- 1International Research Center for Marine Biosciences, Ministry of Science and Technology, Shanghai Ocean University, Shanghai, China
- 2Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-Culture of Aquaculture Animals, Shanghai, China
- 3Southern Marine Science and Engineering Guangdong Laboratory, Guangzhou, China
- 4Graduate School of Fisheries and Environmental Sciences, Nagasaki University, Nagasaki, Japan
Marine invertebrates are the backbone of marine biodiversity and play a pivotal role in the marine ecosystem. The life cycle of most marine invertebrates includes the settlement and metamorphosis stage, which is induced by marine biofilms, but the mechanism is still enigmatic. In the present study, we constructed the capsular polysaccharide (CPS) synthesis gene capC-deleted mutant of Pseudoalteromonas marina by gene knockout and then compared the phenotype, the biofilm-forming ability, the effect on settlement and metamorphosis of Mytilus coruscus, and the exopolysaccharide and CPS levels between the mutant and the wild-type strains to explicate the relationship between bacteria and mussels. The study presented that the phenotype and biofilm-forming ability between the wild-type and ΔcapC strains had no significant difference, but the inducing activity of ΔcapC biofilms on larval settlement and metamorphosis decreased significantly (p < 0.05). Compared with the wild-type, the CPS content of ΔcapC strain significantly decreased by 38.07%, accompanied by the increase of c-di-GMP. Meanwhile, the biomass of α-polysaccharides and β-polysaccharides on ΔcapC biofilms decreased significantly (p < 0.05). Thus, the CPS synthesis gene could modulate c-di-GMP, which regulates bacterial polysaccharide secretion, and then impact larval settlement and metamorphosis of mussels. This work brings an entry point to deeply understand the interaction between bacterial polysaccharide and larval recruitment.
1. Introduction
Marine invertebrates are of great ecological value in the marine biodiversity system (Lotze, 2021). Marine bivalve shellfish, a major representative class of marine invertebrates, are distributed in coastal and estuarine areas of the whole world and often become the dominant group of local biological communities. Mytilus coruscus is a major economic shellfish in East Sea, China (Chang, 2007; Wang et al., 2012). The life history of M. coruscus is similar to that of most marine invertebrates, settlement and metamorphosis are the essential steps for motile larvae to become adults (Cavalcanti et al., 2020), which can contribute to the persistence of M. coruscus. This process is often induced by chemical signals within marine biofilms (Zardus et al., 2008; Hadfield, 2011; Liu et al., 2022).
Biofilms exist in almost all of the matrix surfaces in the marine environment, the chemical cues within biofilms, such as extracellular polymeric substances and c-di-GMP, can induce larvae settlement and metamorphosis (Hadfield, 2011; Cavalcanti et al., 2020; Dobretsov and Rittschof, 2020; Rischer et al., 2022). For instance, the exopolysaccharide secreted by a marine Pseudomonas sp. could promote Ciona intestinalis larvae to metamorphose (Szewzyk et al., 1991). Bao et al. (2007) found that exopolysaccharides or glycoproteins on the biofilm surface may participate in the metamorphosis of Mytilus galloprovincialis by binding with lectins (Bao et al., 2007). Freckelton et al. (2022) found that lipopolysaccharides in the OMVs produced by Cellulophaga lytica could induce the metamorphosis of Hydroides elegans (Freckelton et al., 2022). Moreover, our previous work demonstrated that some polysaccharide biosynthesis genes of marine bacteria promote or inhibit larval settlement and metamorphosis through regulating the content of biofilm extracellular polymeric substances (Zeng et al., 2015; Liang et al., 2020; Peng et al., 2020; Liang et al., 2021). It is worth noting that a point mutation in AT00-17125 of Pseudoalteromonas resulted in a translucent morphology and showed less capsular polysaccharide (CPS) by phenotypic observation, and the biofilm formed by the mutant inhibited larvae metamorphosing to post-larvae (Zeng et al., 2015), but the mechanism of CPS regulating larval settlement and metamorphosis is still unclear.
CPS is an essential component of bacterial capsule in the outer membrane polysaccharides, and it makes a difference in the formation of bacterial biofilm (Badel et al., 2011). CPS can inhibit biofilm formation by preventing adhesion molecules on cell surface (Nagar and Schwarz, 2015). Previous studies have found that the mutants of Vibrio vulnificus (Lee et al., 2013), Pasteurella multocida (Petruzzi et al., 2017) and Bacteroides thetaiotaomicro (Bechon et al., 2020) with defected capsular CPS can enhance the adhesion and cell aggregation capacity of the non-biological surface, and the mutant strain produce more biofilms. However, the interaction between CPS and recruitment of marine benthic animals remains little known.
In this study, we investigated the involvement of CPS synthesis gene capC of Pseudoalteromonas marina (Yang et al., 2013; Peng et al., 2018) in biofilm development and M. coruscus larval settlement and metamorphosis. It provides a supplement for the mechanism of the interaction between bacteria and marine invertebrates and provides a new sight for the study of marine biodiversity conservation from a microscopic perspective.
2. Materials and methods
2.1. Larval culture
Mussel larvae used in larval induction bioassays were all produced in Shengsi Islands (122°76 E; 30°72 N), Zhenjiang, China. The method of larval culture was the same as in a previous study (Liang et al., 2021). Basically, M. coruscus developed into swimming straight-hinge veliger larvae within 2 days after fertilization, and then usually need to be cultivated at 16-20°C for about 20 days to develop into pediveliger stage. During the temporary culture in the laboratory, the larvae was cultured at 18°C in darkness, and fed with Isochrysis zhanjiangensis every day. The larval induction test was organized when the shell length of pediveligers was longer than 300 μm.
2.2. Strains and plasmids
The tested bacterium P. marina was isolated from the natural biofilms and stored at −80°C. The wild-type and ΔcapC were both cultured in Zobell 2216E medium (2216E, Sigma, St Louis, MO, USA) at 25°C (Peng et al., 2018). The Escherichia coli WM3064 was grown in Luria-Bertani (Sigma, St Louis, MO, USA) at 37°C (Peng et al., 2020). When culturing strains containing the pK18mobsacB-ery plasmid, kanamycin and erythromycin should be added to maintain the resistance of the strain (Wang et al., 2015). Other relevant information is shown in Table 1.
