- 1College of Chemistry and Environmental Science, Guangdong Ocean University, Zhanjiang, China
- 2Research Center for Coastal Environmental Protection and Ecological Resilience, Guangdong Ocean University, Zhanjiang, China
- 3School of Humanities, York St. John University, York, United Kingdom
- 4Laboratory for Coastal Ocean Variation and Disaster Prediction, College of Ocean and Meteorology, Guangdong Ocean University, Zhanjiang, China
- 5Key Laboratory of Climate, Resources and Environment in Continental Shelf Sea and Deep Sea of Department of Education of Guangdong Province, Guangdong Ocean University, Zhanjiang, China
- 6Key Laboratory of Space Ocean Remote Sensing and Application, Ministry of Natural Resources, Beijing, China
Dissolved organic matter (DOM) serves as the most active and sensitive organic component in the bay, and its biogeochemical characteristics and reactivity are affected by the properties of terrestrial and marine substances significantly. In this study, in order to study the distribution and characteristics of DOM in a semi-closed bay, 34 water samples from 19 stations were collected from Zhanjiang Bay and analyzed for δ13C of dissolved inorganic carbon (DIC) and fluorescent components of DOM. The results showed that there were many sources of organic matter in the bay, including soil input, algae input, and sewage input. Influenced by freshwater input, DOM in the bay decreased from the upper bay to the outer bay. The organic matter in the bay displayed two characteristics, where the northern bay is composed of terrigenous organic matter mainly with high humus, while the southern bay is more inclined to marine sources with a high biological index (BIX) and low humification index (HIX). The correlation between organic matter with different characteristics and environmental parameters such as salinity, pH, and chlorophyll a was analyzed. The discrepancy may be caused by the weak turbulent mixing in the semi-closed bay.
1 Introduction
Estuaries serve as the important hubs of the global carbon cycle, linking multiple ecosystems such as land, atmosphere, and ocean (Bianchi, 2007). As an ecosystem affected by terrestrial runoff and ocean tides, estuaries have sensitive characteristics of distinct physical–chemical and biological conditions (He et al., 2016; Chen et al., 2018). Estuaries also have frequent biological activities and possess high primary productivity and redox capacity as reaction pools of terrestrial and marine materials (Bianchi, 2011; Gireeshkumar et al., 2013). Driven by climate changes and anthropogenic activities, tremendous alterations in physical–chemical and biological conditions occur in estuaries (Najjar et al., 2010; Kerr, 2011; Canuel et al., 2012; Cabral et al., 2019).
Dissolved organic matter (DOM) serves as the most active and sensitive organic component; its biogeochemical characteristics and reactivity can be affected by the properties of allochthonous and local substances significantly (Murphy et al., 2008; Ya et al., 2015; Lloret et al., 2016; Lee et al., 2020). Being surrounded by frequent human activity and diverse environments, estuarine DOM is provided with complex sources including primary productivity, river input, and sewage input (Hudson et al., 2007; Guo et al., 2014; Kinsey et al., 2018). As a matter of fact, more and more allochthonous DOM is imported into estuarine systems with the increase of anthropogenic activities like industrialization, urbanization, and agricultural practices (Boesch, 2002; Felgate et al., 2021). They will change the quality and quantity of DOM, which in turn impact the biogeochemical cycle, decomposition of organic matter, and recycling of chemical elements in sediments (Spilling et al., 2018; Felgate et al., 2021; García-Martín et al., 2021). Due to the different sources of DOM, their conversion, retention, and output fluxes in biogeochemical processes including flocculation, photochemistry, and biodegradation in estuaries also differ (Carlson and Hansell, 2015; García-Martín et al., 2021). Therefore, discriminating main sources of DOM and their contributions will be an important basis for evaluating the fate of estuarine DOM, which also is of great significance to evaluate the effects of terrestrial runoff and ocean tides on estuarine organic matter (Zhou et al., 2021).
Stable carbon isotope analysis is widely used to identify the origin of DOM because of the selective utilization of HCO3− by algae (Andrews et al., 1998; Beer et al., 2014; Lee et al., 2020). In terms of spectrophotometry, the composition of DOM is evaluated by detecting fluorescent DOM (FDOM) and various established optical indexes, such as fluorescence index (FI), humification index (HIX), and biological index (BIX) (Coble, 1996; Stedmon et al., 2003; Coble et al., 2014; Amaral et al., 2020). Those methods have been widely used to elucidate the contribution of DOM from different sources in estuaries, helping to track the transition and transportation of DOM from a river to an ocean (Coble, 1996; Jørgensen et al., 2011; Coble et al., 2014). A semi-closed bay is usually accompanied by closed topography and weak hydrodynamic conditions (Xue et al., 2021). In this case, Zhanjiang Bay was studied as a semi-closed bay, and the total amount of DOM and its main sources through spatial were investigated, paying attention to the transition and characteristics of DOM from a river to an ocean. The combination of stable carbon isotope and spectrophotometry was used to evaluate the distribution and source of DOM and clarify the characteristics and relationship with environmental factors of DOM in Zhanjiang Bay.
2 Materials and Methods
2.1 Study Area and Sampling Collection
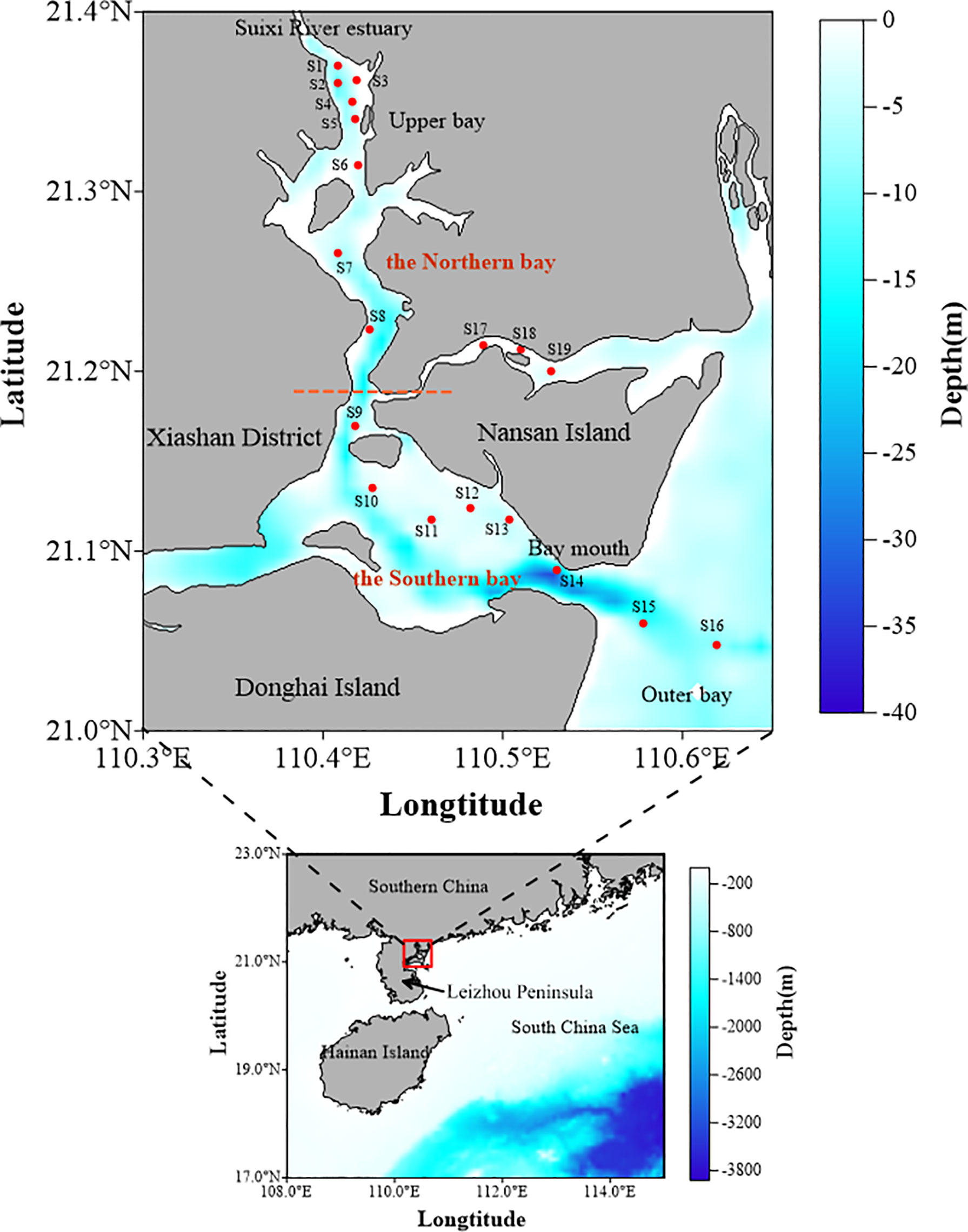
Zhanjiang Bay, located in the east of Leizhou Peninsula in China, is a typical semi-closed bay surrounded by islands and a peninsula. In this study, Zhanjiang Bay is divided into two parts according to the terrain: the northern bay and the southern bay (Figure 1). The northern bay is mainly composed of the estuary of the Suixi River, with shallow water depth. Among them, the Suixi River, the main river of Zhanjiang Bay, is about 80 km long with an annual average flow of 10.4 × 108 m3. The southern bay is the major broad water in the bay, which is connected with the South China Sea through the southeastern bay mouth. Affected by narrow terrain, poor hydrodynamic conditions, and the influence of steady wind–east wind, the half exchange time of water in the northern bay was more than 100 days, and that in the southern bay was 30 days (Li, 2012). Zhanjiang Bay is also a bay deeply affected by human activities, where the degree of eutrophication of the bay is high, and many sewage outlets in the northern bay exist (Yu et al., 2017; Yu et al., 2021; Zhang et al., 2021).
