- 1State Key Laboratory of Marine Environmental Science, College of Ocean and Earth Sciences, Xiamen University, Xiamen, Fujian, China
- 2Fuzhou Ocean and Fishery Technology Center, Fuzhou, Fujian, China
- 3Fishery Resources Monitoring Center of Fujian Province, Fuzhou, Fujian, China
To understand the community structure of benthic molluscs and their relationship under varying environmental and ecological conditions, monthly samplings in April−September 2019 were conducted at 27 stations in an approximate sea area of 20,600 ha (Changle District, Fujian Province, China). Forty-five species were identified, most as food; six dominant species, all bivalves and commercially important, were determined by the index of relative importance > 500. The average abundance and biomass were 308.32 × 103 ± 1,156.24 × 103 ind./km2 and 1,423.71 ± 2,272.37 kg/km2, respectively. Three spatial community groups were identified, named Min River Estuary, Nearshore, and Offshore, with significant differences in species composition and abundance (ANOSIM, p < 0.01). Results of the canonical correlation analysis indicated that the community structure of benthic molluscs was significantly related to water depth, pH, salinity, temperature, phytoplankton abundance and zooplankton abundance (p < 0.1). As the important habitat for benthic molluscs, long-term monitoring in the coastal waters of Changle is needed for sustainable harvest.
Introduction
Macrobenthos are important ecological groups that comprise Mollusca, Polychaeta, Echinodermata, Crustacea and other groups (Herman et al., 1999). They usually settle on the sea bed or bury into sandy and muddy substrates, and are sedentary and sensitive to environmental stress induced by anthropogenic activity and climate change (Mulik et al., 2020; Xu et al., 2021). Therefore, macrobenthos can serve as indicators of aquatic ecological change and play an important role in maintaining ecosystem balance (Rakocinski, 2012; Dietl et al., 2016; Sukumaran et al., 2021). Community structure characteristics, including species composition, diversity, abundance and biomass, have become an increasingly important component in macrobenthic studies to reveal changes in the marine environment and ecological adaptation and to evaluate community health (Rao et al., 2020; Wang et al., 2021a; Wang et al., 2021b). It has been illustrated that community structures of macrobenthos can be affected by environmental factors such as temperature, oxygen and nutrients, and vice versa (McGovern et al., 2020; Wang et al., 2021a; McAfee et al., 2022). The close relationship between community and environment suggests the importance of maintaining a hospitable circumstance with suitable environmental conditions for the sustainability of local macrobenthos.
Researches on marine benthic molluscs, an important component of macrobenthos, and their ecological roles have been increasingly attractive worldwide (Newell, 2004; Kroeker et al., 2014). Many studies were conducted on the abundance and diversity of marine benthic molluscs in different habitats (e.g. nearshore waters, estuaries, mangrove meadows, coral reefs and seagrass beds) and had described the abundance and diversity of benthic molluscs (Zuschin et al., 2001; Hwai et al., 2007; Zvonareva et al., 2015; Anderson et al., 2021) and their community structure under the changing environmental conditions (Martins et al., 2014). Among various habitats, the communities of molluscs in estuaries are characterized by having relatively few species but high abundance because of the rapid change of environment (e.g. salinity) through freshwater input (McLusky and Elliott, 1981).
In China, community structures of benthic molluscs have been evaluated in different locations and habitats, including the Bohai Sea (Zhao et al., 2011), the Yellow Sea (Zhang et al., 2016), the Pearl River Estuary (Li et al., 2012), the mangrove meadows in Fujian Province (Chen et al., 2021) and Hainan Province (Ma et al., 2018), the coastal waters of Hong Kong (Morton, 1996), the coral reefs of the Beibu Gulf (Huang et al., 2020) and the shallow water hydrothermal vents in Taiwan (Chen et al., 2018). Species diversity decline, dominant species shifts, abundance and biomass decline, and size reduction have been documented (Wu et al., 2005; Zhang et al., 2016; Huang et al., 2020).
Fujian Province is in southeastern China, one of the 14 coastal provincial administrative regions. The geological characteristics of Fujian are unique. First, it is the only province mainly facing the Taiwan Strait and close to the south boundary of the East China Sea. Second, Fujian Province has the second longest coastline, only next to Guangdong Province. Third, the coastal waters of Fujian are influenced not only by the China coastal current but also by the South China Sea warm current and the Taiwan warm current (Kang et al., 2021). The coastal waters of Changle District (Fujian Province, China) are influenced by the Min River (the largest river in Fujian Province) and the East China Sea. The semidiurnal tides with a tidal range of approximately 3 m are documented (Liu, 1997). The littoral substrates of the region are suitable for the growth of benthic molluscs. The most commercially important fishery is for the bivalve Mactra antiquata Spengler, 1802 (Liu et al., 2003; Fang, 2013; Lin, 2020). Irrespective of the studies on the reproductive biology and artificial breeding of M. antiquata, the community structures of benthic molluscs in the coastal waters of Changle remain largely unevaluated.
To shed some light on the community features of benthic molluscs in the coastal waters of Changle, this study aims to: (1) investigate the species composition, abundance and biomass of benthic molluscs; (2) obtain the environmental and ecological variables including substrate, water depth, temperature, salinity, pH, dissolved oxygen, chlorophyll a, phytoplankton abundance and zooplankton abundance; and (3) analyze the community structure of benthic molluscs and the relationship with the aforementioned environmental and ecological variables. The results will provide baselines for developing harvest strategies to achieve sustainable use of benthic molluscs as food in the surveyed areas.
Materials and methods
Survey area and collection of benthic molluscs
The survey area (25.81−26.08° N, 119.61−119.80° E) covered approximately 20,600 ha with 27 sampling stations (S1-S27) in the coastal shallow waters (< 20 m) of Changle District, Fujian Province, southern China (Figure 1). To the east, the survey area is connecting to the East China Sea, and to the north, the Min River Estuary is located.
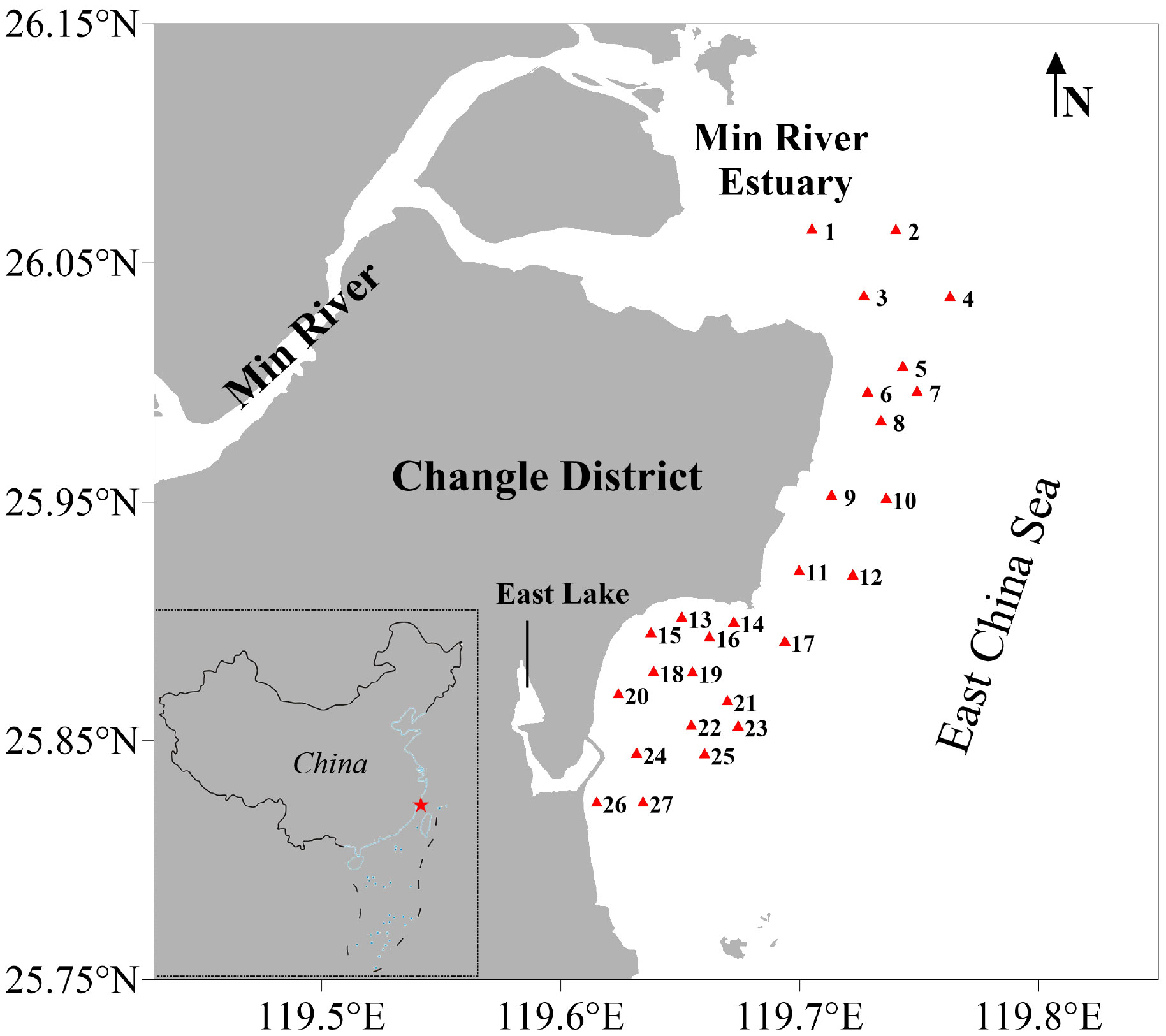
Figure 1 Twenty-seven sampling stations (red triangles) in the coastal waters of Changle District, Fujian Province, China.
