- 1Ocean Conservation, World Wildlife Fund, Washington, DC, United States
- 2Conservation Science Unit, WWF-Indonesia, Jakarta, Indonesia
- 3Yayasan Pemberdayaan Alam, Desa dan Masyarakat Indonesia (PADMI Foundation), Jakarta, Indonesia
- 4Department of Earth and Environmental Science, KU Leuven, Leuven, Belgium
- 5Cabrillo College, Natural and Applied Sciences, Aptos, CA, United States
- 6One People One Reef, Soquel, CA, United States
- 7Ecology Department, Leibniz Centre for Tropical Marine Research (ZMT), Bremen, Germany
- 8Marine Ecology Department, Faculty of Biology and Chemistry (FB2), University of Bremen, Bremen, Germany
- 9Coral Reef Alliance, San Francisco, CA, United States
- 10Duke University Marine Laboratory, Nicholas School of the Environment, Duke University, Beaufort, NC, United States
- 11Department of Biodiversity, Marine Science Program, Biodiversity and Conservation Science, Conservation and Attractions, Kensington, WA, Australia
- 12School of Molecular and Life Sciences, Curtin University, Perth, WA, Australia
- 13Blue Ventures, Conservation, Level 2 Annex, Omnibus Business Centre, London, United Kingdom
- 14Institute for Marine and Antarctic Studies (IMAS), University of Tasmania, Hobart, TAS, Australia
- 15Department of Biological Science, Florida State University, Tallahassee, FL, United States
- 16Global Science, World Wildlife Fund, Washington, DC, United States
- 17Fauna & Flora International, Phnom Penh, Cambodia
Globally, marine protected area (MPA) objectives have increasingly shifted from a primary focus on maintaining ecosystems through prohibiting extractive activities, to more equitable approaches that address the needs of both people and nature. This has led to MPAs with a diverse array of fisheries restrictions and recent debate on the type of restrictions that contribute to achieving biodiversity goals. Here we use a global dataset of 172 MPAs (representing 31 nations) alongside nine detailed case study MPAs (from Australia, Belize, Cambodia, Federated States of Micronesia, Fiji, Indonesia, Madagascar, Solomon Islands, and United States of America), including partially protected areas that allow regulated fishing, to illustrate the many diverse pathways that some MPAs have adopted to protect biodiversity and safeguard the rights and well-being of resource-dependent coastal communities. We group MPAs based on their restrictions and explore four key insights emerging from these groupings using our nine case studies: (i) MPAs use highly diverse approaches to regulate fisheries; (ii) partially protected areas can address gaps in regional fisheries management; (iii) devolving resource management rights to communities influences the chosen fisheries restrictions; and (iv) state-governed MPAs can use highly tailored fisheries restrictions to increase equity in access. We find that partially protected MPAs can offer effective and equitable pathways for biodiversity conservation if tailored to local context. Rather than focusing primarily on fully protected areas for achieving new global MPA targets, we recommend countries use a blend of locally-appropriate protection levels – from fully protected areas to partially protected MPAs to achieve positive biodiversity outcomes.
1 Introduction
Globally, area-based conservation has undergone an evolution from a historical focus on protecting ecosystems through access restrictions, to more equitable approaches for both people and nature (Sandbrook et al., 2011; Mace, 2014; Tallis and Lubchenco, 2014; Garnett et al., 2018; Dawson et al., 2021). With this shift comes an increasing need to recognize a diverse spectrum of approaches to achieve conservation outcomes. The design and implementation of Marine Protected Areas (MPAs), which are commonly used conservation interventions in response to declines in ocean health, have also followed this broader evolution (Pendleton et al., 2018; Campbell and Gray, 2019). For example, those establishing MPAs increasingly engage marine stakeholders in the design and implementation phases and include social considerations in their targets or outcomes (Campbell and Gray, 2019). As many coastal communities depend on coastal ecosystems, there is a need for approaches to marine protection that include and address the diverse needs of marine stakeholders while protecting biodiversity. Many MPAs increasingly have objectives to support building resilient social-ecological systems (Cinner et al., 2012; Mace, 2014).
MPA coverage has rapidly expanded in recent years, in part driven by countries’ commitments under the Convention on Biological Diversity Aichi Target 11 to designate 10% of their marine areas as MPAs by 2020 (Grorud-Colvert et al., 2019). This global commitment to expand MPA area was further reinforced by Goal 14 (‘Life Below Water’) of the UN Sustainable Development Goals, which also adopted a target for nations to designate 10% of their marine areas under protection. MPA coverage increased from 0.5% of global marine area in 2004 (Toropova et al., 2010) to 7.7% in 2020 (UNEP-WCMC and IUCN, 2021)—and has been accelerated by the designation of some very large, isolated (i.e. remote) MPAs (>100,000 km2) (Toonen et al., 2013). The recent adoption of the Kunming-Montreal Global Biodiversity Framework, of which Target 3 calls for nations to ‘ensure and enable that by 2030 at least 30 percent of … coastal and marine areas … are effectively conserved and managed’ seems likely to further accelerate and incentivize global growth in marine protection (CBD, 2022).
IUCN defines an MPA as: ‘A clearly defined geographical space, recognised, dedicated, and managed, through legal or other effective means, to achieve the long-term conservation of nature with associated ecosystem services and cultural values’ (Dudley, 2008). While the IUCN definition is recognized globally, in reality, MPAs are often defined differently in each jurisdiction based on the priorities and legal systems of the country, the national or sub-national legal instruments used in designation, and the naming conventions for protected areas in the country (e.g. Amkieltiela et al., 2022). MPAs are required to set objectives based on achieving positive outcomes for nature to meet the global IUCN definition – such as increases in marine species abundance, biomass, and age-class (Dudley, 2008). MPAs must be implemented in areas where human activities currently or in the future would otherwise be damaging or unsustainable for the marine environment to deliver positive outcomes for nature. Protection levels within MPAs can vary, and the term MPA has always covered a wide range of levels of protection. These range from ‘fully’ protected areas (where all extractive and damaging activities are prohibited) and ‘highly’ protected areas (where only activities with low environmental impact are allowed), to ‘lightly’ protected (with moderate extractive activity, e.g. gear restrictions or periodic harvest), to only ‘minimally’ protected with fewer restrictions on fishing or other extractive activities (Day et al., 2012; Horta e Costa et al., 2016; Grorud-Colvert et al., 2021). A global synthesis suggests that 94% of MPAs allow some form of fishing (Costello and Ballantine, 2015).
There has been debate on the required minimum levels of protection for MPAs to count towards marine protection targets (Agardy et al., 2003; Agardy et al., 2016; Pendleton et al., 2018). On one end of the spectrum, it is argued that only fully or highly protected MPAs (i.e. MPAs that prohibit extractive activities or only allow those with minimal environmental impact) should count towards biodiversity targets (Davis, 2012; Pendleton et al., 2018; Sala and Giakoumi, 2018; Sala et al., 2018). This ‘biodiversity first’ focus, however, risks misalignment with current conservation thinking around inclusivity and equity in conservation and the need to support resilient social-ecological marine systems. While there is strong evidence that effectively managed, isolated, large, older, fully or highly protected MPAs provide the greatest biodiversity outcomes (Lester and Halpern, 2008; Sciberras et al., 2013; Edgar et al., 2014; Sala and Giakoumi, 2018), such strict protection is ill-suited to many areas. In addition, there is a paucity of empirical data to illustrate the efficacy of ‘lightly’ protected areas, many of which are critical to the integrity of social-ecological systems (Cinner et al., 2016; Crane et al., 2017a; Crane et al., 2017b). Some of the most biodiverse ocean areas—and those in most urgent need of protection—are located in places where marine resource use is deeply intertwined with culture (McClanahan et al., 2006; Crane et al., 2017a) and critical for livelihoods and food security (Loper et al., 2008; Cinner et al., 2016). Furthermore, greater returns on investment from MPA establishment for biodiversity can be expected in locations where people are moderately extracting resources, rather than in places that are suitable for fully protected areas (i.e. isolated areas with low extraction rates) (Cinner et al., 2018).
In this paper we discuss how positive biodiversity outcomes can be achieved from MPAs with diverse fishing restrictions. We seek to identify how different and diverse regulations could be combined within MPAs to achieve different forms of partial protection. We refer to MPAs which do not prohibit all fishing as ‘partially protected’ areas (Lester and Halpern, 2008; Sciberras et al., 2013; Zupan et al., 2018) in contrast to MPAs that prohibit all fishing activity known as ‘fully’ protected areas or no-take areas (Grorud-Colvert et al., 2021). We then, through comparative analysis of case studies, evaluate how local context may have influenced MPA regulation choices. Specifically, we use global data and nine case study MPAs—including partially protected areas—to illustrate different MPA implementation strategies that could be used to protect biodiversity while also safeguarding the rights and well-being of resource dependent communities. We first develop and apply a classification framework to explore MPA restrictions across highly diverse contexts. We then focus on fisheries restrictions within MPAs, given these represent a major source of social conflict between local communities and management authorities in many MPAs. We identify common groupings of MPAs based on restrictions and evaluate the restriction choices and local context for the case study MPAs. Through this effort, we illustrate and evaluate potentially locally appropriate marine conservation measures and how they might support more positive and equitable biodiversity outcomes – especially for linked social-ecological systems.
2 Methods
2.1 Protection classifications and definitions
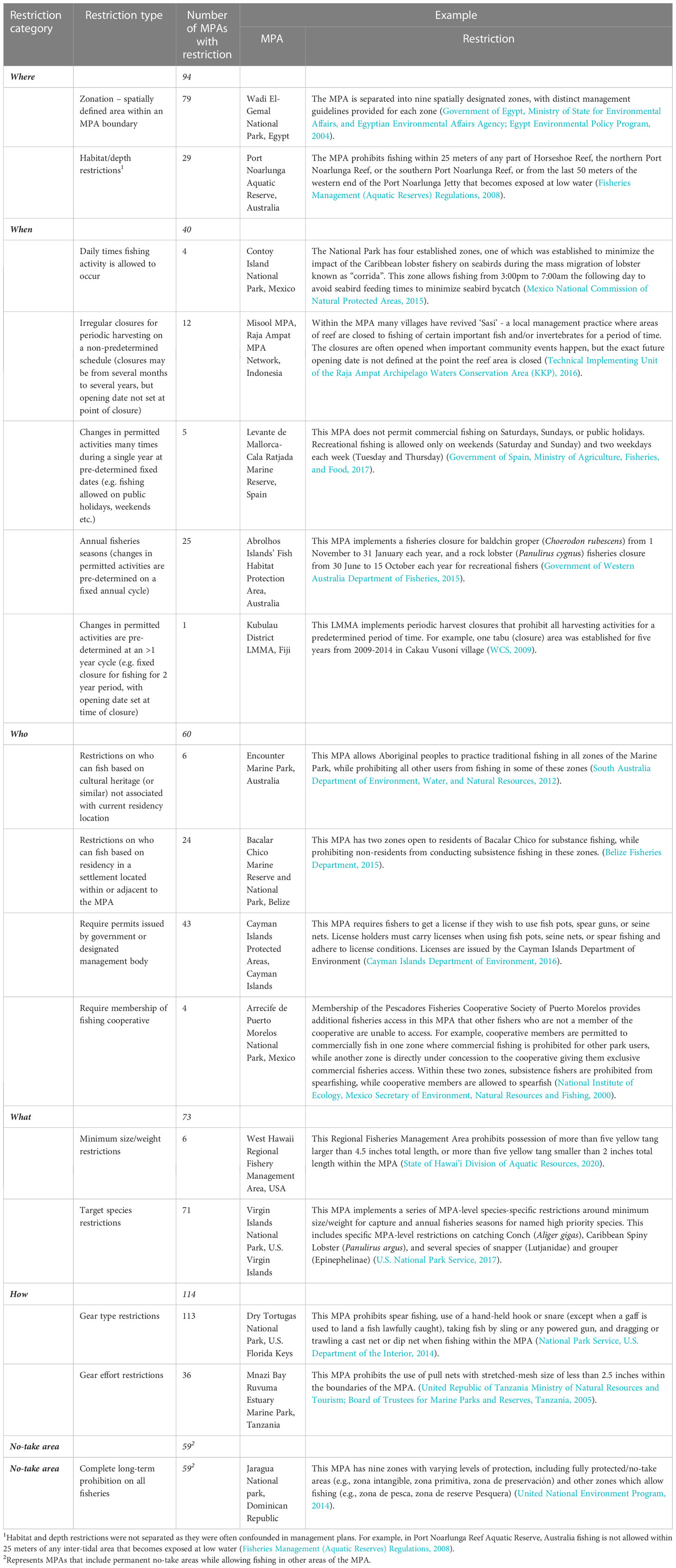
Several typologies have been developed for describing MPAs based on their objectives, regulations, or permitted activities. IUCN defines protected area categories based on the objective of an MPA or zone provided that biodiversity conservation is a primary goal (Day et al., 2012). Other MPA classifications have been proposed, for example, by broadly grouping MPAs into different categories based on fisheries gear restrictions, other human activities (e.g. aquaculture), and accessibility (Horta e Costa et al., 2016), or by level of protection and stage of MPA establishment (Grorud-Colvert et al., 2021). Fisheries restrictions are also often classified based on input rules (e.g. limited entry, time restrictions, gear restrictions), output rules (e.g. allowable catch limits), or technical measures (e.g. size limits, time or area closures) (Selig et al., 2017).
