe H., Ugachi Y., et al. (2020). Physiological changes in off-season smolts induced by photoperiod manipulation in masu salmon (Oncorhynchus masou). Aquaculture 526, 735353. doi: 10.1016/j.aquaculture.2020.735353
Valero Y., García-Alcázar A., Esteban M.Á., Cuesta A., Chaves-Pozo E. (2014). Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax l.). Fish Shell. Immun. 39 (2), 185–187. doi: 10.1016/j.fsi.2014.05.011
van Rijn C. A., Jones P. L., Schultz A. G., Evans B. S., McCormick S. D., Afonso L. O. B. (2020). Atlantic Salmon (Salmo salar) exposed to different preparatory photoperiods during smoltification show varying responses in gill Na+/K+-ATPase, salinity-specific mRNA transcription and ionocyte differentiation. Aquaculture 529, 735744. doi: 10.1016/j.aquaculture.2020.735744
Vindas M. A., Fokos S., Pavlidis M., Höglund E., Dionysopoulou S., Ebbesson L. O. E., et al. (2018). Early life stress induces long-term changes in limbic areas of a teleost fish: the role of catecholamine systems in stress coping. Sci. Rep. 8 (1), 5638. doi: 10.1038/s41598-018-23950-x
Ye Z. Y., Zhao J., He Z. Y., Zhu S. M., Li J. P., Lu H. D. (2016). Behavioral characteristics and statistics-based imaging techniques in the assessment and optimization of tilapia feeding in a recirculating aquaculture system. Trans. ASABE 59 (1), 345–355. doi: 10.13031/trans.59.11406
Keywords: recirculating aquaculture system, steelhead trout, physiology, fish behavior, aquaculture facilities
Citation: Ma Z, Zhang J, Zhang X, Li H, Liu Y and Gao L (2023) Effects of temperature and photoperiod on growth, physiological, and behavioral performance in steelhead trout (Oncorhynchus mykiss) under indoor aquaculture condition. Front. Mar. Sci. 10:1114662. doi: 10.3389/fmars.2023.1114662
Received: 02 December 2022; Accepted: 04 January 2023;
Published: 23 January 2023.
Edited by:
Ce Shi, Ningbo University, ChinaReviewed by:
Shijing Liu, Fishery Machinery and Instrument Research Institute (CAFS), ChinaJian Zhao, Zhejiang University, China
Copyright © 2023 Ma, Zhang, Zhang, Li, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Gao, Z2FvbGVpQGRsb3UuZWR1LmNu
 Zhen Ma
Zhen Ma Jia Zhang1,2
Jia Zhang1,2 Lei Gao
Lei Gao