
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 October 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.981822
This article is part of the Research TopicInnovations in Fishing Technology Aimed at Achieving Sustainable FishingView all 23 articles
With increasing seal populations in the Baltic Sea comes growing interaction between seals and coastal fisheries. The impact of seals, mainly grey seal (Halichoerus grypus), on fisheries can be reduced by implementing of seal-safe fishing gear, which hinders seal access to catches. One successful solution is the introduction of a modified seal-safe trap net, the pontoon trap. In this study, pontoon traps were modified for use in cod (Gadus morhua) fisheries in the southern Baltic Sea. Three aspects of the pontoon trap design were tested for their effects on catch rates: (1) leader net mesh size; (2) leader net length; and (3) fish chamber position. The greatest catch rates were obtained using a leader net with a 100 mm center-knot to center-knot mesh-size on a bottom-set fish chamber while there was no difference in cod catch rates in relation to leader net length. There was no seal-induced damaged cod in the pontoon traps during any of the trials. Cod catch rates using the pontoon trap were also compared to those of the cod gillnet fishery in the same area. The comparison showed that during specific fishing occasions, multiple pontoon traps may have similar catch rates to gillnets.
In the 1970s, Baltic Sea seal populations were on the brink of extinction, but have since recovered to viable population densities (Hårding and Härkönen, 1999; HELCOM, 2010). Today the population of the grey seal (Halichoerus grypus) in the Baltic is stable, with an estimated abundance of between 47 600 and 63 500 individuals (Bäcklin et al., 2016; Swedish Agency for Marine and Water Management, 2019), with a particularly pronounced growth in southern the Baltic Sea (Galatius et al., 2020). With increasing seal populations in the Baltic Sea, has come growing interaction between seals and coastal fishers (Lunneryd et al., 2003; Lunneryd et al., 2005; Bruckmeier and Larsen, 2008; Varjopuro, 2011; Blomquist and Waldo, 2021). Seals can damage fishing gear and eat fish caught in the nets, causing loss of catch (Fjalling, 2005; Königson et al., 2009). Seals also occasionally entangle and drown in fishing gear (Vanhatalo et al., 2014; Königson et al., 2015b; Lyle et al., 2016). Seal-fisheries conflicts are present not only in the Baltic Sea (Bruckmeier et al., 2013), but also in other regions across the globe, such as the eastern and western coasts of North America (Nelson et al., 2006), Scotland (Butler et al., 2011), Australia (Hume et al., 2002), Chile (Sepúlveda and Oliva, 2005), and Japan (Hui et al., 2017).
In the Baltic Sea, it is predominantly grey seals interacting with fishing gear (Jounela et al., 2006; Königson et al., 2013). In the southern and central Baltic Sea, cod fisheries primarily use gillnets, a type of passive fishing gear (Bergenius et al., 2018; Blomquist and Waldo, 2021). The motivation for seals to interact with gillnets is high because they are stationary, often left in the water for several hours or days, and thus offers easily accessible food. Between 2006 and 2017, catch rates in the cod fishery in this region have declined by 80% (Königson, unpublished data). Over the same period, seal-induced damage to gear and catches in the Baltic cod fisheries have increased by 100–150% (Swedish Agency for Marine and Water Management, 2019).
To minimize seal bycatch and mitigate the seal-fisheries conflict, various mitigation strategies have been developed and implemented, with varying degrees of success (Mate et al., 1986; Yurk and Trites, 2000; Königson et al., 2007; Forrest et al., 2009; Königson et al., 2015a; Ljungberg et al., 2020; Lehtonen et al., 2022). One long-lasting and sustainable mitigation measure is to develop and implement seal-safe fishing gear. Seal-safe gear makes it hard for seals to access the catch and will consequently minimize reward, decreasing motivation to raid fishing gear for food (Königson, 2007). To reduce seal impacts in passive gear, pots, traps and trap-nets can be made seal-safe by being designing closed and solid compartments for fish gathering, where seals cannot access the catch.
Trap nets are commonly used within Baltic Sea coastal fisheries targeting salmon (Salmo salar) and white-fish (Coregonus lavaretus). However trap nets tend to provide access to high densities of prey for seals (Nelson et al., 2006). To reduce seal impacts in trap-net fisheries, one successful method is the introduction of a modified seal-safe trap net, the pontoon trap (Lunneryd et al., 2003; Lehtonen and Suuronen, 2004; Hemmingsson et al., 2008). The pontoon trap is a stationary passive fishing gear, in which fish are guided by a leader net and a system of gradually narrowing chambers into the fish holding chamber. The holding chamber is designed to prevent fish escaping. The holding chamber is also equipped with inflatable pontoons to deploy and retrieve the trap. Thus, the trap included the following components: a leader net; wings; adapter; and a seal-safe, pontoon-equipped, rigid-frame fish chamber.
Compared to pots, pontoon traps and other trap-nets are not typically baited, but rely on the fish to follow the leader net into the trap. Pontoon traps are included in the category of low impact and fuel efficient (LIFE) fishing gears and have minimal benthic impacts (Suuronen et al., 2012; Uhlmann and Broadhurst, 2015). The pontoon trap was introduced as a seal-safe alternative in the late 1990s/early 2000s and was originally developed to replace traditional traps nets for salmon and trout (Salmo trutta) (Lunneryd et al., 2003; Suuronen et al., 2006; Hemmingsson et al., 2008; Calamnius et al., 2018), which are susceptible to seal damage. Since then, specific pontoon trap models have been developed for vendace (Coregonus albula), herring (Clupea harengus), and perch (Percha fluventalis) (Lundin et al., 2011; Lundin et al., 2015). This implies that pontoon traps can be made both species and size selective.
With Baltic cod being a predominantly benthic species (Gregory and Anderson, 1997; Grant and Brown, 1998), the pontoon trap was modified to be bottom set. Although traps are excluded from the landing obligation (EU regulation 1380/2013), it is necessary for the sustainability of cod fisheries to reduce the bycatch of cod under minimum conservation reference size (MCRS, EU regulation 1241/2019), which is done by increasing the escape and survival of undersized individuals. This outcomes are important ecologically, to improve long-term prospects for cod populations, and commercially, to reduce handling times for fishers.
This study aimed to determine whether or not modified pontoon traps are a viable gear in coastal cod fisheries in the Baltic Sea. The objective was to maximize cod catch rates along with evaluate the level of seal-induced fish damage in modified pontoon traps. The pontoon traps evaluated included: (1) different leader net mesh size, (2) bottom standing or floating fish chamber, and (3) different leader net length. Finally, a catch rate comparison between the pontoon trap and the commercial landings of cod in gillnet fisheries, was conducted.
Trials were conducted between 2015 and 2018 off Ystad, Sweden, a town located in the southwestern Baltic Sea (Figure 1). In the study area, the shoreline is relatively straight, with a gently sloping bottom, which is generally sandy and partly covered with gravel and rock structures at depths less than 10 m. The trials were performed in collaboration with a commercial fisher contracted by the Swedish University of Agricultural Sciences. Trap deployment depths ranged from 5.5 to 8.0 m along the length of the trap. In salmon fisheries, where similar pontoon traps are used, the leader net and trap net typically stretch from the bottom to the surface, with the buoyed fish chamber floating just below the surface. With cod as target species in this study, the pontoon trap construction was redesigned so that the fish chamber could be placed on the sea floor. This was achieved by removing the buoys from the fish chamber, allowing it to completely sink to the bottom when the pontoons were deflated.
