- 1Shandong Provincial Key Laboratory of Restoration for Marine Ecology, Shandong Marine Resource and Environment Research Institute, Yantai, China
- 2Resource Conservation Department, Yantai Marine Economic Research Institute, Yantai, China
- 3College of Marine Science, Shanghai Ocean University, Shanghai, China
Four cruises in the Bohai Sea and northern Yellow Sea off Yantai, China, in April and August of 2020, collected data on the species composition and community structure characteristics of macrobenthos in these sea areas. Temporal and spatial changes in the dominant macrobenthic species between April and August were also analyzed. The M-AMBI and environmental data were used to assess the benthic ecological quality of the coastal waters off Yantai. The results revealed differences in macrobenthic community structure and ecological quality status between the two sea areas. The main findings are as follows: A total of 7 phyla and 153 macrobenthic species were collected; 113 and 101 species were recorded in the Bohai Sea and northern Yellow Sea areas, respectively, and estimated abundance was 301 ind. m-2 and 598 ind. m-2 (p < 0.01), and biomass was 10.20 g m-2 and 14.65 g m-2, respectively. The dominant species comprised small-sized polychaetes and bivalves. The main dominant species were Glycinde bonhourei, Micronephthys oligobranchia, Sternaspis chinensis, and Moerella hilaris. The number of species differed significantly between seasons (p < 0.05). A comparison of the dominant species and community structure of the macrobenthos between seasons showed that more obvious replacement occurred in the Bohai Sea. There were major differences in community structure of the macrobenthos between the two areas. Macrobenthos abundance was positively correlated with depth, dissolved oxygen, and sand substrate, and negatively correlated with bottom-water temperature, pH, and fine silt or clay substrate. In April, sites with low ecological value were at the ports of Laizhou and Longkou and Yangma Island, with the water quality likely affected by intensive bivalve aquaculture, port activities, and river discharge; in August, low-ecological-value sites were the middle of Laizhou Bay, Longkou Port, west of Daheishan Island, and coastal water between the cities of Yantai and Weihai. Overall, the sea areas off Yantai were generally deemed to be in a “good” state of ecological quality.
Introduction
Benthos are the aquatic animal groups that inhabit bottom substates or sediments during all or part of their life history; those that do not pass through a 0.5-mm mesh screen can be defined as macrobenthos. Macrobenthos are an important part of marine ecosystems. Macrobenthos change sediment particles through bioturbations caused by feeding, burrowing, and tube-building; they can also change the surrounding topography by producing mucus, increasing or decreasing the roughness of the sediment, altering the original structure of the sediment and the biological characteristics of the microorganisms, thereby affecting the marine nutrient environment. At the same time, the macrobenthic community participates in the biogeochemical cycling of elements such as carbon, nitrogen, and sulfur; they provide an important source of food for animals in the bottom-most layer as well as in the whole column of the water, and through feeding and predation they participate in establishing the structure and function of the marine ecosystem (Somerfield et al., 2006; Liu et al., 2007; Liu et al., 2009) . For example, the annual average biomass of macrobenthos in the northern Yellow Sea can reach 99.66 g/m2, which provides a high degree of secondary productivity and biological diversity. At least 10 categories and 800 species of macrobenthos have been recorded in the Yellow Sea and Bohai Sea (Xu and Li, 2021).
Research on macrobenthos as ecological indicators is drawing increasing attention in the field of ecology. Compared with that of zooplankton and fish, benthic fauna have advantages in the ecological indicator function based on their weak movement ability, relatively fixed habitat, and direct vulnerability to environmental changes (Lv et al., 2014). Habitat evaluations focused on benthic fauna have been introduced into environmental monitoring work in many countries, with numerous associated ecological assessment methods recognized (International Organisation for Standardisation (ISO), 1979). Consequently, biotic indices using macrobenthos have been used to evaluate the health of coastal ecosystems (Xu and Li, 2021). The research institute AZTI in Spain (AZTI-Tecnalia) developed a marine biotic index (AMBI) method that is based on the relative habitat density of macrobenthos and divides macrobenthos into five ecological groups, ranging from sensitive to opportunistic, based on their sensitivity to environmental stress. Thus, each sample can obtain a continuous AMBI value and corresponding ecological quality status (Tian, 2012; Liu et al., 2014a). Studies have shown that in some areas, such as characterized by hypoxia, industrial pollution, oil extraction or aquaculture, AMBI can clearly indicate the degree of disturbance to benthic ecosystems, and the evaluation results are more accurate than with other indices affected by the dominant species. However, the AMBI metric can be limited in areas with strong natural pressures or poor biomes, such as inland estuaries or subtidal zones on sandy coasts (Luo and Yang, 2009; Lu et al., 2021). Owing to the pressure of natural factors, although not affected by human activities, samples with fewer species and higher AMBI values may also be defined as denoting poor ecological quality. To overcome this potential weakness and misjudgment problem of AMBI in evaluating ecological status, Muxika et al. (2007) proposed the multivariate AMBI (M-AMBI), which adds biodiversity and species number indicators (Borja et al., 2000). This approach uses a variety of indices to evaluate the quality of the study area, and defines a reference point that can better describe the ecological quality of the study area.
Yantai is a port city on the north coast of the Shandong Peninsula in eastern China and is situated at the junction of the Bohai Sea and Yellow Sea. Since ancient times, the northern sea areas off Yantai have been important spawning and feeding grounds and a migration channel for China’s fishery resources, and the region is an important marine aquaculture base (Liu et al., 2015). In recent years, with the rapid development of the coastal economy at Yantai, the ecological quality of the coastal waters has been seriously affected. The main reasons can be summarized as follows: (1) pollution caused by aquaculture activities, with high-density raft aquaculture altering the environment of the sediments, and aquaculture facilities affecting sea hydrology and current conditions, resulting in organic enrichment and low dissolved oxygen; (2) disturbance caused by high-intensity commercial fishing activities, including excessive trawling and destructive fishing gear, which leads to frequent disturbance of the sea bottom, and simplification and fragmentation of the food web; (3) port- and land-derived pollution sources, such as wastewater, fuel oil, and heavy metals. The coastal waters also receive sewage from domestic, industrial, and agricultural activities. In addition, the northern Yellow Sea and especially the Bohai Sea are relatively closed geographical features, which results in slow water-exchange rates with the ocean and poor self-purification capacity (Liu et al., 2014b).
Research on the community structure of macrobenthos and the ecological quality of sea areas in the region off Yantai has largely focused on areas at a small scale, such as important bays (Li et al., 2013; Wang and Li, 2013; Li et al., 2016) and intertidal zones (Han et al., 2014; Yu and Liu, 2020). Therefore, the purpose of this study was to: (1) identify the community structure characteristics of macrobenthos in large-scale sea areas off Yantai, and analyze the temporal and spatial changes in April and August; (2) combine M-AMBI with environmental factors to assess the benthic ecological quality of the Bohai Sea and northern Yellow Sea areas, and analyze differences in macrobenthic community structure and ecological health between the two sea areas. The findings should improve our understanding of the scope and extent of the human impact on the marine resources off Yantai, China, and contribute to scientifically based evaluation of the health of these coastal waters and sustainable utilization of the biological resources.
Material and methods
Study area
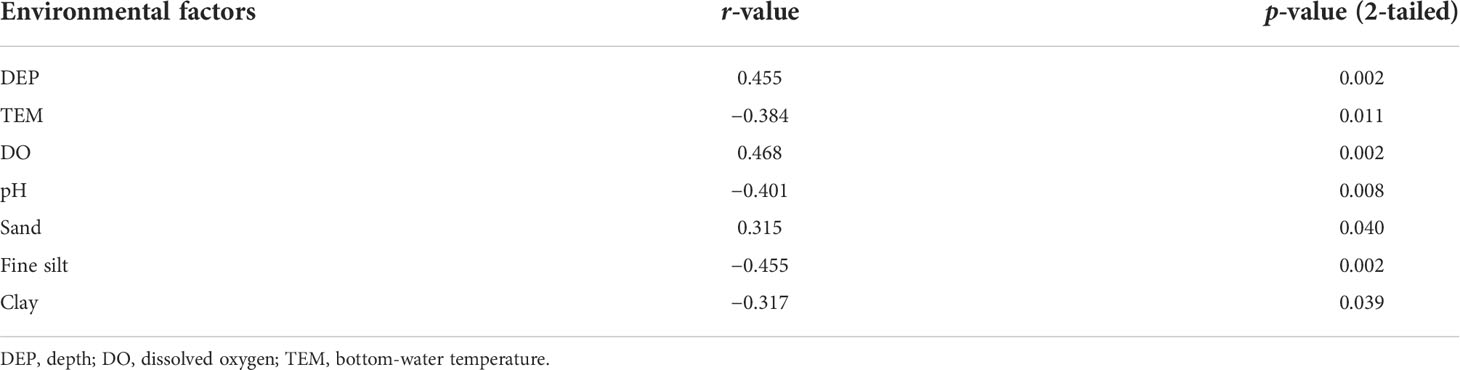
The northern sea areas off Yantai consist of two parts, the Bohai Sea and northern Yellow Sea, which are connected by the Bohai Strait. In this study, the Bohai Sea area off Yantai is taken as the region southeast of Bohai Bay; it has a coastline in China of ~270 km, an average water depth of ~13 m, and includes Laizhou Bay, Longkou Bay, and the mouths of seven major rivers (such as the Huangshui, Yongwen, and Jiehe). The northern Yellow Sea area off Yantai is taken as the southwestern region of the northern part of the Yellow Sea; this area has a coastline of ~280 km and average water depth of ~19 m, and it includes three bays (Taozi, Zhifu, and Sishili), and the months of seven major rivers (such as the Pingchang, Dagujia, and Guangdang). Both sea areas have high-intensity raft aquaculture and commercial fishing areas, as well as five important shipping ports, numerous sewage outlets, and waste-dumping areas. To assess the impact of human activities on the macrobenthos and coastal ecological quality, we surveyed 13 sites in the Bohai Sea (37°12′–38°00′ N, 119°40′–120°30′ E) and 15 sites in the northern Yellow Sea (37°30′–38°00′ N, 121°50′–122°00′ E). Two field surveys were conducted in April and August of 2020, with 21 stations surveyed in April, and 22 stations in August. The survey in April was conducted before implementation of a fishing moratorium, when many fishing boats were operating in both sea areas. The fixed nets set up in the sea, including ground cages and gill nets, prevented the survey ship from approaching the intended sampling sites; therefore, our actual sampling stations differed between the two seasons (Figure 1).
