
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 12 September 2022
Sec. Marine Biogeochemistry
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.968647
This article is part of the Research Topic Chemical and Biogeochemical Processes at Methane and Other Cold Seeps View all 15 articles
With more natural gas hydrate samples recovered and more research approaches applied to hydrate-associated gas studies, data concerning the geochemical characteristics of hydrate-associated gases have been increased significantly in the past decades. Although systematic reviews of hydrocarbons are available, fewer studies have focused on the systematic classification of gas hydrates, yet. In this study, the primary origins and secondary processes that affect the geochemical characteristics of the gases are discussed. The primary origins are affected mainly by the type and /or maturity of the organic matter, which determine the main signature of the gas is microbial gas or thermogenic gas in a broad scheme. Apart from primary origins, secondary processes after gas generation such as migration, mixing, biodegradation and oxidation occur during the migration and/or storage of gases can significantly alter their primary features. Traditional methods such as stable isotope and molecular ratios are basic proxies, which have been widely adopted to identify these primary origins and secondary processes. Isotopic compositions of C2+ gases have been employed to identify the precursor of the gases or source rocks in recent years. Data from novel techniques such as methane clumped isotope and noble gases bring additional insights into the gas origins and sources by providing information about the formation temperature of methane or proxies of mantle contribution. A combination of these multiple geochemical approaches can help to elucidate an accurate delineation of the generation and accumulation processes of gases in a gas hydrate reservoir.
Natural gas hydrates are crystalline compounds composed of water and gases formed under high pressure and low temperature, mainly occurring in permafrost and continental slope sediment (Kvenvolden, 1988; Dickens et al, 1995; Sloan and Sloan, 1998; Buffett and Archer, 2004). As a potential energy resource and an important part of the global carbon cycle, natural gas hydrate has been investigated intensively in recent years [e.g., (Collett et al., 2019; Ye et al., 2019; Li et al., 2021; Zhang et al., 2021b)].
As the gigantic reserve of natural gas hydrate makes it a key part of the carbon cycle with a huge potential environmental effect, understanding the origins of gases can help to constrain the global methane fluxes and potential global climate effects (Nisbet et al., 2014; Schaefer et al., 2016; Schwietzke et al., 2016). Moreover, as an unconventional energy resource, detailed composition information about gas associated with natural gas hydrate can benefit resource evaluation and optimized site selection for production test and prospective commercial exploitation (Li et al., 2018; Ye et al., 2018; Jin et al., 2020; Liang et al., 2022). Furthermore, previous studies indicate that different gas components influence the temperature-pressure phase equilibrium curve of gas hydrate and further affect the thickness of gas hydrate stability zone (GHSZ) (Tréhu AM et al., 2006; Sloan et al., 2010). Hydrates containing heavy hydrocarbon gas may be more thermodynamically stable, so that molecular composition may provide extra clues for hydrate distribution (Xiao et al., 2019).
Compare to the oil and gas which containing long-chain hydrocarbons and biomarkers , the gases associated with hydrate are with relatively simple composition (Kvenvolden, 1988; Dickens et al., 1995; Kvenvolden, 1995; Sloan and Sloan, 1998; Buffett and Archer, 2004). Therefore, the techniques can be used to infer the generation and evolution of the gases are quite limited in the earlier stage of hydrate-associated research, mainly relying on the stable isotope and molecular ratios, thus provide limited information for delineating the definite origins and sources (Bernard et al., 1977; Schoell, 1980; Whiticar, 1999; Milkov and Etiope, 2018). Fortunately, with the increase of energy demand and the need for energy transformation, more attention has been paid to the gas hydrate attribute to its clean signature with huge reserves. More hydrate samples have been obtained in recent years [e.g. (Rodrigues et al., 2019; Ye et al., 2019; Zhang et al., 2019; Lai et al., 2021a)], and several test mining of hydrate area has been implemented [e.g. (Lorenson et al., 2011; Stern et al., 2011; Kida et al., 2015; Ye et al., 2018; Liang et al., 2022)]. Furthermore, thanks to the improvement of mass spectrometry resolution and the deeper understanding of the mechanism of isotope fractionation (Eiler, 2013; Ono et al., 2014; Young et al., 2017; Dong et al, 2020), new techniques such as clumped isotope have been gradually applied to the gas geochemistry research of hydrate (Figure 1), providing valuable information for the formation temperature and kinetic secondary processes of methane (Wang et al, 2015a; Ijiri et al, 2018; Giunta et al., 2021; Zhang et al., 2021a; Lalk et al., 2022). Noble gases, which require higher quality testing methods, have also been used in recent years to study the contribution of deep mantle sources to hydrate reservoirs (Figure 1) (Ruffine et al., 2018; Moore et al., 2020; Snyder et al., 2020). Abundant samples combined with the multi-approaches make it possible to accumulate systematic and valuable data about geochemical characteristics of hydrate-associated gases for a specific area (Figure 1). However, these case studies are required further systematic discussion and summary to excavate more profound insights and provide more uniform criterions and references for future study.
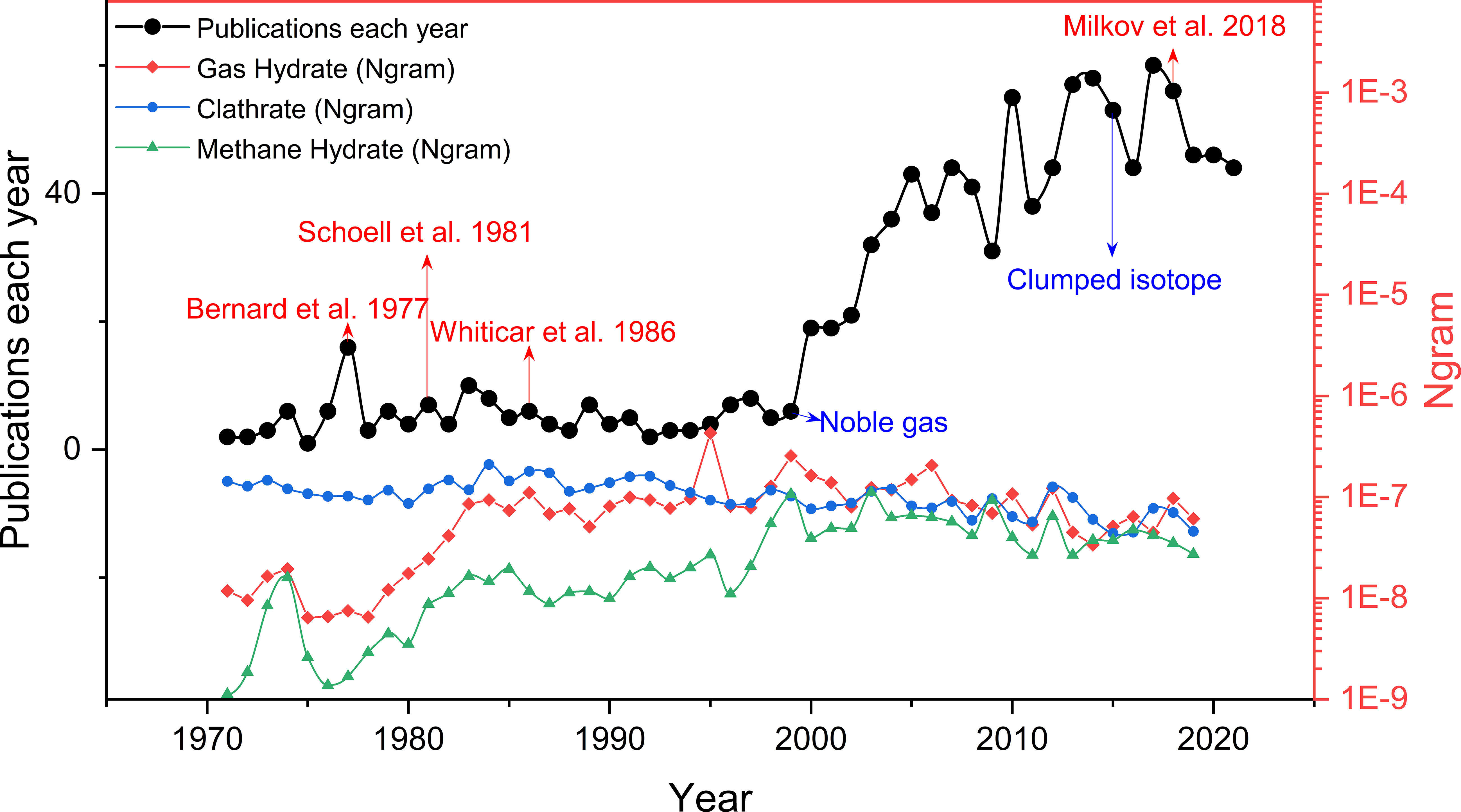
Figure 1 The line of black solid dot represents the number of research articles about gas geochemistry (molecular and/or isotopic composition data) of natural gas hydrate published each year from 1970 to 2021 (data from web of science), modified from Milkov and Etiope (2018). Note that data for about 87% of all samples were published after the genetic diagrams of Bernard et al. (1977); Schoell (1983); Whiticar et al. (1986) and Milkov and Etiope (2018)were proposed. Three line of colored dot represents how those phrases (gas hydrate, clathrate and methane hydrate) have occurred in a corpus of books over the selected years (1970-2019) (data from Google N-gram viewer, (Michel et al., 2011)). We can see that use of “gas hydrate” and “methane hydrate” started to rise in 1980s, match to the publication of the genetic diagrams, while the frequency of the term "clathrate" declined steadily from the 1970s.
In this study, the geochemical data of gases associated with hydrate (including hydrate-bound gas, void gas, headspace gas and venting gas, etc.) published in recent years are compiled, and the effect of processes from the primary generation to the later accumulation of hydrate-associated gas are summarized, the methods of gas source and origin identification in recent years are discussed, to provide profound insights into the understanding of the formation mechanism and accumulation history of gas hydrate system.
The existence of natural gas hydrate requires suitable temperature and pressure conditions, which make them mainly occurring in certain interval (GHSZ) of permafrost and marine sediment. Many factors such as gas composition, pressure gradient, geothermal gradient, and salinity affect gas hydrate stability conditions in nature. The GHSZ profiles for marine setting and permafrost environment are different (Figures 2A, B). The top and bottom of the GHSZ in these two conditions are defined by the intersection of the geothermal gradient (and/or hydrothermal gradient) with the hydrate phase boundary curve.
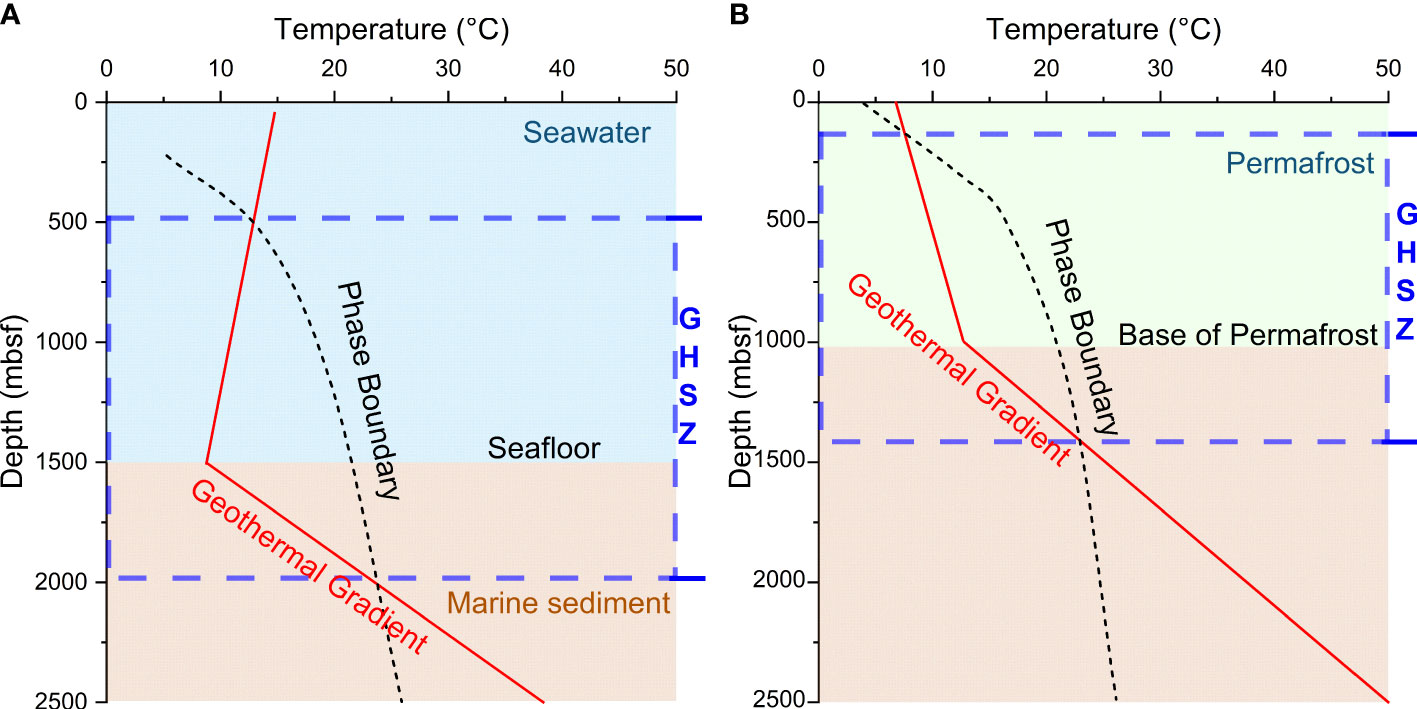
Figure 2 Gas hydrate stability zone (GHSZ) for gas hydrates in (A) marine environment and (B) permafrost environment, modified from (Chen and Merey, 2021).
The dataset of this study came from about 72 studies with more than 1,300 gas samples around the world (Figure 3). Of these, 133 are derived from terrestrial permafrost regions, 114 from terrestrial freshwater lakes (Lake Baikal), and the rest from marine sediments of continental margin. Most of the samples are hydrate-bound gas, void gas, headspace gas or venting gas which associated with natural gas hydrate. Almost all published isotope data of hydrate-associated gases have been compiled in Figure 4. The heaviest δ13C-C1 is found at -22.5‰ in Mountain Qilian (Wang et al., 2015b) and the lightest at -102.2‰ in Amazon Fan from the distribution plot (Rodrigues et al., 2019), with an average of -61.67‰ (Figure 4A). A total of 1193 δ13C-C1 data from 46 area were counted to estimate the δ13C-C1 range of gases associated with natural gas hydrate. The highest frequency range of δ13C-C1 appears in -70~-60‰, especially -70~ -65‰ in the frequency histogram of the δ13C-C1 (Figure 4B), which may indicate that the microbial gas is quantitatively dominant in the current dataset. Samples from permafrost are with higher δ13C-C1 (-55~ -30‰) compared to those from freshwater setting (-70~ 55‰), while samples from marine sediments are with widest distribution of δ13C-C1 range from -102‰ to -30‰. δD-C1 ranges from most enriched at -115‰ in the Gulf of Mexico (Sassen et al., 2001) to most depleted at -326.3‰ in Lake Baikal (Hachikubo et al., 2010), with an average value of -209.75‰ (Figure 4C). A total of 744 δD-C1 data from same area with δ13C-C1 data were counted to estimate the δD-C1 range of gases associated with natural gas hydrate, the high-frequency range is distributed in -200 to -175‰ (Figure 4D). There is also a small high frequency band of δD-C1 around -300‰, which represent the hydrogen isotope signature of methane generated from acetate fermentation in freshwater setting. Similar to the δ13C-C1, the distribution of samples from different environment are with distinct dominated frequency interval in the frequency histogram, which manifest as samples from freshwater are with lowest δD-C1, followed by permafrost regions, and finally marine sediment, which can be ascribed to the different sources of hydrogen in methane.
