
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 18 January 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1075313
 Ernest Obeng Chuku1,2,3*
Ernest Obeng Chuku1,2,3* Kobina Yankson1,2†
Kobina Yankson1,2† Edward Adzesiwor Obodai2
Edward Adzesiwor Obodai2 Emmanuel Acheampong2
Emmanuel Acheampong2 Denis Worlanyo Aheto1,2
Denis Worlanyo Aheto1,2Uncertainties associated with wild harvests of seed and adult oysters due to unknown oceanographic oscillations are a major challenge in oyster fisheries and aquaculture development. In contribution to addressing this challenge, we proffer clarity on the spatiotemporal variations in spatfall (number of spat/m2) of the mangrove oyster Crassostrea tulipa (Lamarck, 1819) in four estuaries along the Gulf of Guinea coast. By monthly deployment of artificial substrates affixed to bamboo racks over 12 months, we find significant differences in spatfall among and within the brackish systems, and across months and seasons. Spatfall regimes were unique in each ecosystem albeit with an overall preponderance of dry season availability of spat. Locations with reef oysters had superior spatfall to mangrove root-adapted-oyster areas. Narkwa, a relatively small lagoon with reef oysters had the highest annual mean spatfall, which was 1.3, 2.5, and 9.8 folds the spatfall in Densu Delta, Benya Lagoon and Whin Estuary, respectively. Spatfall varied significantly by depth as the more frequently exposed top collectors harvested much less spat than submerged collectors. There was a year-round availability of spat, confirming continuous spawning in C. tulipa. Spatfall variability was significantly driven by fluctuations in prevailing dissolved oxygen and salinity. Prevailing dissolved oxygen and salinity levels in the estuaries for optimal spat settlement were 1.68 – 3.40 mg L-1 and 11.00 – 29.33 ppt (parts per thousand), respectively. The findings of this study are recommended as empirical reference points for sustainable seed procurement for aquaculture production and management of C. tulipa fishery in the region of the study.
Mangrove oysters distributed along the shores of the tropical Atlantic Ocean are a vital fishery resource harvested by many communities for subsistence and commercial purposes. The meat of these oysters is a cheap source of animal protein and more so for the vulnerable (women and children) in coastal communities of developing nations. At the same time, the shells are utilised as a building material, as a poultry feed ingredient and in traditional medicine, particularly in West Africa (Yankson, 2004). Active fishing and trade of these oysters occur in South America (Lapegue et al., 2002; zu Ermgassen et al., 2020) and West Africa (Osei et al., 2020; Chuku et al., 2022), where a vast expanse of mangrove vegetation provides suitable habitat for the growth of mangrove oyster species such as Crassostrea rhizophorae and Crassostrea brasiliana in South America and the Caribbean, and Crassostrea tulipa in West Africa. In West Africa, for example, fisheries associated with C. tulipa and other shellfish of estuarine ecosystems were valued at US$ 300 million in 2021, with women being the primary beneficiaries (Chuku et al., 2022).
The continued dependence on this vital resource for food and livelihoods is, however, threatened by climatic factors, mangroves’ degradation for fuelwood use, and other marine stressors, resulting in unpredictable yields. The open-access nature of the fishery in many regions also exposes the animals to overexploitation, thus rendering many populations of the species unsustainable and at risk of total depletion (Cabral et al., 2019; Arthur, 2020; Aguión et al., 2022). Particularly in West Africa, mangrove oysters are not targeted for management on a broad scale (Chuku et al., 2022). To safeguard mangrove oyster populations to ensure communities who depend on the species obtain food security and livelihood opportunities, many stakeholders have adopted to encourage the aquaculture and wild management of the species (Asare et al., 2019; Chuku et al., 2020; Chuku and Osei, 2020; Osei et al., 2021; Mahu et al., 2022). On the other hand, there is also a strong interest in shifting from fed to non-fed aquaculture species as the latter does not require supplemental feeding, hence, has minimal risk of polluting the environment (Fredheim and Reve, 2018; Bhosle et al., 2021; Barrett et al., 2022; McClenachan and Moulton, 2022).
Whereas the threats to the wild fishery and benefits of farming mangrove oysters are well acknowledged, the essential data on biology and ecology needed to manage its fishery and inform the large-scale aquaculture of the endemic species C. tulipa is limited in many coastal areas of the eastern Atlantic coast. Factors influencing the reproduction and successful recruitment of new cohorts into the wild stock of the species are not well researched. Meanwhile, the present exploitation of C. tulipa is faced with fluctuating harvest yields, an occurrence anecdotally attributed to the annual climate variability, i.e., the wet and dry seasons. Oyster fishers have reported low yields during periods of high rainfall, whereas periods after the rains have seen increased yields. The accounts on C. tulipa harvests, consequential of spat availability or stock recruitment, are speculative at present, thus, impeding its exploitation to the full potential. Further, the survival of adult C. tulipa in this region’s myriad of suitable natural habitats is unknown and very little is known of the conditions for optimum seed availability for possible procurement in its aquaculture and the wild management of the species.
This lack of data is limiting the development of C. tulipa in support of coastal management objectives to tackle undernutrition and hunger (SDG 2) and ensure decent jobs and economic growth (SDG 8) through sustainable management of life below water (SDG 14). The literature on mangrove oysters is bereft of data on spatiotemporal spatfall regimes assessed in tandem with the corresponding physico-chemical estuarine conditions to model the effects on spatfall. At present, the species is known to have fast growth reaching table size in approximately seven months in open culture (Asare et al., 2019) and exhibits protandric sequential hermaphroditism as a reproductive adaptation (Yankson, 1996). Considering the foregoing and the fact that the adult stocks are almost fished out in some estuaries during harvesting periods, an understanding of the larval availability is a crucial step toward sustainability interventions, that is, management and aquaculture development.
In this study, we address the data deficit in spatial and temporal spatfall dynamics of C. tulipa by running experiments in different brackish ecosystems selected to reflect different hydrodynamics along the coasts of the Gulf of Guinea. We were particularly interested in ascertaining variations in spatfall at different water depths, as conditions in these different layers were unknown. The monthly and seasonal availability of C. tulipa spat in each brackish system was assessed from November 2017 to October 2018. To depict relative depths at the different spat collection sites and the potential influence on spatfall, bathymetry maps were developed and overlaid with “presence of adult C. tulipa” data. This was necessary as spat availability is a function of larval production which is dependent on adult spawning and successful spat recruitment (Friedman et al., 1998; Soria et al., 2015; Gregori et al., 2019). We further regressed some physicochemical estuarine parameters against the observed spatfall regimes at the different stations and throughout the year to identify the significant hydrographic predictors and their prevailing ranges for optimal C. tulipa spatfall in the open estuary. This knowledge on spatfall regimes highlights the significance of different ecosystems to enable coastal management decisions, including the establishment of protected areas to conserve oyster breeding grounds as demanded by the industry (Kennedy and Roberts, 2006; Laing and Spencer, 2006; Gregori et al., 2019).
Four brackish systems – an open lagoon, an intermittently closed lagoon, a deltaic estuary and a classical fluvial estuary reflecting the hydrography of coastal brackish ecosystems along the Gulf of Guinea – were assessed for C. tulipa spat availability. Sites representing each of these systems were selected in the eastern part of the Gulf of Guinea, on the coast of Ghana. The open and intermittently closed lagoons were represented by Benya (located between 1°20′50″ W, 5°04′59″ N and 1°21′26″ W, 5°05′18″ N), and Nakwa (located between 0°56′22″ W, 5°12′17″ N and 0°54′41″ W, 5°12′32″ N) lagoons, respectively. The Densu Delta represented the deltaic estuary (lies between 0°16′43″ W, 5°34′07″ N and 0°20′02″ W, 5°30′21″ N), and the classical fluvial estuary was represented by Whin (located between 1°46′47″ W, 4°52′52″ N and 1°46′04″ W, 4°52′30″ N). The locations of these water bodies spanned a distance of about 200 km from the easterly Densu delta to the westerly Whin Estuary (Figure 1). These water bodies were selected to represent significant oyster harvesting locations along the Ghana Gulf of Guinea coastline and also to explore the uniqueness of the brackish systems, with respect to hydrology and geomorphology, in the region.
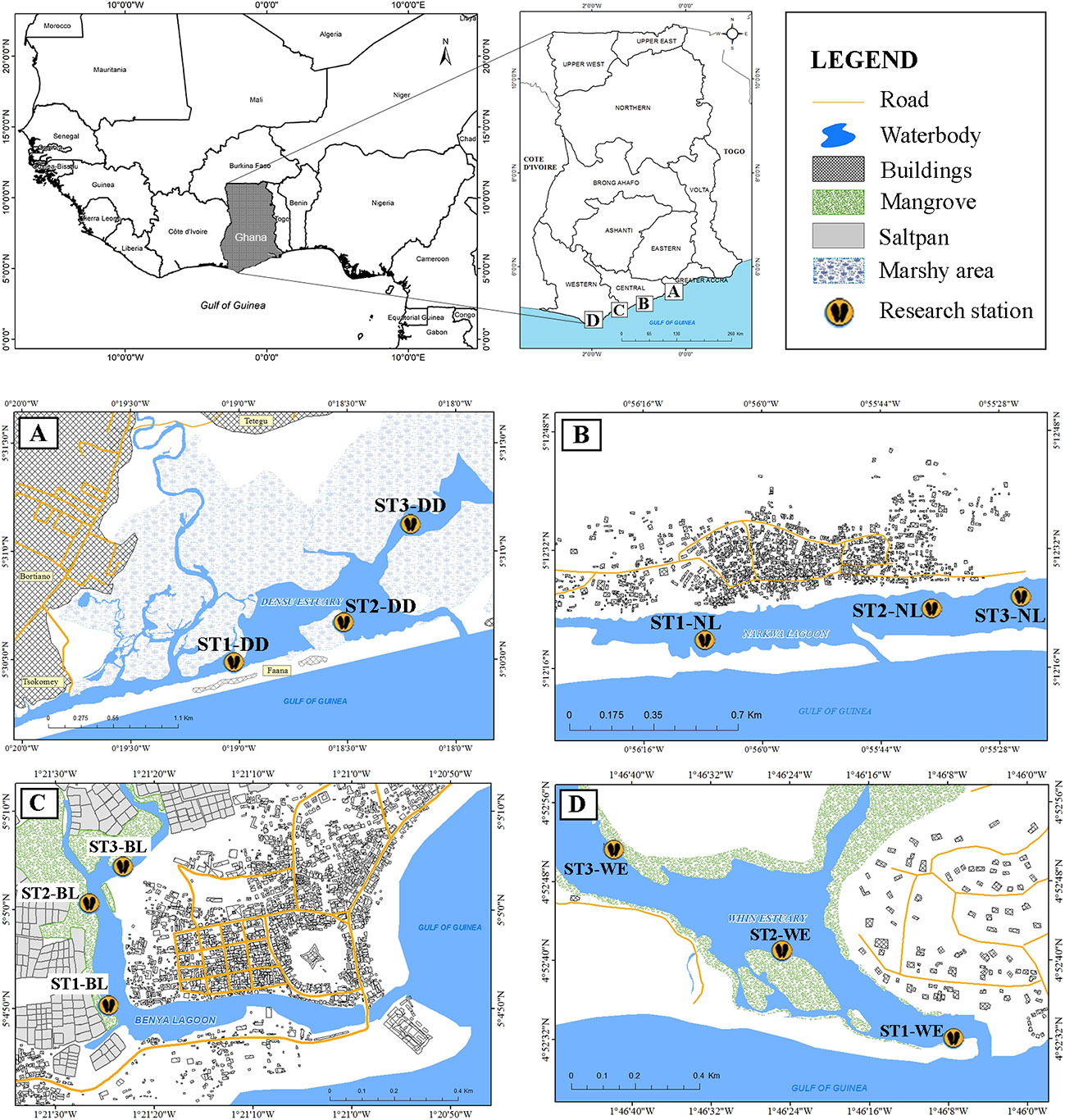
Figure 1 Maps of the water bodies with experimental stations (ST) indicated: (A) Densu Delta - DD, (B) Narkwa Lagoon - NL, (C) Benya Lagoon - BL, and (D) Whin Estuary - WE. Published first in Chuku et al. (2020).
The Densu Delta is a deltaic estuary. The larger Densu River Delta wetland (Figure 1A) is a Ramsar site and covers a vast low-lying land mass encompassing the concession of a salt manufacturing company. Excluding the salt company, the wetland covers an area of about 8.2 km2. The delta is heavily influenced by freshwater from the Densu River, which is dammed upstream by the Weija Dam and emerges as smaller tributaries downstream that empty into the wetland, cumulating in the southerly estuarine section before discharging into the Gulf of Guinea. C. tulipa colonies are found on the bed of the estuary having lost their primary habitat (mangroves) to degradation from cutting for use in the artisanal brush-park fishing in the Densu delta.