Many MPAs do not have a single set of static regulations that are applied in the long-term and across the entire MPA. Instead, many MPAs manage extractive use based on complex interwoven restrictions that we group into five broad restriction categories: who, what, when, where, and how (Tables 1 and 2). These restrictions can be implemented by governments, local communities, or other stakeholders within MPAs, and each of them can be used individually or in combination. For example, an MPA may incorporate zonation (restrictions on where people can fish) that creates fisheries areas subject to gear restrictions (restrictions on how fishing can occur). In addition to fisheries restrictions there can be restrictions on other activities occurring in MPAs, such as aquaculture, bottom exploitation (e.g. sand mining), and non-extractive uses (e.g. tourism) (Horta e Costa et al., 2016).
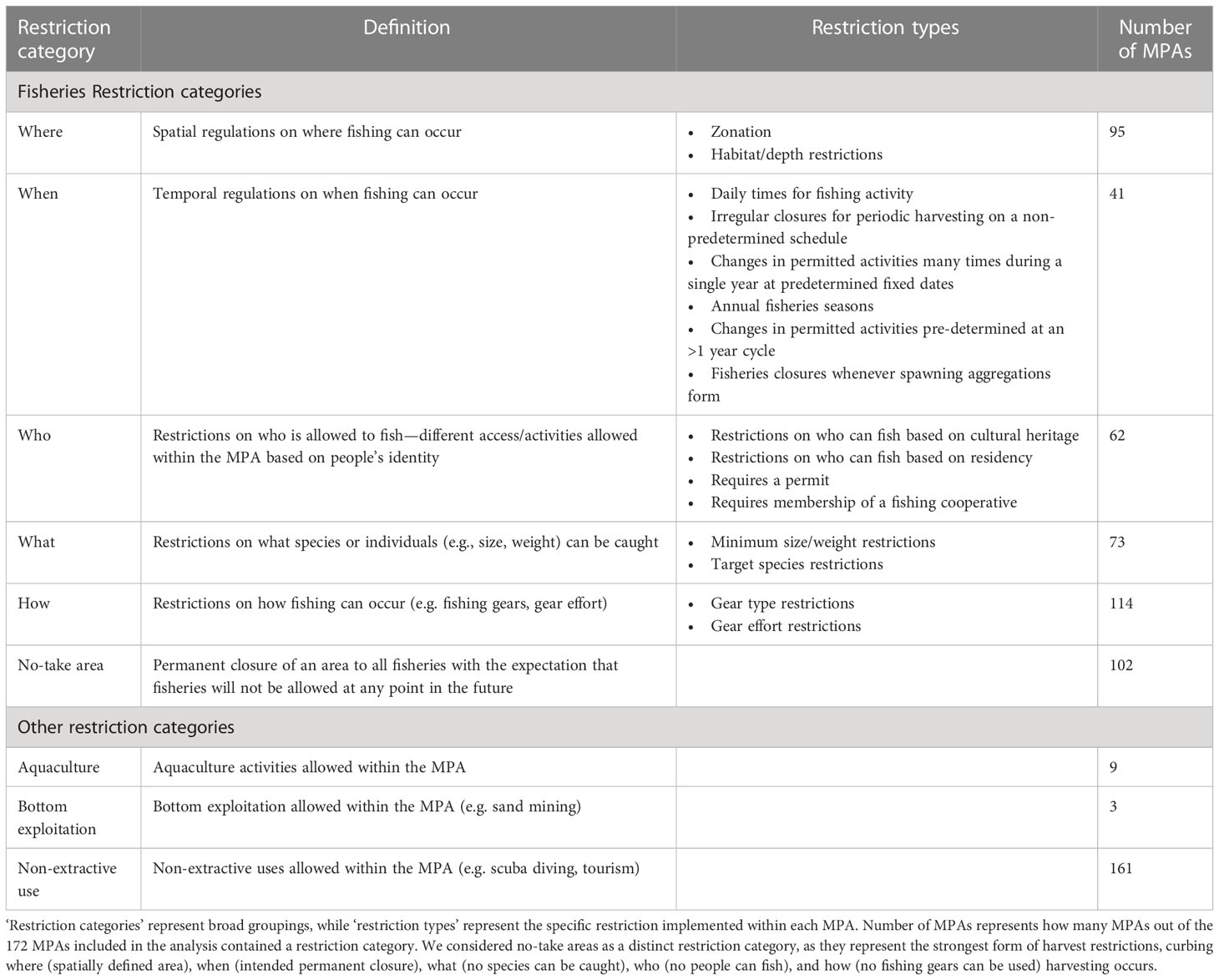
Table 2 Restrictions categories and restriction types used in marine protected areas and definitions.
2.2 Case study MPAs and global dataset
To understand outcomes from a diversity of MPA management and restrictions, we considered nine illustrative case study MPAs (Figure 1; Table 1, S1). These MPAs were selected during a workshop held at the 5th International Marine Conservation Congress in Kuching, Sarawak in June 2018. Case studies were selected based on discussion balancing: (i) diverse governance types and geographical locations, (ii) where detailed knowledge of establishment, management, and regulations were available to the authors/workshop participants, and (iii) where documented assessments of biodiversity outcomes were available. Case study MPAs are: (1) Wakatobi National Park (NP), Indonesia, (2) Kubulau District Locally Managed Marine Area (LMMA), Fiji, (3) Velondriake LMMA, Madagascar, (4) Koh Rong Archipelago Marine Fisheries Management Area (MFMA), Cambodia, (5) Ulithi Atoll and associated islands, Outer Islands of Yap State, Federated States of Micronesia, (6) Cottesloe Reef Fish Habitat Protection Area (FHPA), Australia, (7) Kona Coast Fishery Management Area (FMA), Hawaii, USA, (8) Nusatupe Reef MPA, Solomon Islands, and (9) Half Moon Caye Natural Monument (NM), Belize (Figure 1). Case study MPAs included exclusively fully protected areas, zoned MPAs incorporating partial protection and fully protected areas, and exclusively partially protected MPAs (Table 1). While the majority of case study MPAs do have documented biodiversity benefits (Table 1), we acknowledge that most still face challenges (Table S1). Therefore, these case study MPAs should not be considered ‘fully effective’ and we recognize, as for most MPAs, there is scope for case study MPAs to improve their effectiveness.
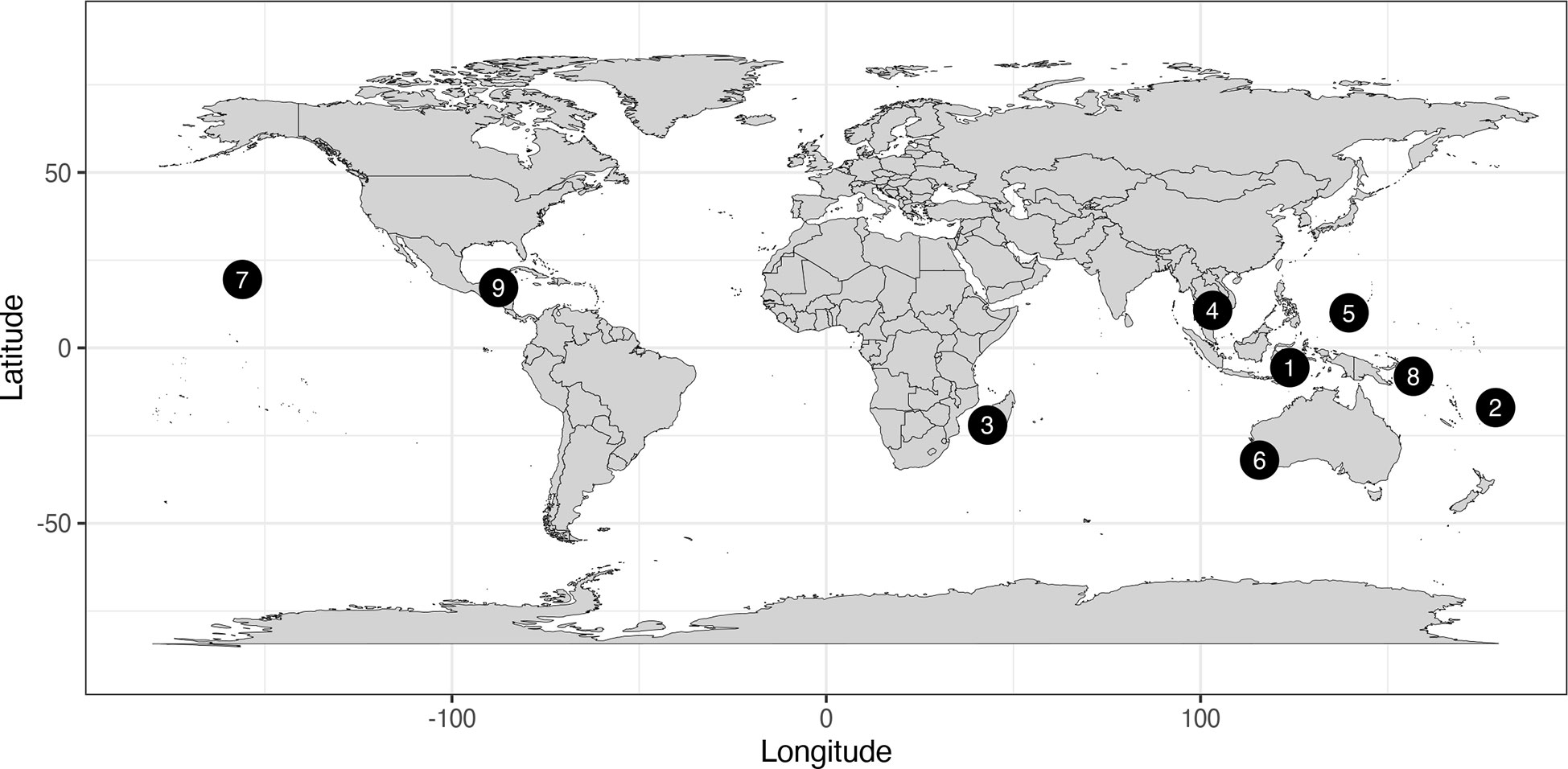
Figure 1 Location of case study MPAs. Case study MPAs are: (1) Wakatobi National Park, Indonesia, (2) Kubulau District Locally Managed Marine Area (LMMA), Fiji, (3) Velondriake LMMA, Madagascar, (4) Koh Rong Archipelago Marine Fisheries Management Area, Cambodia, (5) Ulithi Atoll, Federated States of Micronesia, (6) Cottesloe Reef Fish Habitat Protection Area, Australia, (7) Kona Coast Fishery Management Area, Hawaii, USA, (8) Nusatupe MPA, Solomon Islands, and (9) Half Moon Caye Natural Monument, Belize.
