- 1Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, Sanya Tropical Fisheries Research Institute, Sanya, China
- 2Tropical Aquaculture Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Sanya, China
- 3South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
We evaluated the effect of dietary curcumin supplementation on the antioxidant capacity of the liver and the resistance of the liver and spleen to ammonia stress in the great amberjack (Seriola dumerili). Three isonitrogenous and isolipidic test diets were prepared by supplementing incremental levels of dietary curcumin at 0 mg/kg (CUR0%, control), 100 mg/kg (CUR0.01%), and 200 mg/kg (CUR0.02%), respectively. Each diet was randomly assigned to triplicate groups of 15 fish per tank. At the end of the feeding experiment, dietary curcumin supplementation positively modulated antioxidant-related genes and enzyme activity in liver tissues. After the ammonia challenge, dietary supplementation with the appropriate level of curcumin alleviated ammonia stress in liver tissue by upregulating the relative expression of GSH-Px and downregulating the relative expression of Keap1 and GR. Meanwhile, ammonia stress in spleen tissue could also be alleviated by upregulating the relative expression of CAT, downregulating the relative expression of GR, and increasing the activity of SOD and GSH. After the recovery, dietary supplementation with curcumin still alleviated ammonia stress in the liver tissue by upregulating the relative expression of CAT, downregulating the relative expression of Keap1 and GR, and increasing the activity of SOD and GSH. On the other hand, ammonia stress in spleen tissue was still alleviated by upregulating the relative expression of Mn-SOD and increasing the activity of SOD and GSH. The histological structure results also showed that liver cells in the curcumin-containing groups exhibited a positive impact on cell boundaries, alignment, and nuclei after the ammonia challenge and recovery. Spleen cells in the curcumin-containing groups exhibited greater aggregation of melano-macrophage centers after the ammonia challenge and recovery. These results suggest that dietary curcumin supplementation at 100 mg/kg can promote the health condition and resistance to ammonia stress of the greater amberjack.
1. Introduction
The aquaculture industry is growing rapidly, which provides a valuable source of protein for the increasing global human demand (Gobi et al., 2016). According to the long-term monitoring data from the Food and Agriculture Organization of the United Nations (FAO), marine fishery resources continue to decline, while people’s consumption of edible fish continues to grow at an average annual rate of 3.1% until the latest statistics of 2017 (Stankus, 2021). In recent years, intensive farming systems have become more and more popular due to the characteristics of saving farmland, high yield, easy management, and low risk as the demand for human fish increases. However, intensive farming systems also bring many problems, such as the deterioration of the aquatic environment leading to disease outbreaks (Anderson, 1996; Luis et al., 2019). It has been shown that environmental ammonia concentrations are inevitably raised in intensive farming, even accumulating to unsafe levels due to high stocking densities, overfeeding, low water exchange frequency, and unbalanced diet composition (Randall and Tsui, 2002; Wilson, 2003). When the organism is exposed to high ammonia concentration, it causes abnormal oxidative reactions in aerobic metabolic pathways, forming excess singlet oxygen and other reactive oxygen species (ROS). This can lead to different histopathological changes and a range of physiological effects which can trigger diseases and death, resulting in huge economic losses (Hillaby and Randall, 1979; Qiu et al., 2011). In aquaculture practices, antibiotics and other chemicals are currently used in varying degrees to control diseases. However, as food safety awareness increases, these methods, causing antibiotic resistance due to drug abuse, are gradually becoming less acceptable (Wu et al., 2019). When these negative factors are combined, they have become an important factor limiting aquaculture production (Ahmed et al., 2019).
The greater amberjack (Seriola dumerili) is a marine pelagic carnivorous species that has become a popular fish in the aquaculture industry owing to its excellent taste and nutritional composition, as well as its rapid growth and adaptive characteristics (Jover et al., 1999; Mazzola et al., 2000). Intensive culture is also the main method of culture for the greater amberjack, so it faces the same dilemma as the other fishes. Currently, the addition of immunostimulants to the diet to enhance the innate and adaptive immune response is considered a promising addition to vaccination and selective breeding, which remain the key strategies for the prevention of diseases in fish aquaculture (Djordjevic et al., 2009). In recent years, the extracts of Chinese herbs have received commercial and scientific attention as potential functional materials for immunomodulation and aquaculture due to their effectiveness and fewer negative effects. Many studies have demonstrated the protective effect of Chinese herbal extracts on aquatic animals. Dietary supplementation with glycyrrhetinic acid could protect the liver of Jian carp (Cyprinus carpio var. Jian) by positively modulating the expression of antioxidant- and inflammation-related genes (Cao et al., 2017), and dietary supplementation with garlic could increase the antioxidant capacity of the liver and kidney of tilapia (Oreochromis niloticus) (El-Barbary, 2016).
The Chinese herbal extract we used for this experiment is curcumin which has characteristics of low cost, high availability, and efficiency. Curcumin is the active component of the turmeric rhizome, which has a scavenging effect on oxidative free radicals due to its phenolic structure (Agrawal and Mishra, 2010). Curcumin has also been shown to have various effects such as anti-inflammatory, anticancer, and other functions (Martin et al., 2012; Fu et al., 2014; Farhangi et al., 2015; Shi et al., 2015). Researchers found positive effects of dietary supplementation with curcumin on growth, antioxidant enzymes, and immune response in rainbow trout (Oncorhynchus mykiss) (Kohshahi et al., 2018). Dietary supplementation with 5 g/kg of curcumin significantly enhanced the antioxidant activity of crucian carp (Carassius auratus) in a study by Jiang et al. (2016). However, one previous work has only focused on the effects of curcumin on the serum and intestine of aquatic animals, with few studies on the effects of curcumin on the protective capacity of the fish liver and spleen tissue. The liver and spleen are key frontline immune tissues, playing an important role in both innate and adaptive immunity, and their health condition determines the growth and survival of the fish (Li et al., 2017; Kubes and Jenne, 2018). Furthermore, there are few studies on whether curcumin can still be effective in response to acute high concentrations of ammonia stress in the greater amberjack. Thus, it is necessary to investigate whether dietary curcumin supplementation can enhance the health condition of the liver and spleen of the greater amberjack to resist high ammonia stress. We evaluated the effect of dietary curcumin supplementation on the health and resistance to ammonia stress of the greater amberjack by the antioxidant capacity of the liver after the feeding experiment, as well as the antioxidant capacity and histological structure of the liver and spleen during ammonia stress. This study aimed to assess whether curcumin can be supplemented in the diet of the greater amberjack and also to provide information on the use of herbal extracts as immune additives in aquaculture.
2. Materials and methods
2.1 Experimental diet preparation
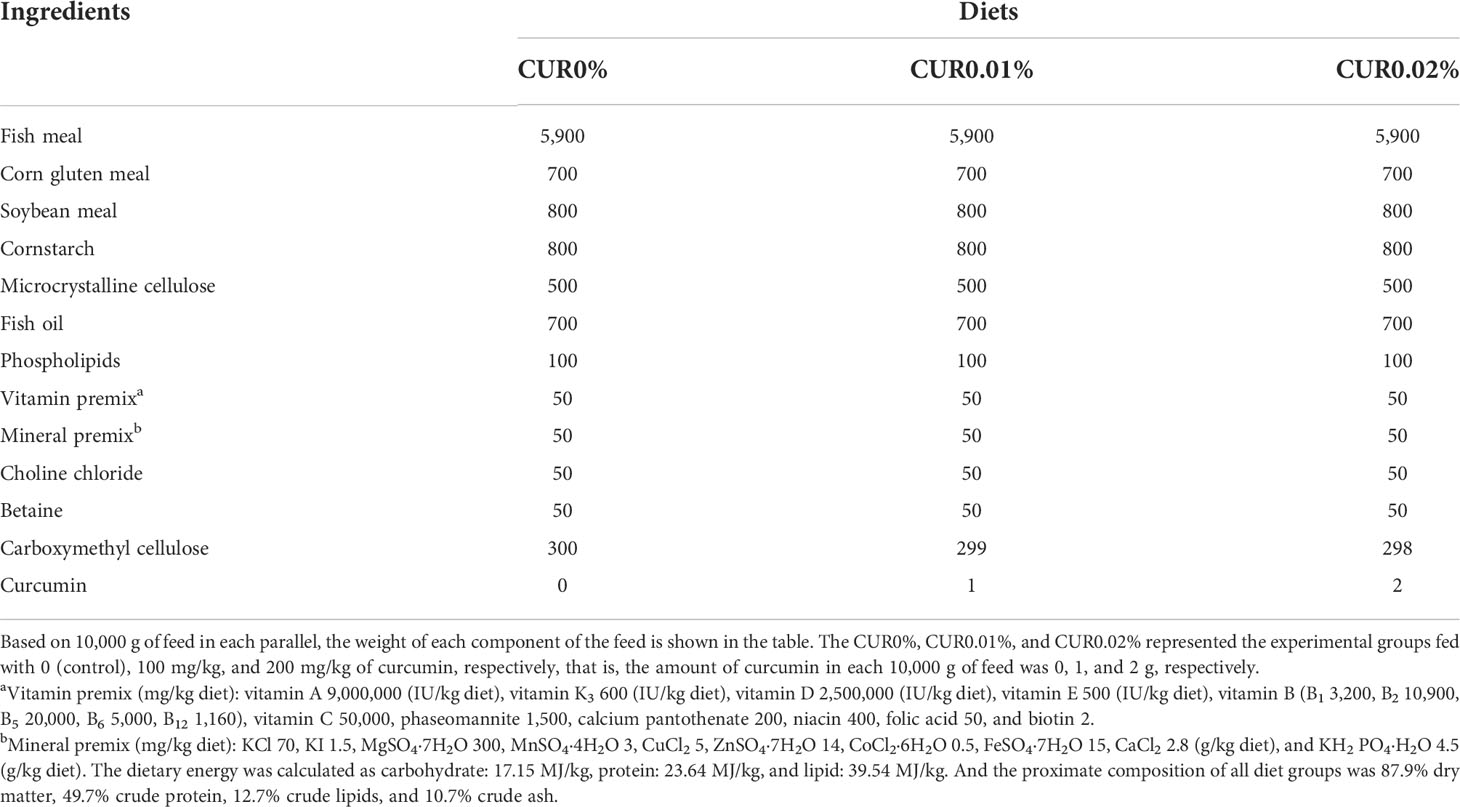
The curcumin (purity > 95%) used in the experiment was supplied by Xi’an Feida Biotechnology Co., Ltd (Xi'an, China). We referenced dietary curcumin levels from previous studies on grass carp (Ctenopharyngodon idella) and common carp (C. carpio) (G. Li et al., 2020; Zhang et al., 2021). Three isonitrogenous (49.7% crude protein) and isolipidic (12.7% crude lipid) experimental diets were formulated to contain different amounts of curcumin (0, 100, and 200 mg/kg, respectively). The formulation and approximate composition of the diets are presented in Table 1. Fish meal, corn gluten meal, and soybean meal were used as protein sources. Fish oil and phospholipids were used as lipid sources. All ingredients were finely ground, weighed, and kneaded until homogeneous. In order to prepare a supplemented diet, the inclusion of curcumin was performed by premixing it with the corn fraction before mixing it with the other ingredients. The mixture of all the ingredients was pelletized through a 1.5-mm diameter die and dried in a forced air circulation oven at 35°C for 24 h. All diets were designed and produced by the Tropical Fisheries Research and Development Center, South China Sea Fisheries Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. All feeds were sealed in vacuum-packed bags and stored at −20°C until use for the feeding trial.
