
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 January 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1105065
This article is part of the Research Topic Integration of Development, Physiology and Responses to Environmental Change in Aquatic Invertebrates View all 13 articles
 Siting Wang1,2†
Siting Wang1,2† Guoliang Ren1,3†
Guoliang Ren1,3† Desheng Li1,2
Desheng Li1,2 Sishao Fan1,2
Sishao Fan1,2 Susu Yan1,2
Susu Yan1,2 Junjie Shi1,2
Junjie Shi1,2 Meimei Liu1,2
Meimei Liu1,2 Zhiguo Dong1,2*
Zhiguo Dong1,2*Residual chlorine is a common by-product of warm drainage in coastal nuclear power plants. when accumulating to some limit, it may threaten marine ecosystem especially for benthic clam. However, there are few studies on the molecular mechanisms related to immunity and antioxidant of residual chlorine stress on clams. In this study, the clam (Cyclina sinensis) was exposed for 96 h at different concentrations (0, 50, 100, 150, 200, 250, 300, 350, 400, 450 and 500 mg/L) of residual chlorine to observe its mortality, measure the activity of antioxidant and immune-related enzymes, and analyses the gene expression level in the hepatopancreas by using the transcriptome sequencing. The results showed that the mortality rate increased with the increase of stress time and concentration, and the mortality rate in the 400, 450 and 500 mg/L groups reached 100% at 96 h. The tolerance to residual chlorine of C. sinensis decreased with the increase of chlorine dioxide concentration, and the LC50 of 96 h was 217.6 mg/L by linear regression method. After residual chlorine stress, the activity of antioxidant-related enzymes (T-AOC and SOD) in the hepatopancreas showed a trend of first increase and then decrease with the extension of stress time. The immune-related enzyme activities of AKP and LZM showed a downward trend between 0 and 96 h, while the ACP enzyme activity showed a trend of first rising and then decreasing. Transcriptome analysis showed that residual chlorine stress significantly changed the expression levels of immune-related molecules associated with signal transduction, prophenoloxidase cascade, cell apoptosis and pattern recognition protein/receptor. Moreover, glutathione S-transferase (GST), heat shock protein (HSP) and other antioxidant-related genes were significantly affected under residual chlorine stress. This study provided valuable information for understanding the effects of residual chlorine stress on survival, physiological metabolism and molecular mechanisms of immune and antioxidant functions of C. sinensis.
Chlorine residual is a common by-product of the warm water discharge from coastal nuclear power stations. In order to prevent seawater organisms from damaging the cooling system, seawater needs to be electrolysed so that the cooling water contains a certain concentration of chlorine, thereby killing all kinds of organisms in the seawater. The seawater enters the cooling system and carries away the waste heat from the nuclear reaction to become warm water, but the residual chlorine in the water also enters the ocean with the warm water. Chlorination of seawater is the main cause of killing organisms in cooling water, while the thermal shock from waste heat in nuclear power plants has less impact (Zeng, 2008). At the same time, in shellfish seedling production, the washing and scrubbing of ponds after transfer, the disinfection of bait cultivation and the daily cleaning of equipment and tools such as nets and bags often involve the use of disinfectants such as bleach. Although the whole process requires strict control, omissions and misuse are inevitable, allowing residual chlorine to flow into the seedling pond to varying degrees, with unpredictable effects on the production of Cyclina sinensis seedling (Xu et al., 2007; Wu et al., 2018).
In response to environmental changes, organisms need to use their own antioxidant and immune systems to counteract external stimuli (Eppley et al., 1976; Dempsey, 1986; Pan et al., 2006; Alexandre et al., 2022). The antioxidant response of aquatic organisms to acute or chronic stimuli has been studied mostly in fish and shrimps (Hegazi et al., 2010; Liang et al., 2016; Liu C et al., 2022). In the available studies, fish death under residual chlorine stress is mainly caused by asphyxiation (Jiang et al., 2009), which is also consistent with the mechanism of toxicity of chlorine damage to the respiratory systems of humans and animals. Residual chlorine causes lesions in the gill tissue of aquatic organisms, such as hyperplasia, hypertrophy and accumulation of mucus (Li et al., 2017; Nikolaivits et al., 2020). Swelling of the gill filaments separates the gill filaments from the capillaries and prevents oxygen from entering the capillaries properly in gill tissue, which causes reduced oxygen supply and lower blood oxygen levels in the organism. This phenomenon increases the respiratory rate in order to provide the oxygen levels required for activity. The free chlorine in residual chlorine has strong oxidizing property and can enter the bloodstream through the gills, where it can undergo redox reactions with haemoglobin in the blood. Thereby it disrupts the oxygen transport role of haemoglobin and reduces the oxygen content in the fish blood, inhibits the ability of the fish blood to transport oxygen (Zeitoun, 1977). The immune and antioxidant enzyme activities of bivalves are important physiological parameters of shellfish. Hepatopancreas are important detoxigenic organs and the first barrier against the interference of the whole organism (Pawert et al., 1996; Opstvedt et al., 2000; Zhao L et al., 2016). However, only a few studies have combined molecular and biochemical procedures to describe the relationship between the transcription of specific immune and antioxidant genes and their activity (Qiu et al., 2018; Chen et al., 2021; Qiao et al., 2022), and the response mechanism of shellfish to residual chlorine stress is also rarely studied.
Cyclina sinensis is a common buried shellfish, also known as the venus clam and black clam, and is one of the main cultured shellfish in China’s aquaculture industry (Dong et al., 2021). The clam is very widely distributed in the coastal areas, from Korea to the north and south coasts of China, mainly in coastal mudflat areas, with river inlets and is a common shellfish species in China’s coastal areas (Dong et al., 2021). Due to the advantages of strong resistance to adversity, tasty meat and high culture yield, the clam has become an ideal cultured shellfish with huge production and sales in China (Zhang, 2014; Shi et al., 2019). As a typical burial-type economic shellfish, C. sinensis, is good bioindicator for coastal pollution investigations and has been widely cultivated and proliferated in China (Wei et al., 2020; Ge et al., 2021; Liao et al., 2022). Therefore, C. sinensis could be used as a model species to understand the potential toxicity effects of marine bivalves exposed to residual chlorine.
In the present study, we observed the mortality of C. sinensis under residual chlorine stress, determined the antioxidant and immune-related enzymes and analysed transcriptome. This study will provide insight into the mechanism of residual chlorine toxicity in C. sinensis and provide some theoretical references for the understanding of the toxic effects of residual chlorine on bivalves.
Juvenile clam (shell length: 1.20 ± 0.10 cm, weight: 0.70 g ± 0.2 g) was collected from a reproduction farm along coastal of Lianyungang China. Juvenile clam was acclimated for one week in an indoor circulating water system. One week before the experiment, we selected the healthy clams, cleaned their surface stains and disinfected, and then temporarily reared them in natural seawater (salinity: 22 ppt, DO: 8.00-9.00 mg/L, pH: 7.40-7.75) and kept aerating and no bait during the temporary rearing period to enable the clam to expel internal dirt. Meanwhile, we removed the deformed and diseased individuals during the temporary rearing period. All clams were treated in strict accordance with the guidelines for the care and use of experimental animals established by the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China and approved by the Committee on Experimental Animal Management of the Jiangsu Ocean University.
The C. sinensis were randomly divided into ten groups (10 individuals in each group) and were subjected to different chlorine dioxide (chlorine dioxide effervescent tablets, South Ranch) concentration levels of 0 mg/L (C0), 50 mg/L (C1), 100 mg/L (C2), 150 mg/L (C3), 200 mg/L (C4), 250 mg/L (C5), 300 mg/L (C6), 350 mg/L (C7), 400 mg/L (C8), 450 mg/L (C9) and 500 mg/L (C10) with C0 set as control. Three parallel experiments were conducted for each chlorine dioxide concentration level, and used a residual chlorine meter (Pocket Colorimeter II, DR 800, HATCH) to measure the residual chlorine concentration in each group. The treatment continued for 96 h.
The mortality of C. sinensis was measured after 24, 48, 72, and 96 h of treatment. The death of individuals in each group was recorded. The mortality rate was calculated as Df × Oi-1 × 100%, where Df is the number of the clams killed at the end of each stress in each group and Oi is the total number of the clams in each group at the start of the stress. The LC50 of chlorine dioxide for C. sinensis at 96 h were analyzed by linear regression analysis on the basis of mortality data. The calculation formula and method were referred from Li et al. (2012). The safe concentration (SC) was calculated as following: SC = 0.1 × 96 h LC50.
Choosing 10%, 25% and 50% of the LC50 of chlorine dioxide at 96 h for C. sinensis obtained from above, i.e., 21.76 mg/L, 54.4 mg/L and 108.8 mg/L as stress concentrations, while as the concentrations could not be set precisely due to the large size of the culture water. So we set three stress concentrations of 20 mg/L (a), 50 mg/L (b) and 100 mg/L (c), and three replicates in each group (10 individuals in each group) in this experiment. The hepatopancreas samples from each individual in groups a, b and c were collected at 0, 3, 6, 12, 24, 48, 72, and 96 h of treatment for enzyme activity determination and only hepatopancreas at 96 h were collected for transcriptomic analysis, with three biological replicates for each experiment.
During sampling, 5 clams from each replicate were pooled together to eliminate any effect of tank, and each clam was collected hepatopancreas at each point of sampling time, immediately frozen in liquid nitrogen and stored at -80°C. The frozen samples were ground in liquid nitrogen, diluted with physiological saline and stored in a refrigerator at 4°C before the assay for subsequent biochemical determination. The activities of enzymes including Lysozyme (LZM), Alkaline phosphatase (AKP), Acid phosphatase (ACP), Superoxide dismutase (SOD) and Total antioxidant capacity (T-AOC) were determined through biochemical analysis kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
Selecting the hepatopancreas of C. sinensis treated with different concentrations (a, b, c) for 96 h in Section 2.3 described as samples, marked as H-96a, H-96b and H-96c, respectively, and the 0 h stress group was used as the control, marked as H-0. Total RNA was extracted from the hepatopancreas using Trizol method, and genomic DNA was removed with DNase I (Takara). Subsequently, the integrity and purity were estimated by a NanoDrop 2000 (Thermo Fisher Scientific Inc., USA), respectively. Only high-quality RNA samples (OD260/OD280 ranged 1.8-2.2, RIN ≥8.0) were used to construct the sequencing library. Then, these libraries were sequenced on the Illumina sequencing platform (HiSeqTM 2500 or Illumina HiSeq X Ten), and 125 bp/150 bp paired-end reads were generated. After removing adaptor and low-quality sequences, the clean reads were assembled into expressed sequence tag clusters (contigs) and de novo assembled into transcripts by using Trinity with the paired-end method (Grabherr et al., 2011). The longest transcript was chosen as an unigene based on the similarity and length of a sequence for subsequent analysis.
The function of assembled unigenes was annotated by alignment of the unigenes with the NCBI nonredundant (NR), SwissProt, and Clusters of Orthologous Groups for Eukaryotic Complete Genomes (KOG) databases using Blastx with a threshold E-value of 10−5. GO and Pfam annotations were performed by Blast2GO and HMMER software, respectively. Database annotation was performed with KEGG. The gene expression level was calculated by using the FPKM (fragments per kb per million reads) method. To identify differentially expressed genes (DEGs) across samples, |log2(fold change)| > 1 and P-value < 0.05 were set to be the thresholds for significantly different expression levels. The gene expression profiles were compared among the four groups, control (H-0, 0 mg/L), a (H-96a, 20mg/L), b, (H-96b, 50 mg/L) and c, (H-96c, 100 mg/L) treatments, and then all DEGs in each comparison were submitted to GO functional and KEGG pathway enrichment analysis using the GO database and KEGG database, respectively.
According to the transcriptome sequencing results, 10 immune and antioxidant related genes were randomly selected to detect Illumina by qRT - PCR. The reliability of sequencing was verified. All the 10 target gene primers were designed using Primer 5.0 and were synthesized by Shanghai Sangon Biological Company. Each pair of primers was validated by gel electrophoresis prior to qPCR. Primers used in the experiment are shown in Table 1.
