- 1Department of Environmental and Marine Biology, Åbo Akademi University, Turku, Finland
- 2Department for Evolutionary Theory, Max Planck Institute for Evolutionary Biology, Plön, Germany
- 3Sorbonne Université-CNRS, Laboratoire d’Océanographie de Villefranche, Villefranche, France
- 4The Swire Institute of Marine Science and School of Biological Sciences, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 5Department of Marine Sciences - Tjärnö, University of Gothenburg, Strömstad, Sweden
- 6Department of Marine Ecology, GEOMAR Helmholtz Centre for Ocean Research, Kiel, Germany
- 7School of Biological Sciences, Victoria University of Wellington, Kelburn, Wellington, New Zealand
- 8Coastal People; Southern Skies, Centre of Research Excellence, Victoria University of Wellington, Kelburn, Wellington, New Zealand
Editorial on the Research Topic
Influence of environmental variability on climate change impacts in marine ecosystems
Phenotypic responses and selection in populations that lead to ecosystem shifts are often driven by environmental variability and extremes (Grant et al., 2017; Al-Janabi et al., 2019). Marine environments, particularly coastal and shallow-water habitats, experience strong fluctuations of abiotic drivers at multiple temporal scales (Boyd et al., 2016; Pansch and Hiebenthal, 2019). This environmental variability can arise from biological activity, irradiance variation, tides, weather-driven changes in water level, waves, and up/downwelling events, but also from seasonal, annual, and semi-decadal cycles such as El Niño and La Niña (Boyd et al., 2016; Choi et al., 2019). Diurnal and seasonal temperature fluctuations (driven by irradiation cycles) form basic cycles on top of which stochastic processes operate (Lima and Wethey, 2012; Wang and Dillon, 2014). These include marine heatwaves (MHWs; Oliver et al., 2018), which can have strong and lasting negative impacts on the physiology of marine species and the composition of entire ecosystems (Pansch et al., 2018; Smale et al., 2019). Likewise, the seawater carbonate system (and oxygen tension) varies in response to the same drivers (Hofmann et al., 2011), although this can be more strongly influenced by the metabolism of organisms in areas with high biomass and high seawater residence times (Rivest et al., 2017; Noisette et al., 2022).
There has been increasing research interest in how organisms respond to environmental variability and how this will interact with future effects of anthropogenic climate change. In habitats where organisms are near, or at, their physiological limits, environmental variability will exacerbate future effects of climate change (Cornwall et al., 2018; Morón Lugo et al., 2020). Alternatively, some fluctuations and cycles can mimic the mean conditions expected due to ongoing ocean warming or acidification, which can precondition resident taxa to greater tolerance (Pansch et al., 2014; Vargas et al., 2017). In all cases, environmental variability can lead to periods where environmental conditions are favorable (Cornwall et al., 2014; Wahl et al., 2018; Vajedsamiei et al., 2021), and variability has therefore been proposed to also provide temporal refugia in a future ocean.
This special issue hosts a range of research that explores concepts relating to environmental variability and climate change. From microbes to corals, and microns to ocean basins, these studies explore the effects of variability in light, temperature, and seawater carbonate chemistry.
Including environmental variability in our projections is essential if we are to accurately assess the impacts of climate change. McClanahan et al. demonstrate that projections of the impacts of MHWs and thermal stress are much more accurate if models include components of variability such as bimodality and standard deviation in temperature, rather than more simplistic assumptions of cumulative thermal stress such as the degree heating day/week models (Maynard et al., 2008; Cornwall et al., 2021). MHWs are complex and comprise different components, such as intensity, duration, and frequency, that will all change in the future (Frölicher et al., 2018). Wolf et al. demonstrate that longer continual MHWs have stronger negative effects on sea star physiology than equivalent MHWs that are broken up into several shorter periods of heat stress (and therefore allow for recovery from stress). Vajedsamiei et al. demonstrate that MHWs may select for mussel recruits with higher heat tolerance and such tolerance may be mediated by lower metabolic demand, but also that mussels’ capacity for beneficial heat acclimation is limited. Samuels et al. indicate that MHW intensity controls responses of diatoms, but that past thermal history and genotypic differences modulate responses further. Collectively, these works exemplify the complexity of MHWs, and show that projections based on traditional research models of slow onset of thermal stress (simulating ocean warming/acidification), or which ignored sequential phases of impact and recovery, should be considered with caution.
