- 1Norwegian Institute for Water Research (NIVA), Oslo, Norway
- 2Department of Biosciences (IBV), University of Oslo, Oslo, Norway
- 3Seaweed Solutions (SES), Trondheim, Norway
- 4SINTEF Ocean, Trondheim, Norway
A growing need for food is causing increased interest for seaweed farming globally. This requires the knowledge of the industry’s effects on the marine environment. We therefore aimed to explore the communities hosted by a kelp farm compared to that of wild kelp forests. The study was performed in mid-western Norway. Kelp associated fauna were collected from farmed kelp (Saccharina latissima and Alaria esculenta), in wild kelp forests (S. latissima, A. esculenta, and Laminaria hyperborea), and from fauna traps in the water column. The study showed that the kelp farm had lower taxa abundance and richness and a lower biodiversity than the wild kelp forests. Nonetheless, the farmed kelp hosted many associated species, with communities different from what was found on ropes without kelp (i.e., in the water column). The fauna communities among the farmed kelp were more similar to what was found in the wild L. hyperborea kelp forest than to its wild counterparts. The difference between the fauna communities of ‘old’ and ‘young’ farmed kelp (grown for 3 and 7 months, respectively) was not significant, but the fauna was dominated by the isopod species Idotea pelagica in the young forest and by amphipods, mainly belonging to the genus Caprella, in the older. The study contributes to our knowledge of kelp farms’ ecological role in the marine environment, which is of importance for today’s management as well as for ensuring a sustainable future development of the kelp farming industry.
1 Introduction
Marine food production is attaining increased focus in the search of fulfilling the need for food for a growing human population (Broch et al., 2019), with the Organization for Economic Co-operation and Development’s expectations of a doubling in the ocean economy in 2030 compared to 2010 (OECD, 2020). Simultaneously, the effort to meet the United Nations Sustainable Development Goals (SDGs) has resulted in a focus on food production at lower trophic levels (Duarte et al., 2009; Duarte et al., 2021). Increased use of macroalgae is considered to be environmentally friendly as it does not require any watering, pesticides, or fertilizers (Hughes et al., 2012) as opposed to terrestrial farming and fish aquaculture. Cultivated macroalgae is already an important resource globally (Food and Agriculture Organization of the United Nations, FAO, 2020), and it is predicted that this industry will potentially exceed that based on terrestrial plants (Creed et al., 2019). Today, 200 different macroalgae species are exploited commercially (FAO, 2020) and used as human food, animal feed, part of the pharmaceutical industry, and fertilizers and in cosmetics and a variety of other products (Olafsen et al., 2012; Mac Monagail et al., 2017). Globally, more than 30 mill. tonnes are harvested from seaweed farms annually, which is an increase of almost 10 mill. tonnes since 2011 (FAO, 2022). For comparison, only approximately 1 mill. tonnes of wild macroalgae are annually harvested from the wild (FAO, 2020). Today, most of the farmed macroalgae are produced in Asia (FAO, 2020). However, the interest is increasing globally (Buschmann et al., 2017) and in the Northern Hemisphere (Stévant et al., 2017).
In Norway, the kelp farming industry started at an experimental level in 2005. The first license for commercial kelp farming was given in 2014. Today, approximately 150 tonnes of kelp are produced, representing a considerable increase from the 60 tonnes produced in 2016 (Fiskeridirektoratet, 2020). The most commonly farmed kelp species in Norway is Saccharina latissima (sugar kelp, 96% of the production, Fiskeridirektoratet, 2020). In the wild, this kelp species commonly grows in sheltered and moderately wave-exposed areas along the Norwegian coast (Bekkby and Moy, 2011). It grows fast and provides high biomass within a short period of time (Handå et al., 2013; Kerrison et al., 2015). The remaining production (4%) is based on Alaria esculenta (winged kelp, Hancke et al., 2018), a species that naturally grows in more wave-exposed areas (Birkett et al., 1998). It has been stated that Norway holds a potential to upscale its kelp cultivation harvest to 20 mill. tonnes by 2050 (Olafsen et al., 2012; Broch et al., 2019).
The impact of kelp cultivation on the marine environment is understudied both in Norway and globally (Walls et al., 2016), and the knowledge of both positive and negative impacts is needed to obtain a knowledge-based management (Campbell et al., 2019) and ensure a sustainable development of the kelp farming industry (Stévant et al., 2017). One important question is related to the kelp farms’ potential role as habitat providers, with the evaluation of the benefits to biodiversity as one of the most pressing needs (Forbes et al., 2022). A kelp farm is placed at the ocean surface, thereby introducing a temporary and artificial ‘kelp forest’ in the water column, where kelp normally does not grow. A wild kelp forest is one of the most productive ecosystems in the world (Steneck et al., 2002; Christie et al., 2003; Teagle et al., 2017), creating a three-dimensional structure and habitat for high numbers of associated species and individuals (Whittick, 1983, Norderhaug et al., 2005). Kelp cultivation in Norway usually consists of kelp seedlings grown on ropes and deployed in the farm between September and February. Harvesting occurs the following spring or summer, typically between April and July (to avoid epiphytic fouling, Andersen et al., 2011), but later in the north, where the cold water allows for a longer growth season (Matsson et al., 2019). The kelp in the farm is consequently a young monoculture growing away from its natural habitat, the seabed. The artificial kelp forest formed by the kelp farm may potentially play a role similar to the wild kelp forest, providing new habitats and increased biodiversity in coastal areas (Walls et al., 2016; Campbell et al., 2019), which will further supply food for fish, seabirds, sea mammals, and others. However, few ecological studies have been done on kelp farms and we therefore lack the knowledge of the ecological function they have (but see Walls et al., 2016; Forbes et al., 2022). Little is also known about the impact of the farmed kelp’s production time (i.e., the duration from sowing to harvesting) on the associated community composition. The aim of this study was therefore to increase the understanding of the ecosystems associated with kelp farms and contribute with the knowledge of relevance for the management of present and future kelp farms. The main focus has been on the potential ecological role of kelp farms as an artificial open-water habitat and to find out if kelp farms host a unique fauna community or resemble that of wild kelp forests. We compared fauna communities associated with farmed kelp with those of wild kelp and artificial substrate in the water column, the latter simulating the rope in the kelp farm. For farmed S. latissima, production had two different growth periods, which allowed us to look into the effect of growth length.
2 Materials and methods
2.1 The sampling area and its surroundings
The data were collected on the east side of Frøya, a large island just outside the Trondheimsfjord on the midwest coast of Norway (63°41’49N 8°49’19E), inside and in close vicinity to the Seaweed Solutions (SES) kelp farm (Figure 1). The landscape is dominated by islands and skerries, providing large shallow (<30 m depth) rocky seabed areas with several species of kelp. Data were collected on 23–27 April 2019, just before the farmed kelp was harvested, to maximize the succession and growth period for the associated fauna. The kelp farm covered an area of 200 × 200 m in a relatively wave exposed area, producing both S. latissima and A. esculenta in separate sections of the farm, on ropes placed at approximately 2 m depth. For S. latissima, one part of the production was sown in September 2018 (i.e., providing a production/growth period equal to 7 months), whereas the other part was sown in January 2019 (i.e., providing a production period of 3 months). A. esculenta was also sown in January, providing a 3-month-old kelp ‘forest’ before harvesting.
