- 1Loggerhead Marinelife Center, Juno Beach, FL, United States
- 2Department of Biological Sciences, Vanderbilt University, Nashville, TN, United States
In the face of modern challenges, analyzing sea turtle nesting trends is critical to better understand impacts to these vulnerable species. The introduction of hard-armoring structures (e.g., seawalls, rock revetments) on sea turtle nesting beaches poses a threat to nesting leatherback (Dermochelys coriacea), loggerhead (Caretta caretta), and green (Chelonia mydas) sea turtles due to habitat loss and turtle interactions with the physical structure. Despite much of Florida’s coastline being protected by some form of hard-armoring technology, research on the impacts of these structures to sea turtles is limited to loggerheads. Our objectives were to (1) examine nest density, nesting success, washout rates, and hatching and emergence success at hard-armoring sites in comparison to a control area and (2) characterize impacts of obstructions encountered by sea turtles nesting in northern Palm Beach County, Florida. Our results indicate that the hard-armoring site showed significantly lower nest density for green turtles and nesting success for loggerheads and green turtles in comparison to a control area. Additionally, nesting success for loggerheads and green turtles that encountered hard-armoring structures was significantly lower in comparison to those that encountered no obstructions or other obstructions (e.g., beach furniture, walkovers, escarpments, etc.). These results suggest that hard-armoring structures negatively impact sea turtle nesting behavior, which could result in loss of energy or other physiological derangements. Green turtles showed the most significant differences between the two sites, likely a result of their typical nest site selection favoring the upper portions of the beach, crawling further distances from the high-water line than loggerheads or leatherbacks. Before additional hard-armoring structures are permitted and installed, governing agencies should first consider more natural methods of protecting shorelines (e.g., dune restoration).
Introduction
Coastal development is a major threat to terrestrial and marine organisms that rely on these dynamic ecosystems for survival (Crain et al., 2009; Dugan et al., 2011; Patrick et al., 2016; Dugan et al., 2018). As the human population encroaches upon the coastline for recreational, economic, cultural, and aesthetic benefits, pressures on these ecosystems are tested (Kittinger and Ayers, 2010; Dugan et al., 2011; Gittman et al., 2016). Natural beaches accrete and erode, changing the shoreline over time (Hanley et al., 2014); however, construction of buildings, roads, and other infrastructure near the coast hampers these natural processes. Coastal squeeze, the degradation or complete loss of coastline as a result of anthropogenic structures restricting shoreline fluctuations, is compounded by sea level rise (Doody, 2004; Fish et al., 2008; Dugan et al., 2011). Coastal development can also have indirect impacts on the environment, including increasing pollution and accumulation of marine debris alongside developed areas (Nordstrom, 1994; Leite et al., 2014). These pressures are ever apparent in Florida, USA, whereby millions of tourists and residents are attracted annually to the nearly 1,300 km of sandy beaches (FDEP, 2021). Current estimates suggest that 83% of Florida’s coastline is critically eroding due to anthropogenic and/or natural impacts that have led to loss of beach to a degree that “upland development, recreational interests, wildlife habitat, or important cultural resources are threatened or lost (FDEP, 2021).”
Florida’s beaches are also a globally important nesting ground for threatened loggerhead sea turtles (Caretta caretta) and a regionally important area for endangered nesting leatherback (Dermochelys coriacea) and threatened green (Chelonia mydas) turtles (Meylan et al., 1995; Stewart et al., 2014; Ceriani et al., 2019). Unfortunately, 94% of Florida’s sea turtle nesting beaches are exposed to cumulative coastal modifications, and the number of permitted alterations continues to grow (Nelson Sella and Fuentes, 2019). Previous studies have evaluated the impacts of various facets of coastal development on sea turtles, including beach nourishment, artificial lighting, and armoring structures (Grain et al., 1995; Salmon et al., 1995; Butler, 1998; Brock et al., 2009; Lopez et al., 2015; Hirsch et al., 2019). In a survey of sea turtle experts across four continents, it was found that beach armoring was perceived as the largest threat to sea turtle nesting beaches in terms of coastal development, which ranked higher than artificial lighting, special events, beach cleaning, and beach sand placement activities (Nelson Sella et al. 2019). Beach armoring (e.g., vertical seawalls, sloped revetments, wooden walls, sandbag/geotextile containers systems) is a termed used to define manmade structures that are erected parallel to the shoreline with the intent of protecting inland development from coastal erosion and storm surge (Griggs, 2005; Dugan et al., 2011). These structures are used in place of a natural dune system, which typically functions to prevent beach erosion through sand accumulation via wind and wave action in the vegetated dune and provides storm protection by dissipating waves (Hanley et al., 2014). Unfortunately, beach armoring, most notably seawalls, increases longshore current intensity, limits how much energy can be converted into sand displacement when waves crash into the dune and flatten the beach, and sharpens and promotes erosion due to the offshore profile cut-off (Pilkey Jr. et al., 1984; Hall and Pilkey, 1991); therefore, these structures threaten the sandy beaches that sea turtles rely on to complete their reproductive cycle.
In addition to habitat loss, barriers that limit access to suitable sandy beach habitat can deter nesting sea turtles from completing the egg-laying process. When a female emerges, but abandons her nesting attempt, she will often return later that night or on subsequent nights to nest (Davis and Whiting, 1977; Talbert et al., 1980; Limpus, 1985; Miller, 1997). This results in additional energy expended by the female (Prange and Jackson, 1976; Mosier, 1998; Burns et al., 2016). Some females may still nest despite encountering an obstacle (i.e., obstruction); however, they may deposit their eggs in sub-optimal habitat. Nesting loggerheads that encountered a portable wall barrier nested just as frequently as those that were given access to the entire beach but laid their nests significantly closer to the waterline (Witherington et al., 2011). Similarly, leatherbacks that encountered steep escarpments due to beach erosion had more abandoned nesting attempts and were more likely to deposit their eggs in high-risk areas (Rivas et al., 2016). Depositing eggs closer to the waterline can increase tidal inundation, negatively impact developing embryos through suffocation, reduce hatching success, alter hatchling fitness, or lead to nest washout (Whitmore and Dutton, 1985; McGehee, 1990; Milton et al., 1994; Martin, 1996; Foley et al., 2006; Caut et al., 2010; Pike et al., 2015; Erb et al., 2018; Fleming et al., 2020; Limpus et al., 2020). Furthermore, if there is less available habitat for nests to be laid, there may be increased incidence of predation, microbial infection, or destruction of nests by other nesting females on high-density nesting beaches (Mazaris et al., 2009).
While coastal armoring is considered a key threat to the preservation of threatened and endangered sea turtles as it may directly impact nesting behavior and reproductive success, few studies aim to quantify these impacts (Horrocks and Scott, 1991; Mosier, 1998; Mosier and Witherington, 2002; Rizkalla and Savage, 2011). Mosier (1998) found that loggerheads emerged from the ocean less often and were less likely to nest in front of a seawall; however, the study was limited temporally (across 25 d of nesting season. The only other in-depth study on coastal armoring impacts also concluded that loggerheads nest less frequently in front of seawalls and nests that were deposited were more susceptible to being washed out during storm events (Rizkalla and Savage, 2011). Both studies focused solely on loggerheads, whose nesting behaviors vary from that of other species (Dodd, 1988). Therefore, the goal of this study was to use a historical dataset to evaluate the impacts of coastal armoring on different species of sea turtle. Because Florida is a hotspot for coastal erosion, beach armoring, and sea turtle nesting (Fuentes et al., 2016), the objectives of this study were to (1) examine nest density, nesting success (i.e., the proportion of successfully laid nests compared to the total number of crawls), washout rates, and hatching and emergence success at hard-armoring sites in comparison to a control area and (2) characterize the impacts of obstructions encountered by leatherback, loggerhead, and green sea turtles nesting in northern Palm Beach County, Florida USA.
Materials and methods
Site description and sea turtle nest monitoring
A 2.97 km stretch of beach located in northern Palm Beach County, Florida is comprised of Tequesta, Coral Cove, and Jupiter Inlet Colony Beaches, which are home to the highest density (nests/km) of loggerhead sea turtle nests in the Western Hemisphere (Ceriani and Meylan, 2017; Nelson Sella and Fuentes, 2019; Ataman et al., 2021) and is a regionally important nesting area for leatherbacks and green turtles. Annual nest numbers for leatherbacks, loggerheads, and green turtles in this area from 2007–2021 are reported in Figure 1. Loggerhead nesting has significantly increased since 2007 (y = -17.50x2 + 70693x – 71408911; r2 = 0.789; p < 0.001; Figure 1B). Leatherback (Figure 1A) and green turtle (Figure 1C) nesting showed no significant trends over time.
