- 1Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan
- 2Department of Aquaculture, National Taiwan Ocean University, Keelung, Taiwan
- 3Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
- 4Doctoral Degree Program in Ocean Resource and Environmental Changes, National Taiwan Ocean University, Keelung, Taiwan
Human activity and global climate change have severely affected marine ecosystems and fishery resources. Habitat conservation and stock enhancement are considered effective methods. Moreover, with the gradual disappearance of fishery resources, fishing ports have become underutilized spaces. Currently, 73 of the 221 fishing ports in Taiwan are underutilized. Therefore, we, for the first time, developed an integrated multitrophic aquaculture (IMTA)-based cage rearing system suitable for stock enhancement and applied it in an optimal underutilized fishing port after the site evaluation and selection of 17 potential fishing ports fishing. We further tested that hypothesis that hatchery-produced organisms can be reared and monitored appropriately in this cage rearing system with good survival and growth as well as less environmental impact and handling stress. Through the collocation of various release organisms of different trophic levels, the cage rearing system can reduce environmental impacts as evidenced by the steady water quality (stable pH and undetectable levels of ammonia nitrogen, nitrates, and nitrites). As for the fish welfare, this semiartificial rearing system could also reduce the discomforts of hatchery-produced organisms after transportation and facilitate their adaptations to the released environments as evidenced by positive growth and high survival rates (94%–98%). The cultured and naturally grown shellfish and algae on the cage nets could provide habitats for hatchery-produced and wild organisms that facilitate habitat conservation and stock enhancement. Taken together, we have demonstrated that it is feasible to implement this novel IMTA-based cage rearing system in an underutilized fishing port required for marine stock enhancement.
1. Introduction
The ocean is critical for human well-being; therefore, its sustainable management is essential. Pollution and habitat destruction have negatively affected global marine biodiversity (Sumaila and Tai, 2020; Yan et al., 2021). Moreover, advancements in fishing techniques have led to the excessive fishing efforts and exhaustion of fishery resources (Palomares and Pauly, 2019). Approximately half of all fish stocks have been deemed to have been fully exploited or overexploited (Pauly and Zeller, 2016; Sumaila and Tai, 2020; Yan et al., 2021). Likewise, a rapid decline of coastal fisheries production in Taiwan has also been evident over the past few decades since the annual production of coastal fisheries in Taiwan has decreased by over 50% from 408,201 tons in 1980 to 174,562 in 2020 (Fisheries Agency, 1981-2021). The lack of marine resource management has resulted in the ineffective use of species habitats and resources. Therefore, developing approaches to ensure the sustainability of fishery resources has become a major topic of research worldwide (Schiermeier, 2002; Pauly, 2009; Sumaila and Tai, 2020).
Marine protected areas (MPAs) are common area-based tools, which through legal or other effective means can contribute to comprehensive ocean management and governance in order to achieve the long-term conservation of nature with associated ecosystem services and cultural values (Jones et al., 2020; Sullivan-Stack et al., 2022). MPAs can provide benefits that extend to local communities, fisheries, and economies (Gurney et al., 2021; Sullivan-Stack et al., 2022). To achieve these benefits, MPAs must be underpinned by positive enabling conditions, such as using appropriate ecological and social design principles and adequate enforcement; including indigenous ecological knowledge; understanding community needs and engagement; performing scientific research; and monitoring key species, ecosystems, and ecosystem services (Jones et al., 2020; Sullivan-Stack et al., 2022). However, Taiwan has only a few complete MPAs, most of which are aquatic organism conservation areas, which could be considered part of other effective area-based conservation measures (OECMs). OECMs may have various objectives (e.g., fisheries), but by definition, they are areas that also effectively conserve biodiversity (Jonas et al., 2014; Gurney et al., 2021).
In addition to some fishing restrictions, two approaches have been applied in Taiwan’s aquatic organism conservation areas, including the placement of artificial reef and release of hatchery-produced organisms (Liu, 2013). To improve marine life habitat through artificial reef placement and construction, since 1974, Taiwan has actively promoted the establishment of artificial reefs to improve the environment of fishing grounds and launched census of the benefits of reef areas in the surrounding waters since 2008. The practical experience of fishermen and the results of field academic studies have demonstrated that the deployment of artificial reefs has positive benefits (Chuang et al., 2010; Liu, 2013). To implement fry release, including stock enhancement and sea ranching, since 2002, Taiwan’s Fisheries Agency has conducted planned large-scale release of hatchery-produced organisms. In the years thereafter, more than 20 species have been released. By 2018, the cumulative number of fish had exceeded 120 million in the areas of release, which covered the entirety of Taiwan along the offshore waters. Some of these species have also been evaluated for molecular markers from 2015 to 2018 (Hsu et al., 2020a; Hsu et al., 2020b; Hsu et al., 2022).
Although these fishery management and resource restoration activities have been implemented, the decline in fishery resources has led to a reduction in fishing boat and fisherman population in Taiwan (Liu, 2013; Hsiao and Chen, 2021). Currently, Taiwan has 221 fishing ports, 73 of which are classified as low-utilization areas. Hence, fishery sustainable management infrastructure projects have been implemented to upgrade these fishing ports to multifunctional areas for fishery production, tourism and leisure, and education and culture (Council of Agriculture, 2020). In addition, some of these fishing ports have been demolished, and the habitats have been restored to their natural habitat. However, some fishing ports have been deemed to be ineligible for conversion or demolition, and the impact of this on the environment and local residents’ needs requires consideration.
IMTA, a polyculture method, aims at the integrated production of aquaculture species belonging to different trophic levels through circular economy approach, which minimizes energy loss and environmental degradation. In IMTA, the uneaten feed and waste of one species are converted to feed, fertilizer, and energy for other species (Fang et al., 2016; Shpigel et al., 2018; Biswas et al., 2020; Chang et al., 2021). While IMTA-based rearing system has emerged as an ideal solution for the sustainable aquaculture, it is not adapted into the marine stock enhancement.
In this study, we aimed to determine whether it is feasible to construct an IMTA-based rearing system in an underutilized fishing ports required for marine stock enhancement. We designed and established an IMTA-based rearing system in an optimal underutilized fishing ports for stock enhancement programs after evaluating 17 potential sites across Taiwan. We further tested the hypothesis that hatchery-produced organism can be reared and monitored appropriately in this cage system with good survival and growth as well as less environmental impact and handling stress. We have shown evidence that various organisms with different trophic levels were reared together that can provide additional natural food sources, minimize effects on the environment, and reduce the accumulation of fish and cuttlefish excrements and organic matters in this IMTA-based cage rearing system. This system could alleviate the discomforts of hatchery-produced organism after transportation and enhance their adaptations to the released environments. The cultured and naturally grown shellfish and algae on the cage nets could provide habitats for both hatchery-produced and wild organisms that facilitate habitat conservation and stock enhancement. To the best of our knowledge, this is the first study to develop and implement an IMTA-based rearing system in an underutilized fishing port effective for marine stock enhancement.