To place our nine case study MPAs in a global context, we used a dataset of 167 MPAs under different forms of governance from 31 nations and tropical and temperate waters originally gathered by Gill et al. (2017). The dataset therefore illustrates variation in MPA restrictions that can be seen at the global scale (see Gill et al., 2017). For each MPA in the global dataset we searched for official management plans online, using the World Database of Protected Areas (www.protectedplanet.net) and also included government documents and non-governmental organization (NGO) reports. Four of our case study MPAs were already included in the global dataset, so in total we obtained information on 172 MPAs. We follow the IUCN MPA definition, which means that some of the MPAs we include in our analysis may not formally be called ‘Marine Protected Areas’ in their countries’ legal system, but they meet the IUCN definition of an MPA.
2.3 MPA classification
For the 172 MPAs we identified the broad ‘restrictions categories’ used within each MPA—i.e. whether there were restrictions based on where, when, what, and how people can fish, who can fish, and other restrictions (Table 2). To better understand how fisheries were being managed, within these restriction categories we identified the specific fisheries ‘restriction types’ that were being implemented in each of the 172 MPAs in the dataset (Tables 2, 3). Where a single management regulation combined multiple restriction categories or restriction types we separated them into their individual components. For example, MPAs that restrict lobster harvesting to a fixed annual season with a minimum size for landings incorporates multiple restriction types. This case includes a species restriction (what—restriction on the specific species allowed to be caught), a size restriction (what—restriction on what sizes of individuals are allowed to be caught), and a temporal restriction (when—annual fishing season). We considered complete permanent bans on all extractive activities (fully protected areas) as a distinct restriction category, as they represent the strictest form of fishery regulation, curbing where (spatially defined area), when (intended permanent closure), what (no species can be caught), who (no people can fish), and how (no fishing gears can be used) fishing occurs. Most MPAs exist within a complex patchwork of fisheries management arrangements, coastal protection, or national management interventions. When classifying MPAs we specifically focused on restrictions implemented within the MPA that are different to surrounding waters. For example, Hawaii has implemented state-wide restrictions on fishing gears (including minimum mesh sizes, time of day restrictions for certain fishing gears, and species-specific protections) that apply to all fisheries in state waters (Department of Land and Natural Resources, 2005; Department of Land and Natural Resources, 2014). While these restrictions apply within all MPAs in the state and may be enforced by MPA management authorities, we did not include them in our MPA restriction analysis as they do not represent MPA-level restrictions. Our analysis of the presence/absence of restriction categories and restriction types is based on written management plans and reports for the MPAs available online. While we have local knowledge of case study MPAs, for some MPAs in our global dataset the restrictions in the management plan may not be fully implemented. This is a broader challenge for protected areas – i.e. ‘paper parks’. This does not affect our analysis or interpretation, which is primarily driven by case studies, with the global dataset providing background context for the types of regulations frequently included in MPA management plans.
2.4 Data analysis
To identify whether there were common groupings of similar restrictions used in MPAs, and evaluate how our case study MPAs aligned with these groupings, we coded the presence or absence of each restriction category or type for all 172 MPAs. We then conducted principal components analysis (PCA), a multivariate statistical method to quantify similarities or differences between individual MPAs based on their restrictions. PCA generates a set of axes (principal components) that are combinations of the original input variables (in this case different MPA restrictions), maximizing the original data variance explained by each axis while minimizing correlations among axes. Each axis can be interpreted based on the strongest correlations to individual variables (restrictions). Correlations can be either positive or negative (between -1 and 1), depending on the direction and magnitude of the results. Here we consider all correlations >|0.3| as significant (following Hoshino et al., 2017). MPA restrictions with strong correlations to principal component axes that explain a large proportion of the data variation are the most important for distinguishing among MPAs. This approach allowed us to first evaluate which restrictions distinguished between MPAs in the global dataset, but then contextualize these clusters identified in the PCA through drawing on qualitative information from the case study MPAs.
We ran two separate PCAs, the first based on all 172 MPAs using the presence or absence of the different broad ‘restriction categories’ in the MPA (i.e., presence/absence matrix of Table 2 restriction categories). This allowed us to explore the relative power to discriminate between MPAs based on broad differences in restrictions. Given the widespread use of partial protection approaches to manage fisheries within MPAs, we then subset the data to the 129 MPAs that allow some form of fishing—thus removing the exclusively no-take MPAs (n=43). We then conducted a PCA based on the individual fisheries ‘restriction types’ (i.e. presence/absence matrix of Table 3 restriction types). PCA analysis was conducted in the vegan package (Oksanen et al., 2020).
To distinguish groups of MPAs based on restrictions we used k-means clustering, which has previously been used to classify MPAs into groups (e.g. Bohorquez et al., 2019). K-means clustering allocates MPAs into a pre-specified number of groups based on the presence/absence of restrictions while minimizing the amount of variation within each group. We selected the number of clusters based on examining a scree plot of the within group sum of squares for 0-15 clusters – i.e. how much variation in MPA restrictions can be explained by the clusters. All analyses were conducted in R (R Core Team, 2020).
3 Results and discussion
3.1 Identified restriction clusters
The majority of MPAs reviewed were partially protected, with 75% (129) of the 172 MPAs allowing some fishing to occur within their boundaries. Fisheries restrictions provided the greatest explanatory power to discriminate among MPAs across the ‘restriction categories’ (Table 2). We found that certain restrictions on fisheries within MPAs were frequently implemented together. For example, when and where fishing can occur were often implemented simultaneously within the same MPA, as were restrictions on who could fish and what could be harvested (Figure 2A). Principal component (PC) 1 explained 37% of variation in MPA restrictions (Figure 2A), and was most strongly driven by how fishing can be conducted (0.52 correlation with PC1), what can be caught (0.48), where fishing can occur (0.47), and who can fish (0.37) (Table S2). PC2 explained 19% of variation (Figure 2A) and was most strongly driven by the presence of no-take areas (0.78 correlation with PC2), in addition to where (0.50) and when (0.33) people can fish (Table S2). Aquaculture, bottom exploitation, and non-extractive recreational uses provided little power to discriminate among MPAs based on their restrictions (Table S2). This was unsurprising given the majority of our restriction categories were focused on fisheries, and fisheries management represents a major objective of most MPAs.
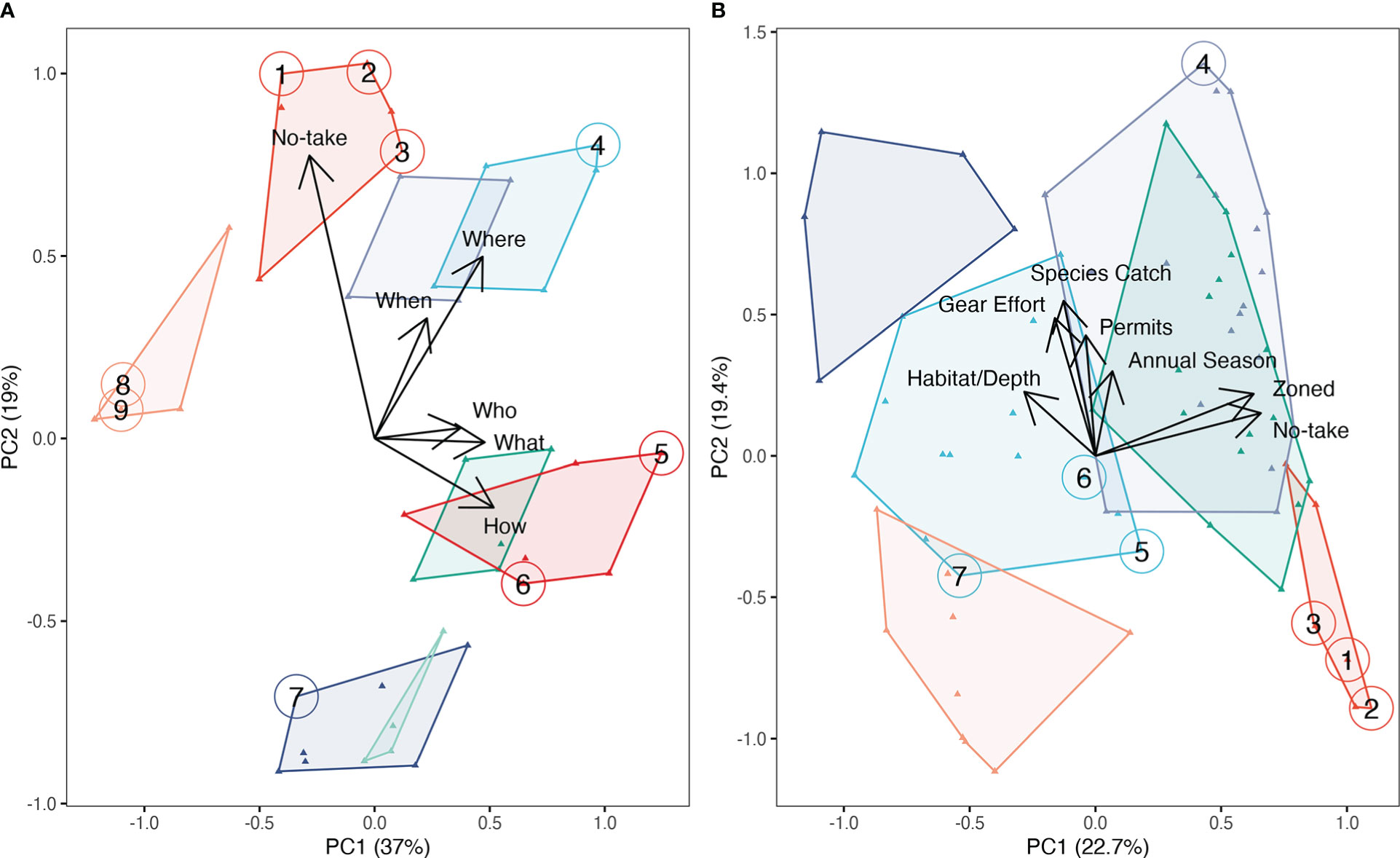
Figure 2 Principal Components Analysis of MPA regulations. (A) All 172 MPAs based on the restriction categories (Table 2), and (B) the 129 MPAs that allow fishing based on the specific fishing restrictions types (Table 3). K-means clustering is used to identify groups of similar MPAs based on (A) eight clusters and (B) six clusters. The nine case study MPAs are colored by cluster group and numbered: (1) Wakatobi National Park, Indonesia, (2) Kubulau District Locally Managed Marine Area (LMMA), Fiji, (3) Velondriake LMMA, Madagascar, (4) Koh Rong Archipelago Marine Fisheries Management Area, Cambodia, (5) Ulithi Atoll, Federated States of Micronesia, (6) Cottesloe Reef Fish Habitat Protection Area, Australia, (7) Kona Coast Fishery Management Area, Hawaii, USA, (8) Nusatupe MPA, Solomon Islands, and (9) Half Moon Caye Natural Monument, Belize. Part (B) shows only MPAs that allow fishing, therefore (8) Nusatupe MPA and (9) Half Moon Caye Natural Monument are not included as these are exclusively no-take MPAs. Of the 172 MPAs included in (A), 43 unique combinations of restriction categories were recorded (43 unique data point locations), while from the 129 MPAs included in (B) 71 unique combinations of specific fishing restrictions from the MPAs were recorded (71 unique data point locations).