Historical variability in temperature can affect species’ responses to MHWs or ongoing ocean warming, such that populations exposed to greater extremes in temperatures often have higher thermal tolerances than populations of the same species not exposed to such extremes (Schoepf et al., 2015; Marshall et al., 2018; Moyen et al., 2020). Kunze et al. demonstrate that responses of phytoplankton to temperature fluctuations vary depending on the frequency of the fluctuations, with faster fluctuations having more positive outcomes. Hennigs et al. indicate that the responses of mysids to temperature fluctuations are dependent on their sex, with females being metabolically more active than males, and female ingestion rates being negatively impacted by temperature fluctuations. Jimenez et al. find sex-related responses to temperature fluctuations in intertidal crabs, where thermal extremes but not latitudinal gradients explain the responses. These three studies collectively reinforce earlier findings that the impacts of thermal variability in experimental work and the impacts of past exposure to temperature variability are complex and context-dependent and that these cannot easily be generalized. This complexity must be well understood and acknowledged if we are to accurately project the impacts of climate change (McClanahan et al.). In this issue, Sylvester et al. support this premise, showing that it is difficult to tease apart the response of krill population growth to ocean warming from responses to the large natural temperature variability in the Southern Ocean. Further complications arise when accounting for the impacts of environmental variability on microbial adaptive plasticity and evolution. The review by Arromrak et al. indicates the importance of these aspects, but also points out the apparent knowledge gaps around the interplay of climate change, environmental variability, and adaptation, as a vast majority of past research has focused on single drivers applying constant environmental regimes.
Variability in drivers besides temperature, such as light, water motion, and seawater carbonate chemistry, are also important for the physiology of marine species. For example, the quality and quantity of fluctuating light can radically alter the physiology of photosynthetic species. Neun et al. demonstrate that responses to light variability of phytoplankton are species-specific. Light also modifies indirectly the seawater chemistry experienced by many marine organisms in nearshore ecosystems. In an extreme example, Houlihan et al. show that increasing light (and therefore photosynthesis) and decreasing water velocity increase pH and the thickness of the diffusion boundary layer (Hurd et al., 2011; Cornwall et al., 2015; Noisette et al., 2022) of crustose coralline algae, which represents µm to mm habitats in which sea urchin larvae settle. However, during the night, respiration processes impose contrasting pH shifts leading to strong diurnal pH variability.
This special issue highlights the complex and context-dependent nature of environmental variability. It shows that responses to variability cannot be generalized from concepts such as Jensen’s inequality and from experimental studies applying constant environmental regimes. In addition, previous experience (carry-over effects, stress memory and cross-stress tolerance, ecological memory, or trans-generational plasticity; Jackson et al., 2021) can all change organisms’ responses to (multiple) fluctuating drivers. Nonetheless, environmental variability is fundamentally important and must be considered in future research that investigates the impacts of climate change on marine taxa.
We propose (Figure 1) future research combining experimental and conceptual/theoretical studies that investigate the effects of multiple (including biotic) fluctuating environmental drivers across relevant spatial and temporal scales on marine organisms and ecosystems. Ideally, drivers would be applied along a species’ performance gradient, i.e., from optimal to stressful conditions. Experiments testing whether performance curves are adaptive in the short- (via phenotypic plasticity) or the long-run (by evolution) will be a highly valuable contribution to the existing literature. Thus, the complexity of this rapidly growing research field may be overcome only by integrating disciplines (from physiology and evolutionary biology to behavioral biology and ecology) and by combining studies from the Petri dish to large-scale mesocosm infrastructure and field observations across environmental gradients.
