- 1Consultant to International Union for Conservation of Nature (IUCN), Gland, Switzerland
- 2International Union for Conservation of Nature (IUCN) Species Survival Commission Shark Specialist Group, Dubai, United Arab Emirates
- 3IUCN Joint Species Survival Commission (SSC)/World Commission on Protected Areas (WCPA) Marine Mammal Protected Areas Task Force, Gland, Switzerland
- 4Tethys Research Institute, Milan, Italy
- 5International Union for Conservation of Nature (IUCN) Ocean Team, Gland, Switzerland
- 6Conservation International, Nairobi, Kenya
- 7National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand
- 8Consultant, Save our Seas Foundation, Geneva, Switzerland
- 9Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, NT, Australia
- 10Conservation Ecology Group, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, Netherlands
- 11College of Science and Engineering, James Cook University, Townsville, Qld, Australia
- 12Institute of Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 13Marine Biological Association of the United Kingdom, The Laboratory, Plymouth, United Kingdom
- 14Ocean and Earth Science, National Oceanography Center Southampton, University of Southampton, Southampton, United Kingdom
- 15Elasmo Project, Dubai, United Arab Emirates
Area-based conservation is essential to safeguard declining biodiversity. Several approaches have been developed for identifying networks of globally important areas based on the delineation of sites or seascapes of importance for various elements of biodiversity (e.g., birds, marine mammals). Sharks, rays, and chimaeras are facing a biodiversity crisis with an estimated 37% of species threatened with extinction driven by overfishing. Yet spatial planning tools often fail to consider the habitat needs critical for their survival. The Important Shark and Ray Area (ISRA) approach is proposed as a response to the dire global status of sharks, rays, and chimaeras. A set of four globally standardized scientific criteria, with seven sub-criteria, was developed based on input collated during four shark, biodiversity, and policy expert workshops conducted in 2022. The ISRA Criteria provide a framework to identify discrete, three-dimensional portions of habitat important for one or more shark, ray, or chimaera species, that have the potential to be delineated and managed for conservation. The ISRA Criteria can be applied to all environments where sharks occur (marine, estuarine, and freshwater) and consider the diversity of species, their complex behaviors and ecology, and biological needs. The identification of ISRAs will guide the development, design, and application of area-based conservation initiatives for sharks, rays, and chimaeras, and contribute to their recovery.
1 Introduction
Biodiversity loss is a global concern and establishing measures to address its conservation is a priority for governments, policy makers, and conservation scientists worldwide (Selig et al., 2014). The accelerating impact of anthropogenic activities on the environment (such as resource use, habitat destruction, and/or climate change) makes protecting and restoring global biodiversity vital to securing Earth’s resilience to further environmental change (Rands et al., 2010; Lockwood et al., 2012). Area-based protection is a cornerstone for halting the loss of biodiversity, and the number of initiatives focused on identifying key sites or seascapes of importance for biodiversity conservation has proliferated in recent decades (Convention on Biological Diversity [CBD], 2010; Donald et al., 2019).
The first such approach, Important Bird and Biodiversity Areas1 (IBAs), was developed in response to knowledge gaps on where the most important sites for birds were located. This program guided the identification of discrete bird and biodiversity sites that could be managed for conservation (Donald et al., 2019). Over time, IBAs have contributed to spatial planning and the design of protected areas specifically adapted to the ecology of birds. IBAs have been widely used to inform the description of other area-based approaches for identifying important sites crucial for preserving biodiversity, such as the Convention on Biological Diversity (CBD) process for describing the oceans’ Ecologically or Biologically Significant Areas2 (EBSAs) (Clark et al., 2014; Dunn et al., 2014; Johnson et al., 2018), and Key Biodiversity Areas3 (KBAs) (Eken et al., 2004; Langhammer et al., 2007; International Union for Conservation of Nature [IUCN], 2016) (Table 1). Both EBSAs and KBAs identify areas of ecological importance for a range of taxa or specific habitats that may require special management considerations, including area-based management that takes species or habitat needs and vulnerability to a range of activities into consideration (Dunn et al., 2014).

Table 1 Definitions of Important Bird and Biodiversity Areas (IBAs) (Donald et al., 2019), Ecologically or Biologically Significant Areas (EBSAs) (Clark et al., 2014), Key Biodiversity Areas (KBAs) (Eken et al., 2004), Important Marine Mammal Areas (IMMAs) (Corrigan et al., 2014), Important Marine Turtle Areas (IMTAs) (Bandimere et al., 2021), and Important Shark and Ray Areas (ISRAs).
These often-complementary approaches support the identification of vital habitats for plant and/or animal species in terrestrial and aquatic ecosystems and have been used to guide implementation of the Strategic Plan for Biodiversity 2011–2020 and its Aichi Biodiversity Targets (Carr et al., 2020). Specifically, Aichi Target 11 stipulates that ‘at least 17% of terrestrial and inland water, and 10% of coastal and marine areas, especially areas of particular importance for biodiversity, are conserved through effectively and equitably managed, ecologically representative, and well-connected systems of protected areas and other effective area-based conservation measures’ (Convention on Biological Diversity [CBD], 2022a). Agreement on these targets was followed by a rapid expansion of protected areas worldwide with, for example, the total marine area covered increasing from 0.67% of the world’s ocean in 2000 to 8.09% in 2022 (UNEP-WCMC 2021).
The CBD is now negotiating a Post-2020 Global Biodiversity Framework to set new conservation targets, with intermediate goals to halt and reverse biodiversity loss by 2030 while achieving recovery and restoration by 2050 (Convention on Biological Diversity [CBD], 2020). This includes negotiations ‘to protect and conserve 30 per cent of land and sea areas through well-connected systems of protected areas and other effective area-based conservation measures by 2030’ (known as the 30x30 initiative; Convention on Biological Diversity [CBD], 2021a; Convention on Biological Diversity [CBD], 2021b). The importance of protected areas for preventing further biodiversity loss is also recognized under other international agreements and United Nations (UN) resolutions including the UN Sustainable Development Goals (SDG) (e.g., SDG 14: ‘conserve and sustainably use the oceans, seas, and marine resources for sustainable development’; United Nations [UN], 2016; UNEP-WCMC 2021). With protected areas seen as a critical component for halting the global biodiversity crisis, targets set in these international agreements have stimulated the increased development of protected area networks worldwide. The knowledge of terrestrial and aquatic biodiversity generated by the IBA, EBSA, and KBA processes has been integral in the identification and design of these areas.
Despite the uptake and contributions of the above approaches to spatial planning, protected areas were still failing to consider the specific habitat needs of some species and often provided insufficient protection for ecosystems (Hoyt, 2005; Notarbartolo di Sciara et al., 2016; Lindegren et al., 2018; Tetley et al., 2022). In particular, global efforts to compile, analyze, and disseminate data on important sites for species groups such as marine mammals and marine turtles lagged behind those for seabirds. Hence, limited representation of these species in spatial planning processes was highlighted as a challenge to conservation (Corrigan et al., 2014; Bandimere et al., 2021). To overcome this, taxon-specific biogeographical approaches, including Important Marine Mammal Areas4 (IMMAs) (Corrigan et al., 2014; Tetley et al., 2022) and Important Marine Turtle Areas5 (IMTAs) (Bandimere et al., 2021), were developed to ensure robust data are available to support conservation planning and inform protection efforts at the species-level across a broader range of taxa (Table 1).
Like marine mammals and marine turtles, the class Chondrichthyes (sharks, rays, and chimaeras [ghost sharks], hereafter ‘sharks’) is a taxonomic group of high conservation concern. The most recent global IUCN Red List of Threatened Species™ (hereafter ‘IUCN Red List’) assessment of sharks estimated that over one third of species (37%, range 32.6–45.5%) are threatened with extinction (i.e., included in the categories Critically Endangered, Endangered, or Vulnerable; Dulvy et al., 2021). The status of certain groups, taxa, or regions is worse. Three quarters of oceanic species are threatened with extinction (Pacoureau et al., 2021) and all but one of the 16 species of wedgefishes (family Rhinidae) and giant guitarfishes (family Glaucostegidae) face an extremely elevated risk of extinction (Kyne et al., 2020). In the Arabian Sea and adjacent waters, over 50% of sharks are considered threatened, a rate much higher than the global average (Jabado et al., 2018). Fishing impacts 89.6% of species (Fowler et al., 2021) and is the primary reason for almost all species listed as threatened having an elevated risk of extinction (Dulvy et al., 2021). A third of all threatened species are also confronted with habitat loss or degradation and impacts from climate change and pollution (Fowler et al., 2021). Exposure to all these threats is greatest in tropical and subtropical coastal waters, which support the highest shark biodiversity (Dulvy et al., 2021). Recent regional biodiversity maps have identified species richness and threatened species hotspots (Dulvy et al., 2021) but, due to their relatively coarse resolution, mapped outputs have not been particularly informative when identifying areas of importance for shark biodiversity conservation at finer spatial scales.
To date, the inclusion of sharks into approaches for identifying important sites for biodiversity like EBSAs and KBAs has been limited, often due to the scarce population and occurrence data required to apply their criteria (Harvey et al., 2021). Of the current existing EBSAs and KBAs, only two EBSAs have been specifically identified for sharks (although many note the occurrence of sharks within their boundaries) and, so far, only three global KBAs have been confirmed for sharks since publication of the KBA Standard in 2016 (BirdLife International, 2022; Convention on Biological Diversity [CBD], 2022b; Figure 1). The limited application of these approaches to sharks has hampered their ability to inform protected area networks for these species. Despite this, significant area-based management for sharks has been implemented and includes marine protected areas (MPAs) and shark sanctuaries (areas that ban commercial shark fishing and often the retention of shark products within a country’s entire Economic Exclusive Zone [EEZ]; Davidson and Dulvy, 2017; Ward-Paige, 2017; Ward-Paige and Worm, 2017; Anonymous, 2018). To date, these designations collectively cover ~12.4%6 of oceans, highlighting rising understanding of the urgent need to protect these increasingly threatened species and the important progress that has been made. The large spatial coverage of shark sanctuaries suggests that they have the potential to benefit highly mobile and resident sharks; however, this largely depends on the extent to which overall fishing mortality is reduced, since other fishing is still allowed within these areas and sharks can be taken as bycatch (Ward-Paige, 2017; MacNeil et al., 2020). No-take MPAs have shown particular success at reducing fishing pressure and have benefited many highly-resident species (e.g., McCook et al., 2010; Bond et al., 2012; Espinoza et al., 2014).