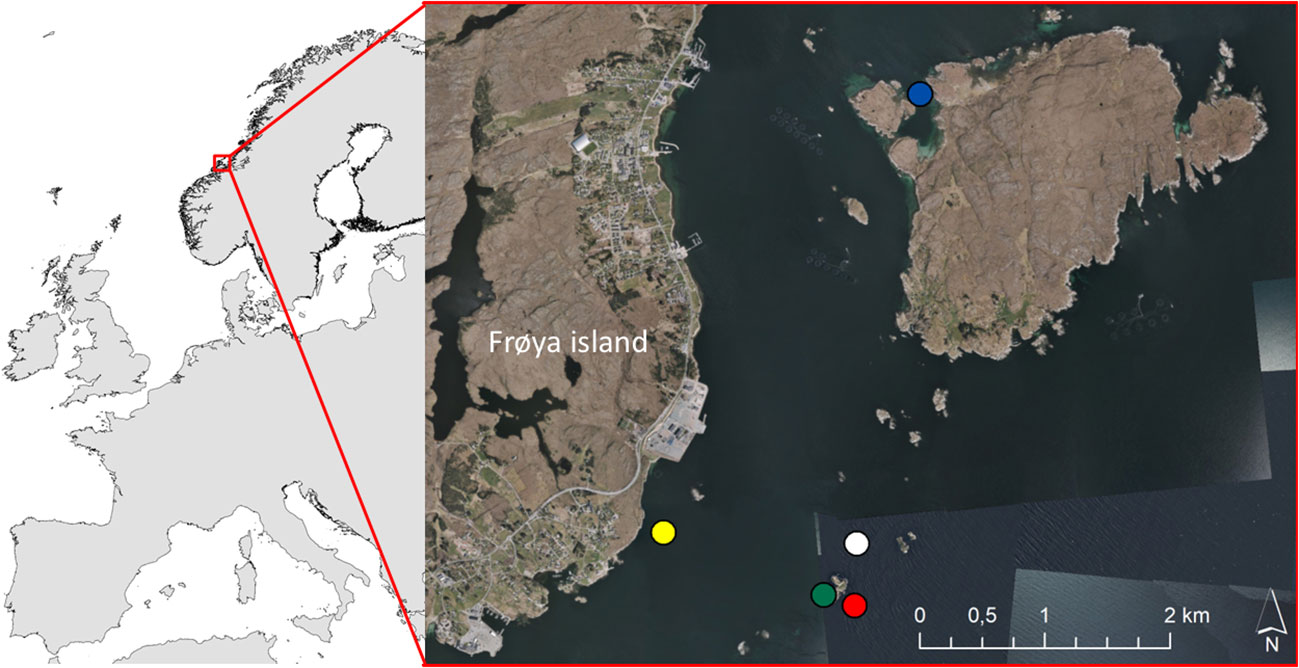
Figure 1 The map (left panel) gives an overview of the location of the study area (63°41’49N 8°49’19E). The figure (right panel) shows the location of the kelp farm (white dot), and the locations of the sampled wild Laminaria hyperborea (red dot), Saccharina latissima (blue dot), and Alaria esculenta (green dot) forests. The yellow dot shows the location of the water column station. Background on the detailed map: ‘Norge i bilder’, provided as orthophoto WMS by the Norwegian Mapping Authority.
2.2 Field sampling of kelp and of associated fauna species
Three replicates of kelp, with the associated fauna, were collected from approximately 2 m depth at each of the locations in the kelp farm (i.e., in the 7-month-old S. latissima forest, 3-month-old S. latissima forest, and A. esculenta section). Three replicates of kelp were also collected in each of the three wild kelp forests (i.e., S. latissima, L. hyperborea, and A. esculenta). The kelps were collected by divers, placing a cotton bag around the whole kelp before sampling, to ensure capturing most of the associated mobile fauna (a well-tested method for seaweeds including kelps, Christie et al., 2009). In addition, mobile invertebrates were sampled using fauna traps, constructed by using a 1-m-long sisal rope (with 8 mm diameter), untwisted into its three parts, and bundled together with a strip (Figure 2). The traps have been designed and tested (see Kraufvelin et al., 2002) to capture an assembly of species representative of the kelp forest–associated mobile invertebrate community, including crustaceans, gastropods, polychaetes, and bivalves (Christie et al., 2007). Fauna traps were deployed at the same locations in the farm and the wild forests where we collected the kelp replicates (i.e., 18 fauna traps in total). We also deployed three replicate fauna traps in the water column to simulate the rope in the kelp farm and thereby control for the effect of introduced substrate, as opposed to the effect of kelp. The traps were also put out at approximately 2 m depth. In the kelp farm, this depth coincided with the middle part of the hanging kelp forest, which was 10–15 m above the seabed. In the wild kelp forest, the traps were deployed close to the seabed, and, in the water column, they were 10–15 m above the seabed, away from the kelp farm and the wild kelp forest (Figure 1). The traps were retrieved after 48 h. The traps in the kelp farm and the wild kelp forests were collected by divers (each trap was put gently into a plastic zip bag), whereas the traps in the open water column were constructed with a cotton bag connected to the fauna trap, thereby enclosing the fauna as the whole construction was lifted up to the surface (Figure 2). Sampled kelps and fauna traps were rinsed in freshwater, and captured fauna was retained in a 250 µm sieve (Christie et al., 2003; Christie et al., 2009) and preserved on 70% ethanol.

Figure 2 Invertebrates were sampled using fauna traps, constructed by using a 1-m-long sisal rope, untwisted into its three parts, and bundled together with a strip (left panel). The traps in the kelp farm and the wild kelp forests were collected by divers (each trap put into a plastic zip bag), whereas the traps in the water column were collected using a cotton bag connected to the fauna trap (right panel), thereby enclosing the fauna as the whole construction was lifted to the surface. Photo: Trine Bekkby and Eli Rinde (NIVA).
2.3 Laboratory work and species determination
The species were identified to the lowest possible taxonomic level using a Leica TL5000 Ergo Transmitted Light Base magnifier, based on the identification literature provided by Lincoln (1979); Enckell (1980); Graham (1988); Hayward and Ryland (1995), and Christiansen (1972). The nomenclature used in the species list is in accordance with the database ‘Worlds Register of Marine Species’. The number of fauna taxa and individuals were counted, and the fauna was weighed. The wet biomass determination was conducted by weighing drip-dried animals, i.e., they were put on absorbent glass microfiber filter for 1 min to remove excess liquid before weighing them on a calibrated Sartorius Research R200D scale with 5-decimal-gram accuracy. The species list, with abundance from kelp and fauna traps shown separate, and the weight data were uploaded to Zenodo (Bekkby et al., 2023a; Bekkby et al., 2023b), an open-access repository developed under the European OpenAIRE program, operated by European Council for Nuclear Research (CERN).
2.4 Statistical analyses
Species composition in the sampled communities were compared using the ordination technique detrended correspondence analysis (DCA, Clarke, 1993). ANOVA (Shaw and Mitchell-Olds, 1993) was used to analyze the variation captured by DCA1 (i.e., the axis that accounts for most of the variation in the ordination) and DCA2 (i.e., the axis that accounts for the second most of the variation) against the location (i.e., kelp farm, wild kelp forest, or water column) and method (data sampled from kelp or by fauna traps). The communities associated with the farmed S. latissima of different growth periods were analyzed separately. ANOVAs were used to analyze the number of individuals (abundance) and the number of taxa (taxa richness), fauna biomass, and species diversity calculated as the Shannon–Wiener index (Shannon, 1948). To reveal the possible effects of the growth periods of the sugar kelp in the farm, we performed the analysis of covariance (ANCOVA) analyses of the abundance and taxa richness, fauna biomass, and species diversity against the growth period. All analyses and visualizations were conducted in RStudio (version 1.1.463—© 2009–2018 Rstudio Inc), using R-version 4.0.3. The ordination and ANOVA analyses were done using the package vegan (version 2.5.7, Oksanen et al., 2020). The plots were done using ggplot2 in the package Tidyverse (version 1.3.1 (Wickham et al., 2019).