Samplings were conducted once per month during the spring tide in April−September 2019. Because of the bad weather and strong current in summer, there were no surveys at S1, S2 and S4 in July−September 2019. Samples were collected by a licensed commercial bottom trawler with the rectangle net opening by 3.5 m in width and 0.4 m in height, a typical fishing gear for benthic molluscs harvest locally. The net mesh size was 22 mm. In each station, the operating time was controlled for 15 min and the vessel speed for 1 kn. The catches were sorted by taxonomic groups on deck and packed in labeled zip-lock bags and kept in ice boxes before transferring to the laboratory for further treatment. Subsampling was performed for numerically abundant taxa; the rest were weighted and released back to the sea.
Benthic molluscs were identified to the lowest taxonomic level as possible based on shell morphology (Wang et al., 1988; Huang, 2006; Dance, 2007; Zhang, 2008; Yang et al., 2017).
Environmental variables measurement
Measurements of six variables were conducted monthly in April−September 2019. Water depth (m) was recorded by onboard equipment. Because the survey area is shallow (< 20 m) and largely influenced by tides, currents and winds, only temperature (°C), salinity, pH and dissolved oxygen (mg/L) of surface water (< 0.5 m) were measured by a precalibrated multi-channel electrochemical device (HACH HQ40d). Substrate samples of S1-S27 were collected using a grab sampler (sample area = 0.1 m2) only in May 2019. The grain sizes, the proportion of sand (%, 1−0.063 mm), silt (%, 0.063−0.004 mm) and clay (%,< 0.004 mm), and the median size (φ, Udden-Wentworth grain-size scale) of substrate were analyzed in the laboratory following the national standard protocol for substrate type (GB/T 12763.8, 2007).
Ecological variables measurement
Measurements of three variables were conducted monthly in April−September 2019. For Chlorophyll a (mg/m3), surface water samples, 1 L per station, were collected, pretreated, and analyzed following the national standard protocol and using a fluorescence spectrophotometer (GB/T 17378.7, 2007). For phytoplankton, surface water samples, 0.5 L per station, were collected and preserved with Lugol’s iodine solution. All cells were counted under a light microscopy in the laboratory after at least 48 hours under still condition. Zooplankton samples were collected by a standard plankton net of 505 μm in mesh size and 0.2 m2 in opening area through vertical tow from bottom to top (GB/T 17378.7, 2007). Each station was applied for one tow, and samples were preserved in 5% formalin buffered with sodium borate. Samples were concentrated and the number of individuals was counted under a stereo-microscopy.
Calculations
For benthic molluscs, the number of individuals and the wet weight, totally or at the lowest taxonomic level, were measured at each station. The abundance (ind./km2) and biomass (kg/km2) of benthic molluscs at each station were calculated as:
where N is the total number of individuals (ind.), W is the total wet weight (kg), A is the bottom area swept (km2), t is the operation time (h), v is the vessel speed (km/h), and w is the net width (i.e. 3.5 m in this study).
The phytoplankton abundance (cells/L) at each station was calculated as:
where N is the number of cells, and V is the water volume (L) collected.
The zooplankton abundance (ind./m3) at each station was calculated as:
where N is the number of individuals per vertical tow (ind.), V is the water volume (m3) filtered into the plankton net, WD is the water depth (m) and NA is the plankton net opening area (i.e. 0.2 m2).
The index of relative importance (IRI) was used to determine the dominant (IRI > 500) and common (100 < IRI ≤ 500) mollusc species (Stevens et al., 1982). The IRI was calculated as:
where N%, W%, and F% are the number proportion, wet weight proportion and station occurrence frequency, respectively.
Statistical analysis
One-way ANOVA was applied to analyze the monthly variation of water depth because Shapiro-Wilk and Bartlett test suggested normality (p > 0.05) and homoscedasticity (p > 0.05). Nonparametric Kruskal-Willis test was applied to other four environmental (temperature, salinity, pH and dissolved oxygen) and all three ecological variables that were not conformed to normality (p < 0.05) and homoscedasticity (p < 0.05). Dunn’s post hoc pairwise comparison was used to compare the seven variables between each month. Analyses were performed using R-3.5.3 (R Core Team, 2019), and p−values ≤ 0.05 were considered statistical significance.
To determine community structure characteristics, multivariate analyses based on the species composition and abundance data were conducted using PRIMER 6. Before analyses, the species with their occurrence frequency ≤ 5% were left out, and the abundance data was lg(x+1) transformed to down-weight the importance of highly abundant species and to reduce the effects of rare species on the similarity matrix (Anderson et al., 2008). Canonical analysis of principal coordinates (CAP) based on Bray-Curtis similarities was used to evaluate spatial variations in community structure (Clarke, 1993). Analysis of Similarities (ANOSIM) was used for significance testing among community groups, after which pairwise comparisons were conducted (Burlakova et al., 2018). Differences in sand and silt proportions of substrate among three spatial community groups were analyzed by one-way ANOVA after meeting the conditions of normality (p > 0.05) and homoscedasticity (p > 0.05). Nonparametric Kruskal-Willis test was applied to clay proportions and median size of substrate that were not conformed to normality (p < 0.05) and homoscedasticity (p < 0.05).
“Similarity Percentage” (SIMPER) procedure was applied to determine the contributions of discriminating species to the similarity within community groups (Gogina et al., 2010). Detrended correspondence analysis (DCA) was performed and the maximum gradient of length was 4.87, so that a unimodal model, canonical correlation analysis (CCA), was chosen (generally, > 4, CCA; 3-4, CCA or RDA; < 3, RDA), to establish the influence of each environmental and ecological parameter in structuring the benthic molluscan communities using CANOCO 5 (Petr and Jan, 2014). A forward selection procedure with 999 permutations was applied to select the variables that exerted a significant effect (p ≤ 0.1) (Godinho and Ferreira, 1998).
Results
Abundance and biomass
The average abundance and biomass of benthic molluscs in the coastal waters of Changle were 308.32 × 103 ± 1,156.24 × 103 ind./km2 and 1,423.71 ± 2,272.37 kg/km2, respectively. Temporal and spatial distribution of abundance and biomass showed similar patterns (Figures 2, 3; Supplementary Table S1). Monthly average abundance and biomass were high in June and low in April and September. For the sampling sites, monthly average abundance and biomass were high at sites S5, S6, S14 and S26, and low at sites S21, S22 and S23.
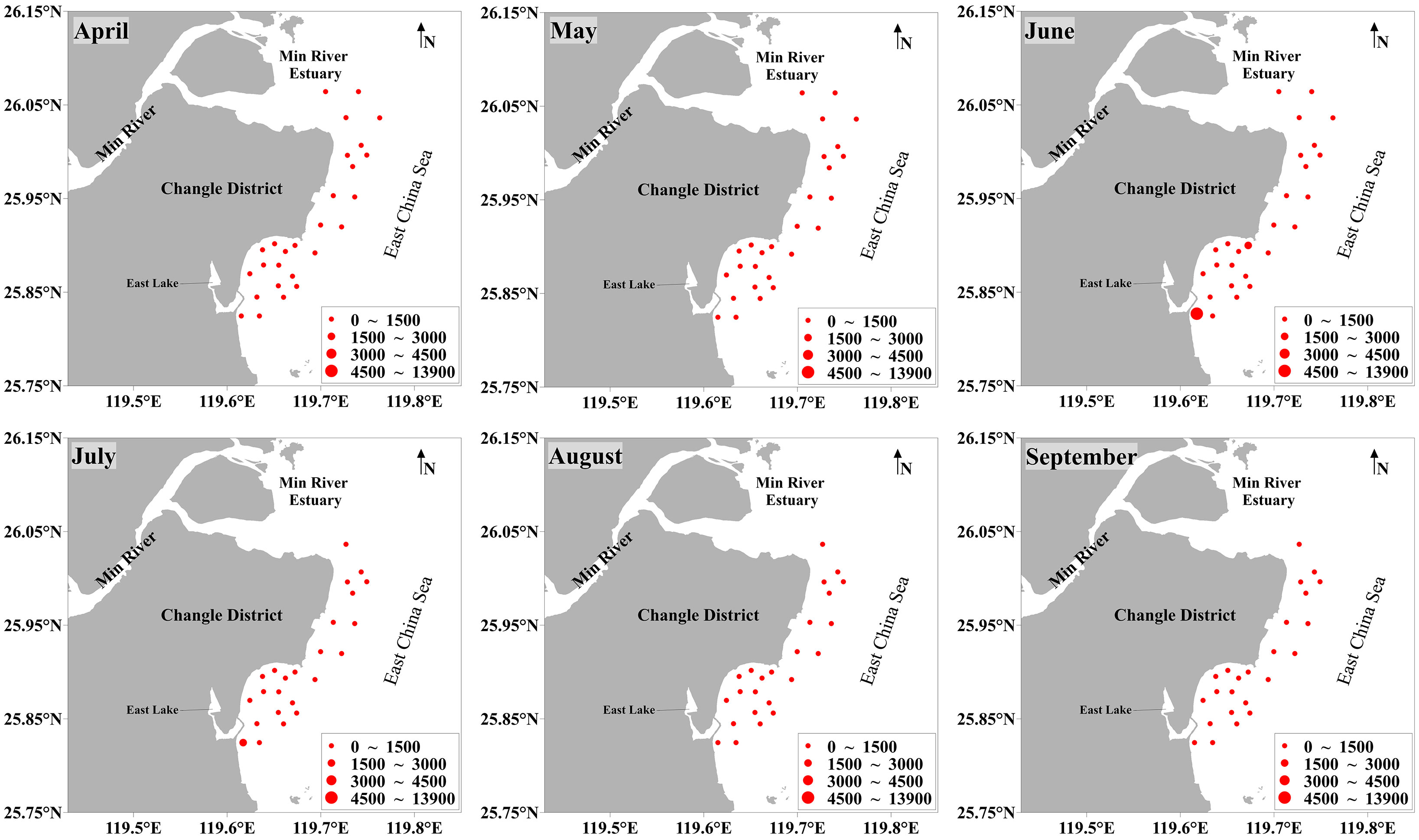
Figure 2 Monthly abundance (× 103 ind./km2) of benthic molluscs at all 27 stations in April−September 2019 in the coastal waters of Changle District, Fujian Province, China.