2.2 Experimental design and fish
The wild-caught juvenile of the greater amberjack was obtained from the Tropical Fisheries Research and Development Center, South China Sea Fisheries Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (Lingshui, Hainan, China). Fish were randomly allocated to circulating mariculture tanks containing 200 L of seawater after 1 week of acclimatization. Fish were fed a basic diet without curcumin until the start of the experiment. The culture environment was maintained with sufficient dissolved oxygen by air stone connected to the central air compressors. The water temperature, salinity, and pH of the aquaculture seawater during the experimental period were maintained at 27°C–31°C, 35, and 7.5–8.0, respectively. The ammonia nitrogen content was lower than 0.1 mg/L, and the nitrite content was kept lower than 0.02 mg/L.
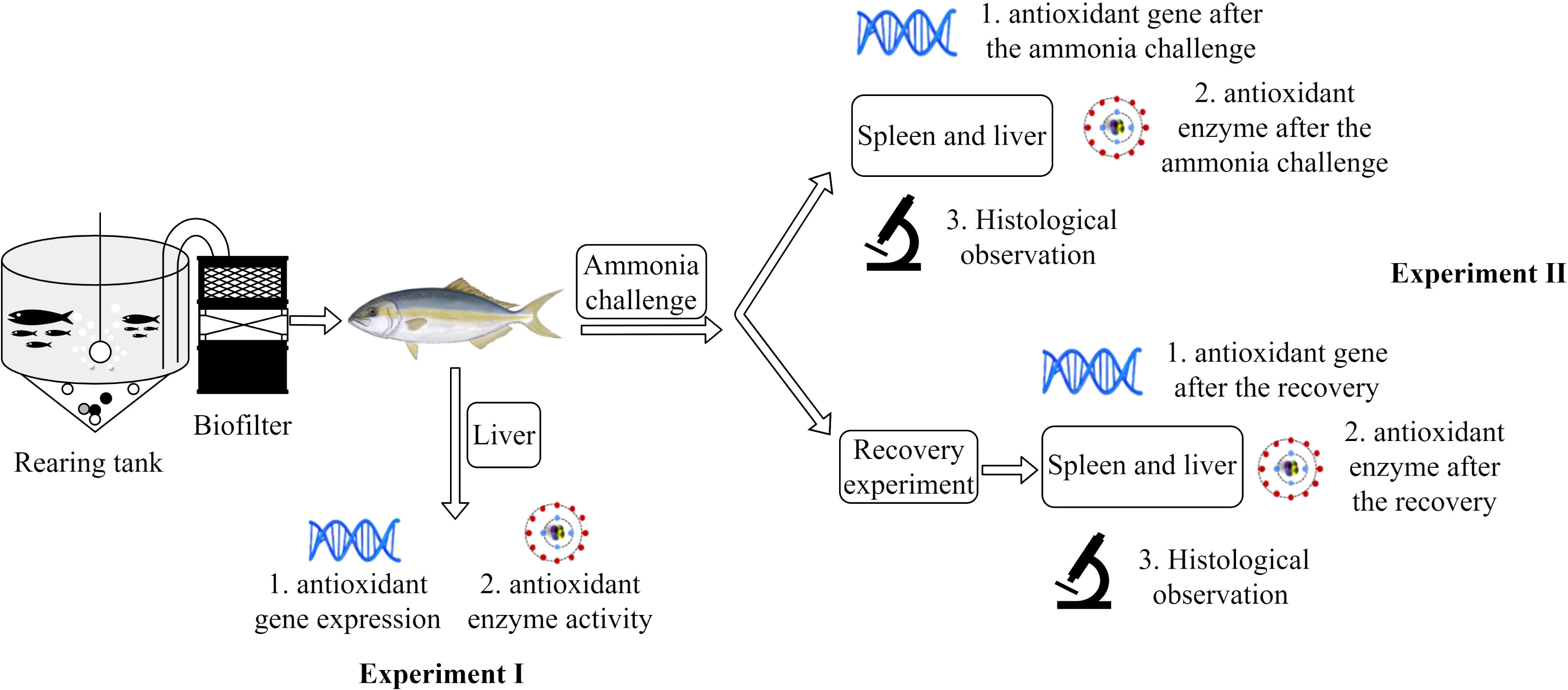
2.2.1 Experiment I
Experiment I was the feeding experiment to determine whether dietary curcumin supplementation could enhance liver health. A total of 135 juvenile greater amberjack were distributed randomly into three groups. Each group had a triplicate of 15 fish. Nine tanks were divided into three treatment groups that were given different diets (the curcumin content was 0, 100, and 200 mg/kg, respectively), and the control group was the non-curcumin supplementation group. Fish were hand-fed two times daily (at 8:00 and 20:00 h) for visual satiation. One hour after feeding, fish feces were cleaned from each tank to prevent pollution of the farming environment. Dead fish within 72 h after stocking was replaced by fish of similar size. Sampling for experiment I was conducted at the end of the 8-week feeding stage.
2.2.2 Experiment II
Experiment II was the ammonia challenge and recovery experiment to determine whether dietary curcumin supplementation improves the resistance of the liver and spleen to ammonia stress. At the end of the sampling stage of experiment I, the remaining fish were first subjected to the ammonia challenge experiment. The farming environment was prepared to the concentration of 1 g/L ammonia by ammonium chloride (NH4Cl). Four fish were randomly selected from each tank when all fish exhibited significant negative effects such as mania or syncope (lasting approximately 6 min according to the pre-experiment). Two of them were sampled for ammonia challenge and the other two fish were released into normal seawater for the recovery experiments. When the fish returned to their normal swimming state, they were anesthetized using a 50-mg/L concentration of eugenol (Shangchi Dental Materials Co., Ltd., Changshu, China), followed by sampling when the fish were in the syncope state. The schematic design of experiments I and II is presented in Figure 1.
2.3 Ethics statement
This study was carried out in strict accordance with the recommendation of the Animal Welfare Committee of the Chinese Academy of Fishery Sciences. No protected species were used during the experiment.
2.4 Sampling
After the feeding stage, the fish were starved for 24 h before sampling and then anesthetized with 50 mg/L of eugenol. We referred to previous studies on the greater amberjack for eugenol concentration (Hossain et al., 2017; Hossain et al., 2018). The livers from four fish (n = 4) per tank were collected for experiment I and immediately aliquoted into a sterile tube, rapidly frozen with liquid nitrogen, and stored at −80°C for analysis of antioxidant gene expression and antioxidant enzyme activity. After the ammonia challenge and recovery experiment, the livers and spleens from six fish (n = 6) per tank were collected for experiment II, respectively. Two of them (n = 2) were fixed in 10% buffered formalin for histological observation, while the other four (n = 4) were rapidly frozen using liquid nitrogen and stored at −80°C for analysis of antioxidant gene expression and antioxidant enzyme activity in the liver and spleen after the ammonia challenge and recovery.