When we examined the more specific ‘restrictions types’ (i.e., the specific rules applied within the broader restriction categories; Table 3) for MPAs that allow fishing, we found frequently co-occurring restrictions. We found that the use of gear effort restrictions, species catch restrictions, permits, and annual fisheries seasons were commonly used together in MPAs (Figure 2B). We also found that the use of zonation and fully protected zones commonly co-occurred—because zonation is required for an MPA to both allow fishing and incorporate permanent fully protected zones. The presence of fully protected zones (0.66 correlation with PC1) and zonation (0.63) had the greatest power to discriminate between MPAs—strongly correlating with PC1 which explained 22% of the variance (Figure 2B and Table S3). We also found that the use of species-specific restrictions (0.55 correlation with PC2), gear effort restrictions (0.49), fisheries permits (0.43), and annual fisheries seasons (0.30) were important for distinguishing between MPAs that allow fishing (Table S3). These restriction types were strong correlates of PC2, which explained 19% of the variation (Figure 2B and Table S3).
We found eight groupings of MPAs based on our cluster analysis of restriction categories, of which five were mapped to our case studies (Figure 2A). To provide a more detailed investigation of how MPAs regulate fisheries, we conducted cluster analysis on the fishing ‘restriction types’ for MPAs that allow fishing. We identified six clusters, three of which were represented by case studies (Figure 2B). Clusters represent groups of MPAs that are implementing similar ‘restriction categories’ or ‘restriction types’. For example, Kubulau District LMMA, Wakatobi NP, and Velondriake LMMA (clustered upper-center of Figure 2A and lower-right of Figure 2B) all use permanent fully protected zones and similar restrictions on where and when fishing can occur, while Nusatupe Reef and Halfmoon Caye NM (clustered center-left of Figure 2A) are exclusively fully/highly protected MPAs. By identifying co-occurring case studies within clusters we can investigate whether MPAs that are implementing similar restriction categories or types are aiming to achieve similar or highly divergent objectives.
3.2 Key insights from case studies
Our comparative analysis of the MPA restrictions and case studies revealed four key insights: (i) MPAs use highly diverse approaches to regulate fisheries; (ii) partially protected areas can address gaps in regional fisheries management; (iii) devolving resource management rights to communities influences the chosen fisheries restrictions; and (iv) state-governed MPAs can use highly tailored fisheries restrictions to increase equity in access. More broadly, across our case studies we found that partial protection approaches are providing an alternative pathway for marine conservation to achieve biodiversity outcomes that can complement fully protected MPAs.
3.2.1 MPAs use highly diverse approaches to regulate fisheries
We found MPAs implement many different restriction combinations to address similar management goals—with spatial zonation particularly important for facilitating diverse fisheries restriction combinations. We identified 16 fisheries ‘restriction types’ (Table 3), which occurred in 73 unique combinations across the 129 MPAs that allowed fishing. This suggests high diversity in how different restrictions can be combined given an MPA’s management objectives and local context. For example, both Koh Rong Archipelago MFMA and Cottesloe Reef FHPA aim to conserve and protect fisheries species and habitats while still allowing sustainable harvesting (Table 1). Cottesloe Reef FHPA uses gear type, habitat and depth restrictions, while Koh Rong Archipelago MFMA uses annual fishing seasons, permits, target species restrictions, and gear effort restrictions (Table 1). Koh Rong Archipelago MFMA had 12 different restriction types—the greatest in our dataset.
Our results highlight the importance of spatial zonation in enabling partial protection approaches for MPAs—both for incorporating no-take areas, but also for spatially allocating other restrictions. Zonation and the use of no-take areas were highly correlated (Figure 2B and Table S3), which is not surprising because zonation is required for an MPA to both allow fishing and incorporate permanent no-take areas. These two variables are not perfectly correlated, however, as some zoned MPAs do not include no-take areas. Some MPAs may also use spatial restrictions without using formal zonation terminology or producing zonation plans. Cottesloe Reef FHPA, for example, does not include no-take areas and is not formally zoned, but has species-specific fisheries restrictions that spatially divide the MPA into two distinct areas based on a visual marker on the coastline (Table 1). Cottesloe Reef FHPA clusters with Ulithi Atoll and Kona Coast FMA in our restriction type analysis (Figure 2B). Similar to Cottesloe Reef FHPA, Ulithi Atoll does not incorporate strict no-take but does include spatial zonation. While Kona Coast FMA also does not incorporate no-take, it does not use spatial zonation. This is likely why Cottesloe Reef FHPA and Ulithi Atoll are located more closely together in our PC analysis (Figure 2B). Our case studies that allowed fishing, therefore, exemplified the diversity of approaches shown by partially protected MPAs to restrict harvesting (Figure 2B).
3.2.2 Partially protected areas can address gaps in regional fisheries management
MPAs exist in seascapes with highly variable national and sub-national fisheries management contexts which are reflected in their MPA-specific fisheries restrictions and management objectives (e.g. Table 1). National or sub-national fisheries management is generally concerned with how fisheries stocks are managed outside of MPAs or other spatially discrete management interventions (Hall and Mainprize, 2004). Fisheries management requires balancing political realities, livelihood needs, and ecological evidence to maintain harvest sustainability and the capacity to monitor and enforce any breaches of fisheries restrictions (Teh et al., 2017). Yet often fisheries management fails because of poor design or implementation, including failure to follow the precautionary principle (Selig et al., 2017). Area-based management approaches—such as MPAs—are amongst the most frequent and most successful tools for small-scale fisheries management (Selig et al., 2017). This is especially true for small-scale fisheries that define success based on ecological and human well-being outcomes, as opposed to profitability and efficiency metrics that are often used to characterize large commercial fisheries (Selig et al., 2017).
Given the low capacity for national or sub-national fisheries management in many coastal areas with urgent conservation needs (Mora et al., 2009; Worm and Branch, 2012; Costello et al., 2016), MPAs often have dual aims of biodiversity conservation and supporting fisheries sustainability (e.g. White et al., 2014). For example, the Koh Rong Archipelago MFMA contains the most restrictions of any MPA in our dataset, in part because there is limited regional fisheries management, a history of open access to fisheries resources, and high community dependence on fisheries (Table 1). Therefore, the multi-use, zoned, Koh Rong Archipelago MFMA must balance supporting and building sustainable fisheries management alongside providing biodiversity outcomes. In a similar way, Wakatobi NP must balance dual objectives of sustainable fisheries with biodiversity conservation (Table 1; Amkieltiela et al., 2022). Indonesia has national fisheries management, however, this mostly excludes small-scale fisheries (vessels < 10 gross tons), which remain largely unmanaged despite representing the majority of fishing vessels (Halim et al., 2019; Tranter et al., 2022). The few national regulations that apply to small-scale fisheries, such as bans on destructive fishing gears and restrictions on some threatened species harvesting (e.g., humphead wrasse; Cheilinus undulatus), are poorly enforced (Amkieltiela et al., 2022; Tranter et al., 2022). To improve fisheries management, Wakatobi NP sustainable fishing zones focus on enforcing these national regulations and limiting fishing to small-scale fishers, but do not implement further gear restrictions.
Where there is greater national fisheries management capacity, MPAs can still be designated to support local-scale fisheries sustainability. Given that MPAs must fit within a complex patchwork of national fisheries management, MPAs established with similar objectives in different countries may use different restrictions based on the national fisheries management context. For example, both Cottesloe Reef FHPA and Kona Coast FMA were established based on the desire from local groups to provide enhanced conservation and protection to fisheries species and habitats above those provided by regional fisheries management in nations with high national fisheries management capacity (Table 1). Both of these MPAs still allow sustainable harvesting, with Kona Coast FMA potentially providing positive biodiversity outcomes (Table 1). Both MPAs grouped together based on ‘restriction types’—including restrictions on what can be fished (Figure 2B). Kona Coast FMA has few fisheries restrictions specifically for the MPA (Table 1), allowing fishing using any ‘legal gear’ for personal consumption. ‘Legal gear’ does not relate to MPA rules, but regional fisheries rules. Cottesole Reef FHPA, in contrast, implements many specific gear restrictions and some species catch restrictions; these supplement regional species catch and temporal restrictions that also apply and are enforced in the FHPA (Table 1).
3.2.3 Devolving resource management rights to communities influences the chosen fisheries restrictions
Marine resource management rights can be devolved in multiple ways to the local level. Four of our case study MPAs (Velondriake LMMA, Wakatobi NP, Kubulau District LMMA, and Ulithi Atoll) that devolve management rights to communities incorporate periodic fisheries closures—and have demonstrated biodiversity benefits (Table 1). Despite different MPA governance characteristics (Table 1), these MPAs all combine spatial management and temporal management, with all except Ulithi Atoll containing permanent no-take areas. Devolved management rights can include a wide range of co-management approaches (Sen and Raakjaer Nielsen, 1996) or governance solely by Indigenous peoples or local communities (Sen and Raakjaer Nielsen, 1996; Borrini et al., 2013). These different approaches allow local groups to make decisions around the management of specific areas within an MPA, a whole MPA, or a larger MPA network.
LMMAs involve local communities in co-management or fully devolve governance to communities (Jupiter et al., 2014; Gardner et al., 2020). In Fiji, for example, there is a strong recognition of Indigenous rights—dividing coastal areas into customary fishing grounds known as qoliqoli (Sloan and Chand, 2016). Communities are required to give up their fishing rights to these areas when incorporated into state-governed MPAs. Hence Fiji has few state-governed MPAs, but instead has extensive LMMAs such as Kubulau District LMMA under co-management between customary owners and an NGO (Table 1; Aswani et al., 2017). Similarly, Velondriake LMMA in Madagascar uses shared governance by local stakeholders and NGOs to achieve biodiversity outcomes (Gardner et al., 2020). These LMMAs can include permanent fully protected areas, but also periodically harvested closures that open on cycles under the control of local village leaders in partnership with NGOs and national LMMA networks (Jupiter et al., 2014). These periodic harvest closures can provide conservation benefits, though they require careful management to avoid biodiversity gains being lost when the area is open to fishing (Goetze et al., 2018).
State-governed MPAs can also use co-management approaches to increase community involvement in marine resource governance. Wakatobi NP, for example, is a government managed MPA that uses formal spatial zonation to designate areas as fully protected, open to small-scale fishing with some restrictions, or for community management (Table 1). These community management areas—known as Kaombo (‘fish banks’)—are located near villages. Local customary institutions control access to Kaombo areas through long-term periodic harvest closures and harvest closure areas that are opened in periods of bad weather when normal fishing grounds are inaccessible (Jack-Kadioglu et al., 2020). While Kaombo areas can lose their conservation gains rapidly when opened to fishing if not well managed, their presence also helps support the implementation of the other fully protected MPA zones.
Exclusively fully protected MPAs that provide biodiversity benefits often owe some of their success to being relatively small, having devolved management rights, or having partial protection in the surrounding seascape. Therefore, positive biodiversity gains may not be scalable with simple expansion of fully protected area extent if this compromises equity. For example, Nusatupe Reef MPA is a small (0.49 km2) exclusively no-take MPA, while Half Moon Caye NM is a larger (39.25 km2) exclusively no-take MPA. While individually these MPAs are fully protected, they are integrated into a much larger network of partially protected MPAs. In the case of Nusatupe, this larger MPA network is governed by a committee comprised of key local stakeholders, NGOs, and local government, and actively promotes both marine conservation and sustainable marine resource use given the high local community dependence (e.g. implementing seasonal closures, rotational closures, and gear restrictions) (Liligeto, 2011). Similarly, Half Moon Caye NM is a relatively small area of high tourism value surrounded by areas under partial protection or open to fisheries (Table 1; Belize Audubon Society, 2007; Belize Audubon Society, 2016). Therefore, successes associated with smaller, fully protected MPAs should be treated cautiously when considering scaling to designate larger, fully protected MPAs, as successes from smaller fully protected areas likely depend, in part, on fisheries access in surrounding areas.
