- 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China
- 3College of Marine Science, University of Chinese Academy of Sciences, Qingdao, China
- 4Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Science, Qingdao, China
- 5South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
Outbreaks of scyphozoan Aurelia coerulea and Nemopilema nomurai in the coastal sea of China are managed in recent years because they have severely jeopardized local socioeconomic development and ecological health. In this study, we propose specific strategies to control these blooms based on the different physio-ecological characteristics of their polyps, which can produce medusae by strobilation. High densities of A. coerulea polyps can survive chronically on the surfaces of some artificial constructions submerged in harbors or bays, China. Through buddings, they can resist the invasion of biofouling organisms and proliferate on the surfaces of some fouling organisms (e.g., ascidians, and bryozoans). However, N. nomurai polyps have not been recorded in natural environment. The in situ experiments found that polyps on settling plates fail to survive via podocysts due to severe biofouling invasion and post-strobilated degeneration in late spring and summer. As a result, the population size following is strongly dependent on the sexual recruitment of medusae during late summer and autumn. Therefore, we suggest that the reasonable governance strategy is to manage polyp populations together with biofouling organisms for A. coerulea blooms, however, with a focus on the medusa stage (particularly young medusae) to decrease the sexual reproduction in N. nomurai blooms. Accordingly, massive occurrences of A. coerulea in Qingdao Middle Port, China were alleviated by eliminating polyps and biofouling organisms on the undersurfaces of floating docks and then brushing the surfaces with modified alloprene paints. Some applicable control measures, including resource utilization of N. nomurai medusae and more severe and earlier summer fishing moratoriums, were used to possibly help restrain outbreaks of N. nomurai in Chinese coastal waters.
Introduction
In recent decades, frequent jellyfish blooms around the globe have had detrimental ecological and socioeconomic nuisance such as stinging bathers, clogging the cooling water intakes of coastal power plants, disturbing normal fishery production, as well as threatening fishery resources (Purcell et al., 2007; Graham et al., 2014). Jellyfish blooms have been frequently claimed to result from anthropogenic disturbances to the coastal environment including climate change, eutrophication, overfishing, development of aquaculture, and habitat modification (Richardson et al., 2009; Purcell, 2012; Duarte et al., 2013; Bayha and Graham, 2014), although robust evidence supporting these assertions is lacking (Pitt et al., 2018). The Chinese coastal waters are also one of the jellyfish blooming hotspots, where the outbreaks of Aurelia coerulea von Lendenfeld, 1884 (Scorrano et al., 2017) and Nemopilema nomurai Kishinouye, 1922 (Omori and Kitamura, 2004) have frequently appeared in the last two decades (Dong et al., 2010; Sun et al., 2015).
A. coerulea occurs mainly in neritic regions, such as harbors and bays, along the Chinese coast (Wang et al., 2012; Zheng et al., 2014; Dong et al., 2015). Frequent outbreaks of A. coerulea had damaged the normal operation of coastal infrastructures and the development of shallow sea aquaculture. In July 2014, a massive aggregation of A. coerulea clogged the cooling water intake of the Hong Yanhe nuclear power plant, Liaoning (Figure 1), causing the outage of two reactors (Li et al., 2017). The biomass of medusae salvaged around the cooling water intake was >10 tons in just 1 week (Li et al., 2017). The clogging cases of the cooling water intakes resulting from medusa aggregations also frequently occurred in several coal-fired power plants located in Qingdao and Qin Huangdao, China (Dong et al., 2010). For example, a serious clog appearing in the Qingdao power plant threatened the normal power supply of partial areas in July 2009 (Ren, 2009). Recently, outbreaks of A. coerulea have also occurred in many artificial sea cucumber culture ponds along the Bohai and Yellow seas (e.g., in Laoting, Dongying, and Rongcheng, Figure 1), which have been regarded as the cause of sea cucumber vomiting because ephyrae could sting them (Dong et al., 2018; Dong, 2019). In addition, A. coerulea blooms might also affect the community structure of zooplankton. In the northern Jiaozhou Bay, A. coerulea medusae frequently appeared in great numbers in June and July (Wan and Zhang, 2012) when the abundance of total zooplankton (except Noctiluca scintillans), in particular, copepods significantly decreased in 2009, a year with a large bloom of A. coerulea, in comparison with 2008 and 2010, which were non-blooming years (Wang et al., 2020a).
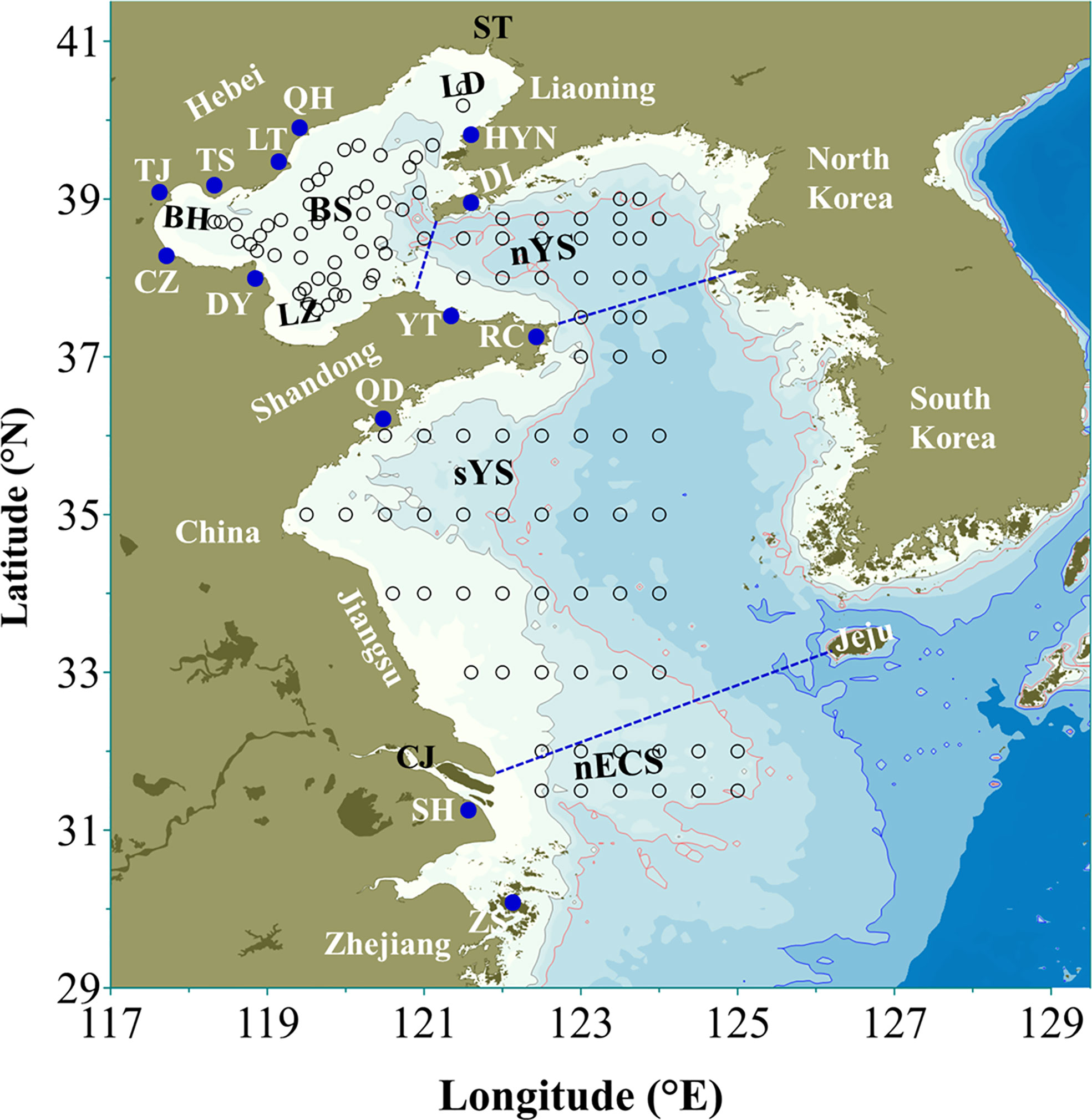
Figure 1 Map of Bohai, Yellow, and northern East China seas. BS: Bohai Sea, nYS: northern Yellow Sea, sYS: southern Yellow Sea, nECS: northern East China Sea, LD: Liaodong Bay, BH: Bohai Bay, LZ: Laizhou Bay, ST: Shuang Taizi River, CJ: Changjiang River, QH: Qin Huangdao, HYN: Hong Yanhe nuclear power plant, LT: Laoting, TS: Tangshan, DL: Dalian, TJ: Tianjin, CZ: Cangzhou, DY: Dongying, YT: Yantai, RC: Rongcheng, QD: Qingdao, SH: Shanghai, ZS: Zhoushan. The blue dots represented the locations of some coastal cities in China mentioned in the study. The black circles denoted the investigation stations of large jellyfish in Bohai, Yellow and northern East China seas in recent years cited from Zhang et al., 2012; Sun et al., 2015 and Wang et al., 2020b.
