- 1School of Marine Sciences, Ningbo University, Ningbo, China
- 2Key Laboratory of Aquacultural Biotechnology Ministry of Education, Ningbo University, Ning bo, China
- 3Guangxi Engineering Technology Research Center of Breeding of New Mariculture Varieties, Guangxi Institute of Oceanology Co., Ltd., Beihai, China
Scylla paramamosain is a high-quality cultivar for saline-alkaline water aquaculture as a euryhaline crustacean species. However, salinity impacts the respiratory metabolism, growth, and survival of marine crustaceans. The metabolic response of crabs adapting to multiple low salinity environments has not been thoroughly studied yet, especially in inland saline-alkaline water. In this study, we analyzed metabolites in the gill and hemolymph of crabs cultured in three different low salinity environments. The results showed that membrane composition (lipids and lipid molecules) and free amino acids played an essential role in the osmoregulation of crabs, and the energy consumption accompanied as well. Meanwhile, S. paramamosain relied on ion transport and energy metabolism under acute/short-term low salinity conditions for osmoregulation. In contrast, amino acids and energy metabolism occupied a leading position in long-term low salinity. Furthermore, taurine and hypotaurine play a vital role in crabs adapting to inland saline-alkaline water. This is the first study to identify the crucial metabolites and key pathways as biomarkers to differentiate the metabolic mechanisms of S. paramamosain under multiple low salinity stress modes based on GC-MS technology, which provided novel insight into the metabolic response of S. paramamosain adapting to inland low salinity saline-alkaline water, and provided theoretical guidance for the aquaculture of S. paramamosain in the inland saline-alkaline water.
Introduction
The problem of soil salinization is globally widespread. The development of saltwater and brackish water aquaculture cannot only make full use of saline land, but also produce better economic benefits and contribute to the improvement of the regional economy. At the same time, the supply of fresh water is severely limited in many countries, and coastal salinization caused by sea-level rise is increasing, which makes it more critical to have salt-tolerant species in aquaculture farms around the world (Zhu et al., 2018).
Scylla paramamosain, also known as mud crab, is a kind of euryhaline crustacean species, which is a suitable species for saline-alkaline water aquaculture. It is mainly distributed in the southeastern coastal area of China, the Indian Ocean, and the western Pacific Ocean (Ma et al., 2014). Because of its fast growth, delicious meat, strong adaptability and high nutritional value, it is an essential species of marine crab in China. (Jiang et al., 2014; Ma et al., 2016). In 2020, the output of S. paramamosain increased to 159,433 tons, accounting for more than half of the total marine crab farming industry in China (China fishery statistical yearbook, 2021). However, the yield cannot meet the ever-increasing market demand (Ma et al., 2016; Shi et al., 2018; Wang et al., 2018b). Therefore, insufficient production capacity is the biggest bottleneck currently in the development of the mud crab industry. The most effective way to break through this bottleneck is to expand the aquaculture space. Under the current situation of saturated coastal aquaculture space, inland saline-alkaline land aquaculture has great potential to solve this problem. At present, our team has built a technical system for cultivating crabs in saline-alkaline water, and successfully cultivated crabs in Henan (with a salinity of 1.6‰) and other low salinity saline-alkali water areas along the Yellow River. Nevertheless, the metabolic response of S. paramamosain adapting to low salinity saline-alkaline water is not clear, and systematic research is urgently needed to improve the theory of crabs’ cultivation in saline-alkaline water.
Salinity is one of the most significant elements that affects the physiological state of aquatic animals. S. paramamosain is an excellent model for studying salinity adaptation mechanisms as a euryhaline species (Chung and Lin, 2006). Changes in environmental salinity are directly related to the capacity of osmoregulation (Fry, 1971). A lot of research work of crustaceans has been done on the morphological structure of osmolality regulation organs (Neufeld et al., 1980), ion transportase (Li et al., 2010), hemolymph osmolality regulation (Péqueux and Gilles, 1978) and neuroendocrine regulation (Pinoni et al., 2005), and many significant achievements have been made. Studies have shown that the gill is a major organ for osmolality and ion regulation in crustaceans (Romano et al., 2014). Crustaceans can adapt to various salinity and other aquatic environmental factors, mainly through the regulation of hemolymph osmolality to maintain the daily activities of the body. The osmolality effectors in hemolymph, such as inorganic ion concentration and free amino acid content, determine the level of hemolymph osmolality in crustaceans (Via, 1986; Chen and Chia, 1997). The amassment of free amino acids in cells is a typical response to changes in environmental salinity in many organisms (Yancey et al., 1982; Hare et al., 2010; Yao et al., 2020). In addition, the metabolic levels of hemolymph components such as protein, blood glucose, lipids, and ammonia also influence osmolality (Via, 1986; Chen and Chia, 1997). The studies on the salinity adaptation mechanism of mud crabs mainly focus on the acute low salinity stress at the laboratory level (Wang et al., 2018a; Wang et al., 2018c; Zhang et al., 2020; Yao et al., 2021). However, the metabolic response of crabs adapting to inland low salinity saline-alkaline water has not been thoroughly studied yet.
In this study, we explored the metabolic response of S. paramamosain under multiple low salinity stress modes based on GC-MS technology, which provided theoretical guidance for the aquaculture of S. paramamosain in the inland low salinity saline-alkaline water.
Materials and methods
Animals and sample collection
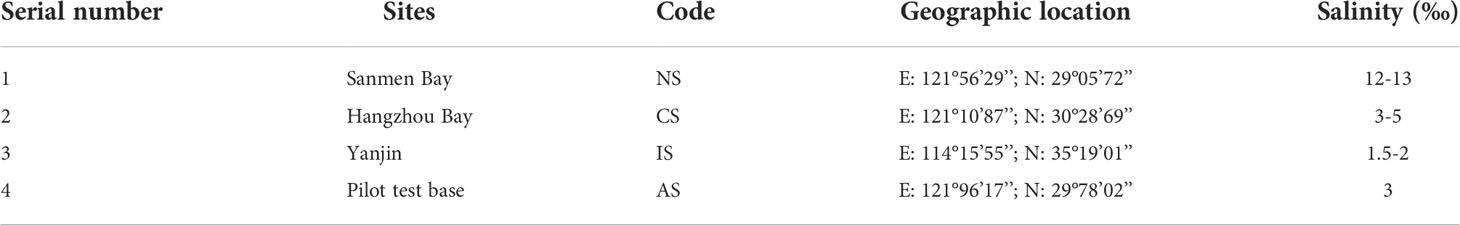
A total of 600 crabs from Sanmen Bay were randomly selected with a bodyweight of 30 ± 5 g. Randomly draw a group of every 50 crabs (n=3) and transferred to the following four aquaculture pools with the same domestication conditions: Normal salinity group (NS), Coastal low salinity group (CS), Inland low salinity saline-alkaline group (IS) and Acute low salinity group (AS). Sanmen Bay, located on the eastern coast of Zhejiang Province, is one of the main producing areas of crabs, with a seawater salinity of 12-18‰, which is suitable for the growth of crabs, and was defined as NS group. Hangzhou Bay, located in the northeastern part of Zhejiang Province, is both the bay and the estuary of the Qiantang River. Affected by river inflow and precipitation, the seawater salinity is low and unstable, about 3-5‰, crabs from NS grow normally here, which is defined as CS group. Yanjin is located on the north bank of the Yellow River, Henan Province, with a large area of saline-alkaline land. The groundwater is saline-alkaline water with a salinity of 1.5-2‰, crabs from NS could grow normally here after gradient desalination and defined as IS group. The pilot test base was an acute low-salinity environment artificially simulated in the laboratory. The seawater was diluted to a salinity of 3‰ with fresh water, and the crabs from NS were kept in it, which is defined as the AS group (Table 1).
Experiments were conducted at ambient temperature and natural photoperiod, and water quality parameters were monitored every morning. The parameters were as follows: water temperature 25°C-30°C, dissolved oxygen≥6.0 mg L-1, pH 7.8-8.6, ammonia nitrogen concentration 0.16-1.58 mg L-1, nitrite concentration 0.009-0.102 mg L-1. Feed the clams once a day at 5:00 pm. After 90 days of culture, adult S. paramamosain (165-297 g body weight) with good vitality, health and uniform bodyweight were obtained from the NS, CS and IS groups in October 2020 (n=5). The AS group was sampled after 120 h according to the previous study (Wang et al., 2018a).The crabs were killed under anesthetize in ice water, then the posterior gill and hemolymph were quickly removed and stored in liquid nitrogen for metabolomic analysis.
Sample preparation
Took out the hemolymph samples stored at -80°C, thawed them at room temperature, transferred 80 μL of the samples to a 1.5 mL EP tube, and vortex for 10 s. Then 240 μL methanol-acetonitrile (V: V=2: 1) mixed solution was added to the tube, vortexed for 30 s, followed by ultrasonic extraction in ice-water bath for 10 min. Then stood them at -20°C for 30 min. After that, centrifuge for 10 min (13,000 rpm, 4°C), and transfer 200 μL of the supernatant into a glass derivatized vial. Quality control samples (QC) were prepared by mixing equal volumes of extracts from all samples, and each QC has the same volume as the sample. After evaporating the sample with a freeze-concentrating centrifugal dryer, add 80 μL of methoxyamine hydrochloride pyridine solution (15 mg/mL) to a glass derivatized vial, vortex for 2 min, and incubate in a shaking incubator at 37°C for 90 min to carry out the oximation reaction. After the samples were taken out, 80 μL of BSTFA (containing 1% TMCS) derivatization reagent, 20 μL of n-hexane and 10 μL internal standards (C8/C9/C10/C12/C14/C16, 0.16 mg/mL; C18/C20/C22/C24/C26, 0.08 mg/mL, all prepared in chloroform) were added, then vortexed for 2 min and reacted at 70°C for 60 min. Finally, the samples were taken out, placed at room temperature for 30 min (Imura et al., 2018), and subjected to GC-MS metabolomic analysis. The AS group had one sample of hemolymph unqualified and therefore only 4 replicates.