2. Materials and methods
2.1. Assessment of fishing ports for cage rearing system
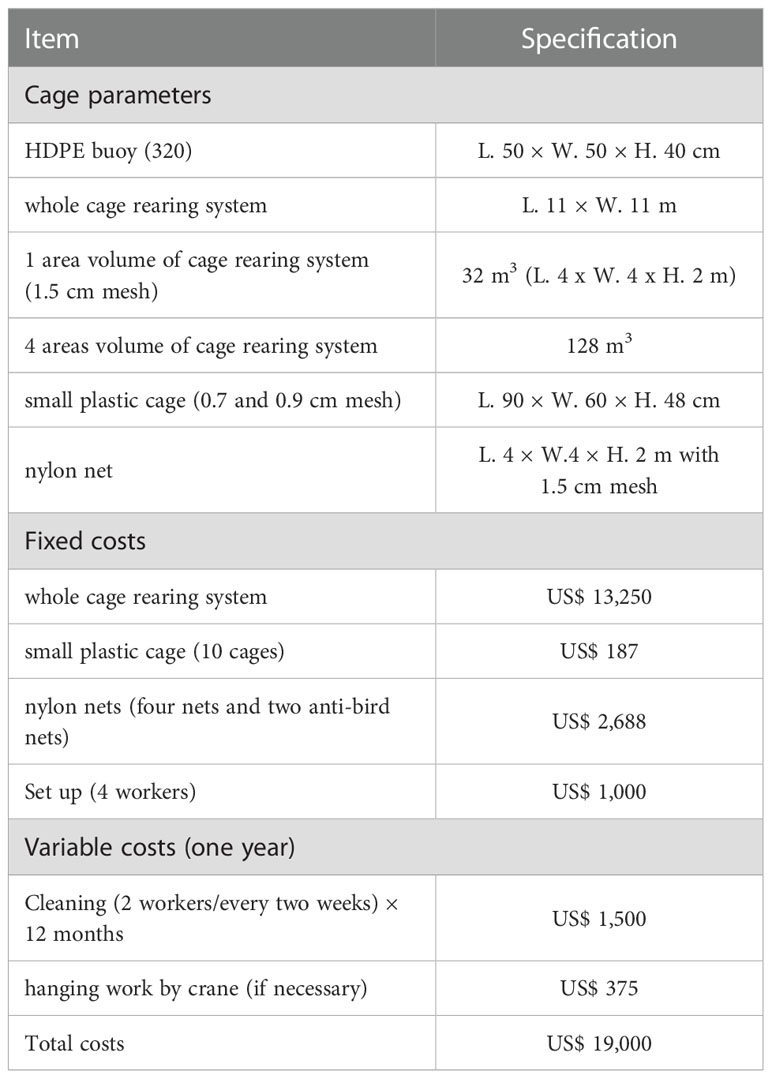
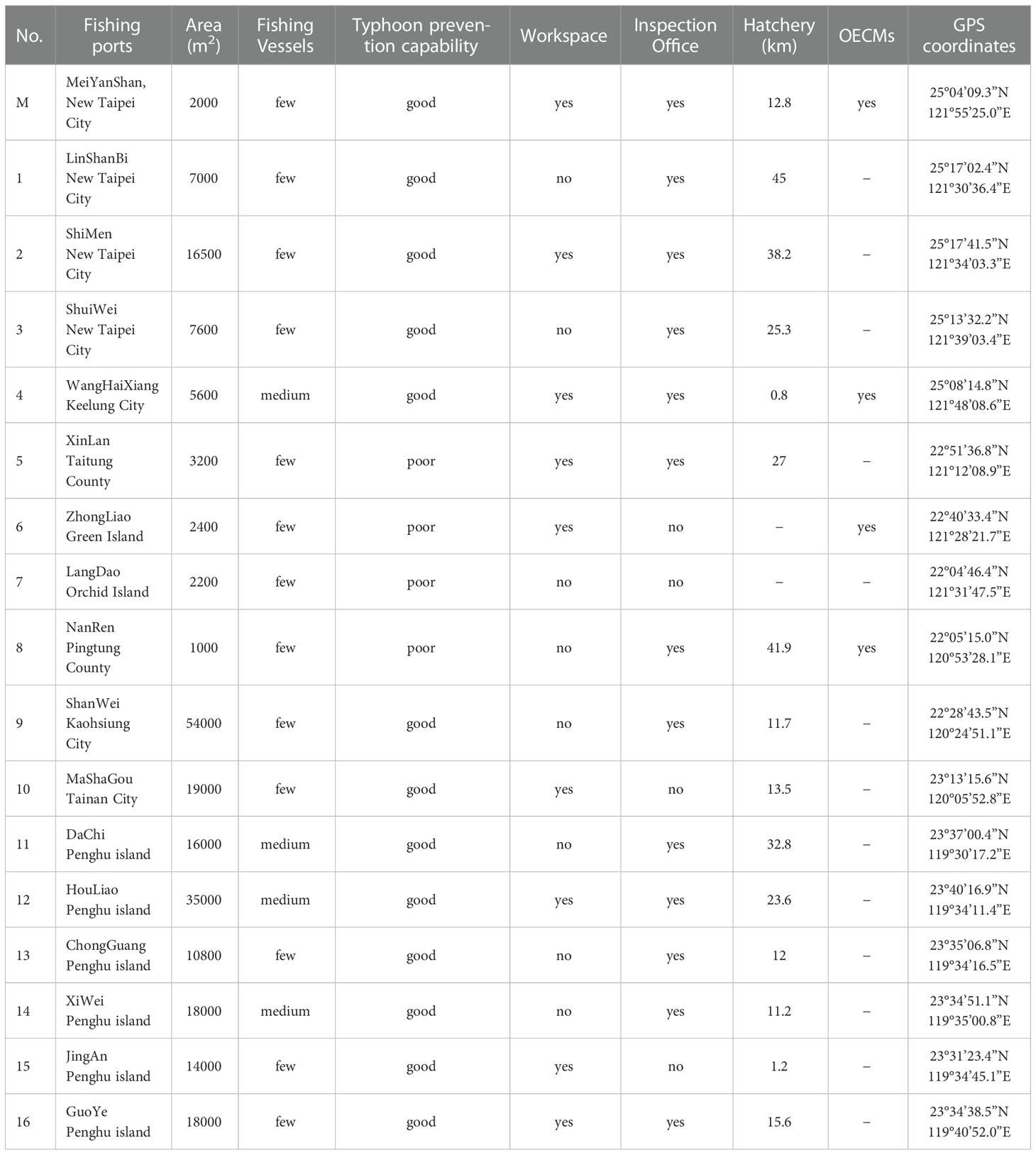
Taiwan has 221 fishing ports, including Penghu, Orchid Island, and Green Island. Of these, 73 have been deemed to be 73 low-utilization fishing ports by Taiwan’s Fisheries Agency on the basis of the number of fishing boats and their current usage (Council of Agriculture, 2020). We assessed these 73 fishing ports for their location, size, number of fishing vessels, typhoon prevention capability, workspace, management (fishing port inspection office), sources of hatchery-produced organisms (official hatchery), and presence of protected areas (i.e., OECMs).
2.2. Experimental location
The Meiyanshan fishing port was selected as the experimental location. This site is located in New Taipei City, on the coast of northeastern Taiwan, within the Northeast National Scenic Area and close to the Gongliao Aqua Center and Maoao Bay aquatic organism conservation area (Figures 1B, C). This area has coral reefs and reef shores, and the fishery activities performed here include coastal fisheries and abalone farming in the intertidal pools in the intertidal zone. Most of the residents are engaged in fishing and tourism. The seawater temperature ranges from 14°C to 32°C (Figure S1). This port can be divided into berths (2000 m2), working platforms (670 m2), towing channels (240 m2), and breakwaters (2560 m2), and it has a security checkpoint of the Coast Guard Administration (Inspection Office, Coast Guard Administration, Ocean Affairs Council; Figure 1D). Our IMTA-based cage rearing system was established in a corner of this port to avoid interference with fishing vessels. We selected a corner close to the working platform, with a relatively high typhoon prevention capability (Figure 1D).