2.3. Construction of ΔcapC mutant strains
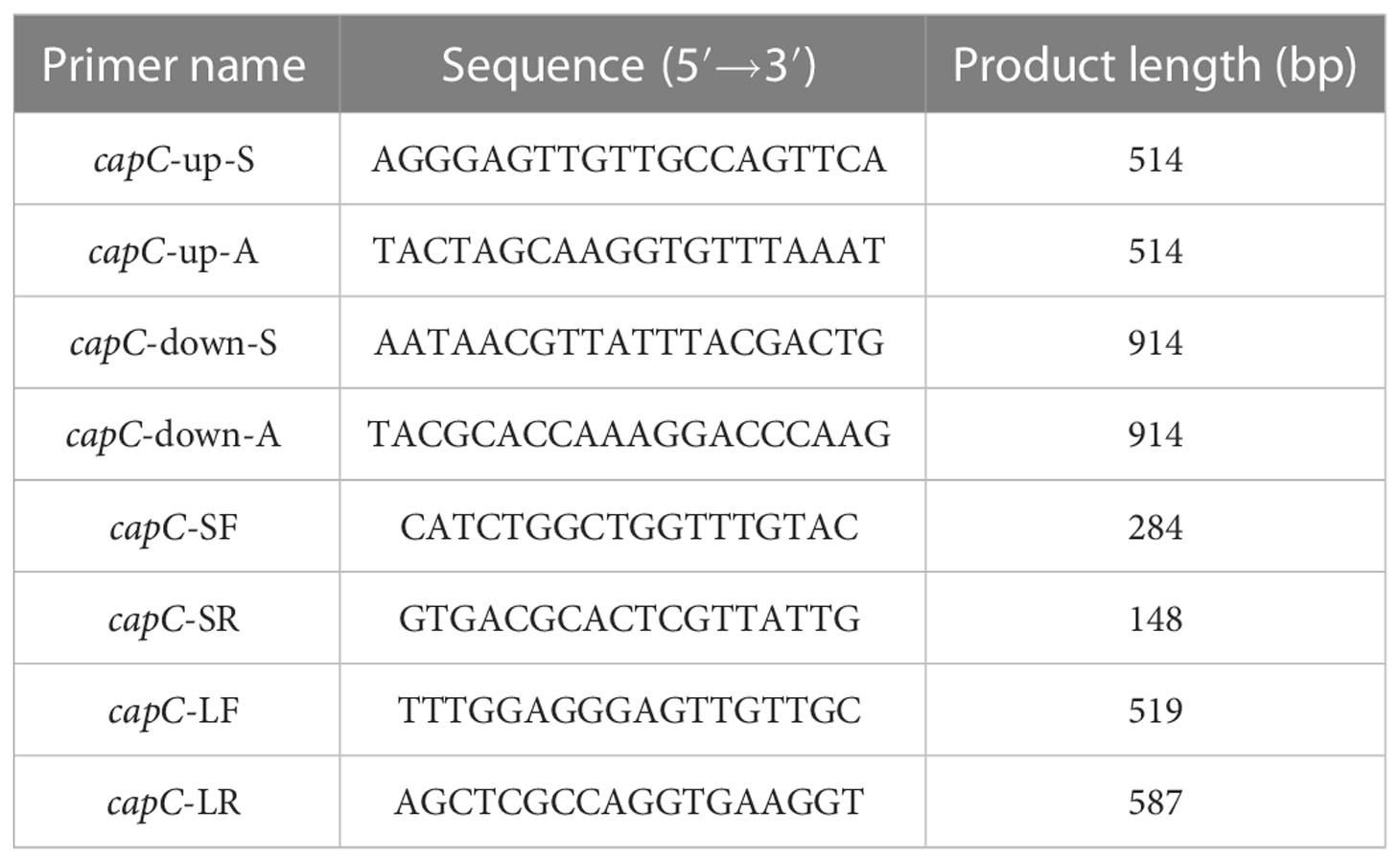
A mutant strain of P. marina with the deletion of capC gene was built by homologous recombination technology (Wang et al., 2015). The upstream and downstream primers used to amplify the target gene capC were shown in Table 2. Recombinant plasmids are built by restriction enzyme ligation and transferred into E. coli WM3064. The suicide plasmid in E. coli WM3064 was transferred into P. marina, and colonies that could grow on Zobell 2216E medium which contained erythromycin were chosen and verified by single-crossover primer pairs capC-SF/LR and capC-LF/SR. The correct ΔcapC strains were screened on 20% sucrose plates and validated by utilizing four primer pairs: capC-SF/capC-LR, capC-LF/capC-SR, capC-SF/capC-SR, capC-LF/capC-LR.
2.4. Effects of capC gene deletion on phenotype of P. marina
The experimental methods referred to Zeng et al. (Zeng et al., 2017) and Hu et al. (2021). P. marina and ΔcapC strains were cultivated in Zobell 2216E for 16 - 18 h (200 r/min, 25°C), diluted and coated the bacterial fluid on Zobell 2216E solid plate according to concentration gradient, and then cultured at 25°C for 2 - 5 days for observation.
2.5. Biofilm formation of experimental strains
The wild-type and ΔcapC biofilms were produced in accordance with previous studies (Wang et al., 2012). The culture conditions of P. marina and ΔcapC strains used to form biofilms were identical to those described previously (Peng et al., 2020). Bacterial cells were obtained by centrifuging at 1600 × g for 15 min, washed with autoclaved filtered seawater (AFSW) and diluted to 50 mL. According to the obtained bacterial density, the corresponding amount of bacterial solution was added to a sterile petri dish (64.0 mm (Φ) × 19.0 mm) which contained sterile glass slide (12.7 mm × 38.1 mm), and the AFSW was added to obtain 1×108, 3×108, 5×108, and 1×109 colony-forming unit (CFU) mL−1 as initial concentrations. The culture dishes were placed at 18°C for 48 h to develop biofilms.
2.6. Cell density of biofilms
The pre-treatment steps of the glasses used to calculate the bacterial density of the biofilm were identical to those described previously (Peng et al., 2020). Briefly, after the biofilms were soaked in 5% formaldehyde solution for 24 h, then rinsed with aseptic saline (0.9% NaCl) once, and stained with acridine orange solution (0.1%) for 5 min. There were 10 fields of view (random observation) were selected under a microscope at 1000 × magnification to calculate the final density of biofilms, and each biofilm pattern contained three duplicates.
2.7. Settlement and metamorphosis bioassays
With regard to detecting whether the larvae have the ability of settlement and metamorphosis, 10-4 mol L−1 of epinephrine (EPI) was used as inducers (Yang et al., 2013). The biofilm slide and 20 larvae were added into a sterile glass Petri dish equipped with 20 mL of AFSW, and the slides without biofilms were built as blank control. Nine repetitions were operated in each test group. After being placed at 18°C without light, the number of post-larvae was recorded at 24, 48, 72, and 96 h.
2.8. Effects of capC gene deletion on growth ability of P. marina
The growth of P. marina and ΔcapC strains was measured by turbidimetry. The two strains were grown overnight for 16 - 18 h. When the OD600 absorbance reached 1.0, 5 μL of bacterial solution was sucked into 5 mL of culture medium respectively (200 r/min, 25°C). Then the OD600 absorbance of 2, 4, 6, 9, 12, 15, 18, and 24 h was measured by a spectrophotometer, respectively.
2.9. Swimming motility assay
P. marina and ΔcapC strains were grown for 16 -18 h in a table concentrator (200 r/min, 25°C). To observe the swimming trajectory, the bacterial solution (1 μL) from P. marina and ΔcapC strains were pointed on the swimming medium (Marine Broth 2216E medium, 0.3% agar), respectively, and kept at 25°C for 18 h. The test consisted of 9 iterations per strain.
2.10. The thickness of biofilms
The morphology of biofilm cells was maintained by 5% formalin solution (30 min). After being dyed with iodide solution (5 g/L) for 20 min in complete darkness, the stained biofilms were washed three times with 0.9% NaCl. To determine the thickness of biofilms, the confocal laser scanning microscopy (CLSM) images were conducted using Leica TCS SP8 with LAS X software. The confocal microscope and software were set to objective magnification of 63 ×, z-step of 0.2 µm and pixels of 1024×1024. Imaging analysis was performed on 9 randomly chosen fields of view.
2.11. Extraction of bacterial CPS
The method of bacterial CPS extraction was identical to that of a previous study (Zhang et al., 2013; Zhang et al., 2017; Lin et al., 2018). Typically, 4 mL of sterile saline was added to each Zobell 2216E agar medium to dissolve the bacterial cells. Bacteria from 24 plates were collected for each strain. The bacterial suspension was centrifugated for 30 min at 12000 × g, then the bacterial sediments were collected for resuspended in 30 mL of sterile buffered saline (10 mM EDTA, 0.9% NaCl). After stored at 4°C for 2 h, the bacterial suspension was centrifugated for 30 min at 12000 × g at 4°C. To eliminate bacteria from the supernatant, the 0.22 μm vacuum filters were utilized to filter the liquid. Next, the filtration liquid was concentrated through 10 kD ultrafiltration and 3 times the volume of anhydrous ethanol was added to the mixture. After cooled at 4°C for 12 h, the mixture was centrifugated (4°C, 1800 × g) for 30 min to remove nucleic acid impurities. Then added 4-times the volume of anhydrous ethanol into the supernatant, and stored at 4°C for 24 h. Next, the suspension was centrifugated for 1h (4°C, 1800 × g), and the polysaccharide precipitation was collected and washed with anhydrous ethanol and acetone twice, respectively. After lyophilized, the polysaccharide was dissolved into a 5% aqueous solution and 1/3 volume of Sevage (Chloroform: N-butanol = 4: 1, v/v) was added. After shaking for 25 min, the mixture was centrifuged for 10 min (4°C, 1800 × g), then the protein precipitation was discarded. The supernatant was dialyzed for 48 h and freeze-dried to obtain CPS.