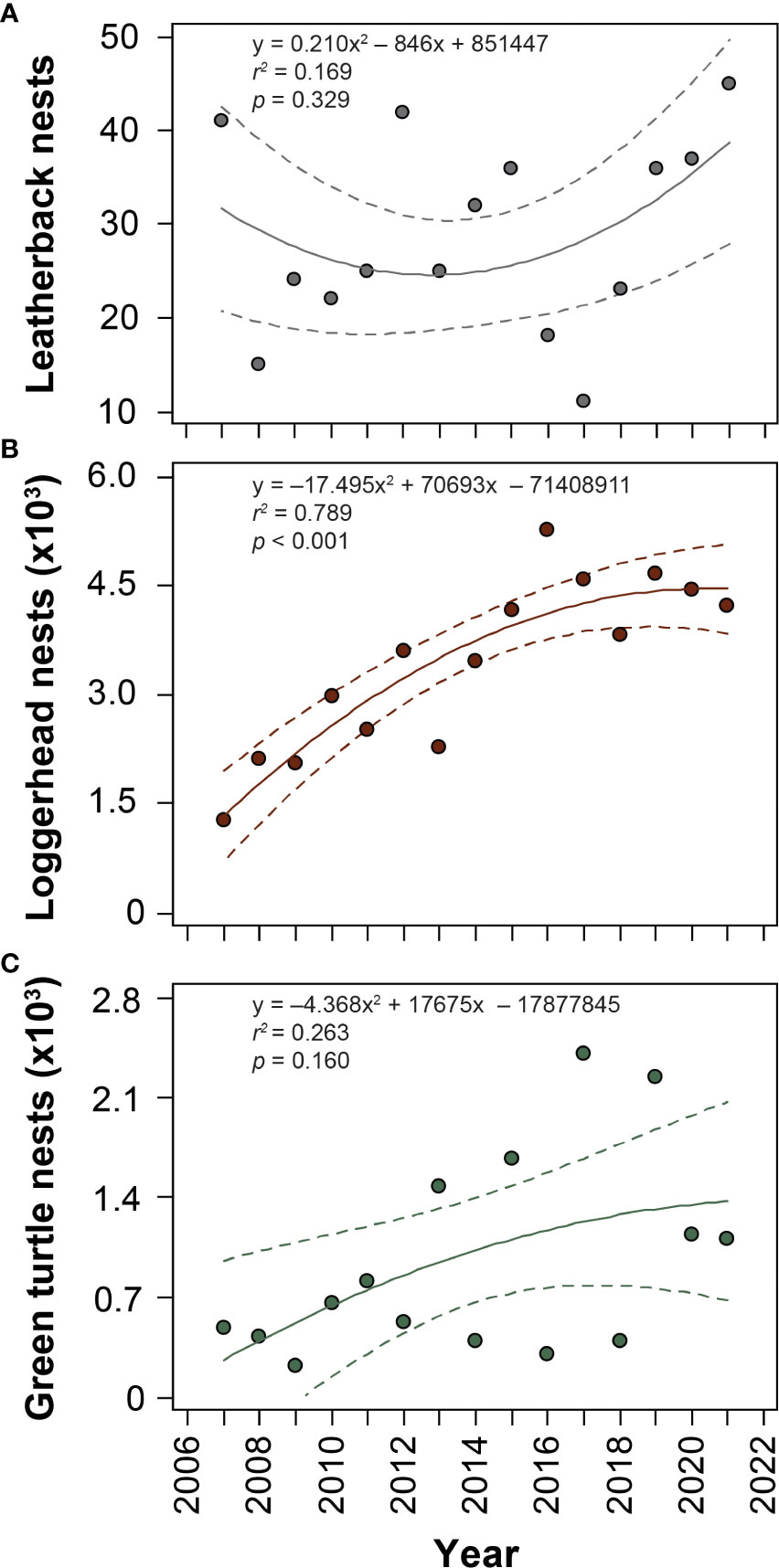
Figure 1 Historical nest numbers from 2007–2021 for (A) leatherback (Dermochelys coriacea), (B) loggerhead (Caretta caretta), and (C) green (Chelonia mydas) sea turtles on Tequesta, Coral Cove, and Jupiter Inlet Colony Beaches (2.97 km). Results of polynomial regression analyses are shown, with loggerheads showing a significant increase in nest numbers during the study period.
Standardized marine turtle nesting surveys were conducted on Tequesta, Coral Cove, and Jupiter Inlet Colony Beaches from 2007–2021 in accordance with statewide nesting beach protocols (FWC, 2016). These beaches were divided into 13 zones, with each zone averaging (± SD) 0.23 ± 0.10 km in distance (range: 0.06–0.37 km). For purposes of this study, five zones located on Tequesta and Jupiter Inlet Colony Beaches were selected for analysis. The control area was composed of three zones of a mostly natural dune system fronting a county park, totaling 0.58 km in length. A dune restoration in the winter of 2013–2014 placed upland mined sand in the dunes, which was then planted with native vegetation. The hard-armoring site was composed of two zones immediately south of the control area totaling 0.59 km in length. The hard-armoring site is made up of a rock revetment, a concrete revetment, and various concrete seawalls that define the dune line for the entire stretch of beach in this area (Figure 2).
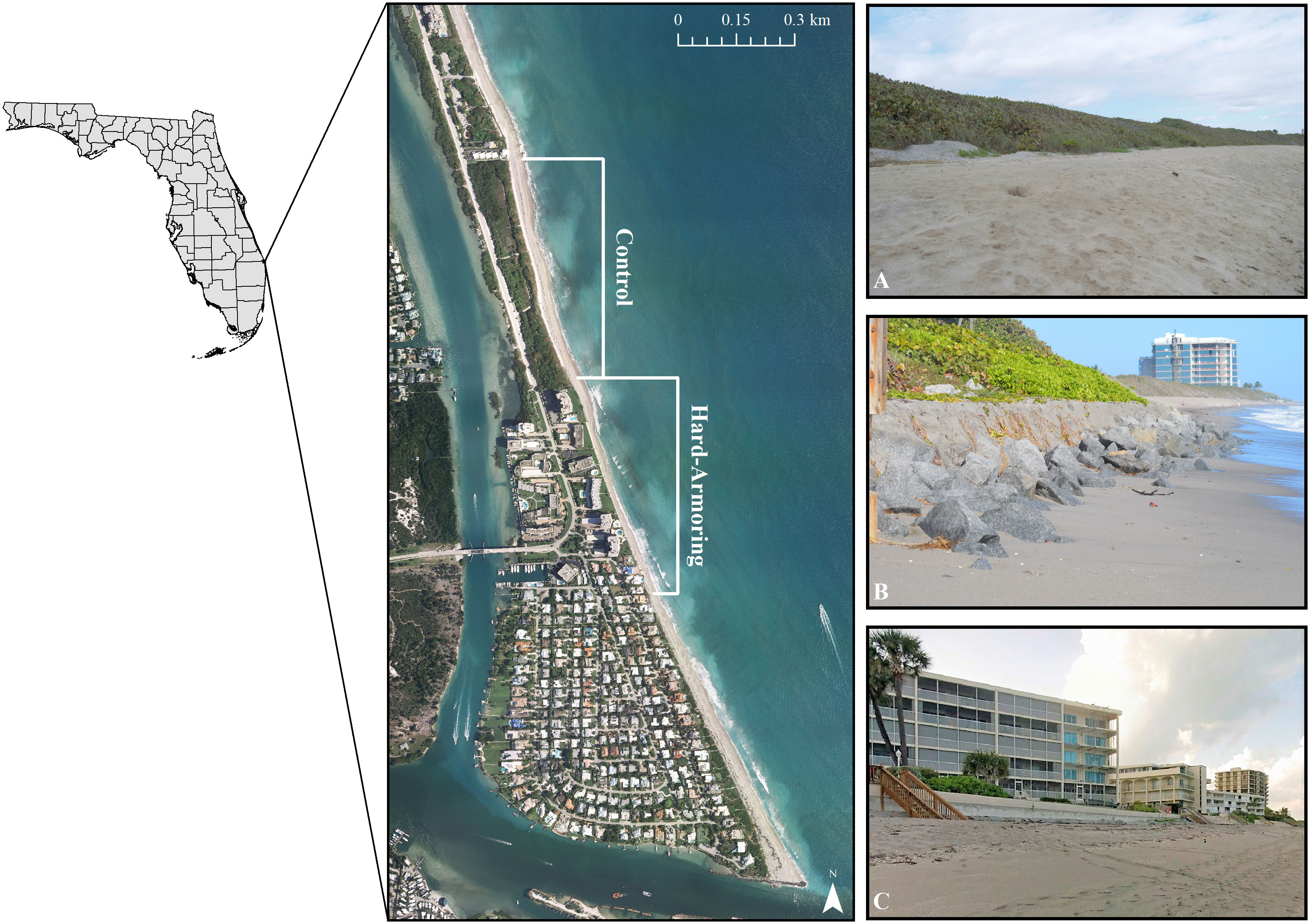
Figure 2 Map showing the location of the control and hard-armoring sites on Tequesta and Jupiter Inlet Colony Beaches in northern Palm Beach County, Florida, USA. The control site is characterized by a mostly natural dune system fronting a county park (A), while the hard-armoring site is composed of a rock revetment (B), a concrete revetment, and concrete seawalls (C).
Daily nesting surveys were conducted at dawn, with varying start and end dates, but always covered the peak of nesting season from 1 May–31 Aug. Surveys conducted from 2013–2021 extended from 1 Mar–31 Oct to cover the entirety of sea turtle nesting season in Florida. Using visual cues left in the sand, each sea turtle emergence above the most recent high tide line was identified to species (i.e., leatherback, loggerhead, or green turtle) and identified as a nest or abandoned nesting attempt. Nesting success was calculated by comparing the ratio of successful nests to the total number of crawls (i.e., nests plus abandoned nesting attempts). Obstruction encounters were noted when the path of a crawl intersected with an object such as beach furniture, boats, concrete seawall/revetment, dead trees or logs, debris, escarpments, live trees, natural rocks, other (e.g., cinderblocks, sprinklers, PVC pipe, rope, etc.), recreational equipment, rock seawall/revetment, sand fencing, trash cans, or beach walkovers.