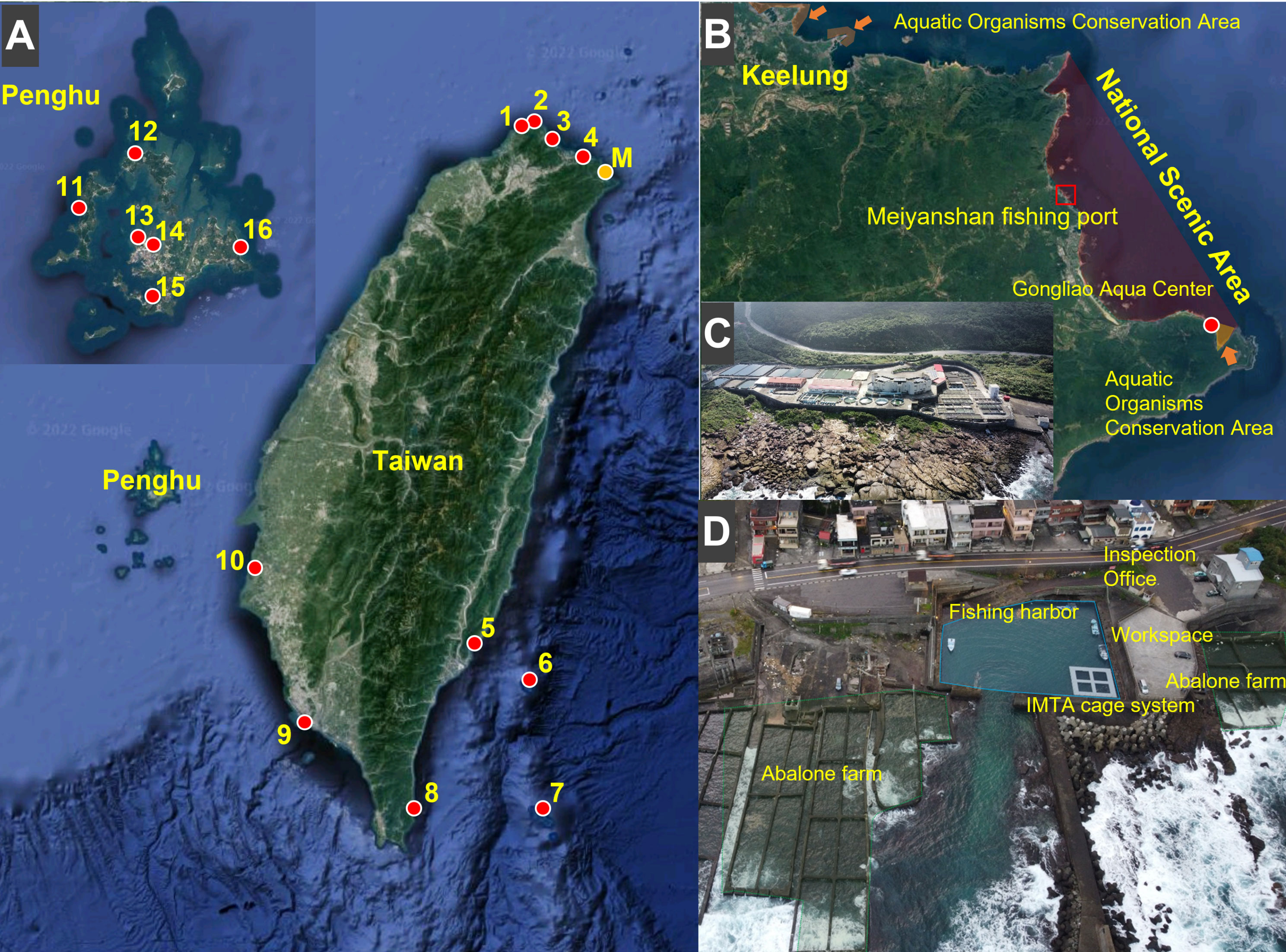
Figure 1 Study site. (A) The 17 potential fishing ports considered for IMTA-based cage rearing system placement. Detailed information is provided in Table 1. (B) Meiyanshan fishing port in New Taipei City in northeastern Taiwan. (C) Gongliao Aqua Center. (D) Aerial view of Meiyanshan fishing port.
2.3. IMTA-based cage rearing system setup
This study was conducted with the approval of New Taipei City Government, Taiwan’s Central Coast Guard and Fisheries Agency, and the local residents. During the experiment, a bulletin board was established, and the inspection office and local residents facilitated the management of the tourists, preventing them from disturbing the experimental area.The cage rearing system, established at the Meiyanshan fishing port in August 2021 (Figure 2A, Table 1), involved using HDPE buoys as the cage frame. Each buoy was 50 cm long and wide and 40 cm tall and had a buoyancy capacity of 93 kg. The cage was 11 m long and wide and consisted of four areas (each 4 m long, 4 m wide, and 2 m tall) with a working aisle. The nets were custom-made nylon nets, which were 4 m long and wide and 2 m tall, with 1.5-cm mesh. Lead bars were used to prevent the buoy platform from falling off because of winds and waves; these bars were attached around the bottom of the nets and fixed on the platform every 50 cm. Antibird nets were added as needed to prevent birds from feeding and fish from jumping out of the cage net. In addition, within the larger cage, 10 smaller plastic cages (90 cm long, 60 cm wide, and 48 cm tall) were placed for all the released organisms. In September 2021, to protect the system from the strong typhoon Chanthu, the cage was dismantled and suspended to the shore using a crane. After the typhoon passed (in approximately 1 week), it was returned to the port for reinstallation (Figure 2B). After the typhoon, several species were introduced to our IMTA-based cage rearing system, which included hair-shaped green alga (Chaetomorpha crassa), red alga (Agardhiella subulata), oyster (Magallana angulata), purple sea urchin (Heliocidaris crassispina), and dog conch (Laevistrombus canarium).
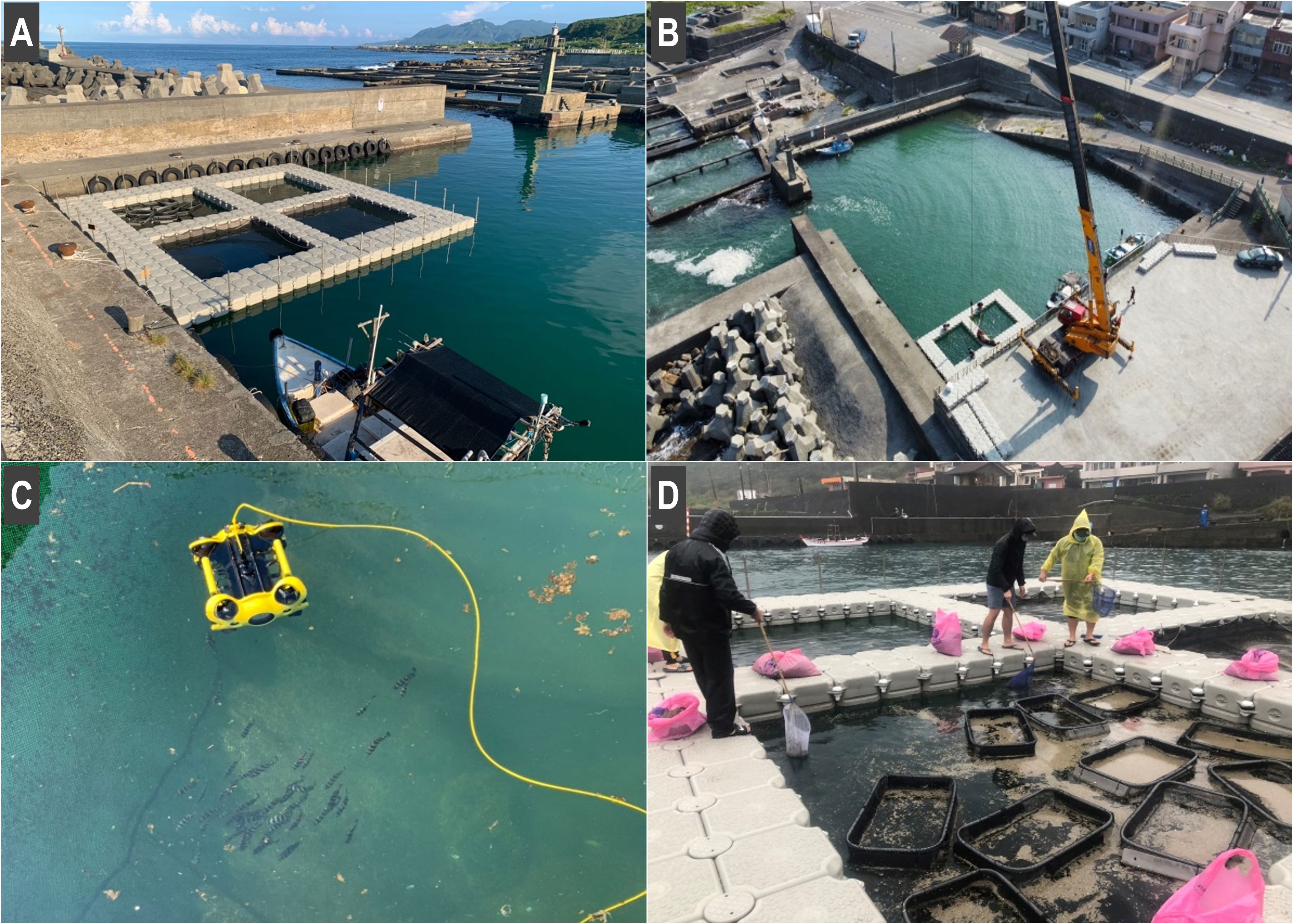
Figure 2 (A) IMTA-based cage rearing system in Meiyanshan fishing port. (B) Crane used for hanging. (C) Observation with remotely operated underwater vehicle. (D) Cleanup of massive amounts of pumice formed during eruption of the Japanese submarine volcano Fukutoku–Okanoba from December 2021 to March 2022.