A cruise was carried out during the summer (June 2021), mainly sampling from the estuary of the Suixi River to the southeastern bay mouth, and 34 water samples from 19 stations were collected from Zhanjiang Bay. The surface water and bottom water were collected with a 10-L Plexiglass water sampler during the cruise. The full-depth profiles of temperature, salinity, and water depth were determined using an SBE37 conductivity–temperature–depth (CTD) meter (Sea-Bird Scientific, Bellevue, WA, USA). The pH was measured on site with a pre-calibrated pH measuring instrument (Thermo Fisher Scientific, Waltham, MA, USA) and then corrected on site with temperature data. The water samples for dissolved oxygen (DO) analysis were collected first. Water from the Plexiglass water sampler was slowly drained into brown glass bottles and allowed to overflow for several minutes. Manganous sulfate and alkaline iodide solutions were then added. Titration was conducted in the laboratory within 24 h after taking the sample. The seawater samples for DOM were filtered using filters (Millipore, Billerica, MA, USA; 0.22 µm, PC Membrane) and stored at −20°C for analysis. For chlorophyll a (Chl-a), water samples (approximately 1,000 ml) were filtered using pre-combusted (450°C, 4 h) glass-fiber filters (Whatman, 0.7 µm, GF/F) and stored at −20°C before further processing and analysis. Water samples for δ13CDIC measurements were collected in 60-ml glass bottles. Soon after collection, the samples were poisoned with HgCl2, sealed with a rubber septum-aluminum cap, and wrapped with thick black paper. In the laboratory, the samples were kept refrigerated until analysis.
2.2 Chemical analysis
In the laboratory, after the sample was extracted in 90% acetone, the concentration of Chl-a was determined using a Turner fluorometer (Lorenzen and Jeffrey, 1980). The concentration of DO was determined using the Winkler method (Montgomery et al., 1964). The stable carbon isotope values of inorganic–organic carbon (δ13CDIC) were determined using an elemental analysis isotope ratio mass spectrometer (GasBench II-IRMS). The precision based on replicate analysis was ±0.1‰ for δ13CDIC measurements.
2.3 Fluorescence Measurements and Excitation–Emission Matrix–PARAFAC Modeling
The FDOM excitation–emission matrices (EEMs) of 34 water samples were determined by an F-7100 fluorescence spectrofluorometer (Hitachi, Tokyo, Japan). The blank sample was prepared using Milli-Q water (Millipore, 18.2 MΩ/cm), and samples were placed into fused silica cells with a 1-cm path length. The EEM fluorescence was obtained by scanning excitation spectra (Ex) at 5-nm intervals of 240–450 nm and the emission wavelength (Em) at 2-nm intervals of 280–600 nm. The blank subtracted, normalized, and PARAFAC models of 34 EEMs were analyzed by the water Raman signals using MATLAB software (R2014b, MathWorks, Natick, MA, USA) (Stedmon and Bro, 2008). The reliability of the PARAFAC model was verified by residual and split-half analyses.
2.4 Optical Index Analysis
The optical indexes could provide effective information for the study of the composition and properties of DOM. FI was the ratio of emission intensity at 450 and 500 nm when the excitation wavelength was 370 nm, which could effectively indicate the source and degradation degree of DOM [Eq. (1)] (McKnight et al., 2001). The BIX implied the relative contributions of fresh DOM by the ratio of the emission at 380 nm to that at 430 nm for an excitation wavelength of 310 nm [Eq. (2)] (Huguet et al., 2009). The HIX could judge the degree of humification of DOM by calculating the ratios of two integrated sections of emission wavelengths (the summed emissions from 435 to 480 nm divided by the summed emissions from 300 to 345 nm) for an excitation wavelength at 254 nm (Zsolnay et al., 1999). Since FDOM was measured every 5 nm for excitation, leading to the excitation wavelength at 254 nm, which was not measured in the study, the adjacent excitation wavelength at 255 nm was used to replace the 254-nm wavelength here Eq. (3).
2.5 Statistical analysis
Hierarchical cluster analysis (HCA) was performed to reveal the intrinsic correlation or similarity between sampling sites of different regions by Origin 2021b. Redundancy analysis (RDA) was applied to investigate the effects of environmental variables on fluorescence components by CANOCO 5.
3 Results
3.1 Distributions of Salinity, Temperature, and pH
As shown in Figure 2, the salinity of Zhanjiang Bay ranged from 19.89 to 31.82 (with an average of 26.71 ± 3.50). A lower salinity was found from the northern bay, particularly in the estuary of the Suixi River, suggesting that it was affected by the coastal freshwater discharge. On the whole, the salinity difference between the surface water and the bottom water was very small, indicating that the water in the bay may be mixed evenly. The water temperature of Zhanjiang Bay ranged from 28.41°C to 32.64°C (with an average of 31.52°C ± 0.93°C), and the temperature in the southern bay was lower than that in the northern bay. The pH ranged from 7.46 to 8.09 (with an average of 7.78 ± 0.18) in Zhanjiang Bay. Similar to salinity, the spatial distribution of pH also showed an obvious increasing trend and the lowest pH value corresponding to the lowest salinity, which indicated that the Suixi River is the region with the lowest pH value in Zhanjiang Bay.
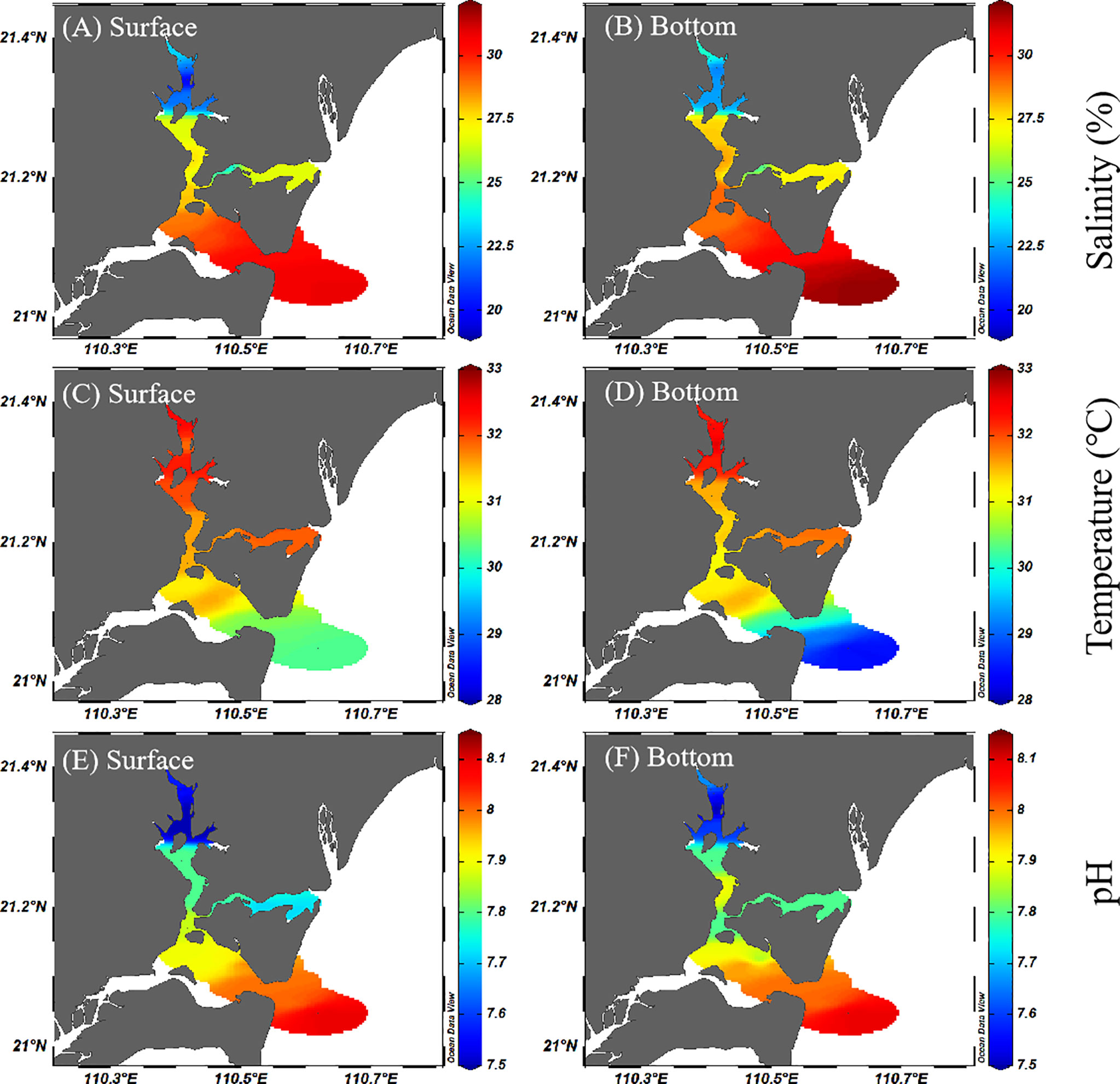
Figure 2 Spatial distributions of salinity (A, B), temperature (C, D) and pH (E, F) in the surface and bottom water of Zhanjiang Bay during the sampling period.
3.2 Distributions of Chlorophyll-A, Dissolved Oxygen, and Stable Isotope
The Chl-a ranged from 0.6 to 10.06 μg L−1 (with an average of 2.00 ± 2.13 μg L−1), and the high-value area was located in the Nansan islands (Figure 3). Compared with salinity and temperature, the spatial distribution of Chl-a did not show an obvious increasing or decreasing trend, and the overall distribution was relatively even. Lower DO values were observed from the estuary of the Suixi River and displayed a seaward increasing trend from the upper bay to the outer bay (3.67–5.79 mg L−1, with an average of 4.79 ± 0.59 mg L−1) (Figure 3). Although the salinity difference between the surface water and the bottom water was very small, the difference between DO was obvious. The correlation between DO and salinity was relatively weak (r2 = 0.56, p< 0.01, n = 34), and that between DO and Chl-a was small (r2 = 0.05, p< 0.01, n = 34), indicating that freshwater input and algae growth did not have a decisive influence on the distribution of DO. Combined with the fact that the DO of Zhanjiang Bay was all in unsaturated states (DO saturation: 57.84%–94.82%) (Figure S1), the DO may be affected by the consumption of organic matter in the degradation process. The concentration of DO near the estuary of the Suixi River was low, and the saturation of DO was also the lowest (Figure S1), which may be due to the fact that it had the highest concentration of DOM and was most severely affected by the degradation of organic matter. The δ13C of dissolved inorganic carbon (DIC) values varied from −4.9‰ to 0.3‰ (with an average of −2.1‰ ± 1.57‰) and showed an increasing trend from the upper bay to the outer bay (Figure 3).