2.5 Gene expression
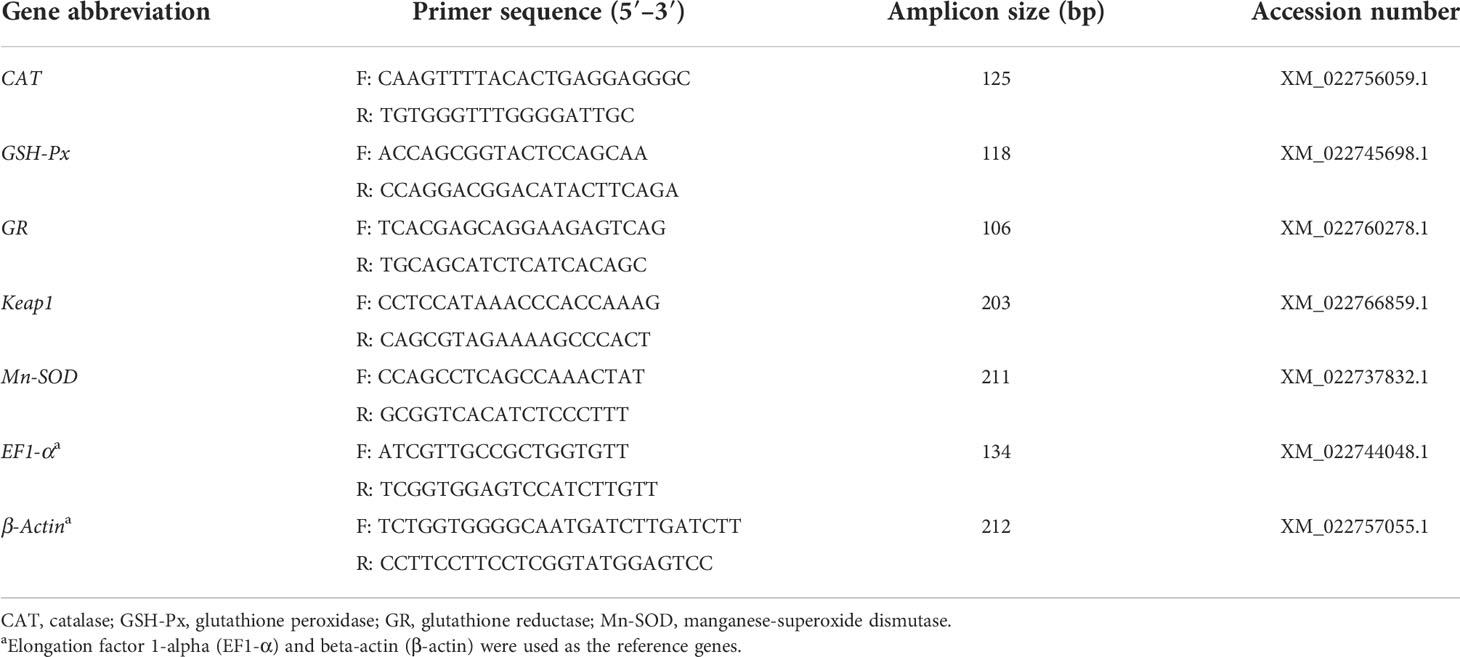
The harvested frozen liver and spleen tissues were homogenized in 1 ml of TRIzol reagent (Invitrogen, Thermo Fisher Scientific Co., Ltd., Shanghai, China) on ice using a handheld homogenizer (GreenPrima Instruments Co., Ltd., UK), and the RNA was separated in a chloroform layer and precipitated with isopropanol. The RNA pellets were washed in 1 ml of 75% ethanol and air-dried before resuspension in RNase-free water. The quantity of the isolated RNA was later determined by measuring their absorbance at 260 and 280 nm using a Nano-300 microspectrophotometer (Hangzhou AllSheng Instruments Co., Ltd., China), and the quality of total RNA was detected by electrophoresis on 1.0% agarose gel. Total RNA was reverse-transcribed using One-Step gDNA Removal and cDNA Synthesis SuperMix (EasyScript, Beijing TransGen Biotech., Ltd., Beijing, China) according to the manufacturer’s instructions, and the first-strand cDNA was synthesized accordingly. Real-time quantitative PCR analysis was conducted using a Real-Time PCR system (Q1000, Hangzhou Longji Scientific Instruments Co., Ltd., Hangzhou, China). The antioxidant-related genes chosen for analysis by qPCR were selected from the Seriola dumerili NCBI database (http://www.ncbi.nlm.nih.go/). The running conditions were as follows: initial denaturation at 95°C for 15 min, 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 30 s. All PCR amplifications were performed in triplicate, and the reactions include 2× RealUniversal PreMix (10 μl), each of the PCR primers (0.6 μl, 10 µmol/L), template cDNA (2 μl), and RNase-free ddH2O with a final volume of 20 μl. We screened the housekeeping genes in the pre-experiment. The β-actin, EF1-α, actb, EEFIA, and GAPDH genes were selected, and the expression of these genes in the liver, gill, spleen, and muscle in different experimental treatments was quantified. In addition, the stability of the measured gene expression was determined using geNorm through the RefFinder portal. The results showed that the CT ranges of β-actin and EF1-α were 20.21 ± 1.77 and 18.81 ± 1.15, respectively, and the M values were in the range of 1–1.5. So, EF1-α and β-actin were used as the reference genes. At the end of each cycle, a melting curve analysis of the primers was performed to ensure that only specific products were obtained with no formation of primer dimers. No template control was included with each assay to verify that PCR master mixes were free of contamination (Fu et al., 2019). The 10-fold serial dilution of each cDNA primer pair was performed to establish a standard curve. The efficiency of PCR reactions ranged from 90% to 110% and Pearson’s coefficient of determination (R2) was >0.97. The primers used for the analysis are shown in Table 2. We determined the relative expression of catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GR), Keap1, and manganese-superoxide dismutase (Mn-SOD) in liver and spleen tissues referring to previous studies that selected antioxidant-related genes (Jiang et al., 2016; Zhao et al., 2021).
2.6 Assay of antioxidant enzyme activity
Liver and spleen tissues were homogenized in ice-cold saline solution (0.86%) in a 1:9 (w/v) ratio. After centrifugation (2,500×g, 10 min, 4°C), the supernatants were separated and kept at 4°C for further analysis. The activities of total superoxide dismutase (T-SOD, E.C. 1.15.1.1) and glutathione peroxidase (GSH-Px, E.C. 1.11.1.9) in the liver and spleen were quantified based on the methods in previous studies (Djordjevic et al., 2011; Dabas et al., 2012). The malondialdehyde (MDA) content in the liver and spleen was determined using the thiobarbituric acid (TBA) method (Kei, 1978). All assays were determined using commercial kits (Nanjing Jiancheng Biotech. Co., Nanjing, China) and performed in triplicate. The protein concentration of the enzyme extracts was measured using the Bradford method (Bradford, 1976).
2.7 Histological observation of the liver and spleen
The fixed liver and spleen samples were wrapped in gauze and rinsed in running water for 24 h. Subsequently, the samples were dehydrated in ethanol and paraffin-embedded accordingly to standard histological procedures. The samples were sliced in a series of transverse sections (5-μm thickness) using a Leica RM 2016 rotary microtome (Shanghai Leica Instrument Co., Ltd., China). Hematoxylin and eosin (H&E) were used for staining and general histological analysis. Each slide with tissue sections was mounted permanently using neutral balsam. The sections were scanned by a Pannoramic 250/MIDI scanner (3DHISTECH Co., Ltd., Hungary), and the samples were photographed by an optical microscope (×100) (DMC4500, Leica, Germany).
2.8 Calculations and statistical analysis
The expression levels of antioxidant-related genes were normalized based on the level of housekeeping genes (EF1-α and β-actin). After verification of PCR efficiency to be around 100%, gene expression was analyzed based on the 2−△△Ct method (Livak and Schmittgen, 2001). All data were presented as the standard deviation (mean ± SD). One-way analysis of variance (ANOVA) was used to test the effect of dietary manipulation. Multiple comparisons of the LSD test were used to compare the means between groups if significance was detected. If significance was detected, the LSD test multiple comparisons were used to compare the means between groups. All statistical analyses were performed using SPSS 23 software. A significant difference was defined as P <0.05.
3. Results
3.1 Antioxidant capacity of the liver in experiment I
3.1.1 Relative expression of antioxidant-related genes after dietary supplementation with curcumin
The relative expression of the antioxidant-related genes in the liver of the greater amberjack is shown in Figure 2. In the fish liver, the relative expression of CAT and GSH-Px was positively associated with the dietary curcumin level, while a significantly (P < 0.05) upregulated relative expression was found in all curcumin-containing groups. A significantly (P < 0.05) upregulated relative expression of GR and Mn-SOD was found in all curcumin-containing groups, with the maximum value found in the CUR0.01% group. There was no significant difference (P > 0.05) in the relative expression of Keap1 among all the groups.
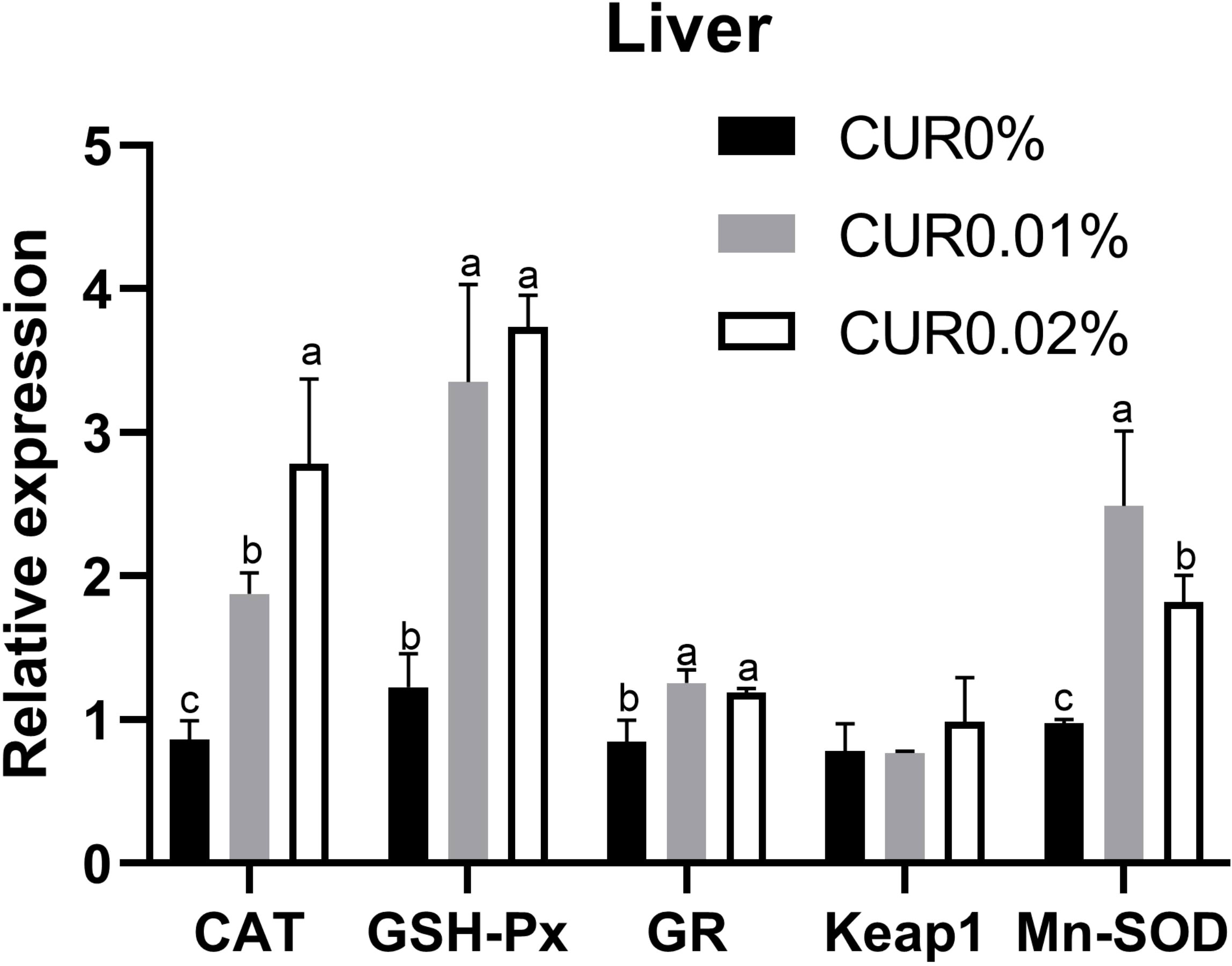
Figure 2 The relative expression of antioxidant genes in liver of the greater amberick after the 8-week feeding experiment in Experiment I. Different letters above bars indicate significant differences at the 0.05 level. Data are expressed as the means ± SD (n=3).