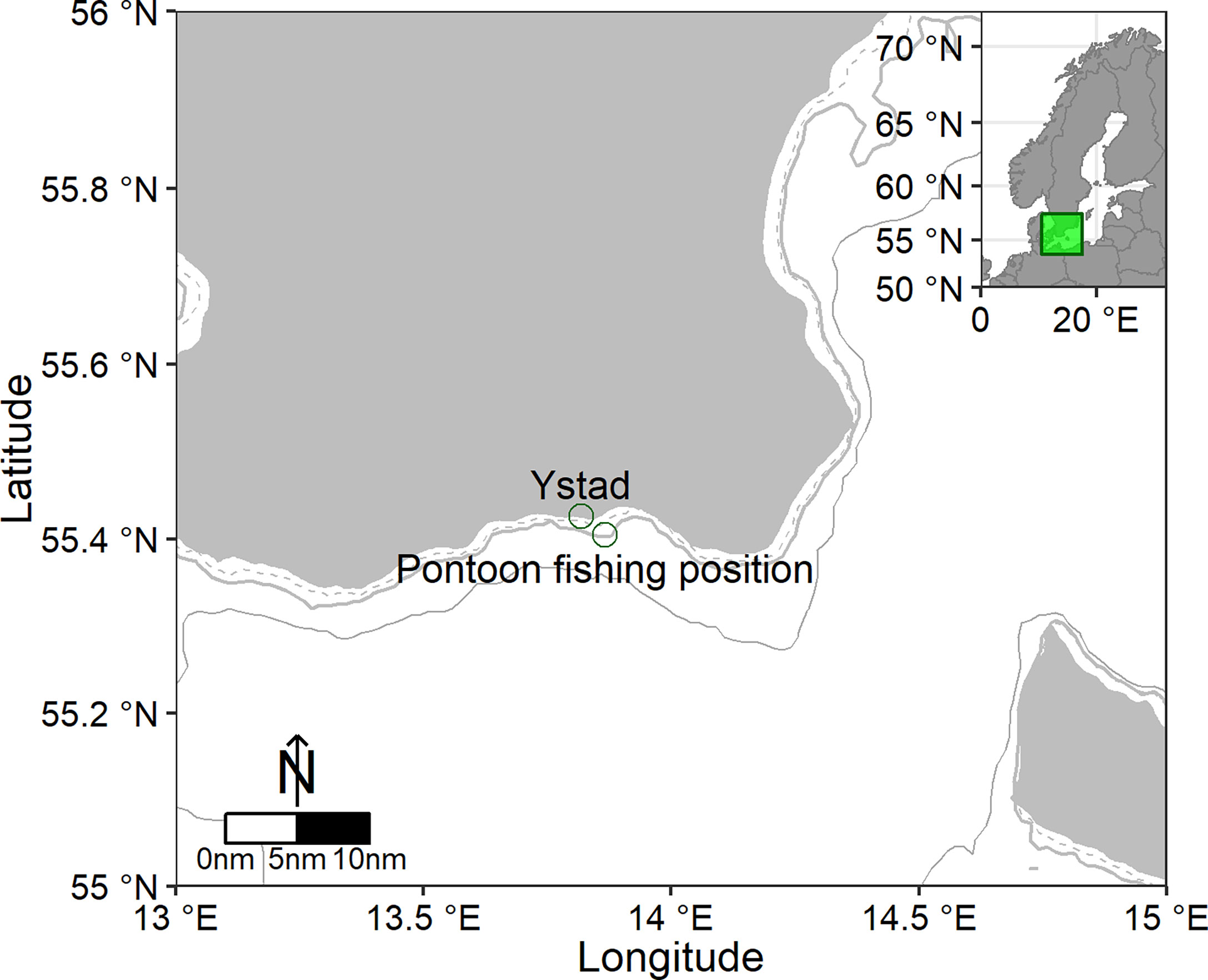
Figure 1 Map of the southern Baltic Sea and surrounding waters. Green circles indicate the port of Ystad and the position of the pontoon trap. Dashed line is the 5 m, thicker grey the 10 m and thinner grey the 20 m depth curve.
The fish chamber used was 1.5 m in diameter a size previously evaluated within perch fisheries (Lundin et al., 2015). One additional, smaller inflatable pontoon was placed on top of the fish chamber so that it could be easily lifted to the surface. At the surface the larger pontoons were inflated to raise the fish chamber above the surface for catch collection. Further, the traditional aluminum chute used for catch collection was replaced with a hose net (Lunneryd, 2018).
The hose net, similar to a trawl codend, is a fine-meshed netting tube with a length of 10 m, in which the fish are lifted from the surface into the boat. Hose nets allow catches to be handled in a selective way, because the fish can be divided by grids into size classes before they are lifted up into the boat or released. Additionally, the hose net is made from knotless net, which minimizes scale loss, and increases the value of collected fish and the survival of discards (Lunneryd, 2018).
Both the trap net/wings and the adapter were equipped with roof netting, because the trap height was less than the water depth at the fishing location (Figure 2). Additionally, the leader net was positively buoyed, allowing it to rise from the bottom, unlike traditional leader nets, which typically hang from the surface.
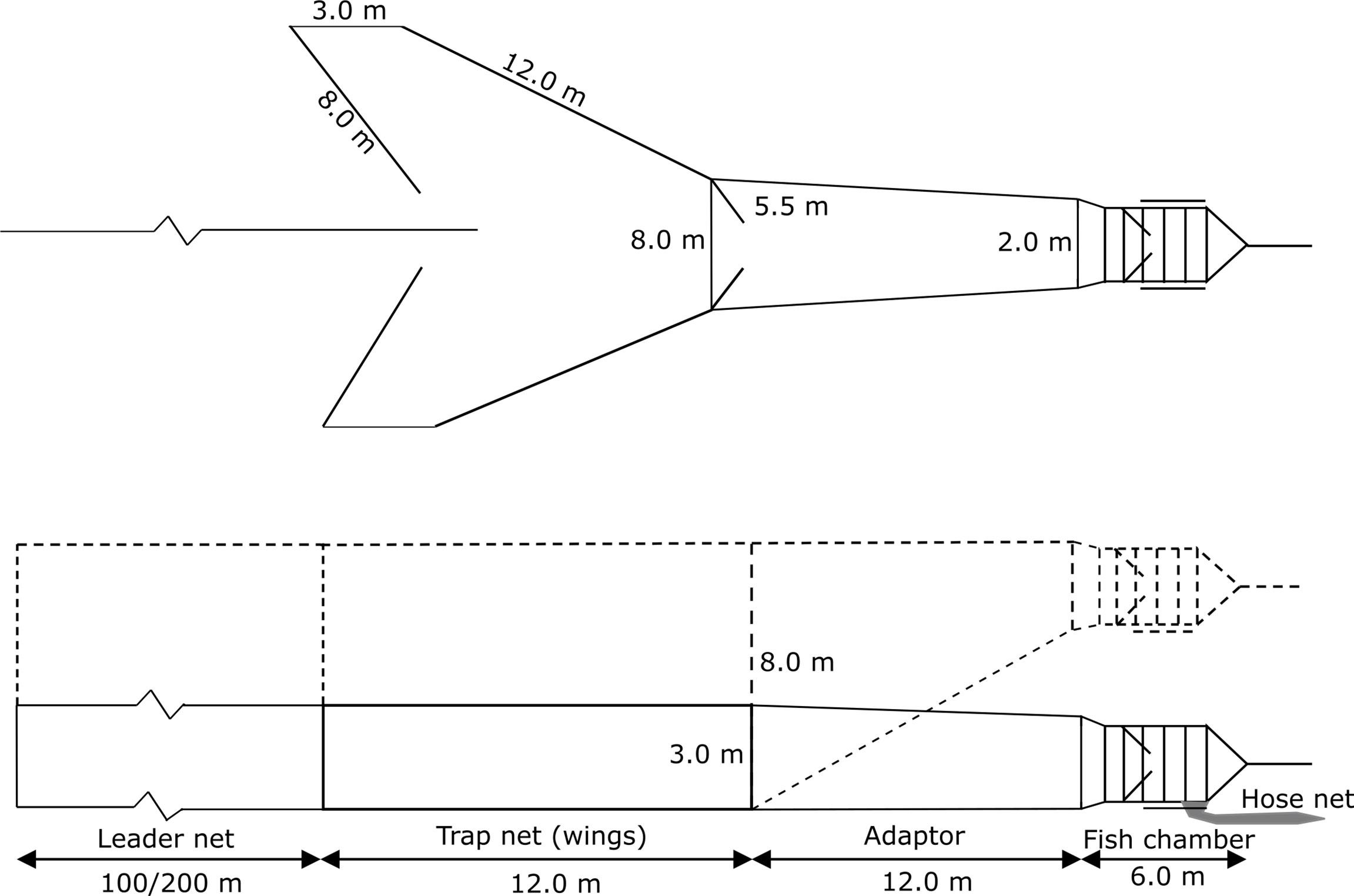
Figure 2 Schematic representation of pontoon-trap leader net, trap net (wings), adapter linking the trap net, pontoon fish chamber and hose net, from Hemmingsson et al. (2008), with measures used in traps parts within this study. The figure illustrates how the adaptor allows the fish chamber to be raised to the surface without requiring the trap net to be raised. Dashed sketch illustrates design of the floating fish chamber pontoon trap, used in trial two.
In the Baltic Sea, gillnets with a 55-mm center-knot to center-knot (CTC) mesh size are commonly used to catch cod above the MCRS of 38 cm TL (Madsen, 2007). Size selectivity, however, functions differently in gillnets than in passive gear with stretched square-mesh panels. Experiments using cod pots have shown that a 40-mm square-mesh panel generally excludes cod under 35 cm TL (Ovegård et al., 2011), which is now the MCRS in Baltic Sea cod and why a selection panel (0.5 x 0.5 m) with a mesh size of 40 mm ± 0.2 mm SE was used within the trials. However, for leader nets, it has been shown that larger than predicted mesh sizes can be used without fish swimming trough the leader net, but rather follow it towards the fish chamber (Lunneryd et al., 2002). Mesh sizes in each part of the trap and for the different trials are presented in Table 1.
