
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 11 July 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1056640
The ridged swimming crab Charybdis natator (Portunidae) is a commercially important crustacean species in China. The purpose of this study is to compare its population structure and reproductive pattern within the same fishing area (the southern Taiwan Strait) from two datasets over 25 years; one from 1994−1996 (the early years of the C. natator fishery) and the other from 2019. The overall sex ratio (male:female) changed from a male bias (1:0.76, p < 0.01) in 1994−1996 to a female bias (1:1.38, p < 0.01) in 2019. Male body sizes (carapace width, CW) were significantly larger than those of females in both datasets (p < 0.05). The average CW and body weight (BW) of males and females in 2019 were significantly smaller (p < 0.01) and lighter (p < 0.01) than those in 1994−1996. The maximum body size and the proportion of large-sized individuals (CW > 10 cm) decreased dramatically over 25 years. One spawning peak season was identified from each dataset, i.e., March−August 1994−1996 and February−April 2019, revealing a 1-month shift. The minimum body sizes for female maturation (carrying eggs) were 6.9 cm CW in 1994−1996 and 6.1 cm CW in 2019, an 11.6% reduction over 25 years. For the first time, the CW at 50% female maturation, the relationship between female absolute fecundity and CW, and egg diameters were obtained from the 2019 dataset, which can be applied in the future comparisons. Recent studies have revealed a consistent spawning peak in February–April for several commercially important crabs in the southern Taiwan Strait. These findings should be considered in crab fishery management. Furthermore, both fishery- and environment-associated factors influencing crustacean population structure and reproductive dynamics merit further investigation.
The decapod crustaceans, including crab, shrimp, and lobster species, are becoming the fastest-growing fishery worldwide, and the increasing global demand is providing opportunities for poverty alleviation and wealth generation (Anderson et al., 2011; Boenish et al., 2022). However, crustaceans are now facing threats from fishing pressure and climate change. Heavy exploitation has led to a decrease in catch per unit effort (CPUE) and individual sizes; the latter is closely associated with low reproductive success rates and fertilization outputs (Orensanz et al., 1998; Carver et al., 2005; Sato et al., 2007). In addition, climate change is known to contribute directly or indirectly to changes in the biomass, reproduction, and distribution patterns of crustaceans (Annala et al., 1980; Aiken and Waddy, 1986; Koeller et al., 2007; Sato et al., 2007; Johnston et al., 2008; Fedewa et al., 2020; Tanaka et al., 2020; Koepper et al., 2021). Under fishing pressure and climate change, sustainable crustacean management is challenging.
The family Portunidae, commonly known as swimming crabs, are important in global crustacean capture fisheries, and more than 1.3 million metric tons (t) of swimming crabs from 52 countries or areas were reported in 2019 (https://www.fao.org/fishery/statisticsquery/en/capture/capture_quantity). China is the most important nation for the swimming crab capture fishery, contributing to over 60% of the global swimming crab production (Lin et al., 2021a). In the China Fishery Statistical Yearbooks, the category “crabs” refers to Portunus species, Scylla species, and Charybdis species. Charybdis species are mainly caught by traps, bottom trawlers, bottom gill nets, and set nets from intertidal to offshore waters, and are usually sold alive in domestic markets or processed as frozen products for international demands (Ng et al., 2008; Liu and Lin, 2020; Naimullah et al., 2022). Statistical data on capture productions of Charybdis species in the China Fishery Statistical Yearbooks are the only resource nation contributing to FAO fishery statistics and have been available since 2003. A declining trend in capture productions of Charybdis species has been noticed from approximately 12% (~72,000 t) of the annual total swimming crab production in 2003 to <4% (~22,000 t) in 2020 (MOA, 2003–2017, MARA, 2018–2021). However, studies from reproductive biology and population structure to fishery patterns on Charybdis species are limited, irrespective of their commercial importance in crustacean fisheries. Data deficiency hinders our understanding of the performance of crab stocks and developing sustainable fishery strategies and effective management measures (Boenish et al., 2022).
As a large-sized Charybdis species, the ridged swimming crab C. natator can reach a maximum of 17 cm carapace width (CW) (Ng, 1998). It is widely distributed in the Indian and Pacific Oceans and commonly found in the East China Sea and South China Sea (Stephenson et al., 1958; Yang et al., 2011). It inhabits rocky-sandy bottoms at depths from 5 to 310 m (Ng, 1998; Yang et al., 2011) and feeds on small demersal invertebrates and fishes (Ye, 1999). Charybdis natator is one of the most important commercial crustacean species in southern China, having a good price of 80–200 RMB/kg (12.5−31.4 USD/kg) (Liu and Lin, 2020). The estimated annual capture production of C. natator in the southern Taiwan Strait was approximately 4,000 t with more than three-quarters of that from bottom trawlers in the early 1990s, making it No. 3 among all crab species caught in the area and contributing to 8%−10% of the total crab capture production in bottom trawl fisheries (Wang et al., 1998; Ye, 1999).
The comparisons of population structure before and after exploitation can provide insight into the impacts of fishery (Weinberg and Keith, 2003). C. natator can be a great candidate to improve our understanding of crustacean population performance in Chinese waters. The fishing pressure in the southern Taiwan Strait has increased from <1,000 bottom trawlers (approximately 0.1 million Kw) in the early 1990s to over 2,000 bottom trawlers (> 0.3 million Kw) since 2005 (Xiao, 2002; Ye et al., 2007). In the early years (1994−1996) of the C. natator fishery in the southern Taiwan Strait, the population structure and reproductive patterns were determined from bottom trawl catches, and data were presented as a 12-month dataset from January to December (Ye, 1999). This study aims to repeat the examination of C. natator catches from bottom trawlers within the same fishing area in 2019 in order to compare its population structure and reproductive pattern over 25 years (between 1994–1996 and 2019). Because of the strict national summer fishing moratorium in Chinese waters, samples were unavailable from May to July 2019, which becomes a common limitation on fishery evaluations. Irrespective of the data gap that exists, this study is still essential for understanding changes in the population structure and reproductive pattern over the 25-year fishery, discussing the fishery and climate change factors that influence the changes and providing recommendations on crab fishery management in the southern Taiwan Strait.
C. natator samples were collected from bottom trawler catches in the southern Taiwan Strait; the fishing areas largely overlapped with 22.00−24.00° N and 117.30−120.00° E in 1994–1996 (Ye, 1999), and 22.00−24.00° N and 116.30−190.00° E in 2019 (this study).
Monthly sampling of C. natator was conducted from January to April and August to December 2019. Approximately 15–35 kg (one to two baskets) of C. natator samples were randomly collected from at least five bottom trawlers each month.
Carapace width (CW, in 0.1 cm) and body weight (BW, in 0.01 g) were measured for each individual (Figure 1A). Based on abdomen morphology, sex was determined (Figures 1B, C). Females carrying eggs on the abdominal pleopods were considered fully mature, with three color groups, namely, red, yellow, and dark-gray, indicating embryonic developmental stages from early to late (Figures 1D, F) (Sumpton, 1990).
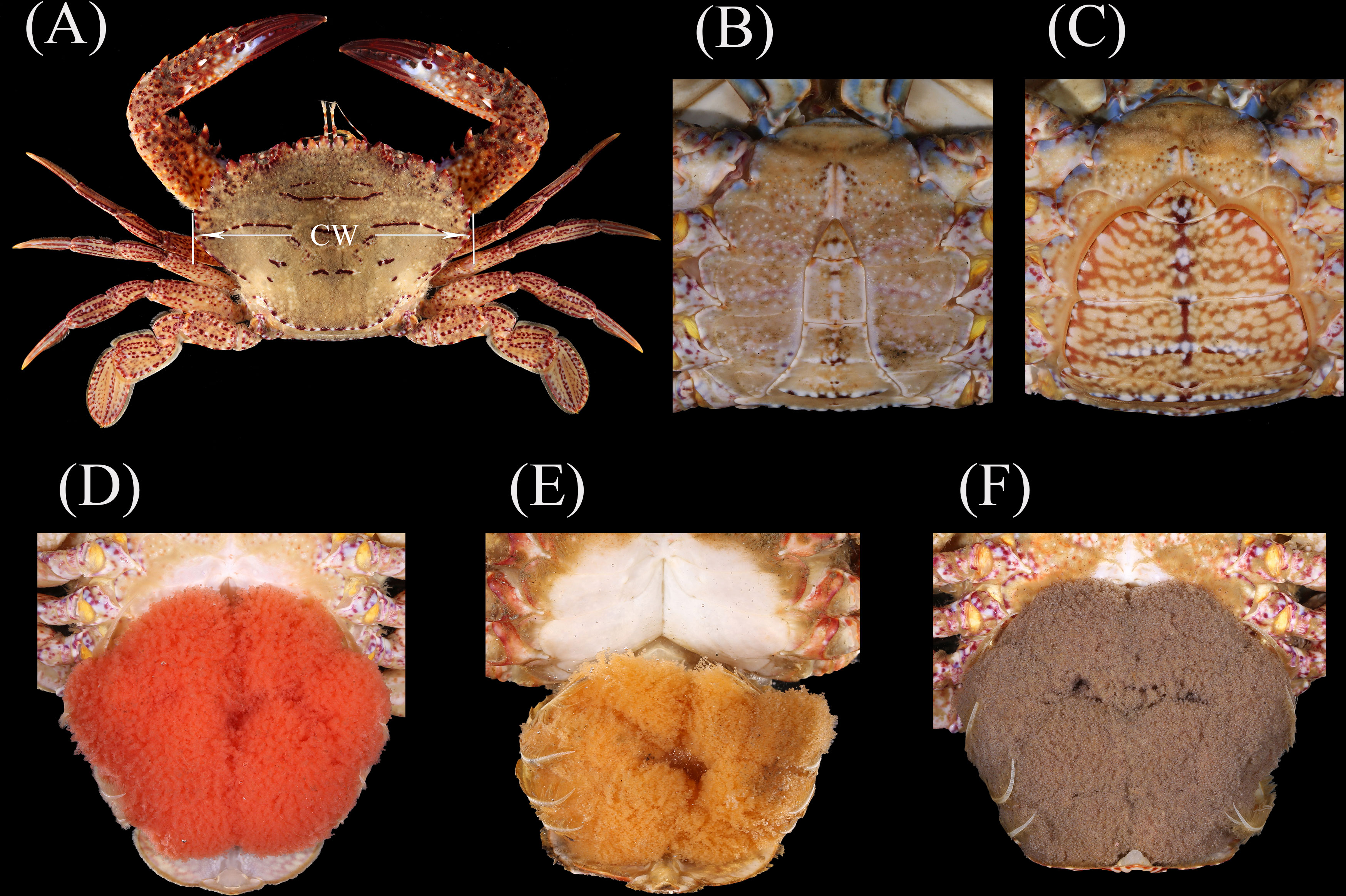
Figure 1 (A) Dorsal view of Charybdis natator for carapace width (CW) measurement. Male (B) and female (C) determination by abdomen morphology. Three egg colors with red (D), yellow (E), and dark gray (F).