3 Results
A total of 575 individuals among 46 different taxa were identified in this study, collected from kelps in the farm and from wild kelp forests, and by the use of fauna traps in all three locations (i.e., the kelp farm, the wild forest, and the water column, Table 1; Supplementary Table 1 and Bekkby et al., 2023a shows a full species list). There was a significant difference between the three locations in both the abundance and the taxa richness (Supplementary Table 2). In total, we found six times as many individuals and almost three times as many species in the wild kelp forest than in the farm (Figure 3, Table 1). In the wild kelp forest, we captured more individuals and taxa from the sampled kelp than from fauna traps, while the opposite was true in the farm, i.e., there, we captured more fauna taxa and individuals using the traps (Figure 3, Supplementary Table 2). We found no difference in biomass between the different locations (Supplementary Table 2), but Table 1 shows that the wild A. esculenta forest had an associated fauna biomass that was much higher than what was found anywhere else. Based on the Shannon–Wiener biodiversity index, there was a significant difference in biodiversity between the locations (Supplementary Table 2). Biodiversity followed the same pattern as for the abundance and taxa richness, with higher biodiversity in the wild kelp forests than in the kelp farm (Figure 4). There was no significant difference related to methods (sampling kelp versus fauna traps) when it came to biodiversity data (Supplementary Table 2, Figure 4).
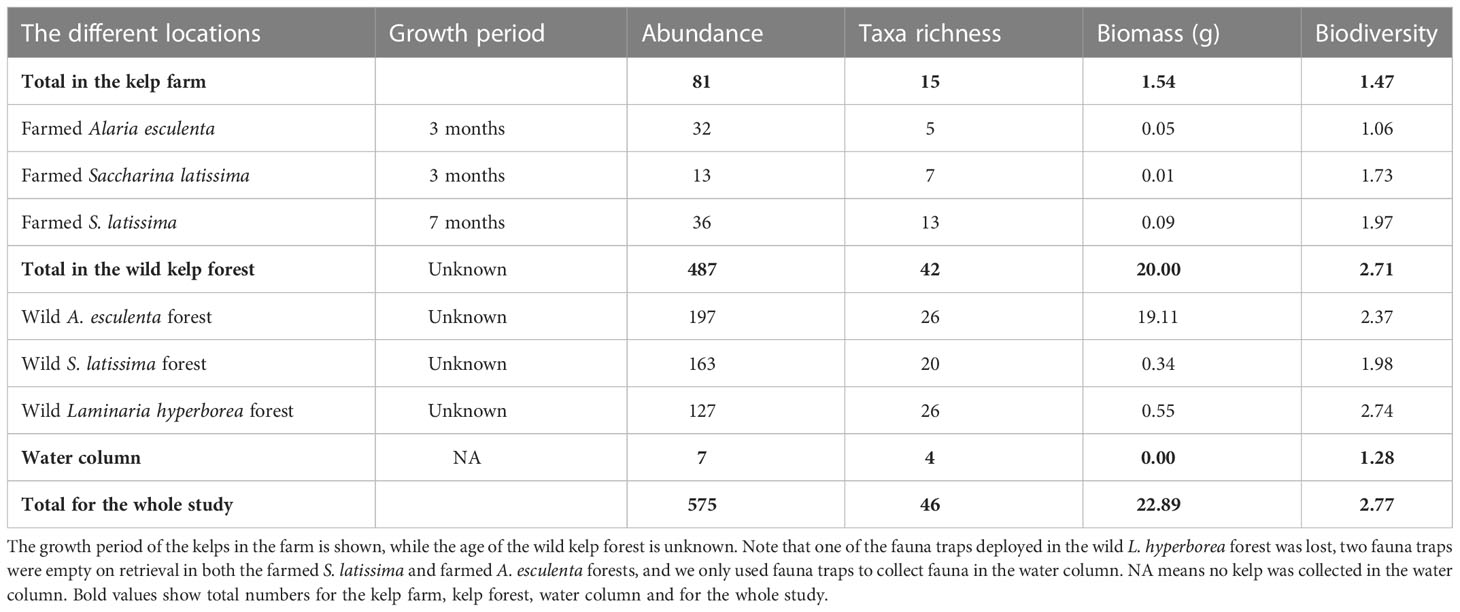
Table 1 The abundance (number of individuals) and taxa richness (number of taxa), biomass (g wet weight) and biodiversity (the Shannon–Wiener index) of fauna associated with the kelps (collected by sampling kelp and deploying fauna traps) at the different locations, i.e., in the farm and the wild kelp forests (of three different kelp species), and within the water column.
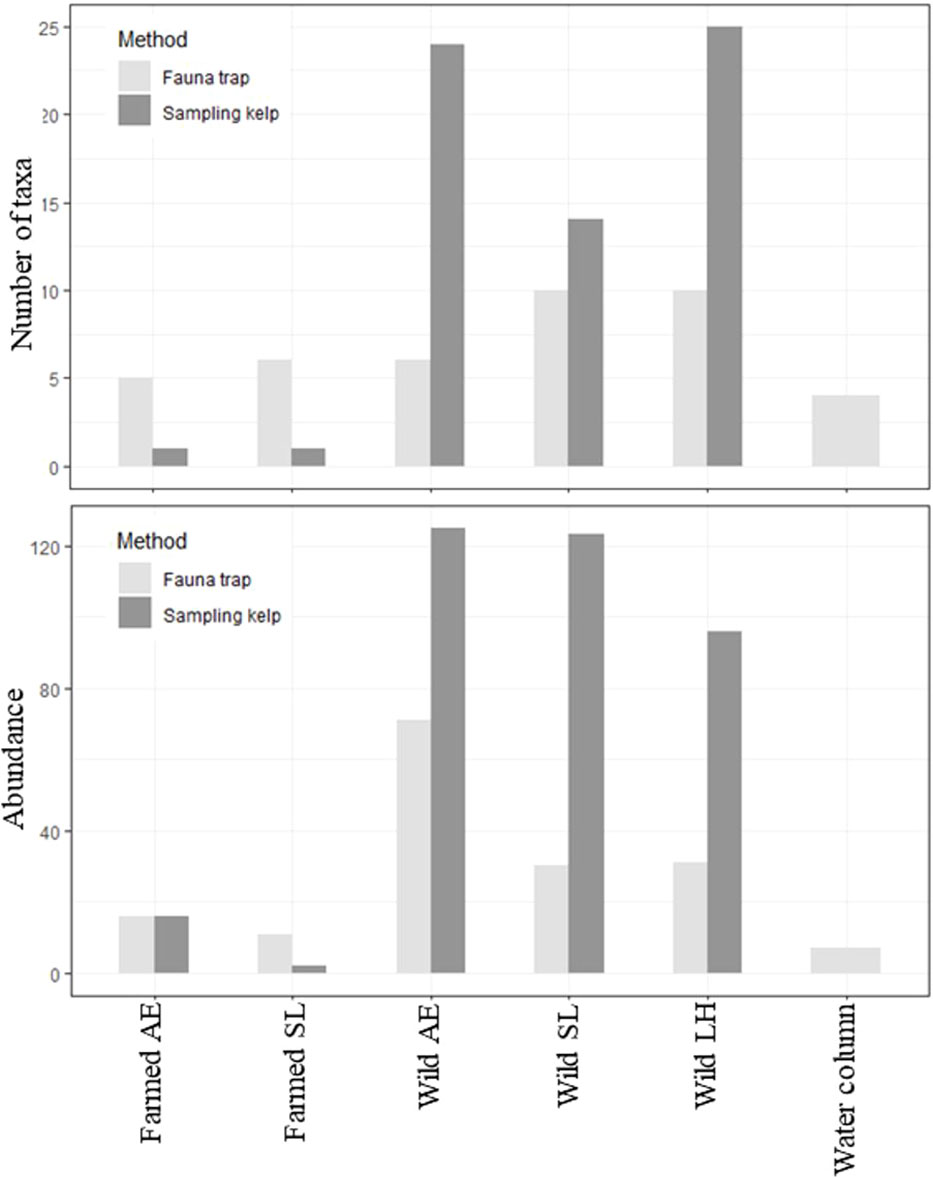
Figure 3 Total number of taxa (taxa richness, upper panel) and total number of individuals (abundance, lower panel), collected by using two different methods (fauna traps or by sampling kelp) at the different locations, i.e., in the kelp farm, wild forests, and the water column (only fauna traps used in the water column). LH, L. hyperborea; SL, S. latissima; AE, A. esculenta. To make the data in the farmed kelp comparable, only kelps with 3-month growth period were included. Note that we only have five replicates from the wild L. hyperborea forest as one fauna trap was lost. Two fauna traps were also empty on retrieval in both the farmed S. latissima and the farmed A. esculenta forest.