Narkwa Lagoon is a complex, intermittent lagoon estuary which lies parallel to the sea and is regularly fed by one of the two major tributaries of the Okye River, the Narkwa-Okye tributary. The lagoon has a somewhat centrally placed mouth usually open to the sea (Figure 1B), which seals off once in several years to form a continuous sand bar (observed during the course of this study) as a result of persistent sand accretion. The sand bar breaks after a few months by the force of water from the riverine source during the rains or mechanically opened to revert resulting floods. The lagoon covers an area of about 0.6 km2. The oysters C. tulipa are adapted to the sandy-mud bottom of the lagoon.
Benya Lagoon is an open tidal lagoon constantly flooded by the sea. Benya Lagoon (Figure 1C) is a man-made open lagoon which maintains contact with the Gulf of Guinea throughout the year (Obodai et al., 1991). Dykes have been constructed at the mouth of the lagoon to maintain a permanent opening. The lagoon is bordered on the east by the densely populated urban community, Elmina, and to the immediate west and north by mangrove vegetation and large saltpans. C. tulipa abounds on the roots of the mangrove fringing the lagoon, with a few found on the muddy bottom. The lagoon receives minimal freshwater inflow during the rainy season.
Whin Estuary (Figure 1D) is a classical river estuary. It lies oblique to the Gulf of Guinea relative to the orientation of the prevailing shoreline at its narrow mouth, which is about 90 m wide compared to further upstream where it spans over 350 m wide. It is relatively shallow and constantly fed with freshwater from the Whin River. The estuary is fringed by mangrove vegetation that provide suitable habitat for the C. tulipa. Although largely shallow with most parts of its sandy bottom exposed at low tide, there is a relatively deep (>1m) rocky section at the western north area which serves as a natural C. tulipa sanctuary. Relatively few oysters are found on the predominantly sandy bottom of the estuary. The estuary receives effluents from near-by industrial area.
The spatial dimensions of the study constituted four coastal water bodies (Densu Delta, Narkwa Lagoon, Benya Lagoon and Whin Estuary) at distances of approximately 65 km apart, from one water body to the next, and three experimental stations (ST1, ST2 and ST3) in each water body. Station 3 at the Densu was, however, eliminated due to conflict with local fishing activities. At each station, a rack made of bamboo stakes with an upper frame measuring approximately 1 × 1 m2 in length and breadth was installed as a holding unit for the study (Figure 2). The height of each rack was adjusted by the position of the upper frames to the level of the observed high tide mark at each station.
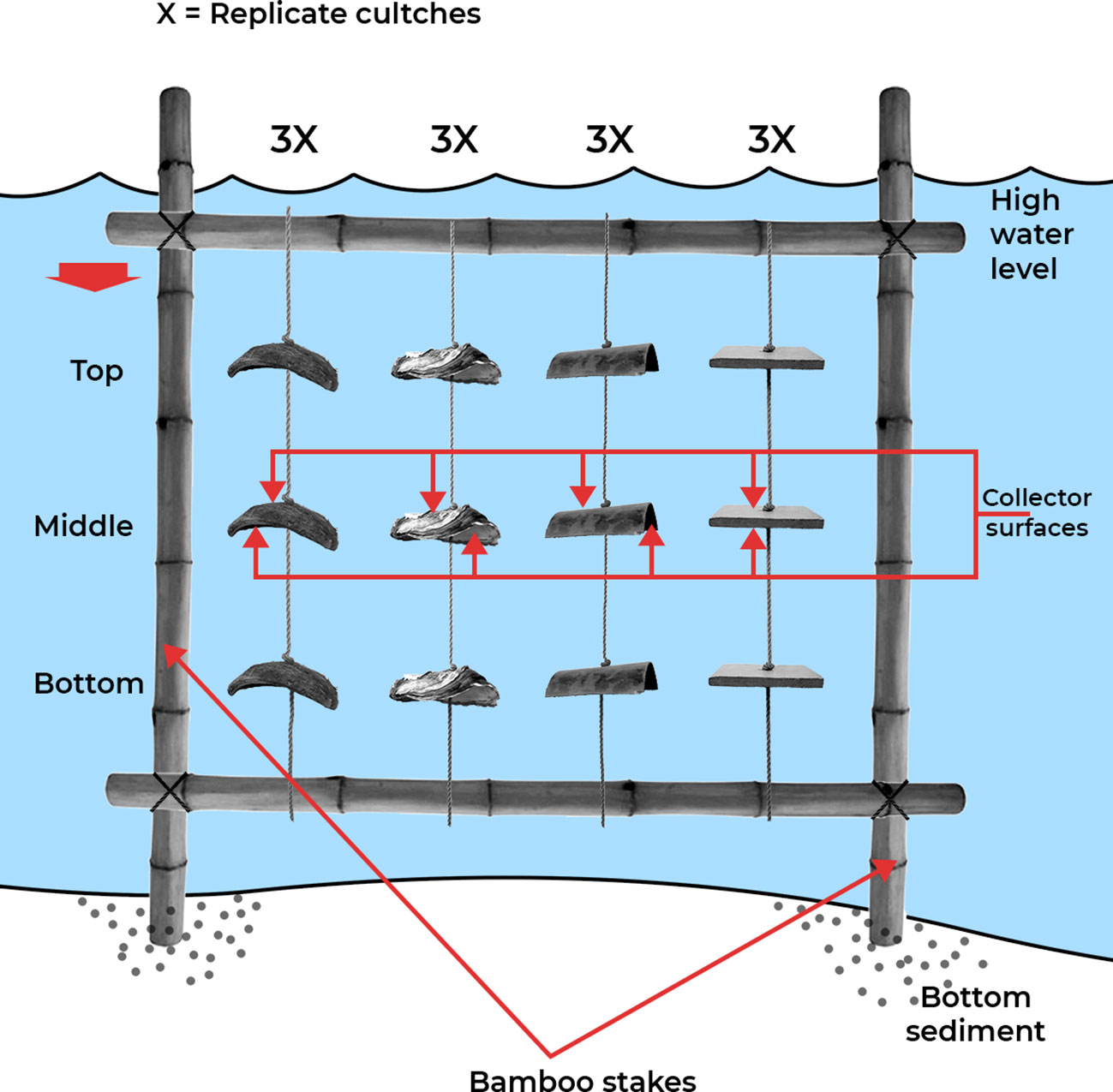
Figure 2 An illustration of the cross-sectional view of the experimental setup at each station (not to scale).
Each rack (holding unit) was strung with collectors of varied materials of the most locally common, affordable and suitable artificial spat collectors (ceramic tiles, coconut shells, oyster shells, and PVC slats) with different spat harvesting capacities (Chuku et al., 2020). The use of different collectors was assumed to mimic the natural occurrence as the species settles on a host of substrates in the open estuary environment. Strings of 3 collectors of the same material, knotted apart (a cultch), were hung vertically on the racks in water with one each at the top, middle and bottom water column (Figure 2). Thirty-six (36) double-sided collectors were deployed at each station with twelve, i.e., three of each of the four different materials, at each water column/depth.
Collectors at the top were adjusted to ≈ 0.1 m below water surface at high water level, bottom ≈ 0.1 m off the bottom, and middle collectors placed equidistant from the two. The depths at the experimental stations are presented in Supplementary Table 1. Although the actual depth in numerical value varied at the stations, the underlying principle of the design was inundation and exposure, such that top collectors were more frequently exposed to open air, middle collectors were moderately exposed/submerged, whereas bottom collectors were only briefly exposed or under constant submergence, by tidal action. This presents a consistent phenomenon of top, middle, and bottom at all experimental stations. Temporally, data were collected at each station monthly for 12 months from November 2017 to October 2018. The sampling months were categorised into the two main annual climatic (dry and wet) seasons using the anomalies from rainfall data obtained for the period of the study from the online global climate database (Tutiempo.net, 2019). Consecutive months of positive anomalies, that is, average total rainfall higher than the annual (12 months study period) mean, were assigned to the wet season category, and vice versa.
At each sampling, spat-laden collectors were removed and carefully arranged, bound and marked according to stations to prevent mixing. A new set of collectors were then installed. The spat-laden collectors were transported to the laboratory where spat were later counted, and spatfall was estimated as the number of spat per square metre of collector surface. The fold-and-trace method was applied for the determination of surface areas of irregular collectors (Morales-Alamo, 1993; Chuku et al., 2020). A combination of localised light and a hand lens was employed to detect very small and translucent spat. Collectors were thereafter cleaned and sun-dried for three days before returning them to the field the following month for replacement. In the course of the study, heavy rains destroyed and drifted the experimental rack at Station 2 of Narkwa Lagoon in June 2018.
Similarly, the experimental setup at Station 3 of Densu Delta suffered repeated destruction due to conflict with intense fishing activities in this section of the delta. These resulted in no spatfall data for Station 2 of Narkwa Lagoon in June 2018 and impeded successful spat collection at Station 3 of Densu Delta entirely. They were uncontrollable limitations of the study albeit not of significant influence on the scientific inferences made from the study. However, these occurrences underscore the challenges with open-water aquaculture experiments.
C. tulipa spatfall was estimated as the spat density on each surface of the collectors (number of spat per unit area of collector surface); hence, spatfall was estimated using the derived equation (Chuku et al., 2020):
Where Sfi = spatfall on individual collector, Ns = Number of spat on collector surface, Ac = Surface area of collector material, = mean spatfall, i = 1, 2, 3 … nth replicate collector, and n = number of replicate collectors/surfaces.
Several previous spatfall studies do not consider surface area of the settlement substrate making it difficult to numerically compare with accuracy spatfall under different conditions. The spatfall estimation used here provides a standardised metric to overcome this challenge.
To relate the observed C. tulipa spatfall with the prevailing estuarine conditions, some physical and chemical parameters of the estuaries and lagoons studied including temperature, salinity, dissolved oxygen (DO), and turbidity were measured in situ at all stations during each sampling, mostly at mid-tide. A multi-parameter water quality instrument (HORIBA U-50®) was used for the reading of temperature (°C), DO (mg L-1), turbidity (NTU) and pH. Salinity [parts per thousand (ppt)] was measured with the E-LINE Refractometer 44-808. Three readings were taken for each of the parameters within the water column at the respective experimental stations without considering the vertical stratification of the water bodies as the brackish systems were shallow, measuring up to about one meter in depth at high water (Supplementary Table 1). With the bottom of some stations completely exposed at low tide and considering the regular inflow and outflow of estuarine water, the systems were assumed to have vertical homogeneous mixing with negligible differences in hydrographic conditions from top to bottom at the stations.
Aerial images of the water bodies were captured with UAV – DJI Phantom 3 Professional and image classification and digitisation were done with the Pix4Dmapper® software. To obtain the depth profile of the water bodies, a depth sounder (3-126-D15) was used to measure depth along grids that were created remotely with ArcGIS software; these grids had geographical positioning system (GPS) location information. The sizes of grid areas varied based on the size of the particular water body under study. Within each grid area, depth was measured, and the specific location was re-marked with a GPS device (Garmin GPSMap64 Handheld Navigator). The data for depth (bathymetry) and the corresponding GPS coordinates were entered into a Microsoft Excel spreadsheet and exported to the ArcMap 10.3 software in comma-separated values (.csv) file format. The data was then converted to shapefiles and interpolated using Inverse Distance Weighting (IDW) tool to create depth profiles overlaid on the maps digitised from the aerial images. Locations with oyster populations within the water bodies were identified and marked through direct observations and also dwelling on local traditional knowledge of oyster harvesting locations. In water bodies with C. tulipa populations on the roots of mangroves, the landward extent of mangrove vegetation with oysters on roots was measured by a horizontal distance using a tape measure.
A nested multi-way Analysis of Variance (ANOVA) was applied in a factorial design to explicate the C. tulipa spatfall variations in the selected Gulf of Guinea brackish systems considering the complex ecosystem interactions across space and time. Spatial factors were the different water bodies (Waterbody), stations within each waterbody (Station) and the categorised depth – top, middle, bottom – in each water body (Depth). The temporal factor was the sampling month (Month) or season (dry season = Nov-17 to April-18; wet season = May-18 to Oct-18). The categorical variable “Station” (Station 1, 2 and 3) was not comparable across systems as the systems were different in configuration with respect to geomorphology and flow. For instance, Densu was deltaic in nature, Narkwa Lagoon had a centrally placed mouth, whereas Benya Lagoon and Whin Estuary had terminal mouths at the end of the watercourse where they meet the ocean. In addition to their varying characteristics, “Station” was less by one at the Densu Delta, presenting an unbalanced design for the variable “Station”.