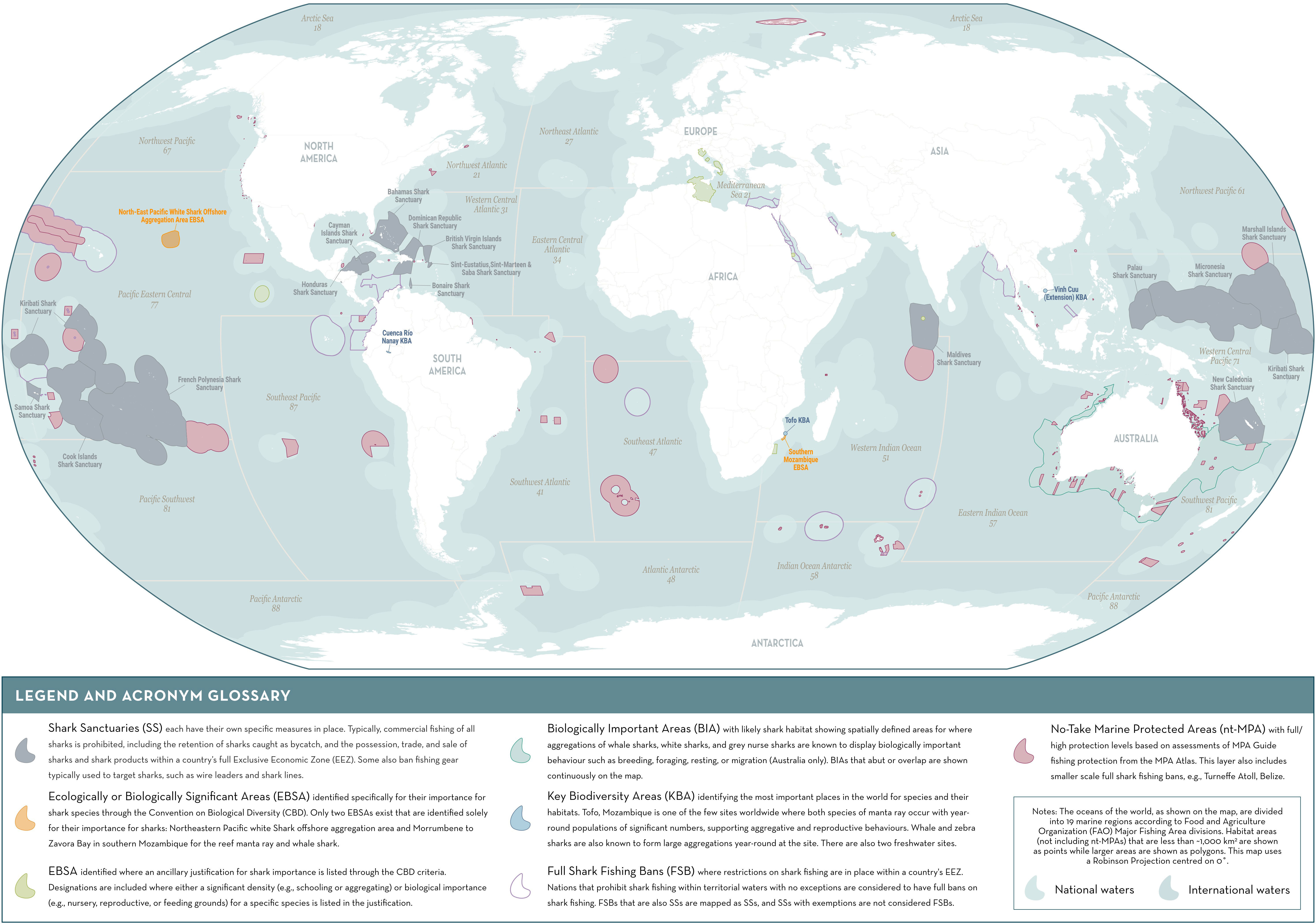
Figure 1 Baseline map of shark area-based conservation. Detailed information on each area delineated is provided in Supplementary Material 1.
Despite these positive steps, many protected areas are failing to adequately provide benefits to sharks (MacKeracher et al., 2019; Dwyer et al., 2020), often because they were not designed for that purpose. In many cases, current protected areas are too small to reduce fishing risk across sufficient proportions of the space used by sharks, do not cover areas that are demographically important, or do not encompass shark biodiversity that requires conservation (Davidson and Dulvy, 2017; Rigby et al., 2019a; Dwyer et al., 2020). When designing effective protected areas for sharks, it is essential to consider species-specific information on life-history, movement patterns, behavior, and habitat use. These can differ across environments (e.g., freshwater, estuarine, or marine) and life-cycle stages (such as presence of newborns, including young-of-the-year, or juveniles in nursery areas; Heupel et al., 2007; Martins et al., 2018). Furthermore, the effectiveness of protected areas would be increased by encompassing areas where known single species aggregations or multispecies assemblages of sharks occur for vital functions, including reproduction, feeding, or resting (García et al., 2008; Francis et al., 2015; Crowe et al., 2018). Given the high diversity of sharks (>1,260 species; Ebert et al., 2021), suitable habitat for many species at different life-cycle stages likely occurs outside of the current protected area network or shark sanctuaries (e.g., for oceanic or deep-water species; Ebert et al., 2021; Finucci et al., 2021), or are not adequately protected by them. Considering the urgent need for increased shark conservation action globally, a taxon-specific approach incorporating the ecological needs of sharks is required to identify critical sites for these species and ensure their integration into area-based approaches.
Inspired by efforts to delineate biogeographical networks of areas important for other marine taxa (i.e., IBAs, IMMAs, and IMTAs; Corrigan et al., 2014; Donald et al., 2019; Bandimere et al., 2021), the IUCN Species Survival Commission (SSC) Shark Specialist Group (SSG) has developed the Important Shark and Ray Area7 (ISRA) approach. The ISRA vision is to ensure that discrete portions of habitats critical to sharks are identified and delineated globally. The aim is to mobilize scientists and conservationists to ensure the ranges of all known shark species are assessed, so that ISRAs can be identified and mapped [International Union for Conservation of Nature Species Survival Commission Shark Specialist Group (IUCN SSC SSG), 2022a]. This will provide decision-makers and other relevant stakeholders with actionable knowledge necessary for the implementation of adequate systematic area-based conservation for sharks. Here, we introduce the ISRA Criteria, the rationale behind their selection, highlight how the ISRA approach is critically needed to ensure shark habitats are considered in area-based management, and how delineated ISRAs can be integrated with other area-based approaches such as the EBSAs and KBAs, ultimately informing protected area expansion and management.
2 Materials and methods
2.1 ISRA criteria
The ISRA Criteria were developed through a consultative process. Between January and April 2022, a series of four workshops were organized by the IUCN SSC Shark Specialist Group (SSG) and the IUCN Ocean Team, with support from the IUCN Marine Mammal Protected Areas Task Force. Online workshop invitations were disseminated through the SSG (>230 global members), and IUCN SSG and Ocean Team networks, and no limits were placed on participation. Two online workshops were held in January 2022, attended by 110 shark and biodiversity experts working across academia, governments, and non-governmental organizations in 47 countries around the world. The objective was to establish an inventory of knowledge regarding the variability of shark biological and ecological needs and gather input on key considerations and data sources to examine in the development of the ISRA Criteria. Registered participants reviewed a preliminary report of four topics related to shark biodiversity and/or conservation requirements. These considered: (1) species and/or populations of conservation concern; (2) sites of occurrence which support species and/or populations; (3) importance for life-history stages; and, (4) areas of biodiversity (richness, diversity, or distinctiveness) and ecological considerations, as well as alignment of these topics to IBA, EBSA, KBA, and IMMA criteria. Focused breakout groups on topics 1–4 were conducted and discussions provided a detailed overview of key considerations for ISRAs. These included: data sources and types (e.g., qualitative vs quantitative, availability of data, global vs regional species status assessments); species groups and habitat considerations (e.g., freshwater, coastal, oceanic, deep-water species); extinction risk status (i.e., the inclusion of IUCN Red List threatened and/or Data Deficient species); and the complexities of life-history attributes (e.g., reproductive modes, aggregations). All participants were provided an opportunity to review the workshop report (Hyde et al., 2022a).
A third online policy-focused workshop was held in February 2022 to complement the prior science-focused workshops in January. Fifty-seven participants from national and regional government and non-government organizations were presented with three questions for open discussion on how to guide the adoption of ISRAs at national and regional scales. These were: (1) What needs to be done to ensure national and regional uptake by governments?; (2) How do we leverage experiences from what was accomplished with IBAs, EBSAs, KBAs, and IMMAs?; and, (3) How can we integrate ISRAs into existing protected areas and spatial planning approaches? (Hyde et al., 2022b). Input from workshop participants formed the basis of a draft Important Shark and Ray Areas (ISRA): Guidance on Criteria Application (International Union for Conservation of Nature Species Survival Commission Shark Specialist Group [IUCN SSC SSG], 2022b). This guidance details the draft ISRA Criteria, first reviewed by the ISRA team, and then by shark and/or biodiversity experts. Following this, a fourth, hybrid (online and in-person) workshop was held by the core ISRA Team in April 2022 at IUCN headquarters in Gland, Switzerland. The focus was to further refine the ISRA Criteria and guidance document. The ISRA Criteria presented here are the result of these four workshops, consultations, and subsequent finalization of the guidance document (International Union for Conservation of Nature Species Survival Commission Shark Specialist Group [IUCN SSC SSG], 2022b).