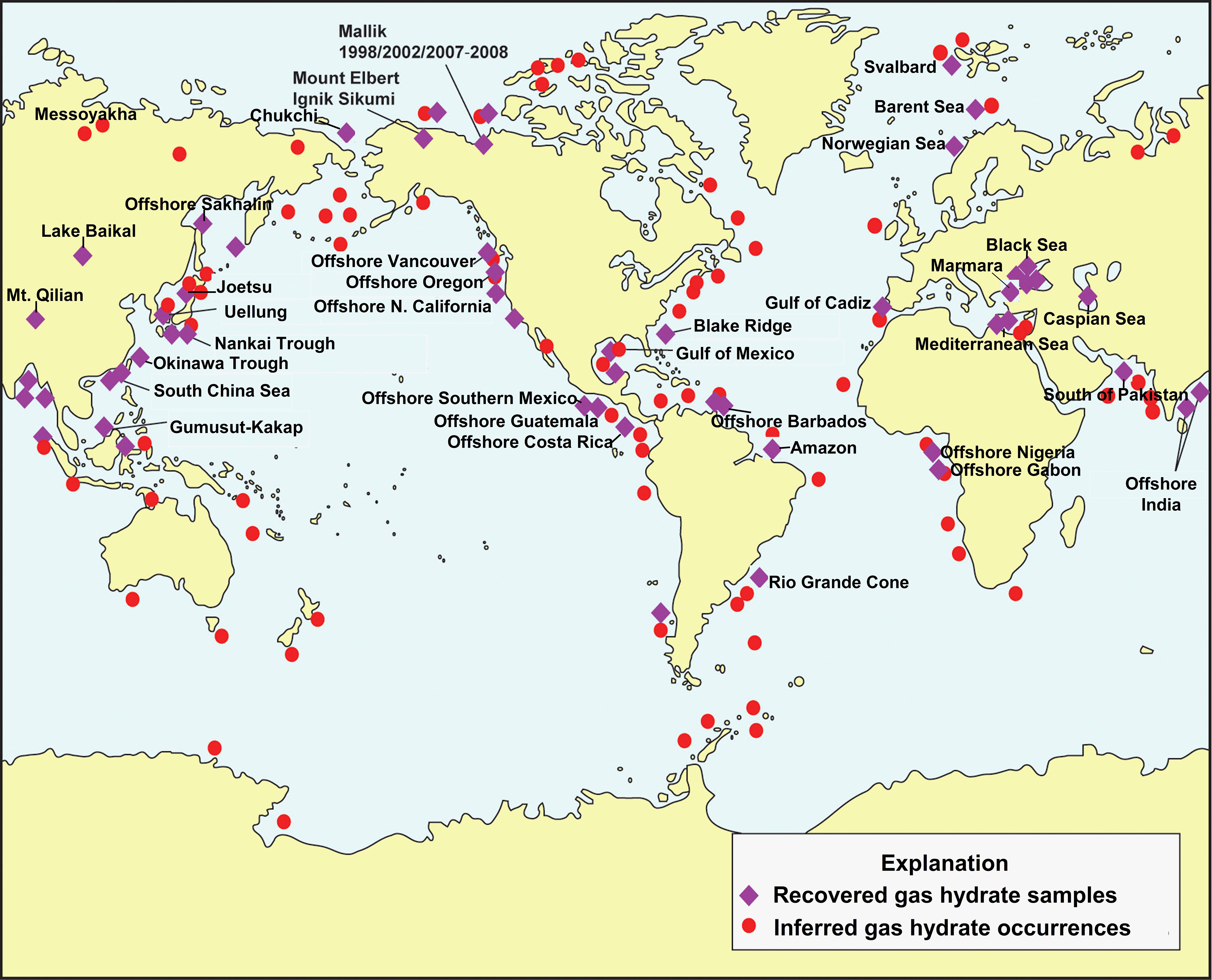
Figure 3 Location of sampled and inferred methane hydrate occurrences in marine sediment of outer continental margins and permafrost regions, modified from (Collett et al., 2009). Data of samples adopted in this study are highlighted with site name on this map.
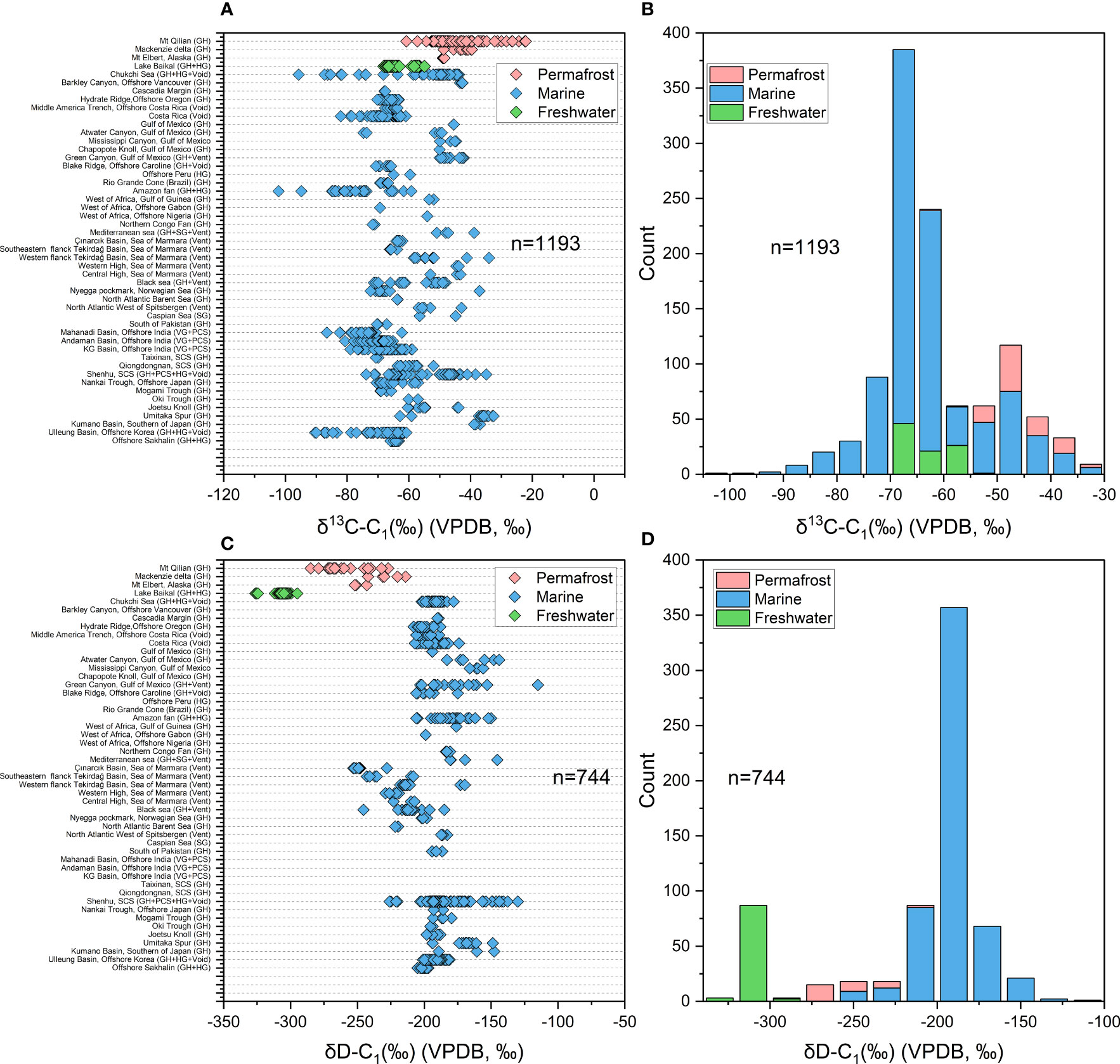
Figure 4 (A) The distribution of δ13C-C1 of hydrate-associated gas from different area around the world. (B) The frequency histogram of δ13C-C1 of hydrate-associated gas counted from 1193 data. (C) The distribution of δD-C1 of hydrate-associated gas from different area. (D) The frequency histogram of δD-C1 of hydrate-associated gas counted from 744 data. GH, Gas Hydrate dissociated gases; Vent, Venting Gases; Void, Void gases; HG, Headspace gases; PCS, gas from Pressure coring systems (PCS).
Continental gas hydrate, has been identified in Messoyakha field of western Siberia, Alaska, Mackenzie delta and Qinghai-Tibet plateau [e.g., (Lorenson et al., 2011; Wang et al., 2018)], which are considered to be a major environmental concern on a number of levels since there are no methane-barrier from marine sediment and seawater compared to marine gas hydrate. As knowledge of permafrost-associated gas hydrates has grown, it has become clear that many permafrost-associated gas hydrates are inextricably linked to an associated conventional petroleum system, and that their formation history (trapping of migrated gas in situ during Pleistocene cooling) is consistent with having been sourced at least partially in nearby thermogenic gas deposits. It can also be found that methane from continental gas hydrate is more enriched in 13C and more depleted in D compared to that from marine settings (Figures 4A, C), which may ascribe to the mixing of microbial methane generated via acetate fermentation (Wang et al., 2018).
The brief description of published geochemical characteristics of hydrate-associated gases around the world are summarized in Table S1. It can be found that hydrate with microbial origin formed by the typical acetate fermentation pathway has been identified in Lake Baikal (Figures 5A, C) (Kida et al., 2006; Hachikubo et al., 2010). The hydrates formed by the typical CO2 reduction pathway occur in India, Blake Ridge, Black Sea, offshore northern California, Nankai Trough, offshore Oregon, Okhotsk Sea, and Ulleung Basin, etc. (Figures 5A–C) (Brooks et al., 1991; Ginsburg and Soloviev, 1997; Waseda and Uchida, 2004; Collett et al., 2008; Choi et al., 2013; Collett et al., 2019). It had been considered that most of the gases contained in natural gas hydrates mainly derived from microbial sources in the early stage of hydrate investigation, and most of the previous gas-hydrate assessments had been carried out based on that natural hydrate was formed from microbial gas. However, more and more thermogenic gas hydrates have also been recovered in later studies, for example from Caspian Sea (Lüdmann and Wong, 2003), Cascadia Margin (Pohlman et al., 2005), Gulf of Mexico (Sassena et al., 1999; Sassena et al., 2001a; Sassena et al., 2001b), Svalbard (Smith et al., 2014), west African province (De Prunel et al., 2017), NW Borneo region of the South China Sea (SCS) (Paganoni et al., 2016; Paganoni et al., 2018), Qiongdongnan of SCS (Ye et al., 2019), Shenhu of SCS (Zhang et al., 2019) and sea of Marmara (Ruffine et al., 2018), etc (Figures 5A–C).
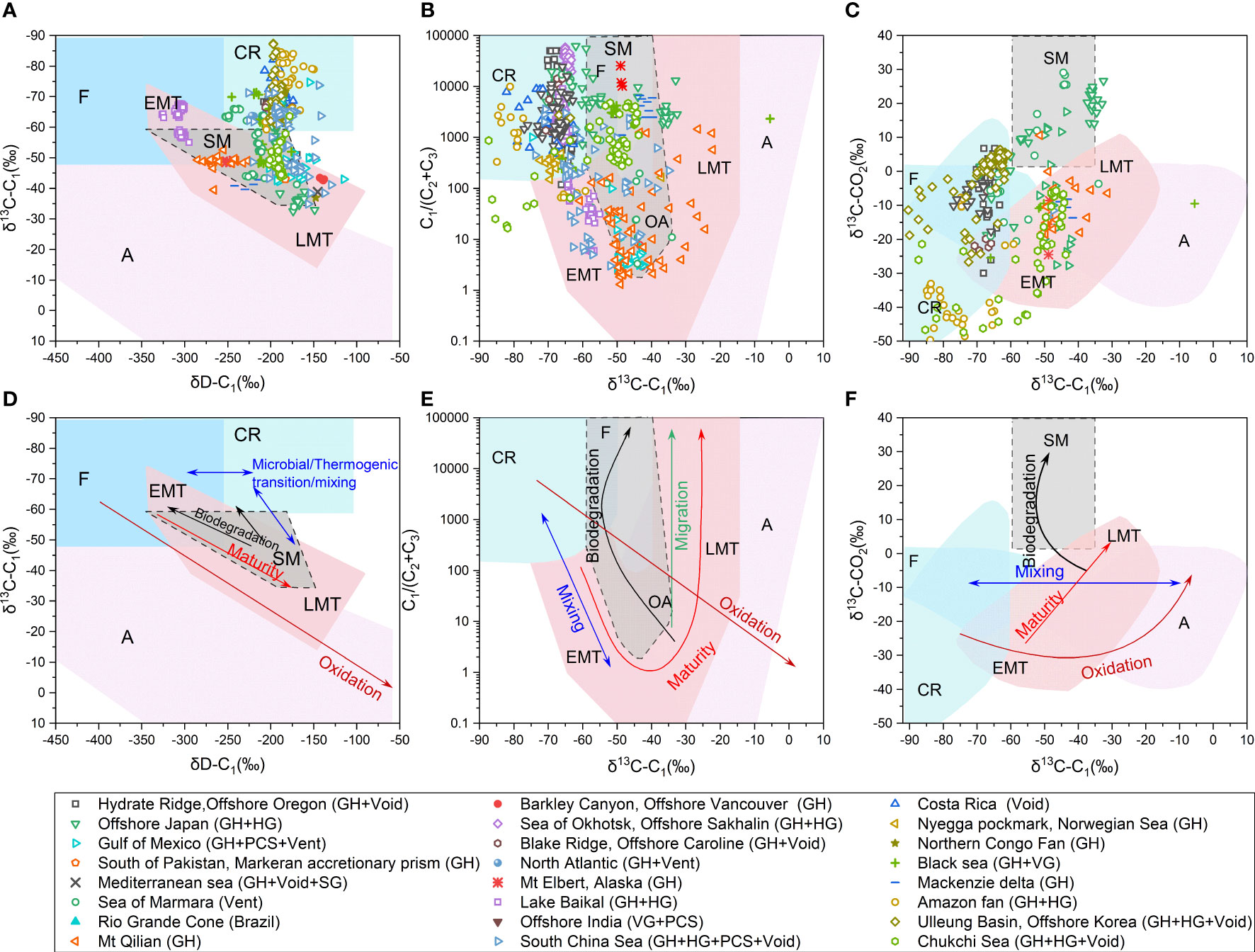
Figure 5 Genetic diagram of δ13C-C1 versus δD-C1 (A); δ13C-C1 vs. C1/(C2 +C3) (B); δ13C-C1 vs. δ13C-CO2 (C) with dataset plotted in. Genetic diagram of δ13C-C1 versus δD-C1 (D); δ13C-C1 vs. C1/(C2 +C3) (E); δ13C-C1 vs. δ13C-CO2 (F) with secondary alteration processes (migration, mixing, biodegradation, oxidation and maturity) plotted, modified from (Milkov and Etiope, 2018). EMT-early mature thermogenic; OA-oil associated; LMT-late mature thermogenic; SM-secondary microbial; A-abiotic; F-fermentation; CR-CO2 reduction.
It can also be seen that with the increase of samples obtained and the diversification of research methods (such as the isotopic composition of C2+, clumped isotopes and noble gases), the understanding of the origin of hydrate-associated gases in a certain region has gradually deepened. For example, the Shenhu area of the SCS, of which the hydrate-associated gas was originally thought to be pure microbial gas (Fu and Lu, 2010; Wu et al., 2011; Liu et al., 2015; Dai et al., 2017), has been gradually realized that it is with structurally controlled mixed gas sources with considerable thermogenic contribution in recent years, and there is a paragenetic relationship between the hydrate-associated gas and surrounding conventional oil and gas reservoirs (Zhang et al., 2019; Lai et al., 2022; Liang et al., 2022). New subtype of gases, such as secondary microbial gases generated by hydrocarbon biodegradation, has been widely recognized in more areas with the deepening of the understanding of the gas generation (Figures 5A–C) [e.g., (Milkov, 2018; Ruffine et al., 2018; Lai et al., 2022; Li et al., 2022)].
Gases bound in hydrate usually formed by biodegradation of organic matter, which includes bacterial gas (or called microbial gas) produced by bacterial processes and thermogenic gas formed through thermochemical reactions (Schoell, 1983). The characteristics of biogenic gas are controlled by the type and thermal maturity of organic matter, i.e. kerogen type, burial history and geothermal gradient (Tissot et al., 1974; Tissot and Welte, 1984; Whiticar, 1994).
The origin material of natural gas is divided into sapropelic type, which are mainly type I/II kerogen and dominated by marine source organic matter, and humic type which are mainly type III kerogen and dominated by terrestrial organic matter (Van Krevelen, 1961). The natural gas generated by sapropelic organic matter is named oil-type gas, and that by humic organic matter is named as coal-type gas. Study of organic matter in hydrate-bearing sediment of Okinawa trough suggested that terrestrial organic matter is more prone to the formation of microbial gases as compared with marine organic matter (Saito and Suzuki, 2007). However, Dai et al. (2017) proposed that most of the gas-forming hydrates are oil-type gas from marine organic matter, and coal-type gas has only been reported in Qilian Mountain and offshore Vancouver island (Wang, 2010; Cao et al., 2012).
The thermal maturation stages of organic matter are labelled (1) Immature (diagenesis), the initial thermal mature stage where microbial gas is dominated; (2) Mature (catagenesis), the intermediate stage where oil and methane are generated from kerogen decomposition; (3) Post-mature (metagenesis), the final stage where almost pure methane (dry gas) formed mainly from thermal cracking of oil and bitumen (Tissot and Welte, 1984; Wiese and Kvenvolden, 1993). Therefore, the natural gas component changes from microbial dry gas to thermogenic wet gas and then to thermogenic dry gas in the immature, mature and over-mature stage of organic matter evolution (Tissot and Welte, 1984; Wiese and Kvenvolden, 1993).
After being generated in source rocks, natural gases may experience a series of secondary processes, such as mixing, migration, oxidation and biodegradation, etc., before the formation of reservoir. The primary geochemical characteristics of the gases can be obscured by these secondary processes, resulting in isotopic and molecular compositional fractionation. As a result, it is necessary to identify these secondary processes and their effects, to properly interpret the origins and sources of the gases.