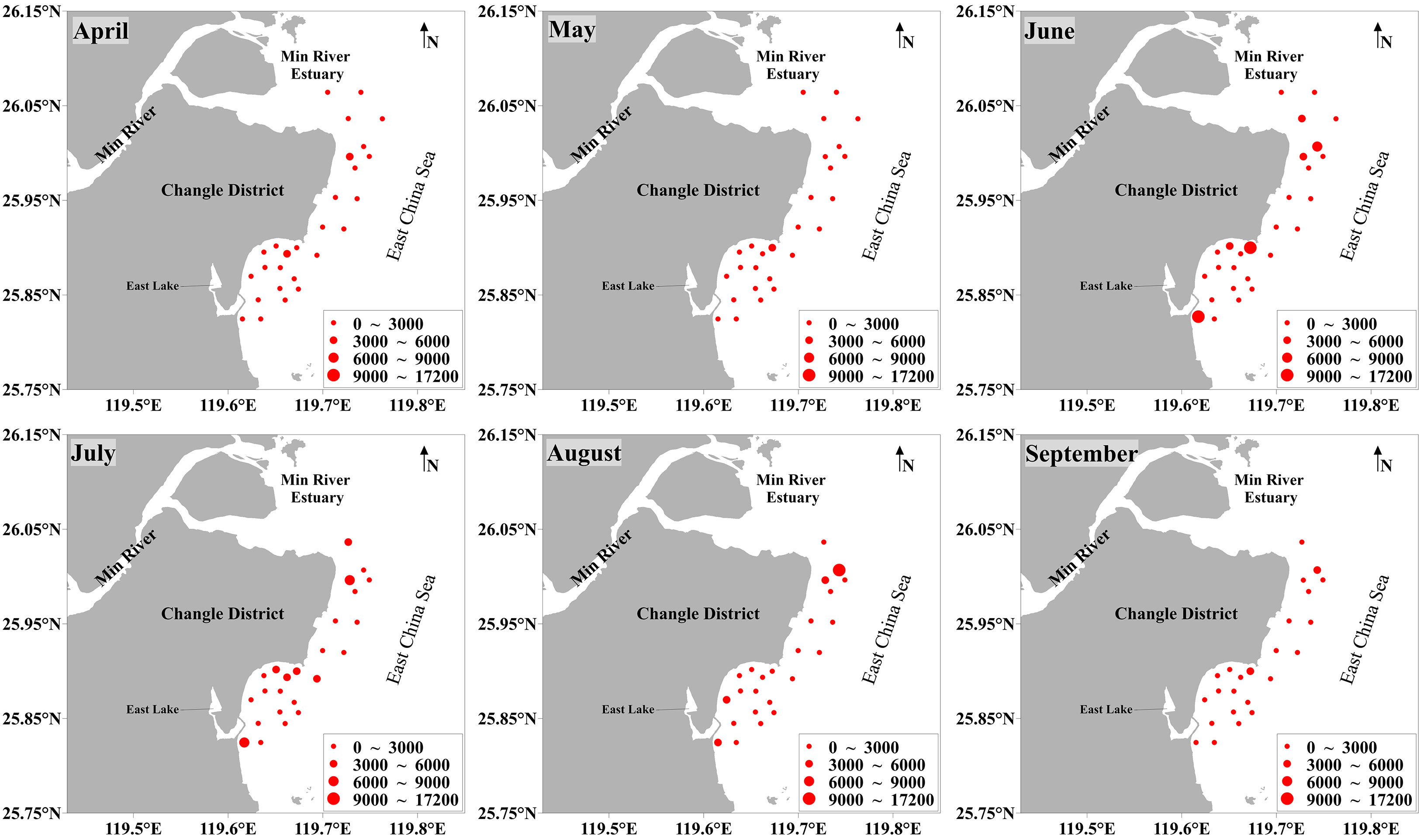
Figure 3 Monthly biomass (kg/km2) of benthic molluscs at all 27 stations in April−September 2019 in the coastal waters of Changle District, Fujian Province, China.
Species composition
A total of 45 species (40 taxa to species level, one to genus level, two to family level and two at class level) were identified from 34,232 individuals collected, including 29 species of benthic bivalves (10 families in 6 orders), and 16 species of benthic gastropods (9 families in 3 orders) (Supplementary Tables S2, S3). The number of species among months was between 24 and 31 and the cumulative species number among stations was between 7 and 29 (Supplementary Table S4; Supplementary Figure S1). High number of species was found at S19 and S20 (> 20 species), and low at S21, S22 and S23 (< 10 species) (Supplementary Figure S2).
Six dominant species, all bivalves, and seven common species including five bivalves and two gastropods were identified (Figure 4; Supplementary Table S5). The six dominant species accounted for 87.70% and 79.68% of the total number of individuals and total wet weight, respectively; the values went up to 96.22% and 96.75% with all dominant and common species (Supplementary Table S3). Psammacoma gubernaculum (Hanley, 1844) and Dosinia japonica (Reeve, 1850) were the only two species that dominated in all months with high IRI, and Anadara broughtonii (Schrenck, 1867), Mactra antiquata Spengler, 1802, Mactra chinensis Philippi, 1846 and Mactra iridescens Kuroda & Habe, 1958 only dominated in certain months.
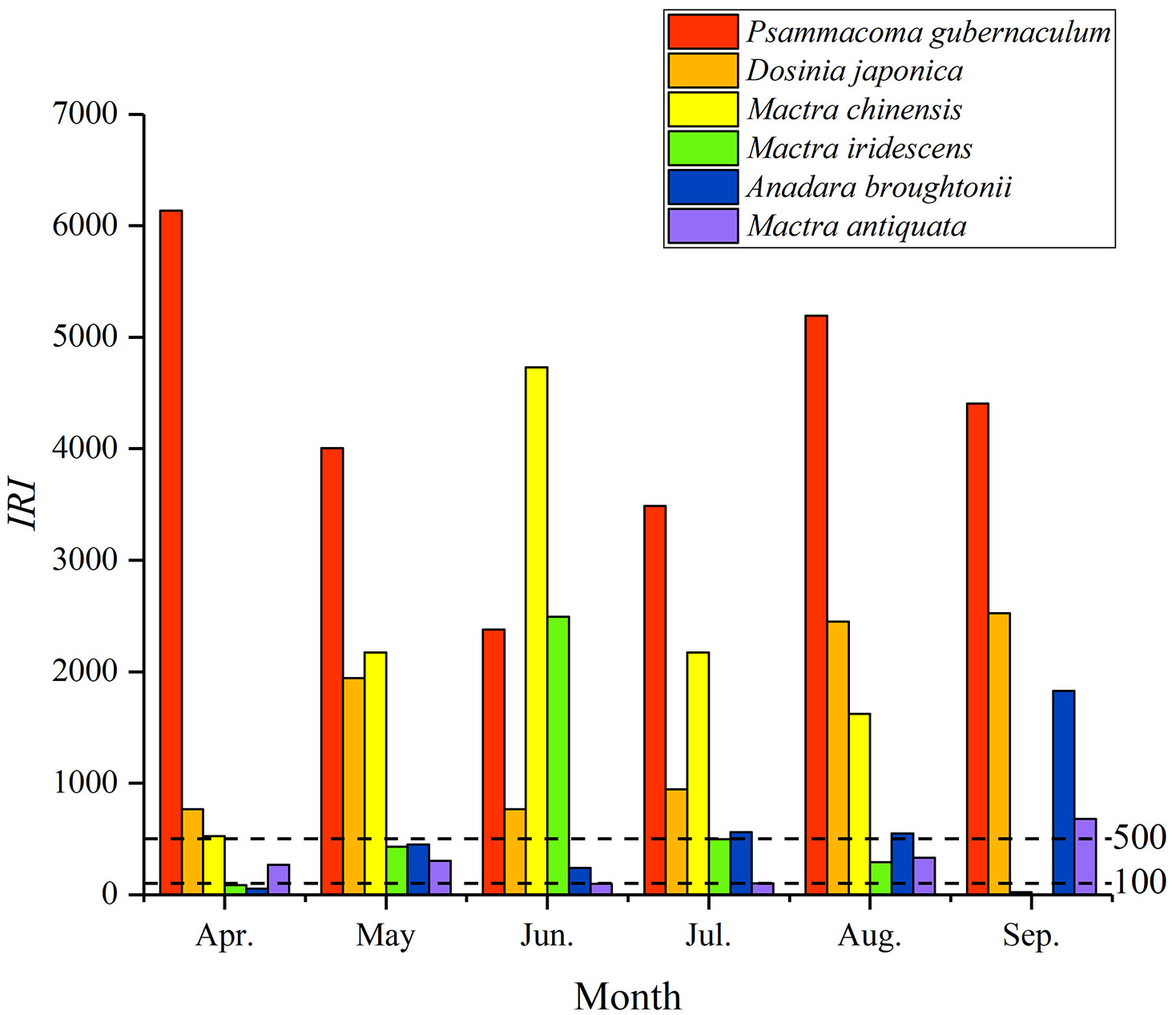
Figure 4 Index of relative importance (IRI) of six dominant species in April−September 2019 in the coastal waters of Changle District, Fujian Province, China.