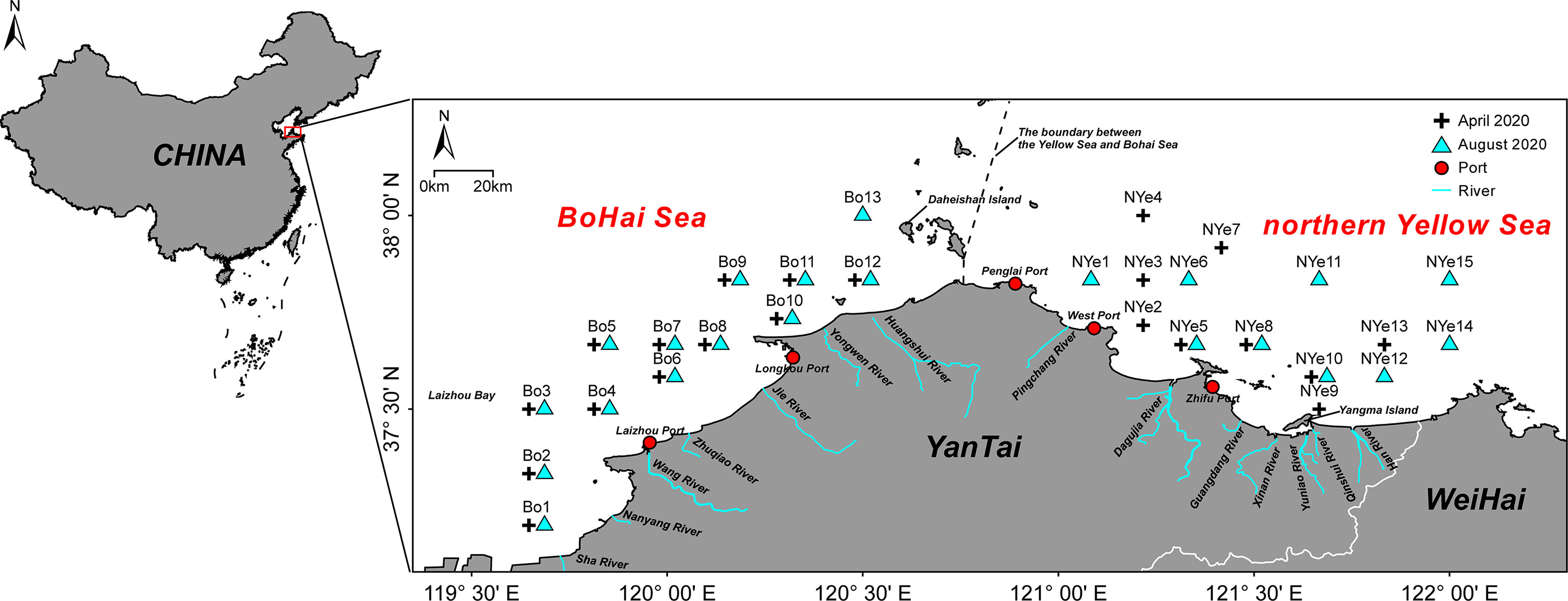
Figure 1 Map showing the study area and locations of the benthic sampling stations in the northern sea areas off Yantai, China.
Biological data
Macrobenthos, bottom seawater, and sediment samples were collected at each survey station. Surveys were performed according to the Specification for Oceanographic Survey (China National Standard GB/T 12763-2007). Five replicate samples were taken at each site using a Van Veen grab (0.05 m2). The grab contents were sieved (0.5-mm mesh screen) in the field, placed into appropriately labeled bottles or bags, and immediately fixed in 5–7% buffered formaldehyde solution. In the laboratory, the macrobenthos were sorted and identified to the lowest possible taxonomic level, and counted and weighed by group. The organisms were categorized as Annelida, Mollusca, Arthropoda, Echinodermata, or ‘Others’ (i.e., miscellaneous phyla that included Nemertea, Turbellaria, and Brachiozoa).
Environmental data
The bottom-water temperature (TEM), salinity (SAL), depth (DEP), pH, dissolved oxygen (DO), dissolved inorganic phosphate (DIP), dissolved inorganic nitrogen (DIN, including NO3-N, NO2-N, and NH4-N), sediment total nitrogen (TN), total phosphorus (TP), total organic carbon (TOC), sulfide (S), petroleum residue (Oil), and heavy metals concentrations (Cu, Pb, Zn, Cd, Hg, As) were recorded for each sampling event. Grain-size analysis was also performed, with the sediment divided into fractions of sand (63–500 μm), fine silt (4–63 μm), and clay (<4 μm); median particle diameter was expressed as D50. The environmental factors TEM, SAL, DEP, pH, and DO were recorded using a profiler (YSI EXO Handheld; Yellow Springs Instrument Co., Inc., USA). To determine DIP and DIN, water was filtered through 40-μm Whatman glass microfiber filters. The filtrate was analyzed by segmented flow analysis. The heavy metals content was determined by a flame atomic adsorption spectrometer (PE4100ZL; Perkin-Elmer Corporation, USA) and an atomic fluorescence spectrophotometer (AF–610A; Beijing Rayleigh Analytical Instrument Co., Ltd., China). Oil residue was measured with a fluorescence spectrophotometer (FL-4500). Sediment grain-size analysis was performed on a laser particle size analyzer (Mastersizer 2000; Malvern, UK).
Data analysis
The biological properties studied were total biomass (B), abundance (A), number of species (S), and the dominance index (Y) calculated as (Xu and Chen, 1989):
where ni is the total abundance of the ith species at all stations; Nt is the total abundance of individuals at all stations; and fi is the frequency of occurrence of the ith species at all stations. Species i is defined as dominant when Y > 0.02.
A station abundance matrix was constructed. The k-means algorithm was used to perform cluster analysis of all stations. The Hartigan–Wong standard algorithm (Hartigan and Wong, 1979) was used to calculate the sum of squares (SS) of the Euclidean distances between each item and centroid:
Each observation (xi) is assigned to a given cluster such that the SS distance of the observation to its assigned cluster centers (μk) is a minimum. The total within-cluster variation is defined as follows:
where xi is a data point belonging to cluster Ck; and μk is the mean value of the points assigned to cluster Ck.
Macrobenthic community composition was calculated using similarity percentage analysis (SIMPER) and analysis of similarity (ANOSIM) in PRIMER 6. The Mantel test was used to test the correlation between the major-benthic-community matrix and the environmental variables matrix for each sea area. The environmental variables and species matrices were transformed by lg(x + 1). All figures were drawn using R 3.6.3 and Surfer 14.
Ecological groups as indicators of ecological health
Based on the species list (updated in December 2020) provided in AMBI v6.0, we divided the macrobenthos collected in the study areas into five ecological groups according to an increasing stress gradient of organic enrichment. These groups represent the initial state of the environment, the transition state to slight imbalance, the state of slight imbalance, the transition state to significant imbalance, and the state of significant imbalance, and can be summarized as follows:
Group I: Species very sensitive to organic enrichment and present only in unpolluted environments; these include obligate carnivores and some sediment-, tube-dwelling polychaetes.
Group II: Species insensitive to organic enrichment, and present in low densities with only slight variations over time; these include suspension feeders, select carnivorous species, and saprophagous species.
Group III: Species that can tolerate excessive organic matter and which may occur under normal conditions but will increase under conditions of organic enrichment; these are often sediment-surface feeders, such as the tube-dwelling spionids.
Group IV: Second-order opportunistic species that tolerate slightly to pronounced unbalanced conditions; these mainly comprise small-sized polychaetes and subsurface sediment feeders.
Group V: First-order opportunistic species that tolerate pronounced imbalances; these are largely deposit feeders that can thrive in degraded sediments.