Microbial methane produced in the GHSZ alone is not sufficient for the accumulation of concentrated gas hydrate in most cases. Most of the allochthonous gas in the GHSZ might have been migrated from the deeper sediments, mainly involving three kinds of processes: (1) diffusion; (2) migration of water-dissolved gas; (3) buoyancy of free gas. Diffusion is quite slow, unlikely to bring up sufficient gas to form highly saturated hydrate reservoirs in most cases (Xu and Ruppel, 1999). However, the latter two are relatively efficient processes.
Diffusion-associated fractionations of isotope and molecular composition of hydrocarbons are expected to occur as a function of mass and are thought to behave “chromatographically”, by which lighter isotopes and hydrocarbons move more quickly than their heavier counterparts (Thompson, 1979; Leythaeuser et al., 1982; Choi et al., 2013). It is also found that 12C-12C bond is more prone to breakage than 12C-13C bond and the compounds with heavy carbon isotope 13C is more easily absorbed by rocks, minerals and organic matters (Chanton, 2005). As a result, 13C-CH4 and D-CH4 for a diffused gas are depleted relative to its source, while C1/(C2+C3) is relatively increased (Coleman et al., 1977; Prinzhofer and Pernaton, 1997; Zhang and Krooss, 2001; Schloemer and Krooss, 2004). Coleman et al. (1977) validated the effect of diffusion on the molecular composition of gases and proposed that C2+ hydrocarbons can be entirely stripped off from the migrated gases. It is proposed that the migration via diffusion can cause measurable carbon isotope fractionation of more than 5‰ (Chen, 1994; Prinzhofer and Pernaton, 1997). Both simulation and experimental results reveal that the fractionation by diffusion is affected by TOC content and porosity and permeability of rock, the migration pathway and the type of migrating gas, etc. (Galimov, 1967; Craig, 1968; Gunter and Gleason, 1971; Stahl, 1977; Chen, 1994; Zhang and Krooss, 2001; Li et al., 2003).
There are still debates on whether isotopic fractionation occurs or not in the process of natural gas migration other than diffusion, although most studies suggest that there is no isotope fractionation during migration (Stahl and Carey, 1975; Coleman et al., 1977; Fuex, 1980; Faber and Stahl, 1984; Zhang and Krooss, 2001). The isotopic compositions of natural gases, recovered from different depths of the same well shows no obvious difference (Stahl and Carey, 1975; Coleman et al., 1977; Faber and Stahl, 1984). Schoell proposed that the migration of natural gas would not cause the change in δ13C-CH4, insteadly the isotopic compositions of natural gases are controlled by the type and thermal evolution of original organic matter (Schoell, 1983; Schoell, 1984). Experiment and numerical simulation conducted by Fuex (1980) showed that migration fractionation of methane was almost negligible, and the most likely cause of this insignificant fractionation was the difference in water solubility between 12CH4 and 13CH4, and in most cases such fractionation would not exceed 1-permil. It is also suggested that depletion in methane carbon isotope is caused by bacterial activities rather than by migration (Fuex, 1980; Faber and Stahl, 1984). Based on the discussions above, migration is expected to cause thermogenic gas zone to shift upward in the C1/(C2+C3) versus δ13C-CH4 diagram, corresponding to an increase in C1/(C2+C3) ratio but no significant change in δ13C-CH4 (Figure 5E) (Bernard et al., 1977).
Mixing of natural gases, which can be from the identical source rock at varying maturity stages or different source rocks, and biogenic and abiogenic origins, is a common phenomenon (Whiticar, 1994). The mixing of microbial and thermogenic gas has been well recognized in gas hydrates in Shenhu area of South China Sea (SCS) (Zhang et al., 2019), Norwegian Sea (Vaular et al., 2010) and Japan Sea (Waseda and Iwano, 2008). In recent years, the mixing of thermogenic gas with secondary biodegraded gas has also been identified in areas such as Western High of Sea of Marmara (Ruffine et al., 2018).
The molecular and isotopic composition of natural gas are adopted to identify mixing and to determine the composition and contribution of each gas-endmember (Schoell, 1983; Chung et al., 1988; Whiticar, 1994; Prinzhofer and Huc, 1995). As shown in Figure 3A, mixing of microbial and thermogenic gas can be identified by the carbon isotope of methane (δ13C-C1) and the dry coefficient of the gas (C1/(C2+C3)) (Figure 5E) (Bernard et al., 1977), and a linear relationship between the two endmembers has been recognized, changing strictly with the mixing ratio in the δ13C-C1 versus δD-C1 diagram (Figure 5D). Prinzhofer and Pernaton (1997) proposed that the mixing of two gas endmembers result in a straight line in the plot of C2/C1 versus δ13C and an exponential line in the plot of log (C2/C1) versus δ13C. Prinzhofer et al. (2000) indicated that in any plot where the ratio of two numerators with a common denominator (for example, δ13C-C1, δ13C-C2 and δ13C-C3) is drawn, the mixing between the two end-members is on a straight line, and the linear relationship becomes more pronounced when isotope of C2+ gases is applied. Chung et al. (1988) proposed “natural gas plot” based on the semi-linear relationships of the carbon isotopic compositions of n-alkanes in pure thermogenic gases, which can help to estimate the relative contribution of microbial gas or thermogenic gas in a two end-member mixing model, after the δ13C of thermogenic methane is obtained by extrapolating the line of isotopic values of C2+ gases. This method has been widely used in hydrate gas studies [e.g., (Hachikubo et al., 2015; Lai et al., 2021a)].
The gas hydrate system has always been in a dynamic equilibrium state and is extremely sensitive to environmental conditions, especially temperature and pressure conditions change, driving forces leading to its dissociation have been primarily ascribed to event such as pressure reduction (e.g., (Teichert et al., 2003, Watanabe et al., 2008), temperature rise [e.g. (Cremiere et al., 2016, Kennett et al., 2000, Phrampus and Hornbach, 2012)], salinity changes (Riboulot et al., 2018) and glacial-interglacial transition (Chen et al., 2019, Deng et al., 2021). Isotopic and molecular fractionation may occur during the hydrate formation, dissolution and dissociation, so it is necessary to clarify whether the isotope fractionation is caused by hydrate crystallization itself. Laboratory studies by Hachikubo et al (2008) found that the δD of hydrate-bound gases is several permil depleted than that of residual gas, while there was no significant difference in δ13C. Nevertheless, the difference in δD is not so significant that affects the determination of gas origin. The effect of temperature on isotopic fractionation was also studied, and it was found that the fractionation was more evident with temperature decreasing (Hachikubo et al (2008), which was confirmed by Kimura et al. (2021). Experimental study from Luzi et al (2011a) has shown that hydrate formation has no significant effect on the carbon isotope ratios for CH4 hydrate, whereas a noticeable δ13C depletion was observed with CO2 hydrates compared to the gas phase. Similar isotope depletion in hydrate phase was observed by Kimura et al. (2021). However, contrary experimental results from Chen et al. (2018) indicates that heavy isotopes tend to preferentially enter the hydrate phase during the formation of CO2 hydrate and the carbon isotope fractionation is less intensive than that of oxygen and hydrogen. A comparison of stable isotope ratios of hydrate-bound and sediment gases, which were collected at the same depth of the same core from Lake Baikal, was performed, revealing that the δ13C-CH4 and δD-CH4 of the hydrate-bound gas were 1-2‰ and 5‰ smaller than the sediment gas, respectively (Kimura et al., 2020). The hydrogen isotope fractionation was in good agreement with the prediction from the experimental results of synthetic methane hydrate (Hachikubo et al., 2008), while the reason for the isotopic fractionation in 13C was still unknown. Lin and Zeng (2010) discovered that the molecular compositions are differentiated during hydrate formation, methane content decreases in hydrate phase as compared with wet gases (C2+) due to the difference in combination ability with water.
Lapham et al. (2012) experimentally studied the possible fractionation during hydrate dissolution or decomposition (two different physical processes), and it was confirmed that there is no isotope fractionation during both processes. Lai et al. (2021b) conducted a step-wise depressurization experiment with hydrate-bearing sediment to study the possible changes in the molecular and isotopic composition of gases released in depressurization process, indicating that no significant change in carbon isotopic composition while heavy hydrocarbons released mainly in the later stage of hydrate dissociation.
When migrating upward into the shallow sediments, thermogenic gases containing certain amount of C2+ components might experience biodegradation with the involvement of microorganisms, and their original characteristics would be changed while yielding secondary methane (Head et al., 2003; Jones et al., 2008; Knittel and Boetius, 2009; Jones et al., 2010; Gao et al., 2013; Mesle et al., 2013; Schlegel et al., 2013). As microorganisms preferentially consume C2+ gases and produce secondary microbial C1 as the terminal product, C1 will be gradually accumulated and the ratio of C1/(C2+C3) will be increased (Figure 5D) (Zeikus, 1977; Larter et al., 2005; Boreham and Edwards, 2008). Generally, methane generated from hydrocarbon biodegradation is relatively enriched in 13C compared to methane from primary methanogenesis (Valentine et al., 2004; Milkov and Dzou, 2007). It is also found that, biodegradation preferentially consumes 12C of propane and results in the enrichment of 13C in residual propane while the isotopic composition of ethane is not changed (James and Burns, 1984). As a result, the carbon isotopic compositions of hydrocarbon components in original thermogenic gases generally display a smooth progressive distribution pattern from C1 to C5, while those of biodegraded gases exhibit a serrated configuration, especially with unique 13C enrichment of propane (James and Burns, 1984). Moreover, as CO2 with 12C derived from biodegraded hydrocarbons is preferentially converted into secondary microbial methane, the residual CO2 becomes more enriched in 13C (> +2‰) (Figures 5C, F) (Lillis and Magoon, 2007). Such significant enrichment in 13C of CO2 is unique, and it can be a proxy of biodegradation degree. Typical gas hydrates, which contain biodegraded-gases with enriched 13C isotope composition of CO2 (+25‰), have been reported in the Western high of Sea of Marmara (Ruffine et al., 2018).
Hydrate-associated gases will be oxidized by anaerobic or aerobic process while upward escaping, and the dominant process depends on the surrounding redox conditions. For aerobic oxidation, both enriched cultures with methane-oxidizing bacteria and analytical results of natural gas samples have suggested that oxidation can lead to the decrease in C1 content and enrichment in 13C and D of residual C1 (Figures 5D–F), because the C1 with lighter isotope is preferentially oxidized during this process (Coleman et al., 1981; Etiope et al., 2011; Daskalopoulou et al., 2018). Coleman et al. (1981) proposed that the extent of isotope fractionation is associated with temperature, and the change in δD value of methane is 8~14 times greater than that of δ13C value. Kinnaman et al. (2007) suggested that the isotope fractionation becomes less intensive with the increment of carbon number (i.e., C1>C2>C3>C4), and the degree of isotope fractionation appears insignificant with substrate decreasing.
For anoxic sediments, the anaerobic oxidation of methane (AOM) predominates with the involvement of microorganism (Barnes and Goldberg, 1976; Reeburgh, 1976). Variation in methane isotopic composition has been observed in active AOM marine sediments, and it has been attributed to the difference in sulfate availability (Borowski et al., 1997; Pohlman et al., 2008; Yoshinaga et al., 2014). Batch enrichment cultures revealed that at seawater sulfate concentrations (28 mM), AOM will induce kinetic isotope fractionations, resulting in the enrichment of 13C and D in the residual methane (Holler et al., 2009; Ono et al., 2021). In contrast, at low sulfate concentrations AOM results in 13C-depletion in the remained methane and were explained as isotopic equilibration between methane and inorganic carbon mediated by AOM (Yoshinaga et al., 2014).
Accurate depict of the formation and accumulation process of the hydrate-associated gas requires information obtained from various aspects. Development of both conventional and novel techniques make it possible to deepen our understanding of the gas geochemistry study. Here, we discuss the hydrate-associated gas geochemistry from three aspects via a complete overview of the state of the art, recent research breakthroughs, and areas of continued controversy.
Stable isotope and molecular composition have always been basic properties of the gases that reflect their origins and sources. The most commonly used method to interpret the origin of gases is the plot of C1/(C2+C3) versus δ13C-CH4, known as “Bernard plot” because it was originally proposed by Bernard et al. (1976). Based on the analytical results of natural gases, Bernard et al. (1976) noticed that the microbial gas always is with a higher dry coefficient (C1/(C2+C3) > 1000) and lighter δ13C-CH4 (δ13C-CH4<-60‰), while thermogenic gas usually is with lower dry coefficient (0< C1/(C2+C3)< 50) and heavier δ13C-CH4 (δ13C-CH4 >-50‰). Schoell first proposed of using the carbon and hydrogen isotopes of methane to recognize the origin of hydrocarbon gases (Schoell, 1980; Schoell, 1983). The genetic diagram includes microbial gases from marine (δ13C< -60‰, δD -200‰ ~ -150‰) and terrestrial environment (δ13C< -60‰, δD -250‰ ~ -200‰), oil-associated thermogenic gases (δ13C -60‰~ -25‰, δD -300‰~ -150‰) and non-oil-associated gases (δD >-150‰). Whiticar further modified the diagram by merging all thermogenic gases into one area, and specified microbial gases into CO2 reduction and acetate fermentation (Whiticar et al., 1986; Whiticar, 1999). Gutsalo and Plotnikov (1981) proposed the first genetic diagram based on the carbon isotopic compositions of methane and carbon dioxide (δ13C-CO2 vs. δ13C-CH4), which grouped the gases into abiogenic gas, microbial gas and thermogenic gas. Milkov, 2011 added to the diagram the genetic region of secondary microbial gases from oil biodegradation, and Etiope et al added new region of abiogenic CH4 to this genetic diagram (Etiope and Lollar, 2013; Etiope and Schoell, 2014; Etiope, 2017).
Milkov and Etiope (2018) revised the three plots based on the data from more than 20,000 natural gas samples published in recent decades, updating a more detailed classification of gas origins (Figure 5). In addition to the above classification diagram, other information from geological geochemical or even experimental studies can benefit to figure out the gas origin. The primary microbial gases are characterized by the composition with C1-C3 hydrocarbons only, which is supported by the results from laboratory experiments that microbes can merely generate these three hydrocarbons (Oremland et al., 1988; Hinrichs et al., 2006). The geological setting can provide boundary condition to identify the microbial gas, such as the absence of oil in the sediment or reservoir. Similarly, thermogenic gases are usually associated with conventional oil reservoir and characterized by the presence of all the methane homologues (i.e., C1-C5).
Most studies of hydrate-related gases stop at distinguishing whether the gas is thermogenic derived from deep sources or microbial from shallow methanogenesis. For gases from deep sources, there are few ways to identify the specific source rocks, which constrain the understanding of the generation and accumulation processes of gas hydrate deposits for a specific area.
Rooney et al. (1995) proposed that the relationship between methane and ethane in the early stages of thermogenic gas generation can be described with the following equation Eq (1):
where δCorg is referred to δ13C of the organic matter; generally -27‰ taken for terrestrial sources, and -20‰ for marine sources (Rooney et al., 1995; Lorenson and Collett, 2018). Lorenson and Collett (2018) studied δC1 and δC2 of hydrate gas offshore India and found that gas from KG basin is mainly microbial, while those from the deep Mahanadi Basin are mainly derived from the gas-prone terrestrial organic matter (Figure 6A). Liang et al. (2022) proposed that microbial and thermogenic gases associated with gas hydrate from the Shenhu area were derived from marine and terrigenous organic matter, respectively, based on the isotopic composition of C1 and C2 (Figure 6A). The integrated isotopic values of C1, C2 and C3 can better identify the gas origins and the types of their precursor organic matter (Figure 6B). Liang et al. (2022) pointed out that most of the hydrate containing gases in Shenhu area fell into the sapropelic gas area (Figure 6B), which is close to conventional gas reservoirs in the plot (area IV in Figure 4), indicating for the first time that the thermogenic gas in hydrate is cogenetic with conventional oil and gas reservoirs in Panyu low uplift of Baiyun Sag. Above studies emphasize the importance of high-precision isotope measurement of C2+ components in hydrate-associated gases, which can provide clues for the identification of the source rocks of the gases.