The cDNA of hepatopancreas of C. sinensis treated with different residual chlorine concentrations for 96 h was used as the template for qPCR. TransGen Biotech’s PerfectStart® Green qPCR SuperMix kit was used for quantitative analysis of gene expression levels. The qRT-PCR assays were performed with three replicates, and the β-actin gene was used as an internal control to normalize the expression level of the target genes The reaction system with a volume of 10 µL was as follows: 2×PerfectStart® Green qPCR SuperMix (5.0µL), Passive Reference Dye (50×) (0.2µL), primer F (0.2µL), primer R (0.2µL), cDNA template (1µL) and Nuclease-free Water (3.4 µL). The qPCR analyses were performed in triplicate on a StepOnePlus Real-Time PCR (Applied Biosystems, USA) as follows: was used, and the reaction procedure was: predenatured at 94 °C for 30 s; followed by 40 cycles of 94 °C for 5 s and 60 °C for 30 s. Using the 2-ΔΔCt method to calculate relative quantification of qRT-PCR data.
SPSS 26.0 software was used for data analysis in this study. Use One-way ANOVA and Duncan’s analysis to compare the difference of the data. Differences were considered significant at P < 0.05.
The mortality rate of C. sinensis after chlorine dioxide treatment and residual chlorine concentration for different chlorine dioxide concentrations are shown in Table 2. As can be seen, the mortality rate in the control group (C0) remained zero during the whole experiment period, indicating the clam individuals used were all in good health.
At the same time, there was a concentration-time effect on the resistance of C. sinensis to residual chlorine. The results showed that the mortality increased with time, and as the concentration of chlorine dioxide increased, the tolerance of C. sinensis to residual chlorine decreased. Among the different concentration groups, only the groups of C8, C9 and C10 showed mortality at 24 h. At 48 h, no death occurred in the C0 group and C1 group, but all the other groups occurred dead individuals, but the mortality was less than 50%. At 72 h the mortality rates of the C8, C9 and C10 groups exceeded 50%, with the mortality rate in the C10 group reached 83.33%, and the mortality rate in the C8, C9 and C10 groups reached 100% at 96 h.
As can be seen from the concentration-mortality curve of chlorine dioxide on clams (Figure 1), using the linear regression method, the 96 h LC50 was 217.6 mg/L, corresponding to a residual chlorine concentration of 0.22 mg/L. The safe concentration (SC) was 21.76 mg/L, corresponding to a residual chlorine concentration of 0.01 mg/L.
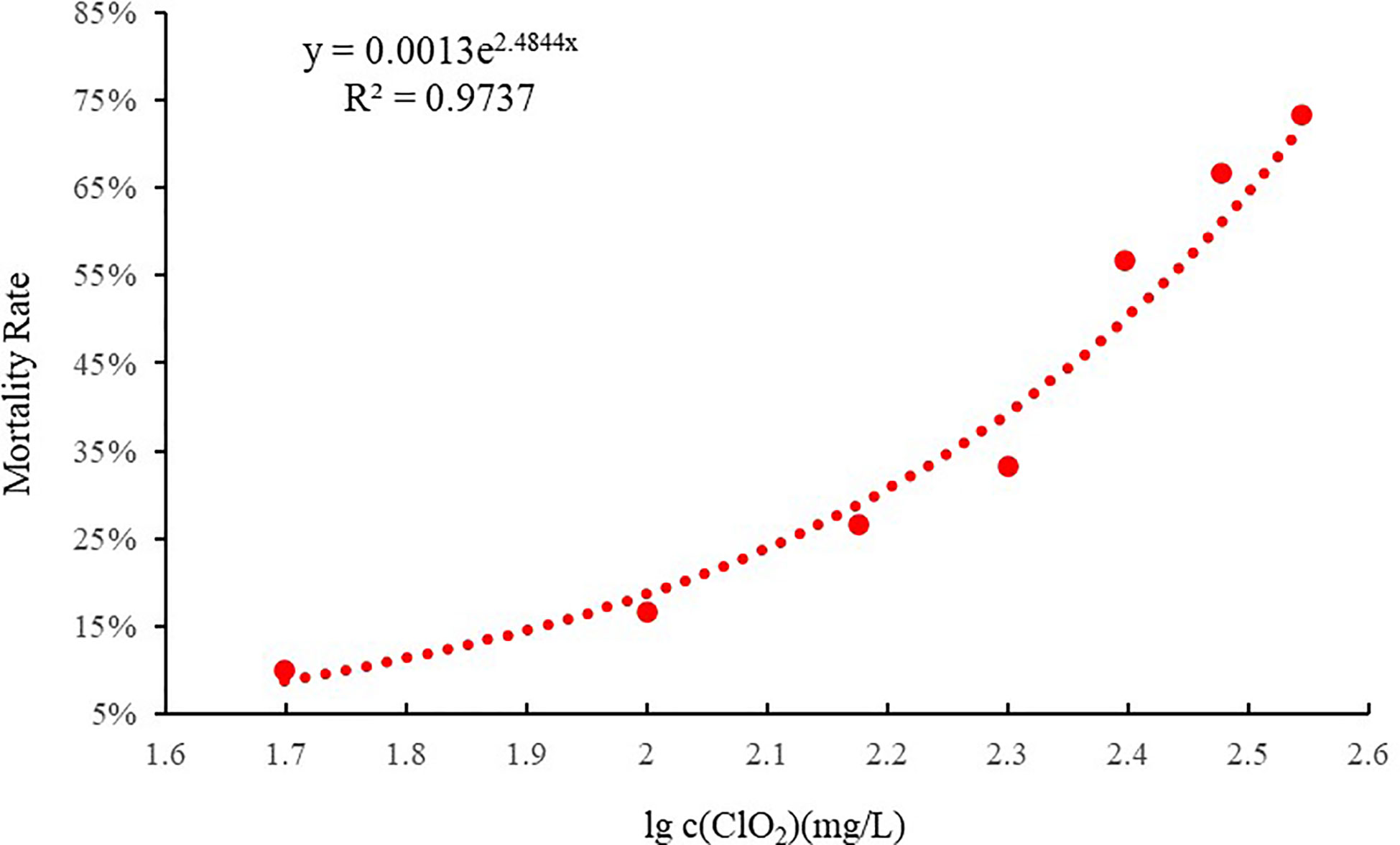
Figure 1 The Concentration - Mortality curve of chlorine dioxide to C. sinensis at 96 h (R2 = 0.9737).
The activities of T-AOC and SOD in hepatopancreas of clams subjected to residual chlorine stress showed a wavy trend from 0 to 96 h (Figure 2). The maximum value of T-AOC in 20 mg/L stress group at 72 h was significantly different from that at 0 h (P < 0.05), the enzyme activity at 96 h was the lowest, and lower than that at 0 h, but the difference was not significant (P > 0.05). The T-AOC activity in 50 mg/L stress group also reached the maximum value at 72 h, and the difference was significant compared with 0 h (P < 0.05), the activity at the 6 h was the lowest, and there was no significant difference from the initial value (P > 0.05). The T-AOC in the 100 mg/L group reached its maximum value at 48 h and was significantly different from that at 0 h (P < 0.05), while it was lowest at 6 h and 96 h with no significant difference from the initial value (P > 0.05). Compared with the other two groups, the maximum T-AOC took a shorter time in the 100 mg/L group than that in the other two groups.
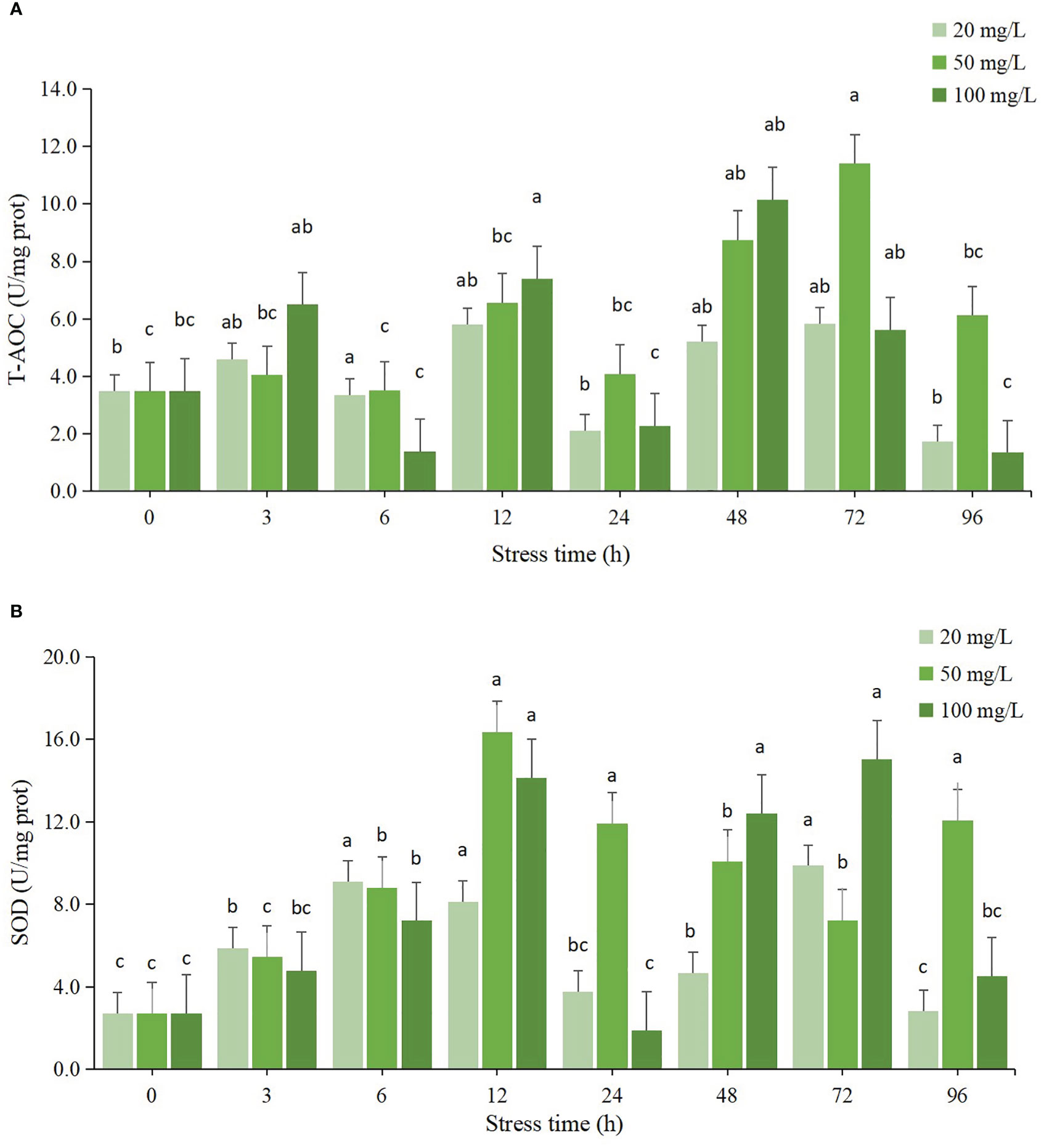
Figure 2 Changes of antioxidant enzyme activities in hepatopancreas of C sinensis at different time points after different residual chlorine stress. (A) T-AOC activitiy, (B) SOD activitiy. Different letters indicate statistically significant differences between different time points for the same treatment using one-way ANOVA and Tukey’s comparison of the means test (P < 0.05).
The SOD enzyme activity of all three concentration groups showed an increasing trend from 0 to 12 h, after which the SOD enzyme activity started to decrease. The SOD enzyme activity of the 20 mg/L group showed an overall increasing trend, and started to decrease at 24 h, and the enzyme activity was still higher than the initial value, but was not significantly different from the initial value (P > 0.05), and started to increase again at 48 h. At 96 h, the SOD enzyme activity decreased significantly (P < 0.05) and reached the lowest value. The SOD enzyme activity in the 50 mg/L group reached its maximum value at 12 h and was significantly different from the initial value (P < 0.05), then the enzyme activity decreased slightly, but was still higher than the initial value and significantly different from the initial value (P < 0.05) and the lowest value was reached at 6 h, which was not significantly different from the initial value (P > 0.05).