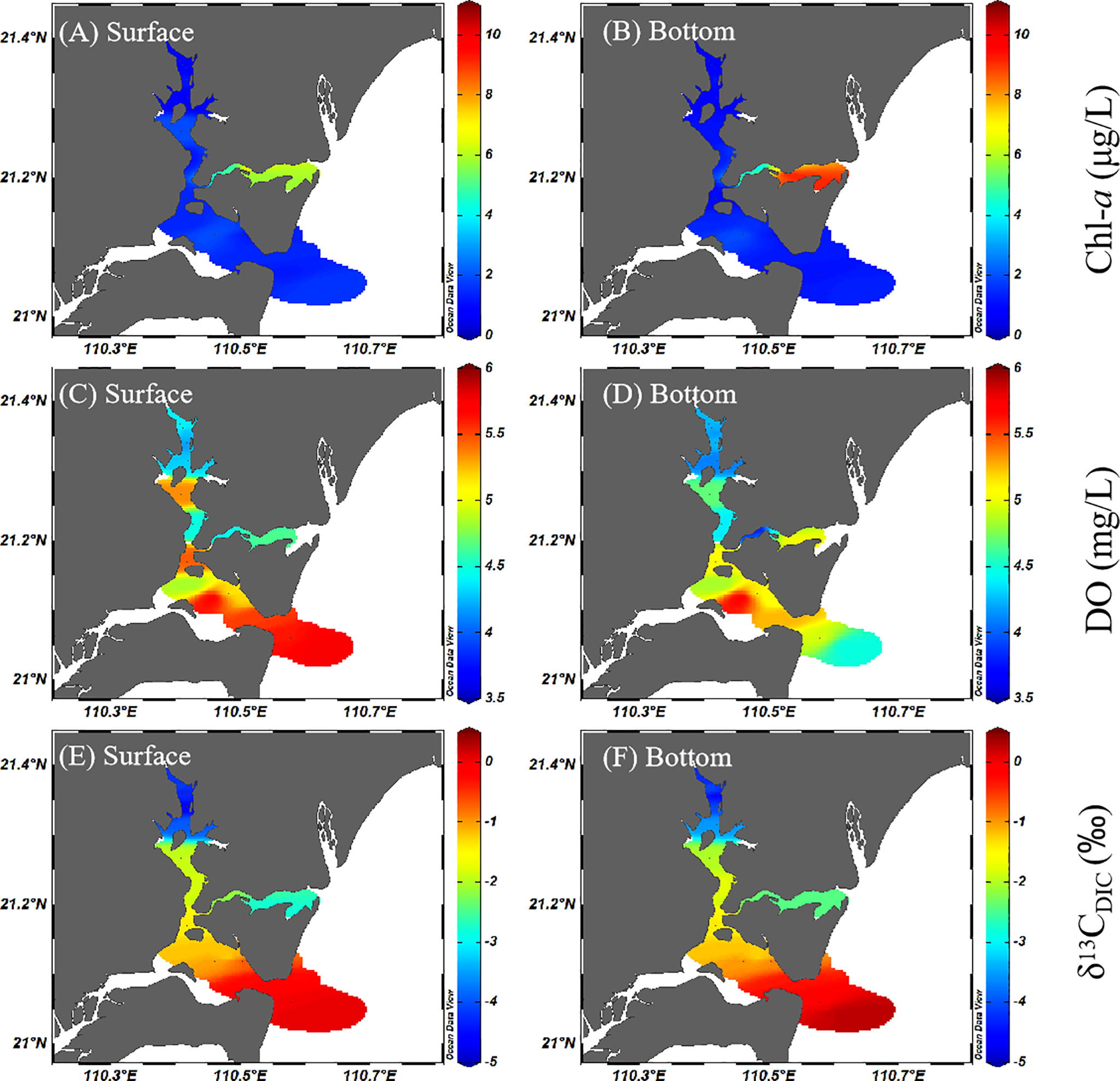
Figure 3 Spatial distributions of Chl-a (A, B), DO (C, D), and stable isotope of DIC (E, F) in surface and bottom water of Zhanjiang Bay during the sampling period. Chl-a, chlorophyll a; DO, dissolved oxygen; DIC, dissolved inorganic carbon.
3.3 Distributions of Fluorescent Dissolved Organic Matter
Two humic-like components (C1–C2) and two protein-like components (C3–C4) were identified using the PARAFAC model in this study (Figure 4). C1 showed an excitation maximum at ≤240 nm and an emission maximum at 400 nm, which had been considered a marine humic-like fluorescence peak M previously (Coble, 1996). C2 displayed an excitation maximum at 250 nm and an emission maximum at 456 nm, which used to be classified into a mixture of terrestrial humic-like fluorescence peak C and peak A (Coble, 1996). C3 has two excitation maxima at ≤240 and 280 nm and an emission maximum at 356 nm, which have previously been reported as tryptophan-like fluorescence peak T (Coble, 1996). C4 had an excitation/emission maximum at 270/324 nm, which was attributed to protein-like fluorescence peak B and peak T (Coble, 1996).
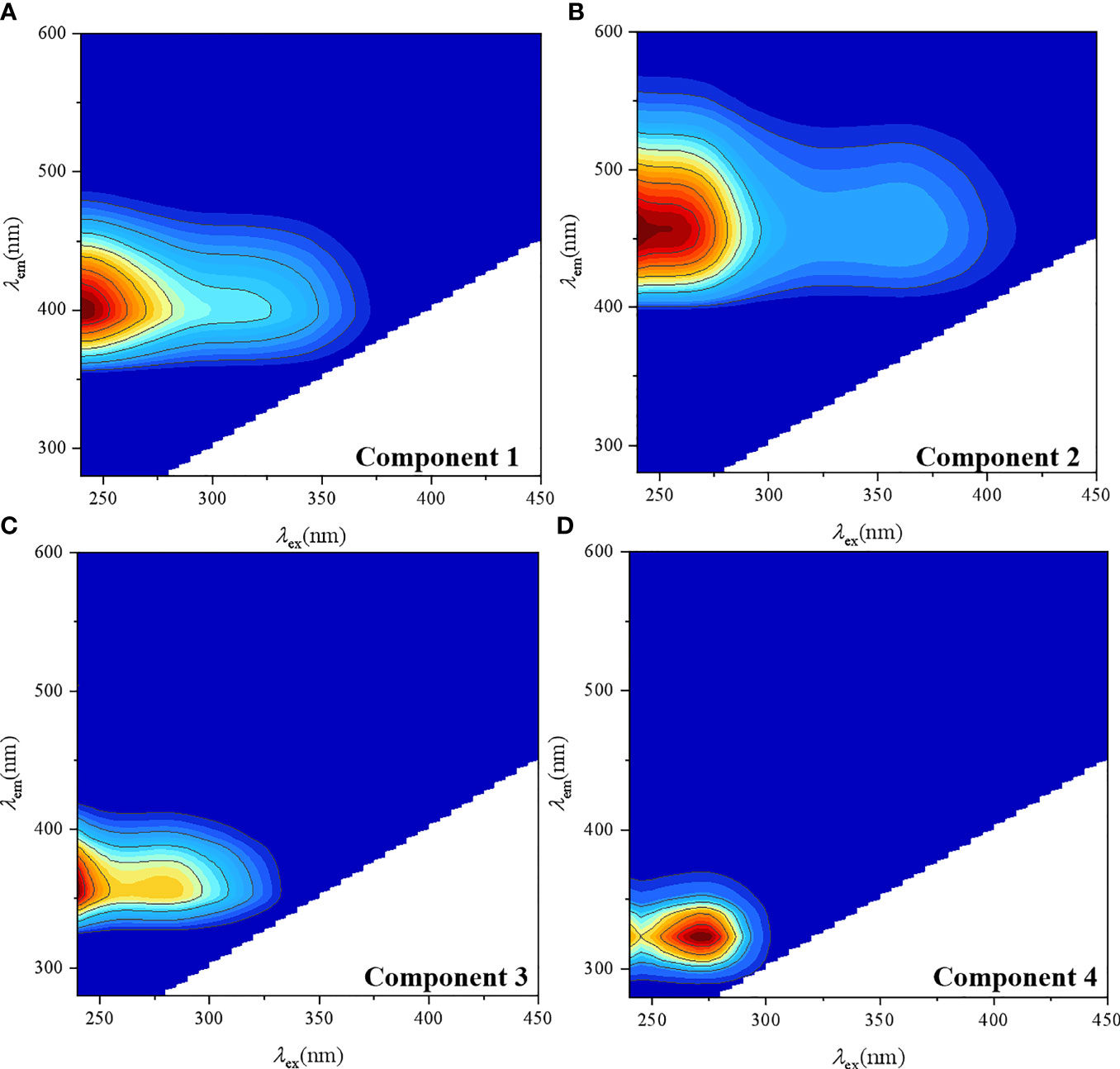
Figure 4 Fluorescence components identified of C1 (A), C2 (B), C3 (C) and C4 (D) by the PARAFAC modeling in Zhanjiang Bay.
