- 1Marine Biology and Biotechnology Program, Department of Life Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
- 2Israel Oceanography and Limnological Research, National Institute of Oceanography, Haifa, Israel
- 3The Interuniversity Institute for Marine Science, Eilat, Israel
- 4Spatial Ecology Lab, Department of Life Sciences, Ben Gurion University of the Negev, Beer-Sheva, Israel
The Trapeziidae constitute a widely distributed and common family of obligatory coral-dwelling crabs on Indo-west Pacific coral reefs, feeding on coral tissue and mucus. In situ nocturnal surveys on Stylophora pistillata (a common branching pocilloporid coral species in the Gulf of Eilat, Red Sea), revealed Trapezia cymodoce and Trapezia digitalis crabs foraging on swimming demersal plankton, including amphipods, copepods, isopods, swimming polychaetes, and occasionally fishes, employing three plankton feeding mechanisms. Laboratory experiments demonstrated that crabs actively searched for Artemia when these were present in the aquaria together with either dead or live coral branches, but did not do so when corals were absent. Overall, the results indicate that trapeziid crabs play a role as planktivorous reef organisms. This suggests the need for further study of the feeding habits of members of this family, considering the potential impacts of their foraging on demersal plankton dynamics in coral reefs.
Introduction
Scleractinian branching corals are essential habitats for diverse macroinvertebrates and fishes. Their living three-dimensional structure provides a variety of ecological services such as food, shelter, mediated competition, breeding grounds, and nurseries for many coral reef residents and pelagic species (Stella et al., 2011a; Coker et al., 2014; Shmuel et al., 2022). Among the common and widely distributed groups of coral-dwelling species are members of the crab genus Trapezia which are obligate symbionts within live pocilloporid corals (Knudsen, 1967; Patton, 1974; Galil, 1987; Castro, 2000; Castro, 2015). Trapezia crabs are considered to be dependent on their coral hosts that provide them with shelter, while exploiting the coral tissue, polyp fragments, mucus, and detritus settled on it as essential components of their daily nutrition (Glynn et al., 1985; Stimson, 1990; Rinkevich et al., 1991). In turn, Trapezia crabs protect the corals from predators and contribute to coral health and survival (Pratchett, 2001; Stewart et al., 2006; Rouzé et al., 2014). Nocturnal surveys on colonies of the pocilloporid coral Stylophora pistillata (Esper, 1792) in Eilat’s (Red Sea) reefs, revealed that Trapezia individuals actively also feed on swimming plankton, a result that led us to further explore the nature of this predation on demersal plankton.
Material and methods
In situ nocturnal observation
Trapezia cymodoce (Herbst, 1801) and Trapezia digitalis (Latreille, 1828) feeding behaviors were observed in situ in Eilat, Red Sea (29°30′ N, 34°56′ E, 3-5m depth) at night (at least half an hour after sunset) using SCUBA (October 2021–April 2022, 8 dives, 30–60 minutes each) and a white LED flashlight (BRETT C15, China), for 2–10 minutes per crab/coral colony. Underwater photos/videos were taken using a camera with a macro lens (Canon G7X; Epoque DML-2, Japan).