For these reasons, “Station” was nested in “Waterbody” during the data analysis to present a scenario of 11 independent sampling stations. The response factor, spatfall (number of spat m−2), was a continuous variable. The data were checked for normality and homogeneity of the variance with Levene’s test, and the spatfall data were transformed to log 10 (x + 1) before ANOVA. The analysis was done using the General Linear Model function in Minitab® 20.3 (64-bit). The critical P-value for all the ANOVA was taken to be 0.05. Tukey’s HSD (α = 0.05) was used as the post-hoc test to determine which pairs of means were significantly different. The relationship between C. tulipa spatfall and depth in the water column was determined in each water body using One-Way ANOVA with depth (top, middle, bottom) as a categorical predictor and spatfall (spat m-2) as response variables. One-Way ANOVA was also used to determine the difference in spatfall among seasons (dry and wet) in general and within the waterbodies and to compare physicochemical estuarine parameters among the water bodies.
The physicochemical estuarine parameters, temperature, dissolved oxygen (DO), salinity, turbidity and pH, were analysed as predictors of spatfall for all water bodies combined for the study period with a multi-linear regression model in Minitab® 20.3 (64-bit) using the “Fit Regression Model” function. The data was checked for homoscedasticity and multicollinearity and transformed for the regression model [log10 (x + 1) for spatfall and salinity; log10 (x) for temperature, DO, turbidity and pH]. Marginal plots generated with the R Statistical Programme were used to visualise the distribution and scattering of the relationship between spatfall and its significant hydrographic markers. We assess the statistical distributions and confidence intervals (CI = 95%) to suggest optimal prevailing conditions for spatfall in the systems. Summary means of hydrographic factors are presented with stations pooled for each water body as many stations were similar.
Variable spatiotemporal spatfall regimes were found in the different Gulf of Guinea brackish systems from which C. tulipa spat was collected in this study. Table 1 presents a summary of the single and combined effects of spatial (i.e., the different water bodies, stations and depths) and temporal (sampling months, and further, seasons) factors on C. tulipa spat availability, showing significant interactions with spatfall (Table 1) at all levels. There were significant monthly variations in spat availability within each of the four water bodies (Table 1).
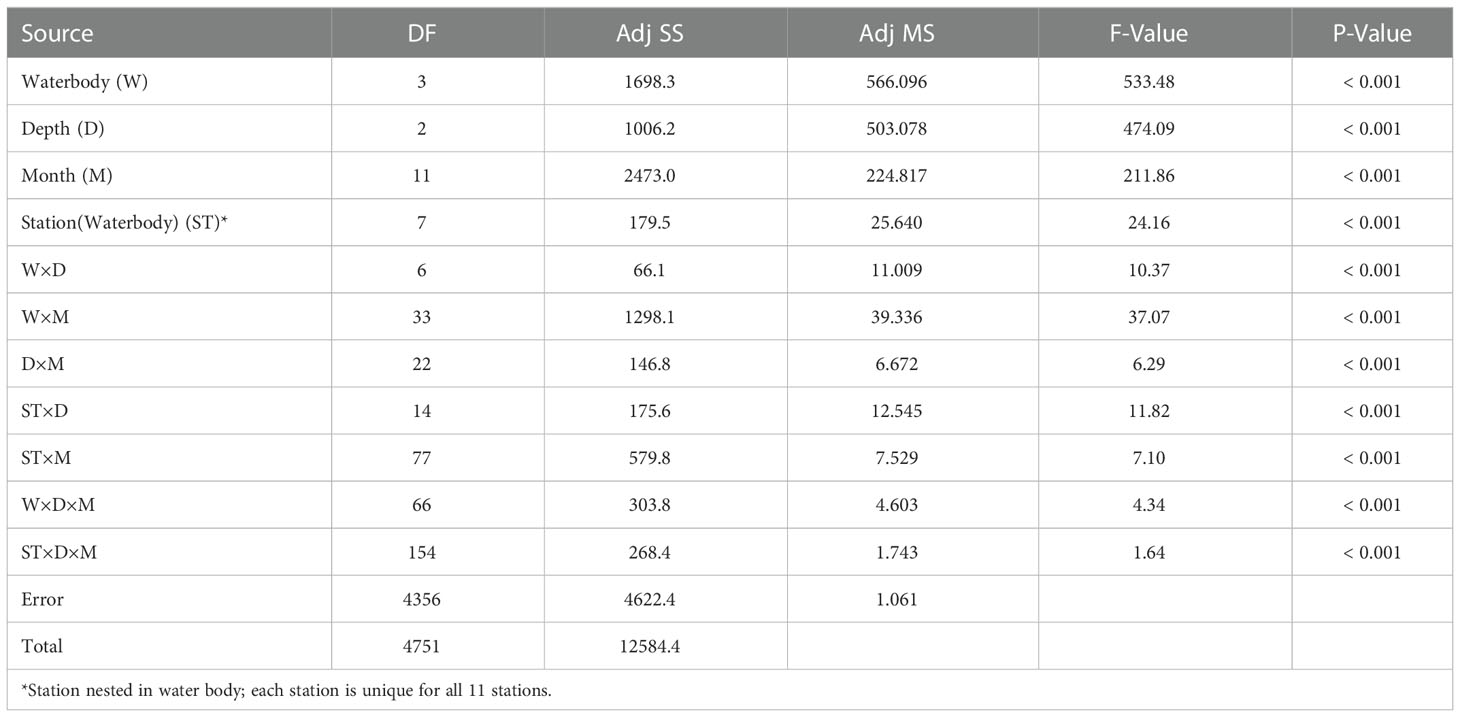
Table 1 Results of multi-way ANOVA of the spatial (water body, stations, and depth) and temporal (monthly) variations in C. tulipa spatfall in the selected coastal water bodies.
Spatfall varied significantly (P < 0.001) depending on the depth at which collectors were placed in the water column (see ANOVA results in Table 1). The overall depiction was a bottom-up decrement in spatfall with bottom water column collectors harvesting most of C. tulipa spat whilst top collectors had the least. This occurrence was not expressed at a few stations, but in most of these cases, the superiority of middle and bottom water column spat availability over the top was maintained (one-way ANOVA). This observation also had slight variations upon nuancing spatfall by water body and season. Charts of station-specific variations in vertical water column (top-middle-bottom) availability of C. tulipa spat are overlaid on composite maps of depth profile and presence of adult C. tulipa in each system in Figures 3–6.
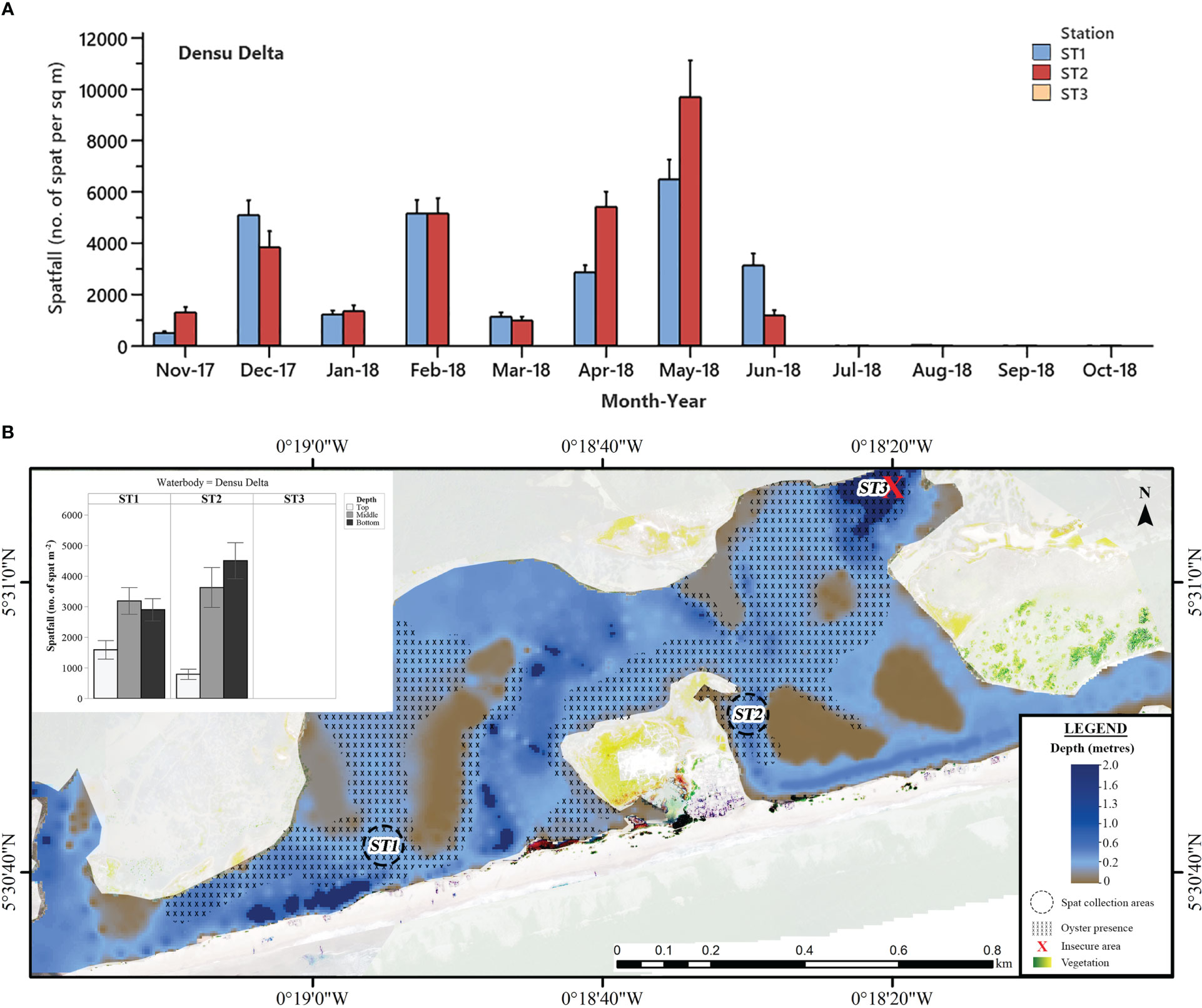
Figure 3 (A) Mean monthly C. tulipa spatfall (spat m-2) at stations (ST) in the Densu Delta pooled from collectors at all depths for each water body. Interval bars are standard errors (S.E.) of the mean. (B) Geomorphological representation of the estuarine section of Densu Delta at low tide showing depth profile, oyster presence and charted C. tulipa spat availability in water column at the experimental spat collection stations. Map generated from orthomosaic image of UAV flights for this study in June 2018.
Spatfall differed significantly for the dry season, i.e., the period from November 2017 to April 2018, and the wet season (May 2018 to October 2018). Overall, mean spatfall for the dry season (2259 ± 60 spat m-2 month-1) was greater than that of the wet season (1037 ± 46 spat m-2 month-1) (One-way ANOVA; P < 0.001); pooled from all the systems studied. There were, however, seasonal discrepancies in the respective brackish systems (Table 2). Spat settlement was significantly higher in the dry season than the wet season (One-way ANOVA) in both Densu Delta (P = 0.003) and Narkwa Lagoon (P < 0.001), the inverse occurred in the Benya Lagoon (P < 0.001), whilst a marginal difference (P = 0.049) was recorded in the Whin Estuary.
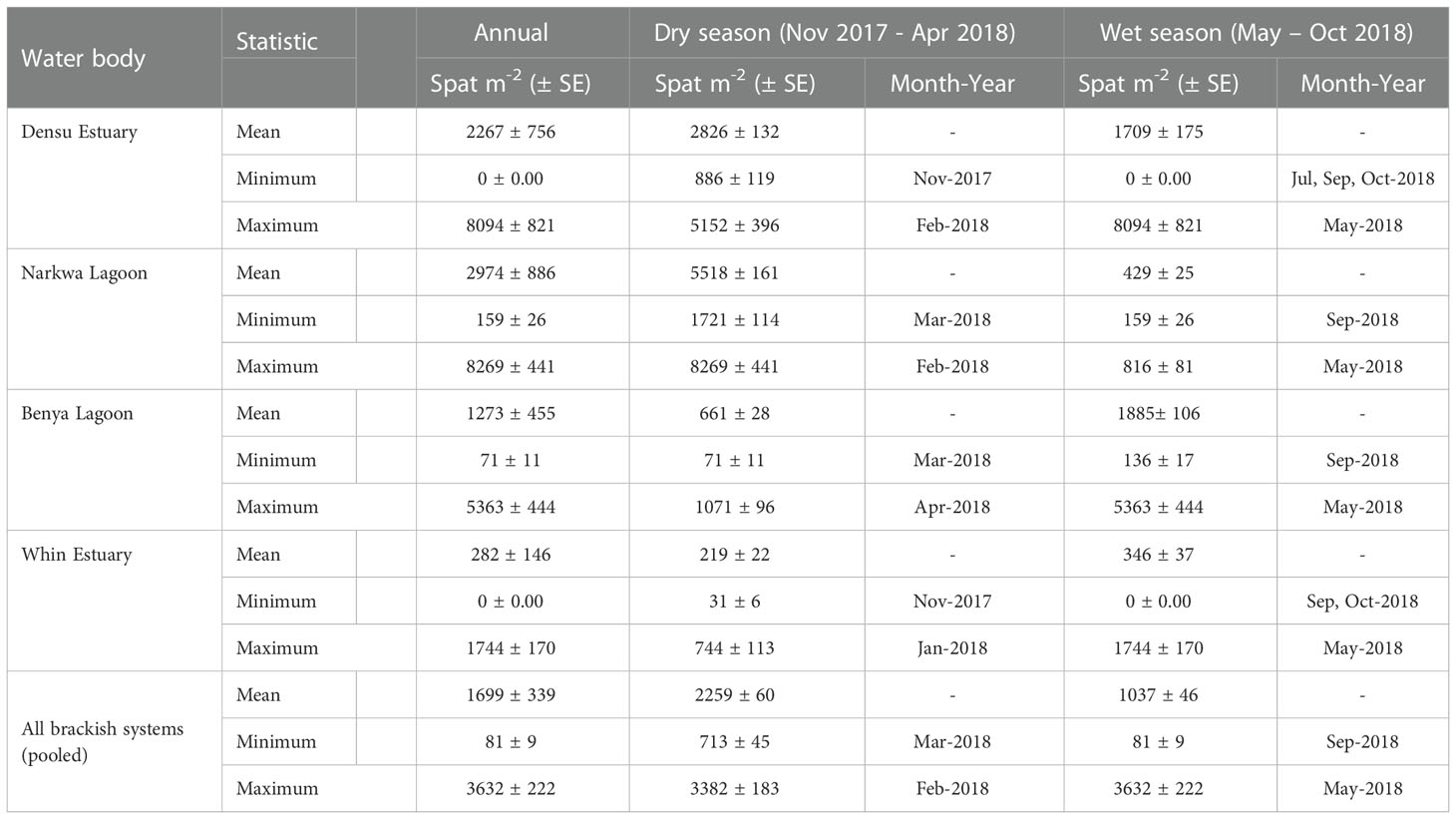
Table 2 Annual and seasonal mean, minimum and maximum spatfall (number of spat/m2) in the Densu, Narkwa, Benya and Whin brackish systems pooled from monthly averages.