2.12. Quantification of bacterial CPS and c-di-GMP
The bacterial CPS quantification method was identical to that of a previous study (Zhang et al., 2017), and the extracted CPS powder was dissolved in 5 mL sterilized distilled water to form a stock solution. The stock solution was diluted 10 folds with distilled water as a test solution. Then, 1 mL distilled water, 1 mL 3% phenol and 5 mL sulfuric were successively added into a sterile tube containing 1 mL test solution. And 2 mL of distilled water served as a blank control. The samples were allowed to react for 20 min at 80°C. Then, the samples were kept at 25°C, and the OD485 values of samples were detected. Three replicates were performed for each sample. The value of the A485 was calculated using a glucose concentration standard curve to calculate the content of the CPS in the sample. The c-di-GMP content was quantitatively analyzed by LC-MA/MS with reference to Peng et al. (2020).
2.13. Analysis of biofilm exopolysaccharide content scanning by CLSM
Biofilms of experimental strains were dyed as in a previous study (Peng et al., 2020; Hu et al., 2021). Confocal images of the processed samples were acquired using CLSM and LAS X software with the same conditions as described in 2.10. Three replicate samples were prepared for wild-type and mutant biofilms, respectively. Each experimental sample consisted of three randomly chosen fields of vision. The Image J software (NIH, Madison, WI, USA) was utilized to analyze confocal images and calculate the content of polysaccharides in extracellular products of biofilms.
2.14. Data statistical analysis
Data analysis was carried out using JMP™ software (Liang et al., 2021).
3. Results
3.1. Effects of capC deletion on phenotype and growth ability of P. marina
To understand the effect of P. marina gene capC on settlement and metamorphosis of M. coruscus larvae, a mutant with deletion of CPS synthesis gene was created (Figure 1A). The phenotypic observation revealed that the formats of the wild-type and ΔcapC strains were smooth and circular (Figures 1B, C). Compared with P. marina, the growth of ΔcapC strain increased significantly during 3 – 12 h (p < 0.05, Figure 1D). When it entered the stationary phase, the growth situation of the two strains was similar.
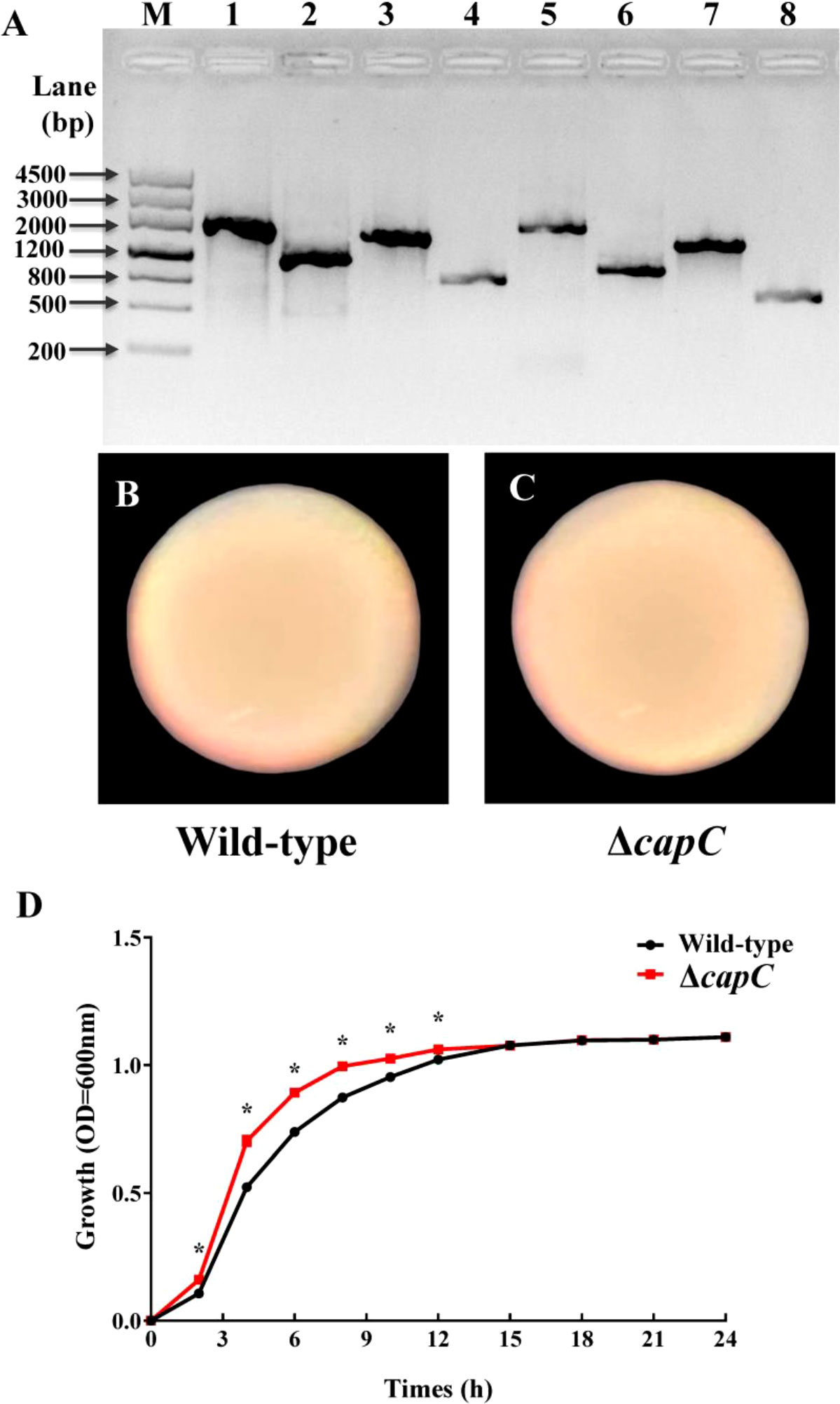
Figure 1 Construction of ΔcapC strain and the colony morphology and growth of wild-type and ΔcapC strains. (A) PCR verified the deletion of the capC gene. M: DNA Maker III; 1: capC-LF/LR, 1 835 bp; 2: ΔcapC-LF/LR, 1 106 bp; 3: capC-LF/SR, 1 396 bp; 4: ΔcapC-LF/SR, 667 bp; 5: capC-SF/LR, 1 600bp; 6: ΔcapC-SF/LR, 871 bp; 7: capC-SF/SR, 1 161 bp; 8: capC-SF/SR, 435bp. The orf (open reading frame) length of capC is 729 bp. (B, C) Bacterial colony characteristics of experimental strains. (D) Growth curve of experimental strains at 25°C. The * indicates a significance level of p < 0.05. The same as below.
3.2. ΔcapC strain reduced larval settlement
The results demonstrated that the inductive ability of ΔcapC biofilms to M. coruscus larvae was significantly reduced at each initial bacterial concentration (p < 0.05). Compared with wild-type biofilms, the largest reduction was 23.1% (p < 0.05, Figure 2A), whereas the bacterial cell density of ΔcapC biofilms did not differ significantly (p > 0.05, Figure 2B). There was no significant correlation between biofilm-inducing activity and bacterial density of biofilms (p > 0.05, Table S1).