Accurately weighed 30 mg of posterior gill tissue samples into a 1.5 mL centrifuge tube, added 600 μL methanol-water (V: V=4: 1) and two small steel balls, put them in a -80°C refrigerator for 2 min, and then ground them in a grinder (60 Hz, 2 min). Added 120 μL of chloroform to the tube, vortexed for 2 min, then ultrasonically extracted in an ice-water bath for 10 min, then stood at -20°C for 30 min. The rest of the steps were the same as for the hemolymph sample.
GC-MS
The derivatived samples were analyzed on an Agilent 7890B gas chromatography system coupled to an Agilent 5977A MSD system (Agilent Technologies Inc., CA, USA). A HP-5MS fused-silica capillary column (30 m ×0.25 mm × 0.25 μm, Agilent J & W Scientific, Folsom, CA, USA) was utilized to separate the derivatives. Helium (>99.999%) was used as the carrier gas at a constant flow rate of 1 mL/min through the column. The injector temperature was maintained at 260°C. Injection volume was 1 μL by splitless mode, and the solvent delay time was set to 6.2 min. The initial temperature of column thermostat is 50°C, keeps 0.5min, ramped to 125°C at a rate of 8°C/min, to 210°C at a rate of 5°C/min, to 270°C at a rate of 10°C/min, to 305°C at a rate of 20°C/min, and finally held at 305°C for 2 min. The temperature of MS quadrupole and ion source (electron impact) was set to 150 and 230°C, respectively. The collision energy was 70 eV. Mass spectrometric data was acquired in a full-scan mode (m/z 50-500).
The QCs were injected at regular intervals (every 8 samples) throughout the analytical run to provide a set of data from which repeatability could be assessed.
Data processing and analysis
The processing of the data was done with MS-DIAL software. GC-MS qualitative analysis was performed using the LUG database (Untarget database of GC-MS from Lumingbio). Qualitative analysis of volatile and similar substances was performed using the NIST database (https://webbook.nist.gov/chemistry/). A combination of multivariate and univariate statistical analysis was used to screen differential metabolites between groups. The criteria for differential metabolite screening were that the VIP value of the first principal component of the OPLS-DA model was >1, and the P -value of the T test was<0.05 (Liu et al., 2021). Principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), heatmaps and bubble charts were analyzed and plotted with R software (version 3.6.2). Metabolic pathway enrichment analysis of differential metabolites was performed based on the KEGG database (http://www.kegg.jp/). Meanwhile, Cytoscape (http://www.cytoscape.org) was adopted to visualize and analyze metabolic pathways and corresponding differential metabolites networks.
In this study, the raw sequences acquired were available through the MetaboLights database (https://www.ebi.ac.uk/metabolights/). The accession number are MTBLS5202 and MTBLS5220.
Results
Metabolic profiling of S. paramamosain in the gill and hemolymph
Metabolites changes in the gill and hemolymph among four groups were detected by GC-MS. The results showed 341 and 285 detected metabolites in the gill and hemolymph, respectively. The detectable metabolites of gill and hemolymph in super class classification were the same except prenol lipids, and the top three were organic acids and derivatives (27.21% and 27.64%), organic oxygen compounds (19.05% and 22.76%), lipids and lipid-like molecules (17.35% and 20.33%) (Figures 1A, B).
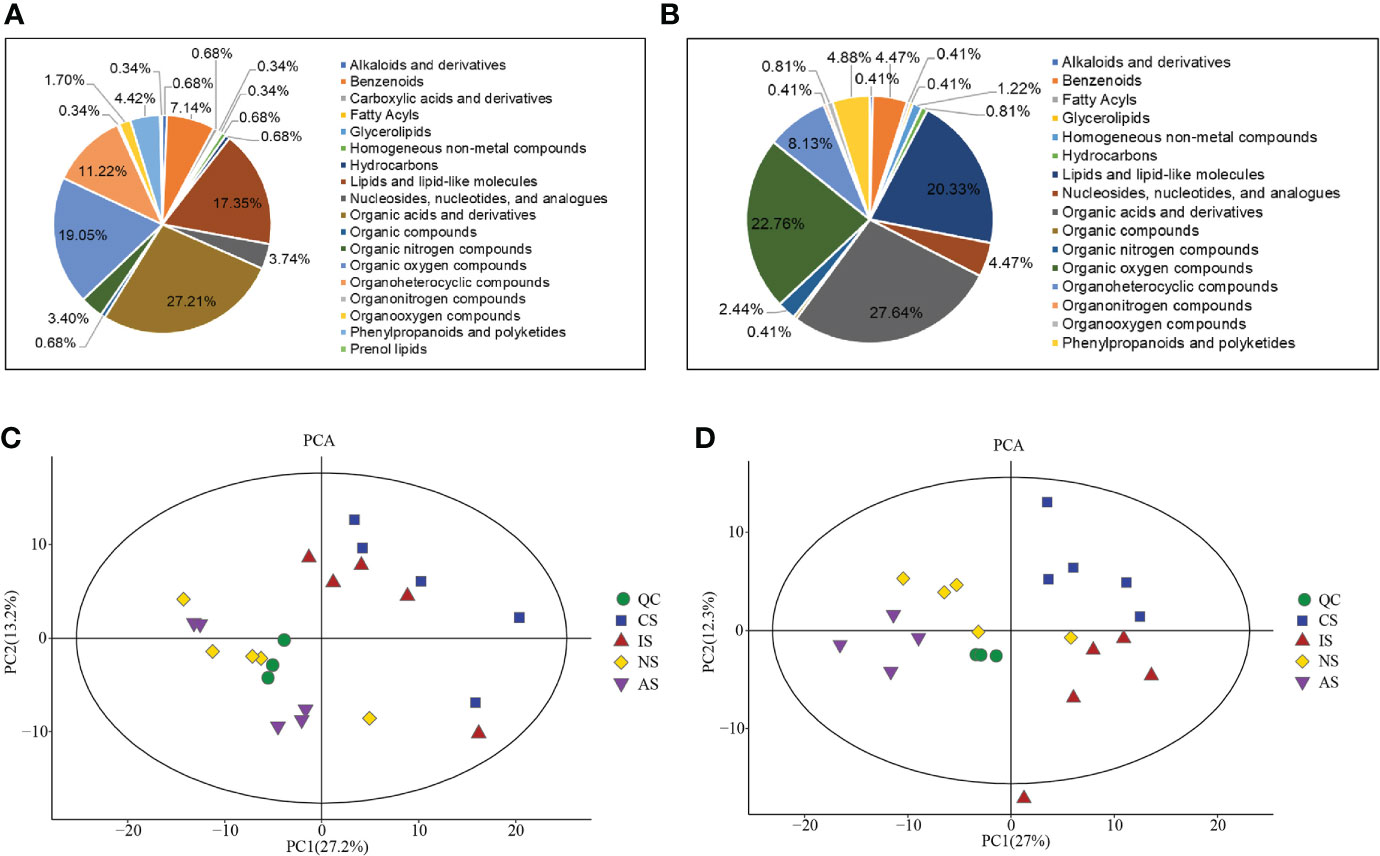
Figure 1 Data overview. Super class classification of total metabolites in gill (A) and hemolymph (B); PCA score plot of metabolites in gill (C) and hemolymph (D).
QC samples were required for reliable and high-quality results (León et al., 2013). The results showed that the QC samples were closely clustered, indicating that the experiment was stable and reproducible and that the data was qualified for statistical analysis. In addition, the PCA showed that all but one outlier sample were within 95% confidence intervals, with scores showing significant separation of metabolite profiles between the four groups (Figures 1C, D). OPLS-DA multivariate statistical analysis revealed significant differences in metabolites between each two groups (Figures S1, S2).
Differential metabolites of S. paramamosain in the gill and hemolymph
To screen the differential metabolites (DMs) among these groups, we integrated the results of the multivariate analysis to identify the DMs between any two groups (VIP > 1, P< 0.05). The metabolites screened under the above conditions had significant differences, and we have screened all the up-regulated and down-regulated DMs (Tables S1-12). In order to exhibit the relationship and the expression differences of metabolites between different samples more intuitively, we performed Hierarchical Clustering on the expression levels of all significantly different metabolites (Figure 2).