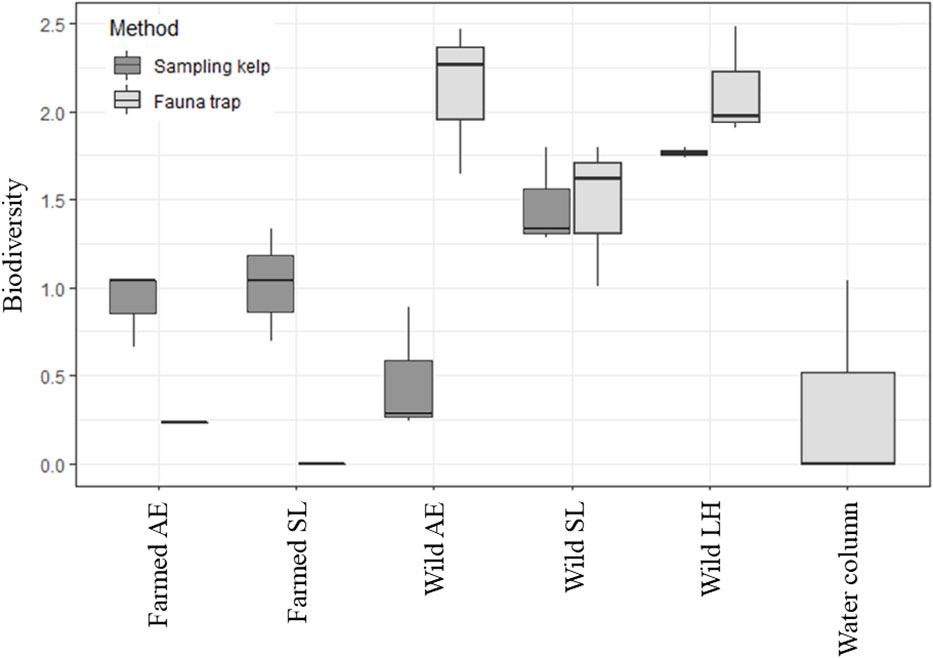
Figure 4 Box-and-whisker plot of the biodiversity (the Shannon–Wiener index), collected by using two different methods (fauna traps or by sampling kelp) at the different locations, i.e., in the kelp farm, the wild forests and the water column (only fauna traps used in the water column). The plot shows the median, the upper and lower quartile, and the maximum and minimum values. LH, L. hyperborea; SL, S. latissima; AE = A. esculenta. To make the data in the farmed kelp comparable, only kelps with the 3-month-long growth period were included. Note that we only have five replicates from the wild L. hyperborea forest as one fauna trap was lost. Two fauna traps were also empty on retrieval in both the farmed S. latissima and the farmed A. esculenta forests.
The DCA and ANOVA show differences in the fauna communities between locations (Figure 5 and Table 2), meaning that the communities in the kelp farm, the wild kelp forests, and the water column were different. The communities established in farmed kelp (i.e., S. latissima and A. esculenta) were, however, quite similar (Figure 5). Further, the communities in farmed kelp (regardless of kelp species) were more similar to the wild L. hyperborea forest that surrounded the farm than to their conspecifics growing in the wild. The communities sampled in the fauna traps in the water column (i.e., not associated with any kelp forest) were different from both the farmed kelp and the wild kelps.
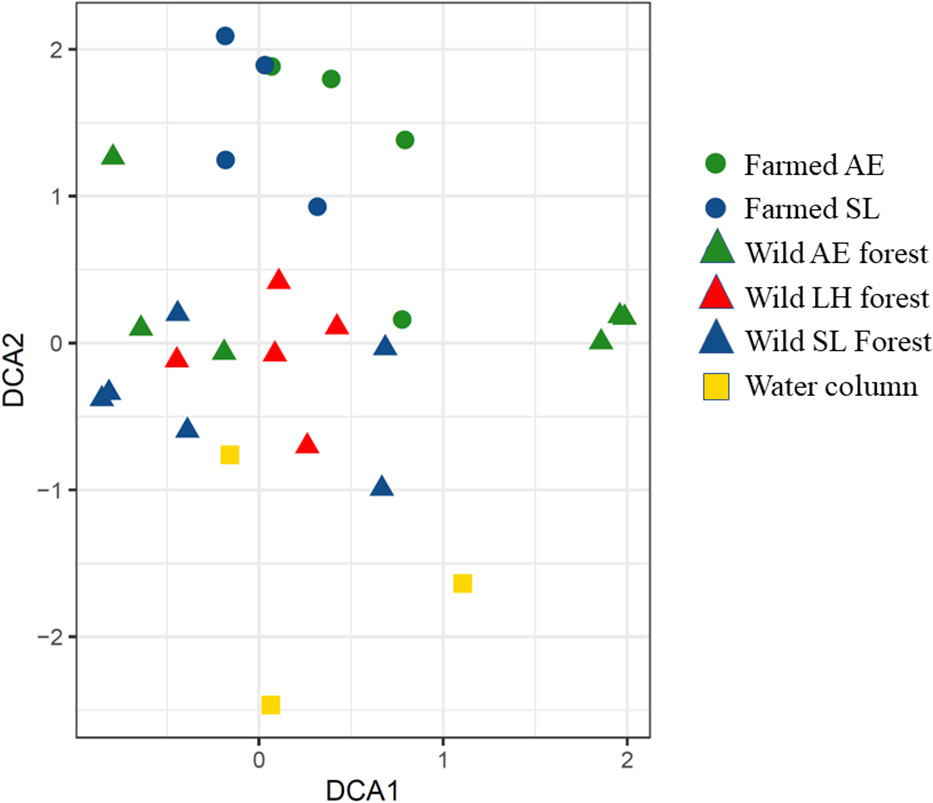
Figure 5 The detrended correspondence analysis ordination of the fauna communities from the kelp farm, the wild kelp forest, and the water column, collected from both the kelp and by using fauna traps (only fauna traps used in the water column). LH, L. hyperborea; SL, S. latissima; AE, A. esculenta. To make the data in the farmed kelp comparable, only kelps with the 3-month-long growth period were included. Note that we only have five replicates from the wild L. hyperborea forest, as one fauna trap was lost. Two fauna traps were also empty on retrieval in both the farmed S. latissima and the farmed A. esculenta forest.

Table 2 ANOVA analysis of detrended correspondence analysis 1 (DCA1, of the ordination in Figure 5), which accounts for most of the variation in the ordination, and DCA2, which accounts for the second most of the variation in the ordination, with respect to the location and method.
The ANOVA analyses of DCA1, i.e., axis 1 of the ordination (Table 2) shows that most of the variation in the ordination is explained by methods, meaning the communities collected with fauna traps were different from those found by collecting kelps.
The most numerous taxonomic groups found in the kelp farm were amphipods, isopods, and gastropods (Table 3). Among these, the juvenile Caprella amphipods (unable to identify species), the amphipod Ischyrocerus anguipes, the isopod Idotea pelagica, and the gastropod Margarites helicinius were the most abundant species (accounting for 72% of the total abundance, Table 4). In the wild kelp forest, the amphipods and gastropods were dominating, whereas almost no isopods were observed (Table 3). The species that were most abundant in the kelp farm were almost absent in the wild forest. The amphipods Dexamine thea and Gammarus spp. and Rissoidae gastropods were the most abundant species in the wild kelps (accounting for 71% of the total abundance, Table 4). In the water column, only a few individuals were found, of which Rissoid gastropods, mysids, the gastropod Lacuna vincta, and scale worms (Polynoidae) were the most dominating taxa (Tables 3, 4). A complete list of the species found in this study is given in Supplementary Table 1. In the kelp farm, the dominating species differed between S. latissima and A. esculenta (Table 3). In S. latissima, amphipods were dominating (58%), whereas gastropods were the most abundant group (accounting for 23% of the total abundance) in A. esculenta. In the wild kelp forest, amphipods were quite abundant in all three types of forests (45%–81%) but most dominant in the A. esculenta forest, whereas gastropods were most common in the L. hyperborea forest (with 36%).