In the Densu Delta, spatfall fluctuated from November 2017 to June 2018 and reduced to extremely low or no settlement from July to October 2018 (Figure 3A). The highest mean monthly spatfall in the Delta was recorded in May 2018 at both Stations (Station 1 = 6484 ± 778 spat m-2; Station 2 = 9703 ± 1426 spat m-2).
Specifically, for the Densu Delta, the bottom (≈ 0.10 m off the water bed and ≈ 0.70 m deep from the surface) and middle collectors (≈ 0.40 m deep) had similar spatfall of 3701 ± 348 spat m-2 month-1 and 3408 ± 391 spat m-2 month-1 respectively (P = 0.792). In contrast, the top collectors (≈ 0.10 m below high-water level) had significantly lower spatfall of 1188 ± 175 spat m-2 month-1 (P = 0.000). The situation was similar for both stations in the delta (Figure 3B). The oysters in this water body are found on the sandy mud bottom substratum and spread out widely in both shallow and deep areas.
Progressively decreasing fluctuations in spatfall were observed at all stations in the Narkwa Lagoon from November 2017 to July 2018 and remained very minimal afterwards (Figure 4A). During this period of minimal spatfall, Stations 1 and 3 maintained spatfall below 400 spat m-2 until October 2018, when Station 3 regained a relatively substantial amount of spatfall. In the same period, there was no spatfall recorded at Station 2 in June, September and October 2018. The highest mean spatfall at Station 1 (10705 ± 918 m-2) of the Narkwa Lagoon occurred in February 2017, whereas Stations 2 and 3 had their respective spatfall peaks of 11011 ± 699 m-2 and 8999 ± 548 m-2 in February 2018.
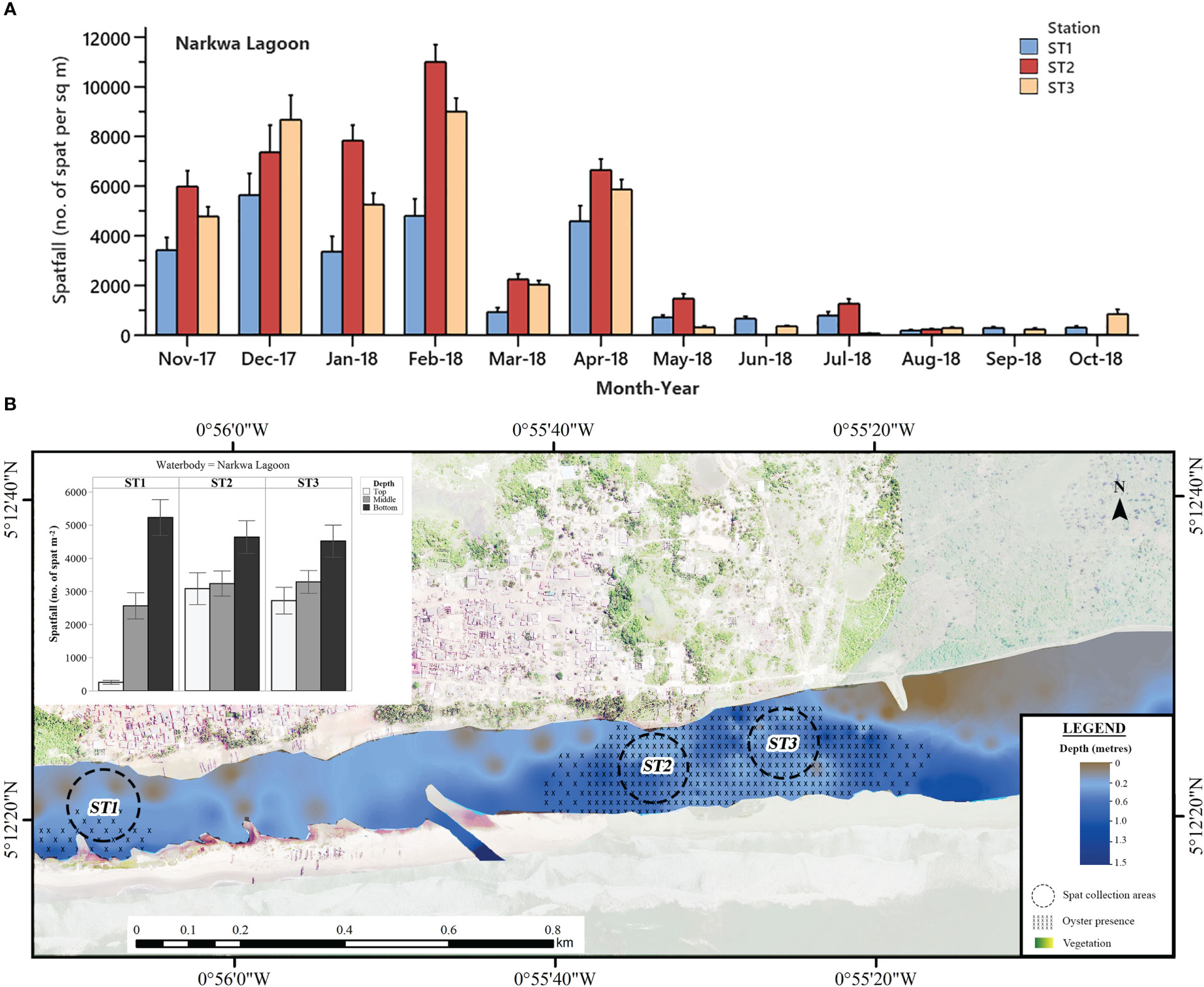
Figure 4 (A) Mean monthly C. tulipa spatfall (spat m-2) at stations (ST) in the Narkwa Lagoon pooled from collectors at all depths for each water body. Interval bars are standard errors (S.E.) of the mean. (B) Geomorphological representation of the Narkwa Lagoon at low tide showing depth profile, oyster presence and charted C. tulipa spat availability in water column at the experimental spat collection stations. Map generated from orthomosaic image of UAV flights for this study in June 2018.
Spatfall in the Narkwa Lagoon followed the general observation of bottom-up C. tulipa spatfall decrement with significant differences at all columns in water, increasing from the top (2022 ± 218 spat m-2 month-1) through the middle (3028 ± 215 spat m-2 month-1) to bottom (4794 ± 293 spat m-2 month-1) (one-way ANOVA; P < 0.001). Probing further into the stations, it was revealed that the western shallows (Station 1) of the Narkwa Lagoon (see Figure 4B), the shallowest of all stations in this study, represented the widest distinctive variation in spatfall among the water columns. Here, spatfall was extremely low at the top (257.7 ± 61.7 spat m-2 month-1) and extremely high at the bottom (5227 ± 544 m-2 month-1), with the middle being intermediate (2560 ± 395 spat m-2 month-1). Stations 2 and 3, on the other hand, had appreciable spatfall in the top water column similar to mid-water spatfall levels [Tukey HSD for top and middle; ST2 (P = 0.969), ST3 (P = 0.599)] whereas the bottom remained highest.
Spat settlement was observed in every month in the Benya Lagoon, although minimal in March, August, September and October 2018. In general, spatfall was relatively low in the Benya Lagoon than that of Densu Delta and Narkwa Lagoon but higher than that of the Whin Estuary. The least spatfall in the Benya Lagoon was recorded in March 2018 for Stations 1 and 2 (Station 1 = 39 ± 11 spat m-2; Station 2 = 104 ± 22 spat m-2) and in August 2018 for Station 3 (52 ± 11 spat m-2). Hikes in spat densities were observed from May to July 2018 in the Benya Lagoon, with a peak at Station 2 in May 2018 (8701 ± 998 spat m-2) (Figure 5A).
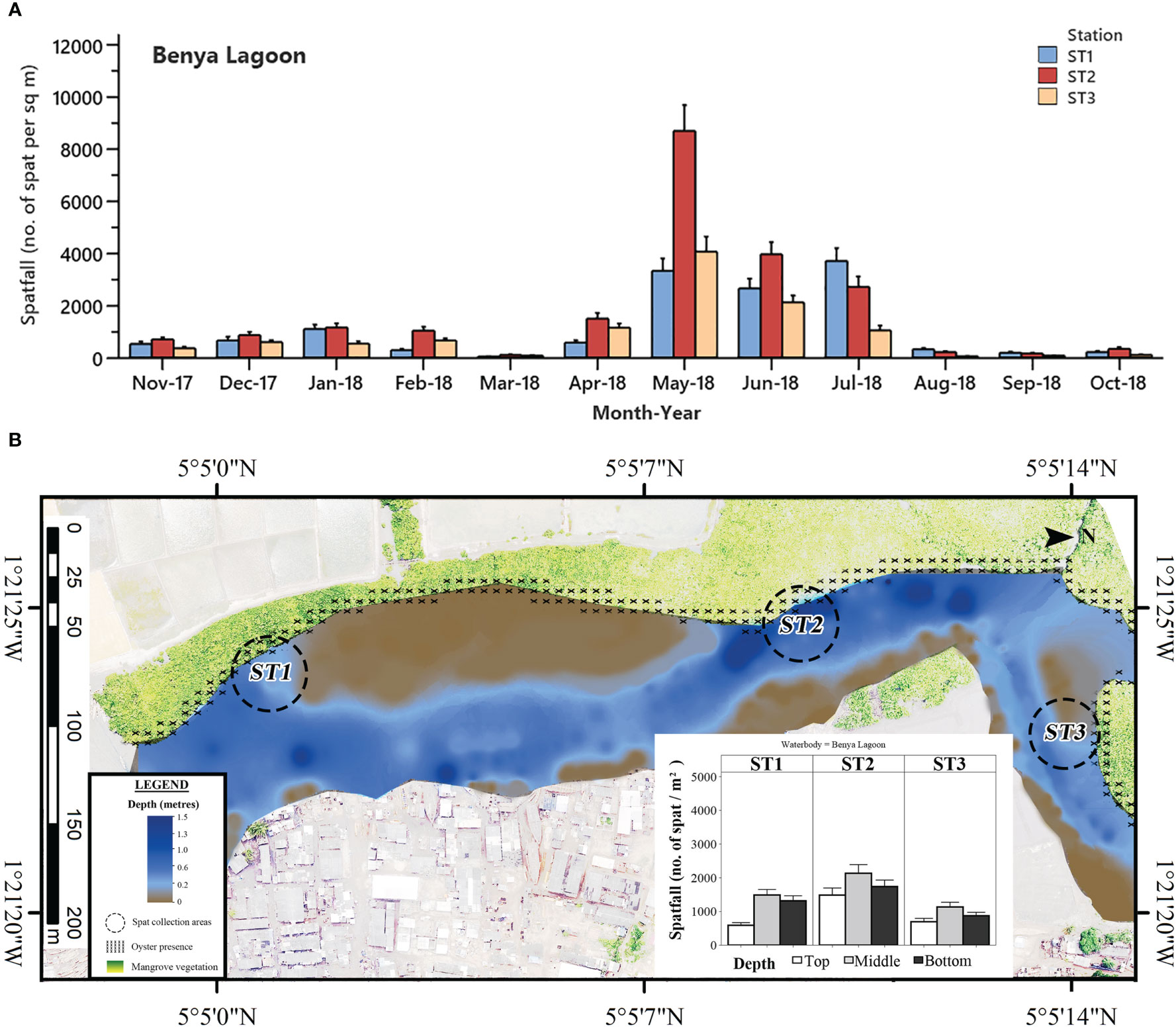
Figure 5 (A) Mean monthly C. tulipa spatfall (spat m-2) at stations (ST) in the Benya Lagoon pooled from collectors at all depths for each water body. Interval bars are standard errors (S.E.) of the mean. (B) Geomorphological representation of the Benya Lagoon at low tide showing depth profile, oyster presence and charted C. tulipa spat availability in the water column at the experimental spat collection stations. Map generated from orthomosaic image of UAV flights for this study in June 2018.