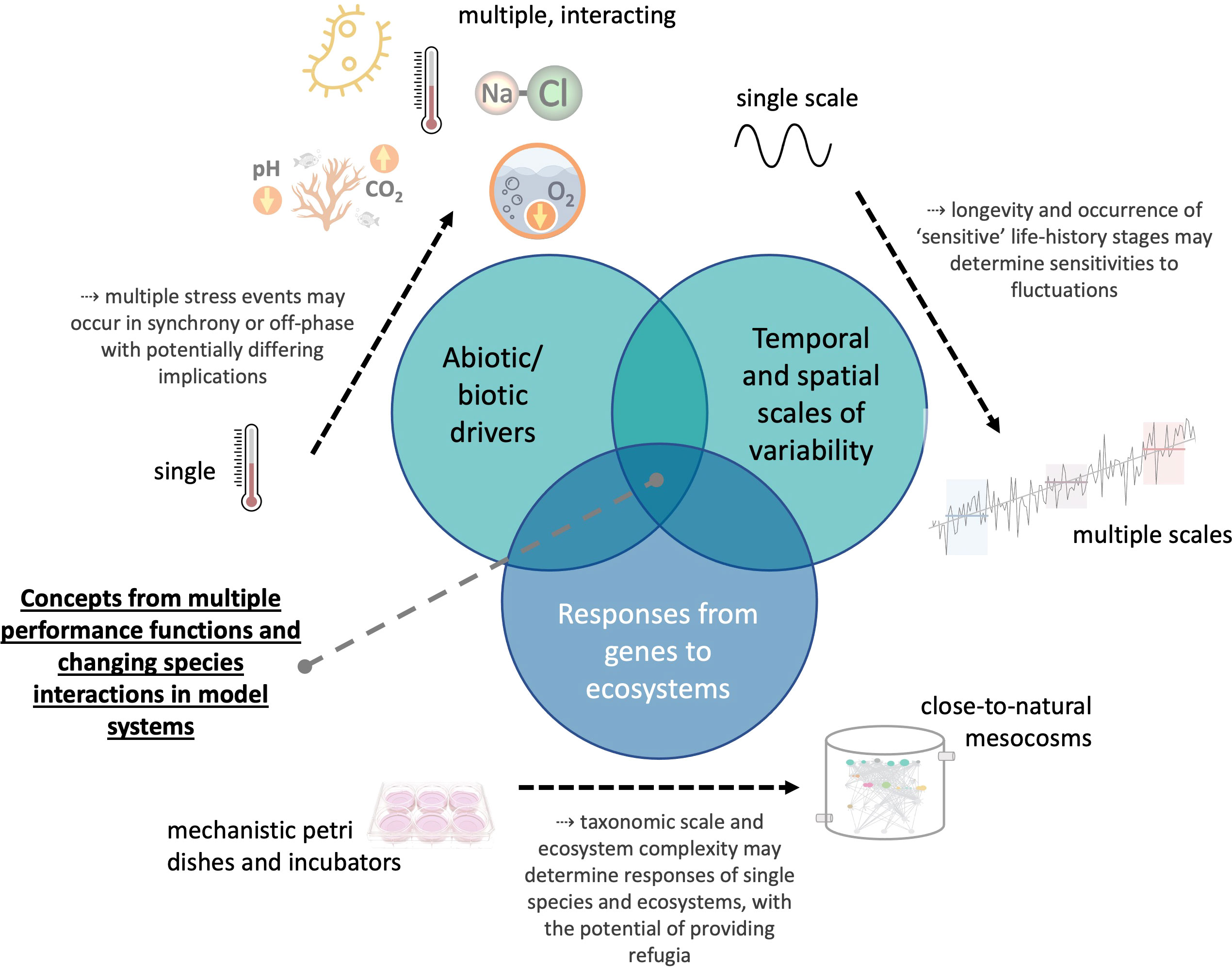
Figure 1 Aspects to be considered in theoretical to experimental research that aims to delineate the consequences of environmental variability and climate change for marine life.
Author contributions
CP initiated this topic. CP and CC framed the text and CP drafted the figure. All authors contributed substantially to the editorial process of this topic and the article and approved the submitted version.