Laboratory feeding experiment
In the laboratory, our test animals comprised 114 T. cymodoce individuals (8–18mm, carapace width), dwelled within Stylophora pistillata colonies (provided by the Israeli Nature and Parks Authority). Prior to the experiment corals with their hosted crabs were kept for up to two days in a running seawater table facility at the Interuniversity Institute for Marine Science in Eilat. Individuals were paired, and each pair was placed in a closed system tank (24.4L) filled with fresh ambient seawater (23-24°C, under room LED light) and presented with one of three treatments: live coral, coral skeleton, and no coral (38 crabs/treatment). Following one hour of acclimatization, each tank was provided with 2-5ml of live Artemia (5 individuals/ml, 7-16 days old). The experiments were conducted repeatedly from 9:00 am to 2:00 am of the next day, with the same live coral or coral skeleton. Observations were made only on a single tank at a time. We defined success/failure outcomes for two feeding behaviors: (1) Reaction - crabs continuously flick their antennules, together with maxilliped beating, accompanied by moving the ambulatory pereiopods along the chela, toward the mouthparts (previously described as mucus collection behavior; Knudsen, 1967); (2) Predation - crabs use their chelipeds to catch and to eat the prey, either by (a) directly catching of passing Artemia, or (b) indirectly, by collecting Artemia trapped on the coral mucus. Each T. cymodoce individual was observed for up to 15 minutes. Differences between treatments were tested using Pearson’s Chi-square followed by post-hoc Fisher exact pairwise tests of independence for nominal data with false discovery rate correction. Analyses were performed using “rcompanion” package (Mangiafico, 2021) in RStudio version 1.3.1093.
Results
In situ nocturnal observation
At night, the T. cymodoce and T. digitalis crabs were observed in the upper coral compartments (Shmuel et al., 2022), with chelipeds extended, continuously moving their maxillipeds (forward, downward, and laterally to the body axis) towards the mouth (Figure 1A; Video S1). When plankton was present, crabs immediately began to stroke the chelipeds towards and away from mouths, simultaneously with fast moving