
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 23 September 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.968838
Immunostimulants are becoming one of the most effective models for healthy and sustainable development against vibriosis, which is a serious threat to the global aquaculture industry. This study evaluated the effects of different concentrations of Ganoderma lucidum polysaccharides (GLPs) on the immunomodulation and disease resistance of pearl gentian groupers. The relative percent survival of the pearl gentian groupers challenged with Vibrio harveyi ZJ0603 by intraperitoneal injection reached 53.3, 60, 70, and 73.3% in the GLPs2, GLPs4, GLPs6, and GLPs8 groups, respectively, which are significantly higher than that of 33.3% in the control group. Meanwhile, the expression levels of immune-relative genes, including IgM, MHC-Iα, MHC2, IL-1β, and TNF-α, were upregulated in the liver, spleen, head kidney, and thymus within 28 days after GLP injection. The total serum protein and the activities of catalase, superoxide dismutase, and lysozymes in serum were significantly upregulated in all GLP groups compared with those in the control. Moreover, the optimal immunity effect was observed with an injection of GLPs at concentrations of 6 or 8 mg/ml. The results demonstrate that GLPs were an effective immunostimulant, enhancing both specific and non-specific immunity as well as disease resistance in pearl gentian grouper.
Vibrio harveyi is a gram-negative opportunistic pathogen found in a wide variety of aquatic and marine environments and has caused serious economics losses to the aquaculture industry in recent decades (Zhang et al., 2020). V. harveyi is responsible for diseases in many aquatic animals in China, including Litopenaeus vannamei (Soto-Rodriguez et al., 2010), Larimichthys crocea (Yang et al., 2021), Epinephelus coioides (Yii et al., 1997), and pearl gentian grouper (Zhu et al., 2018). The presence of V. harveyi, in turn, causes a severe threat to the development of an aquatic industry. Farmed grouper production in China reached 100,000 tons in 2020 (Loh et al., 2020). Pearl gentian grouper is a new hybrid species hybridized by ♀E. fuscoguttatus × ♂E. lanceolatus. The hybrid is widely cultured because of its huge market demand and considerable economic value. However, the development of the grouper farming industry has been severely affected by the outbreak of vibriosis in the past decades (Wei et al., 2020a). In order to decrease the severity of the vibriosis outbreak, antibiotics have been commonly employed. However, the indiscriminate use of antibiotics has caused a buildup of resistance by pathogenic bacteria, and drug resistance problems have emerged (Xiong et al., 2010). Therefore, healthy, effective, and sustainable treatments for combating vibriosis are urgently needed.
Chinese herbs have been used as traditional medicine and immune boosters for human beings for thousands of years in China. In recent years, Chinese herbal medicine has attracted attention in the aquaculture industry. Many countries began to focus on the use of Chinese and Indian herbs for increasing the growth rate and strengthening the immune response of cultivable aquatic animals (Galina et al., 2009). Some medicinal Chinese herbs like Astragalus membranaceus (Ardó et al., 2008), Lonicera japonica (Ardó et al., 2008), plant polysaccharides (Ficus carica polysaccharides, Radix isatidis polysaccharides, and Schisandra chinensis polysaccharides) (Wang et al., 2016), Polyporus umbellatus (Pan et al., 2013), Scutellaria baicalensis (Pan et al., 2013), Ganoderma lucidum polysaccharides (Liu et al., 2015), and Sauropus androgynus L. Merr. (Samad et al., 2014) have been tested and found to enhance the immune response and disease resistance as immunostimulants in fish. Among them, Galla chinensis (Pan and Yan, 2019), S. baicalensis (Pan and Yan, 2019), and S. androgynus L. Merr. (Samad et al., 2014) have achieved beneficial effects against vibriosis infection of aquatic animals.
The fruiting body of G. lucidum was once considered a panacea in ancient China and was said to be able to make the dead come back to life in some old legends and fairy tales (Xu et al., 2011). G. lucidum has long been used as a traditional Chinese herb to treat human disease in China. Various aspects of G. lucidum include polysaccharides (β-D-glucans, heteropolysaccharides, and glycoproteins; concentration of 25.1%), antioxidants, flavonoids, alkaloids, and proteins (Zjawiony, 2004). G. lucidum polysaccharides (GLPs) have been isolated from the fruiting body and are classified as major bioactive components. Many of these components have been shown to possess pharmacological action, including immunomodulation, anti-oxidation, hepatoprotection, anti-proliferation, anti-angiogenesis (Xu et al., 2011; Chithra et al., 2016), and anti-tumor (cervical cancer, lung cancer, and prostate cancer) (Guo et al., 2018; Wu et al., 2018; Zhu et al., 2019). In recent years, GLPs have been applied in aquaculture and found to be capable of significantly enhancing the immune responses and disease resistance in hosts such as Pelteobagrus fulvidraco (Bai et al., 2015), Macrobrachium rosenbergii (Mohan et al., 2019), and E. coioides (Chang et al., 2013). However, there are limited studies focusing on the immunomodulatory and protective effects of GLPs on pearl gentian grouper against V. harveyi.
In our study, pearl gentian groupers were injected with different concentrations of GLPs. The expression pattern of immune-related genes in the liver, spleen, head kidney, and thymus was detected, and the enzyme activities of catalase (CAT), superoxide dismutase (SOD), lysozyme (LZM), and total serum protein in serum were also determined. Finally, the survival rates were measured. These results provide a comprehensive understanding of GLPs as immunostimulants of immune response and disease resistance against V. harveyi infection in pearl gentian grouper.
The pathogenic bacterial strain V. harveyi ZJ0603 was isolated from diseased groupers (Zhanjiang, China) and stored in our laboratory. The bacterium was cultured in tryptic soy broth (Huankai, Guangzhou, China) containing 2% NaCl at 28°C for the following study.
Healthy pearl gentian groupers with body weight of 40.0 ± 4.0 g were purchased from East-island fish farm (Zhanjiang, China). The groupers were cultured in an aerated tank containing seawater (filtered by seawater filter bags) with 27.0 ± 3.0% salinity at 26.0 ± 1.0°C and fed with commercial grouper feed twice a day for 2 weeks. The groupers were fed their respective diets at a level of 3% of body weight once a day. Before the experiments, six fish were randomly chosen for bacterial recovery from the spleen, liver, and head kidney on tryptic soy broth with 1% agar (TSA) plates to ensure that the fish were not infected with V. harveyi or other bacteria. The fish were anesthetized with 100 ng/ml tricaine methanesulfonate (MS-222) before the injection and sample collection. All animal experiments were strictly executed according to ethical standards and approved by the Ethics Committee of Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture.
The GLPs (Yuanye, Shanghai, China) were dissolved in 0.85% saline solution. Then, the GLP solutions were filtrated through a Millipore membrane (0.22 μm) and adjusted to concentrations of 2, 4, 6, and 8 mg/ml. All the GLP solutions were stored at 4°C until further use.