2.2 Mapping
The ISRA baseline map (Figure 1) was created using QGIS version 3.24 (Supplementary Material 1) (QGIS Development Team, 2022). Major fishing area boundaries were downloaded from the Food and Agriculture Organization of the United Nations8 (FAO). All EEZ boundaries were downloaded from the Flanders Marine Institute Maritime Boundaries Geodatabase9. Shark sanctuaries10 and ‘full shark fishing bans’ were mapped based on EEZ boundaries. Key Biodiversity Areas11 were downloaded from the online KBA GIS Database, and maps for EBSAs were downloaded from The Clearing-House Mechanism of the Convention on Biological Diversity (CHM) database12. Biologically Important Areas were downloaded from the Australian Government Department of Climate Change, Energy, the Environment and Water: Species of National Environmental Significance Distributions13. Marine Protected Areas were downloaded from the World Database on Protected Areas14 and manually subset to delineate full/high protection zones based on the MPA Atlas15. Due to the developing nature of the MPA Atlas, this method may result in some MPA designations that are not shown on the baseline map.
3 Results
3.1 Species inclusion in the ISRA process
ISRA Criteria are only applied to sharks at the species level. Lower-level classifications, including subspecies, subpopulations, or stocks, are not considered at this stage due to low levels of such delineations for sharks. Qualifying or Supporting Species are defined as those species that are known to regularly or predictably occur in an area. Qualifying Species satisfy one or more of the ISRA Criteria within the area. Supporting Species are present in the area, but they do not satisfy ISRA Criteria. Species that occurred historically but that no longer occur, or vagrants that do not normally occur in a habitat within the area, are not considered. Only areas with naturally occurring shark species, aggregations (a group of individuals of the same species), or assemblages (a group of individuals of more than one species), can be considered under the ISRA process; shark provisioning sites (e.g., where tourism operators use a variety of stimuli [e.g., chum, food, sound] to attract sharks) are excluded from consideration.
3.2 The ISRA criteria
The ISRA Criteria were developed to provide a science-based framework to objectively identify areas of importance to sharks, crucial for their persistence and, where required, recovery. These criteria can be applied to all environments where sharks occur (marine, estuarine, and freshwater), and consider the diversity of species, their complex behaviors and ecology, and biological needs. The ISRA Criteria are non-hierarchical and address ways in which to identify an ISRA according to the known regular or predictable presence and/or activities of sharks within that area. With the exception of Criterion A (Vulnerability), a single criterion is sufficient to identify an ISRA. However, if appropriate, multiple criteria can be applied. The ISRA Criteria and sub-criteria were developed, to the extent possible, to facilitate alignment with IBA, EBSA, KBA, and IMMA criteria (Supplementary Material 2), but account for particular aspects of shark biology and ecology.
Four criteria incorporating seven sub-criteria were defined (Figure 2). Guiding examples of the application of the ISRA Criteria are provided in Annex A of International Union for Conservation of Nature Species Survival Commission Shark Specialist Group [IUCN SSC SSG], (2022b).
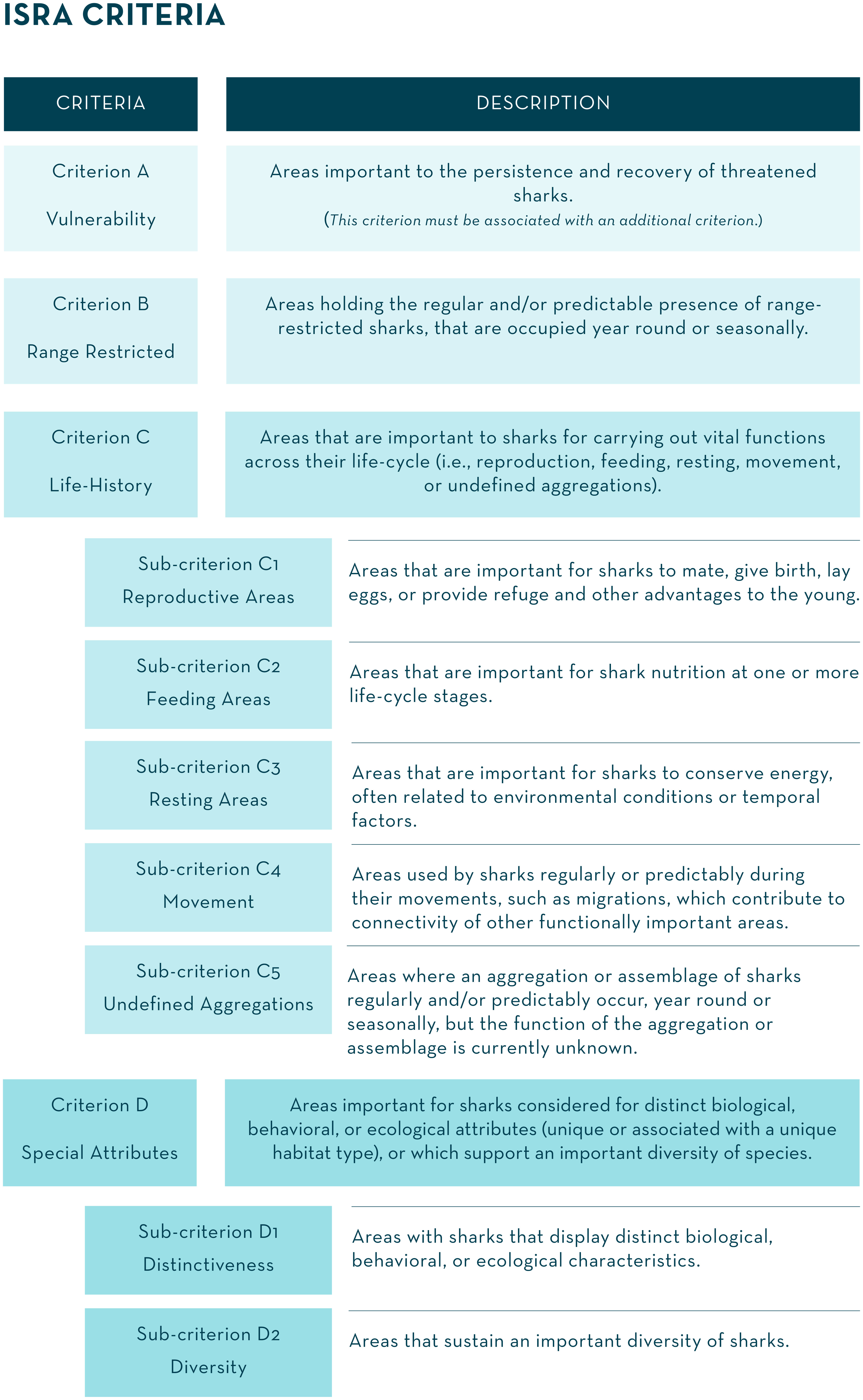
Figure 2 The Important Shark and Ray Areas (ISRA) Criteria. The term ‘sharks’ refers to all species of sharks, rays, and chimaeras.
3.2.1 Criterion A: Vulnerability
Criterion A refers to areas important to the persistence and recovery of threatened sharks. Threatened sharks are those listed on the IUCN Red List as Critically Endangered, Endangered, or Vulnerable (International Union for Conservation of Nature [IUCN], 2022). Under this criterion, ‘threatened’ could also refer to sharks at risk of extinction as reflected in other available assessments (e.g., national regulatory and legal frameworks that assess the extinction risk of species such as the United States Endangered Species Act [ESA] or the Australian Environment Protection and Biodiversity Conservation Act [EPBC]). To ensure its application to areas supporting the persistence and recovery of sharks, and not merely the occurrence of threatened species, Criterion A must also be associated with at least one additional criterion (B, C, or D) describing the type of usage of the area by the species.
3.2.2 Criterion B: Range restricted
Criterion B refers to areas holding the regular and/or predictable presence of range restricted sharks, that are occupied year-round or seasonally. The distribution of sharks may be restricted to those habitats by geographical features (e.g., land masses, bathymetric barriers) or by environmental conditions (e.g., habitat type, temperature, salinity, or depth). Populations of sharks with very restricted natural ranges are especially susceptible to extinction if their natural habitat is eliminated or significantly disturbed. To identify an area based on Criterion B, Large Marine Ecosystems (LMEs) (Sherman and Alexander, 1986) can be used to judge the scale of range restriction appropriate for sharks. Large Marine Ecosystems align with broad biogeographic patterns of fish distribution and many sharks are endemic to a single LME or to two adjoining LMEs. Therefore, under Criterion B, range-restricted sharks are species whose distribution is entirely limited to one LME or two adjoining LMEs. Large Marine Ecosystems have been delineated for continental, polar, and large island/island chain marine waters. However, species that primarily occur outside of delineated LMEs (e.g., oceanic, offshore islands, or inland waters) may also be considered range-restricted. In these cases, if the species’ distribution is similar to, or less than, the spatial extent a single LME, or two adjoining LMEs, then it may be considered range restricted.
3.2.3 Criterion C: Life-history
Criterion C refers to areas that are important to sharks for carrying out vital functions across their life-cycle (i.e., reproduction, feeding, resting, movement, or undefined aggregations). Five sub-criteria were developed to encompass the wide variety and complexity of shark life-histories.
3.2.3.1 Sub-criterion C1: Reproductive areas
Reproductive areas are important for shark mating, birth, egg laying, or providing refuge or other advantages to the young (e.g., predator avoidance or access to food sources), and are therefore critical to reproductive success. These include sites which can be identified as ‘nursery areas’ that are important for newborns, young-of-the-year, or juveniles of viviparous species; or ‘egg nursery areas’ that are important for egg laying and development until hatching and the development of newborns and juveniles of oviparous species. Sub-criterion C1 can also extend to areas where the regular or predictable presence of mature sharks has been recorded for mating, and/or where pregnant females aggregate (e.g., to avoid aggressive males).
3.2.3.2 Sub-criterion C2: Feeding areas
Feeding areas are important for shark nutrition at one or more life-cycle stages. Sub-criterion C2 relates to areas where sharks are known to derive nutrition, and that are supported by the regular and predictable occurrence of prey. This sub-criterion can apply to any life-cycle stage; for example, from the occurrence of newborns or young-of-the-year, or juveniles within inshore and estuarine habitats, to sub-adult or adult sharks which filter-feed and therefore predictably occur in areas of high planktonic productivity (e.g., upwellings). This sub-criterion also applies to areas or conditions (e.g., season, temperature, nutrients, or water activity) where natural aggregations or assemblages of sharks regularly and predictably occur, including where species come to feed during biological or ecological events of a prey species or at geomorphological features (e.g., large species migrations [such as sardine runs], spawning events, marine mammal breeding grounds, submerged reefs, or seamounts). Predictable spatial or temporal dynamic features (e.g., hydrographic features such as fronts and eddies) that are associated with known feeding activities of sharks are also recognized under Sub-criterion C2.