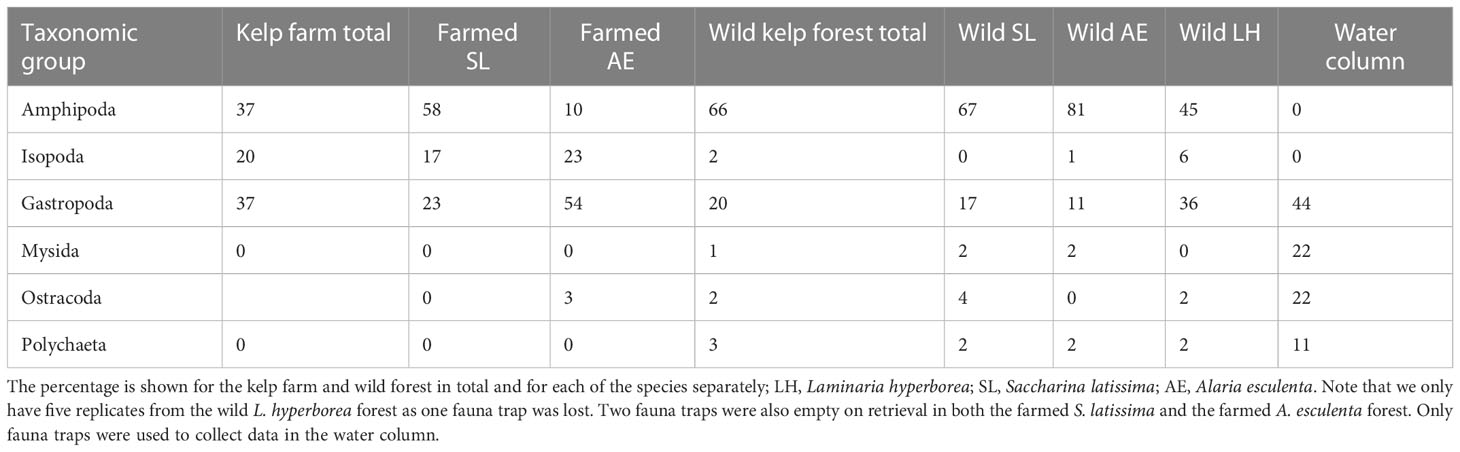
Table 3 The most dominating taxonomic groups, i.e., those that combined constitute minimum 90% of the abundance (number of individuals) within either of the locations, i.e., the kelp farm, the wild kelp forest, and the water column.
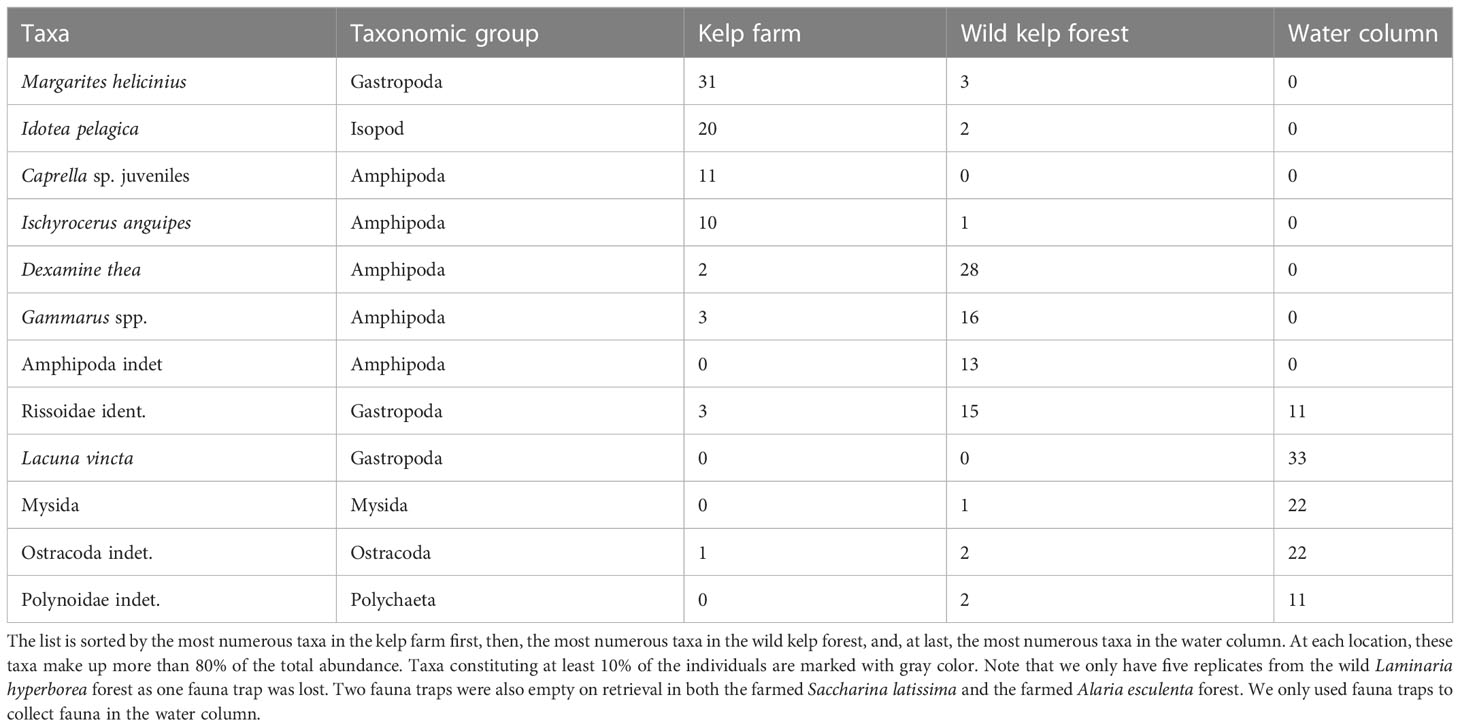
Table 4 The most dominating taxonomic species/taxa, here being those that constitute minimum 10% of the abundance (number of individuals) within either of the locations, i.e., the kelp farm, the wild kelp forest, and the water column.
To assess the potential effect of the length of the growth period of farmed kelp on the fauna communities, we looked in detail at the S. latissima kelp growing in the farm for two different durations, 3 and 7 months, respectively. For the 3-month growing period, a total of 13 individuals and 7 different taxa were identified (Table 1). For the 7-month period, the values almost tripled for the abundance and doubled for the taxa richness, to 36 and 13, respectively. However, the differences between growth period were not significant (p = 0.2081 for abundance, p = 0.1649 for taxa richness, and p = 0.1206 for biodiversity). However, the study provides some knowledge on the fauna species and the abundance of these found in the farmed kelp after 3- and 7-month production periods (Table 5). The isopod species I. pelagica was the most abundant species in the young farmed kelp (38%, as opposed to 9% in the 7-month-old forest). For the 7-month-old kelp, amphipods were dominant, with juvenile Caprella sp. as the single most dominant taxa, followed by the amphipod I. anguipes.