The effect of residual chlorine stress on ACP activity in the hepatopancreas of C. sinensis showed a general trend of increasing and then decreasing, as seen in Figure 3A. In the 20 mg/L group, the ACP activity showed fluctuating changes, reaching two peaks at 6 h and 48 h respectively, with a maximum value at 48 h, which was significantly different from the initial value (P < 0.05), and the lowest ACP activity at 96 h, which was lower than the initial value, but not significantly different from the initial value (P > 0.05). The ACP activity in the 50 mg/L group showed a trend of increasing and then decreasing, and reached a maximum value at 48 h, which was significantly different from the initial value (P < 0.05), and a minimum value at 96 h, which was lower than the initial value, but not significantly different from the initial value (P > 0.05). The ACP activity in the 100 mg/L group showed a tendency to increase and then decrease, and reached a maximum value at 6 h, which was significantly different from the initial value (P < 0.05), and a minimum value at 72 h, which was lower than the initial value but not significantly different from the initial value (P > 0.05).
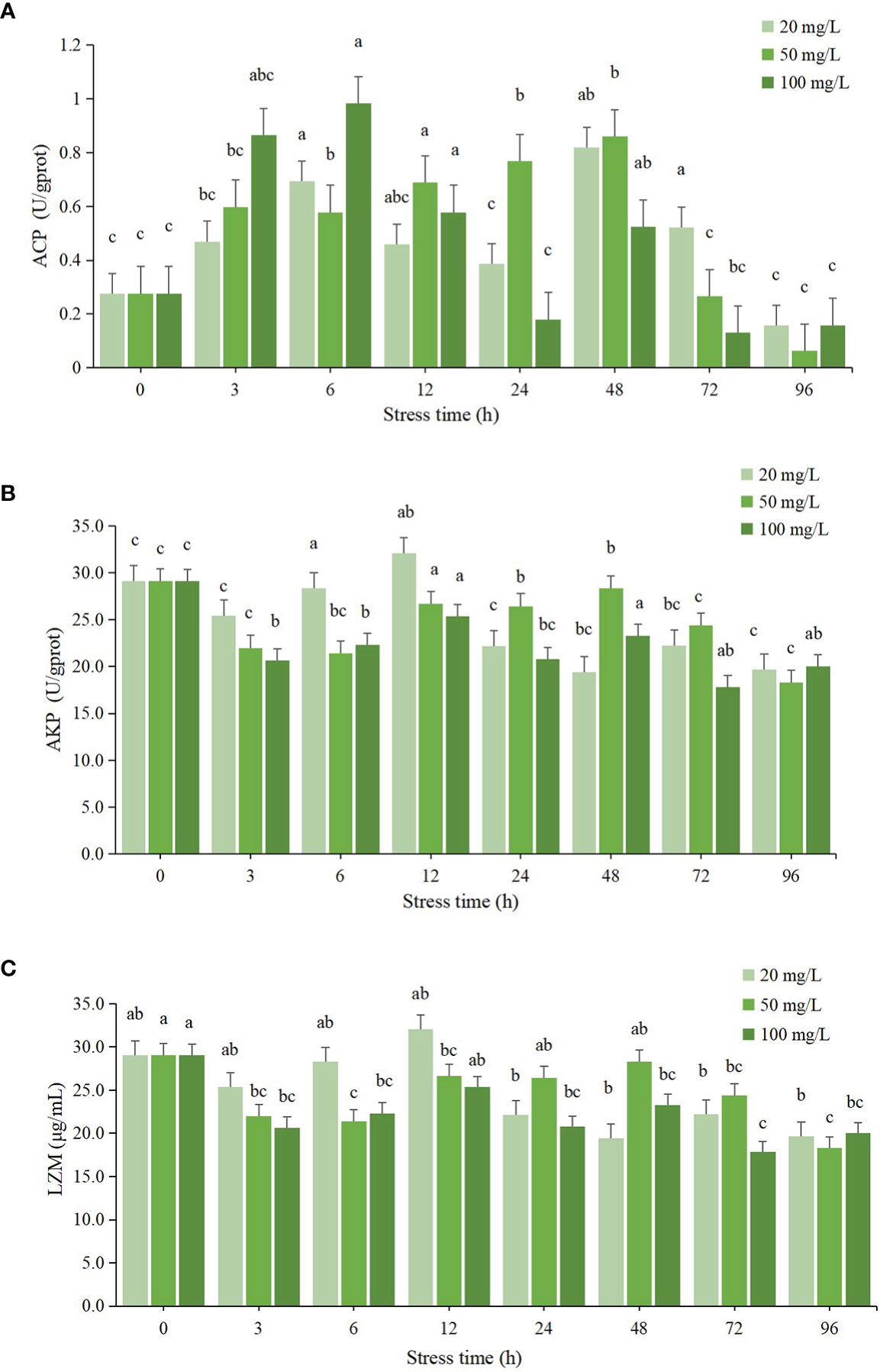
Figure 3 Changes of immune enzyme activities in hepatopancreas of C sinensis at different time points after different residual chlorine stress. (A) ACP activitiy, (B) AKP activitiy and (C) LZM activitiy. Different letters indicate statistically significant differences between different time points for the same treatment using one-way ANOVA and Tukey’s comparison of the means test (P < 0.05).
As seen in Figure 3B, the effect of residual chlorine stress on AKP activity in the hepatopancreas of C. sinensis showed fluctuating changes. The AKP activity in the 20 mg/L group reached its maximum value at 12 h and was significantly different from the initial value (P < 0.05), and reached its lowest value at 48 h and was lower than the initial value and the difference was significant compared with the initial value (P < 0.05). The AKP enzyme activity in the 100 mg/L group also showed fluctuating changes, but the enzyme activity was lower than that of the control group in all period of time and was significantly different from that of the control group (P < 0.05). The lowest value was found at 72 h.
The effect of residual chlorine stress on LZM enzyme activity in the hepatopancreas of C. sinensis showed fluctuating changes as can be seen in Figure 3C. The LZM activity in the 20 mg/L group showed fluctuating changes, reaching a maximum value at 12 h with no significant difference from the initial value (P > 0.05) and a minimum value at 48 h with significant difference (P < 0.05). The LZM activity in the 100 mg/L group was lower than the initial value after 0 h and was significantly different from the initial value (P < 0.05), with the lowest value occurring at 72 h.
In this study, the H-0 group was used as the control, and the number of differential genes in H-96a, H-96b and H-96c groups were counted (Figures 4, 5). The ILLUMINA data were submitted to the National Center for Biotechnology Information (NCBI, PRJNA904083). The results showed that compared with the control group, 13, 17 and 16 unigenes were up-regulated and 23, 34 and 13 unigenes were down-regulated in group H-96a, H-96b and H-96c, respectively. Compared with group H-96a at 96 h of residual chlorine stress, 19 and 23 unigenes were up-regulated and 17 and 16 unigenes were down-regulated in group H-96b and group H-96c, respectively. Finally, 23 and 27 unigenes were up-regulated and down-regulated in group H-96c, respectively, compared with group H-96b at 96 h. Most of the genes in groups H-96a, H-96b, and H-96c were down-regulated compared with the control group, and most of the genes in groups H-96a, H-96b, and H-96c were up-regulated compared with each other.

Figure 4 Statistical chart of DEGs. H-0: Control group; H-96a: concentration of 20 mg/L group; H-96b: concentration of 50 mg/L group; H-96c: concentration of 100 mg/L group. Up- and down-regulated genes are shown in red and blue, respectively.
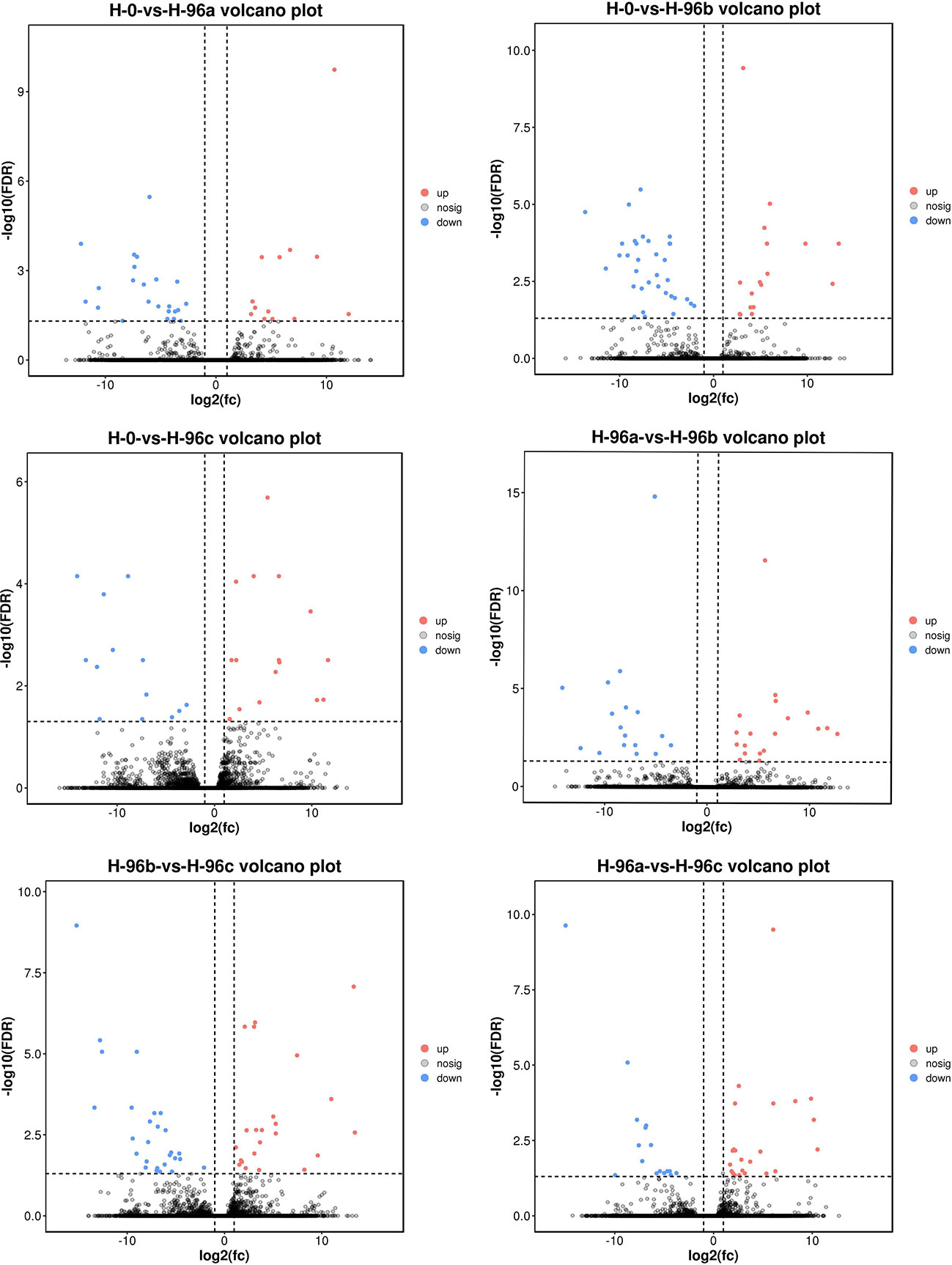
Figure 5 Difference comparison volcano map. H-0: Control group; H-96a: concentration of 20 mg/L group; H-96b: concentration of 50 mg/L group; H-96c: concentration of 100 mg/L group. Up- and down-regulated genes are shown in red and blue, respectively.