There was a slight contrast in the vertical column availability of C. tulipa spat for the stations at Benya compared to those of the other brackish systems studied. Spatfall was highest in the middle water column (2037 ± 195 spat m-2 month-1), which was significantly different from the top (1037 ± 134 spat m-2 month-1; P < 0.001) but statistically similar to the bottom (1670 ± 155 spat m-2 month-1; P = 0.251). Again, collectors at the top had significantly lower spatfall than both middle (P < 0.001) and bottom (P = 0.017) water column ones (one-way ANOVA). The oysters at Benya Lagoon are found on the roots of mangrove vegetation along its fringes, and the deepest parts of the lagoon measure up to 1.5 m at low tide (Figure 5B).
Spatfall in the Whin Estuary was the lowest among the brackish systems studied and was consistently low throughout the study period with two relatively smaller peaks, one in January and the other in May 2018 (Figure 6A) at all three stations. The January peaks were 398 ± 73 spat m-2, 628 ± 182 spat m-2 and 1205 ± 271 spat m-2 for Stations 1, 2 and 3, respectively. More pronounced peaks (Station 1 = 1706 ± 217 spat m-2; Station 2 = 2087 ± 409 spat m-2; Station 3 = 1437 ± 331 spat m-2) were observed in May. There was no spat settlement in September and October 2018 at all three stations.
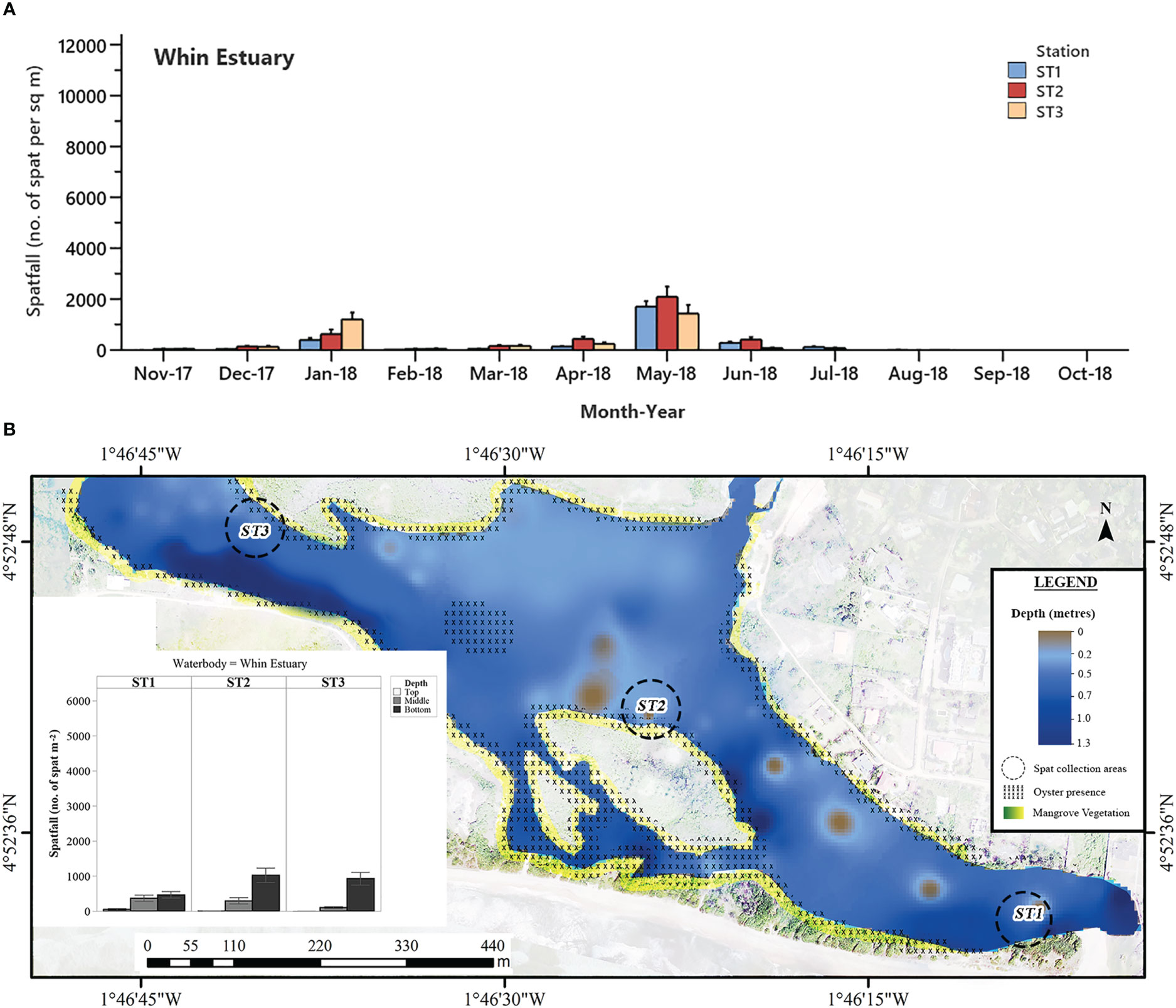
Figure 6 (A) Mean monthly C. tulipa spatfall (spat m-2) at stations (ST) in the Whin Estuary pooled from collectors at all depths for each water body. Interval bars are standard errors (S.E.) of the mean. (B) Geomorphological representation of the Whin Estuary at low tide showing depth profile, oyster presence and charted C. tulipa spat availability in the water column at the experimental spat collection stations. Map generated from orthomosaic image of UAV flights for this study in June 2018.
In general, spatfall differed significantly at all levels from the top (12.35 ± 5.14 m-2 month-1), middle (224.9 ± 34.5 m-2 month-1) to bottom (699.9 ± 68.6 m-2 month-1) in the Whin Estuary. There was, however, no spat settlement on top collectors at Stations 2 and 3 (Figure 6B) for the entire study period. In addition to that, the area near the mouth of the estuary (Station 1), where spatfall was the least and without mangrove vegetation, showed similar spat densities at the middle (≈ 0.50 m deep) and bottom (≈ 0.80 m deep) water columns (P = 0.360).
One-way ANOVA showed similarities and variations in physicochemical estuarine parameters among the water bodies. Annual mean water temperatures were 28.471 ± 0.231°C, 29.048 ± 0.265°C, 28.028 ± 0.204°C and 29.113 ± 0.212°C for the Densu Delta, Narkwa Lagoon, Benya Lagoon and Whin Estuary respectively. Temperatures were similar among the Densu, Narkwa and Whin systems (P > 0.05). The mean temperature in the Benya Lagoon was significantly lower than in the Narkwa and Whin systems (P < 0.01). The general trend in monthly variations (Figure 7A) depicts a fall in temperatures towards the end of the year in December, then a steady rise from January through the highest mean temperatures from February to April 2018 followed by a decline to about 24°C in July and August 2018 (Figure 7A). Water temperature ascended through October, after which temperatures were likely to dip again. The highest mean water temperature of 34.58 ± 0.21°C was recorded at the shallow Station 1 of Narkwa Lagoon in February 2018.
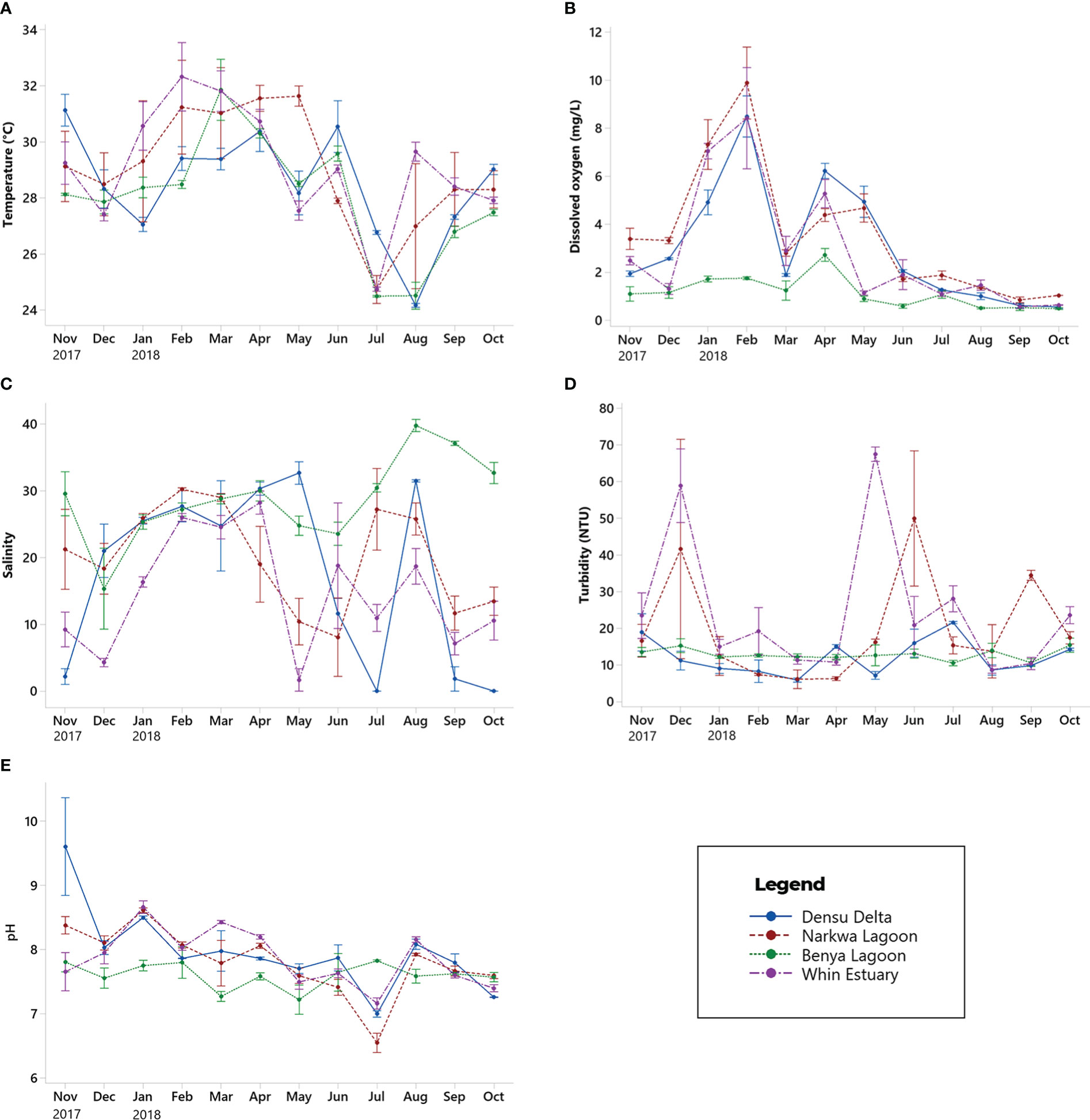
Figure 7 Monthly mean (± SE) concentrations of physico-chemical estuarine parameters - (A) Temperature, (B) Dissolved oxygen, (C) Salinity, (D) Turbidity, and (E) pH - in the Densu Delta, Narkwa Lagoon, Benya Lagoon and Whin Estuary from November 2017 to October 2018. Data were pooled for all stations in each water body.
Mean concentrations of DO were higher in the Densu Delta, Narkwa Lagoon and Whin Estuary than in the Benya Lagoon throughout the study, and followed a similar pattern in these brackish systems (Figure 7B). Annual mean DO concentrations in the three systems with comparable trends were 3.032 ± 0.299 mgL-1, 3.547 ± 0.269 mgL-1 and 2.846 ± 0.263 mgL-1. On the other hand, relatively low DO levels were observed in the Benya Lagoon (1.1421 ± 0.0692 mgL-1). Dissolved oxygen fluctuated, with the highest concentrations recorded in February and April 2018 and dipping in between, whereas minimal concentrations persisted from June to October 2018. For the period of study (November 2017 – October 2018), salinity averaged 17.42 ± 1.55 ppt, 20.021 ± 0.895 ppt, 28.713 ± 0.670 ppt and 14.695 ± 0.928 ppt in the Densu Delta, Narkwa Lagoon, Benya Lagoon and Whin Estuary respectively. Salinity was generally high in the water bodies from January to April 2018 and reduced thereafter, showing no clear pattern after April 2018 (Figure 7C). In the Benya Lagoon, however, higher salinities were recorded after April 2018 with the highest (39.778 ± 0.465 ppt) in August 2018.