3.2.4 State-governed MPAs can use highly fisheries tailored restrictions to increase equity in access
State-governed MPAs can be highly tailored to recognize diverse needs of local communities and increase the likelihood of positive biodiversity outcomes, although overcomplicated regulations risk hindering management effectiveness. Koh Rong Archipelago MFMA contains the most restrictions of all of the case study MPAs—including fully protected areas and restrictions on where, when, who, what, and how fishing can occur—which were defined through spatial prioritization tools and intensive consultation with marine resource users (Boon et al., 2014; Mulligan and Longhurst; Mizrahi et al., 2016; Table 1). This MPA incorporates co-management (Mizrahi et al., 2016), with decisions made by a locally elected committee and a multi-stakeholder group alongside government (Mulligan et al., 2014; Preah Sihanouk Provincial Hall, 2014). Therefore, despite intimate government involvement in Koh Rong Archipelago MFMA, the co-management governance structure and extensive consultation process has led to very different restriction structures to other government-implemented case study MPAs. When considering the ‘restriction categories’, Koh Rong Archipelago MFMA is more similar to community-implemented MPAs such as Ulithi Atoll than to our other MPAs involving government (Figure 2A). However, these similarities disappear when considering the specific ‘restriction types’ implemented (Figure 2B). The high level of tailoring restrictions in Koh Rong MFMA has resulted in an MPA with strong support and positive perceptions by local fishing communities while delivering conservation benefits (Roig-Boixeda et al., 2018). It also, however, has resulted in a complex zoning and regulation system that requires significant and well-communicated demarcation and awareness raising across sectors—especially with the rapidly growing tourism industry (i.e. new site users). The complexity of the regulation system results in an additional management burden that local authorities have been struggling to meet. Too much complexity in partially protected MPA regulations has previously been highlighted as a major challenge for MPA compliance, with the need to simplify restrictions for widespread user adoption (Iacarella et al., 2021).
3.3 Lessons for equitable marine conservation
3.3.1 Partial protection can offer equitable pathways for biodiversity conservation
Given ambitious targets, the diversity of local societal goals and needs, and limited capacity, the ability for the conservation community to deliver on global targets through fully protected MPAs alone is limited. Firstly, a focus on exclusively fully protected MPAs combined with area-based targets for protection will likely lead to prioritization of ‘residual’ sites for MPA establishment—i.e. protecting remote areas that are already at low risk from extractive activities (Devillers et al., 2015; Barnes et al., 2018; Devillers et al., 2020). While in some cases protection of remote sites may be important, given these could face greater risk in the future, their protection results in limited near-term biodiversity gains. Secondly, aligning equity, human well-being, and environmental protection goals is increasingly center stage in conservation (Mace, 2014). This calls into question the appropriateness of fully protected MPAs that have objectives of maintaining or restoring ecosystems to ‘pristine’ condition despite being located in coastal areas with dependent resource users. For MPAs to deliver equitable outcomes they must be well managed with appropriate and inclusive governance structures and regulations for the local context. Therefore, externally imposed, fully protected MPAs will likely be unethical. Thirdly, if implemented, top-down imposed fully protected MPAs are unlikely to generate positive biodiversity outcomes. This is especially in areas where communities are reliant on fisheries or other coastal ecosystem services for human well-being, including food security (Cinner et al., 2012; Klain et al., 2014; Chaigneau et al., 2019). In this context, fully protected MPAs will likely generate social conflict and negative effects on well-being (e.g. Pomeroy et al., 2007; Evans, 2009; Mahajan and Daw, 2016). They would also likely suffer from low compliance (e.g. Campbell et al., 2012), or require substantial resource investment in enforcement to generate any positive biodiversity outcome. Therefore, in addition to being unethical these areas are likely not a cost-effective use of conservation funds. It is therefore important to openly recognize the tradeoffs and tensions that exist between maximizing biodiversity gains while balancing financial realities, issues of equity and food security (e.g. fisheries access), and social cohesion when using MPAs as tools for biodiversity conservation (e.g. Krueck et al., 2019). Fundamentally, MPA protection decisions must be grounded in local context and equity, and decisions on what counts towards protection targets must consider that a sole focus on fully protected areas will devalue the contribution of partially protected areas and risk stalling ocean conservation (Campbell and Gray, 2019).
Partially protected MPAs offer more opportunities for locally relevant tailoring of MPA regulations than exclusively fully protected MPAs. This additional flexibility—especially not fully excluding communities from fishing—can in many cases be perceived as more equitable by stakeholders and generate greater local support (e.g. (Chuenpagdee and Jentoft, 2007; Purwanto et al., 2021). Economic and food security benefits from MPAs often provide tangible outcomes for local stakeholders subjected to MPA restrictions. Furthermore, successful conservation approaches that have community support can rapidly diffuse into adjacent communities (Ehrlich et al., 2012; Gardner et al., 2018; Mills et al., 2019). Therefore, the use of partial protection approaches that include resource users in decision-making may lower the costs of replicating and scaling—potentially leading to overall greater conservation gains. Aligning these considerations with conservation targets can drive progress towards more holistic conservation outcomes that can lead to more sustainable resource governance (Halpern et al., 2013). Therefore, in many contexts, MPAs incorporating partial protection may be better positioned to provide greater return-on-investment benefits for both people and biodiversity than exclusively fully protected MPA approaches (Chuenpagdee and Jentoft, 2007).
More equitable approaches to MPAs are apparent in the increased global recognition of different governance models (Bennett and Dearden, 2014). Equity in protected areas can be thought of in three dimensions: recognition (acknowledged legitimacy of rights/values by stakeholders), procedure (inclusive/effective participation of stakeholders), and distribution (sharing of costs/benefits of management between stakeholders) (Schreckenberg et al., 2016; Zafra-Calvo et al., 2017). All three components must be integrated for equitable MPA establishment and management. By identifying and including different stakeholder groups in finding equitable solutions, it is possible to achieve biodiversity outcomes that minimize disproportionate impacts on particular groups (Gurney et al., 2015). Over the last few decades, momentum has been growing behind building institutional structures that facilitate the co-management of marine resources between government and local communities and/or direct governance by local communities, ensuring local voices can shape MPA management (Clifton, 2003; Schultz et al., 2011). Devolving rights and decision-making authority to resource users through different governance models does not necessarily lead to weaker biodiversity protection, and can lead to more equitable outcomes (Leisher et al., 2007; del Pilar Moreno-Sánchez and Maldonado, 2010; Bennett and Dearden, 2014; Stafford, 2018).
3.3.2 Partial protection approaches can generate biodiversity benefits
Defining fishing regulations within partially protected areas to lead to sustainable fisheries and biodiversity outcomes can be achieved through regulation choice—including fishing gears, appropriate zoning structure, and employing evidence-informed single-species and threshold-based management. Reducing the impact of fishing gears can improve fisheries sustainability and biodiversity outcomes within partially protected areas (Crane et al., 2017a). Different fishing gears have widely variable ecosystem impacts, resulting in variation in their sustainability and the recovery time of ecosystems following use (Horta e Costa et al., 2016; Mbaru et al., 2020). For gears used by small-scale fisheries, destructive fishing practices—such as blast and cyanide fishing—cause damage lasting many decades by destroying reef habitats and killing non-target benthic species (Fox et al., 2019). In contrast, hand line fisheries can have much lower impacts on reef habitat and non-target reef species (Campbell et al., 2018; Mbaru et al., 2020). Restricting fishing within partially protected MPAs to regulated hook-and-line can lead to increased fish biomass (Campbell et al., 2018). In theory, a focus on reducing, and diversifying—but not eradicating—fishing gears and pressure within existing MPAs can result in greater overall biodiversity gains than expanding no-take areas under some contexts (Hopf et al., 2016). Thus, careful planning around gear types allowed, followed by monitoring and evaluation for adaptive management of gear impacts, can be used to balance the trade-off between fisheries and biodiversity outcomes when using partial protection approaches.
Appropriately defined and recognized boundaries are key for partially protected areas to provide both biodiversity and sustainable fisheries benefits. Spatial zonation within MPAs allows the implementation of different regulations in different parts of the MPA. This could include smaller no-take areas as part of a suite of management over a larger area—such as a zoned mixed-use MPA that allows extractive resource use within some areas. Alternatively similar effects could be achieved through a network of MPAs that includes both partial protection and full protection. The effectiveness of partially protected areas can be enhanced by the presence of adjacent fully protected areas (Zupan et al., 2018), and many of the community-governed case study MPAs that reported biodiversity benefits did include permanent no-take areas (Table 1). In other cases, temporal protection approaches can allow some forms of rotational extractive use across the whole MPA improving fisheries sustainability (Carvalho et al., 2019). When combined with species-specific protections for vulnerable species, these temporal protections can also provide longer-term biodiversity protection (Goetze et al., 2016; Carvalho et al., 2019). In all these cases, having clearly defined and recognized internal boundaries is essential for the MPAs to function.
Intuitively, employing evidence-informed single-species and threshold based management can also help partially protected areas to function more effectively. It is important to clearly identify the specific biodiversity objectives of partially protected areas if continued fisheries access is desired. For example, MPAs can focus on protecting key vulnerable species, or rebuilding and maintaining ecological functions or habitats. Ecosystem function approaches can use tools such as biomass thresholds to identify MPA objectives based on maintaining or enhancing fish biomass to certain levels (McClanahan et al., 2011; Karr et al., 2015). These thresholds can be highly variable by species (Brown and Mumby, 2014). For example, maintaining herbivorous reef fish biomass at 50% of the level expected in the absence of fishing retains over 80% of many herbivory functions (MacNeil et al., 2015). Herbivore biomass can be maintained at this level by partial protection approaches—such as bans on specific gears or species catch restrictions (MacNeil et al., 2015). While this results in lower fish biomass than in the absence of fishing, it allows fisheries to continue while still maintaining ecosystem functions. Threshold-based management requires MPA managers to conduct monitoring, evaluation, and learning activities to track fish biomass levels within their MPAs—ideally comparing fully protected, partially protected, and control areas without protection. This information can then be used to adaptively manage MPA-specific fisheries regulations to ensure biomass is maintained above such thresholds.
3.3.3 Looking forward
Our case studies provide diverse examples demonstrating that partial protection approaches within MPAs also have the potential to deliver on biodiversity outcomes while supporting social-ecological resilience when they are well-designed and well-managed. Target 3 of the newly adopted Kunming-Montreal Global Biodiversity Framework calls for 30% of coastal and marine areas to be effectively conserved and managed by 2030 (CBD, 2022). As this global target is translated into new national targets and action plans we encourage countries to consider a blend of locally appropriate protection levels – from fully protected areas to partially protected MPAs – to achieve positive biodiversity outcomes. Fully protected areas remain an important tool for biodiversity protection, and should be implemented where appropriate, either as exclusively fully protected MPAs or as zones within MPAs with differing protection levels. However, because partial protection provides more opportunities to incorporate local access and resource use, it often results in more equitable and effective conservation approaches compared to exclusively fully protected areas (Fidler et al., 2022). We recommend further research into the optimal proportion of fully verses partially protected areas and their appropriate governance models to generate biodiversity outcomes without compromising access to resources, equability, food security, and local rights. A push for exclusively fully protected MPAs as global protection targets are implemented could risk increasing marine resource conflict and undermine social-ecological systems (e.g. Schleicher et al., 2019). We therefore recommend consideration of the full spectrum of MPAs that deliver positive biodiversity outcomes moving forward.
Adoption and recognition of partial protection approaches increases the diversity of regulations available to MPA managers. This helps MPAs become more locally tailored, and thus provides more pathways to achieve equitable governance, effective implementation and therefore build more resilient social-ecological systems. Our regulation classification can help MPA managers consider and design locally relevant MPA regulations, support evaluation of existing MPA regulations, as well as future research efforts on MPA effectiveness. MPAs that embrace contributions from partial protection alongside fully protected zones are therefore a valuable complementary approach to fully protected MPAs. Or, partially protected MPAs may be an important complement to fully protected MPAs within an MPA network. Our in-depth review of partially protected MPA restrictions demonstrates that a diversity of approaches can lead to positive biodiversity outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DA-B, E, HF, DG, NK, and GA contributed to conception and initiation of the study. DAB, LV, Am, NC, Es, HF, DG, JG, NC, SM, MT, and GA participated in the study design workshop. DA-B, LV, and DG analyzed the MPA regulations and organized the dataset. DA-B performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to the submitted article and approved the submitted version.