3.2.3.3 Sub-criterion C3: Resting areas
Resting areas are important for sharks to conserve energy and are often related to environmental conditions or temporal factors. These are areas where an aggregation or assemblage of sharks spends time during daily activity cycles and which can be influenced by environmental conditions (e.g., tidal cycle) or temporal factors (e.g., time of day). Resting areas are a key component of the daily activity of many sharks. They are most relevant to sharks with distinctly diurnal, nocturnal, or crepuscular patterns of activity, and/or sharks that are largely influenced by daily environmental cycles, in particular tidal cycles, which limit access to important habitat. These resting areas are often distinct from areas that are used for reproduction or feeding purposes, and can provide essential refuge for species.
3.2.3.4 Sub-criterion C4: Movement
This sub-criterion identifies areas used by sharks regularly or predictably during their movements, such as migrations, which contribute to the connectivity of important areas. Sub-criterion C4 addresses the predictable movement of sharks, aggregations, or assemblages from one place to another, often related to a seasonal or vital function such as reproduction or feeding. Repeated movements are common in many species of sharks and can encompass a variety of spatial and temporal scales; for example, short tidally-mediated journeys, seasonal movements along coastlines, or transoceanic and trans-equatorial crossings, as well as vertical (in some cases daily) migrations between deeper and shallower water. These areas maintain the connectivity of areas with important life-history functions (Sub-criteria C1, C2, C3, C5) by recognizing that these migratory corridors are critical for sharks.
3.2.3.5 Sub-criterion C5: Undefined aggregations
This sub-criterion identifies areas where an aggregation or assemblage of sharks regularly and/or predictably occurs, year-round or seasonally, but the function of the aggregation is currently unknown. Sub-criterion C5 refers to aggregations or assemblages of sharks in an area which engage in, or display a behavior that is known to occur, but is not (yet) attributed to a known vital function (e.g., reproduction, feeding, resting, or movement) or predator avoidance (e.g., schooling). With further understanding, these aggregations could be attributed to one of the above sub-criteria (C1, C2, C3, C4). Recognizing these aggregations and the areas where they occur is important to ensure that data deficiency does not preclude their consideration in the ISRA process.
3.2.4 Criterion D: Special attributes
Criterion D refers to areas important for sharks considered for distinct biological, behavioral, or ecological attributes (unique or associated with a unique habitat type) or which support an important diversity of species. It consists of two sub-criteria related to distinctiveness and diversity.
3.2.4.1 Sub-criterion D1: Distinctiveness
Sub-criterion D1 identifies areas where sharks display distinct biological, behavioral, or ecological characteristics. The variety of sharks, their unique features, and their adaptations could result in distinctive characteristics. Sharks considered under Sub-criterion D1 for distinctiveness must display such characteristics on a recurrent basis. Sharks which display a non-replicated biological, behavioral, or ecological characteristic should not be considered. These distinct characteristics may have stemmed from a loss of connectivity from the global population (i.e., shark species displaying a different behavior from the same species in other parts of the world). Recognizing areas of distinctiveness is important to ensure the persistence of adaptive unique biological, behavioral, or ecological traits of sharks.
3.2.4.2 Sub-criterion D2: Diversity
Sub-criterion D2 identifies areas that sustain an important diversity of sharks. These are areas that may host a high diversity of sharks (i.e., the diversity of the assemblage of shark species occurring is high or exceptional for that region) and are critical for the persistence of shark diversity. To avoid situations where only peripheral portions of many species’ ranges happen to overlap, care must be taken to ensure these areas contain core habitat for the species being considered. The attribution of Sub-criterion D2 is therefore based on a relative assessment, depending on the broader shark diversity in any particular area. The threshold number of species for the attribution of Sub-criterion D2 is set at a percentage of known regional species diversity within an LME. This threshold can be adjusted in consultation with the SSG, regional experts, and the ISRA Independent Review Panel (see Section 3.3) prior to its application. Sub-criterion D2 is not applicable to areas containing a single species and therefore technically containing 100% of local diversity (e.g., where one freshwater ray species occurs in one river system).
3.3 ISRA identification process
ISRAs are identified through regional expert workshops. These are organized by the IUCN SSC Shark Specialist Group after consultation with its Regional Vice-Chairs. Workshop invitations are extended to regional members and non-members who have knowledge and expertise useful for the identification of ISRAs. Sources of information for consideration and assessment during each workshop are actively sought during an engagement period prior to each regional workshop and become part of the ISRA Inventory of Knowledge (IoK). Based on expert input, preliminary Areas of Interest (pAoI) are examined for the regular or predictable presence of species to which the criteria can be applied. Qualifying or Supporting Species assessed against each of the ISRA Criteria within a pAoI allow for a candidate Important Shark and Ray Area (cISRA) to be justified. Finally, after the workshop, each cISRA is subject to peer-review through an Independent Review Panel. This panel is composed of recognized shark experts who have not been involved in the regional workshops, but who have an in-depth understanding of the species, habitats, and ISRA Criteria (Notarbartolo di Sciara, 2021).
4 Discussion
4.1 ISRAs as a new tool for shark conservation
Given the declining state of many shark populations globally, identifying important sites for sharks that can inform the design and development of protected areas is particularly urgent. Existing area-based management tools (Figure 1) have largely been designed without incorporating the biological, behavioral, and ecological attributes of sharks. To maximize positive outcomes for sharks, systematic planning is required to determine the locations that will provide the greatest conservation benefit. The ISRA process is a new tool addressing this need by delineating discrete areas critical to the demographic success of sharks globally (both marine and inland). Here we discuss the value of the ISRA approach as an independent, science-based, and peer-reviewed model, its need in the context of existing area-based conservation planning initiatives, and outline some of the considerations and justifications for the development of the ISRA Criteria.
One of the most salient properties of the ISRA approach is that it is purely biocentric and relies on the application of science-based criteria. In view of their nature, the identification of ISRAs does not imply a requirement for the adoption of specific policies, management, or conservation measures. Rather, the science-based guidance and actionable knowledge that ISRA identification provides can support management and conservation actions by consolidating and mapping information on sharks (such as occurrence, status, habitat, ecology) into a freely available format for national and regional decision-making. Such actions include marine spatial planning (MSP), environmental impact assessments, fishery spatial management, monitoring, compliance, and surveillance, and, not least, the designation of protected areas. Furthermore, the identification of ISRAs can underpin effective shark conservation by facilitating greater integration of shark species into broader biogeographical conservation planning approaches (e.g., KBAs and EBSAs) through alignment with their criteria. For example, where relevant data are available for sharks and one or more KBA criteria and threshold/s met, a KBA can be proposed through the appropriate processes. Finally, this process can assist in identifying key knowledge gaps (e.g., where instances of data deficiency are noted in regional workshops) that need to be addressed through research on the biology and ecology of sharks, including their potential responses to climate or other environmental changes.
Existing area-based conservation approaches have, so far, largely failed to incorporate sharks. Only two of the 321 EBSAs identified have been justified on the basis of their importance for sharks (Convention on Biological Diversity [CBD], 2021c). Of >18,000 KBAs identified worldwide, of which 4,853 are marine (BirdLife International, 2022), only three global KBAs have been confirmed for shark species based on the 2016 KBA Standard, although 43 other KBAs identified for sharks under previous versions of the KBA Criteria are pending review (International Union for Conservation of Nature [IUCN], 2016). Given the global diversity of sharks (>1,260 species; Ebert et al., 2021), this highlights the under-representation of this group in these tools and stresses the need for an area-based conservation approach specific to sharks. The ISRA approach is designed to incorporate aspects of the biological, behavioral, and ecological characteristics of sharks and account for uncertainty and data deficiency by taking a qualitative direction. For most sharks, quantitative data are insufficient to support application of relevant KBA criteria. Most species-based KBAs are identified based on the proportion of the global population size that regularly or predictably occurs at the site (International Union for Conservation of Nature [IUCN], 2016), information which is unavailable for most sharks.
4.2 The ISRA criteria
The ISRA Criteria were designed to consider species’ vulnerability, range restriction, life-history, distinctiveness, and diversity. The criteria are non-hierarchical and address ways in which to identify an ISRA according to the known regular or predictable presence and/or activities of sharks within an area. Despite the general rule that any ISRA can be identified on the basis of a single criterion, Criterion A (Vulnerability) is the exception. Considering the number of threatened shark species globally, and that many of these have wide geographical ranges (e.g., shortfin mako [Isurus oxyrinchus] assessed as Endangered on the IUCN Red List; Rigby et al., 2019b), applying this criterion alone would lead to vast areas of the ocean delineated as ISRAs. Such an approach would defy the very purpose of ISRAs, which is to focus attention on the places that are most important for the survival of sharks. Additionally, it would diminish the value of ISRAs in assisting future, targeted, and representative spatial planning. Requiring Criterion A to be associated with, at minimum, one other criterion ensures that essential habitats which support threatened sharks are delineated as important areas.
The geographic distribution of sharks varies from globally-ranging cosmopolitan species (e.g., oceanic whitetip shark [Carcharhinus longimanus] found worldwide in tropical and temperate waters; Ebert et al., 2021) to range-restricted species (e.g., ornate sleeper ray [Electrolux addisoni] found only in a small area off eastern South Africa; Last et al., 2016). For wide-ranging species, the risk from threats is spread across their range (Joppa et al., 2016). In contrast, species occupying a limited spatial area face higher risk of population decline or even extinction, since the likelihood of threats acting across their entire range (or a relatively large proportion of their range) is far greater (Joppa et al., 2016; Manes et al., 2021). The ISRA Criterion B (Range Restricted) recognizes this vulnerability and the value of important areas for species occupying small spatial scales.