Based on the AMBI guidelines (Borja and Tunberg, 2011), the value of M-AMBI ranges from 0 to 1, with higher values indicating better ecological health (not degraded). The threshold values for the M-AMBI conditions of ecological quality were as follows: ‘high,’ >0.77; ‘good’ = 0.53–0.77; ‘moderate’ = 0.39–0.53; ‘poor’ = 0.20–0.39; and ‘bad,’ <0.20. To acquire a reference condition, we chose the lowest AMBI value and the highest values of diversity (H) and species richness (S) based on the two surveys (April and August), and then increased these values by 15%. Accordingly, the M-AMBI reference conditions for the two sea areas were: AMBI = 0.17, H = 5.198, and S = 46 for the Bohai Sea, and AMBI = 1.00, H = 5.095, and S = 52 for the northern Yellow Sea. The values of the ‘worst’ possible quality were based on the following values: AMBI = 6, H = 0, and S = 0, representing ecological conditions severely impacted by human activities (Li et al., 2013; Li et al., 2017).
Results
Species numbers, abundance, and biomass
In total, 7 phyla and 153 macrobenthic species were collected across the study area and seasons (April and August). The most species-rich group was polychaetes (60 species), followed by arthropods (43), and mollusks (38). In addition, 7 species of echinoderms, 3 species of nemerteans, 1 planarian, and 1 brachiopod were recorded. A total of 113 species were collected in the Bohai Sea, and 101 species in the northern Yellow Sea, with no significant difference in the numbers of species between the two sea areas (p > 0.05). In both sea areas a higher number of species was collected in April than in August; this difference was significant in the Bohai Sea (p < 0.05) and highly statistically significant in the northern Yellow Sea (p < 0.001) (Figures 2A). The abundance of macrobenthos was 454 ind. m-2 in April, and 399 ind. m-2 in August. Abundance in the northern Yellow Sea was 598 ind. m-2, and in the Bohai Sea it was 301 ind. m-2, and the difference between the two sea areas was extremely significant (p < 0.01) (Figures 2B). The biomass of macrobenthos was 10.86 g m-2 in April, and 13.22 g m-2 August; the biomass was 14.65 g m-2 in the northern Yellow Sea area, and 10.20 g m-2 in the Bohai Sea area; this difference was not significant (p > 0.05) (Figures 2C). In nearshore sites at estuaries and ports, the species numbers and abundance of the macrobenthos were lower. However, owing to the high biomass of Echinocardium cordatum in Longkou Port in the April and August surveys, the waters near the port displayed a high value of macrobenthos biomass (Figure 3).
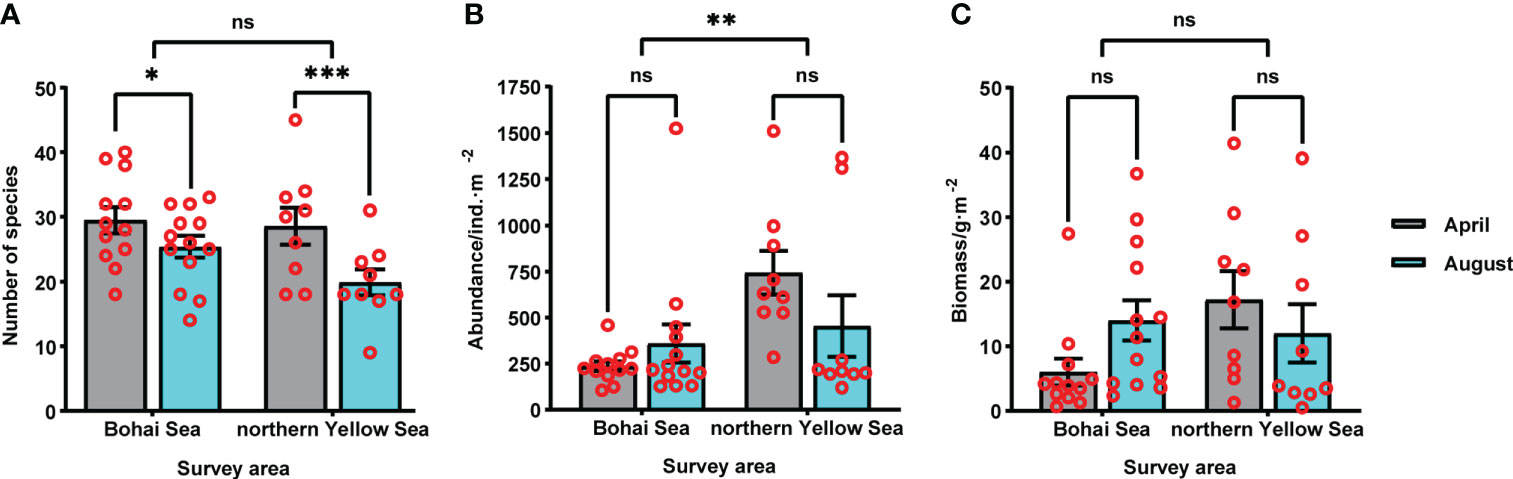
Figure 2 (A) Species numbers, (B) abundance, and (C) biomass of macrobenthos, in April and August of 2020, in the Bohai Sea and northern Yellow Sea areas off Yantai, China. Asterisks denote an highly significant difference (***p < 0.001, **p < 0.01) or a significant difference (*p < 0.05); ns represents no significant difference.
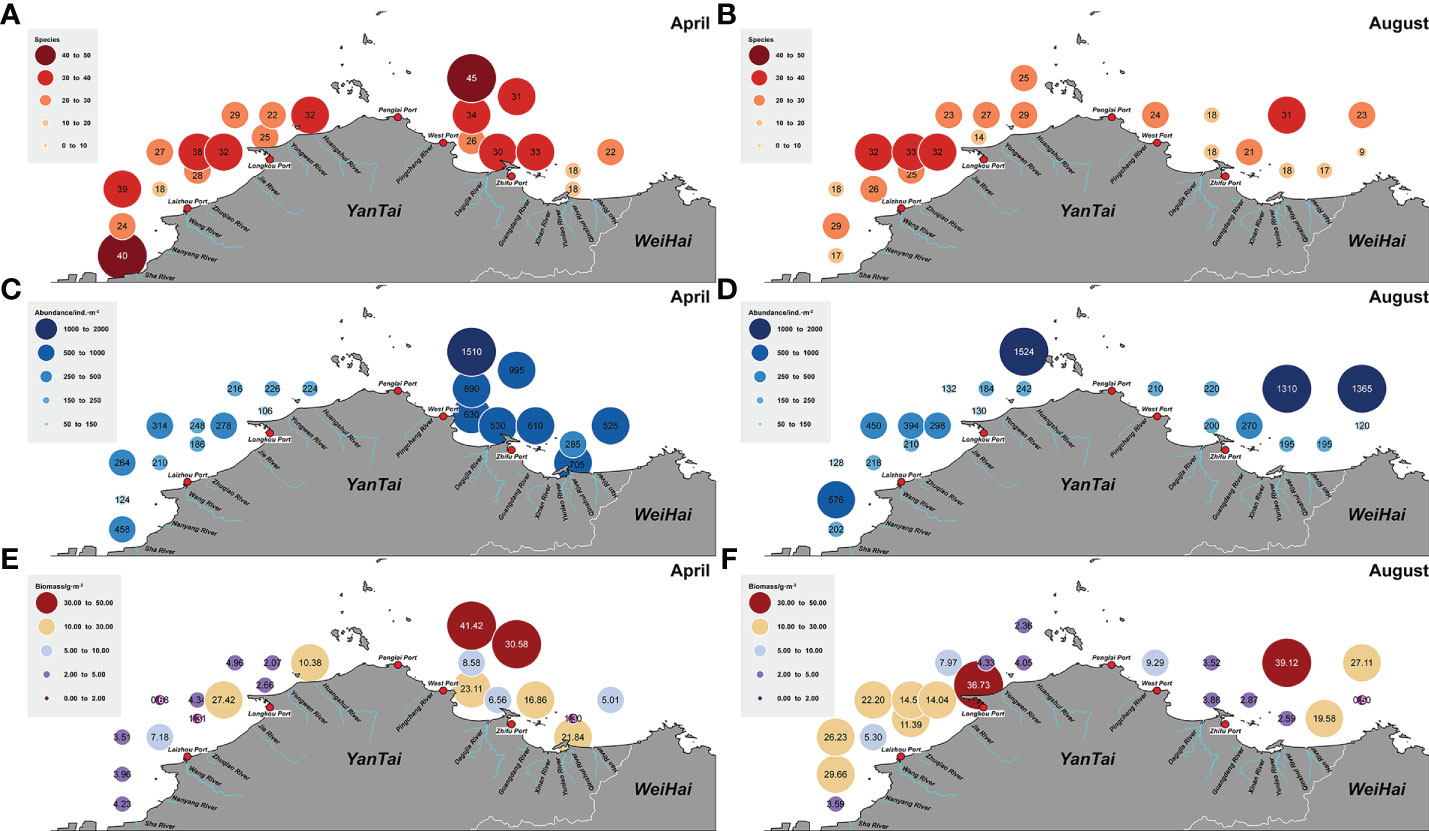
Figure 3 Spatial distribution of (A, B) species numbers, (C, D) abundance, and (E, F) biomass of macrobenthos in the northern sea areas off Yantai, China, in April and August of 2020.