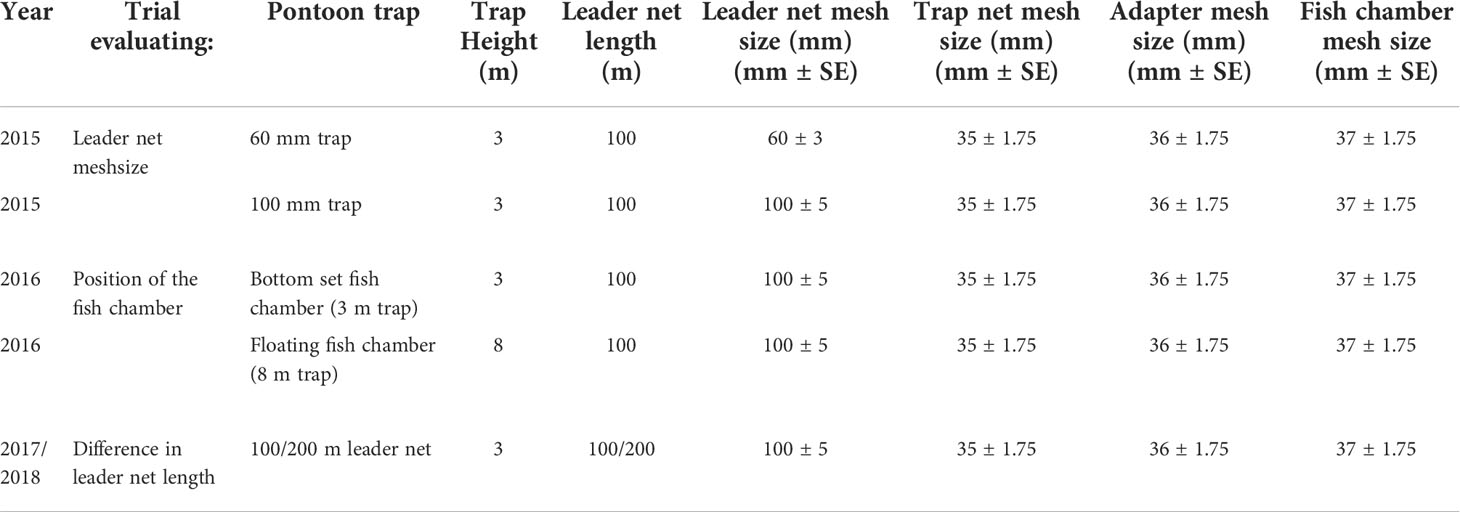
Table 1 Parameters for the different trap parts and running title used in the trials. Mesh sizes indicate the distance between center-knots.
Three separate trials were conducted to evaluate the pontoon trap for cod (Table 1). The first trial evaluated differences in catch rates as a function of leader-net mesh size. The second trial evaluated the position of the fish chamber using both a bottom-set and a traditional surface-set fish chamber. The third trial evaluated the effect of leader net length on catch rates. All within-trial comparisons were done in a pairwise fashion, using either one or two traps (Table 1). The numbers of cod and total catch weights, divided into commercial size classes, for every pontoon trap collection were recorded, along with the date and time. The catch was pooled into commercially sized (> 35 cm TL) and undersized fish (< 35 cm TL). The depth of deployment was kept constant within each trial. Only cod above MCRS (35 cm TL) were kept, while cod below 35 cm TL were immediately released after weighing. If present, dead fish or bites and scratch marks on fish caused by seals were recorded, either by personnel from the project group, or the fisher to evaluate the extent of seal interference with the traps. For all trials, catch rates were measured as weight per unit of effort (WPUE), which was calculated as the total catch weight of cod day-1 trap-1, for both commercial and undersized catch. Finally, catch rates from the pontoon trap were compared to landing data from the commercial gillnet fishery conducted in the same area.
In 2015, two traps were used. The traps were identical in their configuration except for the leader-net mesh size, with the first trap being equipped with a 60 mm ± 3 mm SE (CTC) leader net and the second trap with a 100 mm ± 5 mm SE (CTC) leader net (Table 1). The two traps were set towards each other, with each of the fish chambers positioned on the outer ends. The traps were set with the 60-mm leader towards the shoreline, with the fish chamber at a depth of 5.5 m. The trap with 100-mm leader net was set towards the sea, with the fish chamber at a depth of 8.0 m. The trial ran between 18 April and 19 July, 2015, with both traps being simultaneously used between 18 April and 9 May, and only the 100-mm trap being used from 10 May to 19 July. The pontoons of the 60-mm trap were damaged, and so the trap could not be deployed for the full duration of the experiment. For the same reason, we were unable to switch the position of the traps throughout the trial. Predicted means of catch rates are presented in the Supplementary Table 1.
In 2016, two traps were set (Table 1). The first trap was the same bottom-set trap used for the trials in 2015 (equipped with the 100-mm leader net). The second trap was equipped with a traditional floating fish chamber where the trap net was constructed in a way that fish had to swim up the water column to enter the fish chamber. The reason for using a floating trap was due to the breakdown of one trap the previous year. The breakdown was induced by the trap pontoons being perforated against the rocky bottom.
In the floating trap, the top of the fish chamber was 0.5 m below the surface (with the distance to the sea floor below the fish chamber being 6 m). The trap net had been redesigned so that the fish had to swim at an upward 45° angle to enter the fish chamber (Figure 2). Also, like in traditional pontoon traps, the trap net lacked roofing as it was designed to stretch trough the water column from the bottom to the surface, being bouyed by floats. Both traps where deployed with fish chambers facing the deeper water, with the bottom-set trap having its fish chamber a depth of 7.0 m. The trial period was between 22 March and 21 October, 2016, with both traps being used simultaneously between 5 April and 10 September. Predicted means of catch rates from the two trap models are presented in the Supplementary Table 1.
In 2017 and 2018, one trap was deployed, which was the same bottom-set trap used during previous years, with a 3 m high trap net and leader net with mesh size of 100 mm. Further, an additional 100-meter leader net section with 100-mm mesh size was added to the original 100-m leader net section using carabineers, increasing the total length of the leader from 100 to 200 m (Figure 2). The carabineers allowed the leader net to be divided in the intersection of the two 100 m sections, to evaluate the potential effect of leader net length on catch rates. The division included unhooking the carabineers to fold away and down the intersection-end of the inner leader arm section, hindering fish from the outer section following into the inner section. Leader net length was randomized throughout the trial period and was changed after catch collection. The evaluation of leader net length on catch rate was done for three time periods: spring 2017, autumn 2017 and spring 2018. The first time period, in 2017, was between 9 May and 15 June while the second was between 27 October and 4 December 2017. The third time period was between 26 March and 18 May, 2018. Predicted means of catch rates are presented in the Supplementary Table 1.
With gillnets traditionally being used within the coastal fishery for cod in the Baltic Sea, an evaluation of catch rates of the pontoon trap in relation to the commercial landings of cod in the coastal fisheries was conducted. For licensed commercial Swedish fishers, there is a requirement to report catches in accordance with the official EU logbook system and national requirements. For fishing vessels > 8 m fishing for cod, catches have to be reported daily, along with information on gear type, gear length, and fishing location. For comparison, catch data from gillnet fisheries conducted in the same ICES statistical rectangle (ICES, 2022) as the study area (39G3, total area 3540 km2) were collected from the official EU logbooks, organized in Sweden by Swedish Agency for Marine and Water Management (SwAM).
An estimate of mean daily landings per vessel (kg day-1, WPUE) month-1 were calculated for the same time periods in which the pontoon trials were conducted. Vessel- and day-specific catch estimates were pooled together for all vessels giving a mean catch (WPUE, kg) for each day and month. Since 2017, the amount (WPUE, kg day-1) of seal damaged catch has also been included in the logbook data. For the pontoon trap data, catches from the highest catching gear in each trial period were used; the trap with 100-mm leader net for 2015, the 3-m bottom-set trap for 2016, and for catch data from both 100 and 200 m leader net length in 2017 and 2018 (Table 1). The same time intervals was used also in the gillnet comparison. Mean WPUE of daily catch from the pontoon trap was used to calculate the potential catch day-1 and month-1 in a full-scale commercial pontoon trap fishery. Pontoon trap catches were extrapolated under the assumption that a fisher would be able to handle and collect the catch from five traps within a day based on the time required to collect catch from one trap and using catch data based on soak times from the three conducted trials.