Determining the scale at which the geographic range of species needs to be considered is key. One of the most readily-used metrics for defining restricted geographic range is Extent of Occurrence (EOO). This specifies a threshold of <20,000 km2 for a species to be considered as having a restricted geographic range under the IUCN Red List Categories and Criteria (International Union for Conservation of Nature [IUCN] Standards and Petitions Committee, 2019). This threshold is well below the geographic range of nearly all data-sufficient sharks (some sharks are known only from the original collection site or only from fishery landing sites and therefore do not have an adequately defined geographic range, e.g., Grace et al., 2019; Habib and Islam, 2021). In defining the ISRA Criteria, the thresholds of the IUCN metric EOO were considered to be too narrow to specify range restriction at a scale relevant to sharks. To circumvent this issue, ISRA Criterion B applies Large Marine Ecosystems (LMEs; Sherman and Alexander, 1986) as a measure of range restriction, since these are at a spatial scale more appropriate to what can be considered range restricted in sharks and LMEs generally align with broad biogeographic patterns of fish distribution.
Life-history parameters and ecological characteristics of sharks vary widely (Compagno, 1990; Smith et al., 1998; Pardo et al., 2016). The ISRA Criteria recognize the importance of areas that support shark survival across the varying life-history strategies of all sharks. ISRA Criterion C encompasses reproductive areas, feeding areas, resting areas, areas used for movement, and undefined aggregations (i.e., aggregations or assemblages that occur for unknown purposes but likely linked to vital functions or activities). Some sharks form reproductive aggregations for mating and pupping, sometimes occupying areas seasonally for these vital life-history activities (e.g., Carrier and Pratt, 1998; Chaikin et al., 2020). Identifying places where reproductive functions occur as ISRAs recognizes that they play disproportionately important roles in the demographic success of sharks. Some species regularly visit areas to consume specific prey. For example, tiger sharks (Galeocerdo cuvier) move between areas with seasonally abundant prey such as albatross (Simpfendorfer et al., 2001; Meyer et al., 2010) or turtle rookeries (Fitzpatrick et al., 2012). Resting behavior may vary with respiratory mode. For stationary-respiring species, which possess spiracles allowing water to be actively pumped over the gills, resting on the substrate is common, particularly amongst rays. Resting may also occur in places like caves (e.g., whitetip reef sharks [Triaenodon obesus]; Randall, 1977). For ram-ventilating species, which generally require forward motion to pass water over the gills, resting may involve the use of updrafts to provide lift normally produced by swimming (e.g., gray reef sharks [Carcharhinus amblyrhynchos]; Papastamatiou et al., 2021). The application of ISRA Sub-criteria C1–C3 enables a wide range of life-history characteristics that are vital to sharks to be used to identify important areas.
Advances in understanding movement ecology are providing an improved baseline of knowledge on shark movements and migrations (Carrier et al., 2019). Many sharks are known for their movement patterns, ranging from seasonally-driven limited migrations along coasts to long-distance trans-oceanic movements (e.g., Dudgeon et al., 2013; Guzman et al., 2018). Even in sharks exhibiting site fidelity, there may be considerable movement within individual feeding and breeding grounds (e.g., Chapman et al., 2015). Sub-criterion C4 (Movement) was designed to allow for the identification of regularly used corridors as important areas supporting the connectivity of sharks during predictable migratory movements. The spatial scale of important areas identified through the ISRA process will vary depending on the extent of movement displayed. However, recognizing and mapping migratory connectivity pathways of sharks across the oceans has become increasingly important.
A large number of migratory species have an unfavorable conservation status (Fowler, 2014). Several of these have now been listed on international agreements such as the Convention on the Conservation of Migratory Species of Wild Animals (CMS) and its daughter agreement, the Memorandum of Understanding on the Conservation of Migratory Sharks (Sharks MOU). These treaties highlight the need to contribute to the conservation of migratory species and promote ecological networks and connectivity in the development of conservation measures (e.g., Convention on Migratory Species [CMS], 2020). Without delineating areas of shark movement, it will not be possible to advance regional and international cooperation, including transboundary cooperation, among various stakeholders, or ensure the success of cooperative initiatives between countries. Further, many sharks, particularly epipelagic and mesopelagic species, exhibit regular vertical movements, often on a daily basis (e.g., Coelho et al., 2015; Coffey et al., 2020; Schaber et al., 2022). The three-dimensional lens of the ISRA approach allows the capture of details on non-horizontal movements and migrations. For example, the bluntnose sixgill shark (Hexanchus griseus) undertakes diel vertical migrations from 650 m to 200 m for foraging (Coffey et al., 2020). In this case, the spatial extent of habitat use is sub-surface, and a delineated important area may exclude surface waters, an important consideration of the ISRA identification process.
Ecological knowledge of sharks continues to advance and yet, the purpose of some observed aggregations or assemblages remains unknown. The inclusion of ISRA Sub-criterion C5 (Undefined Aggregations) captures this data deficiency and ensures that potentially important areas are not overlooked due to a lack of information or knowledge relating to specific vital functions or life-history activities. Most aggregations or assemblages of sharks can be assigned to an ecological purpose covered by the ISRA Sub-criterion related to reproduction, feeding, resting, or movement (Sub-criteria C1–C4). In some cases, however, the reason behind predictable and/or regular gatherings of sharks cannot be defined. For example, the precise function of a daytime aggregation of female gray reef sharks (Carcharhinus amblyrhynchos) at Johnston Atoll in the remote Central Pacific Ocean is unknown (Economakis and Lobel, 1998). This aggregation is possibly linked to embryonic development, adult growth, or a navigational ‘landmark’ (Economakis and Lobel, 1998). The reason for the aggregation of white sharks (Carcharodon carcharias) at the ‘white shark café’, a remote area beyond national jurisdiction (ABNJ) in the Pacific Ocean, was initially unknown (Jorgensen et al., 2012); now it is recognized as a feeding aggregation, identified as an EBSA (Northeast Pacific White Shark Offshore Aggregation Area EBSA; Convention on Biological Diversity [CBD], 2016), and proposed for high seas World Heritage Site status (Freestone et al., 2016). While further research may reveal that the gray reef shark aggregation described above is also linked to a vital function or life-history activity, the current uncertainty can be captured in ISRA Sub-criterion C5 (Undefined Aggregations).
With threats to global biodiversity predicted to increase (e.g., overexploitation of species, climate change, habitat destruction; Rands et al., 2010), identifying areas where high biodiversity occurs is a key priority in meeting global conservation targets (Jenkins and Van Houtan, 2016; Carr et al., 2020). ISRA Criterion D (Special Attributes) recognizes areas that house an exceptional diversity of sharks as well as areas where sharks display distinct biological, behavioral, or ecological characteristics. Distinctive sites may include cleaning stations, unique use of habitats, or behavior not observed elsewhere. For example, the Endangered pelagic thresher shark (Alopias pelagicus) visits cleaning stations at a deep-sea seamount off the Philippines (Oliver et al., 2011). This is the only global location where this oceanic shark has so far been recorded to engage in this behavior, suggesting this area (and behavior) might be distinct. Other such distinctive behaviors include mantas and devil rays (Mobula spp.) frequenting cleaning stations (O’Shea et al., 2010; Barr and Abelson, 2019) or skates (e.g., Pacific white skate [Bathyraja spinosissima]) laying eggs at hydrothermal vents (Salinas-de-León et al, 2018). Estuaries harboring the Maugean skate (Dipturus maugeanus) of southwest Tasmania, Australia, may be considered distinct since this species is a Gondwanan relict and the only estuarine-adapted skate (Treloar et al., 2016). Distinct ecological interactions such as cleaning symbiotic relationships are a key component of biodiversity; areas where they occur need to be identified and recognized.
The need to maintain biodiversity of sharks through the viability of populations has long been recognized (e.g., Food and Agriculture Organization of the United Nations [FAO], 1999). Yet, three shark species are already considered Critically Endangered (Possibly Extinct) and have not been seen in over 80 years (Dulvy et al., 2021). These possible extinctions signal that delineating areas that harbor a high diversity of species is essential to ensure an inventory of the most important sites is available. Within the ISRA Criteria, the threshold number of species for Sub-criterion D2 should be set based on known regional species diversity within an LME (this is be adjusted depending upon the region). This is to ensure ISRAs capture the uniqueness of the community structure which contributes to the richness, diversity, and endemicity of an area (e.g., global shark diversity hotspots identified by Lucifora et al., 2011).
4.3 Conclusion
The ISRA approach will directly contribute towards global conservation goals by focusing spatial management where it is most needed for sharks. ISRAs will support the design and implementation of protected area networks through their adoption into national and regional policy frameworks, thus ensuring the inclusion of essential shark habitats and biodiversity features into future conservation and management initiatives. This has already been achieved for essential marine mammal habitats with the inclusion of IMMAs into policies (e.g., the Malaysian National Policy on Biological Diversity 2021–2030, Perlis Integrated Shoreline Management Plan [ISMP], and Mersing Special Area Plan) (as described in Tetley et al., 2022), and national biodiversity and spatial planning initiatives including Australia’s Biologically Important Areas (International Union for Conservation of Nature [IUCN] Marine Mammal Protected Areas Task Force [MMPATF], 2020b). Similarly, ~60% of globally identified KBAs have already been designated as protected areas thereby contributing to Aichi Target 11 (protected areas) (Kullberg et al., 2019).
The delineation of important areas for sharks presents an opportunity to similarly spearhead area-based management for this group of high conservation concern. If ISRAs can follow the successes of established taxa-specific spatial planning approaches (e.g., IBAs, IMMAs), they will not only directly support shark conservation, but also contribute to the EBSA and KBA processes, Aichi targets, the 30x30 initiative, and the CBD Post-2020 Biodiversity Framework. Through the identification of the ecological networks and crucial areas which support and enhance shark species and populations, ISRAs represent a vital and timely step towards improving shark and biodiversity conservation globally, with potentially wide-ranging policy and conservation outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The data presented in this manuscript is available to view and download via the Important Shark and Ray Areas (ISRA) website https://sharkrayareas.org./
Author contributions
RJ and GN conceived and designed the project. CH wrote the manuscript with additional contributions provided by RJ, GN, CB, BF, SF, PK, GL, CS, MT, FW, and LS. CH, RJ, and FW produced all the tables and figures. All authors contributed to, reviewed, and approved the submitted version.
Funding
Funding to conduct this work was received from the Save Our Seas Foundation (Agreement 2021 - 070) and from the German Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection.