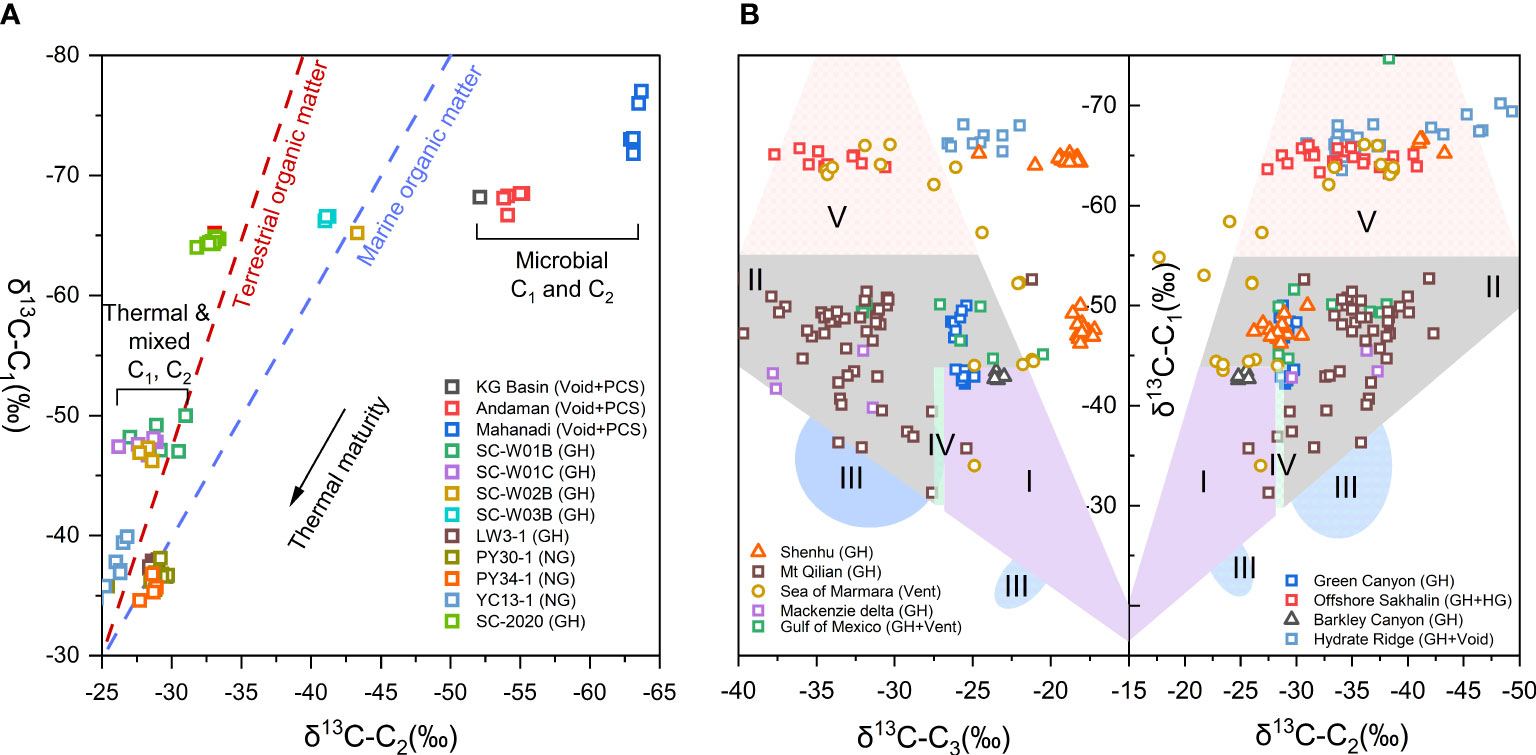
Figure 6 (A) Relationship of different types of organic matter in the source rocks and hydrate-associated gas. The diagonal lines are adapted from (Lorenson and Collett, 2018). VG-Void Gas, PCS-pressure core gas; GH-Gas Hydrate dissociated gases; NG-Natural gas. (B) Plot of the δ13C1, δ13C2, and δ13C3 values of the hydrate-bound gas samples. The genetic diagram is adapted from (Dai, 1992).(I-Humic type gas; II-Sapropel type gas; III-Gas mixture with the reversal of carbon isotope; IV-Humic type gas and sapropel type gas; V-Biogenic gas). .
In addition, detailed organic geochemistry, microbial and geological investigation have been proved to be robust tools to provide extra clues for gas origins and sources in recent years (Lai et al., 2022; Li et al., 2022; Lin et al., 2022). Presence of biomarkers of deeply buried thermogenic hydrocarbons and microbial communities associated with hydrocarbon degradation are remarkable signatures to identify the secondary biodegraded gas. This kind of integrated study should be widely used in the future research of hydrate-associated gases (Lai et al., 2022).
Although several methods have been proposed for the identification of the origins of hydrate-associated gas, sometimes it is still difficult for a specific case because there are unclear boundaries and overlaps between different classifications (Figure 5) (Martini et al., 1996; Prinzhofer and Pernaton, 1997; Martini et al., 1998; Valentine et al., 2004; Etiope and Schoell, 2014; Smith et al., 2014). The molecular and isotopic fractionation caused by post-generation processes makes the identification of gas origins more challenging (Prinzhofer and Pernaton, 1997; Martini et al., 1998; Whiticar, 1999). In addition, empirical diagrams are often proposed through a large amount of statistical data, which may not applicable to hydrate samples from all geological settings. Empirical formulas often ignore the effects from secondary process like mixing, migration and biodegradation. However, these processes often obscure the original signal of the gases, so it is better to interpret the data with other aspects of evidence, such as organic geochemistry of hydrate-bearing sediment, geophysics data, etc.
It is also an unsolved problem to quantitatively identify the contributions of different gas sources. Especially for the mixed-origins gas hydrate, the contribution proportion of shallow in-situ microbial gas and allochthonous thermogenic gas is still a difficult problem. Sun et al. (2020) reconstructed a geological model combining seismic, well and geological interpretation to predict hydrocarbon generation, migration and formation of gas hydrate in Shenhu area. The modeling shows that about 80% of the methane-forming hydrate is with thermogenic source and the total organic carbon (TOC) content is considered to be important factor related to biogenically-sourced gas hydrate distribution. However, TOC and hydrocarbon-generation index (HI) are important parameters that considered in this study, more relevant parameters about source rocks especially biogenic source rocks should be evaluated in the future studies.
Additional proxies are required to help distinguish between different sources and post-generation processes. As a novel technique established in the past 10 years, methane clumped isotopes are with potential to improve our understanding of gas origins. Clumped isotopes are referred to multiply substituted isotopologues with two or more rare isotopes e.g. 13CH3D and 12CH2D2 for methane (Eiler, 2007; Eiler, 2013). The first study of methane clumped isotopes was conducted at Caltech on ultra-high resolution isotope mass spectrometry (Thermo fisher, Ultra), but upon that time only a total Δ18 signal could be available, unable to distinguish 13CH3D from 12CH2D2 (Stolper et al., 2014b). Later, measurement of 13CH3D was also achieved using Tunable Infrared Laser Direct Absorption Spectroscopy (TILDAS) at MIT (Ono et al., 2014). The recognition of both 12CH3D and 13CH3D signals on a single measurement was succeeded for the first time with a higher resolution mass spectrometry (Nu, Panorama) in 2016 at UCLA (Young et al., 2016; Young et al., 2017), although Ultra and TILDAS also achieved this function in the following years (Eldridge et al., 2019; Gonzalez et al., 2019; Dong et al., 2020; Dong et al., 2021). Recently, a commercial instrument (Ultra), which is developed by Thermo-Fisher , has been successful in clumped isotope measurement in Tokyo Institute of Technology, indicative of the maturity of this technology (Zhang et al., 2021a). Methane clumped isotope analysis quantify the abundances of isotopologues of methane relative to the ideal gas state of random distributions of isotopes over methane molecules (Young et al., 2016; Douglas et al., 2017; Young et al., 2017). Clumped isotopes of methane may primarily record its formation or equilibration temperature if the isotopologues are thought thermally equilibrated when both the Δ13CH3D and Δ12CH2D2 values are on the thermodynamic equilibrium curve in Figure 7 (Stolper et al., 2014a; Webb and Miller, 2014; Wang et al., 2015a). This method is established based on the theory of isotopologue exchange reaction [Eq (2)], of which the equilibrium is controlled by temperature.
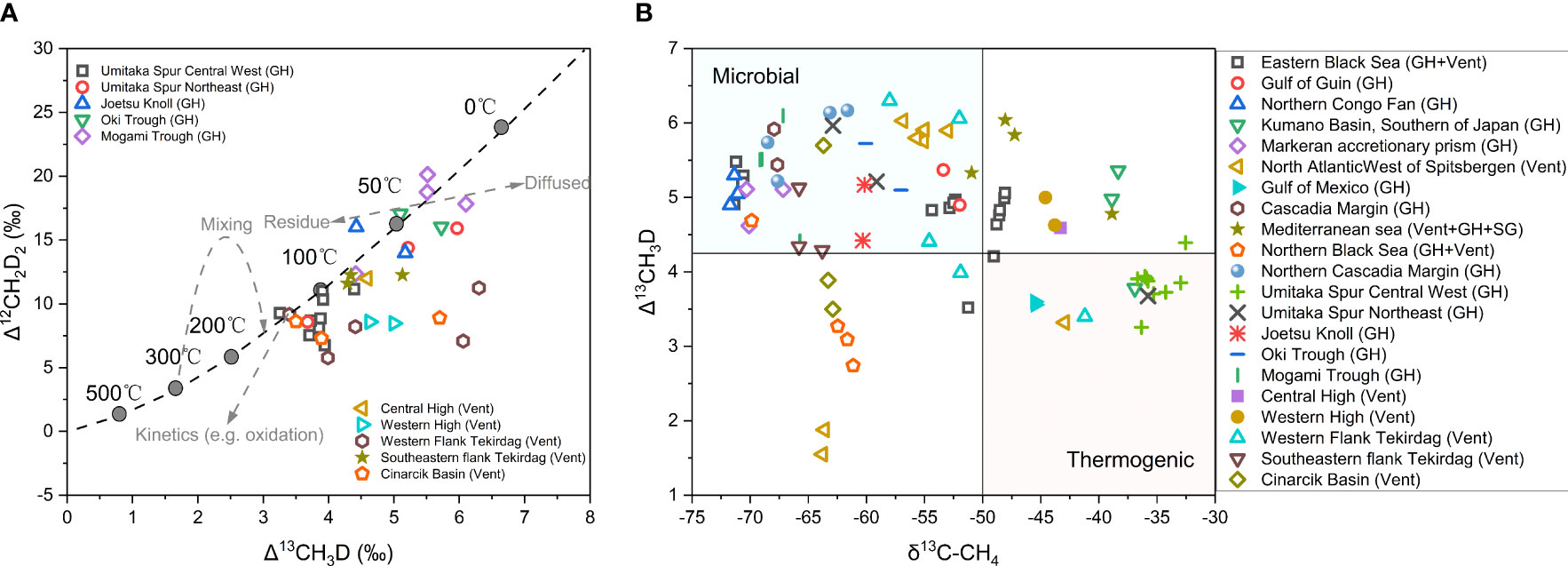
Figure 7 (A) Compile of the published clumped isotope of hydrate associated gases plotted in thermodynamic equilibrium curve in Δ12CH2D2 vs. Δ13CH3D space (both axes are in per mil). Mixing and diffusion trend are adopted from (Douglas et al., 2016), oxidation trend from (Douglas et al., 2017). (B) Cross-plot of the published clumped isotope (Δ13CH3D) and conventional stable isotope (δ13C-C1) of hydrate associated gases (both axes are in per mil), modified from (Lalk et al., 2022). SG-Sedimentary Gases.
The formation temperature of methane obtained by this method provides crucial clues for its origin. Microbial gas is formed at low temperature (usually<75°C) via biogeochemical processes in subsurface sediments, while thermogenic gases generated from pyrolysis of organic matter usually occur at high temperatures (usually>150°C). Abiotic gases involve a variety of reactions, which can be classified as reaction in mantle, magmatic system (~>600°C) or water-rock interaction (~50-500°C).
Clumped isotopologue analyses have been applied to studying hydrate-associated methane from Hydrate Ridge on Cascadia Margin (Wang et al., 2015a), Kumano Basin mud volcano in Nankai accretionary complex (Ijiri et al., 2018), Japan Sea (Zhang et al., 2021a), Sea of Marmara (Giunta et al., 2021), as well as 46 samples from the other 11 regions in the world (Lalk et al., 2022) (Figure 7A, B).
The Δ13CH3D of microbial methane from both porewaters and gas hydrates recovered from sediments in the northern Cascadia margin yielded methane formation temperature ranging from 12°C to 42°C (Figure 7B) (Wang et al., 2015a), which is in accordance with the surrounding environment. Similarly, clumped methane isotopologues of the gases from Kumano forearc basin in Nankai accretionary complex indicate that ~90% of methane is produced microbially at an estimated temperature between 16° and 30°C (Figure 7B), consistent with a relatively shallow microbial source in the sediments of 300-900mbsf (Ijiri et al., 2018). However, from clumped isotopes the formation temperatures of methane are calculated to be 15 to 170°C for the methane gases from the Sea of Japan (Figures 7A, B). By combining clumped isotope results with other traditional approaches, the microbial methane is quantified to be 20-80% compared to the thermogenic (Zhang et al., 2021a). Giunta et al. (2021) measured 13CH3D and 12CH2D2 of methane from cold seeps emanating at the seafloor of Sea of Marmara (SoM) (Figures 7A, B), and it is found that the isotopic characteristics of methane in SoM cannot be simply explained by mixing of multiple reservoirs but appears to be affected to varying degrees by bond re-equilibration with clay minerals (Ruffine et al., 2018). This might imply that the temperature obtained from clumped isotope represents the re-equilibration condition of post-generation rather than the actual formation temperature of methane. Lalk et al. (2022) analyzed 46 gas hydrates and associated gases from seepages of different types (cold seeps, oil seeps, pockmarks, and mud volcanoes) in 11 regions around the world (Figure 7B), discovering that the formation temperatures of methane associated with cold seeps and pockmarks locate in the microbial range between 15°C and 70°C. However, the temperature of methane gases from oil-associated gas hydrates are estimated to be 50°C to 120°C, corresponding to secondary methane generated by oil biodegradation. The methane gases associated with mud volcanoes are with a wide range of Δ13CH3D values, and the correspondent temperatures of methane formation are not consistent with the conditions indicated by conventional proxies, suggesting their diverse origins which could be attributed to the tectonic settings (Lalk et al., 2022).
Clumped isotopes with equilibrium signals can provide important information for temperature and can be used as thermometers (Stolper et al., 2014a). However, not all data measured in reality are ideally in equilibrium. Most of the temperature results calculated with clumped isotopes of thermogenic gases are consistent with those of their surrounding environment (Douglas et al., 2017; Young et al., 2017; Stolper et al., 2018; Giunta et al., 2019; Giunta et al., 2021), indicating that clumped isotope can reflect the formation temperature of methane. However, in laboratory based thermal experiments, such as pyrolysis of shale, coal, or hydrocarbons, significant non-equilibriums in clumped isotopes have been observed (Shuai et al., 2018; Dong et al., 2021), which is considered to be a statistical combinational effect or kinetic isotope effects (KIE) during pyrolysis. Simulating experiments also reveal that hydrocarbons may approach equilibrium in clumped isotope with maturity increasing (Xia and Gao, 2019; Dong et al., 2021).
Compared to thermogenic gas, the values of clumped isotopes of microbial methane are in a wide range. The temperature, deduced from clumped isotopic composition of microbial methane generated in laboratory culture experiments, is much lower than its actual culture condition (Stolper et al., 2015; Wang et al., 2015a; Young et al., 2017; Gruen et al., 2018; Giunta et al., 2019; Douglas et al., 2020). The extremely depletion in 12CH2D2 is mainly ascribed to quantum tunneling effect or combinatorial effects of 4 hydrogen atoms of methane from different reservoirs or experiencing different fractionations (Young et al., 2017; Cao et al., 2019; Young, 2019; Taenzer et al., 2020), while the moderate 13CH3D depletion is mainly attributed to quantum tunneling or kinetic isotope effect of methanogenesis (Wang et al., 2015a; Cao et al., 2019; Young, 2019; Douglas et al., 2020). In surface environments such as cattle rumen and freshwater ecosystems, disequilibrium clumped isotope characteristics of biogenic methane were widely reported, exhibiting obvious kinetic signals, and corresponding temperatures were much higher than the actual ambient condition (Stolper et al., 2015; Wang et al., 2015a; Douglas et al., 2016; Young et al., 2017; Ash et al., 2019; Giunta et al., 2019; Douglas et al., 2020). However, methane gases taken from marine sediments usually show a relatively consistent temperature compared to the ambient condition (Figures 7A, B) (Inagaki et al., 2015; Wang et al., 2015a; Young et al., 2017; Ijiri et al., 2018). The mechanisms for the equilibration of methane clumped isotopes in marine sediments can involve the processes of methanogenesis or oxidation of methane. For methanogenesis, the rates of methane formation and enzyme reversibility are recognized as the two main factors affecting the kinetics of clumped isotope equilibration based on the culturing experiments and observations on natural samples (Stolper et al., 2015; Wang et al., 2015a; Douglas et al., 2016; Douglas et al., 2020). For oxidation of methane, reversible bond reordering in the AOM process was identified as the key process controlling the equilibration of clumped isotopes (Ash et al., 2019; Ono et al., 2021).