All statistical analyses were performed using R (v.4.1.3), (R-Development-Core-Team, 2008) with data analysis included the use of additional R packages ‘psych’ and ‘dplyr’. To evaluate the effects on catch rates of different trap characteristics (mesh size, chamber position and leader net length) on WPUE, linear mixed models (lmm) were fitted using the ‘lmer’-function in the lme4 package (Bates et al., 2015), with separate models for each of the three trials. In all models, the response variable was either WPUE of collected catch (kg day-1) or WPUE of undersized catch (kg day-1). The main predictor variables (fixed) in each three trial base-models were mesh size (60 and 100 mm), chamber position (bottom standing, 3-m and floating), and leader net length (100 and 200 m), respectively. Each model also included soak time (days) as predictor (fixed) variable. In the models with mesh size and chamber position, month were included as random factor. In the model with leader net length, sub-trial period were used as random factor. For the comparison between gillnets and pontoon trap, WPUE of collected catch (kg day-1) was used as predictor (fixed) variable along with month and year as random factors. A Gaussian error distribution was used in all models because WPUE data are continuous. Parameter estimation and inferences were performed on the full model, i.e. including all variables (both fixed and random). Statistical significance of explanatory variables was tested according to Satterthwaite’s method using the ‘lmerTest’-function in R (Kuznetsova et al., 2017).
In total, 32 catch collections were recorded during 2015. Catch data for the 60- and 100-mm traps, used the trial are presented in Table 2 and Figure 3. Taking in regards only the time period between 18– 04–2015 and 15–05–2015, when both traps were used, mean observed WPUE in the 100-mm trap was 50.20 ± 38.48 kg day-1 (n = 9, max = 104.0, min = 0). When evaluating the time period both traps were used, the catch was higher in the 100 mm trap in compared to the 60-mm leader-net trap (β = 46.80 ± 12.02 SE, df = 15, p < 0.01). However, there was no effect on catch by soak time (number of days between catch collections) (β = -16.54 ± 9.01 SE, df = 15, p = 0.086). For the complete trial period, catches were higher in the 100-mm trap then the 60-mm leader net trap (β = 37.98 ± 9.97 SE, df = 24.77, p<0.001). There were also declining catches with increasing soak time, and number of days between catch collections trap (β = -7.13 ± 2.44 SE, df = 15.73, p < 0.05). There were no difference in the proportion of undersized cod between the 60 and 100 mm trap (β = 0.08 ± 0.43 SE, df = 27.00, p = 0.074), but there was an effect of soak time (β = -0.19 ± 0.09 SE, df = 27.0, p < 0.04).
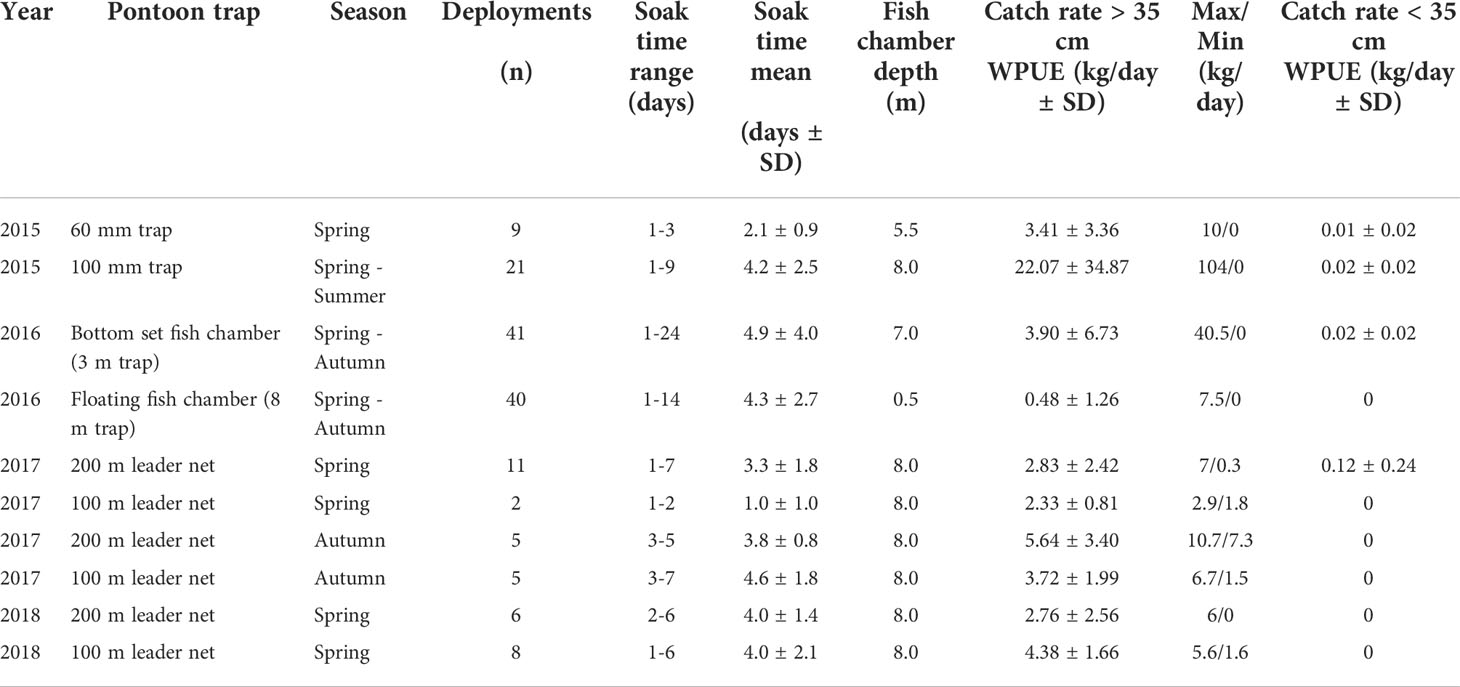
Table 2 Number of deployments for each trap in each trial, range and mean soak time, fish chamber depth (bottom set in all trials except 8 m trap in 2016) mean observed catch (WPUE) of commercial sized cod along with observed maximum and minimum catch and observed catch rates (WPUE) of undersized cod.
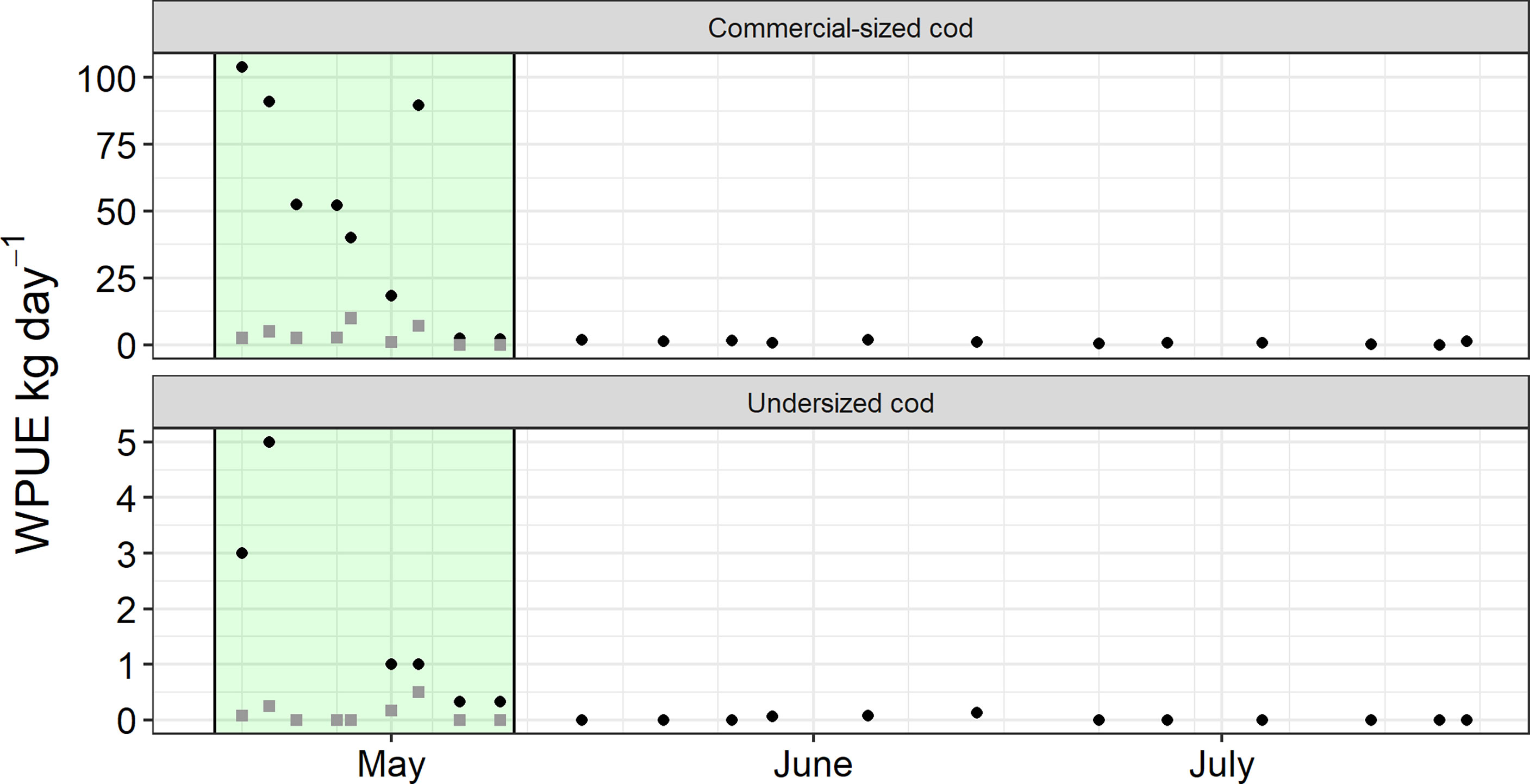
Figure 3 Mean WPUE (kg day-1) of commercial sized and undersized cod (Gadus morhua) in the two traps tested for difference in leader net mesh size. Black circles represent the pontoon trap equipped with 100 mm leader net, while grey squares represent the pontoon trap equipped with 60 mm leader net. Green background indicates the time period when both traps were simultaneously used.