N. nomurai is considered as a long-distance transport species (Takao and Uye, 2018), distributed in East Asian marginal seas (Uye, 2011; Yoon et al., 2014; Sun et al., 2015; Dong et al., 2018; Kitajima et al., 2020). Its blooms (medusa abundance ≥1 ind·100m−2) occurred in 5 out of 10 years from 2000 to 2009 in the southern Yellow Sea (sYS) and northern East China Sea (nECS), but have been relatively modest from 2010 to 2019 (Sun et al., 2015; Zhao et al., 2016; Sun and Zhang, 2017). The bloom only appeared in 2012, and no blooms (medusa abundance <1 ind·100m−2) occurred in the other years. However, the published total hospital-based records of N. nomurai stings have increased more than threefold since 2012 in Qingdao, Weihai, and Qin Huangdao (>7331 cases) in comparison with that before 2007 (about 1950 cases) (Dong et al., 2010; Niu and Wang, 2014; Wen et al., 2014; Huo et al., 2017). In Liaodong Bay, Bohai Sea, N. nomurai also seriously threatened the Hong Yanhe nuclear power plant, with the maximal catches of medusae near its cooling water intakes reaching 986-1160 kg·h-1 per net in August 2015 (Zhang et al., 2019). Similar to reports from Japanese and Korean waters (Kawahara et al., 2006; Yoon et al., 2008; Uye, 2011), the outbreaks of N. nomurai have also negatively affected fishery production in the coastal sea of China by clogging and destroying nets, decreasing fish catches, increasing the amount of labor required to select fish, and stinging fishermen (Cheng et al., 2004; Zheng et al., 2014). In addition, frequent N. nomurai blooms might influence phytoplankton and micro-zooplankton communities as well as fishery stocks. For example, in June 2012 (a blooming year with medusa abundance ≥1 ind·100m−2), in the southern sYS, the size compositions of ciliates, the dominant group of micro-zooplankton, tended to be smaller at the mature stage (i.e., the period that N. nomurai biomass increased to more than 10 t·km−2) than at the developing stage (i.e., the period that N. nomurai biomass was less than 10 t·km−2) of N. nomurai blooms, while the phytoplankton compositions shifted to be fewer diatoms and cryptophytes but more dinoflagellates (Xiao et al., 2019). In N. nomurai-dense regions of the nECS, the number of fish species and catch per unit effort (CPUE) declined in summer 2003 (a blooming year with medusa abundance ≥1 ind·100m−2) compared to 1999-2001 (non-blooming years with medusa abundance <1 ind·100m−2) (Ding and Cheng, 2005).
In order to reduce economic losses and harm to humans, jellyfish blooms have been actively addressed in many areas in recent years. The early warning measures had been widely reported to indirectly relieve the adverse effects of jellyfish blooms. For example, in the Mediterranean coast, the Medjellyrisk Project created a western and central Mediterranean Basin forecasting platform to foresee the probability of a jellyfish bloom arising based on stranded and near-to-coast jellyfish presence data from Spain, Italy, Tunisia, and Malta in combination with Species Distribution Model (Canepa et al., 2016). It was accessible through a free downloadable mobile app (Medjelly) and the project’s webpage, which could help warn beach users of the risk of stings. On the Great Barrier Reef, Australia, blooms of Irukandji jellyfish were forecast based on wind conditions, which largely coincide with relaxation of the prevailing southeasterly trade winds (Gershwin et al., 2014). To alleviate the damages of N. nomurai blooms to Japanese fishery production, Uye (2014) developed a method of forecasting the annual blooming intensity of N. nomurai in Japan based on on-deck sighting surveys from ferries en route from the sYS and nECS to the Sea of Japan in July. This blooming forecast effectively helps fishermen prepare for possible jellyfish encounters well in advance (Uye, 2014).
Comparatively speaking, jellyfish blooms have also been directly controlled by some affected enterprises. For instance, to address the threat of large jellyfish aggregations (e.g., Chrysaora hysoscella) on salmon aquaculture, the bubble curtains were made by releasing compressed air from a perforated tube at depth, forming a plume of bubbles that entrain the water to create a vertical current as the jellyfish barriers in Ireland (Haberlin et al., 2021). To prevent clogging caused by jellyfish aggregations in cooling water intakes, some local power plants in Japan have built structures such as arresting barriers, channel networks, jellyfish conveyers, and crushers to intercept and channel the influx of massive medusae and facilitate the removal of the entrapped medusae (Kazunari, 2014). For the sake of reducing economic losses from A. coerulea blooms in Korean coastal areas, waterjets and scrapers have been used to remove A. coerulea polyps since 2012. This approach decreased the appearance of medusae in Lake Shihwa for 3 consecutive years (Yoon et al., 2018). To reduce the bycatches of N. nomurai in the Japanese fishery production, various jellyfish excluders such as a bottom trawl fishing gear with an intercepting net and a vent in its main net have been applied (Matsushita et al., 2005; Okino et al., 2009). In particular, during the months of the densest N. nomurai aggregations (October-December) in 2009, some modified set nets, consisting of leading nets with an enlarged mesh size, bypass nets, and a partition net, were also introduced to effectively remove entrapped N. nomurai medusae (usually less than a hundred medusae were trapped per net per day) in fish catches (Uye, 2014). On the whole, various control methods have been used to reduce the impacts of jellyfish blooms. But most of them adopted a strategy of disposing of easily-visible medusae, and few were involved in the other stages of jellyfish life cycle with interspecific specificity. In the coastal sea of China, the management of A. coerulea and N. nomurai blooms has also been carried out accompanied by recent studies on the blooming mechanisms to improve the ecological environment inshore and alleviate the economic losses resulting from such blooms. In this review, we suggest specific control strategies based on the different physio-ecological characteristics of their polyps in the life cycles and discuss some current control measures in China.
Specific control strategies
The controls of jellyfish blooms first need to take into account the jellyfish-centered mechanisms of the outbreaks. The life cycle of scyphozoans is characterized by generational alternation, involving a sexually reproducing medusa and an asexually reproducing benthic polyp (Lucas et al., 2012). The polyp is the key stage that could produce medusae through strobilation to realize the transformation from benthic to pelagic living (Lucas et al., 2012). A. coerulea polyps are found most often on the surfaces of some artificial substrates (e.g., floating docks, concrete platforms) submerged in the harbors and bays along the Chinese coasts (Dong et al., 2018; Feng et al., 2021), as is the case in other regions (Miyake et al., 2002; Ishii and Katsukoshi, 2010; Makabe et al., 2014; Yoon et al., 2018). However, N. nomurai polyps have not yet been recorded, although the areas around estuaries, such as the Changjiang estuary and innermost estuaries of northern Liaodong Bay, are considered to be the major habitats of N. nomurai polyps according to the recorded appearances of early pelagic N. nomurai larvae (i.e., ephyrae, metephyrae and juvenile medusae; Toyokawa et al., 2012; Yoon et al., 2014; Sun et al., 2015; Dong et al., 2018).
On the other hand, A. coerulea and N. nomurai polyps show different asexual reproduction modes and ecophysiological responses to environmental changes including temperature, food supply, and biofouling invasion in the natural environment (Han and Uye, 2010; Wang et al., 2015; Feng et al., 2015a; Feng et al., 2018a), despite both generally having to grow and settle on the surfaces of hard substrates (Kawahara et al., 2006; Lucas et al., 2012). A. coerulea polyps have multiple asexual modes for propagation (Schiariti et al., 2015). They are able to produce podocysts under starvation conditions (Thein et al., 2012; Wang et al., 2015), and multiply rapidly via various buddings at warm temperatures (≥18°C) and abundant food supply (Han and Uye, 2010). However, polyps of N. nomurai only proliferate asexually through podocysts (Kawahara et al., 2006; Feng et al., 2015a; Feng et al., 2015b). Under identical temperature and food conditions, polyps with multiple asexual modes could proliferate to higher densities compared with mono-mode polyps (Schiariti et al., 2014). Moreover, through buddings, A. coerulea polyps on the substrates can expand to spaces not occupied by neighboring biofouling organisms (Figure 2A) and even proliferate on the surfaces of some fouling organisms against their invasion (Feng et al., 2017; Feng et al., 2018a). For example, on the undersurface of a floating dock “Haiou” in Jiaozhou Bay, China, A. coerulea polyps were found to grow on the surfaces of many ascidians, bryozoans, and mussels (e.g., Styela clava, Watersipora subtorquata, and Mytilus galloprovincialis) besides on the areas without macro-fouling organisms (Figures 2B-E) like some other Aurelia spp. (Willcox et al., 2008; Di Camillo et al., 2010; Rekstad et al., 2021). However, in situ N. nomurai polyps on the plastic plates cultivated in Jiaozhou Bay were gradually covered by some neighboring fouling organisms (Figure 2F, Feng et al., 2017; Feng et al., 2018a). Ultimately, they could not survive in the combination of severe biofouling invasion and post-strobilated degeneration in late spring and summer (Feng et al., 2017; Feng et al., 2018a; Feng et al., 2020). Thus, the polyp population following strongly depended on the sexual recruitment of medusae in late summer and autumn (Feng et al., 2018a; Feng et al., 2018b). These different ecophysiological characteristics indicate that the focus of A. coerulea bloom control should be on polyps and biofouling organisms, whereas the focus of N. nomurai bloom control should be on medusae (particularly young medusae) to decrease the sexual reproduction rate.