Monthly variation in environmental and ecological variables
The water depth of the survey area in Changle was shallow, no more than 20 m in all surveyed stations and mainly < 10 m (Supplementary Figure S3). Four environmental and three ecological variables had significant differences by month in the coastal waters of Changle (Table 1). Temperature and salinity were high in August and September; pH was high in April and July; dissolved oxygen was high all the time except August; Chlorophyll a was high in April, June and July; phytoplankton abundance was high in July and August; and zooplankton abundance was high in June and July.
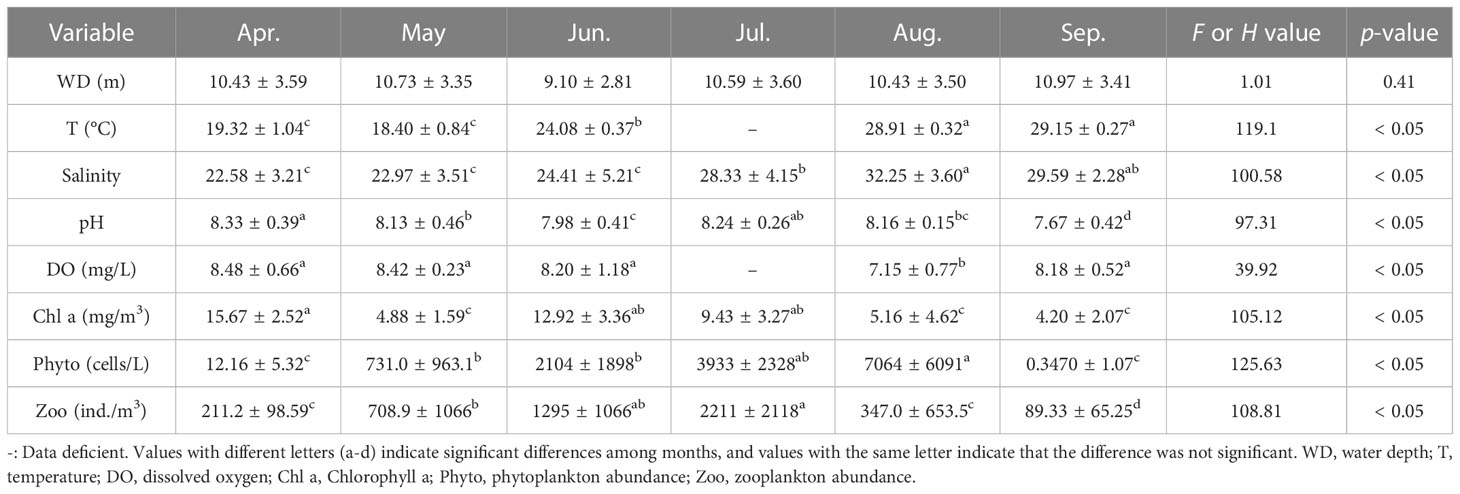
Table 1 Environmental and ecological variables in April−September 2019 in the coastal waters of Changle District, Fujian Province, China.
Community structure of benthic molluscs
Twenty-two species of benthic molluscs with an occurrence frequency higher than 5% were used for further analysis. Three spatial community groups, namely Min River Estuary, Nearshore and Offshore were identified (one-way ANOSIM, Global R = 0.454, p < 0.01), with pairwise tests suggesting that the three spatial groups were significantly different from each other (Min River Estuary vs. Nearshore: R = 0.815, p = 0.001; Min River Estuary vs. Offshore: R = 0.868, p = 0.001; Nearshore vs. Offshore: R = 0.312, p = 0.001). Stations in the Min River Estuary group were farther separated from the stations in the Nearshore and Offshore groups along the CAP axis 1, indicating unique community structure in Min River Estuary, and the latter two groups were separated along the CAP axis 2 (Figure 5).
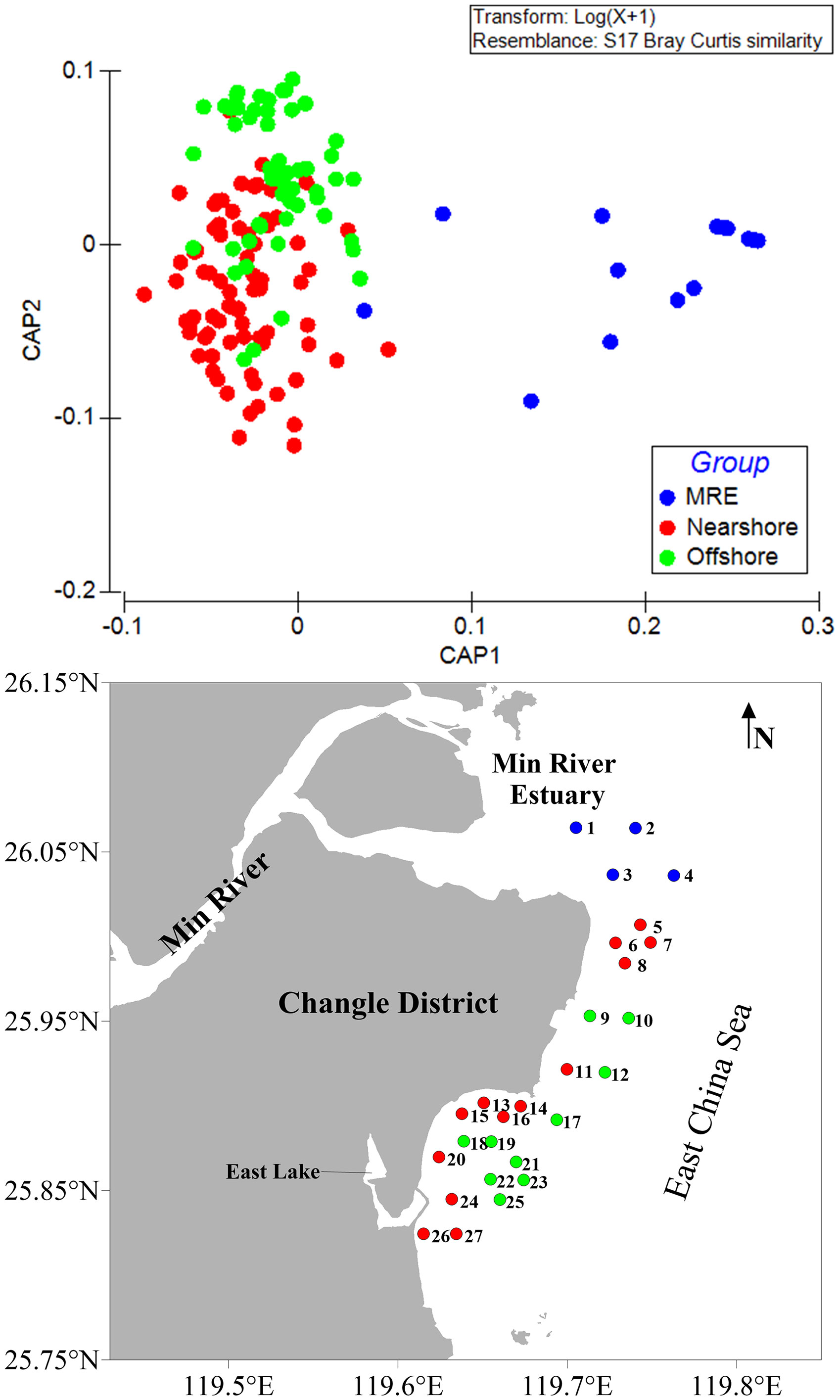
Figure 5 Canonical Analysis of Principal Coordinates (CAP) with the three spatial groups: River Estuary (MRE), Nearshore and Offshore. Bray-Curtis similarity matrix was calculated based on lg(x+1) transformed species abundance data.
Eleven species contributed over 90% of the community similarity in the three spatial community groups (Table 2). The communities in the Min River Estuary group were characterized by three species (Meretrix meretrix (Linnaeus, 1758), Hiatula atrata (Reeve, 1857) and D. japonica) with a total contribution percentage of 97.83%. Eight species in the Nearshore group were P. gubernaculum, D. japonica, M. chinensis, A. brougtonii, Babylonia lutosa (Lamarck, 1816), Neveriat didyma (Röding, 1798), M. iridescens and M. antiquata, contributing to 90.12% in similarity. Five species in the Offshore group were P. gubernaculum, D. japonica, A. brougtonii, B. lutosa and Solen linearis Spengler, 1794, with a cumulative contribution of 91.09% in similarity. P. gubernaculum contributed significantly in both Nearshore and Offshore groups, and D. japonica was the only species contributing significantly to all three groups.