Dominant species and community structure
During the study period, the dominant species of macrobenthos in the northern sea areas off Yantai were mainly small polychaetes and bivalves. There were 19 dominant species recorded (i.e., Y > 0.02): 11 polychaetes, 5 mollusks, 2 arthropods, and 1 echinoderm. Glycinde bonhourei, Micronephthys oligobranchia, Sternaspis chinensis, and Moerella hilaris were the main dominant species in the study area, and appeared in the samples of at least three cruises (Table 1). ANOSIM of community structure showed that the community dissimilarity of the two seas reached more than 60% (global test; R = 0.591, p = 0.001). In the same season, the dominant species and community structure of macrobenthos in the two areas were different. In April, the dominant species in the Bohai Sea were Sigambra bassi and Sternaspis chinensis, and in the northern Yellow Sea they were Scoletoma longifolia and Heteromastus filiformis, with an average community difference of 71.57% (pairwise testing; R = 0.718, p = 0.001). In August, the dominant species in the Bohai Sea was Moerella hilaris, and in the northern Yellow Sea they were L. longifolia, H. filiformis, and Magelona japonica, with an average community difference of 80.48% (pairwise testing; R = 0.692, p = 0.001). Comparing between seasons, replacement of the dominant species was more obvious in the Bohai Sea: small polychaetes dominated in April and small bivalves dominated in August, with an average community difference of 73.63% (pairwise testing; R = 0.469, p = 0.001). In the northern Yellow Sea, the dominant species were similar in the two seasons and comprised small polychaetes, with an average community difference of 59.39% (pairwise testing; R = 0.307, p = 0.002) (Tables 2 , 3).
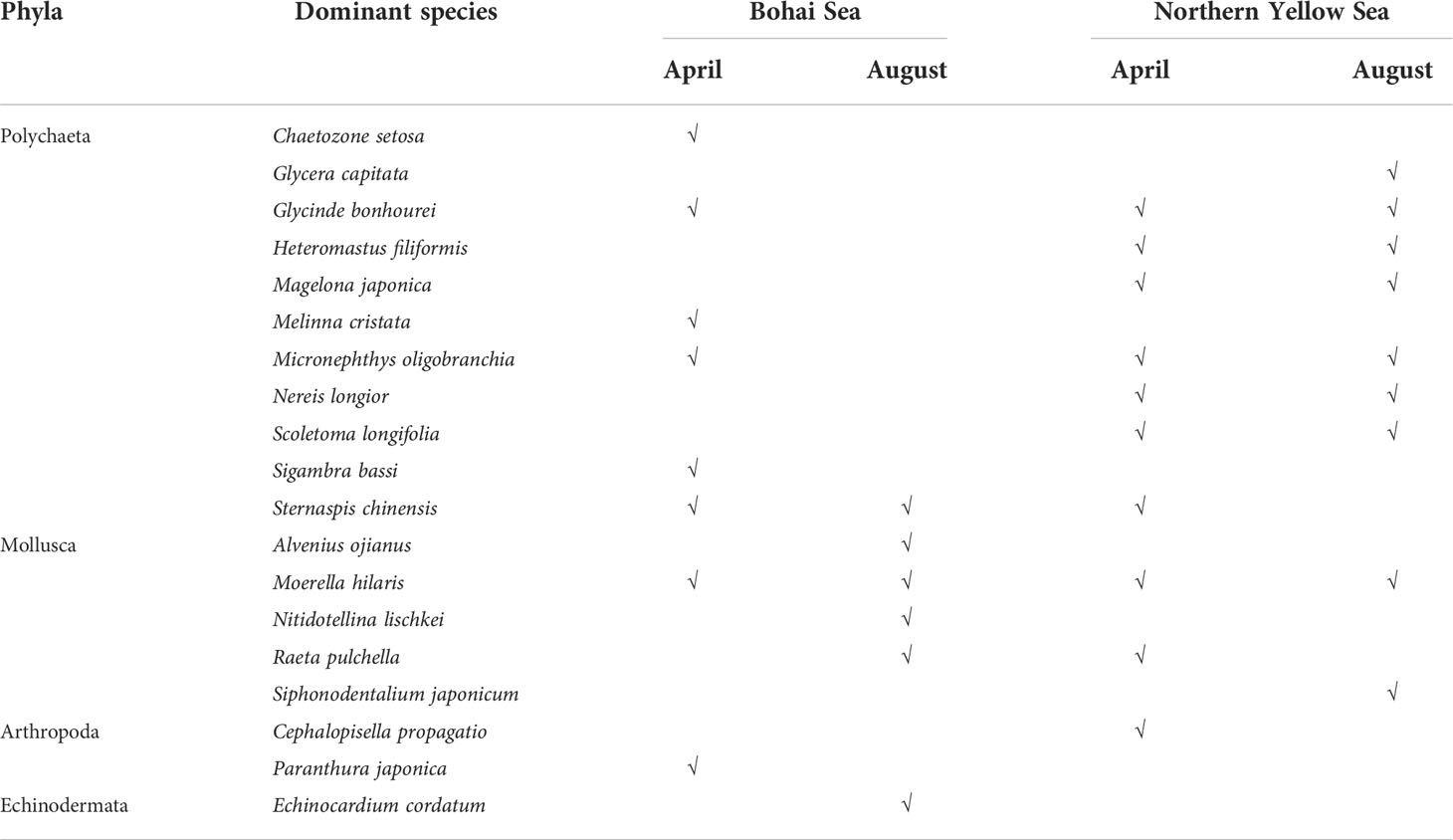
Table 1 Dominant macrobenthic species in two sea areas off Yantai, China, in April and August of 2020.

Table 2 Average macrobenthic community dissimilarity (%) off Yantai, China, based on four surveys, in April and August of 2020.

Table 3 Macrobenthic community composition in the Bohai Sea and northern Yellow Sea areas off Yantai, China, during the two survey months (April and August).
The survey data for April and August were analyzed using k-means clustering. The results showed that the macrobenthic community off Yantai, in both seasons, could be completely divided into Bohai Sea and northern Yellow Sea types; the within-cluster sum of squares was 263.0 and 227.4, respectively (Figure 4).
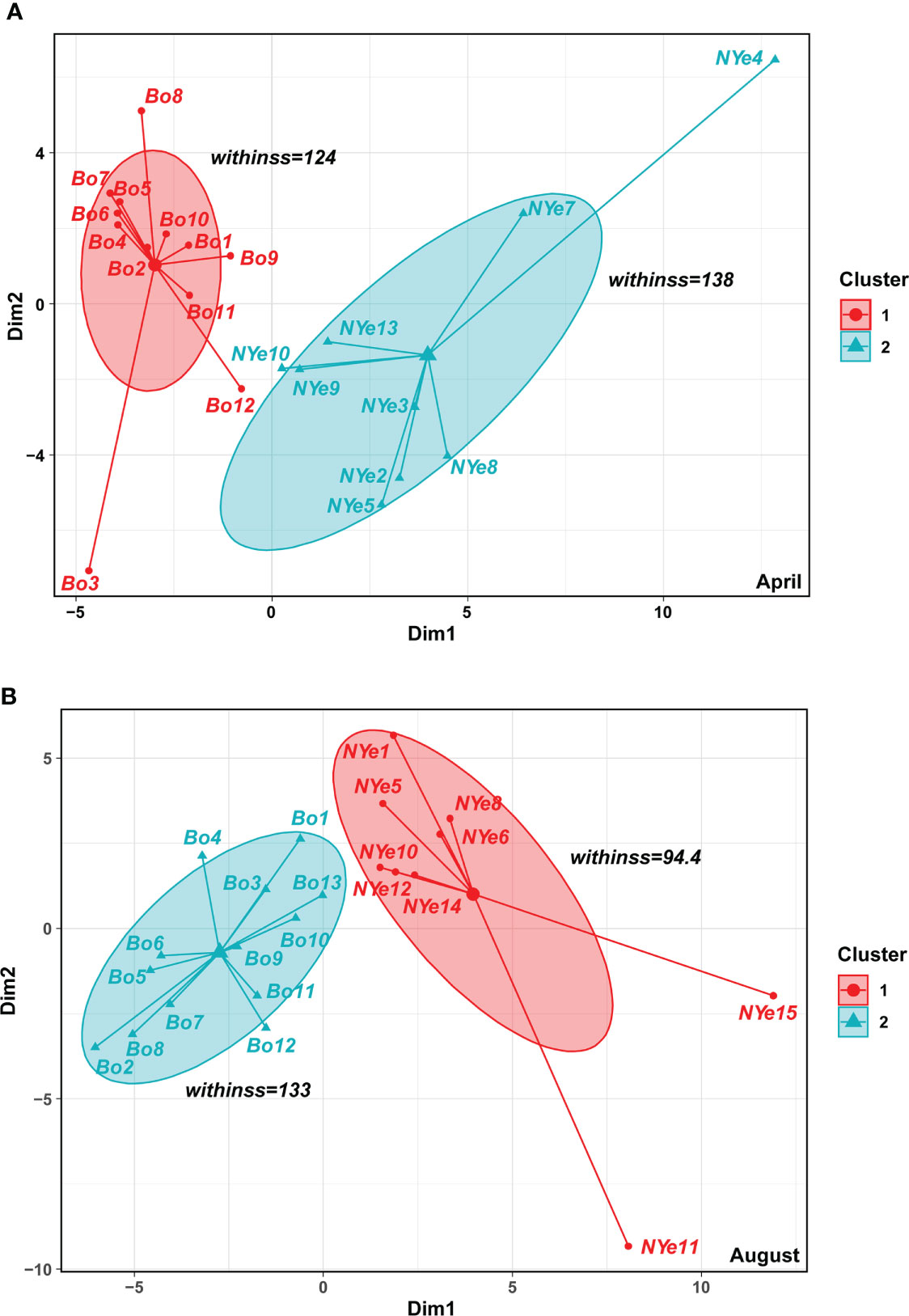
Figure 4 The results of k-means cluster analysis of the macrobenthic community in (A) April and (B) August of 2020, and by sea areas (Bohai Sea and northern Yellow Sea).