All leatherback nests were marked for assessment of hatching success and emergence success. Due to the high nest density for loggerhead and green turtles, a subsample of nests were systematically marked in a manner that reflected the temporal and spatial distribution of nesting in the area. Marked nests were monitored daily to document overwash events, predation, washout, and hatchling emergence. Marked nests were excavated 72 hours after the first sign of hatchling emergence. If no signs of emergence were observed, loggerhead and green turtle nests were evaluated after an incubation period of 70 days, and leatherback nests were evaluated after a period of 80 days in accordance with statewide protocols (FWC, 2016). Hatching success represents the percentage of hatched eggs and was calculated by dividing the number of hatched eggs by total clutch size, while emergence success describes the percentage of hatchlings escaping the nest and was calculated by dividing the number of hatched eggs minus live or dead hatchlings in the egg chamber by total clutch size (Miller, 1997).
Statistical analyses
Statistical analyses were performed using RStudio (v. 4.1.2; R Core Team, 2020). Parametric tests were chosen when the data fit a Gaussian distribution; otherwise, non-parametric tests were selected. Five variables were compared between the hard-armoring and the control sites including (1) nest density (nests/km) using Wilcoxon rank sum test with continuity correction for leatherbacks and green turtles and a Welch’s two-sample t-test for loggerheads; (2) nesting success (%) using a Pearson’s chi-square test with Yates’ continuity correction for all three species; (3) washout rate using a Fisher’s exact test for leatherbacks and Pearson’s chi-square test with Yates’ continuity correction for loggerheads and green turtles; and (4) hatching success (%) and (5) emergence success (%) using a beta regression for all three species. A beta regression was used for hatching success and emergence success as these variables were percentages. Hatching and emergence success were analyzed in two ways, one where washouts (equal to 0% hatching success and emergence success due to nests washing away from extreme tides) were included in the analyses and a second where washouts were excluded. Lastly, Fisher’s exact tests (leatherbacks) or chi-square tests with Yates’ continuity correction (loggerheads and green turtles) were used to compare nesting success at the hard-armoring site between turtles that encountered hard-armoring structures (e.g., concrete seawall/revetment or rock seawall/revetment), other obstructions (e.g., trees, debris, natural rock, escarpments, etc.), or no obstructions.
Results
Nest density
Leatherback nest density was significantly higher (W = 53.5; p = 0.014; Figure 3) and green turtle nest density was significantly lower (W = 196; p = 0.001; Figure 5A) at the hard-armoring structures in comparison to the control site, while loggerhead nest density did not differ (t(23.537) = 0.353; p = 0.728). Mean nest densities for each species at the two sites are shown in Table 1.
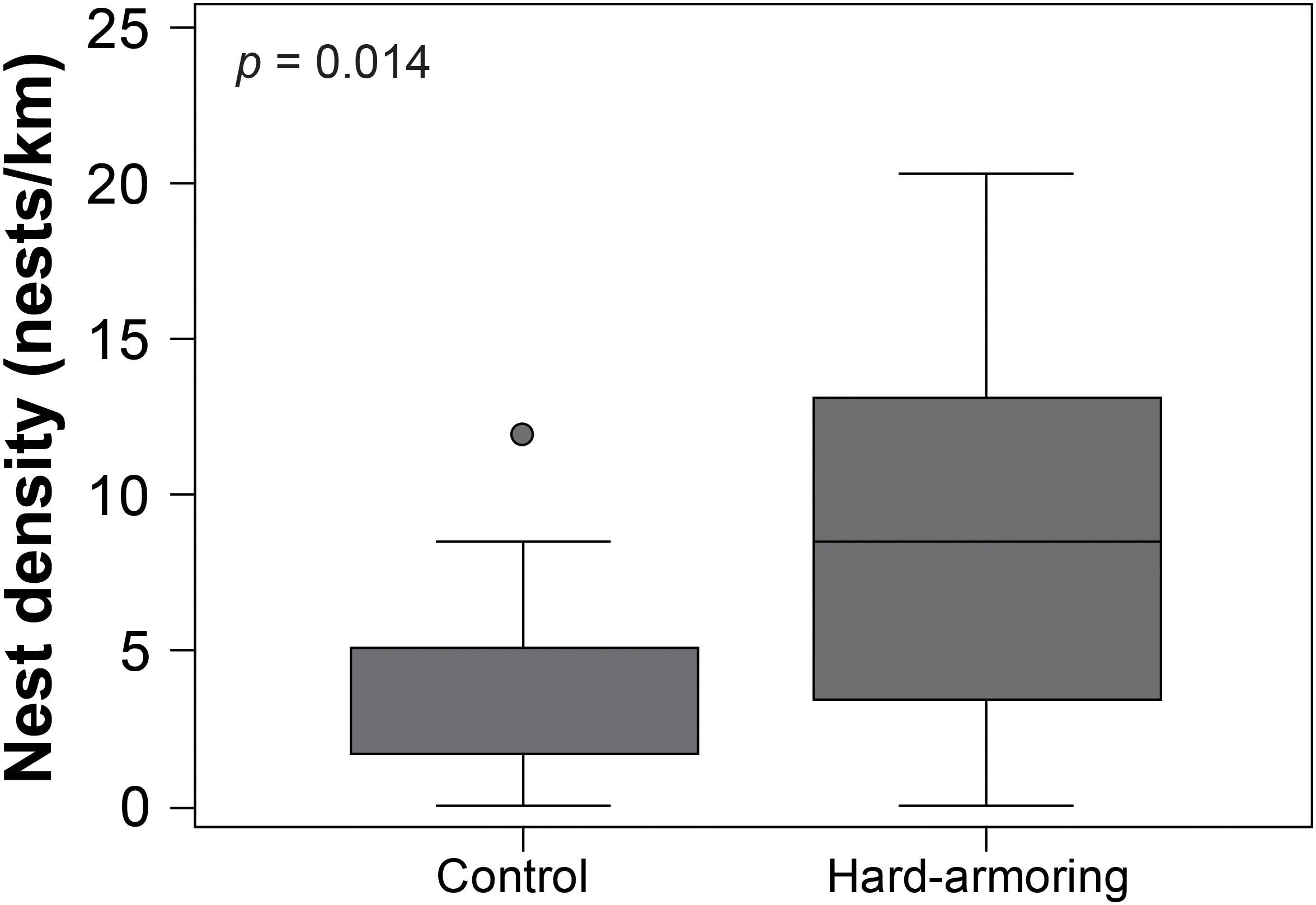
Figure 3 Nest density was significantly higher at the hard-armoring structures in comparison to the control site for nesting leatherback sea turtles (Dermochelys coriacea) on Tequesta and Jupiter Inlet Colony Beaches, Florida, USA. The middle line represents the median, the boxes represent the first and third quartiles, while the whiskers represent the range. The circle is an "outside value" that is greater than 1.5, but less than 3 times the upper quartile.
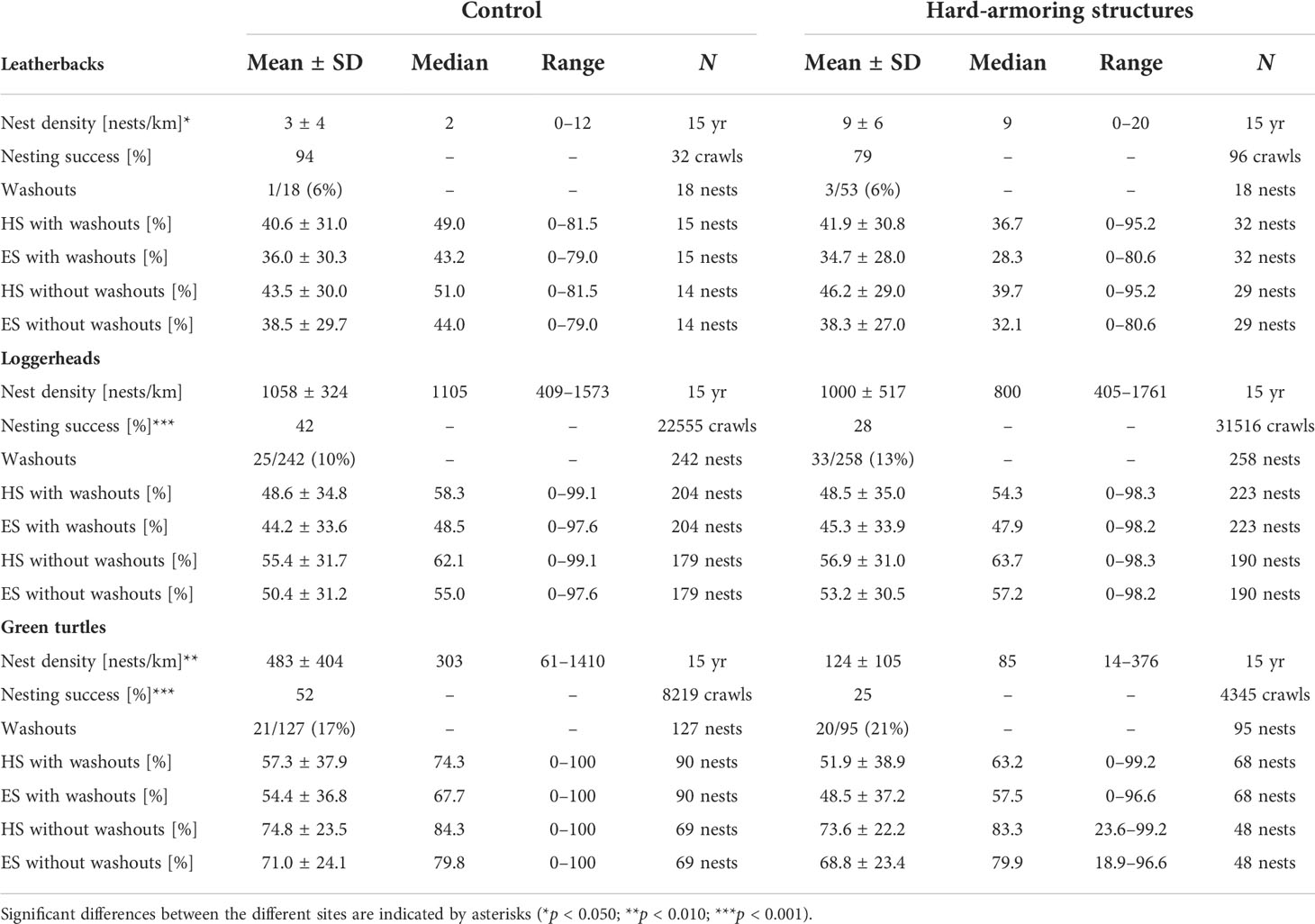
Table 1 Nest density, nesting success, washouts, and hatching success (HS) and emergence success (ES) with and without washouts of leatherback (Dermochelys coriacea), loggerhead (Caretta caretta), and green (Chelonia mydas) sea turtles nesting at the control site in comparison to the hard-armoring structures on Tequesta and Jupiter Inlet Colony Beaches, Florida, USA from 2007–2021.