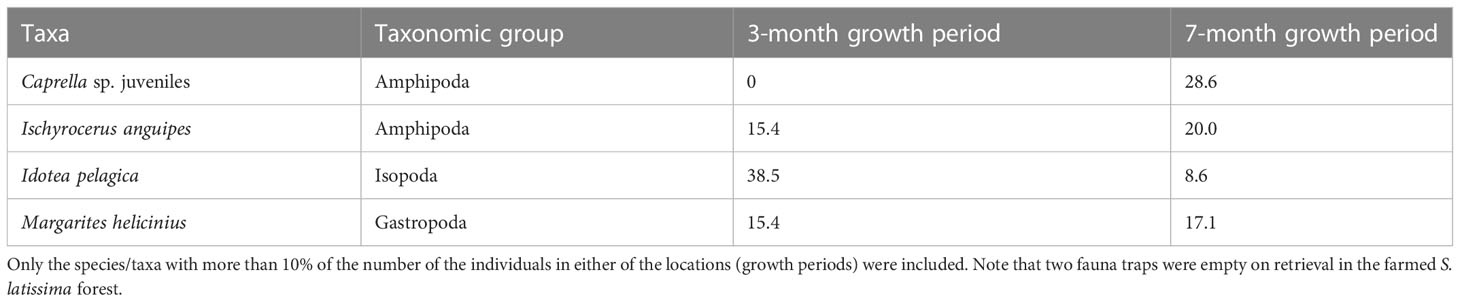
Table 5 The most dominant fauna species/taxa associated with farmed S. latissima, growing for 3 or 7 months within the kelp farm.
4 Discussion
The aim of this study was to deliver knowledge about the ecology of kelp farms with relevance for the management of the industry. The main focus has been on the potential role of kelp farms as an artificial open-water habitat. As part of this aim, we wanted to compare the communities associated with a kelp farm with those of wild kelp forests in order to assess the ecological function that a farm might potentially have in the short period of time in which the kelps are growing there. It is worth mentioning that the abundance and number of data are sometimes low (Table 1), which should be considered when results are discussed and conclusions are drawn.
Our study indicates that the kelp farm has a role as a habitat, a ‘hanging garden’. The findings agree with a recent review (Forbes et al., 2022) that concluded that kelp farms create habitats that enhance biodiversity but that the impact on biodiversity varies with different factors, such as the type of species that is farmed, the environmental conditions, the intensity and scale of the farm, the gear used, and the management practice. We found that the community composition and abundance of species in the kelp farm were different from that of the wild kelp forest, which is also supported by other studies (see Walls et al., 2016; Walls et al., 2019; Forbes et al., 2022). We found lower numbers of individuals and taxa, and a lower biodiversity, in the kelp farm compared to the wild kelp forests. Looking at the community composition (from the ordination analysis), the kelp farm, and the wild kelp forests had some similarities, hosting both amphipods and gastropods (snails), which are found to be the most common taxonomic groups in a kelp forest along the coast of Norway (Christie et al., 2003; Christie et al., 2009). The kelp farm also hosted a lot of isopods, which were almost absent in the wild kelp forests. Even though all these species are found, in high or low numbers, in wild kelp forests (Christie et al., 2003), the species dominating the kelp farm in our study are different from those dominating the wild forests. This is captured by the ordination analysis, showing a difference between the fauna community associated with the kelp forest and that found in the kelp farm. This could be a result of the structural differences between farmed kelp and the wild forests. The kelp in the farm is a young (harvested after 3 and 7 months in this study) monoculture hanging from ropes in the water column, at approximately 2 m depth, and only in winter and spring when fauna abundance is low (Christie et al., 2003). The wild kelp forests, on the other hand, are complex ecosystems that are usually stable over decades and that serve as habitats for a variety of species (Norderhaug et al., 2005; Teagle et al., 2017). A study by Walls et al. (2016) found that the species composition in an oarweed kelp farm (L. digitata) was different from that in a wild kelp forest, but as opposed to this study, they found species richness to be higher in the kelp farm. This indicates that there might be species specific differences in the potential ecological roles of farmed kelps. It is worth noting that in the study by Walls et al. (2016), the kelps were growing for a longer time and developed large holdfasts, which would most likely result in more individuals and species than for younger kelp (Christie et al., 2003; Christie et al., 2007).
In the area surrounding the kelp farm, large areas of wild L. hyperborea kelp forest were recorded, while the S. latissima and A. esculenta kelp forests were observed further away. The short distance between the farm and the wild L. hyperborea forest is a possible explanation to why the communities in the farm, consisting of A. latissima and A. esculenta, were more similar to that of the wild L. hyperborea communities than to the fauna communities of its wild counterpart. More species, more individuals, and a higher species diversity were observed in the L. hyperborea forest than in the kelp farm. Studies by Norderhaug et al. (2002) and Jørgensen and Christie (2003) indicate that kelp-associated fauna can be very mobile and have a high dispersal rate. It is therefore likely that the fauna in the farm may have migrated from the surrounding kelp forest. Based on this assumption, there may be reason to believe that the farm does not represent a distinct habitat, as Walls et al. (2016) suggested but that the community found in a kelp farm is determined by the communities in the surrounding environment and that kelp farms only serve as the ‘receivers’ of species from whatever communities they are surrounded by. The kelp farm in this study was relatively small (approximately 200 m × 200 m), and it is possible that we might have found a different result if the farm was located away from any kelp forest (e.g., more offshore or in areas with a heavy sea urchin grazing of kelp forests, as is found in Northern Norway, Rinde et al., 2014). Another explanation for why the species community in the kelp farm was more similar to that found in the wild L. hyperborea forest than to the wild conspecifics, may be associated with the wave exposure at the farm site. According to Norderhaug et al. (2014), fauna associated with kelp is greatly affected by wave exposure and currents, in addition to the morphology of the epiphytic algae they are associated with. In general, S. latissima grows in sheltered and moderately wave-exposed areas (Bekkby and Moy, 2011), L. hyperborea in areas with higher wave exposure (Bekkby et al., 2009), and A. esculenta in even more exposed areas (Birkett et al., 1998). The wild L. hyperborea forest and the farmed kelp in our study had relatively similar wave exposure (based on the wave exposure model for the area, Bekkby et al., 2009), and it is likely that this contributes to the observed similarities in their associated communities. As the wild S. latissima forest was located in a less wave-exposed area, that may explain why the fauna community in the farmed S. latissima does not resemble that found in the wild S. latissima forest.
According to our study, the community found in the water column (sampled using fauna traps) differs from the other localities (both the kelp farm and the wild kelp forest), with gastropods being the most dominant group, mainly consisting of the species L. vincta. This species has been known to have a high dispersal rate and can create a mucus string for use when drifting around to more favorable sites (Christie et al., 2007), which might explain why it inhabits the water-column fauna traps. In the water column, we found a lower number of species and individuals, and a lower biodiversity compared to the kelp farm and the wild kelp forests. This was also the case when looking at the numbers from the fauna traps alone. We did not expect the communities in the water column and in a kelp forest, wild or farmed, to be similar, as they represent completely different ecosystems. Our study shows that the kelp farm is not just an artificial structure that is placed in the water column; it actually serves as a habitat for kelp-associated fauna, making the farm different that the fauna traps in the water column. However, finding fauna typical for kelp forests also in the water column, far from any kelp beds, supports the results of Jørgensen and Christie (2003) on the horizontal and vertical dispersal of fauna from kelp forests to areas outside the kelp beds, where it serves as food at higher trophic levels.
In the wild, the kelp-associated fauna increases in density and mobility during the warmer seasons (Norderhaug et al., 2002; Christie et al., 2003). We therefore assumed that a longer growth period for the kelp in the farm would give similar findings and that the communities in the kelp farms would change as the cultivated kelp increases in size and complexity (Forbes et al., 2022). However, we found no significant difference between fauna communities in S. latissima farmed for 3 versus 7 months. We might have found differences if the kelp had been able to grow for an even longer period of time in the farm. However, in mid-Norway, kelp is typically harvested in April to May for human food since harvesters want to avoid as much fouling as possible. In a wild kelp forest, Christie et al. (1998) found that it took more than 4–5 years for the species community associated with kelp to return after trawling, which can be a pointer toward the time needed for a kelp farm to function as a true kelp habitat.
Even though we found no significant difference between fauna communities in old and young farmed kelp, some differences were observed. The fauna in the young forest was dominated by the isopod species I. pelagica, whereas the old forest was dominated by amphipods. I. pelagica is a small isopod with high mobility and might be faster at moving from the wild kelp forest to the farm. In the old farmed kelp, amphipods belonging to the genus Caprella was most dominant. As they were juvenile, we did not succeed in determining them to the species level, and thus, we were unable to conclude if we observed any of the Caprella species naturally found in Norway or if this was the alien species Japanese skeleton shrimp (Caprella mutica). C. mutica has often been found on artificial substrates (Ashton et al., 2007), which might explain why we found more Caprella species in the kelp farm than in the wild kelp forest.