The fluorescence intensity of humic-like C1 and C2 ranged from 5.90 to 50.99 (10−2 RU) and 3.90 to 32.95 (10−2 RU) (Figure 5), which had strong correlation between each other (r2 = 0.99, p< 0.01, n = 34). Combined with their similar distribution, C1 and C2 should have the same source. The intensity of tryptophan-like C3 ranged from 4.84 to 28.48 (10−2 RU) (with an average of 13.90 ± 0.07 (10−2 RU)), and the intensity of protein-like C4 ranged from 5.23 to 13.67 (10−2 RU) (with an average of 8.92 ± 0.02 (10−2 RU)). The salinity showed a great negative correlation with the four fluorescent components (C1, r = −0.91, p< 0.01; C2, r = −0.91, p< 0.01; C3, r = −0.92, p< 0.01; C4, r = −0.87, p< 0.01). There were strong negative correlations between salinity and C1–C3 components, indicating that these three components may mainly come from the river, while the correlation between C4 and salinity decreased, indicating that freshwater had weakened the effect for C4 components. The four fluorescent components all showed a decreasing trend from the upper bay to the outer bay, and the decreasing amount was relatively large. In general, the fluorescence intensity of surface water was slightly lower than that of bottom water.
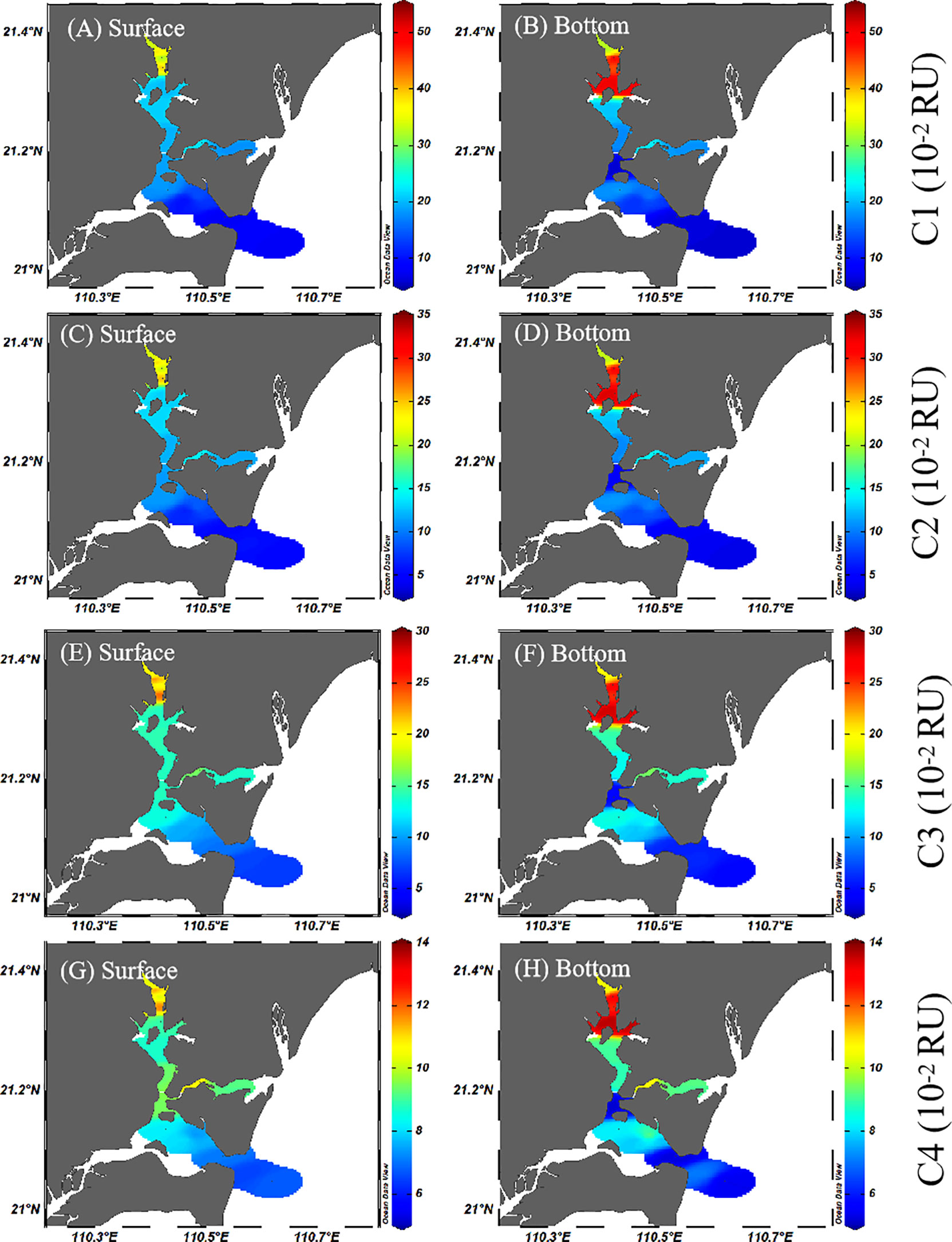
Figure 5 Spatial distributions of fluorescence components C1 (A, B), C2 (C, D), C3 (E, F) and C4 (G, H) in the surface and bottom water of Zhanjiang Bay during the sampling period.
4 Discussion
4.1 Sources and Characteristics of Dissolved Organic Matter in Zhanjiang Bay
Stable carbon isotope (δ13C) was an indicator of the source of organic matter. Algae utilized DIC as a substrate during photosynthesis and distinguished 12C and 13C with a kinetic classification of 20‰–21‰ (Chanton and Lewis, 1999; Hoffman and Bronk, 2006). In general, marine phytoplankton utilized HCO3− (δ13C = 0‰) in water for photosynthesis, while terrestrial plants used atmospheric CO2 (δ13C = −8‰); thus, aquatic microorganisms tended to have heavier δ13C values (Bouillon et al., 2011; Remeikaitė-Nikienė, 2017). The δ13C values of marine phytoplankton were relatively high, ranging from −23‰ to −16‰, while those of freshwater phytoplankton were low, ranging from −33‰ to −25‰ (Wang et al., 2004; Hoffman and Bronk, 2006; Bianchi, 2011; Yang et al., 2014). The variation of DIC stable carbon isotope (δ13CDIC) in summer ranged from −4.9‰ to 0.32‰, displaying an increasing tendency from the upper bay to the outer bay (Figure 3). Assuming the fractionation of algae production was −21‰, the δ13C of organic matter from Zhanjiang Bay in this study ranged from −25.9‰ to −20.68‰, with the mixture of terrigenous (higher plant debris, soil input) and authigenic (phytoplankton input, microbial input) characteristics. There were significant differences in δ13CDIC among the stations, meaning that the source of organic matter may be obviously different. The δ13CDIC increased from the upper bay to the outer bay as a whole; the contribution of terrigenous input gradually decreased from the estuary of the Suixi River to the bay mouth. Among them, the Suixi River, as the largest source of freshwater input to Zhanjiang Bay, inputted a large amount of land-based materials such as higher plant debris and soil humus into Zhanjiang Bay. In the location of stations S1 and S2 closest to the estuary of the Suixi River, the δ13CDIC was relatively low (−4.9‰ to −3.8‰), and the kinetic fractionation of δ13C was −25.9‰ to −24.8‰, which was closer to freshwater phytoplankton (δ13C = −33‰ to −25‰) and soil humus (δ13C = −25‰ to −22‰) (Kendall, 1998; Chanton and Lewis, 1999; Zhang et al., 2018). The δ13CDIC in bay mouth ranged from 0‰ to 0.5‰, which belonged to the range of marine algae, indicating that phytoplankton contributed to the organic matter (Bouillon et al., 2011; Remeikaitė-Nikienė, 2017).
Generally speaking, phytoplankton absorbed 12C preferentially during photosynthesis, resulting in relatively enriched 13C in water (Bouillon et al., 2011). Therefore, phytoplankton photosynthesis in surface water was more intense than in bottom water, and δ13C was relatively positive (Hoffman and Bronk, 2006). However, for some stations (S3–S6) in Zhanjiang Bay, the δ13CDIC of surface water was significantly lower than that of bottom water, which indicated that abiotic processes were more strongly affecting these regions. As this region was near the sewage outlet of Zhanjiang Bay (Zhang et al., 2021), the difference between surface and bottom water may be affected by sewage discharge, and the kinetic fractionation of δ13C in surface water was in the range of sewage (δ13C = −26‰ to −22‰). The C3 component, which was attributed to protein-like fluorescence (Coble, 1996; Ye et al., 2019), also had strong fluorescence intensity in this region at the same time. Furthermore, in abiotic sources of DOM, there was a considerable amount of DOM in surface sediments at times, which may be diffused into the water with the concentration gradient and had a significant impact on the properties of organic matter in the sea (Burdige and Komada, 2015). The δ13CDIC values in this region ranged from −4.9‰ to −3.6‰, and the values of δ13C dynamic fractionation ranged from −25.9‰ to −24.6‰, which were consistent with the previously reported δ13C range of surface sediments in Zhanjiang Bay (Zhou et al., 2021). However, this region had narrow terrain and relatively closed and poor hydrodynamic conditions (Li, 2012; Shi et al., 2015). Moreover, the salinity difference of these stations was relatively small, and the stirring effect of the salt wedge on sediment should be small, so it did not have the dynamic conditions to cause sediment resuspension. In addition, the bay mouth, which was more susceptible to tidal oscillation and had strong hydrodynamic conditions, did not show this characteristic; thus, the possibility of sediment resuspension could be ruled out.
HCA was performed using optical indexes (FI, BIX, and HIX) and fluorescent components (C1 to C4) to explore the source and characteristic similarity or difference of DOM for Zhanjiang Bay (Figure 6). The Ward clustering method of Euclidean distance was selected to generate the tree graph of the samples, and the relationship among the 7 indicators was discussed by the Ward clustering method of Pearson’s correlation coefficient. The data set was standardized before performing HCA, avoiding errors caused by the range of values and units of the original variables.