In total, 81 collections were recorded within the second trial. Catch data for the bottom-set fish chamber and floating fish chamber traps used in the trial are presented in Table 2 and Figure 4. The greater maximum soak time for the bottom-standing trap both in relation to the floating fish-chamber trap in this trial and the soak times used in the two other trials were mainly due to weather conditions at the end of the trial period, precluding trap retrieval. Taking into account the entire trial period, the catch was higher in the bottom standing, 3-m trap than the floating 8-m trap (β = 3.82 ± 1.02 SE, df = 72.95, p < 0.001). However, there was no effect on catch rate by soak time (number of days between catch collections) (β = -0.16 ± 0.17 SE, df = 70.09, p = 0.33). For the time period both traps were used, the results were similar, with a greater catch in the bottom-standing then the floating fish chamber (β = 3.77 ± 1.13 SE, df = 61.38, p < 0.01), with no effect on catch by soak time (β = -0.26 ± 0.24 SE, df = -1.10, p = 0.28). There was a difference in WPUE for undersized catches between the bottom standing and floating trap where the floating pot caught less (β = -0.04 ± 0.02 SE, df = 76.0, p < 0.05) or soak time (β = -0.0009 ± 0.005 SE, df = 76.0, p = 0.74).
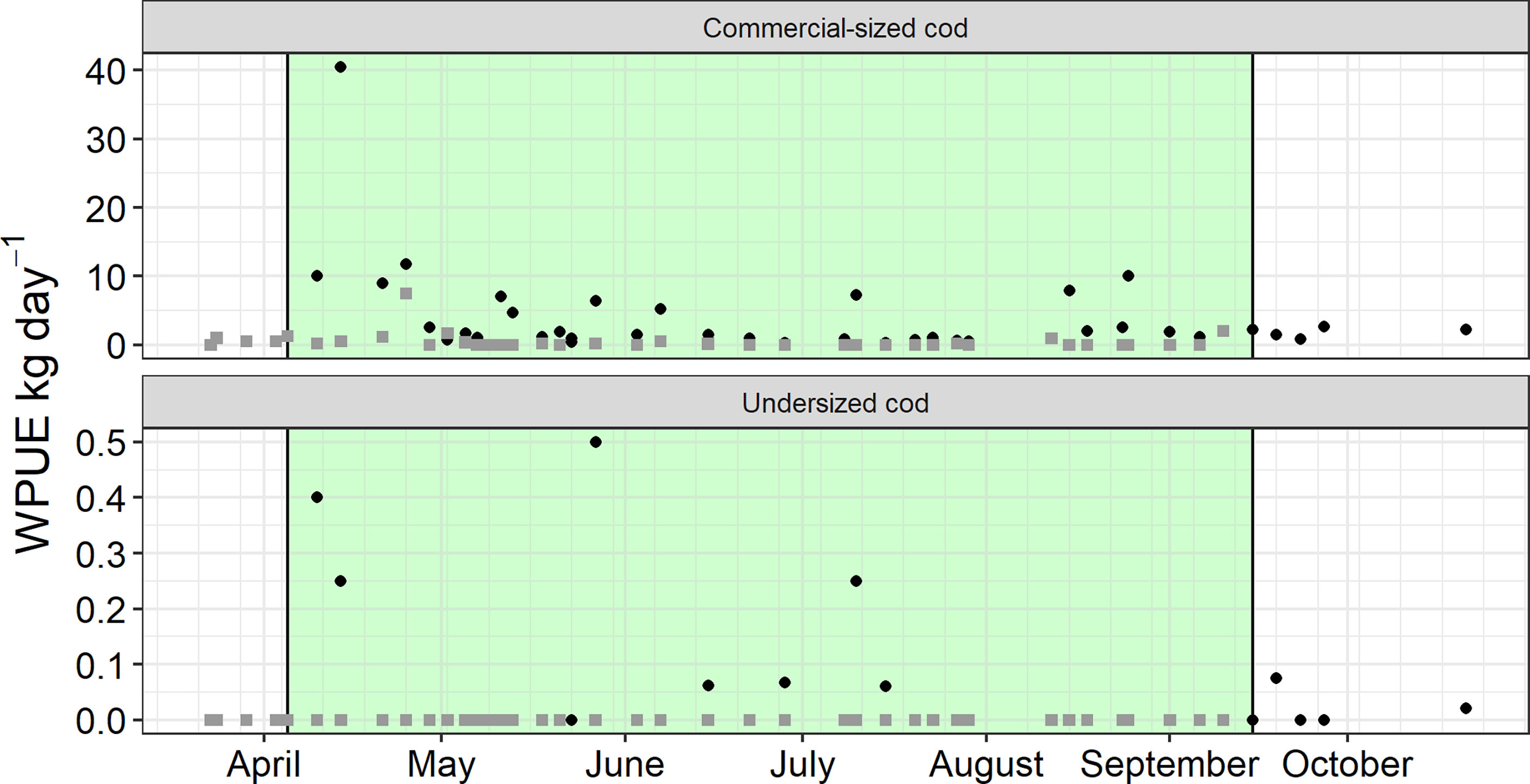
Figure 4 Mean WPUE (kg day-1) of commercial sized and undersized cod (Gadus morhua) in each of the two traps tested for difference in leader net mesh size. Black circles represent the pontoon trap with bottom set fish chamber, while grey squares represent the pontoon trap with floating fish chamber. Green background indicates the time period both traps were simultaneously used.
In total, 37 collections were recorded during the three trial periods. Season-based leader net length dependent trap data are presented in Table 2 and Figure 5. There were no difference in catch rates by leader-net lengths (β = 1.29 ± 0.85 SE, df = 30.90, p = 0.14). Neither were there any effect on catch rates by soak time (number of days between catch collections) (β = -0.26 ± 0.24 SE, df = -1.10, p = 0.28) nor differences between the seasons. The WPUE of undersized cod was not affected by leader net length (β = 0.02 ± 0.05 SE, df = 30.51, p = 0.659) or soak time (β = 0.02 ± 0.01 SE, df = 29.99, p = 0.116).

Figure 5 Mean WPUE (kg day-1) of commercial sized and undersized cod (Gadus morhua) when tested for leader net length. Black circles represent 100 m leader net, while grey squares represent 200 m leader net. Green background indicates the time period when both traps were simultaneously used. The black, vertical dashed line represent the division between 2017 and 2018.
The comparison between trap nets and commercial gillnet fisheries included 96 pontoon trap collections from 2015–2018. Gillnet landing data included 1,397 daily fishing reports in ICES rectangle 39G3, from a total of 27 commercial fishing vessels, with a mean daily (± sd) gillnet length of 7800 ± (2800). The highest amount of daily catch in gillnets was between 100 and 300 kg per day. Further, in 2017 and 2018 there was a high amount of seal damaged catch from gillnets, with this being included in the logbook since 2017 (Figure 6). For the pontoon traps, no seal damaged cod were recorded during any trial. Catches were higher in gillnets than to, the predicted catches using pontoon trap (β = 153.48 ± 21.08 SE, df = 1142.42, p < 0.001).