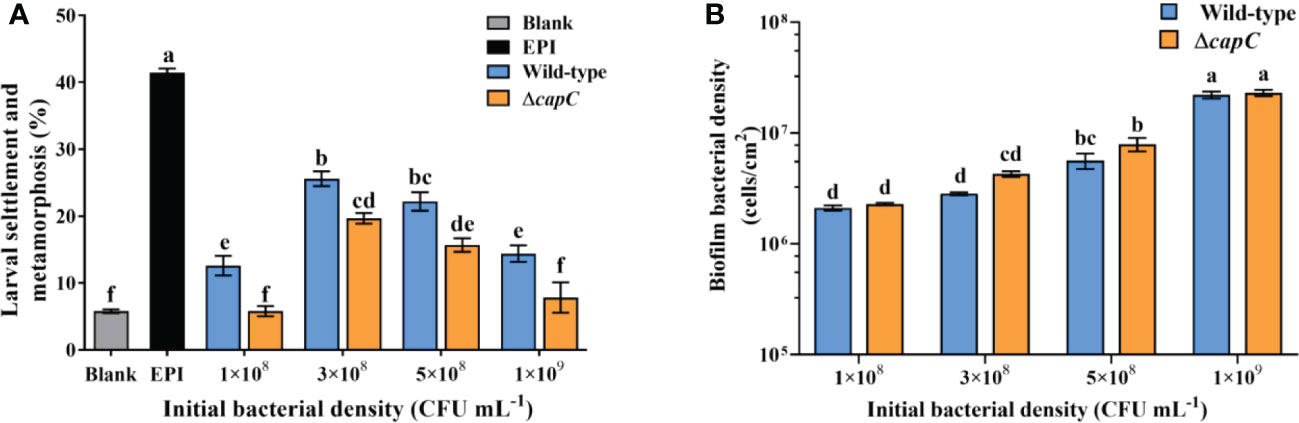
Figure 2 The biofilms of ΔcapC reduced the inducing activity. (A) The effects of biofilms generated by experimental strains on larval settlement and metamorphosis (96h). (B) Biofilm density produced by experimental strains with diverse initial CFU.
3.3. Effects of capC deletion on swimming motility and biofilm thickness
The swimming motility of ΔcapC strain was not significantly different from P. marina by observing (Figure 3A) and calculating the formation of the migration zone (p > 0.05, Figure 3B).
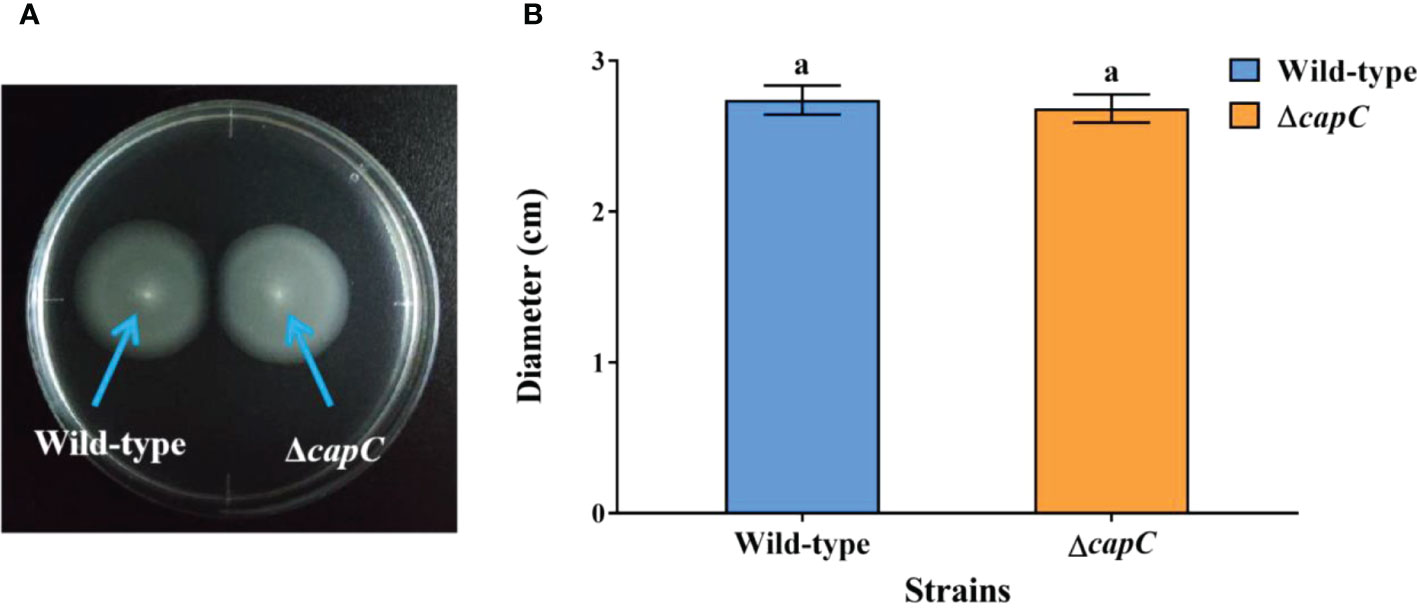
Figure 3 The swimming motility of experimental strains. (A) The swimming motility phenotypes of P. marina and ΔcapC strains. (B) The migration zone diameter of P. marina and ΔcapC strains.
The CLSM scanned data indicated that the bacterial aggregation of P. marina and ΔcapC biofilms was similar (Figures 4A, B). Biofilms were formed separately by the two strains with the initial density of 5×108 cells mL−1 (p > 0.05, Figure 4C). The rate of post-larvae showed no significant correlation with the thickness of biofilms (p > 0.05, Table S1).
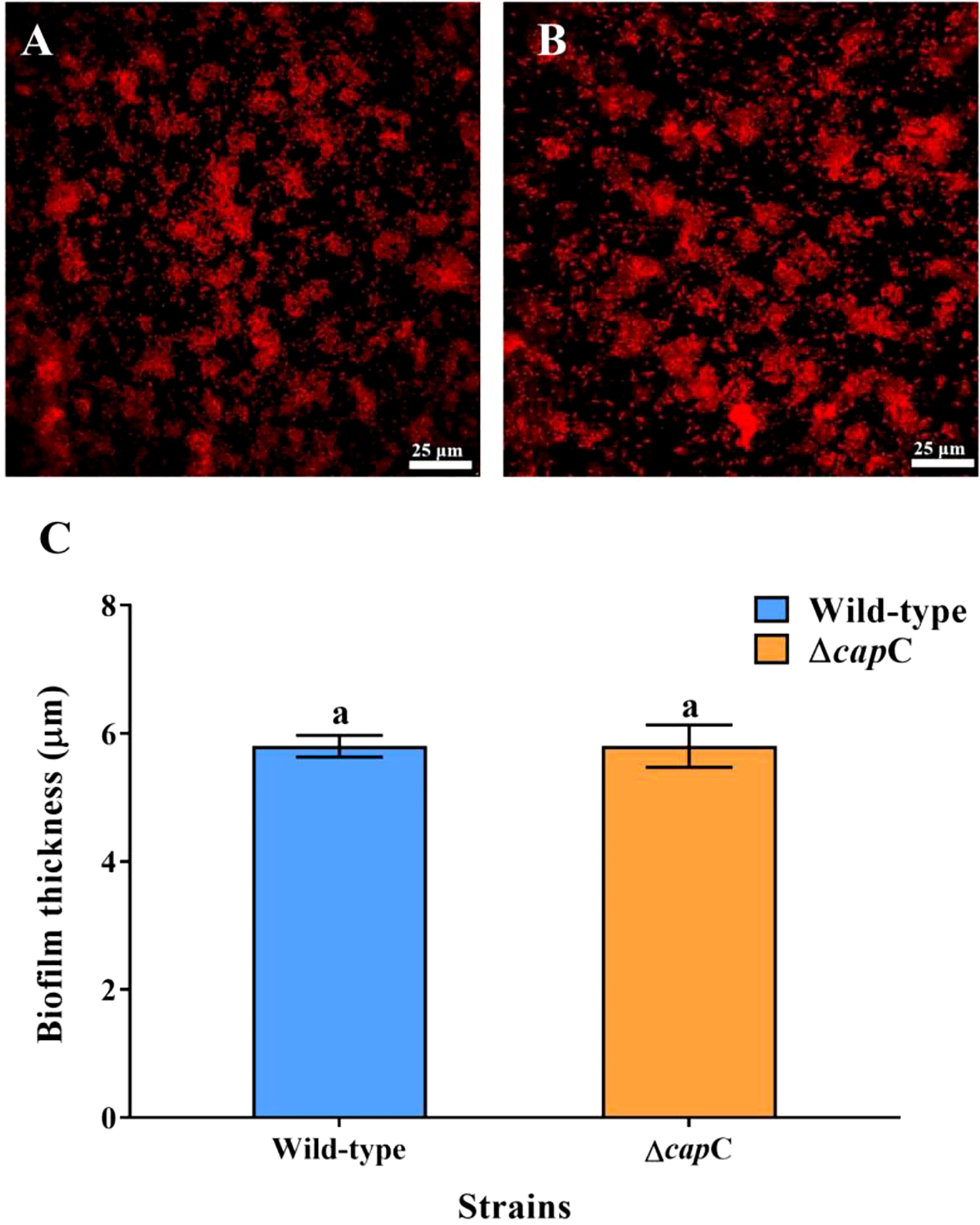
Figure 4 Biofilm-forming ability of experimental strains. (A, B) The CLSM images of wild-type and ΔcapC biofilms. (C) Quantitative results of biofilm thickness of wild-type and ΔcapC biofilms.
