- 1Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture, Fisheries College of Guangdong Ocean University, Zhanjiang, China
- 2Key Laboratory of Control for Diseases of Aquatic Economic Animals of Guangdong Higher Education Institutes, Zhanjiang, China
Vibrio alginolyticus is the main pathogen causing vibriosis in pearl gentian grouper, which has caused significant financial losses to farmers. To develop a sustainable and effective subunit vaccine for the prevention and control of vibriosis, Lrp recombinant protein from V. alginolyticus was expressed and purified in this study. Western Blotting and ELISA demonstrated that Lrp recombinant protein with relatively higher antigenicity in V. alginolyticus can be used as an antigen for the subunit vaccine. Chitosan oligosaccharide (COS) is a very potential aquatic vaccine adjuvant to boost the immunological protection of the vaccine. Therefore, to evaluate the immune response and protection of the subunit vaccine against V. alginolyticus in pearl gentian grouper, we designed the Lrp group and Lrp+COS group as experimental groups with PBS as the control group. Immunological testing revealed that grouper serum from the experimental group had significantly higher levels of the particular antibody IgM, lysozyme (LZM), catalase (CAT), and superoxide dismutase (SOD) than serum from the control group. Additionally, groupers from the experimental group showed higher immune gene expression levels, namely IgM, CD8α, MHC-Iα, IL-1β, IL-16, and TNF-α. After the challenge experiment, the immune protection rates of the Lrp group and Lrp+COS group were respectively enhanced to 60% and 72%. The aforementioned findings demonstrated that the Lrp+COS group’s immunological impact was superior to that of the Lrp group. Therefore, the Lrp+COS subunit vaccine is a promising candidate for the prevention and management of vibrio infection in pearl grouper.
1 Introduction
In China, pearl gentian groupers are a significant marine fish for commerce. It is a hybrid by Epinephelus fuscoguttatus (♀) and Epinephelus lanceolatus (♂), with the head of Epinephelus lanceolatus and the tail of Epinephelus fuscoguttatus, with the advantages of strong disease resistance and fast growth (Chen Y. et al., 2019). It is frequently used in the culinary and ornamental fish industries due to its flavor and decorative value. In recent years, vibriosis has caused huge economic losses to the cultivation of pearl gentian grouper (Wei et al., 2020b). One of the most typical vibriosis pathogens is V. alginolyticus (Supansa et al., 2020). Its pathogenicity is regulated by a variety of virulence factors, which make it can rapidly and efficiently infect the host (Sadat et al., 2020). V. alginolyticus causes outbreaks of vibriosis and high mortality by invading the intestine, gills and epidermis wound of fish (Cai et al., 2019; Ji et al., 2020).
Leucine responsive regulatory protein (Lrp) is one of the seven global transcription factors, which can transcribe and regulate the expression of various genes, and participate in the physiological response of bacteria, and influence the pathogenesis of microbes (Cordone et al., 2005; Kroner et al., 2018; Ziegler and Freddolino, 2021). Lrp not only affected production of bacterial fimbriae in E. coli (Blomfield et al., 1993; Roesch and Blomfield, 1998), but also altered bacterial virulence by repressing virulence genes such as CpxP, PhoP and RpoS (Marshall et al., 1999; Hung et al., 2002). Lrp functioned as a global regulator in Vibrio vulnificus, favorably controlling the ability of the bacteria to iron-acquisition capacity, chemotaxis, and cytotoxicity (Ho et al., 2017). There was a wide range of regulatory functions of Lrp in Clostridium difficile toxin expression, sporulation, motility and pathogenesis (Chen K. Y. et al., 2019).
In order to prevent and treat vibriosis, antibiotics are widely used in aquaculture. Long-term abuse of antibiotics not only causes pollution to the surrounding water environment, but also induces broad-spectrum drug resistance of Vibrio, and it even threatens other higher life forms by accumulating poisonous compounds in edible organisms like fish, shrimp, and shellfish (Cabello, 2006). According to researches, fish vaccines are both safer and more effective than antibiotics at reducing the spread of infectious illnesses in fish used for aquaculture (Alexandra, 2019; Fu et al., 2021). Currently, subunit vaccines have been widely used for the prevention of bacterial diseases in fish and have achieved satisfactory immune effects (Zhang et al., 2014; Duan et al., 2021; Injamamul et al., 2022; Xu et al., 2022). Compared to antigens alone, vaccine adjuvants can enhance the immune response of vaccines and modulate the intrinsic immunogenicity of antigens, thus enhancing protective immunity against target diseases (Jiang et al., 2015). Chitosan oligosaccharide (COS) is a hydrolysis product of chitosan. It has the advantages of low viscosity, anti-inflammatory, antioxidant, anti-tumor, immunomodulatory, antibacterial activity, and easy penetration through the intestinal epithelial barrier (Samad et al., 2018; Guan and Feng, 2022; Meng et al., 2022). Compared with only vaccinated fish, the combination of COS and vaccines can considerably increase the survival rates of turbot and achieve high levels of immunological protection (Liu et al., 2015). Thus, COS is frequently employed as a potential adjuvant candidate for aquaculture vaccinations.
The purpose of this study is to develop a subunit vaccine that can effectively prevent V. alginolyticus in pearl gentian grouper. At first, we used prokaryotic expression and protein affinity chromatography techniques to purify the Lrp recombinant protein. We then prepared mouse anti-Lrp polyclonal antibody to evaluate the immunogenicity of the Lrp recombinant protein. Then we injected pearl gentian grouper with the Lrp recombinant protein intraperitoneally to analyze and evaluate the immunoprotective ability of subunit vaccine. In addition, we selected COS adjuvant to improve the immunological response of the subunit vaccine. We not only detected serum of enzyme activities (CAT, SOD, LZM) and titer of specific antibody IgM, but also detected the expression levels of immune genes, like 1L-1β (interleukin 1β), 1L-16 (interleukin 16), CD8α (cluster of differentiation 8α), IgM (immunoglobulin M), MHC-Iα (major histocompatibility complex Iα) and TNF-α (tumor necrosis factor α) in different tissues. Finally, a challenge experiment was used to investigate the immune protection rate of the subunit vaccination against pearl gentian grouper.
2 Materials and methods
2.1 Bacterial strains and experimental animals
V. alginolyticus HY9901 is a virulent strain isolated from the diseased groupers in Zhanjiang City, Guangdong Province, China, and is now preserved in our laboratory (-80°C).
Two-month-old SPF mice were purchased from the Institute of Agricultural Biotechnology, Guangdong Ocean University. The pearl gentian groupers used in the experiment, with the body length of about 13.0 ± 1.0 cm and the weight of about 20.0 ± 1.0 g, were purchased from Donghai Island Southeast Wharf Farm (Zhanjiang City, Guangdong Province, China). Then, they were temporarily reared in the temporary cement pond of the Marine Biology Research Base of Guangdong Ocean University for a week. We regularly feed grouper feed at 9: 00 a.m. and 18: 00 p.m., and change half of the water every day. Before the experiments, the blood, liver, spleen and kidney of pearl gentian groupers were randomly sampled and coated on BHI solid medium for a bacterial recovery study. The study showed that there was no bacterial infection detected in the sampled grouper tissues.
2.2 Construction and preparation of recombinant plasmid pET-N-His-C-His-Lrp
The primers (Lrp-F: CCGGAATTCATGGCAGACAACTATAAAAAGCCGT, Lrp-R: CCGCTCGAGTTAACGAGTTTTGATCACAAGCTGG) containing EcoR I/Xho I restriction sites and corresponding protective bases were designed according to the Lrp gene sequence of V. alginolyticus HY9901. The pET-N-His-C-His plasmid and the Lrp fragment were digested by restriction enzyme EcoR I/Xho I, and then ligated with T4 ligase at 16°C for 2h, and eventually transferred into BL21(DE3) to obtain the bacterial solution of the recombinant plasmid of pET-N-His-C-His-Lrp. The positive colonies were selected by resistance screening method for PCR identification and sent to Guangzhou Biological Engineering Technology & Services Co., Ltd. (Guangzhou, China). With the aim of inducing protein, the successfully sequenced pET-N-His-C-His-Lrp bacterial broth was cultivated until the OD600 was about 0.4-0.6, 1% isopropyl β-D-thiogalactoside (IPTG) was added to continue the cultivation for 4h and the bacteriophage was collected. Subsequently, it was purified by Ni-column affinity chromatography and identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized after staining with coomassie brilliant blue.