We collected fauna by sampling kelps and by deploying fauna traps. The reason for using these two methods was to collect several types of fauna, i.e., both sessile animals directly associated with kelp and mobile species collected with the fauna traps. In the wild kelp forests, we captured more individuals and taxa from the sampled kelp than from fauna traps. In the kelp farm, the opposite was observed, and, here, most of the fauna was captured by the fauna traps. This might be explained by the fact that the wild kelp forests are complex systems that have had years to establish a fauna community attached to the kelp, while the young kelps in the farm provide a less established and complex habitat, i.e., most of the animals are being and have recently moved in from the surrounding wild forest, therefore being captured by the fauna traps.
To conclude, our results show that despite the kelp farm having fewer individuals and less taxa than wild kelp forests, it has an associated fauna community typically associated with kelp. Thus, kelp farms might serve as ‘hanging gardens’ in the period before harvest, harboring a diversity of organisms that, in turn, can attract fish to the vicinity of the farms. Further work should be done to identify and quantify the abundance of fish and other predators in and around seaweed farms as this would potentially affect the resources of commercial value for local fishers and fishing tourism. Furthermore, the kelp farm tended to house fauna communities more similar to that of the wild kelp forest directly surrounding the farm (in this case, the L. hyperborea) than to the fauna communities of the farmed species’ wild counterparts. All of this considered, it could be essential to consider the surroundings (the presence or absence of wild kelp forests) when evaluating the potential role of kelp farming for local and regional biodiversity. We recommend conducting more studies on the biodiversity and ecological functioning of kelp farms along the Norwegian and European coasts. This will evaluate and increase the robustness of the conclusion from this work and secure a sustainable development of the kelp farming industry.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KH was the KELPPRO project leader, and TB was the work package leader for the work behind this paper. KH, TB, and HC all contributed significantly to the application submitted to the Research Council of Norway. KH and TB developed the sample design and planned the field work together with SF, RT, LG, and LN. RT and LG took their MSci degree on these data, supervised by TB, SF, and HG. KH, TB, RT, LG, ER, MW, HC, and LN all took part in the field sampling. TB, MB, HC, and SF assisted the students in the species determination, and TB, GA, and HG contributed significantly to the statistical analyses. All authors contributed to the article and approved the submitted version.
Funding
The project was funded (2017–2021) by the Research Council of Norway, grant number 267536. Additional funding was provided by the Norwegian Institute for Water Research (NIVA), the University of Oslo and Seaweed Solutions (SES).
Acknowledgments
This work is based on the Master theses of Torstensen (2020) and Grünfeld (2020). Thanks to the Research Council of Norway (project nr. 314314) for funding the project and for Seaweed Solutions (SES), Jon Funderud and Diogo Raposo in particular, for making their farm available for research and helping out with practicalities. We are very grateful to Harry Paulsen (H. Paulsen AS, Hammarvik, Frøya) for letting us use his pier, house, and equipment during field work.
Conflict of interest
Author LN was employed by Seaweed Solutions SES and SINTEF Ocean.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1066101/full#supplementary-material
References
Andersen G. S., Steen H., Christie H., Fredriksen S., Moy F. E. (2011). Seasonal patterns of sporophyte growth, fertility, fouling, and mortality of Saccharina latissima in skagerrak, Norway: implications for forest recovery. J. Mar. Sci. 11, 1–8. doi: 10.1155/2011/690375
Ashton G. V., Willis K. J., Cook E. J., Burrows M. (2007). Distribution of the introduced amphipod, Caprella mutica Schurin, 1935 (Amphipoda: Caprellida: Caprellidae) on the west coast of Scotland and a review of its global distribution. Hydrobiol. 590, 31–41.
Bekkby T., Moy F. E. (2011). Developing spatial models of sugar kelp (Saccharina latissima) potential distribution under natural conditions and areas of its disappearance in skagerrak. Est. Coast. Shelf Sci. 95 (4), 477–483. doi: 10.1016/j.ecss.2011.10.029
Bekkby T., Rinde E., Erikstad L., Bakkestuen V. (2009). Spatial predictive distribution modelling of the kelp species Laminaria hyperborea. ICES J. Mar. Sci. 66, 2106–2115. doi: 10.1093/icesjms/fsp195
Bekkby T., Torstensen R. R. G., Grünfeld L. A. H., Gundersen H., Fredriksen S., Rinde E., et al. (2023a). Fauna species collected in farmed and wild kelp in Norway April 2019 (Data set). Zenodo. doi: 10.5281/zenodo.7575039
Bekkby T., Torstensen R. R. G., Grünfeld L. A. H., Gundersen H., Fredriksen S., Rinde E., et al. (2023b). Weight of fauna collected in farmed and wild kelp in Norway April 2019 (Data set). Zenodo. doi: 10.5281/zenodo.7575121
Birkett D. A., Maggs C. A., Dring M. J., Boaden P. J. S. (1998). Infralittoral reef biotopes with kelp species: an overview of dynamic and sensitivity characteristics for conservation management of marine SACs. natura 2000 report prepared by Scottish association of marine science (SAMS) for the UK marine SACs. Scottish Association of Marine Sciences (UK Marine SACs Project).
Broch O. J., Alver M. O., Bekkby T., Gundersen H., Forbord S., Handå A., et al. (2019). The kelp cultivation potential in coastal and offshore regions of Norway. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00529
Buschmann A. H., Camus C., Infante J., Neori A., Israel Á., Hernández-González M. C., et al. (2017). Seaweed production: overview of the global state of exploitation, farming and emerging research activity. European journal of phycology. Appl. Phycol. Spec. Issue 52 (4), 391–406. doi: 10.1080/09670262.2017.1365175
Campbell I., Macleod A., Sahlmann C., Neves L., Funderud J., Øverland M., et al. (2019). The environmental risks associated with the development of seaweed farming in Europe - prioritizing key knowledge gaps. Front. Mari. Sci. 6. doi: 10.3389/fmars.2019.00107
Christiansen M. E. (1972). Bestemmelsestabell over Crustacea decapoda, tifotkreps (Oslo: Universitetsforlaget).
Christie H., Fredriksen S., Rinde E. (1998). Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway. Int. J. Aquat. Sci. 375-376, 49–58. doi: 10.1023/A:1017021325189
Christie H., Jørgensen N. M., Norderhaug K. M. (2007). Bushy or smooth, high or low; importance of habitat architecture and vertical position for distribution of fauna on kelp. J. Sea Res. 58 (3), 198–208. doi: 10.1016/j.seares.2007.03.006
Christie H., Jørgensen N. M., Norderhaug K. M., Waage-Nielsen E. (2003). Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast. J. Mar. Biol. Ass. UK 83 (4), 687–699. doi: 10.1017/S0025315403007653h
Christie H., Norderhaug K., Fredriksen S. (2009). Macrophytes as habitat for fauna. Mar. Ecol. Progr. Ser. 396, 221–233. doi: 10.3354/meps08351
Clarke K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18 (1), 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Creed J. C., Vieira V. M. N. C. S., Norton T. A., Caetano D. (2019). A meta-analysis shows that seaweeds surpass plants, setting life-on-Earth’s limit for biomass packing. BMC Ecol. 19 (1), 1–11. doi: 10.1186/s12898-019-0218-z
Duarte C. M., Bruhn A., Krause-Jensen D. (2021). A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustainability 5, 185–193. doi: 10.1038/s41893-021-00773-9
Duarte C. M., Holmer M., Olsen Y., Soto D., Marbà N., Guiu J., et al. (2009). Will the oceans help feed humanity? BioScience 59 (11), 967–976. doi: 10.1525/bio.2009.59.11.8
FAO (2020). The state of world fisheries and aquaculture 2020. sustainability in action (Rome). doi: 10.4060/ca9229en
FAO (2022). Global aquaculture production. fisheries and aquaculture division (Rome). Available at: https://www.fao.org/fishery/en/collection/aquaculture?lang=en.