2.4. Hatchery-produced organisms in cage rearing system
The aquatic organisms in this cage rearing system can be divided in two types: IMTA-based species (dog conch, oyster, purple sea urchin, and alga) and hatchery-produced species [silver seabream (Rhabdosargus sarba), red seabream (Pagrus major), javelin grunter (Pomadasys kaakan), striped breakperch (Oplegnathus fasciatus), longfin batfish (Platax teira), and cuttlefish (Sepia pharaonis; subadults, embryos, and larvae)]. The rearing periods were mainly short term (3–7 days), mid-term (≤2 weeks), and long term (≤1 month), adjusted on the basis of biological characteristics and breeding conditions. During the rearing period, a small amount of artificial feed was provided, and the feed amount was reduced gradually before release.
The growth and survival rates of hatchery-produced organisms in this cage rearing system were also estimated. For the estimation of growth rates for hatchery-produced organisms, their initial and ended body size were measured and recorded (n = 30). For the estimation of survival rates, the dead individuals in the cage rearing system and fishing port were regularly checked using a remotely operated underwater vehicle (ROV; Figure 2C). Moreover, the measurements were adjusted for each rearing period to reduce the potential stress caused by the frequent measurements. The survival rate was measured once a day for the organisms with a short-term (3–7-day) rearing period, whereas it was recorded once a week for those with a mid-term (1–2-week) and long-term (2–4-week) rearing period. The rearing density was adjusted for each aquatic organism. Marine debris was removed every 2 weeks (Figure 2D).
2.5. Assessment of water quality in Cage Rearing System with hatchery-produced organisms
In situ measurements of seawater from the system were performed every week during the trials to monitor the parameters of water quality, including the pH and the levels of ammonia nitrogen, nitrates, and nitrites. The pH values of seawater were measured by a portable and waterproof pH meter following the manufacturer’s instructions (Model HI-98194, Hanna Instruments, USA) The level of ammonia nitrogen, nitrates, and nitrites were measured by a VIS-spectrophotometer according to the manufacturer’s manual (Model XD7000, Lovibond, Germany).
3. Results
3.1. Assessment of fishing ports for cage rearing system
As presented in Figure 1A and Table 2, 17 potential fishing ports for the cage rearing system were identified and evaluated. These are distributed on the northern coast of Taiwan (No. 1–4, M), eastern Taiwan and the offshore islands (No. 5–8), the southwestern coast of Taiwan (No. 9 and 10), and Penghu Island (No. 11–16). The area of the fishing ports ranged from 1000 to 54,000 m2. The smallest fishing port is Nanren (No. 8), whereas the largest is Shanwei (No. 9). Water depth is a major factor in the cage rearing system. The Shanwei fishing port, although large, can easily silt up at the entrance, preventing the passage of fishing boats at low tide. By contrast, the Nanren fishing port is too small to set up a long-term rearing system; thus, it may be suitable for smaller, temporary cage rearing systems. On the basis of the number of fishing vessels visiting them, the Wanghaixiang (No. 4), Dachi (No. 11), Houliao (No. 12), and Xiwei (No. 14) fishing ports could be considered the medium-utilization type. At high-utilization fishing ports, establishing a long-term cage rearing system can be difficult but feasible with strong community support.
Typhoon prevention capability is essential for fishing ports, particularly for those in Taiwan. The structure of the Xinlan (No. 5), Zhongliao (No. 6), Langdao (No. 7), and Nanren (No. 8) fishing ports is relatively simple, which can increase typhoon damage risk. Some cage rearing systems may not require a workspace on land, but it can be useful for subsequent entry of the released fish. However, the Linshanbi (No. 1), Shuiwei (No. 3), Langdao (No. 7), Nanren (No. 8), Shanwei (No. 9), Dachi (No. 11), Chongguang (No. 13), and Xiwei (No. 14) fishing ports lack this type of workspace. Fishing port inspection offices may aid in the publicity of the cage systems, thus reducing inference from tourists in the port areas. However, the Zhongliao (No. 6), Langdao (No. 7), Mashagou (No. 10), and Jingan (No. 15) ports do not have an inspection office. Although fish larvae from private hatcheries (most of which are in southern Taiwan) can be transported within 24 hours around Taiwan, official hatcheries or research institutes can provide IMTA species and aid in subsequent observation. The distance between the Meiyanshan fishing port (No. M) and Gongliao Aqua Center is 12.8 km, that between Wanghaixiang (No. 4) and National Museum of Marine Science and Technology (Keelung) is 800 m, that between Xinlan (No. 5) and Fisheries Research Institute (Zhiben, Taitung) is 27 km, that between Shanwei (No. 9) and Fisheries Research Institute (Donggang, Pingtung) is 11.7 km, that between Mashagou and the Fisheries Research Institute (Qigu, Tainan) is 13.5 km, and that between Nanren (No. 8) and National Museum of Marine Biology and Aquarium (Pingtung) is 41.9 km. The distance between fishing ports on the north coast of Taiwan (No. 1–4, M) and Fisheries Research Institute (Keelung) ranges from 25.3 to 45 km, and that between fishing ports in Penghu (No. 11–16) and Marine Life Propagation Station (Penghu) ranges from 1.2 to 32.8 km. Being present in protected areas such as OECMs can facilitate further promotion of the cage rearing system among the community. Currently, only the Meiyanshan (No. M), Wanghaixiang (No. 4), Zhongliao (No. 6), and Nanren (No. 8) fishing ports are located in OECMs. Therefore, because the Meiyanshan (No. M) fishing port satisfied the requirements, we considered it an ideal location for the IMTA-based cage rearing system for our stock enhancement experiment.
3.2. Cage rearing system cost
As presented in Table 1, the total cost of establishing our cage rearing system (US$ 19,000) could be divided into fixed costs (US$ 17,125) and variable costs (US$ 1,875 per year). The fixed costs comprised the cage rearing system (i.e., the cage frame), 10 small plastic cages, nylon nets (4 fishing nets and 2 antibird nets), and set up (by 4 workers/day) whereas the variable costs comprised cleaning (by 2 workers/2 weeks for 12 months) and crane suspension (if necessary).
During the study, most typhoons were not strong enough to warrant the use of a crane. In addition, the system did not require frequent cleaning; however, the large amount of pumice that formed after the Japanese submarine volcano Fukutoku–Okanoba erupted from December 2021 to March 2022 required considerable cleaning (Figure 2D).
3.3. Cage rearing system condition
Through nutrient cycling, production efficiency increases, and to target all nutrient flows, our IMTA-based cage rearing system has four main nutrient levels. The first level includes dissolved inorganic nutrients, which can be absorbed by seaweeds, including naturally growing (Ulva sp.) and artificially introduced hair-shaped green alga (Figure 3L) and red alga (Figure 3M). The second comprises the slow-settling organic particles produced by residual bait or feces, which can be captured by filter-feeding organisms, including the released oysters (Figure 3G) and natural sponges (Figure 3J). The third includes heavier organic solids, also produced from residual bait or feces, which can be consumed by sediment-feeding benthic organisms, including released dog conch (Figures 3H, I) and natural crabs. Finally, the fourth level contains attached organisms with excessive algal epiphytes on the grid of the box net, which warrant restraining and include artificially released purple sea urchin (Figure 3K) and the natural herbivorous fish rabbitfish (Siganus fuscescens).