Figure 2 Aurelia coerulea and Nemopilema nomurai polyps in the field. (A) An A. coerulea polyp on a plate hanging in Jiaozhou Bay, China extending to the unoccupied area by an ascidian Ciona intestinalis via a stolon (red arrow) for proliferation. (B) A. coerulea polyps settled on some areas without macro-fouling organisms (red circles) on the undersurface of the dock “Haiou” in Jiaozhou Bay, China. (C-E) Many A. coerulea polyps growing on the surfaces of an ascidian Styela clava (C), a mussel Mytilus galloprovincialis (D), and a bryozoan Watersipora subtorquata colony (E) on the undersurface of the dock “Haiou” in Jiaozhou Bay, China. (F) A</ca> N. nomurai polyp (red arrow) on a plate hanging in Jiaozhou Bay, China being invaded by an ascidian C. intestinalis. These pictures were photographed with an Olympus waterproof camera.
Control case of A. coerulea blooms in Qingdao middle port harbor
The coastal industries, such as nuclear power plants, in China, have generally addressed local population explosions of A. coerulea by establishing a series of barrier nets and capturing medusae. Recently, the control of A. coerulea blooms by managing A. coerulea polyps and biofouling organisms was successfully conducted in Qingdao Middle Port harbor, China (Figures 3A, B; Feng et al., 2021), where A. coerulea frequently appeared in large numbers from June to July in recent decade (Wang et al., 2012). In 2014, dense A. coerulea polyp colonies were found inhabiting the undersides of some docks at a depth of about 1.8m in Qingdao Middle Port (Feng et al., 2021), indicating that these docks are one of the sources of medusa blooms there.
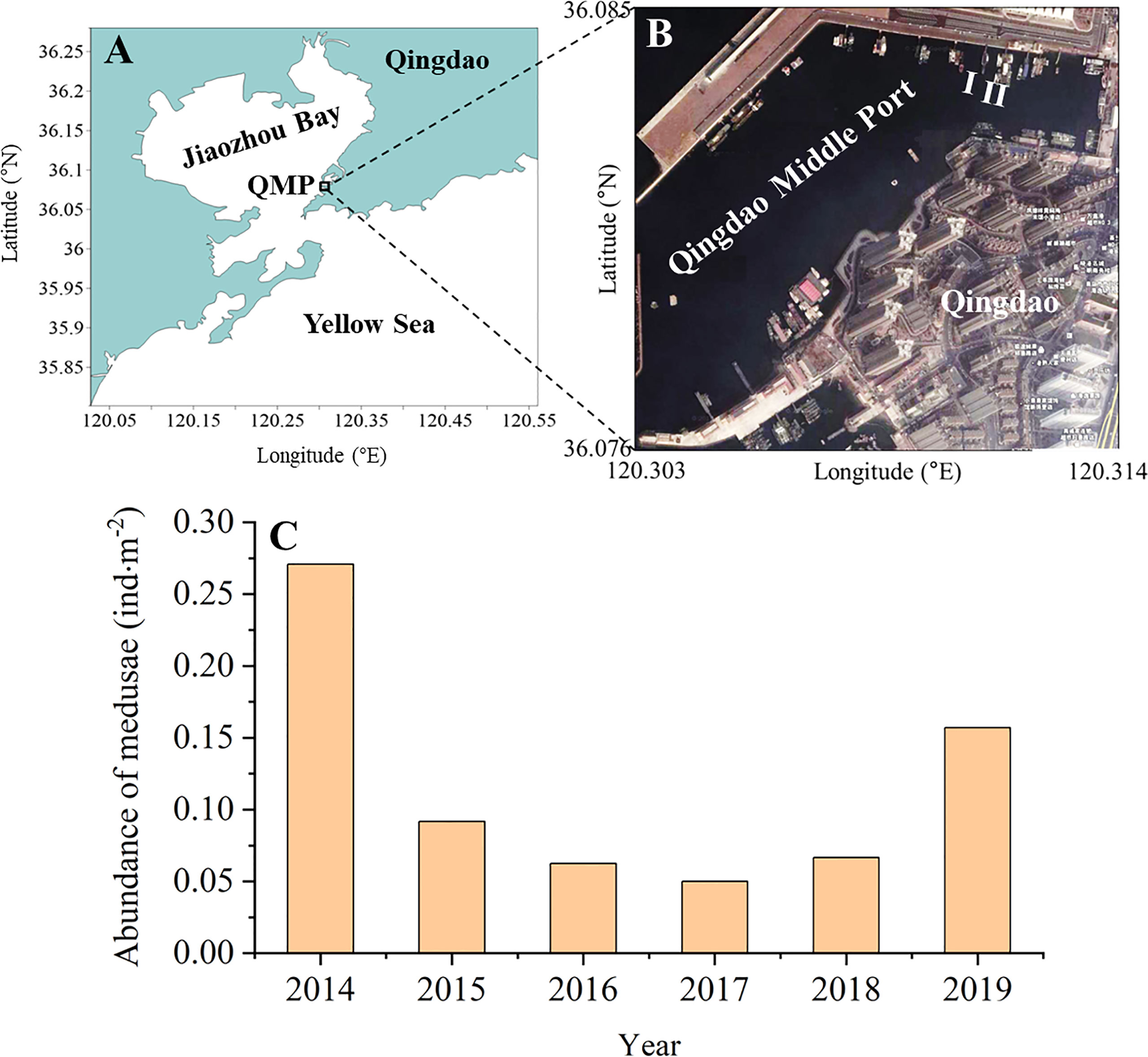
Figure 3 Control of Aurelia coerulea blooms in the Qingdao Middle Port, China (A, B) and changes in the abundance of medusae near the seawater surface in June from 2014 to 2019 (C). Because trawling was banned in the Qingdao Middle Port, the abundance of A. coerulea medusae was monitored through on-deck sighting surveys on the floating docks in the middle of June (the blooming period of medusae) as per the methods described in previous reports (Uye, 2014; Yoon et al., 2014). The number of medusae on both sides of docks was roughly counted by the naked eye from 9:00 to 15:00 in a day. The sighting width of the sea surface was estimated as 4m by a trigonometric function. The abundance of A. coerulea medusae was calculated from the total number of medusae observed on docks divided by the total sighting area of the sea surface. QMP: Qingdao Middle Port.
On the undersurfaces of these floating docks, A. coerulea polyps settled on some areas without macro-fouling organisms as well as on the body surfaces of some fouling organisms (e.g., ascidians, bryozoans, and mussels) (Feng et al., 2021). Two docks (I, II) were the main polyp hotspots, where the average density of polyps reached 2634.63 ± 490.20 ind·m–2 in June 2014 (Feng et al., 2021; Figure 3B). Thus, both docks were selected for the control of A. coerulea blooms in the harbor. Docks I and II were transferred to a nearby shipyard in early April 2015 and 2016, respectively, to avoid the release of massive A. coerulea ephyrae. A. coerulea polyps and biofouling organisms on the undersurfaces of both docks were scraped away completely. Then the modified paint manufactured by the Yantai federal chemical company, China, consisting of alloprene, plasticizer, cuprous oxide, and pigment, was brushed on the undersurface of the dock I in three layers at a thickness of 50-75 μm (Feng et al., 2021). Dock II was left untreated as a control group. After being sun-dried, both docks were then returned to their original positions in July 2015 and 2016, respectively (Feng et al., 2021).