To analyse the functions of these DGEs, GO assignments were made. These DGEs were assigned to major GO categories (level 3), i.e., biological process, cellular component, and molecular function (Figure 6). KO annotation of transcriptome results classified differentially expressed genes (referred to as differential genes) into 5 categories based on metabolic pathways categories, which belong to Metabolism, Organismal systems, Cellular Processes, Genetic information processing, and Environmental information processing. The annotation of the number of differential genes is shown in Figure 7: In KEGG annotation, the most single gene pathways among groups were: cofactors in metabolic pathways and signal transduction in vitamin metabolism and environmental information processing; Transport and catabolism in cellular processes and Signal transduction in environmental information processing.
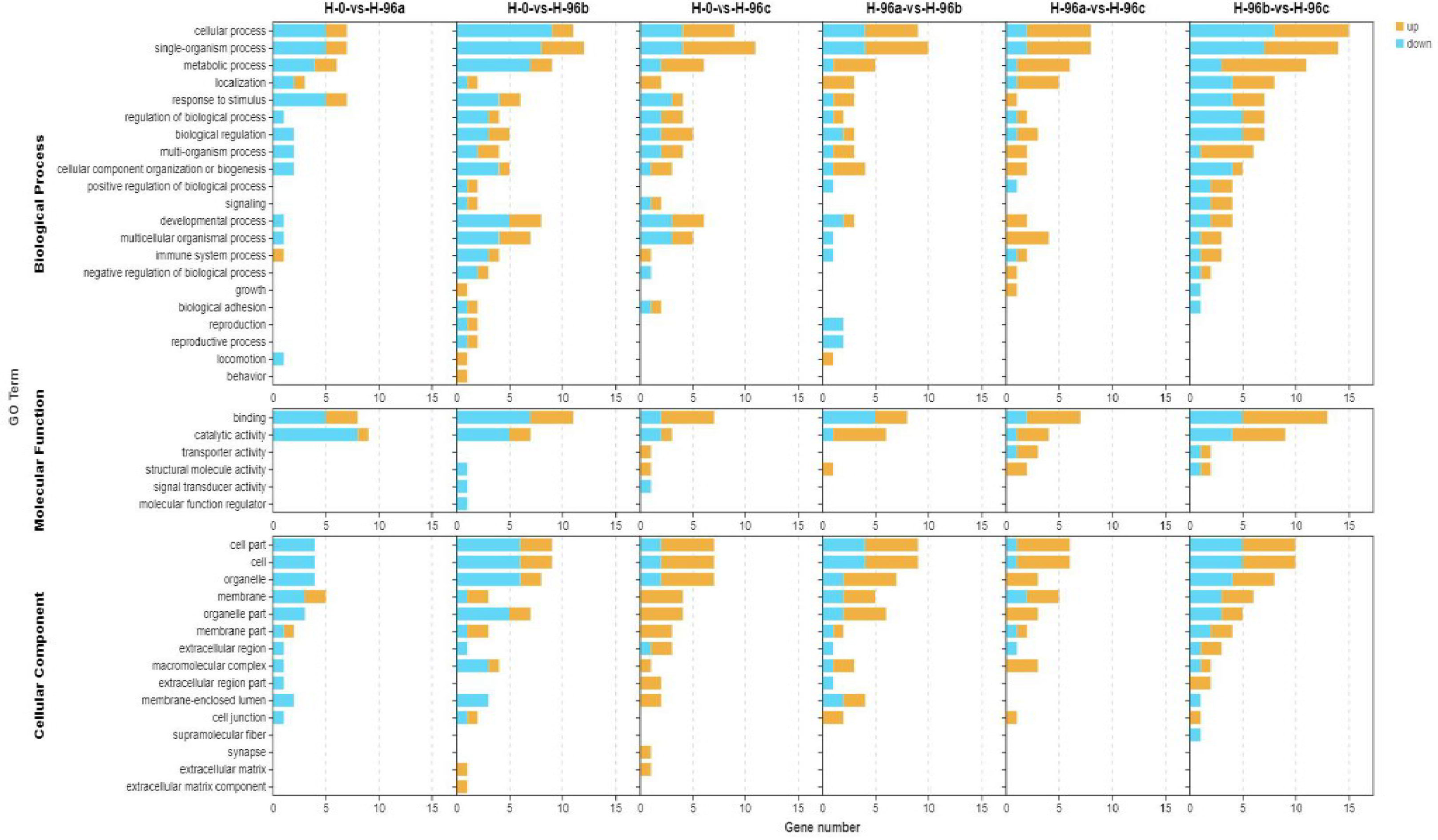
Figure 6 GO enrichment analyses of differentially expressed genes (DEGs) in hemolymph transcriptome. H-0: Control group; H-96a: concentration of 20 mg/L group; H-96b: concentration of 50 mg/L group; H-96c: concentration of 100 mg/L group.
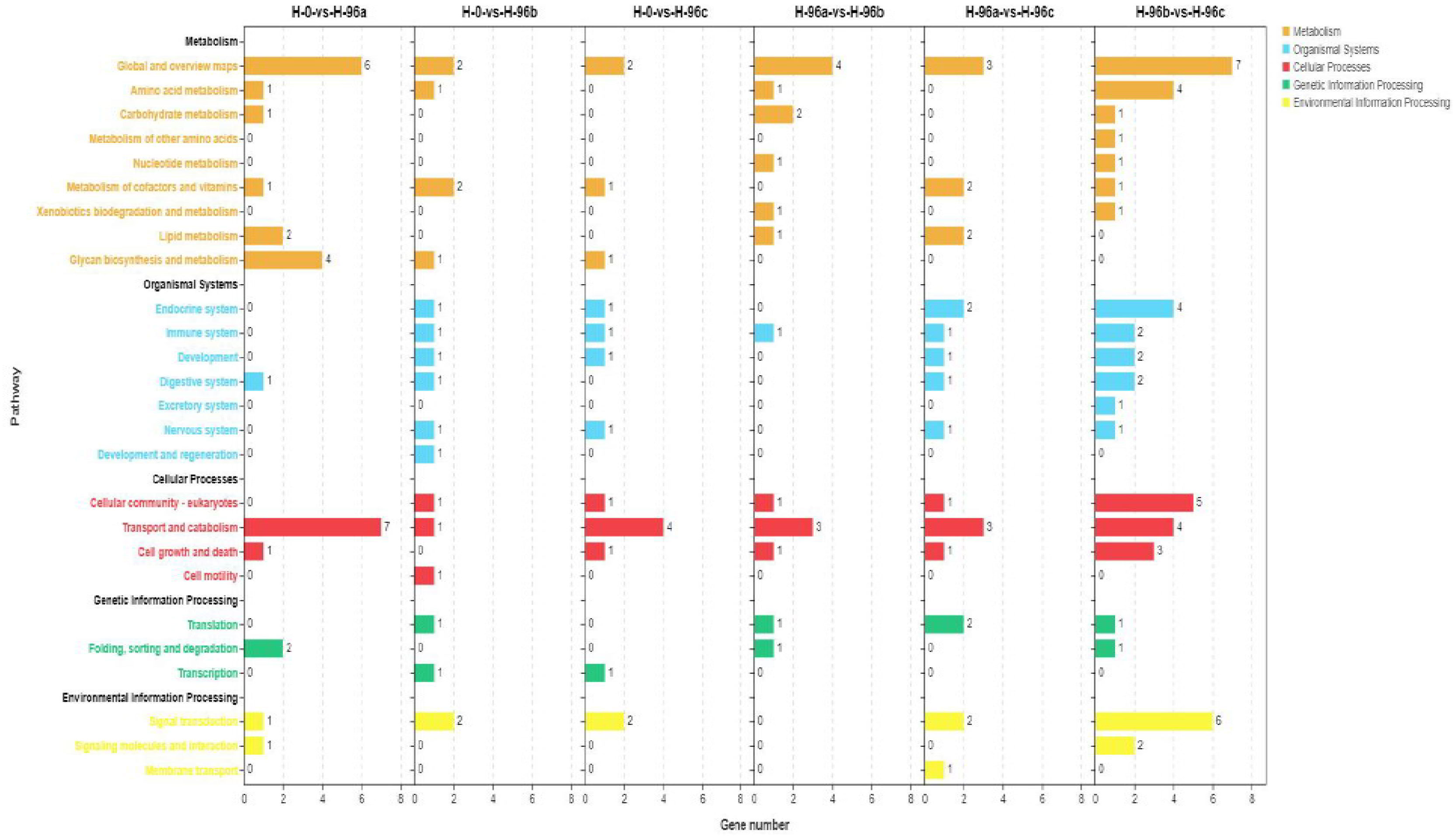
Figure 7 KEGG enrichment analyses of differentially expressed genes (DEGs) in hemolymph transcriptome. H-0: Control group; H-96a: concentration of 20 mg/L group; H-96b: concentration of 50 mg/L group; H-96c: concentration of 100 mg/L group.
In the present study, DEGs associated with immune defence were divided into 5 groups (Table 3). There were 31 genes associated with signal transduction, the most of the 5 groups, followed by other immune molecules (10), pattern recognition proteins/receptors (8), apoptosis (8), antimicrobial peptides (8) and genes associated with the pre-phenol oxidase cascade (3).
Compared with the control group H-0, the expression levels of genes in H-96a related to signal transduction, such as caspase 9 (CASP9), HRAS-like suppressor 3, Thrombospondin-4, Dual oxidase 2-like isoform X2, C-terminal-binding protein-like isoform X1 were significantly up-regulated, while the expression levels of phospholipid phosphatase 3-like, pantetheinase-like, Follistatin, ADP-ribosylation factor-like and other genes were significantly down-regulated. In addition, residual chlorine at group H-96a down-regulated the expression levels of genes in the C-Type Lectin family of pattern recognition proteins/receptors, such as C-type lectin domain family member isoform crab 3, C-type lectin domain family 6 member A, and genes in the prophenoloxidase cascade system, such as serine protease, Kazal-type serine protease genes. The expression levels of Baculoviral IAP repeat-containing protein 7-B were significantly upregulated in genes related to apoptosis, while the expression levels of cathepsin L, inhibitor of apoptosis protein-like were significantly downregulated. In addition, the expression levels of pantetheinase-like, deleted in malignant brain tumors 1 protein-like, gelsolin-like protein 2 and legumain-like were also altered in the concentration treatment group compared to the control group.
Compared to the control group, the expression levels of genes related to signal transduction such as guanylate cyclase soluble subunit beta-2-like, MMP-19 and mitogen-activated protein kinase/p38 were significantly up-regulated in the H-96b group, while the expression levels of phospholipid phosphatase 3-like, ADP-ribosylation factor-like, Protocadherin Fat 4, and fatty acid synthase were significantly down-regulated. At the same time, the expression levels of leptin precursor 4, C-type lectin domain family 6 member A and C-type lectin were significantly down-regulated in genes related to pattern recognition proteins/receptors (PRPs), while the expression levels of mitogen-activated protein kinase/p38 were significantly up-regulated. The expression levels of mitogen-activated protein kinase/p38 were significantly up-regulated. The expression of cathepsin L, Bcl-2 like 2 protein and baculoviral IAP repeat-containing protein 5-like, which are genes related to apoptosis, were all down-regulated. In addition, the expression levels of genes such as guanylate cyclase soluble subunit beta-2-like and Full=G2/mitotic-specific cyclin-B were also altered in the b concentration treatment group.
The expression levels of signal transduction-related protein crumbs homolog 1-like and MASP-related molecule Type 1 genes were significantly down-regulated in group H-96c compared to the H-0, while in the phenol oxidase progenitor system, the expression levels of serine/threonine-protein kinase TBK1 like isoform X1 were significantly up-regulated. Meanwhile, the expression levels of inhibitor of apoptosis 1, a gene related to apoptosis, were significantly up-regulated, while the expression levels of C-type lectin, electin precursor 4 and toll-like receptor 13, a gene related to pattern recognition protein/receptor, were significantly down-regulated. In addition, the c-treatment group also caused deleted in malignant brain tumors 1 protein-like, proteasome activator complex subunit 3-like, Full=G2/mitotic-specific cyclin-B and NRAMP gene expression levels were altered.