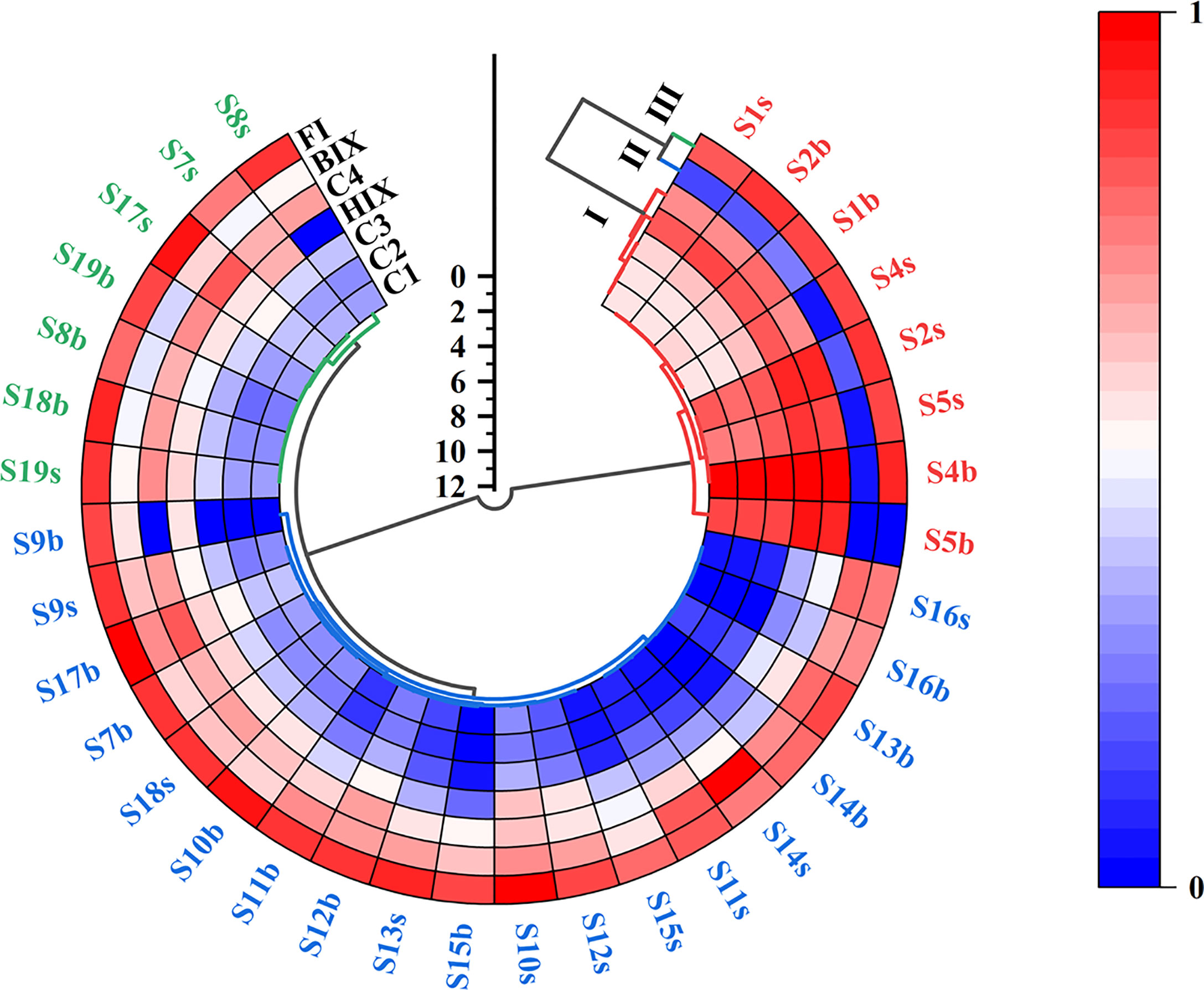
Figure 6 A dendrogram with heatmap of DOM fluorescent components and optical indexes at each sampling site in Zhanjiang Bay. DOM, dissolved organic matter.
The 7 indicators fall into three categories. Cluster I had C1, C2, C3, C4, and HIX, all of which included microbial origin, phytoplanktonic origin, and terrestrial origin, representing the mixed sources of organic matter. Cluster II was the BIX, representing the primary source of DOM, which usually means a large input of fresh antigenic organic matter with a high index (Huguet et al., 2009). Cluster III was composed of the FI, which reflected the relative contribution rate of aromatic amino acids and non-aromatic substances to DOM fluorescence intensity, so it could be used as an indicator of the source of substances and the degradation degree of DOM (Chin et al., 1994; McKnight et al., 2001; Mladenov et al., 2007). DOM was derived from the biological activity and sediment release when the FI was high, and sewage discharge may also be its source (McKnight et al., 2001; Dong and Rosario-Ortiz, 2012; Ye et al., 2019).
In HCA results, the 34 water samples were distinctly divided into three clusters according to their variations of optical indexes and fluorescent components (Figure 6). Among them, 8 water samples were clustered into the red cluster with a higher value of indicators of cluster I, 19 water samples were clustered into the blue cluster with indicators of cluster II exhibiting a higher value, and 7 water samples were clustered in the green cluster with higher value of indicators of cluster III. The red clustering data, accompanying higher values of indicators for cluster I, were mainly concentrated in the upper bay (S1–S5), where the C1 component representing the microbial source, the C2 component representing the terrestrial source, and the C4 component representing phytoplanktonic source all had high fluorescence intensity (Figure 6). Thus, there was a typically mixed source in the red cluster. In addition, the red cluster had the characteristics of high HIX and low BIX, indicating that the region was affected by terrestrial freshwater input and had more terrigenous humus. The high δ13CDIC in the region also supported this view.
BIX of cluster II could be used as an indicator of DOM traceability in water (Chiu et al., 2019), where high values (>0.8) corresponded to DOM of in situin-situ origin, while low BIX (<0.8) indicated allochthonous origin (Catalán et al., 2014). In this study, the blue clustering stations (S9–S16) were mainly concentrated in the southern bay, and the water samples of BIX between 1.1 and 1.19 were mainly distributed here (Figure S1), implying the that highest-value area of BIX was concentrated in the southern bay. These indicated that the input of a large amount of fresh autogenous organic matter made the newly generated DOM into the southern bay, especially protein-like DOM, and had a high proportion in the overall DOM (Huguet et al., 2009). Compared with the red cluster, the fluorescence intensity of C1, C2, and C3 components in the blue cluster was lower, and the HIX was lower at the same time, which means that the terrestrial input was weakened in this region. Moreover, the decreasing trend of C1, C2, and C3 components from the upper bay to the outer bay also confirmed that (Figure 5). In the meantime, higher C4 component and BIX showed a significant role in algal activity in the southern bay, explaining that phytoplankton growth had an important impact on the southern bay. On the whole, although the southern bay was part of the semi-closed bay, its organic matter sources were more marine than terrestrial and displayed a characteristic of the high BIX and low HIX, which tended to be marine water (Huguet et al., 2009).
FI usually had multiple meanings, including that organic matter may be derived from biological activities, sediment release, or sewage discharge (Chin et al., 1994; McKnight et al., 2001; Dong and Rosario-Ortiz, 2012). The green clustering data were mainly concentrated on the Nansan islands, where the area was narrow and the water depth was less than 5 m on the whole (Figure 1), which means that the weak turbulence mixing did not have the dynamic conditions to cause sediment resuspension, so the release of sediment may not be the cause of high FI. The FI in the green cluster showed a decreasing trend from west to east (Figure S1), indicating that the source of the high FI may be at station S17. The lowest salinity at station S17 indicated that freshwater should be coming into a bay here (Figure 2), but there were no rivers in the area; thus, the effects of the river could be excluded. A large number of artificially reclaimed fishponds were distributed on both sides of the green clustering stations, and aquaculture sewage would be discharged from time to time, which seemed to explain the freshwater input. Moreover, the FI in this area ranged from 2.00 to 2.10, with an average of 2.04 ± 0.04, exceeding the range limit of ordinary natural water, which could be from sewage possibly (Dong and Rosario-Ortiz, 2012; Ye et al., 2019). Combined with the high value of Chl-a in this area, it was possible that the FI of green clustering was influenced by sewage input and phytoplankton. In addition, although the C4 component representing protein-like organic matter had a high value in the green cluster and was close to the bay mouth, the phytoplankton growth activity here may be promoted by the high concentration of nutrients brought by sewage instead of by the offshore input, so the maximum value of Chl-a corresponded to the lowest value of salinity. Thus, the green cluster also embodied the dominance of freshwater.
4.2 Interactions Between Fluorescence Parameters of Dissolved Organic Matter and Environmental Variables
RDA was conducted to explain the combined correlation and impacted between response variables (PARAFAC composition and optical index) and environmental variables (water quality index)(Figure 7). In the RDA diagram, the length of the arrow indicated the importance of the factor, and the angle or coordinate axis between the arrow and another arrow indicated the correlation between them (Ouyang et al., 2018). When the angle was smaller, the relationship was stronger. The DOM of the red clustering site and green clustering site may be caused by mixed sources and sewage, respectively; that these areas were affected by human activities was relatively obvious. Therefore, the two clustering data were merged for analysis in RDA, summarized as the northern bay and the blue clustering site for the southern bay, to enhance the credibility of the results. RDA determined the relationship between DOM fluorescence parameters and water quality parameters through Canoco5.0 software. Log(x + 1) conversion was performed on the original optical parameters and water quality parameters before RDA so that these parameters were normally distributed (Ramette, 2007).