A total of 300 fish were randomly divided into five groups (60 fish/group). The control group [phosphate-buffered saline (PBS) group] was intraperitoneally injected with 0.1 ml PBS, while the experimental groups (GLPs2, GLPs4, GLPs6, and GLPs8 groups) were intraperitoneally injected with 0.1 ml of 2, 4, 6, and 8 mg/ml GLPs, respectively. The liver, spleen, head kidney, and thymus were collected once a week from 7 to 28 days and the blood from 7 to 42 days post-injection. The liver, spleen, head kidney, and thymus were washed with sterile saline solution, then collected in cryogenic vials (Thermo Scientific, USA), and quickly frozen in liquid nitrogen. The serum was collected by centrifuging the blood at 3,500 rpm for 20 min at 4°C. The serum, liver, spleen, head kidney, and thymus were stored at -80°C until further use.
At 35 days post-injection, 150 fish from the PBS, GLPs2, GLPs4, GLPs6, and GLPs8 groups (30 fish/group) were intraperitoneally injected with 0.1 ml V. harveyi ZJ0603 at a concentration of 5.6 × 108 CFU/ml (Wei et al., 2020b). The mortality of each group was monitored for 14 days after the bacterium challenge. Relative percent survival (RPS) was calculated using the following formula: RPS (%) = [1 - (mortality of the vaccinated fish/mortality of the control fish)] × 100%. Bacteria from the liver, spleen, and head kidney of dead fish were applied to TSA plates using inoculation loops (Sangon Biotech, Guangzhou, China) to re-isolate the bacteria. The amplification of 16s rDNA (Table 1) was used to identify whether the fish died by V. harveyi ZJ0603 infection. The experiment was repeated three times.
A total of 60 fish from the PBS, GLPs2, GLPs4, GLPs6, and GLPs8 groups (12 fish/group) were used for tissue collection (liver, spleen, head kidney, and thymus) per week for 4 weeks. qRT-PCR was used to analyze the gene expression levels of IgM (immunoglobulin M), MHC-Iα (major histocompatibility complex Iα), MHC2 (major histocompatibility complex II), IL-1β (interleukin 1β), and TNF-α (tumor necrosis factor α), with β-actin used as an internal reference gene. All the primers used are listed in Table 1. Total RNA was extracted from all samples (liver, spleen, head kidney, and thymus) using TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China), and the cDNA was generated using EasyScript® One-Step GLPsA Removal and cDNA Synthesis SuperMix (Takara, Beijing, China). qRT-PCR amplification was performed using PerfectStart® Green qPCR SuperMix (TransGen Biotech, Beijing, China) for gene expression analysis. All data were analyzed by the 2-△△Ct method (Livak and Schmittgen, 2001). Each experiment was repeated three times.
Ninety fish from the PBS, GLPs2, GLPs4, GLPs6, and GLPs8 groups (18 fish/group) were used for serum collection once per week for 6 weeks. The activities of CAT, SOD, LZM, and total serum protein of fish serum samples were detected using a catalase assay kit (Visible light), a superoxide dismutase assay kit (WST-1 method), a lysozyme assay kit, and a total protein quantitative assay kit, respectively. All kits were purchased from Nanjing Jiancheng Bioengineering Institute in China. The experiment was repeated three times.
Data were analyzed by one-way analysis of variance (ANOVA) using SPSS 26.0 software with Duncan’s new multiple-range test. All results were compared and discussed in different concentrations of GLPs at the same time points. The data are shown as mean ± SE, and the differences between data are marked as a, b, c, d, and e as well as when they are statistically significant (p < 0.05).
The efficacy of GLPs was evaluated by fish challenged with V. harveyi at 35 days post-injection. The RPS of the GLPs2, GLPs4, GLPs6, and GLPs8 groups were 53.3, 60, 70, and 73.3%, respectively, which were significantly higher than 33.3% for the PBS group (Figure 1). The results showed that GLPs had better immunoprotection in all experimental groups compared to the PBS group, and GLPs possessed the potency to trigger immune responses in groupers. Therefore, GLPs may act as an effective immunostimulant enhancing the immunity of groupers against V. harveyi infection.
To evaluate the immune response of the pearl gentian grouper immunized with GLPs at different concentrations, the transcriptional levels of IgM, MHC-Iα, MHC2, IL-1β, and TNF-α in the liver, spleen, head kidney, and thymus of groupers were measured at 7, 14, 21, and 28 days post-injection (Figures 2–6). The results showed that all immune-relative genes were upregulated after the GLP injection. IgM and MHC-Iα were significantly more highly expressed in four tissues at all time points in the GLPs6 and GLPs8 groups while only at several time points in the GLPs2 and GLPs4 groups (Figures 2, 3). The expression levels of IL-1β in four tissues had no significant difference in the GLPS2 group but was significantly upregulated in the GLPs4, GLPs6, and GLPs8 groups at most time points (Figure 4). MHC2 was significantly more highly expressed in four tissues at all time points in the GLPs8 group (Figure 5). However, there was no significance in the expression levels of MHC2 in the liver and head kidney at 28 days post-injection in the GLPs2, GLPs4, and GLPs6 groups (Figures 5A, B). Moreover, the expression levels of TNF-α showed different expression patterns in different tissues but were significantly increased compared to those in the PBS group at most of the sampling time points (Figure 6).
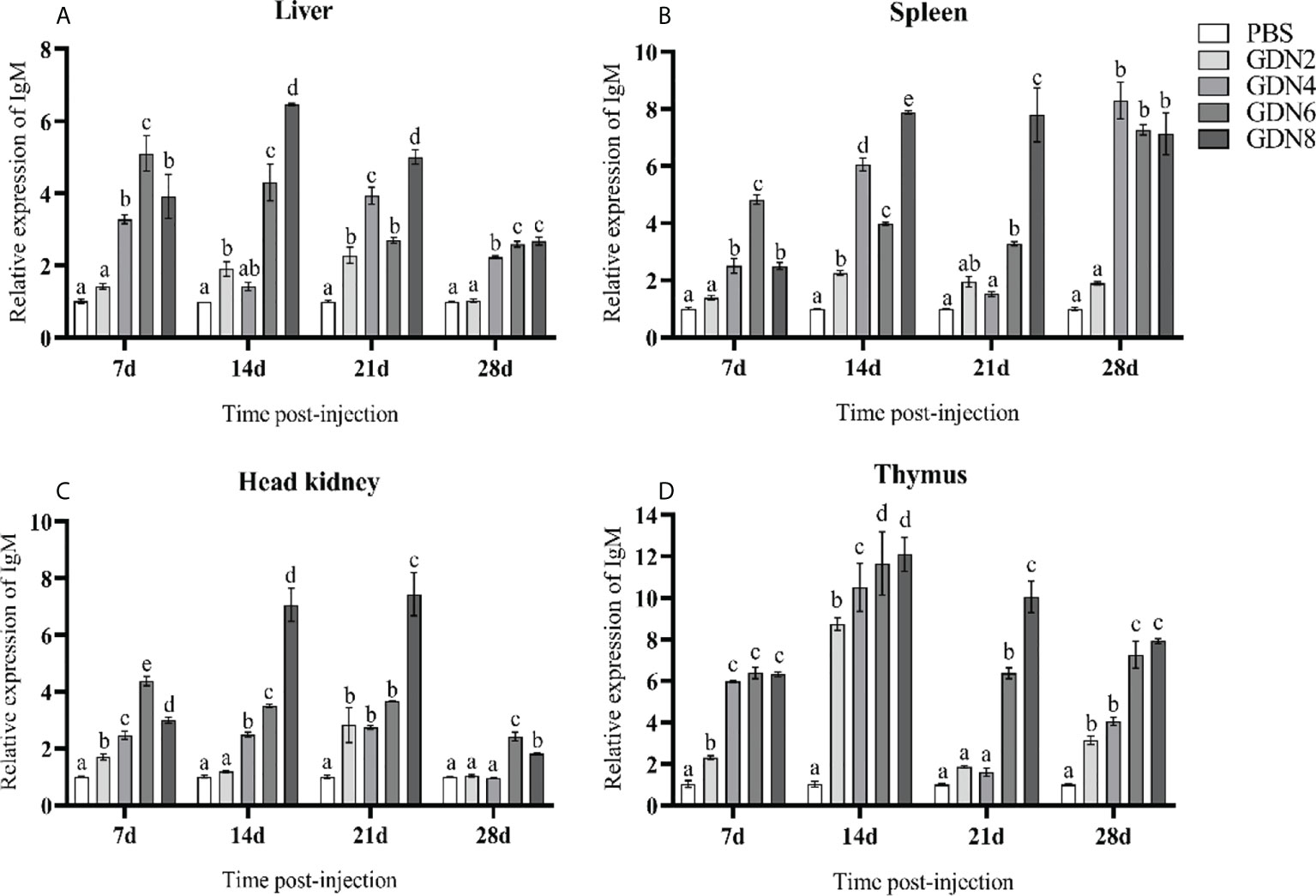
Figure 2 Expression levels of the IgM gene in the four tissues of pearl gentian grouper (A, liver; B, spleen; C, head kidney; D, thymus) by qRT-PCR post-injection. The mRNA levels of the IgM gene were normalized to those of β-actin, and the relative expression levels were calculated. Significant differences (p < 0.05) are marked by different letters (a–e).