Meanwhile, several DEGs are involved in various processes of animal antioxidant mechanisms in the hepatopancreas (Table 4). Compared with the H-0 treated group, the expression levels of omega glutathione S-transferase, glutathione S-transferase and mu class glutathione S-transferase genes associated with antioxidant were significantly up-regulated in the H-96a group, while the expression levels of Thioredoxin-like fold genes were significantly down-regulated in the H-96a group. The expression levels of alanine—glyoxylate aminotransferase 2, pi-class glutathione S-transferase, and thioredoxin genes were significantly up-regulated in the H-96b group, while the expression levels of genes such as ribosyldihydronicotinamide dehydrogenase, thioredoxin-like fold, and asparagine synthetase were significantly downregulated. The expression levels of omega glutathione S-transferase, pi-class glutathione S-transferase, and pi-class glutathione S-transferase were significantly up-regulated in the H-96c group, while the expression levels of Thioredoxin-like fold and peroxiredoxin V genes were significantly down-regulated. As a response to environmental stress, chaperone genes in the organism showed significant variation at the transcriptional or translational level. In the present study, the expression levels of heat shock protein were significantly up-regulated in group H-96a compared to the H-0; in group H-96c, the expression levels of heat shock protein 70 and heat shock protein were significantly up-regulated, while the expression levels of heat shock protein 60 gene were significantly down-regulated. In addition, residual chlorine stress also altered the expression levels of metal-binding proteins, including heavy metal-binding protein HIP-like, metallothionein and ferritin subunit.

Table 4 Summary of differential and non-differentially expressed genes related to antioxidative capability in transcriptome of C. sinensis.
The transcriptome results were verified by qPCR (Figure 8), and selected 10 immune and antioxidant related genes (5 up-regulated and 5 down-regulated) randomly. The verification results were consistent with the sequencing results, indicating that the transcriptome sequencing results were authentic and reliable.
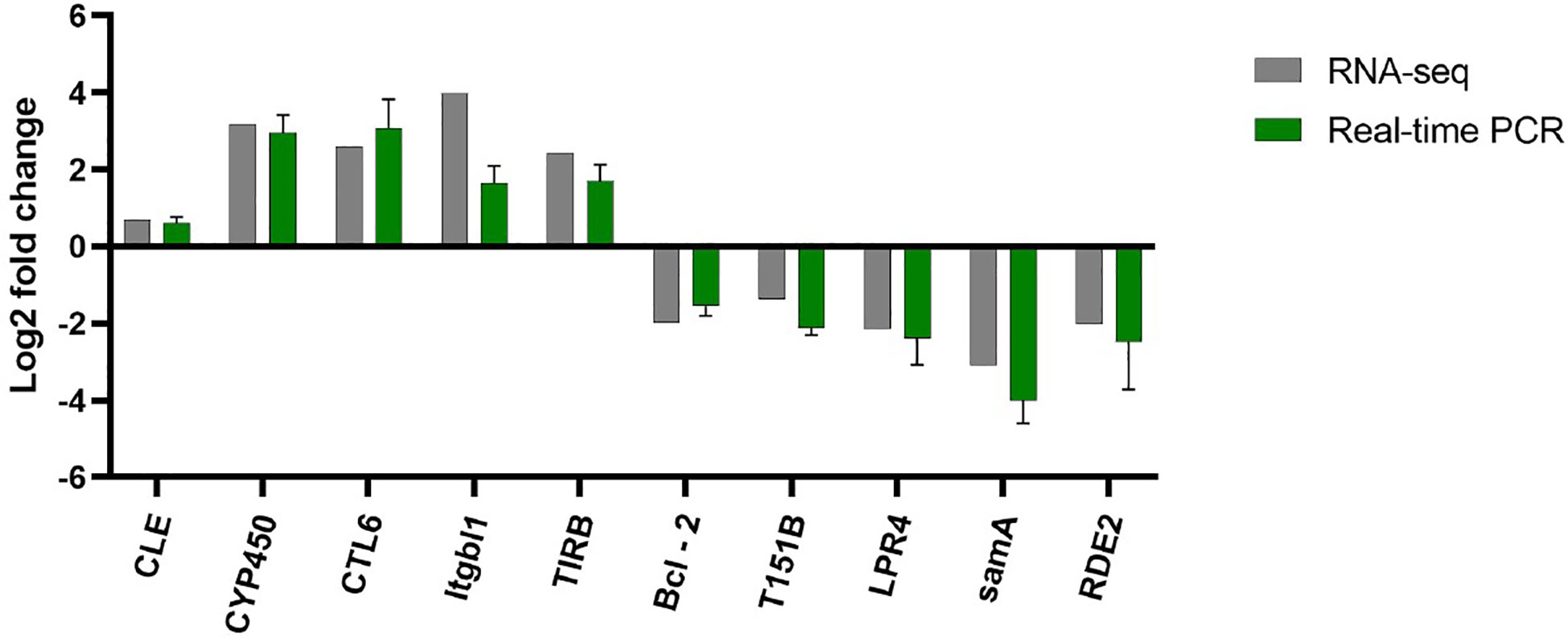
Figure 8 Transcriptome result validation. RNA seq and Real-time PCR are shown in grey and green, respectively.
The mortality of aquatic organisms can be affected by changes in the water environment and chemical stimuli, such as temperature (Casas et al., 2020; Syafaat et al., 2021), salinity (Nie et al., 2018; Zhang Y et al., 2020), pH (Swezey et al., 2020; Liew et al., 2022), and feed (Willer and Aldridge, 2019; Mohan et al., 2019). Chlorine and compounds in water can undergo complex chemical reactions to synthesise a range of compounds under different conditions, such as the chloramine family of compounds. Electrolytic chlorine for the disinfection of cooling water in nuclear power plants is usually free chlorine, which is unstable in its state and very easily dissipated in seawater (Venkatnarayanan et al., 2021). The main sources of chlorine for production activities are several common disinfectants such as bleach, chlorine dioxide and trichloroisocyanuric acid. Also, during the shellfish culture and nursery phase, the cleaning and disinfection of various types of equipment and ponds inevitably involves the use of disinfectants such as bleach and chlorine dioxide, in which residual chlorine is inevitably produced.
The LC50 is an important index to measure the toxin tolerance of an organism. This study calculated the 96-h LC50 of juvenile C. sinensis to chlorine dioxide exposure, based on linear regression method (y = 0.0013e2.4844x, R2 = 0.9737, where y = % mortality and x = lg c (ClO2)) was 217.6 mg/L. The results of the present studies were similar to those reported in other aquatic organisms. The LC50 of residual chlorine (Cl-) in STEL water for brood-stock and 2-mo-old shrimp were 2.3 and 3.2 ppm, respectively (Park et al., 2004). The Manila clam Ruditapes philippinarum exhibited strong tolerance to hypoxia as the 20-day LC50 for dissolved oxygen (DO) was estimated to be 0.57 mg L-1 (Li et al., 2019). The 96-h LC50 at 22 and 26°C values was 28.591 and 11.761 mg/L, respectively, for NiCl2 exposure in the abalone (Min et al., 2015).
Studies on the effects of residual chlorine on mussels showed that the time of death of mussels at the same concentration depended on the size of the mussel, with smaller mussels taking less time to reach 100% mortality than larger mussels. In addition, the death time of mussels with the same size depends on the residual chlorine concentration, with higher concentrations of residual chlorine taking less time to reach 100% mortality than lower concentrations (Zhang et al., 2000; Masilamoni et al., 2002). In the present study, the mortality of bay scallop (Argopectens irradias) parents (Chen, 2009) and yellow clam (Corbicula fluminea) (Zou and Cheng, 2003) showed a gradual increase in mortality with increasing chlorine dioxide concentration in the water column and the extension of soaking time. In this study, chlorine dioxide concentration and duration of stress directly affected the survival and growth of first instar clams, and the mortality rate of clams increased with increasing chlorine dioxide concentration and duration of stress.
The living environment of the C. sinensis is near the estuary of the freshwater river, and the outlet of the warm water drainage of the coastal nuclear power plant is one of the main habitats of C. sinensis. When faced with changes in the external environment, the immune system of the clam undergoes stress behaviour, resulting in the production of large amounts of reactive oxygen species (ROS), and the sharp increase in ROS places a great burden on the organism, generating oxidative stress and causing a decrease in immune function (Liang et al., 2016). The antioxidant system consists of antioxidant enzymes (Lian et al., 2019; Dong et al., 2022; Holen et al., 2022) (e.g., SOD, CAT, T-AOC), which have a strong detoxifying effect on ROS. The activity of antioxidant enzymes also reflects the physiological state of aquatic organisms in response to environmental changes to a certain extent. The immune system of shellfish consists of immune-related enzymes (e.g., ACP, AKP, LZM) and changes in the activity of immune-related enzymes also show changes in immune function.
T-AOC reflects the total antioxidant capacity of organisms. In this study, it can be seen that the T-AOC in the three concentration groups tended to increase significantly at the beginning of the experiment, with the 20 mg/L and 50 mg/L groups reaching their maximum values at 72 h and the 100 mg/L group reaching its maximum value at 48 h. The T-AOC activity of the three concentration groups remained at a higher level at 96 h and was significantly different compared to the initial value (P < 0.05). It is speculated that high concentration of chlorine stimulates the stress response of C. sinensis to produce a large number of ROS. In order to prevent its own damage, the body improves the enzymatic reaction, secretes a large amount of SOD and antioxidant-related enzymes, and improves the total antioxidant capacity. The changes in SOD enzyme activity were the same as those in T-AOC, showing a trend of increasing, then decreasing and then increasing. The SOD activity of all three concentration groups reached its maximum at 12 h, and the activity remained at a high level afterwards, which was significantly different from that of the control group (P < 0.05). This result was also consistent with the results of T-AOC.
ACP and AKP are two common immune-related enzymes, both of which are involved in the detoxification process of the organism when aquatic organisms are affected by external environmental factors (Chi et al., 2019). The study about the ACP and AKP activities in deltamethrin stressed Macrobrachium rosenbergii found that the ACP activity showed a wavy change and the AKP activity showed a trend of increasing and then decreasing after acute stress (Guo et al., 2021; Jiang et al., 2021). A similar conclusion was reached by Peng., in his study on the acute toxicity of the proposed cave green crab (Cyprinus carpio) (Peng et al., 2018). In this study, the ACP activity in the 20 mg/L group showed fluctuating changes, while the ACP activity in the 50 mg/L and 100 mg/L groups both showed an increase followed by a decrease. There was no significant difference between the initial values. It is assumed that the prolonged survival in a high chlorine environment affected the expression of enzyme activity. The AKP activity in this study showed an overall decreasing trend, with all three concentration groups having lower AKP activity than the initial value after 96 h but no significant difference, suggesting that the AKP activity of the C. sinensis organism is inhibited in a high chlorine environment.
LZM protects the organism from foreign bacteria by hydrolyzing the mucopolysaccharides in bacteria (Yin et al., 2022). In a study by Zhou Y., it was found that LZM activity increased during stress after starvation stress in Oratosquilla oratoria and began to decrease beyond a certain range (Zhou, 2014). In the present study, the LZM activity of C. sinensis showed a decrease, then an increase and then a decrease after chlorine stimulation, and all three concentration groups showed this wavelike change. At 96 h, the LZM activities of all three concentration groups were significantly lower than the initial values (P < 0.05). It is hypothesized that the immune function of the C. sinensis was reduced after high chloride stress and the LZM activity was inhibited.
Studies have shown that residual chlorine stress affects the growth and survival of organisms and their physiological changes, but the molecular mechanisms involved are not well understood. In this study, the transcriptome of the hepatopancreas of C. sinensis stressed with residual chlorine for 96 hours was sequenced using RNA-seq technology. These transcriptional data provide a resource for further studies on the molecular mechanisms by which residual chlorine affects immune responses in molluscs. The data from this study indicate that residual chlorine stress has an effect on both the immune and antioxidant systems of the clam.