2.3 Preparation and identification of mouse anti-Lrp polyclonal antibody
We used an ultrafiltration tube to remove imidazole and urea from the purified Lrp recombinant protein solution, and selected the protein with appropriate concentration as an antigen. The Lrp was mixed with Freund’s complete adjuvant at a ratio of 1:1, and the first immunization was carried out after complete emulsification. On 14d, the Lrp was mixed with Freund’s incomplete adjuvant for the second immunization. On 28d and 42d, the third and fourth vaccines were directly injected with Lrp recombinant protein. Every immunization was performed by subcutaneous injection of 200μg into the neck of mice. Phosphate buffered saline (PBS) was injected as negative control. On 56d, blood was collected from the eyeball, and the serum was collected by centrifugation after standing overnight at 4°C and stored at -80°C.
The specificity of mouse anti-lrp polyclonal antibody was detected by Western Blotting. The Lrp protein was subjected to SDS-PAGE and immunoblotted to the PVDF membrane. Then we put the PVDF membrane into the sealing solution, stayed at 4°C overnight. We rinsed it with TBST (TBS buffer added with 0.1% Tween-20, Sangon Biotech, Guangzhou, China) solution for 3 times. Next we incubated the primary antibody for 1h, which was mouse anti-Lrp serum (1:5000, Boster, Wuhan, China). Afterwards we rinsed it again with TBST solution for 3 times. Subsequently, we incubated the secondary antibody for 1h, which was horseradish peroxidase-Tag goat-anti-mouse IgG (1:5000, Boster, Wuhan, China). Finally, we added chromogenic liquid and used automatic chemiluminescence image analysis system (Tanon, USA) to detect the results.
Enzyme-linked immunosorbent assay (ELISA) was used to determine the titer of mouse anti-Lrp polyclonal antibody. We coated the Lrp protein on a 96-well plate with coating solution at 200μL/well, and incubated overnight at 4°C. After washing with PBST (PBS added with 0.05% Tween-20, Sangon Biotech, Guangzhou, China) for 3 times, sealing at 37°C for 30 min, and washing again for 3 times. We used the mouse anti-Lrp serum used as the primary antibody (1:100, then diluted multiple times), incubated at 37°C for 2h. The mouse PBS-injected serum was used as the negative control. After washing with PBST.for 3 times, horseradish peroxidase-Tag goat-anti-mouse IgG (1: 2000, Boster, Wuhan, China) was added as the secondary antibody for 2 hours again. After washing the plates for 3 times, we added TMB (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) to react for 10 min, and then added 2mM H2SO4 to stop the reaction. After color reaction, we read the absorbance at 450 nm by a microplate reader (Thermo fisher scientific, USA).
2.4 Median lethal dose (LD50) experiment
In order to determine LD50 of V. alginolyticus HY9901 strain to experimental fish, we intraperitoneally injected the pearl gentian groupers with 200μL HY9901 in six concentration gradients, including 1.53×105, 4.01×105, 8.40×105, 4.76×106, 7.19×106, 2.08×107 CFU/mL. 10 fish were used in each concentration gradient group, and the control group was injected with the same amount of PBS. Then observed and recorded continuously for 14 days, and calculated LD50 by using the improved version of Karber’s method.
2.5 Preparation and vaccination of vaccine
The ultrafiltered Lrp protein was diluted to 250μg/mL with PBS to obtain the Lrp vaccine. The 1000mg of COS was dissolved in PBS, and the volume was fixed to 100mL, and filtered through a 0.22μm microporous membrane to prepare 10mg/mL COS solution. The ultrafiltered Lrp protein was dissolved in this solution (the final concentration of COS was 10mg/mL, and the final concentration of Lrp protein was 250μg/mL). The vaccines were stored at 4°C.
The pearl gentian groupers were randomly divided into 3 groups, named Lrp group, Lrp+COS group and PBS group. Each group has three parallel barrels, and each barrel has 30 fish. Each fish was intraperitoneally injected with 100μL of the vaccine, and the same dose of PBS was used as the blank control group. During the immunization period, the animals were fed and changed water normally. At 1w, 2w, 3w, 4w, 5w and 6w after vaccination, head kidney, liver, spleen, thymus and blood (n=3) were collected. The tissues were immediately frozen in liquid nitrogen, the blood was left standing at 4°C overnight, centrifuged at 5000 rpm for 10 minutes, and then the serum was separated, and the samples were stored at -80°C for determination of serum non-specific immune index and specific antibody IgM titer. Three parallel groups were set up for each experiment.
2.6 Bacterial challenge experiment
After 6 weeks of immunization of groupers in each group, we randomly selected 30 groupers in each group for challenge experiment. Each grouper was intraperitoneally injected with 200μL of viable V. alginolyticus suspension with a concentration of 10LD50, and the mortality within 14 days of challenge was recorded and observed. The relative immune protection rate (RPS) was calculated according to the formula: RPS= (1-vaccine group mortality/PBS group mortality) ×100%.
2.7 Determination of immunological indexes in serum of grouper
The serum samples were treated according to the instructions of products (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the weekly activities of CAT, LZM and SOD in the serum of groupers were measured.
Antigen-specific serum antibody IgM of grouper serum was determined with a modified ELISA method. In brief, the antigen was coated overnight, then sealed off for 30 min, grouper serum was added (diluted at 1:2, diluted at times), incubated for 1h at 37°C, then rabbit-anti-grouper IgM serum (1:1000, Boster, Wuhan, China) was added, incubated for 1h at 37°C, and then HRP-labeled goat-anti-rabbit IgG (1:2000, Boster, Wuhan, China) was added. Refer to operation 2.3. it needed to be washed with PBST for 3 times after each step of operation.
2.8 Immunofluorescence quantitative analysis
The total RNA of each tissue was extracted (TransGen Biotech, Beijing, China), and then RNA was reverse transcribed into cDNA (Takara, Beijing, China) according to the reagent instructions. Finally, the expression of immune genes in the tissues of pearl gentian grouper was detected by PerfectStart® Green qPCR SuperMix (TransGen Biotech, Beijing, China). The RT-qPCR reaction system was as follows: 40 cycles with 95°C for 5 min, 95°C for 30 s, 60°C for 30 s and 72°C for 30 s, The relative expression of the internal reference gene β-actin and the immune genes 1L-1β, 1L-16, CD8α, IgM, MHC-Iα and TNF-α genes were analyzed by the 2®−ΔΔCt method. The primers are shown in Table 1.
2.9 Statistical analysis
The experimental data were analyzed by one-way analysis of variance (ANOVA) with SPSS statistical 2.1 and illustrated by GraphPad Prism 8.0. Data were presented as the mean ± SD, and significant differences (p < 0.05) between data were marked by a, b, and c.