The percentage of females carrying eggs each month, irrespective of egg color, was calculated as follows: the number of females carrying eggs/the total number of females × 100. The spawning season was defined as the months with females carrying eggs, and the spawning peak season was defined as the month(s) with at least 50% of females carrying eggs or matured (Sadovy, 1996). The minimum size (CW) at female maturity was determined by the smallest female collected that carried eggs. The size at 50% female maturity (CW50) was determined by fitting a logistic regression (probit link) to the percentage of females carrying eggs in 0.5 cm CW size classes using females from the spawning peak month(s).
Female fecundity, defined as the number of eggs carried by a female, was estimated empirically following standard methodologies (Lin et al., 2021a). The absolute fecundity (Fa) was calculated as follows: Fa = W × m/n, where W is the total weight of the entire egg mass for a female, m is the average weight of five egg sub-samples (approximately 0.02 g per each sub-sample), and n is the average number of eggs from the five sub-samples (Johnson et al., 2010; Soundarapandian et al., 2013). The relative fecundity (Fr) was calculated as follows: Fr = Fa/CW and Fr = Fa/BW. Egg diameters (to 1 μm) for all three aforementioned colors were measured using a Leica M165FC fluorescence stereo microscope.
Data from the mid-1990s were exclusively extracted from one publication in Chinese (Ye, 1999). Charybdis natator was collected monthly from July 1994 to June 1996, and the biological data were presented as a 12-month dataset from January to December without annual variation. Sex ratio, CW and BW ranges, and spawning seasons were obtained from the tables directly. Male and female size differences were extracted from figures using the GetData Graph Digitizer. Length (CW)–weight (BW) relationships of males and females and the minimum CW were documented in the main text. CW50, female fecundity, and egg diameters were not available.
Differences in sex ratio from 1:1 monthly and overall were analyzed using a Chi-square test for both the 1994−1996 and the 2019 datasets. Monthly and overall male and female size differences (CW) for 2019 were analyzed via one-way ANOVA or non-parametric analysis (Mann–Whitney U test). Male/female size comparisons (CW) by month and overall between the two datasets were analyzed via Student’s t-test or non-parametric analysis (Wilcoxon test). The relationships of CW–BW in males and females and Fa−CW were all analyzed via one-way ANCOVA. Statistical analyses were conducted using R (R Core Team, 2020) and Microsoft Excel 2019, setting the statistical significance at p ≤ 0.05.
The overall sex ratio of C. natator changed largely from a male bias in 1994−1996 to a female bias in 2019 (Table 1). The overall male:female ratio was 1:0.76 (N = 1,810, with 1,027 males and 783 females) in 1994−1996 (Ye, 1999), which is significantly different from a 1:1 ratio (χ2 = 32.89, df = 1, p < 0.01). In 2019, the overall male:female ratio was 1:1.38 (N = 1,332, with 560 males and 772 females), again, significantly different from a 1:1 ratio (χ2 = 33.37, df = 1, p < 0.01). Monthly variations in sex ratios were different between the two datasets; a significant male bias was found in June, July, and December in 1994−1996 (p < 0.01), and a significant female bias was found in February and March (p < 0.01) and October (p < 0.05) in 2019.

Table 1 Comparisons of Charybdis natator sex ratios (male:female) sampled from bottom trawlers in 1994–1996 (data were extracted from Ye, 1999) and in 2019 in the southern Taiwan Strait, China.
Males were larger and heavier than females, both in 1994−1996 (data were not available for statistical tests) and in 2019 (CW, F = 27.49, df = 1330, p < 0.05; BW, F = 45.43, df = 1,330, p < 0.05) (Table 2; Figure 2). The average CW and BW of males in 2019 were significantly smaller (t = −22.03, df = 559, p < 0.01) and lighter (t = −28.19, df = 559, p < 0.01) than those in 1994−1996, decreasing by 15.28% in CW and by 43.78% in BW. The same trends were found in females over 25 years; the average CW and BW of females in 2019 were significantly smaller (t = -20.58, df = 771, p < 0.01) and lighter (t = -25.39, df = 771, p < 0.01) than those in 1994−1996, decreasing by 9.69% in CW and by 30.57% in BW. The maximum CW and BW of C. natator in 2019 were smaller and lighter than those in 1994−1996, decreasing by approximately 7.5% in CW and more than 32% in BW (Table 2). Over 25 years, the proportions of large individuals (CW > 10 cm) declined from 32.94% (1994−1996) to 10.36% (2019) for males and from 7.53% (1994−1996) to 2.33% (2019) for females. The dominant size groups declined from 8.0−9.9 cm (46.03%, 1994−1996) to 6.5−8.4 cm (55.44%, 2019) for males and from 7.5−9.9 cm (72.97%, 1994−1996) to 6.0−8.9 cm (84.45%, 2019) for females (Figure 2).
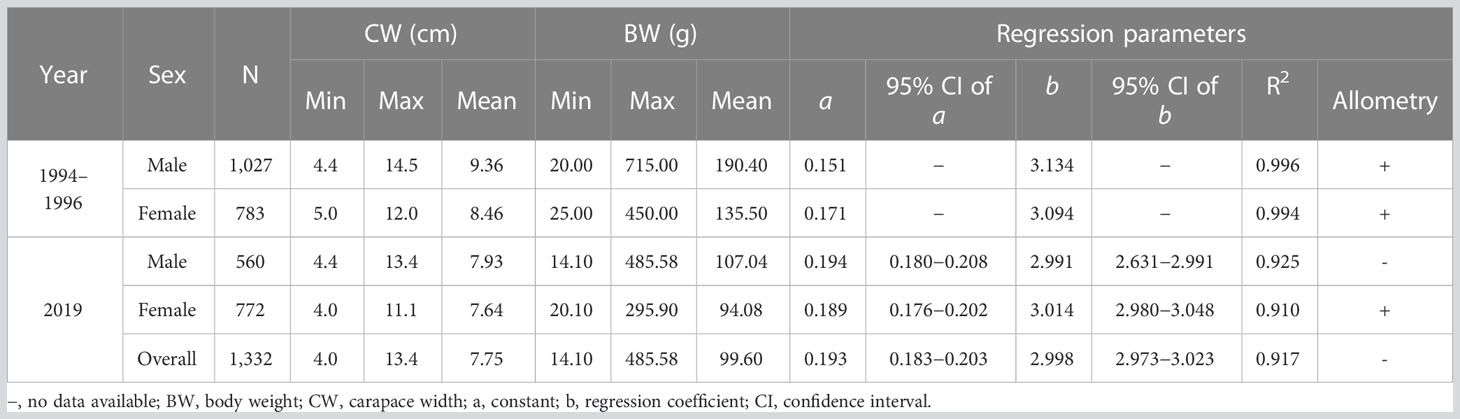
Table 2 Comparisons of regression equations and estimated parameters of length (CW)–weight (BW) relationships for Charybdis natator sampled from bottom trawler catches in 1994–1996 (data were extracted from Ye, 1999) and in 2019 in the southern Taiwan Strait, China.