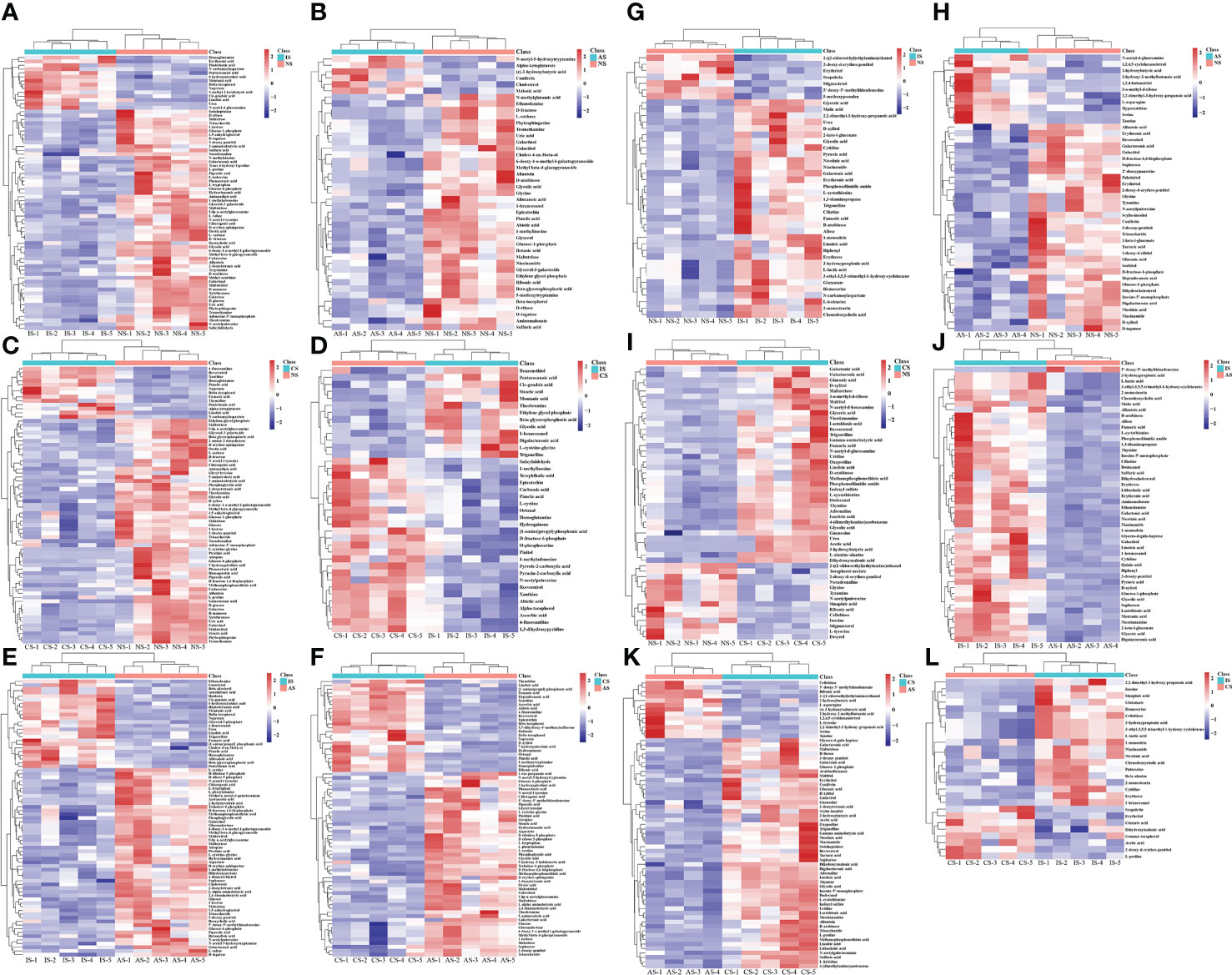
Figure 2 Heat maps of differential metabolites in the gill (A–F) and hemolymph (G–L). (A, G): IS/NS; (B, H): AS/NS; (C, I): CS/NS; (D, L): IS/CS; (E, J): IS/AS; (F, K): CS/AS. The abscissa and ordinate represent sample names and differential metabolites, respectively. Colors from blue to red indicate low to high expression abundance of metabolites.
In the gills, DMs were down-regulated significantly more than up-regulated. We analyzed annotated metabolites and found that 14 DMs were up-regulated and 57 DMs were down-regulated in IS/NS group. The up-regulated DMs were mainly lipids and lipid-like molecules, including δ-tocopherol, montanic acid, linoleic acid, cis-gondoic acid and pentacosanoic acid. Moreover, the most down-regulated DMs were organic oxygen compounds, mainly carbohydrates, including L-sorbose, D-fructose, Glucose-6-phosphate and so on (Figure 2A). As for AS/NS group, we found 6 increased and 37 decreased DMs. The increased metabolites were mainly organic acids and derivatives, while most of the decreased metabolites were organic oxygen compounds, including D-fructose, L-sorbose, D-arabinose etc. (Figure 2B). Among CS/NS group, there were 13 DMs increased and 56 DMs reduced. The increased metabolites were mostly lipids and lipid-like molecules and organic acids and derivatives, including δ-tocopherol, linoleic acid, pimelic acid, fumaric acid, α-ketoglutarate and N-carbamoylaspartate. The dominantly reduced metabolites were organic oxygen compounds (Figure 2C). A total of 38 DMs in IS/CS group were found, among which 13 metabolites were up-regulated and 25 metabolites were down-regulated. The most increased metabolites were lipids and lipid-like molecules, including stearic acid, montanic acid, β-glycerophosphoric acid and cis-gondoic acid. Most of the decreased metabolites were organic acids and derivatives, including N-acetylputrescine, L-cystine, O-phosphoserine and carbamic acid (Figure 2D). There were 13 DMs rose, focusing on lipids and lipid-like molecules, including montanic acid, linoleic acid, cis-gondoic acid and so on. And 51 DMs declined, mainly organic oxygen compounds, including D-fructose-1,6-bisphosphate, glucose-6-phosphate, trehalose-6-phosphate in IS/AS group (Figure 2E). There were 24 DMs up-regulated, mainly lipids and lipid-like molecules, while 48 DMs were down-regulated, predominantly 13 organic oxygen compounds and 13 organic acids and derivatives in CS/AS group (Figure 2F). From the above results, we found that organic oxygen compounds, mainly carbohydrates were significantly down-regulated in the 3 low salinity groups. In addition, lipids and lipid-like molecules, especially δ-tocopherol and linoleic acid were significantly up-regulated in CS and IS compared with NS. Similarly, lipids and lipid-like molecules in IS were significantly up-regulated compared with CS and AS. It was worth noting that montanic acid and cis-gondoic acid were significantly up-regulated in IS compared to the other three groups.
In the hemolymph, the results showed 34 increased and 7 decreased DMs in IS/NS group. Most of the increased metabolites were organic acids and derivatives, including fumaric acid, L-isoleucine, homoserine, glutamate, pyruvic acid, L-lactic acid, etc., while stigmasterol, erythritol, scopoletin, etc. were reduced (Figure 2G). There were 11 DMs up-regulated, mainly organic acids and derivatives, including serine, taurine, 2-hydroxybutyric acid and L-asparagine. And 33 DMs were down-regulated, the majority were organic oxygen compounds, such as erythritol, glucose-1-phosphate, and D-fructose-1-phosphate in the AS/NS group (Figure 2H). There were 36 DMs rose in the CS group, predominantly organic acids and derivatives, such as acetic acid, γ-aminobutyric acid, fumaric acid and isocitric acid, and 14 DMs declined, including glycine compared with the NS group (Figure 2I). There were 47 DMs up-regulated, mainly organic acids and derivatives (including malic acid, fumaric acid, pyruvic acid, L-lactic acid, etc.) and organic oxygen compounds (including D-arabinose, erythrose, D-xylitol, etc.), however, only 5’-deoxy-5’-methylthioadenosine was down-regulated in IS/AS group (Figure 2J). There were 52 DMs increased and 13 DMs reduced in CS/AS group, the most of increased metabolites were organic oxygen compounds (including D-fucose, D-xylitol, maltotriose, etc.) and organic acids and derivatives (including L-histidine, L-proline, γ-aminobutyric acid, etc.), while organic acids and derivatives, such as serine, taurine, L-asparagine and L-tyrosine were decreased (Figure 2K). There were 19 DMs up-regulated and 8 DMs down-regulated in IS/CS group. The up-regulated metabolites were mainly organic acids and derivatives, including L-lactic acid, homoserine and β-alanine, and the primary down-regulated metabolites were also organic acids and derivatives, including L-proline, glutaric acid and acetic acid (Figure 2L). Collectively, these data showed that organic acids and derivatives, mainly amino acids and metabolites related to energy metabolism were significantly up-regulated in the three low salinity groups.
Analysis of metabolic pathway of differential metabolites
To gain further insight into the metabolic changes associated with salinity, a KEGG pathway analysis of all DMs was performed. There were significant differences in various metabolic pathways in different groups, including amino acid metabolism, fatty acid metabolism, tricarboxylic acid (TCA) cycle and other related pathways (Figure 3).