3.1.2 Antioxidant enzyme activity after dietary supplementation with curcumin
The activity of the antioxidant-related enzymes in the liver of the greater amberjack is shown in Figure 3. A significant increase (P < 0.05) in the activity of SOD was found only in the CUR0.01% group. The concentration of MDA was negatively associated with dietary curcumin level, with a significantly lower (P < 0.05) concentration of MDA found in the CUR0.02% group.
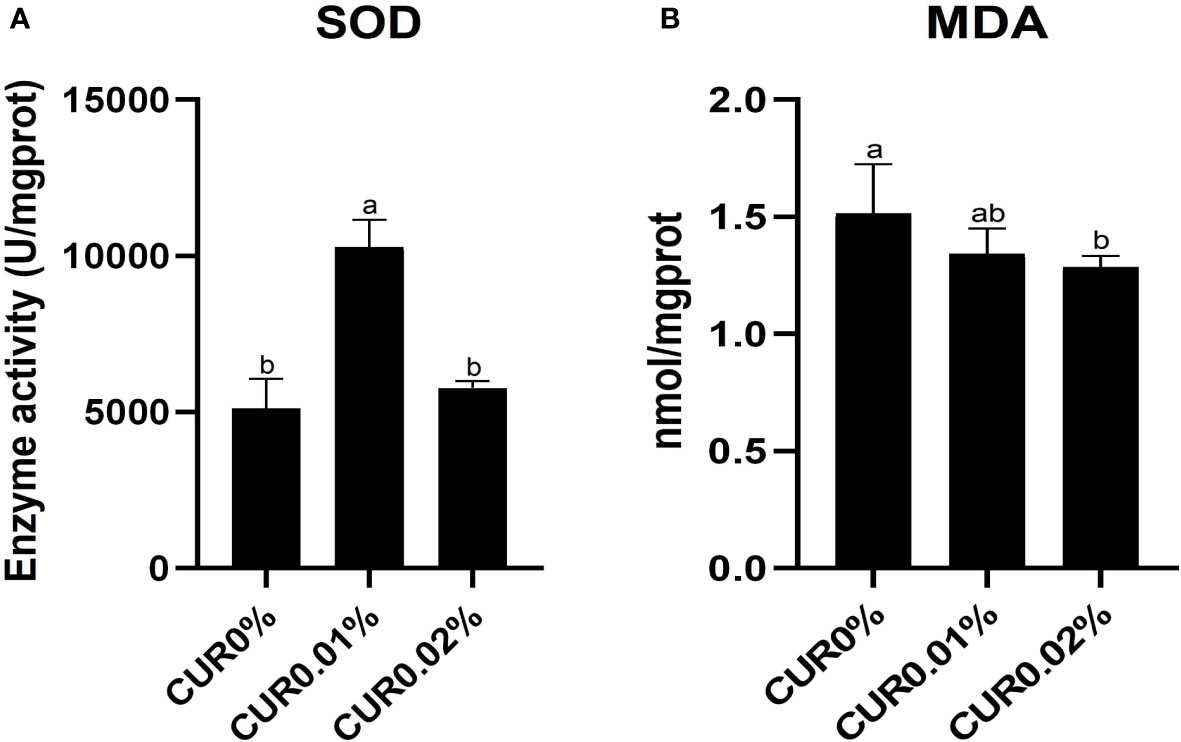
Figure 3 The activity of SOD (A) and the concentration of MDA (B) in liver of the greater amberjack after the 8-week feeding in Experiment I. Different letters above bars indicate significant differences at the 0.05 level. Data are expressed as the means ± SD (n=3).
3.2 Antioxidant capacity of the liver and spleen in experiment II
3.2.1 Antioxidant capacity of the liver after dietary supplementation with curcumin
The gene relative expression and enzyme activity of antioxidant-related genes in the liver of the greater amberjack after the ammonia challenge assay and recovery assay are given in Figure 4. After the ammonia challenge, the relative expression of CAT and GR was significantly downregulated (P < 0.05) in the curcumin-containing groups, with the minimum value found in the CUR0.01% group. The relative expression of Keap1 was negatively associated with dietary curcumin level, with a significantly downregulated (P < 0.05) relative expression found in the CUR0.02% group. A significantly upregulated (P < 0.05) relative expression of GSH-Px was found only in the CUR0.01% group. There were no significant differences (P > 0.05) in the relative expression of Mn-SOD among all the groups. The activity of SOD and GSH was negatively associated with dietary curcumin levels, with a significantly lower (P < 0.05) activity found in all curcumin-containing groups.
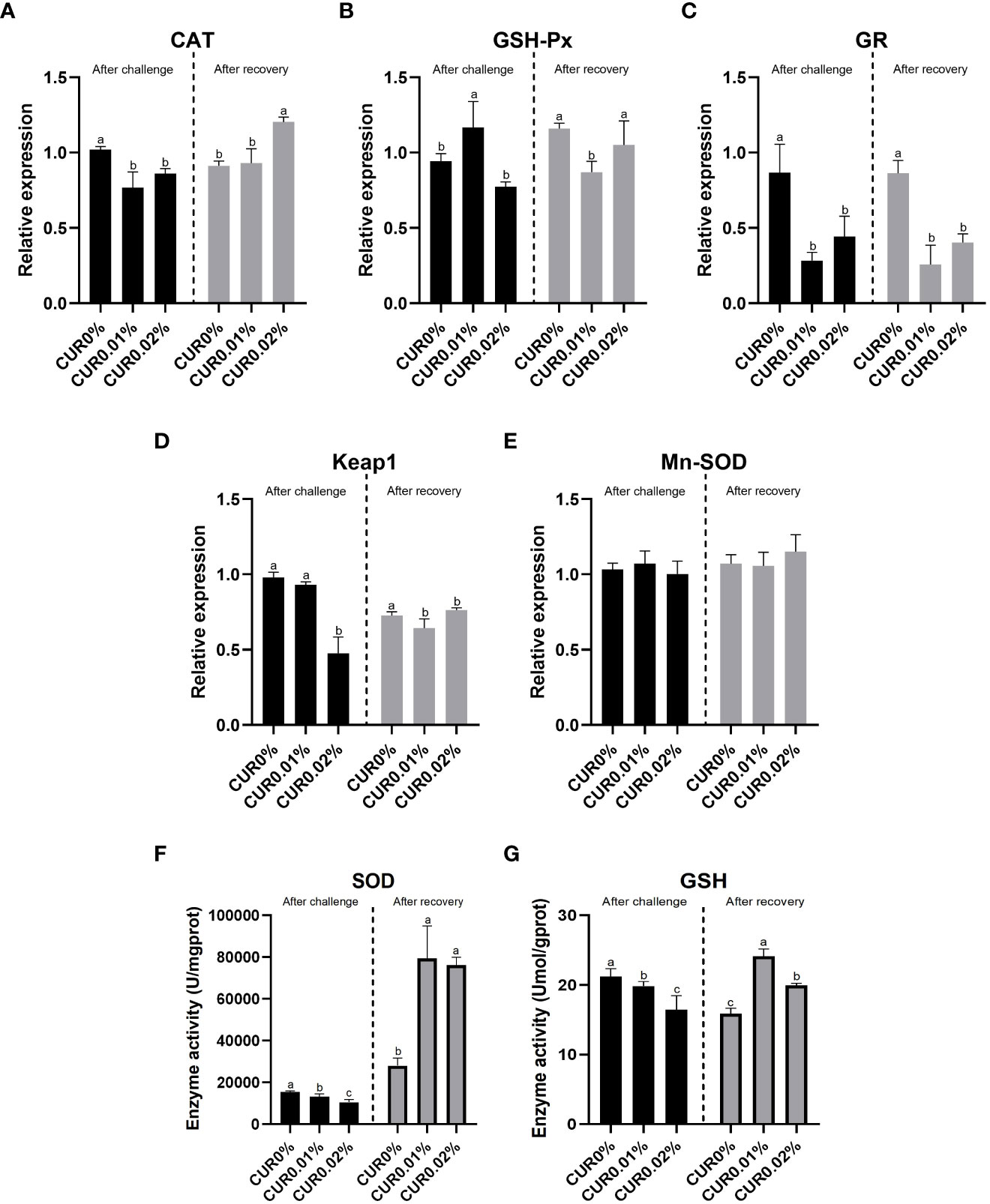
Figure 4 The relative expression of CAT (A), GSH-Px (B), GR (C) Keapl (D) Mn-SOD (E) gene and the enzyme activity of SOD (F), GSH (G) in liver tissue of the greater amberjack subjected to ammonia challenge experiment and recovery experiment. Different letters above bars indicate significant differences at the 0.05 level. Data are expressed as the means ± SD (n=3).
After the recovery, a significantly upregulated (P < 0.05) relative expression of CAT was found only in the CUR0.02% group. A significantly downregulated (P < 0.05) relative expression of GSH-Px was found only in the CUR0.01% group, and a significantly downregulated (P < 0.05) relative expression of Keap1 and GR was found in all curcumin-containing groups, with the minimum value found in the CUR0.01% group. There was no significant difference (P > 0.05) in the relative expression of Mn-SOD among all the groups. A significantly higher (P < 0.05) SOD and GSH activity was found in all curcumin-containing groups, with the maximum value found in the CUR0.01% group.
3.2.2 Antioxidant capacity of the spleen after dietary supplementation with curcumin
The gene relative expression and enzyme activity of antioxidant-related genes in the spleen of the greater amberjack after the ammonia challenge assay and recovery assay are given in Figure 5. After the ammonia challenge, a significantly upregulated (P < 0.05) relative expression of CAT was found in all curcumin-containing groups, with the maximum value found in the CUR0.01% group, and a significantly downregulated (P < 0.05) relative expression of GR was found only in the CUR0.01% group. There was no significant difference (P > 0.05) in the relative expression of GSH-Px, Mn-SOD, and Keap1 among all the groups. A significantly increased (P < 0.05) SOD activity was found in all curcumin-containing groups, with the maximum value found in the CUR0.01% group. The activity of GSH was positively associated with dietary curcumin level, with a significantly increased (P < 0.05) activity found in the CUR0.02% group.