3 Results
3.1 Purification of Lrp recombinant protein
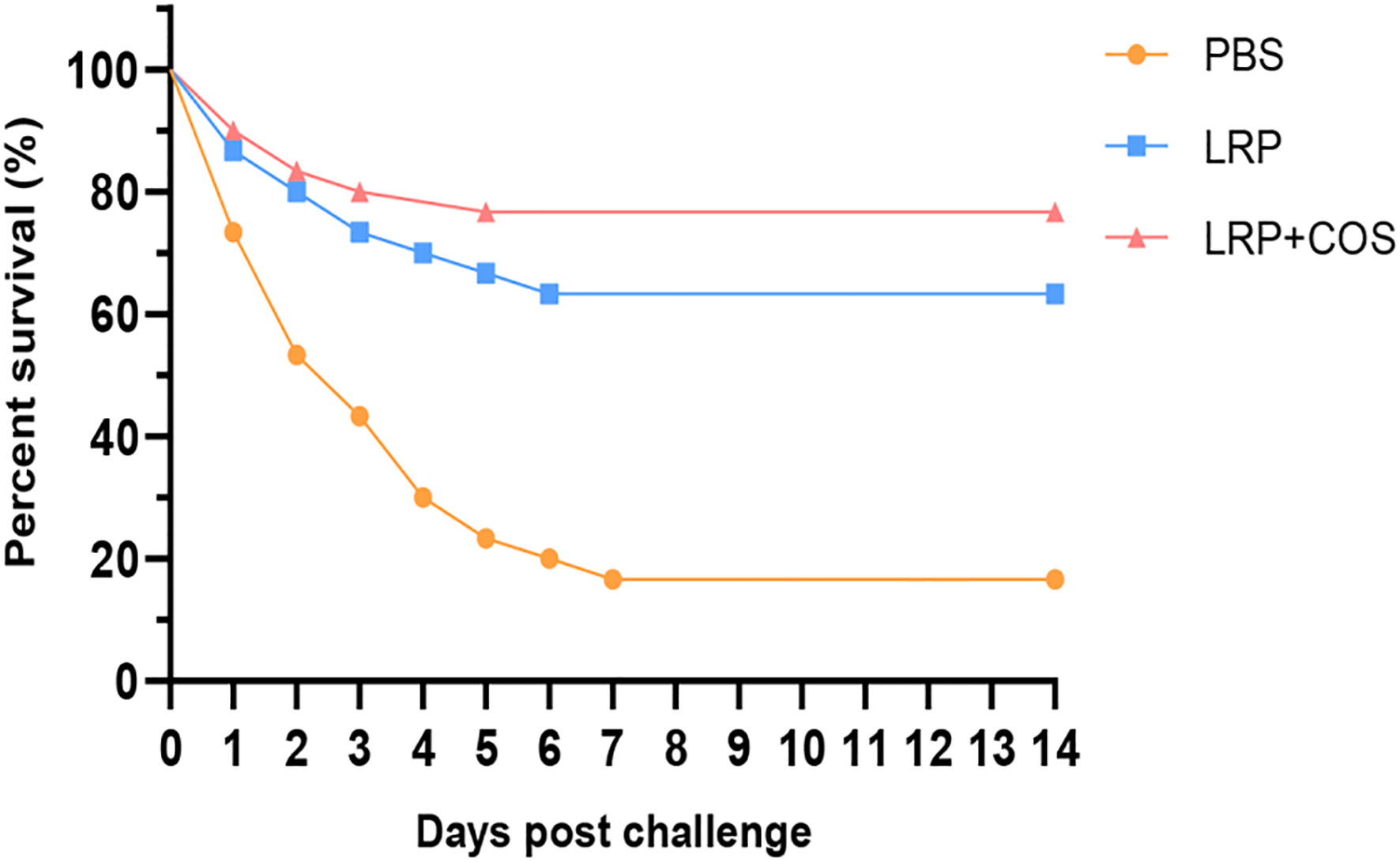
The recombinant plasmid pET-N-His-C-His-Lrp was identified by PCR and sequenced. The sequencing results indicated that the recombinant plasmid was successfully constructed. Compared with the control group, the bacterial solution containing with pET-N-His-C-His-Lrp was induced by IPTG to produce a recombinant protein of about 21.8 kD size, which means that the Lrp recombinant protein was successfully induced to express (Figure 1A). The recombinant Lrp protein was successfully purified by using Ni-column affinity chromatography (Figure 1B).
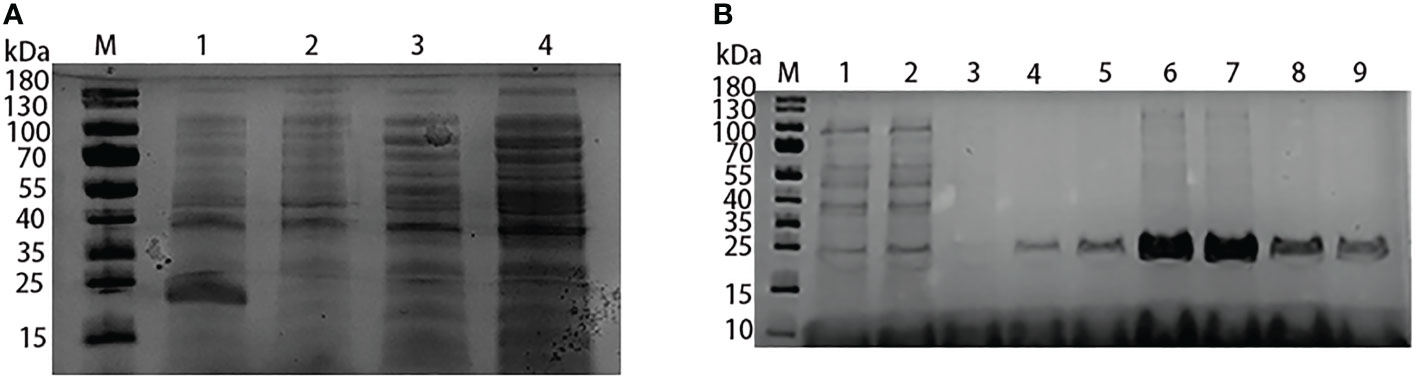
Figure 1 Expression and purification of Lrp recombinant protein. (A) Induced expression of Lrp recombinant protein. M: protein Marker; 1: pET-N-His-C-His-Lrp (induced); 2: pET-N-His-C-His-Lrp (uninduced); 3: pET-N-His-C-His: (induced); 4: pET-N-His-C-His (uninduced). (B) Purification of Lrp recombinant protein Situation. M: protein Marker; 1-2: flow-through solution; 3-5: washing solution; 6-9: elution solution.
3.2 Immunogenicity analysis of mouse anti-Lrp polyclonal antibody
When the mouse anti-Lrp serum was used as the primary antibody and hybridized with goat anti-mouse IgG, Western-Blotting revealed a band of about 21.8 kD (Figure 2A), indicating that the prepared Lrp polyclonal mouse antibody could specifically act on the Lrp recombinant protein with high specificity. By using the formula antibody titer (P/N)= (positive serum OD450- blank control OD450)/(negative serum OD450- blank control OD450) as the reference standard, the titer of the mouse anti-Lrp polyclonal antibody was detected by ELISA (Figure 2B). When the dilution ratio was 1:51200, P/N > 2.1, indicating that the mouse anti-Lrp polyclonal antibody had high titer.
Figure 2 Immunogenicity analysis of mouse anti-Lrp polyclonal antibody. (A) Western-Blotting to detect the specificity of polyclonal antibodies. M: protein Marker; 1: Lrp recombinant protein (B) ELISA to detect the titer of polyclonal antibodies; Positive control: using mouse anti-Lrp serum as primary antibody; Negative control: using mouse PBS-injected serum as primary antibody.
3.3 Determination of LD50
Pearl gentian groupers were artificially infected by V. alginolyticus HY9901 with a certain concentration gradient. The survival rates for each group are represented in Figure 3 as 100%, 80%, 50%, 30%, 20%, and 0%, respectively (Figure 3). A modified version of Kou’s approach was used to determine the LD50 of V. alginolyticus HY9901 on pearl gentian grouper, which was calculated to be 1.18×106 CFU/mL.