Nesting success and obstructions
Loggerhead (χ2(1) = 1050; p < 0.001; Figure 4) and green turtle (χ2(1) = 831.6; p < 0.001; Figure 5B) nesting success were significantly lower at the hard-armoring structures in comparison to the control site, while leatherback nesting success did not differ (Fisher’s exact test; p = 0.063). Mean nesting success for each species at the two sites is shown in Table 1.
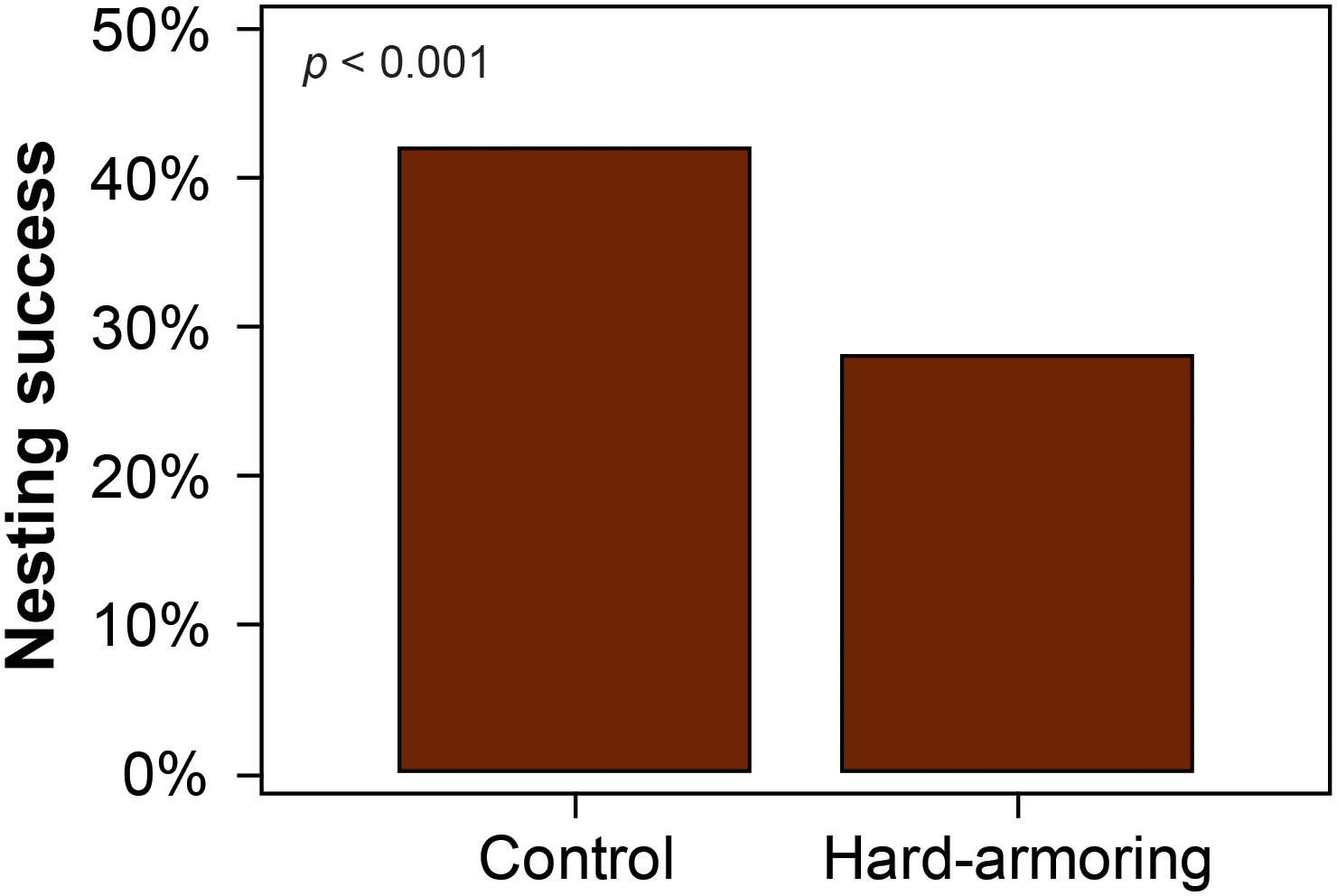
Figure 4 Nesting success was significantly lower at the hard-armoring structures in comparison to the control site for nesting loggerhead sea turtles (Caretta caretta) on Tequesta and Jupiter Inlet Colony Beaches, Florida, USA.
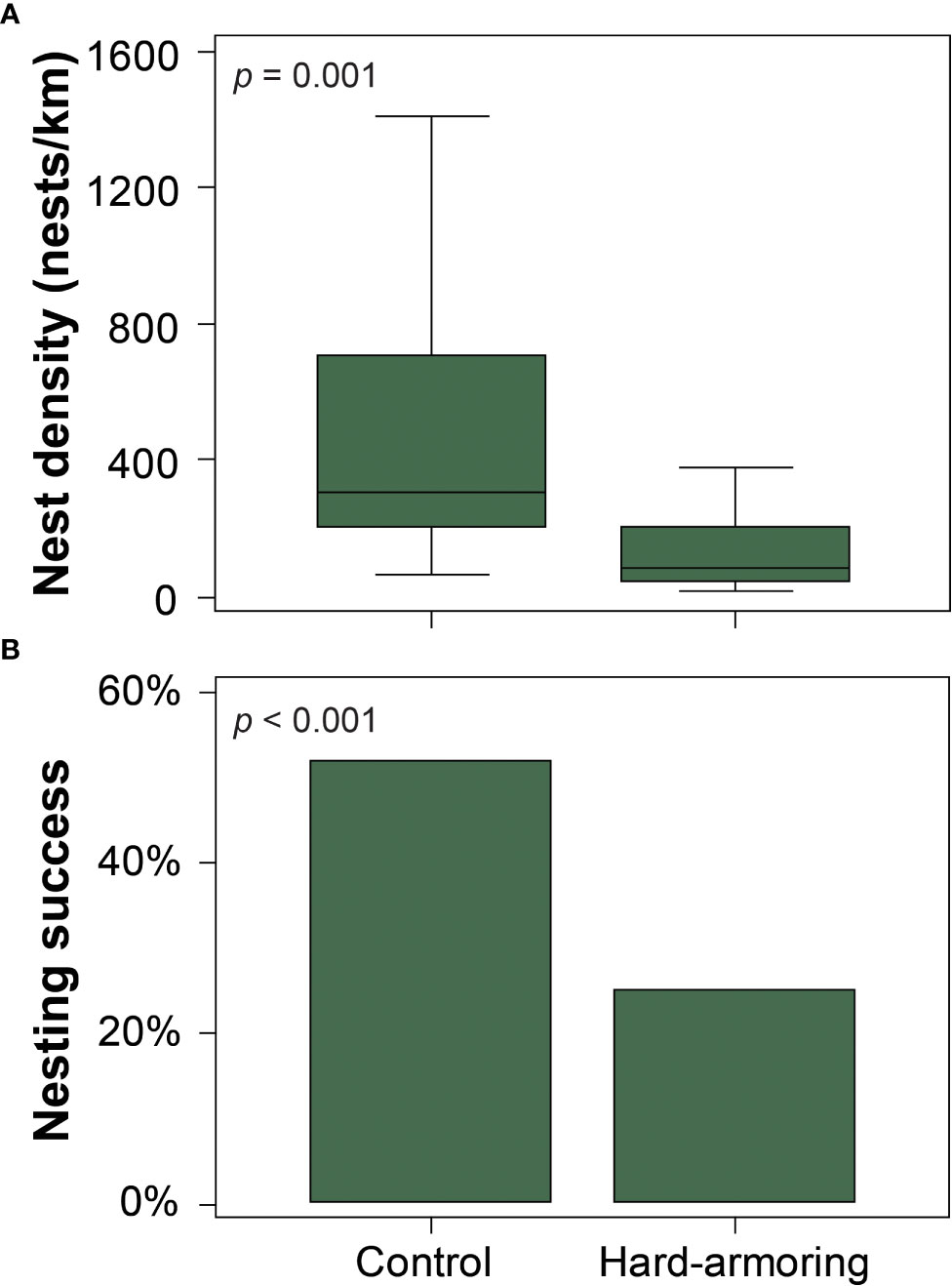
Figure 5 (A) Nest density and (B) nesting success were significantly lower at the hard-armoring structures in comparison to the control site for nesting green sea turtles (Chelonia mydas) on Tequesta and Jupiter Inlet Colony Beaches, Florida, USA. For panel (A), the middle line represents the median, the boxes represent the first and third quartiles, while the whiskers represent the range.
