- 1College of Marine Sciences, Shanghai Ocean University, Shanghai, China
- 2National Engineering Research Center for Oceanic Fisheries, Shanghai Ocean University, Shanghai, China
- 3The Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai, China
- 4Key Laboratory of Oceanic Fisheries Exploration, Ministry of Agriculture and Rural Affairs, Shanghai, China
Introduction: Stable isotope analysis has been widely used in the study of the trophic structure of marine micronekton in recent years.
Methods: In this study, the carbon and nitrogen stable isotope values of fish, cephalopod, shrimp and zooplankton species were measured from samples collected in the Northwest Pacific Ocean in March and September 2019 to construct a continuous trophic spectrum and to compare isotope niches among species. In addition, we compared the variation of isotopic niches of micronekton among different groups and among different species, respectively.
Results: Significant differences of δ13C and δ15N values were detected among fish, cephalopod and shrimp groups with δ13C value ranges of −21.9‰ to −18.7‰, −21.3‰ to −17.7‰ and −20.4‰ to −19.5‰, respectively and the range of δ15N values was 7.0‰ to 12.4‰, 8.2‰ to 12.2‰ and 7.6‰ to 10.6‰, respectively. Using copepods as the baseline for estimating the average trophic level (TL) of micronekton, the TLs ranged from 2.67 to 4.80 and the average TLs for cephalopods, fishes and shrimp were 3.3 ± 0.3, 3.7 ± 0.6 and 4.0 ± 0.3, respectively. Myctophidae and Stomiidae occupied higher TLs. In addition, Oplophorus gracilirostris, Enoploteuthis chunii and Abralia similis had wide isotopic niches.
Discussion: Our results show that Myctophidae, Stomiidae, Oplophorus gracilirostris, Enoploteuthis chunii and Abralia similis play important roles in maintaining the stability of the Kuroshio-Oyashio ecosystem in the Northwest Pacific Ocean.
Introduction
The Northwest Pacific is the largest marine ecosystem on Earth (Brodeur et al., 1999) and is located where two strong western boundary currents (the Kuroshio Current and Oyashio Current) converge; as a result, according to the FAO (Food and Agriculture Organization of the United Nations), this is the location of the richest fishery in the world. Since reaching over 20 million tons in 1984, the total output of the fishery in the Northwest Pacific Ocean has been stable and was about 19.4 million tons in 2020; this accounted for 21.2% of the total global marine catch (FAO, 2022). Many economically important species are found here in abundance, including Pacific sardine (Sardinops sagax), skipjack tuna (Katsuwonus pelamis), Pacific saury (Cololabis saira), Japanese flying squid (Todarodes pacificus) and neon flying squid (Ommastrephes bartramii) (Yatsu et al., 2013; Chang et al., 2019). Smaller but more abundant species are found in mesopelagic waters, typically at depths of 100-1000m (Irigoien et al., 2014). The majority of mesopelagic organisms have a diel migratory feeding habit, migrating upward to the surface region at night and later migrating downward a few hundred meters to the depths where they remain during the day (Salvanes and Kristofersen, 2001; Catul et al., 2011). In recent years, many scholars have conducted research on biological resources and fishing grounds, including research on species such as the neon flying squid and Pacific saury (Chen et al., 2010; Huang and Huang, 2015). In previous studies on the trophic ecology of organisms in the Northwest Pacific Ocean, Lee et al. (2012) focused on the trophic structure and biomass of plankton communities, and found that there was a positive correlation between phytoplankton biomass and both ciliates and mesozooplankton biomass and the increase of mesozooplankton biomass was closely related with ciliate and dinoflagellates biomass. Ohshimo et al. (2019) researched different oceanographic processes as well as trophic ecology and movement of large fishery organisms in the Northwest Pacific Ocean based on stable isotope analysis to map spatial isopleths of top predator prey organisms including fishes and cephalopods and found that the differences in δ13C and δ15N values in the Northwest Pacific might be influenced by production dynamics, ocean currents and upwelling, and the degree of carbon and nitrogen fixation in the atmosphere. Van der Lingen et al. (2009) investigated the trophic ecology and planktonic interactions of small pelagic fishes such as anchovy and Pacific sardine in the Northwest Pacific Ocean, as well as the possible effects of climate on some species, and suggested that changes in zooplankton community structure may cause species alternations between anchovy and sardine, while climate change has more indirect effects.
The trophic structure is one of the most important features of an ecosystem. The production and transfer of biomass by trophic relationships is the core function of an ecosystem, and the relationship between ecosystem productivity and niche size is an important factor in determining ecosystem function. Moreover, an understanding of species distribution and trophic structure gives a better insight into the ecology of a community (Andrade et al., 2016; Lesser et al., 2020). In the study of community trophic structure, stomach content analysis (SCA) and stable isotope analysis (SIA) are often used. SCA can only provide information about how an organism was feeding within the short period of time before it was caught (Ibáñez et al., 2021), whereas SIA can provide longer-term information about its feeding (Li et al., 2014). In addition, different tissues tend to be synthesized and replaced at different rates, which means that SIA can also provide information about temporal variations in individuals’ feeding and TLs. For example, muscle (Malpica-Cruz et al., 2012) can provide isotope information covering periods of a few months to a year, whereas metabolically inert tissues such as fish vertebrae (Carlisle et al., 2015) can usually record biochemical information over the entire period of an individual’s development, from which information about changes in feeding habits and habitat can be derived (Inger and Bearhop, 2008).
Additionally, SIA can be used to study biological carbon sources, energy sources and feeding habits, as well as temporal and spatial trophic relationships and migration, thus revealing how substances cycle through ecosystems and details of the trophic relationship between consumers and producers in the food web (Hansson et al., 1997; Zeng et al., 2008). The carbon stable isotope value (δ13C ) increases negligibly from a consumer’s diet (0-1‰); as it reflects the source of primary production, this value can be used to discriminate between inshore and offshore species or between pelagic and benthic feeders (Kelly et al., 2006; Liu et al., 2019). The stable nitrogen isotope value (δ15N ) is usually used to estimate the trophic level (TL) of consumers due to 15N enrichment with trophic transfer. Compared with the complexity of previous methods such as SCA, SIA, by virtue of its versatility and simplicity, can improve the understanding of elemental and material-energy cycles in ecosystems (Zhang et al., 2019).
The description of a niche derived using the stable carbon and nitrogen isotope values of biological tissues not only gives information about the spatial position occupied by a species within a certain environmental range at a certain time, it also reflects the impact of environmental and other factors on the species as well as the material circulation and energy flow within the ecosystem or community. The niche breadth reflects the diversity of the food resources available to a species: the greater the breadth, the stronger the environmental adaptability of the species. The niche overlap also reflects the degree of competition and the similarity of resource utilization between different species (Hurlbert, 1978; Bearhop et al., 2004). The isotopic niche can reflect variability associated with TL, primary production sources, and possibly isotope baselines in the case of migratory species (Layman et al., 2007; Newsome et al., 2007).
Carbon and nitrogen stable isotope analysis has been widely used in research into trophic structure, trophic levels and seasonal variations in relation to biological communities in fisheries (Jennings et al., 2002). Koichi Yoshii et al. (1999) used carbon and nitrogen SIA to analyze the structural features of the pelagic food web in Lake Baikal and studied the composition of the diets and changes in foraging habits of individual organisms. Huang et al. (2019a) established a continuous trophic spectrum for the main fishery in the South China Sea central and western fishing grounds. However, the number of studies on the trophic structure of micronekton in the Northwest Pacific Ocean has been limited (but see Ohshimo et al., 2019).
Our study was based on micronekton that were collected during a fishery resource survey in the Kuroshio−Oyashio mixing zone in March and September 2019. The stable carbon and nitrogen isotope values of these organisms were measured and their trophic levels estimated. These results were then used to establish a continuous trophic spectrum and combined with isotopic niche to research interspecies differences in community trophic structure and potential impacts on ecosystems.