Figure 2 The size (carapace width, cm) frequency (%) for Charybdis natator collected from bottom trawlers in 1994−1996 (data were extracted from Ye, 1999) (A) and in 2019 (B). Vertical lines indicate the average sizes of males and females.
Monthly CW variations of males and females were found in both datasets (Figure 3). The monthly average CW of males and females in 1994−1996 were significantly larger than those in 2019 (p < 0.01), except in February, August, and September (Figure 3). The length (CW)–weight (BW) relationships of males and females declined from 1994−1996 to 2019 (Table 2; Figure 4). The BW of C. natator larger than 10.0 cm CW was reduced by approximately 10% (41.37 ± 16.90 g) in males and 5% (23.66 ± 19.10 g) in females over 25 years.
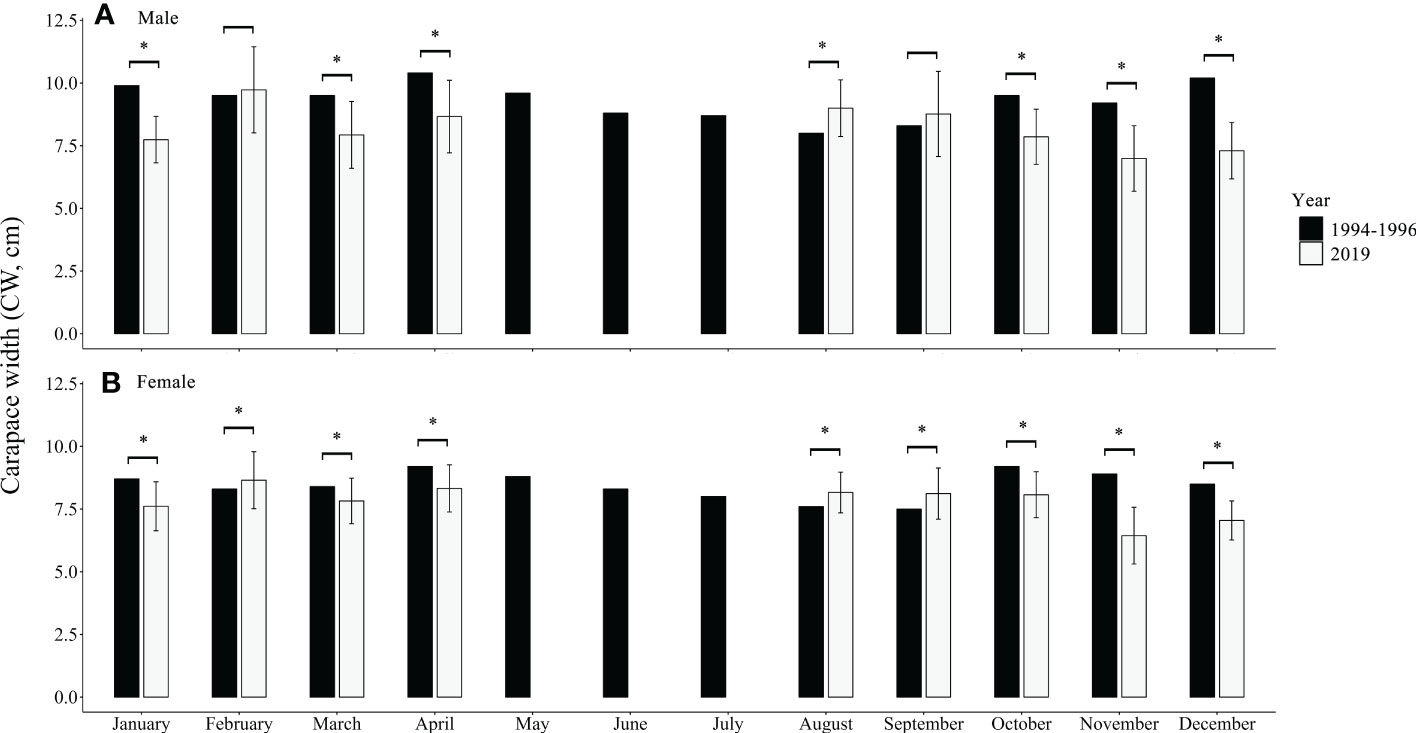
Figure 3 Monthly carapace width (CW, mean ± SD) of Charybdis natator males (A) and females (B) in 1994−1996 (data were extracted from Ye, 1999) and in 2019 (asterisks indicate p < 0.01 in t-test or non-parameter t-test between 1994−1996 and 2019).
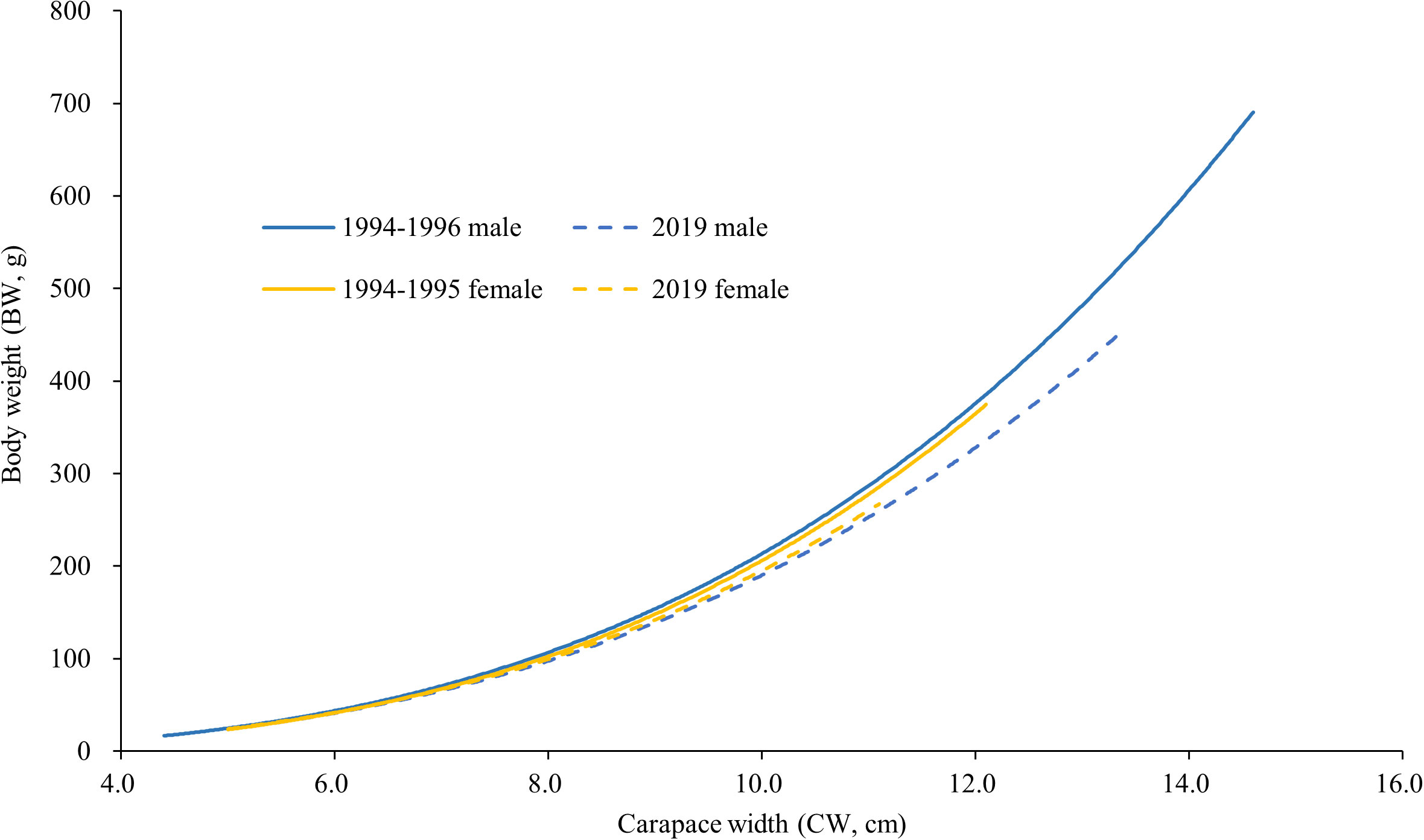
Figure 4 Length (carapace width)–weight (body weight) relationships of male and female Charybdis natator in 1994−1996 (data were extracted from Ye, 1999) and in 2019.
Charybdis natator females carrying eggs were found in most months of the year (except October in 1994−1996 and except August and October in 2019) (Figure 5). One spawning peak season was found for each dataset: March−August in 1994−1996 with a peak in April, and February−April in 2019 with a peak in February (Figure 5). The minimum sizes for females carrying eggs were 6.9 cm CW in 1994−1996 and 6.1 cm CW in 2019. The estimated size at 50% female maturity was 7.7 cm CW in 2019 (Figure 6). The majority size groups for female sexual maturity were 8.0−9.0 cm CW in 1994−1996 (> 50%) and in 2019 (48.94%).