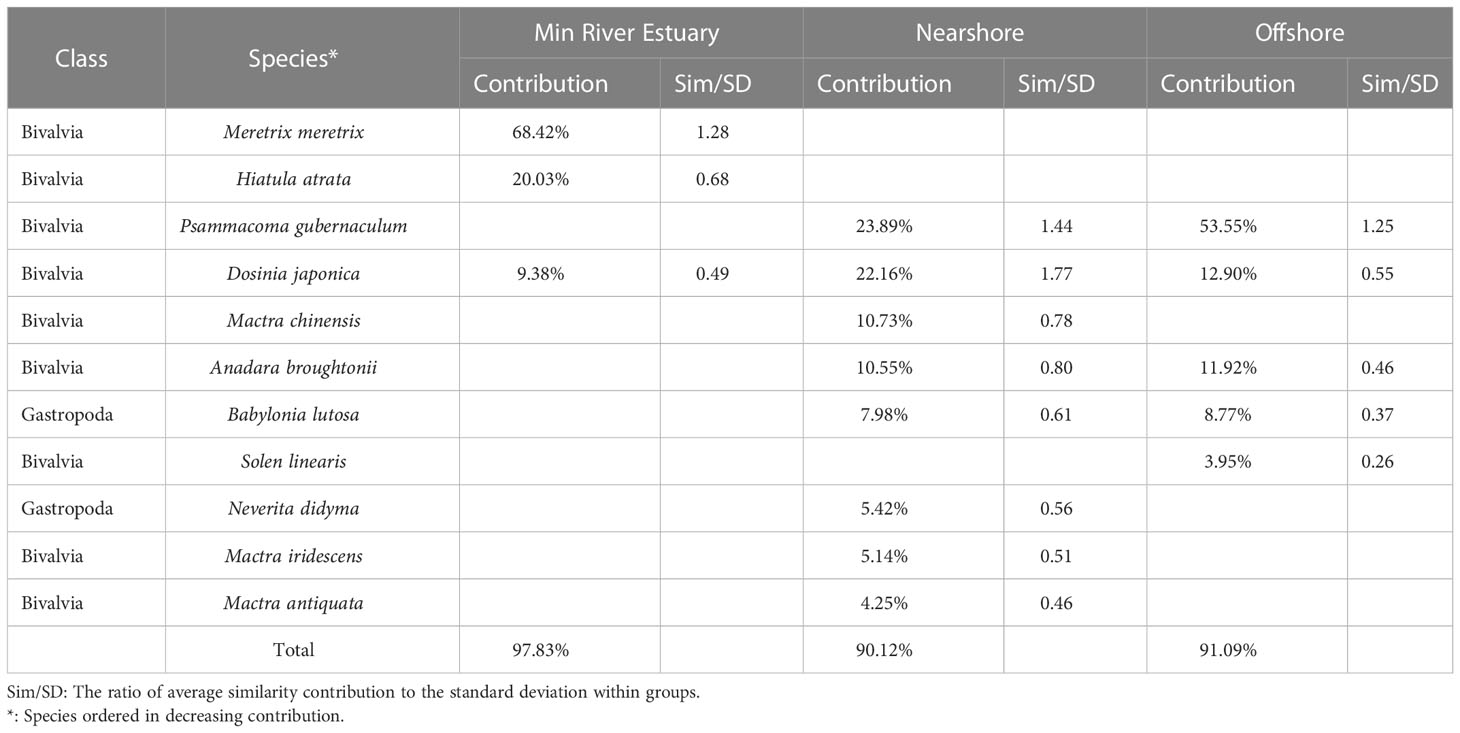
Table 2 Discriminating species and their contributions to average similarity of the three spatial community groups in the coastal waters of Changle District, Fujian Province, China.
The substrate particles included sand, silt and clay, with average proportions of 57.12%, 30.36% and 12.52%, respectively (Supplementary Table S6). Substrate particle types [sand, silt, clay and median size (φ)] in the three spatial community groups were significantly different (p < 0.05) (Figure 6). The Min River Estuary group had the highest sand content (80.70%) and the lowest clay content (5.56%). The Offshore group had the lowest sand content (44.38%) and the highest clay content (16.12%). The average φ in the Min River Estuary, Nearshore and Offshore groups were 2.79, 4.33 and 5.06, respectively.
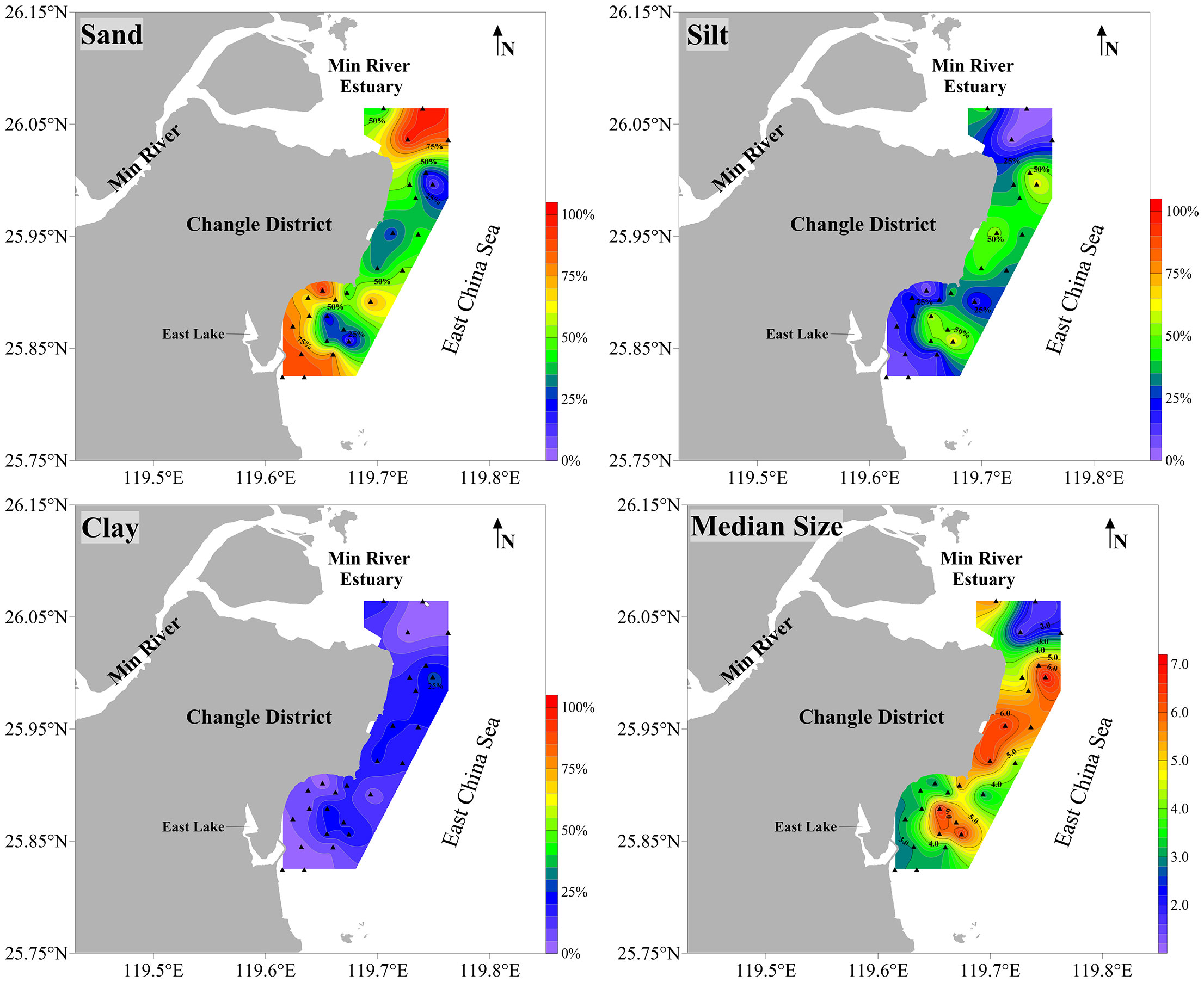
Figure 6 The distribution of sand proportion (%), silt proportion (%), clay proportion (%) and median size (φ) in the coastal waters of Changle District, Fujian province, China.
Community structure in relation to environmental and ecological variables
Six variables were significantly related to the benthic molluscs community structure, including water depth (F = 5.32, p = 0.001), pH (F = 4.65, p = 0.001), salinity (F = 4.77, p = 0.001), phytoplankton (F = 2.12, p = 0.015), zooplankton (F = 1.65, p = 0.068) and temperature (F = 1.49, p = 0.092). Dissolved oxygen and Chlorophyll a were not considered to be correlated with community structure (p > 0.1). The six significant variables showed spatial and temporal patterns (Supplementary Figures S3, S4). Water depth was shallow (< 10 m) in the Min River Estuary and along the coastline of Changle. Temperature had significant temporal variations, increasing from April to September. Salinity and pH shared similar spatial patterns, the lowest in the Min River Estuary. Phytoplankton abundance was higher in May−August than those in April and September, and was higher around the Min River Estuary and southwest. Zooplankton abundance shared similar temporal and spatial patterns to phytoplankton.
The results of CCA (Table 3) with permutation tests on all axes revealed the significance of the constrained ordination model (F = 2.8, p < 0.01). Overall, 15.1% of the total inertia (2.774) was explained by the four environmental and two ecological variables with 10.2% explained by the first two axes. Salinity (-0.3537) and water depth (-0.3966) were negatively correlated with the first axis, while pH (-0.5226) and temperature (0.4363) were negatively and positively correlated with the second axis, respectively. From the Min River Estuary group to the Nearshore and Offshore groups, the benthic molluscan community was increasingly related to higher salinity, higher water depth, lower phytoplankton abundance and lower zooplankton abundance (Figure 7A). The temporal variation of the benthic molluscan community was affected by pH and temperature, with higher pH and lower temperature in April and May than in June, August and September (Figure 7B).