3.4. Comparison of content of bacterial CPS extraction and c-di-GMP
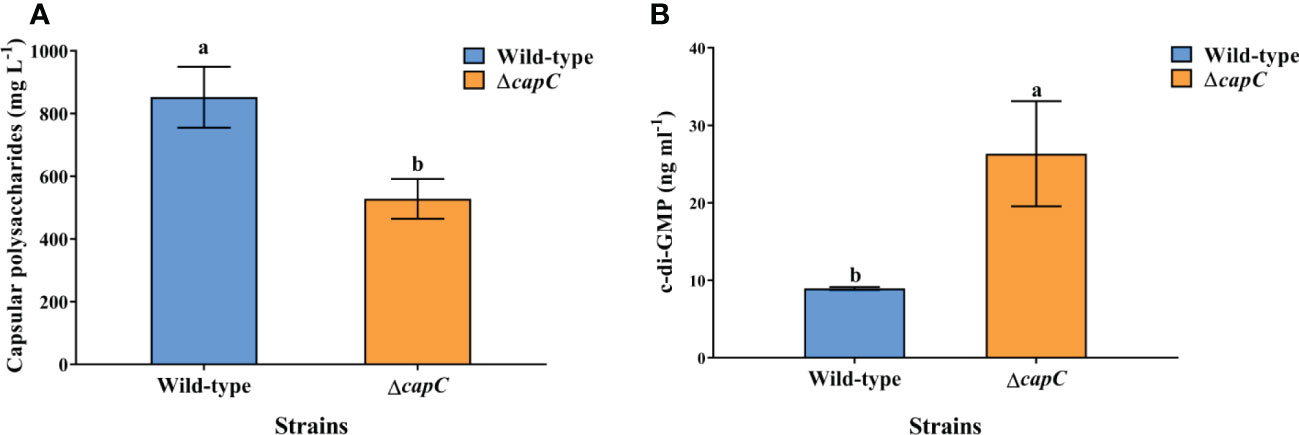
According to the results of the bacterial CPS analysis, the ΔcapC strain generated significantly less CPS (38.07% decrease) than P. marina (p < 0.05, Figure 5A). The CPS content significantly correlated with the inducing capacity of biofilms on the settlement and metamorphosis rate (r = 0.592, p < 0.05, Table S1). The results of bacterial c-di-GMP analysis showed that the c-di-GMP produced by ΔcapC strain was significantly higher than P. marina, and the increase was 1.94 times higher (p < 0.05, Figure 5B).
3.5. CLSM images of ΔcapC biofilms exopolysaccharide
CLSM scanned images exhibited that the production of exopolysaccharide was different between ΔcapC biofilms and wild-type biofilms (Figure 6A). There was also a significant difference in biomass between the two strains (p < 0.05, Figure 6B). The content of α-polysaccharides and β-polysaccharides produced by ΔcapC biofilms was 35.93% and 35.85% less than that in biofilms of P. marina, respectively (p < 0.05, Figure 6B).
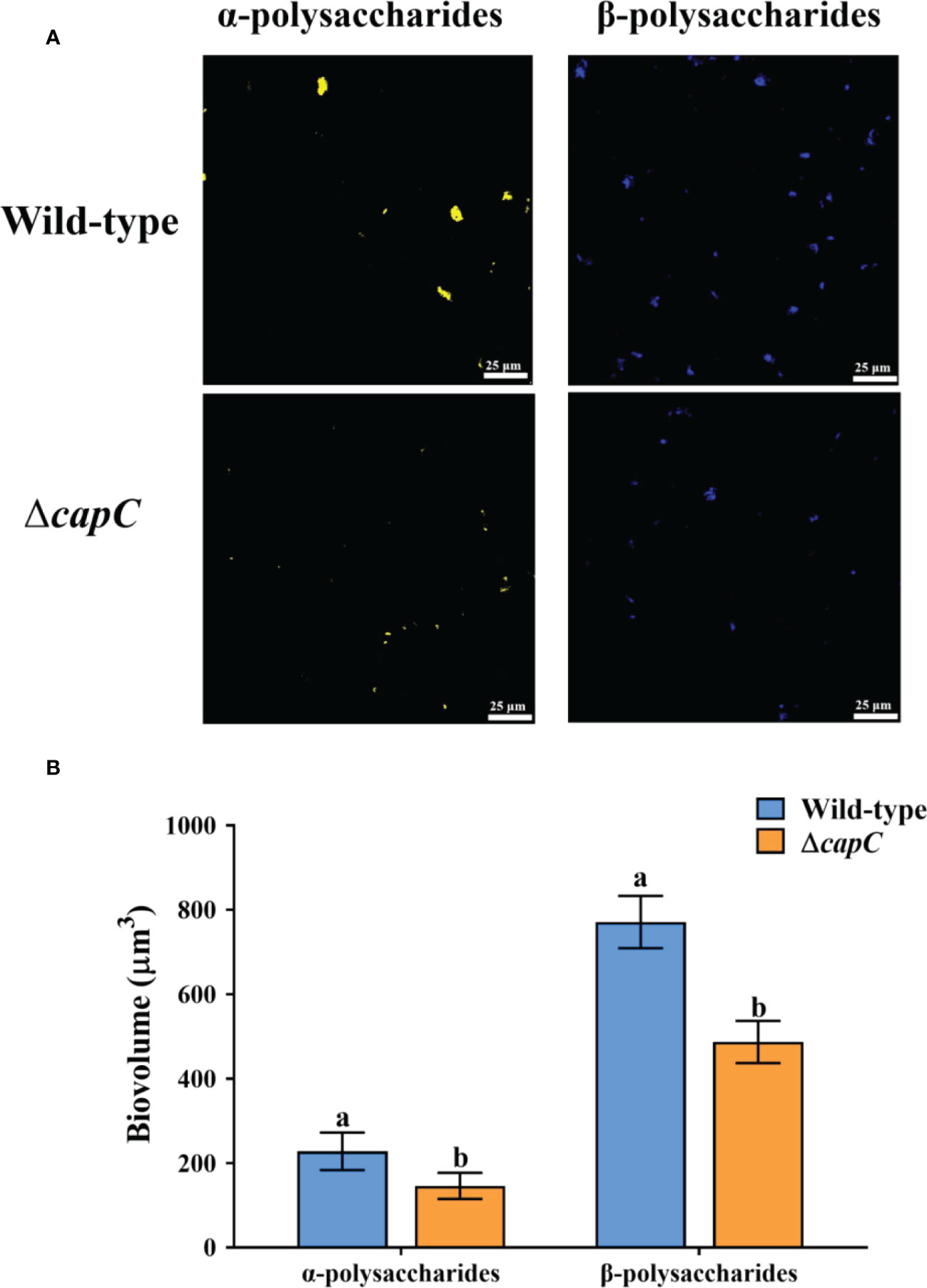
Figure 6 The CLSM analysis of exopolysaccharide in wild-type and ΔcapC biofilms. (A) Distribution of extracellular polysaccharides in two experimental biofilms. (B) Analysis of exopolysaccharide.