maxillipeds, to collect small-size plankton) Videos S1 and S2). The Trapezia crabs actively foraged for a wide range of demersal zooplankton taxa that were frequently passing through the coral compartments, with both chelipeds simultaneously (Figures 1B-E; Figure S1; Video S1). The most common prey items were amphipods (Figure 1C), followed by various copepods, isopods (Figure 1D), polychaetes (Figure 1E), and occasionally fishes [e.g., Spratelloides delicatulus (Bennett, 1832)]. The crabs furthermore collected smaller planktonic organisms that became attached to the fringe of tomentum on the chela (Figures 1A, F), and subsequently transferred to the mouthparts. Plankton trapped on the coral tissues were collected using the chelar fingers (Figure 1C). Larger prey (e.g., amphipods), captured on coral tissues or on crab’s chelar swiftly escaped.
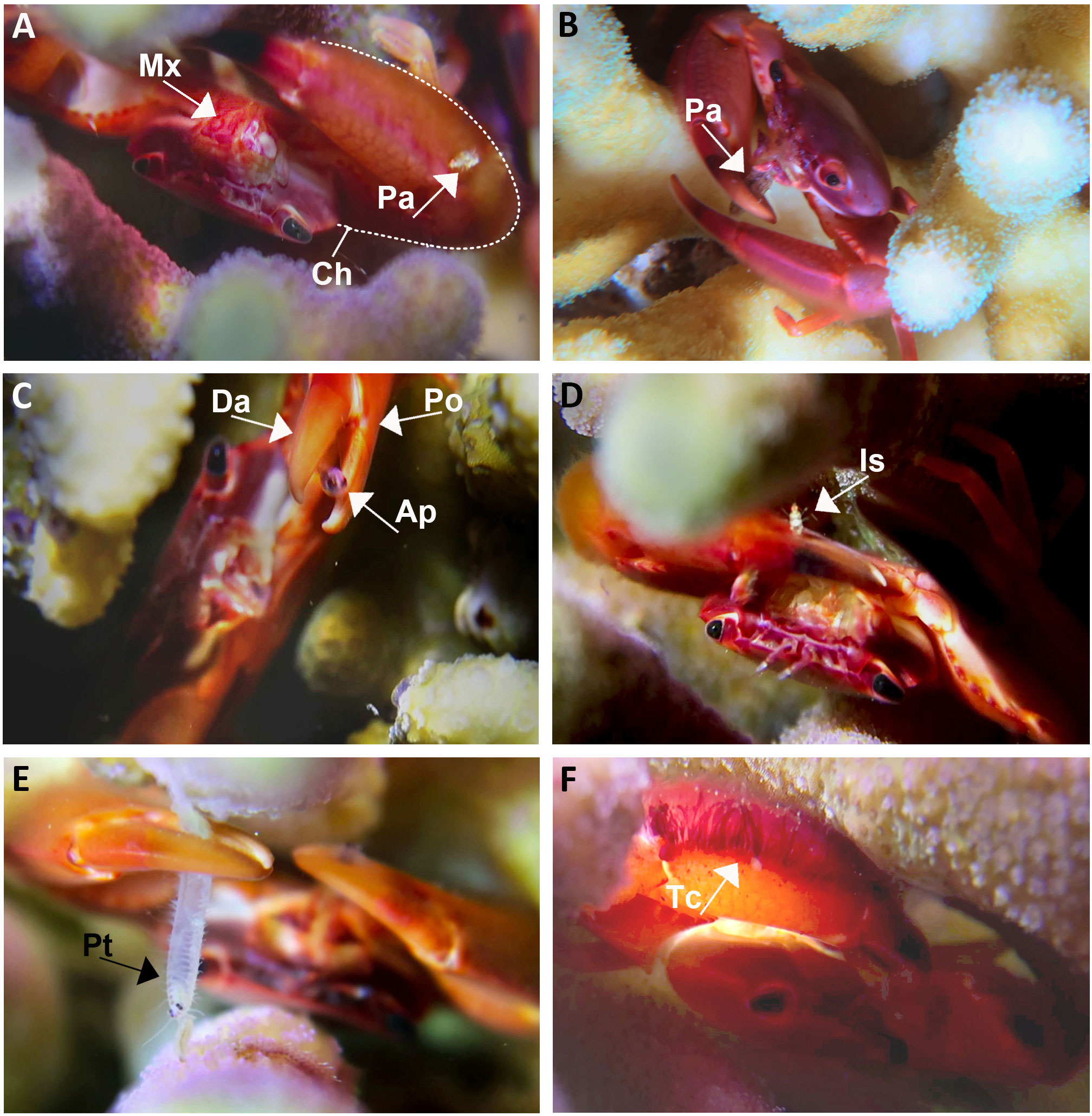
Figure 1 In-situ documentation of Trapezia cymodoce feeding behaviors. (A) Trapezia with extended open chelipeds (Ch) and with continuously moving maxillipeds (Mx), tomentose chela with a captured planktonic animal (Pa). (B) Trapezia actively consuming a planktonic animal (Pa) using its cheliped. (C) An escaping amphipod (Ap) catches between the dactylus (Da) and pollex (Po) of a Trapezia cheliped (also see Video S1). (D) An isopod (Is) captured by Trapezia cheliped (also see Video S1). (E) A polychaete (Pt) caught by the Trapezia chelipeds. (F) Close up on tomentose chela (Tc).
Laboratory feeding experiment
Laboratory experiments with T. cymodoce individuals on live corals and coral skeletons revealed active predation on Artemia, with 92% of the crabs (n=76) successfully catching prey, in less than 15 minutes. Indirect catches from the coral surfaces were observed only for crabs in the live coral setup (7/38 catches), whereas no significant difference was recorded in crab behaviors between the live coral/coral skeleton set-ups, both significantly differed from the non-coral set-up, in which the crabs did not display active predation behavior and ignored the surrounding Artemia (0/38 predation) even when in close vicinity to them [Pearson Chi-square test, X2(2)=90.68, p<0.001, and X2(2)=63.19, p<0.001, predation and reaction, respectively; Table 1, Supplementary S1].