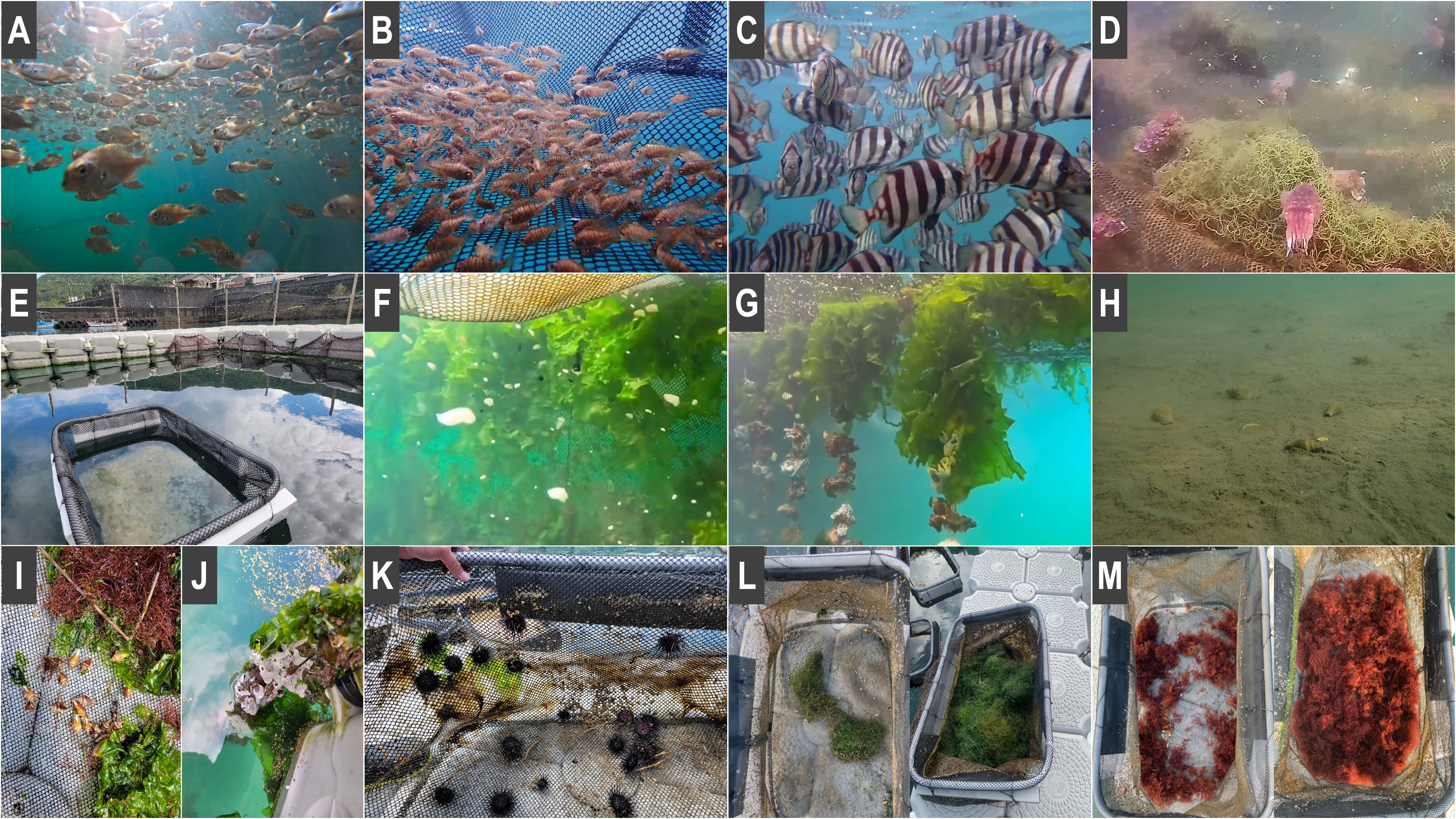
Figure 3 (A) Red seabream (Pagrus major). (B) Javelin grunter (Pomadasys kaakan). (C) Striped breakperch (Oplegnathus fasciatus).(D, E) Cuttlefish (Sepia pharaonis) subadults (D) and embryos (E). (F) Cuttlefish (Sepia pharaonis) hatchlings. (G) Oyster (Magallana angulata). (H, I) Dog conch (Laevistrombus canarium) at the bottom of the cage rearing system (H) and in a small plastic cage (I). (J) Natural sponge. (K) Purple sea urchin (Heliocidaris crassispina). (L) Growth of hair-shaped green algae (Chaetomorpha crassa), before (left) and after (right). (M) Growth of red algae (Agardhiella subulata) before (left) and after (right).
After setting up the cage rearing system, we purchased 20 strings of oysters from an oyster farm (Tainan). These oysters were obtained through wild seed capture, and each string had approximately 60 oysters. Except for a few oysters that fell off during transportation and hanging, the survival rate exceeded 95% after 1 month. From the second month onward, natural sponges and macroalgae began adhering to oyster skewers, which changed with the seasons naturally.
Dog conch was bred by our team in the Gongliao Aqua Center. In total, 1000 individuals with good vitality and complete shell shape were selected and placed at the bottom of the cage rearing system. Moreover, 100 individuals were placed on the umbrella net at the bottom for observation. After 1 day, almost all the dog conches had been moved out of the umbrella net, indicating that they had survived and adapted well. Live dog conches could be observed using our ROV from days 30 to 300 (Figure 3H). Moreover, 30 individuals were coreared in a small plastic cage with macroalgae, and these dog conch survived well until day 300 (90% survival rate).
We purchased purple sea urchin from a private hatchery. Fifty individuals with good vitality were selected and placed in a small plastic cage with macroalgae. After 30 days, the sea urchins ran out of the macroalgae (Figure 3K). After 300 days, the survival rate of the sea urchins was 64%. Compared with other small plastic cages without sea urchins, this small change has extremely few attachments and algae.
Two macroalgae species were used in this experiment: Hair-shaped green algae (Chaetomorpha crassa) and red alga (Agardhiella subulata). These two species could cultivate and maintain all the year in Gongliao Aqua Center. In October, five hundred gram red algae were initially put in the small plastic cages, which increased to 3264g after 30 days (Figure 3L). Initially, 500g of green algae was put into a small plastic cage, which increased to 4253g after 30 days (Figure 3M). However, when the water temperature decreased to 20°C in December, the growth of macroalgae stopped. After January, these two artificial source macroalgae disappeared. From February to June, the natural macroalgae sea lettuce (Ulva lactuca) grows well on the cage nets and oyster strings (Figure 3F, G).
After 1 month of setup, an attractive natural habitat formed on the oyster strings and in the cage nets with sea lettuce, and it included small damselfish species (Abudefduf sordidus, Abudefduf vaigiensis, Abudefduf sexfasciatus, and Pomacentrus coelestis), orbicular batfish (Platax orbicularis), scaled sardine (Sardinella zunasi), spiny pufferfish (Diodon holocanthus), scalpel sawtail (Prionurus scalprum), bigfin reef squid (Sepioteuthis lessoniana), gobies, and wrasse.
3.4. Hatchery-produced organisms in cage rearing system
In total, 200 longfin batfish from private hatcheries were transported and added to the cage rearing system for a short-term (7-day) trial in October. However, 10 longfin batfish died after transportation. No swarming or feeding behavior was observed after 30 minutes. After 1 day, their swarming and feeding behavior was observed, but 24 individuals died. Until release (after 7 days), no more fish died (survival rate = 83%). For the mid-term (14-day) trial, 1000 silver seabream from private hatcheries were transported and added to the cage rearing system in November. No silver seabream died after transportation, and their swarming and feeding behavior could be observed after 30 minutes. Moreover, after 14 days, the silver seabream exhibited a high survival rate (98%) and slight growth (Table 3). For the long-term (30-day) trials, 1000 striped breakperch (October), 5000 red seabream (April), and 3000 javelin grunter (April) individuals from private hatcheries were transported and added to the cage rearing system separately (Figures 3A–C). However, 0 striped breakperch, 5 red seabream, and 16 javelin grunter individuals died after transportation. The swarming and feeding behavior was observed after 30 minutes. After 30 days, all three trials demonstrated high survival rates (94%–98%) and slight growth (Table 3).