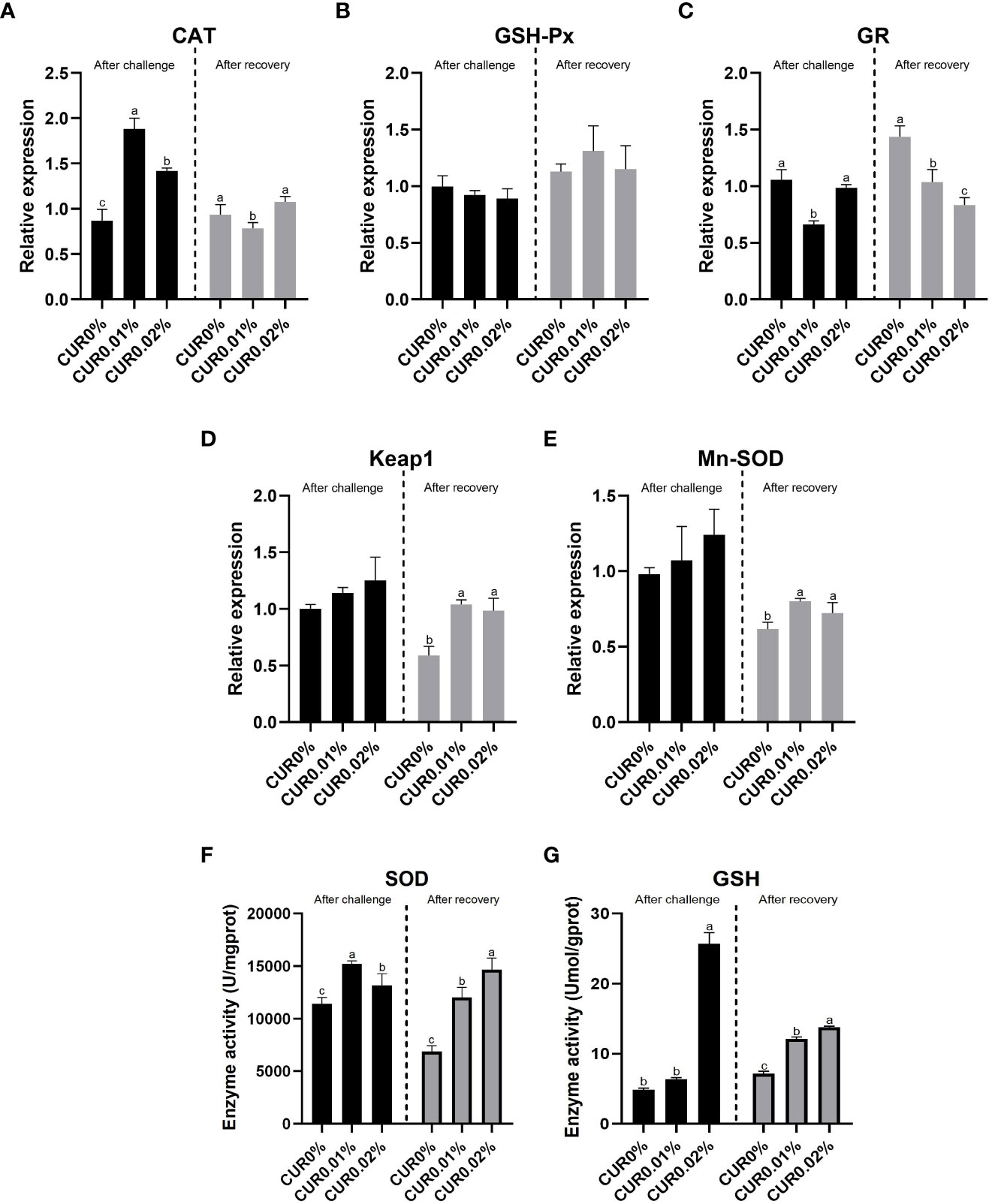
Figure 5 The relative expression of CAT (A), GSH-Px (B), GR (C) Keapl (D) Mn-SOD (E) gene and the enzyme activity of SOD (F), GSH (G) in spleen tissue of the greater amberjack subjected to ammonia challenge experiment and recovery experiment. Different letters above bars indicate significant differences at the 0.05 level. Data are expressed as the means ± SD (n=3).
After the recovery, a significantly downregulated (P < 0.05) relative expression of CAT was found only in the CUR0.01% group, and a significantly upregulated (P < 0.05) relative expression of Keap1 and Mn-SOD was found in all curcumin-containing groups, with the maximum value found in the CUR0.01% group. The relative expression of GR was negatively associated with dietary curcumin level, while a significantly downregulated (P < 0.05) relative expression was found in all curcumin-containing groups. There was no significant difference (P > 0.05) in the relative expression of GSH-Px among all the groups. The activity of SOD and GSH was positively associated with dietary curcumin level, with a significantly increased (P < 0.05) activity found in all curcumin-containing groups.
3.3 Histological structure of the liver and spleen after dietary supplementation with curcumin
After the ammonia challenge, the liver cells in the CUR0.01% and CUR0.02% groups were polygonal in shape and had centrally located nuclei and clear cell boundaries. The liver cells in the CUR0% group exhibited deviated nuclei, enlargement, and blurred and loosely arranged cell borders. After the recovery, the liver showed the same condition just like after the ammonia challenge (Figure 6). There were significant pathological changes in the spleen after the ammonia challenge. In the CUR0.01% and CUR0.02% groups, a greater aggregation of melano-macrophage centers (MMCs) was observed, and splenocytes were more tightly arranged than those of the control group. After the recovery, splenocytes showed the same condition just like after the ammonia challenge (Figure 7).
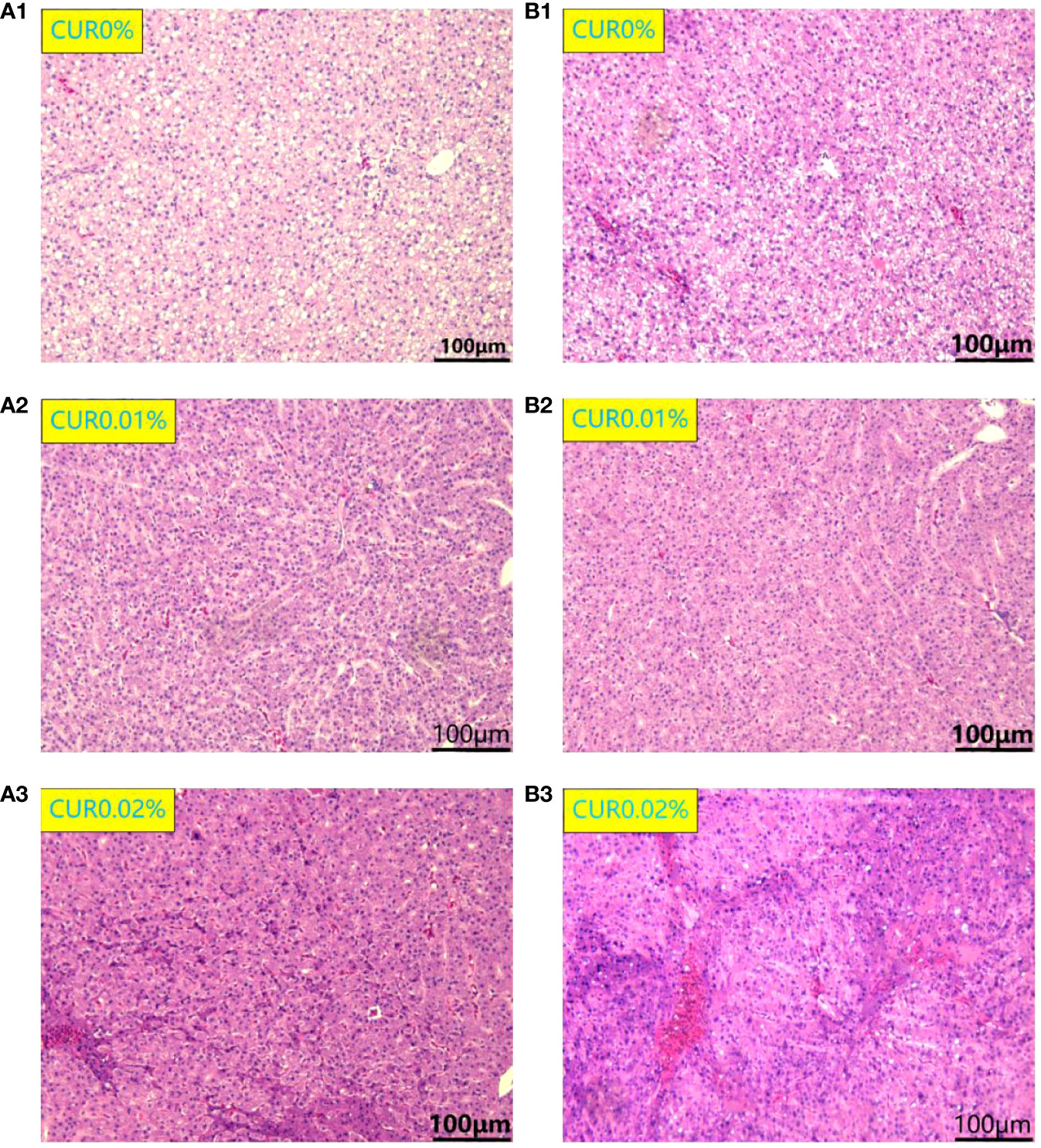
Figure 6 Liver histological structure of different groups of the greater amberjack after ammonia challenge (A1-3) and after recovery (B1-3).