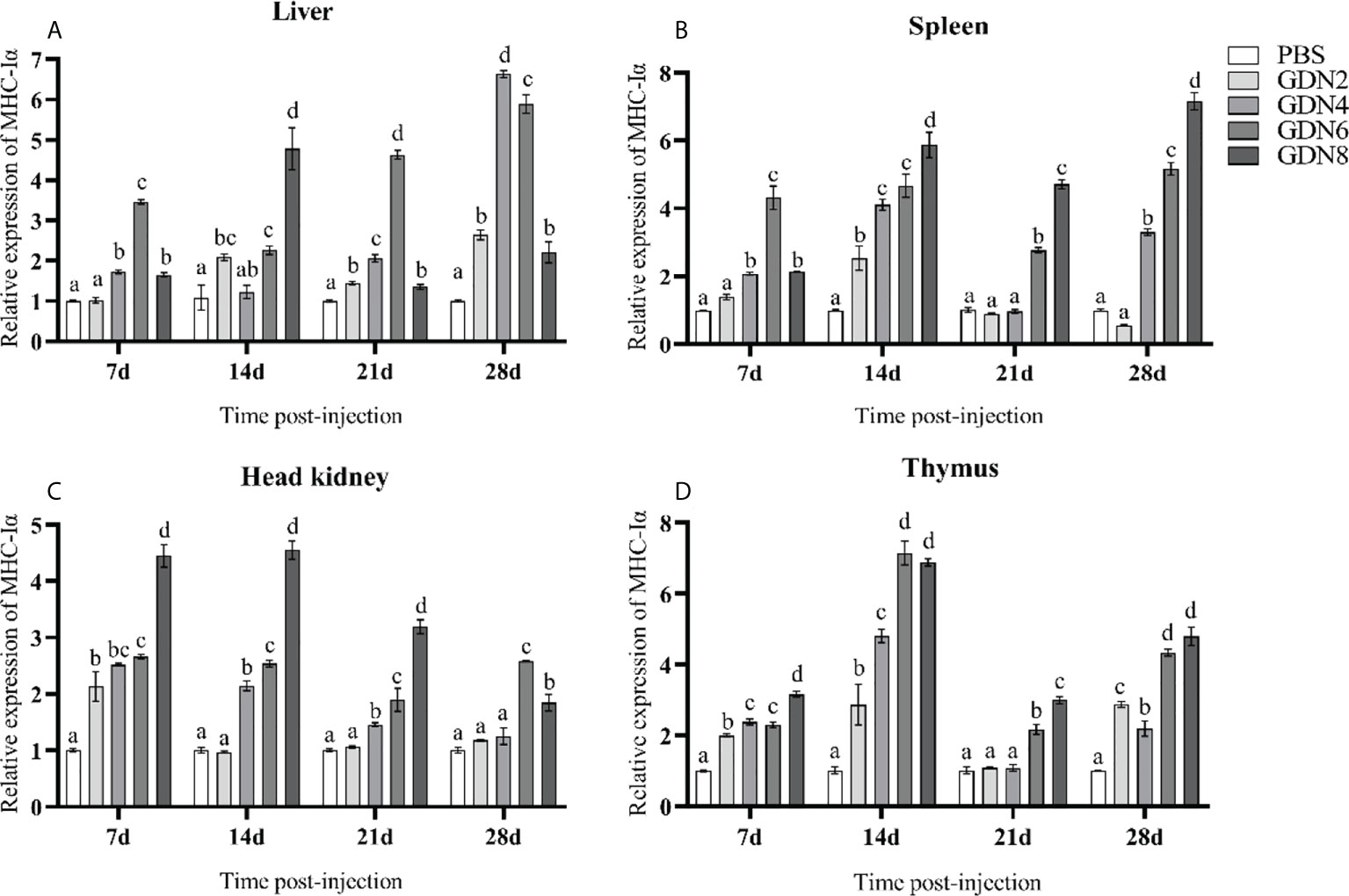
Figure 3 Expression levels of the MHC-Iα gene in the four tissues of pearl gentian grouper (A, liver; B, spleen; C, head kidney; D, thymus) by qRT-PCR post-injection. The mRNA levels of the MHC-Iα gene were normalized to those of β-actin, and the relative expression levels were calculated. Significant differences (p < 0.05) are marked by different letters (a–d).