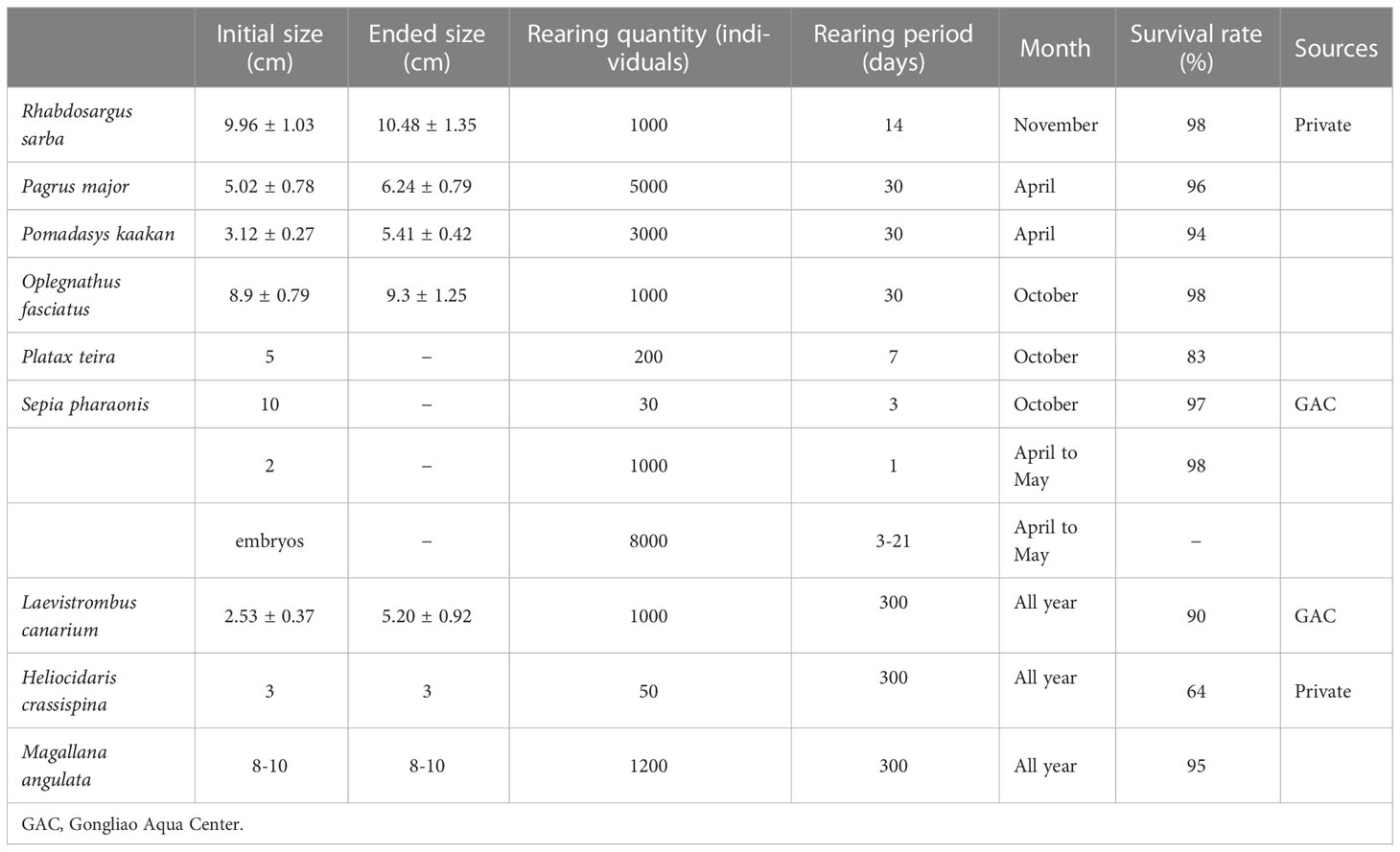
Table 3 Growth and survival of hatchery-produced aquatic organisms in the IMTA-based cage rearing system.
Cuttlefish embryos, larvae (size = 2 cm), and subadults (size = 10 cm) were cultured by our team at the Gongliao Aqua Center. In October, 10 cuttlefish subadults were transported and added to the cage rearing system with hair-shaped green algae (Figure 3D). The cuttlefish settled and hid in the green algae after 1 hour. In the first week, we fed them with fresh fish; in the second week, because the cuttlefish preyed on natural small fish in the cages, we discontinued feeding them. After 14 days, cuttlefish subadults were released, and they adapt well (survival rate = 97%). Our IMTA-based cage rearing system could be used to train the feeding ability of the cuttlefish being reared, facilitating their adaptation to the wild environment. From April to May, 1000 cuttlefish hatchlings and 8000 cuttlefish embryos were reared in a small plastic cage. The hatchlings were released after 1-day adaptation and demonstrated a high survival rate (98%; Figure 3F). The embryos hatched and swam out of their small plastic cage (0.9-cm mesh) naturally in 3–21 days (Figure 3E). We did not observe mass mortality during hatching.
3.5. Water quality for the cage rearing system with hatchery-produced organisms
During the trials, the values of pH were found to be consistently ranged between 8.2 and 8.4. We also did not detect the presence of ammonia nitrogen, nitrates, and nitrites, indicating that the cage rearing system with hatchery-produced organisms did not affect surrounding water quality.
4. Discussion
4.1. Fishing port transformation due to decline in fishing
With climate change, habitat destruction, and overfishing, fishery production, which is the primary income for fishing villages, has decreased sharply worldwide. The younger population that relies solely on traditional fishing has moved out of fishing villages in large numbers, which has resulting in the village communities aging rapidly. The unique natural and cultural resources of coastal fishing villages can aid in meeting the increasing demand for tourism (including ecotourism); the application of a sustainable approach can revitalize these fishing communities and even transform them into multifunctional areas including those for recreation tourism and education (Perry et al., 2011; McClanahan et al., 2015; Chen and Chang, 2017; Lee et al., 2021). Moreover, transforming traditional fishing areas into recreational fishing or oceanic sightseeing areas may increase the income of fishermen (Chen and Chang, 2017).
In addition to fishing villages, the functional transformation of a fishing port has begun to be noticed. Part of the underutilized fishing port can be replanned to meet the needs of tourism and sightseeing, such as waterfront recreation areas and yacht marinas (Huang et al., 2014; Yang et al., 2014) analyzed seven fishing ports in Keelung City, Taiwan, and their results suggested that the fishing ports with high fishing functions and utility should be repaired and maintained; the fishing ports suitable for tourism should be reconstructed in tourism planning. An underutilized fishing port with no function should be abandoned for natural restoration of the related coast.
In practice, however, not all fishing ports are suitable for these purposes. For instance, some fishing ports are close to roads and fishing villages, and thus, they cannot be abandoned for natural restoration. Fishing port areas have been transformed to accommodate aquaculture. In 2016, a stocking area for sea urchin (Mesocentrotus nudus) and sea cucumber (Apostichopus japonicus) was designated using cage culture at Suttsu Fishing Port, Hokkaido, Japan. In addition, longline facilities have been established in fishing ports for temporary oyster and clam cultivation (Sakurai et al., 2021). However, to the best of our knowledge, this is the first study globally to develop an IMTA-based cage rearing system and implement it at a fishing port; our results indicate that underutilized fishing ports can be utilized for stock enhancement.
4.2. Site selection and evaluation at an underutilized fishing port
In Taiwan, coastal fishery production has decreased by >50% to around 150,000 tons since 1990 according to the fisheries statistical yearbooks (Taiwan, Kinmen, and Matsu area). Most of Taiwan’s coastal fishery communities are eager to restore their fishery resources; therefore, the government has prioritized restoration policies, including large-scale stock enhancement, artificial reef creation, and further delineation of aquatic organism conservation areas. However, concerns over the recovery of coastal fishery resources include poor enforcement of illegal fishing, conflicts of interest between those promoting commercial fisheries and those promoting fishery resource recovery, and a lack of production statistics. Several fishery stakeholders have agreed that the sustainable development of fishing communities requires active community-oriented economic growth with ecological and marine resource sustainability (Hsiao and Chen, 2021).
On the northeast coast of Taiwan, the fishery production was 75,857 t in 2018, accounting for 41.14% of offshore fishery production in Taiwan (Hsiao and Chen, 2021). However, the fishing communities on the northeast coast gradually declined as catches declined. Moreover, this area is a crucial national scenic area with a unique natural landscape and has several aquatic organism conservation areas for stock enhancement as well as an MPA; therefore, it is a complex area compatible with fishery, tourism, and ecological conservation. Moreover, the residents in this area are engaged in fishing, collecting natural seaweed, and abalone farming; they also perform activities related to tourism and diving and other watersports. This area also has fishing ports with high utility, which have been transformed for tourism planning; some of the fishing ports have also been abandoned to restore the nature of the coast.
However, the Meiyanshan fishing port, used as the current study location, does satisfy these criteria. It has a small hinterland and few community residents, and it is close to a high-utility fishing port (approximately 2 km away), which has a nearly complete set of functions and thus has few fishing vessels docked. Moreover, the Meiyanshan fishing port is close to the main road and is surrounded by intertide farms for abalone, and it is unfeasible for sightseeing and demolition. In our assessment of fishing ports around Taiwan, this fishing port demonstrated comprehensive advantages along with strong consensus among the local government, fishermen’s associations, and local residents attributable to the distinct background of the area.
4.3. Importance and advantages of cage rearing system
Short-term acclimation in the cage net before fish release can improve the efficiency and survival of released fish (Fairchild et al., 2008; Rosenberger et al., 2013; Connerton et al., 2022). Because the hatchery environment often differs from that in the wild, typical factors include physical environmental features (e.g., temperature and water currents), nutrition, and feeding practices (Tanaka et al., 1998). A rearing system in the fishing port can enable hatchery-produced organisms to grow and adapt to the offshore environment and significantly reduce land-based farming costs. For instance, the high demand for live food in the cuttlefish hatchling stage and its domestication effects can be reduced through natural hatching and release. Moreover, most hatchery-produced organisms are sourced from private hatcheries in Southern Taiwan and often transported to the northern and outer islands of Taiwan for release. During the high-density, long-term transportation, transport and release associated stress may increase mortality. Short-term observation and acclimation in the rearing system can reduce mortality or prevent abnormal behavior. Moreover, moderate feeding before release can increase survival. In our study, short-term rearing led to good fry health, whereas long-term rearing enabled the fry to continue growing and adapting to the environment with high viability.