From June 2015 to June 2018, the abundance of A. coerulea medusae observed near the seawater surface in the Qingdao Middle Port declined dramatically by 66–81% compared with 2014, reaching its lowest value in 2017 (Feng et al., 2021; Figure 3C). In June 2019, the medusa abundance was only 42% less than that in 2014. Only a few biofilms developed on the undersurface of Dock I 1 month later (Figure 4A); after 2 years, a few macro-fouling organisms were found. They mainly included barnacles, mussels, and ascidians with densities of 238.10 ± 64.70 ind·m-2, 92.00 ± 75.94 ind·m-2, and 33.55 ± 27.61 ind·m-2, respectively (Feng et al., 2021; Figure 4B). A. coerulea polyps occurred at a very low density of 1.08 ± 3.02 ind·m-2 in the third year post-treatment, when ascidians and mussels were the dominating biofouling organisms (densities of 155.84 ± 87.35 ind·m-2 and 309.52 ± 120.85 ind·m-2, respectively; Figure 4C). In contrast, many biofouling organisms were found on the undersurface of Dock II 1 year later, with ascidians, oysters, and mussels being the main taxa (densities of 179.30 ± 127.07 ind·m-2, 106.06 ± 83.33 ind·m-2, and 55.56 ± 50.44 ind·m-2, respectively; Figure 4D). After 2 years, numerous A. coerulea polyps were discovered in September, the density of which had exceeded the initial level (3512.31 ± 2229.78 ind·m-2) (Feng et al., 2021). The dominating biofouling organisms were ascidians, barnacles, and mussels, with densities of 284.10 ± 170.09 ind·m-2, 252.53 ± 257.32 ind·m-2, and 150.25 ± 201.47 ind·m-2, respectively. Thus, applying modified alloprene paint to the undersurface of Dock I effectively inhibited the resettlement of A. coerulea polyps on there in comparison with Dock II during 3 years. It follows that the long-term control of A. coerulea blooms in the harbor, where polyps massively inhabit the surfaces of movable floating docks, may be accomplished by regular maintenance of these structures using modified alloprene paint.
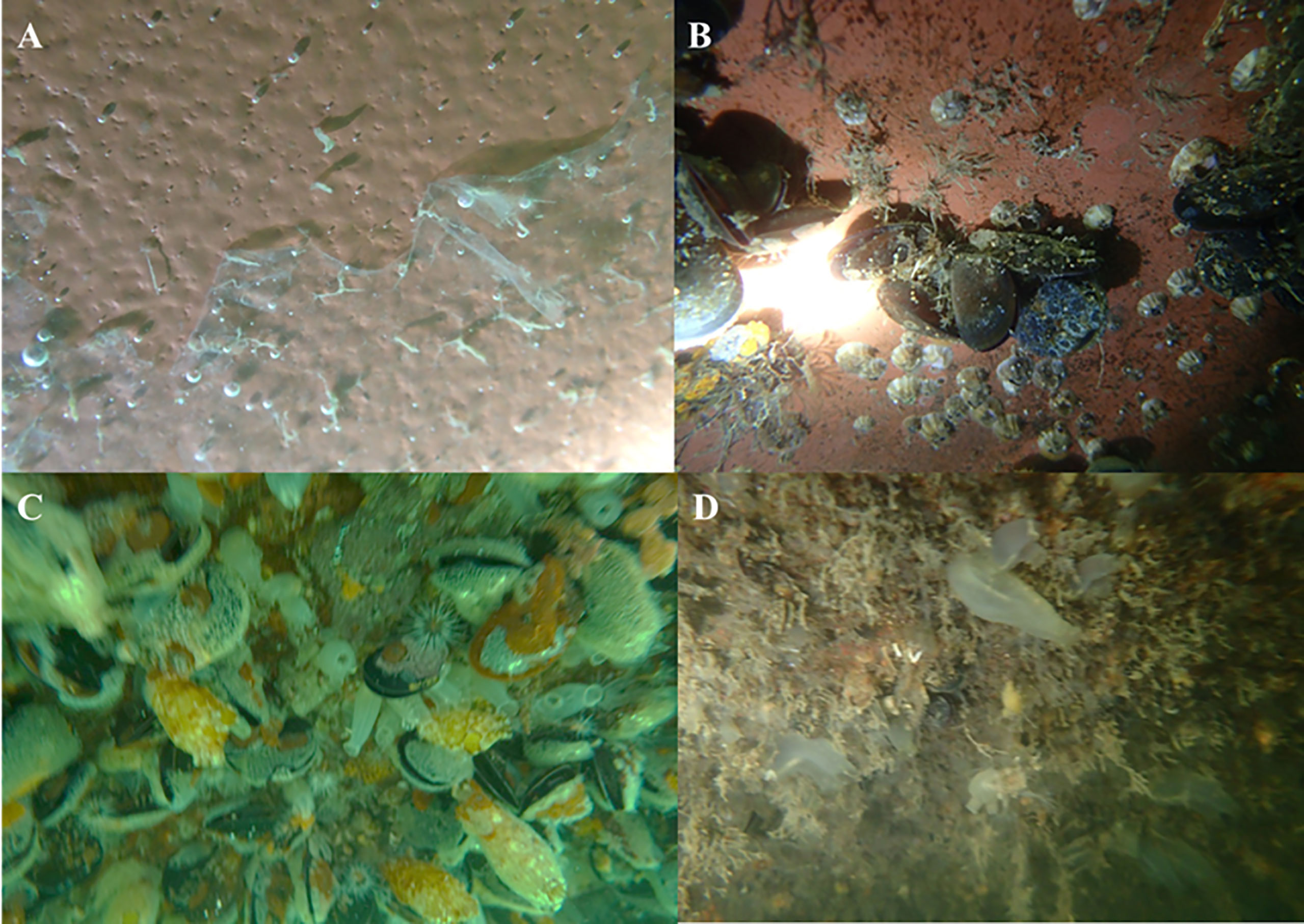
Figure 4 Resettlement photographs of the main fouling organisms including ascidians, mussels ,barnacles, etc. on the undersurfaces of Docks I and II over 3 years following their initial removal. (A) Some thin biofilms appeared on the undersurface of Dock I 1 month later. (B) A</ca> few macro-fouling organisms, such as Mytilus galloprovincialis and Balanus spp., appeared on the undersurface of Dock I after 2 years. (C) A</ca> large number of macro-fouling organisms including M. galloprovincialis, Styela spp., etc. settled on the undersurface of Dock I in the third year. (D) Many macro-fouling organisms (e.g., Ciona intestinalis) resettled on the undersurface of Dock II 1 year later. To monitor the resettlement of A. coerulea polyps and other macro-fouling organisms on the undersurfaces of Docks I and II, 10-15 quadrats, each with an area of 600cm2, on the undersurface of the dock were chosen randomly and photographed via scuba diving using an Olympus waterproof camera in September. The number of A. coerulea polyps and other macro-fouling organisms in each quadrat image was counted. Their density on the undersurface of the dock was calculated from the average of the corresponding number divided by the area of each quadrat.
In addition, A. coerulea polyps are also capable of settling in large numbers on the permanent infrastructures submerged in the sea, such as bridge piers and fixed platforms (Ishii and Katsukoshi, 2010; Yoon et al., 2018). For these immovable constructions, physical methods of eliminating polyps, such as waterjets or mechanical scraping, could be adopted (Yoon et al., 2018; Feng et al., 2021), although their inhibition on the resettlement of A. coerulea polyps on substrate surfaces may be less effective than antifouling paint (Feng et al., 2021). Compared to capturing medusae and establishing a series of arresting nets to solve A. coerulea blooms, methods of eliminating polyps have a lower cost (Yoon et al., 2018), and have the potential to prevent the formation of A. coerulea blooms at their source and decrease the risks of outbreaks in affected areas ahead of time.
Applicable measures to suppress N. nomurai blooms
Resource utilization of N. nomurai medusae
In China, the edible species Rhopilema esculentum is popular seafood with a long history and good market value, which has been an important Chinese jellyfish fishery (Dong et al., 2009). In recent decades, as the production of R. esculentum sharply declined (Dong et al., 2014; Li et al., 2014) and N. nomurai frequently bloomed in the coastal sea of China (Sun and Zhang, 2017; Dong et al., 2018), the Chinese fishermen have started to massively capture N. nomurai medusae in the nearshore waters of many coastal cities, where jellyfish manufacturing districts have been established to process them into new edible jellyfish products alternative to R. esculentum for improving the local fishery economy. For example, in Huangshan village, Qingdao, massive N. nomurai medusae were captured nearby by a kind of specialized jellyfish net with a mesh size of about 18cm from July to October. Then they were made into various jellyfish products (e.g., salted jellyfish, instant jellyfish, and other dishes). Thus, the market share of N. nomurai has exceeded 95% of all jellyfish production in some cities, such as Qingdao and Tangshan (Zheng et al., 2014). According to statistics, the annual catch of N. nomurai has increased continuously in the Bohai and Yellow seas from 2009 to 2013 (Figure 5A; Li et al., 2014). In particular, in Tangshan, Hebei Province, China (Figure 1), since 2009, N. nomurai catches have been more than twice annually compared with those in 2008 (Figure 5B, Zheng et al., 2014). This resource utilization of N. nomurai medusae can conduce to decreasing the number of medusae and their sexual reproduction in the adjacent seas, which may be one of the potential reasons for the decline in N. nomurai blooms in the 2010s as compared with that in the 2000s. Thus, the long-term persistence of this measure might reduce the possibility of N. nomurai blooms in the coastal seas of China.