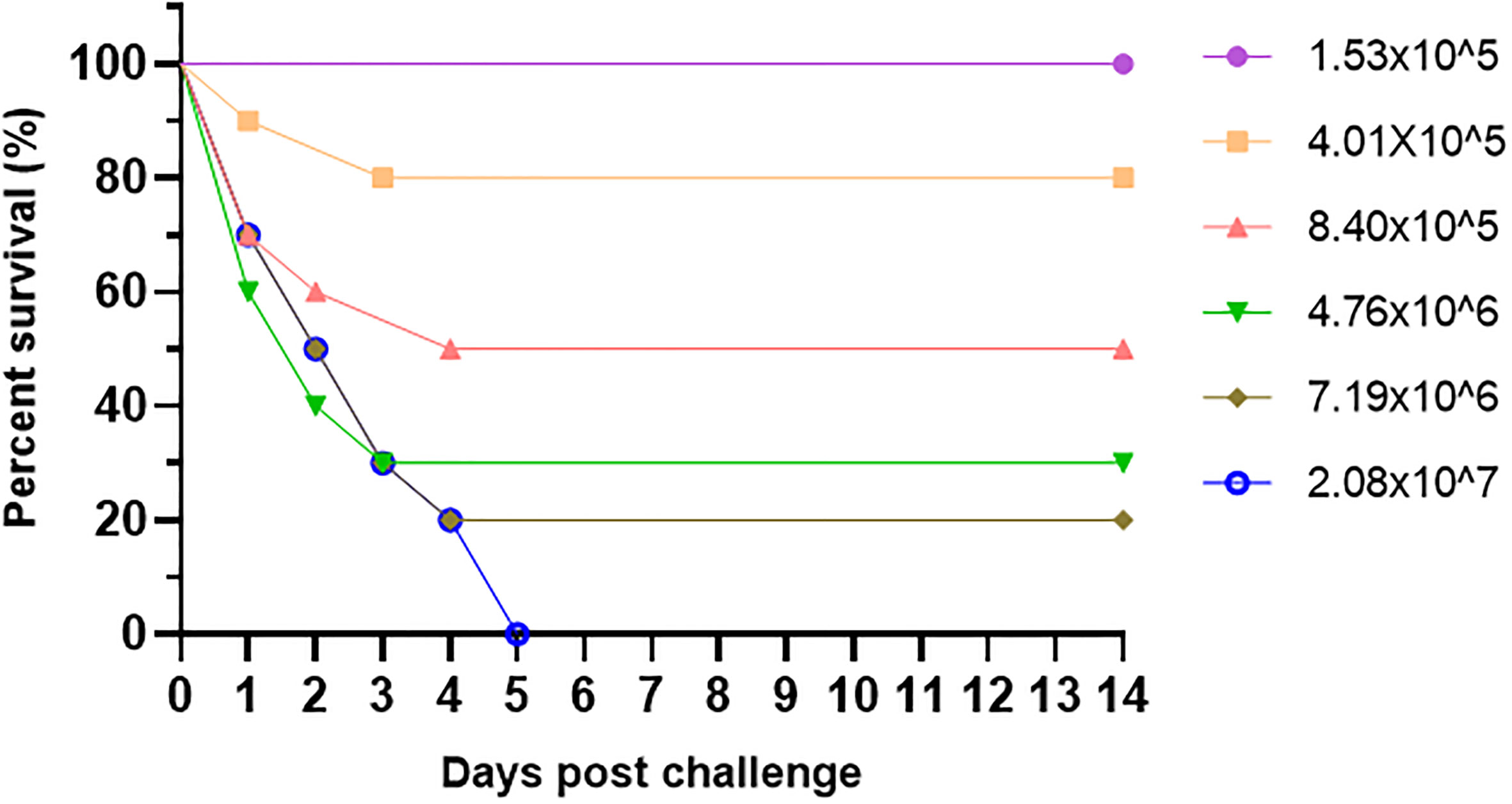
Figure 3 Relative survival rate of V. alginolyticus HY9901 infected with pearl gentian grouper at different concentrations.
3.4 Immunoprotective effect of subunit vaccine
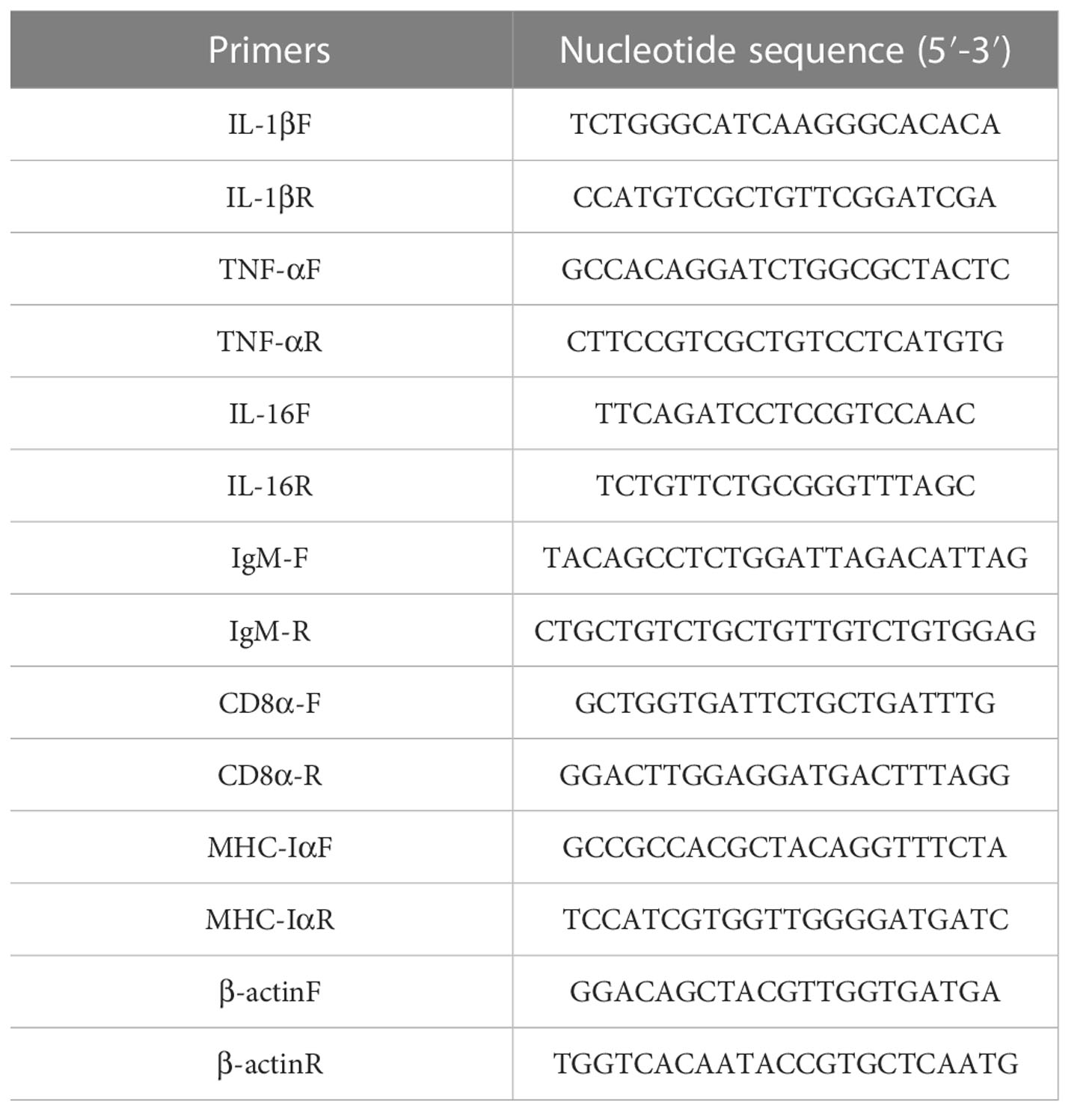
After 6 weeks of immunization, we injected the pearl gentian groupers with 200μL of 1.18×107 CFU/mL V. alginolyticus HY9901 to test the immune protection effect of subunit vaccine. As shown in Figure 4, groupers died partly in all groups on the first day after challenging, with the PBS group accounting for the greatest deaths and ending them by the seventh day. Following a 14-day artificial infection, the survival rates for PBS, Lrp and Lrp+COS groups were respectively 16.67%, 63.3% and 76.67%, and the survival rates of the Lrp and Lrp+COS groups were significantly higher than those of the PBS group. The RPS values of the Lrp and Lrp+COS groups were calculated respectively to be 60% and 72%. The results indicated that Lrp, as a subunit vaccine, could produce strong protection against V. alginolyticus in pearl grouper, and the COS adjuvant could enhance the immune protection of the vaccine against V. alginolyticus.