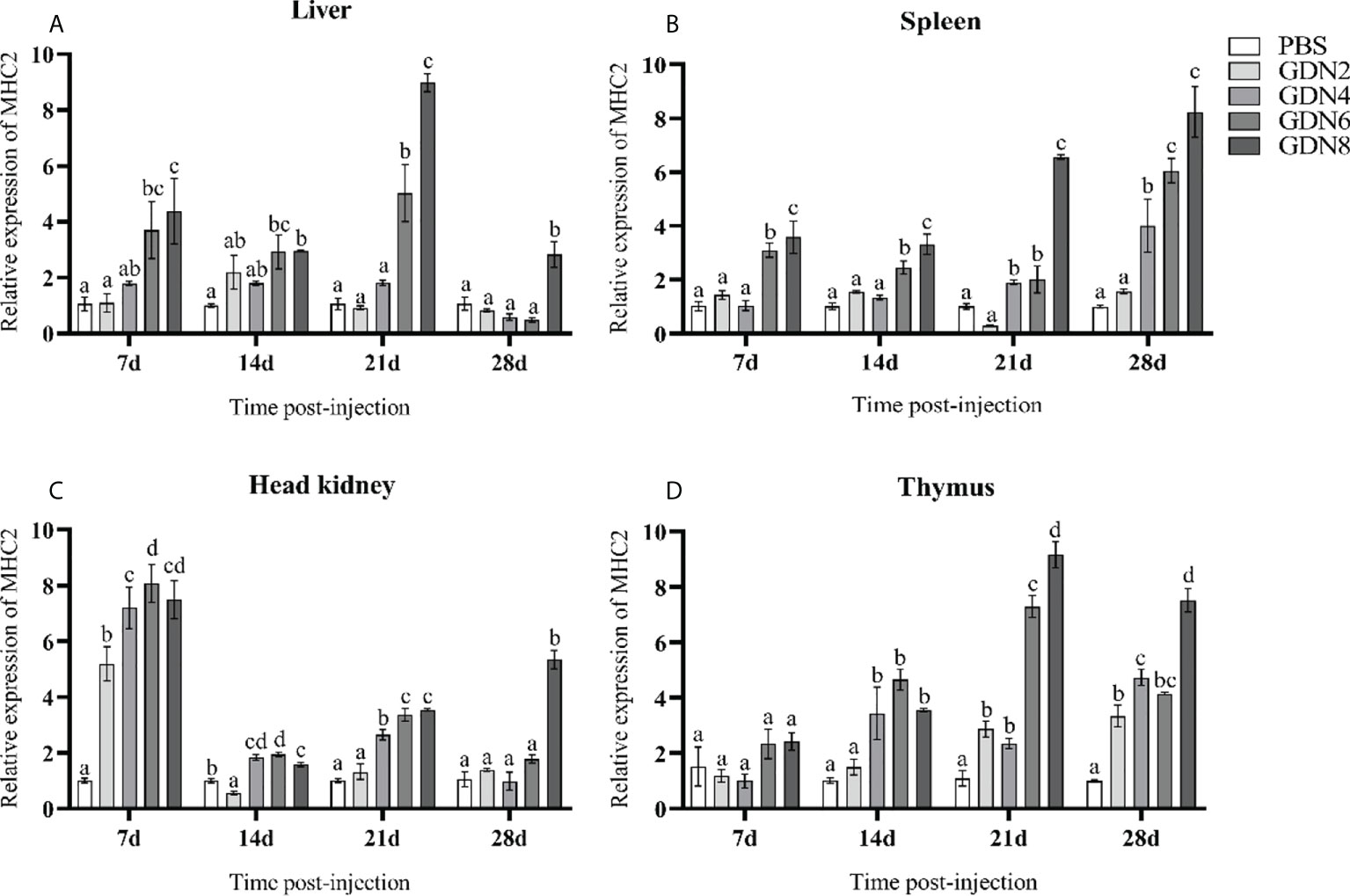
Figure 4 Expression levels of the IL-1β gene in the four tissues of pearl gentian grouper (A, liver; B, spleen; C, head kidney; D, thymus) by qRT-PCR post-injection. The mRNA levels of the IL-1β gene were normalized to those of β-actin, and the relative expression levels were calculated. Significant differences (p < 0.05) are marked by different letters (a–d).
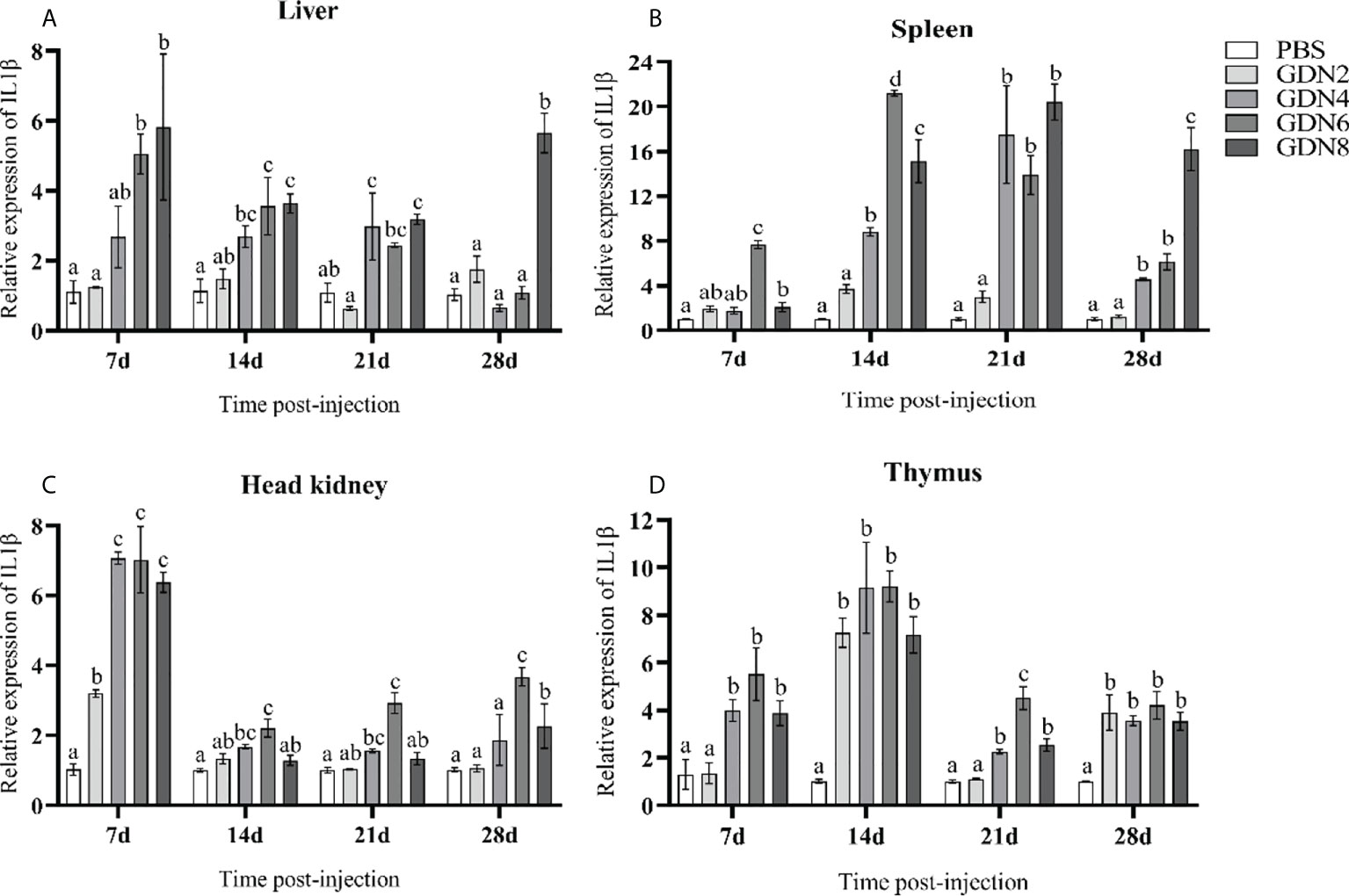
Figure 5 Expression levels of the MHC2 gene in the four tissues of pearl gentian grouper (A, liver; B, spleen; C, head kidney; D, thymus) by qRT-PCR post-injection. The mRNA levels of the MHC2 gene were normalized to those of β-actin, and the relative expression levels were calculated. Significant differences (p < 0.05) are marked by different letters (a–d).