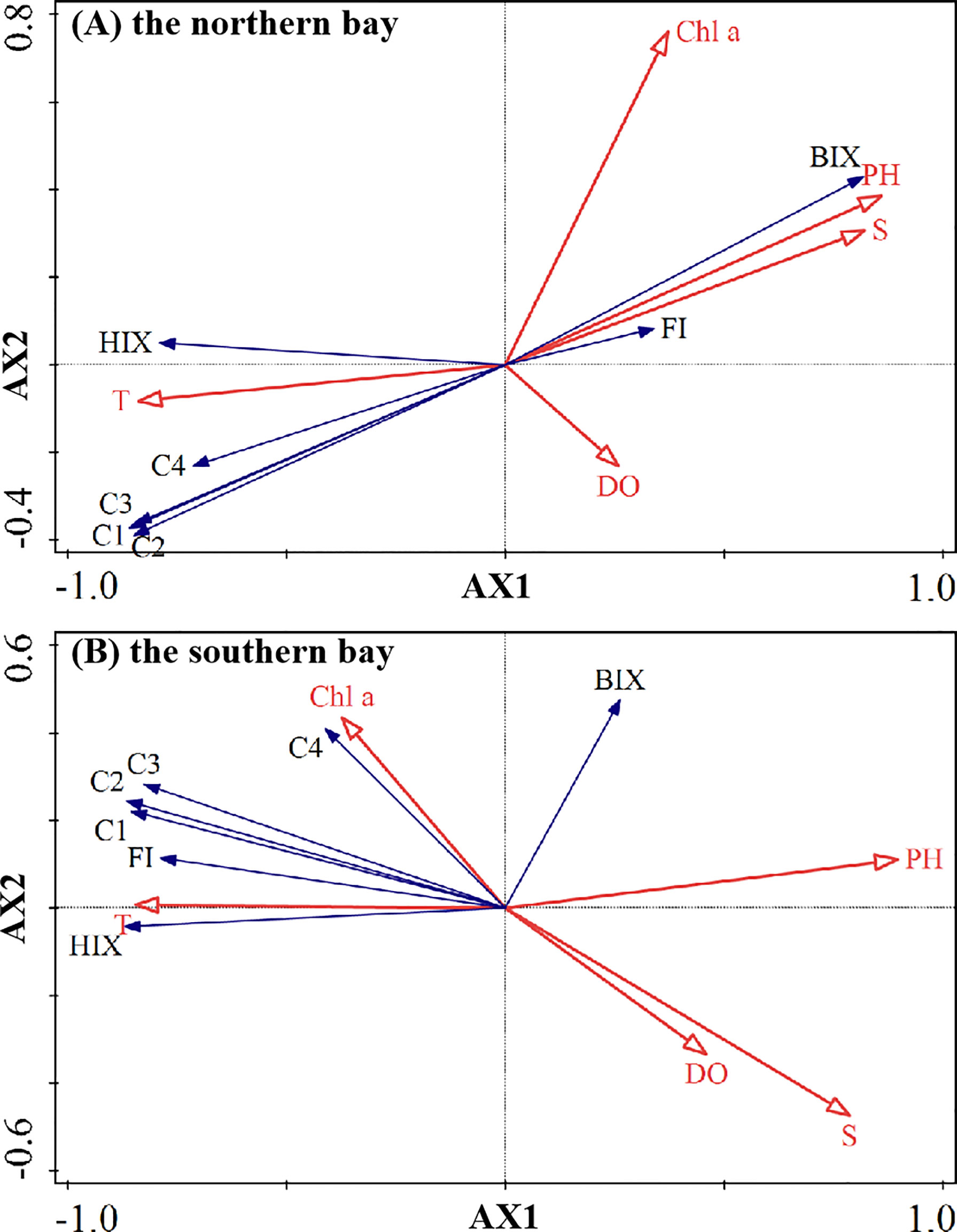
Figure 7 The plots based on redundancy analysis in Zhanjiang Bay (the solid arrows with blue fonts are the response variables, and the hollow arrows with red fonts are the environmental variables).
In general, freshwater inputs from the land were rich in organic matter and served as important sources of DOM in estuaries. The salinity in the northern and southern bays was opposite to the direction of the arrow of the four fluorescent components, and the included angle of the extension line was small, indicating that terrigenous input served as the important source of Zhanjiang Bay. Among them, the angle between C1–C4 fluorescence components and reverse extension line of salinity in the southern bay was slightly larger than that in the northern bay, signifying that the terrigenous input had weakened in the southern bay. Compared with the upper bay, the fluorescence intensity of C1–C4 components in the bay mouth decreased by 80%, 79%, 69%, and 42%, respectively, on average, further indicating that terrigenous organic matter was greatly diluted from the bay mouth and the concentration of organic matter decreased significantly. Terrigenous DOM in Zhanjiang Bay had limited influence on the outside bay, which may be due to the fact that Zhanjiang Bay served as a typical semi-closed bay with a narrow bay mouth of only 2 km, where only a small amount of water could flow out of the bay to participate in water exchange each time (Shi et al., 2015), or it may also be affected by seawater intrusion.
The pH value of seawater was mainly controlled by the dissociation equilibrium of dissolved inorganic carbonate (Zeebe and Wolf-Gladrow, 2001), so any action which could change the dissolution equilibrium of inorganic carbonate also was able to further affectting the pH value of seawater. In theory, many processes were equipped to affect the relative concentration of carbonate in seawater systems, such as temperature, hydrostatic pressure, salinity, and consumption by life activities, as well as to change the pH value of seawater ulteriorly (Zeebe and Wolf-Gladrow, 2001). The pH of the northern bay was in the same direction as the arrow of salinity, and the angle between pH and salinity was less than 20°, existing as a strong positive correlation; that is, the change of pH was affected by salinity. The pH value of the northern bay increased with the increase of the salinity of the mixed water, showing a trend of moving from the upper bay to the outer bay. They were strongly correlated and presented a conservative mixing condition, which means that the mechanical mixing of water played a leading role in the changing trend of pH in the northern bay. In the southern bay, the pH was in the same direction as the arrow of salinity, and the included angle with salinity was greater than 60°, which means that the positive correlation between pH and salinity was not as strong as that in the northern bay, and mechanical mixing of water may not play a leading role in the changing trend of pH in the southern bay. Moreover, the angle between pH, HIX, and reverse extension line of temperature was small, which signified that humus organic matter and temperature may have an important influence on pH change.
The direction of Chl-a and BIX in the northern bay was the same as that of the arrow of salinity but opposite to that of the arrow of each fluorescence component. The included angle between BIX and salinity was small, and the occurrence of high Chl-a values at sites with high salinity also indicated this, which seemed to signify that seawater had a relatively important influence on the input of fresh antigenic organic matter. In fact, the correlation was more likely due to sewage discharge in the green cluster. The angle between the BIX and salinity arrow was 90° nearly in the southern bay, and the BIX showed the characteristics of neither marine sources nor terrigenous sources, which may be due to two reasons. On the one hand, the contribution of freshwater to nutrients in Zhanjiang Bay (about 54%–90%) was much higher than that in the seawater (about 18%–45%) in summer (unpublished data from our previous survey). However, freshwater from rivers accounts for only a small proportion in summer, about 7% (unpublished data from our previous survey). When the salinity of seawater increased, the terrigenous signal was weakened by the dilution of seawater. As the result, the influence of terrigenous organic matter in the southern bay could not be as strong as that in the northern bay. On the other hand, as the salinity of seawater increased, the autogenous contribution of phytoplankton in the ocean gradually became dominant (Seidel et al., 2014; Medeiros et al., 2015), at which time organic matters may show characteristics of the high BIX and low HIX (Huguet et al., 2009; Milbrandt et al., 2010). Therefore, with the weakening of terrestrial organic matter and the enhancement of marine organic matter, the BIX of the southern bay may be affected by the organic matter at both ends.
4.3 Weak Turbulent Mixing of Dissolved Organic Matter in Semi-Closed Bay
Influenced by freshwater input, the DOM of Zhanjiang Bay displayed a decreasing trend from the upper bay to the outer bay on the whole. However, the organic matter in the bay displayed different characteristics, and the correlation between organic matter with different characteristics and environmental parameters was also different. This phenomenon may be caused by the fact that Zhanjiang Bay was a typical semi-closed bay with weak turbulent mixing.
Meanwhile, with the tidal driving effect, seawater as the medium could effectively transport the terrigenous matter from the estuary to the outer bay at low tide (Lin et al., 2019; Iglesias et al., 2020). The northern part of Zhanjiang Bay had a long and narrow terrain, while the southern part had a small bay mouth and a large stomach. Coupled with the influence of summer monsoon easterly winds, the turbulent mixing was weak, while the semi-exchange time of water in the northern bay was more than 100 days, and that in the southern bay was 30 days (Li, 2012). Thus, the tide may not dilute and replace the water in the northern bay in time. Terrigenous organic matter was mainly trapped in the northern narrow sea area and had limited influence on the southern bay. Most of the marine source features in the southern bay came from algae, which may be produced in situ by seawater inside the bay, brought in by seawater outside the bay during high tide, or both.
As a general rule, more and more terrestrial DOM is being imported from the river into the estuary and then into the ocean, with the increase of anthropogenic activities (Hudson et al., 2007; Guo et al., 2014). However, the weak turbulent mixing of semi-closed bay may not be effective in facilitating the dispersal of terrigenous material all the time, even during the summer when river flows were at their greatest. In seasons or years with less rainfall, changes in organic matter characteristics of semi-closed bays and their response to the marine environment are worthy of our continued attention in future research.