In addition to the above disequilibrium condition, secondary post-generation processes such as oxidation, migration and mixing of methane can also alter the original clumped isotope signal (Stolper et al., 2015; Wang et al., 2015a; Yeung et al., 2015; Young et al., 2017; Giunta et al., 2019; Labidi et al., 2020; Giunta et al., 2021; Warr et al., 2021). Mixing may lead to a non-linearity trajectory in the Δ13CH3D-Δ12CH2D2 diagram (Figure 7A) (Douglas et al., 2016; Young et al., 2016), with a curvature depending on bulk isotopic compositions (δ13C and δD) of end-members. The curvature of the mixing line is negligible when the values of end-member δD and δ13C are close, but it becomes progressively significant when the values of end-member δD and δ13C become more different (Stolper et al., 2015; Douglas et al., 2016). Diffusion of methane, either in the condition of vacuum or interaction with particles, is predicted to favor the light isotopologues to be enriched in diffused gas as compared with the residual gas (Figure 7A). Two variants of Δ13CH3D and Δ12CH2D2 should be with an integer mass ratio of 18/16, so any fractionation caused by molecular mass, like diffusion, should display a 1:1 slope in this space (Douglas et al., 2017). Douglas et al. (2017) proposed that atmospheric chemical reactions of methane with OH.-, Cl.- would increase the abundance of 12CH2D2 and 13CH3D (Figure 7A), but their enrichment degrees are different. Microbial methanotrophy, including aerobic and anaerobic oxidation of methane, can change initial clumped isotope signatures of methane as well (Wang et al. 2015a; Ash et al., 2019; Young, 2019; Ono et al., 2021). For aerobic oxidation of methane, Wang et al. (2016) found a decrease in Δ13CH3D and a significant increase in δD and δ13C values of CH4 in bio-simulation experiments under aerobic condition. While for anaerobic oxidation of methane (AOM), reversible bond re-ordering is proposed to be the key process leading to near-equilibrium values based on observations in natural samples (Ash et al., 2019). Laboratory culture experiments (Young, 2019; Ono et al., 2021) qualitatively confirm the role of AOM in modifying methane clumped isotope signatures, but some of these experimental results do not quantitatively agree with the observations in natural samples. Ono et al. (2021) reported that Δ13CH3D values of residual CH4 were 3.1‰ higher than the expected equilibrium values, which might be associated with KIE resulted from rapid AOM rates during incubation, while Young (2019) observed distinct bond re-ordering trends in Δ13CH3D vs. Δ12CHD2 plot under changing sulfate concentrations. The specific mechanism of how AOM catalyzes bond re-ordering requires further investigation.
Noble gases are chemically inert, so their elemental ratios can be changed only by physical processes but not by microbial activities and chemical reactions. Based on these specific properties, methods are established to decipher complex geochemical processes (Winckler et al., 2000). Noble gases are well constrained in different units of the earth surface system, coming from three-endmember sources: (i) atmosphere, or air-saturated water (ASW): e.g., 20Ne, 36Ar, 38Ar, 84Kr, 132Xe; (ii) 3He-rich mantle; and (iii) crustal, rich in radiogenic noble gases accumulated by radioactive decay, e.g. U + Th → 4He*, 21Ne*, 136Xe* ; 40K → 40Ar* (Ballentine and Burnard, 2002).
The elemental and isotopic compositions of noble gases facilitate additional constraints on the genetic origins of and semiquantitative estimation of the biogenic and thermogenic contributions of natural gas. Noble gases are also employed to study the dynamics of fluid migration, estimate the residence time of hydrocarbon gases in reservoir, and specify post-genetic processes that might have modified hydrocarbon composition (Zhou et al., 2005; Zhou and Ballentine, 2006; Schlegel et al., 2011; Hunt et al., 2012; Darrah et al., 2014; Darrah et al., 2015; Wen et al., 2015; Barry et al., 2016; Harkness et al., 2017).
Previous experimental and theoretical studies have demonstrated that formation of hydrate will result in fractionation to certain degree between noble gases, as reflected by the enrichment of heavier noble gases in gas hydrate (Nikitin, 1937; Barrer and Stuart, 1957; Hunt et al., 2013). Noble gases studies of gas hydrates from Blake Ridge show that the formation of hydrate is characterized by the Xe enrichment in hydrate phase due to noble gas fractionation (Dickens and Kennedy, 2000). Natural gas hydrates from Hydrate Ridge are almost with no He and Ne, but contain high Ar and Kr and Xe, suggesting that the heavier noble gases were preferentially entered gas hydrate structure compared with the lighter noble gases, which could be ascribed to the thermodynamic effect or solubility difference (Winckler et al., 2002). The experimental results of synthesizing hydrate of mixed methane and noble gases (He, Ne, Ar, Kr and Xe) reveal that Kr and Xe are enriched relative to Ar but without detectable He and Ne in hydrate, also indicative of the effect of mass fractionation of noble gases in hydrate formation process (Hunt et al., 2011; Hunt et al., 2013).
As discussed in previous sections, traditional methods are with limitations to delineate gas origins, noble gases can provide additional clues about the thermal maturity of natural gases and the migration of allothogenic fluids. Crustal noble gases like 4He, 21Ne and 40Ar are present in high concentrations in thermogenic gases, whereas recently formed microbial gases are nearly devoid of radiogenic noble gases (Hunt et al., 2012; Darrah et al., 2015; Wen et al., 2015; Harkness et al., 2017). On the contrary, the increase of atmospherically derived gases like 20Ne and 36Ar seems to correspond to an elevated proportion of microbial gas (Ballentine et al., 2002; Ballentine and Burnard, 2002). Furthermore, the 3He/4He (shown as R/Ra, where R is the 3He/4He in a gas sample and Ra is the 3He/4He of air, 1.39 × 10−6), provides proxies to identify the participation of abiotic gases from the mantle or continental crust after eliminating the contribution of the atmosphere by referring to 4He/20Ne ratio (Figure 8) (Graham, 2002). High R/Ra and high 4He/20Ne ratios are the indication of the input of mantle-derived gases (Figure 8) (Oxburgh et al., 1986; Poreda et al., 1986).
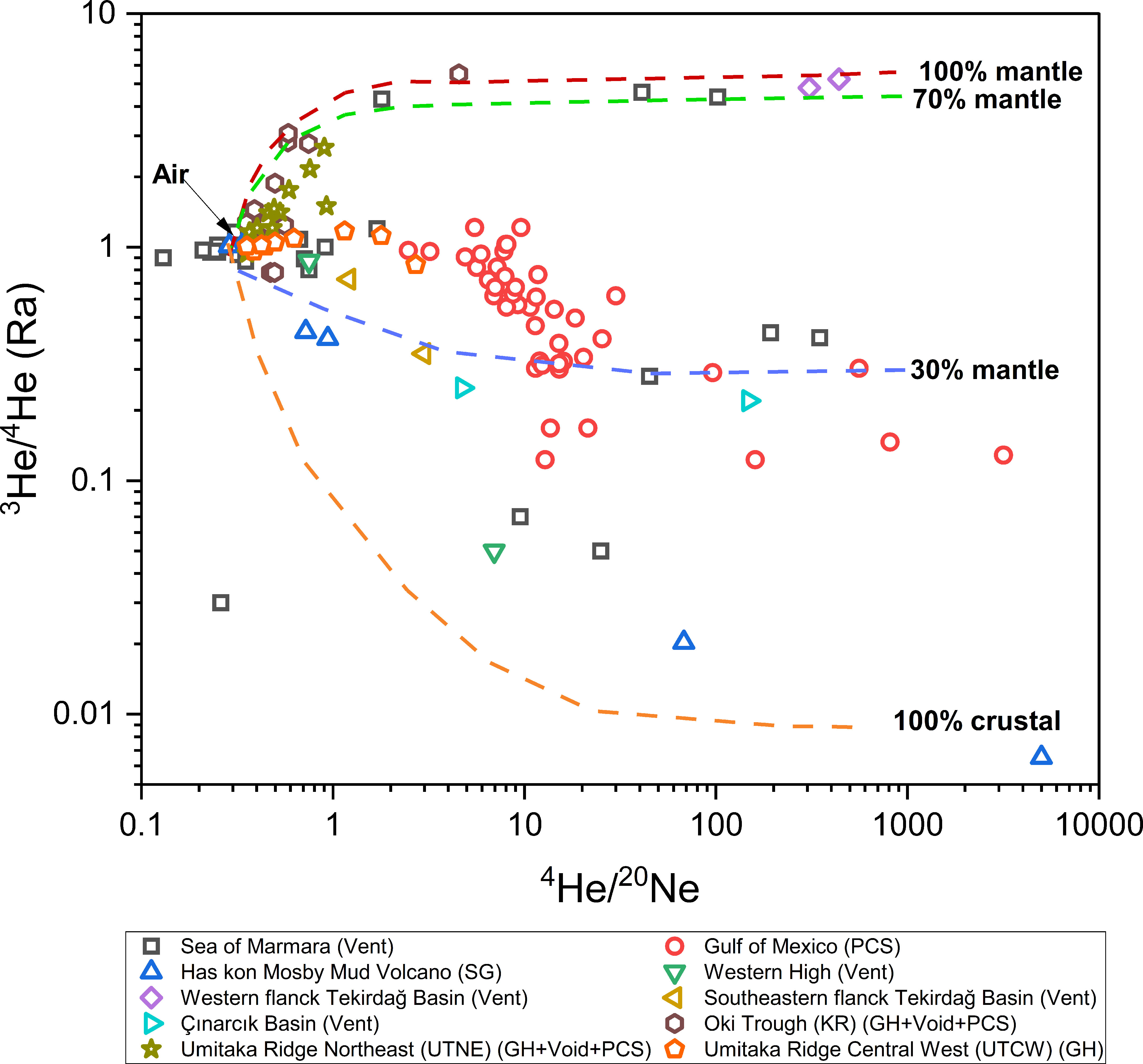
Figure 8 Compiled of published noble gases geochemical data of hydrate associated gases plotted in He isotope versus 4He/20Ne ratio.
The content and isotope ratios of noble gases in hydrate-bearing sediments from the Haskon Mosby Mud Volcano reveal that all the samples are with non-atmospheric He, which is considered to be crustal/radiogenic origin, and transported to hydrate stability zone through mud volcano (Figure 8) (Prasolov et al., 1999). One of these sites is with higher isotopic 3He/4He ratio, which probably contains the contribution from mantle. The 3He/4He ratio of hydrate-associated venting gas in Sea of Marmara is as high as 5.25 Ra (Figure 8) (Ruffine et al., 2018), and similar high 3He/4He ratio was also found in the southern part of the Ganos fault in the same area (Burnard et al., 2012), indicating a possible mantle contribution through large-scale fault systems (Gautheron and Moreira, 2002). Snyder et al. (2020) reported noble gas results from gas hydrate in the Japan sea and found that hydrate gas is mainly of a mixture of thermogenic/crustal source, biogenic gases and mantle source. The existence of hydrate with mantle 3He/4He characteristics indicates that fluids enriched in mantle gases are the main cause of the mobilization of thermogenic gas in the active chimney structures of the Japan Sea (Figure 8).
Fractionation of noble gases during the hydrate formation, combined with the episodic cycle of hydrate melting and refreezing, complicated the scenarios for noble gases studies associated with gas hydrate. For example, high 3He/4He ratios, which are generally related to mantle source gas, are found in hydrate-associated gases which were determined as biogenic origin by δ13C-CH4 in samples from Sea of Japan. However, low 3He/4He ratios are observed in hydrate-associated gases with thermogenic carbon isotope signature (Snyder et al., 2020). The authors proposed that hydrates which were formed from thermogenic gas in chimney experienced decomposition and reformation, and helium was lost in this process because it is difficult to be encaged in hydrate by its small molecular size (Snyder et al., 2020). Therefore, the melting and reprecipitation effect of gas hydrate may prevent the identification of the magmatic or radiogenic contribution.
Air contamination and gas-water interactions in the pretreatment process prevent accurate measurement of noble gases degassing from hydrate-bearing pressurized core, Moore et al. (2020) proposed the modified quantitative degassing method which can minimize these effects and applied this technique to samples from the Gulf of Mexico (Figure 8), which may be important for future hydrate-associated noble gases study. Although this modified method reduces the impact of air pollution, it still requires complex procedures and large sample quantity, so it is still in need of a better pre-treatment scheme for noble gas detection.
Many natural gas hydrate samples have been collected and studied through multiple approaches during the past decades. Based on the data published, the factors affecting the geochemical characteristics of hydrate-associated gases have been summarized, and the methods for identifying gas origins and delineating the post-generation processes have been reviewed.
1. Apart from the gas of abiotic origin, most of the hydrate-associated gases are derived from the degradation of organic matter. Different types of organic matter are with distinct hydrocarbon-generation profile in the course of thermal evolution, as indicated by the molecular and isotopic compositions of their products. Oil-type gas and coal-type gas are the products of different types of organic matter, while microbial gases and thermogenic gases are the product in different evolution stages of organic matter in the burial history of sediments.
2. The characteristics of hydrate-associated gases are affected not only by the primary properties of organic materials such as organic type and thermal maturity but also associated with the secondary processes in gas accumulation processes after generation. Among the secondary processes, migration, mixing and biodegradation are common in hydrate-bearing area.
3. Clumped isotopes with equilibrium signals can be a robust proxy to estimate the formation temperature of methane, whereas the data without equilibrium signals can reflect the secondary processes that lead to the deviation from the equilibrium state. Most published methane clumped isotopes from marine sediments are found locating in a reasonable temperature zone which are generally consistent to their surroundings.
4. Noble gases can play an important role in recognizing the dynamics of thermogenic hydrocarbon generation generated by interactions between hydrothermal fluids and deeply buried organic matter. As such, noble gas helium isotopic signatures provide an indicator of large-scale deep migration pathways.
Conceptualization, HL; writing—original draft preparation, YL; writing—review and editing, CJ and HL; supervision, HL; funding acquisition, HL. All authors have read and agreed to the published version of the manuscript.
This work was supported by China Geological Survey (No. DD20221703).
We would like to express our gratitude to researchers who focus on the gas geochemical characteristics of natural gas hydrate, their previous work laid the foundation of this review. We also appreciate the efforts of the editors and reviewers, who gave constructive comments and suggestions which greatly improved this manuscript and inspired in-depth discussion.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.968647/full#supplementary-material
Ash J. L., Egger M., Treude T., Kohl I., Cragg B., Parkes R. J., et al. (2019). Exchange catalysis during anaerobic methanotrophy revealed by 12CH2D2 and 13CH3D in methane. Geochemi. Perspect. Lett. 10, 26–30. doi: 10.7185/geochemlet.1910
Ballentine C. J., Burgess R., Marty B. (2002). Tracing fluid origin, transport and interaction in the crust. Rev. Mineral. Geochem. 47 (1), 539–614. doi: 10.2138/rmg.2002.47.13
Ballentine C. J., Burnard P. G. (2002). Production, release and transport of noble gases in the continental crust. Rev. Mineral. Geochem. 47 (1), 481–538. doi: 10.2138/rmg.2002.47.12
Barnes R. O., Goldberg E. D. (1976). Methane production and consumption in anoxic marine sediments. Geology 4, 297–300. doi: 10.1130/0091-7613(1976)4<297:MPACIA>2.0.CO;2
Barrer R. M., Stuart W. I. (1957). Non-stoicheiometric clathrate compounds of water. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 243, 172–189. doi: 10.1098/rspa.1957.0213
Barry P. H., Lawson M., Meurer W. P., Warr O., Mabry J. C., Byrne D. J., et al. (2016). Noble gases solubility models of hydrocarbon charge mechanism in the sleipner vest gas field. Geochim. Cosmochim. Acta 194, 291–309. doi: 10.1016/j.gca.2016.08.021
Bernard B. B., Brooks J. M., Sackett W. M. (1976). Natural gas seepage in the gulf of Mexico. Earth Planet. Sci. Lett. 31, 48–54. doi: 10.1016/0012-821X(76)90095-9
Bernard B., Brooks J. M., Sackett W. M. (1977). A geochemical model for characterization of hydrocarbon gas sources in marine sediments. Offshore Technol. Conf. 435–438. doi: 10.4043/2934-MS
Boreham C. J., Edwards D. S. (2008). Abundance and carbon isotopic composition of neo-pentane in Australian natural gases. Org. Geochem. 39 (5), 550–566. doi: 10.1016/j.orggeochem.2007.11.004
Borowski W. S., Paull C. K., Ussle W. (1997). Carbon cycling within the upper methanogenic zone of continental rise sediments: An example from the methane-rich sediments overlying the Blake ridge gas hydrate deposits. Mar. Chem. 57, 299–311. doi: 10.1016/S0304-4203(97)00019-4
Brooks J. M., Michael E. F., XXXM.C. K. I. (1991). Observations of gas hydrates in marine sediments, offshore northern California. Mar. Geol. 96, 103–109. doi: 10.1016/0025-3227(91)90204-H
Buffett B., Archer D. (2004). Global inventory of methane clathrate: sensitivity to changes in the deep ocean. Earth Planet. Sci. Lett. 227 (3-4), 185–199. doi: 10.1016/j.epsl.2004.09.005
Burnard P., Bourlange S., Henry P., Geli L., Tryon M. D., Natal'in B., et al. (2012). Constraints on fluid origins and migration velocities along the marmara main fault (Sea of marmara, Turkey) using helium isotopes. Earth Planet. Sci. Lett. 341-344, 68–78. doi: 10.1016/j.epsl.2012.05.042
Cao X., Bao H., Peng Y. (2019). A kinetic model for isotopologue signatures of methane generated by biotic and abiotic CO2 methanation. Geochim. Cosmochim. Acta 249, 59–75. doi: 10.1016/j.gca.2019.01.021
Cao D. Y., Wang D., LI J., Dou X. Q. (2012). Gas source analysis of natural gas hydrate of muli coalfield in qilian mountain permafrost, qinghai province, China. J. China Coal Soc. 37, 1364–1368. doi: CNKI:SUN:MTXB.0.2012-08-020
Chanton J. P. (2005). The effect of gas transport on the isotope signature of methane in wetlands. Org. Geochem. 36 (5), 753–768. doi: 10.1016/j.orggeochem.2004.10.007
Chen A. D. (1994). Origin and migration of natual gas in Ordovician reservoir in shang ganning basin central gas field. Acta Petrolei Sin. 15, 1–10. doi: 10.1007/BF02943584
Chen F., Wang X., Li N., Cao J., Bayon G., Peckmann J., et al (2019). Gas Hydrate Dissociation During Sea‐Level Highstand Inferred From U/Th Dating of Seep Carbonate From the South China. Sea. Geophys. Res. Lett. 46 (23), 13928–13938. doi: 10.1029/2019gl085643
Chen M., Deng X. B., Liu C. L., Ren H. B., Yin X. J., Zhang A. M. (2018). Experimental study on carbon isotopic composition changes during the formation of gas hydrates. Geoscience 32, 205–212.