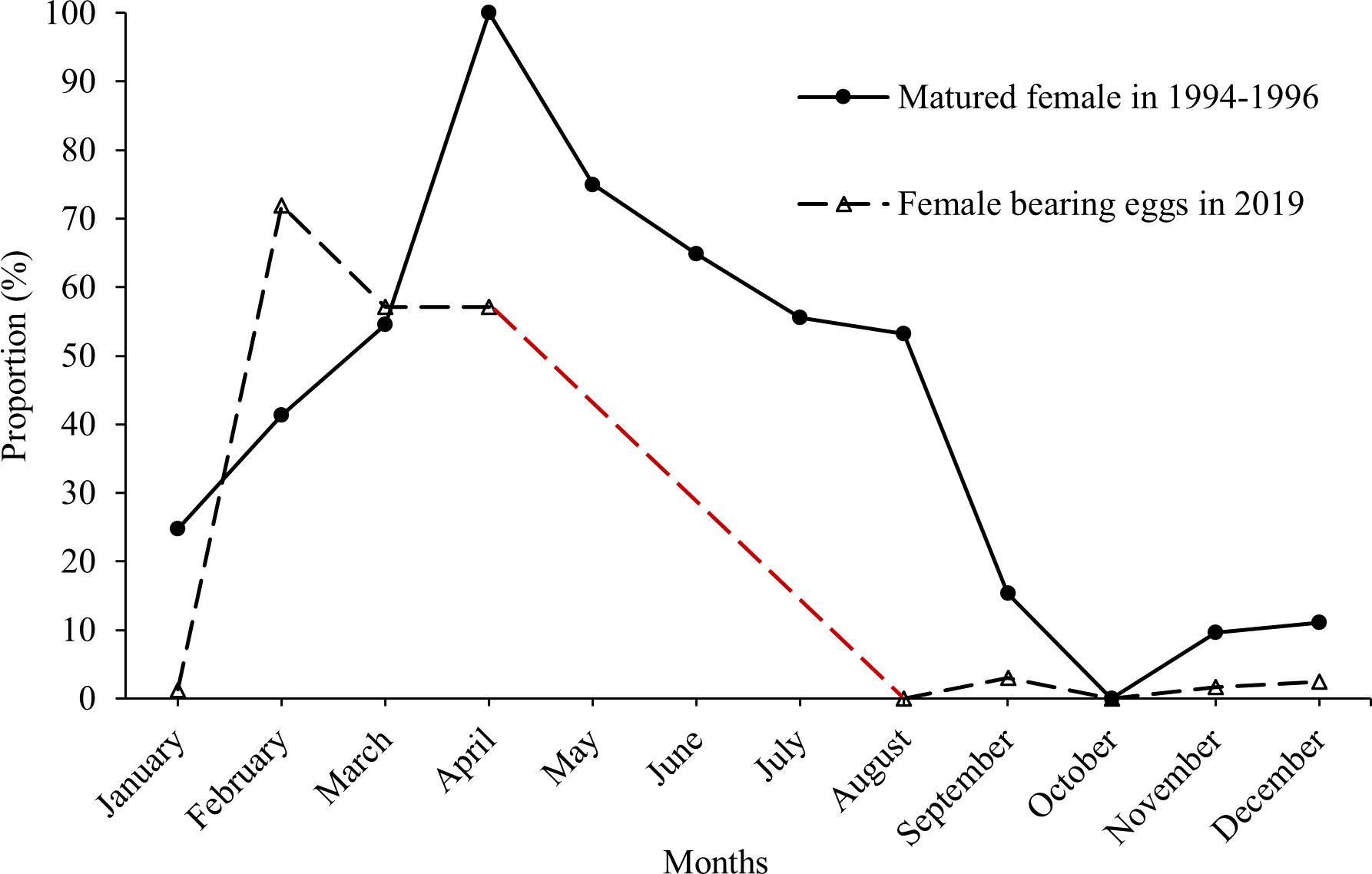
Figure 5 Percentages of matured female Charybdis natator by month in 1994−1996 (data were extracted from Ye, 1999) and in 2019 (data no available in May-July). The red dashed line indicates the trend estimation in 2019 based on the trend pattern in 1994–1996. The higher percentages of mature females in 1994−1996 might come from the inclusion of both females bearing eggs and with vitellogenic stage of oocytes.
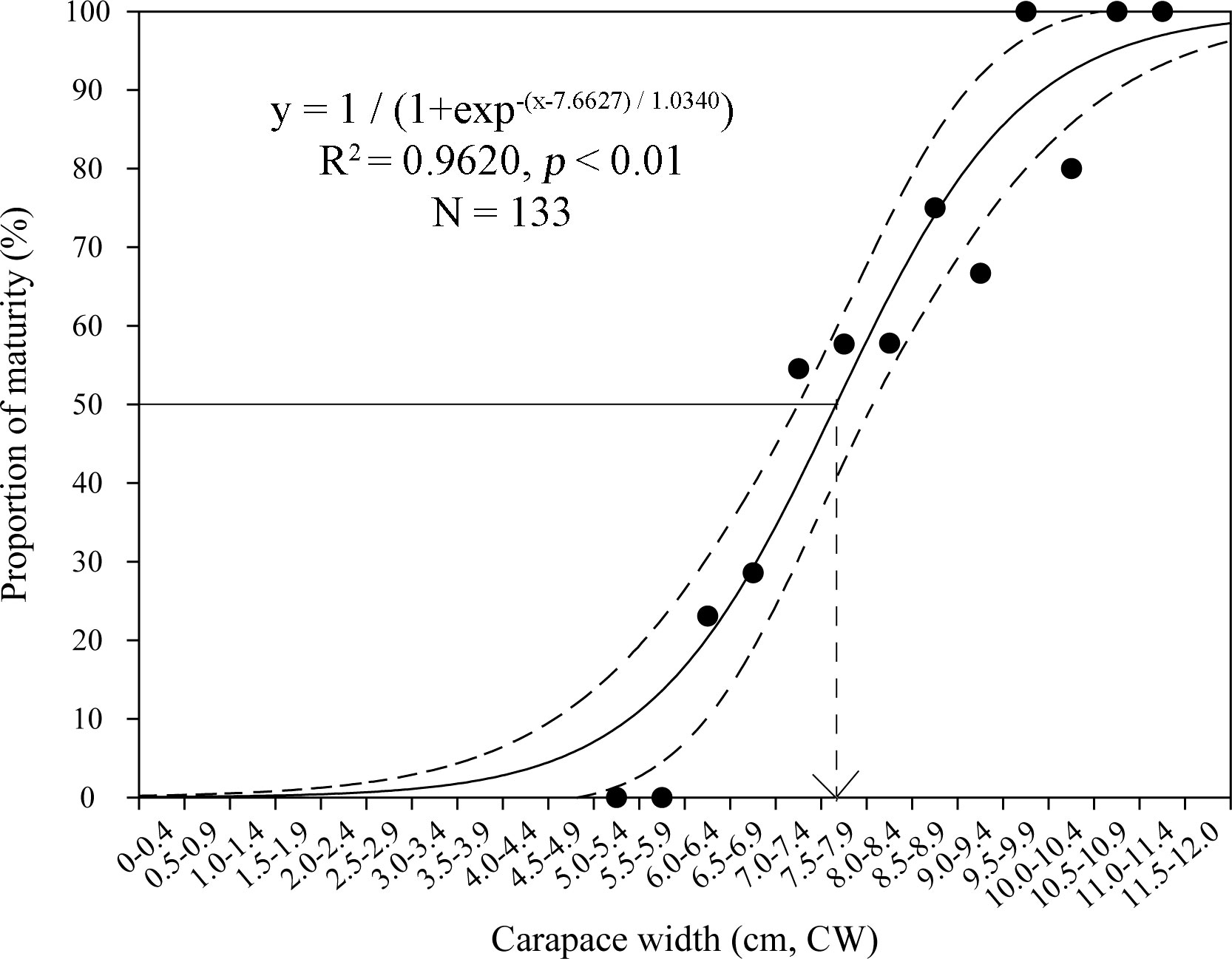
Figure 6 Size (carapace width, CW) at 50% female maturity for Charybdis natator based on all females sampled from the spawning peak (February−April) in 2019. Bootstrapped 95% confidence intervals shown with dashed lines (N = 9,999).
A total of 15 females carrying eggs with 6.9−10.1 cm CW (seven with red eggs, three with yellow eggs, and five with dark-gray eggs) were selected for fecundity and egg size measurements. Fa was between 287,597 and 973,417 eggs (575,349 ± 157,478 N = 15). Fr was between 41,681 and 94,398 eggs cm−1 CW (66,009 ± 12,906, N = 15), and 2,933 and 5,423 eggs g−1 BW (3,769 ± 650, N = 15). A typical power relationship between Fa and CW was found (p < 0.01) (Figure 7). Egg diameters were 283–371 μm (326 ± 16, N = 150), 317 ± 10 μm for red eggs, 320 ± 14 μm for yellow eggs, and 338 ± 14 μm for dark-gray eggs, showing a general increase in egg diameters from early to pre-hatching stage (F = 34.84, df = 147, p < 0.01).