Relationship with environmental factors
Pearson correlation between 24 environmental factors and macrobenthos abundance used the data of four cruises. The results showed that species abundance was positively correlated with DEP (p < 0.01), DO (p < 0.01), and Sand (p < 0.05), and negatively correlated with TEM (p < 0.05), pH (p < 0.01), Fine silt (p < 0.01), and Clay (p < 0.05) (Table 4).
The correlation matrix between environmental variables and abundance of the major macrobenthic communities in four surveys was analyzed using a Mantel test, and correlations among environmental factors was also analyzed using a Pearson’s test. In April, DEP, SAL, and Hg were the main environmental factors affecting the macrobenthic community in the Bohai Sea area (p < 0.05) (Figure 5A); the concentration of Hg in the bottom-water samples was significantly correlated with both major communities I and II (p < 0.05). Correspondingly, DEP, pH, NO2-N, and Sand were the main environmental factors in the northern Yellow Sea area (p < 0.05), where DEP and NO2-N were significantly correlated with both major communities I and II (p < 0.01) (Figure 6A). In August, DEP, Fine silt, Clay, and D50 in the Bohai Sea area were the main environmental factors (p < 0.05), with Fine silt and Clay significantly correlated with both major communities I and II (p < 0.01) (Figure 5B). In the northern Yellow Sea area, DEP was the main environmental factor in August (like in April), followed by NO2-N (p < 0.05) (Figure 6B).
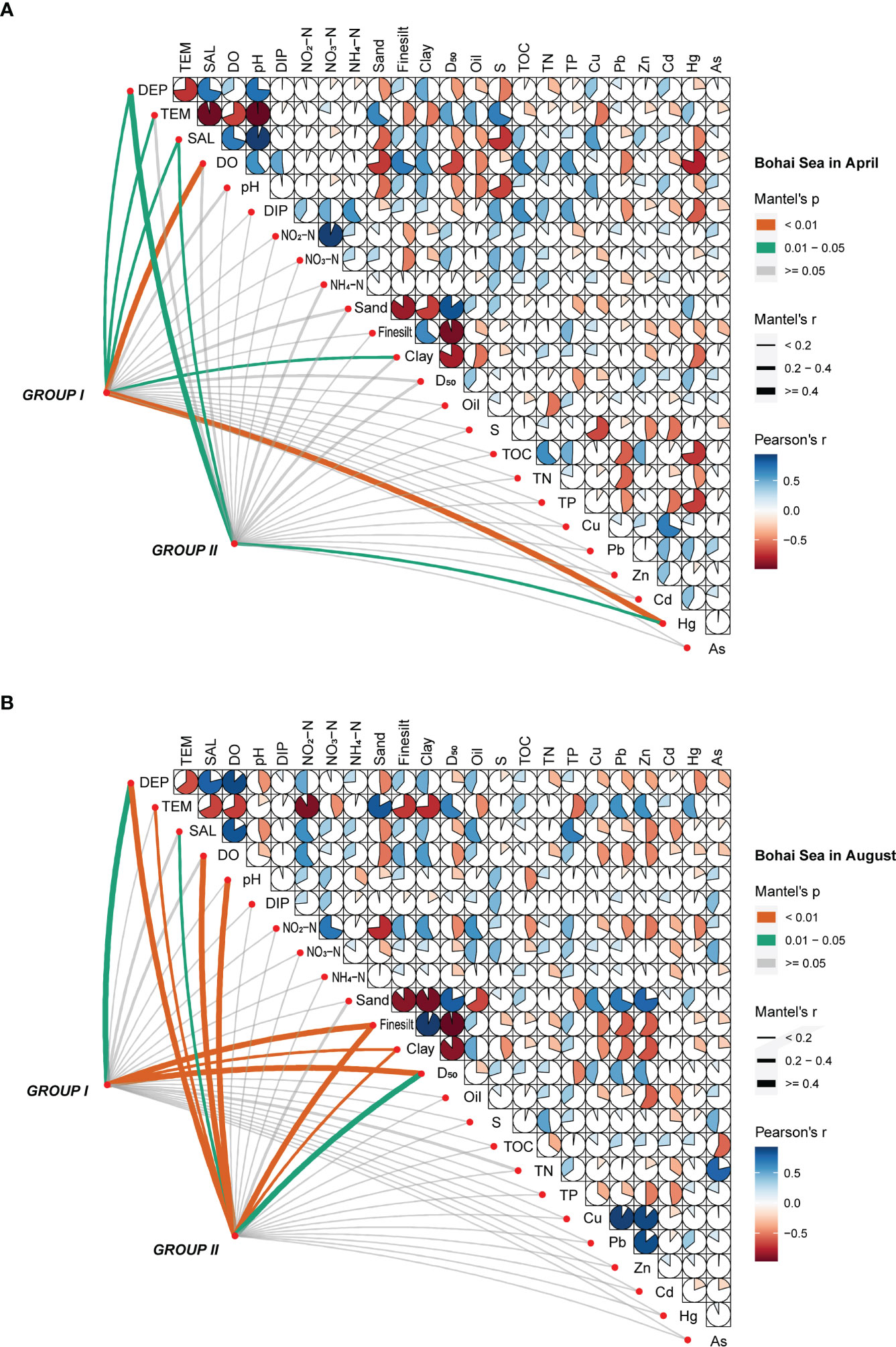
Figure 5 Correlations between the major macrobenthic communities and environmental factors, by month, in (A) April and (B) August of 2020, in the Bohai Sea area.
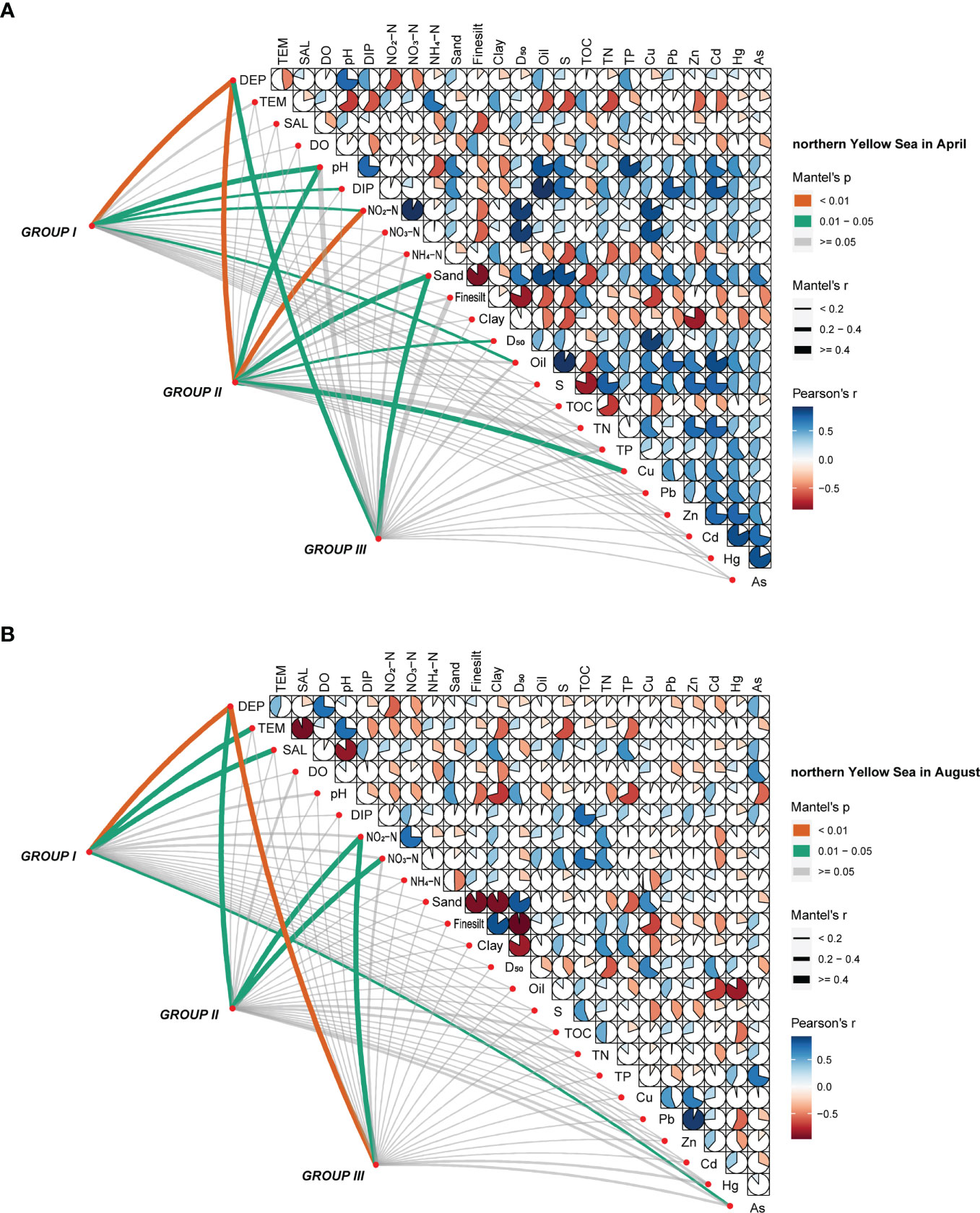
Figure 6 Correlations between the major macrobenthic communities and environmental factors by month, in (A) April and (B) August of 2020, in the northern Yellow Sea area.