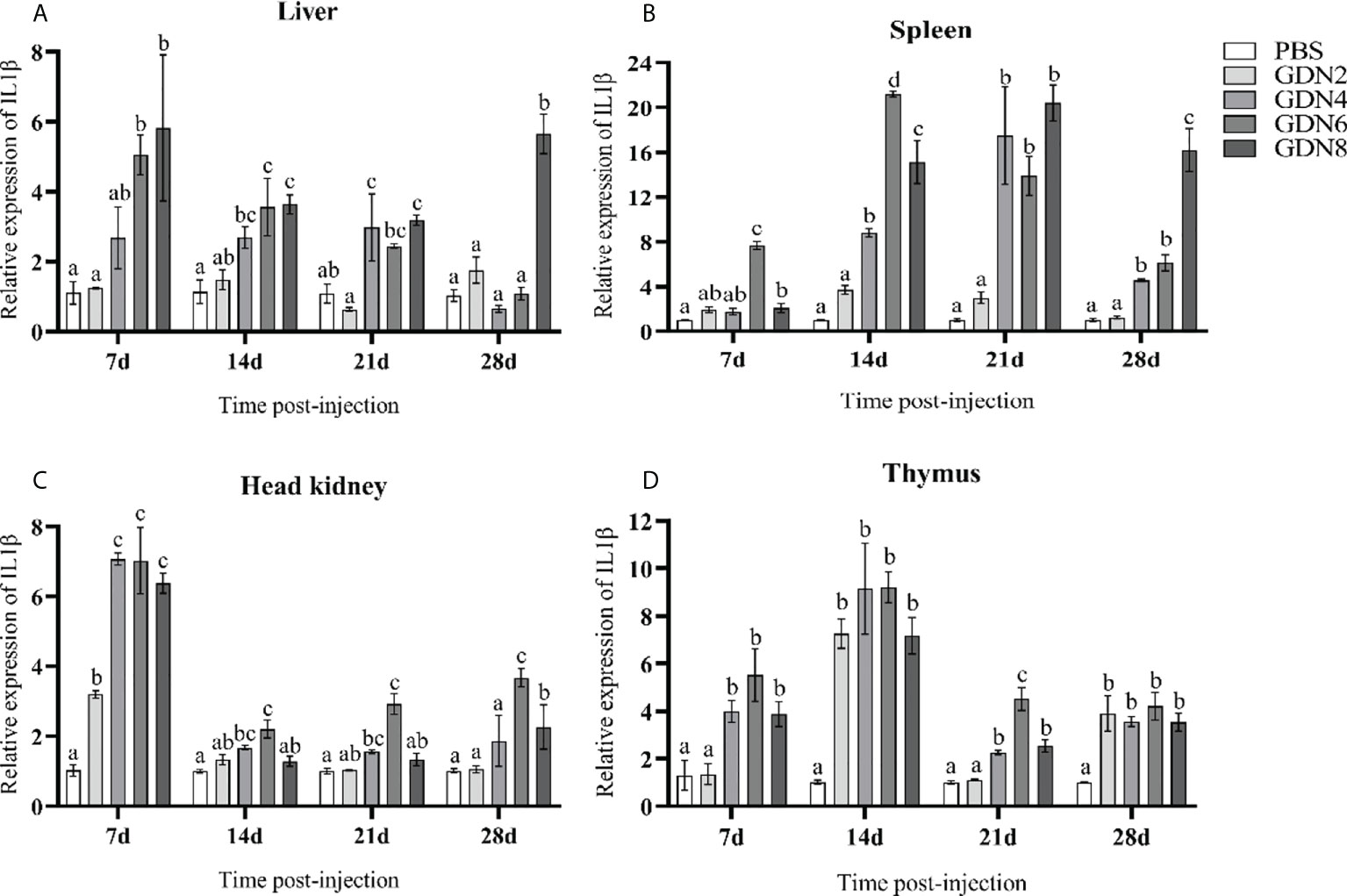
Figure 6 Expression levels of the TNF-α gene in the four tissues of pearl gentian grouper (A, liver; B, spleen; C, head kidney; D, thymus) by qRT-PCR post-injection. The mRNA levels of the TNF-α gene were normalized to those of β-actin, and the relative expression levels were calculated. Significant differences (p < 0.05) are marked by different letters (a–d).
The enzyme activities and total serum protein in fish serum from 7 to 42 days were determined (Figure 7). CAT activities were significantly upregulated during 7 to 14 days in the GLPs6 group and from 7 to 35 days in the GLPs8 group, while there was no significant difference at all time points in the GLPs2 and GLPs4 groups (Figure 7A). The SOD activities significantly increased during 14 to 35 days in the GLPs6 and GLPs8 groups, and the highest activity appeared at 28 days in the GLPs6 group (Figure 7B). LZM activities were significantly upregulated from 7 to 35 days in all experimental groups, and the highest activity appeared at 28 days in the GLPs6 group (Figure 7C). Total serum protein gradually increased and reached its peak at 28 days in all experimental groups and then decreased to normal levels after 35 days in all experimental groups (Figure 7D).