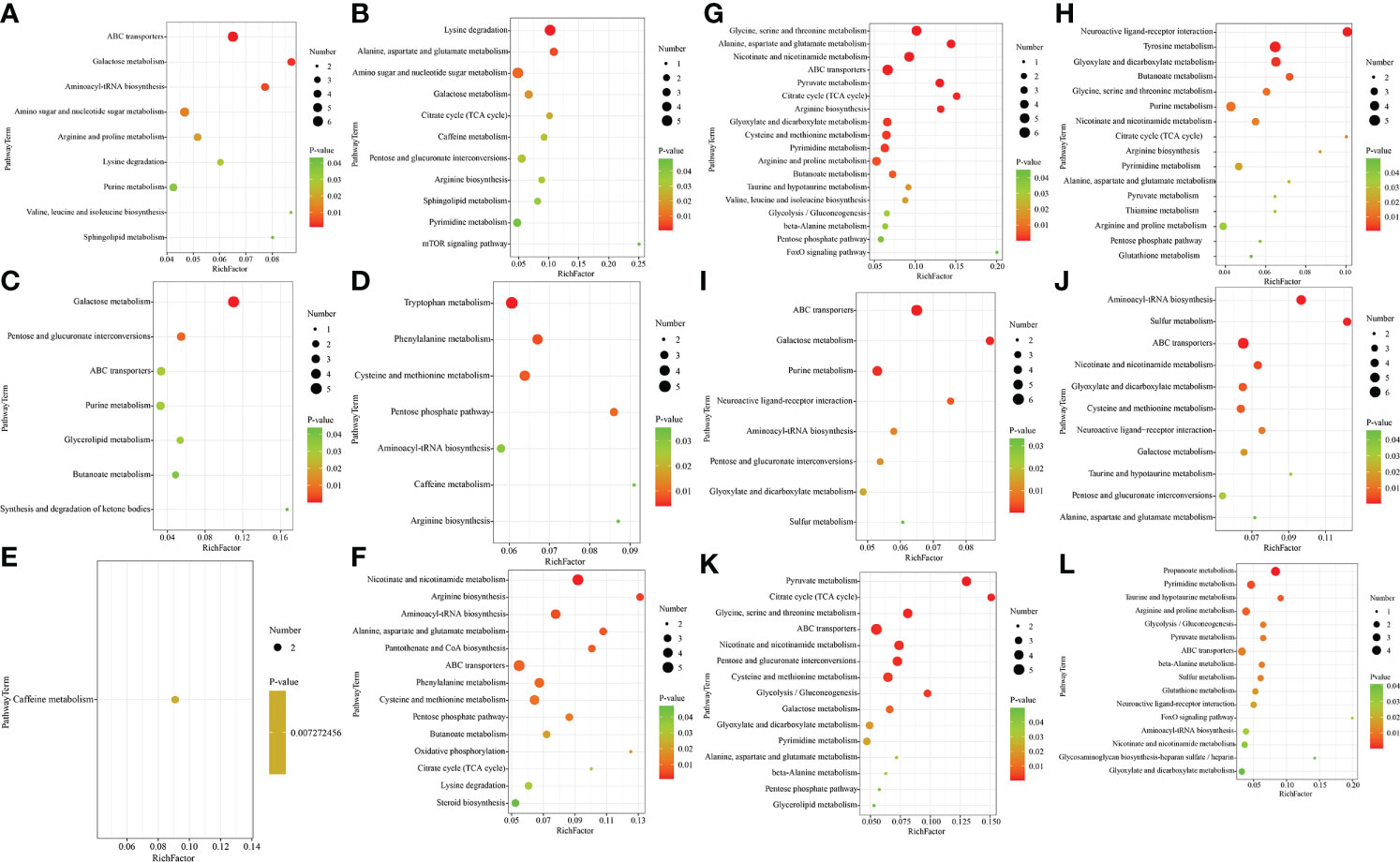
Figure 3 Bubble diagram of the significantly enriched metabolic pathways in the gill (A–F) and hemolymph (G–L) (p< 0.05). (A), G:IS/NS; (B), H:CS/NS; (C), I:AS/NS; (D, J): CS/AS; (E, K):IS/CS; (F, L): IS/AS. The abscissa represents the Rich factor (Rich factor=number of significantly different metabolites/total number of metabolites in the pathway), and the ordinate represents the metabolic pathway name. The greater the Rich factor, the greater the degree of enrichment; The color from red to green indicates that the p-value decreases in turn; The larger the point, the greater the number of metabolites enriched on the pathway.
In the gill, 37 metabolic pathways were found in IS/NS group through DMs metabolic pathway enrichment analysis, of which 9 were significantly enriched (P< 0.05) and 28 were non-significantly enriched (P > 0.05). The 9 significant enriched metabolic pathways were mainly amino acid metabolism. In addition, pathways related to purine metabolism and phingolipid metabolism were also included. Particularly, ABC transporters, galactose metabolism and aminoacyl-tRNA biosynthesis have extremely significant differences (Figure 3A, P< 0.01). The analysis indicated that there were 42 metabolic pathways of DMs annotated in CS/NS group. Among them, amino sugar and nucleotide sugar metabolism, lysine degradation, alanine, aspartate and glutamate metabolism were extremely significant enrichment (P< 0.01), while galactose metabolism, TCA cycle, caffeine metabolism, pentose and glucuronate interconversions, arginine biosynthesis, sphingolipid metabolism, pyrimidine metabolism, mTOR signaling pathway were significant enrichment (Figure 3B, P< 0.05). 38 metabolic pathways were enriched in AS/NS group, and 7 of them were significant enrichment (P< 0.05). Galactose metabolism and pentose and glucuronate interconversions were extremely significant enrichment (Figure 3C, P< 0.01). There were 41 metabolic pathways of DMs enriched in CS/AS group, including 4 extremely significant enrichment pathways (P< 0.01) and 3 significant enrichment (P< 0.05) pathways (Figure 3D). There were 21 metabolic pathways enriched, and only caffeine metabolism was significantly enriched in IS/CS group (Figure 3E, P< 0.05). We found 47 metabolic pathways, of which 14 were significantly enriched in IS/AS group (P< 0.05). It is worth noting that arginine biosynthesis, alanine, aspartate and glutamate metabolism, and ABC transporters showed extremely significant enrichment (Figure 3F, P< 0.01).
In the hemolymph, the data indicated 45 metabolic pathways enrichment of DMs in IS/NS group. In particular, seven pathways including glycine, serine and threonine metabolism, alanine, aspartate and glutamate metabolism, TCA cycle, arginine biosynthesis and so on were extremely significant enriched (Figure 3G, P< 0.01). For CS/NS group, there were 41 metabolic pathways enrichment of DMs, including 4 extremely significant enrichment pathways (neuroactive ligand-receptor interaction, tyrosine metabolism, glyoxylate and dicarboxylate metabolism and butanoate metabolism, P< 0.01) and 12 significant enrichment pathways (glycine, serine and threonine metabolism, TCA cycle, alanine, aspartate and glutamate metabolism, arginine, proline metabolism, etc., P< 0.05) (Figure 3H). 30 metabolic pathways were enriched in AS/NS group, of which 5 pathways were extremely significant enriched (P< 0.01), including ABC transporters, galactose metabolism, purine metabolism, neuroactive ligand-receptor interaction and aminoacyl-tRNA biosynthesis, and three pathways were significant enriched (Figure 3I, P< 0.05). As for CS/AS group, there were 40 metabolic pathways enrichment, 6 pathways including cysteine and methionine metabolism were extremely significant enriched (P< 0.01), and 6 pathways including taurine and hypotaurine metabolism were significant enriched (Figure 3J, P< 0.05). For IS/CS group, there were 33 metabolic pathways enrichment of DMs, including 4 extremely significant enrichment pathways (propanoate metabolism, pyrimidine metabolism, taurine and hypotaurine metabolism, arginine and proline metabolism) (P< 0.01) and 12 significant enrichment pathways (glycolysis/gluconeogenesis, pyruvate metabolism, ABC transporters, β-alanine metabolism, etc.) (Figure 3K, P< 0.05). There were 38 metabolic pathways of DMs enriched in IS/AS group, of which 9 pathways such as pyruvate metabolism, glycine, serine and threonine metabolism and glycolysis/gluconeogenesis were extremely significant enriched (P< 0.01), and 6 pathways including alanine, aspartate and glutamate metabolism, β-alanine metabolism, etc. were significant enriched (Figure 3L, P< 0.05).