5 Conclusion
By measuring δ13C of DIC and fluorescent components of DOM, the distribution and characteristics of DOM in Zhanjiang Bay were discussed. The following conclusions were drawn based on the results presented in this study: 1) there were several organic matter sources in Zhanjiang Bay, including river input, algae input, and sewage input, but freshwater inputs serve as an important source of DOM on the whole. Among them, the DOM in the bay displayed two characteristics in different parts. The DOM in the northern bay was composed of terrigenous organic matter mainly with high humus, while that in the southern bay was more inclined to marine sources with high BIX and low HIX. 2) The correlation between DOM with different characteristics and environmental parameters was also different. On the one hand, the salinity, pH, and Chl-a in the northern bay were correlated with DOM strongly, indicating that freshwater input had a profound impact on the relationship between organic matter and the environment. On the other hand, the correlation in the southern bay was weakened or even disappeared, meaning that with decreased freshwater input, DOM had changed from being dominated by freshwater input to being affected by the joint action of terrace and ocean. 3) The discrepancy between bays may be caused by the weak turbulent mixing in the semi-closed bay, where terrigenous organic matter was mostly trapped in the northern bay because of the narrow terrain, causing the southern bay to be affected by terrigenous and marine organic matter.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YZ, HZ, and CW conceived of the presented idea. HZ and CW performed the analysis of the manuscript. YZ processed the data, drafted the manuscript, and designed the figures. GP was involved in planning and supervising the funding of this work. All authors listed made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The present research is supported by the National Natural Science Foundation of China (No. 42076162), Natural Science Foundation of Guangdong Province, China (No. 2020A1515010496), Postgraduate Education Innovation Project of Guangdong Ocean University (202145), and a project supported by Innovation Group Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (No. 311020004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.956930/full#supplementary-material
References
Amaral V., Romera-Castillo C., Forja J. (2020). Dissolved Organic Matter in the Gulf of Cádiz: Distribution and Drivers of Chromophoric and Fluorescent Properties. Front. Mar. Sci. 7, 126. doi: 10.3389/fmars.2020.00126
Andrews J. E., Greenaway A. M., Dennis P. F. (1998). Combined Carbon Isotope and C/N Ratios as Indicators of Source and Fate of Organic Matter in a Poorly Flushed, Tropical Estuary: Hunts Bay, Kingston Harbour, Jamaica. Estuarine. Coast. Shelf. Sci. 46 (5), 743–756. doi: 10.1006/ecss.1997.0305
Beer S., Björk M., Beardall J. (2014). Photosynthesis in the Marine EnvironmentNew Jersey: Wiley-Blackwell.
Bianchi T. S. (2011). The Role of Terrestrially Derived Organic Carbon in the Coastal Ocean: A Changing Paradigm and the Priming Effect. Proc. Natl. Acad. Sci. U.S.A. 108 (49), 19473–19481. doi: 10.1073/pnas.1017982108
Boesch D. F. (2002). Challenges and Opportunities for Science in Reducing Nutrient Over-Enrichment of Coastal Ecosystems. Estuaries 25 (4), 886–900. doi: 10.1007/BF02804914
Bouillon S., Connolly R. M., Gillikin D. P. (2011). 7.07 Use of Stable Isotopes to Understand Food Webs and Ecosystem Functioning in Estuaries. Treatise. Estuar. Coast. Sci. 7 (13-14):143–173. doi: 10.1016/B978-0-12-374711-2.00711-7
Burdige D. J., Komada T. (2015). “Sediment Pore Waters,” in Biogeochemistry of Marine Dissolved Organic Matter: Academic Press.
Cabral H., Fonseca V., Sousa T., Costa Leal M. (2019). Synergistic Effects of Climate Change and Marine Pollution: An Overlooked Interaction in Coastal and Estuarine Areas. Int. J. Environ. Res. Public Health 16 (15), 2737. doi: 10.3390/ijerph16152737
Canuel E. A., Cammer S. S., McIntosh H. A., Pondell C. R. (2012). Climate Change Impacts on the Organic Carbon Cycle at the Land-Ocean Interface. Annu. Rev. Earth Planet. Sci. 40(685), 2012. doi: 10.1146/annurev-earth-042711-105511
Carlson C. A., Hansell D. A. (2015). DOM Sources, Sinks, Reactivity, and Budgets. Biogeochem. Mar. Dissolved Org. Matter, 65–126. doi: 10.1016/B978-0-12-405940-5.00003-0
Catalán, Núria, Obrador Biel, Ll Pretus Joan, (2014). "Ecosystem processes drive dissolved organic matter quality in a highly dynamic water body." Hydrobiologia 728(1) 111–124. doi: 10.1007/S10750-014-1811-Y
Chanton J. P., Lewis F. G. (1999). Plankton and Dissolved Inorganic Carbon Isotopic Composition in a River-Dominated Estuary: Apalachicola Bay, Florida. Estuaries 22 (3), 575–583. doi: 10.2307/1353045
Chen N., Krom M. D., Wu Y., Yu D., Hong H. (2018). Storm Induced Estuarine Turbidity Maxima and Controls on Nutrient Fluxes Across River-Estuary-Coast Continuum. Sci. Total. Environ. 628, 1108–1120. doi: 10.1016/j.scitotenv.2018.02.060
Chin Y. P., Aiken G., O’Loughlin E. (1994). Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 28 (11), 1853–1858. doi: 10.1021/es00060a015
Chiu T. P., Huang W. S., Chen T. C., Yeh Y. L. (2019). Fluorescence Characteristics of Dissolved Organic Matter (DOM) in Percolation Water and Lateral Seepage Affected by Soil Solution (SS) In A Lysimeter Test. Sensors, 19(18), 4016. doi: 10.3390/s19184016
Coble P. G. (1996). Characterization of Marine and Terrestrial DOM in Seawater Using Excitation-Emission Matrix Spectroscopy. Mar. Chem. 51 (4), 325–346. doi: 10.1016/0304-4203(95)00062-3
Coble P. G., Lead J., Baker A., Reynolds D. M., Spencer R. G. (Eds.)(2014). Aquatic Organic Matter Fluorescence. ( Cambridge University Press).
Dong M. M., Rosario-Ortiz F. L. (2012). Photochemical Formation of Hydroxyl Radical From Effluent Organic Matter. Environ. Sci. Technol. 46 (7), 3788–3794. doi: 10.1021/es2043454
Felgate S. L., Barry C. D., Mayor D. J., Sanders R., Carrias A., Young A., et al. (2021). Conversion of Forest to Agriculture Increases Colored Dissolved Organic Matter in a Subtropical Catchment and Adjacent Coastal Environment. J. Geophys. Res.: Biogeosci. 126 (6), e2021JG006295. doi: 10.1029/2021JG006295
García-Martín E. E., Sanders R., Evans C. D., Kitidis V., Lapworth D. J., Rees A. P., et al. (2021). Contrasting Estuarine Processing of Dissolved Organic Matter Derived From Natural and Human-Impacted Landscapes. Global Biogeochem. Cycles 35 (10), e2021GB007023. doi: 10.1029/2021GB007023
Gireeshkumar T. R., Deepulal P. M., Chandramohanakumar N. (2013). Distribution and Sources of Sedimentary Organic Matter in a Tropical Estuary, South West Coast of India (Cochin Estuary): A Baseline Study. Mar. pollut. Bull. 66 (1-2), 239–245. doi: 10.1016/j.marpolbul.2012.10.002
Guo W., Yang L., Zhai W., Chen W., Osburn C. L., Huang X., et al. (2014). Runoff-Mediated Seasonal Oscillation in the Dynamics of Dissolved Organic Matter in Different Branches of a Large Bifurcated Estuary—The Changjiang Estuary. J. Geophys. Res.: Biogeosci. 119 (5), 776–793. doi: 10.1002/2013JG002540
He W., Chen M., Schlautman M. A., Hur J. (2016). Dynamic Exchanges Between DOM and POM Pools in Coastal and Inland Aquatic Ecosystems: A Review. Sci. Total. Environ. 551, 415–428. doi: 10.1016/j.scitotenv.2016.02.031
Hoffman J. C., Bronk D. A. (2006). Interannual Variation in Stable Carbon and Nitrogen Isotope Biogeochemistry of the Mattaponi River, Virginia. Limnol. Oceanogr. 51 (5), 2319–2332. doi: 10.4319/lo.2006.51.5.2319
Hudson N., Baker A., Reynolds D. (2007). Fluorescence Analysis of Dissolved Organic Matter in Natural, Waste and Polluted Waters—a Review. River. Res. Appl. 23 (6), 631–649. doi: 10.1002/rra.1005
Huguet A., Vacher L., Relexans S., Saubusse S., Froidefond J. M., Parlanti E. (2009). Properties of Fluorescent Dissolved Organic Matter in the Gironde Estuary. Org. Geochem. 40 (6), 706–719. doi: 10.1016/j.orggeochem.2009.03.002
Iglesias I., Almeida C. M. R., Teixeira C., Mucha A. P., Magalhães A., Bio A., et al. (2020). Linking Contaminant Distribution to Hydrodynamic Patterns in an Urban Estuary: The Douro Estuary Test Case. Sci. Total. Environ. 707, 135792. doi: 10.1016/j.scitotenv.2019.135792
Jørgensen L., Stedmon C. A., Kragh T., Markager S., Middelboe M., Søndergaard M. (2011). Global Trends in the Fluorescence Characteristics and Distribution of Marine Dissolved Organic Matter. Mar. Chem. 126 (1-4), 139–148. doi: 10.1016/j.marchem.2011.05.002
Kendall C. (1998). “Tracing Nitrogen Sources and Cycling in Catchments,” in Isotope Tracers in Catchment Hydrology Amsterdam: Elsevier, 519–576.
Kerr R. A. (2011). Humans are Driving Extreme Weather; Time to Prepare. Science 334 (6059), 1040. doi: 10.1126/science.334.6059.1040
Kinsey J. D., Corradino G., Ziervogel K., Schnetzer A., Osburn C. (2018). Formation of Chromophoric Dissolved Organic Matter by Bacterial Degradation of Phytoplankton-Derived Aggregates. Front. Mar. Sci. 4, 430. doi: 10.3389/fmars.2017.00430
Lee S. A., Kim T. H., Kim G. (2020). Tracing Terrestrial Versus Marine Sources of Dissolved Organic Carbon in a Coastal Bay Using Stable Carbon Isotopes. Biogeosciences 17 (1), 135–144. doi: 10.5194/bg-17-135-2020
Li X. ,. (2012). Numerical Study on the Water Exchange of a Semi-Closed Bay. Mar. Sci. Bull. 31 (03), 248–254.