The impact and control of biofouling in cage culture can reduce water exchange efficiency and increase disease risk. However, the goals and management of an IMTA-based cage rearing system differ from those of cage aquaculture. Our IMTA-based cage rearing system included macroalgae, oysters, sea urchins, and dog conch, which effectively prevented residual bait and ammonia nitrogen waste pollution caused by feeding and reduce the need for physical removal. In the early stage, we mainly introduced macroalgae artificially, whereas in the later stage, natural macroalgae and sponges grew. Biofouling in an IMTA-based cage rearing system can provide a natural environment; however, when a considerable amount of biofouling occurs on the cage net and the small cage, physical removal is still required.
Because typhoons affect Taiwan annually, typhoon prevention capabilities can reduce the risk of damage to rearing cage systems at fish ports. For maintaining the IMTA system and reducing management costs, oysters and macroalgae should remain attached to the cage net. Moreover, during the rearing period for stock enhancement, the system should be suspended using a crane during typhoon season. We suspended the system once, and it did not affect our results even after the typhoon passed.
To the best of our knowledge, ours is the first IMTA-based cage rearing system used for stock enhancement at fishing ports worldwide. Its benefits could be extended to habitat creation, particularly for the relatively homogenous environment at fishing ports. Oysters are easy to establish and provide a habitat and foraging environment for fish; thus, they are ideal for habitat creation and complexity (Gilby et al., 2018). IMTA not only has little effect on the environment but also provides good shelter and habitat for released fish in the port and increases native species diversity. Therefore, introducing additional species of different trophic levels in the future is required to determine the most suitable combination in different regions and thus increase IMTA-based cage rearing system efficiency and function.
4.4. Transformation of fishing port of marine ecology from destruction to restoration
MPAs are essential for biodiversity conservation, with the primary goal of long-term protection of the environment; however, some MPAs may not afford identical ecological and social benefits (Sullivan-Stack et al., 2022). At present, MPA establishment is often considerably hindered by the activities of fishermen. Governments and other similar organizations worldwide have attempted to implore fishermen to seek alternative jobs or transition to conservation-related work; however, these attempts have failed mainly because most fishermen prefer to continue fishing (Cinner et al., 2009; Schuhbauer and Koch, 2013) and consider that alternative job opportunities unrelated to fishing may drive them away from their culture and roots (Cinner et al., 2009). Therefore, the goal of promoting the establishment of OECMs targeting fisheries is more feasible.
The support and involvement of local communities is essential for studies similar to ours (Ferse et al., 2010; Thornton and Scheer, 2012; Bennett and Dearden, 2014; Islam et al., 2017). To ensure that the local fishermen did not reject our project at the Meiyanshan fishing port because of protected area demarcation or conservation-related issues, we explained our aims and processes to all the local fishermen through the local fishery officials and fishermen’s associations at three different instances. We invited residents and fishermen to observe our cage rearing system and accepted their suggestions related to species for stock enhancement (e.g., striped breakperch). After checking and observing the rearing process voluntarily, the fishermen provided assistance in patrolling the facilities and fish. In addition, we sought assistance from the inspection office to make Tourists and anglers believe that our cage rearing system is official.
In the future, the participation of local residents and fishermen can be expanded to the management of cage rearing systems to increase their sense of identity and attention. Our IMTA-based cage rearing system, developed for the first time in this study for stock enhancement at fishing ports, may serve as a model for future application in other regions. Fishing ports also serve as a main location to perform life release–related activities for many religious locals; in the traditional Buddhist practice, life release is the practice of saving the lives of beings destined for slaughter. Our cage rearing system can also be used as a centralized life release location, which can be managed efficiently. As such, our system can increase the activities at underutilized fishing ports and create additional habitats for stock enhancement. Moreover, these fishing ports can move toward sustainable utilization and increase fishery benefits and marine resources.
5. Conclusion
In the present study, we have proposed a novel IMTA-based cage rearing system required for marine stock enhancement. Unlike the conventional rearing system, multiple species with different trophic levels are reared in this novel rearing system that can reduce organic matter and environmental impact accumulation as evidenced by the steady water quality (stable pH and undetectable levels of ammonia nitrogen, nitrates, and nitrites). More importantly, it also could provide better fish welfare by reducing the discomfort after transportation and facilitating adaptation to the wild conditions as evidenced by positive growth and high survival rates (94%–98%). Furthermore, we have demonstrated it is feasible to implement this novel rearing system specifically in those fishing harbors with low utilization rates. With this novel rearing system, the release of hatchery-produced organisms can be monitored more appropriately, including their behavior, growth, and survival.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of National Taiwan Ocean University (NTOU).
Author contributions
Conceptualization, Y-CC; Methodology, Y-CC, H-TL and T-HH; Software, Y-CC, H-TL and T-HH; Validation, Y-CC, H-TL, C-HL and T-HH; Formal analysis, Y-CC, H-TL, and T-HH; Investigation, Y-CC and H-TL; Resources, C-HL and T-HH; Data curation, H-TL and T-HH; Writing—original draft preparation, H-TL and T-HH Writing—review and editing, H-TL and T-HH; Visualization, Y-CC, H-TL and T-HH; Supervision, C-HL and T-HH; Project administration, C-HL and T-HH; and Funding acquisition, C-HL and T-HH; All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the New Taipei City Council (110325A, 1100603A) and the Center of Excellence for the Oceans (National Taiwan Ocean University), which were financially supported by the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education, ROC (Taiwan). This work was also supported by the Taiwan Ocean Conservation and Fisheries Sustainability Foundation (110toffrest001, 111toffrest001).
Acknowledgments
We would like to acknowledge the comments on the development of IMTA–based cage rearing system from Professor Kuo-Tien Lee. We also thank Yi-Chun Yu and all colleagues of Gongliao Aqua Center for their encouragements and supports of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.998198/full#supplementary-material
Supplementary Figure 1 | Seawater temperature in Northeast National Scenic Area (source: Central Weather Bureau, Taiwan).
References
Bennett N. J., Dearden P. (2014). Why local people do not support conservation: Community perceptions of marine protected area livelihood impacts, governance and management in Thailand. Mar. Policy 44, 107–116. doi: 10.1016/j.marpol.2013.08.017
Biswas G., Kumar P., Ghoshal T. K., Kailasam M., De D., Bera A., et al. (2020). Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity and economic return in brackishwater ponds. Aquaculture 516, 734626. doi: 10.1016/j.aquaculture.2019.734626
Chang Y.-C., Ma C.-H., Lee H.-T., Hsu T.-H. (2021). Polyculture of juvenile dog conch laevistrombus canarium reveals high potentiality in integrated multitrophic aquaculture (IMTA). Biology 10, 812. doi: 10.3390/biology10080812
Chen C.-L., Chang Y.-C. (2017). A transition beyond traditional fisheries: Taiwan’s experience with developing fishing tourism. Mar. Policy 79, 84–91. doi: 10.1016/j.marpol.2017.02.011
Chuang C.-T., Guinea H. E., Kuo H. H., Hsu C.-H. (2010). Evaluating the performance of artificial reef deployment projects in Taiwan. J. Fisheries Soc. Taiwan 37, 217–230. doi: 10.29822/JFST.201009.0006
Cinner J. E., Daw T., McClanahan T. R. (2009). Socioeconomic factors that affect artisanal fishers’ readiness to exit a declining fishery. Conserv. Biol., 23 (1), 124–130. doi: 10.1111/j.1523-1739.2008.01041.x
Connerton M. J., Lantry J. R., Bronte C. R., Lapan S. R. (2022). Origin, postrelease survival, and imprinting of pen-acclimated and direct-stocked Chinook salmon in lake Ontario. North Am. J. Fisheries Manage. 42, 713–740. doi: 10.1002/nafm.10756
Council of Agriculture. (2020). 110-113 Fishery Sustainable Management Infrastructure Project. Taipei, Taiwan: Council of Agriculture. Available at: https://www.coa.gov.tw/ws.php?id=2508891
Fairchild E. A., Rennels N., Howell W. H. (2008). Predators are attracted to acclimation cages used for winter flounder stock enhancement. Rev. Fisheries Sci. 16, 262–268. doi: 10.1080/10641260701678181
Fang J., Zhang J., Xiao T., Huang D., Liu S. (2016). Integrated multi-trophic aquaculture (IMTA) in sanggou bay, China. Aquaculture Environ. Interact. 8, 201–205. doi: 10.3354/aei00179
Ferse S. C., Costa M. M., Manez K. S., Adhuri D. S., Glaser M. (2010). Allies, not aliens: increasing the role of local communities in marine protected area implementation. Environ. Conserv. 37, 23–34. doi: 10.1017/S0376892910000172
Fisheries Agency. (1981–2021) Fisheries statistical yearbook 1980–2020, Taiwan, kinmen and matsu area (Taipei: Fisheries Agency).