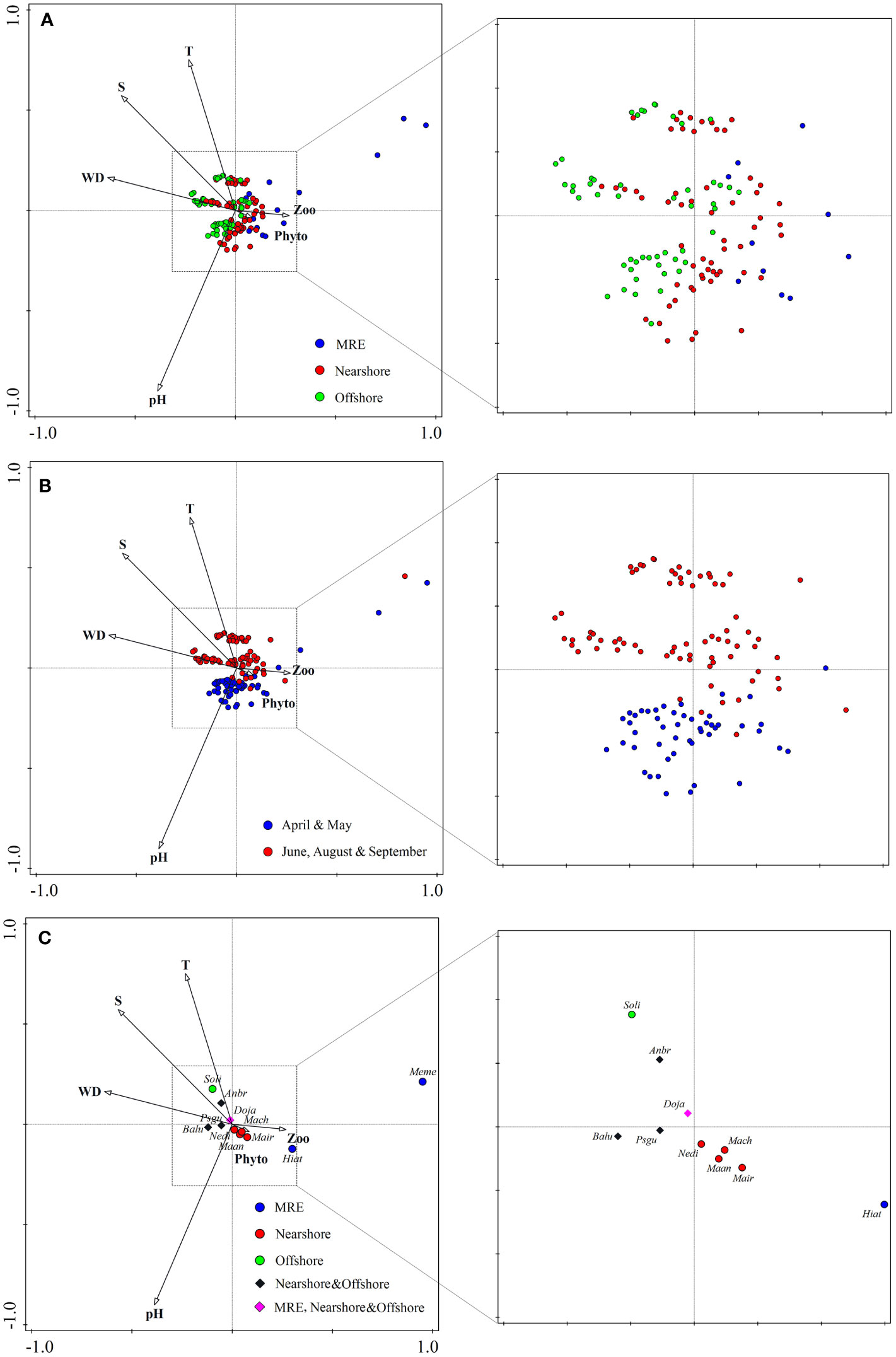
Figure 7 Biplots of canonical correlation analysis (CCA) showing the surveyed stations (A, B)/discriminating species (C) and the significant explanatory environmental and ecological variables in the coastal waters of Changle District, Fujian Province, China. The communities were classified by the three spatial community groups (A) or by months (B) to indicate spatial and temporal variances separately. Discriminating species of different groups were represented in (C). Meme, Meretrix meretrix; Hiat, Hiatula atrata; Maan, Mactra antiquata; Mair, Mactra iridescens; Mach, Mactra chinensis; Nedi, Neverita didyma; Anbr, Anadara broughtonii; Balu, Babylonia lutosa; Doja, Dosinia japonica; Psgu, Psammacoma gubernaculum; Soli, Solen linearis.
Different discriminating species in the three spatial community groups showed different preferences to environmental and ecological variables (Figure 7C). M. meretrix and H. atrata in the Min River Estuary group preferred lower water depth, higher phytoplankton abundance and higher zooplankton abundance. S. linearis in the Offshore group was closely related to lower temperature, deeper water depth and higher saline waters. D. japonica was typical in all three spatial groups and it was the closest to the origin in the CCA plot, showing no obvious preference to environmental conditions.
Discussion
More bivalves than gastropods in Changle
The coastal waters of Changle are shallow with a depth range< 20 m, and the substrates mainly contain sand. There are more bivalve species (N = 29 species) than gastropod species (N = 16 species), many as food (Wu et al., 2019; Hu, 2021). The number of bivalve species recorded in this study corresponded to 32% of the recorded bivalve species in the region (Liu and Zhu, 2009). The bivalves attributed to 96.38% of the total number of individuals and 96.86% of the total wet weight (Supplementary Table S3). The same pattern, i.e. benthic bivalves contributed more than gastropods in abundance and biomass, was found in the Yellow River Estuary (Zhang et al., 2021).
The higher species number in bivalves is likely associated with their diet habit. Gastropods are usually scavengers and feed on polychaetes, crustaceans and fish carrion, and bivalves are more phytoplankton (e.g. diatoms) feeders with highly developed gills (Morton, 1990; Palomares and Pauly, 2021). High nutrients in the estuaries enhanced the proliferation of phytoplankton, providing rich food resources for bivalves. (McLusky and Elliott, 1981). In this study, no gastropods were dominant species. Only two gastropods (B. lotosa and N. didyma) were common species (Supplementary Table S5); both were also in eleven discriminating species determined by SIMPER, indicating the importance of bivalves in the coastal waters of Changle (Table 2).
Species composition and abundance changed temporally and spatially
Dominant species changed over time in the coastal waters of Changle (Figure 4; Supplementary Table S5). Only P. gubernaculum and D. japonica were high abundant and widespread, with the occurrence rate of 77.12% and 69.28%, respectively (Supplementary Figures S5, S6). The distribution of P. gubernaculum was away from the Min River Estuary and inhabited in the south of the survey area, and D. japonica had relatively high abundance in the Min River Estuary. M. antiquata, the most high-valued mollusc food species in Fujian waters, had high abundance in the Min River Estuary with a core area of approximately 1,000 ha, with an occurrence rate of 33.33% (Supplementary Figure S8). A. broughtonii had high abundance in the south of the survey area, away from the Min River Estuary (Supplementary Figure S7). Two species of genus Mactra, M. chinensis and M. iridescens, showed the same pattern; their IRI were the highest in June and the lowest in September, with their high abundance in the south of the survey area, far away from the Min River Estuary influence (Supplementary Figures S9, S10). These temporal and spatial changes might be associated with seasonal migration and reproductive requirements (Hiddink and Wolff, 2002; Ni et al., 2011).
The environmental variable gradients can largely influence the spatial patterns of species distribution, such as in estuarine and coastal waters (Li et al., 2020). The most important discriminating species in the Min River Estuary community group is M. meretrix (bivalve) (Table 2). The species can habit in waters shallower than 1 m and tolerate abrupt salinity change (Narasimham et al., 1988). The shallow water depth and the low salinity in the north of the survey area influenced by the Min River, fully supported the inhabit pattern of M. meretrix. As the representative species in the Offshore and Nearshore community groups, A. broughtonii (bivalve), was adapted to higher salinity and its growth rate in juvenile stage increased with high salinity and intermediate temperature (26 °C) (Wang et al., 2017a). In this study, the highest abundance of A. broughtonii in the south of the survey area and the water temperature of approximate 26 °C in June highly support its distribution pattern (Supplementary Figures S3, S7).
Compared to grabs, bottom trawling is less commonly used in macrobenthos surveys. However, bottom trawling method has at least two advantages. First, bottom trawling is an efficient method for sampling large-sized and rare species, contributing to comprehensive surveys of large-scale seabed area (Blanchard et al., 2004). Covering a larger survey area can increase the accuracy of density estimation for benthic molluscs. Second, bottom trawling can be effective for harvest macrobenthos in shallow, sandy and muddy bottoms (McConnaughey et al., 2000). In Changle District, bottom trawling is commercially used in harvesting large benthic molluscs. Therefore, bottom trawling method was selected in this study.
Environmental and ecological factors influence community structure of benthic molluscs
The spatial structure of molluscan communities appears to be driven by the combined influence of a series of environmental factors in the estuaries and coastal waters (McLusky and Elliott, 1981; El Asri et al., 2021). In the Min River Estuary, the physic-chemical properties of waters are affected by freshwater discharge from the Min River. These oscillations of environmental variables, mainly salinity, usually form a barrier that prevents marine organisms from entering and stop estuarine and freshwater species from exiting (Costa-Dias et al., 2010). In this study, the community structure of benthic molluscs in the Min River Estuary was also confirmed to be unique. In the Pearl River Estuary (China), total nitrogen and organic matter in the sediment were the main environmental factors influencing the benthic communities (Li et al., 2012). In the Yellow Sea (China), temperature, water depth and salinity were the major factors affecting the benthic community (Zhang et al., 2016). In the Portuguese continental shelf, the main factors influencing the benthic community structure were water depth, hydrodynamic regime and grain sizes (Martins et al., 2014). In the ports of Mumbai (India), temperature, sediment texture and suspended particulate matter were the environmental factors that influenced the benthic community structure (Mandal and Harkantra, 2013). This study revealed that both environmental and ecological factors, including water depth, pH, salinity, temperature, phytoplankton abundance and zooplankton abundance, shaped the benthic molluscan community structures in the coastal waters of Changle (China).
The influence of plankton abundance on the molluscan community structure is an interesting topic to further explore. Phytoplankton and zooplankton are important food sources for benthic molluscs, especially those suspension-feeding bivalves (Cloern, 1982). In this study, the phytoplankton samples were comprised of 6 taxonomic groups, including diatom (the dominant group), dinoflagellate, cyanophyta, chrysophyceae, chlorella and xanthophyceae (detailed data not shown here). The zooplankton samples were comprised of 14 taxonomic groups, mainly including copepod and planktonic larva (detailed data not shown here). Our results provided a valid evidence that phytoplankton abundance and zooplankton abundance had an influence on the community structure of benthic molluscs. Some dominant species in this study, e.g. M. chinensis and M. antiquata, are mainly phytoplankton filters (Palomares and Pauly, 2021). It merits further evaluation on relationship between plankton species composition and community structure of benthic molluscs.