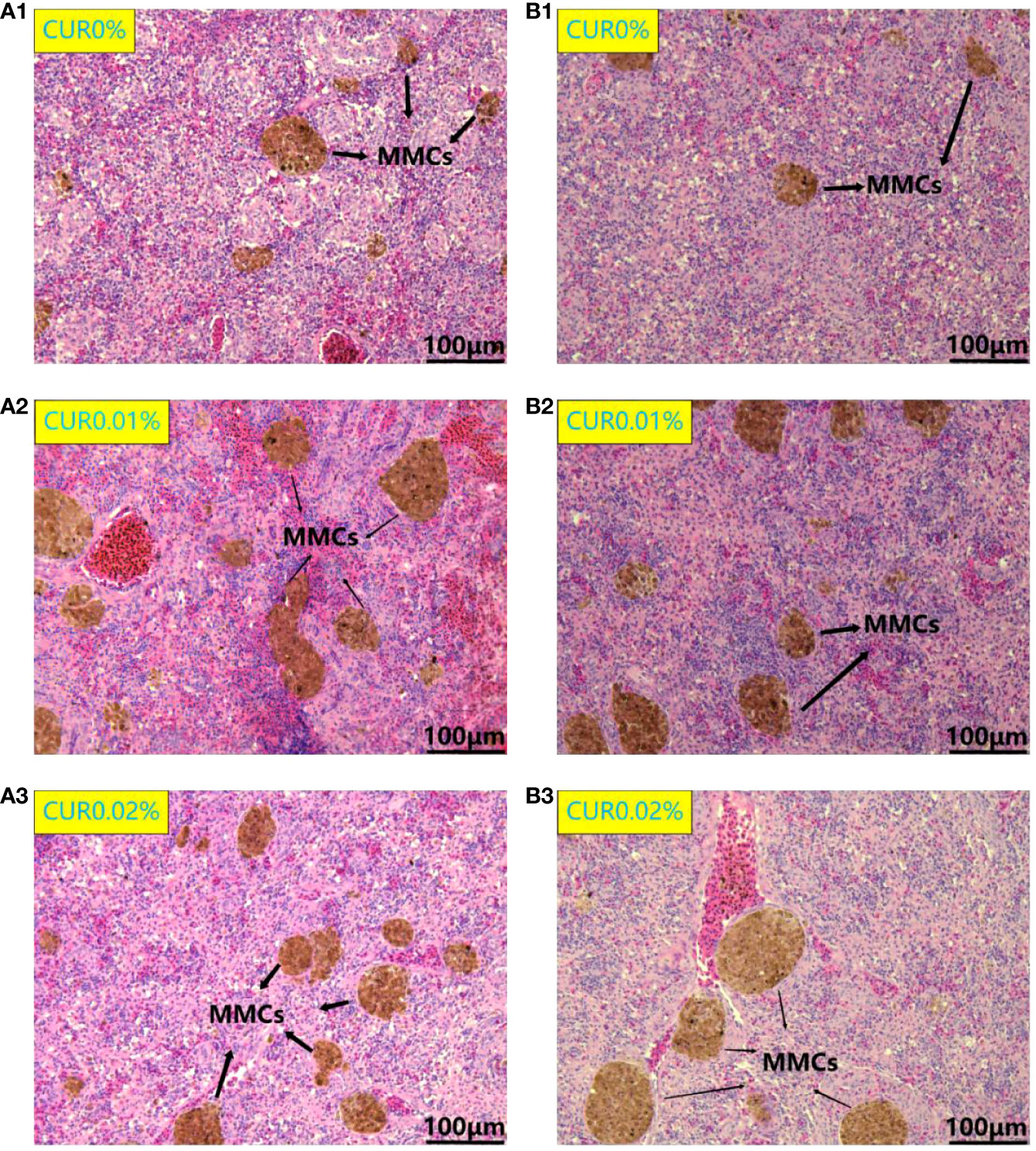
Figure 7 Spleen histological structure of different groups of the greater amberjack after ammonia challenge (A1–3) and after recovery (B1–3). MMCs, melano-macrophage centers.
4. Discussion
4.1 The effects on antioxidant capacity of the liver after dietary supplementation with curcumin
The results of this experiment showed that dietary curcumin supplementation enhanced the health condition of aquatic animals by increasing their antioxidant capacity. This result has been demonstrated in previous studies. For example, the SOD, GR, CAT, and GPx activities in the intestine of crucian carp (C. auratus) were positively associated with the content of curcumin supplemented in the diet (Cao et al., 2015). Dietary supplementation with curcumin modulated liver SOD activity, glutathione (GSH) metabolism, and the peroxisome proliferator-activated receptor (PPAR) signaling pathway to enhance the health of Nile tilapia (O. niloticus) (Li et al., 2020). The antioxidant systems of fish are composed of non-enzymatic compounds, such as SOD, CAT, GSH-Px, and GR. These antioxidant compounds and enzymes convert and decompose superoxide anions and hydrogen peroxide to protect cells from damage caused by the accumulation of ROS (Monaghan et al., 2009). In addition, ROS can activate transcription factors to control antioxidant enzymes, such as Nrf2/Keap1, which binds to the cytosolic chaperone Keap1 to keep the relatively inhibitory activity under physiological conditions. When ROS accumulates in excess, Nrf2 activates a series of downstream antioxidant enzymes, such as SOD and CAT (Motohashi and Yamamoto, 2004; Sykiotis and Bohmann, 2008). These antioxidant indicators are closely linked to the entire immune defense system. In this experiment, a positive effect of relative expression of antioxidant-related genes and enzyme activity in the liver was found when the diet was supplemented with 100 mg/kg of curcumin, while there was no significant difference in SOD activity when the diet was supplemented with 200 mg/kg of curcumin. Thus, dietary supplementation with 100 mg/kg of curcumin was more suitable for the greater amberjack considering the antioxidant capacity of the liver.
Dietary supplementation with curcumin can inhibit lipid peroxidation in the liver tissue by increasing antioxidant capacity. Previous studies have shown that the liver is involved in the immunity of teleostean and is also a vital organ with the capacity to digest and absorb nutrients (Hoekstra et al., 2013). The appropriate lipid content in the diet can improve dietary efficiency, conserve protein, and enhance the immunity system of aquatic animals (Castell and Covey, 1976; López et al., 2006), but the high frequency of feeding in intensive aquaculture often leads to oxidative damage because of the high polyunsaturated fatty acid content in fish diets and such fatty acids could release lipid free radicals easily inducing oxidative damage (National Research Council, 2011; Zhang et al., 2013; Mahmoud et al., 2017). In many liver diseases of aquatic animals, various enzymes and free radicals in the liver are closely related to lipid peroxidation. The present study showed that the positive effect of antioxidant-related genes and enzymes represented that lipid peroxidation was inhibited, and the decrease of MDA concentration in the liver also proved that. Similar results were found in Nile tilapia and Jian carp, whose liver tissues showed significantly higher antioxidant enzyme activity and significantly lower MDA levels when the diet was supplemented with curcumin (Cao et al., 2015; Mahmoud et al., 2017). These results suggest that dietary curcumin supplementation has an important role in protecting liver tissue from lipid peroxidation and restoring cell membrane function. In earlier studies, curcumin was found to be eight times more effective than vitamin E in preventing lipid peroxidation (Toda et al., 1985). In addition, dietary supplementation with curcumin may be a potential method to save protein by reducing the negative effects of more lipid content.
4.2 The effects on the resistance of the liver and spleen to ammonia stress and recovery after dietary supplementation with curcumin
In intensive aquaculture, the concentration of ammonia in the aquaculture environment may rise sharply in a short-term period due to many factors, thus causing acute ammonia stress injury to aquatic animals. Non-ionic ammonia can enter the body easily through organs such as the gills, causing a large number of free radicals to be generated in different organs (Widman et al., 2008).
Under normal conditions, there is a balance between the production of ROS and antioxidant defense systems, and the presence of ROS can prevent the invasion of pathogens (Aikawa et al., 2010). Changes in environmental factors such as salinity, temperature, oxygen levels, ammonia, nitrite, and pH may disturb the dynamic balance and induce excessive production or accumulation of ROS, even leading to oxidative stress (Canakci et al., 2009; Lushchak, 2011). This can lead to pathological changes in the aquatic animals’ tissues, disruption of the immune system, and even death (Monaghan et al., 2009). In recent years, an increasing number of studies have shown that ammonia exposure can trigger immune responses, oxidative stress, and apoptosis in shrimps (Wei et al., 2017; Yu et al., 2019), fish (Yu et al., 2019), and crabs (Cheng et al., 2019). To determine whether dietary supplementation with curcumin improves resistance to ammonia stress, we analyzed the histological structure and antioxidant capacity of the liver and spleen after the ammonia challenge and recovery.
The antioxidant capacity of the liver and spleen in the curcumin-containing groups was significantly higher than that of the control group after the ammonia challenge, suggesting that dietary curcumin supplementation alleviated oxidative stress in the liver and spleen. Previous studies have shown that curcumin alleviates ethanol-induced oxidative stress in the brain of rats by enhancing antioxidant capacity (Ikram et al., 2019). Curcumin enhances the liver antioxidant capacity of Jian carp to reduce CCl4-induced oxidative stress (Cao et al., 2015). However, the results showed that the antioxidant enzymes in the liver after the ammonia challenge were negatively related to the dietary curcumin level. We believe that this phenomenon is related to the deposition of curcumin in the liver. Curcumin is highly lipophilic that may bind to lipids in the diet (Hocking et al., 2018). Curcumin deposited in the liver also has an antioxidant function when oxidative stress occurs; thus, the activity of SOD and GSH is inhibited. In addition, we found that curcumin supplementation significantly inhibited the relative expression of GR in the liver and spleen. Previous studies have shown that elevated GR activity usually occurs during the initial phase of liver tissue damage and inflammation induced by external factors (Djordjevic et al., 2011; Huo et al., 2011). Thus, dietary supplementation with curcumin could alleviate tissue damage and inflammatory response induced by a high concentration of ammonia. Our other study also showed that dietary curcumin supplementation inhibits pro-inflammatory cytokine and promotes anti-inflammatory cytokine to improve the intestinal immune condition of the greater amberjack.