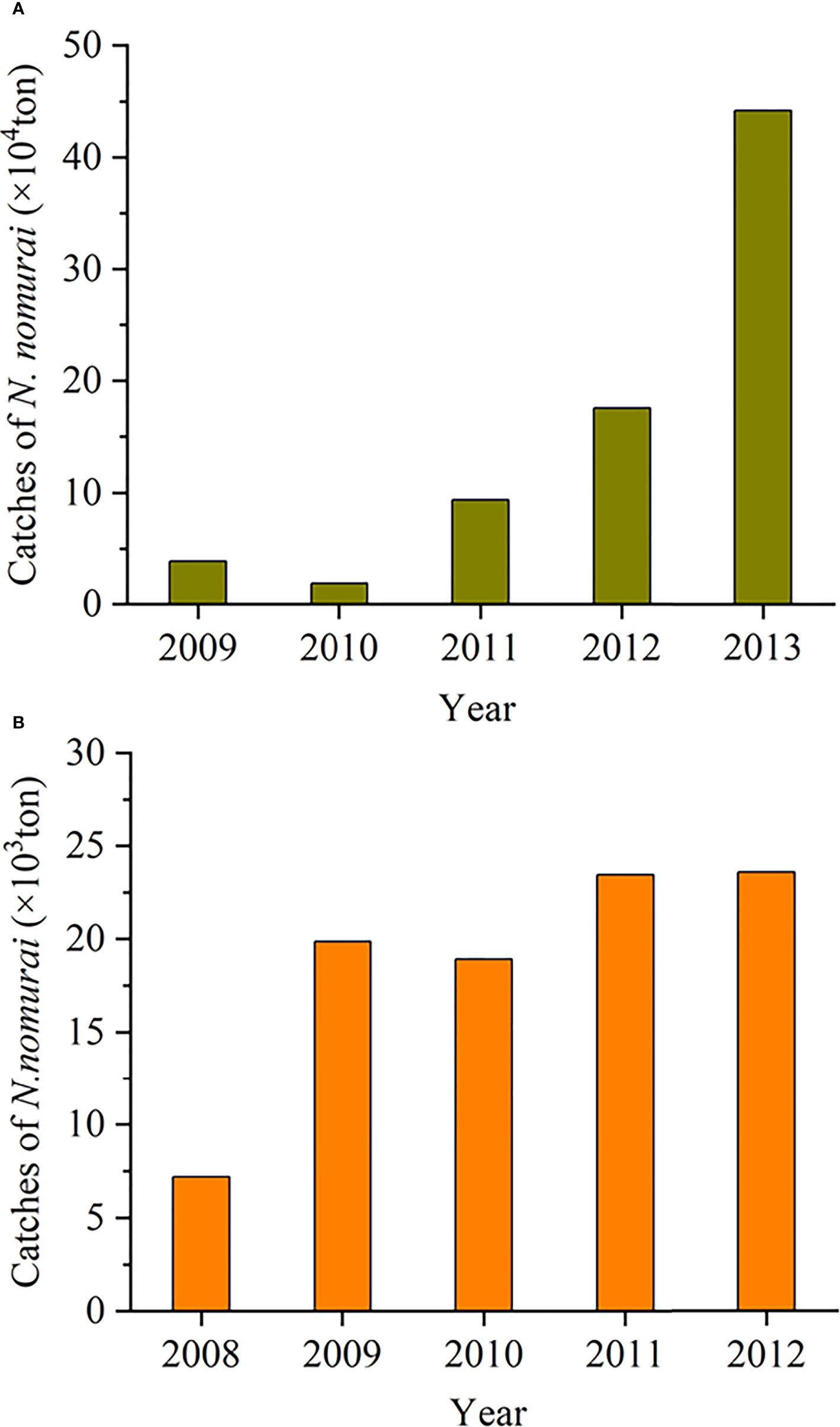
Figure 5 Changes in the annual catches of Nemopilema nomurai in the Bohai and Yellow seas from 2009 to 2013 (A) and in Tangshan, Hebei Province, China from 2008 to 2012 (B). The data are from Report on China’s marine fishing situation, Li et al., 2014, and Zheng et al., 2014. The location of Tangshan is marked in Figure 1.
More severe and earlier summer fishing moratorium
In the marine ecosystem, jellyfish and fish were generally adversaries (Uye, 2011). N. nomurai could compete with fish for copepods (Uye, 2008). They also ingest fish eggs and larvae from species such as Paralichthys olivaceus and Sebastes schlegeli (Liu et al., 2016). In addition, some fish (e.g., Thamnaconus septentrionalis and Pampus argenteus) also prey on N. nomurai (Ding and Cheng, 2005; Tong et al., 2013; Liu et al., 2014). Thus, changes in the governance of fishery resources also affect the abundance of N. nomurai in the Bohai Sea, Yellow Sea, and nECS. To maintain fish stocks and enhance fishery production, a summer fishing moratorium came into effect in 1995. Its duration was gradually prolonged in different regions over the last two decades. For example, in the 27-35°N areas of Chinese coastal seas, which include the major birthplaces and high-density areas (31.5-35°N) of N. nomurai in the sYS and nECS (Zhang et al., 2012; Sun et al., 2015; Zuo et al., 2016), the summer fish moratorium extended from July 1 to August 31 in 1995, then from June 16 to September 15 in 1998, and from June 1 to September 16 in 2009 (Chen and Bao, 2010). In Bohai Sea, the summer fish moratorium also changed from June 16 to September 1 in 2004 into from June 1 to September 1 in 2009 (Hu, 2020). Since 2017, the longest summer fishing moratorium was imposed with tougher enforcement from May 1 to September 16 in the 26.5-35°N areas and from May 1 to September 1 in the north of 35°N, respectively (Yan et al., 2019a; Hu, 2020). The advancement of the moratorium onset time to May 1 might play an important role in regulating both the biomass of N. nomurai and the fishery resource. When the fishing ban is implemented later than early June, the overfishing of adult and juvenile fish (e.g., P. argenteus, Larimichthys polyactis, and Trichiurus lepturus; Chen and Bao, 2010; Wang, 2012) might create more suitable conditions in spring (e.g., abundant zooplankton bait and reduced fish predation rate) for the development of early pelagic N. nomurai larvae, which are weakly competitive compared with medusae. Therefore, these larvae can grow well into medusae within 1 month (Kawahara et al., 2006; Sun et al., 2015). When the fishing ban began to be implemented more rigorously and forcefully in early May 2017, these conditions favorable to early pelagic N. nomurai larvae may reduce as the annual fish catches decreased (Figure 6) and fishery resources were forecast to improve during the summer fishing moratorium (Yan et al., 2019a; Yan et al., 2019b; Xu et al., 2022). Thus this might contribute to the weakening of outbreaks of N. nomurai in these years.