Material and methods
Sample collection
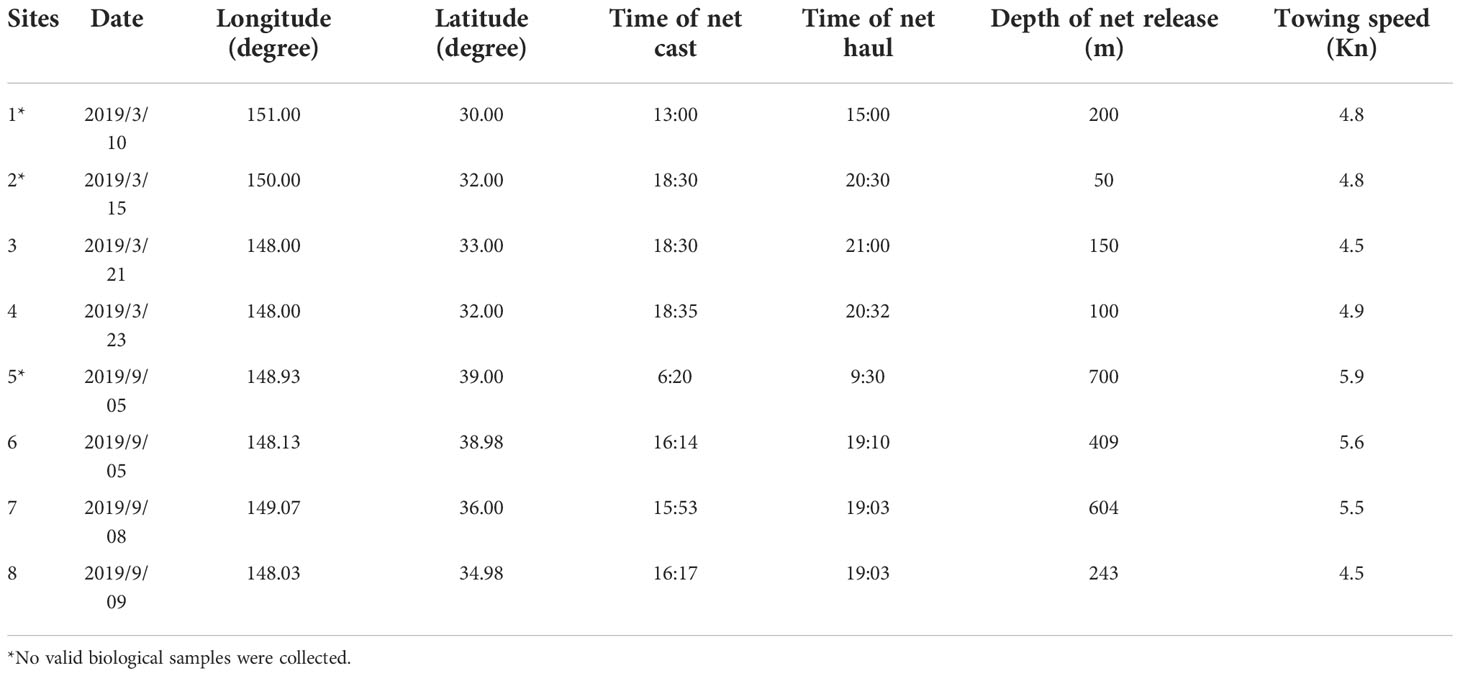
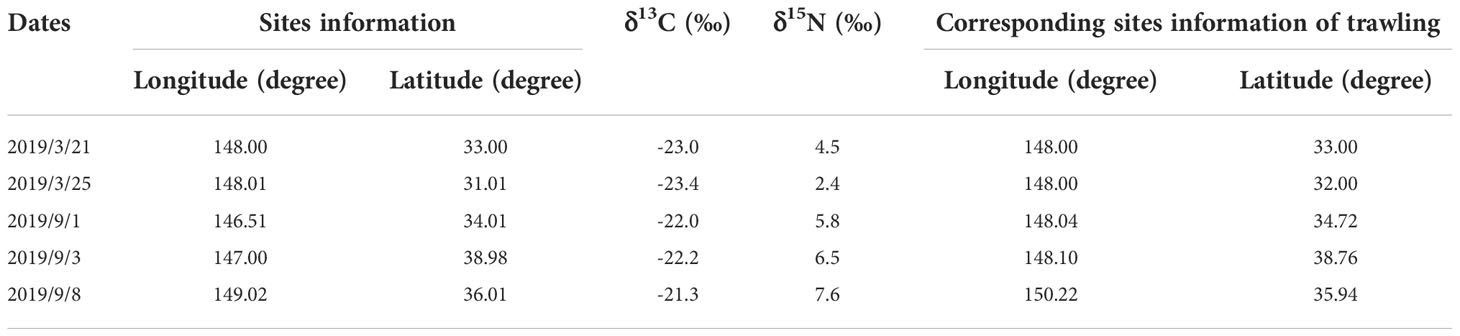
The sampling vessel used in this study was Shanghai Ocean University’s oceanic fisheries resources survey vessel “Song Hang”. This vessel has a total length of 85m, a moulded depth of 8.7 m and a displacement of 3271.4 tonnes when fully loaded. A four piece mesopelagic trawl net with a single-capsule structure was used for all collections; the overall dimensions of the net were 434 m × 97.1m (44.98 m). The net port part had large mesh and the net body part had a machine-woven mesh. Double-leaf mesh board had single-hand steel connection. Zooplankton were collected using a Bongo net following the same collection protocol for each survey. Specific sampling information on the trawl and sampled zooplankton is available in Tables 2 and 3.
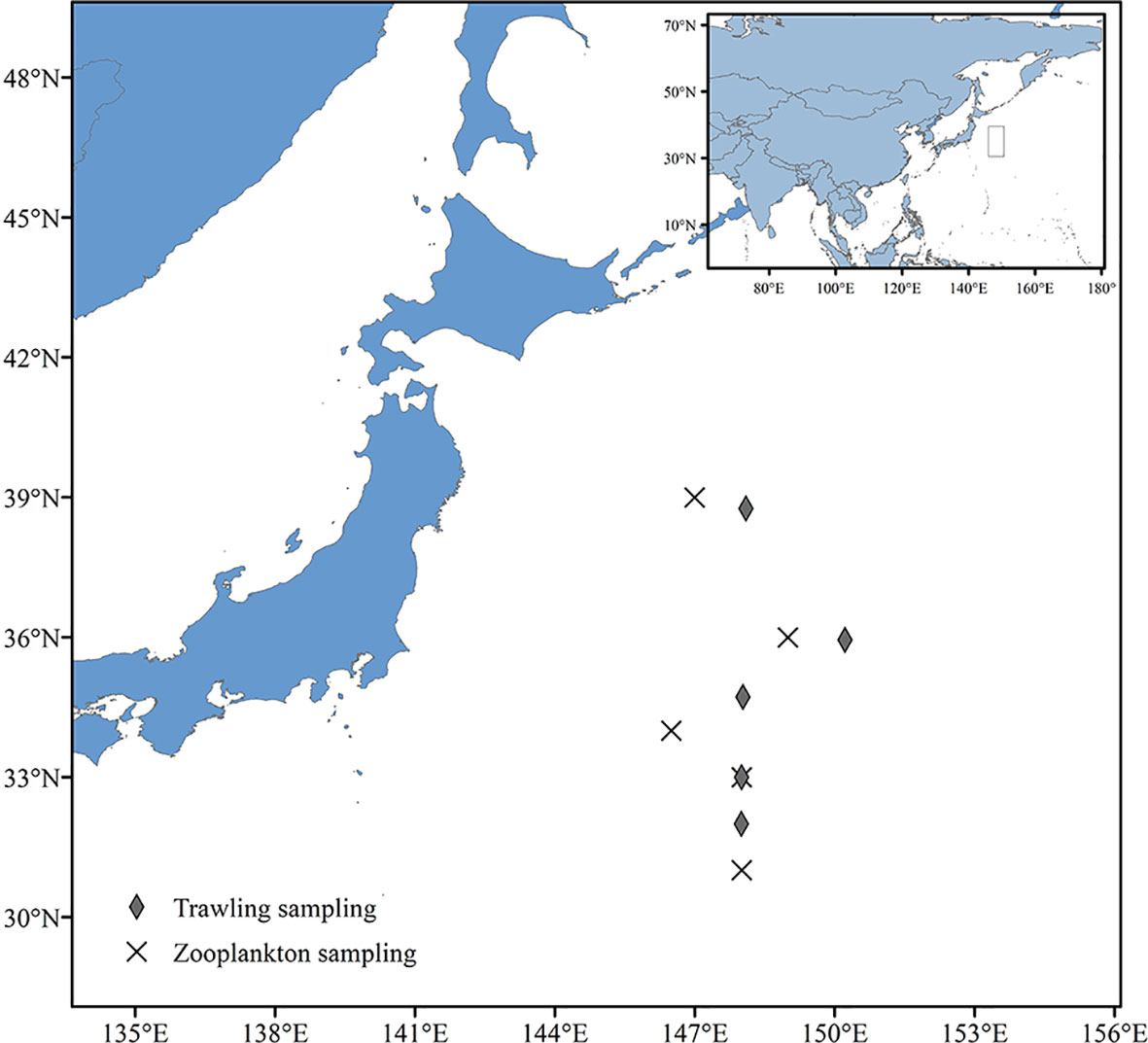
Fish, cephalopod and shrimp samples were collected by trawling within the Kuroshio−Oyashio mixing zone in the Northwest Pacific from March 21st to March 23rd and from September 5th to September 9th, 2019. The individual sampling sites were located at 148°E, 33°N; 148°E, 31°59′N; 148°5′E, 38°45′N; 150°13′E, 35°56′N and 148°2′E, 34°43′N (Table 1 and Figure 1) with tow depths of 150m, 100m, 409m, 604m and 243m, respectively (Table 2). After classification and identification at sea, the samples were stored in a freezer at −20°C.
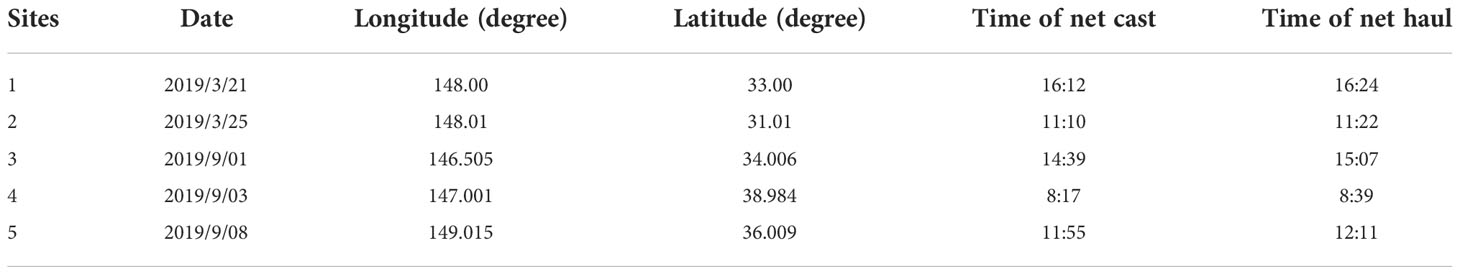
The zooplankton samples were obtained by vertical trawling using a large plankton net at a depth of 100 m. The trawling speed was 0.5−0.8 m/s. Trawling was carried out for 30 minutes at each of the five sites listed above (Table 3 and Figure 1). Trawl-collected samples were washed into 1000ml jars using seawater, and then filtered through a 100 um mesh sieve to end up with approximately 450ml of remaining zooplankton, which were also stored in a freezer at -20°C.