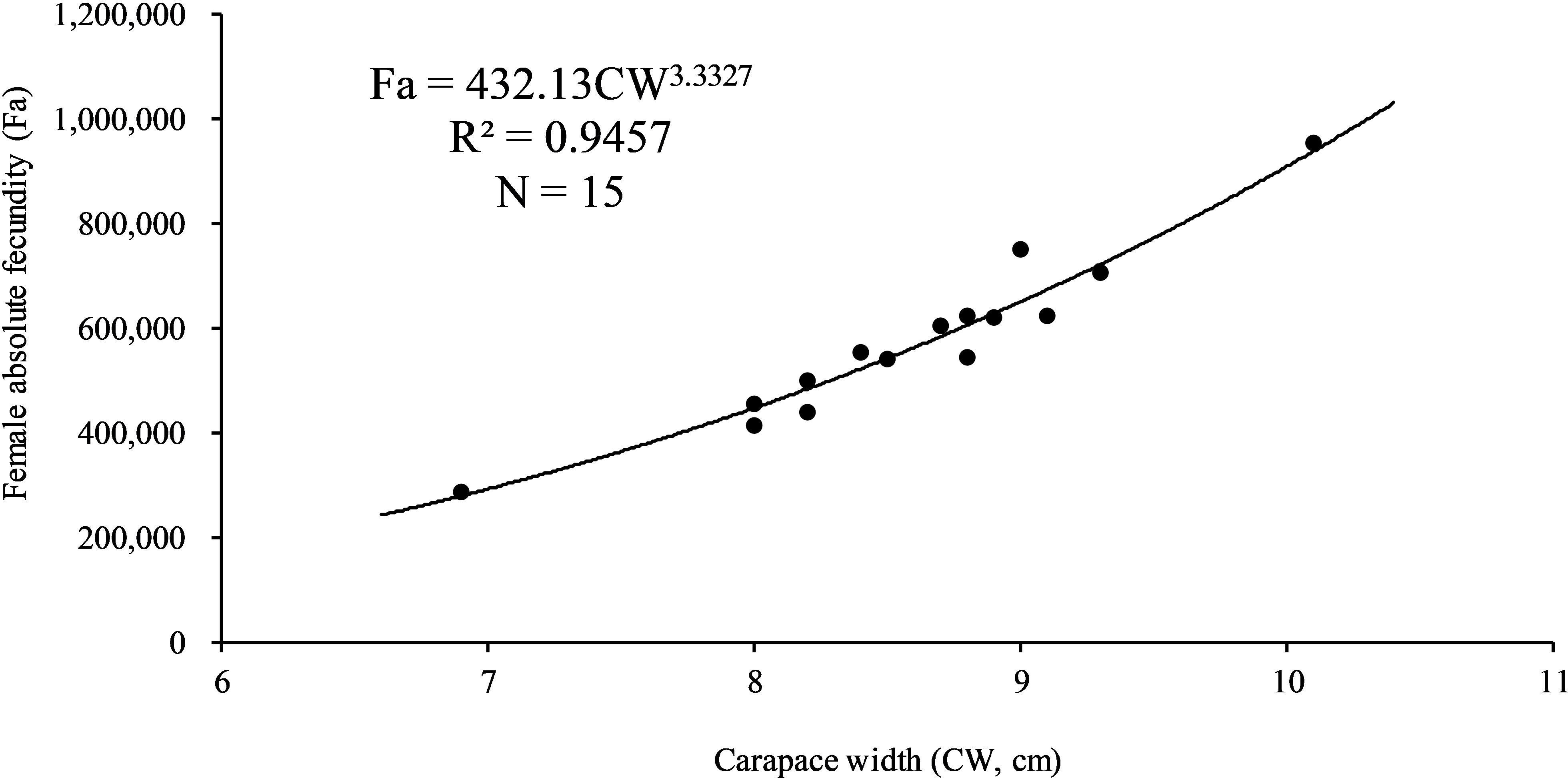
Figure 7 Absolute fecundity (Fa) and size (carapace width, CW) relationship for Charybdis natator females carrying eggs (N = 15).
An increasing abundance of one sex usually occurs in specific sex-only and size-selective crustacean fisheries (Carver et al., 2005; Sato et al., 2007; Wahle et al., 2008). In this study, however, a significant change from a male- to a female-bias sex ratio was noticed in the non-selective, bottom trawling fishery in the southern Taiwan Strait over 25 years. In the area, non-selective fishing gears such as bottom trawlers and crab traps have been the main fishing gears for crab catches, and there are no sex or size regulations in action.
The impact of long-term, non-selective fishery on the crustacean sex ratio merits further discussion and investigation. First, marine crustaceans commonly have a skewed sex ratio due to environmental differences, such as salinity and temperature (Wenner, 1972; Jury et al., 2019; Koepper et al., 2021). In the southern Taiwan Strait, bottom trawling has developed since the 1970s and changed the biodiversity, biomass, and habitat of the benthic ecosystem (Xiao, 2002). The changed habitat may impact the benthic communities and the food compositions, which particularly favor C. natator females. Second, the changing climate has been reported to influence the spatial-temporal distribution, migration pattern, and sex ratio of marine crustaceans (Sato, 2011; Wahle et al., 2015; Greenan et al., 2019; Fedewa et al., 2020; Tanaka et al., 2020; Naimullah et al., 2021). For example, under the warming seawater, the American lobster females moved toward farther offshore waters, and the large males were more likely to inhabit warmer shallow waters (Tanaka et al., 2020; Koepper et al., 2021). In the southern Taiwan Strait, the surface sea temperature has increased dramatically (Belkin and Lee, 2014), and C. natator males might stay at nearshore shallow water where the non-selective bottom trawl fisheries operate. More studies are needed to understand the sex ratio patterns of C. natator under changing climate and exploration conditions in the future.
Long-term harvesting can truncate the size structure of a population or a stock (Melville-Smith, 1988; NEFSC, 1993; NEFSC, 1998). This phenomenon is not only observed in marine crustaceans (Jamieson et al., 1998; Wahle et al., 2008; Sato, 2012) but also in marine fishes and other invertebrate species (Roy et al., 2003; Harvey et al., 2006). This study also detected a reduction in the proportion of large individuals (>10 cm CW), average size, and body weight in C. natator male and female catches in the southern Taiwan Strait over a 25-year fishery. The larger males and females found in February 2019 might be relative to the spawning activity of C. natator in the southern Taiwan Strait. A similar phenomenon also occurred in the peaking spawning month (April) in 1994−1996. The significantly larger C. natator males and females observed in August and September 2019 may be due to China’s summer fishing moratorium management strategy, as they reflect the first and second months’ catches right after the restricted three-and-a-half-month fishing ban. A bottom trawl ban management regulation would significantly increase the mean weight and size and the proportion of large-sized individuals in crustaceans (Tao et al., 2018).
The relationship between average carapace width (CW) and body weight (BW) declined between 1994−1996 and 2019 datasets. The exponential value “b” showed that male C. natator shifted from positive allometry (b = 3.13398) in the 1994−1996 dataset to negative allometry (b = 2.9913) in the 2019 dataset. The same phenomenon was also recorded in Chaceon quinquedens in the northwest Atlantic after nearly 30-year commercial fishing (Weinberg and Keith, 2003). The reduction in average male body weight would reflect a negative impact on the availability or quality of the prey and the success rate of male crustaceans mating (Sato, 2012; Kolts et al., 2013). The impact of lighter male body weight in a population of C. natator needs further investigation.
A 1-month shift was found in C. natator’s spawning peak season over 25 years, changing from March−August in the 1994−1996 dataset to February−April in the 2019 dataset in the southern Taiwan Strait. Because of the national summer fishing moratorium, there was a 3-month data gap in 2019 (May−July), and it is unknown whether the spawning peak season could extend into May−July. Small juveniles (1.8−2.2 cm CW) collected in October 2019 (Liu and Lin, 2020) indicate that spawning activity is likely to occur at least in July, as fertilized C. natator eggs usually take approximately 3 months to reach 2.0 cm CW (Sumpton, 1990; Arshad et al., 2006).
The causes for the 1-month shift in C. natator’s spawning peak in the Taiwan Strait have not completely been understood. The earlier spawning season might be caused by the increased seawater temperatures, smaller maturation size, earlier maturation age, or interaction effect. The higher temperature stimulates early ovarian development and accelerates ovarian maturity to spawn early (Annala et al., 1980). It is known that the reproductive season of the spiny king crab Paralithodes brevipes in Hamanaka Bay in 2004 started 1 month earlier than in 2003, owing to the higher seawater temperature in 2004 (Sato, 2012). In the Taiwan Strait, the annual overall sea surface temperature has increased more than 1°C from 1957 to recent times, and long-term warming was more strongly enhanced in winter with the highest average warming of 3.8°C in February (Belkin and Lee, 2014). The increased seawater temperature in the southern Taiwan Strait is considered to be a possible factor promoting the earlier spawning of C. natator. On the other hand, the warmer seawater and intensive fishing pressure can reduce the size at maturity and cause mature individuals to spawn earlier in marine crustaceans (Hines, 1989; Zheng, 2008; Hines et al., 2010; Hjelset et al., 2012; Green et al., 2014). The smallest female bearing eggs decreased from 6.9 cm CW in 1994−1996 to 6.1 cm CW in 2019 (Ye, 1999; this study). Likewise, cancer crab females, including Cancer irroratus and Cancer pagurus, in warm waters matured earlier and spawned at smaller sizes than those in cool waters (Shields, 1991). The size at maturity for tanner crab Chionoecetes bairdi decreased significantly due to the exploitation (Zheng, 2008). Therefore, higher temperatures and smaller matured sizes result in earlier spawning peak season of C. natator in the southern Taiwan Strait.