Chen L., Merey S. (2021). Oceanic methane hydrates: fundamentals, technological innovations, and sustainability. (Cambridge, MA, Gulf Professional) 1–37.
Choi J., Kim J.-H., Torres M. E., Hong W.-L., Lee J.-W., Yi B. Y., et al. (2013). Gas origin and migration in the ulleung basin, East Sea: Results from the second ulleung basin gas hydrate drilling expedition (UBGH2). Mar. Petroleum Geol. 47, 113–124. doi: 10.1016/j.marpetgeo.2013.05.022
Chung H. M., Gormly J. R., Squires R. M. (1988). Origin of gaseous hydrocarbons in subsurface environments: theoretical considerations of carbon isotope distribution. Chem. Geol. 71, 97–104. doi: 10.1016/0009-2541(88)90108-8
Coleman D. D., Meents W. F., Liu C. L., Keogh R. A. (1977). Isotopic identification of leakage gas from underground storage reservoirs. progress report. Ill., State Geol. Surv., Ill. Pet. 111, 1–10. doi: 10.2118/6491-MS
Coleman D. D., Risatti J. B., Schoell M. (1981). Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria. Geochim. Cosmochim. Acta 45, 1033–1037. doi: 10.1016/0016-7037(81)90129-0
Collett T. S., Boswell R., Waite W. F., Kumar P., Roy S. K., Chopra K., et al. (2019). India National gas hydrate program expedition 02 summary of scientific results: Gas hydrate systems along the eastern continental margin of India. Mar. Pet. Geol. 108, 39–142. doi: 10.1016/j.marpetgeo.2019.05.023
Collett T. S., Johnson A. H., Knapp C. C., Boswell R. (2009). Natural gas hydrates: A review. AAPG Memoir 89, 149–219. doi: 10.1306/13201142M891602
Collett T. S., Riedel M., Cochran J. R., Boswell R., Kumar P., Sathe A. V. (2008). “India Continental margin gas hydrate prospects: results of the Indian national gas hydrate program (NGHP) expedition 01,” in Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008).
Craig H. (1968). Isotope separation by carrier diffusion. Science 159, 93–96. doi: 10.1126/science.159.3810.93
Cremiere A., Lepland A., Chand S., Sahy D., Condon D. J., Noble S. R., et al (2016). Timescales of methane seepage on the Norwegian margin following collapse of the Scandinavian Ice Sheet. Nat Commun. 7, 11509. doi: 10.1038/ncomms11509
Dai J. X. (1992). The discrimination of various of alkane gases. Sci. Sin. 22, 185–193. doi: 10.1360/zb1992-22-2-185
Dai J. X., Ni Y. Y., Huang S. P., Peng W. L., Han W. X., Gong D. Y., et al. (2017). Genetic types of gas hydrates in China. Pet. Explor. Dev. 44, 837–848. doi: 10.11698/PED.2017.06.01
Darrah T. H., Jackson R. B., Vengosh A., Warner N. R., Whyte C. J., Walsh T. B., et al. (2015). The evolution of Devonian hydrocarbon gases in shallow aquifers of the northern Appalachian basin: Insights from integrating noble gas and hydrocarbon geochemistry. Geochim. Cosmochim. Acta 170, 321–355. doi: 10.1016/j.gca.2015.09.006
Darrah T. H., Vengosh A., Jackson R. B., Warner N. R., Poreda R. J. (2014). Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett shales. Proc. Natl. Acad. Sci. 111 (39), 14076–14081. doi: 10.1073/pnas.1322107111
Daskalopoulou K., Calabrese S., Grassa F., Kyriakopoulos K., Parello F., Tassi F., et al. (2018). Origin of methane and light hydrocarbons in natural fluid emissions: A key study from Greece. Chem. Geol. 479, 286–301. doi: 10.1016/j.chemgeo.2018.01.027
De Prunel A., Ruffine L., Riboulot V., Peters C. A., Croguennec C., Guyader V., et al. (2017). Focused hydrocarbon-migration in shallow sediments of a pockmark cluster in the Niger delta (Off Nigeria). Geochem., Geophys., Geosyst. 18, 93–112. doi: 10.1002/2016GC006554
Deng Y., Chen F., Guo Q., Hu Y., Chen D., Yang S., et al. (2021). Possible Links Between Methane Seepages and Glacial-Interglacial Transitions in the South China Sea. Geophys. Res. Lett. 48 (8), e2020GL091429. doi: 10.1029/2020gl091429
Dickens G. R., Kennedy B. M. (2000). “"Noble gases in methane hydrate from the Blake ridge",” in Proceedings of the ocean drilling program. scientific results. ocean drilling program. 164 (1), 187–192. doi: 10.2973/odp.proc.sr.164.211.2000
Dickens G. R., O'Neil J. R., Rea D. K., Owen R. M. (1995). Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography 10, 965–971. doi: 10.1029/95PA02087
Dong G., Xie H., Eiler J., Zhang N., Mayuko N., Yoshida N., et al. (2020). “Clumped isotope analysis of methane using HR-IRMS: New insights into origin and formation mechanisms of natural gases and a potential geothermometer,” in Thermo scientific white paper WP30767.
Dong G., Xie H., Formolo M., Lawson M., Sessions A., Eiler J. (2021). Clumped isotope effects of thermogenic methane formation: Insights from pyrolysis of hydrocarbons. Geochim. Cosmochim. Acta 303, 159–183. doi: 10.1016/j.gca.2021.03.009
Douglas P. M. J., Gonzalez Moguel R., Walter Anthony K. M., Wik M., Crill P. M., Dawson K. S., et al. (2020). Clumped isotopes link older carbon substrates with slower rates of methanogenesis in northern lakes. Geophy. Res. Lett. 47 (6), 1–10. doi: 10.1029/2019gl086756
Douglas P. M. J., Stolper D. A., Eiler J. M., Sessions A. L., Lawson M., Shuai Y., et al. (2017). Methane clumped isotopes: Progress and potential for a new isotopic tracer. Org. Geochem. 113, 262–282. doi: 10.1016/j.orggeochem.2017.07.016
Douglas P. M. J., Stolper D. A., Smith D. A., Walter Anthony K. M., Paull C. K., Dallimore S., et al. (2016). Diverse origins of Arctic and subarctic methane point source emissions identified with multiply-substituted isotopologues. Geochim. Cosmochim. Acta 188, 163–188. doi: 10.1016/j.gca.2016.05.031
Eiler J. M. (2007). “Clumped-isotope” geochemistry–the study of naturally-occurring, multiply-substituted isotopologues. Earth Planet. Sci. Lett. 262 (3-4), 309–327. doi: 10.1016/j.epsl.2007.08.020
Eiler J. M. (2013). The isotopic anatomies of molecules and minerals. Annu. Rev. Earth Planet. Sci. 41 (1), 411–441. doi: 10.1146/annurev-earth-042711-105348
Eldridge D. L., Korol R., Lloyd M. K., Turner A. C., Webb M. A., Miller T. F., et al. (2019). Comparison of experimental vs theoretical abundances of 13CH3D and 12CH2D2 for isotopically equilibrated systems from 1 to 500 °C. ACS Earth Space Chem. 3 (12), 2747–2764. doi: 10.1021/acsearthspacechem.9b00244
Etiope G. (2017). Abiotic methane in continental serpentinization sites: An overview. Proc. Earth Planet. Sci. 17, 9–12. doi: 10.1016/j.proeps.2016.12.006
Etiope G., Baciu C. L., Schoell M. (2011). Extreme methane deuterium, nitrogen and helium enrichment in natural gas from the homorod seep (Romania). Chem. Geol. 280 (1-2), 89–96. doi: 10.1016/j.chemgeo.2010.10.019
Etiope G., Lollar B. S. (2013). Abiotic methane on earth. Rev. Geophysics 51 (2), 276–299. doi: 10.1002/rog.20011
Etiope G., Schoell M. (2014). Abiotic gas: Atypical, but not rare. Elements 10 (4), 291–296. doi: 10.2113/gselements.10.4.291
Faber E., Stahl W. J. (1984). Geochemical surface exploration for hydrocarbons in north Sea. AAPG Bull. 68, 363–386. doi: 10.1306/AD460A26-16F7-11D7-8645000102C1865D
Fuex A. N. (1980). Experimental evidence against an appreciable isotopic fractionation of methane during migration. Org. Geochem. 12, 725–732. doi: 10.1016/0079-1946(79)90153-8
Fu S. Y., Lu J. A. (2010). The characteristics and origin of gas hydrate in shenhu area, south China Sea. Mar. Geol. Lett. 26, 6–10. doi: 10.16028/j.1009
Galimov E. M. (1967). 13C enrichment of methane during passage through rocks. Geochem. Int. 4, 1180–1181.
Gao L., Brassell S. C., Mastalerz M., Schimmelmann A. (2013). Microbial degradation of sedimentary organic matter associated with shale gas and coalbed methane in eastern Illinois basin (Indiana), USA. Int. J. Coal Geol. 107, 152–164. doi: 10.1016/j.coal.2012.09.002
Gautheron C., Moreira M. (2002). Helium signature of the subcontinental lithospheric mantle. Earth Planet. Sci. Lett. 199, 39–47. doi: 10.1016/S0012-821X(02)00563-0
Ginsburg G. D., Soloviev V. A. (1997). Methane migration within the submarine gas-hydrate stability zone under deep-water conditions. Mar. Geol. 137, 49–57. doi: 10.1016/S0025-3227(96)00078-3
Giunta T., Labidi J., Kohl I. E., Ruffine L., Donval J. P., Géli L., et al. (2021). Evidence for methane isotopic bond re-ordering in gas reservoirs sourcing cold seeps from the Sea of marmara. Earth Planet. Sci. Lett. 553, 116619. doi: 10.1016/j.epsl.2020.116619
Giunta T., Young E. D., Warr O., Kohl I., Ash J. L., Martini A., et al. (2019). Methane sources and sinks in continental sedimentary systems: New insights from paired clumped isotopologues 13CH3D and 12CH2D2. Geochim. Cosmochim. Acta 245, 327–351. doi: 10.1016/j.gca.2018.10.030
Gonzalez Y., Nelson D. D., Shorter J. H., McManus J. B., Dyroff C., Formolo M., et al. (2019). Precise measurements of (12)CH2D2 by tunable infrared laser direct absorption spectroscopy. Anal. Chem. 91 (23), 14967–14974. doi: 10.1021/acs.analchem.9b03412
Graham D. W. (2002). Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: Characterization of mantle source reservoirs. Rev. Mineral. Geochem. 47 (1), 247–317. doi: 10.2138/rmg.2002.47.8
Gruen D. S., Wang D. T., Könneke M., Topçuoğlu B. D., Stewart L. C., Goldhammer T., et al. (2018). Experimental investigation on the controls of clumped isotopologue and hydrogen isotope ratios in microbial methane. Geochim. Cosmochim. Acta 237, 339–356. doi: 10.1016/j.gca.2018.06.029
Gunter B. D., Gleason J. D. (1971). Isotope fractionation during gas chromatographic separations. J. Chromatographic Sci. 9, 191–192. doi: 10.1093/chromsci/9.3.191
Gutsalo L. K., Plotnikov A. M. (1981). Carbon isotopic composition in the CH4-CO2 system as a criterion for the origin of methane and carbon dioxide in earth natural gases (in Russian). Doklady Akademii Nauk SSSR 259, 470–473.
Hachikubo A., Khlystov O., Krylov A., Sakagami H., Minami H., Nunokawa Y., et al. (2010). Molecular and isotopic characteristics of gas hydrate-bound hydrocarbons in southern and central lake baikal. Geo-Marine Lett. 30 (3-4), 321–329. doi: 10.1007/s00367-010-0203-1
Hachikubo A., Ozeki T., Kosaka T., Sakagami H. (2008a). “Isotopic fractionation of guest gas at the formation of methane and ethane hydrates,” in Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008).
Hachikubo A., Yanagawa K., Tomaru H., Lu H. L., Matsumoto R. (2015). Molecular and isotopic composition of volatiles in gas hydrates and in sediment from the joetsu basin, Eastern margin of the Japan Sea. Energies 8 (6), 4647–4666. doi: 10.3390/en8064647
Harkness J. S., Darrah T. H., Warner N. R., Whyte C. J., Moore M. T., Millot R., et al. (2017). The geochemistry of naturally occurring methane and saline groundwater in an area of unconventional shale gas development. Geochim. Cosmochim. Acta 208, 302–334. doi: 10.1016/j.gca.2017.03.039
Head l.M., Jones D. M., Larter S. R. (2003). Biological activity in the deep subsurface and the origin of heavy oil. Nature 426, 344–352. doi: 10.1038/nature02134
Hinrichs K.-U., Hayes J. M., Bach W., Spivack A. J., Hmelo L. R., Holm N. G., et al. (2006). Biological formation of ethane and propane in the deep marine subsurface. Proc. Natl. Acad. Sci. 103, 14684–14689. doi: 10.1073/pnas.0606535103
Holler T., Wegener G., Knittel K., Boetius A., Brunner B., Kuypers M. M., et al. (2009). Substantial (13) C/(12) c and D/H fractionation during anaerobic oxidation of methane by marine consortia enriched in vitro. Environ. Microbiol. Rep. 1 (5), 370–376. doi: 10.1111/j.1758-2229.2009.00074.x
Hunt A. G., Darrah T. H., Poreda R. J. (2012). Determining the source and genetic fingerprint of natural gases using noble gas geochemistry: A northern Appalachian basin case study. AAPG Bull. 96 (10), 1785–1811. doi: 10.1306/03161211093
Hunt A. G., Pohlman J., Stern L., Ruppel C., Moscati R. J., Landis G. P., et al. (2011). “Observations of mass fractionation of noble gases in synthetic methane hydrate,” in Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, United Kingdom.