Annual mean turbidity values were 12.177 ± 0.641 NTU, 19.83 ± 2.00 NTU, 12.865 ± 0.277 NTU and 24.84 ± 1.91 NTU in the Densu Delta, Narkwa Lagoon, Benya Lagoon and Whin Estuary respectively. It is seen in Figure 7D that the Narkwa Lagoon and Whin Estuary had wider fluctuations in turbidity with peaks in December 2017, May and June 2018. On the other hand, Densu Delta and Benya Lagoon had relatively low fluctuations in turbidity. In the Narkwa and Whin systems, high turbidity was observed in December 2017 and from May to September 2018. Annual mean pH in the water bodies (Densu = 7.96 ± 0.08, Narkwa = 7.81 ± 0.05, Benya = 7.60 ± 0.03 and Whin = 7.8652 ± 0.05) indicates slight alkalinity in the coastal water bodies studied. Densu Estuary had the highest pH of 9.61 ± 0.44 in November 2017 whereas the least pH (6.55 ± 0.08) for the study period was recorded at Narkwa Lagoon in July 2018 (Figure 7E).
Results of the multi-linear regression model provided enough evidence to adduce that dissolved oxygen and salinity were the significant (P < 0.001) predictors (hydrographic markers) of spatfall among all observed hydrographic parameters in the brackish systems studied, explaining 18.64% and 10.26% of the respective data variations in the model. A regression coefficient of 32.77% significant at P < 0.001 was obtained for the model (Table 3). The two hydrographic markers were individually regressed against spatfall, and their models were used to predict data within the observed range of values (see red trend line) and overlaid with a scatter plot of the actual data as shown in the marginal plots (Figure 8). The distribution of the actual data is visualised on the axial margins of the marginal plots as histograms, boxplots and density plots. There was a moderately positive but statistically significant relationship between the two predictors and spatfall [DO (r = 0.47, R2 = 0.22); Salinity (r = 0.42, R2 = 0.18); P < 0.001].

Figure 8 Marginal plots with histogram, boxplot and density plot visualisations for the relationship between spatfall and the most significant hydrographic parameters, salinity and dissolved oxygen, in the ecosystems studied. A moderately positive statistically significant relationship exists between the two predictors and spatfall [Dissolved oxygen (r = 0.47, R2 = 0.44); Salinity (r = 0.42, R2 = 0.18); P < 0.001]. The dark red line is the model-predicted data, the salmon-red ribbon is the 95% Confidence Interval region, and the cornflower-blue points are observed data points.
The median values for DO and salinity were 1.68 mg/L and 25 ppt, respectively. Dissolved oxygen ranged from 0.32 to 12.79, and salinity from 0.00 to 41.00 ppt. The corresponding interquartile ranges were 2.54 (1.68 – 3.40) mg/L and 18.33 ppt (11.00 – 29.33). The means and other descriptive statistics are shown in Supplementary Table 3. The data distribution provides further insight into the prevailing conditions in the systems based on frequencies. The interquartile ranges of the axial distribution plots (the box plot helps with the illustration here) for DO and salinity represent the spectra of the most occurring data points (i.e., prevailing estuarine conditions) and the consequential spatfall. There is also greater confidence in the predicted data traceable to this region of the interquartile range (see salmon-red 95% CI ribbon in Figure 8), thus, inferred to be the conducive conditions prevailing in the systems and supporting most of the observed spatfall.
The results of this study provide enough evidence to adduce spatiality and temporality in the availability of C. tulipa spat in the tropical sub-Saharan coastal systems studied. Spatially, the highest mean monthly spatfall among the water bodies studied, recorded at Narkwa Lagoon, was 1.3, 2.5, and 9.8 times those of Densu Delta, Benya Lagoon and Whin Estuary, respectively. Spatfall variations were also confirmed within each water body at the different stations and depths. Temporally, a generally greater spatfall in the dry season (November 2017 to April 2018; total precipitation ≈ 448 mm; average temperature ≈ 28.2°C) than in the wet season (May to October 2018; total precipitation ≈ 1390 mm; average temperature ≈ 26.5°C) was observed, in addition to monthly fluctuations. Whereas the observed general seasonal spatfall trend resonated with the occurrence in the Narkwa Lagoon and Densu Delta, the reverse was true for Benya Lagoon whilst Whin Estuary showed no conspicuous seasonal variation. The preponderance of dry season availability of C. tulipa spat was accentuated in the Narkwa Lagoon, where there was a drastic and persistent decline from the onset of rains until the end of the wet season as major spat settlement occurred from November to April in the following year.
Narkwa Lagoon presented the most favourable sections for harvesting spat among all 11 stations, particularly Stations 2 and 3 yielding an average of 3592 ± 252 spat m-2 and 3360 ± 227 spat m-2, respectively, in a month. The relatively low spatfall at Station 1 at Narkwa Lagoon is most probably due to the sharp contrast in estuarine conditions compared to the other two. Unlike stations 2 and 3, which were located at the eastern part of the lagoon with similar depth (≈ 1 m at high water), Station 1 was very shallow (≈ 0.5 m at high water) and exposed for longer periods with muddy substratum and only a few adult oysters visible on the bed at low tide. The hydrodynamics of the Narkwa lagoon also appears to create a relatively narrower salinity regime (11 – 32 ppt) at Station 1 than at Stations 2 and 3 (1.4 – 34 ppt), engendering a more stable environment for larvae to develop and settle on spat collectors successfully.
The Densu Delta presents an alternative site for spat harvesting after the Narkwa Lagoon. When estuarine conditions were favourable, C. tulipa spat were available in the Densu Delta from November 2017 to June 2018, with a major settlement at Station 2 during April-May 2018. The apparent absence of spat settlement at Densu from July to October 2018 is a probable effect of freshwater intrusion in the delta due to the flushing of an upstream river retention dam, the Weija Dam. This annual intervention is carried out to protect the integrity of the dam when it exceeds the threshold retention capacity, which occurs after periods of high and prolonged rains in the rainy season. Such major flooding events diminish the quality of estuarine water and further endanger populations of oysters by causing mass mortalities (Pruett et al., 2021). Stations 1 and 2 had a similar abundance of C. tulipa spat, whilst Station 3 was not secure for spat collection due to persistent interference with other fishing activities.
In contrast with the two previous accounts, the wet season had increased salinity in the Benya Lagoon, possibly caused by the washing of salt from the numerous saltpans located at the periphery into the lagoon system coupled with the daily tidal seawater inflow. This period coincided with the major spat settlement season for C. tulipa in this lagoon, from May to July 2018. Consequently, seasonal variation in salinity appears to determine temporality in C. tulipa spat availability in the Benya Lagoon. The longest continuous peaks previously observed in Benya Lagoon (April to June 1996) (Obodai, 2007) corroborate the findings of this study. The Benya Lagoon presents the most stable marine condition and would be suitable for C. tulipa seed banking. Deviating from the other systems, the Whin Estuary showed no distinct seasonal pattern in the availability of C. tulipa spat but had two relatively small peaks occurring separately in January and in May 2018. This pattern is not easy to explain, but it should be noted that spatfall in the Whin was the least among the water bodies studied. The uniform but low spat settlement observed at the Whin Estuary could be due to the similar hydrographic conditions at all the stations and the shallowness of that water body. The estuary appears to be at a higher altitude, compounded by the high intrusion of sea sand. A summary visualisation of the observed spatfall is depicted by the heatmap in Figure 9.
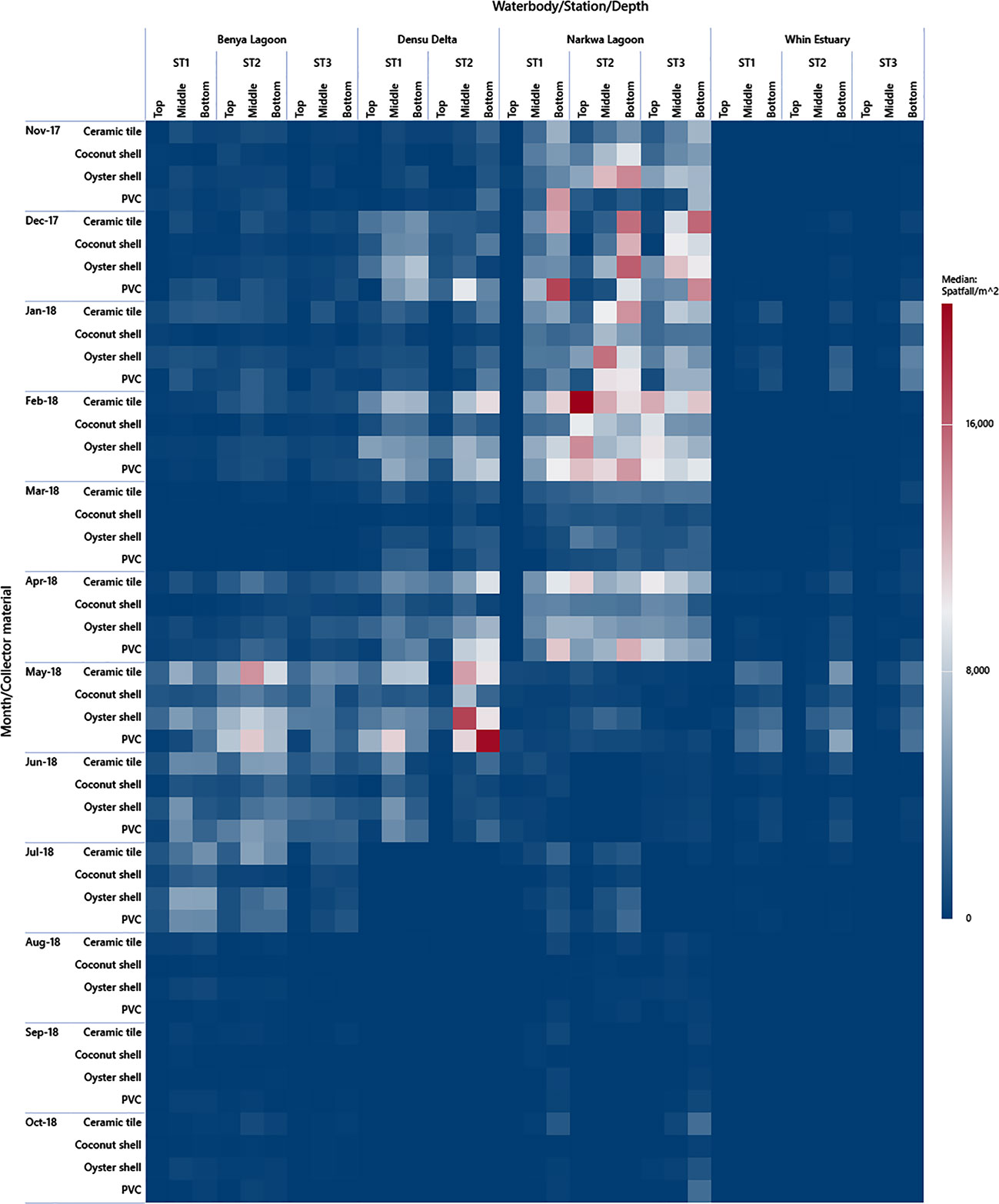
Figure 9 Heat map plot of spat availability in the brackish systems by season (dry season = Nov-17 to April-18; wet season = May-18 to Oct-18) and by the depth at stations in the water bodies. Individual spat collector spatfall estimates are used for this graph to give a detailed chequer of the heatmap. The results of the effectiveness of the different collectors are published in Chuku et al. (2020). Note that Station 3 of Densu Delta was eliminated from the study.
The observed year-round availability of spat in the Narkwa and Benya Lagoons in this study reinforces continuous spawning by C. tulipa and corroborates findings from Obodai (2007). C. tulipa populations from the Ghanaian coast are reported to exhibit protandric sequential hermaphroditism as a reproductive behaviour, differentiating first as male and later as female (Yankson, 1996). This reproductive strategy is a key survival adaptation that enhances breeding success as the species may differentiate faster to female and spawn quickly in response to unfavourable environmental conditions (Yankson, 1996). The success of this reproductive strategy is, however, contingent on food availability. It is worthy of note that accounts of the sexual differentiation of Crassostrea species are conflicting and remain debated in oyster biology (Enríquez-Díaz et al., 2009; Santerre et al., 2012; Yasuoka and Yusa, 2016; Parker et al., 2018). Also, in the course of this study, an instigation to elevate fishing pressure by the local harvesters at the onset of the rainy season was driven by the local ecological knowledge of the advent of mass mortalities in C. tulipa from freshwater intrusion. The events of increased fishing pressure and rapidly changing estuarine conditions at the onset of the rainy season appeared to trigger synchronous spawning in the oyster populations, thus, the record of maximum mean spatfall in May. The differences in temporal spatfall patterns among the systems indicate varied spawning potentials and patterns that require further investigation.