Sample processing
After using a microscope to pick out copepods in the laboratory, the copepod samples were filtered using GF/C filter membranes. They were then wrapped in tinfoil and dried to a constant mass in a drying oven at 60°C before being ground to a fine, homogeneous powder using a RETSCH MM200 automatic ball mill in the laboratory. After sieving, between 1 and 2 mg of this powder was then put into a 0.3-mg tin capsule for preservation before further testing. Before being dried, the GF/C filter membranes used for concentrating the samples were heated in a muffle furnace at 450°C for 4 hours to remove any organic matter present.
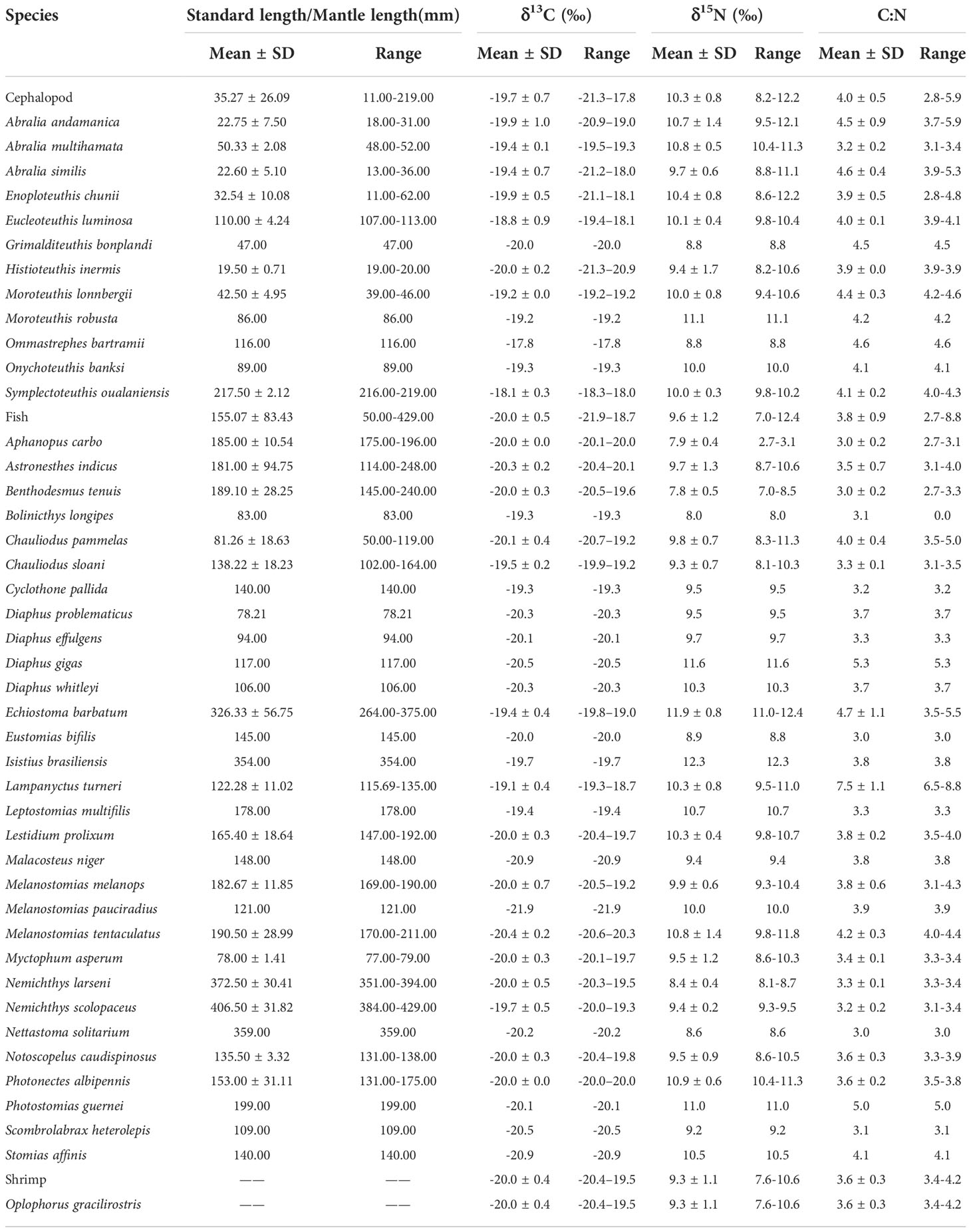
A total of 90 fishes, 162 cephalopods and 5 shrimp were collected (Table 1). After thawing and cleaning the trawl-caught organisms in the laboratory, the determination of biological data and muscle sample collection were carried out in accordance with the Specifications for Oceanographic Survey——Part 6: Marine biological survey (GB/T12763.6-2007) (China National Standardization Management Committee, 2007). Standard (fishes) and mantle (cephalopods) length ( ± 0.01 mm) and weight ( ± 0.01 g) were measured and recorded for each organism. Approximately 10 g of soft tissue (dorsal muscle for fish, abdomen muscle for shrimp and mantle muscle for squid) were obtained from selected individuals to determine the δ13C and δ15N values. All of the selected samples were rinsed with Milli-Q ultrapure water for 5 minutes to remove possible contaminants; they were then freeze-dried in German Christ Alpha 1-4 LSG freeze-dryers at −50°C for at least 24 hours before being ground to a fine, homogeneous powder using an automatic RETSCH MM200 ball mill. Between 1 and 2 mg of this powder was then put into a 0.3-mg tin capsule for preservation before further testing.
Stable carbon and nitrogen isotope analysis
The stable isotope values were determined at the Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai, China, using an ISOPRIME 100 isotope-ratio mass spectrometer (Isoprime Corporation, Cheadle, UK) and a vario ISOTOPE cube elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The standard substance used for determining δ13C was Vienna Pee Dee Belemnite (V-PDB) and the standard substance used for determining δ15N was atmospheric nitrogen (N2). During the sample testing, in order to ensure the stability of the instrument and the accuracy of the test results, three standard isotope samples were added to every tenth sample for testing with an analytical precision of ±0.06‰. The values of δ13C , δ15N , %C and %N were measured and the δ13C and δ15N measurements were made with a precision of 0.2‰. Stable isotope values were calculated using the following formula:
where X is 13C or 15N and δ is the ratio of the heavy isotope to the light isotope in the sample. Rsample and Rstandard are the atomic ratios of 13C/12C and 15N/14N in the sample and standard substance, respectively.
Considering that micronekton are often lipid rich, we chose the correction model developed by Hoffman and Sutton (2010) for deep-sea fishes to correct the δ13C values of micronekton with C: N >3.76 in this study.
Trophic level calculation
The trophic level of each sample was estimated and a continuous trophic spectrum was constructed. The formula used to estimate the trophic level was
where TLbase represents the trophic level of the primary consumer; its value was set as 2 (Du et al., 2020). The nitrogen stable isotope values of the consumer and copepod baseline are represented by δ15Nconsumer and δ15Nbase, respectively. In this study, copepods obtained near the same site location and during the same season as the consumer species were selected as the baseline organisms (Table 4). We chose to use copepods as the baseline organism to reduce variability associated with other zooplankton components that could affect baseline value estimates (e.g., size, trophic position, biochemical composition). TEF is the trophic enrichment factor. In this study, the TEF was assumed to be 3.4‰ (Minagawa and Wada, 1984; Post, 2002).
Isotopic niche estimation
The SIBER package (Jackson et al., 2011) in R 4.0.4 (R Core Team, 2022) was used to draw the isotopic niches of the micronekton. The niche areas were then calculated to determine the niche breadths, and the SIAR package in R 4.0.4 was used to calculate the isotopic niche overlap. The standard ellipse area corrected for small sample sizes (SEAc) contained about 40% of the stable carbon and nitrogen isotope data, which meant that it could be used to represent the core niche of each individual and also to calibrate the sample sizes (Layman et al., 2007; Layman et al., 2012). It could also be used to estimate the breadth of the isotopic niches and the overlap between the isotopic niches of different species. The overlap values ranged from 0 to 1 using a scale proposed by Langton (1982) in which values from 0 to 0.29 indicate a low degree of overlap, values from 0.30 to 0.60 indicate medium overlap and values higher than 0.60 indicate high overlap.
Data analysis
Excel, Sigmaplot 12.5 and R were used for the data analysis, drawing and calculations. The difference in δ13C and δ15N between the different groups of micronekton was tested using analysis of variance (ANOVA). Prior to data analysis, normality and homogeneity of variance tests were run. If the results of the aforementioned tests were significant, nonparametric tests were run to determine the levels of significance of the differences. Multiple comparisons were then carried out for fishes, cephalopods and shrimp including Bonferroni corrections. The correlation between isotope values and standard length (SL; fishes) or mantle length (ML; cephalopods) were tested using linear regression.