Acknowledgments
We would like to thank the Save Our Seas Foundation and the German Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection for support through grants to the International Union for Conservation of Nature (IUCN) and IUCN Species Survival Commission (SSC) Shark Specialist Group. Special thanks to members of the IUCN SSC Shark Specialist Group and IUCN Marine Mammal Protected Area Task Force, along with other workshop participants, for contributions to the development of the ISRA Criteria.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.968853/full#supplementary-material
Supplementary Material 1 | Information database (Excel spreadsheet) of areas delineated on the ISRA Baseline map of shark area-based conservation.
Supplementary Material 2 | Important Shark and Ray Area Criteria alignment with other area-based conservation approaches criteria of Important Bird and Biodiversity Areas (Donald et al., 2019), Ecologically or Biologically Significant Areas (Clarke et al., 2014), Key Biodiversity Areas (Eken et al., 2004), and Important Marine Mammal Areas (Corrigan et al., 2014).
Footnotes
- ^ Important Bird and Biodiversity Areas (IBAs). Available online at: https://www.birdlife.org/ [Accessed April 15, 2022].
- ^ Ecologically or Biologically Significant Areas (EBSAs). Available online at: https://www.cbd.int/ebsa/ [Accessed May 2, 2022].
- ^ Key Biodiversity Areas (KBAs). Available online at: https://www.keybiodiversityareas.org/kba-data/request [Accessed March 10, 2022].
- ^ Important Marine Mammal Areas (IMMAs). Available online at: https://www.marinemammalhabitat.org/immas/ [Accessed May 2, 2022].
- ^ Important Marine Turtle Areas (IMTAs). Available online at: https://static1.squarespace.com/static/5e4c290978d00820618e0944/t/61e0557f9c2cdd4c4bec8037/1642091906570/IMTA+Guidelines+1.0.pdf [Accessed April 27, 2022].
- ^ United Nations Environment Programme. Available online at: www.unep.org/publications-data [Accessed June 7, 2022].
- ^ Important Shark and Ray Areas (ISRAs). Available online at: https://sharkrayareas.org/ [Accessed May 5, 2022].
- ^ Food and Agriculture Organization of the United Nations (FAO). Available online at: (https://www.fao.org/fishery/en/area/search) [Accessed February 10, 2022].
- ^ Flanders Marine Institute Maritime Boundaries Geodatabase. Available online at: www.marineregions.org/downloads.php [Accessed February 10, 2022].
- ^ Anonymous. (2022). Shark Sanctuaries Around the World. Pew Charitable Trusts. Philadelphia, Pennsylvania, United States. Available online at: https://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2016/03/shark-sanctuaries-around-the-world [Accessed March 2, 2022].
- ^ KBA GIS Database. Available online at: www.keybiodiversityareas.org/kba-data/request [Accessed March 15, 2022].
- ^ The Clearing-House Mechanism of the Convention on Biological Diversity (CBD CHM) database. Available online at: chm.cbd.int/database [Accessed March 15, 2022].
- ^ Department of Climate Change, Energy, the Environment and Water, Species of National Environmental Significance Distributions. Available online at: https://www.awe.gov.au/environment/environmental-information-data/databases-applications/snes [Accessed March 15, 2022].
- ^ World Database on Protected Areas. Available online at: https://www.protectedplanet.net/en/thematic-areas/marine-protected-areas [Accessed October 17, 2021].
- ^ MPA Atlas. Available online at: https://mpatlas.org/ [Accessed June 9, 2022].
References
Anonymous (2018). Shark sanctuaries around the world (Philadelphia, Pennsylvania, United States: Pew Charitable Trusts). Available at: https://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2016/03/shark-sanctuaries-around-the-world (Accessed March 2, 2022).
Bandimere A., Brenner H., Casale P., DiMatteo A., Hurley B., Hutchinson B., et al. (2021). Important Marine Turtle Areas. In: Guidelines 1.0. IUCN SSC Marine Turtle Specialist Group. Available at: https://static1.squarespace.com/static/5e4c290978d00820618e0944/t/61e0557f9c2cdd4c4bec8037/1642091906570/IMTA+Guidelines+1.0.pdf (Accessed February 15, 2022).
Barr Y., Abelson A. (2019). Feeding-cleaning trade-off: Manta ray “decision-making” as a conservation tool. Front. Mar. Sci. 6, 88. doi: 10.3389/fmars.2019.00088
BirdLife International (2022). The World Database of Key Biodiversity Areas. Developed by the KBA Partnership: BirdLife International, International Union for the Conservation of Nature, Amphibian Survival Alliance, Conservation International, Critical Ecosystem Partnership Fund, Global Environment Facility, Global Wildlife Conservation, NatureServe, Rainforest Trust, Royal Society for the Protection of Birds, Wildlife Conservation Society and World Wildlife Fund. Available at: www.keybiodiversityareas.org. [Accessed June 2022].
Bond M. E., Babcock E. A., Pikitch E. K., Abercrombie D. L., Lamb N. F., Chapman D. D. (2012). Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the mesoamerican barrier reef. PloS One 7 (3), e32983. doi: 10.1371/journal.pone.0032983
Carr H., Abas M., Boutahar L., Caretti O. N., Chan W. Y., Chapman A. S. A., et al. (2020). The Aichi biodiversity targets: achievements for marine conservation and priorities beyond 2020. PeerJ 8, e9743. doi: 10.7717/peerj.9743
Carrier J. C., Heithaus M. R., Simpfendorfer C. A. (2019). Shark research: Emerging technologies and applications for the field and laboratory (Boca Raton, United States: CRC Press). doi: 10.1201/b21842
Carrier J. C., Pratt H. L. (1998). Habitat management and closure of a nurse shark breeding and nursery ground. Fish. Res. 39 (2), 209–213. doi: 10.1016/S0165-7836(98)00184-2
Chaikin S., Belmaker J., Barash A. (2020). Coastal breeding aggregations of threatened stingrays and guitarfish in the Levant. Aquat. Conserv. 30 (6), 1160–1171. doi: 10.1002/aqc.3305
Chapman D. D., Feldheim K. A., Papastamatiou Y. P., Hueter R. E. (2015). There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Ann. Rev. Mar. Sci. 7, 547–570. doi: 10.1146/annurev-marine-010814-015730
Clark M. R., Rowden A. A., Schlacher T. A., Guinotte J., Dunstan P. K., Williams A., et al. (2014). Identifying Ecologically or Biologically Significant Areas (EBSA): A systematic method and its application to seamounts in the South Pacific Ocean. Ocean. Coast. Manage. 91, 65–79. doi: 10.1016/j.ocecoaman.2014.01.016
Coelho R., Fernandez-Carvalho J., Santos M. N. (2015). Habitat use and diel vertical migration of bigeye thresher shark: Overlap with pelagic longline fishing gear. Mar. Environ. Res. 112 (B), 91–99. doi: 10.1016/j.marenvres.2015.10.009
Coffey D. M., Royer M. A., Meyer C. G., Holland K. N. (2020). Diel patterns in swimming behavior of a vertically migrating deepwater shark, the bluntnose sixgill (Hexanchus griseus). PloS One 15 (1), e0228253. doi: 10.1371/journal.pone.0228253
Compagno L. J. V. (1990). Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes. 28, 33–75. doi: 10.1007/BF00751027
Convention on Biological Diversity [CBD] (2010). CBD/COP/DEC/X/2 2010, decision adopted by the conference of the parties to the Convention on Biological Diversity at its tenth meeting, Nagoya, Japan, 18–29 October 2010. In: The Strategic Plan for Biodiversity 2011–2020 and the Aichi biodiversity targets. Available at: https://www.cbd.int/doc/decisions/cop-10/cop-10-dec-02-en.pdf (Accessed May 22, 2022).
Convention on Biological Diversity [CBD] (2016). Convention on Biological Diversity CHM Ecologically or Biologically Significant Areas (EBSAs) northeast Pacific white shark aggregation area fact sheet. Available at: https://chm.cbd.int/database/record?documentID=204043 (Accessed June 10, 2022).
Convention on Biological Diversity [CBD] (2020). Zero draft of the post-2020 Global Biodiversity Framework. Available at: https://www.cbd.int/article/zero-draft-update-august-2020 (Accessed April 4, 2022).
Convention on Biological Diversity [CBD] (2021a). Strategic Plan for Biodiversity 2011–2020, including Aichi biodiversity targets. Available at: https://www.cbd.int/sp/ (Accessed April 4, 2022).
Convention on Biological Diversity [CBD] (2021b). COP-15 Kunming Declaration. Available at: https://www.cbd.int/doc/c/df35/4b94/5e86e1ee09bc8c7d4b35aaf0/kunmingdeclaration-en.pdf (Accessed April 4, 2022).
Convention on Biological Diversity [CBD] (2021c). Special places in the ocean: A decade of describing Ecologically or Biologically Significant Marine Areas. Available at: https://www.cbd.int/marine/ebsa/booklet-ebsa-impact-en.pdf (Accessed May 20, 2022).
Convention on Biological Diversity [CBD] (2022a). Launch of the fifth edition of the Global Biodiversity Outlook. Available at: https://www.cbd.int/conferences/sbstta24-sbi3-prep/sbstta-24-prep (Accessed May 9, 2022).
Convention on Biological Diversity [CBD] (2022b). Clearing-house mechanism of the Convention on Biological Diversity Database. Available at: https://chm.cbd.int/database (Accessed March 15, 2022).
Convention on Migratory Species (CMS) (2020). UNEP/CMS/Resolution 12.7. the role of ecological networks in the conservation of migratory species. Bonn (CMS). Available at: https://www.cms.int/sites/default/files/document/cms_cop13_res.12.7_rev.cop13_e.pdf (Accessed June 1, 2022).