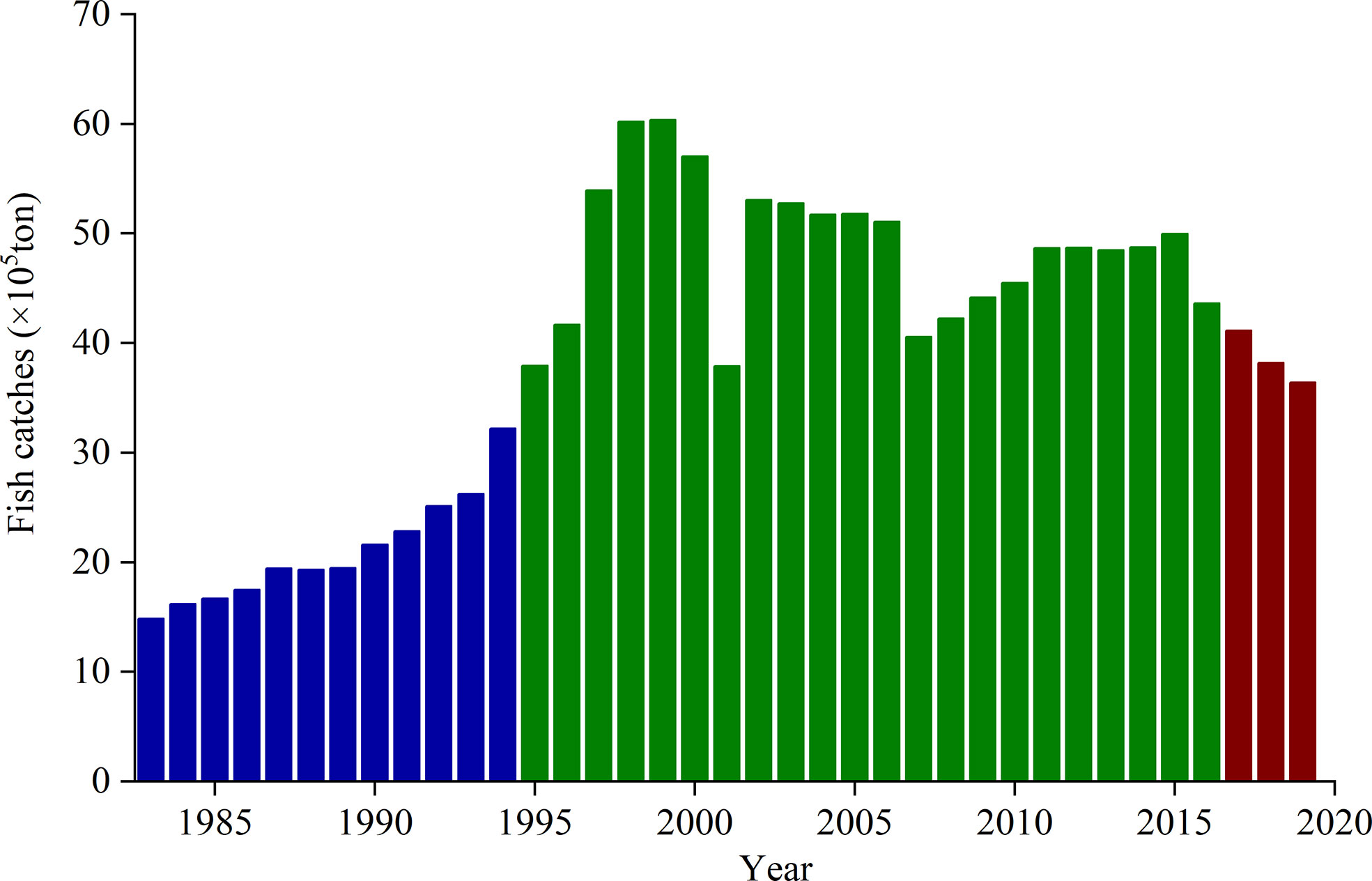
Figure 6 Changes in annual fish catches in Bohai, Yellow, and northern East China seas. The data are from Chinese fishery statistics, which were collected from Liaoning, Hebei, Tianjin, Shandong, Jiangsu, Shanghai, and Zhejiang. The blue column reflects the annual fish catches before the summer fishing moratorium was established. The green column represents the annual fish catches from 1995 to 2016, when the summer fishing moratorium started after early June. The red column represents the annual fish catches after 2017, when the fishing ban was enforced more rigorously and forcefully on May 1.
Other experimental control measures
Some additional physical and chemical measures have been examined experimentally in the laboratory to solve the problems of jellyfish blooms along the Chinese coasts. Liu et al. (2017) designed a monolithic trawl net 5m long with a mouth of 2m×2m, at the bottom of which a circular steel ring 50cm in diameter with 16 crossed steel wires inside was installed. The medusae are captured and crushed while the net is towed at sea. However, this equipment awaits further testing in terms of its feasibility and efficiency in destroying medusae. Some drugs, such as “tea saponin”, comprising saponins extracted from plants, have been reported to be capable of killing the polyps, ephyrae, or medusae of jellyfish at low concentrations (Dong et al., 2017; Liu et al., 2017). However, because of their lethal effects on many microorganisms, algae, and zooplankton (Liu et al., 2017), the method that treats jellyfish blooms by using a large amount of the drugs, might produce serious secondary damage to the marine ecosystem. Therefore, these drugs have limitations concerning field application.
Prospects
It is a general perception that coastal anthropogenic stressors (e.g., climate change, eutrophication, increase of man-made structures, and overfishing) are responsible for jellyfish blooms (Richardson et al., 2009; Purcell, 2012; Uye, 2014). However, the particular causes of medusa outbreaks differ among species (Pitt et al., 2018). For example, the frequent outbreaks of A. coerulea are closely associated with the increase of artificial substrates in the coastal sea of China (Feng et al., 2017; Dong et al., 2018; Feng et al., 2021). Therefore, to decrease A. coerulea blooms, government administration policies relevant to inshore artificial constructions need to be formulated. For example, agencies that manage marine development should require the regular application of antifouling paint to the undersurfaces of floating constructions and the elimination of A. coerulea polyps on the surfaces of fixed platforms in the harbors. In contrast, the control of N. nomurai blooms may require an expansion of medusa utilization as a food source or as other beneficial products and strict enforcement of the current summer fishing moratorium. Thus, for the health of the marine ecosystem in China, reasonable management of these two jellyfish blooms will require a long-term commitment and perseverance.
Author contributions
SF carried out the field investigation, and data analysis, and drafted the manuscript. SS, CL, and FZ contributed to the project administration, conceptualization, and paper editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (grant no. 42176136, 42130411, and 42076166), the Natural Science Foundation of Shandong Province (grant no. ZR2020KE047), Mount Tai Scholar Climbing Plan to SS, and Huiquan Scholar to SF.
Acknowledgments
We are very grateful to Pro. Jiansheng Li for providing the data on N. nomurai catches in the Bohai and Yellow seas for this study. We also thank Associate Pro. Huilian Liu for helping us identify the species of fouling organisms.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bayha M. K., Graham W. M. (2014) . “Nonindigenous marine jellyfish: Invasiveness, invasibility and impacts,” in Jellyfish blooms. Eds. Lucas C. H., Pitt K. A., (Dordrecht, Heidelberg, New York, London:Springer) p 45–p 77. doi: 10.1007/978-94-007-7015-7
Canepa A., Fuentes M., Marambio M., Lopez L., Deidun A., Yahia O., et al. (2016). “Forecasting jellyfish blooms in the Mediterranean Sea: The med-JellyRisk project,” in 5th International Jellyfish Bloom Symposium Barcelona Spain: Institut de Ciències del Mar - CSIC.
Cheng J., Li S., Ding F., Yan L. (2004). Primary analysis on the jellyfish bloom and its cause in the East China Sea and the yellow Sea. Modern Fish. Inform. 19, 10–12.
Di Camillo C. G. D., Betti F., Bo M., Martinelli M., Puce S., Bavestrello G. (2010). Contribution to the understanding of seasonal cycle of Aurelia aurita (Cnidaria: Scyphozoa) scyphopolyps in the northern Adriatic Sea. J. Mar. Biol. Assoc. UK 90, 1105–1110. doi: 10.1017/S0025315409000848
Ding F. Y., Cheng J. H. (2005). The aanalysis on fish stock characteristics in the distribution areas of Large jellyfish during summer and autumn in the East China Sea region. Mar. Fish. 27, 120–128.