Results and analysis
Stable carbon and nitrogen isotope values
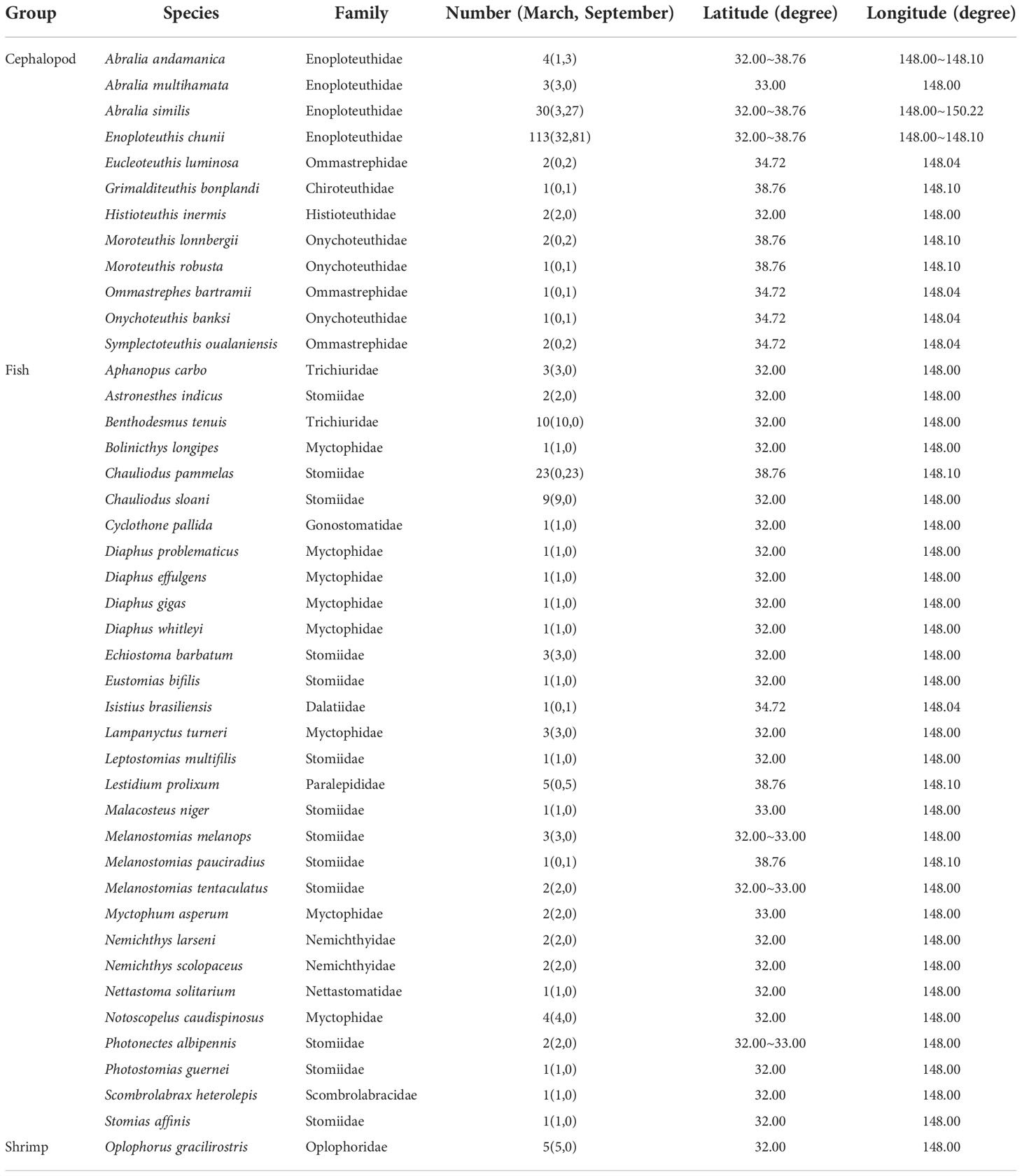
A total of 43 micronekton species from 16 families and 33 genera were collected, including 30 fish species, 12 cephalopod species and 1 shrimp species. Enoploteuthidae were the most abundant, accounting for 58.37% of all samples and the micronekton in Stomiidae were the most diverse, accounting for 30.23% of all micronekton. The most common species found in the samples were the fishes Benthodesmus tenuis, Chauliodus pammelas, Chauliodus sloani, Lestidium prolixum, the cephalopods Enoploteuthis chunii, Abralia similis and the shrimp Oplophorus gracilirostris (Table 1).
The δ13C values ranged from −21.9‰ to −17.9‰ (−19.8‰ ± 0.6‰) and the δ15N values from 7.0‰ to 12.4‰ (10.0‰ ± 1.0‰). For the fish samples, δ13C ranged from −21.9‰ to −18.7‰ (−20.0‰ ± 0.5‰) and δ15N ranged from 7.0‰ to 12.4‰ (9.6‰ ± 1.2‰); for the cephalopods, δ13C ranged from −21.3‰ to −17.7‰ (−19.7‰ ± 0.7‰) and δ15N ranged from 8.2‰ to 12.2‰ (10.3‰ ± 0.8‰); and for the shrimp, δ13C ranged from −20.4‰ to −19.5‰ (−20.0‰ ± 0.4‰) and δ15N ranged from 7.6‰ to 10.6‰ (9.3‰ ± 1.1‰) (Table 5). In addition, the copepod δ13C values ranged from −23.4‰ to −21.0‰ (−23.2‰ ± 0.2‰) and the δ15N values from 2.4‰ to 4.5‰ (3.5‰ ± 1.1‰). Detailed information of each micronekton is presented in Table 5.
Population variance of δ13C and δ15N values violated the assumptions of homogeneity of variance (PC=0.013<0.05; PN=0.001<0.05), and the results of the non-parametric tests showed that the difference in δ13C and δ15N values between the fish, cephalopod and shrimp groups were significant (FC=10.076, PC=0.006<0.05; FN=32.472, PN=0.000<0.05). Multiple comparisons showed significant differences in δ13C and δ15N values between fish and cephalopod groups (PC=0.006<0.05, PN=0.000<0.05), while δ13C and δ15N values between shrimp and fish groups (PC=1.000>0.05, PN=1.000>0.05) and between shrimp and cephalopod groups (PC=0.922>0.05, PN=0.121>0.05) were not significant. In addition, for cephalopods, there was a significant correlation between ML and δ13C values (F=22.652, P=0.000<0.05), while the correlation between δ15N values and ML was not significant (P=0.585>0.05); for fishes, neither δ13C or δ15N values were significantly correlated with SL (PC=0.074>0.05; PN=0.849>0.05).
Trophic levels and continuous trophic spectrum
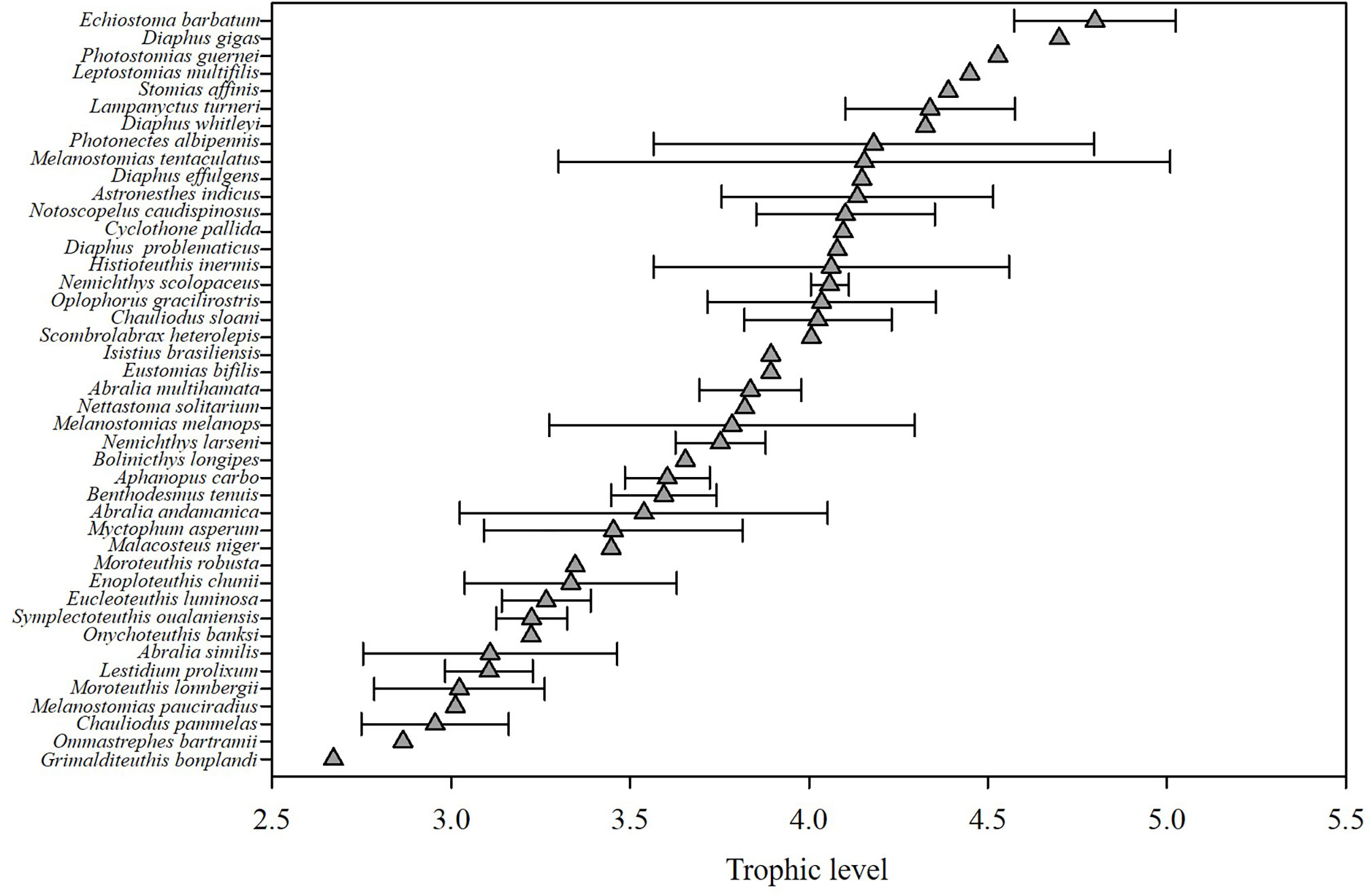
The continuous trophic spectrum that was constructed (Figure 2) showed that, for the samples obtained, the TL values ranged from 2.67 (for Grimalditeuthis bonplandi) to 4.80 (for Echiostoma barbatum); the average TL value for cephalopods was 3.30 ± 0.34, for fishes it was 3.66 ± 0.59 and for shrimp it was 4.04 ± 0.32. A total of twenty-one species (48.8%) had TL values ranging from 3.0 to 4.0. The TL values of 19 species were greater than 4.0 − these species included seventeen species of fish (among which were Scombrolabrax heterolepis and Chauliodus sloani), one species of cephalopod (Histioteuthis inermis), as well as the shrimp O. gracilirostris. In addition, there were three species with TL values greater than 4.5: Photostomias guernei, Diaphus gigas and Echiostoma barbatum.