Serine proteases (SP) are a group of widely distributed protein hydrolases that perform a range of important physiological functions, including participation in digestion, blood coagulation, embryonic development and immune response processes (Yin S et al., 2022; Liu H et al., 2022), and play an important role in the innate immune defence of invertebrates (Hu et al., 2018; Xu et al., 2018). When invertebrates are stimulated or infected by microorganisms (Faisal et al., 1998; Xue et al., 2006), SP in their bodies can rapidly activate the body’s immune system to produce the corresponding immune effects and kill the invading pathogens. However, the benzoquinone and ROS produced during the reaction can cause cellular damage, and serine protease inhibitors (SPI) can precisely regulate this process to avoid serious damage to the body (Ding et al., 2011; Chen et al., 2020). In this study, SP expression level was down-regulated at safe concentrations compared to the control group, while the expression levels of SP in the high residual chlorine stress group were not significantly different from the control group, which is consistent with previous findings (Zhang et al., 2016; Mao et al., 2018), indicating that that residual chlorine stress induces SP in the clam to protect the organism by activating cascade processes and phenol oxidase pathways.
The C-type lectin family is an important class of pattern recognition receptors that trigger processes such as agglutination of microorganisms, induction of phagocytosis and activation of complement by recognizing glycan structures on the surface of microorganisms (Vasta et al., 2004; Osorio and Sousa, 2011) and participate in the regulation of specific immunity with the emergence of specific immunity (van Vliet et al., 2008), playing an important role in shellfish innate immunity (Wang et al., 2007; Zhu et al., 2008; Hu et al., 2011), but has rarely been studied in C. sinensis. The results of the present study showed that the expression levels of C-type lectins were significantly down-regulated in both groups a and b after 96 h of residual chlorine stress compared to the control group, with no significant difference in expression levels in group c. This is consistent with the previous findings that the expression of the C-type lectin gene tends to decrease when the stimulation time exceeds 72 hours (Shen et al., 2018), indicating that the clam is affected by residual chlorine and adapts to the environment by regulating the secretion of C-type lectin in the body to protect itself from the stimulus. The regulatory mechanism of the body becomes slower when the stimulation time is too long, and the higher the concentration of residual chlorine, the higher the level of gene expression.
Cathepsin L (Cts L) is a major member of the papain C1 family of cysteine proteases. Studies have shown that Cts L is involved in a variety of physiological and pathological processes in the organism, such as antigen presentation, hormone protein maturation and processing, tumour invasion and metastasis, apoptosis, bone resorption, individual development and tissue differentiation. In addition, Cts L may also mediate the immune defence of the organism (Ma et al., 2010; Peng et al., 2012). Consistent with previous studies (Wang et al., 2013; Liu S et al., 2014) in the present study, Cts L expression was significantly down-regulated in both groups a and b after 96 h of residual chlorine stimulation compared to the control group, while no significant difference was observed in group c. This may imply that residual chlorine stress induced an inflammatory response in the organism, which was in a post-infection recovery period after 96 h.
It has been shown that apoptosis is a highly regulated programmed cell death and that the Caspase family plays a pivotal role in apoptosis, with Caspase 9 (CASP9) being the initiator of apoptosis (Yu, 2017; Lu et al., 2021; Zhu et al., 2022). In the present study, CASP9 expression was up-regulated at a and b stress concentrations compared to the control, while no significant difference was observed in group c. This suggests that residual chlorine stress initiated an apoptotic response, but the initiation of the response became slower with increasing residual chlorine concentrations after 96 hours of stress. Previous studies on stress in aquatic animals such as carp (Ctenopharyngodon idella) (Luan et al., 2022), yellow catfish (Pelteobagrus fulvidraco) (Li et al., 2020) and grouper bream (Megalobrama amblycephala) (Zhang, 2015) are consistent with the results of this experiment, suggesting that CASP9 is associated with apoptosis induced by stress.
The effects of Glutathione S-transferase (GST) molecule are both antidotal and antioxidant. When shellfish are stimulated by environmental pollutants, the GST activity in their organism is significantly increased (Sandamalika et al., 2019) and protects cells from external stressful environmental damage by catalyzing the redox reaction of hydrogen peroxide and scavenging oxygen radicals and peroxides generated by metabolic reactions in the body (Samaraweera et al., 2019). pi type is a typical representative of many GST isoforms and is widely involved in the metabolism of toxins in immune cells and the production of lipophilic compounds and their derivatives (Liu et al., 2020). The GST in marine shellfish is basically of the pi type (Liu H et al., 2014). Under the stimulation of pathogenic bacteria, the respiratory burst of cells increases significantly and induces the production of large amounts of ROS. ROS protect the organism from oxidative damage by killing bacteria (Zhang et al., 2017). The antibacterial process of ROS refers to that overly high concentration of ROS breaks the antioxidant defense mechanism in cells and reacts with genetic materials, enzymes, proteins and other substances in bacteria to cause oxidative damage, or reacts with structural components of cell membrane (wall) to cause lipid peroxidation in cells, causing damage and death of bacteria (Zhou et al., 2021). However, excess ROS can cause damage to host cell structure and function, oxidizing cell membrane phospholipids and leading to alterations in cell membrane fluidity and permeability. GSTs, as an important antioxidant enzyme, play an important role in detoxification of exogenous organisms and protection of cells from excess effects, and ROS regulate cell function by affecting the end products formed from macromolecules damaged by oxidative stress. Therefore, the antioxidant function of GSTs is essential for the survival of molluscs, as these enzymes (Hu et al., 2012) can rapidly scavenge excess ROS. In the present study, the expression of GST was significantly higher in the H-96a, H-96b and H-96c treated groups than in the control group, which is consistent with previous findings (Zheng et al., 2019; Zhang H et al., 2020). This suggests that C. sinensis are sensitive to residual chlorine stress and that GST are involved in the detoxification process of residual chlorine, thus maintaining the stability of the organism.
Heat shock proteins (Hsp), also known as stress proteins, are a group of highly structurally conserved peptide protein family molecules that perform important physiological functions under both normal and stressful conditions. Hsp70s are members of a family of 70 kDa heat shock proteins that are widely present in prokaryotic and eukaryotic cells (Gong et al., 2020). Hsp70 is an inducibly expressed protein that is one of the first protective molecules to appear in response to cellular injury, which helps denatured proteins to recuperate and clear permanently denatured proteins, and its expression increases significantly in response to stimulation by contaminants (Guo et al., 2020; Wang et al., 2020; Cui et al., 2022; Roh and Kim, 2022). In this study, the expression level of Hsp70 gene was significantly increased in the H-96c group compared to the control group, while there was no significant change in the other two groups, which is consistent with the results of previous studies (Shao and Zhang, 2015; Zhao H et al., 2016; Jing et al., 2019), indicating that the stress response of hepatopancreatic cells increased with the increase of residual chlorine concentration and the expression level of Hsp70 gradually increased.
This study showed that residual chlorine stress significantly altered the expression of antioxidant and immune-related genes in the hepatopancreas of C. sinensis, providing evidence at the gene transcriptional level for a potential mechanism by which excessive residual chlorine residues in the culture environment affect basal immunity in molluscs. Among the DEGs between the control and three different residual chlorine concentration treatment groups, 68 DEGs were associated with immune responses and 29 DEGs were involved in antioxidant mechanisms. Specifically, genes associated with the proPO cascade system, PRRs and signal transduction play an important role in residual chloride-stimulated immune defence to maintain the normal physiological state of the green clam. In addition, molecules associated with the GST-dependent antioxidant system are highly involved in antioxidant defence against residual chlorine stress. Thus, these results not only provide some evidence that residual chlorine affects immune homeostasis in aquatic animals, but also contribute to further understanding of the molecular mechanisms and potential pathways of immune function and antioxidant capacity in larval C. sinensis under residual chlorine stress.
The study showed that the mortality rate of the C. sinensis increased with the increase of stress time and concentration. The antioxidant-related T-AOC and SOD activities and immune-related ACP, AKP and LZM enzyme activities of hepatopancreas of C. sinensis after residual chlorine stress were significantly changed between 0 and 96 h (P < 0.05). Meanwhile, transcriptome analysis showed that residual chlorine stress significantly altered the expression levels of immune and antioxidant related genes. Thus, this study provided valuable information for understanding the effects of residual chlorine stress on survival, physiological metabolism and molecular mechanisms of immune and antioxidant functions of the C. sinensis and provided some theoretical references for the understanding of the toxic effects of residual chlorine on bivalves.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
All clams were treated in strict accordance with the guidelines for the care and use of experimental animals established by the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China and approved by the Committee on Experimental Animal Management of the Jiangsu Ocean University.
SW: validation, writing - original draft, visualization, data curation. GR: formal analysis, data curation. DL: data curation, writing - review and editing. SF: data curation, writing - review and editing. SY: data curation, writing - review and editing. JS: data curation, writing - review and editing. ML: supervision, project administration. ZD: data curation, writing - review and editing, funding acquisition, project administration, conceptualization. All authors contributed to the article and approved the submitted version.
This work was supported by Jiangsu Natural Resources Development Special-Marine Science and Technology Innovation Project (JSZRHYKJ202008); Jiangsu Modern Agriculture Independent Innovation Project (CX (20) 3150); The “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS〔2021〕034); Modern Agro-industry Technology Research System (CARS-49) and Jiangsu Graduate Research and Practice Innovation Program (KYCX2021-035, KYCX22_3388).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alexandre L., Christelle C., Olivier B., Jean-Marc L., Antoine L., Katherine C., et al. (2022). Effects of chronic exposure of metals released from the dissolution of an aluminium galvanic anode on the pacific oyster Crassostrea gigas. Aquat. Toxicol. 249, 106223. doi: 10.1016/j.aquatox.2022.106223
Casas S., La, Peyre J. (2020). Heat shock protein 70 levels and post-harvest survival of eastern oysters following sublethal heat shock in the laboratory or conditioning in the field. Cell Stre. Chaper 25 (2), 369–378. doi: 10.1007/s12192-019-01056-1
Chen J. (2009). Tolerance of chlorine dioxide in parent, larvae and juvenile scallops of the bay scallop (Scallopus spp.). Chin. Agron. Bull. 07), 279–283.
Chen D., Guo L., Yi C., Wang S., Ru Y., Wang H. (2021). Hepatopancreatic transcriptome analysis and humoral immune factor assays in red claw crayfish (Cherax quadricarinatus) provide insight into innate immunomodulation under vibrio parahaemolyticus infection. Ecotoxicol. Environ. Saf. 217, 112266. doi: 10.1016/j.ecoenv.2021.112266
Chen S., Liang J., Zhang R. (2020). Cloning and expression of the serine protease inhibitor gene pfser1 from the hepu mother-of-pearl shell. J. GD. Ocean Univer. 40 (01), 1–7.
Chi C., Yun S., Giri S., Kim H., Kim S., Kang J., et al. (2019). Effect of the algicide thiazolidinedione 49 on immune responses of bay scallop argopecten irradians. Molecules 24 (19), 3579. doi: 10.3390/molecules24193579
Cui Y., Zhao N., Wang C., Long J., Chen Y., Deng Z., et al. (2022). Acute ammonia stress-induced oxidative and heat shock responses modulated by transcription factors in Litopenaeus vannamei. Fish Shellfish Immunol. 128, 181–187. doi: 10.1016/j.fsi.2022.07.060
Dempsey C. (1986). The exposure of herring postlarvae to chlorine in coastal power stations. Mari. Environ. Res. 20 (4), 279–290. doi: 10.1016/0141-1136(86)90053-X
Ding J., Zhang G., Li L. (2011). Cloning and expression analysis of a serine protease inhibitory factor from abalone cuniculus. Aquatic. Sci. 30 (12), 731–738. doi: 10.16378/j.cnki.1003-1111.2011.12.001
Dong Z., Duan H., Zheng H., Ge H., Wei M., Liu M., et al. (2021). Research progress on germplasm, culture and exploitation of Cyclina sinensis. J. Aquac. 12), 2083–2098.