Dong Z. (2019). “Blooms of the moon jellyfish aurelia: Causes, consequences and controls,” in World seas: An environmental evaluation: Volume III: Ecological issues and environmental impacts, (London, San Diego, Cambridge and Oxford:Elsevier) 163–170. doi: 10.1016/B978-0-12-805052-1.00008-5
Dong J., Jiang L., Tan K., Liu H., Purcell J. E., Li P., et al. (2009). Stock enhancement of the edible jellyfish (Rhopilema esculentum kishinouye) in liaodong bay, China: A review. Hydrobiologia 616, 113–118. doi: 10.1007/s10750-008-9592-9
Dong Z., Lie Z., Liu D. (2015). Genetic characterization of the scyphozoan jellyfish Aurelia spp. in Chinese coastal waters using mitochondrial markers. Biochem. Syst. Ecol. 60, 15–23. doi: 10.1016/j.bse.2015.02.018
Dong Z., Liu D., Keesing J. K. (2010). Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 60, 954–963. doi: 10.1016/j.marpolbul.2010.04.022
Dong Z., Liu D., Keesing J. K. (2014). “Contrasting trends in populations of rhopilema esculentum and aurelia aurita in Chinese waters,” in Jellyfish blooms. Eds. Pitt K. A., Lucas C. H., (Dordrecht, Heidelberg, New York, London:Springer) 207–217. doi: 10.1007/978-94-007-7015-7
Dong Z., Sun T., Liang L., Wang L. (2017). Effect of tea saponin on ephyrae and polyps of the moon jellyfish Aurelia Sp.1. PloS One 12, 1–9. doi: 10.1371/journal.pone.0182787
Dong J., Wang B., Duan Y., Yoon W. D., Wang A., Liu X., et al. (2018). Initial occurrence, ontogenic distribution-shifts and advection of Nemopilema nomurai (Scyphozoa: Rhizostomeae) in liaodong bay, China from 2005-2015. Mar. Ecol. Prog. Ser. 591, 185–197. doi: 10.3354/meps12272
Dong Z., Wang L., Sun T., Liu Q., Sun Y. (2018). Artificial reefs for Sea cucumber aquaculture confirmed as settlement substrates of the moon jellyfish Aurelia coerulea. Hydrobiologia 818, 223–234. doi: 10.1007/s10750-018-3615-y
Duarte C. M., Pitt K. A., Lucas C. H., Purcell J. E., Uye S., Robinson K., et al. (2013). Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 11, 91–97. doi: 10.1890/110246
Feng S., Lin J., Sun S., Zhang F., Li C. (2018b). Hyposalinity and incremental microzooplankton supply in early-developed Nemopilema nomurai polyp survival, growth, and podocyst reproduction. Mar. Ecol. Prog. Ser. 591, 117–128. doi: 10.3354/meps12204
Feng S., Lin J., Sun S., Zhang F., Li C., Xian W. (2020). Combined effects of seasonal warming and hyposalinity on strobilation of Nemopilema nomurai polyps. J. Exp. Mar. Biol. Ecol. 524, 151316. doi: 10.1016/j.jembe.2020.151316
Feng S., Wang S., Sun S., Zhang F., Zhang G., Liu M., et al. (2018a). Strobilation of three scyphozoans (Aurelia coerulea, nemopilema nomurai, and Rhopilema esculentum) in the field at jiaozhou bay, China. Mar. Ecol. Prog. Ser. 591, 141–153. doi: 10.3354/meps12276
Feng S., Wang S. W., Zhang G. T., Sun S., Zhang F. (2017). Selective suppression of In situ proliferation of scyphozoan polyps by biofouling. Mar. pollut. Bull. 114, 1046–1056. doi: 10.1016/j.marpolbul.2016.10.062
Feng S., Zhang F., Lin J., Wang M., Wang S., Sun S. (2021). Method for controlling Aurelia spp. blooms in harbors. United States Patent. Patent number: US10959419B2. Pg. 1–12.
Feng S., Zhang F., Sun S., Wang S., Li C. (2015a). Effects of duration at low temperature on asexual reproduction in polyps of the scyphozoan Nemopilema nomurai (Scyphozoa: Rhizostomeae). Hydrobiologia 754, 97–111. doi: 10.1007/s10750-015-2173-9
Feng S., Zhang G., Sun S., Zhang F., Wang S. (2015b). Effects of temperature regime and food supply on asexual reproduction in Cyanea nozakii and Nemopilema nomurai. Hydrobiologia 754, 201–214. doi: 10.1007/s10750-015-2279-0
Gershwin L., Condie S. A., Mansbridge J. V., Richardson A. J. (2014). Dangerous jellyfish blooms are predictable. J. R. Soc Interface 11, 20131168. doi: 10.1098/rsif.2013.1168
Graham W. M., Gelcich S., Robinson K. L., Duarte C. M., Brotz L., Purcell J. E., et al. (2014). Linking human well-being and jellyfish: Ecosystem services, impacts and social responses. Front. Ecol. Environ. 12, 515–523. doi: 10.1890/130298
Haberlin D., McAllen R., Doyle T. K. (2021). Field and flume tank experiments investigating the efficacy of a bubble curtain to keep harmful jellyfish out of finfish pens. Aquaculture 531, 735915. doi: 10.1016/j.aquaculture.2020.735915
Han C. H., Uye S. I. (2010). Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita S.L. Plankton Benthos Res. 5, 98–105. doi: 10.3800/pbr.5.98
Hu Z. (2020). Evaluating the effects of summer fishing moratorium in the bohai Sea and its influence on the population dynamics of Japanese anchovy (Engraulis japonicus) (Shanghai, China:Shanghai Ocean University) .
Huo S., Tian Y., Zhang Y., Su X., Liu J. (2017). Epidemiological analysis of jellyfish stings in 2577 cases in qinhuangdao. J. Hebei Med. Univ. 38, 1141–1157.
Ishii H., Katsukoshi K. (2010). Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the innermost part of Tokyo bay. J. Oceanogr. 66, 329–336. doi: 10.1007/s10872-010-0029-5
Kawahara M., Uye S., Ohtsu K., Izumi H. (2006). Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar. Ecol. Prog. Ser. 307, 161–173. doi: 10.3354/meps307161
Kazunari K. (2014). Manual of countermeasures against biofoulings in power plants. Japan Nucl. Power Eng. Assoc., (Keisei Corporation publisher) 1–356.
Kitajima S., Hasegawa T., Nishiuchi K., Kiyomoto Y., Taneda T., Yamada H. (2020). Temporal fluctuations in abundance and size of the giant jellyfish Nemopilema nomurai medusae in the northern East China Sea 2006-2017. Mar. Biol. 167, 75. doi: 10.1007/s00227-020-03682-1
Li J. S., Ling J. Z., Cheng J. H. (2014). On utilization of two edible macro-jellyfish and evaluation of the biomass of Nemopilema nomurai in China Sea. Mar. Fish. 36, 202–207.
Li J., Liu X., Zhang J., Meng Y. (2017). Research on marine biological detection technology to improve the safety of cooling water source in nuclear power plants. Electrical Secur. 10, 32–37.
Liu Y., Song L., Song Y. G., Yang S. (2017). Studies on the emergency disposal technologies of giant jellyfish blooms. Hebei Fish 2, 6–10.
Liu C., Zhuang Z., Chen S., Liu C., Zhao P., Chen Z. (2014). Navodon septentrionalis predation of four species of giant jellyfish. Fish. Pro. 35, 30–38.
Liu C., Zhuang Z., Chen S., Yan J. (2016). Predation of three juvenile scyphomedusa species to Paralichthys olivaceus and Sebastes schlegeli larvae. Chin. Fish. Sci. 23, 436–446.
Lucas C. H., Graham W. M., Widmer C. (2012). Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 63, 133–196. doi: 10.1016/B978-0-12-394282-1.00003-X
Makabe R., Furukawa R., Takao M., Uye S. (2014). Marine artificial structures as amplifiers of Aurelia aurita S.L. blooms: A case study of a newly installed floating pier. J. Oceanogr. 70, 447–455. doi: 10.1007/s10872-014-0249-1
Matsushita Y., Honda N., Kawamura S. (2005). Design and tow trial of JET (Jellyfish excluder for towed fishing gear). Nippon Suisan Gakkaishi 71, 965–967. doi: 10.2331/suisan.71.965
Miyake H., Terazaki M., Kakinuma Y. (2002). On the polyps of the common jellyfish Aurelia aurita in Kagoshima bay. J. Oceanogr. 58, 451–459. doi: 10.1023/A:1021628314041
Niu S. J., Wang W. L. (2014). Clinical analysis of 1136 stinging cases by Nemopilema nomurai. Clin. Focus 29, 188–189.
Okino A., Murayama T., Inoue Y. (2009). Development of fishing gear to exclude and release giant jellyfishes from an offshore trawl net. Nippon Suisan Gakkaishi 75, 6–18. doi: 10.2331/suisan.75.6
Omori M., Kitamura M. (2004). Taxonomic review of three Japanese species of edible jellyfish (Schyphozoa: Rhizostomeae). Plankton Biol. Ecol. 51, 36–51.
Pitt K., Lucas C. H., Condon R. H., Duarte C. M., Stewart-Koster B. (2018). Claims that anthropogenic stressors facilitate jellyfish blooms have been amplified beyond the available evidence: A systematic review. Front. Mar. Sci. 451. doi: 10.3389/fmars.2018.00451
Purcell J. E. (2012). Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu. Rev. Mar. Sci. 4, 209–235. doi: 10.1146/annurev-marine-120709-142751
Purcell J. E., Uye S., Lo W. T. (2007). Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 350, 153–174. doi: 10.3354/meps07093
Rekstad M. E., Majaneva S., Borgersen Å. L., Aberle N. (2021). Occurrence and habitat characteristics of Aurelia sp. polyps in a high-latitude fjord. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.684634
Ren L. (2009). Jellyfish attacked on a power plant in Qingdao possibly resulting in the shutdown or affecting the power supply of 1/3 areas. Qingdao News 2009-7–8. (in Chinese).