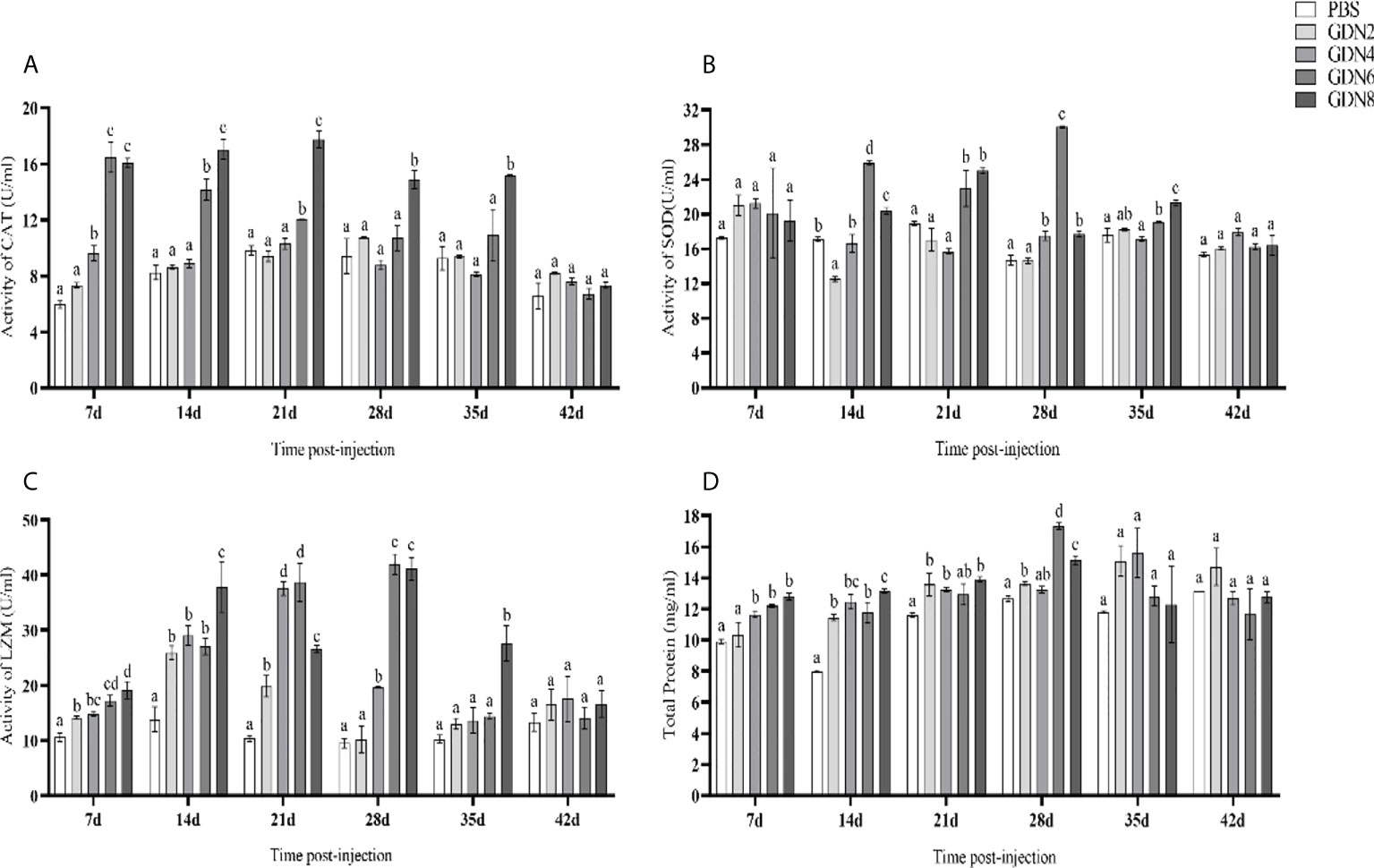
Figure 7 Catalase (CAT), superoxide dismutase (SOD), and lysozyme (LZM) activities and total serum protein of pearl gentian grouper at 7, 14, 21, 28, 35, and 42 days post-injection (mean ± SE; n = 3). (A) CAT activity, (B) SOD activity, (C) LZM activity, and (D) total serum protein. Significant differences (p < 0.05) are marked by different letters (a–d).
In recent years, the outbreak of vibriosis has impeded the development of the aquaculture industry. There is an urgent need for a healthy and sustainable treatment method against the occurrence of vibriosis. The application of medicinal plant products as immunostimulants and therapeutic measures that modulate the immune response and are anti-bacterial has raised the interest of extensive scientific investigation (Peters, 1985). Ardó et al. (2008) showed previously that Astragalus membranaceus significantly enhanced the phagocytic and respiratory burst activity of blood phagocytic cells and moderately enhanced the lysozyme levels in the plasma in feeding tilapia. Wang et al. (2016) showed that three plant polysaccharides (Ficus carica polysaccharides, Radix isatidis polysaccharides, and Schisandra chinensis polysaccharides) significantly enhanced the innate immune response, including leukocyte phagocytosis activity, serum bactericidal activity, lysozyme activity, total protein level, complement C3, and superoxide dismutase activity of crucian carp. Liu et al. (2015) showed that G. lucidum polysaccharides could increase the mRNA expressional levels of TNF-α, IL-1β, caspase-3, caspase-8, and the enzyme activities of glutamate pyruvate transaminase, glutamate oxalate transaminase, lactate dehydrogenase, malondialdehyde, and superoxide dismutase in hepatocytes from Cyprinus carpio L. The above-mentioned results reveal that Chinese herbal medicine has the potential to improve the immune responses and disease resistance of aquaculture animals.
The effectiveness of mushrooms and mushroom-derived compounds as prebiotics in aquaculture has been reviewed by Mohan et al. (2022). GLPs, as one of the bioactive components isolated from the G. lucidum fruiting body, has been confirmed to affect immune cells and immune-related cells, including B lymphocytes, T lymphocytes, dendritic cells, macrophages, and natural killer cells (Xu et al., 2011). Both cellular immunity and humoral immunity were motivated by intraperitoneally injecting the mice with GLPs (Zhu et al., 2007; Zhang et al., 2010; Xu et al., 2011). In fish, dietary GLPs could also enhance the immune responses and disease resistance (Yin et al., 2009; Bai et al., 2015; Mohan et al., 2019). However, few studies have focused on the immune effects of intraperitoneally injecting fish with GLPs.
This study was conducted to examine the efficacy of injecting fish with GLPs at concentrations of 2, 4, 6, and 8 mg/ml for stimulating the immune response of pearl gentian groupers against V. harveyi. It was observed that injecting the pearl gentian groupers with GLPs at concentrations of 4, 6, and 8 mg/ml could effectively increase their protection against V. harveyi. Both the non-specific immunity index (enzyme activities, total serum protein, and innate response related genes) and specific immunity index (adaptive response-related genes) of the fish were enhanced following the GLP injection. Furthermore, optimal immunity effect was observed with the injection of GLPs at concentrations of 6 and 8 mg/ml. In this study, groupers injected with GLPs showed increasing RPS and protection against V. harveyi. The improvement of specific and non-specific immunomodulation and disease resistance in pearl gentian groupers injected with GLPs suggests the possibility of GLPs as effective immunostimulants with potential future applications.
GLPs were shown to be able to modulate the immune response. Immunomodulatory action requires the participation and communication of immune organs, immune-related cells, and immune-related factors. As immune organs, the liver, spleen, head kidney, and thymus play important roles in the immune process. The liver is an active lymphopoietic organ that mainly functions in innate immunity, metabolism, and growth (Zapata and Amemiya, 2000; Causey et al., 2018). The spleen is a hematopoietic organ in fish (Zapata et al., 2006). The head kidney is an important hematopoietic organ with key regulatory functions and the central organ for immune–endocrine interactions (Geven and Klaren, 2017). The thymus, as a lymphoid organ situated near the opercular cavity in teleosts, produces T lymphocytes and stimulates phagocytosis and antibody production by B cells (Zapata and Amemiya, 2000). The thymus, head kidney, and spleen are the major lymphoid organs in fish. The expression of immune-related genes IgM, MHC-Iα, MHC2, IL-1β, and TNF-α in these immune organs was determined in this study. IgM is the earliest antibody induced by a primary response that participates in humoral adaptive immunity (Bag et al., 2009). MHC class II and MHC class I on antigen-presenting cells bind with CD4 and CD8 (co-receptor of TCR on helper T cell), and they altogether transduce T cell activation signals (Holling et al., 2004; Chaplin, 2010). The interleukin-1 (IL-1) family of cytokines produced by macrophages and monocytes consists of major mediators of innate immune reactions, and IL-1α or IL-1β has proinflammatory activities (Weber et al., 2010; Dinarello, 2011). TNF is mainly produced by activated macrophages, NK cells, and T lymphocytes, can induce apoptotic pathways, and possesses potent proinflammatory and antibacterial activities (Chaplin, 2010). In our study, both specific-related genes (IgM, MHC-Iα, and MHC2) and non-specific-related genes (IL-1β and TNF-α) were upregulated in the four immune organs from 7 to 28 days post-injection, suggesting that GLPs can regulate both the innate immunity and adaptive immunity of pearl gentian grouper.