4. Discussion
Marine invertebrates and marine microorganisms are crucial components of marine ecosystems (Falkowski, 1998; Paredes et al., 2021), and participate in the maintenance of marine biodiversity. Many studies have shown that the larval settlement and metamorphosis of various marine invertebrates are influenced by the chemical cue on marine biofilms (Dworjanyn and Pirozzi, 2008; Tamburri et al., 2008; Webster et al., 2011; Whalan and Webster, 2014). CPS, as a bacterial polysaccharide, has been demonstrated to affect the formation of bacterial biofilms (Petruzzi et al., 2017; Eberle et al., 2020), whether CPS synthesis gene regulates biofilm formation of marine bacterial biofilm and larval settlement and metamorphosis is still undefined. Here, a capC deletion strain of P. marina was created, and it was found that the ΔcapC strain with lower CPS production and lower exopolysaccharide on ΔcapC biofilms significantly prevented mussel settlement and metamorphosis. This revealed that capC gene regulates the production of bacterial CPS, which participate in larval settlement and metamorphosis (Figure 7).
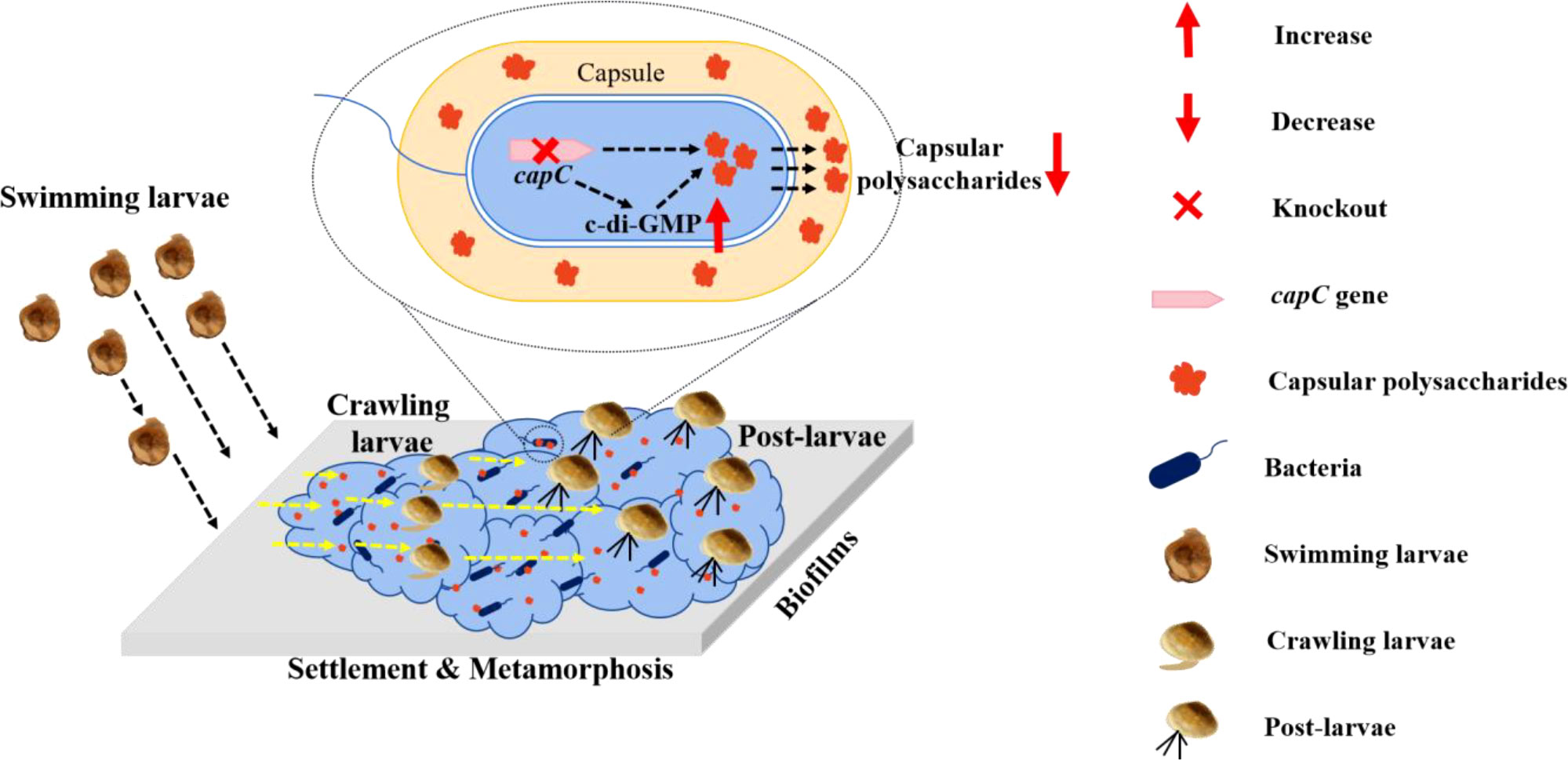
Figure 7 Model of capC regulation on larval settlement and metamorphosis: the capsular polysaccharide synthesis gene could modulate bacterial c-di-GMP which regulates the secretion of capsular polysaccharide, and then impact mussel larval settlement and metamorphosis. Once capC is deficient, bacterial biofilm biomass including cell density and thickness shows no change and capsular polysaccharide secretion is reduced accompanied with upregulation of c-di-GMP.
CPS is a major structural component outside the bacterial cell wall, appearing in many Gram-positive bacteria and Gram-negative bacteria (Whitfield, 2006; Cuthbertson et al., 2009). The general biosynthesis pathway of CPS is wzy dependent pathway, monosaccharide passes through the flipper enzyme to the outer periplasmic, and the high-level polymerization of CPS requires the alternation of phosphorylation of tyrosine kinase and homologous phosphatase (Wugeditsch et al., 2001; Hagelueken et al., 2009). It is reported that in E. coli, tyrosine kinase wzc and homologous phosphatase wzb have been proved to be pivotal genes during CPS synthesis and assembly (Wugeditsch et al., 2001; Cuthbertson et al., 2009). In Streptococcus Pneumoniae, the cpsCD complex is considered to be similar to wzc, and phosphatase cpsB is its corresponding homologous phosphatase (Grangeasse et al., 2007). In this study, homologous sequence alignment found that the capC gene of P. marina was similar to cpsB, and the deletion of capC gene led to a significant reduction in CPS content. Thus, it can be speculated that the capC gene plays a role in homologous phosphorylation in the P. marina CPS synthesis.
Previous studies have shown that the deletion of CPS synthesis gene can promote the formation of biofilms (Petruzzi et al., 2017; Eberle et al., 2020). The deletion of wza gene of V. vulnificus CPS transfer protein caused that the CPS in bacterial capsule cannot be secreted to extracellular surface, and the formation of biofilms was significantly increased. (Wright et al., 1999; Wright et al., 2001). During the formation of S. Pneumoniae bacterial biofilm, the decrease of cps3A gene expression led to the reduction of CPS content and promoted the formation of biofilms (Hall-Stoodley et al., 2008). Nevertheless, this study showed that the deletion of capC gene of P. marina did not alter the final growth ability, motility, colony morphology and bacterial density of biofilms, as well as biofilm thickness. It was showed that the capC gene of P. marina did not influence the ability of biofilm formation. Considering previous studies, it was speculated that the function of CPS synthesis gene is different in various bacteria. This situation may be caused by various bacterial characteristics and living environments. Furthermore, the analysis of CLSM images confirmed that the deletion of capC gene of P. marina resulted in a significant decline in α-polysaccharides and β-polysaccharides of biofilms. These findings showed that the effects of capsular polysaccharide biosynthesis genes on biofilm formation ability were different from those found in previous studies, the deletion of capC gene in this study only altered the exopolysaccharide secretion of P. marina biofilms, without affecting the ability of bacteria to aggregate.