Corrigan C. M., Ardron J. A., Comeros-Raynal M. T., Hoyt E., Notarbartolo Di Sciara G., Carpenter K. E. (2014). Developing Important Marine Mammal Area criteria: learning from Ecologically or Biologically Significant Areas and Key Biodiversity Areas. Aquat. Conserv. 24 (S2), 166–183. doi: 10.1002/aqc.2513
Crowe L. M., O’Brien O., Curtis T. H., Leiter S. M., Kenney R. D., Duley P., et al. (2018). Characterization of large basking shark Cetorhinus maximus aggregations in the western north Atlantic ocean. J. Fish. Biol. 92 (5), 1371–1384. doi: 10.1111/jfb.13592
Davidson L. N., Dulvy N. K. (2017). Global marine protected areas to prevent extinctions. Nat. Ecol. Evol. 1, 0040. doi: 10.1038/s41559-016-0040
Donald P. F., Fishpool L. D. C., Ajagbe A., Bennun L. A., Bunting G., Burfield I. J., et al. (2019). Important Bird and Biodiversity Areas (IBAs): The development and characteristics of a global inventory of key sites for biodiversity. Bird. Conserv. Int. 29 (2), 177–198. doi: 10.1017/S0959270918000102
Dudgeon C. L., Lanyon J. M., Semmens J. M. (2013). Seasonality and site fidelity of the zebra shark, Stegostoma fasciatum, in southeast Queensland, Australia. Anim. Behav. 85 (2), 471–481. doi: 10.1016/j.anbehav.2012.12.013
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31 (21), 4773–4787. doi: 10.1016/j.cub.2021.08.062
Dunn D. C., Ardron J., Bax N., Bernal P., Cleary J., Cresswell I., et al. (2014). The Convention on Biological Diversity’s Ecologically or Biologically Significant Areas: Origins, development, and current status. Mar. Policy 49, 137–145. doi: 10.1016/j.marpol.2013.12.002
Dwyer R. G., Krueck N. C., Udyawer V., Heupel M. R., Chapman D., Pratt H. L. Jr., et al. (2020). Individual and population benefits of marine reserves for reef sharks. Curr. Biol. 30 (3), 480–489. doi: 10.1016/j.cub.2019.12.005
Ebert D. A., Dando M., Fowler S. (2021). Sharks of the world: A complete guide (New Jersey, USA: Princeton University Press). doi: 10.1515/9780691210872
Economakis A. E., Lobel P. S. (1998). Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean. Environ. Biol. Fish. 51, 129–139. doi: 10.1023/A:1007416813214
Eken G., Bennun L., Brooks T. M., Darwall W., Fishpool L. D. C., Foster M., et al. (2004). Key Biodiversity Areas as site conservation targets. Bioscience 54 (12), 1110–1118. doi: 10.1641/0006-3568(2004)054[1110:KBAASC]2.0.CO;2
Espinoza M., Cappo M., Heupel M. R., Tobin A. J., Simpfendorfer C. A. (2014). Quantifying shark distribution patterns and species-habitat associations: implications of marine park zoning. PloS One 9 (9), e106885. doi: 10.1371/journal.pone.0106885
Finucci B., Cheok J., Ebert D. A., Herman K., Kyne P. M., Dulvy N. K. (2021). Ghosts of the deep – biodiversity, fisheries, and extinction risk of ghost sharks. Fish. Fish. 22 (2), 391–412. doi: 10.1111/faf.12526
Fitzpatrick R., Thums M., Bell I., Meekan M. G., Stevens J. D., Barnett A. (2012). A comparison of the seasonal movements of tiger sharks and green turtles provides insight into their predator-prey relationship. PloS One 7 (12), e51927. doi: 10.1371/journal.pone.0051927
Food and Agriculture Organization of the United Nations [FAO] (1999). International plan of action for reducing incidental catch of seabirds in longline fisheries. International plan of action for the conservation and management of sharks. In: International plan of action for the management of fishing capacity (Rome, Italy: FAO). Available at: https://www.fao.org/3/X3170E/X3170E03.HTM (Accessed June 12, 2022).
Fowler S. (2014). The conservation status of migratory sharks (Bonn, Germany: UNEP/CMS Secretariat). Available at: https://www.cms.int/sites/default/files/publication/The%20Conservation%20Status%20of%20Migratory%20Sharks.pdf (Accessed January 22, 2022).
Fowler S., Bräutigam A., Okes N., Sant G. (2021). Conservation, fisheries, trade and management status of CITES-listed sharks [Germany: Federal Agency for Nature Conservation (BfN)]. Available at: https://www.bfn.de/sites/default/files/2021-08/Skript607.pdf (Accessed January 22, 2022). BfN-Skripten 607.
Francis M. P., Duffy C., Lyon W. (2015). Spatial and temporal habitat use by white sharks (Carcharodon carcharias) at an aggregation site in southern New Zealand. Mar. Freshw. Res. 66 (10), 900–918. doi: 10.1071/MF14186
Freestone D., Laffoley D., Douvere F., Badman T. (2016). World heritage in the high seas: an idea whose time has come (Switzerland and Paris, France: IUCN and UNESCO. Gland). Available at: https://unesdoc.unesco.org/ark:/48223/pf0000245467https://whc.unesco.org/en/highseas (Accessed June 10, 2022).
García V. B., Lucifora L. O., Myers R. A. (2008). The importance of habitat and life history to extinction risk in sharks, skates, rays, and chimaeras. Proc. R. Soc B. Biol. Sci. 275 (1630), 83–89. doi: 10.1098/rspb.2007.1295
Grace M. A., Doosey M. H., Denton J. S. S., Naylor G., Bart H. L. Jr., Maisey J. G. (2019). A new western north Atlantic Ocean kitefin shark (Squaliformes: Dalatiidae) from the Gulf of Mexico. Zootaxa 4619 (1), 109–120. doi: 10.11646/ZOOTAXA.4619.1.4
Guzman H. M., Gomez C. G., Hearn A., Eckert S. A. (2018). Longest recorded trans-Pacific migration of a whale shark (Rhincodon typus). Mar. Biodivers. Rec. 11 (1), 8. doi: 10.1186/s41200-018-0143-4
Habib K. A., Islam M. J. (2021). Description of a new species of giant guitarfish, Glaucostegus younholeei sp. nov. (Rhinopristiformes: Glaucostegidae) from the northern bay of Bengal, Bangladesh. Zootaxa 4995 (1), 129–146. doi: 10.11646/zootaxa.4995.1.7
Harvey M. S., Ralph G. M., Polidoro B. A., Maxwell S. M., Carpenter K. E. (2021). Identifying Key Biodiversity Areas as marine conservation priorities in the greater Caribbean. Biodivers. Conserv. 30, 4039–4059. doi: 10.1007/s10531-021-02291-8
Heupel M. R., Carlson J. K., Simpfendorfer C. A. (2007). Shark nursery areas: concepts, definition, characterization, and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Hoyt E. (2005). Marine protected areas for whales, dolphins, and porpoises. 1st edn (London: Earthscan).
Hyde C. A., Sorrentino L., Notarbartolo di Sciara G., Leurs G., Boyd C., Dulvy N., et al. (2022a). Report of the first workshop for the development of Important Shark and Ray Areas (ISRA) selection and review criteria (IUCN SSC Shark Specialist Group and IUCN Ocean Team: Dubai, United Arab Emirates).
Hyde C. A., Sorrentino L., Notarbartolo di Sciara G., Leurs G., Jabado R. W. (2022b). Report of the second workshop on uptake of Important Shark and Ray Areas (ISRAs) into national and regional policy (IUCN SSC Shark Specialist Group and IUCN Ocean Team: Dubai, United Arab Emirates).
International Union for Conservation of Nature [IUCN] (2016). A global standard for the identification of Key Biodiversity Areas, version 1.0. first edition (Gland, Switzerland: IUCN). Available at: https://portals.iucn.org/library/sites/library/files/documents/2016-048.pdf (Accessed May 10, 2022).
International Union for Conservation of Nature [IUCN] (2022). IUCN Red List of Threatened Species. version 2021-3. Available at: http://www.iucnredlist.org (Accessed May 13, 2022).
International Union for Conservation of Nature [IUCN] Marine Mammal Protected Areas Task Force [MMPATF] (2020b). Final report of the sixth IMMA workshop: Important Marine Mammal Area regional workshop for Australia-New Zealand and south East Indian Ocean (Perth, WA). Available at: https://www.marinemammalhabitat.org/downloads (Accessed June 6, 2022).
International Union for Conservation of Nature [IUCN] Standards and Petitions Committee (2019). Guidelines for using the IUCN Red List categories and criteria. Version 14. prepared by the Standards and Petitions Committee. Available at: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (Accessed March 10, 2022).
International Union for Conservation of Nature Species Survival Commission Shark Specialist Group (IUCN SSC SSG) (2022a). Important Shark and Ray Areas brochure (Gland, Switzerland: IUCN). Available at: https://sharkrayareas.org/resources/ (Accessed June 6, 2022).
International Union for Conservation of Nature Species Survival Commission Shark Specialist Group [IUCN SSC SSG] (2022b). Important Shark and Ray Areas (ISRA): Guidance on criteria application. Version 1 (Dubai: IUCN SSC Shark Specialist Group).
Jabado R. W., Kyne P. M., Pollom R. A., Ebert D. A., Simpfendorfer C. A., Ralph G. M., et al. (2018). Troubled waters: threats and extinction risk of the sharks, rays, and chimaeras of the Arabian Sea and adjacent waters. Fish. Fish. 19, 1043–1062. doi: 10.1111/faf.12311
Jenkins C. N., Van Houtan K. S. (2016). Global and regional priorities for marine biodiversity protection. Biol. Conserv. 204, 333–339. doi: 10.1016/j.biocon.2016.10.005
Johnson D. E., Barrio Froján C., Turner P. J., Weaver P., Gunn V., Dunn D. C., et al. (2018). Reviewing the EBSA process: Improving on success. Mar. Policy 88, 75–85. doi: 10.1016/j.marpol.2017.11.014
Joppa L. N., Butchart S. H. M., Hoffmann M., Bachman S. P., Akçakaya H. R., Moat J. F., et al. (2016). Impact of alternative metrics on estimates of extent of occurrence for extinction risk assessment. Conserv. Biol. 30 (2), 362–370. doi: 10.1111/cobi.12591
Jorgensen S. J., Arnoldi N. S., Estess E. E., Chapple T. K., Rückert M., Anderson S. D., et al. (2012). Eating or meeting? cluster analysis reveals intricacies of white shark (Carcharodon carcharias) migration and offshore behavior. PloS One 7, e47819. doi: 10.1371/journal.pone.0047819
Kullberg P., Di Minin E., Moilanen A. (2019). Using Key Biodiversity Areas to guide effective expansion of the global protected area network. Glob. Ecol. Conserv. 20, e00768. doi: 10.1016/j.gecco.2019.e00768
Kyne P. M., Jabado R. W., Rigby C. L., Gore M. A., Pollock C. M., Herman K.B., et al. (2020). The thin edge of the wedge: extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat. Conserv. 30, 1337–1361. doi: 10.1002/aqc.3331
Langhammer P. F., Bakarr M. I., Bennun L., Brooks T. M., Clay R.P., Darwall W.R.T., et al. (2007). Identification and gap analysis of Key Biodiversity Areas: targets for comprehensive protected area systems (Gland, Switzerland: IUCN).