M-AMBI
In April, areas of low-value ecological quality were: north of Laizhou Port, northeast of Longkou Port, and north of Yangma Island (Table 5). The most dominant species in the low-value areas belonged to ecological groups III and IV, such as Sigambra bassi, Sternaspis chinensis, Heteromastus filiformis, and Capitella capitata (Figure 7A). In August, low-value areas were: the center of Laizhou Bay, Longkou Port, west of Daheishan Island, and the coastal water between Yantai and Weihai. These sites were characterized by a low number of species and a low biodiversity index. Single dominant species that were profuse in low-value areas were Echinocardium cordatum, Alveinus ojianus, and Amphioplus japonicus (Figure 7B).

Table 5 Values of M-AMBI and the corresponding ecological quality status, by month, in the Bohai Sea and northern Yellow Sea areas off Yantai, China.
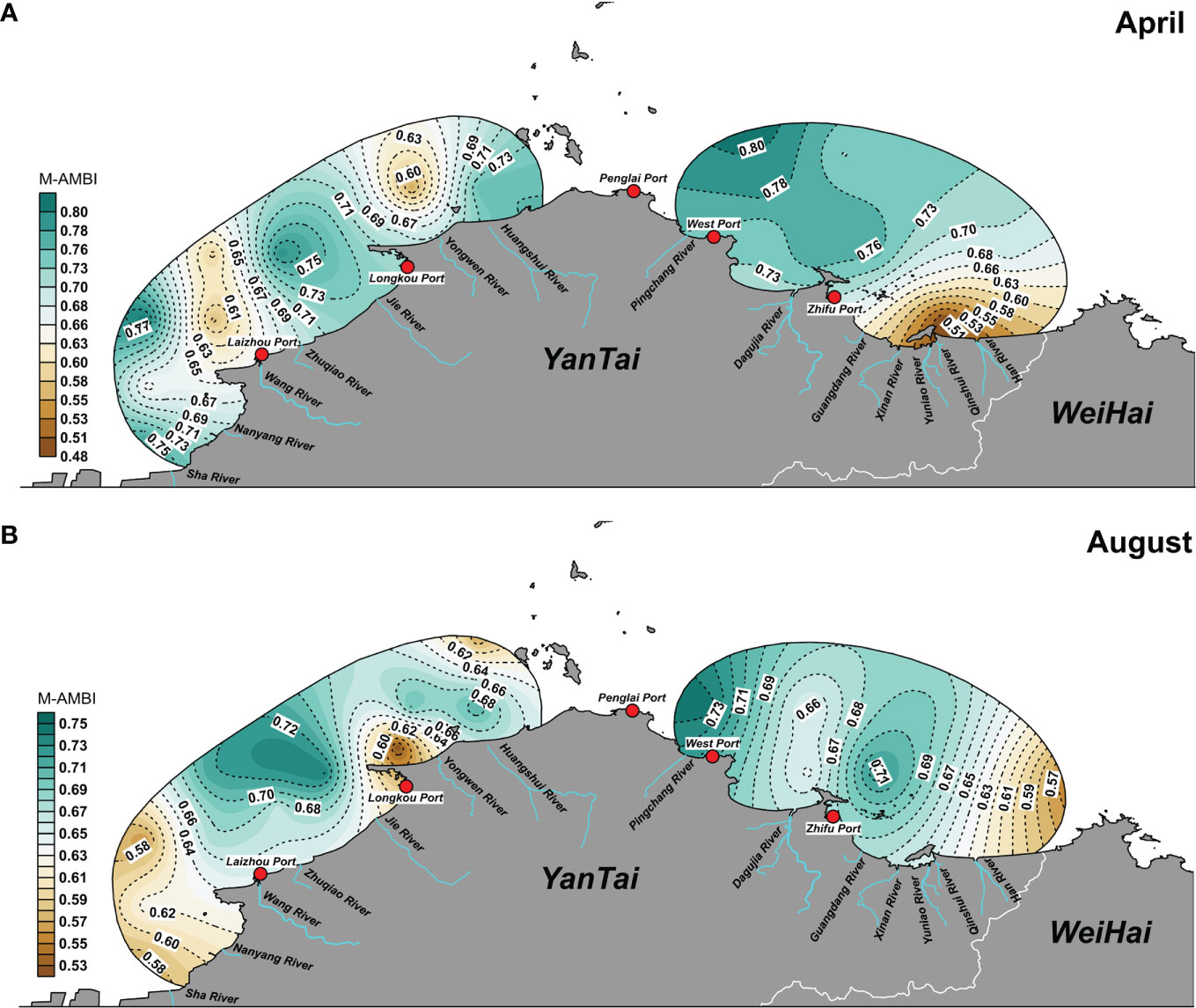
Figure 7 Spatial distribution of the ecological quality status in the two northern sea areas surveyed at Yantai, China, in April (A) and August (B) of 2020.
Discussion
Spatial and temporal changes in the macrobenthic community in the Bohai Sea and northern Yellow Sea
The spatial distribution of macrobenthos is closely related to environmental factors, such as the water depth and sediments (Coleman et al., 1978; Levin and Gage, 1998). This study found that the major communities of macrobenthos off Yantai in April and August were closely related to water depth. Li et al. (2013) surveyed macrobenthos in Taozi Bay and Shili Bay and found that the distribution of macrobenthos in the bays was clearly affected by the proximity of ports, waterways, and pollutants in rivers entering the sea. Liu et al. (2021) reported that the macrobenthos in scallop-farming areas and near heavily used waterways were affected by these human activities. Their distribution is locally random. This study found that the number of species, abundance, and biomass of macrobenthos showed characteristically low values for nearshore sites and high values for offshore sites. The benthos in the Bohai Sea area was likely affected by the ports of Longkou and Laizhou, as well as the marine aquaculture area on the west side of Daheishan Island. For example, the August survey coincided with large-scale raft farming of scallops in the southern part of the Bohai Sea. To a certain extent, intensive marine aquaculture activities will hinder the exchange capacity of the water body; the metabolites and excrement from marine aquaculture can also greatly impact the marine sediment environment, further disturbing the community structure of benthic fauna (Zhang et al., 2012; Zhang et al., 2013).
The macrobenthic community structure in the Bohai Sea and Yellow Sea has been investigated on a temporal scale since the 1990s. Macrobenthos biomass and abundance were reportedly high in the Bohai Sea in 1997–1999, with 306 species recorded (abundance: 2,575 ind. m-2; biomass: 42.59 g m-2). In the Bohai Strait, the abundance and biomass were as high as 3,968 ind. m-2 and 103.27 ind. m-2, respectively (Han et al., 2001). The annual abundance and biomass have since shown a downward trend, with higher declines in species biomass than in species abundance (Liu et al., 2014; Yuan et al., 2020). Changes in the dominant species show that K-strategy species with large-size individuals contribute less to biomass as they are replaced by R-strategy species with a small size, high fecundity, and short life span (Chen et al., 2016). In a 1997 survey in the northern Yellow Sea, Li (2003) reported a dominant community of Goniada maculata–Thyasira tokunagai–Ophiura sarsii vadicola. A 2007 survey Qu (2010), reported that the dominant macrobenthic species in the coastal areas of Shandong Peninsula were mainly Sternaspis chinensis, Lumbrineris cruzensis, Notomastus latericeus, and Magelona cineta. These were likewise dominant or important species in our study. Comparing the results of historical studies with the present study reveals that the benthic community structure in the northern Yellow Sea has changed little compared with that in the Bohai Sea. In terms of seasonal variation, there were no significant differences in macrobenthic abundance and biomass between the April and August surveys in this study. However, the dominant species in the Bohai Sea have changed from polychaetes to filter-feeding mollusks, consistent with the findings of Chen et al. (2016) regarding macrobenthos in the southern Bohai Sea. This trend may be related to the increase in organic content and primary productivity in coastal waters of the Bohai Sea.