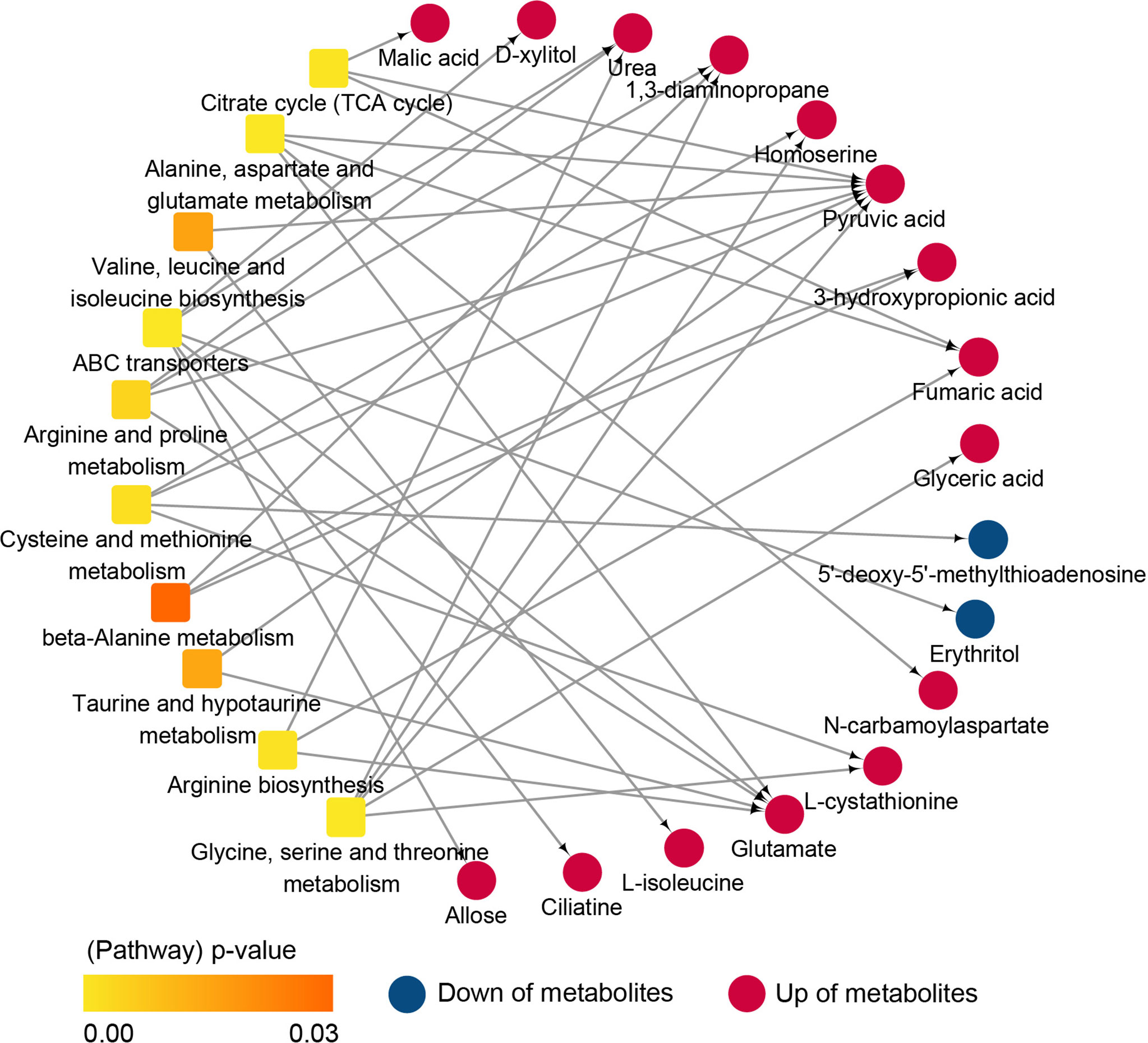
The above results showed that significant metabolic pathways were mainly related to amino acids, energy metabolism, and signal transduction. In the gills, compared with NS, it was found that galactose metabolism and lipid metabolism (phingolipid and glycerolipid) metabolic pathways were all significantly enriched in IS, CS and AS, while amino acids (especially lysine degradation) metabolic pathways were significantly enriched in IS and CS, and ABC transporters metabolic pathways were significantly enriched in IS and AS. In the hemolymph, compared with NS, the results revealed that amino acids (glycine, serine and threonine metabolism, alanine, aspartate and glutamate metabolism, arginine and proline metabolism) and TCA cycle metabolic pathways were significantly enriched in IS and CS. Compared with CS, NS and AS, alanine, pyruvate metabolism, ABC transporters, pyrimidine metabolism, nicotinate and nicotinamide metabolism pathways were significantly enriched in IS. Particularly, taurine and hypotaurine metabolism were significantly enriched in IS compared with CS and NS. In this study, taurine and hypotaurine metabolism directly regulated the synthesis of taurine, which significantly increased the levels of pyruvate and glutamate (intermediate metabolites of taurine) in the IS group (Figure 4). The significantly different amino acid pathways, ABC transporters, TCA cycle and corresponding differential metabolites in IS/NS group were then combined to form an interaction network diagram (Figure 5). The results indicated that these changed metabolites might play an essential role in the response of S. paramamosain to low-salinity saline-alkaline water stress.
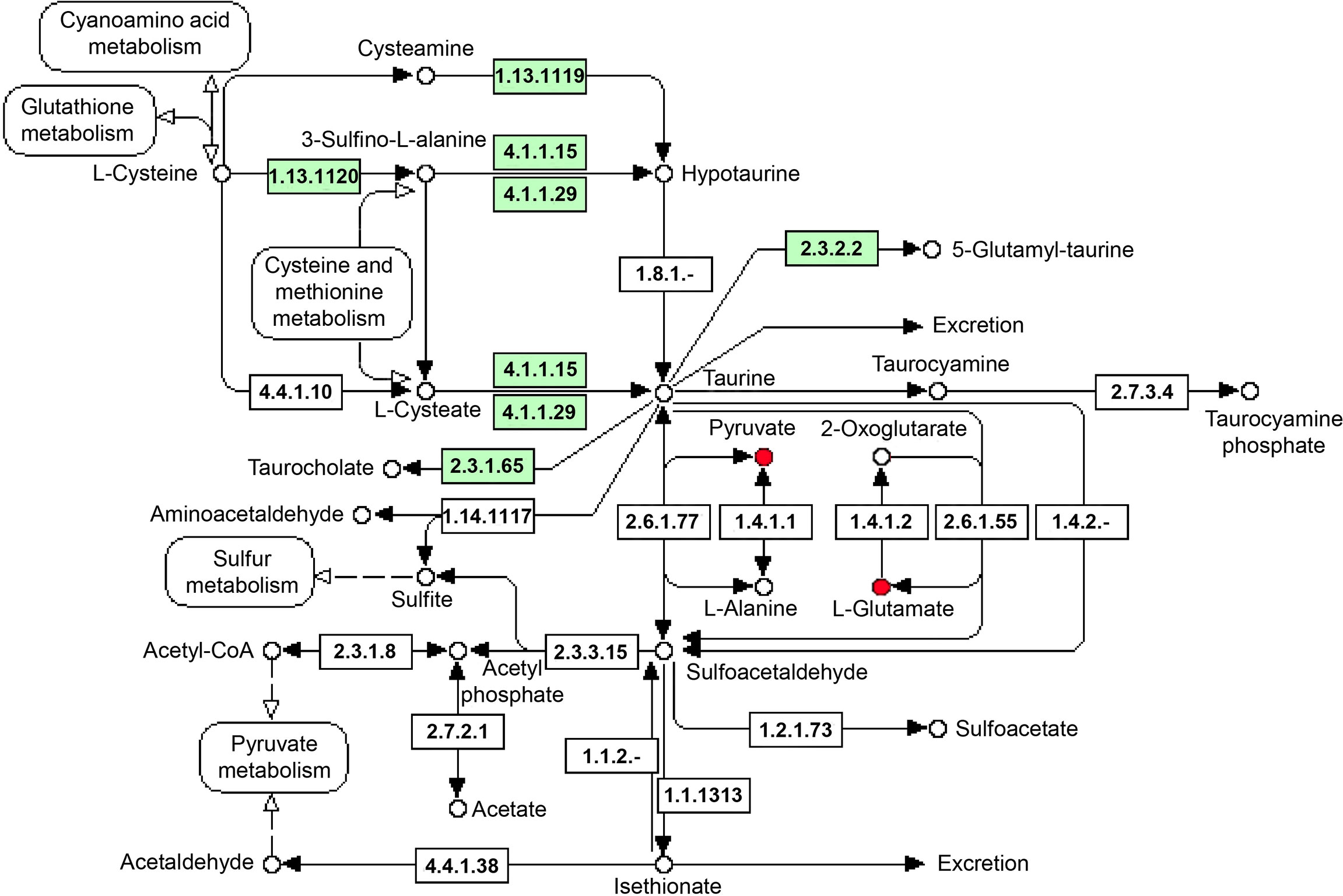
Figure 4 Taurine and hypotaurine metabolism KEGG pathway. The red indicates the up-regulated compound in this study, the green squares indicate enzymes unique to the species.
Discussion
Gills play a crucial role in osmotic and ionic regulation in crustaceans (Romano et al., 2012; Lv et al., 2016). Previous studies have proved that the anterior gills were specialized for gas exchange, whereas the main function of the posterior gills is osmoregulation (Péqueux, 1995). Thus, the posterior gill was used as the research material in this study. The function of posterior gills on osmotic and ion regulation depends on ion transport enzymes, especially Na+/K+-ATPase, and transporters (Henry et al., 2003; Henry et al., 2012). The results showed that lipids and lipid-like molecules (especially δ-tocopherol and linoleic acid) were the main metabolites, which were significantly up-regulated in IS and CS, while cholesterol was significantly up-regulated in AS. At the same time, lipid metabolism (phingolipid and glycerolipid) metabolic pathways were significantly enriched in IS, CS and AS compared with NS. Phospholipids are known to be major components of cell membranes, maintaining the shape and fluidity of cells (Lavie et al., 1999). Meanwhile, cholesterol is also an essential component of cell membranes. It is embedded between the phospholipid bilayers of cell membranes, making the cell membrane structure fluid and playing a vital role in maintaining normal cell function. The analysis revealed that lipids and lipid-like molecules play a critical role in the osmolality regulation of crab, which was consistent with Yao et al. (Yao et al., 2021).
Previous studies have shown that crustaceans, including crabs, responded to changes in environmental salinity by altering the fatty acid composition in different tissues, especially gills (Romano et al., 2014). Moreover, the lipid composition and the intra-/intercellular ion concentration of S. serrata varied significantly with salinity (Bhoite and Roy, 2013). The total phospholipid content in the hind gills of Eriocheir sinensis significantly increased after salinity changes (Chapelle et al., 1976). Previous studies have found that S. paramamosain regulates osmolality by transporting Na+, K+ and Cl- into cells via cotransporters (Gagnon et al., 2003; Gamba, 2005; Xu et al., 2017). These results indicated that membrane components of gill cells were associated with the osmoregulation of S. paramamosain.