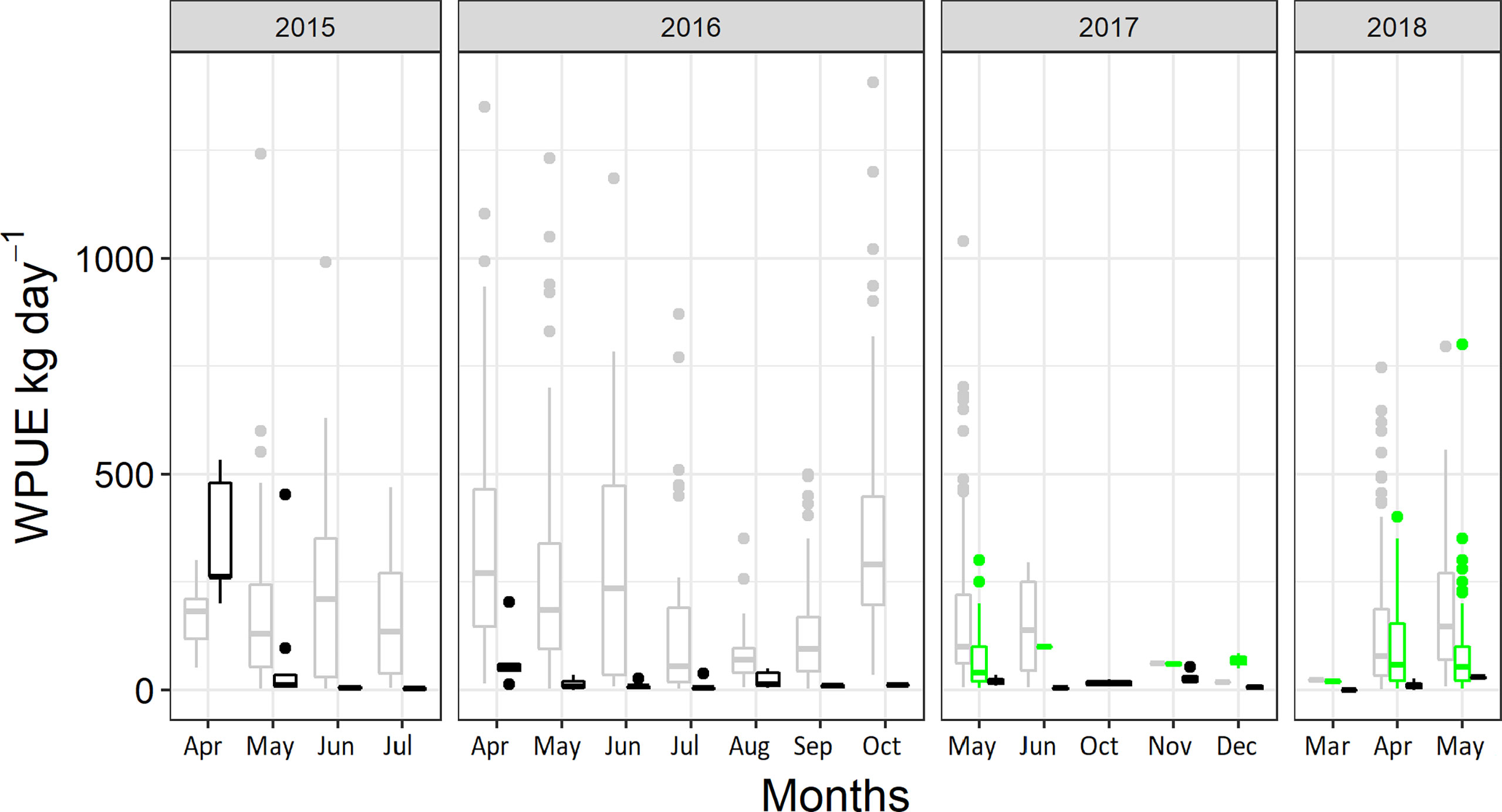
Figure 6 Mean catch, WPUE (kg day-1), of commercial sized cod (Gadus morhua) in pontoon trap, extrapolated to the potential catch from five simultaneously used traps (black), mean commercial landings from the gillnet fisheries in the same area (light grey) and seal damaged catch (green).
This study evaluated the effectiveness of pontoon traps for use in coastal cod fisheries. Initially, the pontoon traps where redesigned from being used to catch salmon so that they might be suitable for use in coastal cod fisheries. This included using of a 40-mm square-mesh panel in the fish chamber and of a hose net for releasing of unwanted catch. However, with the potential implementation of a new gear type in an existing fishery comes the need for evaluating gear specific parts that may influence catch rates of the target species. In cod fisheries, there is a need to understand the effect of leader net mesh size in order to lead fish into the fish chamber. With other fish species having shown to follow leader nets even with mesh sizes large enough for unobstructed passage (Lunneryd et al., 2002), the effect of leader net mesh size on catch rate was evaluated.
There were differences in catch rates between the larger, 100 mm mesh size and the smaller, 60 mm mesh size. Both mesh sizes tested were large enough for cod to swim through, but a static gear deployed and left for an extended time period in shallow water will eventually become overgrown and gather free-floating vegetation. An overgrown leader net may affect fish behavior, because it may be perceived as an obstacle so that the fish will be deterred from swimming nearby. The vegetation is also likely to affect the buoyancy of the gear and eventually cause it to sink it towards the bottom, ultimately affecting its effectiveness why the use of a larger mesh size would be beneficial over a smaller. The strong difference in catch rates between both traps types could however also be a result of the differing positions within the water. The two traps were placed with their trap nets and fish chambers on opposite sides, forcing fish to swim towards more shallow waters to be caught in the 60 mm trap, while the 100 mm trap was situated in a way that the fish were swimming to deeper waters before being trapped. To compensate for the setup with cod having to swim shallower, the two traps were to be switched during the trial. However, because the shallower set 60-mm trap broke before switching, this was not possible, consequently, the mechanism between cod specific mesh size dependent catch rates deserves further future attention.
The second trial evaluated the difference between traps with either bottom set (3 m trap) or floating fish chambers (8 m trap). To avoid confounding effects due to cod not following the trap net into shallower water, both traps were placed with the fish chamber facing deeper waters. The fish chamber was also positioned slightly above the bottom, as opposed to standing on the bottom, so that the stressors from waves and currents on the frame of the fish chamber could be reduced. With catch rates of cod in the bottom standing trap greater than those in the floating trap, indicates an unwillingness for cod to move up the water column. These results are consistent with conclusions from studies evaluating cod behavior in active gears, such as trawls, where conspecifics entering a trawl are known to mainly follow the lower netting panels unless they are stimulated to raise their vertical position (Ferro et al., 2007; Krag et al., 2009; Rosen et al., 2012).
The third trial evaluated the effect of leader-net length on catch rates. Traditional salmon traps are often equipped with a 100 to 120 m leader net, while 80 to 120 m leader nets have been used for herring and 60 m leader nets is commonly used for perch, (Lundin et al., 2011; Lundin et al., 2015). With pontoon traps traditionally being set from shore in limnic-marine transition areas to catch salmon, leader net lengths may affect catch rate when traps are deployed in an open environment. There were no difference in catch rates between the 100 and 200 m leader-net lengths. This indicates that catch rate is not regulated by leader net lengths in the interval of 100 to 200 m at least in non-estuarine coastal environments. Further studies are needed to conclude optimal leader net length. Pontoon traps are deployed and retrieved using smaller boats than those in gillnet fisheries, (which are typically between 5 to 8 m). As such, gear handling ease is an important factor in pontoon-trap fisheries. Apart from the lower gear costs, the use of shorter leader nets opens up for exchange of leader net within fishing periods, something that will lower the effect of buoyancy issues due to overgrowth and sinking due to increased weigh. Along with leader-net length, the positioning of the trap may be improved by optimal placement, such as near underwater pinnacles, which naturally attract more fish.
In the comparison between extrapolated catch in pontoon traps based on full-time fishery and traditional gillnet fishery, catch rates in gillnets exceeded those of pontoon traps throughout the study except during the initial period, when the extrapolated catch in the pontoon trap was expected to be greater than in gillnets (Figure 6). Therefore, bottom standing pontoon traps targeting cod are currently not an alternative to traditional gillnets. However, the advantages of the pontoon trap may be found in the comparison of seal-damaged catch and in a multi-species fishery. Since gear development is an iterative process, it is important to carefully evaluate the results of this study to be able to take the next step towards increasing pontoon catch rates when targeting cod.
One potential explanation for the lower catch rates in pontoon traps may be due to the positioning of the trap. Pontoon traps are large static gears with low potential to be moved within a fishery. Swedish fishing regulations stipulate that static gear with a height exceeding 1.5 m requires fishers to apply for a precise placement authorization. Therefore deployment positions are often limited to one or few spots. Further, pontoon traps can only be set in shallow waters, with a maximum deployment depth in these trials of 8 m. Shallow waters in the Baltic Sea are predominantly used as feeding grounds by cod during winter and early spring months, because both water temperature and food resources regulate their presence (Ljungberg, 2013). This probably leads to higher catch during these periods, as indicated by the greater catch obtained here in the spring. For the gillnet fishery, data for comparison were obtained for all fisheries performed in the current ICES statistic rectangle (39G3), independent of fishing depth. The comparison between pontoon and gillnets have to be put into perspective of a seasonality effect in catch rates because fishers target cod at various depth during the season in the evaluated area. With grey seal numbers and distributions increasing in the Baltic Sea, it is necessity to find alternative gears to gillnets, which are highly affected by seal inflicted damage. The amount of seal damaged catch during the trial period ranged between 20−100% of the catches in. For the pontoon trap there were no seal damaged catch during the trial period even though seal presence, visible through logbook data, was high. Fish caught in traps also have an increased probability of being of good quality, because they are live-caught with minimum stress (Uhlmann and Broadhurst, 2015; Meintzer et al., 2018). Other live-catching gear have however shown variation in catch quality and value, why this has to be further studied also in pontoon traps (Ovegård et al., 2012; Ljungberg et al., 2020).