The spatiotemporal variations in C. tulipa spat in this study could be influenced by a myriad of physical and chemical estuarine factors such as temperature (Flores-Vergara et al., 2004; Cognie et al., 2006; Saucedo et al., 2007) and other conditions of the aquatic environment, i.e., freshwater inflow, microalgal diet and total seston (Hofmann et al., 1992; Monteforte et al., 1995; Dekshenieks et al., 2000; Kimmel and Newell, 2007). This study underscores the significance of DO and salinity, among the other parameters, to spat availability in the Gulf of Guinea brackish systems. By inference from the data distribution analysis and with recourse to the confidence intervals, the prevailing DO and salinity conditions for optimal spatfall in the open brackish systems were 1.68 – 3.40 mg/L and 11.00 – 29.33 ppt, respectively. As observed for the Densu and Narkwa systems, it would appear that periods after heavy rains are accompanied by low salinity due to freshwater incursions with deleterious effects on adult C. tulipa and, consequently, spat availability. A reduction in activities for acquiring energy whilst concentrating on expending more energy to regulate cell volume and to prevent osmotic shock (Solan and Whiteley, 2016) as a survival strategy may have compromised spawning at very low salinities. Further, fertilisation of successfully spawned gametes, if any, and survival of zygotes would not be achieved in salinity less than 10 ppt (Pechenik et al., 2007; Allen and Pechenik, 2010; Fang et al., 2016). Oscillations in physicochemical estuarine conditions prior to and during spatfall could affect setting success (Laing, 1995) and may influence juvenile abundance and reproductive activity of the oysters (O’Connor and Lawler, 2004). In addition, the relatively higher dissolved oxygen concentration in the Narkwa Lagoon could be a major contributor to the high spatfall observed.
The general increase in abundance of C. tulipa spat with increasing depth in all the four water bodies observed in this study could be attributed to differences in exposure and hence food availability or active feeding time. In an experiment conducted by Laing (1995), setting success was affected by the amount, type and concentration of algal diet supplied to larvae of O. edulis and C. gigas before and during spatfall. The spat collectors’ contact time with the water medium for the nourishment of the newly attached pediveliger larvae is therefore important for the successful setting. The apparent interminable inundation of most bottom collectors in such fixed rack systems as the one used in this study could also lead to the faster formation of biofilms providing suitable conditioning of collector surfaces, attractive to larvae. Biofilms play a vital role in larval settlement in oysters by increasing settlement rate and metamorphosis (Tanyaros, 2011; Tanyaros and Chuseingjaw, 2016). Longer tidal exposure limited conditioning time for top collectors than those at the lower levels. In a deep estuary, this occurrence could be influenced by stratification at the different depths, however, the unlikely assumption of vertical hydrographic advection in these shallow systems studied rules out differences in physical and chemical oceanic parameters as significant determinants of C. tulipa spat availability at the different depths.
Genetic variations in the reproductive capacity of different populations or pedigrees could also be a reason for spatial differences in spat availability of Crassostrea species (Taris et al., 2006; Han et al., 2022), providing cues to the possibility of different sub-populations of C. tulipa along the Gulf of Guinea coast. Indeed, this observation inspired an assessment of the genetic diversity and population structure in wild populations of C. tulipa which revealed that the populations of C. tulipa occurring along the coast of Ghana were potentially of multiple populations (Edekor et al., 2022). From the spatfall data in this study, bottom-sediment-adapted C. tulipa populations appeared to be more prolific than their mangrove root-adapted counterparts. Gamete quality, interactions between sperm and eggs at fertilisation, and differential viability among larval genotypes all contribute to variance in reproductive success in C. gigas (Boudry et al., 2022) but not well researched for C. tulipa on the Gulf of Guinea coast. Adult C. tulipa in the Benya and Whin systems were mostly settled on mangrove roots coinciding with relatively low spatfall suggesting a lower fecundity adaptation of the oysters. Further, the respective beds of these systems had muddy and sandy substrata, respectively, unsuitable for the survival and growth of the species.
Geomorphological evolution of the systems potentially changed the hydrodynamics within, altering the estuarine conditions at the different stations and the resultant spat settlement success as well as local larval transport. In this light, an important observation in this study was the slight natural revamping of C. tulipa spat in October 2018 in the Narkwa Lagoon; Stations 1 and 3 appeared to be regaining, whereas Station 2, which hitherto yielded more spat, had none. The relocation of the mouth of the lagoon close to Station 2 after three months of the closure of the original mouth resulted in the deposition of large volumes of sea sand into this section due to escalated erosion rate on the banks of the new mouth of the lagoon. The impact of such changes in the geomorphology of coastal intertidal areas, brought about by artificial openings in the sand bar, on C. tulipa populations has not been assessed. However, the observation in this study reveals potential negative consequences on the availability of spat, possibly from adult C. tulipa being smothered under sand deposits. A similar event of the mechanical opening of the sand bar as flood control was observed in the Densu, which led to a massive sand accretion in the system that blocked away the section west of Station 1. The distribution of competent larvae by flow and currents would be hampered under such events. Potentially suitable sites for harvesting C. tulipa spat may therefore be endangered by the process of creating artificial openings in sand bars close to oyster beds, hence, requires detailed investigation. Further, in a system with nearly consistent physicochemical estuarine conditions at all three stations like the Benya lagoon, geomorphology could steer the duration of submergence of the vertically distributed spat collectors due to undulated estuary beds, hence, driving the differences in spat availability among stations. Consequently, deeper portions (Station 2), where middle and bottom collectors were inundated even at low tide, had about twice as much spat as higher beds (Stations 1 and 3), where all collectors were exposed at low tide.
Pollution could account for the relatively suppressed spat availability at the Benya and Whin. Microplastics significantly negatively impact the larvae of oysters by upsetting their reproductive cycle and causing alterations in their feeding patterns (Sussarellu et al., 2016) and ecotoxic compounds such as persistent organic pollutants and metals, for example copper, have dire developmental effects on oyster larvae (Jo et al., 2008; Gamain et al., 2016). The Benya and Whin systems had copious amounts of plastic and industrial waste discharge that could consequently exacerbate physiological stress and hamper successful reproduction. Whin estuary receives effluents from hospitality and industrial establishments on the north-eastern boundary. The Benya has a notable heap of refuse and a pig farm and houses the largest artisanal fishing canoe fleet along the coast of Ghana. Paint fragments are increasingly becoming a big part of the ocean microplastics pollution (Gaylarde et al., 2021) and the artisanal canoe fleet in the Benya are a likely source of such pollution.
The data and discussion on the spatiotemporal dynamics of C. tulipa spatfall and the potential (and empirically assessed for physico-chemical estuarine parameters) controlling factors presented in this study highlight the need for management interventions to conserve the species along the Gulf of Guinea coast. This will safeguard the livelihood, food and nutrition security of several thousands of vulnerable coastal inhabitants in the region and guarantee aquaculture production reliant on wild seed stock. Management interventions could be seasonalised. During the period of superior spat availability in the year, that is, the dry season, interventions that focus on the harvest sizes and quotas will ensure that maturing stocks are not fished out, thereby enhancing the spawning activity of the population and culminating in sustainable exploitation of the stocks. This period also presents the ideal time of the year to deploy suitable substrates in order to provide adequate surfaces of attachment for the settlement and growth of competent larvae. Of particular use of this practice is the procurement of seed oysters for aquaculture either within the same ecosystem or for transplant to other suitable systems of desired growing conditions with the consideration of any biosecurity concerns. The Densu and Narkwa systems are the most suitable for oyster seed supply, and the Benya suitable for holding and conditioning. Spat collectors should be placed near the bottom to achieve the best spatfall. Other spat collector placement techniques may be useful for optimising the available surface area for spat settlement and minimising wastage of collector material (Chuku et al., 2020; Chuku and Osei, 2020). In addition, a proactively controlled spillage of the Weija dam is suggested to regulate the salinity regime of the Densu delta and minimise the present effect on C. tulipa. Instituting sanitation programmes around estuaries with mangrove oysters will also reduce pollution and improve reproductive success.
The dynamics of wild spat availability in one of the most significant endemic regions for C. tulipa globally are described in this study. Clarity is proffered on the annual trend of seed availability and the ecosystem-specific variations in spatfall. In summary, C. tulipa spat availability varied monthly through the year and was generally greater in periods of drought whereas with elevated precipitation came suppressed spatfall. The success of spat collection differed from one brackish system to another, and further within each system, there was significant variation among locations. Vertical progression in spat availability was recorded from top to bottom, suggesting that longer inundation periods present a greater chance of harvesting more spat. Among the four brackish systems studies, the Densu Delta and Narkwa Lagoon, which had reef oysters, are recommended for seed procurement for aquaculture. There was enough statistical evidence to adduce that dissolved oxygen and salinity were the significant markers of spat availability in the open estuary environment. Extremely low levels of these two parameters possibly hampered the physiological processes of the oyster, culminating the observed unsuccessful settlement of spat. Management and policy actions should be geared towards sustainable extraction including harvest quotas and size limits during the dry season for the benefit of investments, local economic development and job creation. Systems such as the Benya Lagoon or sections within particular systems with minimal DO and salinity fluctuation could be held as sanctuaries and managed under protected area arrangements. The results of this study could support criteria for granting harvest quotas and rights at different seasons and the balancing of social equity which currently has women at the core. Considering that ocean conditions may differ from one ecosystem to another and patterns may shift over time, we recommend that spatfall assessments be conducted for specific ecosystems of interest for management, conservation and the establishment of growing leases. Assessments should be conducted from time to time to ascertain possible shifts in spatfall regimes spatially and temporally.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
EOC, KY and EAO contributed to the conceptualization, study design and development of methodology for the study. EOC performed the formal analysis, investigation, writing of the original draft, and data visualization. KY and EAO supervised the study and validated the approach and results. EA and DWA contributed to editing and rewriting sections. All authors contributed the article and approved the submitted version.
Financial support for the fieldwork of this study was provided by the United States Agency for International Development (USAID) - University of Cape Coast (UCC) Fisheries and Coastal Management Capacity Building Support Project (Grant No.: 641-A18- FY14-IL#007). Additional funding for the publication of this work was provided by the Africa Centre of Excellence in Coastal Resilience Project (No. 6389-GH).
To the late Professor Emeritus Kobina Yankson (a.k.a. Prof. Yankson) of the University of Cape Coast, who supervised this research and to whom the authors are deeply indebted for his commitment to marine biology andecology in West Africa, we dedicate this scientific piece. Prof. Yankson was the primary supervisor of the lead author EOC during his Master of Philosophy (Aquaculture) thesis research which investigated strategies for optimising seed collection and growth of Crassostrea tulipa in coastal water bodies. Through his exceptional mentoring and guidance this study serves as a significant scientific contribution to the ecology of C. tulipa as part of efforts towards the development of an aquaculture industry, as well as the sustainable management of this important seafood resource.