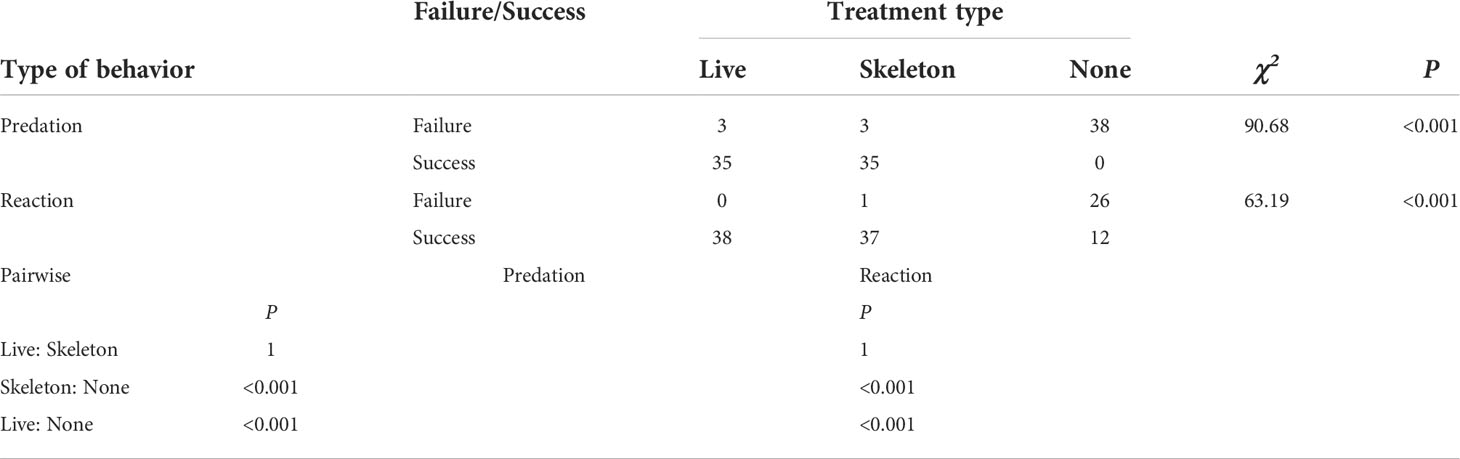
Table 1 Pearson’s Chi-square followed by Fisher exact pairwise tests of independence for nominal data analyses of predation and reaction behaviors (failure/success) of Trapezia cymodoce individuals subjected to three experimental set-ups: with live corals (‘live’); with coral skeletons (‘skeleton’); and without corals (‘none’), following the introduction of live Artemia.
Discussion
Here we document, for the first time, active nocturnal feeding of Trapezia crabs on plankton in situ and in laboratory settings. The crabs employ their chelipeds to directly catch both large (Amphipoda, Copepoda, Isopoda, Polychaeta) and small (including eggs and larvae) plankton, while these organisms freely move between the coral branches and compartments or when they were attached to the coral mucus. These results add both to our understanding of the trophic webs existing within coral canopies and to previous studies, documenting the ingestion of coral tissue mucus, including mucus-trapped small particles. The nocturnal feeding behaviors of Trapezia exposed here suggest that their relationships with their coral hosts are not fully understood, interactions that can potentially range from parasitic to mutualistic. Our finding reveals a previously undescribed planktivory group inhabiting the coral reefs (Trapezia crabs), an outcome that should be considered when describing nocturnal plankton predation in coral reefs.
Our observations further reveal that the Trapezia may use the fringe of tomentum on its chelae to trap planktonic prey items, and use the whole chelipeds to function as paddles, moving plankton towards the mouthparts. This study supplements earlier observations where finer planktonic particles were trapped by means of constant maxilliped movements, a type of suspension-feeding previously described for other crabs (Abelson et al., 1991; Trager and Genin, 1993; Patton, 1994; Valdivia and Stotz, 2006). Of particular interest is the feeding behavior of active trapping of large demersal plankton organisms by using the tips of the chelae fingers.