Reactive oxygen species (ROS) are normal byproducts of cellular metabolism produced by living cells. However, the large quantities of ROS damage organelle structures and biomolecules, leading to severe inflammatory responses (He et al., 2017). As important antioxidants, SOD and CAT can jointly eliminate the damaging effects of free radicals in the body, and they play an important role in the whole defense strategy of animals (Ighodaro and Akinloye, 2019). Lysozyme can lyse gram-negative bacteria by catalyzing the hydrolysis of β(1–4)-linked disaccharides of N-acetylglucosamine and N-acetylmuramic acid in the bacterial cell wall. In addition, it also has prominent anti-inflammatory properties (Ogundele, 1998). The total serum protein contains albumin and total globulin, and these are collective indicators of enhanced immunity (Peters, 1985; Busher, 1990). In this study, CAT, SOD, LZM, and total serum protein were significantly upregulated post-injection, demonstrating that the non-specific immunity of groupers could be induced by GLPs.
This study demonstrated that GLPs can resist disease caused by V. harveyi. RPS is an important and relative straightforward indicator in disease resistance of immunostimulants. The results of our study showed that the RPS of groupers injected with GLPS at concentrations of 2, 4, 6, and 8 mg/ml was significantly higher by 53.3, 60.0, 70.0, and 73.3%, respectively, than 33.3% for the control. These results are consistent with those of GLPs resisting disease in P. fulvidraco (Bai et al., 2015), M. rosenbergii (Mohan et al., 2019), and E. coioides (Chang et al., 2013).
In conclusion, the effects of different concentrations of GLPs as immunostimulants were evaluated in pearl gentian grouper. The results indicate that an injection with GLPs at concentrations of 6 or 8 mg/ml could effectively enhance both the specific immunity and the non-specific immunity and disease resistance against V. harveyi of pearl gentian grouper. Therefore, GLPs have great potential as effective immunostimulants against V. harveyi.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All animal experiments were strictly executed according to ethical standards and approved by Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals Ethics Committee.
Experimental design: SC, JJ, and YH. Sampling: YZ. Analyses: YZ and JZ. Supervision: SC and JJ. Writing—original draft: YZ. Writing, review, and editing: SC, JJ, and YH. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Area Research and Development Program of Guangdong Province (no. 2021B0202040002) and Guangdong Natural Science Foundation (no. 2021A1515010532).
We would like to thank all teachers and schoolfellow for the skillful organization of sampling and technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ardó L., Yin G., Xu P., Váradi L., Szigeti G., Jeney Z., et al. (2008). Chinese Herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275 (1-4), 26–33. doi: 10.1016/j.aquaculture.2007.12.022
Bag M. R., Makesh M., Rajendran K. V., Mukherjee S. C. (2009). Characterization of IgM of Indian major carps and their cross-reactivity with anti-fish IgM antibodies. Fish Shellfish Immunol. 26 (2), 275–278. doi: 10.1016/j.fsi.2008.11.009
Bai D. Q., Xu H. L., Wu X., Zhai S. L., Yang G., Qiao X. T., et al. (2015). Effect of dietary Ganoderma lucidum polysaccharides (GLP) on cellular immune responses and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Israeli J. Aquaculture- Bamidgeh 67, 1–9. Available at: http://hdl.handle.net/10524/49207
Busher J. T. (1990). “Serum albumin and globulin,” in Clinical methods: The history, physical, and laboratory examinations. Eds. Walker H. K., Hall W. D., Hurst J. W. (Boston: Butterworths).
Causey D. R., Pohl M. A. N., Stead D. A., Martin S. A. M., Secombes C. J., Macqueen D. J. (2018). High-throughput proteomic profiling of the fish liver following bacterial infection. BMC Genomics 19 (1), 719. doi: 10.1186/s12864-018-5092-0
Chang C. S., Huang S. L., Chen S., Chen S. N. (2013). Innate immune responses and efficacy of using mushroom beta-glucan mixture (MBG) on orange-spotted grouper, Epinephelus coioides, aquaculture. Fish Shellfish Immunol. 35 (1), 115–125. doi: 10.1016/j.fsi.2013.04.004
Chaplin D. D. (2010). Overview of the immune response. J. Allergy Clin. Immunol. 125 (2 Suppl 2), S3–23. doi: 10.1016/j.jaci.2009.12.980
Chithra E., Padmanaban A. M., Mohan K. (2016). Potential use of Ganoderma lucidum polysaccharides as a feed supplement in diets on survival and growth performance of the grass carp, Ctenopharyngodon idella. Int. J. Fisheries Aquat. Stud. 4 (5), 328–333. doi: 10.1016/j.jep.2018.02.005
Dinarello C. A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117 (14), 3720–3732. doi: 10.1182/blood-2010-07-273417
Galina J., Yin G., Ardo L., Jeney Z. (2009). The use of immunostimulating herbs in fish. an overview of research. Fish Physiol. Biochem. 35 (4), 669–676. doi: 10.1007/s10695-009-9304-z
Geven E. J. W., Klaren P. H. M. (2017). The teleost head kidney: Integrating thyroid and immune signalling. Dev. Comp. Immunol. 66, 73–83. doi: 10.1016/j.dci.2016.06.025
Guo J., Yuan C., Huang M., Liu Y., Chen Y., Liu C., et al. (2018). Ganoderma lucidum-derived polysaccharide enhances coix oil-based microemulsion on stability and lung cancer-targeted therapy. Drug Deliv. 25 (1), 1802–1810. doi: 10.1080/10717544.2018.1516006
He L., He T., Farrar S., Ji L., Liu T., Ma X. (2017). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 44 (2), 532–553. doi: 10.1159/000485089
Holling T. M., Schooten E., Elsen P. J. (2004). Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 65 (4), 282–290. doi: 10.1016/j.humimm.2004.01.005
Ighodaro O. M., Akinloye O. A. (2019). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54 (4), 287–293. doi: 10.1016/j.ajme.2017.09.001
Liu Y. J., Du J. L., Cao L. P., Jia R., Shen Y. J., Zhao C. Y. (2015). Anti-inflammatory and hepatoprotective effects of ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio l.). Int. Immunopharmacology 25 (1), 112–120. doi: 10.1016/j.intimp.2015.01.023
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Loh J. Y., Chan H. K., Yam H. C., In L. L. A., Lim C. S. Y. (2020). An overview of the immunomodulatory effects exerted by probiotics and prebiotics in grouper fish. Aquaculture Int. 28, 729–750. doi: 10.1007/s10499-019-00491-2