Furthermore, the authors express utmost gratitude to Dr Noble Asare of the Department of Fisheries and Aquatic Sciences, University of Cape Coast, for approving additional logistics for the study. We are grateful to Messers Richard Adade and Bernard Ekumah for their assistance in creating maps and to Eunice Efua Boahemaa Kobil for her immense laboratory assistance during the data collection period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1075313/full#supplementary-material
Aguión A., Ojea E., García-Flórez L., Cruz T., Garmendia J. M., Davoult D., et al. (2022). Establishing a governance threshold in small-scale fisheries to achieve sustainability. Ambio 51, 652–665. doi: 10.1007/s13280-021-01606-x
Allen J. D., Pechenik J. A. (2010). Understanding the effects of low salinity on fertilization success and early development in the sand dollar Echinarachnius parma. Biol. Bull. 218, 189–199. doi: 10.1086/BBLv218n2p189
Arthur R. I. (2020). Small-scale fisheries management and the problem of open access. Mar. Policy 115, 1–7. doi: 10.1016/j.marpol.2020.103867
Asare B., Obodai E. A., Acheampong E. (2019). Mangrove oyster farming: Prospects as supplementary livelihood for a ghanaian fishing community. J. Fish. Coast. Manage. 1, 7–14. doi: 10.5455/jfcom.20190311090846
Barrett L. T., Theuerkauf S. J., Rose J. M., Alleway H. K., Bricker S. B., Parker M., et al. (2022). Sustainable growth of non-fed aquaculture can generate valuable ecosystem benefits. Ecosyst. Serv 56, 1–16. doi: 10.1016/j.ecoser.2021.101396
Bhosle R. V., Kumar S. S., Lingam R. S. S. (2021). Non-fed aquaculture – an alternative livelihood option for fisherman. Int. J. Curr. Microbiol. Appl. Sci. 10, 3181–3188. doi: 10.20546/ijcmas.2021.1002.349
Boudry P., Collet B., Cornette F., Hervouet V., Bonhomme F. (2022). High variance in reproductive success of the pacific oyster (Crassostrea gigas, thunberg) revealed by microsatellite-based parentage analysis of multifactorial crosses. Aquaculture 204, 283–296. doi: 10.1016/S0044-8486(01)00841-9
Cabral R. B., Halpern B. S., Lester S. E., White C., Gaines S. D., Costello C. (2019). Designing MPAs for food security in open-access fisheries. Sci. Rep. 9, 1–10. doi: 10.1038/s41598-019-44406-w
Chuku E. O., Effah E., Adotey J., Abrokwah S., Adade R., Okyere I., et al. (2022). Spotlighting women-led fisheries livelihoods toward sustainable coastal governance: The estuarine and mangrove ecosystem shellfisheries of West Africa. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.884715
Chuku E. O., Osei I. (2020). Estimating accurate rope length to minimize wastage in cultch construction for mangrove oyster farming. J. Fish. Coast. Manage. 2, 34–40. doi: 10.5455/jfcom.20200407063121
Chuku E. O., Yankson K., Obodai E. A., Acheampong E., Boahemaa-Kobil E. E. (2020). Effectiveness of different substrates for collecting wild spat of the oyster crassostrea tulipa along the coast of Ghana. Aquac. Rep. 18, 1–11. doi: 10.1016/j.aqrep.2020.100493
Cognie B., Haure J., Barillé L. (2006). Spatial distribution in a temperate coastal ecosystem of the wild stock of the farmed oyster Crassostrea gigas (Thunberg). Aquaculture 259, 249–259. doi: 10.1016/j.aquaculture.2006.05.037
Dekshenieks M. M., Hofmann E. E., Klinck J. M., Powell E. N. (2000). Quantifying the effects of environmental change on an oyster population: A modeling study. Estuaries 23, 593–610. doi: 10.2307/1352887
Edekor J. A. M., Obodai E. A., Mireku K. K. (2022). Assessment of genetic diversity and population structure in wild populations of crassostrea tulipa in Ghana. Sci. Afr. 18, 1–10. doi: 10.1016/j.sciaf.2022.e01420
Enríquez-Díaz M., Pouvreau S., Chávez-Villalba J., le Pennec M. (2009). Gametogenesis, reproductive investment, and spawning behavior of the pacific giant oyster crassostrea gigas: Evidence of an environment-dependent strategy. Aquac. Int. 17, 491–506. doi: 10.1007/s10499-008-9219-1
Fang A. N. P., Peng T. C., Yen P. K., Yasin Z., Hwai A. T. S. (2016). Effect of salinity on embryo and larval development of oyster crassostrea iredalei. Trop. Life Sci. Res. 27, 23–29. doi: 10.21315/tlsr2016.27.3.4
Flores-Vergara C., Cordero-Esquivel B., Cerón-Ortiz A. N., Arredondo-Vega B. O. (2004). Combined effects of temperature and diet on growth and biochemical composition of the pacific oyster Crassostrea gigas (Thunberg) spat. Aquac. Res. 35, 1131–1140. doi: 10.1111/j.1365-2109.2004.01136.x
Fredheim A., Reve T. (2018). “Future prospects of marine aquaculture,” in OCEANS 2018 MTS/IEEE Charleston (Charleston, SC, USA: IEEE), 1–8. doi: 10.1109/OCEANS.2018.8604735.
Friedman K. J., Bell J. D., Tiroba G. (1998). Availability of wild spat of the blacklip pearl oyster, Pinctada margaritifera, from “open” reef systems in Solomon Islands. Aquaculture 167, 283–299. doi: 10.1016/S0044-8486(98)00286-5
Gamain P., Gonzalez P., Cachot J., Pardon P., Tapie N., Gourves P. Y., et al. (2016). Combined effects of pollutants and salinity on embryo-larval development of the pacific oyster, crassostrea gigas. Mar. Environ. Res. 113, 31–38. doi: 10.1016/j.marenvres.2015.11.002.
Gaylarde C. C., Neto J. A. B., da Fonseca E. M. (2021). Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull 162, 1–9. doi: 10.1016/j.marpolbul.2020.111847.
Gregori M., Villón J., Jara F., Gonzabay-Tomalá P., Freites L. (2019). Spatial and temporal spatfall of the winged pearl oyster Pteria sterna (Gould 1851), in equatorial coasts. Aquaculture 511, 734258. doi: 10.1016/j.aquaculture.2019.734258.
Han Z., Guo X., Lu Z., Song Y., Chen R., Han X., et al. (2022). Heritability estimates for gonadal development traits and their genetic correlations with growth and heat tolerance traits in the fujian oyster crassostrea angulata. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.986441
Hofmann E. E., Powell E. N., Klinck J. M., Wilson E. A. (1992). Modeling oyster populations III critical feeding periods, growth and reproduction. J. Shellfish Res. 11, 399–416.
Jo Q., Choy E. J., Kang C. K., Moon H. B., Lee S. J., Kim D. H., et al. (2008). Effects of the coastal sediment elutriates containing persistent organic pollutants (POPs) on early reproductive outputs of the pacific oyster, crassostrea gigas. J. Environ. Biol. 294 (4), 507–512.
Kennedy R. J., Roberts D. (2006). Commercial oyster stocks as a potential source of larvae in the regeneration of ostrea edulis in strangford lough, northern Ireland. J. Mar. Biol. Assoc. UK 86, 153–159. doi: 10.1017/S0025315406012963
Kimmel D. G., Newell R. I. E. (2007). The influence of climate variation on eastern oyster (Crassostrea virginica) juvenile abundance in Chesapeake bay. Limnol. Oceanogr. 52, 959–965. doi: 10.4319/lo.2007.52.3.0959
Laing I. (1995). Effect of food supply on oyster spatfall. Aquaculture 131, 315–324. doi: 10.1016/0044-8486(94)00368-X.
Laing I., Spencer B. E. (2006). Bivalve cultivation: criteria for selecting a site (No. 136), CEFAS science series technical report. Dorset, 25.
Lapegue S., Boutet I., Leitao A., Heurtebise S., Cia G. A. R. (2002). Trans-Atlantic distribution of a mangrove oyster species revealed by 16s mtDNA and karyological analyses. Biol. Bull. 202, 232–242. doi: 10.2307/1543473
Mahu E., Sanko S., Kamara A., Chuku E. O., Effah E., Sohou Z., et al. (2022). Climate resilience and adaptation in West African oyster fisheries: An expert-based assessment of the vulnerability of the oyster crassostrea tulipa to climate change. Fishes 7, 205. doi: 10.3390/fishes.
McClenachan L., Moulton A. (2022). Transitions from wild-caught fisheries to shellfish and seaweed aquaculture increase gender equity in Maine. Mar. Policy 146, 1–7. doi: 10.1016/j.marpol.2022.105312.
Monteforte M., Kappelmana E., Lopez-Espinosa B. (1995). Spatfall of pearl oyster, Pteria sterna (Gould), on experimental collectors at bahía de la paz, south Baja California, Mexico. Aquac. Res. 26, 497–511. doi: 10.1111/j.1365-2109.1995.tb00940.x.
Morales-Alamo R. (1993). Estimation of oyster shell surface-area using regression equations derived from aluminum foil molds. J. Shellfish Res. 12, 15–19.
Obodai E. A. (2007). Effects of recruitment period on the performance of cultured oysters in Ghana. J. Ghana Sci. Assoc. 9, 28–34.
Obodai E. A., Yankson K., Blay J. J. (1991). Seasonal changes in hydrographic factors and breeding in two populations of Crassostrea tulipa (Lamarck). Ghana J. Sci. 31, 45–51.
O’Connor W. A., Lawler N. F. (2004). Reproductive condition of the pearl oyster, pinctada imbricata, roding, in port stephens, new south Wales, Australia. Aquac. Res. 35, 385–396. doi: 10.1111/j.1365-2109.2004.01027.x.
Osei I. K., Yankson K., Obodai E. A. (2020). Demographic and profitability analyses of the West African mangrove oyster. J. Fish. Coast. Manage. 2, 12–22. doi: 10.5455/jfcom.20190528122752.
Osei I. K., Yankson K., Obodai E. A., Okyere I. (2021). Implications of overlooked seasonal growth dynamics in tropical fisheries assessment: A test case of an oyster (Crassostrea tulipa) fishery in the densu delta, Ghana. Fish. Res. 244, 1–8. doi: 10.1016/j.fishres.2021.106118.
Parker L. M., O’Connor W. A., Byrne M., Dove M., Coleman R. A., Pörtner H. O., et al. (2018). Ocean acidification but not warming alters sex determination in the Sydney rock oyster, saccostrea glomerata. Proc. R. Soc. B: Biol. Sci. 285, 1–9. doi: 10.1098/rspb.2017.2869
Pechenik J. A., Pearse J. S., Qian P. Y. (2007). Effects of salinity on spawning and early development of the tube-building polychaete Hydroides elegans in Hong Kong: Not just the sperm’s fault? Biol. Bull. 212, 151–160. doi: 10.2307/25066592
Pruett J. L., Pandelides A. F., Willett K. L., Gochfeld D. J. (2021). Effects of flood-associated stressors on growth and survival of early life stage oysters (Crassostrea virginica). J. Exp. Mar. Biol. Ecol. 544, 151615. doi: 10.1016/j.jembe.2021.151615
Santerre C., Sourdaine P., Martinez A.-S. (2012). Expression of a natural antisense transcript of cg-Foxl2 during the gonadic differentiation of the oyster Crassostrea gigas: First demonstration in the gonads of a lophotrochozoa species. Sexual Dev. 6, 210–221. doi: 10.1159/000338085
Saucedo P. E., Ormart-Castro P., Osuna-García M. (2007). Towards development of large-scale hatchery cultivation of larvae and spat of the pearl oyster Pinctada mazatlanica in Mexico. Aquaculture 273, 478–486. doi: 10.1016/j.aquaculture.2007.10.029
Solan M., Whiteley N. M. (2016). Stressors in the marine environment: Physiological and ecological responses; societal implications. 1st Ed (Oxford: Oxford Unversity Press).
Soria G., Lavín M. F., Cudney-Bueno R.. (2015). Spat availability of commercial bivalve species recruited on artificial collectors from the northern Gulf of California. Seasonal changes in species composition. Aquac. Res. 46, 2829–2840. doi: 10.1111/are.12435.
Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M. E. J., et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U.S.A. 113, 2430–2435. doi: 10.1073/pnas.1519019113
Tanyaros S. (2011). The effect of substrate conditioning on larval settlement and spat growth of the cupped oyster, Crassostrea belcheri (Sowerby), in a hatchery. Kasetsart J. - Natural Sci. 45, 629–635.
Tanyaros S., Chuseingjaw S. (2016). A partial substitution of microalgae with single cell detritus produced from seaweed (Porphyra haitanensis) for the nursery culture of tropical oyster (Crassostrea belcheri). Aquac. Res. 47, 2080–2088. doi: 10.1111/are.12662
Taris N., Ernande B., McCombie H., Boudry P. (2006). Phenotypic and genetic consequences of size selection at the larval stage in the pacific oyster (Crassostrea gigas). J. Exp. Mar. Biol. Ecol. 333, 147–158. doi: 10.1016/j.jembe.2005.12.007
Tutiempo.net (2019) Climate data on Ghana. Available at: https://en.tutiempo.net/climate/ghana.html (Accessed 1.15.19).
Yankson K. (1996). Sexual differentiation of crassostrea tulipa in two contrasting brackishwater environments. J. Molluscan Stud. 62, 135–137. doi: 10.1093/mollus/62.1.135
Yankson K. (2004). Fish from the shell: Its potential in the quest for adequate protein in Ghana, inaugral lecture. Cape Coast, 40 pp.
Yasuoka N., Yusa Y. (2016). Effects of size and gregariousness on individual sex in a natural population of the pacific oyster crassostrea gigas. J. Molluscan Stud. 82, 485–491. doi: 10.1093/mollus/eyw020
Keywords: oyster, aquaculture, spat, spatial and temporal, estuarine ecology, Crassostrea, Gulf of Guinea
Citation: Chuku EO, Yankson K, Obodai EA, Acheampong E and Aheto DW (2023) Spatiotemporal spatfall dynamics and prevailing estuarine conditions for optimal oyster (Crassostrea tulipa) spat availability in selected Gulf of Guinea brackish systems. Front. Mar. Sci. 10:1075313. doi: 10.3389/fmars.2023.1075313
Received: 20 October 2022; Accepted: 02 January 2023;
Published: 18 January 2023.
Edited by:
Cornelia E. Nauen, Mundus Maris, BelgiumReviewed by:
Moslem Sharifinia, Iranian Fisheries Science Research Institute, IranCopyright © 2023 Chuku, Yankson, Obodai, Acheampong and Aheto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ernest Obeng Chuku, ZW9iZW5nY2h1a3VAdWNjLmVkdS5naA==
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.