The fecundity and recruitment of a population or a stock may decline as a result of reduced abundance and individual size and skewed female sex ratios (Murua et al., 2003; Hjelset et al., 2012). Male size is an important factor in mating efficiency and success rate due to their handling and guarding behavior (Paul and Paul, 1997). The decreased abundance and male size can result in sperm competition, which potentially reduced the female’s reproductive output and success rate in crustaceans (Sato et al., 2007; Sato, 2011; Ogburn et al., 2014; Pardo et al., 2015; Pardo et al., 2017). At biased sex ratios or in species for which females preferentially mate with large males, females may have reduced reproductive success because they are unable to find suitable mates due to the prevalence of smaller males (Rowe and Hutchings, 2003; Rains et al., 2018).
Although altered sex ratios related to male-only or size-selective fisheries have been observed for a variety of crustaceans, the impacts on reproductive output at the C. natator population level under non-selective bottom trawl fishery is unclear. No historical data are available on fecundity and egg size to directly understand the impacts of fishery on C. natator.
Around the world, decapod crustacean capture fisheries are growing faster than other major fisheries, and more attention needs to be paid to their scientific research and sustainable management (Boenish et al., 2022). The “3S” (legal catchable size, sex, and season) management strategy was applied to achieve sustainable use in crustacean fisheries (Otto, 1986; Kruse, 1993). In China, one national and two provincial (Zhejiang and Fujian Provinces) regulations were released in 2015 and 2018, respectively, detailing minimum catch sizes and landing proportions of juveniles for 36 commercially important species including 28 fishes, two shrimps, four crabs (excluding C. natator), and two cephalopods (http://hyyyj.fujian.gov.cn/; http://www.zj.gov.cn/; http://www.moa.gov.cn/). The implementation of these new regulations, however, is challenging. Fisheries operations in Chinese economic exclusive zones are mainly multi-species fisheries, and approximately 90% of the total marine catch is from non-selective trawlers, purse seines, gill nets, and set nets (Kang et al., 2018). A recent study showed that 62% of Monomia haanii caught in bottom trawlers operating in the southern Taiwan Strait were smaller than the legal minimum catch size (8 cm CW) (Lin et al., 2021a).
A closed season to protect juveniles and females bearing eggs would help the recovery of the recruitment and spawning stocks to achieve the maximum sustainable yield for biological and economic objectives (Johnston et al., 2011; Dichmont et al., 2014). Management measures on protecting mature female crabs and juveniles are still limited in China. Currently, Portunus trituberculatus females carrying eggs and juveniles (individuals < 70 g) are banned at port landings and in food markets in Zhejiang Province between 1 April and 16 September. This regulation seeks to prevent the capture of bearing females and small juveniles by trap vessels and even extends beyond the fishing moratorium period in Zhejiang Province (1 April−1 August) (http://nynct.zj.gov.cn/art/2021/2/26/art_1694969_58931103.html). Recent studies revealed a consistent spawning peak (February−April) in the southern Taiwan Strait for C. natator, M. haanii, and Calappa philargius, indicating that the national summer fishing moratorium cannot protect the spawning stocks of these commercially important crabs (Lin et al., 2021a; Lin et al., 2021b; this study). Because of the dominance of multi-species fisheries in China, it is not easy to amend the closure season for specific species or species groups. However, a recent bio-economic modeling analysis of M. hannii in the southern Taiwan Strait demonstrated that the current national fishing moratorium can provide better biological and economic benefits compared with closing early to protect spawning peaks (Boenish et al., 2021). In the future, samples from May−July are needed for better assessment and management improvement.
In the early 1990s in Chinese waters, the fishing pressure in terms of fishing power (kw) and the number of trawlers have increased more than twofold since the early 1990s (Kang et al., 2018). In Dongshan County, the estimated capture production of C. natator from bottom trawlers has decreased from 3,100−3,800 t/year in the early 1990s (Ye, 1999) to approximately 2,500 t in 2019 (Liu and Lin, 2020). The CPUE of M. haanii in the bottom trawl fishery has decreased by 50% from the early 1990s to 2018−2019 in the southern Taiwan Strait (Zhang, 1997; Liu and Lin, 2020). Therefore, the CPUE decline trend of C. natator in the southern Taiwan Strait was estimated to be under the same situation as M. haanii. Since the 1990s, fishery management strategies in China, including vessel buyback programs, fishermen relocation programs, closed seasons and zones, the total allowable catch system, and zero and minus growth targets, have been implemented to control the effects of fishing and catch landing to prevent the decline of fishery stocks (Cao et al., 2017). In the future, an adaptive science-based fisheries management strategy for C. natator and other fishery species should include gathering information, setting goals, predicting outcomes, and monitoring and evaluating results toward obtaining such outcomes, then reviewing what worked and what failed, in order to lead to more economically and socially optimal outcomes under changing conditions.
In C. natator, body sizes (maximum, average, dominant group, and minimum at female sexual maturity) and body weights have reduced, and the spawning peak season has shifted to 1 month earlier in the southern Taiwan Strait over the last 25 years. The intensive fishing pressure is considered to be the trigger for these reductions and changes. The current national summer fishing moratorium regulation (May–mid-August) cannot protect the consistent spawning peak (February–April) of C. natator and other commercially important crabs (C. philargius, M. haanii, Portunus pelagicus, and Portunus sanguinolentus) in southern China. Such reproductive patterns for crabs should be taken into account in the region to achieve integrated fisheries management for maximum biological and economic output. It is worth noting that the impact of climate change and sea sand mining on the physiological regulation and population structure of C. natator and other marine crustaceans in China merits further investigations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
B-AL wrote the first draft. B-AL and YJ performed the data analyses. B-AL, YJ, and ML conducted commercial sampling, interviews, and measurement. ML supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
The study was funded by Ocean Outcomes (USA), Qingdao Marine Conservation Society (China), and Society of Entrepreneurs and Ecology Foundation (China).
We thank Si-qi Cai, Sheng-yao Sun, Jing Wang, Xin-ya Xu, Jing Zhang, and Zhe-hua Guang for facility and logistics support; Qing Xu and Guo-han Yang for sample treatment; and many fishermen and traders for sample collection assistance. Thanks to Emily King for the grammar corrections on the manuscript. Comments and suggestions from reviewers and Dr. Carlo Pipitone were much appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aiken D. E., Waddy S. L. (1986). Environmental influence on recruitment of the American lobster, Homarus americanus: a perspective. Can. J. Fisheries Aquat. Sci. 43, 2258–2270. doi: 10.1139/f86-277
Anderson J., Olsen Z., Glen Sutton T. W., Gelpi C., Topping D. (2017). Environmental drivers of the spatial and temporal distribution of spawning blue crabs Callinectes sapidus in the Western gulf of Mexico. North Am. J. Fisheries Manage. 37, 920–934. doi: 10.1080/02755947.2017.1335255
Annala J. H., McKoy J. L., Booth J. D., Pike R. B. (1980). Size at the onset of sexual maturity in female Jasus edwardsii (Decapoda, palinuridae) in New Zealand. New Z. J. Mar. Freshw. Res. 14, 217–227. doi: 10.1080/00288330.1980.9515864
Arshad A., Efrizal E., Kamarudin M. S., Saad C. R. (2006). Study on fecundity, embryology and larval development of blue swimming crab Portunus pelagicus (Linnaeus 1758) under laboratory conditions. Res. J. Fisheries Hydrobiology 1 (1), 3544. Available at: https://www.researchgate.net/publication/286016868_Study_on_fecundity_embryology_and_larval_development_of_blue_swimming_crab_Portunus_pelagicus_Linnaeus_1758_under_laboratory_conditions.