Isotopic niches
Among different groups of micronekton, the fish group had the highest SEAc value − 1.80‰2. Cephalopod and shrimp groups had relatively low values of 1.59‰2 and 1.73‰2, respectively (Figure 3A). There was a moderate amount of trophic overlap between two of the three groups, with the trophic overlap between cephalopod and fish being the greatest (cepholopod-fish: 0.55; cephalopod-shrimp: 0.33; fish-shrimp: 0.45).

Figure 3 Isotopic niche of different groups and different species of micronekton. (A) Different groups of micronekton, (B) Different species of micronekton.
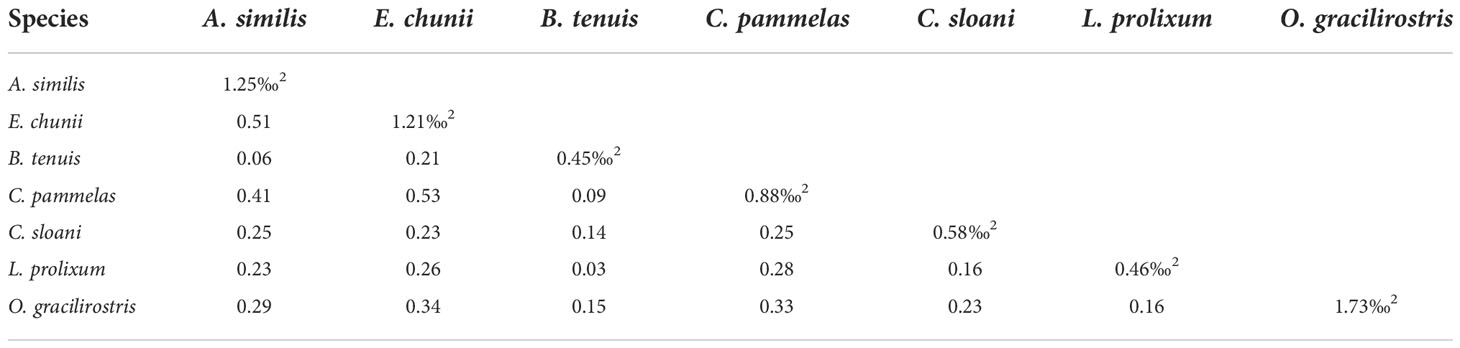
We selected seven species with relatively large sample sizes (n≥5) among all sampled species, which included two cephalopods, four fishes and a shrimp, to compare their isotopic niche. Among different species, O. gracilirostris had the largest SEAc value of 1.73‰2, whereas B. tenuis and Lestidium prolixum again had low values of 0.45‰2 and 0.46‰2, respectively. In this case, Enoploteuthis chunii and Chauliodus pammelas (0.53) or Abralia similis (0.51) had a greater degree of overlap. Trophic overlap was moderate between Chauliodus pammelas and E. chunii or A. similis or O. gracilirostris, and between E. chunii and O. gracilirostris, respectively. In addition, there was relatively low trophic overlap between B. tenuis and A. similis or C. pammelas or L. prolixum (Table 6 and Figure3B).
Discussion
Stable carbon and nitroge n isotope values
δ13C can be used to gain information about the sources of carbon and energy used by organisms (Peterson and Fry, 1987; Fry, 2006). The range of δ13C values (CR) can be used to estimate the diversity of food sources in the food web; the higher its value, the greater the diversity (Madurell et al., 2008; Jackson et al., 2011). In this study, the range of δ13C values was 4.1‰, meaning that a wide range of food sources was available. The confluence of the Kuroshio Current and Oyashio Current brings a rich composition of nutrients and species (Nishikawa et al., 2020), thus the range of food sources was complex and the degree of feeding specialization by the different types of fishes, cephalopods and shrimp was relatively low. As a result, the differences in δ13C among fishes, cephalopods and shrimp were significant. In addition, in this study, the difference in δ13C among fishes, cephalopods and shrimp may be related to different vertical and horizontal ranges of some species (Sherwood and Rose, 2005).
The range of δ15N values (NR) mainly reflects the trophic length of the community: the greater NR is, the greater the trophic length of the community and the more TLs there will be in the community (Jackson et al., 2011). Ohshimo et al. (2019) investigated a total of 84 species of pelagic forage fish and squid in the Northwest Pacific Ocean. CR had a range of 6.3‰ (from −21.5‰ to −15.2‰) and NR had a range of 10.5‰ (from 4.3‰ to 14.8‰). In comparison, the ranges of CR values in our study were relatively low, which may be related to the smaller benthic sample size and small sampling range that was used. Benthic organisms tend to have higher δ13C values than pelagic organisms (Le Loc'h et al., 2008; Kopp et al., 2015). The range of NR values was also quite small due to the lack of large predators.
Trophic levels
When stable isotope techniques are used to investigate micronekton food webs and TLs, the selection of the δ15N baseline and the trophic enrichment factor (TEF) affects the determination of the trophic positions. In recent years, primary consumers, including zooplankton, have mainly been chosen as baseline species (Post, 2002). Chiba et al. (2012) estimated the average copepod (Neocalanus cristatus, Neocalanus flemingeri and Neocalanus plumchrus) δ15N values in Oyashio and the results gave values of 7‰−10‰ with δ15N variation often associated with shifts in feeding strategies (i.e., between herbivorous and omnivorous/carnivorous). In our study, the average δ15N values of copepods obtained in March (3.5‰) and September (6.7‰) were lower than Chiba et al. (2012) estimates. Copepods sampled by Chiba et al. (2012) may have included more omnivorous individuals that would be feeding at a higher TL compared to the copepods in our study. In addition, the low sea surface temperature (SST) in March compared with September (SSTMar: 18.00°C; SSTSep: 26.37°C) (https://resources.marine.copernicus.eu/products), could affect photosynthesis and nitrogen absorption as well as the fractionation of phytoplankton (Takai et al., 2000). As zooplankton feed on various kinds of phytoplankton, this will ultimately be reflected in the difference of copepod δ15N values between March and September in this study (Xu et al., 2010).
In this study, the sampled micronekton corresponded to three TLs with the average TL value being 3.44. Other studies have shown that, on average, there are between four and six TLs between the primary predators and top predators in marine ecosystems (Arreguín-Sánchez et al., 1993; Bulman et al., 2002), and the average TL value of the global marine ecosystem based on δ15N has been estimated as 4.0 ± 0.5 (Vander Zanden and Fetzer, 2007). Compared with these results, the samples in this study corresponded to a lower number of TLs and the average TL value was also lower. This can be explained by the fact that the samples mainly consisted of micronekton and did not include large fishes from high TLs.
In addition, some of the listed TL estimates are a bit surprising given the physiology of the species and their relative TL estimates, especially some of the lowest estimated TLs. For example, the deep-sea squid Grimalditeuthis bonplandi has the lowest estimated TL of< 3.0 whereas Hoving et al. (2013) noted that this species was believed to prey on crustaceans and cephalopods. Relatedly, Ommastrephes bartramii had the second lowest TL estimate of the dataset but others have observed high TL for this species (Sthenoeuthis oualaniensis occupied trophic level about 2.72~3.08 and adult O. bartramii were 1.3TLs higher than S. oualaniensis) (Parry, 2008; Huang et al., 2019b). Combined with the sampling location and sampling date of the O. bartramii in this study, as well as its migratory route throughout the life cycle (Ichii et al., 2009), we believe that the O. bartramii in this study is in the juvenile stage. In this stage the species tends to prey not only on zooplankton, but also small fishes and cephalopods (Watanbe et al., 2004). As the individual grows, its ML and beak size increase, which means that they can prey on fishes and cephalopods with higher TLs (Jin and Chen, 2014). Size explains the lower TL of O. bartramii in this study compared with other studies, but the TL estimated in this study for O. bartramii is still lower than what would be expected with their presumed diet. O. bartramii migrations cross different habitats throughout its life cycle (Yu et al., 2015), which could create a mismatch between the baseline isotope value used for our TL calculation and the baseline reflected in O. bartramii mantle tissue. The use of sampling site baseline values introduces some spatial bias if the subjects migrate to the sampling sites within a short period of time before being caught, which also introduces some error in the TL measurements (Young et al., 2015). In addition, Ammonical cephalopod species (e.g., G. bonplandi and O. bartramii) may also have different tissue biochemical composition that could bias TL estimates for these species as has been observed with urea retention in elasmobranchs (Kim and Koch, 2012).