Free amino acids (FAAs) are important osmotic regulators used by crustaceans under salinity stress (Huong et al., 2001; Li et al., 2014; Yao et al., 2020). In euryhaline crustaceans, FAAs constitute most of the organic permeate that accumulates in response to hypertonic stress. In the gills, amino acids (especially lysine degradation) metabolic pathways were significantly enriched and N-carbamoylaspartate was significantly up-regulated in IS and CS compared with NS, indicating that free amino acids, especially lysine and N-carbamoylaspartate participated in the osmolality regulation of S. paramamosain. Hemolymph has the function of maintaining homeostasis, including pH, osmolarity, water balance, and ion composition. Studies have indicated that various inorganic ions, especially Na+ and Cl-, are major contributors to hemolymph osmotic pressure in crustaceans (Chen and Chen, 2000; Wang et al., 2012).
Besides, FAAs are also considered to be key factors in osmoregulation, which play an essential role in intracellular osmoregulation in crustaceans, although their concentrations are lower than those of inorganic ions (Abe, 1999; Huong et al., 2001). In the hemolymph, more amino acids were taking part in the regulating osmolality of S. paramamosain. Oxoproline, γ-aminobutyric acid, L-isoleucine, homoserine and glutamate were up-regulated, and arginine and proline metabolism, alanine, aspartate and glutamate metabolism, glycine, serine and threonine metabolism were significantly enriched in IS and CS compared with NS. Studies have shown that glycine, proline and alanine widely function as osmoeffectors in several crustacean species (Fujimori and Abe, 2002; Liu et al., 2008; Faria et al., 2011; Mazzarelli et al., 2015). In addition, lysine and arginine were specific osmolality effectors in the hemolymph of Litopenaeus vannamei (Liu et al., 2008). Glutamate is known to play a key role in the biosynthesis of non-essential amino acids, serving as a precursor for L-proline and L-glutamine and as an amino donor for glycine and L-alanine (Schoffeniels and Reid, 1976). Thus, the glutamate metabolism was enriched in our study. Aspartic acid was known to be a critical osmoregulatory factor in S. paramamosain (Yao et al., 2020) and Eriocheir sinensis (Wang et al., 2004). From the above results, it was found that FAAs occupied an important position in the regulation of osmotic in the S. paramamosain.
The osmoregulation process of crustaceans is physiologically energy-consuming under low salinity conditions (Ye et al., 2009; Dirk et al., 2009; Wang et al., 2018c; Yao et al., 2020). Carbohydrate metabolism plays a critical part in the energy required for ionic and osmoregulation (Tseng and Hwang, 2008). In this study, the significantly down-regulated metabolites in the gill of CS, IS and AS were mainly organic oxygen compounds, most of which were carbohydrates, including galactose, glucose-6-phosphate, D-fructose-1,6-bisphosphate, D-fructose, maltotriose and so on. At the same time, galactose metabolism and lipid metabolism metabolic pathways were significantly enriched in IS, CS and AS. On the contrary, most carbohydrates and organic acids (related to energy metabolism) were significantly up-regulated in the hemolymph of CS and IS groups, especially fumaric acid, pyruvic acid and lactic acid. As we all know, fumaric acid takes part in the TCA cycle (Ma et al., 2017). Pyruvate is converted into acetyl-CoA by dehydrogenase and then enters the TCA cycle, and lactic acid is converted into pyruvate under the catalysis of lactate dehydrogenase and then enters the TCA cycle. TCA is a key link between carbohydrate, protein and lipid metabolism (Zalis et al., 2019). Furthermore, changes in TCA metabolites indicate alterations in cellular bioenergetics (Koundal et al., 2015). Additionally, lactic acid level reflects anaerobic metabolism level (Venkatesh and Ramalingam, 2007). This is consistent with the significant enrichment of the TCA cycle and pyruvate metabolic pathway in this study. Up- and down-regulation of carbohydrates and organic acids were possibly related to the dynamic energy changes of S. paramamosain in response to low salinity. Lipid and amino acid metabolism were also vital pathways for energy production (Amer et al., 2017). Osmotic stress could induce physiological responses such as increased dissolved oxygen consumption (Spanopoulos-Hernández et al., 2005) and ammonia excretion (Chen and Lin, 1994; Silvia et al., 2004). It has been reported that crustaceans can detoxify elevated ammonia levels in their body fluids by converting it into urea (Ren et al., 2015; Zimmer et al., 2017; Dong et al., 2020). This explains the significant up-regulation of urea in CS and IS groups of hemolymphs in this study.
There were significant differences in the metabolites of S. paramamosain in different low salinity areas, indicating certain differences in their osmotic regulation mechanisms. In the gills, ABC transporters’ metabolic pathways were significantly enriched in IS and AS compared with NS, while amino acids’ metabolic pathways were significantly enriched in IS and CS. In the hemolymph, amino acids and energy metabolism related metabolites were significantly up-regulated and amino acids and TCA cycle metabolic pathways were significantly enriched in IS and CS compared with NS, while energy metabolism related metabolites were significantly down-regulated and ABC transporters, galactose metabolism, purine metabolism were extremely significantly enriched in AS. The ABC transporter super family is one of the largest types of transporters, their function is to use the binding and hydrolysis of ATP to promote the translocation of various substrates from ions to macromolecules across membrane (Rees et al., 2009). These results indicated that the osmoregulation of AS group was dominated by ion transport and energy metabolism, while IS and CS were dominated by amino acid and energy metabolism. The AS group was in an acute low salinity environment artificially simulated in the laboratory. The seawater was diluted to a salinity of 3‰ with fresh water, and the crabs were kept for only one week. While IS and CS were natural low salinity environments, crabs have adapted to the local environment after a long period of cultivation. Therefore, we speculate that under acute/short-term low salinity conditions, S. paramamosain relied on ion transport and energy metabolism for osmoregulation, while amino acids and energy metabolism occupied a leading position in long-term low salinity.
Unlike the other three groups, IS belongs to the inland low salinity saline-alkaline water environment, the type of saline-alkaline type is SO2+4, and the ion composition in the water is obviously different from that of seawater. It was also the first-time marine crabs were successfully cultured in inland low salinity saline-alkaline water. Thus, the IS group received more tremendous salinity stress and consumed more energy to regulate osmolality balance. As a result, there were more free amino acids and energy metabolism-related metabolites of IS than CS and AS compared to NS, such as L-isoleucine, homoserine, glutamate, malic acid, pyruvic acid and L-lactic acid. Besides, the metabolic pathways of arginine and proline metabolism, valine, leucine and isoleucine biosynthesis were significantly enriched in IS. Taurine and hypotaurine metabolism were significantly enriched in IS compared with NS, while extremely significantly enriched compared with CS. This indicated that taurine and hypotaurine play an important role in the response of crabs to inland saline-alkaline water stress. Previous studies have shown that taurine was an important osmoregulatory factor in S. paramamosain (Yao et al., 2020) and Eriocheir sinensis (Long et al., 2017). Therefore, in the culturing process, adding a certain amount of taurine and hypotaurine to the feed, or strengthening the metabolic pathway through related products, may improve the ability of S. paramamosain adapting to inland low salinity saline-alkaline water and improve their survival rate.
Conclusion
In conclusion, we investigated the metabolic response of S. paramamosain under multiple low salinity stress modes based on GC-MS technology. The structure and permeability of gill cell membranes play an essential role in osmotic regulation in crustaceans to maintain hemolymph osmotic pressure/ion and survive under salinity stress (Freire et al., 2008). In this study, lipids and lipid molecules accounted for most of the metabolites of gills, while amino acids took possession of a large proportion of metabolites of hemolymph. Thus, our results suggested that in the low salinity environments, the metabolic mechanisms of osmoregulation of crabs maybe improve their ability to transport ions, amino acids and carbohydrates through biomembranes by altering the membrane composition of the gill cells, and then the free amino acids content in the hemolymph is increased. This process is accompanied by consuming energy substances such as carbohydrates and lipids. However, the mechanism still requires further study. On the other hand, there were specific differences in the metabolic mechanisms of osmotic regulation of crabs under acute low salinity and long-term low salinity stress, and taurine and hypotaurine play an important role in the adaptation of crabs to inland saline-alkaline water.
In this work, we used metabolomics analysis to elucidate the osmoregulation mechanism of S. paramamosain in multiple low salinity stress modes, which provided novel insight into the metabolic mechanism of S. paramamosain adapting to inland low salinity saline-alkaline water, and provided theoretical guidance for the cultivation of S. paramamosain in inland saline-alkaline water.
Data availability statement
The data presented in the study are deposited in the Metabolights repository, accession number MTBLS5202 and MTBLS5220.
Author contributions
HW conceived and designed the study. MN, GG, KQ, XL and YC took samples of experimental animals. MN, GL, HW, CW, CM and QS performed and analyzed all the other experiments. MN wrote the manuscript with support from all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R & D Program of China (2020YFD0900203); Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02069-6); China Agriculture Research System of MOF and MARA; “Three Rurals and Six Parties” Science and Technology Cooperation Plan of Zhejiang Province (2021SNLF029); and the K. C. Wong Magna Fund in Ningbo University. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. And we are grateful to Shanghai Luming Biology Co., Ltd. for their assistance in metabolomic data analysis.