The coastal waters in Changle is shallow (< 20 m), and there are no significant variances among months (p > 0.40, Table 1). Considering the shallow water environment and strong effects of winds, tides and currents, we assumed that there was no temporary stratification that could cause significantly abiotic and biotic variances between surface and bottom waters. Therefore, we only used surface water parameters to assess the sea water properties in this study.
For sustainable harvest of benthic molluscs
The coastal waters of Changle provide suitable habitats for the growth of benthic molluscs, many as food species. The most valuable species is M. antiauqta. The annual catch of M. antiquata in the coastal waters of Changle had experienced precipitous decline from 150 t in the 1960s to only about 5 t after 2001, mainly due to overexploitation (Liu, 1997; Liu and Zhu, 2009). The operation of adding water cannons to the sea bed is commonly seen to collect benthic molluscs in the coastal waters of Changle District. The bottom trawlers are equipped with a high-pressure water component to stir the sandy bottom down to half a meter to collect the infaunal molluscs. The destructive effects of bottom trawls on benthic infauna and habitats have been noted in studies from both tropical and temperate waters (Hiddink et al., 2017; Wang et al., 2021a). More environmental-friendly fishing methods and regulations and enforcement to achieve sustainable harvest of benthic molluscs in Changle District are needed.
Estuaries are usually the regions with active economic development, facing environmental problems such as pollution and eutrophication (Zhou and Cai, 2010; Wang et al., 2017b; Qiu et al., 2018). As the largest estuary in Fujian Province, the Min River Estuary is also facing the same problems caused by the rapid development of industrialization and urbanization along the Min River Basin and coastal waters (Sun et al., 2017). Appropriate harvest management measures and environmental protection are essential to help improve the eco-environment and maintain sustainable development of benthic molluscs.
Conclusion
This study reported the species composition, abundance and biomass of benthic molluscs, and analyzed the environmental and ecological variables as well as their correlation with molluscan community structure in the coastal waters of Changle, Fujian Province, China. Six bivalve species (i.e. P. gubernaculum, M. chinensis, D. japonica, M. iridescens, A. broughtonii, M. antiquata) were dominant in the region. Three spatial community groups were identified, and the community structure was best correlated with four environmental and two ecological variables including water depth, pH, salinity, temperature, phytoplankton abundance and zooplankton abundance. As a suitable habitat for the growth of commercial important benthic molluscs, the coastal waters of Changle deserve effective protection and appropriate management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Fujian Province Ocean and Fisheries Bureau of China and Xiamen University of China.
Author contributions
C-LL and QX wrote the first draft. QX, X-BJ, Q-QR, J-HS and G-MD conducted sample collections. C-LL, ZW, QX, Q-QR and ML conducted sample sorting, identification and measurement, and data analyses. C-LL, ZW and ML revised the manuscript. ML received funding and designed the surveys. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Fujian Province Ocean and Fisheries Bureau of China (grant no. [3500]HTZB[GK]2019007-1-1). The funder had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors thank the fisheries authorities of Fujian Province and Fuzhou City for sampling permits, and graduate and undergraduate students from Xiamen University for sample treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1045393/full#supplementary-material
References
Anderson M. P., Fenberg P. B., Griffiths H. J., Linse K. (2021). Macrobenthic mollusca of the prince Gustav channel, eastern Antarctic peninsula: An area undergoing colonisation. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.771369
Anderson M. J., Gorley R. N., Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to software and statistical methods (Plymouth, United Kingdom: PRIMER-E Ltd).
Blanchard F., LeLoc'h F., Hily C., Boucher J. (2004). Fishing effects on diversity, size and community structure of the benthic invertebrate and fish megafauna on the bay of Biscay coast of France. Mar. Ecol. Prog. Ser. 280, 249260. doi: 10.3354/meps280249
Burlakova L. E., Barbiero R. P., Karatayev A. Y., Daniel S. E., Hinchey E. K., Warren G. J. (2018). The benthic community of the laurentian great lakes: Analysis of spatial gradients and temporal trends from 1998 to 2014. J. Great Lakes Res. 44 (4), 600617. doi: 10.1016/j.jglr.2018.04.008
Chen C., Chan T.-Y., Chan B. K. K. (2018). Molluscan diversity in shallow water hydrothermal vents off kueishan island, Taiwan. Mar. Biodiv. 48 (1), 709714. doi: 10.1007/s12526-017-0804-2
Chen G., Wang W., Gu X., Hong W., Zhao K., Xin W., et al. (2021). Patterns and mechanisms of coexistence of benthic mollusks in mangrove forests in fujian delta zone. Wetland Sci. Manage. 17 (1), 913. doi: 10.3969/j.issn.1673-3290.2021.01.02
Clarke K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18 (1), 117143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Cloern J. E. (1982). Does the benthos control phytoplankton biomass in south San Francisco bay. Mar. Ecol. Prog. Ser. 9 (2), 191202.
Costa-Dias S., Freitas V., Sousa R., Antunes C. (2010). Factors influencing epibenthic assemblages in the minho estuary (NW Iberian peninsula). Mar. pollut. Bull. 61 (4-6), 240–246. doi: 10.1016/j.marpolbul.2010.02.020
Dietl G. P., Durham S. R., Smith J. A., Tweitmann A. (2016). Mollusk assemblages as records of past and present ecological status. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00169
El Asri F., Errhif A., Tamsouri M., Nhhala H., Maanan M., Zidane H. (2021). Temporal and spatial variation of the molluscan community structure in oualidia lagoon, Moroccan Atlantic coast. African. J. Mar. Sci. 43 (4), 471481. doi: 10.2989/1814232X.2021.1987985
Fang M. (2013). Preliminary investigation on the resource of Coelomactra antiquata in changle coastal waters. J. Fujian fish. 35 (4), 312316. doi: 10.14012/j.cnki.fjsc.2013.04.004
Godinho F. N., Ferreira M. T. (1998). The relative influences of exotic species and environmental factors. Environ. Biol. Fish. 51 (1), 4151.
Gogina M., Glockzin M., Zettler M. L. (2010). Distribution of benthic macrofaunal communities in the western Baltic Sea with regard to near-bottom environmental parameters. 1. causal analysis. J. Mar. Syst. 79 (12), 112123. doi: 10.1016/j.jmarsys.2009.07.006
Herman P., Middelburg J., Van de Koppel J., Heip C. (1999). Ecology of estuarine macrobenthos. Adv. Ecol. Res. 29 (780), 195240. doi: 10.1016/S0065-2504(08)60194-4
Hiddink J. G., Jennings S., Sciberras M., Szostek C. L., Hughes K. M., Ellis N., et al. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. PNAS 114 (31), 83018306. doi: 10.1073/pnas.1618858114
Hiddink J., Wolff W. (2002). Changes in distribution and decrease in numbers during migration of the bivalve Macoma balthica. Mar. Ecol. Prog. Ser. 233, 117130. doi: 10.7717/peerj.1240
Hu L. (2021). Species diversity and geographical distribution of marine, benthic, shell-bearing mollusks on the coast and adjacent area of pingtan island, fujian province. Biodiv. Sci. 29 (10), 14031410. doi: 10.17520/biods.2021114
Huang W., Wen Z., Hu L., Ma P., Xu M., Huang X., et al. (2020). Community characteristics of benthic shellfish in the coral reef of the weizhou island, beibu gulf. Acta Oceanol. Sin. 42 (6), 6269. doi: 10.3969/j.issn.0253–4193.2020.06.008
Hwai A. S., Karim N.-n., Yasin Z. (2007). Diversity of mollusc communities in the seagrass bed in pulau gazumbo, penang, Malaysia. Mar. Res. Indones 32 (2), 123127. doi: 10.14203/mri.v32i2.445
Kang B., Pecl G. T., Lin L., Sun P., Zhang P., Li Y., et al. (2021). Climate change impacts on china’s marine ecosystems. Rev. Fish. Biol. Fisher. 31 (3), 599629. doi: 10.1007/s11160-021-09668-6
Kroeker K. J., Sanford E., Jellison B. M., Gaylord B. (2014). Predicting the effects of ocean acidification on predator-prey interactions: a conceptual framework based on coastal molluscs. Biol. Bull. 226 (3), 211222. doi: 10.1086/BBLv226n3p211
Li B., Jiang S., Lü J., Chen L., Yan L., Liu C., et al. (2020). Species composition and long-term variation of macrobenthos in intertidal zone and offshore areas of the yellow river delta. Biodiv. Sci. 28 (12), 15111522. doi: 10.17520/biods.2020164
Lin J. (2020). Current stock status and protection efficiency for Mactra antiquata in changle, fujian. J. @ Appl. Oceanogr 39 (04), 551558. doi: 10.3969/J.ISSN.2095-4972.2020.04.011
Liu D. (1997). The declining resources of Coelomactra antiquata and the countermeasures. Stud. Mar. Sin. 39 (39), 6570.
Liu D., Wang J., Xiao H., Xiao S., Huang T., Chi B. (2003). Studies on the growth of Coeloactra antiquata. J. Zhanjiang Ocean Univ. 23 (1), 1721.
Liu D., Zhu S. (2009). Study on edible lamellibranch in changle inshore and their sustainable utilization. J. Econ. Anim. 13 (04), 229236+241. doi: 10.13326/j.jea.2009.04.013
Li Y.-F., Xu R.-L., Wang C.-F. (2012). The community structure of molluscs in three different wetland types in the qi'ao-dan'gan island mangrove nature reserve at qi'ao island, pearl river estuary, China. Zool. Stud. 51 (6), 745754.