Hunt A. G., Stern L., Pohlman J. W., Ruppel C., Moscati R. J., Landis G. P. (2013). Mass fractionation of noble gases in synthetic methane hydrate: Implications for naturally occurring gas hydrate dissociation. Chem. Geol. 339, 242–250. doi: 10.1016/j.chemgeo.2012.09.033
Ijiri A., Inagaki F., Kubo Y., Adhikari R. R., Hattori S., Hoshino T., et al. (2018). Deep-biosphere methane production stimulated by geofluids in the nankai accretionary complex. Sci. Adv. 4, eaao4631. doi: 10.1126/sciadv.aao4631
Inagaki F., Hinrichs K. U., Kubo Y., Bowles M. W., Heuer V. B., Hong W. L., et al. (2015). Exploring deep microbial life in coal-bearing sediment down to similar to 2.5 km below the ocean floor. Science 349, 420–424. doi: 10.1126/science.aaa6882
James A. T., Burns B. J. (1984). Microbial alteration of subsurface natural gas accumulations. AAPG Bull. 68, 957–960. doi: 10.1306/AD46169C-16F7-11D7-8645000102C1865D
Jin J., Wang X., Guo Y., Li J., Li Y., Zhang X., et al. (2020). Geological controls on the occurrence of recently formed highly concentrated gas hydrate accumulations in the shenhu area, south China Sea. Mar. Pet. Geol. 116, 104294. doi: 10.1016/j.marpetgeo.2020.104294
Jones D. M., Head I. M., Gray N. D., Adams J. J., Rowan A. K., Aitken C. M., et al. (2008). Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451 (7175), 176–180. doi: 10.1038/nature06484
Jones E. J., Voytek M. A., Corum M. D., Orem W. H. (2010). Stimulation of methane generation from nonproductive coal by addition of nutrients or a microbial consortium. Appl. Environ. Microbiol. 76 (21), 7013–7022. doi: 10.1128/AEM.00728-10
Kennett J. P., Cannariato K. G. H., Hendy I. L., Behl R. J.. (2000). Carbon Isotopic Evidence for Methane Hydrate Instability During Quaternary Interstadials. Science 288, 128–133. doi: 10.1126/science.288.5463.12
Kida M., Jin Y., Watanabe M., Konno Y., Yoneda J., Egawa K., et al. (2015). Chemical and crystallographic characterizations of natural gas hydrates recovered from a production test site in the eastern nankai trough. Mar. Pet. Geol. 66, 396–403. doi: 10.1016/j.marpetgeo.2015.02.019
Kida M., Khlystov O., Zemskaya T., Takahashi N., Minami H., Sakagami H., et al. (2006). Coexistence of structure I and II gas hydrates in lake baikal suggesting gas sources from microbial and thermogenic origin. Geophy. Res. Lett. 33 (24), L24603. doi: 10.1029/2006gl028296
Kimura H., Fuseya G., Takeya S., Hachikubo A. (2021). Carbon isotope fractionation during the formation of CO2 hydrate and equilibrium pressures of (12)CO2 and (13)CO2 hydrates. Molecules 26 (14), 4215. doi: 10.3390/molecules26144215
Kimura H., Hachikubo A., Sakagami H., Minami H., Yamashita S., Khlystov O., et al. (2020). Isotopic difference between hydrate-bound and sediment gases retrieved at lake baikal. Limnol. Freshw. Biol. 4), 924–925. doi: 10.31951/2658-3518-2020-a-4-924
Kinnaman F. S., Valentine D. L., Tyler S. C. (2007). Carbon and hydrogen isotope fractionation associated with the aerobic microbial oxidation of methane, ethane, propane and butane. Geochim. Cosmochim. Acta 71 (2), 271–283. doi: 10.1016/j.gca.2006.09.007
Knittel K., Boetius A. (2009). Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334. doi: 10.1146/annurev.micro.61.080706.093130
Kvenvolden K. A. (1988). Methane hydrate — a major reservoir of carbon in the shallow geosphere? Chem. Geol. 71, 41–51. doi: 10.1016/0009-2541(88)90104-0
Kvenvolden K. A. (1995). A review of the geochemistry of methane in natural gas hydrate. Org. Geochem. 23, 997–1008. doi: 10.1016/0146-6380(96)00002-2
Labidi J., Young E. D., Giunta T., Kohl I. E., Seewald J., Tang H., et al. (2020). Methane thermometry in deep-sea hydrothermal systems: Evidence for re-ordering of doubly-substituted isotopologues during fluid cooling. Geochim. Cosmochim. Acta 288, 248–261. doi: 10.1016/j.gca.2020.08.013
Lai H., Fang Y., Kuang Z., Ren J., Liang J., Lu J.a., et al. (2021a). Geochemistry, origin and accumulation of natural gas hydrates in the qiongdongnan basin, south China Sea: Implications from site GMGS5-W08. Mar. Pet. Geol. 123 (2021), 104774. doi: 10.1016/j.marpetgeo.2020.104774
Lai H., Fang Y., Kuang Z., Xing D., Li X., Kang D., et al. (2021b). Molecular and carbon isotopic characteristics during natural gas hydrate decomposition: Insights from a stepwise depressurization experiment on a pressure core. Energy Fuels 35 (19), 15579–88. doi: 10.1021/acs.energyfuels.1c01978
Lai H., Qiu H., Kuang Z., Ren J., Fang Y., Liang J., et al. (2022). Integrated signatures of secondary microbial gas within gas hydrate reservoirs: A case study in the shenhu area, northern south China Sea. Mar. Pet. Geol. 136 (2022), 105486. doi: 10.1016/j.marpetgeo.2021.105486
Lalk E., Pape T., Gruen D. S., Kaul N., Karolewski J. S., Bohrmann G., et al. (2022). Clumped methane isotopologue-based temperature estimates for sources of methane in marine gas hydrates and associated vent gases. Geochim. Cosmochim. Acta. doi: 10.1016/j.gca.2022.04.013
Lapham L. L., Wilson R. M., Chanton J. P. (2012). Pressurized laboratory experiments show no stable carbon isotope fractionation of methane during gas hydrate dissolution and dissociation. Rapid Commun. Mass Spectrom 26 (1), 32–36. doi: 10.1002/rcm.5290
Larter S. R., Head I. M., Huang H., Bennett B., Jones M., Aplin A. C., et al. (2005). “Biodegradation, gas destruction and methane generation in deep subsurface petroleum reservoirs: an overview,” in Pet. geology: Northwest Europe and global perspectives. Eds. Dore A. G., Vining B. (London: Geological Society). Proceedings of the 6th Petroleum Geology Conference.
Leythaeuser D., Schaefer R. G., Yukler A. (1982). Role of diffusion in primary migration of hydrocarbons. AAPG Bull. 66, 408–429. doi: 10.1306/03B59B2A-16D1-11D7-8645000102C1865D
Liang Q., Xiao X., Zhao J., Zhang W., Li Y., Wu X., et al. (2022). Geochemistry and sources of hydrate-bound gas in the shenhu area, northern south China sea: Insights from drilling and gas hydrate production tests. J. Pet. Sci. Eng. 208 (2022), 109459. doi: 10.1016/j.petrol.2021.109459
Li J., Liu Z. L., Li Z. S., Hu G. Y., Yan Q. T., Shan X. Q., et al. (2003). Experiment investigation on the carbon isotope and composition fractionation of methane during gas migration by diffusion. Natural Gas Geosci. 14, 463–468. doi: 10.3969/j.issn.1672-1926.2003.06.008
Li A., Li Q., Xu C., Cai F., Wang H., Zhang W. (2021). Review of methane seepages in the Okinawa trough: Progress and outlook. Geofluids 2021, 1–13. doi: 10.1155/2021/5539893
Lillis P. G., Warden A., Claypool G. E., Magoon L. B. (2007). “Petroleum systems ofthe San Joaquin basin province – geochemical characteristics of gas types. In:Hosford Scheirer A (ed) Petroleum systems and geologic assessment of oil and gas in the San Joaquin Basin Province, California. (Energy Resources Program, U.S. Geological Survey, Reston: US Geological Survey professional paper) vol 1713.
Lin G., Lu J., Luo K., Fang Y., Liu J., Ji X., et al. (2022). Characterization of bacterial and archaeal community structure in deep subsurface sediments in the shenhu area, northern south China Sea. Mar. Pet. Geol. 136 (2022), 105468. doi: 10.1016/j.marpetgeo.2021.105468
Lin X. Y., Zeng J. H. (2010). Gas composition differentiation during natural gas hydrate formation and its geological significance. Geoscience 24, 1157–1163. doi: 10.3969/j.issn.1000-8527.2010.06.018
Li Y., Pang L., Wang Z., Meng Q., Guan P., Xu X., et al. (2022). Geochemical characteristics and significance of organic matter in hydrate-bearing sediments from shenhu area, south China Sea. Molecules 27 (8), 2533. doi: 10.3390/molecules27082533
Liu C., Meng Q., He X., Li C., Ye Y., Zhang G., et al. (2015). Characterization of natural gas hydrate recovered from pearl river mouth basin in south China Sea. Mar. Pet. Geol. 61, 14–21. doi: 10.1016/j.marpetgeo.2014.11.006
Li J.-f., Ye J.-l., Qin X.-w., Qiu H.-j., Wu N.-y., Lu H.-l., et al. (2018). The first offshore natural gas hydrate production test in south China Sea. China Geol. 1 (1), 5–16. doi: 10.31035/cg2018003
Lorenson T. D., Collett T. S. (2018). National gas hydrate program expedition 01 offshore india; gas hydrate systems as revealed by hydrocarbon gas geochemistry. Mar. Pet. Geol. 92, 477–492. doi: 10.1016/j.marpetgeo.2017.11.011
Lorenson T. D., Collett T. S., Hunter R. B. (2011). Gas geochemistry of the mount Elbert gas hydrate stratigraphic test well, Alaska north slope: Implications for gas hydrate exploration in the Arctic. Mar. Pet. Geol. 28 (2), 343–360. doi: 10.1016/j.marpetgeo.2010.02.007
Lüdmann T., Wong H. K. (2003). Characteristics of gas hydrate occurrences associated with mud diapirism and gas escape structures in the northwestern Sea of Okhotsk. Mar. Geol. 201 (4), 269–286. doi: 10.1016/s0025-3227(03)00224-x
Luzi M., Schicks J. M., Erzinger J. (2011a). “"Carbon isotopic fractionation of synthetic methane and carbon dioxide hydrates",” in Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011).
Martini A. M., Budai J. M., Walter L. M., Schoell M. (1996). Microbial generation of economic accumulations of methane within a shallow organic-rich shale. Nature 383, 155–158. doi: 10.1038/383155a0
Martini A., Walter L., Budai J., Ku T., Kaiser C., Schoell M. (1998). Genetic and temporal relations between formation waters and biogenic methane: Upper Devonian Antrim shale, Michigan basin, USA. Geochim. Cosmochim. Acta 62, 1699–1720. doi: 10.1016/S0016-7037(98)00090-8
Mesle M., Dromart G., Oger P. (2013). Microbial methanogenesis in subsurface oil and coal. Res. Microbiol. 164 (9), 959–972. doi: 10.1016/j.resmic.2013.07.004
Michel J. B., Shen Y. K., Aiden A. P., Veres A., Gray M. K., Team G. B., et al. (2011). Quantitative analysis of culture using millions of digitized books. Science 331, 176–182. doi: 10.1126/science.1199644
Milkov A. V. (2011). Worldwide distribution and significance of secondary microbial methane formed during petroleum biodegradation in conventional reservoirs. Org. Geochem. 42 (2), 184–207. doi: 10.1016/j.orggeochem.2010.12.003
Milkov A. V. (2018). Secondary microbial gas In: Wilkes H. (Ed.), Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate. Springer, Cham. 1–10. doi: 10.1007/978-3-319-54529-5_22-1
Milkov A. V., Dzou L. (2007). Geochemical evidence of secondary microbial methane from very slight biodegradation of undersaturated oils in a deep hot reservoir. Geology 35 (5), 455. doi: 10.1130/g23557a.1
Milkov A. V., Etiope G. (2018). Revised genetic diagrams for natural gases based on a global dataset of > 20,000 samples. Org. Geochem. 125, 109–120. doi: 10.1016/j.orggeochem.2018.09.002
Moore M. T., Phillips S. C., Cook A. E., Darrah T. H. (2020). Improved sampling technique to collect natural gas from hydrate-bearing pressure cores. Appl. Geochem. 122 (2020), 104773. doi: 10.1016/j.apgeochem.2020.104773
Nisbet E. G., Dlugokencky E. J., Bousquet P. (2014). Methane on the rise-again. Science 343, 493–494. doi: 10.1126/science.1247828
Ono S., Rhim J. H., Gruen D. S., Taubner H., Kölling M., Wegener G. (2021). Clumped isotopologue fractionation by microbial cultures performing the anaerobic oxidation of methane. Geochim. Cosmochim. Acta 293, 70–85. doi: 10.1016/j.gca.2020.10.015
Ono S., Wang D. T., Gruen D. S., Sherwood Lollar B., Zahniser M. S., McManus B. J., et al. (2014). Measurement of a doubly substituted methane isotopologue, (1)(3)CH(3)D, by tunable infrared laser direct absorption spectroscopy. Anal. Chem. 86 (13), 6487–6494. doi: 10.1021/ac5010579
Oremland R. S., Whiticar M. J., Strohmaier F. E., Kiene R. P. (1988). Bacterial ethane formation from reduced, ethylated sulphur compounds in anoxic sediments. Geochim. Cosmochim. Acta 52, 1895–1904. doi: 10.1016/0016-7037(88)90013-0
Oxburgh E. R., O'nions R. K., Hill R. I. (1986). Helium isotopes in sedimentary basins. Nature 324, 632–635. doi: 10.1038/324632a0
Paganoni M., Cartwright J. A., Foschi M., Shipp R. C., Van Rensbergen P. (2016). Structure II gas hydrates found below the bottom-simulating reflector. Geophy. Res. Lett. 43 (11), 5696–5706. doi: 10.1002/2016gl069452
Paganoni M., Cartwright J. A., Foschi M., Shipp C. R., Van Rensbergen P. (2018). Relationship between fluid-escape pipes and hydrate distribution in offshore sabah (NW Borneo). Mar. Geol. 395, 82–103. doi: 10.1016/j.margeo.2017.09.010
Phrampus B. J., Hornbach M. J. (2012). Recent changes to the Gulf Stream causing widespread gas hydrate destabilization. Nature 490 (7421), 527–530. doi: 10.1038/nature11528
Pohlman J. W., Canuel E. A., Chapman N. R., Spence G. D., Whiticar M. J., Coffin R. B. (2005). The origin of thermogenic gas hydrates on the northern cascadia margin as inferred from isotopic (13C/12C and D/H) and molecular composition of hydrate and vent gas. Org. Geochem. 36 (5), 703–716. doi: 10.1016/j.orggeochem.2005.01.011
Pohlman J. W., Ruppel C., Hutchinson D. R., Downer R., Coffin R. B. (2008). Assessing sulfate reduction and methane cycling in a high salinity pore water system in the northern gulf of Mexico. Mar. Pet. Geol. 25 (9), 942–951. doi: 10.1016/j.marpetgeo.2008.01.016
Poreda R. J., Jenden P. D., Kaplan I. R., Craig H. (1986). Mantle helium in Sacramento basin naturalgas wells. Geochim. Cosmochim. Acta 50, 2847–2853. doi: 10.1016/0016-7037(86)90231-0
Prasolov E. M., Tokarev I. V., Ginsburg G. D., Soloviev V. A., Eltsova G. M. (1999). Helium and other noble gases in gas-hydrate sediments of the has kon mosby mud volcano. Geo-Marine Lett. 19, 84–88. doi: 10.1007/s003670050096
Prinzhofer A. A., Huc A. Y. (1995). Genetic and post-genetic molecular and isotopic fractionations in natural gases. Chem. Geol. 126, 281–290. doi: 10.1016/0009-2541(95)00123-9
Prinzhofer A., Mello M. R., Freitas L., Takaki T. (2000). New geochemical characterization of natural gas and its use in oil and gas evaluation. AAPG Bull. 73, 107–19. doi: 10.1016/s0304-3959(02)00346-9.
Prinzhofer A., Pernaton E. (1997). Isotopically light methane in natural gas: bacterial imprint or diffusive fractionation? Chem. Geol. 142, 193–200. doi: 10.1016/S0009-2541(97)00082-X
Reeburgh W. S. (1976). Methane consumption in cariaco trench waters and sediments. Earth Planet. Sci. Lett. 28, 337–344. doi: 10.1016/0012-821X(76)90195-3
Riboulot V., Ker S., Sultan N., Thomas Y., Marsset B., Scalabrin C., et al (2018). Freshwater lake to salt-water sea causing widespread hydrate dissociation in the Black Sea. Nat Commun 9 (1), 117. doi: 10.1038/s41467-017-02271-z
Rodrigues L., Ketzer J., Oliveira R., dos Santos V., Augustin A., Cupertino J., et al. (2019). Molecular and isotopic composition of hydrate-bound, dissolved and free gases in the Amazon deep-Sea fan and slope sediments, Brazil. Geosciences 9 (2), 73. doi: 10.3390/geosciences9020073
Rooney M. A., Claypool G. E., Chung H. M. (1995). Modeling thermogenic gas generation using carbon isotope ratios of natural gas hydrocarbons. Chem. Geol. 126, 219–232. doi: 10.1016/0009-2541(95)00119-0
Ruffine L., Donval J.-P., Croguennec C., Burnard P., Lu H., Germain Y., et al. (2018). Multiple gas reservoirs are responsible for the gas emissions along the marmara fault network. Deep Sea Res. Part II: Topical Stud. Oceanog. 153, 48–60. doi: 10.1016/j.dsr2.2017.11.011
Saito H., Suzuki N. (2007). Terrestrial organic matter controlling gas hydrate formation in the nankai trough accretionary prism, offshore Shikoku, Japan. J. Geochem. Explor. 95 (1-3), 88–100. doi: 10.1016/j.gexplo.2007.05.007
Sassena R., Joye S., Sweeta S. T., DeFreitasa D. A., Milkov A. V., MacDonald I. R. (1999). Thermogenic gas hydrates and hydrocarbon gases in complex chemosynthetic communities, gulf of Mexico continental slope. Org. Geochem. 30, 485–497. doi: 10.1016/S0146-6380(99)00050-9
Sassena R., Losh S. L., Iii L. C., Roberts H. H., Whelan J. K., Milkov A. V., et al. (2001a). Massive vein-filling gas hydrate: relation to ongoing gas migration from the deep subsurface in the gulf of mexico. Mar. Pet. Geol. 18, 551–560. doi: 10.1016/S0264-8172(01)00014-9
Sassena R., Sweet S. T., DeFreitas D. A., Morelosb J. A., Milkov A. V. (2001b). Gas hydrate and crude oil from the mississippi fan foldbelt, downdip gulf of mexico salt basin: significance to petroleum system. Org. Geochem. 32, 999–1008. doi: 10.1016/S0146-6380(01)00064-X
Sassen R., Sweet S. T., Milkov A. V., DeFreitas D. A., Kennicutt II, M.C. (2001). Thermogenic vent gas and gas hydrate in the gulf of Mexico. Geology 29, 107–110. doi: 10.1130/0091-7613(2001)029<0107:TVGAGH>2.0.CO;2
Schaefer R. G., Fletcher S. E. M., Veidt C., Lassey K. R., Brailsford (2016). A 21st-century shift from fossil-fuel to biogenic methane emissions indicated by 13CH4. Science 352, 80–84. doi: 10.1126/science.aad2705
Schlegel M. E., McIntosh J. C., Petsch S. T., Orem W. H., Jones E. J. P., Martini A. M. (2013). Extent and limits of biodegradation by in situ methanogenic consortia in shale and formation fluids. Appl. Geochem. 28, 172–184. doi: 10.1016/j.apgeochem.2012.10.008
Schlegel M. E., Zhou Z., McIntosh J. C., Ballentine C. J., Person M. A. (2011). Constraining the timing of microbial methane generation in an organic-rich shale using noble gases, Illinois basin, USA. Chem. Geol. 287 (1-2), 27–40. doi: 10.1016/j.chemgeo.2011.04.019
Schloemer S., Krooss B. M. (2004). Molecular transport of methane, ethane and nitrogen and the influence of diffusion on the chemical and isotopic composition of natural gas accumulations. Geofluids 4, 81–108. doi: 10.1111/j.1468-8123.2004.00076.x
Schoell M. (1980). The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 44, 649–661. doi: 10.1016/0016-7037(80)90155-6
Schoell M. (1983). Genetic characterization of natural gases. AAPG Bull. 22, 229–230. doi: 10.1306/AD46094A-16F7-11D7-8645000102C1865D
Schoell M. (1984). Recent advances in petroleum isotope geochemistry. Org. Geochem. 6, 645–663. doi: 10.1016/0146-6380(84)90086-X
Schwietzke S., Sherwood O. A., Bruhwiler L. M., Miller J. B., Etiope G., Dlugokencky E. J., et al. (2016). Upward revision of global fossil fuel methane emissions based on isotope database. Nature 538 (7623), 88–91. doi: 10.1038/nature19797
Shuai Y., Douglas P. M. J., Zhang S., Stolper D. A., Ellis G. S., Lawson M., et al. (2018). Equilibrium and non-equilibrium controls on the abundances of clumped isotopologues of methane during thermogenic formation in laboratory experiments: Implications for the chemistry of pyrolysis and the origins of natural gases. Geochim. Cosmochim. Acta 223, 159–174. doi: 10.1016/j.gca.2017.11.024
Sloan E. D., Koh C. A., Sum A. K. (2010). Gas hydrate stability and sampling: The future as related to the phase diagram. Energies 3 (12), 1991–2000. doi: 10.3390/en3121991
Sloan E. D. (1998). Clathrate hydrates of natural gas, second ed. (New York: Marcel Dekker Inc,), 726.