It is worth mentioning that the liver and spleen of the curcumin-containing groups of fish maintained a higher antioxidant capacity after the recovery. This implies that dietary supplementation with curcumin can restore fish to fully normal physiological conditions more quickly. Previous studies have shown that oxidatively stressed aquatic animals can recover to basic physiological conditions in the non-ammonia environment for a short period of time, but it still takes some time to recover to a fully normal condition (Wei et al., 2010). A recovery period of 10 days is not sufficient to restore the internal homeostasis of Brazilian flounder (Paralichthys orbignyanus) (Lucas et al., 2017). In practice production, it is necessary to reduce feeding and water exchange to avoid secondary oxidative stress when aquatic animals have already experienced oxidative stress, but this method can lead to a reduction in the growth rate of aquatic animals. Therefore, dietary supplementation with curcumin may be a potential way to shorten the recovery time and avoid further financial losses.
The histological structure results showed that dietary curcumin supplementation significantly alleviated the stress damage to the histological structure of the liver and spleen caused by external factors. Similar results were found in previous studies, such as the ability of curcumin to normalize the function of the liver and kidney in paracetamol-induced rats by protecting the histological structure of the liver and kidney (Yousef et al., 2010). Cao et al. (2015) found that curcumin supplementation alleviated CCl4-induced liver tissue injury in Jian carp. In addition, we found a positive correlation between dietary curcumin level and the area of MMCs in the spleen histological structure result. Previous studies have found that MMCs in teleostean were surrounded by a layer of lymphocytes containing antigen-forming cells and leukocytes, with immunoglobulins on the surface (Gregori et al., 2014). Therefore, it is generally accepted that MMCs have an important role in dealing with the recycling and degradation of various substances in vivo. When the farming environment is contaminated, the free melano-macrophage engulfs necrotic cells and aggregates to form MMCs, further enhancing phagocytosis. Mela et al. found that water-soluble copper caused the liver of Rhamdia quelen to show a larger area of MMCs (Mela et al., 2013), while cadmium significantly increased the amount of MMCs in the head kidney and spleen of European seabass (Suresh, 2009). These studies are consistent with our results. Dietary curcumin supplementation may be able to stimulate the rapid aggregation of MMCs, thereby alleviating the organ damage caused by high ammonia concentrations.
5. Conclusion
We assessed whether dietary curcumin supplementation could improve the health and resistance to ammonia stress of the greater amberjack through histological structure, antioxidant-related gene relative expression, and enzyme activity. According to the results of the study, it can be confirmed that dietary curcumin supplementation improves the antioxidant capacity of the liver and has a positive effect in terms of resistance to ammonia stress. The dietary curcumin supplementation level of 100 mg/kg is more suitable for practical application in the greater amberjack considering the health of the liver and spleen. We believe that our research contributes to the healthy and intensive farming of the greater amberjack through supplementation with the appropriate levels of curcumin. In addition, it also provides a better reference for future practical applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Welfare Committee of the Chinese Academy of Fishery Sciences.
Author contributions
YH designed and performed the whole experiment with the help of ZM. YH drafted the manuscript. ZF assisted in data analysis. GY and SD helped in field experimental work. ZM revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Central Public-Interest Scientific Institution Basal Research Fund (CAFS No. 2020TD55) and Guangxi Innovation Driven Development Special Fund Project (grant no. Guike AA18242031) and the Asian Cooepration Fund Program on Modern Fishery Cooperation between China and Other Coastal States of the South China Sea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.961783/full#supplementary-material
References
Agrawal D. K., Mishra P. K. (2010). Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 30 (5), 818–860. doi: 10.1002/med.20188
Ahmed N., Thompson S., Glaser M. (2019). Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manage. 63 (2), 159–172. doi: 10.1007/s00267-018-1117-3
Aikawa C., Nozawa T., Maruyama F., Tsumoto K., Nakagawa I. (2010). Reactive oxygen species induced by Streptococcus pyogenes invasion trigger apoptotic cell death in infected epithelial cells. Cell. Microbiol. 12 (6), 814–830. doi: 10.1111/j.1462-5822.2010.01435.x
Anderson D. P. (1996). Environmental factors in fish health: immunological aspects. The fish immune system: organism, pathogen, and environment. 15, 289–310. doi: 10.1016/s1546-5098(08)60277-0
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. [Journal article; research support, U.S. gov't, P.H.S.]. Anal. Biochem. 72, 248–254. doi: 10.1006/abio.1976.9999
Canakci C. F., Cicek Y., Yildirim A., Sezer U., Canakci V. (2009). Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur. J. Dentistry. 3 (2), 100–106. doi: 10.1055/s-0039-1697415
Cao L., Ding W., Du J., Jia R., Liu Y., Zhao C., et al. (2015). Effects of curcumin on antioxidative activities and cytokine production in jian carp (Cyprinus carpio var. jian) with CCl4-induced liver damage. [Journal article; randomized controlled trial; research support, non-U.S. gov't]. Fish Shellfish Immunol. 43 (1), 150–157. doi: 10.1016/j.fsi.2014.12.025
Cao L., Ding W., Jia R., Du J., Wang T., Zhang C., et al. (2017). Anti-inflammatory and hepatoprotective effects of glycyrrhetinic acid on CCl4-induced damage in precision-cut liver slices from jian carp (Cyprinus carpio var. jian) through inhibition of the nf-k pathway. Fish Shellfish Immunol. 64, 234. doi: 10.1016/j.fsi.2017.03.007
Castell J. D., Covey J. F. (1976). Dietary lipid requirements of adult lobsters, Homarus americanus (M.E.). J. Nutr. 106 (8), 1159–1165. doi: 10.1093/jn/106.8.1159
Cheng C. H., Ma H. L., Su Y. L., Deng Y. Q., Feng J., Xie J. W., et al. (2019). Ammonia toxicity in the mud crab ( Scylla paramamosain ): The mechanistic insight from physiology to transcriptome analysis. Ecotox. Environ. Safe. 179, 9–16. doi: 10.1016/j.ecoenv.2019.04.033
National Research Council (2011). Nutrient requirements of fish and shrimp (Vol. 26) (Portland: National Academies Press).
Dabas A., Nagpure N. S., Kumar R., Kushwaha B., Lakra P. K. S. (2012). Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol&Biochem. 38 (2), 469–482. doi: 10.1007/s10695-011-9527-7
Djordjevic J., Djordjevic A., Adzic M., Elakovic I., Matic G., Radojcic M. B. (2011). Fluoxetine affects antioxidant system and promotes apoptotic signaling in wistar rat liver. Eur. J. Pharmacol. 659 (1), 61–66. doi: 10.1016/j.ejphar.2011.03.003
Djordjevic B., Škugor S., Jørgensen S. M., Øverland M., Mydland L. T., Krasnov A. (2009). Modulation of splenic immune responses to bacterial lipopolysaccharide in rainbow trout (Oncorhynchus mykiss) fed lentinan, a beta-glucan from mushroom Lentinula edodes. Fish Shellfish Immun. 26 (2), 201–209. doi: 10.1016/j.fsi.2008.10.012
El-Barbary M. I. (2016). Detoxification and antioxidant effects of garlic and curcumin in oreochromis niloticus injected with aflatoxin b-1 with reference to gene expression of glutathione peroxidase (GPx) by RT-PCR. Fish Physiol. Biochem. 42 (2), 617–629. doi: 10.1007/s10695-015-0164-4
Farhangi B., Alizadeh A. M., Khodayari H., Khodayari S., Dehghan M. J., Khori V., et al. (2015). Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur. J. Pharmacol. 758, 188–196. doi: 10.1016/j.ejphar.2015.03.076
Fu Y., Gao R., Cao Y., Guo M., Wei Z., Zhou E., et al. (2014). Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in mice. [Journal article; research support, non-U.S. gov't]. Int. Immunopharmacol. 20 (1), 54–58. doi: 10.1016/j.intimp.2014.01.024
Fu Z., Yang R., Chen X., Qin J. G., Ma Z. (2019). Dietary non-protein energy source regulates antioxidant status and immune response of barramundi (Lates calcarifer). Fish Shellfish Immun. 95, 697–704. doi: 10.1016/j.fsi.2019.11.018
Gobi N., Ramya C., Vaseeharan B., Malaikozhundan B., Vijayakumar S., Murugan K., et al. (2016). Oreochromis mossambicus diet supplementation with psidium guajava leaf extracts enhance growth, immune, antioxidant response and resistance to Aeromonas hydrophila. Fish Shellfish Immun. 58, 572–583. doi: 10.1016/j.fsi.2016.09.062
Gregori M., Miragliotta V., Leotta R., Cecchini S., Prearo M., Abramo F. (2014). Morphometric evaluation of interrenal gland and kidney macrophages aggregates in normal healthy rainbow trout (Oncorhynchus mykiss) and after bacterial challenge with yersinia ruckeri. Vet. Med. Int. 2014, 210625. doi: 10.1155/2014/210625
Hillaby B. A., Randall D. J. (1979). Acute ammonia toxicity and ammonia excretion in rainbow trout (Salmo gairdneri). J. Fish Res. Board Canada. 36 (6), 621–629. doi: 10.1139/f79-090
Hocking A. J., Elliot D., Hua J., Klebe S. (2018). Administering fixed oral doses of curcumin to rats through voluntary consumption. J. Am. Assoc. Lab. Anim. Sci. 57 (5), 508–512. doi: 10.30802/AALAS-JAALAS-17-000143
Hoekstra L. T., de Graaf W., Nibourg G. A., Heger M., Bennink R. J., Stieger B., et al. (2013). Physiological and biochemical basis of clinical liver function tests: A review. [Journal article; review]. Ann. Surg. 257 (1), 27–36. doi: 10.1097/SLA.0b013e31825d5d47
Hossain S., Koshio S., Ishikawa M., Yokoyama S., Sony N. M., Islam J., et al. (2018). Substitution of dietary fishmeal by soybean meal with inosine administration influences growth, digestibility, immunity, stress resistance and gut morphology of juvenile amberjack Seriola dumerili. Aquacult. 488, 174–188. doi: 10.1016/j.aquaculture.2018.01.037
Hossain M. S., Koshio S., Ishikawa M., Yokoyama S., Sony N. M., Kader M. A., et al. (2017). Effects of dietary administration of inosine on growth, immune response, oxidative stress and gut morphology of juvenile amberjack, Seriola dumerili. Aquacult. 468, 534–544. doi: 10.1016/j.aquaculture.2016.11.020
Huo H. Z., Wang B., Liang Y. K., Bao Y. Y., Gu Y. (2011). Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Inter. J. Mol. Sci. 12 (10), 6529–6543.