3.5 Analysis of serum enzyme activity (CAT, SOD and LZM) and IgM antibody titer
Figure 5 showed the enzyme activities in the serum of groupers post-vaccination measured at different immunization periods. The CAT enzyme activity was peaked at 4w in the Lrp group, while it was peaked at 3w in the Lrp+COS group (Figure 5A). The SOD and LZM enzyme activities was peaked at 3w in both the Lrp and Lrp+COS groups (Figures 5B, C). The overall trend of each enzyme activity was to increase first and then gradually decrease to a stable level. The CAT, SOD and LZM activities were significantly higher in the experimental group than in the control group (PBS) (p<0.05), and the activity in the Lrp+COS group was higher than that in the Lrp group.
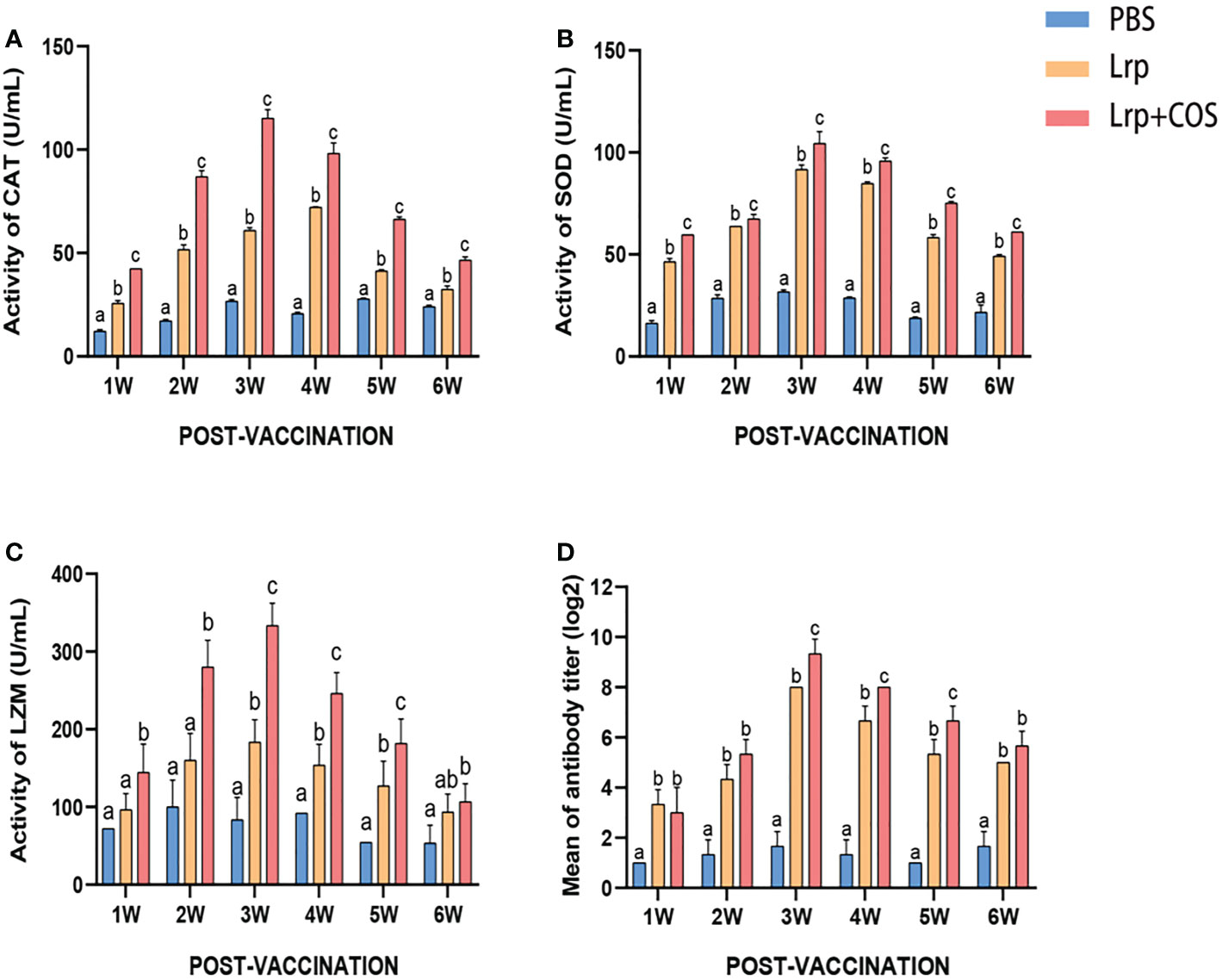
Figure 5 Determination of serum nonspecific immune index and IgM titer of specific antibody. (A) Detection of CAT activity in serum post-vaccination. (B) Detection of SOD activity in serum post-vaccination. (C) Detection of LZM activity in serum post-vaccination. (D) Detection of the IgM titer in serum post-vaccination by ELISA. Bars represented the mean relative expression (n=3) and error bars represented standard errors. Significant differences (p <0.05) were marked by a,b and c.
Following vaccination with Lrp and Lrp+COS, groupers developed specific serum antibodies at 1w post-vaccination. Subsequently, antibody titers gradually rose, peaking at 3w post-vaccination with titers of 1:256 and 1:512, respectively (Figure 5D). At every time point, groupers post-vaccination with Lrp+COS had higher antibody levels than groupers post-vaccination with Lrp alone.
3.6 Expression of related immune genes in different tissues
In this study, qRT-PCR was used to analyze the transcription levels of IgM, CD8α, MHC-Iα, IL-1β, IL-16 and TNF-α in the head kidney, thymus, liver and spleen of pearl grouper post-vaccination. According to Figure 6, both the Lrp and Lrp+COS experimental groups had considerably higher expression levels of immune-related genes than the PBS group, with the Lrp+COS group outperforming the Lrp group. The transcription of IgM peaked in the head kidney (15.502-folds), thymus (19.786-folds) and spleen (15.411-folds) at 4w, while that of the liver (13.256-folds) was at 3w. The transcription of CD8α peaked in the head kidney (12.420-folds), thymus (17.496-folds) and liver (5.765-folds) at 3w, while that of the spleen (11.234-folds) was at 4w. The transcription of MHC-Iα peaked in the head kidney (14.390-folds), liver (9.445-folds),thymus (14.728-folds) and spleen (12.003-folds) at 3w. The transcription of IL-1β peaked at 3w in the head kidney (14.042-folds), thymus (14.888-folds) and spleen (9.034-folds) while that of the liver (6.761-folds) was at 4w. The transcription of IL-16 peaked at 4w in the head kidney (12.180-folds), thymus (13.089-folds) and liver (6.894-folds) while that of the spleen (14.609-folds) was at 3w. The transcription of TNF-α peaked at 3w in the head kidney (15.144-folds), liver (8.597-folds) and spleen (11.189-folds) while that of the thymus (20.945-folds) was at 4w.