Dong X., Yang Z., Liu Z., Wang X., Yu H., Peng C., et al. (2022). Metabonomic analysis provides new insights into the response of zhikong scallop (Chlamys farreri) to heat stress by improving energy metabolism and antioxidant capacity. Antioxidants (Basel) 11 (6), 1084. doi: 10.3390/antiox11061084
Eppley R., Renger E., Williams P. (1976). Chlorine reactions with seawater constituents and the inhibition of photosynthesis of natural marine phytoplankton. Estua. Coast. Mar. Sci. 4 (2), 147–161. doi: 10.1016/0302-3524(76)90039-6
Faisal M., MacIntyre E., Adham K., Tall B., Kothary M., La, et al. (1998). Evidence for the presence of protease inhibitors in eastern (Crassostrea virginica) and pacific (Crassostrea gigas) oysters. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 121 (2), 161–168. doi: 10.1016/S0305-0491(98)10084-6
Ge H., Liang X., Liu J., Cui Z., Guo L., Sun Y., et al. (2021). Effects of acute ammonia exposure on antioxidant and detoxification metabolism in clam Cyclina sinensis. Ecotoxicol. Environ. Saf. 211, 111895. doi: 10.1016/j.ecoenv.2021.111895
Gong Y., Kong T., Ren X., Chen J., Lin S., Zhang Y., et al. (2020). Exosome-mediated apoptosis pathway during WSSV infection in crustacean mud crab. PloS. Pathog. 16 (5), e1008366. doi: 10.1371/journal.ppat.1008366
Grabherr M., Haas B., Yassour M., Levin J., Thompson D., Amit I., et al. (2011). Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29 (7), 644–652. doi: 10.1038/nbt.1883
Guo J., Pu Y., Zhong L., Wang K., Duan X., Chen D. (2021). Lead impaired immune function and tissue integrity in yellow catfish (Peltobargus fulvidraco) by mediating oxidative stress, inflammatory response and apoptosis. Ecotoxicol. Environ. Saf. 226, 112857. doi: 10.1016/j.ecoenv.2021.112857
Guo K., Ruan G., Fan W., Wang Q., Fang L., Luo J., et al. (2020). Immune response to acute heat stress in the intestine of the red swamp crayfish, Procambarus clarkii. Fish Shellfish Immunol. 100, 146–151. doi: 10.1016/j.fsi.2020.03.017
Hegazi M., Attia Z., Ashour O. (2010). Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 99 (2), 118–125. doi: 10.1016/j.aquatox.2010.04.007
Holen E., Espe M., Larsen A., Olsvik P. (2022). Dietary chlorpyrifos-methyl exposure impair transcription of immune, detoxification and redox signaling genes in leukocytes isolated from cod (Gadus morhua). Fish Shellfish Immunol. 127, 549–560. doi: 10.1016/j.fsi.2022.06.060
Hu J., Chen Y., Duan X., Jin T., Li Y., Zhang L., et al. (2018). Involvement of clip-domain serine protease in the anti-vibrio immune response of abalone (Haliotis discus hannai)-molecular cloning, characterization and functional analysis. Fish Shellfish Immunol. 72, 210–219. doi: 10.1016/j.fsi.2017.10.062
Hu B., Deng L., Wen C., Yang X., Pei P., Xie Y., et al. (2012). Cloning, identification and functional characterization of a pi-class glutathione-s-transferase from the freshwater mussel Cristaria plicata. Fish Shellfish Immunol. 32 (1), 51–60. doi: 10.1016/j.fsi.2011.10.018
Hu Y., Zhang D., Cui S., Guo H., Chen M., Jiang S., et al. (2011). Sequence features and functional analysis of the c-type lectin gene(PoLEC1)from pearl oyster Pinctada fucata. J. Fish. China 35 (9), 1327–1336.
Jiang Q., Jiang Z., Ao S., Gao X., Zhu X., Zhang Z., et al. (2021). Multi-biomarker assessment in the giant freshwater prawn Macrobrachium rosenbergii after deltamethrin exposure. Ecotoxicol. Environ. Saf. 214, 112067. doi: 10.1016/j.ecoenv.2021.112067
Jiang Z., Liao Y., Gao A., Chen Q., Zeng J. (2009). Progress in the study of residual chlorine effects on fish toxicity. Oceano. Res. 27 (04), 86–94.
Jing Y., Zhang T., Liu E., Chen Q., Sun M., Guo W., et al. (2019). Effect of cadmium on the expression of heat shock protein 70 gene in different tissues of pacific oysters at different salinities. J. GX Acad. Sci. 35 (04), 332–336. doi: 10.13657/j.cnki.gxkxyxb.20191129.002
Liang Z., Liu R., Zhao D., Wang L., Sun M., Wang M., et al. (2016). Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 54, 523–528. doi: 10.1016/j.fsi.2016.05.009
Lian S., Zhao L., Xun X., Lou J., Li M., Li X., et al. (2019). Genome-wide identification and characterization of sods in zhikong scallop reveals gene expansion and regulation divergence after toxic dinoflagellate exposure. Mar. Drugs 17 (12), 700. doi: 10.3390/md17120700
Liao X., Sun Z., Cui Z., Yan S., Fan S., Xia Q., et al. (2022). Effects of different sources of diet on the growth, survival, biochemical composition and physiological metabolism of clam (Cyclina sinensis). Aquac. Res. 00, 1–10. doi: 10.1111/are.15886
Liew H., Rahmah S., Tang P., Waiho K., Fazhan H., Rasdi N., et al. (2022). Low water pH depressed growth and early development of giant freshwater prawn Macrobrachium rosenbergii larvae. Heliyon 8 (7), e09989. doi: 10.1016/j.heliyon.2022.e09989
Li Q., Sun S., Zhang F., Wang M., Li M. (2019). Effects of hypoxia on survival, behavior, metabolism and cellular damage of Manila clam (Ruditapes philippinarum). PloS. One 14 (4), e0215158. doi: 10.1371/journal.pone.0215158
Liu H., He J., Zhao R., Xue C. (2014). Analysis of glutathione s-transferase gene expression in the thick-shelled mussel Mytilus edulis under heavy metal stress. Ocean Lake Marsh 45 (02), 274–280.
Liu H., Yang R., Chen Y., Zhang L., Sun L., Liu G., et al. (2022). Cloning, expression and properties of myogenic fibril-binding serine protease from rhubarb fish. J. Aquac. 46 (07), 1154–1166.
Liu H., Zhang H., Cheng D., Tan K., Ye T., Ma H., et al. (2020). Differential responses of a pi-class glutathione s-transferase (CnGSTp) expression and antioxidant status between golden and brown noble scallops under pathogenic stress. Fish Shellfish Immunol. 105, 144–151. doi: 10.1016/j.fsi.2020.07.004
Liu C., Zhang W., Jiang P., Lin Y., Lu W. (2022). The anti-stress effect of taurine in fish: Assessments based on repeat acute stress and animal individuality. Aquaculture 561, 738685. doi: 10.1016/j.aquaculture.2022.738685
Liu S., Zhao T., Zhang H., Pan B. (2014). Expression and recombinant protein activity analysis of the histone l gene of the clam (Cyclina sinensis). Mar. Lake. Marsh. 01), 134–140.
Li C., Wu M., Wang H. (2012). LC50 caculated by kochi, probit analysis and linear regression methods. Prog. Veteri. Medi. 33 (09), 89–92. doi: 10.16437/j.cnki.1007-5038.2012.09.012
Li M., Zhang M., Qian Y., Shi G., Wang R. (2020). Ammonia toxicity in the yellow catfish (Pelteobagrus fulvidraco): The mechanistic insight from physiological detoxification to poisoning. Fish Shellfish Immunol. 02, 195–202. doi: 10.1016/j.fsi.2020.04.042
Li Y., Zhang X., Yang M., Liu J., Li W., Graham N., et al. (2017). Three-step effluent chlorination increases disinfection efficiency and reduces DBP formation and toxicity. Chemosphere 168, 1302–1308. doi: 10.1016/j.chemosphere.2016.11.137
Luan P., Zhang H., Zhang X., Hu G., Zhang Z. (2022). Cadmium regulates FKBP5 through miR-9-5p and induces carp lymphocyte apoptosis. Fish Shellfish Immunol. 120, 353–359. doi: 10.1016/j.fsi.2021.12.006
Lu J., Zhang J., Zhou G., Wang P., Qing H. (2021). Effects of acute high temperature stress on liver apoptosis-related enzyme activity and gene expression in largemouth bass (Percus macrocephalus). Freshw. Fisher 02), 81–86. doi: 10.13721/j.cnki.dsyy.2021.02.010
Mao Z., Lin Q., Wang B., Zhang C., Chen C., Jian S., et al. (2018). Effects of Cu~(2+) stress on toxic effects and immune-related gene expression in the Chinese mitten crab Eriocheir sinensis. J. JX Agricul. Univer. 40 (02), 358–364. doi: 10.13836/j.jjau.2018047
Masilamoni G., Jesudoss K., Nandakumar K., Satapathy K., Azariah J., Nair K. (2002). Lethal and sub-lethal effects of chlorination on green mussel Perna viridis in the context of biofouling control in a power plant cooling water system. Mar. Environ. Res. 53 (1), 65–76. doi: 10.1016/s0141-1136(01)00110-6
Ma J., Zhang D., Jiang J., Cui S., Pu H., Jiang S. (2010). Molecular characterization and expression analysis of cathepsin L1 cysteine protease from pearl oyster Pinctada fucata. Fish Shellfish Immunol. 29 (3), 501–507. doi: 10.1016/j.fsi.2010.05.006
Min E., Cha Y., Kang J. (2015). Effects of waterborne nickel on the physiological and immunological parameters of the pacific abalone haliotis discus hannai during thermal stress. Environ. Sci. pollut. Res. Int. 22 (17), 13546–13555. doi: 10.1007/s11356-015-4597-1
Mohan K., Ravichandran S., Muralisankar T., Uthayakumar V., Chandirasekar R., Seedevi P., et al. (2019). Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish Immunol. 86, 1177–1193. doi: 10.1016/j.fsi.2018.12.072
Nie H., Zuo S., Li L., Tian C., Cao C., Yan X. (2018). Physiological and biochemical responses of Dosinia corrugata to different thermal and salinity stressors. J. Exp. Zool A Ecol. Integr. Physiol. 329 (1), 15–22. doi: 10.1002/jez.2152
Nikolaivits E., Agrafiotis A., Baira E., Le Goff G., Tsafantakis N., Chavanich S., et al. (2020). Degradation mechanism of 2,4-dichlorophenol by fungi isolated from marine invertebrates. Int. J. Mol. Sci. 21 (9), 3317. doi: 10.3390/ijms21093317
Opstvedt J., Mundheim H., Nygrd E., Aase H., Pike I. (2000). Reduced growth and feed consumption of Atlantic salmon (Salmo salar l.) fed fish meal made from stale fish is not due to increased content of biogenic amines. Aquaculture 188 (3-4), 323–337. doi: 10.1016/S0044-8486(00)00343-4
Osorio F., Sousa C. (2011). Myeloid c-type lectin receptors in pathogen recognition and host defense. Immunol. 34 (5), 651–664. doi: 10.1016/j.immuni.2011.05.001
Pan L., Ren J., Liu J. (2006). Responses of antioxidant systems and LPO level to benzo (a) pyrene and benzo (k) fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. pollut. 141 (3), 443–451. doi: 10.1016/j.envpol.2005.08.069
Park J., Seok S., Cho S., Baek M., Lee H., Kim D., et al. (2004). Safety and protective effect of a disinfectant (STEL water) for white spot syndrome viral infection in shrimp. Dis. Aquat Organ. 60 (3), 253–257. doi: 10.3354/dao060253
Pawert M., Triebskorn R., Gräff S., Berkus M., Schulz J., Köhler H. (1996). Cellular alterationsin collembolan midgut cellsasa marker of heavy metal exposure: ultrastructure and intracellular metal distribution. Sci. Total Environ. 181 (3), 187–200. doi: 10.1016/0048-9697(95)05009-4
Peng J., Chen L., Cheng C., Feng J., Ma H., Guo Z. (2018). Acute toxicity of ammonia nitrogen to the caveman green crab (Cyprinus carpio) and effects on its serum immune-related enzyme activity. Adv. Fish. Sci. 05), 114–121. doi: 10.19663/j.issn2095-9869.20170907001
Peng K., Wang J., Liu T., Sheng J., Shi J., Shao P., et al. (2012). Tissue expression and immune stress analysis of the histone l gene in the pond mussel, Phyllostomus pelagicus. J. Aquatic. Bio. 36 (06), 128–1134.