Exopolysaccharide is the main component of bacterial biofilms, and it has great biological activity (Sutherland, 2001; Flemming et al., 2016; Moradali and Rehm, 2020). Many studies have found that a variety of exopolysaccharides in biofilms can regulate the larval settlement and metamorphosis in marine invertebrates (see Table S2) (Kirchman et al., 1982; Liang et al., 2021; Freckelton et al., 2022). The settlement and metamorphosis of Janua brasiliensis larvae is thought to be caused by the associated exopolysaccharides on the bacterial surface, rather than the water-soluble metabolites of the bacteria (Kirchman, 1982). Szewzyk et al. (1991) found that the decrease in the content of exopolysaccharide in Pseudomonas sp. (strain S9) reduced the settlement response of the larvae of C. intestinalis. Holmström et al. (1992) predicted the involvement of exopolysaccharide in larval settlement by analyzing the effects of 40 strains of marine bacteria on Balanus amphitrite and Ciona integrinalis (Holmström et al., 1992). And in the mutant strain of Pseudoalteromonas lipolytica AT00-17125 gene, the bacterial capsule became thinner, the content of colanic acid decreased, and significantly reduced the inducing activity on M. coruscus (Zeng et al., 2015). These above studies indicated that different polysaccharides have different inducing effects on the settlement and metamorphosis of marine invertebrate larvae.
This current investigation demonstrated that the knockout of CPS synthesis gene capC caused the increasing the bacterial c-di-GMP and reduction of the secretion of CPS and exopolysaccharide of biofilms, and the inducing activity of ΔcapC biofilms to M. coruscus larvae decreased significantly. Our previous work also found that bacterial colanic acid concentration nearly quadrupled after deleting gene 01912 associated to polysaccharide biosynthesis, accompanied with increasing c-di-GMP caused the significant upregulation in mussel larval settlement and metamorphosis (Peng et al., 2020). In addition, knockout cellulose synthesis gene bcsQ caused significantly a reduction in exopolysaccharide (including cellulose) and c-di-GMP, and inhibited the settlement of M. coruscus larvae (Liang et al., 2021). Thus, these findings suggested that bacterial c-di-GMP mediates polysaccharide secretion and subsequent changed the inducing capability of biofilm on larval recruitment. Further molecular basis is needed.
5. Conclusion
The present findings indicated that deletion of the gene capC in P. marina bring about the decrease in exopolysaccharides and CPS of biofilms, upregulation of bacterial c-di-GMP and significantly lower levels of biofilm-inducing activity. Thus, the CPS synthesis gene could modulate mussel recruitment via c-di-GMP and CPS. This study contributes a fresh insight to our understanding of the molecular processes that lead to the production of bacterial exopolysaccharides and recruitment of marine invertebrates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by The Experimental Animal Ethics Committee of Shanghai Ocean University.
Author contributions
J-LY and XL: supervision, funding acquisition, conceptualization, validation, data curation, writing original draft preparation, writing review and editing, visualization. C-HH and WZ: methodology, software, formal analysis, investigation, data curation, writing original draft preparation. X-MH: methodology, formal analysis, data curation, writing original draft preparation. AY and KO: writing original draft preparation, writing review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (41876159), Program of Shanghai Academic Research Leader (20XD1421800), Shanghai Foreign High-End Talent Project (22WZ2504500), and Science and technology innovation action plan “The Belt and Road international joint laboratory construction projects”, Science and Technology Commission of Shanghai (19590750500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1089024/full#supplementary-material
References
Badel S., Bernardi T., Michaud P. (2011). New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 29 (1), 54–66. doi: 10.1016/j.biotechadv.2010.08.011
Bao W. Y., Yang J. L., Satuito C. G., Kitamura H. (2007). Larval metamorphosis of the mussel Mytilus galloprovincialis in response to Alteromonas sp. 1: evidence for two chemical cues? Mar. Bio. 152 (3), 657–666. doi: 10.1007/s00227-007-0720-2
Bechon N., Mihajlovic J., Vendrell-Fernandez S., Chain F., Langella P., Beloin C., et al. (2020). Capsular polysaccharide cross-regulation modulates Bacteroides thetaiotaomicron biofilm formation. mBio 11 (3), e00729–e00720. doi: 10.1128/mBio.00729-20
Cavalcanti G. S., Alker A. T., Delherbe N., Malter K. E., Shikuma N. J. (2020). The influence of bacteria on animal metamorphosis. Annu. Rev. Microbiol. 74, 137–158. doi: 10.1146/annurev-micro-011320-012753
Chang Y. Q. (2007). Stock enhancement and culture in mollusks (in Chinese) (Beijing: China Agriculture Press).
Cuthbertson L., Mainprize I. L., Naismith J. H., Whitfield C. (2009). Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol. Mol. Biol. Rev. 73 (1), 155–177. doi: 10.1128/MMBR.00024-08
Dobretsov S., Rittschof D. (2020). Love at first taste: Induction of larval settlement by marine microbes. Int. J. Mol. Sci. 21 (3), 731. doi: 10.3390/ijms21030731
Dworjanyn S. A., Pirozzi I. (2008). Induction of settlement in the sea urchin tripneustes gratilla by macroalgae, biofilms and conspecifics: A role for bacteria? Aquaculture 274 (2-4), 268–274. doi: 10.1016/j.aquaculture.2007.11.030
Eberle K. C., Hau S. J., Luan S. L., Weinert L. A., Stasko J. A., Wang J., et al. (2020). Generation and evaluation of a Glaesserella (Haemophilus) parasuis capsular mutant. Infect. Immun. 88 (5), e00879–e00819. doi: 10.1128/iai.00879-19
Falkowski P. G. (1998). Biogeochemical controls and feedbacks on ocean primary production. Science 281 (5374), 200–206. doi: 10.1126/science.281.5374.200
Flemming H. C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14 (9), 563–575. doi: 10.1038/nrmicro.2016.94
Freckelton M. L., Nedved B. T., Cai Y.-S., Cao S., Turano H., Alegado R. A., et al. (2022). Bacterial lipopolysaccharide induces settlement and metamorphosis in a marine larva. PNAS 119 (18), e2200795119. doi: 10.1073/pnas.2200795119
Grangeasse C., Cozzone A. J., Deutscher J., Mijakovic I. (2007). Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem. Sci. 32 (2), 86–94. doi: 10.1016/j.tibs.2006.12.004
Hadfield M. G. (2011). Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann. Rev. Mar. Sci. 3, 453–470. doi: 10.1146/annurev-marine-120709-142753
Hagelueken G., Huang H., Mainprize I. L., Whitfield C., Naismith J. H. (2009). Crystal structures of wzb of Escherichia coli and CpsB of Streptococcus pneumoniae, representatives of two families of tyrosine phosphatases that regulate capsule assembly. J. Mol. Biol. 392 (3), 678–688. doi: 10.1016/j.jmb.2009.07.026
Hall-Stoodley L., Nistico L., Sambanthamoorthy K., Dice B., Nguyen D., Mershon W. J., et al. (2008). Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC. Microbiol. 8, 173. doi: 10.1186/1471-2180-8-173
Holmström C., Rittschof D., Kjelleberg S. (1992). Inhibition of settlement by larvae of Balanus amphitrite and Ciona intestinalis by a surface-colonizing marine bacterium. Appl. Environ. Microbiol. 58 (7), 2111–2115. doi: 10.1128/aem.58.7.2111-2115.1992
Hu X. M., Zhang J. B., Ding W. Y., Liang X., Wan R., Dobretsov S., et al. (2021). Reduction of mussel metamorphosis by inactivation of the bacterial thioesterase gene via alteration of the fatty acid composition. Biofouling 37 (8), 911–921. doi: 10.1080/08927014.2021.1981882
Kirchman D. (1982). Bacteria induce settlement and metaorphosis of jannu (Dexiospira) brasiliensis (Grude). J. Exp. Mar. Biol. Ecol. 56 (2–3), 153–163. doi: 10.1016/0022-0981(81)90186-6
Kirchman D., Graham S., Reish D., Mitchell R. (1982). Lectins may mediate in the settlement and metamorphosis of Janua (Dexiospira) brasiliensis grube (Polychaeta: Spirorbidae). Mar. Biol. Lett. 3, 131–142.