Moreover, in the trophic level results (Figure 2), we also found that the TLs of some mesopelagic fishes (3.45 to 4.80) were almost the same as those of large oceanic predators like tunas (3.0~4.8) (Sarà and Sarà, 2007) or sharks (3.8~4.1) (Cortés, 1999), which implies that the TLs may be overestimated in this study. Richards et al. (2020) indicated that the δ15N values in POM increase with depth and we assume that baseline δ15N values at depth may be higher than surface waters. Considering the copepods used in our study largely reflect the δ15N values of surface waters, using copepods as the TL baseline could result in artificially high TL estimates for mesopelaic species.
Myctophidae and Stomiidae are considered by Choy et al. (2012) to be the two dominant groups of meso-pelagic fish consumers. Kenaley (2012) showed that Stomiidae is capable of consuming prey whose SLs are greater than 50% of their SLs due to their large jaw and wide gape, which also explains that in our study, compared with other species of the same size such as Aphanopus carbo, B. tenuis and Lestidium prolixum, Stomiidae occupied higher trophic positions. Myctophids undertake daily vertical migrations and have a diverse prey composition including copepods, seston, fishes, and chaetognaths (Eduardo et al., 2021). Olivar et al. (2019) estimated the TL of lanternfishes (Myctophidae) in the tropical and equatorial Atlantic and estimated lanternfish TL to be between 2 and 4. The higher TL estimates of myctophids in our study suggest that they fed on a higher proportion of prey like fishes rather than zooplankton.
Community isotopic niches
In this study, among different groups of micronekton, fishes had a larger SEAc than cephalopods and shrimp. In this study, there is no significant correlation between fish δ13C values and SL, indicating that the carbon source did not change significantly throughout the ontogenetic process. It is therefore considered that the baseline values of their feeding environment did not change significantly (Chen et al., 2021). However, the majority of the fishes included in this study are meso- and bathypelagic species. According to Brierley (2014), most marine pelagic communities exhibit diel vertical migration. Compared with cephalopods and shrimp, the fishes in this study had a greater SEAc, which could be due to their broader range of SLs among species and corresponding foraging diversity. Additionally, the medium overlap among fish, cephalopod and shrimp groups suggests a more competitive relationship between them regarding the use of resources as well as a certain degree of similarity.
Among different species of micronekton, O. gracilirostris had the largest SEAc. According to Burdett et al. (2017), the Oplophorid shrimp species are extensively dispersed throughout the meso-pelagic zone of every ocean. Shrimp are omnivorous and their main sources of food include algae, organic detritus, phytoplankton, zooplankton and zoobenthos as well as some types of fish and other shrimp (Wilcox and Jeffries, 1974) and also serve as important prey organisms for top predators such as tunas and squid (Hopkins et al., 1994). O. gracilirostris is a vertically migrating deep-sea species which means that they can easily adapt to the environment of different habitats and have a large area of activity (Chan and Yu, 1986). B. tenuis is a benthopelagic species whose low SEAc value may be explained by a relatively constrained habitat and diet (Nakamura and Parin, 1993). The SEAc of C. sloani was small, which is consistent with the conclusion drawn by Eduardo et al. (2020) that the value of C. sloani’s SEAc is limited by the diversity of the species’ prey: they mainly feed on zooplanktivorous fishes, of which myctophids account for more than 50%. C. sloani are meso- and bathypelagic fish. Temperature affects the vertical migration mode of C. sloani, limiting its food sources and habitat to a certain extent. L. prolixum are small to medium-sized deep-sea fish whose small SEAc value may be related to its relatively narrow SL range.
The values of the niche overlap between B. tenuis and L. prolixum were low; the same was also true for the overlap between B. tenuis and C. pammelas or A. similis. These patterns indicate that there is a certain degree of feeding separation between B. tenuis and the other three species (L. prolixum, C. pammelas and A. similis) in the ecosystem, thus the degree of competition for resources and habitats between these species is low (Wang et al., 2018). However, the higher niche overlap between E. chunii and A. similis or C. pammelas reflect that there is a greater degree of interspecific resource competition between E. chunii and these two other species.
Conclusion and caveats
In this study, finding an appropriate baseline species for the purposes of TL estimates was challenging and is a major potential source of error in TL calculations. Both temporal and spatial (vertical and horizontal) variation in the baseline used can have an impact on the calculated TL values. The copepods used in this study may artificially overestimate the TL of mesopelagic species due to vertical baseline differences. In addition, ammonium accumulation in deep water cephalopod muscle tissues may affect δ15N values and then influence TL estimates.
Due to the limits on the trawling frequency, the trawling equipment used and the number of sampling sites this study did not sample all of the existing biological communities present. Sample sizes of the species that were collected were often small, so even the species represented in this study were not always sampled comprehensively. Samples in this study were collected during different months before pooling. In the future we will expand the temporal and spatial range of the sampling, as well as the number of trawling sites, to collect larger sample sizes over a larger regional scale. Considering that individual copepod size was not measured at each site, and that only one stable isotope value of copepods was measured at each site, in future studies we will collect a larger number of zooplankton, and then conduct species identification of zooplankton at each site. Given the potential errors associated with TL estimates based on bulk δ15N and surface zooplankton baseline samples, future studies would benefit from the inclusion of deep water particulate organic matter (POM) as an alternative baseline or the use of amino acid compound specific stable isotope analysis (AA-CSIA) of micronekton tissues as an alternative approach to TL calculation (Popp et al., 2007; McMahon and Newsome, 2019).
Despite these caveats, our study provides general trophic information on the micronekton community of the Northwest Pacific Ocean. Our results have shown that the degree of feeding specialization by the different types of fishes, cephalopods and shrimp was relatively low. As the two dominant groups of meso-pelagic fish consumers, the species of Myctophidae and Stomiidae occupied higher TLs while Oplophorus gracilirostris, Enoploteuthis chunii and Abralia similis also played important roles in the transfer of energy and materials in the meso-pelagic ecosystem.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional animal care and use Committee of Shanghai Ocean University.
Author contributions
JZ contributed to the idea and experimental design. BL provided samples. JZ completed the experiments and statistical analyses and data interpretation with the help of BL and wrote the manuscript with the help of BL, SH. YG provided the data of zooplankton stable carbon and nitrogen values. All authors contributed to the article and approved the submitted version.
Funding
This work was sponsored by Program on the Survey, Monitoring and Assessment of Global Fishery Resources (Comprehensive scientific survey of fisheries resources at the high seas) sponsored by the Ministry of Agriculture and Rural Affairs; the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning under contract 0810000243.
Acknowledgments
We thank those people for helping to collect, process samples, and analyze the isotopic signatures. We thank Project on the Survey and Monitor-Evaluation of Global Fishery Resources sponsored by Ministry of Agriculture and Rural Affairs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.971649/full#supplementary-material
References
Andrade C., Ríos C., Gerdes D., Brey T. (2016). Trophic structure of shallow-water benthic communities in the sub-Antarctic strait of Magellan. Polar Biol. 39 (12), 2281–2297. doi: 10.1007/s00300-016-1895-0
Arreguín-Sánchez F., Valero-Pacheco E., Chávez E. A. (1993). “A trophic box model of the coastal fish communities of the southwestern gulf of Mexico,” in ICLARM Conf. Proc. (Makati, Metro Manila: ICLARM Conf. Proc) 26, 197–205.
Bearhop S., Adams C. E., Waldron S., Fuller R. A., MacLeod H. (2004). Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73 (5), 1007–1012. doi: 10.1111/j.0021-8790.2004.00861.x
Brodeur R., McKinnell S., Nagasawa K., Pearcy W., Radchenko V., Takagi S. (1999). Epipelagic nekton of the north pacific subarctic and transition zones. Prog. Oceanography 43 (2-4), 365–397. doi: 10.1016/S0079-6611(99)00013-0
Bulman C. M., He X., Koslow J. A. (2002). Trophic ecology of the mid-slope demersal fish community off southern Tasmania, Australia. Mar. Freshw. Res. 53 (1), 59–72. doi: 10.1071/MF01057
Burdett E. A., Fine C. D., Sutton T. T., Cook A. B., Frank T. M. (2017). Geographic and depth distributions, ontogeny, and reproductive seasonality of decapod shrimps (Caridea: Oplophoridae) from the northeastern gulf of Mexico. Bull. Mar. Sci. 93 (3), 743–767. doi: 10.5343/bms.2016.1083
Carlisle A. B., Goldman K. J., Litvin S. Y., Madigan D. J., Bigman J. S., Swithenbank A. M., et al. (2015). Stable isotope analysis of vertebrae reveals ontogenetic changes in habitat in an endothermic pelagic shark. Proc. R. Soc. B: Biol. Sci. 282 (1799), 20141446. doi: 10.1098/rspb.2014.1446
Catul V., Gauns M., Karuppasamy P. K. (2011). A review on mesopelagic fishes belonging to family myctophidae. Rev. Fish Biol. Fisheries 21 (3), 339–354. doi: 10.1007/s11160-010-9176-4
Chang Y. J., Lan K. W., Walsh W. A., Hsu J., Hsieh C. H. (2019). Modelling the impacts of environmental variation on habitat suitability for pacific saury in the northwestern pacific ocean. Fisheries Oceanography 28 (3), 291–304. doi: 10.1111/fog.12408
Chan T. Y., Yu H. P. (1986). The deep-sea shrimps of the family oplophoridae (Crustacea: Decapoda) from Taiwan. Asian Mar. Biol. 3, 89–99.