Last P., White W., de Carvalho M., Séret B., Stehmann M., Naylor G. (2016). Rays of the world (Clayton: CSIRO Publishing). doi: 10.1080/17451000.2017.1336246
Lindegren M., Holt B. G., MacKenzie B. R., Rahbek C. (2018). A global mismatch in the protection of multiple marine biodiversity components and ecosystem services. Sci. Rep. 8 (1), 4099. doi: 10.1038/s41598-018-22419-1
Lockwood M., Davidson J., Hockings M., Haward M., Kriwoken L. (2012). Marine biodiversity conservation governance and management: Regime requirements for global environmental change. Ocean. Coast. Manage. 69, 160–172. doi: 10.1016/j.ocecoaman.2012.07.015
Lucifora L. O., García V. B., Worm B. (2011). Global diversity hotspots and conservation priorities for sharks. PloS One 6 (5), e19356. doi: 10.1371/journal.pone.0019356
MacKeracher T., Diedrich A., Simpfendorfer C. A. (2019). Sharks, rays, and marine protected areas: A critical evaluation of current perspectives. Fish. Fish. 20 (2), 255–267. doi: 10.1111/faf.12337
MacNeil M. A., Chapman D. D., Heupel M., Simpfendorfer C. A., Heithaus M., Meekan M., et al. (2020). Global status and conservation potential of reef sharks. Nature 583 (7818), 801–806. doi: 10.1038/s41586-020-2519-y
Manes S., Costello M. J., Beckett H., Debnath A., Devenish-Nelson E., Grey K.-A., et al. (2021). Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 257, 109070. doi: 10.1016/j.biocon.2021.109070
Martins A. P. B., Heupel M. R., Chin A., Simpfendorfer C. A. (2018). Batoid nurseries: definition, use and importance. Mar. Ecol. Prog. Ser. 595, 253–267. doi: 10.3354/meps12545
McCook L. J., Ayling T., Cappo M., Choat J. H., Evans R. D., De Freitas D. M., et al. (2010). Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. PNAS 107 (43), 18278–18285. doi: 10.1073/pnas.0909335107
Meyer C. G., Papastamatiou Y. P., Holland K. N. (2010). A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and Galapagos sharks (Carcharhinus galapagensis) at French Frigate Shoals, Hawaii. Mar. Biol. 157 (8), 1857–1868. doi: 10.1007/s00227-010-1457-x
Notarbartolo di Sciara G. (2021). Towards an Important Shark and Ray Area (ISRA) process: implementation strategy (Report to IUCN Species Survival Commission Shark Specialist Group). Available at: https://sharkrayareas.org/resources/meeting-workshop-reports/ (Accessed March 2, 2022).
Notarbartolo di Sciara G., Hoyt E., Reeves R., Ardron J., Barr B. (2016). Place-based approaches to marine mammal conservation. Aquat. Conserv. 26 (S2), 85–100. doi: 10.1002/aqc.2642
Oliver S. P., Hussey N. E., Turner J. R., Beckett A. J. (2011). Oceanic sharks clean at coastal seamount. PloS One 6 (3), e14755. doi: 10.1371/journal.pone.0014755
O’Shea O. R., Kingsford M. J., Seymour J. (2010). Tide-related periodicity of manta rays and sharks to cleaning stations on a coral reef. Mar. Fresh. Res. 61 (1), 65–73. doi: 10.1071/MF08301
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589 (7843), 567–571. doi: 10.1038/s41586-020-03173-9
Papastamatiou Y. P., Iosilevskii G., Di Santo V., Huveneers C., Hattab T., Planes S., et al. (2021). Sharks surf the slope: Current updrafts reduce energy expenditure for aggregating marine predators. J. Anim. Ecol. 90 (10), 2302–2314. doi: 10.1111/1365-2656.13536
Pardo S. A., Kindsvater H. K., Reynolds J. D., Dulvy N. K. (2016). Maximum intrinsic rate of population increase in sharks, rays, and chimaeras: the importance of survival to maturity. Can. J. Fish. Aquat. Sci. 73 (8), 1159–1163. doi: 10.1139/cjfas-2016-0069
QGIS Development Team (2022). QGIS geographic information system. Open source geospatial foundation project. version 3.24. Available at: https://qgis.org/ (Accessed April 1, 2022).
Randall J. E. (1977). Contributions to the biology of the whitetip reef shark (Triaenodon obesus). Pac. Sci. 31 (2), 143–164. Available at: https://scholarspace.manoa.hawaii.edu/server/api/core/bitstreams/89357213-40a0-4544-8a72-2df54207d112/content (Accessed April 8, 2022).
Rands M. R. W., Adams W. M., Bennun L., Butchart S. H. M., Clements A., Coomes D., et al. (2010). Biodiversity conservation: challenges beyond 2010. Science 329 (5997), 1298–1303. doi: 10.1126/science.1189138
Rigby C. L., Barreto R., Carlson J., Fernando D., Fordham S., Francis M. P., et al. (2019b). Isurus oxyrinchus. IUCN Red List of Threatened Species. 2019, e.T39341A2903170. doi: 10.2305/IUCN.UK.2019-1.RLTS.T39341A2903170.en
Rigby C. L., Simpfendorfer C. A., Cornish A. (2019a). A practical guide to effective design and management of MPAs for sharks and rays (Gland, Switzerland: WWF).
Salinas-de-León P., Phillips B., Ebert D., Shivji M., Cerutti-Pereyra F., Ruck C., et al. (2018). Deep-sea hydrothermal vents as natural egg-case incubators at the Galapagos rift. Sci. Rep. 8, 1788. doi: 10.1038/s41598-018-20046-4
Schaber M., Gastauer S., Cisewski B., Hielscher N., Janke M., Peña M., et al. (2022). Extensive oceanic mesopelagic habitat use of a migratory continental shark species. Sci. Rep. 12 (1), 2047. doi: 10.1038/s41598-022-05989-z
Selig E. R., Turner W. R., Troëng S., Wallace B. P., Halpern B. S., Kaschner K., et al. (2014). Global priorities for marine biodiversity conservation. PloS One 9 (1), e82898. doi: 10.1371/journal.pone.0082898
Sherman K., Alexander L. M. (1986). “Chapter 1: Introduction to parts one and two: Large Marine Ecosystems as tractable entities for measurement and management.,” in In part one: Impact of perturbations on the productivity of renewable resources in Large Marine Ecosystems. AAAS selected symposium 99 (Boulder, Colorado, USA: Westview Press).
Simpfendorfer C. A., Goodreid A. B., McAuley R. B. (2001). Size, sex, and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environ. Biol. Fishes. 61 (1), 37–46. doi: 10.1023/A:1011021710183
Smith S. E., Au D. W., Show C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Fresh. Res. 49, 663–678. doi: 10.1071/MF97135
Tetley M. J., Braulik G. T., Lanfredi C., Minton G., Panigada S., Politi E., et al. (2022). The Important Marine Mammal Area Network: a tool for systematic spatial planning in response to the marine mammal habitat conservation crisis. Front. Mar. Sci. 9, 841789. doi: 10.3389/fmars.2022.841789
Treloar M. A., Barrett N. S., Edgar G. J. (2016). Biology and ecology of Zearaja maugeana, an Endangered skate restricted to two south-western Tasmanian estuaries. Mar. Fresh. Res. 68, 821–830. doi: 10.1071/MF15478
United Nations [UN] (2016). The Sustainable Development Goals report 2016 (United Nations, New York). Available at: https://unstats.un.org/sdgs/report/2016/the%20sustainable%20development%20goals%20report%202016.pdf (Accessed June 1, 2022).
United Nations Environment Program - World Conservation Monitoring Center, [UNEP-WCMC] (2021). Protected Planet Report 2020. UNEP-WCMC and IUCN: Cambridge UK; Gland, Switzerland
Ward-Paige C. A. (2017). A global overview of shark sanctuary regulations and their impact on shark fisheries. Mar. Policy 82, 87–97. doi: 10.1016/j.marpol.2017.05.004
Keywords: biodiversity, chimaeras, conservation, Important Shark And Ray Areas (ISRA), marine spatial planning (MSP), protected areas, rays (fish), threatened species
Citation: Hyde CA, Notarbartolo di Sciara G, Sorrentino L, Boyd C, Finucci B, Fowler SL, Kyne PM, Leurs G, Simpfendorfer CA, Tetley MJ, Womersley F and Jabado RW (2022) Putting sharks on the map: A global standard for improving shark area-based conservation. Front. Mar. Sci. 9:968853. doi: 10.3389/fmars.2022.968853
Received: 14 June 2022; Accepted: 10 August 2022;
Published: 13 September 2022.
Edited by:
Salvatore Siciliano, Fundação Oswaldo Cruz (Fiocruz), BrazilReviewed by:
David Shiffman, Arizona State University, United StatesCharles Bangley, Dalhousie University, Canada
Copyright © 2022 Hyde, Notarbartolo di Sciara, Sorrentino, Boyd, Finucci, Fowler, Kyne, Leurs, Simpfendorfer, Tetley, Womersley and Jabado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ciaran A. Hyde, Y2lhcmFuLmh5ZGVAb3V0bG9vay5jb20=; Rima W. Jabado, cmltYWphYmFkb0Bob3RtYWlsLmNvbQ==
 Ciaran A. Hyde
Ciaran A. Hyde Giuseppe Notarbartolo di Sciara
Giuseppe Notarbartolo di Sciara Lynn Sorrentino
Lynn Sorrentino Charlotte Boyd
Charlotte Boyd Brittany Finucci
Brittany Finucci Sarah L. Fowler
Sarah L. Fowler Peter M. Kyne
Peter M. Kyne Guido Leurs
Guido Leurs Colin A. Simpfendorfer
Colin A. Simpfendorfer Michael J. Tetley
Michael J. Tetley Freya Womersley
Freya Womersley Rima W. Jabado
Rima W. Jabado