Our laboratory experiments confirm the field observations, revealing that Trapezia crabs actively react and catch plankton, and this feeding behavior is not affected when placed on bare coral skeleton. Yet, studies on coral bleaching events showed that the coral health status affects crabs’ densities and fecundities (Tsuchiya et al., 1992; Stella et al., 2011b), research outcomes suggesting that the coral tissues, as well as the released mucus, may have positive nutritional impacts (Bythell and Wild, 2011) and that Trapezia crabs recede from diseased or stressed coral colonies (Glynn et al., 1985). Results further revealed that regardless of day/night hours, the introduction of prey did not induce plankton-feeding behaviors by resting crabs, when corals are absent (either alive or bare skeletons). Furthermore, Trapezia crabs in the reef are actively feeding only at nighttime, when they usually move between colonies (Castro, 1978). It is highly possible that daytime feeding is not activated due to the existence of predatory fishes such as wrasses, hawkfishes, and sweepers (Stella et al., 2011a) and we may expect that in predation relax sites, Trapezia crabs would be observed feeding also during daytime hours.
Conclusion
Trapezia crabs actively feed on plankton. The three documented foraging methods include seizing swimming plankton, collecting trapped plankton either from the coral tissue or by the tomentum chelae, and suspension-feeding. Trapezia cymodoce foraging activity depends on the presence of branching corals (live/dead) and the three-dimension structure formed by the coral astogeny. Future studies should elucidate the impacts of Trapezia and other planktivorous coral-dwelling species on the food-web dynamics in coral reefs, the nature of Trapezia-coral relationship, and the dynamic usage (day/night; presence/absence of prey) of the coral canopy’s compartments by Trapezia crabs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YS, sample collection, experiment design, data processing, photography, and writing original draft. YZ and BR, project administration, supervision, writing- review, and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We wish to thank B.V. Farstey and T. Guy-Haim for taxonomic identification, T. Marcus for photo editing, and N. Paz for editing the manuscript. We would like to thank the Interuniversity Institution for Marine Sciences (IUI) and the Underwater Observatory Marine Park in Eilat for logistical support. We also thank the reviewers who greatly improved the final manuscript. This manuscript is dedicated to the memory of Ziggy Livnat who was a true warrior of the marine environment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.964725/full#supplementary-material
References
Abelson A., Galil B., Loya Y. (1991). Skeletal modifications in stony corals caused by indwelling crabs: hydrodynamical advantages for crab feeding. Symbiosis 1, 233–248.
Bythell J. C., Wild C. (2011). Biology and ecology of coral mucus release. J. Exp. Mar. Biol. Ecol. 408, 88–93. doi: 10.1016/j.jembe.2011.07.028
Castro P. (1978). Movements between coral colonies in Trapezia ferruginea (Crustacea brachyura), an obligate symbiont of scleractinian corals. Mar. Biol. 46, 237–245. doi: 10.1007/BF00390685
Castro P. (2000). “Biogeography of trapeziid crabs (Brachyura, trapeziidae) symbiotic with reef corals and other cnidarians,” in The biodiversity crisis and crustacea proceedings of the fourth international crustacean congress, vol. 2(12). Eds. von Vaupel Klein J. C., Schram F. R. (Amsterdam: CRC Press). p 65–75.
Castro P. (2015). “Symbiotic brachyura,” in Decapoda: Brachyura, treatise on zoology – anatomy, taxonomy, biology, 9C-I, vol. 71–10 . Eds. Castro P., Davie P., Guinot D., Schram F. R., von Vaupel Klein J. C. (Leiden and Boston: Brill), 543–581.
Coker D. J., Wilson S. K., Pratchett M. S. (2014). Importance of live coral habitat for reef fishes. Rev. Fish. Biol. Fish. 24, 89–126. doi: 10.1007/s11160-013-9319-5
Galil B. (1987). The adaptive functional structure of mucus-gathering setae in trapezid crabs symbiotic with corals. Symbiosis 4, 75–86.
Glynn P. W., Perez M., Gilchrist S. L. (1985). Lipid decline in stressed corals and their crustacean symbionts. Biol. Bull. 168, 276–284. doi: 10.2307/1541240
Knudsen J. (1967). Trapezia and Tetralia (Decapoda, brachyura, xanthidae) as obligate ectoparasites of pocilloporid and acroporid corals. Pac. Sci. 21, 50–57.