Lee K. J., Kim J. A., Hwang W., Park S. J., Lee K. H. (2013). Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol. Microbiol. 90 (4), 841–857. doi: 10.1111/mmi.12401
Liang X., Zhang X. K., Peng L. H., Zhu Y. T., Yoshida A., Osatomi K., et al. (2020). The flagellar gene regulates biofilm formation and mussel larval settlement and metamorphosis. Int. J. Mol. Sci. 21 (3), 710. doi: 10.3390/ijms21030710
Liang X., Zhang J. B., Shao A. Q., Hu X. M., Wan R., Yang J. L. (2021). Bacterial cellulose synthesis gene regulates cellular c-di-GMP that control biofilm formation and mussel larval settlement. Int. Biodeter. Biodegr. 165, 105330. doi: 10.1016/j.ibiod.2021.105330
Lin H., Wang C., Zhao H., Chen G., Chen X. (2018). Interaction between copper and extracellular nucleic acids in the EPS of unsaturated Pseudomonas putida CZ1 biofilm. Environ. Sci. pollut. Res. Int. 25 (24), 24172–24180. doi: 10.1007/s11356-018-2473-5
Liu X. B., Yang J. L., Rittschof D., Maki J. S., Gu J. D. (2022). Redirecting marine antibiofouling innovations from sustainable horizons. Trends Ecol. Evol. 37 (6), 469–472. doi: 10.1016/j.tree.2022.02.009
Lotze H. K. (2021). Marine biodiversity conservation. Curr. Biol. 31 (19), 1190–1195. doi: 10.1016/j.cub.2021.06.084
Moradali M. F., Rehm B. H. A. (2020). Bacterial biopolymers: from pathogenesis to advanced materials. Nat. Rev. Microbiol. 18 (4), 195–210. doi: 10.1038/s41579-019-0313-3
Nagar E., Schwarz R. (2015). To be or not to be planktonic? self-inhibition of biofilm development. Environ. Microbiol. 17 (5), 1477–1486. doi: 10.1111/1462-2920.12583
Paredes E., Heres P., Anjos C., Cabrita E. (2021). Cryopreservation of marine invertebrates: From sperm to complex larval stages. Methods Mol. Biol. 2180, 413–425. doi: 10.1007/978-1-0716-0783-1_18
Peng L. H., Liang X., Chang R. H., Mu J. Y., Chen H. E., Yoshida A., et al. (2020). A bacterial polysaccharide biosynthesis-related gene inversely regulates larval settlement and metamorphosis of Mytilus coruscus. Biofouling 36 (7), 753–765. doi: 10.1080/08927014.2020.1807520
Peng L. H., Liang X., Guo X. P., Yoshida A., Osatomi K., Yang J. L. (2018). Complete genome of Pseudoalteromonas marina ECSMB14103, a mussel settlement-inducing bacterium isolated from the East China Sea. Mar. Genom. 41, 46–49. doi: 10.1016/j.margen.2018.04.001
Petruzzi B., Briggs R. E., Tatum F. M., Swords W. E., De Castro C., Molinaro A., et al. (2017). Capsular polysaccharide interferes with biofilm formation by Pasteurella multocida serogroup a. mBio 8 (6), e01843–e01817. doi: 10.1128/mBio.01843-17
Rischer M., Guo H. J., Beemelmanns C. (2022). Signalling molecules inducing metamorphosis in marine organisms. Nat. Prod. Rep. 39 (9), 1833–1855. doi: 10.1039/d1np00073j
Sutherland I. W. (2001). The biofilm matrix–an immobilized but dynamic microbial environment. Trends Microbiol. 9 (5), 222–227. doi: 10.1016/s0966-842x(01)02012-1
Szewzyk U., Holmstrom C., Wrangstadh M., Maki J. S., Kjelleberg S. (1991). Relevance of the exopolysaccharide of marine Pseudomonas sp. strain S9 for the attachment of Ciona intestinalis larvae. Mar. Ecol. Prog. Se. 75 (2-3), 259–265. doi: 10.3354/meps075259
Tamburri M. N., Luckenbach M. W., Breitburg D. L., Bonniwell S. M. (2008). Settlement of crassostrea ariakensis larvae: Effects of substrate, biofilms, sediment and adult chemical cues. J. Shellfish Res. 27 (3), 601–608. doi: 10.2983/0730-8000(2008)27[601:Socale]2.0.Co;2
Wang C., Bao W. Y., Gu Z. Q., Li Y. F., Liang X., Ling Y., et al. (2012). Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to natural biofilms. Biofouling 28 (3), 249–256. doi: 10.1080/08927014.2012.671303
Wang P., Yu Z., Li B., Cai X., Zeng Z., Chen X., et al. (2015). Development of an efficient conjugation-based genetic manipulation system for Pseudoalteromonas. Microb. Cell. Fact. 14, 11. doi: 10.1186/s12934-015-0194-8
Webster N. S., Soo R., Cobb R., Negri A. P. (2011). Elevated seawater temperature causes a microbial shift on crustose coralline algae with implications for the recruitment of coral larvae. Isme J. 5 (4), 759–770. doi: 10.1038/ismej.2010.152
Whalan S., Webster N. S. (2014). Sponge larval settlement cues: the role of microbial biofilms in a warming ocean. Sci. Rep. 4, 4072. doi: 10.1038/srep04072
Whitfield C. (2006). Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Ann. Rev. Biochem. 75 (1), 39. doi: 10.1146/annurev.biochem.75.103004.142545
Wright A. C., Powell J. L., Kaper J. B., Morris J. G. Jr (2001). Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69 (11), 6893–6901. doi: 10.1128/iai.69.11.6893-6901.2001
Wright A. C., Powell J. L., Tanner M. K., Ensor L. A., Karpas A. B., Morris J. G. Jr., et al. (1999). Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67 (5), 2250–2257. doi: 10.1128/iai.67.5.2250-2257.1999
Wugeditsch T., Paiment A., Hocking J., Drummelsmith J., Forrester C., Whitfield C. (2001). Phosphorylation of wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 276 (4), 2361–2371. doi: 10.1074/jbc.M009092200
Yang J. L., Li S. H., Li Y. F., Liu Z. W., Liang X., Bao W. Y., et al. (2013). Effects of neuroactive compounds, ions and organic solvents on larval metamorphosis of the mussel Mytilus coruscus. Aquaculture 396, 106–112. doi: 10.1016/j.aquaculture.2013.02.039
Zardus J. D., Nedved B. T., Huang Y., Tran C., Hadfield M. G. (2008). Microbial biofilms facilitate adhesion in biofouling invertebrates. Biol. Bull. 214 (1), 91–98. doi: 10.2307/25066663
Zeng Z. S., Guo X. P., Cai X. S., Wang P. X., Li B. Y., Yang J. L., et al. (2017). Pyomelanin from pseudoalteromonas lipolytica reduces biofouling. Microb. Biotechnol. 10 (6), 1718–1731. doi: 10.1111/1751-7915.12773
Zeng Z., Guo X. P., Li B., Wang P., Cai X., Tian X., et al. (2015). Characterization of self-generated variants in Pseudoalteromonas lipolytica biofilm with increased antifouling activities. Appl. Microbiol. Biotechnol. 99 (23), 10127–10139. doi: 10.1007/s00253-015-6865-x
Zhang P., He M., Yin L., Ge Q. (2013). Deproteinization of the crude polysaccharide from pomegranate peel with sevage method. Food. Sci. Technol. 38 (12), 219–222+231. doi: 10.13684/j.cnki.spkj.2013.12.049
Keywords: Mytilus coruscus, settlement and metamorphosis, biofilm, capsular polysaccharide, capC gene
Citation: He C-H, Zhang W, Hu X-M, Yoshida A, Osatomi K, Liang X and Yang J-L (2023) Larval settlement and metamorphosis of Mytilus coruscus in response to varying bacterial capsular polysaccharide. Front. Mar. Sci. 10:1089024. doi: 10.3389/fmars.2023.1089024
Received: 03 November 2022; Accepted: 10 January 2023;
Published: 23 January 2023.
Edited by:
Tao Zhang, Institute of Oceanology (CAS), ChinaReviewed by:
Shugeng Cao, University of Hawaii at Hilo, United StatesXian Li, Ocean University of China, China
Copyright © 2023 He, Zhang, Hu, Yoshida, Osatomi, Liang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Liang, x-liang@shou.edu.cn; Jin-Long Yang, jlyang@shou.edu.cn
†These authors have contributed equally to this work and share first authorship
 Chu-Han He
Chu-Han He