Belkin I. M., Lee M. A. (2014). Long-term variability of sea surface temperature in Taiwan strait. Climatic Change 124, 821–834. doi: 10.1007/s10584-014-1121-4
Boenish R., Lin B.-A., Kritzer J. P., Wilberg M. J., Shen C., Jiang Y., et al. (2021). A bioeconomic approach towards improved fishery management of Monomia haanii in the southern Taiwan strait, China. Fisheries Res. 240, 105969. doi: 10.1016/j.fishres.2021.105969
Boenish R., Kritzer J. P., Kleisner K., Steneck R. S., Werner K. M., Zhu W., et al. (2022). The global rise of crustacean fisheries. Front. Ecol. Environ. 20 (2), 102–110. doi: 10.1002/fee.2431
Cao L., Chen Y., Dong S., Hanson A., Huang B., Leadbitter D., et al. (2017). Opportunity for marine fisheries reform in China. Proc. Natl. Acad. Sci. 114 (3), 435–442. doi: 10.1073/pnas.1616583114
Carver A. M., Wolcott T. G., Wolcott D. L., Hines A. H. (2005). Unnatural selection: effects of a male-focused size-selective fishery on reproductive potential of a blue crab population. J. Exp. Mar. Biol. Ecol. 319 (1–2), 29–41. doi: 10.1016/j.jembe.2004.06.013
Dichmont C. M., Jarrett A., Hill F., Brown M. (2014). Harvest strategy for the northern prawn fishery under input control (Canberra: Australian Fisheries Management Authority).
Fedewa E. J., Jackson T. M., Richar J. I., Gardner J. L., Litzow M. A. (2020). Recent shifts in northern Bering Sea snow crab (Chionoecetes opilio) size structure and the potential role of climate-mediated range contraction. Deep Sea Res. Part II: Topical Stud. Oceanography 181-182, 104878. doi: 10.1016/j.dsr2.2020.104878
Green B. S., Gardner C., Hochmuth J. D., Linnane A. (2014). Environmental effects on fished lobsters and crabs. Rev. Fish Biol. Fisheries 24 (2), 613–638. doi: 10.1007/s11160-014-9350-1
Greenan B. J. W., Shackell N. L., Ferguson K., Greyson P., Cogswell A., Brickman D., et al. (2019). Climate change vulnerability of American lobster fishing communities in Atlantic Canada. Front. Mar. Sci. 6, 579. doi: 10.3389/fmars.2019.00579
Harvey C. J., Tolimieri N., Levin P. S. (2006). Changes in body size, abundance, and energy allocation in rockfish assemblages of the Northeast Pacific. Ecol. Appl. 16, 1502–1515. doi: 10.1890/1051-0761(2006)016[1502:CIBSAA]2.0.CO;2
Hines A. H. (1989). Geographic variation in size at maturity in brachyuran crabs. Bull. Mar. Sci. 45, 356–368. Available at: https://www.ingentaconnect.com/content/umrsmas/bullmar/1989/00000045/00000002/art00012#.
Hines A. H., Johnson E. G., Darnell M. Z., Rittschof D., Miller T. J., Bauer L. J., et al. (2010). “Predicting effects of climate change on blue crabs in Chesapeake bay,” in Biology and management of exploited crab populations under climate change. Eds. Kruse G. H., Eckert G. L., Foy R. J., Lipcius R. N., Sainte-Marie B., Stram D. L., Woodby D. (Alaska Sea Grant, University of Alaska Fairbanks). doi: 10.4027/bmecpcc.2010.22
Hjelset A. M., Nilssen E. M., Sundet J. H. (2012). Reduced size composition and fecundity related to fishery and invasion history in the introduced red king crab (Paralithodes camtschaticus) in Norwegian waters. Fisheries Res. 121, 73–80. doi: 10.1016/j.fishres.2012.01.010
Jamieson G. S., Phillips A., Smith B. D. (1998). Implications of selective harvests in dungeness crab (Cancer magister) fisheries. Can. J. Fisheries Aquat. Sci. 125, 309–321.
Johnson D. D., Gray C. A., Macbeth W. G. (2010). Reproductive biology of Portunus pelagicus in a south-east Australian estuary. J. Crustacean Biol. 30 (2), 200–205. doi: 10.1651/08-3076.1
Johnston D., Melville-Smith R., Hendriks B., Phillips B. (2008). Growth rates and survival of western rock lobster (Panulirus cygnus) at two temperatures (ambient and 23°C) and two feeding frequencies. Aquaculture 279, 77–84. doi: 10.1016/j.aquaculture.2008.03.048
Johnston D. J., Harris D., Caputi N., Thomson P. (2011). Decline of a blue swimmer crab (Portunus pelagicus) fishery in Western Australia–history, contributing factors and future management strategy. Fisheries Res. 109 (1), 119–130. doi: 10.1016/j.fishres.2011.01.027
Jury S. H., Pugh T. L., Henninger H., Carloni J. T., Watson W. H. (2019). Patterns and possible causes of skewed sex ratios in American lobster (Homarus americanus) populations. Invertebrate Reprod. Dev. 63 (3), 189–199. doi: 10.1080/07924259.2019.1595184
Kang B., Liu M., Huang X. X., Li J., Yan Y. R., Han C. C., et al. (2018). Fisheries in Chinese seas: what can we learn from controversial official fisheries statistics? Rev. Fish Biol. Fisheries 28, 503–519. doi: 10.1007/s11160-018-9518-1
Koeller P. A., Fuentes-Yaco C., Platt T. (2007). Decreasing shrimp (Pandalus borealis) sizes off Newfoundland and Labrador–environment or fishing? Fish. Oceanogr. 16 (2), 105–115. doi: 10.1111/j.1365-2419.2006.00403.x
Koepper S., Revie C. W., Stryhn H., Clark K. F., Scott-Tibbetts S., Thakur K. K. (2021). Spatial and temporal patterns in the sex ratio of American lobsters (Homarus americanus) in southwestern Nova Scotia, Canada. Sci. Rep. 11, 24100. doi: 10.1038/s41598-021-03233-8
Kolts J., Lovvorn J., North C., Grebmeier J., Cooper L. (2013). Effects of body size, gender, and prey availability on diets of snow crabs in the northern Bering Sea. Mar. Ecol. Prog. Ser. 483, 209–220. doi: 10.3354/meps10292
Kruse G. H. (1993). “Biological perspectives on crab management in Alaska,” in Proceedings of the international symposium on management strategies for exploited fish populations. Eds. Kruse G. H., Eggers D. M., Marasco R. J., Pautzke C., Quinn T. J. II (University of Alaska), 355–384. Available at: https://www.arlis.org/docs/vol1/RIR/77522224.pdf.
Lin B.-a., Boenish R., Kritzer J. P., Jiang Y., Wang S.-L., Liu M. (2021a). Reproductive dynamics of a swimming crab (Monomia haanii) in the world’s crab basket. Fisheries Res. 236, 105828. doi: 10.1016/j.fishres.2020.105828
Lin B.-a., Jiang Y., Boenish R., Xu Q., Liu M. (2021b). Population, reproductive and fishery dynamics of spotted box crab (Calappa philargius), a new claw-only fishery species, in the southern Taiwan strait, China. Front. Mar. Sci. 8, 751790. doi: 10.3389/fmars.2021.751790
Liu M., Lin B. (2020). Chinese Red swimming crab (Monomia haanii) fishery improvement project (FIP) in Zhangzhou City, Fujian Province, China (January–December 2019) (Xiamen University), 93.
Melville-Smith R. (1988). The commercial fishery for and population dynamics of red crab Geryon maritae off southwest Africa 1976-1986. South Afr. J. Mar. Sci. 6, 79–95. doi: 10.2989/025776188784480681
Murua H., Kraus G., Sabrido-Rey F., Witthames P. R., Thorsen A., Junquera S. (2003). Procedure to estimate fecundity of marine fish species in relation to their reproductive strategy. J. Northwest Atlantic Fishery Sci. 33, 33–54. doi: 10.2960/J.v33.a3
Naimullah M., Wu Y.-L., Lee M.-A., Lan K.-W. (2021). Effect of the El niño–southern oscillation (ENSO) cycle on the catches and habitat patterns of three swimming crabs in the Taiwan strait. Front. Mar. Sci. 8, 763543. doi: 10.3389/fmars.2021.763543
Naimullah M., Lee W. Y., Wu Y. L., Chen Y. K., Huang Y. C., Liao C. H., et al. (2022). Effect of soaking time on targets and bycatch species catch rates in fish and crab trap fishery in the southern East China Sea. Fisheries Res. 250, 106258. doi: 10.1016/j.fishres.2022.106258
NEFSC (1993). “C. gulf of Maine-georges bank redfish update,” in Report of the 15th Northeast Regional Stock Assessment Workshop (15th SAW). Stock Assessment Review Committee (SARC) Consensus Summary of Assessments. 34–50, Northeast Fisheries Science Center Ref. Doc. 93-06. Available at: https://repository.library.noaa.gov/view/noaa/8639.
NEFSC (1998). “D. spiny dogfish,” in 26th Northeast Regional Stock Assessment Workshop (26th SAW). Stock Assessment Review Committee (SARC) Consensus Summary of Assessments. 224–283, Northeast Fisheries Science Center Ref. Doc. 98-03. Available at: https://repository.library.noaa.gov/view/noaa/5241.
Ng P. K. L. (1998). “Crabs,” in FAO species identification guide for fishery purposes. the living marine resources of the Western Central Pacific, vol. 2 . Eds. Carpenter K. E., Niem V. H. (Rome: Food and Agriculture Organization), 1045–1155.
Ng P. K. L., Guinot D., Davie P. J. F. (2008). Systema brachyurorum part i. an annotated checklist of extant brachyuran crabs of the world. Raffles Bull. Zool. 17, 1–286.