Mangiafico S. (2021). “Package ‘rcompanion’ functions to support extension education program evaluation,” in R package version 1.0.1. Cran. Available at: https://CRAN.R-project.org/package=rcompanion.
Patton W. K. (1974). “Community structure among the animals inhabiting the coral pocillopora damicornis at heron island, Australia,” in Symbiosis in the Sea. Ed. Vernberg W. B. (Columbia: University of South Carolina Press), 210–243.
Patton W. K. (1994). Distribution and ecology of animals associated with branching corals (Acropora spp.) from the great barrier reef, Australia. Bull. Mar. Sci. 55, 193–211.
Pratchett M. S. (2001). Influence of coral symbionts on feeding preferences of crown-of-thorns starfish Acanthaster planci in the western pacific. Mar. Ecol. Prog. Ser. 214, 111–119. doi: 10.3354/meps214111
Rinkevich B., Wolodarsky Z., Loya Y. (1991). Coral-crab association: a compact domain of a multilevel trophic system. Hydrobiologia 216/217, 279–284. doi: 10.1007/BF00026475
Rouzé H., Lecellier G., Mills S. C., Planes S., Berteaux-Lecellier V., Stewart H. (2014). Juvenile trapezia spp. crabs can increase juvenile host coral survival by protection from predation. Mar. Ecol. Prog. Ser. 515, 151–159. doi: 10.3354/meps10970
Shmuel Y., Ziv Y., Rinkevich B. (2022). Strahler ordering analyses on branching coral canopies: Stylophora pistillata as a case study. J. Mar. Sci. Eng. 10, 121. doi: 10.3390/jmse10010121
Stella J. S., Munday P. L., Jones G. P. (2011b). Effects of coral bleaching on the obligate coral-dwelling crab Trapezia cymodoce. Coral Reefs 30, 719–727. doi: 10.1007/s00338-011-0748-0
Stella J. S., Pratchett M. S., Hutchings P. A., Geoffrey J. P. (2011a). Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. 49, 43–104. doi: 10.1111/gcb.12569
Stewart H. L., Holbrook S. J., Schmitt R. J., Brooks A. J. (2006). Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 25, 609–615. doi: 10.1007/s00338-006-0132-7
Stimson J. (1990). Stimulation of fat-body production in the polyps of the coral Pocillopora damicornis by the presence of mutualistic crabs of the genus Trapezia. Mar. Biol. 106, 211–218. doi: 10.1007/BF01314802
Trager G., Genin A. (1993). Flow velocity induces a switch from active to passive suspension feeding in the porcelain crab Petrolisthes leptocheles (Heller). Biol. Bull. 185, 20–27. doi: 10.2307/1542127
Tsuchiya M., Yamauchi Y., Moretzsohn F., Tsukiji M. (1992). “Species composition and some population traits of obligate symbiotic xanthid crabs, Trapezia and Tetralia, associated with bleached corals,” in Proceedings of the 7th International Coral Reef Symposium, Eds. Richmond R.H. (Micronesia: University of Guam) Vol. 1. 56–63.
Keywords: Trapezia, foraging, demersal plankton, nocturnal, coral reef
Citation: Shmuel Y, Ziv Y and Rinkevich B (2022) Coral-inhabiting Trapezia crabs forage on demersal plankton. Front. Mar. Sci. 9:964725. doi: 10.3389/fmars.2022.964725
Received: 08 June 2022; Accepted: 09 August 2022;
Published: 26 August 2022.
Edited by:
Sancia Van Der Meij, University of Groningen, NetherlandsReviewed by:
Henrique Bravo, University of Groningen, NetherlandsRobert Lasley, Florida Museum of Natural History, United States
Copyright © 2022 Shmuel, Ziv and Rinkevich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaniv Shmuel, eWFuaXZzaG1AcG9zdC5iZ3UuYWMuaWw=
 Yaniv Shmuel
Yaniv Shmuel Yaron Ziv
Yaron Ziv Baruch Rinkevich
Baruch Rinkevich