Ikram M., Saeed K., Khan A., Muhammad T., Khan M. S., Jo M. G., et al. (2019). Natural dietary supplementation of curcumin protects mice brains against ethanol-induced oxidative stress-mediated neurodegeneration and memory impairment via Nrf2/TLR4/RAGE signaling. Nutrients. 11 (5), 1082. doi: 10.3390/nu11051082
Jiang J., Wu X., Zhou X., Feng L., Liu Y., Jiang W., et al. (2016). Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquacult. 463, 174–180. doi: 10.1016/j.aquaculture.2016.05.040
Jover M., Garcıa-Gómez A., Tomás A., de la Gándara F., Pérez L. (1999). Growth of mediterranean yellowtail (Seriola dumerilii) fed extruded diets containing different levels of protein and lipid. Aquacult. 179 (1), 25–33. doi: 10.1016/S0044-8486(99)00149-0
Kei S. (1978). Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 90 (1), 37–43. doi: 10.1016/0009-8981(78)90081-5
Kohshahi A. J., Sourinejad I., Sarkheil M., Johari S. A. (2018). Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): Influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 45 (2), 793–804. doi: 10.1007/s10695-018-0585-y
Kubes P., Jenne C. (2018). Immune responses in the liver. Annu. Rev. Immunol. 36 (1), 247–277. doi: 10.1146/annurev-immunol-051116-052415
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods (San Diego Calif.). 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Li L., Zhang Z., Huang Y. (2020). Integrative transcriptome analysis and discovery of signaling pathways involved in the protective effects of curcumin against oxidative stress in tilapia hepatocytes. Aquat. Toxicol. 224, 105516. doi: 10.1016/j.aquatox.2020.105516
Li G., Zhao Y., Wang J., Liu B., Sun X., Guo S., et al. (2017). Transcriptome profiling of developing spleen tissue and discovery of immune-related genes in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 60, 400–410. doi: 10.1016/j.fsi.2016.12.012
Li G., Zhou X., Jiang W., Wu P., Liu Y., Jiang J., et al. (2020). Dietary curcumin supplementation enhanced growth performance, intestinal digestion, and absorption and amino acid transportation abilities in on-growing grass carp (Ctenopharyngodon idella). Aquac. Res. 51 (12), 4863–4873. doi: 10.1111/are.14777
López L. M., Torres A. L., Durazo E., Drawbridge M., Bureau D. P. (2006). Effects of lipid on growth and feed utilization of white seabass (Atractoscion nobilis) fingerlings. Aquacult. 253 (1), 557–563. doi: 10.1016/j.aquaculture.2005.08.007
Lucas, Campos, Maltez, Giovanna, Rodrigues, Stringhetta, et al. (2017). Ammonia exposure and subsequent recovery trigger oxidative stress responses in juveniles of Brazilian flounder Paralichthys orbignyanus. Fish Physiol. Biochem. 43 (6), 1747–1759. doi: 10.1007/s10695-017-0406-8
Luis A. I. S., Campos E. V. R., Oliveira J. L., Fraceto L. F. (2019). Trends in aquaculture sciences: From now to use of nanotechnology for disease control. Rev. Aquacult. 11 (1), 119–132. doi: 10.1111/raq.12229
Lushchak V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 101 (1), 13–30. doi: 10.1016/j.aquatox.2010.10.006
Mahmoud H. K., Al-Sagheer A. A., Reda F. M., Mahgoub S. A., Ayyat M. S. (2017). Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquacult. 475, 16–23. doi: 10.1016/j.aquaculture.2017.03.043
Martin R. C., Aiyer H. S., Malik D., Li Y. (2012). Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. [Journal article]. Food Chem. Toxicol. 50 (2), 227–231. doi: 10.1016/j.fct.2011.10.070
Mazzola A., Favaloro E., Sarà G. (2000). Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso 1810), in submerged cages in the Western Mediterranean Sea. Aquacult. 181 (3), 257–268. doi: 10.1016/S0044-8486(99)00243-4
Mela M., Guiloski I. C., Doria H. B., Rabitto I. S., Da S. C., Maraschi A. C., et al. (2013). Risks of waterborne copper exposure to a cultivated freshwater Neotropical catfish (Rhamdia quelen). Ecotoxicol Environ. Saf. 88, 108–116. doi: 10.1016/j.ecoenv.2012.11.002
Monaghan P., Metcalfe N. B., Torres R. (2009). Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. [Journal article; research support, non-U.S. gov't; review]. Ecol. Lett. 12 (1), 75–92. doi: 10.1111/j.1461-0248.2008.01258.x
Motohashi H., Yamamoto M. (2004). Nrf2-Keap1 defines a physiologically important stress response mechanism. [Journal article; research support, non-U.S. gov't; review]. Trends Mol. Med. 10 (11), 549–557. doi: 10.1016/j.molmed.2004.09.003
Qiu J., Wang W. N., Wang L. J., Liu Y. F., Wang A. L. (2011). Oxidative stress, DNA damage and osmolality in the pacific white shrimp, Litopenaeus vannamei exposed to acute low temperature stress. [Journal article; research support, non-U.S. gov't]. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 154 (1), 36–41. doi: 10.1016/j.cbpc.2011.02.007
Randall D. J., Tsui T. K. N. (2002). Ammonia toxicity in fish. Mar. Pollut. Bull. 45 (1), 17–23. doi: 10.1016/S0025-326X(02)00227-8
Shi X., Zheng Z., Li J., Xiao Z., Qi W., Zhang A., et al. (2015). Curcumin inhibits abeta-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. [Journal article; research support, non-U.S. gov't]. Neurosci. Lett. 594, 105–110. doi: 10.1016/j.neulet.2015.03.045
Stankus A. (2021). State of world aquaculture 2020 and regional reviews: FAO webinar series. FAO Aquacult Newslett. 63), 17–18.
Suresh N. (2009). Effect of cadmium chloride on liver, spleen and kidney melano macrophage centres in tilapia mossambica. J. Environ. Biol. 30 (4), 505–508. doi: 10.2112/JCOASTRES-D-09-00027.1
Sykiotis G. P., Bohmann D. (2008). Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in drosophila. Dev. Cell. 14 (1), 76–85. doi: 10.1016/j.devcel.2007.12.002
Toda S., Miyase T., Arichi H., Tanizawa H., Takino Y. (1985). Natural antioxidants. III. antioxidative components isolated from rhizome of curcuma longa l. [Journal article]. Chem. Pharm. Bull. (Tokyo). 33 (4), 1725–1728. doi: 10.1248/cpb.33.1725
Wei Y., Xiang F., Sun H., Chen Y., Minter E., Zhou Y. (2010). Changes in the selected hematological parameters and gill Na(+)/K(+) ATPase activity of juvenile crucian carp Carassius auratus during elevated ammonia exposure and the post-exposure recovery. Biochem. Syst Ecol. 38 (4), 557–562. doi: 10.1016/j.bse.2010.06.005
Wei W., Yang S., Wang C., Shi L., Chan S. (2017). Gill transcriptomes reveal involvement of cytoskeleton remodeling and immune defense in ammonia stress response in the banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 71, 319. doi: 10.1016/j.fsi.2017.10.028
Widman J. J., Meseck S. L., Sennefelder G., Veilleux D. J. (2008). Toxicity of un-ionized ammonia, nitrite, and nitrate to juvenile bay scallops, Argopecten irradians. Arch. Environ. Contam Toxicol. 54 (3), 460–465. doi: 10.1007/s00244-007-9051-z
Wilson R. P. (2003). “3 - amino acids and proteins,” in Fish nutrition (Third edition), vol. 143-179) . Eds. Halver J. E., Hardy R. W. (San Diego: Academic Press).
Wu J., Su Y., Deng Y., Guo Z., Cheng C., Ma H., et al. (2019). Spatial and temporal variation of antibiotic resistance in marine fish cage-culture area of guangdong, China. Environ. pollut. 246, 463–471. doi: 10.1016/j.envpol.2018.12.024
Yousef M. I., Omar S. A., El-Guendi M. I., Abdelmegid L. A. (2010). Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. [Journal article]. Food Chem. Toxicol. 48 (11), 3246–3261. doi: 10.1016/j.fct.2010.08.034
Yu J., Sun J., Zhao S., Wang H., Zeng Q. (2019). Transcriptome analysis of oriental river prawn (Macrobrachium nipponense) hepatopancreas in response to ammonia exposure. Fish Shellfish Immun. 93, 223–231. doi: 10.1016/j.fsi.2019.07.036
Zhang S. P., Li J. F., Wu X. C., Zhong W. J., Xian J. A., Liao S. A., et al. (2013). Effects of different dietary lipid level on the growth, survival and immune-relating genes expression in pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 34 (5), 1131–1138. doi: 10.1016/j.fsi.2013.01.016
Zhang Y., Song L., Guo H., Wu J., Wang X., Yao F. (2021). Effects of curcumin on growth and liver-protection in common carp, Cyprinus carpio. Pak. J. Zool. 53 (4), 1211. doi: 10.17582/journal.pjz/20200409120439
Zhao H., Peng K., Wang G., Mo W., Huang Y., Cao J. (2021). Metabolic changes, antioxidant status, immune response and resistance to ammonia stress in juvenile yellow catfish (Pelteobagrus fulvidraco) fed diet supplemented with sodium butyrate. Aquacult. 536, 736441. doi: 10.1016/j.aquaculture.2021.736441
Keywords: antioxidant-related genes, antioxidant-related enzyme, oxidative stress, histological structure, liver, spleen
Citation: He Y, Fu Z, Dai S, Yu G and Ma Z (2022) Dietary curcumin supplementation can enhance health and resistance to ammonia stress in the greater amberjack (Seriola dumerili). Front. Mar. Sci. 9:961783. doi: 10.3389/fmars.2022.961783
Received: 05 June 2022; Accepted: 29 August 2022;
Published: 16 September 2022.
Edited by:
Benjamin Costas, University of Porto, PortugalReviewed by:
Khalil Eslamloo, Memorial University of Newfoundland, CanadaMo Peng, Jiangxi Agricultural University, China
Copyright © 2022 He, Fu, Dai, Yu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Ma, emhlbmh1YS5tYUBob3RtYWlsLmNvbQ==
 Yuhang He1,2
Yuhang He1,2 Zhengyi Fu
Zhengyi Fu Zhenhua Ma
Zhenhua Ma