Macrobenthic response to human impacts and environmental changes
The impact of human activities along the coast at Yantai as well as nutrient inputs from runoff into estuaries can create an imbalance of N/P and cause local eutrophication in the coastal water. In April, at the low-value ecological area of Yangma Island, the concentration of nitrogen was 0.450 mg/L-1, equating to class-IV seawater quality (medium-polluted) by the Chinese national standard. This level of pollution is certainly attributable to domestic and industrial sewage discharged into the Xin’an River and from sewage plants on the Yuniao and Qinshui rivers. Eutrophication and the N/P imbalance near estuaries have reduced the species diversity of benthic organisms. Therefore, the proximity of river mouths affects the community structure and ecological functions of the macrobenthos. Furthermore, we speculated that determination of such impacts can vary depending on the locations of survey sites and the survey scale. Most previous surveys have investigated smaller areas around individual bays or at Zhifu Island (Li et al., 2013; Wang and Li, 2013; Li et al., 2016; Liu, 2017). The influence of rivers entering the sea was more obvious in the above studies.
Numerous studies have reported on the relationship between the distribution of benthos and the sediment type. The distribution of benthic communities has been correlated mostly with the grain size of sediments. Moreover, sediment type is understood as a primary determinant of the composition and distribution of benthic communities (Sanders, 1958). For example, in terms of habitat selection by benthic fauna, Long and Lewis (1987) and Coleman et al. (2007) found that the abundance and diversity of benthos positively correlated with the proportion of coarse gravel in the sediment. Li et al. (2020) found that most macrobenthos prefer high D50 and low clay content. In terms of nutrient use by benthic fauna, Shou et al. (2018) asserted that it is difficult for macrobenthos to utilize nutrients in sediments with small-sized particles, and that spatial heterogeneity could provide more suitable habitats for benthos in coarse-sized sediments. Our study found that the abundance of macrobenthos in the two sea areas off Yantai was significantly positively correlated with the sand content (r = 0.315), and extremely significantly negatively correlated with the silt (r = −0.455) and clay (r = −0.317) contents, which is consistent with the results of previous studies. In contrast, through correlation analysis of the macrobenthic communities and sediment types in Laizhou Bay (southern Bohai Sea) in summer, Liu et al. (2014) found that fine sediment particles and high organic content in sediment were conducive to the survival of benthic fauna. This may be related to the feeding types of the dominant species in the study area, as well as a combination of environmental factors suitable to the benthic fauna in the area.
Ecological quality status based on AMBI analysis
The AMBI and M-AMBI are widely used ecological indices with good applicability for large-scale ecological risk assessments. Notably, the following two points will affect the accuracy of evaluations with the AMBI index. First, AMBI values are closely related to the survey method; for example, benthic fauna typically have weak locomotion and are vulnerable to the direct stress of sediment pollution. Thus, mud traps should be used as far as possible to sample and quantify the benthic fauna. In contrast, the use of bottom trawling survey methods, such as Agassiz trawl samples, can bias the evaluation (Li et al., 2017). Second, the application of AMBI requires adequate species assignments. The species list provided in the current AMBI system (updated December 2020) is applicable to most European regions, but numerous species elsewhere in the world have not yet been assigned, and the same species may be classified differently in different regions. Therefore, the worldwide list of species needs to be expanded by experts (Luo and Yang, 2009; Li et al., 2013).
Based on the results of k-means clustering and the M-AMBI analysis, the macrobenthos community structure clearly differed between the Bohai Sea and the northern Yellow Sea areas, with lower ecological quality shown for the latter area. A Mantel test showed no correlation between the major communities of macrobenthos and the concentration of nitrogen in the Bohai Sea in the two seasons, but revealed a significant correlation between them in the northern Yellow Sea area. The dominant species in the major communities was Heteromastus filiformis. Pearson and Rosenberg (1978) identified opportunistic species of polychaetes, such as Capitella capitata, Heteromastus filiformis, and Cirratulus cirratus. Species of Capitellidae and Cirratulidae can efficiently replenish their populations and reproduce in large numbers in a short period. The growth and distribution of these pioneer benthic fauna follow environmental pollution or human disturbance; hence, they are often used as indicator species of marine organic pollution, as they play key roles in the succession of benthic communities in disturbed soft-bottom sediments (Zhang et al., 2012).
Unlike opportunistic polychaete species, amphipods (particularly members of the suborder Gammaridea) are keystone organisms in the environment. Because they promote oxidation and nitrification processes on the seafloor, they are often used as indicators for environmental remediation efforts (Goméz-Gesteira and Dauvin, 2000). At the sites with low ecological value in this study, the average abundance of Heteromastus filiformis and Capitella capitata could reach 52 ind. m-2, and the highest estimated abundance was 355 ind. m-2, whereas the average abundance of all 10 species of amphipods was only 14 ind. m-2 at these sites. In contrast, at the high-ecological-quality sites, the average abundance of opportunistic species was 17 ind. m-2, while that of amphipods was 39 ind. m-2. Thus, these results demonstrate that a low abundance of amphipods and a high abundance of opportunistic polychaete species are important ecological indicators of marine pollution.
Conclusion
A total of 153 macrobenthic species were collected in two northern sea areas off Yantai, China, surveyed in April and August 2020. These included 113 species from the Bohai Sea and 101 species from the northern Yellow Sea; the abundance was 301 ind. m-2 and 598 ind. m-2, and the biomass was 10.20 g m-2 and 14.65 g m-2, respectively. The dominant species were small-sized polychaetes and bivalves. Glycinde bonhourei, Micronephthys oligobranchia, Sternaspis chinensis, and Moerella hilaris were the main dominant species. The average community difference between the two sea areas was 71.57% in April, and 80.48% in August. Replacement of the dominant macrobenthic species between seasons was more obvious in the Bohai Sea: small-size polychaetes dominated in April, and small-size bivalves dominated in August. In the northern Yellow Sea, the dominant species were similar in the two seasons and comprised small-size polychaetes. The results of k-means clustering showed that the macrobenthic community off Yantai could be effectively divided into Bohai Sea and northern Yellow Sea types. The abundance of macrobenthos was significantly positively correlated with depth, dissolved oxygen, and sandy substrate, and negatively correlated with bottom-water temperature, pH, fine silt, and clay. Likely owing to the impacts of intensive bivalve aquaculture, the water quality of river discharge, and port activities, the low-value ecological areas in April were at Yangma Island and the ports of Laizhou and Longkou; in August, areas of low ecological quality were the middle of Laizhou Bay, Longkou Port, west of Daheishan Island, and the coastal water between Yantai and Weihai. The overall average ecological quality of the northern sea areas off Yantai was generally in a “good” state.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, with reasonable request. Inquiries should be directed to the corresponding author.
Author contributions
FL, YM, SL, XS, and TW: manuscript writing and funding acquisition; YM: project administration; XW: statistical analysis; XZ and ZS: sampling and analysis. All authors contributed to the writing, review, and editing, and have approved the final version of the manuscript.
Funding
This work was supported by the Science and Technology Innovation Development Program of Yantai (contracts 2020MSGY061, 2020MSGY056, 2021MSGY031 and 2021XDHZ053), and the Qingdao National Laboratory for Marine Science and Technology, Shandong Province Special Funds (2021QNLM050103).
Acknowledgments
We thank all of our colleagues who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Borja A., Franco J., Pérez V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. pollut. Bull. 40, 1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Borja A., Tunberg B. G. (2011). Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and m-AMBI. Ecol. Indic. 11, 295–303. doi: 10.1016/j.ecolind.2010.05.007
Chen L. L., Wang Q., Li X. J., Zhou Z. Q., Li B. Q. (2016). Long-term trends of macrobenthos in southern bohai Sea, China, in relation to environmental changes. Sci. China 46, 1121–1134. doi: 10.1360/N052016-00063
Coleman N., Cuff W., Drummond M., Kudenov J. (1978). A quantitative survey of the macrobenthos of Western port, Victoria. Mar. Freshw. Res. 29, 445–466. doi: 10.1071/MF9780445
Coleman N., Cuff W., Moverley J., Anne S., Gason H. (2007). Depth, sediment type, biogeography and high species richness in shallow-water benthos. Mar. Freshw. Res. 58, 293–305. doi: 10.1071/MF06098
Goméz-Gesteira J. L., Dauvin J. C. (2000). Amphipods are good bioindicators of the impact of oil spills on soft-bottom macrobenthic communities. Mar. pollut. Bull. 40, 1017–1027. doi: 10.1016/S0025-326X(00)00046-1
Han Q. X., Yuan Z. Y., Chen B. J., Wang Y. J., Shi Y. J., Liu D. Y. (2014). The community structure and distribution pattern of intert-idal macrobenthos in the intertidal zone of yantai. Mar. Sci. 38, 59–68. doi: 10.11759/hykx20130704001
Han J., Zhang Z. N., Yu Z. S. (2001). Study on the macrobenthic abundance and biomass in bohai Sea. J. Ocean Univ. Qingdao 31, 889rnal. doi: 10.3969/j.issn.1672-5174.2001.06.011
Hartigan J. A., Wong M. A. (1979). “Algorithm AS 136: A K-means clustering algorithm”. J. R. Stat. Soc. 28, 100–108. doi: 10.2307/2346830
International Organisation for Standardisation (ISO) (1979). Assessment of the biological quality of rivers by a macroinvertebrate “score”. ISO/TC 147/SC 5/WG 6/N5 (Geneva: International Organisation for Standardisation, ISO) 18.