Lin Y., Li Y., Zheng B., Yin X., Wang L., He J., et al. (2019). Evolution of Sedimentary Organic Matter in a Small River Estuary After the Typhoon Process: A Case Study of Quanzhou Bay. Sci. Total. Environ. 686, 290–300. doi: 10.1016/j.scitotenv.2019.05.452
Lloret E., Dessert C., Buss H. L., Chaduteau C., Huon S., Alberic P., et al. (2016). Sources of Dissolved Organic Carbon in Small Volcanic Mountainous Tropical Rivers, Examples From Guadeloupe (French West Indies). Geoderma 282, 129–138. doi: 10.1016/j.geoderma.2016.07.014
Lorenzen C. J., Jeffrey S. W. (1980). Determination of Chlorophyll in Seawater. Unesco. tech. pap. Mar. Sci. 35 (1), 1–20. doi: 10.1556/Nano.2008.00001
McKnight D. M., Boyer E. W., Westerhoff P. K., Doran P. T., Kulbe T., Andersen D. T. (2001). Spectrofluorometric Characterization of Dissolved Organic Matter for Indication of Precursor Organic Material and Aromaticity. Limnol. Oceanogr. 46 (1), 38–48. doi: 10.4319/lo.2001.46.1.0038
Medeiros P. M., Seidel M., Ward N. D., Carpenter E. J., Gomes H. R., Niggemann J., et al. (2015). Fate of the Amazon River Dissolved Organic Matter in the Tropical Atlantic Ocean. Global Biogeochem. Cycles 29 (5), 677–690. doi: 10.1002/2015GB005115
Milbrandt E. C., Coble P. G., Conmy R. N., Martignette A. J., Siwicke J. J. (2010). Evidence for the Production of Marine Fluorescent Dissolved Organic Matter in Coastal Environments and a Possible Mechanism for Formation and Dispersion. Limnol. Oceanogr. 55 (5), 2037–2051. doi: 10.4319/lo.2010.55.5.2037
Mladenov N., McKnight D. M., Macko S. A., Norris M., Cory R. M., Ramberg L. (2007). Chemical Characterization of DOM in Channels of a Seasonal Wetland. Aquat. Sci. 69 (4), 456–471. doi: 10.1007/s00027-007-0905-2
Montgomery H., Thom N. S., Cockburn A. (1964). Determination of Dissolved Oxygen by the Winkler Method and the Solubility of Oxygen in Pure Water and Sea Water. J. Appl. Chem. 14 (7). 280–296 doi: 10.1002/jctb.5010140704
Murphy K. R., Stedmon C. A., Waite T. D., Ruiz G. M. (2008). Distinguishing Between Terrestrial and Autochthonous Organic Matter Sources in Marine Environments Using Fluorescence Spectroscopy. Mar. Chem. 108 (1-2), 40–58. doi: 10.1016/j.marchem.2007.10.003
Najjar R. G., Pyke C. R., Adams M. B., Breitburg D., Hershner C., Kemp M., et al. (2010). Potential Climate-Change Impacts on the Chesapeake Bay. Estuarine. Coast. Shelf. Sci. 86 (1), 1–20. doi: 10.1016/j.ecss.2009.09.026
Ouyang W., Yang W., Tysklind M., Xu Y., Lin C., Gao X., et al. (2018). Using River Sediments to Analyze the Driving Force Difference for non-Point Source Pollution Dynamics Between Two Scales of Watersheds. Water Res. 139, 311–320. doi: 10.1016/j.watres.2018.04.020
Ramette A. (2007). Multivariate Analyses in Microbial Ecology. FEMS Microbiol. Ecol. 62 (2), 142–160. doi: 10.1111/j.1574-6941.2007.00375.x
Remeikaitė-Nikienė N. (2017). Distribution of Organic Matter and Metals in the South-Eastern Baltic Sea (Lithuanian Zone) Lithuania: ( Doctoral dissertation, Vilniaus universitetas).
Seidel M., Beck M., Riedel T., Waska H., Suryaputra I. G., Schnetger B., et al. (2014). Biogeochemistry of Dissolved Organic Matter in an Anoxic Intertidal Creek Bank. Geochim. Cosmochim. Acta 140, 418–434. doi: 10.1016/j.gca.2014.05.038
Shi Y., Zhang Y., Sun X. (2015). Spatiotemporal Distribution of Eutrophication and Its Relationship With Environmental Factors in Zhanjiang Sea Bay Area. Environ. Sci. Technol. 38 (12), 90–96+122. doi: CNKI:SUN:FJKS.0.2015-12-017
Spilling K., Olli K., Lehtoranta J., Kremp A., Tedesco L., Tamelander T., et al. (2018). Shifting Diatom—Dinoflagellate Dominance During Spring Bloom in the Baltic Sea and its Potential Effects on Biogeochemical Cycling. Front. Mar. Sci. 5, 327. doi: 10.3389/fmars.2018.00327
Stedmon C. A., Bro R. (2008). Characterizing Dissolved Organic Matter Fluorescence With Parallel Factor Analysis: A Tutorial. Limnol. Oceanogr.: Methods 6 (11), 572–579. doi: 10.4319/lom.2008.6.572
Stedmon C. A., Markager S., Bro R. (2003). Tracing Dissolved Organic Matter in Aquatic Environments Using a New Approach to Fluorescence Spectroscopy. Mar. Chem. 82 (3-4), 239–254. doi: 10.1016/S0304-4203(03)00072-0
Wang X. C., Chen R. F., Gardner G. B. (2004). Sources and Transport of Dissolved and Particulate Organic Carbon in the Mississippi River Estuary and Adjacent Coastal Waters of the Northern Gulf of Mexico. Mar. Chem. 89(1-4), 241–256. doi: 10.1016/j.marchem.2004.02.014
Xue Y., Zhang Z. M., Zhang R. R., Li Y. Q., Sun A. L., Shi X. Z., et al. (2021). Aquaculture-Derived Distribution, Partitioning, Migration, and Transformation of Atrazine and its Metabolites in Seawater, Sediment, and Organisms From a Typical Semi-Closed Mariculture Bay. Environ. pollut. 271, 116362. doi: 10.1016/j.envpol.2020.116362
Ya C., Anderson W., Jaffé R. (2015). Assessing Dissolved Organic Matter Dynamics and Source Strengths in a Subtropical Estuary: Application of Stable Carbon Isotopes and Optical Properties. Cont. Shelf. Res. 92, 98–107. doi: 10.1016/j.csr.2014.10.005
Yang J., Gao J., Liu B., Zhang W. (2014). Sediment deposits and organic carbon sequestration along mangrove coasts of the Leizhou Peninsula, Southern China. Estuarine, Coastal Shelf Sci. 136, 3–10. doi: 10.1016/j.ecss.2013.11.020
Ye Q., Zhang Z. T., Liu Y. C., Wang Y. H., Zhang S., He C., et al. (2019). Spectroscopic and Molecular-Level Characteristics of Dissolved Organic Matter in a Highly Polluted Urban River in South China. ACS Earth Space. Chem. 3 (9), 2033–2044. doi: 10.1021/acsearthspacechem.9b00151
Yu G., Fu D., Zhong Y., Luo Y. (2021). Optical Characteristics of Colored Dissolved Organic Matter in Zhanjiang Bay and Its Adjacent Sea Areas. J. Guangdong Ocean. Univ. 41 (01), 55–62. doi: 10.3969/j.issn.1673-9159.2021.01.008
Yu G., Liao S., Fu D., Li X. (2017). Remote Sensing Retrieval of Colored Dissolved Organic Matter in Zhanjiang Coastal Area. J. Guangdong Ocean. Univ. 37 (04), 123–127. doi: 10.3969/j.issn.1673-9159.2017.04.019
Zeebe R. E., Wolf-Gladrow D. (2001). CO2 in Seawater: Equilibrium, Kinetics, Isotopes, Amsterdam: Elsevier.
Zhang J., Wei L., Lai Y., Dai P., Chen Y., Zhang J. (2021). Concentration, Composition and Fluxes of Land-Based Nitrogen and Phosphorus Source Pollutants Input Into Zhanjiang Bay in Summer. J. Guangdong Ocean. Univ. 39 (4), 63–72. doi: 10.3969/j.issn.1673-9159.2019.04.010
Zhang M., Zhi Y., Shi J., Wu L. (2018). Apportionment and Uncertainty Analysis of Nitrate Sources Based on the Dual Isotope Approach and a Bayesian Isotope Mixing Model at the Watershed Scale. Sci. Total. Environ. 639, 1175–1187. doi: 10.1016/j.scitotenv.2018.05.239
Zhou Y., He D., He C., Li P., Fan D., Wang A., et al. (2021). Spatial Changes in Molecular Composition of Dissolved Organic Matter in the Yangtze River Estuary: Implications for the Seaward Transport of Estuarine DOM. Sci. Total. Environ. 759, 143531. doi: 10.1016/j.scitotenv.2020.143531
Zhou X., Jin G., Li J., Song Z., Zhang S., Chen C., et al. (2021). Effects of Typhoon Mujigae on the Biogeochemistry and Ecology of a Semi-Enclosed Bay in the Northern South China Sea. J. Geophys. Res.: Biogeosci. 126 (7), e2020JG006031. doi: 10.1029/2020JG006031
Keywords: dissolved organic matter, characteristic, stable isotope, Zhanjiang Bay, semi-closed bay
Citation: Zhong Y, Pan G, Zhao H and Wang C (2022) Characteristics of Dissolved Organic Matter in a Semi-closed Bay in Summer: Insights from Stable Isotope and Optical Analyses. Front. Mar. Sci. 9:956930. doi: 10.3389/fmars.2022.956930
Received: 30 May 2022; Accepted: 20 June 2022;
Published: 26 July 2022.
Edited by:
Meilin Wu, South China Sea Institute of Oceanology (CAS), ChinaReviewed by:
Yong Zhang, Shandong University, ChinaDongliang Lu, Beibu Gulf University, China
Wen-zhuo Zhu, Zhejiang Ocean University, China
Copyright © 2022 Zhong, Pan, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhao, aHVpemhhbzE5NzhAMTYzLmNvbQ==; Chao Wang, Y2hhb3dhbmdAZ2RvdS5lZHUuY24=
 Yafeng Zhong
Yafeng Zhong Gang Pan
Gang Pan Hui Zhao
Hui Zhao Chao Wang4,5,6*
Chao Wang4,5,6*