At the hard-armoring site, nesting success for leatherbacks did not significantly differ between those turtles that encountered hard-armoring structures, encountered other obstructions, or encountered no obstructions (χ2(2) = 5.053; p = 0.080; Table 2). At the hard-armoring site, nesting success for loggerheads significantly differed between those turtles that encountered hard-armoring structures, encountered other obstructions, or encountered no obstructions (χ2(2) = 1040.328; p < 0.001). Post-hoc tests indicated that nesting success was significantly lower for loggerheads that encountered hard-armoring structures compared to those that encountered no obstructions (p < 0.001) or encountered other obstructions (p < 0.001). Additionally, nesting success in loggerheads was significantly lower for turtles that encountered no obstructions versus those that encountered other obstructions (p < 0.001). Lastly, at the hard-armoring site, nesting success for green turtles significantly differed between those turtles that encountered hard-armoring structures, encountered other obstructions, or encountered no obstructions (χ2(2) = 65.059; p < 0.001; Table 2). Post-hoc tests indicated that nesting success was significantly lower for green turtles that encountered hard-armoring structures compared to those that encountered no obstructions (p < 0.001) and encountered other obstructions (p < 0.001); however, nesting success did not differ for turtles that encountered no obstructions versus those that encountered other obstructions (p = 0.282; Table 2).
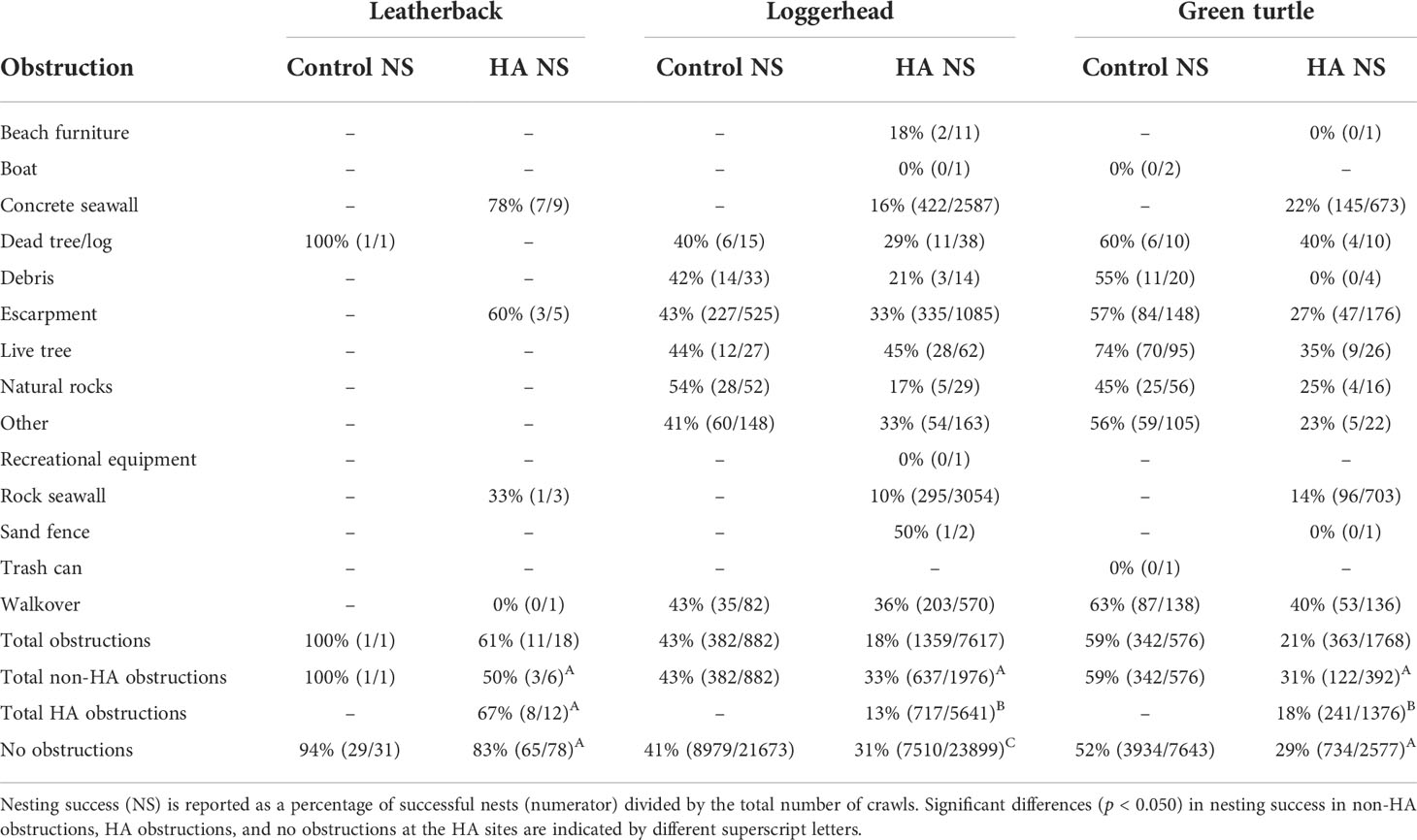
Table 2 Obstructions encountered by nesting leatherback (Dermochelys coriacea), loggerhead (Caretta caretta), and green (Chelonia mydas) sea turtles in Tequesta and Jupiter Inlet Colony Beaches, Florida, USA from 2007–2021 at the control site and the hard-armoring (HA) structures.
Washout rates
Leatherback (Fisher’s exact test; p > 0.999), loggerhead (χ2(1) = 0.517; p = 0.472), and green turtle (χ2(1) = 0.634; p = 0.426) washout rates did not significantly differ between the hard-armoring structures and the control site. Mean washout rates for the three species at the two sites are shown in Table 1.
Hatching and emergence success
Reproductive success did not differ between nests laid at the control site and at the hard-armoring structures when washouts were included or were removed from analyses for leatherbacks (washouts included: hatching success, p = 0.970; emergence success, p = 0.860; washouts excluded: hatching success, p = 0.628, emergence success, p = 0.745), loggerheads (washouts included: hatching success, p = 0.969; emergence success, p = 0.782; washouts excluded: hatching success, p = 0.359, emergence success, p = 0.209), and green turtles (washouts included: hatching success, p = 0.448; emergence success, p = 0.357; washouts excluded: hatching success, p = 0.982, emergence success, p = 0.718). Mean hatching and emergence success for the three species at the two sites, with and without washouts, are shown in Table 1.
Discussion
Previous studies examining the impacts of hard-armoring structures have almost exclusively focused on loggerheads (Mosier, 1998; Mosier and Witherington, 2002; Rizkalla and Savage, 2011). Therefore, the information presented here improves upon our understanding of the effects of coastal armoring to include different species of sea turtles at a high-density nesting beach. While the general nesting process is similar for leatherback, loggerhead, and green turtles, these three species do exhibit some variation in their nesting behaviors (Miller, 1997) that likely led to some of the varying results found in this study. Leatherbacks tend to lay their nests on the open beach (Whitmore and Dutton, 1985; Nordmoe et al., 2004), are less sensitive to disturbances than other sea turtle species, and typically complete the nesting process if they emerge onto the beach (Carr and Ogren, 1959; Hughes et al., 1967; Tucker, 1988; Meylan et al., 1995; Reina et al., 2002). Nest abandonment in leatherbacks likely has a higher energetic cost than smaller sea turtle species; therefore, unsuccessful nesting attempts could have been selected against throughout their evolutionary history to conserve energy. Loggerheads usually lay their nests between the open beach and the supra-littoral vegetation, abandon nesting attempts when disturbed by lighting or movement, and have nesting success rates of ~50% (Dodd, 1988). Green turtles also nest successfully ~50% of the time but tend to lay their nests on the upper portion of the nesting beach, in or near vegetation, and are particularly sensitive to movement, artificial light, and vibrations (Hendrickson, 1958; Bustard and Greenham, 1969; Weishampel et al., 2003; Witherington et al., 2006).
Leatherback nesting success was not significantly impacted by the hard-armoring structures; however, nest density was significantly higher at the hard-armoring structures in comparison to the control site. These results reflect high nesting success rates of leatherbacks and their tendency to nest despite facing disturbances (Carr and Ogren, 1959; Hughes et al., 1967; Tucker, 1988; Meylan et al., 1995; Reina et al., 2002). Numerous obstacles to nesting besides hard-armoring structures are present on Tequesta and Jupiter Inlet Colony Beaches; therefore, we were also interested in evaluating how nesting success was impacted by various other obstructions to help elucidate if the physical barrier may be the primary reason for nest abandonment. We found that leatherback nesting success was not significantly different for turtles that physically encountered obstructions. Our findings support previous literature that leatherbacks are likely to nest when emerging onto the beach, regardless of encountering obstructions. There were no differences in washout rates or hatching success and emergence success between the two sites for leatherbacks – possibly a result of timing of nesting, as leatherbacks nest earlier in the season and may therefore be exposed to less storm activity and subsequent tidal overwash (Stewart and Johnson, 2006; Dewald and Pike, 2014). Leatherback nesting was similar between the control and hard-armoring sites, which suggests that seawalls may not hinder the nesting process for this species. The results presented here should be interpreted cautiously as sample sizes for leatherbacks were low (1–19 nests/year across both sites) and similar analyses on higher-density nesting beaches are warranted.