Although the catch rates of cod was lower than the traditional gillnet fishery, the rigid construction of the pontoon trap helps to protect the catches whereas in gillnet fisheries catches remain unprotected. A rigid construction has been shown to be important to reduce seal damage. Even though seal damage is one of the main reasons for this fisheries decline, the poor state of the cod population also cause fisheries to decline (Casini et al., 2016). Due to this negative development, commercial fishing for cod in most of the Baltic Sea was banned in 2019 as the EU Commission announced emergency-measures to save the eastern Baltic cod stock from collapse. Although the current cod gillnet fishery is limited, there is an urgent need to develop and implement seal-safe and selective alternative fishing gear for a future sustainable coastal cod fisheries. In relation to this, bottom-set pontoon traps show the potential to catch large amounts of cod. The disadvantage is that the large rigid aluminum construction is inappropriate in an open coastline because wave and current actions strongly affect the gear. Also, the large construction makes the pontoon difficult to deploy and retrieve in an open sea environment, with increased difficulty with greater deployment depth. However understanding the catchability of bottom set pontoon traps may be used to improve the potential for a future coastal fishery using pontoon traps not only for cod but also in multi-target species fishery. The future perspective in gear development of leading gears in open coastal environment should focus on the use of rigid construction with less bottom contact along with the ability to move the gears depending on season to have them in the area where cod are currently located. There is also a need of further understanding about optimal leader net length depending on target species along with the potential of deploying the gear at various depth in order to target cod.
The datasets presented in this article are not readily available because the datasets are the property of the Seal and Fisheries Program in Sweden. Requests to access the datasets should be directed to Sven-Gunnar Lunneryd, c3Zlbi1ndW5uYXIubHVubmVyeWRAc2x1LnNl.
This study was reviewed and approved by Swedish Agency for Marine and Water Management (SwAM).
PL and S-GL did the conception and design of the study. SK did the extraction of the logbook data. PL performed the statistical analyses. PL wrote the first draft of the manuscript. SK wrote sections in the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
We thank the participating fisher Bengt Andersson for his efforts in making this study possible, along with Jerome Chladek. Edmond Sacre and Henrik Pärn for helpful discussions and comments on early versions of the manuscript. We also thank Matt Broadhurst and two anonymous reviewers for review. This study was funded by the European Structural Fund for Fisheries and the Program Seal and Fishery with economical support from the Swedish Agency for Marine and Water Management (SwAM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.981822/full#supplementary-material
Supplementary Table 1 | Predicted means of catch weights (WPUE) from the mixed effects models based on trial and pontoon trap.
Bäcklin B.-M., Moraeus C., Strömberg A., Karlsson O., Härkönen T. (2016). Sälpopulationer och sälhälsa. sealpopulations and seal health. havet 2015/2016: Om miljötillståndet i svenska havsområden the Sea 2015/2016. about the environmental condition in Swedish marine areas. (Gothenburg: Swedish Agency for Marine and Water Management).
Bates D., Machler M., Bolker B. M., Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 (1), 1–48. doi: 10.18637/jss.v067.i01
Bergenius M., Ringdahl K., Sundelöf A., Carlshamre S., Wennhage H., Valentinsson D. (2018). “Atlas över svenskt kust- och havsfiske 2003-205,” in Sveriges lantbruksuniversitet (Drottningholm Lysekil Öregrund: Institutionen för akvatiska resurser).
Blomquist J., Waldo S. (2021). Seal interactions and exits from fisheries: insights from the Baltic Sea cod fishery. ICES J. Mar. Sci. 78 (8), 2958–2966. doi: 10.1093/icesjms/fsab173
Bruckmeier K., Larsen C. H. (2008). Swedish Coastal fisheries - from conflict mitigation to participatory management. Mar. Policy 32 (2), 201–211. doi: 10.1016/j.marpol.2007.09.005
Bruckmeier K., Westerberg H., Varjopuro R. (2013). “Baltic seal reconciliation in practice. the seal conflict and its mitigation in Sweden and finland,” in Human-wildlife conflicts in Europe fisheries and fish-eating vertebrates as a model case. Eds. Klenke R. A., Ring I., Kranz A., Jepsen N., Rauschmayer F., Henle K. (Heidelberg: Springer Berlin), 15–48.
Butler J. R. A., Middlemas S. J., Graham I. M., Harris R. N. (2011). Perceptions and costs of seal impacts on Atlantic salmon fisheries in the Moray firth, Scotland: Implications for the adaptive co-management of seal-fishery conflict. Mar. Policy 35 (3), 317–323. doi: 10.1016/j.marpol.2010.10.011
Calamnius L., Lundin M., Fjalling A., Konigson S. (2018). Pontoon trap for salmon and trout equipped with a seal exclusion device catches larger salmons. PloS One 13 (7), 1–13. doi: 10.1371/journal.pone.0201164
Casini M., Kall F., Hansson M., Plikshs M., Baranova T., Karlsson O., et al. (2016). Hypoxic areas, density-dependence and food limitation drive the body condition of a heavily exploited marine fish predator. R. Soc. Open Sci. 3, 1–15. doi: 10.1098/rsos.160416
Ferro R. S. T., Jones E. G., Kynoch R. J., Fryer R. J., Buckett B. E. (2007). Separating species using a horizontal panel in the Scottish north Sea whitefish trawl fishery. Ices J. Mar. Sci. 64 (8), 1543–1550. doi: 10.1093/icesjms/fsm099
Fjalling A. (2005). The estimation of hidden seal-inflicted losses in the Baltic Sea set-trap salmon fisheries. ICES J. Mar. Sci. 62 (8), 1630–1635. doi: 10.1016/j.icesjms.2005.02.015
Forrest K. W., Cave J. D., Michielsens C. G. J., Haulena M., Smith D. V. (2009). Evaluation of an electric gradient to deter seal predation on salmon caught in gill-net test fisheries. North Am. J. Fish. Manage. 29 (4), 885–894. doi: 10.1577/M08-083.1
Galatius A., Teilmann J., Dahne M., Ahola M., Westphal L., Kyhn L. A., et al. (2020). Grey seal halichoerus grypus recolonisation of the southern Baltic Sea, Danish straits and kattegat. Wildl. Biol. 2020 (4), 1–10. doi: 10.2981/wlb.00711
Grant S. M., Brown J. A. (1998). Diel foraging cycles and interactions among juvenile Atlantic cod (Gadus morhua) at a nearshore site in newfoundland. Can. J. Fish. Aquat. Sci. 55 (6), 1307–1316. doi: 10.1139/f97-291
Gregory R. S., Anderson J. T. (1997). Substrate selection and use of protective cover by juvenile Atlantic cod gadus morhua in inshore waters of Newfoundland. Mar. Ecol. Prog. Ser. 146 (1-3), 9–20. doi: 10.3354/meps146009
Hårding K. C., Härkönen T. J. (1999). Development in the Baltic grey seal (Halichoerus grypus) and ringed seal (Phoca hispida) populations during the 20th century. AMBIO 28 (7), 619–627.
HELCOM (2010). Hazardous substances in the Baltic Sea–an integrated thematic assessment of hazardous substances in the baltc Sea. (Helsinki: Helsinki Commission).
Hemmingsson M., Fjälling A., Lunneryd S.-G. (2008). The pontoon trap: Description and function of a seal-safe trap-net. Fish. Res. 93 (3), 357–359. doi: 10.1016/j.fishres.2008.06.013
Hui T. C. Y., Morita Y., Kobayashi Y., Mitani Y., Miyashita K. (2017). Dietary analysis of harbour seals (Phoca vitulina) from faecal samples and overlap with fisheries in erimo, Japan. Mar. Ecol. Evol. Persp. 38 (3), 1–12. doi: 10.1111/maec.12431
Hume F., Pemberton D., Gales R., Brothers N., Greenwood M. T. (2002). Trapping and relocating seals from salmonid fish farms in tasmania 1990–2000: was it a success? Pap Proc. R Soc. Tasman. 136, 1–6. doi: 10.26749/rstpp.136.1
ICES (2022) Copenhagen. Available at: https://www.ices.dk/data/maps/Pages/ICES-statistical-rectangles.aspx.
Jounela P., Suuronen P., Millar R. B., Koljonen M.-L. (2006). Interactions between grey seal (Halichoerus grypus), Atlantic salmon (Salmo salar), and harvest controls on the salmon fishery in the gulf of bothnia. ICES J. Mar. Sci. 63 (5), 936–945. doi: 10.1016/j.icesjms.2006.02.005
Königson S. (2007). Seal behaviour around fishing gear and its impact on Swedish fisheries.Licentiate thesis (Göteborg, Sweden: Göteborg University).