Richardson A. J., Bakun A., Hays G. C., Gibbons M. J. (2009). The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 24, 312–322. doi: 10.1016/j.tree.2009.01.010
Schiariti A., Melica V., Kogovšek T., Malej A. (2015). Density−dependent effects control the reproductive strategy and population growth of Aurelia aurita S.L. scyphistomae. Mar. Biol. 162, 1665–1672. doi: 10.1007/s00227-015-2704-y
Schiariti A., Morandini A. C., Jarms G., von Glehn Paes R., Franke S., Mianzan H. (2014). Asexual reproduction strategies and blooming potential in scyphozoa. Mar. Ecol. Prog. Ser. 510, 241–253. doi: 10.3354/meps10798
Scorrano S., Aglieri G., Boero F., Dawson M. N., Piraino S. (2017). Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. Lond. 180 (2), 243–267. doi: 10.1111/zoj.12494
Sun S., Zhang F. (2017). “Spatio-temporal variations of biomass and bloom conditions in regional seas: Chinese coast,” in Report of working group 26 on jellyfish blooms around the north pacific rim: Causes and consequences (Edited by Shin-ichi Uye and Richard D. Brodeur), Sidney, Canada: North Pacific Marine Science Organization., (Sidney, Canada).107–116.
Sun S., Zhang F., Li C., Wang S., Wang M., Tao Z., et al. (2015). Breeding places, population dynamics, and distribution of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in the yellow Sea and the East China Sea. Hydrobiologia 754, 59–74. doi: 10.1007/s10750-015-2266-5
Takao M., Uye S. I. (2018). Effects of low salinity on the physiological ecology of planulae and polyps of scyphozoans in the East Asian marginal seas: Potential impacts of monsoon rainfall on Medusa population size. Hydrobiologia 815, 165–176. doi: 10.1007/s10750-018-3558-3
Thein H., Ikeda H., Uye S. (2012). The potential role of podocysts in perpetuation of the common jellyfish Aurelia aurita S.L. (Cnidaria: Scyphozoa) in anthropogenically perturbed coastal waters. Hydrobiologia 690, 157–167. doi: 10.1007/s10750-012-1045-9
Tong Y., Li Z., Guo X. (2013). Feeding ecology of Pampus argenteus in the southern waters of the yellow Sea. Prog. Fish. Sci. 34, 19–28.
Toyokawa M., Shibata M., Cheng J., Li H., Ling J. Z., Lin N., et al. (2012). First record of wild ephyrae of the giant jellyfish Nemopilema nomurai. Fish. Sci. 78, 1213–1218. doi: 10.1007/s12562-012-0550-0
Uye S. I. (2008). Blooms of the giant jellyfish Nemopilema nomurai: A threat to the fisheries sustainability of the East Asian marginal seas. Plankton Benthos Res. 3, 125–131. doi: 10.3800/pbr.3.125
Uye S. I. (2011). Human forcing of the copepod-Fish-Jellyfish triangular trophic relationship. Hydrobiologia 666, 71–83. doi: 10.1007/s10750-010-0208-9
Uye S. I. (2014). “The giant jellyfish nemopilema nomurai in East Asian marginal seas,” in Jellyfish blooms. Eds. Pitt K. A., Lucas C. H. (Heidelberg: Springer), p 185–p 205. doi: 10.1007/978-94-007-7015-7
Wan A, Zhang G (2012). Annual Occurrence of Moon Jellyfish Aurelia Sp.1 in the Jiaozhou Bay and Its Impacts on Zooplankton Community. Oceanol. et Limnol. Sin. 43(3):494–501.
Wang X. Y. (2012). Marine ecology fishing: the optimization direction of summer closed fishing in China. Chin. Fish. Econ. 2, 16–22.
Wang P., Zhang F., Sun S., Wang W., Wan A., Li C. (2020a). Experimental clearance rates of Aurelia coerulea ephyrae and medusae, and the predation impact on zooplankton in jiaozhou bay. J. Oceanol. Limnol. 38, 1256–1269. doi: 10.1007/s00343-020-0024-7
Wang S., Zhang G., Sun S., Wang Y., Zhao Z. (2012). Population dynamics of three scyphozoan jellyfish species during summer of 2011 in jiaozhou bay. Oceanol. Limnol. Sin. 43, 471–479.
Wang P., Zhang F., Sun S., Yang T. (2020b). Distribution of giant jellyfish in the bohai Sea in June 2018. Oceanol. Limnol. Sin. 51 (1), 85–94.
Wang Y. T., Zheng S., Sun S., Zhang F. (2015). Effect of temperature and food type on asexual reproduction in Aurelia Sp.1 polyps. Hydrobiologia 754, 169–178. doi: 10.1007/s10750-014-2020-4
Wen Z. Y., Liu J. L., Kang X. H., Zhang Q., Wu Q. (2014). Clinical analysis to 630 stinging cases of children by Nemopilema nomurai. Shandong Med. 54, 53–55.
Willcox S., Moltschaniwskyj N. A., Crawford C. M. (2008). Population dynamics of natural colonies of Aurelia sp. scyphistomae in Tasmania, Australia. Mar. Biol. 154, 661–670. doi: 10.1007/s00227-008-0959-2
Xiao W., Zeng Y., Liu X., Huang X., Chiang K. P., Mi T. Z., et al. (2019). The impact of giant jellyfish Nemopilema nomurai blooms on plankton communities in a temperate marginal Sea. Mar. pollut. Bull. 149, 110507. doi: 10.1016/j.marpolbul.2019.110507
Xu L., Song P., Xie B., Huang L., Li Y., Zheng X., et al. (2022). Estimating the impact of a seasonal fishing moratorium on the East China Sea ecosystem from 1997 to 2018. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.865645
Yan L., Liu Z., Jin Y., Cheng J. (2019a). Effects of prolonging the trawl net summer fishing moratorium period in the East China Sea on the conservation of fishery resources. J. Fishery Sci. China 26, 118–123. doi: 10.3724/SP.J.1118.2019.18243
Yan L., Liu Z., Jin Y., Cheng J. (2019b). Effects of prolonging summer fishing moratorium in the East China Sea on the increment of fishery resources. Mar. Fisheries 41 (5), 513–519.
Yoon W., Chae J., Koh B.-S., Han C. (2018). Polyp removal of a bloom forming jellyfish, Aurelia coerulea, in Korean waters and its value evaluation. Ocean Sci. J. 53, 499–507. doi: 10.1007/s12601-018-0015-1
Yoon W. D., Lee H. E., Han C., Chang S. J., Lee K. (2014). Abundance and distribution of Nemopilema nomurai (Scyphozoa, rhizostomeae) in Korean waters in 2005-2013. Ocean Sci. J. 49, 183–192. doi: 10.1007/s12601-014-0018-5
Yoon W. D., Yang J. Y., Shim M. B., Kang H. K. (2008). Physical processes influencing the occurrence of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around jeju island, Korea. J. Plankton Res. 30, 251–260. doi: 10.1093/plankt/fbm102
Zhang C., Guan C., Xu P., Liu G., Xu Q., Ye J., et al. (2019). Analysis on risk organisms for the cold source water of nuclear power plant in the Eastern waters of liaodong bay. Mar. Environ. Sci. 38, 41–45.
Zhang F., Sun S., Jin X. S., Li C. L. (2012). Associations of Large jellyfish distributions with temperature and salinity in the yellow Sea and East China Sea. Hydrobiologia 690, 81–96. doi: 10.1007/s10750-012-1057-5
Zhao L., Li X., Zhang F. (2016). On relationship between inter-annual variation in water temperature regime and Nemopilema nomurai abundance in the yellow Sea. Oceanol. Limnol. Sin. 47, 564–571.
Zheng X. R., Li Y., Rao Q., Mu J. (2014). Causes of giant jellyfish in the coastal Sea of qin huangdao, China. Hebei Fish. 2, 16–20.
Keywords: jellyfish blooms, control, biofouling organisms, polyps, medusae
Citation: Feng S, Sun S, Li C and Zhang F (2022) Controls of Aurelia coerulea and Nemopilema nomurai (Cnidaria: Scyphozoa) blooms in the coastal sea of China: Strategies and measures. Front. Mar. Sci. 9:946830. doi: 10.3389/fmars.2022.946830
Received: 18 May 2022; Accepted: 09 September 2022;
Published: 27 September 2022.
Edited by:
Yuanyuan Feng, Shanghai Jiao Tong University, ChinaReviewed by:
Jinho Chae, Marine Environmental Research and Information Laboratory, South KoreaDoojin Hwang, Chonnam National University, South Korea
Copyright © 2022 Feng, Sun, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Sun, c3Vuc29uZ0BxZGlvLmFjLmNu; Fang Zhang, emhhbmdmYW5nQHFkaW8uYWMuY24=
 Song Feng
Song Feng Song Sun1,2,3,4*
Song Sun1,2,3,4* Chaolun Li
Chaolun Li Fang Zhang
Fang Zhang