Ogburn M. B., Roberts P. M., Richie K. D., Johnson E. G., Hines A. H. (2014). Temporal and spatial variation in sperm stores in mature female blue crabs Callinectes sapidus and potential effects on brood production in Chesapeake bay. Mar. Ecol. Prog. Ser. 507, 249–262. doi: 10.3354/meps10869
Orensanz J. M., Armstrong J., Armstrong D., Hilborn R. (1998). Crustacean resources are vulnerable to serial depletion–the multifaceted decline of crab and shrimp fisheries in the greater gulf of Alaska. Rev. Fish Biol. Fisheries 8, 117–176. doi: 10.1023/A:1008891412756
Otto R. S. (1986). Management and assessment of eastern Bering Sea king crab stocks. Can. Special Publ. Fisheries Aquat. Sci. 92, 83–106.
Pardo L. M., Rosas Y., Fuentes J. P., Riveros M. P., Chaparro O. R. (2015). Fishery induces sperm depletion and reduction in male reproductive potential for crab species under male-biased harvest strategy. PLoS One 10, e0115525. doi: 10.1371/journal.pone.0115525
Pardo L. M., Riveros M. P., Fuentes J. P., Pinochet R., Cardenas C., Sainte-Marie B. (2017). High fishing intensity reduces females’ sperm reserve and brood fecundity in a eubrachyuran crab subject to sex- and size-biased harvest. ICES J. Mar. Sci. 74, 2459–2469. doi: 10.1093/icesjms/fsx077
Paul A. J., Paul J. M. (1997). Breeding success of large male red king crab Paralithodes camtschatica with multiparous mates. J. Shellfish Res. 16, 379–381. Available at: https://ia802802.us.archive.org/28/items/cbarchive_109357_breedingsuccessoflargemaleredk1981/breedingsuccessoflargemaleredk1981.pdf.
Rains S. A. M., Wilberg M. J., Miller T. J. (2018). Evaluation of fishery-induced sperm limitation in Chesapeake Bay blue crab using an individual-based model. Mar. Ecol. Prog. Ser. 596, 127–142. doi: 10.3354/meps12595
R Core Team (2020). R: a language and environment for statistical computing (Vienna: R Foundation for Statistical Computing). Available at: http://www.R-project.org/.
Rowe S., Hutchings J. A. (2003). Mating systems and the conservation of commercially exploited marine fish. Trends Ecol. Evol. 18, 567–572. doi: 10.1016/j.tree.2003.09.004
Roy K., Collins A. G., Becker B. J., Begovic E., Engle J. M. (2003). Anthropogenic impacts and historical decline in body size of rocky intertidal gastropods in southern California. Ecol. Lett. 6, 205–211. doi: 10.1046/j.1461-0248.2003.00419.x
Sadovy Y. J. (1996). “Reproduction of reef fishery species,” in Reef fisheries. Eds. Polunin N. V. C., Roberts C. M. (London: Chapman & Hall), 1559.
Sato T., Ashidate M., Jinbo T., Goshima S. (2007). Does male-only fishing influence reproductive success of female spiny king crab, Paralithodes brevipes? Can. J. Fisheries Aquat. Sci. 64, 735–742. doi: 10.1139/f07-044
Sato T. (2011). Plausible causes for sperm-store variations in the coconut crab Birgus latro under large selective harvesting. Aquat. Biol. 13, 11–19. doi: 10.3354/ab00350
Sato T. (2012). Impacts of large male-selective harvesting on reproduction: illustration with large decapod crustacean resources. Aqua-BioScience Monogr. 5, 67–102. doi: 10.5047/absm.2012.00503.0067
Shields J. D. (1991). “Reproductive ecology and fecundity of cancer crabs,” in Crustacean egg production, crustacean issues, vol. 7 . Eds. Wenner A., Kuris A. (Rotterdam: A.A. Balkema).
Soundarapandian P., Varadharajan D., Boopathi A. (2013). Reproductive biology of the commercially important portunid crab, Portunus sanguinolentus (Herbst). J. Mar. Sci. Res. Dev. 3 (2), 1–9. doi: 10.4172/2155-9910.1000124
Stephenson W., Hudson J. J., Campbell B. (1958). The Australian portunidae (Crustacea: Portunidae). II. the genus Charybdis. Aust. J. Mar. Freshw. Res. 8 (4), 491–507. doi: 10.1071/MF9570491
Sumpton W. (1990). Biology of the rock crab Charybdis natator (Herbst) (Brachyura: portunidae). Bull. Mar. Sci. 46 (2), 425–431. doi: 10.1016/s0198-0254(06)80476-0
Tanaka K. R., Torre M. P., Saba V. S., Stock C. A., Chen Y. (2020). An ensemble high-resolution projection of changes in the future habitat of American lobster and sea scallop in the northeast US continental shelf. Diversity Distribution 26 (8), 987–1001. doi: 10.1111/ddi.13069
Tao L. S. R., Lui K. K. Y., Lau E. T. C., Ho K. K. Y., Mak Y. K. Y., Sadovy de Mitcheson Y., et al. (2018). Trawl ban in a heavily exploited marine environment: responses in population dynamics of four stomatopod species. Sci. Rep. 8, 17876. doi: 10.1038/s41598-018-35804-7
Wahle R. A., Bergeron C. E., Chute A. S., Jacobson L. D., Chen Y. (2008). The Northwest Atlantic deep-sea red crab (Chaceon quinquedens) population before and after the onset of harvesting. ICES J. Mar. Sci. 65 (6), 862–872. doi: 10.1093/icesjms/fsn058
Wahle R. A., Dellinger L., Olszewski S., Jekielek P. (2015). American lobster nurseries of southern New England receding in the face of climate change. ICES J. Mar. Sci. 72, i69–i78. doi: 10.1093/icesjms/fsv093
Wang W. Y., Ye S. Z., Ye Q. T. (1998). Investigation on resources in the surrounding sea of Taiwan bank. J. Fujian Fisheries 78 (3), 8–12. doi: 10.14012/j.cnki.fjsc.1998.03.018
Weinberg J. R., Keith C. (2003). Population size–structure of harvested deep-sea red crabs (Chaceon quinquedens) in the Northwest Atlantic ocean. Crustaceana 76, 819–833. doi: 10.1163/15685400360730606
Wenner A. M. (1972). Sex ratio as a function of size in marine Crustacea. Am. Nat. 106, 3250. doi: 10.1086/282774
Xiao F. S. (2002). Problem and management countermeasure of otter trawling operation in fishing grounds of Minnan and Taiwan bank. Modern Fisheries Inf. 17 (12), 19–21. Available at: https://chn.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2002&filename=XYYZ200212005&uniplatform=OVERSEA&v=P2vHZlWH0SZ76mUPc3tFESOHnybNlkigQCCUgdNLSRYA7kxbg85TtgqwDwNFNDaH.
Yang S. L., Chen H. L., Dai A. Y. (2011). “Fauna sinica. invertebrates,” in Crustacea, Decapoda, Portunidae, vol. 49. (Beijing, China: Science Press), 417.
Ye Q. T. (1999). Biological characteristic and resource status of Charybdis natator in Minnan-Taiwan shallow shoal fishing ground. Mar. Fisheries 1999 (3), 107–111. Available at: https://chn.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9899&filename=HTYY199903004&uniplatform=OVERSEA&v=CW8HVFdX8qhHZUi_kQPDKP2gW5IegnMmJS9zIR2MvFAOUEKmPcjJ8V87kfvXQAJ6.
Ye S. Z., Zhang Z. L., Ye Q. T., Zhang C. M. (2007). The monitoring investigation of single trawler fishery in Minnan and Taiwan bank fishing ground. J. Fujian Fisheries 114 (3), 3538. doi: 10.14012/j.cnki.fjsc.2007.03.006
Zhang Z. (1997). The fisheries and biological characteristics of Portunus (Amphitrite) gladiator in south Fujian-Taiwan bank fishing ground. Mar. Fisheries 1, 17–21. Available at: https://chn.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9697&filename=HTYY199701005&uniplatform=OVERSEA&v=wSNoRfe3KbTkjq0_JzWRxTdGmgxF6jX91Pthc9Ww9lDQAk5iiL2lZRLoTLviQyko.
Keywords: Portunidae, crab fishery, spawning season, population demographic structure, fecundity, Taiwan Strait, Chinese waters
Citation: Lin B-a, Jiang Y and Liu M (2023) Population structure and reproductive dynamics of the ridged swimming crab Charybdis natator in the southern Taiwan Strait of China: significant changes within 25 years. Front. Mar. Sci. 10:1056640. doi: 10.3389/fmars.2023.1056640
Received: 29 September 2022; Accepted: 07 June 2023;
Published: 11 July 2023.
Edited by:
Carlo Pipitone, CNR - IAS Institute of Anthropic Impacts and Sustainability in Marine Environment, ItalyReviewed by:
Khor Waiho, University of Malaysia Terengganu, MalaysiaCopyright © 2023 Lin, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, bWlubGl1eG1AeG11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.