Mandal S., Harkantra S. N. (2013). Changes in the soft-bottom macrobenthic diversity and community structure from the ports of Mumbai, India. Environ. Monit. Assess. 185 (1), 653672. doi: 10.1007/s10661-012-2582-4
Martins R., Sampaio L., Quintino V., Rodrigues A. (2014). Diversity, distribution and ecology of benthic molluscan communities on the Portuguese continental shelf. J. Sea Res. 93, 7589. doi: 10.1016/j.seares.2013.11.006
Ma W., Wang M., Wang W., Liu Y., Luo L., Tang C. (2018). Biodiversity of mangrove mollusks in the west coast of hainan island, China. Biodiv. Sci. 26 (7), 707716. doi: 10.17520/biods.2018104
McAfee D., Bishop M. J., Williams G. A. (2022). Temperature-buffering by oyster habitat provides temporal stability for rocky shore communities. Mar. Environ. Res. 173, 105536. doi: 10.1016/j.marenvres.2021.105536
McConnaughey R., Mier K., Dew C. (2000). An examination of chronic trawling effects on soft-bottom benthos of the eastern Bering Sea. ICES J. Mar. Sci. 57 (5), 13771388. doi: 10.1006/jmsc.2000.0906
McGovern M., Poste A. E., Oug E., Renaud P. E., Trannum H. C. (2020). Riverine impacts on benthic biodiversity and functional traits: A comparison of two sub-Arctic fjords. Estuar Coast. Shelf Sci. 240, 106774. doi: 10.1016/j.ecss.2020.106774
McLusky D., Elliott M. (1981). The feeding and survival strategies of estuarine molluscs. Feeding survival srategies Estuar. organisms, 15, 109–121. doi: 10.1007/978-1-4613-3318-0_9
Morton B. (1990). The physiology and feeding behaviour of two marine scavenging gastropods in Hong Kong: the subtidal Babylonia lutosa (Lamarck) and the intertidal Nassarius festivus (Powys). J. Moll. Stud. 56 (2), 275288. doi: 10.1093/mollus/56.2.275
Morton B. (1996). The subsidiary impacts of dredging (and trawling) on a subtidal benthic molluscan community in the southern waters of Hong Kong. Mar. pollut. Bull. 32 (10), 701710. doi: 10.1016/0025-326X(96)00002-1
Mulik J., Sukumaran S., Srinivas T. (2020). Factors structuring spatio-temporal dynamics of macrobenthic communities of three differently modified tropical estuaries. Mar. pollut. Bull. 150, 110767. doi: 10.1016/j.marpolbul.2019.110767
Narasimham K., Muthiah P., Sundararajan D., Vaithinathan N. (1988). Biology of the great clam, Meretrix meretrix in the korampallam creek, tuticorin. Indian J. Fish. 35 (4), 288293.
Newell R. I. (2004). Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: a review. J. Shellfish Res. 23 (1), 5162.
Ni L., Li Q., Kong L. (2011). Microsatellites reveal fine-scale genetic structure of the Chinese surf clam Mactra chinensis (Mollusca, bivalvia, mactridae) in northern China. Mar. Ecol. 32 (4), 488497. doi: 10.1080/17386357.2007.9647339
Palomares M. L. D., Pauly D. (2021) SeaLifeBase (World Wide Web electronic publication). Available at: www.sealifebase.org,version (Accessed 04/2022).
Petr Š., Jan L. (2014). Multivariate analysis of ecological data using CANOCO 5 (USA: Cambridge University Press, New York).
Qiu B., Zhong X., Liu X. (2018). Assessment of the benthic ecological status in the adjacent waters of Yangtze river estuary using marine biotic indices. Mar. pollut. Bull. 137, 104112. doi: 10.1016/j.marpolbul.2018.10.006
Rakocinski C. F. (2012). Evaluating macrobenthic process indicators in relation to organic enrichment and hypoxia. Ecol. Indic. 13 (1), 112. doi: 10.1016/j.ecolind.2011.04.031
Rao Y., Cai L., Chen B., Chen X., Zheng L., Lin S. (2020). How do spatial and environmental factors shape the structure of a coastal macrobenthic community and meroplanktonic larvae cohort? evidence from daya bay. Mar. pollut. Bull. 157, 111242. doi: 10.1016/j.marpolbul.2020.111242
R Core Team (2019). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for 572 Statistical Computing). Available at: http://www.R-project.org/.
Stevens B., Armstrong D., Cusimano (1982). Feeding habits of the dungeness crab cancer magister as determined by the index of relative importance. Mar. Biol. 72 (2), 135145. doi: 10.1007/BF00396914
Sukumaran S., Vijapure T., Mulik J., Ridha H. (2021). Marine macrobenthos of Northwest India-reviewing the known and unknown. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.671245
Sun Z., Li J., He T., Ren P., Zhu H., Gao H., et al. (2017). Spatial variation and toxicity assessment for heavy metals in sediments of intertidal zone in a typical subtropical estuary (Min river) of China. Environ. Sci. pollut. Res. 24 (29), 2308023095. doi: 10.1007/s11356-017-9897-1
Wang Z., Leung K. M. Y., Li X., Zhang T., Qiu J.-W. (2017b). Macrobenthic communities in Hong Kong waters: Comparison between 2001 and 2012 and potential link to pollution control. Mar. pollut. Bull. 124 (2), 694700. doi: 10.1016/j.marpolbul.2017.04.026
Wang Z., Leung K. M. Y., Sung Y. H., Dudgeon D., Qiu J.-W. (2021a). Recovery of tropical marine benthos after a trawl ban demonstrates linkage between abiotic and biotic changes. Commu. Biol. 4 (1), 212. doi: 10.1038/s42003-021-01732-y
Wang R., Qu X., Cai Y., Zhang H. (1988). Coloured illustrations of aquatic mollusks in China (Zhejiang, China: Zejiang Publishing House of Science and Technology).
Wang Z., Wang H., Fan S., Xin M., Sun X. (2021b). Community structure and diversity of macrobenthos in jiaozhou bay. Mar. pollut. Bull. 171, 112781. doi: 10.1016/j.marpolbul.2021.112781
Wang Q., Xie X., Zhang M., Teng W., Liang M., Kong N., et al. (2017a). Effects of temperature and salinity on survival and growth of juvenile ark shell Anadara broughtonii. Fish. Sci. 83 (4), 619624. doi: 10.1007/s12562-017-1095-z
Wu H., Fu S., Cai X., Chen Q. (2019). Composition and distribution characteristics of macroinvertebrates in subtidal zone of the main marine bays in fujian province, China. Chin. J. Appl. Ecol. 30 (12), 42404248. doi: 10.13287/j.1001-9332.201912.036
Wu J., Liang C., Chen L., Chen S., Li X. (2005). A study on the sustainable utilization of benthic marine Mollusca living in soft substrate in China (in Chinese with English abstract). Trans. Oceanol. Limnol. 02), 9398.
Xu J., Lu X., Liu X. (2021). Patterns of species and functional diversity of macrofaunal assemblages and the bioassessment of benthic ecological quality status in the southern yellow Sea. Mar. pollut. Bull. 171, 112784. doi: 10.1016/j.marpolbul.2021.112784
Yang W., Cai Y., Kuang X. (2017). Color atlas of molluscs of the south China Sea (Beijing, China: China Agriculture Press).
Zhang T., Guo W., Jing Y., Liu E., Hu F., Wang S., et al. (2021). Characteristics of macrobenthos assemblages in national special marine protected area at dongying estuary shallow Sea shellfish ecology. Mar. Sci. 45 (03), 113. doi: 10.11759/hykx20200520002
Zhang J., Zhang S., Zhang S., Du Y., Xu F. (2016). What has happened to the benthic mollusks of the yellow Sea in the near half century? comparison on molluscan biodiversity between 1959 and 2007. Cont. Shelf Res. 113, 2129. doi: 10.1016/j.csr.2015.12.004
Zhao Y., Zhao W., Yan X., Yang Z. (2011). Coastal shellfish resources survey of gaojia beach in liaodong bay, bohai Sea. J. Dalian Ocean Univ. 26 (05), 471474. doi: 10.16535/j.cnki.dlhyxb.2011.05.003
Zhou X., Cai L. (2010). Coastal and marine environmental issues in the pearl river delta region, China. Int. J. Environ.Stud. 67 (2), 137145. doi: 10.1080/00207231003683549
Zuschin M., Hohenegger J., Steininger F. (2001). Molluscan assemblages on coral reefs and associated hard substrata in the northern red Sea. Coral Reefs 20 (2), 107116. doi: 10.1007/s003380100140
Keywords: bivalves, gastropods, community structure, environmental variables, ecological variables, China
Citation: Liu C-l, Xu Q, Wang Z, Jiang X-b, Ding G-m, Ren Q-q, Song J-h and Liu M (2023) Community structure of benthic molluscs shaped by environmental and ecological variables in the coastal waters of Changle, Fujian Province, China. Front. Mar. Sci. 10:1045393. doi: 10.3389/fmars.2023.1045393
Received: 15 September 2022; Accepted: 27 January 2023;
Published: 09 February 2023.
Edited by:
Çetin Keskin, Istanbul University, TürkiyeReviewed by:
Jongseong Ryu, Anyang University, Republic of KoreaSusete Wambier Christo, Universidade Estadual de Ponta Grossa, Brazil
Copyright © 2023 Liu, Xu, Wang, Jiang, Ding, Ren, Song and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, bWlubGl1eG1AeG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Cai-lian Liu
Cai-lian Liu Qing Xu
Qing Xu Zhi Wang
Zhi Wang Xiao-bin Jiang2
Xiao-bin Jiang2 Qing-qiang Ren
Qing-qiang Ren Jia-hao Song
Jia-hao Song Min Liu
Min Liu