Fiskeridirektoratet (2020). Akvakulturstatistikk (tidsserier) (Alger). Available at: www.fiskeridir.no/Akvakultur/Tall-og-analyse/Akvakulturstatistikk-tidsserier/Alger.
Forbes H., Shelamoff V., Visch W., Layton C. (2022) Farms and forests: evaluating the biodiversity benefits of kelp aquaculture. J. Appl. Phycol. 34, 3059–3067. doi: 10.1007/s10811-022-02822-y
Graham (1988). Molluscs: Prosobranch and pyramidellid gastropods: keys and notes for the identification of the species. 2nd edition. Synopses of the British Fauna 2. E.J. Brill/W. Backhuys: London, UK. ISBN 90-04-08771-0. VII, 662 pp.
Hancke K., Bekkby T., Gilstad M., Chapman A., Christie H. (2018). Taredyrking - mulige miljøeffekter, synergier og konflikter med andre interesser i kystsonen. NIVA Rep. 7265, 37 p.
Handå A., Forbord S., Wang X., Broch O. J., Dahle S. W., Størseth T. R., et al. (2013). Seasonal- and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquacult 414-415, 191–201. doi: 10.1016/j.aquaculture.2013.08.006
Hayward P. J., Ryland J. S. (1995). Handbook of the marine fauna of north-West Europe (Oxford: Oxford University Press).
Hughes A. D., Black K. D., Campbell I., Davidson K., Kelly M. S., Stanley M. S. (2012). Does seaweed offer a solution for bioenergy with biological carbon capture and storage? GHG Sci. Technol. 2 (6), 402–407. doi: 10.1002/ghg.1319
Jørgensen N., Christie H. (2003). Diurnal, horizontal and vertical dispersal of kelp-associated fauna. Int. J. Aquat. Sci. 503 (1-3), 69–76. doi: 10.1023/B:HYDR.0000008491.89382.e5
Kerrison P. D., Stanley M. S., Edwards M. D., Black K. D., Hughes A. D. (2015). The cultivation of European kelp for bioenergy: Site and species selection. Biom. Bioenerg. 80, 229–242. doi: 10.1016/j.biombioe.2015.04.035
Kraufvelin P., Christie H., Olsen M. (2002). Littoral macrofauna (secondary) responses to experimental nutrient addition to rocky shore mesocosms and a coastal lagoon. Hydrobiol 484, 149.166. doi: 10.1007/978-94-017-3190-4_13
Lincoln R. J. (1979). British Marine amphipoda: Gammaridea (London: British Museum Natural History).
Mac Monagail M., Cornish L., Morrison L., Araújo R., Critchley A. T. (2017). Sustainable harvesting of natural seaweed resources. Eur. J. Phycol. 52 (4), 371–390. doi: 10.1080/09670262.2017.1365273
Matsson S., Christie H., Fieler R. (2019). Variation in biomass and biofouling of kelp, Saccharina latissima, cultivated in the Arctic, Norway. Aquacult 506, 445–452. doi: 10.1016/j.aquaculture.2019.03.068
Norderhaug K. M., Christie H., Fosså J. H., Fredriksen S. (2005). Fish–macrofauna interactions in a kelp (Laminaria hyperborea) forest. J. Mar. Biol. Assoc. UK 85, 1279–1286. doi: 10.1017/S0025315405012439
Norderhaug K. M., Christie H., Rinde E. (2002). Colonisation of kelp imitations by epiphyte and holdfast fauna; a study of mobility patterns. Mar. Biol. 141, 965–973. doi: 10.1007/s00227-002-0893-7
Norderhaug K. M., Christie H., Rinde E., Gundersen H., Bekkby T. (2014). Importance of wave and current exposure to fauna communities in Laminaria hyperborea kelp forests. Mar. Ecol. Progr. Ser. 502, 295–301. doi: 10.3354/meps10754
OECD (2020). OECD work in support of a sustainable ocean (Paris: OECD Publishing). Available at: https://www.oecd.org/ocean/OECD-work-in-support-of-a-sustainable-ocean.pdf.
Oksanen J., Blanchet F., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2020) Community ecology package. Available at: https://github.com/vegandevs/vegan.
Olafsen T., Winther U., Olsen Y., Skjermo J. (2012) Verdiskaping basert på produktive hav i 2050. Available at: www.sintef.no/globalassets/upload/fiskeri_og_havbruk/publikasjoner/verdiskaping-basert-pa-produktive-hav-i-2050.pdf.
Rinde E., Christie H., Fagerli C. W., Bekkby T., Gundersen H., Norderhaug K. M., et al. (2014). The influence of physical factors on kelp and sea urchin distribution in previously and still grazed areas in the NE Atlantic. PloS One 9, e0100222. doi: 10.1371/journal.pone.0100222
Shannon C. E. (1948). A mathematical theory of communication. Bell System Tech. J. 27, 623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x
Shaw R. G., Mitchell-Olds T. (1993). ANOVA for unbalanced data: an overview. Ecol 74 (6), 1638–1645. doi: 10.2307/1939922
Steneck R. S., Graham M. H., Bourque B. J., Corbett D., Erlandson J. M., Estes J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environm. Conserv. 29 (4), 436–459. doi: 10.1017/S0376892902000322
Stévant P., Rebours C., Chapman A. (2017). Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquacult. Int. 25 (4), 1373–1390. doi: 10.1007/s10499-017-0120-7
Teagle H., Hawkins S. J., Moore P. J., Smale D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 492, 81–98. doi: 10.1016/j.jembe.2017.01.017
Walls A. M., Edwards M. D., Firth L. B., Johnson M. P. (2019). Ecological priming of artificial aquaculture structures: kelp farms as an example. J. Mar. Biol. Assoc. UK 99, 729–740. doi: 10.1017/S0025315418000723
Walls A., Kennedy R., Fitzgerald R., Blight A., Johnson M., Edwards M. (2016). Potential novel habitat created by holdfasts from cultivated Laminaria digitata: assessing the macroinvertebrate assemblages. Aquacult. Environm. Interact. 8, 157–169. doi: 10.3354/aei00170
Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., et al. (2019). Welcome to the tidyverse. J. Open Source Software 4 (43), 1686. doi: 10.21105/joss.01686
Keywords: kelp farm, kelp forest, seaweed, species composition, abundance, diversity, invertebrates
Citation: Bekkby T, Torstensen RRG, Grünfeld LAH, Gundersen H, Fredriksen S, Rinde E, Christie H, Walday M, Andersen GS, Brkljacic MS, Neves L and Hancke K (2023) ‘Hanging gardens’—comparing fauna communities in kelp farms and wild kelp forests. Front. Mar. Sci. 10:1066101. doi: 10.3389/fmars.2023.1066101
Received: 10 October 2022; Accepted: 30 January 2023;
Published: 20 February 2023.
Edited by:
Katerina Vasileiadou, Hellenic Centre for Marine Research (HCMR), GreeceReviewed by:
José M. Rico, University of Oviedo, SpainJason Michael Hall-Spencer, University of Plymouth, United Kingdom
Copyright © 2023 Bekkby, Torstensen, Grünfeld, Gundersen, Fredriksen, Rinde, Christie, Walday, Andersen, Brkljacic, Neves and Hancke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trine Bekkby, dHJpbmUuYmVra2J5QG5pdmEubm8=
 Trine Bekkby
Trine Bekkby Ragnhild Ryther Grimm Torstensen1,2
Ragnhild Ryther Grimm Torstensen1,2 Hege Gundersen
Hege Gundersen Stein Fredriksen
Stein Fredriksen Eli Rinde
Eli Rinde Hartvig Christie
Hartvig Christie Luiza Neves
Luiza Neves Kasper Hancke
Kasper Hancke