Chen X., Tian S., Chen Y., Liu B. (2010). A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommostrephes bartramii) in the Northwest Pacific Ocean. Fish. Bull. 108 (1), 1–14. doi: 10.1093/icesjms/fsq063
Chen Z. A., Wu F., Dai X. J., Li Y. K. (2021). Trophic niche partitioning of four pelagic shark species in the tropical Atlantic based on multi-tissue stable isotopes ratios. Chin. J. Appl. Ecol. 32 (06), 2014–2020. doi: 10.13287/j.1001-9332.202106.027
Chiba S., Sugisaki H., Kuwata A., Tadokoro K., Kobari T., Yamaguchi A., et al. (2012). Pan-north pacific comparison of long-term variation in Neocalanus copepods based on stable isotope analysis. Prog. Oceanography 97, 63–75. doi: 10.1016/j.pocean.2011.11.007
China National Standardization Management Committee (2007). Specifications for oceanographic survey–part 6: Marine biological survey. (Beijing: China Standards Press).
Choy C. A., Davison P. C., Drazen J. C., Flynn A., Gier E. J., Hoffman J. C., et al. (2012). Global trophic position comparison of two dominant mesopelagic fish families (Myctophidae, stomiidae) using amino acid nitrogen isotopic analyses. PloS One 7 (11), e50133. doi: 10.1371/journal.pone.0050133
Cortés E. (1999). Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 56 (5), 707–717. doi: 10.1006/jmsc.1999.0489
Du J., Makatipu P. C., Tao L. S., Pauly D., Cheung W. W., Peristiwady T., et al. (2020). Comparing trophic levels estimated from a tropical marine food web using an ecosystem model and stable isotopes. Estuarine Coast. Shelf Sci. 233, 106518. doi: 10.1016/j.ecss.2019.106518
Eduardo L. N., Bertrand A., Mincarone M. M., Martins J. R., Frédou T., Assunção R. V., et al. (2021). Distribution, vertical migration, and trophic ecology of lanternfishes (Myctophidae) in the southwestern tropical Atlantic. Prog. Oceanography 199, 102695. doi: 10.1016/j.pocean.2021.102695
Eduardo L. N., Lucena-Frédou F., Mincarone M. M., Soares A., Le Loc’h F., Frédou T., et al. (2020). Trophic ecology, habitat, and migratory behaviour of the viperfish Chauliodus sloani reveal a key mesopelagic player. Sci. Rep. 10 (1), 1–13. doi: 10.1038/s41598-020-77222-8
FAO (2022). “Fishery and aquaculture statistics. global capture production 1950-2020 (FishStatJ),” in FAO fisheries and aquaculture division. (Rome: FAO). Available at: www.fao.org/fishery/statistics/software/fishstatj/en.
Hansson S., Hobbie J. E., Elmgren R., Larsson U., Fry B., Johansson S. (1997). The stable nitrogen isotope ratio as a marker of food-web interactions and fish migration. Ecology 78 (7), 2249–2257. doi: 10.1890/0012-9658(1997)078[2249:TSNIRA]2.0.CO;2
Hoffman J. C., Sutton T. T. (2010). “Lipid correction for carbon stable isotope analysis of deep-sea fishes,” in Deep Sea research part I: Oceanographic research papers. (United Kingdom, Kidlington: Elsevier) 57, 956–964. doi: 10.1016/j.dsr.2010.05.003
Hopkins T. L., Flock M. E., Gartner J. V. Jr., Torres J. J. (1994). Structure and trophic ecology of a low latitude midwater decapod and mysid assemblage. Mar. Ecol. Prog. Ser. 109, 143–156. doi: 10.3354/meps109143
Hoving H. J., Zeidberg L. D., Benfield M. C., Bush S. L., Robison B. H., Vecchione M. (2013). First in situ observations of the deep-sea squid Grimalditeuthis bonplandi reveal unique use of tentacles. Proc. R. Soc. B: Biol. Sci. 280 (1769), 20131463. doi: 10.1098/rspb.2013.1463
Huang J. X., Gong Y. Y., Xu S. N. (2019a). Characteristics of stable carbon and nitrogen isotopes of major fishery organisms in the fishing ground of central western south China Sea. J. Trop. Oceanography 38 (1), 76–84.
Huang J. X., Gong Y. Y., Xu S. N., Chen Z. Z., Zhang J., Yu W. M. (2019b). Trophic niche of medium-form and dwarf-form of purple flying squid Sthenoeuthis oualaniensis in the central and western south China sea. ying yong sheng tai xue bao=. J. Appl. Ecol. 30 (8), 2822–2828. doi: 10.13287/j.1001-9332.201908.021
Huang W. B., Huang Y. C. (2015). Maturity characteristics of pacific saury during fishing season in the Northwest pacific. J. Mar. Sci. Technol. 23 (5), 27. doi: 10.6119/JMST-015-0630-1
Hurlbert S. H. (1978). The measurement of niche overlap and some relatives. Ecology 59 (1), 67–77. doi: 10.2307/1936632
Ibáñez C. M., Riera R., Leite T., Díaz-Santana-Iturrios M., Rosa R., Pardo-Gandarillas M. C. (2021). Stomach content analysis in cephalopods: past research, current challenges, and future directions. Rev. Fish Biol. Fisheries 31 (3), 505–522. doi: 10.1007/s11160-021-09653-z
Ichii T., Mahapatra K., Sakai M., Okada Y. (2009). Life history of the neon flying squid: effect of the oceanographic regime in the north pacific ocean. Mar. Ecol. Prog. Ser. 378, 1–11. doi: 10.3354/meps07873
Inger R., Bearhop S. (2008). Applications of stable isotope analyses to avian ecology. Ibis 150 (3), 447–461. doi: 10.1111/j.1474-919X.2008.00839.x
Irigoien X., Klevjer T. A., Røstad A., Martinez U., Boyra G., Acuña J. L., et al. (2014). Large Mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5 (1), 1–10. doi: 10.1038/ncomms4271
Jackson A. L., Inger R., Parnell A. C., Bearhop S. (2011). Comparing isotopic niche widths among and within communities: SIBER–stable isotope Bayesian ellipses in r. J. Anim. Ecol. 80 (3), 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
Jennings S., Greenstreet S., Hill L., Piet G., Pinnegar J., Warr K. J. (2002). Long-term trends in the trophic structure of the north Sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Mar. Biol. 141 (6), 1085–1097. doi: 10.1007/s00227-002-0905-7
Jin Y., Chen X. (2014). Information in beaks of Ommastrephes bartramii in the north pacific ocean using stable isotope technology. Chin. J. Ecol. 33 (8), 2101–2107. doi: 10.13292/j.1000-4890.2014.0193
Kelly M. H., Hagar W. G., Jardine T. D., Cunjak R. A. (2006). Nonlethal sampling of sunfish and slimy sculpin for stable isotope analysis: how scale and fin tissue compare with muscle tissue. North Am. J. Fisheries Manage. 26 (4), 921–925. doi: 10.1577/M05-084.1
Kenaley C. P. (2012). Exploring feeding behaviour in deep-sea dragonfishes (Teleostei: Stomiidae): jaw biomechanics and functional significance of a loosejaw. Biol. J. Linn. Soc. 106 (1), 224–240. doi: 10.1111/j.1095-8312.2012.01854.x
Kim S. L., Koch P. L. (2012). Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ. Biol. Fishes 95 (1), 53–63. doi: 10.1007/s10641-011-9860-9
Kopp D., Lefebvre S., Cachera M., Villanueva M. C., Ernande B. (2015). Reorganization of a marine trophic network along an inshore–offshore gradient due to stronger pelagic–benthic coupling in coastal areas. Prog. Oceanography 130, 157–171. doi: 10.1016/j.pocean.2014.11.001
Langton R. W. (1982). Diet overlap between Atlantic cod, Gadus morhua, silver hake, Merluccius bilinearis, and fifteen other northwest Atlantic finfish. Fishery Bull. 80 (4), 745–759.