Funding
CEC was funded by a Rutherford Discovery Fellowship from the Royal Society of New Zealand Te Apārangi.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Janabi B., Wahl M., Karsten U., Graiff A., Kruse I. (2019). Sensitivities to global change drivers may correlate positively or negatively in a foundational marine macroalga. Sci. Rep. 9, 14653. doi: 10.1038/s41598-019-51099-8
Boyd P. W., Cornwall C. E., Davison A., Doney S. C., Fourquez M., Hurd C. L., et al. (2016). Biological responses to environmental heterogeneity under future ocean conditions. Global Change Biol. 22, 2633–2650. doi: 10.1111/gcb.13287
Choi F., Gouhier T., Lima F., Rilov G., Seabra R., Helmuth B. (2019). Mapping physiology: biophysical mechanisms define scales of climate change impacts. Conserv. Physiol. 7, coz028. doi: 10.1093/conphys/coz028
Cornwall C. E., Boyd P. W., McGraw C. M., Hepburn C. D., Pilditch C. A., Morris J. N., et al. (2014). Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PloS One 9, e97235. doi: 10.1371/journal.pone.0097235
Cornwall C. E., Comeau S., DeCarlo T. M., Moore B., D'Alexis Q., McCulloch M. T. (2018). Resistance of corals and coralline algae to ocean acidification: physiological control of calcification under natural pH variability. Proc. R. Soc. B: Biol. Sci. 285, 20181168. doi: 10.1098/rspb.2018.1168
Cornwall C. E., Comeau S., Kornder N. A., Perry C. T., van Hooidonk R., DeCarlo T. M., et al. (2021). Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc. Natl. Acad. Sci. 118, e2015265118. doi: 10.1073/pnas.2015265118
Cornwall C. E., Pilditch C. A., Hepburn C. D., Hurd C. L. (2015). Canopy macroalgae influence understorey corallines' metabolic control of near-surface pH and oxygen concentration. Mar. Ecol. Prog. Ser. 525, 81–95. doi: 10.3354/meps11190
Frölicher T. L., Fischer E. M., Gruber N. (2018). Marine heatwaves under global warming. Nature 560, 360–364. doi: 10.1038/s41586-018-0383-9
Grant P. R., Grant B. R., Huey R. B., Johnson M. T. J., Knoll A. H., Schmitt J. (2017). Evolution caused by extreme events. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160146. doi: 10.1098/rstb.2016.0146
Hofmann G. E., Smith J. E., Johnson K. S., Send U., Levin L. A., Micheli F., et al. (2011). High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PloS One 6, e28983. doi: 10.1371/journal.pone.0028983
Hurd C. L., Cornwall C. E., Currie K. I., Hepburn C. D., McGraw C. M., Hunter K. A., et al. (2011). Metabolically-induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Global Change Biol. 17, 3254–3262. doi: 10.1111/j.1365-2486.2011.02473.x
Jackson M. C., Pawar S., Woodward G. (2021). The temporal dynamics of multiple stressor effects: From individuals to ecosystems. Trends Ecol. Evol. 36, 402–410. doi: 10.1016/j.tree.2021.01.005
Lima F. P., Wethey D. S. (2012). Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 3, 704. doi: 10.1038/ncomms1713
Marshall D. J., Brahim A., Mustapha N., Dong Y., Sinclair B. J. (2018). Substantial heat tolerance acclimation capacity in tropical thermophilic snails, but to what benefit? J. Exp. Biol. 221, jeb187476. doi: 10.1242/jeb.187476
Maynard J. A., Turner P. J., Anthony K. R. N., Baird A. H., Berkelmans R., Eakin C. M., et al. (2008). ReefTemp: An interactive monitoring system for coral bleaching using high-resolution SST and improved stress predictors. Geophysical Res. Lett. 35, L05603. doi: 10.1029/2007GL032175
Morón Lugo S. C., Baumeister M., Nour O. M., Wolf F., Stumpp M., Pansch C. (2020). Warming and temperature variability determine the performance of two invertebrate predators. Sci. Rep. 10, 6780. doi: 10.1038/s41598-020-63679-0