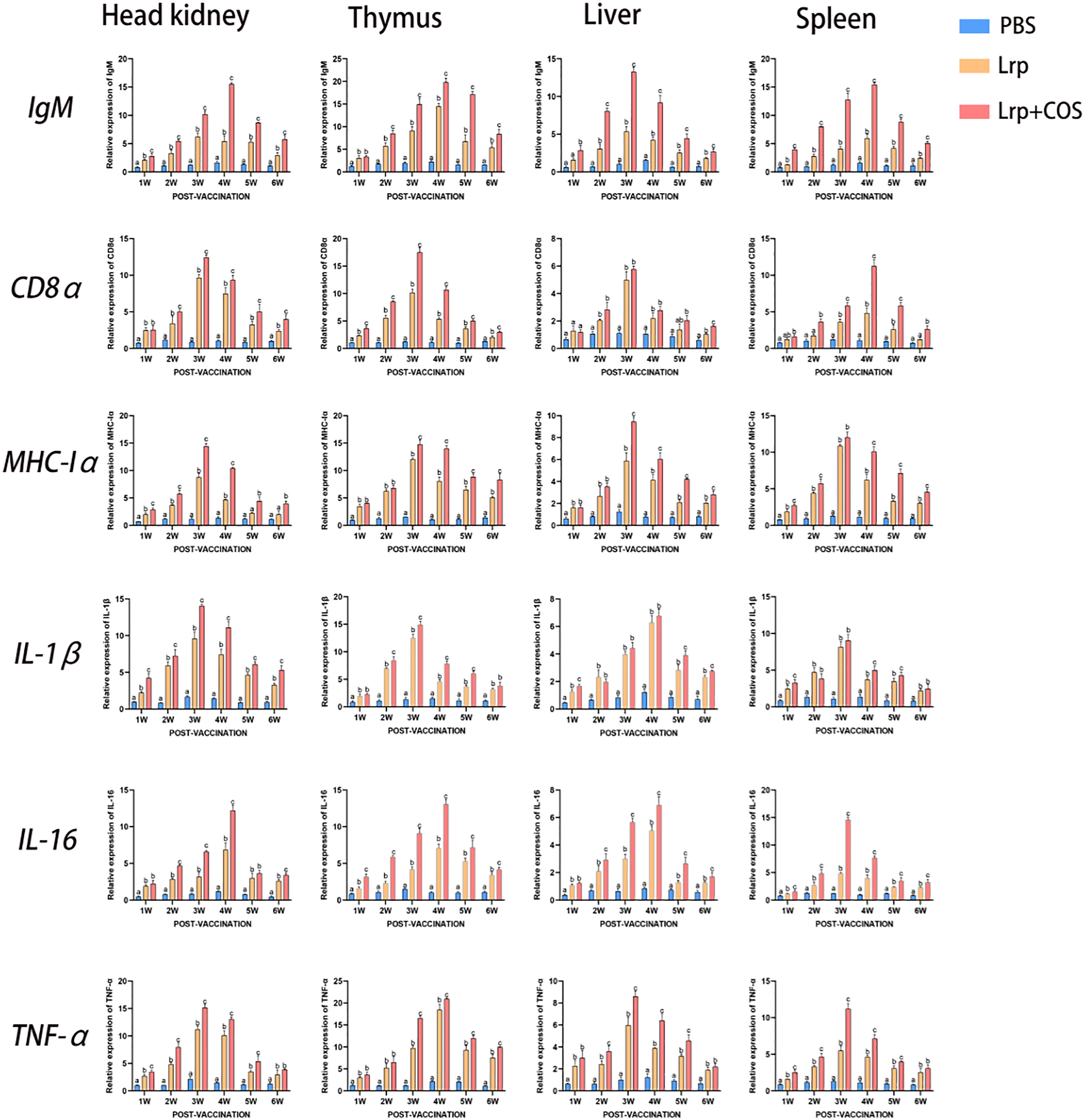
Figure 6 qRT-PCR to analyze the transcript levels of IgM, CD8α, MHC-Iα, IL-1β, IL-16 and TNF-α in the kidney, thymus, liver and spleen of pearl grouper weekly post-vaccination. The mRNA levels of each immune-related gene were normalized to the mRNA levels of β-actin, and the relative expression was calculated. Bars represented the mean relative expression (n=3) and error bars represented standard errors. Significant differences (p < 0.05) were marked by a,b and c.
All immune genes studied demonstrated a time-dependent connection in various organs. The transcription level firstly increased gradually, and then decreased gradually after reaching the maximum transcription level in the 3rd or 4th week, when it was still higher than that in the PBS group until the end of the experiment. The results of this experiment also showed that IgM, CD8α, MHC-Iα, IL-1β, IL-16 and TNF-α were all highly expressed in the head kidney, thymus, liver and spleen. However, the expression level was highest in the thymus and lowest in the liver tissue when compared to other tissues.
4 Discussion
Compared with traditional vaccines, subunit vaccines have the advantages of simultaneously causing humoral and cellular immune reactions, with higher biological safety and ideal immune effect (Gao F. X. et al., 2021). At present, there are more and more reports about the use of recombinant subunit vaccines in aquaculture to prevent and control effectively fish bacterial diseases. Orange-spotted groupers (Epinephelus coioides) vaccinated with recombinant OmpK produced specific antibodies and were extremely resistant to infection by virulent V. harveyi (Li et al., 2008). Oral recombinant Bacillus subtilis VP56-2 subunit vaccine can effectively control grass carp reovirus (GCRV) infection (Gao Y. et al., 2021). Zhang et al. immunized European eel (Anguilla anguilla) with OmpF and OmpK recombinant proteins of Aeromonas hydrophila, which proved that subunit vaccine prepared from the outer membrane protein of Gram-negative bacteria can effectively prevent and treat related bacterial diseases (Zhang et al., 2019).
Lrp plays an significant function in bacterial infection, not only affecting the synthesis of bacterial fimbriae, but also acting as a virulence inhibitor of pathogenic bacteria (Baek et al., 2009; McFarland et al., 2008; Schachterle and Sundin, 2019). Therefore, we selected Lrp as the antigen of subunit vaccine to prevent vibriosis caused by V. alginolyticus. We cloned and purified Lrp recombinant protein (Figure 1), and prepared the mouse anti-Lrp polyclonal antibody (Figure 2), which proved that Lrp recombinant protein has good antigenicity in Vibrio alginolyticus and a high titer.
RPS can clearly show the effect of vaccine. In this study, the relative protection rate of Lrp+COS group is 72%, which is 12% higher than that of Lrp group (Figure 4). It proved that the Lrp vaccine could induce a strong antibody production, and COS as an adjuvant could significantly enhance the immune protection of grouper. The immune protection rate of the Lrp+COS group was higher than that of Lrp alone, which was similar to previous studies (Wei et al., 2020a).
For the purpose of investigating the immune effect of the vaccine on groupers, we examined the activity of various enzymes and the antigen-specific serum antibody IgM in the serum of grouper at different immunization periods. LZM is an alkaline protein, which destroys and eliminates foreign bodies invading the body by hydrolyzing bacterial peptidoglycan, and takes the defense function of the body (Zhao et al., 2022). SOD is a crucial component of antioxidant enzymes, as an active oxygen scavenger, which can scavenge free radicals in the body and enhance the phagocytosis of macrophages. CAT is essential for resisting the harm caused by peroxidation (Yuan et al., 2021). These enzymes are involved in the defense of bacteria. The activities of CAT, LZM and SOD in the experimentally vaccinated group were higher than those of the PBS group, and the promoting effect of Lrp+COS was significantly higher than that of the Lrp group (Figures 5A-C), which indicated that subunit vaccines could induce non-specific immunity in grouper. Specific antibody IgM, as a key component of the fish humoral immune response, plays a major role in the specific immunity and against bacterial infection (Tan et al., 2022). Grouper can produce IgM with high expression levels after vaccined (Figure 5D). The aforementioned experiments showed that the addition of COS to the subunit vaccine could enhance the level of immune-specific antibodies in the serum of pearl grouper and the activity of serum enzymes, thereby improving the immunity and viability of pearl gentian groupers against V. alginolyticus.
Furthermore, the head kidney, thymus, liver and spleen are siginificant immune organs of grouper (Gong et al., 2021; Baharum et al., 2022). By detecting the expression of the immune gene by qRT-PCR technology, we can further understand the immune effective of subunit vaccine. IgM is the primary immunoglobulin produced by B lymphocytes, which reflects the humoral immunity of fish (Abós et al., 2018). MHC is a group of genes that can encode major histocompatibility systems, and its encoded products are widely involved in immune response (Takuya and Johannes, 2019). CD8α is an auxiliary receptor of MHC-Iα recognition antigen that can improve the impact of antigen presentation (Chen et al., 2008). IL-1β, IL-16 and TNF-α are secreted by immunological cells such as macrophages, and regulate the production of various chemokines and induce neutrophils and macrophages to migrate to infected sites to play an immunological function (Ramirez et al., 2013; Minerva et al., 2020). Therefore, the increase of immune gene expression is of great significance for fish to react swiftly and fend against pathogen invasion. Compared with PBS group, the immune genes of the Lrp and Lrp+COS group reached the highest value at the 3rd or 4th week after vaccination. In the immune period, the immune effect of Lrp+COS group is superior to that of Lrp group (Figure 6). This study showed that subunit vaccine caused cellular immunity and humoral immunity of grouper, and COS adjuvant improved the immune protection of vaccine against grouper. Besides, the expression of immune genes is dependent on the tissues and organs (Gong et al., 2021). In this study, the transcription level of thymus is higher that others, which indicates in the molecular level that the thymus is also the main organ producing specific antibodies in grouper.