Acknowledgments
We thank the organizers of the 5th International Marine Conservation Congress in Kuching, Sarawak where a workshop that supported this study was held.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1099579/full#supplementary-material
References
Agardy T., Bridgewater P., Crosby M. P., Day J., Dayton P. K., Kenchington R., et al. (2003). Dangerous targets? unresolved issues and ideological clashes around marine protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 13, 353–367. doi: 10.1002/aqc.583
Agardy T., Claudet J., Day J. C. (2016). ‘Dangerous targets’ revisited: Old dangers in new contexts plague marine protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 7–23. doi: 10.1002/aqc.2675
Amkieltiela, Handayani C. N., Andradi-Brown D. A., Estradivari, Ford A. K., Beger M., et al. (2022). The rapid expansion of Indonesia’s marine protected area requires improvement in management effectiveness. Mar. Policy 146, 105257. doi: 10.1016/j.marpol.2022.105257
Andriamalala G., Gardner C. J. (2010). The use of the dina as a tool for natural resource governance: lessons learned from velondriake, southwestern Madagascar. Trop. Conserv. Sci. 3, 447–472. doi: 10.1177/194008291000300409
Aswani S., Albert S., Love M. (2017). One size does not fit all: Critical insights for effective community-based resource management in Melanesia. Mar. Policy 81, 381–391. doi: 10.1016/j.marpol.2017.03.041
Barnes M. D., Glew L., Wyborn C., Craigie I. D. (2018). Preventing perverse outcomes from global protected area policy. Nature Ecol. Evol.2, 759–762. doi: 10.1038/s41559-018-0501-y
Barrett L. T., de Lima A., Goetze J. S. (2018). Evidence of a biomass hotspot for targeted fish species within Namena Marine Reserve, Fiji. Pac. Conserv. Biol. 25, 204. doi: 10.1071/PC18034
Benbow S., Humber F., Oliver T., Oleson K., Raberinary D., Nadon M., et al. (2014). Lessons learnt from experimental temporary octopus fishing closures in south-west Madagascar: benefits of concurrent closures. Afr. J. Mar. Sci. 36, 31–37. doi: 10.2989/1814232X.2014.893256
Bennett N. J., Dearden P. (2014). From measuring outcomes to providing inputs: Governance, management, and local development for more effective marine protected areas. Mar. Policy 50, 96–110. doi: 10.1016/j.marpol.2014.05.005
Bohorquez J. J., Dvarskas A., Pikitch E. K. (2019). Categorizing global MPAs: A cluster analysis approach. Mar. Policy 108, 103663. doi: 10.1016/j.marpol.2019.103663
Boon P. Y., Mulligan B., Benbow S. L. P., Thorne B. V., Leng P., Longhurst K. (2014). Zoning cambodia’s first marine fisheries management area. Cambodian J. Nat. Hist. 2014, 55–65.
Borrini-Feyerabend G., Dudley N., Jaeger T., Lassen B., Pathak Broome N., Phillips A., et al. (2013). Governance of protected areas: from understanding to action. (No. 20), best practice protected area guidelines (Gland, Switzerland: IUCN) xvi + 124pp.
Brown C. J., Mumby P. J. (2014). Trade-offs between fisheries and the conservation of ecosystem function are defined by management strategy. Front. Ecol. Environ. 12, 324–329. doi: 10.1890/130296
Campbell S. J., Edgar G. J., Stuart-Smith R. D., Soler G., Bates A. E. (2018). Fishing-gear restrictions and biomass gains for coral reef fishes in marine protected areas. Conserv. Biol. 32, 401–410. doi: 10.1111/cobi.12996
Campbell L. M., Gray N. J. (2019). Area expansion versus effective and equitable management in international marine protected areas goals and targets. Mar. Policy 100, 192–199. doi: 10.1016/j.marpol.2018.11.030
Campbell S. J., Hoey A. S., Maynard J., Kartawijaya T., Cinner J., Graham N. A. J., et al. (2012). Weak compliance undermines the success of no-take zones in a large government-controlled marine protected area. PloS One 7, e50074. doi: 10.1371/journal.pone.0050074
Capitini C. A., Tissot B. N., Carroll M. S., Walsh W. J., Peck S. (2004). Competing perspectives in resource protection: The case of marine protected areas in West Hawai’i. Soc Nat. Resour. 17, 763–778. doi: 10.1080/08941920490493747
Carvalho P. G., Jupiter S. D., Januchowski-Hartley F. A., Goetze J., Claudet J., Weeks R., et al. (2019). Optimized fishing through periodically harvested closures. J. Appl. Ecol. 56, 1927–1936. doi: 10.1111/1365-2664.13417
Cayman Islands Department of Environment (2016). Rules for Cayman Islands Protected Areas (National Conservation Laws & Regulations).
CBD (2022). Kunming-Montreal Global Biodiversity Framework. Convention on Biological Diversity (UN Environment Programme).
Chaigneau T., Brown K., Coulthard S., Daw T. M., Szaboova L. (2019). Money, use and experience: Identifying the mechanisms through which ecosystem services contribute to wellbeing in coastal Kenya and Mozambique. Ecosyst. Serv. 38, 100957. doi: 10.1016/j.ecoser.2019.100957
Chuenpagdee R., Jentoft S. (2007). Step zero for fisheries co-management: What precedes implementation. Mar. Policy 31, 657–668. doi: 10.1016/j.marpol.2007.03.013
Cinner J. E., Huchery C., MacNeil M. A., Graham N. A. J., McClanahan T. R., Maina J., et al. (2016). Bright spots among the world’s coral reefs. Nature 535, 416–419. doi: 10.1038/nature18607
Cinner J. E., Maire E., Huchery C., MacNeil M. A., Graham N. A. J., Mora C., et al. (2018). Gravity of human impacts mediates coral reef conservation gains. Proc. Natl. Acad. Sci. 115, E6116–E6125. doi: 10.1073/pnas.1708001115
Cinner J. E., McClanahan T. R., MacNeil M. A., Graham N. A. J., Daw T. M., Mukminin A., et al. (2012). Comanagement of coral reef social-ecological systems. Proc. Natl. Acad. Sci. 109, 5219–5222. doi: 10.1073/pnas.1121215109
Clifton J. (2003). Prospects for co-management in Indonesia’s marine protected areas. Mar. Policy 27, 389–395. doi: 10.1016/S0308-597X(03)00026-5
Clifton J. (2013). Refocusing conservation through a cultural lens: Improving governance in the Wakatobi National Park, Indonesia. Mar. Policy 41, 80–86. doi: 10.1016/j.marpol.2012.12.015
Costello M. J., Ballantine B. (2015). Biodiversity conservation should focus on no-take marine reserves. Trends Ecol. Evol. 30, 507–509. doi: 10.1016/j.tree.2015.06.011
Costello C., Ovando D., Clavelle T., Strauss C. K., Hilborn R., Melnychuk M. C., et al. (2016). Global fishery prospects under contrasting management regimes. Proc. Natl. Acad. Sci. 113, 5125–5129. doi: 10.1073/pnas.1520420113
Cox C., Valdivia A., McField M., Castillo K., Bruno J. (2017). Establishment of marine protected areas alone does not restore coral reef communities in Belize. Mar. Ecol. Prog. Ser. 563, 65–79. doi: 10.3354/meps11984
Crane N. L., Nelson P., Abelson A., Precoda K., Rulmal J., Bernardi G., et al. (2017b). Atoll-scale patterns in coral reef community structure: Human signatures on Ulithi Atoll, Micronesia. PloS One 12, e0177083. doi: 10.1371/journal.pone.0177083
Crane N. L., Rulmal J. B., Nelson P. A., Paddack M. J., Bernardi G. (2017a). “Collaborating with indigenous citizen scientists towards sustainable coral reef management in a changing world. The One People One Reef program,” in Citizen science for coastal and marine conservation, earthscan oceans (London; New York: Routledge, Taylor & Francis Group).
Davis J. (2012). What counts as a marine protected area (MPA News). Available at: https://octogroup.org/news/what-counts-marine-protected-area.
Dawson N. M., Coolsaet B., Sterling E. J., Loveridge R., Gross-Camp N. D., Wongbusarakum S., et al. (2021). The role of indigenous peoples and local communities in effective and equitable conservation. Ecol. Soc. 26 (3), 19. doi: 10.5751/ES-12625-260319
Day J., Dudley N., Hockings M., Holmes G., Laffoley D., Stolton S., et al. (2012). Guidelines for applying the IUCN protected area management categories to marine protected areas, best practice protected area guidelines (Gland, Switzerland: IUCN, CBD Secretariat, IUCN-WCPA, Protected Planet, UEP-WCMC).
del Pilar Moreno-Sánchez R., Maldonado J. H. (2010). Evaluating the role of co-management in improving governance of marine protected areas: An experimental approach in the Colombian Caribbean. Ecol. Econ. 69, 2557–2567. doi: 10.1016/j.ecolecon.2010.07.032
Department of Fisheries (2001). Plan of management for the Cottesloe Reef Fish Habitat Protection Area (No. 155), Fisheries Management Paper (Department of Fisheries, Western Australia).
Department of Fisheries (2010). Cottesloe Reef Fish Habitat Protection Area. Information Pamphlet. (Department of Fisheries, Western Australia)
Department of Land and Natural Resources (2005). Hawaii Administrative rules title 13, subtitle 4 (Fisheries, part II: Marine fisheries management areas) (No. chapter 58 (Kona Coast, Hawaii)) (Department of Land and Natural Resources).
Department of Land and Natural Resources (2014). Report on the findings and recommendations of effectiveness of the West Hawaii regional fishery management area (Department of Land and Natural Resources).
Devillers R., Pressey R. L., Grech A., Kittinger J. N., Edgar G. J., Ward T., et al. (2015). Reinventing residual reserves in the sea: are we favouring ease of establishment over need for protection? Aquat. Conserv. Mar. Freshw. Ecosyst. 25, 480–504. doi: 10.1002/aqc.2445
Devillers R., Pressey R. L., Ward T. J., Grech A., Kittinger J. N., Edgar G. J., et al. (2020). Residual marine protected areas five years on: Are we still favouring ease of establishment over need for protection? Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 1758–1764. doi: 10.1002/aqc.3374
Dudley N. (2008). Guidelines for applying protected area management categories (Gland, Switzerland: IUCN). doi: 10.2305/IUCN.CH.2008.PAPS.2.en
Edgar G. J., Stuart-Smith R. D., Willis T. J., Kininmonth S., Baker S. C., Banks S., et al. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. doi: 10.1038/nature13022
Ehrlich P. R., Kareiva P. M., Daily G. C. (2012). Securing natural capital and expanding equity to rescale civilization. Nature 486, 68–73. doi: 10.1038/nature11157
Evans L. S. (2009). Understanding divergent perspectives in marine governance in Kenya. Mar. Policy 33, 784–793. doi: 10.1016/j.marpol.2009.02.013
Fairclough D., Keay I., Johnson C., Lai E. (2008). “West Coast demersal scalefish fishery status report,” in State of the fisheries report 2007/08. Eds. Fletcher W. J., Santoro K. (Perth, Western Australia: Department of Fisheries), 68–76.
Fairclough D., Lai E., Bruce C., Moore N., Syers C. (2011). “West Coast demersal scalefish resource status,” in State of the fisheries and aquatic resources report 2010/11. Eds. Fletcher W. J., Santoro K. (Perth, Western Australia: Department of Fisheries), 96–106.