Almost all studies evaluating impacts of coastal armoring on sea turtles focus on loggerheads (Mosier, 1998; Mosier and Witherington, 2002; Rizkalla and Savage, 2011). We found that loggerhead nest density did not differ between the control and hard-armoring structures, which contradicts findings from loggerheads nesting in Indian River County, Florida (Rizkalla and Savage, 2011). These dissimilarities may be due to differences in beach width and hard-armoring type (e.g., rock revetment, vertical seawall, etc.). The present study evaluated impacts from a range of sloped revetments and vertical seawalls, whereas the Indian River County study only evaluated vertical seawalls (Mosier and Witherington, 2002), which may be perceived differently from gradual sloped structures. In the current study, nesting success was significantly lower at the hard-armoring structures, similar to loggerheads nesting at three study areas from Melbourne Beach to Wabasso Beach, Florida (Mosier, 1998). We also found that nest densities were similar between the two sites, indicating that more loggerheads attempted to nest at the hard-armoring site than at the control site. It is still unclear as to what the turtles react to in terms of beach-based structures, as loggerheads that were presented with a portable wall barrier nested just as successfully as those that were allowed to access the entire beach. Witherington et al. (2011) concluded that loggerheads may be reacting to visual topographical cues prior to interaction with a physical barrier.
Loggerheads from this study that physically encountered hard-armoring structures had significantly lower nesting success in comparison to those that encountered other obstructions or no obstructions, indicating a negative effect of hard-armoring structures on loggerhead nesting behavior. Surprisingly, nesting success was also 2% lower for turtles that did not encounter any obstructions in comparison to those that encountered other obstructions. The reason for this difference is unclear; however, nest site selection is poorly understood in sea turtles and is likely affected by a combination of factors including physical obstructions and barriers, elevation, temperature, moisture, and sand characteristics (Miller et al., 2003). Lastly, there were no differences in washout rate or hatching and emergence success at the hard-armoring structures compared to the control site for loggerheads. No data were available on whether large erosive events took place during the study period, so we are uncertain if washout events and hatching and emergence success may have been negatively impacted by erosion. These data were not available during our study period and this question requires further investigation and increased sample sizes.
In the present study, green turtles were seemingly most affected by the hard-armoring structures, showing significantly lower nest density and nesting success at the hard-armoring structures in comparison to the control site. Because green turtles prefer to use upper portions of the beach (Brock et al., 2009), they were more likely to interact with these structures. Overall, 32% of the green turtles emerging onto the beach physically interacted with the hard-armoring structures, compared to just 18% of loggerheads and 13% of leatherbacks. Additionally, green turtle nesting success was significantly lower for those turtles that encountered the concrete or rock seawall compared to those that encountered other obstructions or no obstructions; however, no difference in nesting success was observed between green turtles that encountered other obstructions (e.g., trees, debris, natural rock, escarpments, etc.) or no obstructions. This suggests that hard-armoring structures reduce nesting success in green turtles due to their preference to nest in vegetated areas high on the beach (Bustard and Greenham, 1969; Brock et al., 2009), features that are minimal or absent at the hard-armoring site. Abandoned nesting attempts expend limited energy reserves and can result in turtles laying nests in high-risk habitats (Mosier, 1998). However, we found no difference in washout rates or hatching and emergence success for green turtle nests at the hard-armoring site; therefore, while green turtles deposited fewer nests in this area, those that were laid successfully were not more susceptible to lower hatching or emergence success.
It is important to note that there are several differences between the control and hard-armoring sites in this study that could have confounded our results. The zones where the hard-armoring structures occur are more residentially developed and nesting behavior may also be influenced by artificial lighting, although Palm Beach County regulates beachfront lighting through their Sea Turtle Protection and Sand Preservation Ordinance requiring lights to be shielded and of long wavelengths (Unified Land Development Code Article 14, Chapter A). Additionally, fine-scale nest-site selection can be influenced by a variety of environmental cues including temperature, moisture content, salinity, and beach slope, in addition to beach width and obstructions (Wood and Bjorndal, 2000), all of which were not evaluated in this study. The control and hard-armoring sites were adjacent to each other, and so abiotic variations were likely minimal. However, because the sites were in proximity, turtles that emerge in one treatment may have ultimately ended up crawling into the other treatment area. A limitation of field-based studies is the inability to control for numerous factors and before-after-control-impact (BACI) designs (e.g., Hirsch et al., 2019) can help limit confounding factors by evaluating both control and study sites before and after the habitat alterations. A BACI design was not possible in the current study as a comprehensive sea turtle nesting dataset was not available prior to the installation of the hard-armoring structures. Future studies should also aim to determine the driving factors for sea turtle nesting behavior, in addition to comparing how different armoring structures (e.g., vertical seawalls, sloped revetments, geotextiles containers, etc.) impact nesting, since these influence approach to the nesting beach and nest site selection (Schroeder and Mosier, 2000).
Coastal armoring is increasing in frequency on sea turtle nesting beaches around the globe; therefore, the data presented here provide a better understanding into the impacts that these structures have on leatherback and green turtles, which have not previously been evaluated. Additionally, this study evaluated a longer temporal and spatial scale for loggerheads than previous studies (Mosier, 1998; Mosier and Witherington, 2002; Rizkalla and Savage, 2011). Identifiable changes in population size for sea turtles can often take decades due to the time it takes for sea turtles to reach reproductive maturity (Mazaris et al., 2017). Since 2007, we have seen increases in loggerhead nesting on Tequesta, Coral Cove, and Jupiter Inlet Colony beaches; however, Florida’s statewide nesting population of leatherbacks, loggerheads, and green turtles have shown population declines (NALWG, 2018), stable trends (Ceriani et al., 2019), and significant increases in nest numbers, respectively, although in recent years the nesting trends of green turtles have become less predictable (FWC, 2021). Green turtles were most impacted by coastal armoring, as they use the upper portions of the beach where these types of structures are likely to be installed, increasing the chance of physical interaction with the structure, and ultimately resulting in a reduction in nesting success. Decreases in nesting success can lead to individual turtles constructing sub-optimal egg chambers on subsequent nesting attempts or release of the eggs in the water (Mosier, 1998). Additionally, the impacts of the increased energy expenditure/exertion from multiple unsuccessful nesting attempts to sea turtle physiology are unknown and could possibly lead to metabolic alterations (Phillips et al., 2015; Innis et al., 2010), especially considering the unique physiologic state and decreased nutritional condition of nesting sea turtles (Plot et al., 2013; Stacy et al., 2013; Perrault et al., 2014; Perrault and Stacy, 2018; Page-Karjian et al., 2020), particularly those that are further along in nesting season (i.e., have laid more clutches). Because hard-armoring structures often lead to habitat loss, coastal managers should carefully consider impacts to nesting turtles and setback limits (i.e., how far away the structure is from the waterline) of these structures if they are to permit their installation; however, more natural methods (e.g., dune restoration) to beach armoring should first be considered.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Florida Fish and Wildlife Conservation Commission on Marine Turtle Permit #154.
Author contributions
Conceptualization: SEH and JRP; methodology: SEH, JDR, SRH, and JRP; validation: SEH, JDR, and SRH; formal analysis: SEH and JRP; data curation: SEH, JDR, and SRH; writing – original draft preparation: SEH, MT, and JRP; writing – review and editing: SEH, MT, JDR, SRH, and JRP; funding acquisition: SEH and JRP. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project was acquired, in part, from the Florida Department of Environmental Protection (#20-PBL, #21-PBL), Palm Beach County Environmental Resource Management, and Loggerhead Marinelife Center.
Acknowledgments
The authors wish to thank the staff and volunteers of Loggerhead Marinelife Center for assistance with data collection. We also thank Meghan Koperski of Florida Fish and Wildlife Conservation Commission for assistance with permitting.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ataman A., Gainsbury A. M., Manire C. A., Hoffmann S. L., Page-Karjian A., Hirsch S. E., et al. (2021). Evaluating prevalence of external injuries on nesting loggerhead sea turtles Caretta caretta in southeastern Florida, USA. Endanger. Spec. Res. 46, 137–146. doi: 10.3354/esr01149
Brock K. A., Reece J. S., Ehrhart L. M. (2009). The effects of artificial beach nourishment on marine turtles: differences between loggerhead and green turtles. Restor. Ecol. 17, 297–307. doi: 10.1111/j.1526-100X.2007.00337.x
Burns T. J., Davidson H., Kennedy M. (2016). Large-Scale investment in the excavation and “camouflaging” phases by nesting leatherback turtles (Dermochelys coriacea). Can. J. Zool. 94, 443–448. doi: 10.1139/cjz-2015-0240
Bustard H. R., Greenham P. (1969). Nesting behavior of the green sea turtle on a Great Barrier Reef island. Herpetologica 25, 93–102.
Carr A., Ogren L. (1959). The ecology and migrations of sea turtles, 3 Dermochelys in Costa Rica. Am. Mus. Novit. 1958, 1–29.