Moyen N. E., Crane R. L., Somero G. N., Denny M. W. (2020). A single heat-stress bout induces rapid and prolonged heat acclimation in the California mussel, Mytilus californianus. Proc. R. Soc. B: Biol. Sci. 287, 20202561. doi: 10.1098/rspb.2020.2561
Noisette F., Pansch C., Wall M., Wahl M., Hurd C. L. (2022). Role of hydrodynamics in shaping chemical habitats and modulating the responses of coastal benthic systems to ocean global change. Global Change Biol. 28, 3812–3829. doi: 10.1111/gcb.16165
Oliver E. C. J., Donat M. G., Burrows M. T., Moore P. J., Smale D. A., Alexander L. V., et al. (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9, 1324. doi: 10.1038/s41467-018-03732-9
Pansch C., Hiebenthal C. (2019). A new mesocosm system to study the effects of environmental variability on marine species and communities. Limnol Oceanography-Methods 17, 145–162. doi: 10.1002/lom3.10306
Pansch C., Schaub I., Havenhand J., Wahl M. (2014). Habitat traits and food availability determine the response of marine invertebrates to ocean acidification. Global Change Biol. 20, 765–777. doi: 10.1111/gcb.12478
Pansch C., Scotti M., Barboza F. R., Al-Janabi B., Brakel J., Briski E., et al. (2018). Heat waves and their significance for a temperate benthic community: A near-natural experimental approach. Global Change Biol. 24, 4357–4367. doi: 10.1111/gcb.14282
Rivest E. B., Comeau S., Cornwall C. E. (2017). The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Climate Change Rep. 3, 271–281. doi: 10.1007/s40641-017-0082-x
Schoepf V., Stat M., Falter J. L., McCulloch M. T. (2015). Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17639–17639. doi: 10.1038/srep17639
Smale D. A., Wernberg T., Oliver E. C. J., Thomsen M., Harvey B. P., Straub S. C., et al. (2019). Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Climate Change 9, 306–312. doi: 10.1038/s41558-019-0412-1
Vajedsamiei J., Melzner F., Raatz M., Morón Lugo S. C., Pansch C. (2021). Cyclic thermal fluctuations can be burden or relief for an ectotherm depending on fluctuations’ average and amplitude. Funct. Ecol. 35, 2483–2496. doi: 10.1111/1365-2435.13889
Vargas C. A., Lagos N. A., Lardies M. A., Duarte C., Manríquez P. H., Aguilera V. M., et al. (2017). Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 1, 0084. doi: 10.1038/s41559-017-0084
Wahl M., Covacha S. S., Saderne V., Hiebenthal C., Muller J. D., Pansch C., et al. (2018). Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol Oceanography 63, 3–21. doi: 10.1002/lno.10608
Keywords: multiple drivers, environmental variability, Climate change, marine heatwaves, stress memory, Ecological memory, Thermal performance curves, acclimation
Citation: Pansch C, Raatz M, Comeau S, Hui TTY, Havenhand JN, Vajedsamiei J and Cornwall CE (2022) Editorial: Influence of environmental variability on climate change impacts in marine ecosystems. Front. Mar. Sci. 9:994756. doi: 10.3389/fmars.2022.994756
Received: 15 July 2022; Accepted: 27 July 2022;
Published: 11 August 2022.
Edited and Reviewed by:
Rui Rosa, University of Lisbon, PortugalCopyright © 2022 Pansch, Raatz, Comeau, Hui, Havenhand, Vajedsamiei and Cornwall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Pansch, Y2gucGFuc2NoQGdtYWlsLmNvbQ==
 Christian Pansch
Christian Pansch Michael Raatz
Michael Raatz Steeve Comeau
Steeve Comeau Tommy T. Y. Hui
Tommy T. Y. Hui Jonathan N. Havenhand
Jonathan N. Havenhand Jahangir Vajedsamiei
Jahangir Vajedsamiei Christopher E. Cornwall
Christopher E. Cornwall