Conflict of interest
Author QS is employed by Guangxi Institute of Oceanography Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.977599/full#supplementary-material
Abbreviations
NS, normal salinity group; CS, coastal low salinity group; IS, inland low salinity saline-alkaline water group; AS, acute low salinity group; QC, quality control sample; PCA, principal component analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; DMs, differentially metabolites; TCA cycle, tricarboxylic acid cycle.
References
Abe H. (1999). Effects of seawater acclimation on the levels of free d- and l-alanine and other osmolytes in the Japanese mitten crab Eriocheir japonicus. Fish. Sci. 65 (6), 949–954. doi: 10.2331/fishsci.65.949
Amer B., Clausen M. R., Bertram H. C., Bohl M., Nebel C., Zheng H., et al. (2017). Consumption of whey in combination with dairy medium-chain fatty acids (MCFAs) may reduce lipid storage due to urinary loss of tricarboxylic acid cycle intermediates and increased rates of MCFAs oxidation. Mol. Nutr. Food Res. 61 (12), 1601048. doi: 10.1002/mnfr.201601048
Bhoite S., Roy R. (2013). Role of membrane lipid in osmoregulatory processes during salinity adaptation: a study with chloride cell of mud crab, Scylla serrata. Mar. Freshw. Behav. Physiol. 46 (5-6), 287–300. doi: 10.1080/10236244.2013.832525
Chapelle S., Drifosse G., Zwingelstein G. (1976). Metabolism of phospholipids of anterior or posterior gills of the crab eriocheir sinensis m. EDW, during the adaptation of this animal to media of various salinitie. Int. J. Biochem. 7 (6-7), 343–351. doi: 10.1016/0020-711x(76)90097-5
Chen J. M., Chen J. C. (2000). Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle of penaeus monodon exposed to elevated ambient ammonia. Aquat. Toxicol. 50 (1-2), 27–37. doi: 10.1016/S0166-445X(99)00095-8
Chen J. C., Chia P. G. (1997). Osmotic and ionic concentrations of Scylla serrata (Forskal) subjected to different salinity levels. Comp. Biochem. Physiol. 117 (2), 239–244. doi: 10.1016/S0300-9629(96)00237-X
Chen J. C., Lin J. L. (1994). Osmolality and chloride concentration in the hemolymph of subadult penaeus chinensis subjected to different salinity levels. Aquaculture 125 (1-2), 167–174. doi: 10.1016/0044-8486(94)90293-3
China fishery statistical yearbook (2021). China Fishery statistical yearbook (Beijing: China Agriculture Press).
Chung K. F., Lin H. C. (2006). Osmoregulation and Na,K-ATPase expression in osmoregulatory organs of Scylla paramamosain. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 144 (1), 48–57. doi: 10.1016/j.cbpa.2006.02.003
Dirk W., Michael P. W., Patrick J. W. (2009). Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 212 (17), 2879. doi: 10.1242/jeb.036103
Dong X. X., Liu Q., Kan D. Q., Zhao W. H., Guo H. S., Lv L. L. (2020). Effects of ammonia-n exposure on the growth, metabolizing enzymes, and metabolome of Macrobrachium rosenbergii. Ecotoxicol. Environ. Saf. 189, 110046. doi: 10.1016/j.ecoenv.2019.110046
Faria S., Augusto A. S., Mcnamara J. C. (2011). Intra- and extracellular osmotic regulation in the hololimnetic caridea and anomura: a phylogenetic perspective on the conquest of fresh water by the decapod Crustacea. J. Comp. Physiol. B. Biochem. Syst. Environ. Physiol. 181 (2), 175. doi: 10.1007/s00360-010-0522-6
Freire C. A., Onken H., Mcnamara J. C. (2008). A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 151 (3), 272–304. doi: 10.1016/j.cbpa.2007.05.008
Fry F. (1971). The effect of environmental factors on the physiology of fish. Fish. Physiol. 6, 1–98. doi: 10.1016/S1546-5098(08)60146-6
Fujimori T., Abe H. (2002). Physiological roles of free d- and l-alanine in the crayfish Procambarus clarkii with special reference to osmotic and anoxic stress responses. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 131 (4), 893–900. doi: 10.1016/S1095-6433(02)00006-5
Gagnon E., Forbush B., Caron L., Isenring P. (2003). Functional comparison of renal Na-K-Cl cotransporters between distant species. Am. J. Physiol. Cell Physiol. 284 (2), C365–C370. doi: 10.1152/ajpcell.00262.2002
Gamba G. (2005). Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 85 (2), 423–493. doi: 10.1152/physrev.00011.2004
Hare P. D., Cress W. A., Staden J. V. (2010). Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 21 (6), 535–553. doi: 10.1046/j.1365-3040.1998.00309.x
Henry R. P., Gehnrich S., Weihrauch D., Towle D. W. (2003). Salinity-mediated carbonic anhydrase induction in the gills of the euryhaline green crab, Carcinus maenas. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 136 (2), 243–258. doi: 10.1016/S1095-6433(03)00113-2
Henry R. P., Lucu C. E., Onken H., Weihrauch D. (2012). Multiple functions of the crustacean gill: osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 3, 431. doi: 10.3389/fphys.2012.00431
Huong D., Yang W. J., Okuno A., Wilder M. N. (2001). Changes in free amino acids in the hemolymph of giant freshwater prawn Macrobrachium rosenbergii exposed to varying salinities: relationship to osmoregulatory ability. Comp. Biochem. Physiol. A. 128 (2), 317–326. doi: 10.1016/S1095-6433(00)00310-X
Imura M., Iwakiri R., Bamba T., Fukusaki E. (2018). Metabolomics approach to reduce the crabtree effect in continuous culture of Saccharomyces cerevisiae. J. Biosci. Bioeng. 126 (2), 183–188. doi: 10.1016/j.jbiosc.2018.02.008
Jiang W., Ma H. Y., Ma C. Y., Li S. J., Liu Y. X., Qiao Z. G., et al. (2014). Characteristics of growth traits and their effects on body weight of G1 individuals in the mud crab (Scylla paramamosain). Genet. Mol. Res. 13 (3), 6050–6059. doi: 10.4238/2014.August.7.19
Koundal S., Gandhi S., Kaur T., Mazumder A., Khushu S. (2015). "Omics" of high altitude biology: A urinary metabolomics biomarker study of rats under hypobaric hypoxia. OMICS.: A. J. Integr. Biol. 19 (12), 757–765. doi: 10.1089/omi.2015.0155
Lavie Y., Fiucci G., Czarny M., Liscovitch M. (1999). Changes in membrane microdomains and caveolae constituents in multidrug-resistant cancer cells. Lipids 34 (S1), S57–S63. doi: 10.1007/BF02562229
León Z., García-Caaveras J. C., Donato M. T., Lahoz A. (2013). Mammalian cell metabolomics: Experimental design and sample preparation. ELECTROPHORESIS 34 (19), 2762–2775. doi: 10.1002/elps.201200605
Li T., Roer R., Vana M., Pate S., Check J. (2010). Gill area, permeability and na+, k+-ATPase activity as a function of size and salinity in the blue crab, callinectes sapidus. J. Exp. Zool. Part A. Comp. Exp. Biol. 305A (3), 233–245. doi: 10.1002/jez.a.248
Liu J., Cheng A., Wang M., Liu M., Zhu D., Yang Q., et al. (2021). Comparative genomics and metabolomics analysis of Riemerella anatipestifer strain CH-1 and CH-2. Sci. Rep. 11 (1), 616. doi: 10.1038/s41598-020-79733-w
Liu H. Y., Pan L. Q., Zheng D. B. (2008). Injection of biogenic amines modulates osmoregulation of Litopenaeus vannamei: Response of hemolymph osmotic pressure, ion concentration and osmolality effectors. Comp. Biochem. Physiol. Part A.: Mol. Integr. Physiol. 151 (2), 191–197. doi: 10.1016/j.cbpa.2008.06.021
Li E., Wang S., Li C., Wang X., Chen K., Chen L. (2014). Transcriptome sequencing revealed the genes and pathways involved in salinity stress of Chinese mitten crab, Eriocheir sinensis. Physiol. Genomics 46 (5), 177. doi: 10.1152/physiolgenomics.00191.2013
Long X., Wu X., Zhao L., Ye H., Cheng Y., Zeng C. (2017). Effects of salinity on gonadal development, osmoregulation and metabolism of adult male Chinese mitten crab, Eriocheir sinensis. PloS One 12 (6), e0179036. doi: 10.1371/journal.pone.0179036
Lv J. J., Liu P., Gao B. Q., Li J. (2016). The identification and characteristics of salinity-related microRNAs in gills of Portunus trituberculatus. Cell Stress Chaperones. 21, 63–74. doi: 10.1007/s12192-015-0641-9
Ma Q. Q., Chen Q., Shen Z. H., Li D. L., Han T., Qin J. G., et al. (2017). The metabolomics responses of Chinese mitten-hand crab (Eriocheir sinensis) to different dietary oils. Aquaculture 479, 188–199. doi: 10.1016/j.aquaculture.2017.05.032
Ma H. Y., Li S. J., Feng N. N., Ma C. Y., Ma L. B. (2016). First genetic linkage map for the mud crab (Scylla paramamosain) constructed using microsatellite and AFLP markers. Genet. Mol. Res. Gmr. 15 (2), 15026929. doi: 10.4238/gmr.15026929