Mohan K., Karthick Rajan D., Muralisankar T., Ramu Ganesan A., Marimuthu K., Sathishkumar P. (2022). The potential role of medicinal mushrooms as prebiotics in aquaculture: A review. Rev. Aquaculture 14 (3), 1300–1332. doi: 10.1111/raq.12651
Mohan K., Muralisankar T., Uthayakumar V., Chandirasekar R., Karthick Rajan D. (2019). Dietary Ganoderma lucidum polysaccharides to enhance the growth, immune response and disease resistance of freshwater prawn Macrobrachium rosenbergii. Aquaculture Rep 14, 100203. doi: 10.1016/j.aqrep.2019.100203
Ogundele M. O. (1998). A novel anti-inflammatory activity of lysozyme: modulation of serum complement activation. Mediators Inflammation 7 (5), 363–365. doi: 10.1080/09629359890893
Pan T., Yan M. (2019). The screening of traditional Chinese herbs on nonspecific immune response and protection of pacific white shrimp (Litopenaeus vannamei) from Vibrio harveyi infection. Aquaculture Int. 28 (2), 767–776. doi: 10.1007/s10499-019-00493-0
Pan T., Yan M., Chen S., Yin X. (2013). Effects of ten traditional Chinese herbs on immune response and disease resistance of sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae). Acta Ichthyologica Et Piscatoria 43 (1), 41–49. doi: 10.3750/aip2013.43.1.06
Samad A. P., Santoso U., Lee M. C., Nan F. H. (2014). Effects of dietary katuk (Sauropus androgynus l. merr.) on growth, non-specific immune and diseases resistance against Vibrio alginolyticus infection in grouper epinephelus coioides. Fish Shellfish Immunol. 36 (2), 582–589. doi: 10.1016/j.fsi.2013.11.011
Soto-Rodriguez S. A., Gomez-Gil B., Lozano R. (2010). 'Bright-red' syndrome in pacific white shrimp Litopenaeus vannamei is caused by Vibrio harveyi. Dis. Aquat. Organisms 92 (1), 11–19. doi: 10.3354/dao02274
Wang E., Chen X., Wang K., Wang J., Chen D., Geng Y., et al. (2016). Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol 59, 196–202. doi: 10.1016/j.fsi.2016.10.039
Weber A., Wasiliew P., Kracht M. (2010). Interleukin-1 (IL-1) pathway. Sci. Signaling 3 (105), cm1. doi: 10.1126/scisignal.3105cm1
Wei G., Cai S., Wu Y., Ma S., Huang Y. (2020a). Immune effect of Vibrio harveyi formalin-killed cells vaccine combined with chitosan oligosaccharide and astragalus polysaccharides in ♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus. Fish Shellfish Immunol. 98, 186–192. doi: 10.1016/j.fsi.2020.01.015
Wei G., Tan H., Ma S., Sun G., Zhang Y., Wu Y., et al. (2020b). Protective effects of β-glucan as adjuvant combined inactivated Vibrio harveyi vaccine in pearl gentian grouper. Fish Shellfish Immunol. 106, 1025–1030. doi: 10.1016/j.fsi.2020.09.027
Wu K., Na K., Chen D., Wang Y., Pan H., Wang X. (2018). Effects of non-steroidal anti-inflammatory drug-activated gene-1 on Ganoderma lucidum polysaccharides-induced apoptosis of human prostate cancer PC-3 cells. Int. J. Oncol. 53 (6), 2356–2368. doi: 10.3892/ijo.2018.4578
Xiong X., Wang C., Ye M., Yang T., Peng X., Li H. (2010). Differentially expressed outer membrane proteins of Vibrio alginolyticus in response to six types of antibiotics. Mar. Biotechnol. (New York N.Y.) 12 (6), 686–695. doi: 10.1007/s10126-009-9256-4
Xu Z., Chen X., Zhong Z., Chen L., Wang Y. (2011). Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 39 (1), 15–27. doi: 10.1142/S0192415X11008610
Yang A., Li W., Tao Z., Ye H., Xu Z., Li Y., et al. (2021). Vibrio harveyi isolated from marine aquaculture species in eastern China and virulence to the large yellow croaker (Larimichthys crocea). J. Appl. Microbiol. 131 (4), 1710–1721. doi: 10.1111/jam.15070
Yii K. C., Yang T. I., Lee K. K. (1997). Isolation and characterization of Vibrio carchariae, a causative agent of gastroenteritis in the groupers, Epinephelus coioides. Curr. Microbiol. 35 (2), 109–115. doi: 10.1007/s002849900221
Yin G., Ardo L., Thompson K. D., Adams A., Jeney Z., Jeney G. (2009). Chinese Herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol. 26 (1), 140–145. doi: 10.1016/j.fsi.2008.08.015
Zapata A., Amemiya C. T. (2000). Phylogeny of lower vertebrates and their immunological structures. Curr. Topics Microbiol. Immunol. 248, 67–107. doi: 10.1007/978-3-642-59674-2_5
Zapata A., Diez B., Cejalvo T., Gutierrez-de Frias C., Cortes A. (2006). Ontogeny of the immune system of fish. Fish Shellfish Immunol. 20 (2), 126–136. doi: 10.1016/j.fsi.2004.09.005
Zhang Y., Tan H., Wei G., Huang Y., Jian J., Cai S. (2020). The effect of chitosan oligosaccharide as an immune enhancer against Vibrio harveyi in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Aqua Res. 52 (2), 541–546. doi: 10.1111/are.14912
Zhang J., Tang Q., Zhou C., Jia W., Da Silva L., Nguyen L. D., et al. (2010). GLIS, a bioactive proteoglycan fraction from ganoderma lucidum, displays anti-tumour activity by increasing both humoral and cellular immune response. Life Sci. 87 (19-22), 628–637. doi: 10.1016/j.lfs.2010.09.026
Zhu X. L., Chen A. F., Lin Z. B. (2007). Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacology 111 (2), 219–226. doi: 10.1016/j.jep.2006.11.013
Zhu Z. M., Dong C. F., Weng S. P., He J. G. (2018). The high prevalence of pathogenic Vibrio harveyi with multiple antibiotic resistance in scale drop and muscle necrosis disease of the hybrid grouper, Epinephelus fuscoguttatus (♀) × E. lanceolatus (♂), in China. J. Fish Dis. 41 (4), 589–601. doi: 10.1111/jfd.12758
Zhu J., Xu J., Jiang L. L., Huang J. Q., Yan J. Y., Chen Y. W., et al. (2019). Improved antitumor activity of cisplatin combined with Ganoderma lucidum polysaccharides in U14 cervical carcinoma-bearing mice. Kaohsiung J. Med. Sci. 35 (4), 222–229. doi: 10.1002/kjm2.12020
Keywords: Vibrio harveyi, Ganoderma lucidum polysaccharides, immune enhancer, pearl gentian grouper, intraperitoneal injection
Citation: Zhang Y, Zhong J, Huang Y, Jian J and Cai S (2022) Effect of Ganoderma lucidum polysaccharides as immunostimulants against Vibrio harveyi in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Front. Mar. Sci. 9:968838. doi: 10.3389/fmars.2022.968838
Received: 14 June 2022; Accepted: 24 August 2022;
Published: 23 September 2022.
Edited by:
Seyyed Morteza Hoseini, Iranian Fisheries Science Research Institute (IFSRI), IranReviewed by:
Baoliang Liu, Yellow Sea Fisheries Research Institute (CAFS), ChinaCopyright © 2022 Zhang, Zhong, Huang, Jian and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuanghu Cai, Y2Fpc2hAZ2RvdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.