Caut S., Guirlet E., Girondot M. (2010). Effect of tidal overwash on the embryonic development of leatherback turtles in French Guiana. Mar. Environ. Res. 69, 254–261. doi: 10.1016/j.marenvres.2009.11.004
Ceriani S. A., Casale P., Brost M., Leone E. H., Witherington B. E. (2019). Conservation implications of sea turtle nesting trends: elusive recovery of a globally important loggerhead population. Ecosphere 10, e02936. Available at: https://www.iucnredlist.org/species/84131194/119339029
Ceriani S. A., Meylan A. B. (2017). Caretta caretta (North West Atlantic subpopulation) (amended version of 2015 assessment). The IUCN Red list of Threatened Species 2017. doi: 10.2305/IUCN.UK.2017-2.RLTS.T84131194A119339029.en
Crain C. M., Halpern B. S., Beck M. W., Kappel C. V. (2009). Understanding and managing human threats to the coastal marine environment. Ann. N.Y. Acad. Sci. 1162, 39–62. doi: 10.1111/j.1749-6632.2009.04496.x
Davis G. E., Whiting M. C. (1977). Loggerhead sea turtle nesting in Everglades National Park, Florida, USA. Herpetologica 33, 18–28.
Dewald J. R., Pike D. A. (2014). Geographical variation in hurricane impacts among sea turtle populations. J. Biogeogr. 41, 307–316. doi: 10.1111/jbi.12197
Dodd C. K. (1988). “Synopsis of the biological data on the loggerhead sea turtle Caretta caretta (Linnaeus 1758),” in US Fish and Wildlife Service Biological Report 88, 110. Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj_0p-26YD6AhUCVTABHTZ_Ab4QFnoECAcQAQ&url=http%3A%2F%2Fwww.seaturtle.org%2Fdocuments%2FDodd_1988_Loggerhead.pdf&usg=AOvVaw3JKGqmKjlb_EgJ5AR4lWVV
Doody J. P. (2004). ‘Coastal squeeze’ — an historical perspective. J. Coast. Conserv. 10, 129–138. doi: 10.1652/1400-0350(2004)010[0129:CSAHP]2.0.CO;2
Dugan J. E., Airoldi L., Chapman M. G., Walker S. J., Schlacher T. (2011). Estuarine and coastal structures: Environmental effects, a focus on shore and nearshore structures. Treatise Estuar. Coast. Sci. 11, 17–41. doi: 10.1016/B978-0-12-374711-2.00802-0
Dugan J. E., Emery K. A., Alber M., Alexander C. R., Byers J. E., Gehman A. M., et al. (2018). Generalizing ecological effects of shoreline armoring across soft sediment environments. Estuaries Coast. 41, 180–196. doi: 10.1007/s12237-017-0254-x
Erb V., Lolavar A., Wyneken J. (2018). The role of sand moisture in shaping loggerhead sea turtle (Caretta caretta) neonate growth in southeast Florida. Chelonian Conserv. Biol. 17, 245–251. doi: 10.2744/CCB-1301.1
Fish M. R., Côté I. M., Horrocks J. A., Mulligan B., Watkinson A. R., Jones A. P. (2008). Construction setback regulations and sea-level rise: Mitigating sea turtle nesting beach loss. Ocean Coast. Manage. 51, 330–341. doi: 10.1016/j.ocecoaman.2007.09.002
Fleming K. A., Perrault J. R., Stacy N. I., Coppenrath C. M., Gainsbury A. M. (2020). Heat, health and hatchlings: Associations of in situ nest temperatures with morphological and physiological characteristics of loggerhead sea turtle hatchlings from Florida. Conserv. Physiol. 8, coaa046. doi: 10.1093/conphys/coaa046
Florida Department of Environmental Protection (FDEP) (2021) Critically eroded beaches in Florida (Bureau of Beaches and Coastal Systems Division of Water Resource Management). Available at: https://floridadep.gov/sites/default/files/FDEP-Critically-Eroded-Beaches-07-2021_0_1.pdf (Accessed 4 Jan 2022).
Florida Fish and Wildlife Conservation Commission (FWC) (2016) Marine turtle conservation handbook (Tallahassee, Florida). Available at: https://myfwc.com/media/3133/fwc-mtconservationhandbook.pdf (Accessed 7 Dec 2021).
Foley A. M., Peck S. A., Harman G. R. (2006). Effects of sand characteristics and inundation on the hatching success of loggerhead sea turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest Florida. Chelonian Conserv. Biol. 5, 32–41. doi: 10.2744/1071-8443(2006)5[32:EOSCAI]2.0.CO;2
Fuentes M. M. P. B., Gredzens C., Bateman B. L., Boettcher R., Ceriani S. A., Godfrey M. H., et al. (2016). Conservation hotspots for marine turtle nesting in the United States based on coastal development. Ecol. Appl. 26, 2708–2719. doi: 10.1002/eap.1386
FWC (2021) Index nesting beach survey totals: 1989–2021. Available at: https://myfwc.com/research/wildlife/sea-turtles/nesting/beach-survey-totals/.
Gittman R. K., Scyphers S. B., Smith C. S., Neylan I. P., Grabowski J. H. (2016). Ecological consequences of shoreline hardening: A meta-analysis. Bioscience 1, 763–773. doi: 10.1093/biosci/biw091
Grain D. A., Bolten A. B., Bjorndal K. A. (1995). Effects of beach nourishment on sea turtles: review and research initiatives. Restor. Ecol. 3, 95–104. doi: 10.1111/j.1526-100X.1995.tb00082.x
Hall M. J., Pilkey O. H. (1991). Effects of hard stabilization on dry beach width for New Jersey. J. Coast. Res. 7, 771–785.
Hanley M. E., Hoggart S. P. G., Simmonds D. J., Bichot A., Colangelo M. A., Bozzeda F., et al. (2014). Shifting sands? Coastal protection by sand banks, beaches and dunes. Coast. Eng. 87, 136–146. doi: 10.1016/j.coastaleng.2013.10.020
Hendrickson J. R. (1958). The green sea turtle, Chelonia mydas (Linn.) in Malaya and Sarawak. Proc. Zool. Soc Lond. 130, 455–535. doi: 10.1111/j.1096-3642.1958.tb00583.x
Hirsch S. E., Kedzuf S., Perrault J. R. (2019). Impacts of a geotextile container dune core on marine turtle nesting in Juno Beach, Florida, United States. Restor. Ecol. 27, 431–439. doi: 10.1111/rec.12878
Horrocks J. A., Scott N.McA (1991). Nest site location and nest success in the hawksbill turtle Eretmochelys imbricata in Barbados, West indies. Mar. Ecol. Prog. Ser. 69, 1–8.
Hughes G. R., Bass A. J., Mentis M. T. (1967). Further studies on marine turtles in Tongaland I. Lammergeyer 7, 5–54.
Innis C., Merigo C., Dodge K., Tlusty M., Dodge M., Sharp B., et al. (2010). Health evaluation of leatherback turtles (Dermochelys coriacea) in the northwestern Atlantic during direct capture and fisheries gear entanglement. Chelonian Conserv. Biol. 9, 205–222. doi: 10.2744/CCB-0838.1
Kittinger J. N., Ayers A. L. (2010). Shoreline armoring, risk management, and coastal resilience under rising seas. Coast. Manage. 38, 634–653. doi: 10.1080/08920753.2010.529038
Leite A. S., Santos L. L., Costa Y., Hatje V. (2014). Influence of proximity to an urban center in the pattern of contamination by marine debris. Mar. Pollut. Bull. 81, 242–247. doi: 10.1016/j.marpolbul.2014.01.032
Limpus C. J. (1985). A study of the loggerhead sea turtle, Caretta caretta (Eastern Australia. Brisbane, Queensland, Australia: University of Queensland).
Limpus C. J., Miller J. D., Pfaller J. B. (2020). Flooding-induced mortality of loggerhead sea turtle eggs. Wildl. Res. 48, 142–151. doi: 10.1071/WR20080
Lopez G. G., Saliés E. D. C., Lara P. H., Tognin F., Marcovaldi M. A., Serafini T. Z. (2015). Coastal development at sea turtles nesting grounds: efforts to establish a tool for supporting conservation and coastal management in northeastern Brazil. Ocean Coast. Manage. 116, 270–276. doi: 10.1016/j.ocecoaman.2015.07.027
Martin R. E. (1996). Storm impacts on loggerhead turtle reproductive success. Mar. Turtle Newsl. 73, 10–12.
Mazaris A. D., Matsinos G., Pantis J. D. (2009). Evaluating the impacts of coastal squeeze on sea turtle nesting. Ocean Coast. Manage. 52, 139–145. doi: 10.1016/j.ocecoaman.2008.10.005
Mazaris A. D., Schofield G., Gkazinou C., Almpanidou V., Hays G. C. (2017). Global sea turtle conservation successes. Sci. Adv. 3, e1600730. doi: 10.1126/sciadv.1600730
McGehee M. A. (1990). Effects of moisture on eggs and hatchlings of loggerhead sea turtles (Caretta caretta). Herpetologica 46, 251–258.
Meylan A. B., Schroeder B. A., Mosier A. (1995). Sea turtle nesting activity in the state of Florida 1979–1992. Florida marine research publications number 52 (St. Petersburg, FL, USA: Florida Marine Research Institute), 51 p.
Miller J. D. (1997). “Reproduction in sea turtles,”, in The Biology of Sea turtles, vol. Volume I . Eds. Lutz P. L., Musick J. A. (Boca Raton, FL: CRC Press), 51–81.
Miller J. D., Limpus C. J., Godfrey M. H. (2003). “Nest site selection, oviposition, eggs, development, hatching, and emergence of loggerhead Turtles,”, in Loggerhead Sea Turtles. Eds. Bolton A. B., Witherington B. E. (Washington, DC: Smithsonian Institution Press), 125–143.
Milton S. L., Leone-Kabler S., Schulman A. A., Lutz P. L. (1994). Effects of hurricane Andrew on the sea turtle nesting beaches of south Florida. Bull. Mar. Sci. 54, 974–981.