Qiao Y., Zhou L., Qu Y., Lu K., Han F., Li E. (2022). Effects of different dietary β-glucan levels on antioxidant capacity and immunity, gut microbiota and transcriptome responses of white shrimp (litopenaeus vannamei) under low salinity. Antioxidants (Basel) 11 (11), 2282. doi: 10.3390/antiox11112282
Qiu L., Shi X., Yu S., Han Q., Diao X., Zhou H. (2018). Changes of ammonia-metabolizing enzyme activity and gene expression of two strains in shrimp Litopenaeus vannamei under ammonia stress. Front. Physiol. 9. doi: 10.3389/FPHYS.2018.00211
Roh H., Kim D. (2022). Identification, classification and functional characterization of HSP70s in rainbow trout (Oncorhynchus mykiss) through multi-omics approaches. Fish Shellfish Immunol. 121, 205–214. doi: 10.1016/j.fsi.2021.12.059
Samaraweera A., Sandamalika W., Liyanage D., Lee S., Priyathilaka T., Lee J. (2019). Molecular characterization and functional analysis of glutathione s-transferase kappa 1 (GSTκ1) from the big belly seahorse (Hippocampus abdominalis): Elucidation of its involvement in innate immune responses. Fish Shellfish Immunol. 92, 356–366. doi: 10.1016/j.fsi.2019.06.010
Sandamalika W., Priyathilaka T., Lee S., Yang H., Lee J. (2019). Immune and xenobiotic responses of glutathione s-transferase theta (GST-θ) from marine invertebrate disk abalone (Haliotis discus discus): With molecular characterization and functional analysis. Fish Shellfish Immunol. 91, 159–171. doi: 10.1016/j.fsi.2019.04.004
Shao C., Zhang Z. (2015). Response of the heat shock protein 70 gene to tributyltin in the near-river oyster (Oyster sinensis). Environ. Sci. Res. 28 (05), 745–751. doi: 10.13198/j.issn.1001-6929.2015.05.11
Shen S., Zhu L., Li J., Xue S., Li Y., Chen Q., et al. (2018). Cloning and expression analysis of the cDNA of c-type lectin gene in the ark clam (Arca inflata). Adv. Fish Sci. 39 (01), 128–136.
Shi P., Bi X., Dong S., Song L., Dai W., Zhang S. (2019). Preparation of baited microalgae slow release cake and its preliminary application in Cyclina sinensis pond culture. J. Dalian Ocean Uni. 03), 310–315. doi: 10.16535/j.cnki.dlhyxb.2019.03.002
Swezey D., Boles S., Aquilino K., Stott H., Bush D., Whitehead A., et al. (2020). Evolved differences in energy metabolism and growth dictate the impacts of ocean acidification on abalone aquaculture. Proc. Natl. Acad. Sci. U S A. 117 (42), 26513–26519. doi: 10.1073/pnas.2006910117
Syafaat M., Azra M., Mohamad F., Che-Ismail C., Amin-Safwan A., Asmat-Ullah M., et al. (2021). Thermal tolerance and physiological changes in mud crab, Scylla paramamosain crablet at different water temperatures. Ani. (Basel) 11 (4), 1146. doi: 10.3390/ani11041146
van Vliet S., Garcíavallejo J., Van K. (2008). Dendritic cells and c-type lectin receptors: coupling innate to adaptive immune responses. Immun. Cell Bio. 86 (7), 580–587. doi: 10.1038/icb.2008.55
Vasta G., Ahmed H., Odom E. (2004). Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr. Opin. Struc. Bio. 14 (5), 617–630. doi: 10.1016/j.sbi.2004.09.008
Venkatnarayanan S., Murthy P., Kirubagaran R., Veeramani P., Venugopalan V. (2021). Response of green mussels (Perna viridis) subjected to chlorination: investigations by valve movement monitoring. Environ. Monit. Assess. 193 (4), 202. doi: 10.1007/s10661-021-09008-y
Wang Z., Jian J., Lu Y., Ding Y., Wang B., Chen G., et al. (2013). Cloning and expression analysis of the histone l gene from the mother-of-pearl mussel (Pinctada fucata). Mar. Lake 06), 1604–1611.
Wang H., Song L., Li C., Zhao J., Zhang H., Ni D., et al. (2007). Cloning and characterization of a novel c-type lectin from Chlamys farreri. Mole Immun. 44 (5), 722–731. doi: 10.1016/j.molimm.2006.04.015
Wang Z., Zhou J., Li J., Zou J., Fan L. (2020). The immune defense response of pacific white shrimp (Litopenaeus vannamei) to temperature fluctuation. Fish Shellfish Immunol. 103, 103–110. doi: 10.1016/j.fsi.2020.04.053
Wei M., Ge H., Shao C., Yan X., Nie H., Duan H., et al. (2020). Chromosome-level genome assembly of the Venus clam, Cyclina sinensis, helps to elucidate the molecular basis of the adaptation of buried life. iScience 23 (6), 101148. doi: 10.1016/j.isci.2020.101148
Willer D., Aldridge D. (2019). Microencapsulated diets to improve growth and survivorship in juvenile European flat oysters (Ostrea edulis). Aquaculture 505, 256–262. doi: 10.1016/j.aquaculture.2019.02.072
Wu Y., Chen A., Zhang Y., Cao Y., Chen S., Zhang Z. (2018). Study on the toxicity and median lethal concentration of residual chlorine on larval razor clams. Aquat. Sci. Techno. Inform 03), 158–161. doi: 10.16446/j.cnki.1001-1994.2018.03.009
Xue Q., Waldrop G., Schey K., Itoh N., Ogawa M., Cooper R., et al. (2006). A novel slow-tight binding serine protease inhibitor from eastern oyster (Crassostrea virginica) plasma inhibits perkinsin, the major extracellular protease of the oyster protozoan parasite perkinsus marinus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 145 (1), 16–26. doi: 10.1016/j.cbpb.2006.05.010
Xu Y., Liao D., Zhang C., Qin S. (2007). Research on clam purification technology in the Philippines. Fish. Modern 04), 9–12.
Xu X., Liu J., Wang Y., Si Y., Wang X., Wang Z., et al. (2018). Kunitz-type serine protease inhibitor is a novel participator in anti-bacterial and anti-inflammatory responses in Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 80, 22–30. doi: 10.1016/j.fsi.2018.05.058
Yin S., He Y., Yu J., Zhang Y., Liu Y., Geng X., et al. (2022). Cloning and immune response characteristics of the serine protease homologue gene (Lv-SPH) in Penaeus vannamei. J. Aquac. 46 (05), 815–824.
Yin Z., Nie H., Jiang K., Yan X. (2022). Molecular mechanisms underlying vibrio tolerance in ruditapes philippinarum revealed by comparative transcriptome profiling. Front. Immunol. 13. doi: 10.3389/fimmu.2022.879337
Yu Q. (2017). Effects of copper, cadmium and their combined exposure on oxidative stress and apoptosis in rare minnow (Xianyang: MAster's thesis, Northwest Agriculture and Forestry University).
Zeitoun I. (1977). The effect of chlorine toxicity on certain blood parameters of adult rainbow trout (Salmo gairdneri). Environ. Bio. Fish. 1 (2), 189–195. doi: 10.1007/BF00000410
Zeng J. (2008). Study on the ecological impact of coastal power plant warm water drainage on subtropical seas (Hangzhou: Doctoral dissertation, Zhejiang University).
Zhang X. (2014). Cyclina sinensis and shrimp pond mixed culture technology. Hebei Fishery 09), 31–32.
Zhang Q. (2015). Full-length cDNA cloning and expression analysis of g-type lysozyme and caspase family genes in g. regia under ammonia nitrogen stress (Master’s thesis (Nanjing: Nanjing Agricultural Universit).
Zhang H., Dong Y., Yao H., Yu K., Hu Y., Lin Z. (2020). Cloning of GST and HSP90 genes in razor clams (Sinonovacula constricta) and characterization of their expression under ammonia nitrogen stress. J. Oceano. 04), 66–78.
Zhang S., Huang H., Chen H., Peng Y., Wang Z., Fang Z., et al. (2000). Impact of residual chlorine discharge from daya bay nuclear power station on the environment of adjacent sea areas. Mar. Environ. Sci. 19 (2), 14–18.
Zhang Z., Lv Z., Shao Y., Qiu Q., Zhang W., Duan X., et al. (2017). Microsomal glutathione transferase 1 attenuated ROS-induced lipid peroxidation in Apostichopus japonicus. Dev. Comp. Immunol. 73, 79–87. doi: 10.1016/j.dci.2017.03.011
Zhang J., Wei K., Zhao T. (2016). Effect of Cu~(2+) stress on the activity of phenol oxidase pro-activation system in crayfish (Procambarus clarkii). J. Agricul. Environ. Sci. 35 (05), 865–870.
Zhang Y., Wu Q., Fang S., Li S., Zheng H., Zhang Y., et al. (2020). mRNA profile provides novel insights into stress adaptation in mud crab megalopa, Scylla paramamosain after salinity stress. BMC Genomics 21 (1), 559. doi: 10.1186/s12864-020-06965-5
Zhao H., Li W., Wan L., Yang D., Monday B. (2016). Effect of cd (II) and Cu (II) stress on heat shock protein 70 (HSP70) gene expression in the double-toothed fence silkworm, silkworm (Serratia marcescens). J. DL Ocean Univer. 31 (02), 156–161. doi: 10.16535/j.cnki.dlhyxb.2016.02.007
Zhao L., Yang X., Cheng Y., Yang S. (2016). Effect of dietary histamine supplementation on growth, digestive enzyme activities and morphology of intestine and hepatopancreas in the Chinese mitten crab Eriocheir sinensis. Springerplus. 5, 552. doi: 10.1186/s40064-016-2105-9
Zheng S., Liu Q., Zhang X., Xie Y. (2019). Analysis of GST mRNA expression and enzyme activity in the viscera of river clams under heavy metal stress. J. Eco. Environ. 02), 369–375. doi: 10.16258/j.cnki.1674-5906.2019.02.019
Zhou Y. (2014). Preliminary study on the characteristics of digestive enzymes and immune factors in the mouth shrimp, phyllostomus spp. (Dalian: Master’s thesis, Dalian Ocean University).
Zhou Z., Li B., Liu X., Li Z., Zhu S., Liang Y., et al. (2021). Recent progress in photocatalytic antibacterial. ACS Appl. Bio Mater. 4 (5), 3909–3936. doi: 10.1021/acsabm.0c01335
Zhu L., Song L., Xu W., Qian P. (2008). Molecular cloning and immune responsive expression of a novel c-type lectin gene from bay scallop Argopecten irradians. Fish Shellfish Immunol. 25 (3), 231–238. doi: 10.1016/j.fsi.2008.05.004
Zhu P., Sun Y., Wang H., Ji X., Zeng Y. (2022). Molecular insight into the hepatopancreas of oriental river prawn (Macrobrachium nipponense) in response to residual chlorine stimulus. Aquat. Toxicol. 243, 106052. doi: 10.1016/j.aquatox.2021.106052
Keywords: Cyclina sinensis, residual chlorine, immunity, antioxidants, transcriptome
Citation: Wang S, Ren G, Li D, Fan S, Yan S, Shi J, Liu M and Dong Z (2023) Transcriptome reveals the immune and antioxidant effects of residual chlorine stress on Cyclina sinensis. Front. Mar. Sci. 10:1105065. doi: 10.3389/fmars.2023.1105065
Received: 22 November 2022; Accepted: 02 January 2023;
Published: 17 January 2023.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Yan Fang, Ludong University, ChinaCopyright © 2023 Wang, Ren, Li, Fan, Yan, Shi, Liu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Dong, ZHpnNzcxMkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.