Layman C. A., Araujo M. S., Boucek R., Hammerschlag-Peyer C. M., Harrison E., Jud Z. R., et al. (2012). Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol. Rev. 87 (3), 545–562. doi: 10.1111/j.1469-185X.2011.00208.x
Layman C. A., Arrington D. A., Montaña C. G., Post D. M. (2007). Can stable isotope ratios provide for community-wide measures of trophic structure? Ecol. 88 (1), 42–48. doi: 10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2
Lee C. R., Choi K. H., Kang H. K., Yang E. J., Noh J. H., Choi D. H. (2012). Biomass and trophic structure of the plankton community in subtropical and temperate waters of the northwestern pacific ocean. J. Oceanography 68 (3), 473–482. doi: 10.1007/s10872-012-0111-2
Le Loc'h F., Hily C., Grall J. (2008). Benthic community and food web structure on the continental shelf of the bay of Biscay (North Eastern Atlantic) revealed by stable isotopes analysis. J. Mar. Syst. 72 (1-4), 17–34. doi: 10.1016/j.jmarsys.2007.05.011
Lesser J. S., James W. R., Stallings C. D., Wilson R. M., Nelson J. A. (2020). Trophic niche size and overlap decreases with increasing ecosystem productivity. Oikos 129 (9), 1303–1313. doi: 10.1111/oik.07026
Li Y. K., Gong Y., Chen X. J. (2014). Applications of stable isotope analysis in the trophic ecology studies of cephalopods. J. Appl. Ecol. 25 (5), 1541–1546. doi: 10.13287/j.1001-9332.2014.0022
Liu B., Chen X., Qian W., Jin Y., Li J. (2019). δ13C and δ15N in Humboldt squid beaks: understanding potential geographic population connectivity and movement. Acta Oceanologica Sin. 38 (10), 53–59. doi: 10.1007/s13131-019-1487-2
Madurell T., Fanelli E., Cartes J. E. (2008). Isotopic composition of carbon and nitrogen of suprabenthic fauna in the NW Balearic islands (western Mediterranean). J. Mar. Syst. 71 (3-4), 336–345. doi: 10.1016/j.jmarsys.2007.03.006
Malpica-Cruz L., Herzka S. Z., Sosa-Nishizaki O., Lazo J. P. (2012). Tissue-specific isotope trophic discrimination factors and turnover rates in a marine elasmobranch: empirical and modeling results. Can. J. Fisheries Aquat. Sci. 69 (3), 551–564. doi: 10.1139/f2011-172
McMahon K. W., Newsome S. D. (2019). Amino acid isotope analysis: a new frontier in studies of animal migration and foraging ecology. in tracking animal migration with stable isotopes (London, U.K.: Academic Press), 173–190. doi: 10.1016/B978-0-12-814723-8.00007-6
Minagawa M., Wada E. (1984). Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica cosmochimica Acta 48 (5), 1135–1140. doi: 10.1016/0016-7037(84)90204-7
Nakamura I., Parin N. V. (1993). “FAO species catalogue. vol. 15. snake mackerels and cutlassfishes of the world (families gempylidae and trichiuridae). An annotated and illustrated catalogue of the snake mackerels, snoeks, escolars, gemfishes, sackfishes, domine, oilfish, cutlassfishes,. scabbardfishes, hairtails, and frostfishes known to date,” in FAO fish. synop (Rome: FAO) 125, 136 p.
Newsome S. D., Martinez del Rio C., Bearhop S., Phillips D. L. (2007). A niche for isotopic ecology. Front. Ecol. Environ. 5 (8), 429–436. doi: 10.1890/060150.1
Nishikawa H., Nishikawa S., Ishizaki H., Wakamatsu T., Ishikawa Y. (2020). Detection of the oyashio and kuroshio fronts under the projected climate change in the 21st century. Prog. Earth Planetary Sci. 7 (1), 1–12. doi: 10.1186/s40645-020-00342-2
Ohshimo S., Madigan D. J., Kodama T., Tanaka H., Komoto K., Suyama S., et al. (2019). Isoscapes reveal patterns of δ13C and δ15N of pelagic forage fish and squid in the Northwest pacific ocean. Prog. Oceanography 175, 124–138. doi: 10.1016/j.pocean.2019.04.003
Olivar M. P., Bode A., López-Pérez C., Hulley P. A., Hernández-León S. (2019). Trophic position of lanternfishes (Pisces: Myctophidae) of the tropical and equatorial Atlantic estimated using stable isotopes. ICES J. Mar. Sci. 76 (3), 649–661. doi: 10.1093/icesjms/fsx243
Parry M. (2008). Trophic variation with length in two ommastrephid squids, ommastrephes bartramii and sthenoteuthis oualaniensis. Mar. Biol. 153, 249–256. doi: 10.1007/s00227-007-0800-3
Peterson B. J., Fry B. (1987). Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Systematics, 18 (1), 293–320. doi: 10.1146/annurev.es.18.110187.001453
Popp B. N., Graham B. S., Olson R. J., Hannides C. C., Lott M. J., López-Ibarra G. A., et al. (2007). Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. Terrestrial Ecol. 1, 173–190. doi: 10.1016/S1936-7961(07)01012-3
Post D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83 (3), 703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
R Core Team. (2022) “R: A language and environment for statistical computing,” in R foundation for statistical computing(Vienna, Austria).
Richards T. M., Sutton T. T., Wells R. D. (2020). Trophic structure and sources of variation influencing the stable isotope signatures of meso-and bathypelagic micronekton fishes. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.507992
Salvanes A. G. V., Kristofersen J. B. (2001). Mesopelagic fishes (San Diego, CA: Academic Press), 1711–1717).
Sarà G., Sarà R. (2007). Feeding habits and trophic levels of bluefin tuna Thunnus thynnus of different size classes in the Mediterranean Sea. J. Appl. Ichthyol 23 (2), 122–127. doi: 10.1111/j.1439-0426.2006.00829.x
Sherwood G. D., Rose G. A. (2005). Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web. Estuarine Coast. Shelf Sci. 63 (4), 537–549. doi: 10.1016/j.ecss.2004.12.010
Takai N., Onaka S., Ikeda Y., Yatsu A., Kidokoro H., Sakamoto W. (2000). Geographical variations in carbon and nitrogen stable isotope ratios in squid. J. Mar. Biol. Assoc. United Kingdom 80 (4), 675–684. doi: 10.1017/S0025315400002502
Van der Lingen C., Bertrand A., Bode A., Brodeur R., Cubillos L., Espinoza P., et al. (2009). Trophic dynamics of small pelagic fish. Center Mar. Environ. Stud. 29, 31.
Vander Zanden M. J., Fetzer W. W. (2007). Global patterns of aquatic food chain length. Oikos 116 (8), 1378–1388. doi: 10.1111/j.0030-1299.2007.16036.x
Wang J., Chapman D., Xu J., Wang Y., Gu B. (2018). Isotope niche dimension and trophic overlap between bigheaded carps and native filter-feeding fish in the lower Missouri river, USA. PloS One 13 (5), e0197584. doi: 10.1371/journal.pone.0197584
Watanabe H., Kubodera T., Ichii T., Kawahara S. (2004). Feeding habits of neon flying squid Ommastrephes bartramii in the transitional region of the central North Pacific. Mar. Ecol. Prog. Ser. 266, 173–184. doi: 10.3354/meps266173
Wilcox J. R., Jeffries H. P. (1974). Feeding habits of the sand shrimp Crangon septemspinosa. Biol. Bull. 146 (3), 424–434. doi: 10.2307/1540416
Xu J., Zhang M., Xie P. (2010). Variability of stable nitrogen isotopic baselines and its consequence for trophic modeling. J. Lake Sci. 22 (1), 8–20.
Yatsu A., Chiba S., Yamanaka Y., Ito S. I., Shimizu Y., Kaeriyama M., et al. (2013). Climate forcing and the Kuroshio/Oyashio ecosystem. ICES J. Mar. Sci. 70 (5), 922–933. doi: 10.1093/icesjms/fst084
Yoshii K., Melnik N. G., Timoshkin O. A., Bondarenko N. A., Anoshko P. N., Yoshioka T., et al. (1999). Stable isotope analyses of the pelagic food web in lake baikal. Limnol Oceanography 44 (3), 502–511. doi: 10.4319/lo.1999.44.3.0502
Young J. W., Olson R. J., Ménard F., Kuhnert P. M., Duffy L. M., Allain V., et al. (2015). Setting the stage for a global-scale trophic analysis of marine top predators: a multi-workshop review. Rev. Fish Biol. Fisheries 25 (1), 261–272. doi: 10.1007/s11160-014-9368-4
Yu W., Chen X., Yi Q., Tian S. (2015). A review of interaction between neon flying squid (Ommastrephes bartramii) and oceanographic variability in the north pacific ocean. J. Ocean Univ. China 14 (4), 739–748. doi: 10.1007/s11802-015-2562-8
Zeng Q. F., Kong F. X., Zhang E. L., Qian S. Q. (2008). Assessment of sample processing methods for stable isotope analysis of aquatic food webs. J. Lake Sci. 20 (1), 13–20.
Keywords: Northwest Pacific Ocean, stable carbon isotope values, stable nitrogen isotope values, trophic level, isotope niche
Citation: Zhang J, Liu B, Hu S and Gong Y (2022) Trophic structure of micronekton in the Northwest Pacific Ocean. Front. Mar. Sci. 9:971649. doi: 10.3389/fmars.2022.971649
Received: 17 June 2022; Accepted: 14 November 2022;
Published: 01 December 2022.
Edited by:
John Logan, Massachusetts Division of Marine Fisheries, United StatesReviewed by:
Felipe Galván-Magaña, Centro Interdisciplinario de Ciencias Marinas (IPN), MexicoXiujuan Shan, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2022 Zhang, Liu, Hu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bi Lin Liu, YmwtbGl1QHNob3UuZWR1LmNu
 Jiaqi Zhang1
Jiaqi Zhang1 Bilin Liu
Bilin Liu Song Hu
Song Hu Yi Gong
Yi Gong