Fidler R. Y., Ahmadia G. N., Amkieltiela A., Cox C., Estradivari, Glew L., et al. (2022). Participation, not penalties: Community involvement and equitable governance contribute to more effective multiuse protected areas. Sci. Adv. 8, eabl8929. doi: 10.1126/sciadv.abl8929
Firmansyah F., Musthofa A., Estradivari, Damora A., Handayani C., Ahmadia G. N., et al. (2016). Satu dekade pengelolaan Taman Nasional Wakatobi: Keberhasilan dan tantangan konservasi laut. (World Wide Fund for Nature, Jakarta, Indonesia) doi: 10.6084/M9.FIGSHARE.6987083.V2
Fisheries Administration (2016). Management plan for the Koh Song Archipelago Marine Fisheries Management Area 2016-2020 (Phnom Penh, Cambodia: Fisheries Administration, Royal Government of Cambodia.).
Foale S., Manele B. (2003). “Privatising fish? barriers to the use of marine protected areas for conservation and fishery management in Melanesia,” in Resource management in Asia-pacific working paper no. 47 (Resource Management in Asia-Pacific Program, Research School of Pacific and Asian Studies, The Australian Naitonal University, Canberra).
Fox H. E., Harris J. L., Darling E. S., Ahmadia G. N., Estradivari, Razak T. B. (2019). Rebuilding coral reefs: success (and failure) 16 years after low-cost, low-tech restoration. Restor. Ecol. Rec 27, 862–869. doi: 10.1111/rec.12935
Gardner C. J., Cripps G., Day L. P., Dewar K., Gough C., Peabody S., et al. (2020). A decade and a half of learning from Madagascar’s first locally managed marine area. Conserv. Sci. Pract. 2, e298. doi: 10.1111/csp2.298
Gardner C. J., Nicoll M. E., Birkinshaw C., Harris A., Lewis R. E., Rakotomalala D., et al. (2018). The rapid expansion of madagascar’s protected area system. Biol. Conserv. 220, 29–36. doi: 10.1016/j.biocon.2018.02.011
Garnett S. T., Burgess N. D., Fa J. E., Fernández-Llamazares Á., Molnár Z., Robinson C. J., et al. (2018). A spatial overview of the global importance of indigenous lands for conservation. Nat. Sustain 1, 369–374. doi: 10.1038/s41893-018-0100-6
Gilchrist H., Rocliffe S., Anderson L. G., Gough C. L. A. (2020). Reef fish biomass recovery within community-managed no take zones. Ocean Coast. Manage. 192, 105210. doi: 10.1016/j.ocecoaman.2020.105210
Gill D. A., Mascia M. B., Ahmadia G. N., Glew L., Lester S. E., Barnes M., et al. (2017). Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543, 665–669. doi: 10.1038/nature21708
Glue M., Teoh M. (2020). Koh Rong Marine National Park: Coral reef status report (Phnom Penh, Cambodia: Fauna & Flora International).
Glue M., Teoh M., Duffy H. (2020). Community-led management lays the foundation for coral reef recovery in Cambodian marine protected areas. Oryx 54, 599–599. doi: 10.1017/S0030605320000587
Goetze J. S., Claudet J., Januchowski-Hartley F., Langlois T. J., Wilson S. K., White C., et al. (2018). Demonstrating multiple benefits from periodically harvested fisheries closures. J. Appl. Ecol. 55, 1102–1113. doi: 10.1111/1365-2664.13047
Goetze J., Langlois T., Claudet J., Januchowski-Hartley F., Jupiter S. D. (2016). Periodically harvested closures require full protection of vulnerable species and longer closure periods. Biol. Conserv. 203, 67–74. doi: 10.1016/j.biocon.2016.08.038
Government of Egypt, Ministry of State for Environmental Affairs, and Egyptian Environmental Affairs Agency; Egypt Environmental Policy Program (2004). Management plan for Wadi el Gemal National Park.
Government of Spain, Ministry of Agriculture, Fisheries, and Food (2017). Marine reserves in Spain: Levante de Mallorca - Cala Rajada.
Government of Western Australia Department of Fisheries (2015). The Abrolhos Islands information guide.
Grorud-Colvert K., Constant V., Sullivan-Stack J., Dziedzic K., Hamilton S. L., Randell Z., et al. (2019). High-profile international commitments for ocean protection: Empty promises or meaningful progress? Mar. Policy 105, 52–66. doi: 10.1016/j.marpol.2019.04.003
Grorud-Colvert K., Sullivan-Stack J., Roberts C., Constant V., Horta e Costa B., Pike E. P., et al. (2021). The MPA guide: A framework to achieve global goals for the ocean. Science 373, eabf0861. doi: 10.1126/science.abf0861
Gurney G. G., Pressey R. L., Ban N. C., Álvarez-Romero J. G., Jupiter S., Adams V. M. (2015). Efficient and equitable design of marine protected areas in Fiji through inclusion of stakeholder-specific objectives in conservation planning: Social factors in conservation planning. Conserv. Biol. 29, 1378–1389. doi: 10.1111/cobi.12514
Halim A., Wiryawan B., Loneragan N. R., Hordyk A., Sondita M. F. A., White A. T., et al. (2019). Developing a functional definition of small-scale fisheries in support of marine capture fisheries management in Indonesia. Mar. Policy 100, 238–248. doi: 10.1016/j.marpol.2018.11.044
Hall S. J., Mainprize B. (2004). Towards ecosystem-based fisheries management. Fish Fish. 5, 1–20. doi: 10.1111/j.1467-2960.2004.00133.x
Halpern B. S., Klein C. J., Brown C. J., Beger M., Grantham H. S., Mangubhai S., et al. (2013). Achieving the triple bottom line in the face of inherent trade-offs among social equity, economic return, and conservation. Proc. Natl. Acad. Sci. 110, 6229–6234. doi: 10.1073/pnas.1217689110
Harris A. (2007). “To live with the sea” development of the velondriake community - managed protected area network, southwest Madagascar. Madag. Conserv. Dev. 2, 43–49. doi: 10.4314/mcd.v2i1.44129
Hopf J. K., Jones G. P., Williamson D. H., Connolly S. R. (2016). Synergistic effects of marine reserves and harvest controls on the abundance and catch dynamics of a coral reef fishery. Curr. Biol. 26, 1543–1548. doi: 10.1016/j.cub.2016.04.022
Horta e Costa B., Claudet J., Franco G., Erzini K., Caro A., Gonçalves E. J. (2016). A regulation-based classification system for marine protected areas (MPAs). Mar. Policy 72, 192–198. doi: 10.1016/j.marpol.2016.06.021
Hoshino E., van Putten E. I., Girsang W., Resosudarmo B. P., Yamazaki S. (2017). Fishers’ perceived objectives of community-based coastal resource management in the kei islands, Indonesia. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00141
Iacarella J. C., Clyde G., Bergseth B. J., Ban N. C. (2021). A synthesis of the prevalence and drivers of non-compliance in marine protected areas. Biol. Conserv. 255, 108992. doi: 10.1016/j.biocon.2021.108992
Jack-Kadioglu T., Pusparini N. K. S., Lazuardi M. E., Estradivari, Rukma A., Campbell S. J., et al. (2020). “Community involvement in marine protected area governance,” in Kementerian Kelautan dan Perikanan (Jakarta, Indonesia: Management of Marine Protected Areas in Indonesia: Status and Challenges. Kementerian Kelautan dan Perikanan and Yayasan WWF Indonesia). doi: 10.6084/m9.figshare.13341476.v1
Jupiter S. D., Cohen P. J., Weeks R., Tawake A., Govan H. (2014). Locally-managed marine areas: multiple objectives and diverse strategies. Pac. Conserv. Biol. 20, 165. doi: 10.1071/PC140165
Jupiter S. D., Egli D. P. (2010). Ecosystem-based management in Fiji: Successes and challenges after five years of implementation. J. Mar. Biol. 2011, 1–14. doi: 10.1155/2011/940765
Karr K. A., Fujita R., Halpern B. S., Kappel C. V., Crowder L., Selkoe K. A., et al. (2015). Thresholds in Caribbean coral reefs: implications for ecosystem-based fishery management. J. Appl. Ecol. 52, 402–412. doi: 10.1111/1365-2664.12388
Klain S. C., Beveridge R., Bennett N. J. (2014). Ecologically sustainable but unjust? negotiating equity and authority in common-pool marine resource management. Ecol. Soc. 19, art52. doi: 10.5751/ES-07123-190452
Krueck N. C., Abdurrahim A. Y., Adhuri D. S., Mumby P. J., Ross H. (2019). Quantitative decision support tools facilitate social-ecological alignment in community-based marine protected area design. Ecol. Soc. 24, art6. doi: 10.5751/ES-11209-240406
Leisher C., van Beukering P., Scherl L. M. (2007). Nature’s investment bank: how marine protected areas contributed to poverty reduction (Carlton, Victoria, Australia: The Nature Conservancy, Arlington, VA, USA).
Lester S. E., Halpern B. S. (2008). Biological response in marine no-take reserves versus partially protected areas. Mar. Ecol. Prog. Ser. 367, 49–56. doi: 10.3354/meps07599
Liligeto W. (2011). Gizmo Environment Livelihood Conservation Association (GELCA) resource management plan (Jakarta, Indonesia: Coral Triangle Initiative on Coral Reefs, Fisheries and Food Security).
Loper C., Pomeroy R., Hoon V., McConney P., Pena M., Sanders A., et al. (2008). Socioeconomic conditions along the world’s tropical coast (Townsville, Australia: NOAA, Global Coral Reef Monitoring Network, Conservation Internationa).
MacNeil M. A., Graham N. A. J., Cinner J. E., Wilson S. K., Williams I. D., Maina J., et al. (2015). Recovery potential of the world’s coral reef fishes. Nature 520, 341–344. doi: 10.1038/nature14358
Mahajan S. L., Daw T. (2016). Perceptions of ecosystem services and benefits to human well-being from community-based marine protected areas in Kenya. Mar. Policy 74, 108–119. doi: 10.1016/j.marpol.2016.09.005
Mbaru E. K., Graham N. A. J., McClanahan T. R., Cinner J. E. (2020). Functional traits illuminate the selective impacts of different fishing gears on coral reefs. J. Appl. Ecol. 57, 241–252. doi: 10.1111/1365-2664.13547
McClanahan T. R., Graham N. A. J., MacNeil M. A., Muthiga N. A., Cinner J. E., Bruggemann J. H., et al. (2011). Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc. Natl. Acad. Sci. 108, 17230–17233. doi: 10.1073/pnas.1106861108
McClanahan T. R., Marnane M. J., Cinner J. E., Kiene W. E. (2006). A comparison of marine protected areas and alternative approaches to coral-reef management. Curr. Biol. 16, 1408–1413. doi: 10.1016/j.cub.2006.05.062
Mexico National Commission of Natural Potected Areas (2015). Contoy Island National Park Management Program.
Mills M., Bode M., Mascia M. B., Weeks R., Gelcich S., Dudley N., et al. (2019). How conservation initiatives go to scale. Nat. Sustain. 2, 935–940. doi: 10.1038/s41893-019-0384-1
Mitchell B. A., Walker Z., Walker P. (2017). A governance spectrum: Protected areas in Belize. PARKS 23, 45–60. doi: 10.2305/IUCN.CH.2017.PARKS-23-1BAM.en
Mizrahi M., Ouk V., West K. (2016). Management plan for the koh rong archipelago marine fisheries management area 2015-2019 (Fisheries Administration (FiA) & Fauna & Flora International (FFI) (Cambodia: Phnom Penh).