Gilby B. L., Olds A. D., Peterson C. H., Connolly R. M., Voss C. M., Bishop M. J., et al. (2018). Maximizing the benefits of oyster reef restoration for finfish and their fisheries. Fish Fisheries 19, 931–947. doi: 10.1111/faf.12301
Gurney G. G., Darling E. S., Ahmadia G. N., Agostini V. N., Ban N. C., Blythe J., et al. (2021). Biodiversity needs every tool in the box: use OECMs. Nature 595, 646–649. doi: 10.1038/d41586-021-02041-4
Hsiao Y.-J., Chen J.-L. (2021). Different perspectives of stakeholders on the sustainable development of fishery-based communities in northeast Taiwan. Mar. Policy 130, 104576. doi: 10.1016/j.marpol.2021.104576
Hsu T.-H., Huang C.-W., Lee H.-T., Kuo Y.-H., Liu K.-M., Lin C.-H., et al. (2020a). Population genetic analysis for stock enhancement of silver sea bream (Rhabdosargus sarba) in Taiwan. Fishes 5, 19. doi: 10.3390/fishes5020019
Hsu T.-H., Huang C.-W., Lin C.-H., Lee H.-T., Pan C.-Y. (2020b). Tracing the origin of fish without hatchery information: genetic management of stock enhancement for mangrove red snapper (Lutjanus argentimaculatus) in Taiwan. Fisheries Aquat. Sci. 23, 1–7. doi: 10.1186/s41240-020-00156-9
Hsu T.-H., Lee H.-T., Lu H.-J., Liao C.-H., Gong H.-Y., Huang C.-W. (2022). Maintenance of genetic diversity of black Sea bream despite unmonitored and Large-scale hatchery releases. Biology 11, 554. doi: 10.3390/biology11040554
Huang W.-C., Kao S.-K., Lin W.-H. (2014). Research on the transformation in Taiwan fishing ports: A case study of keelung city. J. Fisheries Soc. Taiwan 41, 145–163. doi: 10.29822/JFST.201409_41(3).0001
Islam G. M. N., Tai S. Y., Kusairi M. N., Ahmad S., Aswani F. M. N., Senan M. K. A. M., et al. (2017). Community perspectives of governance for effective management of marine protected areas in Malaysia. Ocean Coast. Manage. 135, 34–42. doi: 10.1016/j.ocecoaman.2016.11.001
Jonas H. D., Barbuto V., Jonas H. C., Kothari A., Nelson F. (2014). New steps of change: looking beyond protected areas to consider other effective area-based conservation measures. Parks 20, 111–128. doi: 10.2305/IUCN.CH.2014.PARKS-20-2.HDJ.en
Jones K. R., Klein C. J., Grantham H. S., Possingham H. P., Halpern B. S., Burgess N. D., et al. (2020). Area requirements to safeguard earth’s marine species. One Earth 2, 188–196. doi: 10.1016/j.oneear.2020.01.010
Lee S., Kim D., Park S., Lee W. (2021). A study on the strategic decision making used in the revitalization of fishing village tourism: Using A’WOT analysis. Sustainability 13, 7472. doi: 10.3390/su13137472
Liu W.-H. (2013). Managing the offshore and coastal fisheries in Taiwan to achieve sustainable development using policy indicators. Mar. Policy 39, 162–171. doi: 10.1016/j.marpol.2012.11.001
McClanahan T., Allison E. H., Cinner J. E. (2015). Managing fisheries for human and food security. Fish Fisheries 16, 78–103. doi: 10.1111/faf.12045
Palomares M., Pauly D. (2019). On the creeping increase of vessels’ fishing power. Ecol. Soc. 24 (3), 31. doi: 10.5751/ES-11136-240331
Pauly D. (2009). Beyond duplicity and ignorance in global fisheries. Sci. Mar. 73, 215–224. doi: 10.3989/scimar.2009.73n2215
Pauly D., Zeller D. (2016). Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7, 1–9. doi: 10.1038/ncomms10244
Perry R. I., Ommer R. E., Barange M., Jentoft S., Neis B., Sumaila U. R. (2011). Marine social–ecological responses to environmental change and the impacts of globalization. Fish Fisheries 12, 427–450. doi: 10.1111/j.1467-2979.2010.00402.x
Rosenberger S. J., Connor W. P., Peery C. A., Milks D. J., Schuck M. L., Hesse J. A., et al. (2013). Acclimation enhances postrelease performance of hatchery fall Chinook salmon subyearlings while reducing the potential for interaction with natural fish. North Am. J. Fisheries Manage. 33, 519–528. doi: 10.1080/02755947.2013.768567
Sakurai I., Mori T., Hisatomi Y. (2021). Experimental study on the possibility of a suspended culture of the Japanese littleneck clam ruditapes philippinarum in suttsu fishing port, Hokkaido. Fisheries Sci. 87, 239–251. doi: 10.1007/s12562-021-01494-w
Schiermeier Q. (2002). Fisheries science: how many more fish in the sea? Nature 419, 662–666. doi: 10.1038/419662a
Schuhbauer A., Koch V. (2013). Assessment of recreational fishery in the Galapagos marine reserve: Failures and opportunities. Fisheries Res. 144, 103–110. doi: 10.1016/j.fishres.2013.01.012
Shpigel M., Shauli L., Odintsov V., Ben-Ezra D., Neori A., Guttman L. (2018). The sea urchin, paracentrotus lividus, in an integrated multi-trophic aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 490, 260–269. doi: 10.1016/j.aquaculture.2018.02.051
Sullivan-Stack J., Aburto-Oropeza O., Brooks C. M., Cabral R., Caselle J. E., Chan F., et al. (2022). A scientific synthesis of marine protected areas in the united states: status and recommendations. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.849927
Sumaila U. R., Tai T. C. (2020). End overfishing and increase the resilience of the ocean to climate change. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00523
Tanaka M., Seikai T., Yamamoto E., Furuta S. (1998). Significance of larval and juvenile ecophysiology for stock enhancement of the Japanese flounder, paralichthys olivaceus. Bull. Mar. Sci. 62, 551–571.
Thornton T. F., Scheer A. M. (2012). Collaborative engagement of local and traditional knowledge and science in marine environments: a review. Ecol. Soc. 17 (3), 8. doi: 10.5751/ES-04714-170308
Yang C.-M., Lai C.-C., Wu L.-J., Li J.-J. (2014). Assessing fishing port locations for adaption into yacht marinas in Taiwan. J. Mar. Sci. Technol. 22, 10. doi: 10.6119/JMST-013-1029-1
Keywords: hatchery release, religious release, restoration, coastal fisheries, growth, survival
Citation: Lee H-T, Chang Y-C, Liao C-H and Hsu T-H (2022) Development of integrated multitrophic aquaculture–based cage rearing system in an underutilized fishing port and its application in marine stock enhancement. Front. Mar. Sci. 9:998198. doi: 10.3389/fmars.2022.998198
Received: 19 July 2022; Accepted: 05 December 2022;
Published: 19 December 2022.
Edited by:
MD Wahab, WorldFish, BangladeshReviewed by:
J. Emmett Duffy, Smithsonian Environmental Research Center (SI), United StatesCicilia Selviane Kambey, Sea Six Energy Bali Indonesia, Indonesia
Copyright © 2022 Lee, Chang, Liao and Hsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Te-Hua Hsu, cmVhbGdpZ2lAbWFpbC5udG91LmVkdS50dw==
†These authors have contributed equally to this work
 Hung-Tai Lee
Hung-Tai Lee Yung-Cheng Chang
Yung-Cheng Chang Cheng-Hsin Liao1,4
Cheng-Hsin Liao1,4 Te-Hua Hsu
Te-Hua Hsu