Königson S., Fjälling A., Berglind M., Lunneryd S.-G. (2013). Male Gray seals specialize in raiding salmon traps. Fish. Res. 148, 117–123. doi: 10.1016/j.fishres.2013.07.014
Königson S., Fredriksson R. E., Lunneryd S. G., Strömberg P., Bergström U. M. (2015a). Cod pots in a Baltic fishery: are they efficient and what affects their efficiency? Ices J. Mar. Sci. 72 (5), 1545–1554. doi: 10.1093/icesjms/fsu230
Königson S., Hemmingsson M., Lunneryd S. G., Lundström K. (2007). Seals and fyke nets: An investigation of the problem and its possible solution. Mar. Biol. Res. 3 (1), 29–36. doi: 10.1080/17451000601072596
Königson S., Lövgren J., Hjelm J., Ovegård M., Ljunghager F., Lunneryd S. G. (2015b). Seal exclusion devices in cod pots prevent seal bycatch and affect their catchability of cod. Fish. Res. 167, 114–122. doi: 10.1016/j.fishres.2015.01.013
Königson S., Lunneryd S.-G., Stridh H., Sundqvist F. (2009). Grey seal predation in cod gillnet fisheries in the central Baltic Sea. J. Northwest Atl. Fish. Sci. 42 (4), 41–47. doi: 10.2960/J.v42.m654
Krag L. A., Holst R., Madsen N. (2009). The vertical separation of fish in the aft end of a demersal trawl. Ices J. Mar. Sci. 66 (4), 772–777. doi: 10.1093/icesjms/fsp034
Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82 (13), 1–26. doi: 10.18637/jss.v082.i13
Lehtonen E., Lehmonen R., Kostensalo J., Kurkilahti M., Suuronen P. (2022). Feasibility and effectiveness of seal deterrent in coastal trap-net fishing-development of a novel mobile deterrent. Fish. Res. 252, 1–6. doi: 10.1016/j.fishres.2022.106328
Lehtonen E., Suuronen P. (2004). Mitigation of seal-induced damage in salmon and whitefish trapnet fisheries by modification of the fish bag. ICES J. Mar. Sci. 61 (7), 1195–1200. doi: 10.1016/j.icesjms.2004.06.012
Ljungberg P. (2013). Habitat choice and foraging behaviour in temperate coastal environments (Lund, Sweden: Lund University).
Ljungberg P., Ovegard M., Ohman K., Konigson S. (2020). Correlation between catch method, condition, and diet patterns in Atlantic cod (Gadus morhua). Ices J. Mar. Sci. 77 (1), 267–277. doi: 10.1093/icesjms/fsz167
Lundin M., Calamnius L., Hillström L., Lunneryd S.-G. (2011). Size selection of herring (Clupea harengus membras) in a pontoon trap equipped with a rigid grid. Fish. Res. 108 (1), 81–87. doi: 10.1016/j.fishres.2010.12.001
Lundin M., Calamnius L., Lunneryd S.-G., Magnhagen C. (2015). The efficiency of selection grids in perch pontoon traps. Fish. Res. 162, 58–63. doi: 10.1016/j.fishres.2014.09.017
Lunneryd S. G. (2018). “Skonsam vittjning av pushup-fällor för lax och sik (in Swedish),” in Sekretariatet för selektiv fiske-rapportering av 2014 års verksamhet. aqua reports 2018:2, vol. 63 . Ed. Nilsson H. (Lysekil: Sveriges lantbruksuniversitet, Institutionen för akvatiska resurser).
Lunneryd S. G., Fjalling A., Westerberg H. (2003). A large-mesh salmon trap: a way of mitigating seal impact on a coastal fishery. ICES J. Mar. Sci. 60 (6), 1194–1199. doi: 10.1016/s1054-3139(03)00145-0
Lunneryd S.-G., Hemingsson M., Tärnlund S. (2005). “A voluntary logbook scheme as a method of monitoring the by-catch of seals in Swedish coastal fisheries,” in ICES annual science conference location. (Copenhagen: ICES).
Lunneryd S. G., Westerberg H., Wahlberg M. (2002). Detection of leader net by whitefish coregonus lavaretus during varying environmental conditions. Fish. Res. 54 (3), 355–362. doi: 10.1016/S0165-7836(01)00271-5
Lyle J. M., Willcox S. T., Hartmann K. (2016). Underwater observations of seal-fishery interactions and the effectiveness of an exclusion device in reducing bycatch in a midwater trawl fishery. Can. J. Fish. Aquat. Sci. 73 (3), 436–444. doi: 10.1139/cjfas-2015-0273
Madsen N. (2007). Selectivity of fishing gears used in the Baltic Sea cod fishery. Rev. Fish Biol. Fish. 17 (4), 517–544. doi: 10.1007/s11160-007-9053-y
Mate B. R., Brown R. F., Greenlaw C. F., Harvey J. T., Temte J. (1986). “An acoustic harassment technique to reduce seal predation on salmon,” in Acoustical deterrents in marine mammal conflicts with fisheries. Eds. Mate B. R., Harvey J. T. (Corvallis: Oregon Sea Grant), 23–36.
Meintzer P., Walsh P., Favaro B. (2018). Comparing catch efficiency of five models of pot for use in a Newfoundland and Labrador cod fishery. PloS One 13 (6), 1–18. doi: 10.1371/journal.pone.0199702
Nelson M. L., Gilbert J. R., Boyle K. J. (2006). The influence of siting and deterrence methods on seal predation at Atlantic salmon (Salmo salar) farms in Maine 2001–2003. Can. J. Fish. Aquat. Sci. 63 (8), 1710–1721. doi: 10.1139/f06-067
Ovegård M., Berndt K., Lunneryd S. G. (2012). Condition indices of Atlantic cod (Gadus morhua) biased by capturing method. Ices J. Mar. Sci. 69 (10), 1781–1788. doi: 10.1093/icesjms/fss145
Ovegård M., Königson S., Persson A., Lunneryd S. G. (2011). Size selective capture of Atlantic cod (Gadus morhua) in floating pots. Fish. Res. 107, 239–244. doi: 10.1016/j.fishres.2010.10.023
R-Development-Core-Team (2008). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Rosen S., Engas A., Ferno A., Jorgensen T. (2012). The reactions of shoaling adult cod to a pelagic trawl: Implications for commercial trawling. Ices J. Mar. Sci. 69 (2), 303–312. doi: 10.1093/icesjms/fsr199
Sepúlveda M., Oliva D. (2005). Interactions between south American sea lions otaria flavescens (Shaw) and salmon farms in southern Chile. Aquacult. Res. 36, 1062–1068. doi: 10.1111/j.1365-2109.2005.01320.x
Suuronen P., Chopin F., Glass C., Løkkeborg S., Matsushita Y., Queirolo D., et al. (2012). Low impact and fuel efficient fishing–looking beyond the horizon. Fish. Res., 119–120. doi: 10.1016/j.fishres.2011.12.009
Suuronen P., Siira A., Kauppinen T., Riikonen R., Lehtonen E., Harjunpää H. (2006). Reduction of seal-induced catch and gear damage by modification of trap-net design: Design principles for a seal-safe trap-net. Fish. Res. 79 (1-2), 129–138. doi: 10.1016/j.fishres.2006.02.014
Swedish Agency for Marine and Water Management (2019). Nationell förvaltningsplan för gråsäl (Halichoerus grypus) i Östersjön. report 2019:24. (Gothenburg: Swedish Agency for Marine and Water Management)
Uhlmann S. S., Broadhurst M. K. (2015). Mitigating unaccounted fishing mortality from gillnets and traps. Fish Fish. 16 (2), 183–229. doi: 10.1111/faf.12049
Vanhatalo J., Vetemaa M., Herrero A., Aho T., Tiilikainen R. (2014). By-catch of grey seals (Halichoerus grypus) in Baltic fisheries-a Bayesian analysis of interview survey. PloS One 9 (11), 1–16. doi: 10.1371/journal.pone.0113836
Varjopuro R. (2011). Co-Existence of seals and fisheries? adaptation of a coastal fishery for recovery of the Baltic grey seal. Mar. Policy 35, 450–456. doi: 10.1016/j.marpol.2010.10.023
Keywords: seal-fisheries conflict, passive gear development, selective gear, pontoon trap, seal-safe gear
Citation: Ljungberg P, Königson S and Lunneryd S-G (2022) An evolution of pontoon traps for cod fishing (Gadus morhua) in the southern Baltic Sea. Front. Mar. Sci. 9:981822. doi: 10.3389/fmars.2022.981822
Received: 29 June 2022; Accepted: 15 September 2022;
Published: 03 October 2022.
Edited by:
Alessandro Lucchetti, National Research Council (CNR), ItalyReviewed by:
Matt Broadhurst, NSW Government, AustraliaCopyright © 2022 Ljungberg, Königson and Lunneryd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Ljungberg, cGV0ZXIubGp1bmdiZXJnQHNsdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.