Mora C., Myers R. A., Coll M., Libralato S., Pitcher T. J., Sumaila R. U., et al. (2009). Management effectiveness of the world’s marine fisheries. PloS Biol. 7, e1000131. doi: 10.1371/journal.pbio.1000131
Muawanah U., Habibi A., Lazuardi M. E., Yusuf M., Andradi-Brown D., Krueck N. C., et al. (2020). “Fisheries and marine protected areas,” in Management of marine protected areas in Indonesia: Status and challenges (Jakarta, Indonesia: WWF-Indonesia). doi: 10.6084/m9.figshare.13341476.v1
Mulligan B., Longhurst K. (2014). Research and recommendations for a proposed marine fisheries management area in the Koh Rong Archipelago (Surrey, UK: Fauna & Flora International Cambodia Programme, Coral Cay Conservation, Phnom Penh, Cambodia).
National Institute of Ecology, Mexico Secretary of Environment, Natural Resources and Fishing (2000). Management plan of Arrecife de Puerto Morelos National Park, Mexico.
Oksanen J., Blanchet G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2020). Community ecology package (R package).
Pendleton L. H., Ahmadia G. N., Browman H. I., Thurstan R. H., Kaplan D. M., Bartolino V. (2018). Debating the effectiveness of marine protected areas. ICES J. Mar. Sci. 75, 1156–1159. doi: 10.1093/icesjms/fsx154
Pomeroy R. S., Mascia M. B., Pollnac R. B. (2007) Marine protected areas: The social dimension (FAO Epert Workshop on Marine Protected Areas and Fisheries Management: Review of Issues and Considerations, FAO Fisheries Report No. 825. Rome).
Preah Sihanouk Provincial Hall (2014). The creation of technical working group for the Koh Rong Archipelago Marine Fisheries Management Area of Preah Sihanouk province (Preah Sihanouk, Cambodia: Preah Sihanouk Provincial Hall).
Purwanto, Andradi-Brown D. A., Matualage D., Rumengan I., Awaludinnoer, Pada D., et al. (2021). The Bird’s Head Seascape Marine Protected Area Network–preventing biodiversity and ecosystem service loss amidst rapid change in Papua, Indonesia. Conserv. Sci. Pract. 3. doi: 10.1111/csp2.393
R Core Team (2020). R: A language and environment for statistical computing (R Foundation for Statistical Computing).
Roig-Boixeda P., Chea P., Brozovic R., You R., Neung S., San T., et al. (2018). Using patrol records and local perceptions to inform management and enforcement in a marine protected area in Cambodia. Cambodian J. Nat. Hist. 2018, 9–23.
Rossiter J. S., Levine A. (2014). What makes a “successful” marine protected area? the unique context of Hawaii′s Fish Replenishment Areas. Mar. Policy 44, 196–203. doi: 10.1016/j.marpol.2013.08.022
Sala E., Giakoumi S. (2018). No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 75, 1166–1168. doi: 10.1093/icesjms/fsx059
Sala E., Lubchenco J., Grorud-Colvert K., Novelli C., Roberts C., Sumaila U. R. (2018). Assessing real progress towards effective ocean protection. Mar. Policy 91, 11–13. doi: 10.1016/j.marpol.2018.02.004
Sandbrook C., Scales I. R., Vira B., Adams W. M. (2011). Value plurality among conservation professionals: Value plurality in conservation. Conserv. Biol. 25, 285–294. doi: 10.1111/j.1523-1739.2010.01592.x
Schleicher J., Zaehringer J. G., Fastré C., Vira B., Visconti P., Sandbrook C. (2019). Protecting half of the planet could directly affect over one billion people. Nat. Sustain. 2, 1094–1096. doi: 10.1038/s41893-019-0423-y
Schreckenberg K., Franks P., Martin A., Lang B. (2016). Unpacking equity for protected area conservation. PARKS 22, 11–28. doi: 10.2305/IUCN.CH.2016.PARKS-22-2KS.en
Schultz L., Duit A., Folke C. (2011). Participation, adaptive co-management, and management performance in the world network of biosphere reserves. World Dev. 39, 662–671. doi: 10.1016/j.worlddev.2010.09.014
Sciberras M., Jenkins S. R., Kaiser M. J., Hawkins S. J., Pullin A. S. (2013). Evaluating the biological effectiveness of fully and partially protected marine areas. Environ. Evid. 2, 4. doi: 10.1186/2047-2382-2-4
Sedberry G. R., Carter H. J., Barrick P. A. (1999). A comparison of fish communities between protected and unprotected areas of the Belize reef ecosystem: implications for conservation and management, in: Gulf and Caribbean Fisheries Institute proceedings. Presented at Proc. Gulf Caribbean Fisheries Institute 45, 95–127.
Selig E. R., Kleisner K. M., Ahoobim O., Arocha F., Cruz-Trinidad A., Fujita R., et al. (2017). A typology of fisheries management tools: using experience to catalyse greater success. Fish Fish. 18, 543–570. doi: 10.1111/faf.12192
Sen S., Raakjaer Nielsen J. (1996). Fisheries co-management: a comparative analysis. Mar. Policy 20, 405–418. doi: 10.1016/0308-597X(96)00028-0
Sloan J., Chand K. (2016). An analysis of property rights in the Fijian qoliqoli. Mar. Policy 72, 76–81. doi: 10.1016/j.marpol.2016.06.019
South Australia Department of Environment, Water, and Natural Resources (2012). Encounter marine park management plan summary.
Stafford R. (2018). Lack of evidence that governance structures provide real ecological benefits in marine protected areas. Ocean Coast. Manage. 152, 57–61. doi: 10.1016/j.ocecoaman.2017.11.013
Stevenson T. C., Tissot B. N. (2013). Evaluating marine protected areas for managing marine resource conflict in Hawaii. Mar. Policy 39, 215–223. doi: 10.1016/j.marpol.2012.11.003
Stevenson T. C., Tissot B. N., Walsh W. J. (2013). Socioeconomic consequences of fishing displacement from marine protected areas in Hawaii. Biol. Conserv. 160, 50–58. doi: 10.1016/j.biocon.2012.11.031
Tallis H., Lubchenco J. (2014). Working together: A call for inclusive conservation. Nature 515, 27–28. doi: 10.1038/515027a
Tam C. L. (2019). Branding Wakatobi: marine development and legitimation by science. Ecol. Soc. 24, art23. doi: 10.5751/ES-11095-240323
Technical Implementing Unit of the Raja Ampat Archipelago Waters Conservation Area (KKP) (2016). MPA profile: Misool MPA. Available at: https://rajaampatmarinepark.com/misool-islands-mpa/.
Teh L. S., Cheung W. W., Christensen V., Sumaila U. (2017). Can we meet the target? status and future trends for fisheries sustainability. Curr. Opin. Environ. Sustain. 29, 118–130. doi: 10.1016/j.cosust.2018.02.006
Thorne B. V., Mulligan B., Mag Aoidh R., Longhurst K. (2015). Current status of coral reef health around the koh rong archipelago. Cambodian J. Nat. Hist 2015, 98–113.
Tissot B. N., Hallacher L. E. (2003). Effects of aquarium collectors on coral reef fishes in Kona, Hawaii. Conserv. Biol. 17, 1759–1768. doi: 10.1111/j.1523-1739.2003.00379.x
Toonen R. J., Wilhelm T., Aulani, Maxwell S. M., Wagner D., Bowen B. W., et al. (2013). One size does not fit all: The emerging frontier in large-scale marine conservation. Mar. pollut. Bull. 77, 7–10. doi: 10.1016/j.marpolbul.2013.10.039
Toropova C., Meliane I., Laffoley D., Matthews E., Spalding M. (2010). Global ocean protection present status and future possibilities (Gland, Switzerland: International Union for Conservation of Nature and Natural Resources).
Tranter S. N., Estradivari, Ahmadia G. N., Andradi-Brown D. A., Muenzel D., Agung F., et al. (2022). The inclusion of fisheries and tourism in marine protected areas to support conservation in Indonesia. Mar. Policy 146, 105301. doi: 10.1016/j.marpol.2022.105301
UNEP-WCMC and IUCN (2021). Protected planet report 2020 (Gland, Switzerland: UNEP-WCMC and IUCN, Cambridge UK).
United National Environment Program (2014). Annotated format for presentation reports for Jaragua National Park (Dominican Republic: UNEP).
United Republic of Tanzania Ministry of Natural Resources and Tourism; Board of Trustees for Marine Parks and Reserves, Tanzania (2005). General management plan (Mnazi Bay Ruvuma Estuary Marine Park).
U.S. National Park Service (2017). Virgin Islands fishing information (Virgin Islands National Park: U.S. National Park Service).
von Heland F., Clifton J. (2015). Whose threat counts? conservation narratives in the Wakatobi National Park, Indonesia. Conserv. Soc 13, 154. doi: 10.4103/0972-4923.164194
WCS (2009). Ecosystem-based management plan: Kubulau District, Vanua Levu, Fiji (Suava, Fiji: Wildlife Conservation Society).
Weeks R., Jupiter S. D. (2013). Adaptive comanagement of a marine protected area network in Fiji. Conserv. Biol. 27, 1234–1244. doi: 10.1111/cobi.12153
West K., Teoh M. (2016). Cambodia’s first large-scale marine protected area declared in the Koh Ring Archipelago. Cambodian J. Nat. Hist. 2016, 82–83.
White A. T., Aliño P. M., Cros A., Fatan N. A., Green A. L., Teoh S. J., et al. (2014). Marine protected areas in the Coral Triangle: Progress, issues, and options. Coast. Manage. 42, 87–106. doi: 10.1080/08920753.2014.878177
Williams I. D., Walsh W. J., Claisse J. T., Tissot B. N., Stamoulis K. A. (2009). Impacts of a Hawaiian marine protected area network on the abundance and fishery sustainability of the yellow tang, Zebrasoma Flavescens. Biol. Conserv. 142, 1066–1073. doi: 10.1016/j.biocon.2008.12.029
Worm B., Branch T. A. (2012). The future of fish. Trends Ecol. Evol. 27, 594–599. doi: 10.1016/j.tree.2012.07.005
Zafra-Calvo N., Pascual U., Brockington D., Coolsaet B., Cortes-Vazquez J. A., Gross-Camp N., et al. (2017). Towards an indicator system to assess equitable management in protected areas. Biol. Conserv. 211, 134–141. doi: 10.1016/j.biocon.2017.05.014
Keywords: marine protected area (MPA), partial protection, fisheries regulation, marine management, biodiversity targets, MPA
Citation: Andradi-Brown DA, Veverka L, Amkieltiela, Crane NL, Estradivari, Fox HE, Gill D, Goetze J, Gough C, Krueck NC, Lester SE, Mahajan SL, Rulmal J Jr., Teoh M and Ahmadia GN (2023) Diversity in marine protected area regulations: Protection approaches for locally appropriate marine management. Front. Mar. Sci. 10:1099579. doi: 10.3389/fmars.2023.1099579
Received: 15 November 2022; Accepted: 19 January 2023;
Published: 01 February 2023.
Edited by:
Catarina Frazão Santos, University of Lisbon, PortugalReviewed by:
Stuart James Kininmonth, The University of Queensland, AustraliaAlfonso Aguilar-Perera, Universidad Autónoma de Yucatán, Mexico
Copyright © 2023 Andradi-Brown, Veverka, Amkieltiela, Crane, Estradivari, Fox, Gill, Goetze, Gough, Krueck, Lester, Mahajan, Rulmal, Teoh and Ahmadia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominic A. Andradi-Brown, ZG9taW5pYy5hbmRyYWRpLWJyb3duQHd3ZnVzLm9yZw==
 Dominic A. Andradi-Brown
Dominic A. Andradi-Brown Laura Veverka1
Laura Veverka1 Amkieltiela
Amkieltiela Nicole L. Crane
Nicole L. Crane Estradivari
Estradivari David Gill
David Gill Jordan Goetze
Jordan Goetze Charlotte Gough
Charlotte Gough Sarah E. Lester
Sarah E. Lester Shauna L. Mahajan
Shauna L. Mahajan Gabby N. Ahmadia
Gabby N. Ahmadia