Smith A. J., Mienert J., Bünz S., Greinert J. (2014). Thermogenic methane injection via bubble transport into the upper Arctic ocean from the hydrate-charged vestnesa ridge, Svalbard. Geochem. Geophysics Geosyst. 15, 1945–1959. doi: 10.1002/2013GC005179
Snyder G. T., Sano Y., Takahata N., Matsumoto R., Kakizaki Y., Tomaru H. (2020). Magmatic fluids play a role in the development of active gas chimneys and massive gas hydrates in the Japan Sea. Chem. Geol. 535 (2020), 119462. doi: 10.1016/j.chemgeo.2020.119462
Stahl W. J. (1977). Carbon and nitrogen isotopes in hydrocarbon research and exploration. Chem. Geol. 20, 121–149. doi: 10.1016/0009-2541(77)90041-9
Stahl W. J., Carey B. D. (1975). Source-rock identification by isotope analyses of natural gases from fields in the val verde and delaware basins, west texas. Chem. Geol. 16, 257–267. doi: 10.1016/0009-2541(75)90065-0
Stern L. A., Lorenson T. D., Pinkston J. C. (2011). Gas hydrate characterization and grain-scale imaging of recovered cores from the mount Elbert gas hydrate stratigraphic test well, Alaska north slope. Mar. Pet. Geol. 28 (2), 394–403. doi: 10.1016/j.marpetgeo.2009.08.003
Stolper D. A., Lawson M., Davis C. L., Ferreira A. A., Santos Neto E. V., Ellis G. S., et al. (2014a). Gas formation. formation temperatures of thermogenic and biogenic methane. Science 344 (6191), 1500–1503. doi: 10.1126/science.1254509
Stolper D. A., Lawson M., Formolo M. J., Davis C. L., Douglas P. M. J., Eiler J. M. (2018). The utility of methane clumped isotopes to constrain the origins of methane in natural gas accumulations. Geological Society London Special Publications 468 (1), 23–52. doi: 10.1144/sp468.3
Stolper D. A., Martini A. M., Clog M., Douglas P. M., Shusta S. S., Valentine D. L., et al. (2015). Distinguishing and understanding thermogenic and biogenic sources of methane using multiply substituted isotopologues. Geochim. Cosmochim. Acta 161, 219–247. doi: 10.1016/j.gca.2015.04.015
Stolper D. A., Sessions A. L., Ferreira A. A., Santos Neto E. V., Schimmelmann A., Shusta S. S., et al. (2014b). Combined 13C–d and d–d clumping in methane: Methods and preliminary results. Geochim. Cosmochim. Acta 126, 169–191. doi: 10.1016/j.gca.2013.10.045
Sun L., Wang X., He M., Jin J., Li J., Yuanping L., et al. (2020). Thermogenic gas controls high saturation gas hydrate distribution in the pearl river mouth basin: Evidence from numerical modeling and seismic anomalies. Ore Geol. Rev. 127 (2020), 103846. doi: 10.1016/j.oregeorev.2020.103846
Taenzer L., Labidi J., Masterson A. L., Feng X., Rumble D., Young E. D., et al. (2020). Low Δ12CH2D2 values in microbialgenic methane result from combinatorial isotope effects. Geochim. Cosmochim. Acta 285, 225–236. doi: 10.1016/j.gca.2020.06.026
Teichert B. M. A., Eisenhauer A., Bohrmann G., Haase-Schramm A., Bock B., and Linke P., et al (2003). U/Th systematics and ages of authigenic carbonates from Hydrate Ridge, Cascadia Margin: Recorders of fluid flow variations. Geochim. Cosmochim. Acta 67 (20), 3845–3857. doi: 10.1016/s0016-7037(03)00128-5
Thompson K. F. M. (1979). Light hydrocarbons in subsurface sediments. Geochim. Cosmochim. Acta 43, 657–672. doi: 10.1016/0016-7037(79)90251-5
Tissot B., Durand B., Espitalie J., Combaz A. (1974). Influence of nature and diagenesis of organic matter in formation of petroleum. AAPG Bull. 58 (3), 499–506. doi: 10.1306/83D91425-16C7-11D7-8645000102C1865D
Tissot B. P., Welte D. H. (1984). Petroleum Formation and Occurrence. A New Approach to Oil and Gas Exploration (Berlin: Springer-Verlag), 538. doi.org/10.1007/978-3-642-87813-8
Tréhu A. M., Ruppel C., Holland M., Dickens G. R., Torres M. E., Collett T. S., et al. (2006). Gas hydrates in marine sediments: lessons from scientific ocean drilling. Oceanography 19, 124–142. doi: 10.5670/oceanog.2006.11
Valentine D. L., Chidthaisong A., Rice A., Reeburgh W. S., Tyler S. C. (2004). Carbon and hydrogen isotope fractionation by moderately thermophilic methanogens 1 1Associate editor: N. e. ostrom. Geochim. Cosmochim. Acta 68 (7), 1571–1590. doi: 10.1016/j.gca.2003.10.012
Van Krevelen D. W. (1961). Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 29, 269–283.
Vaular E. N., Barth T., Haflidason H. (2010). The geochemical characteristics of the hydrate-bound gases from the nyegga pockmark field, Norwegian Sea. Org. Geochem. 41 (5), 437–444. doi: 10.1016/j.orggeochem.2010.02.005
Wang T. (2010). Gas hydrate resource potential and its exploration and development prospect of the muli coalfield in the northeast Tibetan plateau. Energy Explor. Exploit. 28, 147–158. doi: 10.1260/0144-5987.28.3.147
Wang D. T., Gruen D. S., Sherwood L. B., Hinrichs K.-U., Stewart L. C., Holden J. F., et al (2015a). Nonequilibrium clumped isotope signals in microbial methane. Science 348, 428–431. doi: 10.1126/science.aaa43
Wang P., Huang X., Pang S., Zhu Y., Lu Z., Zhang S., et al (2015b). Geochemical dynamics of the gas hydrate system in the qilian mountain permafrost, qinghai, Northwest China. Mar. Pet. Geol. 59, 72–90. doi: 10.1016/j.marpetgeo.2014.07.009
Wang D. T., Welander P. V., Ono S. (2016). Fractionation of the methane isotopologues 13CH4, 12CH3D, and 13CH3D during aerobic oxidation of methane by methylococcus capsulatus (Bath). Geochim. Cosmochim. Acta 192, 186–202. doi: 10.1016/j.gca.2016.07.031
Wang P., Zhu Y., Lu Z., Bai M., Huang X., Pang S., et al. (2018). Research progress of gas hydrates in the qilian mountain permafrost, qinghai, Northwest China: Review. SCIENTIA Sin. Physica Mechanica Astronomica 49 (3), 034606. doi: 10.1360/sspma2018-00133
Warr O., Young E. D., Giunta T., Kohl I. E., Ash J. L., Sherwood Lollar B. (2021). High-resolution, long-term isotopic and isotopologue variation identifies the sources and sinks of methane in a deep subsurface carbon cycle. Geochim. Cosmochim. Acta 294, 315–334. doi: 10.1016/j.gca.2020.12.002
Waseda A., Iwano H. (2008). Characterization of natural gases in Japan based on molecular and carbon isotope compositions. Geofluids 8 (4), 286–292. doi: 10.1111/j.1468-8123.2008.00222.x
Waseda A., Uchida T. (2004). The geochemical context of gas hydrate in the Eastern nankai trough. Resource Geol. 54, 69–78. doi: 10.1111/j.1751-3928.2004.tb00188.x
Watanabe Y., Nakai S. i., Hiruta A., Matsumoto R., Yoshida K. (2008). U–Th dating of carbonate nodules from methane seeps off Joetsu, Eastern Margin of Japan Sea. Earth Planet. Sci. Lett. 272 (1–2), 89–96. doi: 10.1016/j.epsl.2008.04.012
Webb M. A., Miller T. F. (2014). Position-specific and clumped stable isotope studies: Comparison of the urey and path-integral approaches for carbon dioxide, nitrous oxide, methane, and propane. J. Phys. Chem. A 118 (2), 467–474. doi: 10.1021/jp411134v
Wen T., Castro M. C., Ellis B. R., Hall C. M., Lohmann K. C. (2015). Assessing compositional variability and migration of natural gas in the Antrim shale in the Michigan basin using noble gas geochemistry. Chem. Geol. 417, 356–370. doi: 10.1016/j.chemgeo.2015.10.029
Whiticar M. J. (1994). Correlation of natural gases with their sources. AAPG Memoir 60, 261–283. doi: 10.1306/M60585C16
Whiticar M. J. (1999). Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314. doi: 10.1016/S0009-2541(99)00092-3
Whiticar M. J., Faber E., Schoell M. (1986). Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation–isotope evidence. Geochim. Cosmochim. Acta 50, 693–709. doi: 10.1016/0016-7037(86)90346-7
Wiese K., Kvenvolden K. A. (1993). Introduction to microbial and thermal methane. United States Geological Survey Prof. Paper 1570, 13–20.
Winckler G., Aeschbach-Hertig W., Holocher J., Kipfer R., Levin I., Poss C., et al. (2002). Noble gases and radiocarbon in natural gas hydrates. Geophy. Res. Lett. 29 (10), 63–61-63-64. doi: 10.1029/2001gl014013
Winckler G., Kipfer R., Aeschbach–Hertig W., Botz R., Schmidt M., Schuler S., et al. (2000). Sub Sea floor boiling of red sea brines: new indication from noble gas data. Geochim. Cosmochim. Acta 64, 1567–1575. doi: 10.1016/S0016-7037(99)00441-X
Wu N. Y., Zhang H. Q., Yang S. X., Zhang G. X., Liang J. Q., Lu J. A., et al. (2011). Gas hydrate system of shenhu area, northern south China Sea: Geochemical results. J. Geological Res. 2011, 1–10. doi: 10.1155/2011/370298
Xia X., Gao Y. (2019). Kinetic clumped isotope fractionation during the thermal generation and hydrogen exchange of methane. Geochim. Cosmochim. Acta 248, 252–273. doi: 10.1016/j.gca.2019.01.004
Xiao K., Zou C., Yang Y., Zhang H., Li H., Qin Z. (2019). A preliminary study of the gas hydrate stability zone in a gas hydrate potential region of China. Energy Sci. Eng. 8 (4), 1080–1091. doi: 10.1002/ese3.569
Xu W., Ruppel C. (1999). Predicting the occurrence, distribution, and evolution of methane gas hydrate in porous marine sediments. J. Geophy. Res.: Solid Earth 104 (B3), 5081–5095. doi: 10.1029/1998jb900092
Ye J., Qin X., Qiu H., Xie W., Lu H., Lu C., et al. (2018). Data report: Molecular and isotopic compositions of the extracted gas from china’s first offshore natural gas hydrate production test in south China Sea. Energies 11 (10), 2793. doi: 10.3390/en11102793
Yeung L. Y., Ash J. L., Young E. D. (2015). Biological signatures in clumped isotopes of O2. Science 348, 431–434. doi: 10.1126/science.aaa6284
Ye J., Wei J., Liang J., Lu J., Lu H., Zhang W. (2019). Complex gas hydrate system in a gas chimney, south China Sea. Mar. Pet. Geol. 104, 29–39. doi: 10.1016/j.marpetgeo.2019.03.023
Yoshinaga M. Y., Holler T., Goldhammer T., Wegener G., Pohlman J. W., Brunner B., et al. (2014). Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane. Nat. Geosci. 7 (3), 190–194. doi: 10.1038/ngeo2069
Young E. D. (2019). A two-dimensional perspective on CH4 isotope clumping. Deep Carbon 1029, 388–414. doi: 10.1017/9781108677950
Young E. D., Kohl I. E., Lollar B. S., Etiope G., Rumble D., Li S., et al. (2017). The relative abundances of resolved 12CH2D2 and 13CH3D and mechanisms controlling isotopic bond ordering in abiotic and biotic methane gases. Geochim. Cosmochim. Acta 203, 235–264. doi: 10.1016/j.gca.2016.12.041
Young E. D., Rumble D., Freedman P., Mills M. (2016). A large-radius high-mass-resolution multiple-collector isotope ratio mass spectrometer for analysis of rare isotopologues of O2, N2, CH4 and other gases. Int. J. Mass Spectrometry 401, 1–10. doi: 10.1016/j.ijms.2016.01.006
Zeikus J. G. (1977). The biology of methanogenic bacteria. Bacteriol. Rev. 41, 514–541. doi: 10.1128/br.41.2.514-541.1977
Zhang T., Krooss B. M. (2001). Experimental investigation on the carbon isotope fractionation of methane during gas migration by diffusion through sedimentary rocks at elevated temperature and pressure. Geochim. Cosmochim. Acta 65, 2723–2742. doi: 10.1016/S0016-7037(01)00601-9
Zhang W., Liang J., Liang Q., Wei J., Wan Z., Feng J., et al. (2021b). Gas hydrate accumulation and occurrence associated with cold seep systems in the northern south China Sea: An overview. Geofluids 2021, 1–24. doi: 10.1155/2021/5571150
Zhang W., Liang J. Q., Wei J. G., Su P. B., Lin L., Huang W. (2019). Origin of natural gases and associated gas hydrates in the shenhu area, northern south China Sea: Results from the China gas hydrate drilling expeditions. J. Asian Earth Sci. 183, 1–16. doi: 10.1016/j.jseaes.2019.103953
Zhang N., Snyder G. T., Lin M., Nakagawa M., Gilbert A., Yoshida N., et al. (2021a). Doubly substituted isotopologues of methane hydrate (13CH3D and 12CH2D2): Implications for methane clumped isotope effects, source apportionments and global hydrate reservoirs. Geochim. Cosmochim. Acta 315, 127–151. doi: 10.1016/j.gca.2021.08.027
Zhou Z., Ballentine C. J. (2006). 4He dating of groundwater associated with hydrocarbon reservoirs. Chem. Geol. 226 (3-4), 309–327. doi: 10.1016/j.chemgeo.2005.09.030
Keywords: gas hydrate, thermogenic gas, microbial gas, clumped isotope, noble gas
Citation: Li Y, Chang J and Lu H (2022) Geochemical characteristics of gases associated with natural gas hydrate. Front. Mar. Sci. 9:968647. doi: 10.3389/fmars.2022.968647
Received: 14 June 2022; Accepted: 24 August 2022;
Published: 12 September 2022.
Edited by:
Dong Feng, Shanghai Ocean University, ChinaReviewed by:
Zhilei Sun, Qingdao Institute of Marine Geology (QIMG), ChinaCopyright © 2022 Li, Chang, Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Lu, aGx1QHBrdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.