In summary, pearl grouper developed a robust immunological response to the Lrp subunit vaccination, and COS adjuvant could effectively increase its resistance to Vibrio infection by boosting the subunit’s immune defense. Therefore, Lrp+COS subunit is a strong candidate for a vaccine that can prevent and treat Vibrio infection in pearl grouper.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture.
Author contributions
Experimental design, Data Curation, Writing, MYW. Project administration, Writing - Review & Editing, YD. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Guangdong Provincial Natural Science Foundation Project(NO.2022A1515012203; NO.2014A030313604) and Guangdong Ocean University Graduate Education Innovation Program Grant Project(NO.201724).
Acknowledgments
We would like to thank all teachers and schoolfellow for the skillful organization of sampling and technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abós B., Bird S., Granja A. G., Morel E., More B. J., Barreda D. R., et al. (2018). Identification of the first teleost CD5 molecule: Additional evidence on phenotypical and functional similarities between fish IgM+ b cells and mammalian B1 cells. J. Immunol. (Baltimore Md 1950) 201 (2), 465–480. doi: 10.4049/jimmunol.1701546
Alexandra A. (2019). Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 90, 210–214. doi: 10.1016/j.fsi.2019.04.066
Baek C. H., Wang S. F., Roland K., Curtiss R. (2009). Leucine-responsive regulatory protein (Lrp) acts as a virulence repressor in salmonella enterica serovar typhimurium. J. Bacteriol. 191 (4), 1278–1292. doi: 10.1128/JB.01142-08
Baharum S. N., Mayalvanan Y., Natnan E., Azizan A., Bunawan H., Him N. N., et al. (2022). LC–qTOF-MS analysis of fish immune organs reveals the distribution of amino acids in response to metabolic adaptation of the survival phenotype in grouper against vibrio infection. 3 Biotech. 12 (9), 206–206. doi: 10.1007/S13205-022-03269-1
Blomfield I. C., Calie P. J., Eberhardt K. J., McClain M. S., Eisenstein B. I. (1993). Lrp stimulates phase variation of type 1 fimbriation in escherichia coli K-12. J. Bacteriol. 175 (1), 27–36. doi: 10.1128/jb.175.1.27-36.1993
Cabello F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 8 (7), 1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x
Cai J., Zou Z. Z., Wei S. N., Zheng Q., Xu Y. X., Lu Y. S., et al. (2019). Identification of beclin-1 from orange-spotted grouper (Epinephelus coioides) involved in viral infection. Fish Shellfish Immunol. 94, 336–345. doi: 10.1016/j.fsi.2019.09.029
Chen Y., Ma T., Huang H., Zhong H. G., Juan L. J. (2019). Effects of the replacement of fishmeal by soy protein concentrate on growth performance, apparent digestibility, and retention of protein and amino acid in juvenile pearl gentian grouper. PloS One 14 (12), e0222780. doi: 10.1371/journal.pone.0222780
Chen K. Y., Rathod J., Chiu Y. C., Chen J. W., Tsai P. J., Huang I. H. (2019). The transcriptional regulator lrp contributes to toxin expression, sporulation, and swimming motility in clostridium difficile. Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00356
Chen Q., Yan Q. P., Wang K. J., Zhuang Z. X., Wang X. R. (2008). Portal of entry for pathogenic vibrio alginolyticus into large yellow croaker pseudosciaena crocea, and characteristics of bacterial adhesion to mucus. Dis. Aquat. Organisms 80 (3), 181–188. doi: 10.3354/dao01933
Cordone A., Mauriello E. M. F., Pickard D. J., Dougan G., De F. M., Ricca E. (2005). The lrp gene and its role in type I fimbriation in citrobacter rodentium. J. Bacteriol. 187 (20), 7009–7017. doi: 10.1128/JB.187.20.7009-7017.2005
Duan H. X., Zhao Z., Jin Y. J., Wang Z. L., Deng J. F., He J., et al. (2021). PEG-modified subunit vaccine encoding dominant epitope to enhance immune response against spring viraemia of carp virus. J. Fish Dis. 44 (10), 1587–1594. doi: 10.1111/JFD.13481
Fu Y., Zhang Y. A., Shen J. Y., Tu J. G. (2021). Immunogenicity study of OmpU subunit vaccine against vibrio mimicus in yellow catfish, pelteobagrus fulvidraco. Fish Shellfish Immunol. 108, 80–85. doi: 10.1016/j.fsi.2020.11.030
Gao F. X., Huang J. J., Li T. T., Hu C., Shen M. Y., Mu S., et al. (2021). A highly conserved peptide vaccine candidate activates both humoral and cellular immunity against SARS-CoV-2 variant strains. Front. Immunol. 12. doi: 10.3389/FIMMU.2021.789905
Gao Y., Huo X. C., Wang Z. S., Yuan G. L., Liu X. L., Ai T., et al. (2021). Oral administration of bacillus subtilis subunit vaccine significantly enhances the immune protection of grass carp against GCRV-II infection. Viruses 14 (1), 30–30. doi: 10.3390/V14010030
Gong H., Wang Q., Lai Y. T., Zhao C. C., Sun C. W., Chen Z. H., et al. (2021). Study on immune response of organs of epinephelus coioides and carassius auratus after immersion vaccination with inactivated Vibrio harveyi vaccine. Front. Immunol. 11. doi: 10.3389/FIMMU.2020.622387
Guan Z. W., Feng Q. (2022). Chitosan and chitooligosaccharide: The promising non-Plant-Derived prebiotics with multiple biological activities. Int. J. Mol. Sci. 23 (12), 6761–6761. doi: 10.3390/IJMS23126761
Ho Y. C., Hung F. R., Weng C. H., Li E. T., Chuang T. H., Liu T. L., et al. (2017). Lrp, a global regulator, regulates the virulence of vibrio vulnificus. J. Biomed. Sci. 24 (1), 54. doi: 10.1186/s12929-017-0361-9
Hung S. P., Baldi P., Hatfield G. W. (2002). Global gene expression profiling in escherichia coli K-12. the effects of leucine-responsive regulatory protein. J. Biol. Chem. 277 (43), 40309–40323. doi: 10.1074/jbc.M204044200
Injamamul I. S., Jahan M. M., Sanjida S. (2022). Application of reverse vaccinology to design a multi-epitope subunit vaccine against a new strain of aeromonas veronii. J. Genet. Eng. Biotechnol. 20 (1), 118–118. doi: 10.1186/S43141-022-00391-8
Ji Q. Y., Wang S. Y., Ma J. F., Liu Q. (2020). A review: Progress in the development of fish vibrio spp. vaccines. Immunol. Lett. 226 (prepublish), 46–54. doi: 10.1016/j.imlet.2020.07.002
Jiang J., Zheng Z. L., Wang K. Y., Wang J., He Y., Wang E., et al. (2015). Adjuvant immune enhancement of subunit vaccine encoding pSCPI of streptococcus iniae in channel catfish (Ictalurus punctatus). Int. J. Mol. Sci. 16 (12), 28001–28013. doi: 10.3390/ijms161226082
Kroner G. M., Wolfe M. B., Freddolino P. L. (2018). Escherichia coli lrp regulates one-third of the genome via direct, cooperative, and indirect routes. J. Bacteriol. 201 (3), e00411–e00418. doi: 10.1128/JB.00411-18