Ma H. Y., Ma C. Y., Li S. J., Jiang W., Li X. C., Liu Y. X., et al. (2014). Transcriptome analysis of the mud crab (Scylla paramamosain) by 454 deep sequencing: Assembly, annotation, and marker discovery. PloS One 9 (7), e102668. doi: 10.1371/journal.pone.0102668
Mazzarelli C., Santos M., Amorim R., Augusto A. (2015). Effect of salinity on the metabolism and osmoregulation of selected ontogenetic stages of an Amazon population of Macrobrachium amazonicum shrimp (Decapoda, palaemonidae). Braz. J. Biol. = Rev. Brasleira. Biol. 75 (2), 372–379. doi: 10.1590/1519-6984.14413
Neufeld G. J., Holliday C. W., Pritchard J. B. (1980). Salinity adaption of gill Na, K-ATPase in the blue crab, Callinectes sapidus. J. Exp. Zool. Part A. Ecol. Genet. Physiol. 211 (2), 215–224. doi: 10.1002/jez.1402110210
Péqueux A. (1995). Osmotic regulation in crustaceans. J. Crustacean. Biol. 15 (1), 1–60. doi: 10.1163/193724095x00578
Péqueux A., Gilles R. (1978). Osmoregulation of the euryhaline chinese crab Eriocheir sinensis: ionic transports across isolated perfused gills as related to the salinity of the environment. Physiol. Behav. Mar. Organisms. 17 (5), 105–111. doi: 10.1016/B978-0-08-021548-8.50019-X
Pinoni S. A., Goldemberg A. L., Mañanes A. A. L. (2005). Alkaline phosphatase activities in muscle of the euryhaline crab Chasmagnathus granulatus: Response to environmental salinity. J. Exp. Mar. Biol. Ecol. 326 (2), 217–226. doi: 10.1016/j.jembe.2005.06.004
Rees D., Johnson E., Lewinson O. (2009). ABC Transporters: the power to change. Nat. Rev. Mol. Cell Biol. 10 (3), 218–227. doi: 10.1038/nrm2646
Ren Q., Pan L., Zhao Q., Si L. (2015). Ammonia and urea excretion in the swimming crab portunus trituberculatus exposed to elevated ambient ammonia-n. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 187, 48–54. doi: 10.1016/j.cbpa.2015.04.013
Romano N., Wu X. G., Zeng C. S., Genodepa J., Elliman J. (2014). Growth, osmoregulatory responses and changes to the lipid and fatty acid composition of organs from the mud crab, Scylla serrata, over a broad salinity range. Mar. Biol. Res. 10 (5), 460–471. doi: 10.1080/17451000.2013.819981
Romano N., Zeng C. (2012). Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 334–337) 12–23. doi: 10.1016/j.aquaculture.2011.12.035
Schoffeniels E., Reid B. L. (1976). Anti-chance : A reply to monod's chance and necessity. Oxford: Pergammon Press. doi: 10.1016/C2013-0-02803-4
Shi X., Waiho K., Li X., Ikhwanuddin M., Miao G., Lin F., et al. (2018). Female-specific SNP markers provide insights into a WZ/ZZ sex determination system for mud crabs Scylla paramamosain, s. tranquebarica and S. serrata with a rapid method for genetic sex identification. BMC Genomics 19 (1), 981. doi: 10.1186/s12864-018-5380-8
Silvia G. J., Abel Antonio U. R., Francisco V. O., Georgina H. W. (2004). Ammonia efflux rates and free amino acid levels in litopenaeus vannamei postlarvae during sudden salinity changes. Aquaculture 233 (1-4), 573–581. doi: 10.1016/j.aquaculture.2003.09.050
Spanopoulos-Hernández M., Martínez-Palacios C., Vanegas-Pérez R., Rosas C., Ross L. G. (2005). The combined effects of salinity and temperature on the oxygen consumption of juvenile shrimps litopenaeus stylirostris (Stimpson 1874). Aquaculture 244 (1-4), 341–348. doi: 10.1016/j.aquaculture.2004.11.023
Tseng Y. C., Hwang P. P. (2008). Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. Toxicol. Pharmacol. Cbp. 148 (4), 419–429. doi: 10.1016/j.cbpc.2008.04.009
Venkatesh C., Ramalingam K. (2007). Lactic acid, pyruvic acid and lactate/pyruvate ratio in the anoplocephalid tapeworm stilesia globipunctata infecting sheep (Ovis aries). Vet. Parasitol. 144 (1), 176–179. doi: 10.1016/j.vetpar.2006.09.024
Via G. (1986). Salinity responses of the juvenile penaeid shrimp (prawn) Penaeus japonicus. 1. oxygen consumption and estimations of productivity. Aquaculture 55 (4), 297–306. doi: 10.1016/0044-8486(86)90170-5
Wang Y., Li E. C., Yu N., Wang X. D., Cai C. F., Tang B. P., et al. (2012). Characterization and expression of glutamate dehydrogenase in response to acute salinity stress in the Chinese mitten crab, Eriocheir sinensis. PloS One 7 (5), e37316. doi: 10.1371/journal.pone.0037316
Wang H., Tang L., Wei H. L., Lu J. K., Mu C. K., Wang C. L. (2018a). Transcriptomic analysis of adaptive mechanisms in response to sudden salinity drop in the mud crab, Scylla paramamosain. BMC Genomics 19 (1), 421. doi: 10.1186/s12864-018-4803-x
Wang W. N., Wang A. L., Bao L., Wang J., Liu Y., Sun R. Y. (2004). Changes of protein-bound and free amino acids in the muscle of the freshwater prawn macrobrachium nipponense in different salinities. Aquaculture 233 (1-4), 561–571. doi: 10.1016/j.aquaculture.2003.09.042
Wang H., Wei H. L., Tang L., Lu J. K., Mu C. K., Wang C. L. (2018b). A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene 677, 119–131. doi: 10.1016/j.gene.2018.07.059
Wang H., Wei H. L., Tang L., Lu J. K., Mu C. K., Wang C. L. (2018c). Identification and characterization of miRNAs in the gills of the mud crab (Scylla paramamosain) in response to a sudden drop in salinity. BMC Genomics 19 (1), 609. doi: 10.1186/s12864-018-4981-6
Xu B. P., Tu D. D., Yan M. C., Shu M. A., Shao Q. J. (2017). Molecular characterization of a cDNA encoding Na+/K+/2Cl cotransporter in the gill of mud crab (Scylla paramamosain) during the molt cycle: Implication of its function in osmoregulation. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 203, 115–125. doi: 10.1016/j.cbpa.2016.08.019
Yancey P., Clark M., Hand S., Bowlus R., Somero G. (1982). Living with water stress: evolution of osmolyte systems. Science 217 (4566), 1214–1222. doi: 10.1126/science.7112124
Yao H. Z., Li X., Chen Y. H., Liang G. L., Gao G., Wang H., et al. (2021). Metabolic changes in Scylla paramamosain during adaptation to an acute decrease in salinity. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.734519
Yao H. Z., Li X., Tang L., Wang H., Wang C. L., Mu C. K., et al. (2020). Metabolic mechanism of the mud crab (Scylla paramamosain) adapting to salinity sudden drop based on GC-MS technology. Aquacult. Rep. 18, 100533. doi: 10.1016/j.aqrep.2020.100533
Ye L., Jiang S., Zhu X. M., Yang Q. B., Wen W. G., Wu K. C. (2009). Effects of salinity on growth and energy budget of juvenile. Penaeus. Monodon. Aquacult. 290 (1-2), 140–144. doi: 10.1016/j.aquaculture.2009.01.028
Zalis E. A., Nuxoll A. S., Manuse S., Clair G., Lewis K. (2019). Stochastic variation in expression of the tricarboxylic acid cycle produces persister cells. mBio 10 (5), e01930–e01919. doi: 10.1128/mBio.01930-19
Zhang Y., Wu Q., Fang S., Li S., Ma H. Y. (2020). mRNA profile provides novel insights into stress adaptation in mud crab megalopa, Scylla paramamosain after salinity stress. BMC Genomics 21 (1), 559. doi: 10.1186/s12864-020-06965-5
Zhu H., Liu Z., Gao F., Lu M., Liu Y., Su H., et al. (2018). Characterization and expression of Na+/K+-ATPase in gills and kidneys of the teleost fish Oreochromis mossambicus, Oreochromis urolepis hornorum and their hybrids in response to salinity challenge. Comp. Biochem. Physiol. Part A.: Mol. Integr. Physiol. 224, 1–10. doi: 10.1016/j.cbpa.2018.05.017
Keywords: Scylla paramamosain, metabolic response, low salinity stress, osmoregulation, inland saline-alkaline water
Citation: Niu MM, Gao G, Qin KX, Chen YH, Wang H, Li X, Liang GL, Wang CL, Mu CK and Su Q (2022) Multiple low salinity stress modes provided novel insight into the metabolic response of Scylla paramamosain adapting to inland saline-alkaline water. Front. Mar. Sci. 9:977599. doi: 10.3389/fmars.2022.977599
Received: 24 June 2022; Accepted: 22 July 2022;
Published: 15 August 2022.
Edited by:
Youji Wang, Shanghai Ocean University, ChinaReviewed by:
Linlan Lv, Yancheng Institute of Technology, ChinaGuoxing Nie, Henan Normal University, China
Copyright © 2022 Niu, Gao, Qin, Chen, Wang, Li, Liang, Wang, Mu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlin Wang, wangchunlin@nbu.edu.cn; Huan Wang, wanghuan1@nbu.edu.cn
 Mingming Niu1
Mingming Niu1