Levin L. A., Gage J. D. (1998). Relationships between oxygen, organic matter and the diversity of bathyal macrofauna. Deep-Sea Res. Part II 45, 129–163. doi: 10.1016/S0967-0645(97)00085-4
Li R. G. (2003). Macrobenthos of the Chinese sea shelf and adjacent waters. Beijing: China Ocean Press, 9–10.
Li B. Q., Li X. J., Bouma T. J., Soissons L. M., Cozzoli F., Wang Q. C., et al. (2017). Analysis of macrobenthic assemblages and ecological health of yellow river delta, China, using AMBI & m-AMBI assessment method. Mar. pollut. Bull. 119, 23–32. doi: 10.1016/j.marpolbul.2017.03.044
Li S. W., Li F., Song X. K., Zhang M. L. (2020). The influence of water-sediment regulation on macrobenthic community structures in the huanghe River(Yellow river) estuary during 2012–2016. Acta Oceanol. Sin. 39, 120–128. doi: 10.1007/S13131-020-1664-3
Li S., Lv T. T., Han Q. G., Zheng J. Y., Han Q. X. (2017). Comparison of composition and structure of macrobenthic communities in the yellow Sea and the bohai Sea in may 2010 and June 2013. J. Mar. Sci. 35, 86–94. doi: 10.3969/j.issn.1001-909X.2017.01.011(inChinese
Liu T. T. (2017). Effects of sewage discharge on the macrobenthic community offshore area of yantai, Shandong province. Zhejiang Ocean Univ., 5–7.
Liu X. S., Fan Y., Shi S. J., Hua E., Zhang Z. N. (2014b). Studies on the species composition and community structure of macrofauna in the bohai Sea,China. Acta Oceanol. Sin. 36, 53–66. doi: 10.3969/j.issn.0253-4193.2014.12.005
Liu L. S., Li B. Q., Lin K. X., Cai W. Q., Wang Q. C. (2014a). Assessing benthic ecological status in coastal area near changjiang river estuary using AMBI and m-AMBI. Chin. J. Oceanol. Limnol. 32, 290–305. doi: 10.1007/s00343-014-3125-3
Liu L. S., Li Z. Y., Meng W., Zheng B. H., Hu X. A. (2007). The community structure of zoobenthos and bioassessment of water quality in the lower reaches of the songhua river. Res. Environ. Sci. 20, 81–86. doi: 10.13198/j.res.2007.03.83.liuls.013
Liu L. S., Meng W., Li X. Z., Li Z. C., Li Z. Y. (2009). Studies on macrobenthos in the northern waters of liaodong bay: II. biodiversity and community structure. Res. Environ. Sci. 22, 155–161. doi: 10.13198/j.res.2009.02.33.liuls.002(inChinese
Liu X. D., Wang J. S., Sun L. E., Cui W. L., Li Z. (2021). Community structure and diversity of macrozoobenthos at bohai Sea off Shandong. Mar. Environ. Sci. 40, 929–936. doi: 10.13634/j.cnki.mes.2021.06.018(inChinese
Liu X. S., Shi S. J., Zhou H., Hua E., Zhang Z.N. (2015). Macrofaunal Diversity and Its Relationship with Environmental Factors in the Bohai Sea. Guangxi Sciences 22, 540–548. doi: 10.13656/j.cnki.gxkx.20151027.009
Liu X. S., Zhao R., Hua E., Lu L., Zhang Z. N. (2014). Macrofaunal community structure in the laizhou bay in summer and the comparison with historical data. Mar. Sci. Bull. 33, 46–55. doi: 10.11840/j.issn.1001-6392.2014.03.006(inChinese
Li B. Q., Wang Q. C., Li B. J. (2013). Assessing the benthic ecological status in the stressed coastal waters of yantai, yellow Sea, using AMBI and m-AMBI. Mar. pollut. Bull. 75, 53–61. doi: 10.1016/j.marpolbul.2013.08.007
Li X. J., Zhou Z. Q., Chen L. L., Li B. Q. (2016). Characteristics of macrobenthic communities in the estuary of dagujia river and its adjacent water areas in yantai, Shandong. Biodiv. Sci. 24, 157–165. doi: 10.17520/biods.2015217(inChinese
Long B., Lewis J. B. (1987). Distribution and community structure of the benthic fauna of the north shore of the gulf of st. Lawrence described by numerical methods of classification and ordination. Mar. Biol. 95, 93–101. doi: 10.1007/BF00447490
Luo X. X., Yang J. Q. (2009). Progress in researches on benthic indices of assessing marine ecosystem health. Mar. Sci. Bull. 28, 106–112. doi: 10.3969/j.issn.1001-6392.2009.03.016(inChinese
Lu X., Xu J., Xu Z. D., Liu X. S. (2021). Assessment of benthic ecological quality status using multi-biotic indices based on macrofaunal assemblages in a semi-enclosed bay. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.734710
Lv W., Ma C. A., Huang Y., Yang Y., Ji Y., Zhang M., et al. (2014). Macrobenthic diversity in protected, disturbed, and newly formed intertidal wetlands of a subtropical estuary in China. Mar. pollut. Bull. 89, 259–266. doi: 10.1016/j.marpolbul.2014.09.051
Muxika I., Borja A., Bald J. (2007). Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European water framework directive. Mar. pollut. Bull. 55, 16–29. doi: 10.1016/j.marpolbul.2006.05.025
Pearson T. H., Rosenberg R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanog. Mar. Biol. Annu. Rev. 16, 229–231. doi: 10.1111/j.1610-0387.2007.06252.x
Qu F. Y. (2010). The ecological study of macrobenthos in north yellow Sea in spring and autumn. Ocean Univ. China, 11–66.
Sanders H. (1958). Benthic studies in buzzards bay. i. animal-sediment relationships. Limnol. Oceanog. 3, 245–258. doi: 10.4319/lo.1958.3.3.0245
Shou L., Liao Y. B., Tang Y. B., Chen J. F., Jiang Z. B., Gao A. G., et al. (2018). Seasonal distribution of macrobenthos and its relationship with environmental factors in yellow Sea and East China Sea. Chin. J. Oceanol. Limnol. 36, 772–782. doi: 10.1007/s00343-018-6271-1
Somerfield P. J., Cochrane S. J., Dahle S., Pearson T. H. (2006). Free-living nematodes and macrobenthos in a high-latitude glacial fjord. J. Exp. Mar. Biol. Ecol. 330, 284–296. doi: 10.1016/j.jembe.2005.12.034
Tian J. (2012). The ecological quality evaluation of yellow river estuary and adjacent waters based on the macrobenthos. Ocean Univ. China, 12–16.
Wang Q. C., Li B. Q. (2013). Community structure of macrobenthos in coastal water off yantai, east China. Oceanol. Limnol. Sin. 44, 1667–1676. doi: 10.11693/hyhz20130300003
Xu Z. L., Chen Y. Q. (1989). Aggregated intensity of dominate species of zooplankton in autumn in the East China Sea and the yellow Sea. J. Ecol. 8, 13–15. doi: 10.13292/j.1000-4890.1989.0055
Xu Y., Li X. Z. (2021). Macrobenthos and offshore ecosystem health evaluation. Science 73, 30–34. doi: 10.3969/j.issn.0368-6396.2021.04.008
Yuan W., Wang J., Zuo T., Niu M. X., Luan Q. S., Shi Y. Q., et al. (2020). Characteristics of community structure and the dynamic changes of macrobenthos in the laizhou bay. Haiyang Xuebao 42, 52–61. doi: 10.3969/j.issn.0253
Yu B., Liu Z. D. (2020). Investigation on the zoobenthos in the intertidal zone of the intertidal zone of yudaishan bay, yantai. Environ. Dev. 32, 205–206. doi: 10.16647/j.cnki.cn15-1369/X.2020.01.113
Zhang Y., Li S. W., Lv Z. B., Ma Y. Q., Liu Y. J., Wei Z. H., et al. (2013). Application of polychaete in ecological environment evaluation of laizhou bay. Acta Ecol. Sin. 33, 2522–2530. doi: 10.5846/stxb201207311086
Keywords: macrobenthos, community structure, ecological quality, M-AMBI, Bohai Sea, Northern Yellow Sea, dominant species
Citation: Li F, Ma Y, Song X, Li S, Zhang X, Wang X, Wang T and Sun Z (2022) Community structure and ecological quality assessment of macrobenthos in the coastal sea areas of Northern Yantai, China. Front. Mar. Sci. 9:989034. doi: 10.3389/fmars.2022.989034
Received: 08 July 2022; Accepted: 16 August 2022;
Published: 06 September 2022.
Edited by:
Xiaoshou Liu, Ocean University of China, ChinaCopyright © 2022 Li, Ma, Song, Li, Zhang, Wang, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaowen Li, bGlzaGFvd2VuQHNoYW5kb25nLmNu
†These authors have contributed equally to this work and share first authorship
 Fan Li1†
Fan Li1† Shaowen Li
Shaowen Li