Li N. Q., Bai J., Wu S. Q., Fu X. Z., Lao H. H., Ye X., et al. (2008). An outer membrane protein, OmpK, is an effective vaccine candidate for vibrio harveyi in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 25 (6), 829–833. doi: 10.1016/j.fsi.2008.09.007
Liu X. H., Zhang H., Gao Y., Zhang Y., Wu H. Z., Zhang Y. X. (2015). Efficacy of chitosan oligosaccharide as aquatic adjuvant administrated with a formalin-inactivated vibrio anguillarum vaccine. Fish Shellfish Immunol. 47 (2), 855–860. doi: 10.1016/j.fsi.2015.10.012
Marshall D. G., Sheehan B. J., Dorman C. J. (1999). A role for the leucine-responsive regulatory protein and integration host factor in the regulation of the salmonella plasmid virulence (spv) locus in 1Salmonella typhimurium. Mol. Microbiol. 34 (1), 134–145. doi: 10.1046/j.1365-2958.1999.01587.x
McFarland K. A., Lucchini S., Hinton J. C., Dorman C. J. (2008). The leucine-responsive regulatory protein, lrp, activates transcription of the fim operon in salmonella enterica serovar typhimurium via the fimZ regulatory gene. J. Bacteriol. 190 (2), 602–612. doi: 10.1128/JB.01388-07
Meng L. Y., Ma J. Q., Liu C. H., Mao X. Z., Li J. (2022). The microbial stress responses of escherichia coli and staphylococcus aureus induced by chitooligosaccharide. Carbohydr. Polymers 287, 119325–119325. doi: 10.1016/J.CARBPOL.2022.119325
Minerva N. M., Brenda P. L., Paulina A. A., Ruth L., Sobeida S. N., Manuel I. G. P., et al. (2020). Sub-Basal increases of GABA enhance the synthesis of TNF-α, TGF-β, and IL-1β in the immune system organs of the Nile tilapia. J. Neuroimmunol. 348, 577382–577382. doi: 10.1016/j.jneuroim.2020.577382
Ramirez V., Macias M. A., Ortiz G. G., Pacheco M. F., Torres E. D., Sorto T. E., et al. (2013). Efficacy of fish oil on serum of TNF α , IL-1β , and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev. 2013, 709493. doi: 10.1155/2013/709493
Roesch P. L., Blomfield I. C. (1998). Leucine alters the interaction of the leucine-responsive regulatory protein (Lrp) with the fim switch to stimulate site-specific recombination in escherichia coli. Mol. Microbiol. 27 (4), 751–761. doi: 10.1046/j.1365-2958.1998.00720.x
Sadat A., ElSherbiny H., Zakaria A., Ramadan H., Awad A. (2020). Prevalence, antibiogram and virulence characterization of vibrio isolates from fish and shellfish in Egypt: A possible zoonotic hazard to humans. J. Appl. Microbiol. 131 (1), 485–498. doi: 10.1111/jam.14929
Samad R., Yuan X., Wang L., Lu K., Song K., Zhang C. X. (2018). Chitooligosaccharide supplementation in low-fish meal diets for pacific white shrimp (Litopenaeus vannamei ): Effects on growth, innate immunity, gut histology, and immune-related genes expression. Fish Shellfish Immunol. 80, 405–415. doi: 10.1016/j.fsi.2018.06.025
Schachterle J. K., Sundin G. K. (2019). The leucine-responsive regulatory protein lrp participates in virulence regulation downstream of small RNA ArcZ in erwinia. amylovora. mBio 10 (3), e00757–e00719. doi: 10.1128/mBio.00757-19
Supansa B., Nattarika C., Teng J. L. L., Huih L. H., Woo P. C. Y., Decha S., et al. (2020). Outer membrane protein a (OmpA) is a potential virulence factor of vibrio alginolyticus strains isolated from diseased fish. J. Fish Dis. 43 (2), 275–284. doi: 10.1111/jfd.13120
Takuya Y., Johannes W. D. (2019). Major histocompatibility complex (MHC) genes and disease resistance in fish. Cells 8 (4), 378–378. doi: 10.3390/cells8040378
Tan H. M., Da F., Lin G. X., Wan X. J., Cai S. H., Cai J., et al. (2022). Construction of a phosphodiesterase mutant and evaluation of its potential as an effective live attenuated vaccine in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Fish Shellfish Immunol. 124, 543–551. doi: 10.1016/J.FSI.2022.04.016
Wei G. B., Cai S. H., Wu Y. Z., Ma S. H., Huang Y. C. (2020a). Immune effect of vibrio harveyi formalin-killed cells vaccine combined with chitosan oligosaccharide and astragalus polysaccharides in ♀Epinephelus fuscoguttatus×♂Epinephelus lanceolatus. Fish Shellfish Immunol. 98 (C), 186–192. doi: 10.1016/j.fsi.2020.01.015
Wei G. B., Tan H. M., Ma S. H., Sun G. R., Zhang Y. L., Yuan Y. Z., et al. (2020b). Protective effects of β-glucan as adjuvant combined inactivated vibrio harveyi vaccine in pearl gentian grouper. Fish Shellfish Immunol. 106, 1025–1030. doi: 10.1016/j.fsi.2020.09.027
Xu F. F., Jiang F. Y., Zhou G. Q., Xia J. Y., Yang F., Zhu B. (2022). The recombinant subunit vaccine encapsulated by alginate-chitosan microsphere enhances the immune effect against micropterus salmoides rhabdovirus. J. Fish Dis. 45 (11), 1757–1765. doi: 10.1111/JFD.13697
Yuan J. M., Wang Z. J., Wang B., Mei H. Q., Zhai X. L., Zhuang Z. H., et al. (2021). Non-specific immunity associated gut microbiome in aristichthys nobilis under different rearing strategies. Genes 12 (6), 916–916. doi: 10.3390/GENES12060916
Zhang W. N., Liao Z. C., Hu F. X., Chen X. H., Huang X. H. (2019). Protective immune responses of recombinant outer membrane proteins OmpF and OmpK of aeromonas hydrophila in European eel (Anguilla anguilla). Aquacult. Res. 50 (12), 3559–3566. doi: 10.1111/are.14311
Zhang Y. Q., Zhang T. T., Li J. N., Liu X. L. (2014). Design and evaluation of a tandemly arranged outer membrane protein U (OmpU) multi-epitope as a potential vaccine antigen against vibrio mimicus in grass carps (Ctenopharyngodon idella). Vet. Immunol. Immunopathol. 160 (1-2), 61–69. doi: 10.1016/j.vetimm.2014.03.016
Zhao C. Y., Wen H. G., Huang S. S., Weng S. P., He J. G. (2022). A novel disease (Water bubble disease) of the giant freshwater prawn macrobrachium rosenbergii caused by citrobacter freundii: Antibiotic treatment and effects on the antioxidant enzyme activity and immune responses. Antioxidants 11 (8), 1491–1491. doi: 10.3390/ANTIOX11081491
Keywords: Vibrio alginolyticus, pearl gentian grouper, leucine responsive regulatory protein (Lrp), subunit vaccine, chitosan oligosaccharide (COS)
Citation: Wan M and Ding Y (2023) Study on immunogenicity of Lrp subunit vaccine against Vibrio alginolyticus in pearl gentian grouper(♀Epinephelus fuscoguttatus ×♂Epinephelus lanceolatus). Front. Mar. Sci. 10:1098816. doi: 10.3389/fmars.2023.1098816
Received: 15 November 2022; Accepted: 10 February 2023;
Published: 22 February 2023.
Edited by:
Ana Maulvault, Portuguese Institute for Sea and Atmosphere (IPMA), PortugalReviewed by:
Zaohe Wu, Zhongkai University of Agriculture and Engineering, ChinaXiuzhen Sheng, Ocean University of China, China
Copyright © 2023 Wan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Ding, ZGluZ3lAZ2RvdS5lZHUuY24=
 Mingyue Wan
Mingyue Wan Yu Ding
Yu Ding