Mosier A. E. (1998). The impact of coastal armoring structures on Sea turtle nesting behavior at three beaches on the East coast of Florida (Tampa, FL, USA: University of South Florida).
Mosier A. E., Witherington B. E. (2002). “Documented effects of coastal armoring structures on sea turtle nesting behavior,” in Proceedings of the 20th International Sea Turtle Symposium. Eds. Mosier A., Foley A., Brost B. (NOAA Tech Memo. NMFS-SEFSC-477) p. 304–306. Available at: https://internationalseaturtlesociety.org/wp-content/uploads/2021/02/20-turtle.pdf
Nelson Sella K. A., Fuentes M. M. P. B. (2019). Exposure of marine turtle nesting grounds to coastal modifications: Implications for management. Ocean Coast. Manage. 169, 182–190. doi: 10.1016/j.ocecoaman.2018.12.011
Nelson Sella K. A., Sicius L., Fuentes M. M. P. B. (2019). Using expert elicitation to determine the relative impact of coastal modifications on marine turtle nesting grounds. Coast. Manage 47, 492–506. doi: 10.1080/08920753.2019.1642176
Nordmoe E. D., Sieg A. E., Sotherland P. R., Spotila J. R., Paladino F. V., Reina R. D. (2004). Nest site fidelity of leatherback turtles at Playa Grande, Costa Rica. Anim. Behav. 68, 387–394. doi: 10.1016/j.anbehav.2003.07.015
Nordstrom K. F. (1994). Beaches and dunes of human-altered coasts. Prog. Phys. Geogr. 18, 497–516. doi: 10.1177/030913339401800402
Northwest Atlantic Leatherback Working Group (NALWG) (2018). Northwest Atlantic leatherback turtle (Dermochelys coriacea) status assessment. Eds. Wallace B., Eckert K. (Godfrey, Illinois, USA: Conservation Science Partners and the Wider Caribbean Sea Turtle Conservation Network (WIDECAST), 36 p. Available at: http://www.cspinc.org/public/16-NWA-leatherback-status-report-FINAL.pdf.
Page-Karjian A., Chabot R., Stacy N. I., Morgan A. S., Valverde R. A., Stewart S., et al. (2020). Comprehensive health assessment of green turtles Chelonia mydas nesting in southeastern Florida, USA. Endanger. Spec. Res. 42, 21–35. doi: 10.3354/esr01036
Patrick C. J., Weller D. E., Ryder M. (2016). The relationship between shoreline armoring and adjacent submerged aquatic vegetation in Chesapeake Bay and nearby Atlantic coastal bays. Estuaries Coast. 39, 158–170. doi: 10.1007/s12237-015-9970-2
Perrault J. R., Stacy N. I. (2018). Note on the unique physiologic state of loggerhead sea turtles (Caretta caretta) during nesting season as evidenced by a suite of health variables. Mar. Biol. 165, 71. doi: 10.1007/s00227-018-3331-1
Perrault J. R., Wyneken J., Page-Karjian A., Merrill A., Miller D. L. (2014). Seasonal trends in nesting leatherback turtle (Dermochelys coriacea) further verify capital breeding hypothesis. Conserv. Physiol. 2, cou002. doi: 10.1093/conphys/cou002
Phillips B. E., Cannizzo S. A., Godfrey M. H., Stacy B. A., Harms C. A. (2015). Exertional myopathy in a juvenile green sea turtle (Chelonia mydas) entangled in a large mesh gillnet. Case Rep. Vet. Med. 2015, 604320. doi: 10.1155/2015/604320
Pike D. A., Roznik E. A., Bell I. (2015). Nest inundation from sea-level rise threatens sea turtle population viability. R. Soc Open Sci. 2, 150127. doi: 10.1098/rsos.150127
Pilkey O. H. Jr., Sharma O. H., Wanless H. R., Doyle L. J., Pilkey O. H. Sr., Neal W. J., et al. (1984). Living with the East Florida Shore (Durham: Duke University Press), 259p.
Plot V., Jenkins T., Robin J.-P., Fossette S., Georges J.-Y. (2013). Leatherback turtles are capital breeders: Morphometric and physiological evidence from longitudinal monitoring. Physiol. Biochem. Zool. 86, 385–397. doi: 10.1086/671127
Prange H. D., Jackson D. C. (1976). Ventilation, gas exchange and metabolic scaling of a sea turtle. Resp. Physiol. 27, 369–377. doi: 10.1016/0034-5687(76)90065-7
R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/org/.
Reina R. D., Mayor P. A., Spotila J. R., Piedra R., Paladino F. V. (2002). Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque National Marino Las Baulas, Costa Rica: 1988–1989 to 1999–2000. Copeia 3, 653–664. doi: 10.1643/0045-8511(2002)002[0653:NEOTLT]2.0.CO;2
Rivas M. L., Santidrían Tomillo P., Diéguez-Uribeondo J., Marco A. (2016). Potential effects of dune scarps caused by beach erosion on the nesting behavior of leatherback turtles. Mar. Ecol. Progr. Ser. 551, 239–248. doi: 10.3354/meps11748
Rizkalla C. E., Savage A. (2011). Impact of seawalls on loggerhead sea turtle (Caretta caretta) nesting and hatching success. J. Coast. Res. 27, 166–173. doi: 10.2112/JCOASTRES-D-10-00081.1
Salmon M., Tolbert M. G., Painter D. P., Goff M., Reiners R. (1995). Behavior of loggerhead sea turtles on an urban beach. II. Hatchling orientation. J. Herpetol. 29, 568–576. doi: 10.2307/1564740
Schroeder B. A., Mosier A. E. (2000). “Between a rock and a hard place: coastal armoring and marine turtle nesting habitat in Florida,” in Proceedings of the 18th International Sea Turtle Symposium. Eds. Abreu-Grobois F. A., Briseno-Duenas R., Marquez R., Sarti L. (NOAA Tech Memo. NMFS-SEFSC-436), p. 290–292. Available at: https://internationalseaturtlesociety.org/wp-content/uploads/2021/02/18-turtle.pdf
Stacy B. A., Foley A., Garner M. M., Mettee N. (2013). Yolk embolism associated with trauma in vitellogenic sea turtles in Florida (USA): A review of 11 cases. J. Zoo Wildl. Med. 44, 1043–1048. doi: 10.1638/2013-0074R.1
Stewart K., Johnson C. (2006). Dermochelys coriacea – leatherback sea turtle. in: Meylan, P.A. (ed.), biology and conservation of Florida turtles. Chelonian Res. Monogr. 3, 144–157.
Stewart K. R., Martin K. J., Johnson C., Desjardin N., Eckert S. A., Crowder L. B. (2014). Increased nesting, good survival and variable site fidelity for leatherback turtles in Florida, USA. Biol. Conserv. 176, 117–125. doi: 10.1016/j.biocon.2014.05.008
Talbert O. R. Jr., Stancyk S. E., Dean J. M., Will J. M. (1980). Nesting activity of the loggerhead turtle (Caretta caretta) in south Carolina I: A rookery in transition. Copeia 1980, 709–719. doi: 10.2307/1444448
Tucker A. D. (1988). A summary of leatherback turtle, Dermochelys coriacea, nesting at Culebra, Puerto Rico, from 1984–1987 with management recommendations. Unpublished Research Report to the U.S. Fish and Wildlife Service, Washington, DC. 24 p.
Weishampel J. F., Bagley D. A., Ehrhart L. M., Rodenbeck B. L. (2003). Spatiotemporal patterns of annual sea turtle nesting behaviors along an east central Florida beach. Biol. Conserv. 110, 295–303. doi: 10.1016/S0006-3207(02)00232-X
Whitmore C. P., Dutton P. H. (1985). Infertility, embryonic mortality, and nest-site selection in leatherback and green sea turtles in Suriname. Biol. Conserv. 34, 251–272. doi: 10.1016/0006-3207(85)90095-3
Witherington B., Bresette M., Herren R. (2006). Chelonia mydas – green turtle. in: Meylan, P.A. (ed.), Biology and Conservation of Florida Turtles. Chelonian Res. Monogr. 3, 90–104.
Witherington B., Hirama S., Mosier A. (2011). Sea turtle responses to barriers on their nesting beach. J. Exper. Mar. Biol. Ecol. 401, 1–6. doi: 10.1016/j.jembe.2011.03.012
Keywords: leatherback (Dermochelys coriacea), loggerhead (Caretta caretta), green turtle (Chelonia mydas), false crawl, beach armoring, nesting success, abandoned nesting attempt, seawall
Citation: Hirsch SE, Toonder M, Reilly JD, Hoover SR and Perrault JR (2022) Responses of three nesting sea turtle species to hard-armoring structures. Front. Mar. Sci. 9:980715. doi: 10.3389/fmars.2022.980715
Received: 28 June 2022; Accepted: 26 August 2022;
Published: 23 September 2022.
Edited by:
Salvatore Siciliano, Fundação Oswaldo Cruz (Fiocruz), BrazilReviewed by:
Paolo Casale, University of Pisa, ItalyBlair Witherington, Inwater Research Group, United States
Nicolas James Pilcher, Marine Research Foundation, Malaysia
Copyright © 2022 Hirsch, Toonder, Reilly, Hoover and Perrault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah E. Hirsch, c2hpcnNjaEBtYXJpbmVsaWZlLm9yZw==
†Present address: Shelby R. Hoover, Gumbo Limbo Nature center, Boca Raton, FL, United States
 Sarah E. Hirsch
Sarah E. Hirsch Madison Toonder
Madison Toonder Jennifer D. Reilly1
Jennifer D. Reilly1 Justin R. Perrault
Justin R. Perrault